Abstract
Background
Telangiectasias (spider veins) and reticular veins on the lower limbs are very common, increase with age, and have been found in 41% of women. The cause is unknown and the patients may be asymptomatic or can report pain, burning or itching. Treatments include sclerotherapy, laser, intense pulsed light, microphlebectomy and thermoablation, but none is established as preferable.
Objectives
To assess the effects of sclerotherapy, laser therapy, intensive pulsed light, thermocoagulation, and microphlebectomy treatments for telangiectasias and reticular veins.
Search methods
The Cochrane Vascular Information Specialist searched the Cochrane Vascular Specialised Register, CENTRAL, MEDLINE, Embase, AMED and CINAHL databases, and the World Health Organization International Clinical Trials Registry Platform and ClinicalTrials.gov trials registers to 16 March 2021. We undertook additional searches in LILACS and IBECS databases, reference checking, and contacted specialists in the field, manufacturers and study authors to identify additional studies.
Selection criteria
We included randomised controlled trials (RCTs) and quasi‐RCTs that compared treatment methods such as sclerotherapy, laser therapy, intensive pulsed light, thermocoagulation, and microphlebectomy for telangiectasias and reticular veins in the lower limb. We included studies that compared individual treatment methods against placebo, or that compared different sclerosing agents, foam or laser treatment, or that used a combination of treatment methods.
Data collection and analysis
Three review authors independently performed study selection, extracted data, assessed risks of bias and assessed the certainty of evidence using GRADE. The outcomes of interest were resolution or improvement (or both) of telangiectasias, adverse events (including hyperpigmentation, matting), pain, recurrence, time to resolution, and quality of life.
Main results
We included 3632 participants from 35 RCTs. Studies compared a variety of sclerosing agents, laser treatment and compression. No studies investigated intensive pulsed light, thermocoagulation or microphlebectomy. None of the included studies assessed recurrence or time to resolution. Overall the risk of bias of the included studies was moderate. We downgraded the certainty of evidence to moderate or low because of clinical heterogeneity and imprecision due to the wide confidence intervals (CIs) and few participants for each comparison.
Any sclerosing agent versus placebo
There was moderate‐certainty evidence that sclerosing agents showed more resolution or improvement of telangiectasias compared to placebo (standard mean difference (SMD) 3.08, 95% CI 2.68 to 3.48; 4 studies, 613 participants/procedures), and more frequent adverse events: hyperpigmentation (risk ratio (RR) 11.88, 95% CI 4.54 to 31.09; 3 studies, 528 participants/procedures); matting (RR 4.06, 95% CI 1.28 to 12.84; 3 studies, 528 participants/procedures). There may be more pain experienced in the sclerosing‐agents group compared to placebo (SMD 0.70, 95% CI 0.06 to 1.34; 1 study, 40 participants; low‐certainty evidence).
Polidocanol versus any sclerosing agent
There was no clear difference in resolution or improvement (or both) of telangiectasias (SMD 0.01, 95% CI −0.13 to 0.14; 7 studies, 852 participants/procedures), hyperpigmentation (RR 0.94, 95% CI 0.62 to 1.43; 6 studies, 819 participants/procedures), or matting (RR 0.82, 95% CI 0.52 to 1.27; 7 studies, 859 participants/procedures), but there were fewer cases of pain (SMD −0.26, 95% CI −0.44 to −0.08; 5 studies, 480 participants/procedures) in the polidocanol group. All moderate‐certainty evidence.
Sodium tetradecyl sulphate (STS) versus any sclerosing agent
There was no clear difference in resolution or improvement (or both) of telangiectasias (SMD −0.07, 95% CI −0.25 to 0.11; 4 studies, 473 participants/procedures). There was more hyperpigmentation (RR 1.71, 95% CI 1.10 to 2.64; 4 studies, 478 participants/procedures), matting (RR 2.10, 95% CI 1.14 to 3.85; 2 studies, 323 participants/procedures) and probably more pain (RR 1.49, 95% CI 0.99 to 2.25; 4 studies, 409 participants/procedures). All moderate‐certainty evidence.
Foam versus any sclerosing agent
There was no clear difference in resolution or improvement (or both) of telangiectasias (SMD 0.04, 95% CI −0.26 to 0.34; 2 studies, 187 participants/procedures); hyperpigmentation (RR 2.12, 95% CI 0.44 to 10.23; 2 studies, 187 participants/procedures) or pain (SMD −0.10, 95% CI −0.44 to 0.24; 1 study, 147 participants/procedures). There may be more matting using foam (RR 6.12, 95% CI 1.04 to 35.98; 2 studies, 187 participants/procedures). All low‐certainty evidence.
Laser versus any sclerosing agent
There was no clear difference in resolution or improvement (or both) of telangiectasias (SMD −0.09, 95% CI −0.25 to 0.07; 5 studies, 593 participants/procedures), or matting (RR 1.00, 95% CI 0.46 to 2.19; 2 studies, 162 participants/procedures), and maybe less hyperpigmentation (RR 0.57, 95% CI 0.40 to 0.80; 4 studies, 262 participants/procedures) in the laser group. All moderate‐certainty evidence. High heterogeneity of the studies reporting on pain prevented pooling, and results were inconsistent (low‐certainty evidence).
Laser plus sclerotherapy (polidocanol) versus sclerotherapy (polidocanol)
Low‐certainty evidence suggests there may be more resolution or improvement (or both) of telangiectasias in the combined group (SMD 5.68, 95% CI 5.14 to 6.23; 2 studies, 710 participants), and no clear difference in hyperpigmentation (RR 0.83, 95% CI 0.35 to 1.99; 2 studies, 656 participants) or matting (RR 0.83, 95% CI 0.21 to 3.28; 2 studies, 656 participants). There may be more pain in the combined group (RR 2.44, 95% CI 1.69 to 3.55; 1 study, 596 participants; low‐certainty evidence).
Authors' conclusions
Small numbers of studies and participants in each comparison limited our confidence in the evidence. Sclerosing agents were more effective than placebo for resolution or improvement of telangiectasias but also caused more adverse events (moderate‐certainty evidence), and may result in more pain (low‐certainty evidence). There was no evidence of a benefit in resolution or improvement for any sclerosant compared to another or to laser. There may be more resolution or improvement of telangiectasias in the combined laser and polidocanol group compared to polidocanol alone (low‐certainty evidence). There may be differences between treatments in adverse events and pain. Compared to other sclerosing agents polidocanol probably causes less pain; STS resulted in more hyperpigmentation, matting and probably pain; foam may cause more matting (low‐certainty evidence); laser treatment may result in less hyperpigmentation (moderate‐certainty evidence). Further well‐designed studies are required to provide evidence for other available treatments and important outcomes (such as recurrence, time to resolution and delayed adverse events); and to improve our confidence in the identified comparisons.
Keywords: Female, Humans, Pruritus, Pruritus/drug therapy, Sclerotherapy, Telangiectasis, Telangiectasis/therapy, Veins
Plain language summary
Treatment for telangiectasias and reticular veins
What are telangiectasias and reticular veins?
Telangiectasias (spider veins) are small dilated blood vessels near the skin surface measuring less than 1.0 mm in diameter. Reticular veins have a diameter of less than 3.0 mm and are deeper in the skin. The cause is unknown, and they can be solely cosmetic, or can result in pain, burning or itching. Telangiectasias and reticular veins on the legs are very common, increase with age, and have been found in 41% of women over the age of 50 years. Risk factors include family history, pregnancy, local trauma, obesity and hormonal factors
How are telangiectasias and reticular veins treated?
There are several treatments, such as sclerotherapy, laser, intense pulsed light, microphlebectomy and thermoablation, but none is established as preferable. Unwanted side effects of treatments include hyperpigmentation (skin darkening), matting (new telangiectasis after treatment), allergy and pain. It is therefore important to know the effects of these treatments to help doctors and patients decide which is the best option for them.
What did we do?
We searched for studies where patients were randomly selected to receive one treatment for spider veins compared to a sham treatment, or to another type of treatment. We then compared the results and summarised the evidence from all the studies. Finally, we assessed how certain we are of the evidence. We considered factors such as the way studies were conducted, study sizes, and consistency of findings across studies. Based on our assessments, we categorised the evidence as being of very low, low, moderate or high certainty.
What did we find?
We found 35 studies with a combined total of 3632 participants (searched to 16 March 2021). Some studies compared one treatment on one leg to another treatment on the other leg. Studies used a variety of different treatments and none of them reported on all of our outcomes. Not all available treatments were investigated: no studies investigated intensive pulsed light, thermocoagulation or microphlebectomy.
Sclerosing agents improved telangiectasias and reticular veins resolution when compared to sham treatment, but resulted in more unwanted side effects (hyperpigmentation and matting). There was no benefit seen in one sclerosing agent compared to another, or compared to laser, for improving telangiectasias. There may be differences between treatments in adverse events and pain. Compared to other agents, polidocanol may result in less pain. Sodium tetradecyl sulphate (STS) may cause more hyperpigmentation, matting and probably more pain; foam may result in more matting; laser treatment may cause less hyperpigmentation. Combined laser plus sclerotherapy may result in better resolution compared to only sclerotherapy, but may cause more pain.
How reliable are these results?
We are not very confident in these results. We downgraded the certainty of the evidence by one or two levels (from high to moderate or low). This was because of the differences in the designs of the studies, which meant that only small numbers of studies and participants provided information for each treatment comparison.
Conclusion
Further well‐designed studies are needed to improve our confidence in the comparisons identified in this review, for other treatments available, and for other important outcomes, such as recurrence, time to resolution and long‐term side effects.
Summary of findings
Summary of findings 1. Sclerotherapy compared to placebo for treatment of telangiectasias and reticular veins.
| Sclerotherapy compared to placebo for treatment of telangiectasias and reticular veins | ||||||
| Patient or population: people with telangiectasias and reticular veins Setting: outpatient Intervention: sclerotherapy (any) Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants/procedures* (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with sclerotherapy | |||||
|
Resolution or improvement of telangiectasias (follow‐up: 4 ‐ 12 weeks) |
SMD 3.08 higher (2.68 higher to 3.48 higher) | ‐ | 613 (4 RCTs) | ⊕⊕⊕⊝ MODERATEa | ‐ | |
|
Adverse events ‐ hyperpigmentation (follow‐up: 4 ‐ 12 weeks) |
Study population | RR 11.88 (4.54 to 31.09) | 528 (3 RCTs) | ⊕⊕⊕⊝ MODERATEb | ‐ | |
| 25 per 1000 | 299 per 1000 (114 to 784) | |||||
|
Adverse events ‐ matting (follow‐up: 4 ‐ 12 weeks) |
Study population | RR 4.06 (1.28 to 12.84) | 528 (3 RCTs) | ⊕⊕⊕⊝ MODERATEb | ‐ | |
| 17 per 1000 | 68 per 1000 (22 to 216) | |||||
|
Pain (follow‐up: 1 day) |
SMD 0.7 higher (0.06 higher to 1.34 higher) | ‐ | 40 (1 RCT) | ⊕⊕⊝⊝ LOWc | ‐ | |
| Recurrence | See comment | ‐ | ‐ | ‐ | ‐ | The studies in this comparison did not assess this outcome |
| Time to resolution | See comment | ‐ | ‐ | ‐ | ‐ | The studies in this comparison did not assess this outcome |
| Quality of life | See comment | ‐ | ‐ | ‐ | ‐ | The studies in this comparison did not assess this outcome |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio; SMD: standardised mean difference | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
* Three studies used participants as the unit of analysis and one study used the number of procedures as the unit of analysis for each comparison. aWe downgraded by one level due to high clinical heterogeneity of the included studies. bWe downgraded by one level due to high clinical heterogeneity of the included studies and wide CI of the included studies (imprecision). c We downgraded by two levels due to high clinical heterogeneity of the included studies and only one included study with few participants.
Summary of findings 2. Sclerotherapy (polidocanol) compared to sclerotherapy (any sclerosant) for treatment of telangiectasias and reticular veins.
| Sclerotherapy (polidocanol) compared to sclerotherapy (any sclerosant) for treatment of telangiectasias and reticular veins | ||||||
| Patient or population: people with telangiectasias and reticular veins Setting: outpatient Intervention: sclerotherapy (polidocanol) Comparison: sclerotherapy (any sclerosant) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants/procedures* (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with sclerotherapy (any sclerosant agent) | Risk with sclerotherapy (polidocanol) | |||||
|
Resolution or improvement of telangiectasias (follow‐up: 4 ‐ 16 weeks) |
SMD 0.01 higher (0.13 lower to 0.14 higher) | ‐ | 852 (7 RCTs) | ⊕⊕⊕⊝ MODERATEa | ‐ | |
|
Adverse events ‐ hyperpigmentation (follow‐up: 4 ‐ 16 weeks) |
Study population | RR 0.94 (0.62 to 1.43) | 819 (6 RCTs) | ⊕⊕⊕⊝ MODERATEa | ‐ | |
| 476 per 1000 | 447 per 1000 (295 to 680) | |||||
|
Adverse events ‐ matting (follow‐up: 4 ‐ 16 weeks) |
Study population | RR 0.82 (0.52 to 1.27) | 859 (7 RCTs) | ⊕⊕⊕⊝ MODERATEa | ‐ | |
| 144 per 1000 | 118 per 1000 (75 to 183) | |||||
|
Pain (follow‐up: 1 day) |
SMD 0.26 lower (0.44 lower to 0.08 lower) | ‐ | 480 (5 RCTs) | ⊕⊕⊕⊝ MODERATEa | ‐ | |
| Recurrence | See comment | ‐ | ‐ | ‐ | ‐ | The studies in this comparison did not assess this outcome |
| Time to resolution | See comment | ‐ | ‐ | ‐ | ‐ | The studies in this comparison did not assess this outcome |
| Quality of life | See comment | ‐ | ‐ | ‐ | ‐ | The studies in this comparison did not assess this outcome |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio; SMD: standardised mean difference | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
* Three studies used participants as the unit of analysis and four studies used the number of procedures as the unit of analysis for each comparison. aWe downgraded by one level due to wide CIs.
Summary of findings 3. Sclerotherapy (STS) compared to sclerotherapy (any sclerosant) for treatment of telangiectasias and reticular veins.
| Sclerotherapy (STS) compared to sclerotherapy (any sclerosant) for treatment of telangiectasias and reticular veins | ||||||
| Patient or population: people with telangiectasias and reticular veins Setting: outpatient Intervention: sclerotherapy (STS) Comparison: sclerotherapy (any sclerosant) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants/procedures* (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with sclerotherapy (any sclerosant) | Risk with sclerotherapy (STS) | |||||
|
Resolution or improvement of telangiectasias (follow‐up: 4 ‐ 16 weeks) |
SMD 0.07 lower (0.25 lower to 0.11 higher) | ‐ | 473 (4 RCTs) | ⊕⊕⊕⊝ MODERATEa | ‐ | |
|
Adverse events ‐ hyperpigmentation (follow‐up: 4 ‐ 24 weeks) |
Study population | RR 1.71 (1.10 to 2.64) | 478 (4 RCTs) | ⊕⊕⊕⊝ MODERATEa | ‐ | |
| 371 per 1000 | 634 per 1000 (408 to 979) | |||||
|
Adverse events ‐ matting (follow‐up: 4 ‐ 24 weeks) |
Study population | RR 2.10 (1.14 to 3.85) | 323 (2 RCTs) | ⊕⊕⊕⊝ MODERATEa | ‐ | |
| 82 per 1000 | 173 per 1000 (94 to 318) | |||||
|
Pain (follow‐up: 1 day) |
Study population | RR 1.49 (0.99 to 2.25) | 409 (4 RCTs) | ⊕⊕⊕⊝ MODERATEa | ‐ | |
| 275 per 1000 | 410 per 1000 (273 to 619) | |||||
| Recurrence | See comment | ‐ | ‐ | ‐ | ‐ | The studies in this comparison did not assess this outcome |
| Time to resolution | See comment | ‐ | ‐ | ‐ | ‐ | The studies in this comparison did not assess this outcome |
| Quality of life | See comment | ‐ | ‐ | ‐ | ‐ | The studies in this comparison did not assess this outcome |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio; SMD: standardised mean difference | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
* Two studies used participants as the unit of analysis and four studies used the number of procedures as the unit of analysis for each comparison. aWe downgraded by one level due to wide CIs and small number of participants.
Summary of findings 4. Sclerotherapy (hypertonic saline) compared to sclerotherapy (any sclerosant) for treatment of telangiectasias and reticular veins.
| Sclerotherapy (hypertonic saline) compared to sclerotherapy (any sclerosant) for treatment of telangiectasias and reticular veins | ||||||
| Patient or population: people with telangiectasias and reticular veins Setting: outpatient Intervention: sclerotherapy (hypertonic saline) Comparison: sclerotherapy (any sclerosant) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants/procedures* (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with sclerotherapy (any sclerosant) | Risk with sclerotherapy (hypertonic saline) | |||||
|
Resolution or improvement of telangiectasias (follow‐up: 4 ‐ 12 weeks) |
‐ | SMD 0.01 higher (0.2 lower to 0.22 higher) | ‐ | 348 (3 RCTs) | ⊕⊕⊕⊝ MODERATEa | ‐ |
|
Adverse events ‐ hyperpigmentation (follow‐up: 8 ‐ 12 weeks) |
Study population | RR 0.74 (0.59 to 0.93) | 288 (2 RCTs) | ⊕⊕⊕⊝ MODERATEb |
‐ | |
| 493 per 1000 | 365 per 1000 (291 to 459) | |||||
|
Adverse events ‐ matting (follow‐up: 8 ‐ 12 weeks) |
Study population | RR 0.89 (0.58 to 1.36) | 288 (2 RCTs) | ⊕⊕⊕⊝ MODERATEb | ‐ | |
| 215 per 1000 | 192 per 1000 (125 to 293) | |||||
|
Pain (follow‐up: 1 day) |
‐ | SMD 6.22 higher (5.7 higher to 6.73 higher) | ‐ | 348 (3 RCTs) | ⊕⊕⊕⊝ MODERATEc | ‐ |
| Recurrence | See comment | ‐ | ‐ | ‐ | ‐ | The studies in this comparison did not assess this outcome |
| Time to resolution | See comment | ‐ | ‐ | ‐ | ‐ | The studies in this comparison did not assess this outcome |
| Quality of life | See comment | ‐ | ‐ | ‐ | ‐ | The studies in this comparison did not assess this outcome |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio; SMD: standard mean difference | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
* All studies used the number of procedures as the unit of analysis for each comparison. aWe downgraded by one level because of high risk of other bias in the included studies. bWe downgraded by one level because of wide CIs. cWe downgraded by one level because of clinical heterogeneity between included studies.
Summary of findings 5. Sclerotherapy (chromated glycerin) compared to sclerotherapy (any sclerosant) for treatment of telangiectasias and reticular veins.
| Sclerotherapy (chromated glycerin) compared to sclerotherapy (any sclerosant) for treatment of telangiectasias and reticular veins | ||||||
| Patient or population: people with telangiectasias and reticular veins Setting: outpatient Intervention: sclerotherapy (chromated glycerin) Comparison: sclerotherapy (any sclerosant) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants/procedures* (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with sclerotherapy (any sclerosing agent) | Risk with sclerotherapy (chromated glycerin) | |||||
|
Resolution or improvement of telangiectasias (follow‐up: 5 ‐ 24 weeks) |
‐ | SMD 0.45 higher (0.11 lower to 1.02 higher) | ‐ | 125 (2 RCTs) | ⊕⊕⊝⊝ LOWa | ‐ |
|
Adverse events ‐ hyperpigmentation (follow‐up: 5 ‐ 24 weeks) |
Study population | RR 0.49 (0.09 to 2.50) | 125 (2 RCTs) | ⊕⊕⊝⊝ LOWa | ‐ | |
| 66 per 1000 | 32 per 1000 (6 to 164) | |||||
|
Adverse events ‐ matting (follow‐up: 5 ‐ 24 weeks) |
Study population | RR 0.31 (0.01 to 7.53) | 99 (1 RCT) | ⊕⊕⊝⊝ LOWa | ‐ | |
| 21 per 1000 | 6 per 1000 (0 to 157) | |||||
|
Pain (follow‐up: 1 day) |
Study population | RR 1.50 (0.30 to 7.55) |
26 (1 RCT) | ⊕⊕⊝⊝ LOWa | ‐ | |
| 154 per 1000 | 231 per 1000 (46 to 1000) | |||||
| Recurrence | See comment | ‐ | ‐ | ‐ | ‐ | The studies in this comparison did not assess this outcome |
| Time to resolution | See comment | ‐ | ‐ | ‐ | ‐ | The studies in this comparison did not assess this outcome |
| Quality of life | See comment | ‐ | ‐ | ‐ | ‐ | The studies in this comparison did not assess this outcome |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio; SMD: standardised mean difference | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
* One study used participants as the unit of analysis and one study used the number of procedures as the unit of analysis for each comparison. a We downgraded by two levels due to few included studies and participants.
Summary of findings 6. Foam compared to sclerotherapy (any sclerosant) for treatment of telangiectasias and reticular veins.
| Foam compared to sclerotherapy (any sclerosant) for telangiectasias and reticular veins | ||||||
| Patient or population: people with telangiectasias and reticular veins Setting: outpatient Intervention: foam Comparison: sclerotherapy (any sclerosant) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants/procedures* (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with sclerotherapy (any sclerosing agent) | Risk with foam | |||||
|
Resolution or improvement of telangiectasias (follow‐up: 5 ‐ 10 weeks) |
‐ | SMD 0.04 higher (0.26 lower to 0.34 higher) | ‐ | 187 (2 RCTs) | ⊕⊕⊝⊝ LOWa | ‐ |
|
Adverse events ‐ hyperpigmentation (follow‐up: 5 ‐ 10 weeks) |
Study population | RR 2.12 (0.44 to 10.23) | 187 (2 RCTs) | ⊕⊕⊝⊝ LOWa | ‐ | |
| 26 per 1000 | 55 per 1000 (11 to 265) | |||||
|
Adverse events ‐ matting (follow up: 5 ‐ 10 weeks) |
Study population | RR 6.12 (1.04 to 35.98) | 187 (2 RCTs) | ⊕⊕⊝⊝ LOWa |
‐ | |
| 9 per 1000 | 53 per 1000 (9 to 310) | |||||
|
Pain (follow up: 1 day) |
SMD 0.1 lower (0.44 lower to 0.24 higher) | ‐ | 147 (1 RCT) | ⊕⊕⊝⊝ LOWa | ‐ | |
| Recurrence | See comment | ‐ | ‐ | ‐ | ‐ | The studies in this comparison did not assess this outcome |
| Time to resolution | See comment | ‐ | ‐ | ‐ | ‐ | The studies in this comparison did not assess this outcome |
| Quality of life | See comment | ‐ | ‐ | ‐ | ‐ | The studies in this comparison did not assess this outcome |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio; SMD: standardised mean difference | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
* One study used participants as the unit of analysis and one study used the number of procedures as the unit of analysis for each comparison. aWe downgraded by two levels due to wide CIs and few participants in the included studies.
Summary of findings 7. Laser compared to sclerotherapy for treatment of telangiectasias and reticular veins.
| Laser compared to sclerotherapy for treatment of telangiectasias and reticular veins | ||||||
| Patient or population: people with telangiectasias and reticular veins Setting: outpatient Intervention: laser Comparison: sclerotherapy | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants/procedures* (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with sclerotherapy | Risk with laser | |||||
|
Resolution or improvement of telangiectasias (follow‐up: 4 ‐ 24 weeks) |
‐ | SMD 0.09 lower (0.25 lower to 0.07 higher) | ‐ | 593 (5 RCTs) | ⊕⊕⊕⊝ MODERATEa | ‐ |
|
Adverse events ‐ hyperpigmentation (follow‐up: 4 ‐ 24 weeks) |
Study population | RR 0.57 (0.40 to 0.80) | 262 (4 RCTs) | ⊕⊕⊕⊝ MODERATEa | ‐ | |
| 328 per 1000 | 187 per 1000 (131 to 263) | |||||
|
Adverse events ‐ matting (follow‐up: 16 ‐ 24 weeks) |
Study population | RR 1.00 (0.46 to 2.19) | 162 (2 RCTs) | ⊕⊕⊕⊝ MODERATEa | ‐ | |
| 123 per 1000 | 123 per 1000 (57 to 270) | |||||
|
Pain (follow‐up: 1 day) |
Study population | ‐ | 100 (2 RCTs) | ⊕⊝⊝⊝ LOWb | We were not able to pool the data due to high heterogeneity | |
| See comment | ‐ | |||||
| Recurrence | See comment | ‐ | ‐ | ‐ | ‐ | The studies in this comparison did not assess this outcome |
| Time to resolution | See comment | ‐ | ‐ | ‐ | ‐ | The studies in this comparison did not assess this outcome |
| Quality of life | See comment | ‐ | ‐ | ‐ | ‐ | The studies in this comparison did not assess this outcome |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio; SMD: standardised mean difference | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
* Two studies used participants as the unit of analysis and three studies used the number of procedures as the unit of analysis for each comparison
aWe downgraded by one level due to wide CIs. bWe downgraded by two levels because of few included participants.
Summary of findings 8. Laser plus sclerotherapy compared to sclerotherapy for treatment of telangiectasias and reticular veins.
| Laser plus sclerotherapy compared to sclerotherapy for treatment of telangiectasias and reticular veins | ||||||
| Patient or population: people with telangiectasias and reticular veins Setting: outpatient Intervention: laser plus sclerotherapy Comparison: sclerotherapy | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants/procedures* (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with sclerotherapy | Risk with laser plus sclerotherapy | |||||
|
Resolution or improvement of telangiectasias (follow‐up: 12 ‐ 16 weeks) |
‐ | SMD 5.68 higher (5.14 higher to 6.23 higher) | ‐ | 710 (2 RCTs) | ⊕⊕⊝⊝ LOWa | ‐ |
|
Adverse events ‐ Hyperpigmentation (follow‐up: 12 ‐ 16 weeks) |
Study population | RR 0.83 (0.35 to 1.99) | 656 (2 RCTs) | ⊕⊕⊝⊝ LOWa | ‐ | |
| 64 per 1000 | 53 per 1000 (22 to 128) | |||||
|
Adverse events ‐ matting (follow‐up: 12 ‐ 16 weeks) |
Study population | RR 0.83 (0.21 to 3.28) | 656 (2 RCTs) | ⊕⊕⊝⊝ LOWa | ‐ | |
| 18 per 1000 | 15 per 1000 (4 to 60) | |||||
|
Pain (follow‐up: 1 day) |
Study population | RR 2.44 (1.69 to 3.55) | 596 (1 RCT) | ⊕⊕⊝⊝ LOWb | ‐ | |
| 266 per 1000 | 649 per 1000 (449 to 944) | |||||
| Recurrence | See comment | ‐ | ‐ | ‐ | ‐ | The studies in this comparison did not assess this outcome |
| Time to resolution | See comment | ‐ | ‐ | ‐ | ‐ | The studies in this comparison did not assess this outcome |
| Quality of life | See comment | ‐ | ‐ | ‐ | ‐ | The studies in this comparison did not assess this outcome |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio; SMD: standardised mean difference | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
* All studies used participants as the unit of analysis. aWe downgraded by two levels because of clinical heterogeneity in the included studies and the fact that the two studies were conducted by the same group of investigators. bWe downgraded by two levels due to having one included study.
Summary of findings 9. Sclerotherapy (hypertonic glucose plus polidocanol) compared to sclerotherapy (hypertonic glucose).
| Sclerotherapy (hypertonic glucose plus polidocanol) compared with sclerotherapy (hypertonic glucose) for telangiectasias and reticular veins | ||||||
|
Patient or population: people with telangiectasias and reticular veins Settings: outpatient Intervention: sclerotherapy (hypertonic glucose plus POL) Comparison: sclerotherapy (hypertonic glucose) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No of Participants* (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with hypertonic glucose | Risk with hypertonic glucose plus POL | |||||
|
Resolution or improvement of telangiectasias (follow‐up: 12 ‐ 16 weeks) |
‐ | SMD 0.79 higher (0.50 higher to 1.09 higher) |
191 (2 RCTs) |
⊕⊕⊕⊝ MODERATEa | ‐ | |
|
Adverse events ‐ hyperpigmentation (follow‐up: 16 weeks) |
Study population | RR 0.79 (0.62 to 1.01) |
191 (2 RCTs) |
⊕⊕⊕⊝ MODERATEa | ‐ | |
| 649 per 1000 | 513 per 1000 (403 to 656) | |||||
|
Adverse events ‐ matting (follow‐up: 16 weeks) |
Study population | RR 0.78 (0.51 to 1.20) |
191 (2 RCTs) |
⊕⊕⊕⊝ MODERATEa | ‐ | |
| 351 per 1000 | 273 per 1000 (179 to 421) | |||||
|
Pain (follow‐up: 16 weeks) |
Study population | RR 1.02 (0.83 to 1.24) |
191 (2 RCTs) |
⊕⊕⊕⊝ MODERATEa |
‐ | |
| 443 per 1000 | 442 per 1000 (359 to 537) |
|||||
| Recurrence | See comment | ‐ | ‐ | ‐ | ‐ | The studies in this comparison did not assess this outcome |
| Time to resolution | See comment | ‐ | ‐ | ‐ | ‐ | The studies in this comparison did not assess this outcome |
| Quality of life | See comment | ‐ | ‐ | ‐ | ‐ | The studies in this comparison did not assess this outcome |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; POL: polidocanol; RCT: randomised controlled trial; RR: risk ratio; SMD: standardised mean difference | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect | ||||||
* All studies used participants as the unit of analysis. aWe downgraded one level because of few participants in included studies.
Summary of findings 10. Sclerotherapy plus compression compared to sclerotherapy alone for telangiectasias and reticular veins.
| Sclerotherapy plus compression compared to sclerotherapy alone for telangiectasias and reticular veins | ||||||
| Patient or population: people with telangiectasias and reticular veins Setting: outpatient Intervention: sclerotherapy plus compression Comparison: sclerotherapy | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies)* | Certainty of the evidence (GRADE) | Comments | |
| Risk with sclerotherapy | Risk with sclerotherapy plus compression | |||||
|
Resolution or improvement of telangiectasias (follow‐up: 4 ‐ 8 weeks) |
‐ | SMD 0.09 higher (0.19 lower to 0.37 higher) | ‐ | 196 (2 studies) | ⊕⊕⊕⊝ MODERATEa |
‐ |
|
Adverse events ‐ hyperpigmentation (follow‐up:4 ‐ 8 weeks) |
Study population | RR 0.93 (0.41 to 2.07) | 196 (2 studies) | ⊕⊕⊕⊝ MODERATEa |
‐ | |
| 112 per 1000 | 104 per 1000 (46 to 232) | |||||
|
Adverse events ‐ matting (follow‐up: 8 weeks) |
Study population | RR 1.84 (0.17 to 19.62) | 96 (1 study) | ⊕⊕⊝⊝ LOWb | ‐ | |
| 22 per 1000 | 40 per 1000 (4 to 427) | |||||
| Pain | See comment | ‐ | ‐ | ‐ | ‐ | The studies in this comparison did not assess this outcome |
| Recurrence | See comment | ‐ | ‐ | ‐ | ‐ | The studies in this comparison did not assess this outcome |
| Time to resolution | See comment | ‐ | ‐ | ‐ | The studies in this comparison did not assess this outcome | |
|
Quality of life (follow up: 8 weeks) |
SMD 0.02 lower (0.42 lower to 0.39 higher) | ‐ | 93 (1 study) | ⊕⊕⊝⊝ LOWb | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio; SMD: standard mean difference | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect | ||||||
*All studies used participants as the unit of analysis. aWe downgraded one level because of few participants in included studies. bWe downgraded two levels because of few participants and only one included study.
Background
Description of the condition
Telangiectasias, or spider veins, are dilated venules or arterioles (small superficial veins) measuring less than 1.0 mm in diameter and occurring predominantly in the lower extremities (Thomson 2016). Reticular veins have a diameter less than 3.0 mm and are often tortuous and located in the subdermal or subcutaneous tissue (Eklof 2004; Porter 1995). Their cause is unknown. Patients may be asymptomatic or can report pain, burning or itching. Risk factors include family history, pregnancy, local trauma, obesity and hormonal factors (Goldman 2002).
The diagnoses of telangiectasias and reticular veins are clinical and made according to the Clinical, Etiological, Anatomical and Pathophysiological (CEAP) classification system for chronic venous disorders in the lower limb. CEAP classification comprises seven main categories: C0 to C6, and telangiectasias are classified as C1 (Eklof 2004; Lurie 2020).
C0 ‐ no visible or palpable signs of venous disease C1 ‐ telangiectasia or reticular veins (thread veins) C2 ‐ varicose veins (diameter of 3.0 mm or more) C3 ‐ oedema C4 ‐ changes in the skin and subcutaneous tissue: pigmentation (C4a), eczema (C4a), lipodermatosclerosis (C4b), atrophic blanche (C4b), or corona phlebectatica (C4c) C5 ‐ healed venous ulcer C6 ‐ active venous ulcer
The incidence of telangiectasias increases with age (Schwartz 2011). Telangiectasias on the lower limbs are very common and have been found in 41% of women over the age of 50 years (Mujadzic 2015). They can be considered an important aesthetic or cosmetic problem (Hercogova 2002). The presence of telangiectasias may be associated with the insufficiency of major venous systems; approximately 50% to 62% of insufficient perforating veins are found in the presence of telangiectasias (Andrade 2009).
Description of the intervention
Treatments for telangiectasias and reticular veins include sclerotherapy, laser therapy, intense pulsed light treatment, microphlebectomy and thermocoagulation. These techniques can be used in combination to maximise the effects and avoid any damage from the individual techniques. The most common treatment for telangiectasias is sclerotherapy (Schwartz 2011), which is a technique or group of techniques for the destruction of spider veins via the injection of a medication that destroys the vein endothelium, leading to occlusion and subsequent fibrosis. Sclerosing agents are injected into the vein using hypodermic needles until the area around the puncture site blanches, or resistance can be felt. The injection is immediately discontinued if there is extravasation. Individual injections use between 0.1 mL and 0.5 mL of sclerosing agent for each telangiectasias area, although larger volumes of the sclerosing agent are required for larger veins (Worthington‐Kirsch 2005). There are many sclerosing agents and they are generally categorised as detergents or osmotic or chemical irritants. These agents cause endothelial damage that results in blocking the vein (vessel occlusion) and the subsequent disappearance of the vessel being treated (Vitale‐Lewis 2008). Foam sclerotherapy mixes gas and fluid sclerosing agents between two syringes (Tessari 2001). Foam with detergent sclerosants have a more efficient effect as a result of increasing both dwell time and contact area. This increase in efficiency also allows for lower sclerosing doses (Worthington‐Kirsch 2005). Foam is associated with side effects such as microthrombi, matting and transient visual disturbance (Kern 2004). These adverse effects may also occur with conventional sclerotherapy.
Laser therapy is used for the treatment of telangiectasias in people with veins of a diameter less than a 30 gauge needle. Patients with a phobia to needles or allergy to certain sclerosing agents can also benefit from this technique. There are several types of lasers for the treatment of telangiectasias, with varying wavelengths between 532 nm to 1064 nm (Meesters 2014). Treatment with a Nd:YAG 1064 nm laser has shown similar results to sclerotherapy (Parlar 2015). The side effects of laser therapy in the treatment of telangiectasias include erythema, crusting, swelling, and blistering (Tierney 2009). Laser therapy may cause less pain but may also result in complications such as spotting (Mujadzic 2015).
Intense Pulsed Light (IPL) is similar to laser therapy, as high‐intensity light sources emit polychromatic light ranging within the 515 to 1200 nm wavelength spectrum. The treatment of vascular lesions with IPL depends on the type and size of vessels, with angiomas and spider veins demonstrating the best response (Goldberg 2012). There are many clinical indications for treatment with IPL (Raulin 2003). IPL is indicated for the treatment of unwanted hair growth, vascular lesions, pigmented lesions, acne vulgaris, photo damage and skin rejuvenation (Babilas 2010). The negative side effects of IPL include vesicles, burns, erosions, blisters and crust formation, and hypo‐ and hyperpigmentations are also common (Stangl 2008).
Microphlebectomy is performed using hooks which enable venous extraction through minimal skin incisions or even needle punctures. Ambulatory microphlebectomy is indicated in varicose veins in any part of the body, such as arms, the periorbital, abdomen and dorsum (Ramelet 2002).
Thermocoagulation or the radiofrequency energy method is another technique for the treatment of telangiectasias or reticular veins. The method is based on the production of high‐frequency waves, at 4 ΜΗz, transmitted through a thin needle, which causes thermal damage in the veins (Chadornneau 2012).
How the intervention might work
All the above techniques cause lesions in the vascular endothelium and consequently result in the disappearance of the target vessel.
In sclerotherapy, the ideal sclerosant causes full destruction of the vessel wall and minimal thrombus formation. Incomplete destruction of the wall or local thrombosis may lead to recanalisation. The ideal agent would also be nontoxic, easily manipulated, and painless (Worthington‐Kirsch 2005).
Laser and IPL therapies are alternative options but have a high cost compared to sclerotherapy. Both techniques act by exposing the red elements of blood to light energy. Oxyhaemoglobin is the major chromophore in blood vessels, with two absorption bands in the visible light spectrum at 542 nm and 577 nm. Following absorption by oxyhaemoglobin, light energy is converted to thermal energy, which diffuses in the blood vessel, causing photocoagulation, mechanical injury, and finally thrombosis and occlusion of the target vessel (Micali 2016).
Different laser wavelengths can be successfully used to treat vascular lesions. Each type of laser has advantages specific to its wavelength, pulse duration, spot size, and cutaneous cooling profile. The 532 to 595 nm lasers have multiple applications, treating not only telangiectasias, but also pigmentation and even fine wrinkles. The main advantage of using a 1064 nm laser is that its longer wavelength can penetrate more deeply, allowing the effective thermosclerosis of spider veins (Goldman 2004).
A possible advantage of IPL is selective photothermolysis, in which thermal damage is confined to specific epidermal or dermal pigmented targets. Tissues surrounding these targeted structures are spared, potentially reducing nonspecific, widespread thermal injury. There are three main chromophores: haemoglobin, water, and melanin. They have broad absorption peaks of light energy, allowing them to be targeted by a range, as well as a specific wavelength of light (Goldberg 2012).
The advantage of microphlebectomy is minimal or no scarring, no skin necrosis and no residual hyperpigmentation (Ramelet 2002).
Thermocoagulation is a relatively new technology with advantages such as the immediate disappearance of veins, no allergic manifestations, no pigmentation and necrosis, and applicability to all skin types (Chadornneau 2012).
Why it is important to do this review
There is a high prevalence of telangiectasias, or spider veins, and the most common age for presentation is between 30 and 50 years (Ruckley 2008). The incidence increases with age and is an important aesthetic problem (Hercogova 2002). In Brazil, the incidence of telangiectasias in young women is 50% and represents a cosmetic problem for them (Scuderi 2002). A research report from Poland, including women aged between 18 and 60, found a telangiectasias incidence of 27% (Karch 2002). Sclerotherapy, the treatment most often used for telangiectasias, has low costs but is not free from complications. Laser therapy is a safe and efficacious treatment for telangiectasias and can be achieved with multiple lasers (McCoppin 2011). IPL is versatile, which allows the treatment of both vascular and pigmented lesions (Wall 2007). IPL may offer an advantage due to its selective photothermolysis but has a high cost compared to sclerotherapy. Currently, there is a lack of evidence about which of these methods is more effective in the treatment of telangiectasias. There has been a previous Cochrane Review on sclerotherapy for telangiectasias (Schwartz 2011), but none has addressed other methods for the treatment of telangiectasias. This review reports on the evidence available to enable healthcare professionals and consumers to choose the most appropriate treatment method for telangiectasias and reticular veins.
Objectives
To assess the effects of sclerotherapy, laser therapy, intensive pulsed light (IPL), thermocoagulation, and microphlebectomy treatments for telangiectasias and reticular veins.
Methods
Criteria for considering studies for this review
Types of studies
We searched and considered for inclusion all randomised controlled trials (RCTs) and quasi‐RCTs that compared treatment methods for telangiectasias and reticular veins in the lower limb. We included studies that compared individual treatment methods against placebo, or that compared different sclerosing agent, or foam or laser treatment. We also included studies that used a combination of methods.
Types of participants
We considered all participants, both male and female and of all ages, with telangiectasias and reticular veins in the lower limb, confirmed by either the CEAP C1 classification or the clinical assessment of a physician. We excluded people with hereditary haemorrhagic telangiectasias (HHT), mucous telangiectasias, people treated for telangiectasias or superficial vein reflux within the previous 30 days, and people undergoing a simultaneous treatment for telangiectasias and superficial vein reflux.
Types of interventions
We evaluated the following interventions:
Sclerotherapy with any sclerosing agent of any dose or duration (with or without compression treatment);
Laser therapy applied directly to the telangiectasias or reticular veins (any wavelength, any treatment regimen);
Intensive Pulsed Light (IPL) applied directly to the telangiectasias or reticular veins (any wavelength, any treatment regimen);
Thermocoagulation applied directly to the telangiectasias or reticular veins;
Microphlebectomy in reticular veins.
Comparisons:
Sclerotherapy versus placebo;
Sclerotherapy versus sclerotherapy;
Sclerotherapy versus laser therapy;
Sclerotherapy versus IPL;
Sclerotherapy versus thermocoagulation;
Sclerotherapy versus microphlebectomy;
Laser therapy versus placebo;
Laser therapy versus laser therapy;
Laser therapy versus IPL therapy;
Laser therapy versus thermocoagulation;
Laser therapy versus microphlebectomy;
IPL versus placebo;
IPL versus IPL therapy;
IPL versus thermocoagulation;
IPL versus microphlebectomy;
Thermocoagulation versus placebo;
Thermocoagulation versus microphlebectomy;
Any combination of the above treatments versus any combination.
Types of outcome measures
Primary outcomes
Clinically‐ or photographically‐assessed resolution or improvement (or both) of telangiectasias: resolution or improvement were measured by clear diagnostic scales, e.g. vessel clearance < 20%, 20 to 40%, 40 to 60%, 60 to 80%, > 80% (Shamma 2005) or study definitions
Adverse events (including hyperpigmentation, matting, allergy, bruising, anaphylaxis, necrosis of the skin)
Secondary outcomes
Pain during procedure and post‐procedure: pain was measured by clear diagnostic scales during the procedure and 24 hours post‐procedure, e.g. visual analogue pain scale (VAS), used for determining the pain level during laser treatment. Pain is graded by the participant with the help of a coloured gradient and graduated line from 1 to 10 (Kozarev 2011)
Recurrence: recurrence was measured by clear diagnostic scales until 30 days after the procedure, e.g. vessel clearance < 20%, 20 to 40%, 40 to 60%, 60 to 80%, > 80% (Shamma 2005)
Time to resolution (time unit: days)
Quality of life: any scale of quality of life, e.g. Aberdeen Varicose Vein Severity Score (AVVSS) (Smith 1999)
Search methods for identification of studies
Electronic searches
The Cochrane Vascular Information Specialist (CIS) conducted systematic searches of the following databases for RCTs without language, publication year or publication status restrictions:
Cochrane Vascular Specialised Register via the Cochrane Register of Studies (CRS‐Web) (searched 16 March 2021);
Cochrane Central Register of Controlled Trials (CENTRAL; 2021, Issue 2) via the Cochrane Register of Studies Online (CRSO);
MEDLINE (Ovid MEDLINE Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE Daily and Ovid MEDLINE) (searched 16 March 2021);
Embase Ovid (searched 16 March 2021);
AMED Ovid (searched 16 March 2021);
CINAHL Ebsco (searched 16 March 2021).
The CIS modelled search strategies for other databases on the search strategy designed for CENTRAL. Where appropriate,we combined them with adaptations of the highly sensitive search strategy designed by the Cochrane Collaboration for identifying RCTs and controlled clinical trials (as described in the Cochrane Handbook for Systematic Reviews of Interventions Chapter 6, Lefebvre 2011). Search strategies for major databases are provided in Appendix 1.
The Information Specialist searched the following trial registries on 16 March 2021:
World Health Organization International Clinical Trials Registry Platform (who.int/trialsearch);
ClinicalTrials.gov (clinicaltrials.gov).
The authors performed additional searches in LILACS and IBECS databases. The search strategy was designed by the authors and checked by the Cochrane Information Specialist of Cochrane Brazil. See Appendix 2 for details of the search strategy used for the authors' search (searched 17 March 2021).
Searching other resources
We checked the bibliographies of included trials for further references to relevant trials. We contacted specialists in the field, manufacturers and authors of the included trials for any possible unpublished data.
Data collection and analysis
Selection of studies
We examined the titles and abstracts to select the relevant reports after merging the search results and removing duplicate records. Three review authors (LCUN, DGC and RLGF) independently evaluated the trials to determine if they were appropriate to include. We resolved disagreements by discussion within the review team. We then retrieved and examined the full text of the relevant trials for compliance with eligibility criteria. Where a trial did not meet the eligibility criteria, we excluded the trial and documented the reason for exclusion.
Data extraction and management
Three review authors (LCUN, DGC and RLGF) independently extracted and collected data on paper data extraction forms. We resolved disagreements by discussion within the review team. We collected the following information.
Study features: publication details (e.g. year, country, authors); study design; population data (e.g. age, comorbidities, severity of telangiectasias, duration, history of treatments, and responses); details of intervention (e.g. manufacture, material, site of insertion, additional procedures); number of participants randomised into each treatment group; the number of participants in each group who failed treatment; the numbers of participants lost to follow‐up; the duration of follow‐up; cost of treatment; sources of funding; study authors’ potential conflicts of interest.
Outcomes: types of outcomes measured; timing of outcomes.
Assessment of risk of bias in included studies
Three review authors (LCUN, DGC and RLGF), independently assessed the included studies for risks of bias, using Cochrane's risk of bias tool, described in Section 8.5 of the Cochrane Handbook for Systematic Reviews of interventions (Higgins 2011). We planned to resolve disagreements by discussion within the review team, if necessary.
We assessed the following domains and rated them at low, unclear, or high risk of bias:
random sequence generation;
adequate concealment of allocation;
blinding of participants and personnel;
blinding of outcome assessment;
incomplete outcome data;
selective outcome reporting; and
other potential threats to validity.
We reported the assessments for each individual study in the risk of bias tables located in the 'Characteristics of included studies' section. We planned to contact the study author(s) to seek clarification in cases of uncertainty over data.
Measures of treatment effect
We used the risk ratio (RR) for dichotomous data and mean difference (MD) for continuous data with the same scale, or standardised mean difference (SMD) for continuous data with different scales, all with 95% confidence intervals (CIs).
Unit of analysis issues
We considered each participant as the unit of analysis. For trials that considered multiple interventions in the same group, we analysed only the partial data of interest. Studies with a split‐body design were treated as cross‐over trials as recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2021a).
Dealing with missing data
We analysed only the available data and contacted the trial authors to request missing data. We reported dropout rates in the 'Characteristics of included studies' tables of the review, and we used intention‐to‐treat analysis.
Assessment of heterogeneity
We inspected forest plots visually to consider the direction and magnitude of effects and the degree of overlap between confidence intervals. We quantified inconsistency among the pooled estimates using the I2 statistic (where I2 = ((Q ‐ df)/Q) x 100% where Q is the Chi2 statistic, and 'df' represents the degree of freedom). This illustrates the percentage of the variability in effect estimates resulting from heterogeneity rather than sampling error (Deeks 2019). We interpreted the thresholds for the I2 statistic as follows: 0 to 30% = low heterogeneity; 30% to 60% = moderate heterogeneity; 60% to 90% = substantial heterogeneity, and more than 90% = considerable heterogeneity (Deeks 2019).
Assessment of reporting biases
We planned to assess the presence of publication bias and other reporting bias using funnel plots if we identified sufficient studies (more than 10) for inclusion in the meta‐analysis (Higgins 2021b).
Data synthesis
We synthesised the data using Review Manager 5 (Review Manager 2020). We planned to use the fixed‐effect model to synthesise the data if there were low to moderate levels of heterogeneity. If there was substantial heterogeneity, we planned to use a random‐effects model. If there was considerable heterogeneity, we planned not to undertake a meta‐analysis but to describe the data narratively in the text. As we identified clinical heterogeneity due to differences in, for example, study designs or sclerosing agents, we used a random‐effects model to synthesize the data.
Subgroup analysis and investigation of heterogeneity
If sufficient data were available, we planned to perform subgroup analyses for the following:
interventions: types of sclerosant, IPL and laser wave lengths; and combination of methods;
participant characteristics: age (e.g. youth (15 years to 24 years), adults (25 years to 64 years) and seniors (65 years and over)), gender and race.
Sensitivity analysis
If an adequate number of studies were available, we planned to perform sensitivity analysis based on allocation concealment (high, low, or unclear) and blinding of outcome assessment (high, low, or unclear). We planned to carry out sensitivity analyses by excluding those trials that we judged to be at high risk of bias according to Higgins 2021b. We were not able to do this, as comparisons did not include sufficient studies.
Summary of findings and assessment of the certainty of the evidence
We prepared summary of findings tables to provide the key information presented in the review comparing treatments in participants with telangiectasias and reticular veins. We prepared summary of findings tables for each comparison at one time point, using the outcomes described in Types of outcome measures:
clinically‐ or photographically‐assessed resolution or improvement, or both, of telangiectasias;
adverse events (hyperpigmentation and matting);
pain during procedure and post‐procedure;
recurrence;
time to resolution;
quality of life.
We assessed the certainty of the evidence for each outcome as high, moderate, low or very low, based on the criteria of risk of bias, inconsistency, indirectness, imprecision, and publication bias, using the GRADE approach (Grade 2004). We based the tables on methods described in Chapters 11 and 12 of the Cochrane Handbook, and justified any departures from the standard methods (Grade 2004; Higgins 2021b).
Results
Description of studies
Results of the search
The searches in the literature databases and trial registries identified 2649 reports, which we reduced to 2279 potentially relevant records after deduplication. We assessed 48 full‐text articles for eligibility, and identified 35 studies which met the review inclusion criteria (Figure 1). We excluded 10 studies and identified three ongoing studies.
1.
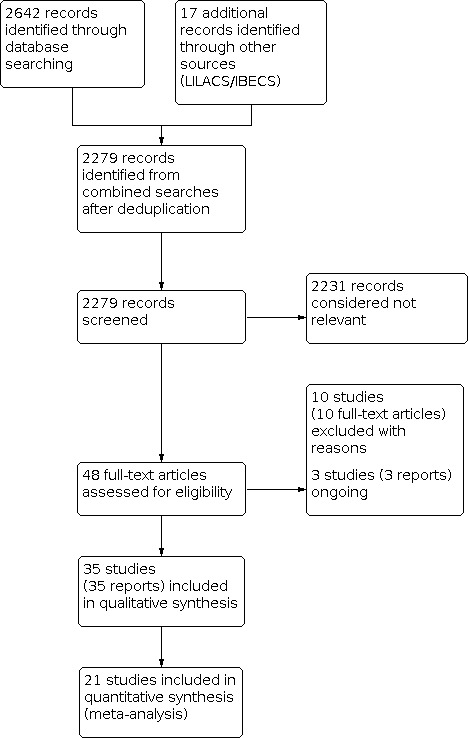
Study flow diagram.
Included studies
See Characteristics of included studies.
Type of study
The characteristics of the 35 included studies are shown in Characteristics of included studies. All 35 included studies were RCTs published between 1987 and 2021 (Alos 2006; Bayer 2021; Benigni 1999; Bertanha 2017; Bertanha 2021; Carlin 1987; Christiansen 2015; Goldman 2002; Hamel‐Desnos 2009; Hoss 2020; Ianosi 2019; Kahle 2006; Kern 2004; Kern 2007; Kern 2011; Kern 2012; Klein 2013; Leach 2003; Lupton 2002; McCoy 1999; Moreno‐Moraga 2013; Moreno‐Moraga 2014; Munia 2012; Nguyen 2020; Norris 1989; Ochoa 2021; Ozden 2011; Parlar 2015; Peterson 2012a; Peterson 2012b; Prescott 1992; Rabe 2010; Rao 2005; Schul 2011; Zhang 2012).
Of the 35 studies, nine evaluated participants with telangiectasias (Carlin 1987; Kahle 2006; Leach 2003; Lupton 2002; McCoy 1999; Moreno‐Moraga 2013; Munia 2012; Norris 1989; Ozden 2011), and 26 studied participants with telangiectasias and reticular varicose veins up to 3.0 mm in diameter (Alos 2006; Bayer 2021; Benigni 1999; Bertanha 2017; Bertanha 2021; Christiansen 2015; Goldman 2002; Ochoa 2021; Hamel‐Desnos 2009; Hoss 2020; Ianosi 2019; Kern 2004; Kern 2007; Kern 2011; Kern 2012; Klein 2013; Moreno‐Moraga 2014; Nguyen 2020; Parlar 2015; Peterson 2012a; Peterson 2012b; Prescott 1992; Rabe 2010; Rao 2005; Schul 2011; Zhang 2012).
Only five studies presented a sample size calculation (Bertanha 2017; Kern 2004; Kern 2007; Kern 2011; Kern 2012).
Setting
Ten RCTs were conducted in the USA (Carlin 1987; Goldman 2002; Hoss 2020; Leach 2003; Lupton 2002; Norris 1989; Peterson 2012a; Peterson 2012b; Rao 2005, Schul 2011), five in Switzerland (Kern 2004; Kern 2007; Kern 2011; Kern 2012; Parlar 2015), three in Spain (Alos 2006; Moreno‐Moraga 2013; Moreno‐Moraga 2014), three in Brazil (Bertanha 2017; Bertanha 2021; Munia 2012); four in Germany (Bayer 2021; Kahle 2006; Klein 2013; Rabe 2010), two in France (Benigni 1999; Hamel‐Desnos 2009), one each in China (Zhang 2012), in Turkey (Ozden 2011), in Australia (McCoy 1999), in Canada (Prescott 1992), in Denmark (Christiansen 2015), in Romania (Ianosi 2019), in Vietnam (Nguyen 2020) and in Mexico (Ochoa 2021).
Unit of analysis
Of the 35 included studies, 18 used a split‐body design, comparing groups in an opposite leg or a lower limb quadrant (Benigni 1999; Carlin 1987; Christiansen 2015; Hoss 2020; Ianosi 2019; Kern 2012; Klein 2013; Leach 2003; Lupton 2002; McCoy 1999; Munia 2012; Nguyen 2020; Norris 1989; Ozden 2011; Peterson 2012a; Peterson 2012b; Prescott 1992; Rao 2005). The remaining 17 studies used the participant as the unit of analysis (Alos 2006; Bayer 2021; Bertanha 2017; Bertanha 2021; Goldman 2002; Ochoa 2021; Hamel‐Desnos 2009; Kahle 2006; Kern 2004; Kern 2007; Kern 2011; Moreno‐Moraga 2013; Moreno‐Moraga 2014; Parlar 2015; Rabe 2010; Schul 2011; Zhang 2012).
Study participants
The 35 included studies provided data for 3632 participants. The smallest study included 13 participants (Leach 2003) and the largest included 720 participants (Ochoa 2021). Thirteen studies included up to a maximum of 30 participants (Leach 2003, n = 13; Christiansen 2015, n = 14; Klein 2013, n = 15; Carlin 1987, n = 20; Norris 1989, n = 20; Lupton 2002, n = 20; Nguyen 2020, n = 20; Rao 2005, n = 20; Peterson 2012a, n = 20; Ozden 2011, n = 21; Benigni 1999, n = 24; Hoss 2020, n = 30; Munia 2012, n = 30). Twelve studies included up to 100 participants (Bertanha 2017, n = 93; Kahle 2006, n = 48; Bayer 2021, n = 50; Kern 2012, n = 53; Parlar 2015, n = 56; Schul 2011, n = 58; Prescott 1992, n = 60; Moreno‐Moraga 2013, n = 90; Peterson 2012b, n = 63; Alos 2006, n = 75; McCoy 1999, n = 81; Kern 2007, n = 100). Ten studies included more than 100 participants (Hamel‐Desnos 2009, n = 105; Kern 2011, n = 110; Bertanha 2021, n = 115; Goldman 2002, n = 129; Kern 2004, n = 150; Ianosi 2019, n= 285; Zhang 2012, n = 288; Rabe 2010, n = 316; Moreno‐Moraga 2014, n = 320; Ochoa 2021, n = 720).
All included studies evaluated participants with CEAP C1, telangiectasias or reticular veins (diameter less than 3.0 mm) in the lower limb. Three studies included participants classified CEAP C2, but these data are not included in this review (Goldman 2002; Rao 2005; Zhang 2012).
Most studies (n = 25) evaluated only women (Benigni 1999; Bertanha 2017; Bertanha 2021; Carlin 1987; Christiansen 2015; Hamel‐Desnos 2009; Hoss 2020; Ianosi 2019; Kern 2004; Kern 2007; Kern 2011; Kern 2012; Klein 2013; Leach 2003; Lupton 2002; Moreno‐Moraga 2013; Moreno‐Moraga 2014; McCoy 1999; Munia 2012; Norris 1989; Ozden 2011; Parlar 2015; Peterson 2012a; Prescott 1992; Schul 2011). Eight studies analysed men and women (Alos 2006; Bayer 2021; Ochoa 2021; Nguyen 2020; Peterson 2012b; Rabe 2010; Rao 2005; Zhang 2012). Two studies did not report the gender of the participants (Goldman 2002; Kahle 2006).
Five includes studies did not provide data about the age of the participants (Bayer 2021; Goldman 2002; Kahle 2006; Kern 2011; Rao 2005), and another 10 studies reported the age range without the mean (Bertanha 2017; Bertanha 2021; Carlin 1987; Moreno‐Moraga 2013; Norris 1989; Peterson 2012a; Peterson 2012b; Prescott 1992; Rabe 2010; Zhang 2012). The age of participants ranged from 17 to 80 years.
Twelve studies reported data on the skin photo type by Fitzpatrick’s classification: Photo type I to III (Benigni 1999; Christiansen 2015; Ozden 2011; Parlar 2015); Photo type I to IV (Alos 2006; Bertanha 2017; Klein 2013; Munia 2012; Peterson 2012b); Photo type IV (Moreno‐Moraga 2013; Nguyen 2020); Photo type II to IV (Moreno‐Moraga 2014).
Interventions
There were six sclerosing agents in the included studies: polidocanol (0.25% to 3%), sodium tetradecyl sulfate (STS) (0.25% to 1%), hypertonic saline (20% to 23.4%), chromated glycerin (72%), hypertonic glucose (70%), and dextrose.
Four studies compared any sclerosing agent versus placebo (Carlin 1987; Kahle 2006; Rabe 2010; Zhang 2012). Zhang 2012 and Kahle 2006 compared polidocanol versus placebo. Carlin 1987 compared polidocanol versus STS versus hypertonic saline versus placebo; and Rabe 2010 compared polidocanol versus STS versus placebo).
Nine studies compared sclerosing liquid versus sclerosing liquid (Norris 1989 ‐ polidocanol (0.25%) versus polidocanol (0.50%) versus polidocanol (0.75%) versus polidocanol (1%); Prescott 1992 ‐ hypertonic dextrose versus STS, McCoy 1999 – hypertonic saline versus polidocanol; Goldman 2002 – STS versus polidocanol; Leach 2003 – chromated glycerin versus STS; Rao 2005 – STS versus polidocanol; Peterson 2012b – hypertonic saline versus polidocanol; Bertanha 2017 and Bertanha 2021 – hypertonic glucose versus hypertonic glucose plus polidocanol).
Five studies compared any form of foam (Alos 2006; Benigni 1999; Hoss 2020; Kern 2004; Peterson 2012a). Benigni 1999 and Alos 2006 compared foam versus polidocanol. Kern 2004 compared foam versus polidocanol versus chromated glycerin and Hoss 2020 and Peterson 2012a compared two types of foam.
Ten studies compared laser treatment (Christiansen 2015; Ianosi 2019; Klein 2013; Lupton 2002; Moreno‐Moraga 2013; Moreno‐Moraga 2014; Munia 2012; Nguyen 2020; Ozden 2011; Parlar 2015). Four types of laser were used for the treatment of telangiectasias and reticular veins: long pulsed 1064 nm Nd:YAG laser with different spot sizes, fluency and pulse duration; pulsed dye laser (PDL; 595 nm), potassium titanyl phosphate laser (KTP; 532 nm) and long pulsed 755 nm Nd:YAG. Six studies compared laser versus sclerotherapy (Lupton 2002 – laser versus STS, Munia 2012 – laser versus hypertonic glucose, Moreno‐Moraga 2013 – laser versus polidocanol (foam) versus laser plus polidocanol (foam), Moreno‐Moraga 2014 – laser plus polidocanol (foam) versus polidocanol (foam) and Parlar 2015 – laser versus polidocanol and Ianosi 2019 – laser versus polidocanol versus hypertonic saline). Four studies compared laser versus laser (Klein 2013 – PDL versus Nd:YAG, Christiansen 2015 – Nd:YAG versus Nd‐YAG, Ozden 2011 – KTP versus Nd:YAG, and Nguyen 2020 – Nd:YAG 1064 versus Nd:YAG 755).
Six studies compared additional therapy to the sclerosing agent or different treatment techniques of injecting sclerosing agent: Bayer 2021 and Kern 2007 – sclerotherapy versus sclerotherapy plus compression; Kern 2011 – chromated glycerin versus chromated glycerin plus lidocaine; Kern 2012 – chromated glycerin (standard technique) versus chromated glycerin (two‐step technique), Hamel‐Desnos 2009 – sclerotherapy plus warfarin versus sclerotherapy plus low molecular weight heparin, and Ochoa 2021 – sclerotherapy versus sclerotherapy plus sulodexide.
One study compared sclerotherapy versus compression stockings (Schul 2011).
We did not find eligible studies of the other techniques identified in our protocol (Nakano 2017): Intensive Pulsed Light (IPL), microphlebectomy, or thermocoagulation.
Outcomes
Thirty studies evaluated our primary outcome of improvement or resolution of telangiectasias using photographs and external examination (Alos 2006; Bayer 2021; Bertanha 2017; Bertanha 2021; Carlin 1987; Christiansen 2015; Goldman 2002; Hoss 2020; Ianosi 2019; Kahle 2006; Kern 2004; Kern 2007; Kern 2011; Kern 2012; Klein 2013; Lupton 2002; McCoy 1999; Moreno‐Moraga 2013; Moreno‐Moraga 2014; Munia 2012; Nguyen 2020; Norris 1989; Ozden 2011; Parlar 2015; Peterson 2012b; Prescott 1992; Rabe 2010; Rao 2005; Zhang 2012). Three studies evaluated improvement by direct clinical access (Benigni 1999; Leach 2003; Peterson 2012a). Nineteen studies included participant satisfaction as an outcome (Alos 2006; Benigni 1999; Carlin 1987; Christiansen 2015; Goldman 2002; Kahle 2006; Kern 2004; Kern 2007; Kern 2011; Klein 2013; McCoy 1999; Moreno‐Moraga 2013; Moreno‐Moraga 2014; Munia 2012; Parlar 2015; Peterson 2012b; Rabe 2010; Rao 2005; Zhang 2012).
Two included studies did not mention adverse effects (Kahle 2006; Kern 2011). The main adverse events reported by the other 33 studies are allergy, blistering, bruising, ecchymosis, hyperpigmentation, matting, microthrombosis, necrosis, scarring, swelling, transient neurological abnormality and urticaria. Four studies classified adverse events using different scales (Christiansen 2015; Klein 2013; McCoy 1999; Peterson 2012a).
Twenty‐three studies reported pain as an outcome, using different scales (Alos 2006; Bayer 2021; Bertanha 2017; Bertanha 2021; Carlin 1987; Christiansen 2015; Hoss 2020; Kern 2011; Kern 2012; Klein 2013; Leach 2003; Lupton 2002; McCoy 1999; Moreno‐Moraga 2014; Munia 2012; Nguyen 2020; Norris 1989; Ozden 2011; Parlar 2015; Peterson 2012b; Prescott 1992; Rao 2005; Zhang 2012).
Hamel‐Desnos 2009 did not report any outcomes of interest, as they studied prophylaxis of deep venous thrombosis. We report this as an adverse event.
Only Kern 2007 and Schul 2011 reported quality of life (QoL) as an outcome.
None of the 35 included studies reported on the outcomes of recurrence or time to resolution.
Excluded studies
See Characteristics of excluded studies.
Of the 10 excluded studies, eight were not randomised (Alora 1999; Conrad 1995; Gillet 2017; McDaniel 1999; Omura 2003; Sadick 2003; Weiss 1990; Woo 2003). Spendel 2002 was excluded for not comparing techniques. Dinsdale 2014 was excluded for not separating telangiectasias of the face and limbs.
Ongoing studies
We identified three ongoing studies (NCT04132323; NCT04690803; Zaleski‐Larsen 2017). See Characteristics of ongoing studies.
Risk of bias in included studies
See Figure 2 and Figure 3 for the risk of bias of all included studies summary, and the risk of bias tables of the Characteristics of included studies for further details.
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Random sequence generation
We ranked random sequence generation (selection bias) at low risk of bias in 15 studies (Alos 2006; Bayer 2021; Benigni 1999; Bertanha 2017; Bertanha 2021; Goldman 2002; Kern 2007; Kern 2011; Kern 2012; Klein 2013; Leach 2003; Moreno‐Moraga 2014; Ozden 2011; Rabe 2010; Schul 2011). We rated two studies at high risk of bias because scheduled appointments for randomisation were used (Moreno‐Moraga 2013), and legs laterality right and left were used (Nguyen 2020). The remaining 18 included studies were considered at unclear risk of bias due to lack of information (Carlin 1987; Christiansen 2015; Ochoa 2021; Hamel‐Desnos 2009; Hoss 2020; Ianosi 2019; Kahle 2006; Kern 2004; Lupton 2002; McCoy 1999; Munia 2012; Norris 1989; Parlar 2015; Peterson 2012a; Peterson 2012b; Prescott 1992; Rao 2005; Zhang 2012).
Allocation concealment
We rated only five included studies at low risk of bias (Bertanha 2017; Bertanha 2021; Goldman 2002; Klein 2013; Moreno‐Moraga 2014). We considered the remaining 30 studies to have an unclear risk of bias, due to lack of information.
Blinding
Blinding of participants and personnel
We judged 13 of the studies to be at low risk of bias for blinding of participants and personnel (Alos 2006; Bertanha 2017; Bertanha 2021; Carlin 1987; Goldman 2002; Hoss 2020; Kahle 2006; Kern 2011; Norris 1989; Peterson 2012b; Rabe 2010; Rao 2005; Zhang 2012). We rated nine studies at unclear risk of bias due to lack of information (Christiansen 2015; Ochoa 2021; Klein 2013; Moreno‐Moraga 2013; Nguyen 2020; Lupton 2002; Ozden 2011; Peterson 2012a; Prescott 1992), and 13 studies were considered to have a high risk of bias because the participants were not blinded (Bayer 2021; Benigni 1999; Hamel‐Desnos 2009; Ianosi 2019; Kern 2004; Kern 2007; Kern 2012; Leach 2003; McCoy 1999; Moreno‐Moraga 2014; Munia 2012; Parlar 2015; Schul 2011).
Blinding of outcome assessment
We judged 26 studies to have a low risk of bias (Alos 2006; Bayer 2021; Bertanha 2017; Bertanha 2021; Christiansen 2015; Goldman 2002; Ochoa 2021; Hoss 2020; Kahle 2006; Kern 2004; Kern 2007; Kern 2011; Klein 2013; Leach 2003; Lupton 2002; McCoy 1999; Moreno‐Moraga 2013; Munia 2012; Nguyen 2020; Ozden 2011; Parlar 2015; Peterson 2012a; Peterson 2012b; Rabe 2010; Rao 2005; Zhang 2012). Eight studies were considered to have an unclear risk of bias due to lack of information (Benigni 1999; Carlin 1987; Hamel‐Desnos 2009; Ianosi 2019; Moreno‐Moraga 2014; Norris 1989; Prescott 1992; Schul 2011). One study was considered at high risk of bias because outcome assessment was not blinded (Kern 2012).
Incomplete outcome data
We rated 24 studies at low risk of bias (Alos 2006; Bertanha 2017; Bertanha 2021; Carlin 1987; Christiansen 2015; Ianosi 2019; Kern 2004; Kern 2007; Kern 2011; Kern 2012; Klein 2013; Leach 2003; Lupton 2002; Moreno‐Moraga 2013; Moreno‐Moraga 2014; Munia 2012; Norris 1989; Parlar 2015; Peterson 2012a; Peterson 2012b; Rabe 2010; Rao 2005; Schul 2011; Zhang 2012). Nine studies were at unclear risk of bias (Bayer 2021; Goldman 2002;Hamel‐Desnos 2009Ochoa 2021; Hoss 2020; Kahle 2006; McCoy 1999; Nguyen 2020; Prescott 1992). We judged two studies to have a high risk of bias, because four people were "lost of view", as per personal communication with author (Benigni 1999), and because three participants were lost to follow‐up, and two left because of intolerance to pain in Ozden 2011.
Selective reporting
Only Leach 2003 was considered at low risk of bias. We considered two studies to be at high risk of bias, as some adverse events were not statistically analysed by sclerosing agent used and descriptive data are not provided (Carlin 1987; Prescott 1992). We judged the remaining 32 included studies to have an unclear risk of bias due to lack of information.
Other potential sources of bias
Only Carlin 1987 was considered to have a high risk of bias because the participants received separate simultaneous treatments and analysis, meaning that the outcomes could have been impacted due to the carry‐over effect. The remaining 34 studies had no clear evidence of other bias.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6; Table 7; Table 8; Table 9; Table 10
Sclerotherapy (any sclerosing agent) versus placebo
Four studies compared sclerotherapy versus placebo (Carlin 1987; Kahle 2006; Rabe 2010; Zhang 2012). The sclerosing agents used in these four studies were: polidocanol; sodium tetradecyl sulfate (STS); hypertonic saline. We were able to pool the data for these four studies in meta‐analysis. Carlin 1987 was a split‐body study and the data were reported by procedure. See Table 1.
Resolution or improvement of telangiectasias
All four included studies showed improvement or resolution of telangiectasias individually, and this benefit from sclerotherapy was also seen on pooling the data (SMD 3.08, 95% CI 2.68 to 3.48; I2 = 51%; 613 participants/procedures; moderate‐certainty evidence) (Analysis 1.1). We used a random‐effects model because of the clinical heterogeneity due to different agents used.
1.1. Analysis.

Comparison 1: Sclerotherapy (any sclerosing agent) versus placebo, Outcome 1: Resolution or improvement of telangiectasias
Adverse events
Adverse events were studied in three of the included studies (Carlin 1987; Rabe 2010; Zhang 2012). Results showed that hyperpigmentation was more frequent in the group of sclerosing agents compared to the placebo group (RR 11.88, 95% CI 4.54 to 31.09; I2 = 0%; 528 participants/procedures; moderate‐certainty evidence). Matting was more frequent in the group of sclerosing agents compared to the placebo group (RR 4.06, 95% CI 1.28 to 12.84; I2 = 0%; 528 participants/procedures; moderate‐certainty evidence) (Analysis 1.2).
1.2. Analysis.

Comparison 1: Sclerotherapy (any sclerosing agent) versus placebo, Outcome 2: Adverse events
Studies did not report on bruising, anaphylaxis or necrosis of the skin.
Pain during procedure and post‐procedure
Only Carlin 1987 assessed the outcome pain. There was more pain experienced in the sclerotherapy group compared to the placebo group (SMD 0.70, 95% CI 0.06 to 1.34; 40 procedure; low‐certainty evidence) (Analysis 1.3).
1.3. Analysis.

Comparison 1: Sclerotherapy (any sclerosing agent) versus placebo, Outcome 3: Pain
The outcomes 'recurrence', 'time to resolution' and 'quality of life (QoL)' were not reported by any of the four studies in this comparison.
Sclerotherapy (polidocanol) versus any sclerosing agent
Seven studies compared polidocanol versus another sclerosing agent (Carlin 1987; Goldman 2002; Kern 2004; McCoy 1999; Peterson 2012b; Rabe 2010; Rao 2005). Polidocanol was compared to STS (Carlin 1987; Goldman 2002; Rabe 2010; Rao 2005), to chromated glycerin (Kern 2004), and to hypertonic saline (Carlin 1987; McCoy 1999; Peterson 2012b). One study compared different concentrations of polidocanol without a control group (Norris 1989). All included studies were split‐body studies and the data were reported by procedure, except for Goldman 2002, who reported by participant. See Table 2.
Resolution or improvement of telangiectasias
We found no clear difference between the polidocanol group compared to the group of other sclerosing agents, for improvement or resolution (SMD 0.01, 95% CI −0.13 to 0.14; I2 = 0%; 7 studies, 852 participants/procedures; moderate‐certainty evidence) (Analysis 2.1).
2.1. Analysis.

Comparison 2: Sclerotherapy (polidocanol) versus sclerotherapy (any sclerosing agent), Outcome 1: Resolution or improvement of telangiectasias
Adverse events
There was no clear difference between the polidocanol group and other sclerosing‐agent groups in cases of hyperpigmentation (RR 0.94, 95% CI 0.62 to 1.43; I2 = 84%; 6 studies, 819 participants; moderate‐certainty evidence), or matting (RR 0.82, 95% CI 0.52 to 1.27; I2 = 21%; 7 studies, 859 participants/procedures; moderate‐certainty evidence). There were no clear differences in bruising (RR 0.77, 95% CI 0.56 to 1.06; I2 = 72%; 4 studies, 558 participants/procedures), microthrombosis (RR 0.96, 95% CI 0.69 to 1.34; I2 = 0%; 4 studies, 394 participants/procedures); or allergy between the groups (RR 0.68, 95% CI 0.23 to 2.01; I2 = 20%; 4 studies, 472 participants/procedures). There was less necrosis in the polidocanol group compared to the other sclerosing agents group (RR 0.07, 95% CI 0.02 to 0.29; I2 = 0%; 4 studies, 558 participants) (Analysis 2.2).
2.2. Analysis.

Comparison 2: Sclerotherapy (polidocanol) versus sclerotherapy (any sclerosing agent), Outcome 2: Adverse events
Pain during procedure and post‐procedure
There was less pain in the polidocanol group compared to other sclerosing agent group (SMD −0.26, 95% CI −0.44 to −0.08; I2 = 0%; 5 studies, 480 participants/procedures; moderate‐certainty evidence) (Analysis 2.3).
2.3. Analysis.

Comparison 2: Sclerotherapy (polidocanol) versus sclerotherapy (any sclerosing agent), Outcome 3: Pain
The outcomes 'recurrence', 'time to resolution' and 'QoL' were not available in the seven studies in this comparison.
Sclerotherapy (sodium tetradecyl sulfate (STS)) versus any sclerosing agent
Six studies compared sclerotherapy (sodium tetradecyl sulfate (STS)) with another sclerosing agent (Carlin 1987; Goldman 2002; Leach 2003; Prescott 1992; Rabe 2010; Rao 2005). Sodium tetradecyl sulphate (STS) was compared to polidocanol (Carlin 1987; Goldman 2002; Rabe 2010; Rao 2005), to chromated glycerin (Leach 2003) and to hypertonic dextrose (Prescott 1992). Carlin 1987; Leach 2003; Prescott 1992; Rao 2005 were split‐body studies and the data were reported by procedure. See Table 3.
Resolution or improvement of telangiectasias
There was no clear difference in improvement or resolution between the STS group or other agents group (SMD −0.07, 95% CI −0.25 to 0.11; I2 = 0%; 4 studies, 473 participants/procedures; moderate‐certainty evidence) (Analysis 3.1).
3.1. Analysis.

Comparison 3: Sclerotherapy (STS) versus sclerotherapy (any sclerosing agent), Outcome 1: Resolution or improvement of telangiectasias
Adverse events
There were more cases of hyperpigmentation (RR 1.71, 95% CI 1.10 to 2.64; ; I2 = 65%; 4 studies, 478 participants/procedures; moderate‐certainty evidence), and matting (RR 2.10, 95% CI 1.14 to 3.85; I2 = 0%; 2 studies, 323 participants; moderate‐certainty evidence) in the STS group compared with the other sclerosing agents group. There was more bruising (RR 1.62, 95% CI 1.14 to 2.30; I2 = 53%; 3 studies, 418 participants/procedures) and necrosis (RR 16.31, 95% CI 3.14 to 84.79; I2 = 0%; 2 studies, 392 participants/procedures) in the STS group. There was little or no difference in reports of allergy (RR 1.38, 95% CI 1.01 to 1.88; I2 = 0%; 3 studies, 452 participants/procedures) or microthrombosis (RR 1.04, 95% CI 0.78 to 1.39; 1 study, 129 participants/procedures) (Analysis 3.2).
3.2. Analysis.

Comparison 3: Sclerotherapy (STS) versus sclerotherapy (any sclerosing agent), Outcome 2: Adverse events
Pain during procedure and post‐procedure
STS probably results in more pain compared with other sclerosing agents (RR 1.49, 95% CI 0.99 to 2.25; I2 = 45%; 4 studies, 409 participants; moderate‐certainty evidence) (Analysis 3.3).
3.3. Analysis.

Comparison 3: Sclerotherapy (STS) versus sclerotherapy (any sclerosing agent), Outcome 3: Pain
The outcomes 'recurrence', 'time to resolution' and 'QoL' were not reported in the six studies in this comparison.
Sclerotherapy (hypertonic saline) versus any sclerosing agent
We included three studies in this comparison (Carlin 1987; McCoy 1999; Peterson 2012b). The sclerosing agent hypertonic saline was compared to STS (Carlin 1987) and to polidocanol (Carlin 1987; McCoy 1999; Peterson 2012b). All included studies were split‐body studies and the data were reported by procedure. See Table 4.
Resolution or improvement of telangiectasias
There was no clear difference in improvement or resolution of telangiectasias between the hypertonic saline group and the other sclerosing agent group (SMD 0.01, 95% CI −0.20 to 0.22; I2 = 0%; 3 studies, 348 participants/procedures; moderate‐certainty evidence) (Analysis 4.1).
4.1. Analysis.

Comparison 4: Sclerotherapy (hypertonic saline) versus sclerotherapy (any sclerosing agent), Outcome 1: Resolution or improvement of telangiectasias
Adverse events
There were fewer cases of hyperpigmentation in the hypertonic saline group than in another‐sclerosing‐agent group or the polidocanol subgroup (RR 0.74, 95% CI 0.59 to 0.93; I2 = 0%; 2 studies, 288 participants/procedures; moderate‐certainty evidence) (Analysis 4.2).
4.2. Analysis.

Comparison 4: Sclerotherapy (hypertonic saline) versus sclerotherapy (any sclerosing agent), Outcome 2: Adverse events
There was no clear difference in matting between the hypertonic‐saline group and another‐sclerosing‐agent group (RR 0.89, 95% CI 0.58 to 1.36; 2 studies; I2 = 0%; 288 participants/procedures; moderate‐certainty evidence) (Analysis 4.2).
No other adverse effects have been reported in the included studies.
Pain during procedure and post‐procedure
More pain was reported in the hypertonic‐saline group than in another‐sclerosing‐agent group (SMD 6.22, 95% CI 5.70 to 6.73; I2 = 0%; 3 studies, 348 participants/procedures; moderate‐certainty evidence) (Analysis 4.3).
4.3. Analysis.

Comparison 4: Sclerotherapy (hypertonic saline) versus sclerotherapy (any sclerosing agent), Outcome 3: Pain
The outcomes 'recurrence', 'time to resolution' and 'QoL' were not reported in the three studies in this comparison.
Sclerotherapy (chromated glycerin) versus any sclerosing agent
Four studies used chromated glycerin (Kern 2004; Kern 2011; Kern 2012; Leach 2003). Two studies analysed chromated glycerin as a sclerosing agent and compared it with POL (Kern 2004) and STS (Leach 2003). Kern 2012 and Leach 2003 were both split‐body studies and the data were reported by procedure. See Table 5.
The two further studies compared chromated glycerin versus chromated glycerin with different techniques, and assessed only pain, and so were not part of the meta‐analysis (Kern 2011; Kern 2012).
Resolution or improvement of telangiectasias
There was no difference in improvement or resolution of telangiectasias in the chromated glycerin group compared to the other sclerosing agent group (SMD 0.45, 95% CI −0.11 to 1.02; I2 = 44%; 2 studies, 125 participants/procedures; low‐certainty evidence) (Analysis 5.1).
5.1. Analysis.

Comparison 5: Sclerotherapy (chromated glycerin) versus sclerotherapy (any sclerosing agent), Outcome 1: Resolution or improvement of telangiectasias
Adverse events
There were no clear differences in hyperpigmentation (RR 0.49, 95% CI 0.09 to 2.50; I2 = 0%; 2 studies, 125 participants/procedures; low‐certainty evidence), or matting (RR 0.31, 95% CI 0.01 to 7.53; 1 study, 99 participants/procedures; low‐certainty evidence) between the chromated‐glycerin group compared to the other‐sclerosing‐agent group. There were no differences in bruising (RR 0.14, 95% CI 0.02 to 1.00; 1 study, 26 participants/procedures) or microthrombosis (RR 1.32, 95% CI 0.45 to 3.87; 1 study, 99 participants/procedures) between analysed groups (Analysis 5.2).
5.2. Analysis.

Comparison 5: Sclerotherapy (chromated glycerin) versus sclerotherapy (any sclerosing agent), Outcome 2: Adverse events
Pain during procedure and post‐procedure
There were no clear differences in pain (RR 1.50, 95% CI 0.30 to 7.55; 1 study, 26 participants/procedures; low‐certainty evidence) between the chromated glycerin group compared to another sclerosing agent (Analysis 5.3).
5.3. Analysis.

Comparison 5: Sclerotherapy (chromated glycerin) versus sclerotherapy (any sclerosing agent), Outcome 3: Pain
Kern 2011 studied pure chromated glycerin versus chromated glycerin plus 1% lidocaine, and demonstrated that a combination of chromated glycerin plus lidocaine resulted in less pain than chromated glycerin alone. Kern 2012 studied conventional sclerotherapy with chromated glycerin versus sclerotherapy with chromated glycerin in two steps. Kern 2012 concluded that the two‐step technique resulted in less pain than the conventional sclerotherapy technique.
The outcomes 'recurrence', 'time to resolution' and 'QoL' were not available in the studies in this comparison.
Foam versus sclerotherapy (any sclerosant agent)
Foam was compared to polidocanol in three studies (Alos 2006; Benigni 1999; Kern 2004).
We were able to pool data from two studies with 187 participants (Benigni 1999; Kern 2004). Benigni 1999 was a split‐body study and the data were reported by procedure. See Table 6.
Resolution or improvement of telangiectasias
There was no clear difference in improvement or resolution of telangiectasias between the foam group and the other‐sclerosing‐agents group (SMD 0.04, 95% CI −0.26 to 0.34; I2 = 0%; 2 studies, 187 participants/procedures; low‐certainty evidence) (Analysis 6.1).
6.1. Analysis.

Comparison 6: Foam versus sclerotherapy (any sclerosing agent), Outcome 1: Resolution or improvement of telangiectasias
Alos 2006 studied 75 participants (150 procedures) comparing polidocanol and foam. Three months after treatment, total occlusion of the vein was observed in 94% of foam interventions and 54% of polidocanol interventions (P < 0.001). Differences in the percentages of total efficacy for the two study groups were reported as statistically significant.
Adverse events
There was no clear difference in hyperpigmentation between the foam group and the other‐sclerosing‐agents group (RR 2.12, 95% CI 0.44 to 10.23; I2 = 0%; 2 studies, 187 participants/procedures; low‐certainty evidence). There were more cases of matting in the foam group compared with the other sclerosing agents group (RR 6.12, 95% CI 1.04 to 35.98; I2 = 0%; 2 studies, 187 participants/procedures; low‐certainty evidence). There was no difference in bruising (RR 0.60, 95% CI 0.35 to 1.04; 1 study, 40 participants/procedures), or microthrombosis (RR 1.39, 95% CI 0.70 to 2.76; I2 = 0%; 2 studies, 187 participants/procedures;) in the included studies (Analysis 6.2).
6.2. Analysis.

Comparison 6: Foam versus sclerotherapy (any sclerosing agent), Outcome 2: Adverse events
Alos 2006 reported that no complications occurred at the time of sclerotherapy. Inflammation was present in 25.3% of the foam group and 9.5% of the polidocanol liquid intervention group (P = 0.08). This study reported that the percentage of pigmentation was significantly higher at all follow‐up intervals for the foam group.
Pain during procedure and post‐procedure
There was no clear difference in pain between the foam group compared to the other‐sclerosing‐agents group (SMD −0.10, 95% CI −0.44 to 0.24; 1 study, 147 participants/procedures; low‐certainty evidence) (Analysis 6.3).
6.3. Analysis.

Comparison 6: Foam versus sclerotherapy (any sclerosing agent), Outcome 3: Pain
Benigni 1999, Kern 2004 and Alos 2006 did not report 'recurrence', 'time to resolution' or 'QoL'.
Laser versus sclerotherapy (any sclerosing agent)
Five studies were included in this comparison (Ianosi 2019; Lupton 2002; Moreno‐Moraga 2013; Munia 2012; Parlar 2015). Laser was compared to STS (Lupton 2002), hypertonic glucose, (Munia 2012), and polidocanol (Ianosi 2019; Moreno‐Moraga 2013; Parlar 2015). Ianosi 2019; Lupton 2002 and Munia 2012 were split‐body studies and the data were reported by procedure. See Table 7.
Resolution or improvement of telangiectasias
There were no clear differences in improvement or resolution of telangiectasias in the laser group compared to the any‐sclerosing‐agent group (SMD −0.09, 95% CI −0.25 to 0.07; I2 = 0%; 5 studies, 593 participants/procedures;moderate‐certainty evidence) (Analysis 7.1).
7.1. Analysis.

Comparison 7: Laser versus sclerotherapy (any sclerosing agent), Outcome 1: Resolution or improvement of telangiectasias
Adverse events
There were fewer hyperpigmentation events in the laser group than in the any‐sclerosing‐agent group (RR 0.57, 95% CI 0.40 to 0.80; I2 = 0%; 4 studies, 262 participants/procedures; moderate‐certainty evidence) (Analysis 7.2).
7.2. Analysis.

Comparison 7: Laser versus sclerotherapy (any sclerosing agent), Outcome 2: Adverse events
There were no clear differences between the laser group compared to the any‐sclerosing‐agent group in matting (RR 1.00, 95% CI 0.46 to 2.19; I2 = 0%; 2 studies, 162 participants/procedures;moderate‐certainty evidence). There were no differences in bruising (RR 0.79, 95% CI 0.60 to 1.04; 1 study, 40 participants/procedures), or necrosis (RR 1.60, 95% CI 0.20 to 12.74; I2 = 0%; 3 studies, 202 participants/procedures) in the included studies (Analysis 7.2).
Pain during procedure and post‐procedure
Due to the high heterogeneity among the included studies (I2 = 94%), we present the results qualitatively (Analysis 7.3).
7.3. Analysis.

Comparison 7: Laser versus sclerotherapy (any sclerosing agent), Outcome 3: Pain
In Lupton 2002, 70% of 20 participants reported mild treatment pain associated with both methods (laser and conventional sclerotherapy). Munia 2012 reported mild treatment pain in 7/30 participants in the laser group versus 26/30 in the sclerotherapy group; very painful in 20/30 participants versus 4/30 participants respectively in laser and sclerotherapy groups; and extremely painful in 3/30 participants in the laser group versus 0/30 in the sclerotherapy group.
The outcomes 'recurrence', 'time to resolution' and 'QoL' were not reported by the four studies in this comparison.
Laser plus sclerotherapy (polidocanol) versus sclerotherapy (polidocanol)
Moreno‐Moraga 2013 and Moreno‐Moraga 2014 compared a combination technique with laser neodymium: YAG (Nd:YAG) plus polidocanol (foam) sclerotherapy versus only sclerotherapy with polidocanol (foam). See Table 8.
Resolution or improvement of telangiectasias
There was more improvement or resolution in telangiectasias and reticular veins in the laser‐plus‐sclerotherapy group compared to the sclerotherapy group (SMD 5.68, 95% CI 5.14 to 6.23; I2 = 19%; 2 studies, 710 participants; low‐certainty evidence) (Analysis 8.1).
8.1. Analysis.

Comparison 8: Laser plus sclerotherapy (polidocanol) versus sclerotherapy (polidocanol), Outcome 1: Resolution or improvement of telangiectasias
Adverse events
There were no clear differences in hyperpigmentation (RR 0.83, 95% CI 0.35 to 1.99; I2 = 0%; 2 studies, 656 participants; low‐certainty evidence), or matting (RR 0.83, 95% CI 0.21 to 3.28; I2 = 0%; 2 studies, 656 participants; low‐certainty evidence) in the combination‐technique group compared to the sclerosing‐agent‐alone group. Studies did not report on bruising, anaphylaxis or necrosis of the skin (Analysis 8.2).
8.2. Analysis.

Comparison 8: Laser plus sclerotherapy (polidocanol) versus sclerotherapy (polidocanol), Outcome 2: Adverse events
Pain during procedure and post‐procedure
Only Moreno‐Moraga 2014 reported on pain. There was more pain in the combination‐technique group compared to the sclerosing‐agent‐alone group (RR 2.44, 95% CI 1.69 to 3.55; 1 study, 596 participants; low‐certainty evidence).
Moreno‐Moraga 2013 and Moreno‐Moraga 2014 did not report 'recurrence', 'time to resolution' or 'QoL'.
Sclerotherapy (hypertonic glucose plus polidocanol) versus hypertonic glucose
Two studies (191 participants) analysed hypertonic glucose as a sclerosing agent and compared hypertonic glucose plus polidocanol versus hypertonic glucose (Bertanha 2017; Bertanha 2021). See Table 9.
Resolution or improvement of telangiectasias
There was more improvement or resolution of telangiectasias in the combination‐technique group (polidocanol plus hypertonic glucose) when compared with the hypertonic‐glucose group (SMD 0.79, 95% CI 0.50 to 1.09; I2 = 0%; 2 studies, 191 participants; moderate‐certainty evidence) (Analysis 9.1).
9.1. Analysis.

Comparison 9: Sclerotherapy (polidocanol plus glucose) versus sclerotherapy (glucose), Outcome 1: Resolution or improvement of telangiectasias
Adverse events
There were no clear differences in hyperpigmentation (RR 0.79, 95% CI 0.62 to 1.01; I2 = 0%; 2 studies, 191 participants; moderate‐certainty evidence); matting (RR 0.78, 95% CI 0.51 to 1.20; I2 = 0%; 2 studies, 191 participants; moderate‐certainty evidence) when comparing the combination technique (polidocanol plus hypertonic glucose) with the hypertonic‐glucose group (Analysis 9.2).
9.2. Analysis.

Comparison 9: Sclerotherapy (polidocanol plus glucose) versus sclerotherapy (glucose), Outcome 2: Adverse events
No other adverse events were reported.
Pain during procedure and post‐procedure
There were no clear differences in pain (RR 1.02, 95% CI 0.83 to 1.24; I2 = 0%; 2 studies, 191 participants; moderate‐certainty evidence), when comparing the combination‐technique group (polidocanol plus hypertonic glucose) with the hypertonic‐glucose group (Analysis 9.3).
9.3. Analysis.

Comparison 9: Sclerotherapy (polidocanol plus glucose) versus sclerotherapy (glucose), Outcome 3: Pain
Bertanha 2017 and Bertanha 2021 did not evaluate 'recurrence', 'time to resolution' or 'QoL'.
Compression after sclerotherapy
Kern 2007 and Bayer 2021 studied the effect of compression after sclerotherapy. Both groups underwent sclerotherapy, then one group was randomised to go without compression stockings (WCS) and one group was randomised to use compression stocking (23 to 32 mmHg).
Resolution or improvement of telangiectasias
There was no difference in improvement or resolution in the compression‐after‐sclerotherapy group compared to the WCS group (SMD 0.09, 95% CI −0.19 to 0.37; I2 = 0%; 2 studies, 196 participants; moderate‐certainty evidence) (Analysis 10.1).
10.1. Analysis.

Comparison 10: Sclerotherapy plus compression versus sclerotherapy alone, Outcome 1: Resolution or improvement of telangiectasias
Adverse events
There were no clear differences in adverse events between the compression‐after‐sclerotherapy group and the WCS group for hyperpigmentation (RR 0.93, 95% CI 0.41 to 2.07; ; I2 = 0%; 2 studies, 196 participants; moderate‐certainty evidence); or matting (RR 1.84, 95% CI 0.17 to 19.62; 1 study, 96 participants; low‐certainty evidence) (Analysis 10.2).
10.2. Analysis.

Comparison 10: Sclerotherapy plus compression versus sclerotherapy alone, Outcome 2: Adverse events
Quality of life (QoL)
There was no difference in QoL scores (SF‐36 questionnaires) between the compression‐after‐sclerotherapy group and the WCS group (SMD −0.02, 95% CI −0.42 to 0.39; 1 study, 93 participants; low‐certainty evidence) (Analysis 10.3).
10.3. Analysis.

Comparison 10: Sclerotherapy plus compression versus sclerotherapy alone, Outcome 3: Quality of life
The outcomes 'pain', 'recurrence' and 'time to resolution' were not reported by Kern 2007 or Bayer 2021.
Foam (STS plus air) versus foam (STS plus CO2)
Only Peterson 2012a studied different types of foam combined with air or CO2.
Resolution or improvement of telangiectasias
Peterson 2012a reported no difference in improvement or resolution in reticular veins when the STS‐plus‐air group (20 participants) was compared with the STS‐plus‐CO2 group (20 participants). The study authors did not explore the resolution of telangiectasias, since these veins were treated with glycerin solution.
Adverse events
Peterson 2012a reported there was no clear difference in adverse events between the STS‐plus‐air group and the STS‐plus‐CO2 group. Coagulums were presented in 55% of CO2 foam and 60% of RA foam (P = 0.75).
Peterson 2012a did not report 'pain', 'recurrence', 'time to resolution' or 'QoL'.
Foam (POL plus 1:2 air) versus foam (POL plus 1:4 air)
Only Hoss 2020 studied foam with polidocanol combined with different proportions of air (1:2 and 1:4).
Resolution or improvement of telangiectasias
The study authors reported a mean improvement between 0% and 50% at day 21 and 26% to 75% at day 90, with no significant difference in the resolution or improvement of the reticular veins between the 1:2 ratio versus the 1:4 ratio groups.
Adverse events
The study authors found no statistically significant difference for adverse events between the 1:2 and 1:4 ratio groups at any time point. Most participants rated pain during injection (1.73 vs 1.70), current pain (0.80 vs 1.07), itching (0.57 vs 0.83), swelling (0.93 vs 1.37), and redness (1.53 vs 1.83) as none, minimal, or mild in both the 1:2 and 1:4 ratio groups, respectively.
Laser versus laser
We included four studies for a qualitative analysis (Christiansen 2015; Klein 2013; Nguyen 2020; Ozden 2011). We did not pool data in a meta‐analysis because they were isolated studies using different laser techniques that cannot be pooled.
Ozden 2011 studied potassium‐titanyl‐phosphate (KTP) versus Nd:YAG.
Study authors reported a significant positive correlation in the Nd:YAG group compared to the KTP laser group for outcome improvement or resolution in veins of 1.0 mm to 3.0 mm. There was no difference between KTP and Nd:YAG laser for telangiectasias and veins of less than 1.0 mm. They reported that both laser treatments were well‐tolerated by all participants, with no reported serious adverse events. Urticaria appeared in most participants immediately after treatment, but resolved within a few hours. The average level of pain reported by participants during treatment with the KTP laser was 3.1 (95% CI 2.23 to 3.97) compared with 6.89 (95% CI 5.63 to 8.15) for the Nd:YAG treatment.
Klein 2013 studied indocyanine green (ICG)‐augmented diode laser therapy (808 nm) versus diode laser without ICG and pulsed dye laser (PDL).
The mean clearance rate of resolution of telangiectasias for PDL therapy after three months was 2.07 (95% CI 1.07 to 3.07) (moderate clearance), as rated by the participants, and 0.78 (95% CI −0.11 to 1.67) (no difference) as rated by the blinded investigator. The rate of resolution of telangiectasias for ICG diode laser therapy alone was 0.3, as rated by the blinded investigator. Hypopigmentation and hyperpigmentation were seen in 32% of participants.
Christiansen 2015 studied different parameters of Nd:YAG laser. The application of Nd;YAG laser with fixed (FF) and adjustable (JF) parameters has been studied. There was no difference in improvement or resolution with FF or JF parameters.
There was a higher incidence of adverse events (hyperpigmentation) at 39.3% versus 28.6% in the FF group (P = 0.05).
Pain was not mentioned by Christiansen 2015.
Nguyen 2020 studied long pulsed 1064 nm (LP 1064) versus 755 nm (LP 755). There was no difference in improvement or resolution of telangiectasias or reticular veins (71.87% in LP 1064 versus 71.69% in LP 755, P = 0.99). All participants reported painful sensation. The study reported that pain caused by LP 1064 with a median of 7 (range 2 to 8) was significantly higher than pain caused by LP 755, with a median of 5 (range 2 to 8; P = 0.001). Hyperpigmentation occurred in half or more of the participants at one month of observation, with no significant difference between the two groups (63.64% and 50% for LP 1064 and LP 755, respectively; P = 0.36).
The preplanned outcomes 'recurrence', 'time to resolution' and 'QoL' were not reported by the studies in this comparison.
Different concentrations of polidocanol
Norris 1989 compared different concentrations of polidocanol, 0.25%, 0.50%, 0.75%, and 1.0% in 20 participants. There were no differences among the four dosages for improvement or resolution of telangiectasias, itching, or neovascularisation. Polidocanol concentrations of 0.75% and 1% showed more hyperpigmentation (P = 0.15 and P = 0.07, respectively).
Compression versus sclerotherapy
Schul 2011 compared compression stockings (20 to 30 mmHg) versus sclerotherapy in 58 participants with symptomatic reticular veins and telangiectasias. They only reported on quality of life, measured using an Aberdeen Varicose Vein Questionnaire. The study reported that compression stockings can offer relief of aching (P < 0.001), pain (P = 0.002); and cramping (P = 0.003) in participants with isolated refluxing reticular veins and telangiectasias. Sclerotherapy of these smaller vessels offers superior relief of aching (P < 0.001), pain (P < 0.001), swelling (P < 0.001), leg cramps (P < 0.05), and presence of symptoms at rest.
Sclerotherapy plus sulodexide versus sclerotherapy
Ochoa 2021 compared sclerotherapy with POL plus sulodexide versus sclerotherapy in 609 participants with telangiectasias and reticular veins. The study authors reported there was no difference in improvement or resolution between sulodexide group and control group after three months (76% in sulodexide group and 73% in control group, P = 0.61). There was less hyperpigmentation in the sulodexide group compared with the control group at one month (10.7% in sulodexide group and 18.2% control group; P < 0.01).
Sclerotherapy plus warfarin versus sclerotherapy plus nadroparin
Hamel‐Desnos 2009 compared sclerotherapy with POL plus warfarin versus sclerotherapy with POL plus nadroparin in 105 participants with thrombophilia. There was no thromboembolic event in any group. The rate of inflammatory reactions (1.5%) and superficial thrombophlebitis (1.5%) did not exceed that found in the general population (4.5%). They did not report on any other outcomes of interest.
Reporting bias, subgroup and sensitivity analyses
We were unable to assess reporting bias using funnel plots, as none of the meta‐analyses included 10 or more studies.
We were unable to perform the planned subgroup analyses for interventions of participant characteristics, because there were no data available in the selected studies.
We planned to carry out sensitivity analyses by excluding those trials that we judged to be at high risk of bias for allocation concealment or blinding of outcome assessment. No studies were at high risk of allocation bias. Kern 2012 was at high risk from blinding of outcome assessment, but was not included in any meta‐analysis.
Discussion
Summary of main results
We identified 35 randomised controlled trials (RCTs) with a total of 3632 participants, which used a variety of different methods to treat telangiectasias and reticular veins. None of the included studies reported on recurrence or time to resolution, and most did not report on quality of life (QoL).
There is moderate‐certainty evidence that sclerosing agents are more effective for resolution or improvement of telangiectasias compared to a placebo, but that they also have more adverse effects. The observed adverse effects were relatively minor, such as hyperpigmentation and matting. There may be increased pain caused by the sclerosing agent that cannot be attributed solely to the injection of the agent (low‐certainty evidence). See Table 1.
We did not find reliable evidence for the superiority of any of the various sclerosing agents studied for resolution or improvement of telangiectasias (moderate‐certainty evidence). See Table 2. Polidocanol and hypertonic saline probably cause less necrosis and hyperpigmentation respectively, when compared to other sclerosing agents (moderate‐certainty evidence). STS resulted in more hyperpigmentation, matting (moderate‐certainty evidence), bruising and necrosis, and probably more pain(moderate‐certainty evidence), than the other sclerosing agents. See Table 3. Hypertonic saline may result in more pain compared to other sclerosing agents (moderate‐certainty evidence). See Table 4. There were few studies with glycerin and glucose and no differences in resolution or improvement of telangiectasias, adverse events or pain were detected (low‐certainty evidence). See Table 5.
The use of foam did not affect resolution compared to liquid polidocanol (low‐certainty evidence), and no clear differences were detected in hyperpigmentation, bruising, microthrombus or pain (low‐certainty evidence). There may be more matting in the foam group compared to other sclerosing agents (low‐certainty evidence). See Table 6.
Laser treatment had similar improvement or resolution of telangiectasias compared to the any‐sclerosing‐agent group (moderate‐certainty evidence). Laser treatment may result in less hyperpigmentation compared to any sclerosing agents (moderate‐certainty evidence), but no differences were detected for matting, bruising or necrosis compared to other sclerosing agents. Due to the high heterogeneity among the included studies, the pain data are presented qualitatively. See Table 7.
The combination technique laser plus polidocanol may be more effective to treat telangiectasias and reticular veins compared to polidocanol alone (low‐certainty evidence). There were no differences in hyperpigmentation and matting (low‐certainty evidence), but more pain may occur after laser plus polidocanol (low‐certainty evidence). See Table 8.
The combination technique hypertonic glucose plus polidocanol was probably more effective to treat telangiectasias and reticular veins compared to hypertonic glucose alone (moderate‐certainty evidence). There were no differences in hyperpigmentation, matting or pain (moderate‐certainty evidence). See Table 9.
The combination technique sclerotherapy plus compression did not affect improvement or resolution of telangiectasias compared to sclerotherapy alone (moderate‐certainty evidence). No clear differences were detected for hyperpigmentation (moderate‐certainty evidence), matting or QoL (low‐certainty evidence). See Table 10.
Overall completeness and applicability of evidence
We did not find any studies comparing intensive pulsed light (IPL), thermocoagulation and microphlebectomy with other techniques for treatment of telangiectasias and reticular veins. The included studies did not present data on time to resolution, recurrence or QoL. We found different study designs using either body parts (opposite leg or a lower limb quadrant), or the individual participant as a research unit, which makes it difficult to analyse the data together.
Quality of the evidence
We used GRADE to evaluate the certainty of the evidence (Grade 2004).
Sclerotherapy versus placebo
See Table 1. The certainty of the evidence for the outcome 'resolution or improvement of telangiectasias' was downgraded by one level to moderate because of inconsistency due to the clinical heterogeneity of the included studies. We downgraded by one level to moderate for adverse events (hyperpigmentation and matting) because of clinical heterogeneity and imprecision due to the wide of confidence intervals (CIs). The certainty of the evidence for pain was downgraded by two levels to low because of clinical heterogeneity, the small sample size and the data being from a single study.
Polidocanol versus any sclerosing agent
See Table 2. The certainty of the evidence for the outcomes 'resolution or improvement of telangiectasias', adverse events (hyperpigmentation and matting), and pain was downgraded by one level due to a wide CI.
STS versus any sclerosing agent
See Table 3. The certainty of the evidence for the outcomes 'resolution or improvement of telangiectasias', adverse events (hyperpigmentation and matting), and pain was downgraded by one level to moderate because of imprecision, due to the wide CI and few included participants.
Hypertonic saline versus any sclerosing agent
See Table 4. The certainty of the evidence for the outcome 'resolution or improvement of telangiectasias' was downgraded by one level to moderate because of risk of bias in the included studies. The certainty of the evidence for adverse events (hyperpigmentation and matting) was downgraded by one level to moderate because of imprecision due to the wide CI. Pain was downgraded by one level to moderate due to the clinical heterogeneity of the included studies.
Chromated glycerin versus any sclerosing agent
See Table 5. The certainty of the evidence for the outcomes 'resolution or improvement of telangiectasias', adverse events (hyperpigmentation, matting) and pain, was downgraded by two levels to low because of few included participants.
Foam versus any sclerosing agent
See Table 6. The certainty of the evidence for the outcomes 'resolution or improvement of telangiectasias', adverse events (hyperpigmentation and matting), and pain was downgraded by two levels to low because of imprecision, due to the wide CI and few participants in the included studies.
Laser versus sclerotherapy
See Table 7. The certainty of the evidence for the outcome 'resolution or improvement of telangiectasias', adverse events (hyperpigmentation and matting) was downgraded by one level to moderate because of imprecision due to the wide CI. Pain was downgraded by two levels because of few participants in the included studies.
Laser plus POL versus POL
See Table 8. The certainty of the evidence for the outcome 'resolution or improvement of telangiectasias', and adverse events (hyperpigmentation and matting), was downgraded by two levels to low because of inconsistency due to the clinical heterogeneity between the included studies and the fact that the two studies in this comparison were conducted by the same group of investigators. Pain was downgraded by two levels to low because there was only one included study.
Hypertonic glucose plus POL versus hypertonic glucose
See Table 9. The certainty of the evidence for all outcomes,'resolution or improvement of telangiectasias', adverse events (hyperpigmentation and matting) and pain was downgraded by one level to moderate because of few participants in the included studies.
Sclerotherapy plus compression versus sclerotherapy
See Table 10. The certainty of the evidence for 'resolution or improvement of telangiectasias' and hyperpigmentation was downgraded by one level to moderate because of few participants in the included studies. Matting and QoL were downgraded two levels because of few participants and only one included study.
Potential biases in the review process
We have attempted to include all available RCTs in this review, but it is possible that some studies have not been included, especially from the grey literature. We adhered to the inclusion and exclusion criteria prespecified in the protocol in order to limit subjectivity (Nakano 2017). We made efforts to obtain additional relevant data from study authors but were unable to do so for all. If we can source supplementary data, we will consider them in future updates. Two review authors selected studies in duplicate, independently, to reduce potential bias of the review process. Three review authors independently extracted and collected data, and assessed risks of bias of the included studies to reduce potential bias in the review process. We were not able to include all studies in a meta‐analysis.
Agreements and disagreements with other studies or reviews
Schwartz 2011 studied sclerosing agents for the treatment of telangiectasias, but they evaluated neither reticular veins nor adverse events in their review. In agreement with our findings, Schwartz 2011 reported that no sclerosing agent studied was more effective than the others, with a low quality of evidence due to a lack of eligible studies.
Smith 2015 studied the management of reticular veins and telangiectasias of the lower limb, by sclerotherapy, radiofrequency and laser. They concluded that sclerotherapy was the most effective method for the treatment of reticular veins and telangiectasias. This was a narrative rather than a systematic review, and included all types of studies.
In the Management of Chronic Venous Disease Clinical Practice Guidelines of the European Society for Vascular Surgery (ESVS), Wittens 2015 recommended that liquid sclerotherapy should be considered for treating telangiectasias and reticular veins. This recommendation was made based on studies that were included in our review (Kahle 2006, Rabe 2010, Zhang 2012). European guidelines for sclerotherapy in chronic venous disorders (Rabe 2014) also recommends sclerotherapy as a treatment for telangiectasias.
Yiannakopoulou 2016 studied the adverse events of sclerosing agents. Hyperpigmentation and matting were the most frequently‐reported local adverse events in sclerotherapy, a finding supported by our review.
Authors' conclusions
Implications for practice.
Sclerosing agents were more effective than placebo for resolution or improvement of telangiectasias, but also cause more adverse events and pain (moderate and low‐certainty evidence). There was no evidence that any sclerosing agent resulted in more resolution or improvement of telangiectasias compared to another or to laser, but there may be some differences in adverse events and pain between some treatments: polidocanol probably causes less necrosis; hypertonic saline probably causes less hyperpigmentation but more pain compared to other sclerosing agents (moderate‐certainty evidence); STS resulted in more hyperpigmentation, matting and probably more pain than other sclerosing agents (moderate‐certainty evidence). Foam agents compared to liquid did not improve resolution, but there may be more matting (moderate‐certainty evidence). Laser treatment may result in less hyperpigmentation compared to any sclerosing agents (moderate‐certainty evidence); combining laser with polidocanol may be more effective to treat telangiectasias and reticular veins compared to polidocanol alone (low‐certainty evidence), but more pain may occur (low‐certainty evidence). There was more improvement of telangiectasias in combining polidocanol with hypertonic glucose compared with hypertonic glucose alone (moderate‐certainty evidence), and no clear differences in adverse events or pain (moderate‐certainty evidence). There was no clear difference in improvement, adverse events or quality of life when comparing sclerotherapy plus compression with sclerotherapy alone. Small numbers of studies and participants in each comparison limited our confidence in the evidence.
Implications for research.
Although the treatment of telangiectasias and reticular veins has been conducted by vascular surgeons for several years, there is limited high‐certainty evidence. The lack of standardisation in studies also makes it difficult to analyse and summarise the evidence. We suggest that future trials use a standard methodology. For the intervention standard, the number of sessions is critical for access and to compare data. As most trials evaluated only one intervention session, we suggest that it should be standardised as a unique session to facilitate analysis of the outcome.
Another important topic is the design of the study: some authors compared different individuals and others compare similar regions on different limbs in the same participant. In our view the best design for the study of telangiectasias and reticular veins is the split‐body design, since we are analysing different treatments in the same individual, and the response obtained can be inferred more appropriately for each treatment; we acknowledge that a split‐body design could aggravate the randomisation process. Different study designs can be considered, provided that they are well‐standardised and specified.
We suggest using a single scale to facilitate the interpretation of results for the 'improvement or resolution' outcome and later inclusion of studies in a meta‐analysis. Most of the reviewed studies used a scale of 0 to 4, so we suggest the use of this scale in future trials, with specifications in percentages in order to infer the results obtained with more precision.
When evaluating adverse events it is important for future studies to establish whether they are immediate or late effects. We suggest that immediate adverse events should be evaluated just after the specific session and delayed adverse events arising three to six months after the procedure should also be evaluated in order to study their evolution.
In the studies reviewed here, pain was analysed using the analogue pain scale (0 to 100 mm), and we suggest that standardising the VAS for pain is important for the comparison of results.
History
Protocol first published: Issue 7, 2017
Notes
Parts of the Methods section of this review are based on a standard template established by Cochrane Vascular.
Acknowledgements
We would like to thank Cochrane Vascular, Cochrane Brazil and the Division of Vascular and Endovascular Surgery of the Federal University of Sao Paulo, Brazil for their methodological support. We also thank the authors that have provided additional information of the included studies: JP Benigni, MP Goldman and J Zhang.
The review authors and the Cochrane Vascular Editorial base wish to thank the following peer reviewers for their comments: István B. Bálint, MD, PhD, Department of Surgery and Vascular Surgery, Zala County Saint Rafael Hospital, Hungary; Dr Jean‐Jérôme Guex, Président European Board of Phlebology UEMS, Editor in Chief Phlébologie – Annales Vasculaires, Nice, France; Professor Philip Coleridge‐Smith, British Vein Institute, Amersham, UK; M Dulce Estêvão, School of Health ‐ University of Algarve, Portugal.
Appendices
Appendix 1. Sources searched and search strategies
| Source | Search strategy | Hits retrieved |
| VASCULAR REGISTER IN CRSW | 1. telang* AND INREGISTER 2. Spider AND INREGISTER 3. reticular near3 vein* AND INREGISTER 4. #1 OR #2 OR #3 |
29.8.17 ‐ 63 21.1.19 ‐ 2 16.3.21 ‐ 82 |
| CENTRAL via CRSW | #1 MESH DESCRIPTOR Telangiectasis EXPLODE ALL TREES 146 #2 telangiectas*:TI,AB,KY 551 #3 microvaric*:TI,AB,KY 1 #4 (reticular near3 vein*):TI,AB,KY 27 #5 (reticular near3 varic* ):TI,AB,KY 1 #6 (reticular near3 venous ):TI,AB,KY 0 #7 (thread near3 vein* ):TI,AB,KY 0 #8 (thread near3 varic* ):TI,AB,KY 1 #9 (thread near3 venous ):TI,AB,KY 0 #10 (spider near3 vein* ):TI,AB,KY 10 #11 (spider near3 varic* ):TI,AB,KY 1 #12 (spider near3 venous ):TI,AB,KY 1 #13 angioectasias:TI,AB,KY 6 #14 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 574 #15 01/01/2017 TO 22/01/2019:CD 324775 #16 #14 AND #15 177 |
29.8.17 ‐ 547 21.1.19 ‐ 117 16.3.21 ‐ 228 |
| Clinicaltrials.gov | Telangiectasis OR telangiectatic OR "reticular veins" OR "reticular vein" OR "spider veins" OR "spider vein" OR "thread veins" OR "thread vein" OR angioectasias | Interventional Studies | 29.8.17 ‐ 136 21.1.19 ‐ 18 16.3.21 ‐ 30 |
| ICTRP Search Portal | Telangiecta* OR reticular vein* OR spider vein* OR thread vein* OR angioectasias | 29.8.17 ‐ 131 21.1.19 ‐ 31 16.3.21 ‐ 43 |
| Ovid MEDLINE Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE Daily and Ovid MEDLINE 1946 to Present | 1 exp Telangiectasis/ 2 telangiectas*.ti,ab. 3 microvaric*.ti,ab. 4 (reticular adj3 varic*).ti,ab. 5 (reticular adj3 vein*).ti,ab. 6 (reticular adj3 venous).ti,ab. 7 (thread adj3 vein*).ti,ab. 8 (thread adj3 varic*).ti,ab. 9 (thread adj3 venous).ti,ab. 10 (spider adj3 vein*).ti,ab. 11 (spider adj3 varic*).ti,ab. 12 (spider adj3 venous).ti,ab. 13 angioectasias.ti,ab. 14 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 15 limit 14 to yr="2017" 16 randomized controlled trial.pt. 17 controlled clinical trial.pt. 18 randomized.ab. 19 placebo.ab. 20 drug therapy.fs. 21 randomly.ab. 22 trial.ab. 23 groups.ab. 24 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 25 exp animals/ not humans.sh. 26 24 not 25 27 15 and 26 |
29.8.17 ‐ 45 21.1.19 ‐ 235 16.3.21 ‐ 230 |
| Embase Ovid | 1 exp Telangiectasis/ 2 telangiectas*.ti,ab. 3 microvaric*.ti,ab. 4 (reticular adj3 varic*).ti,ab. 5 (reticular adj3 vein*).ti,ab. 6 (reticular adj3 venous).ti,ab. 7 (thread adj3 vein*).ti,ab. 8 (thread adj3 varic*).ti,ab. 9 (thread adj3 venous).ti,ab. 10 (spider adj3 vein*).ti,ab. 11 (spider adj3 varic*).ti,ab. 12 (spider adj3 venous).ti,ab. 13 angioectasias.ti,ab. 14 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 15 limit 14 to yr="2017" 16 randomized controlled trial/ 17 controlled clinical trial/ 18 random$.ti,ab. 19 randomization/ 20 intermethod comparison/ 21 placebo.ti,ab. 22 (compare or compared or comparison).ti. 23 ((evaluated or evaluate or evaluating or assessed or assess) and (compare or compared or comparing or comparison)).ab. 24 (open adj label).ti,ab. 25 ((double or single or doubly or singly) adj (blind or blinded or blindly)).ti,ab. 26 double blind procedure/ 27 parallel group$1.ti,ab. 28 (crossover or cross over).ti,ab. 29 ((assign$ or match or matched or allocation) adj5 (alternate or group$1 or intervention$1 or patient$1 or subject$1 or participant$1)).ti,ab. 30 (assigned or allocated).ti,ab. 31 (controlled adj7 (study or design or trial)).ti,ab. 32 (volunteer or volunteers).ti,ab. 33 trial.ti. 34 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 35 15 and 34 |
29.8.17 ‐ 99 21.1.19 ‐ 581 16.3.21 ‐ 487 |
| AMED (Allied and Complementary Medicine) | 1. telangiectas*.ti,ab. 2. microvaric*.ti,ab. 3. (reticular adj3 varic*).ti,ab. 4. (reticular adj3 vein*).ti,ab. 5. (reticular adj3 venous).ti,ab. 6. (thread adj3 vein*).ti,ab. 7. (thread adj3 varic*).ti,ab. 8. (thread adj3 venous).ti,ab. 9. (spider adj3 vein*).ti,ab. 10. (spider adj3 varic*).ti,ab. 11. (spider adj3 venous).ti,ab. 12. angioectasias.ti,ab. 13. or/1‐12 14. exp CLINICAL TRIALS/ 15. RANDOM ALLOCATION/ 16. DOUBLE BLIND METHOD/ 17. Clinical trial.pt. 18. (clinic* adj trial*).tw. 19. ((singl* or doubl* or trebl* or tripl*) adj (blind* or mask*)).tw. 20. PLACEBOS/ 21. placebo*.tw. 22. random*.tw. 23. PROSPECTIVE STUDIES/ 24. or/14‐23 25. 13 and 2 |
29.8.17 ‐ 0 21.1.19 ‐ 0 16.3.21 ‐ 0 |
| CINAHL | S30 S28 AND S29 S29 EM 2017 OR EM 2018 OR 2019 EM S28 S14 AND S27 S27 S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24 OR S25 OR S26 S26 MH "Random Assignment" S25 MH "Single‐Blind Studies" or MH "Double‐Blind Studies" or MH "Triple‐Blind Studies" S24 MH "Crossover Design" S23 MH "Factorial Design" S22 MH "Placebos" S21 MH "Clinical Trials" S20 TX "multi‐centre study" OR "multi‐center study" OR "multicentre study" OR "multicenter study" OR "multi‐site study" S19 TX crossover OR "cross‐over" S18 AB placebo* S17 TX random* S16 TX trial* S15 TX "latin square" S14 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 S13 TX angioectasias S12 TX spider venous S11 TX spider varic* S10 TX spider vein* S9 TX thread venous S8 TX thread varic* S7 TX thread vein* S6 TX reticular venous S5 TX reticular varic* S4 TX reticular vein* S3 TX telangiectas* S2 (MH "Telangiectasis+") S1 TX Telangiectasis |
29.8.17 ‐ 49 21.1.19 ‐ 41 16.3.21 ‐ 23 |
Appendix 2. LILACS/BECS search strategy
((MH: "Telangiectasis" OR MH: "Telangiectasia" OR MH: "Telangiectasia" OR "Spider Veins") AND (MH: "Lasers" OR MH: "Rayos Láser" OR MH: "Lasers" OR "Masers" OR E07.632.490$ OR E07.710.520$ OR SP4.011.087.698.384.075.166.027$ OR VS2.006.002.009$ OR MH: "Laser Coagulation" OR MH: "Coagulación con Láser" OR MH: "Fotocoagulação a Laser" OR "Laser Thermocoagulation" OR "Thermocoagulation, Laser" OR E02.520.745.410$ OR E02.594.530$ OR E04.014.520.530$ OR E04.350.750.410$ OR E04.540.630.410$ OR MH: "Low‐Level Light Therapy" OR MH: "Terapia por Luz de Baja Intensidad" OR MH: "Terapia com Luz de Baixa Intensidade" OR "Laser Therapy, Low‐Level" OR "Laser Biostimulation" OR "Laser Irradiation, Low‐Power" OR "LLLT" OR E02.594.540$ OR E02.774.500$ OR MH: "Laser Therapy" OR MH: "Terapia por Láser" OR MH: "Terapia a Laser" OR "Laser Knife" OR "Laser Scalpel" OR "Surgery, Laser" OR "Vaporization, Laser" OR E02.594$ OR E04.014.520$ OR MH: "Lasers, Gas" OR MH: "Láseres de Gas" OR MH: "Lasers de Gás" OR "Argon Ion Lasers" OR "Carbon Dioxide Lasers" OR "CO2 Lasers" OR "Copper Vapor Lasers" OR "Gas Laser" OR "Gas Lasers" OR "Gold Vapor Lasers" OR "Helium Lasers" OR "Helium Neon Gas Lasers" OR "Metal Vapor Lasers" OR "Nitrogen Lasers" OR "Xenon Ion Lasers" OR E07.632.490.367$ OR E07.710.520.367$ OR MH: "Intense Pulsed Light Therapy" OR "Tratamiento de Luz Pulsada Intensa" OR "Terapia de Luz Pulsada Intensa" OR MH: "Sclerotherapy" MH: "Escleroterapia" MH: "Escleroterapia" OR MH: "Sclerosing Solutions" OR MH: "Soluciones Esclerosantes" OR MH: "Soluções Esclerosantes" OR "Injections, Sclerosing" OR "Sclerosing Agents" OR D26.776.708.822$ OR D27.505.954.411.700$ OR D27.505.954.578.822$ OR D27.720.752.822$)) AND (DB:("IBECS" OR "LILACS"))
Appendix 3. Glossary
| acne vulgaris | skin disease caused by overactivity of sebaceous glands |
| ambulatory | people treated out of the hospital setting |
| angiomas | dilatation or new formation of blood vessels |
| arterioles | small branches of an artery |
| atrophic blanche | small smooth ivory‐white areas on the skin with hyperpigmented borders and telangiectasias |
| chromophore | chemical group that absorbs light at a specific frequency |
| dermal | relating to skin and specially to the dermis |
| dorsum | the dorsal part of an organism such as human body |
| endothelium | tissue that forms a single layer of cells lining various organs |
| epidermal | non sensitive layer of the skin |
| erythema | superficial reddening of the skin |
| extravasation | escape of blood from a vessel into the tissues |
| fibrosis | the thickening and scarring of connective tissue |
| hypopigmentation | decreased pigmentation of skin area |
| hyperpigmentation | increased pigmentation of skin area |
| lipodermatosclerosis | chronic fibrosing panniculitis associated with venous insufficiency |
| matting | new telangiectasis after treatment |
| melanin | pigment responsible for determining skin and hair colours |
| microthrombi | small thrombus (blood clot formed in situ within the vascular system) |
| necrosis | death of most or all of the cells in an organ or tissue |
| occlusion | blockage of blood vessel |
| oedema | excess of watery fluid collecting in the tissue of the body and outside of blood vessels |
| osmotic | diffusion of fluid through a semipermeable membrane |
| oxyhaemoglobin | substance formed by the combination of haemoglobin with oxygen |
| periorbital | tissues surrounding or lining the orbit of the eye |
| photocoagulation | coagulation of tissue using a laser or other intense light source |
| photothermolysis | a method of laser skin resurfacing |
| polychromatic | various wavelengths or frequencies |
| recanalisation | process of restoring flow of the blood vessels |
| subcutaneous | situated or applied under the skin |
| subdermal | situated or lying under the skin |
| thermocoagulation | coagulation of tissue with high‐frequency currents |
| thermosclerosis | coagulation of blood vessels for heat |
| thrombosis | local coagulation or clotting of the blood in a part of circulatory system |
| vascular | relating to blood vessels |
| venous | relating to a vein |
| venules | very small veins |
| vesicles | small fluid‐filled bladders, sacs, or cysts |
Data and analyses
Comparison 1. Sclerotherapy (any sclerosing agent) versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Resolution or improvement of telangiectasias | 4 | 613 | Std. Mean Difference (IV, Random, 95% CI) | 3.08 [2.68, 3.48] |
| 1.2 Adverse events | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.2.1 Hyperpigmentation | 3 | 528 | Risk Ratio (M‐H, Random, 95% CI) | 11.88 [4.54, 31.09] |
| 1.2.2 Matting | 3 | 528 | Risk Ratio (M‐H, Random, 95% CI) | 4.06 [1.28, 12.84] |
| 1.3 Pain | 1 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected |
Comparison 2. Sclerotherapy (polidocanol) versus sclerotherapy (any sclerosing agent).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Resolution or improvement of telangiectasias | 7 | 852 | Std. Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.13, 0.14] |
| 2.2 Adverse events | 8 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.2.1 Hyperpigmentation | 6 | 819 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.62, 1.43] |
| 2.2.2 Matting | 7 | 859 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.52, 1.27] |
| 2.2.3 Bruising | 4 | 558 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.56, 1.06] |
| 2.2.4 Microthrombosis | 4 | 394 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.69, 1.34] |
| 2.2.5 Allergy | 4 | 472 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.23, 2.01] |
| 2.2.6 Necrosis | 4 | 558 | Risk Ratio (M‐H, Random, 95% CI) | 0.07 [0.02, 0.29] |
| 2.3 Pain | 5 | 480 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.26 [‐0.44, ‐0.08] |
Comparison 3. Sclerotherapy (STS) versus sclerotherapy (any sclerosing agent).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Resolution or improvement of telangiectasias | 4 | 473 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐0.25, 0.11] |
| 3.2 Adverse events | 5 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 3.2.1 Hyperpigmentation | 4 | 478 | Risk Ratio (IV, Random, 95% CI) | 1.71 [1.10, 2.64] |
| 3.2.2 Matting | 2 | 323 | Risk Ratio (IV, Random, 95% CI) | 2.10 [1.14, 3.85] |
| 3.2.3 Bruising | 3 | 418 | Risk Ratio (IV, Random, 95% CI) | 1.62 [1.14, 2.30] |
| 3.2.4 Microthrombosis | 1 | 129 | Risk Ratio (IV, Random, 95% CI) | 1.04 [0.78, 1.39] |
| 3.2.5 Allergy | 3 | 452 | Risk Ratio (IV, Random, 95% CI) | 1.38 [1.01, 1.88] |
| 3.2.6 Necrosis | 2 | 392 | Risk Ratio (IV, Random, 95% CI) | 16.31 [3.14, 84.79] |
| 3.3 Pain | 4 | 409 | Risk Ratio (M‐H, Random, 95% CI) | 1.49 [0.99, 2.25] |
Comparison 4. Sclerotherapy (hypertonic saline) versus sclerotherapy (any sclerosing agent).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Resolution or improvement of telangiectasias | 3 | 348 | Std. Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.20, 0.22] |
| 4.2 Adverse events | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.2.1 Hyperpigmentation | 2 | 288 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.59, 0.93] |
| 4.2.2 Matting | 2 | 288 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.58, 1.36] |
| 4.3 Pain | 3 | 348 | Std. Mean Difference (IV, Random, 95% CI) | 6.22 [5.70, 6.73] |
Comparison 5. Sclerotherapy (chromated glycerin) versus sclerotherapy (any sclerosing agent).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 Resolution or improvement of telangiectasias | 2 | 125 | Std. Mean Difference (IV, Random, 95% CI) | 0.45 [‐0.11, 1.02] |
| 5.2 Adverse events | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.2.1 Hyperpigmentation | 2 | 125 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.09, 2.50] |
| 5.2.2 Matting | 1 | 99 | Risk Ratio (M‐H, Random, 95% CI) | 0.31 [0.01, 7.53] |
| 5.2.3 Bruising | 1 | 26 | Risk Ratio (M‐H, Random, 95% CI) | 0.14 [0.02, 1.00] |
| 5.2.4 Microthrombosis | 1 | 99 | Risk Ratio (M‐H, Random, 95% CI) | 1.32 [0.45, 3.87] |
| 5.3 Pain | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 6. Foam versus sclerotherapy (any sclerosing agent).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 6.1 Resolution or improvement of telangiectasias | 2 | 187 | Std. Mean Difference (IV, Random, 95% CI) | 0.04 [‐0.26, 0.34] |
| 6.2 Adverse events | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.2.1 Hyperpigmentation | 2 | 187 | Risk Ratio (M‐H, Random, 95% CI) | 2.12 [0.44, 10.23] |
| 6.2.2 Matting | 2 | 187 | Risk Ratio (M‐H, Random, 95% CI) | 6.12 [1.04, 35.98] |
| 6.2.3 Bruising | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.35, 1.04] |
| 6.2.4 Microthrombosis | 2 | 187 | Risk Ratio (M‐H, Random, 95% CI) | 1.39 [0.70, 2.76] |
| 6.3 Pain | 1 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected |
Comparison 7. Laser versus sclerotherapy (any sclerosing agent).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 7.1 Resolution or improvement of telangiectasias | 5 | 593 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.09 [‐0.25, 0.07] |
| 7.2 Adverse events | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 7.2.1 Hyperpigmentation | 4 | 262 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.40, 0.80] |
| 7.2.2 Matting | 2 | 162 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.46, 2.19] |
| 7.2.3 Bruising | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.60, 1.04] |
| 7.2.4 Necrosis | 3 | 202 | Risk Ratio (M‐H, Random, 95% CI) | 1.60 [0.20, 12.74] |
| 7.3 Pain | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only |
Comparison 8. Laser plus sclerotherapy (polidocanol) versus sclerotherapy (polidocanol).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 8.1 Resolution or improvement of telangiectasias | 2 | 710 | Std. Mean Difference (IV, Random, 95% CI) | 5.68 [5.14, 6.23] |
| 8.2 Adverse events | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 8.2.1 Hyperpigmentation | 2 | 656 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.35, 1.99] |
| 8.2.2 Matting | 2 | 656 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.21, 3.28] |
| 8.3 Pain | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
8.3. Analysis.

Comparison 8: Laser plus sclerotherapy (polidocanol) versus sclerotherapy (polidocanol), Outcome 3: Pain
Comparison 9. Sclerotherapy (polidocanol plus glucose) versus sclerotherapy (glucose).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 9.1 Resolution or improvement of telangiectasias | 2 | 191 | Std. Mean Difference (IV, Random, 95% CI) | 0.79 [0.50, 1.09] |
| 9.2 Adverse events | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 9.2.1 Hyperpigmentation | 2 | 191 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.62, 1.01] |
| 9.2.2 Matting | 2 | 191 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.51, 1.20] |
| 9.3 Pain | 2 | 191 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.83, 1.24] |
Comparison 10. Sclerotherapy plus compression versus sclerotherapy alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 10.1 Resolution or improvement of telangiectasias | 2 | 196 | Std. Mean Difference (IV, Random, 95% CI) | 0.09 [‐0.19, 0.37] |
| 10.2 Adverse events | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 10.2.1 Hyperpigmentation | 2 | 196 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.41, 2.07] |
| 10.2.2 Matting | 1 | 96 | Risk Ratio (M‐H, Random, 95% CI) | 1.84 [0.17, 19.62] |
| 10.3 Quality of life | 1 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Alos 2006.
| Study characteristics | ||
| Methods |
Study design: a double‐blind, controlled clinical trial study Method of randomisation: "Regions were randomly assigned one or other procedure as follows; the right limb (if both legs were involved) or the upper region (if only one leg was involved) were always treated first. The assignment of the first region to be treated to liquid or foam was performed according to a list of 38 random numbers from 0 to 75 created by an specific software. Each patient had an identification number according strict chronological recruitment order, if this number was in the list of random numbers then the patient received first foam and if not received liquid sclerosant first." Blinding: participant ‐ yes; treating doctor ‐ no; outcome assessors ‐ yes Power calculation: no details provided Total number of participants: 75 people with reticular or postoperative varices were enrolled and sclerotherapy was performed with liquid and with foam (Tessari method) using the same quantity of sclerosant for homogeneous varicose regions, to a total of 150 procedures Total number of procedures: 150 (sclerotherapy with liquid polidocanol and sclerotherapy with foam of polidocanol) Treatment localisation: lower limbs Number of exclusions post‐randomisation: none Number of withdrawals and reasons: 4 had left the study by the 90‐day follow‐up interval and 12 by the 365‐day assessment |
|
| Participants |
Setting: outpatient site Country: Spain Gender: 6 men and 69 women Age: mean 59 years (range: 23 – 78) Inclusion criteria: people with primary reticular varices (those of more than 2 mm of diameter) or postoperative varices in more than one region that did not involve the saphenofemoral junction Exclusion criteria: patients with truncal varices with junctional (terminal valve) and extra‐junctional incompetence, postoperative varices that involved the saphenofemoral junction, post‐thrombotic varices with occluded deep veins, varices secondary to arteriovenous fistulas, bilateral varices of asymmetric calibre, unilateral varices with asymmetric calibre between regions, chronic ischaemia of the lower limbs, severe arterial hypertension (blood pressure > 180/95 mmHg), and those being treated with anticoagulants and anti‐inflammatories and/or diuretics for other pathologies, to avoid these affecting the appearance or degree of possible secondary effects |
|
| Interventions |
Technique: all participants underwent 1 session of sclerotherapy in which both sclerosants (foam and liquid polidocanol) were given. Participants received the sclerotherapy in both regions by the same doctor, whether in different limbs or the same limb in different regions Treatment 1: 2 mL injection of foam into only 1 varicose vein. The concentration depended on the diameter of the vein measured with Duplex ultrasound with the participant standing. Foam was obtained from 0.5 mL of liquid polidocanol mixed with air at a ratio of 1:4, using the Tessari method which uses a 3‐way stopcock to mix the sclerosant. So 2 mL of foam contained 0.5 mL of polidocanol. After the sclerosant was injected, the sclerosed vein was compressed for 48 h with stockings at a pressure of 25 – 35 mmHg, while participants resumed a normal life style and regularly applied heparinoid ointment (3 times a day) Treatment 2: 0.5 mL liquid polidocanol injection in only one varicose vein in the corresponding region. An antegrade injection technique was used with the same kind of material (a 2 mL syringe and a 25 G, 5/8 needle) and the same postoperative care with compression and heparinoid as the study group. The same quantity of sclerosant was used in each injection in the foam and liquid sclerosant groups Duration of follow‐up: 365 days Use of compression: stockings 25 ‐ 35 mmHg |
|
| Outcomes |
Efficacy: assessed according to whether sclerosis of the vein was complete, as shown by duplex ultrasound. Sclerosis was considered complete when the lumen of the vein was sealed and the vein occluded. Total efficacy corresponded to complete sclerosis and partial efficacy to incomplete sclerosis when obliteration of the lumen was less than 100% The length of the sclerosed vein, measured in cm with a tape measure Side effects: pain in the treated region graded on an ordinal scale (absent, mild, moderate, or severe); requirement for analgesic treatment and type of analgesic given; inflammation in the treated region and degree of severity of inflammation (mild, moderate, severe) according to clinical criteria; appearance of skin pigmentation in the sclerosis region; formation of bulla, cutaneous necrosis and other effects Efficacy was assessed at 15, 30, and 90 days after the sclerotherapy and safety was evaluated at 15, 30, 90, and 365 days |
|
| Funding sources | No details provided | |
| Declarations of interest | No details provided | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The assignment of the first region to be treated to liquid or foam was performed according to a list of 38 random numbers from 0 to 75 created by an specific software. Each patient had an identification number according strict chronological recruitment order, if this number was in the list of random numbers then the patient received first foam and if not received liquid sclerosant first." |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Both the participant and the research assistant who assessed the clinical and ultrasound results were blind to the type of treatment applied in each area. The doctor who performed the treatment was not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Clinical and ultrasound results were assessed by a member of the research team other than the doctor who had performed the treatment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Of the 75 original participants, four had left the study by the 90‐day follow‐up interval and 12 by the 365‐day assessment |
| Selective reporting (reporting bias) | Unclear risk | No details given |
| Other bias | Unclear risk | No details given |
Bayer 2021.
| Study characteristics | ||
| Methods |
Study design: a randomised, controlled clinical trial study Method of randomisation: participants were allocated to 1 of the 2 study subgroups by selection of a sealed envelope Blinding: participant ‐ no; treating doctor ‐ no; outcome assessors ‐ yes Power calculation: no details provided Total number of participants: 50 people with telangiectasias or reticular veins, 100 legs for procedure Total number of procedures: 100 (sclerotherapy with polidocanol and compression stocking) Treatment localisation: lower limbs Number of exclusions post‐randomisation: none Number of withdrawals and reasons: none |
|
| Participants |
Setting: outpatient site Country: Germany Gender: 4 men and 46 women Age: not mentioned Inclusion criteria: people with telangiectasias or reticular varices Exclusion criteria: pregnancy, classification CEAP higher than stage C2, allergy to sclerosing agent, walking range < 200 m |
|
| Interventions | Sclerotherapy with polidocanol. Group A received sclerotherapy only, i.e. eccentric compression using low‐stretch bandages over rolled gauze for 24 hrs Group B received an additional week of concentric stockings of 18 to 20 mmHg above the ankle | |
| Outcomes | Improvement or resolution of telangiectasias or reticular veins Adverse events: pain, itching, burning Hyperpigmentation |
|
| Funding sources | No details provided | |
| Declarations of interest | None declared | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sealed envelope |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of participants |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of 2 experts to analyse the photography after procedure |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No details given |
| Selective reporting (reporting bias) | Unclear risk | No details given |
| Other bias | Unclear risk | No details given |
Benigni 1999.
| Study characteristics | ||
| Methods |
Study design: multicentre, split‐body, randomised comparative trial (a pilot study) Method of randomisation: drawing of lots Blinding: participant ‐ no, treating doctor ‐ no Power calculation: no Total number of participants randomised: 24 Total number of participants analysed: 20 Total number of procedures: 40 Treatment localisation: lower limbs Number of exclusions post‐randomisation: 4 (information obtained through personal correspondence with Jean‐Patrick Benigni, MD) Number of withdrawals and reasons: none |
|
| Participants |
Setting: 4 outpatient centres (information obtained through personal correspondence with Jean‐Patrick Benigni, MD) Country: France Gender: women Age: mean 37.7 (range 18 ‐ 65) Inclusion criteria: "clear photo type" (classifications I to III on the Fitzpatrick classification scale), "roughly symmetrical reticular veins and telangiectases of the lateral thigh surface", reflux of reticular varices of at least 1 point on continuous Doppler Exclusion criteria: incompetence of the large saphenous veins or their collaterals, incompetence of the deep veins, perineal varices, skin condition of the lateral face of the thigh, obesity (BMI > 30), pregnancy, psychiatric trouble, a contraindication to sclerotherapy, hormonal treatment within 6 months of enrolment |
|
| Interventions |
Treatment 1: 1 thigh was treated with polidocanol foam 0.25% by the Monfreux method Treatment 2: the other thigh was treated with polidocanol solution 0.25% Duration of follow‐up: 5 sessions every 15 ± 2 days, and a control visit 15 days after the last session |
|
| Outcomes | Efficacy: appearance of telangiectasias on visual analogue scales (0 to 10) as assessed by the treating doctors (days 0, 15, 30, 45, 60 & 75) and by participants (days 0 and 75). 4 criteria were assessed: colour, density, relief and surface Adverse effects: as assessed by the treating physician: ecchymosis, thrombi, microthrombi, matting, pigmentation, necrosis Number of points of injection The quantity of sclerosant injected |
|
| Funding sources | No details provided | |
| Declarations of interest | No details provided | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Drawing of lots |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding is not mentioned for participants or treating doctors. Foam and liquid sclerosants have distinct appearances on injection |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details given |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 4 people were "lost of view" as per personal communication with Dr. Benigni. No explanation was provided in the published article |
| Selective reporting (reporting bias) | Unclear risk | No details given |
| Other bias | Unclear risk | No details given |
Bertanha 2017.
| Study characteristics | ||
| Methods |
Study design: prospective, randomised, triple‐blind, controlled, parallel‐group clinical trial Method of randomisation: "Participants were randomly assigned using an open‐source, web‐based randomisation software (Stat Trek, http://stattrek.com/statistics/random‐number‐generator.aspx) to 1 of 2 treatment groups: group 1 to receive 0.2% polidocanol diluted in 70% hypertonic glucose; group 2 to receive 75% hypertonic glucose. The computer generated allocation sequence was kept by an independent nurse, who prepared opaque, sealed envelopes for each group. The nurse prepared the medications in a room separate from the treatment room." Blinding: participant ‐ yes; treating doctor ‐ yes; outcome assessors ‐ yes Power calculation: yes Total number of participants: 93 Total number of procedures: 93 Treatment localisation: lower limbs Number of exclusions post‐randomisation: none Number of withdrawals and reasons: none |
|
| Participants |
Setting: outpatient site Country: Brazil Gender: women. "The study population consisted of a calculated sample of adult women consecutively recruited among patients seeking treatment at the specialized out patient clinic of our institution" Age: range 24 ‐ 62 Inclusion criteria; eligible participants were all women aged 18 to 69 years who had at least 1 reticular vein with a minimum length of 10 cm in 1 of their lower limbs with mild venous disease classified as CEAP C1, and all were available to attend the appointments Exclusion criteria: venous disease with a CEAP clinical class other than C1, pregnancy or puerperium, allergy to polidocanol or glucose, restricted mobility, PAD, diabetes mellitus, uncontrolled systemic disorders, dermatitis at the treatment site, asthma, migraine, previous DVT, family history of DVT, acute thrombophlebitis, known thrombophilia or any hypercoagulable state, and use of anticoagulants. Patients who failed to attend the treatment session or the follow‐up visits were also excluded |
|
| Interventions |
Treatment 1: 0.2% polidocanol diluted in 70% hypertonic glucose Treatment 2: 75% hypertonic glucose The treatment area on the participant’s lower limb was defined as a rectangle of approximately 600 cm2 (25 cm long × 15 cm wide) on the lateral aspect of the distal midthigh and the proximal and middle leg of 1 of the limbs. The lower limbs were photographed before and after treatment using a high‐definition digital camera All sclerotherapy procedures were performed by the same physician. Both medications were in liquid form and identical in appearance within the syringes (colourless, odourless, and with similar viscosity). The maximum volume per puncture was 0.3 mL, and punctures were performed until whitening of the reticular veins occurred in the treatment area. After the procedure, elastic compression bandages (Atadress; Adamed) were applied directly over the treated area for 24 hours Duration of follow‐up: 60 days Use of compression: elastic compression bandage |
|
| Outcomes | The primary efficacy end point was complete elimination of the reticular veins by 60 days after sclerotherapy treatment with the study medications. To assess this outcome, the reticular veins were measured on images obtained before treatment (day 0) and after treatment (day 60) using ImageJ software. The linear measurements of reticular veins before treatment and residual veins after treatment were captured in pixels, which were then converted to millimetres. Each image was analysed by 2 independent observers who were blinded to the medication used. The safety outcomes were analysed at each post‐treatment visit for the occurrence of serious adverse events (chest pain, transient neurological abnormalities, anaphylaxis, accidental arterial puncture, tissue necrosis, DVT, and PE), minor adverse events (scars, cough, superficial thrombophlebitis, telangiectatic matting, allergies, lipothymia, and scotomas), and particularly pigmentation running the course of the treated veins, which was analysed based on direct measurements performed on post‐treatment images using ImageJ software Secondary outcomes: skin colour, number of punctures, volume of medication used, occurrence of haematomas, residual veins in relation to pigmentation, comparison of current pain with pain at the time of previous treatments, treatment‐related cough, migraine, oedema, early (day 7) and late (day 60) phlebitis, telangiectatic matting, and lipothymia |
|
| Funding sources | No details provided | |
| Declarations of interest | None declared | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants randomly assigned in a 1:1 ratio using open‐source, web‐based randomisation software (Stat Trek, http://stattrek.com/statistics/random‐number‐generator.aspx) |
| Allocation concealment (selection bias) | Low risk | Quote: "The computer generated allocation sequence was kept by an independent nurse,who prepared opaque, sealed envelopes for each group. The nurse prepared the medications in a room separate from the treatment room." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Both medications were in liquid form and identical in appearance within the syringes (colourless, odourless, and with similar viscosity) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Each image was analysed by 2 independent observers who were blinded to the medication used. Still blindly, the safety outcomes were analysed at each post treatment visit for the occurrence of serious adverse events." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Of 106 eligible participants, 51 were randomised to receive 0.2% polidocanol diluted in 70% hypertonic glucose; in this group 1 did not receive allocated intervention and 7 lost to follow‐up; and 55 to receive 75% hypertonic glucose alone, in this group 1 did not receive allocated intervention and 4 lost to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | No details given |
| Other bias | Unclear risk | No details given |
Bertanha 2021.
| Study characteristics | ||
| Methods |
Study design: prospective, randomised, triple‐blind, controlled trial Method of randomisation: "Participants were randomly assigned using an open‐source, web‐based randomisation software (Stat Trek, http://stattrek.com/statistics/random‐number‐generator.aspx) to 1 of 2 treatment groups: group 1 to receive 0.2% polidocanol diluted in 70% hypertonic glucose; group 2 to receive 75% hypertonic glucose. The computer generated allocation sequence was kept by an independent nurse, who prepared opaque, sealed envelopes for each group. The nurse prepared the medications in a room separate from the treatment room." Blinding: participant ‐ yes; treating doctor ‐ yes; outcome assessors ‐ yes Power calculation: yes Total number of participants: 115 Total number of procedures: 98 Treatment localisation: lower limbs Number of exclusions post‐randomisation: 17 Number of withdrawals and reasons: 17 lost to follow‐up |
|
| Participants |
Setting: outpatient site Country: Brazil Gender: women. "The study population consisted of a calculated sample of adult women consecutively recruited among patients seeking treatment at the specialized out patient clinic of our institution." Age: range 34 ‐ 50 Inclusion criteria; eligible participants were all women aged 18 to 69 years who had at least 1 reticular vein with a minimum length of 10 cm in 1 of their lower limbs with mild venous disease classified as CEAP C1, and all were available to attend the appointments. Exclusion criteria: venous disease with a CEAP clinical class other than C1, pregnancy or puerperium, allergy to polidocanol or glucose, restricted mobility, PAD, diabetes mellitus, uncontrolled systemic disorders, dermatitis at the treatment site, asthma, migraine, previous DVT, family history of DVT, acute thrombophlebitis, known thrombophilia or any hypercoagulable state, and use of anticoagulants. Patients who failed to attend the treatment session or the follow‐up visits were also excluded |
|
| Interventions |
Treatment 1: 0.2% polidocanol diluted in 70% hypertonic glucose Treatment 2: 75% hypertonic glucose The treatment area on the participant’s lower limb was defined as a rectangle of approximately 600 cm2 (25 cm long × 15 cm wide) on the lateral aspect of the distal midthigh and the proximal and middle leg of 1 of the limbs. The lower limbs were photographed before and after treatment using a high‐definition digital camera. All sclerotherapy procedures were performed by the same physician. Both medications were in liquid form and identical in appearance within the syringes (colourless, odourless, and with similar viscosity). The maximum volume per puncture was 0.3 mL, and punctures were performed until whitening of the reticular veins occurred in the treatment area. After the procedure, elastic compression bandages (Atadress; Adamed) were applied directly over the treated area for 24 hours Duration of follow‐up: 60 days Use of compression: elastic compression bandage |
|
| Outcomes | The primary efficacy end point was complete elimination of the reticular veins by 60 days after sclerotherapy treatment with the study medications. To assess this outcome, the reticular veins were measured on images obtained before treatment (day 0) and after treatment (day 60) using ImageJ software. The linear measurements of reticular veins before treatment and residual veins after treatment were captured in pixels, which were then converted to millimetres. Each image was analysed by 2 independent observers who were blinded to the medication used. The safety outcomes were analysed at each post‐treatment visit for the occurrence of serious adverse events (chest pain, transient neurological abnormalities, anaphylaxis, accidental arterial puncture, tissue necrosis, DVT, and PE), minor adverse events (scars, cough, superficial thrombophlebitis, telangiectatic matting, allergies, lipothymia, and scotomas), and particularly pigmentation running the course of the treated veins, which was analysed based on direct measurements performed on post‐treatment images using ImageJ software. Secondary outcomes: skin colour, number of punctures, volume of medication used, occurrence of haematomas, residual veins in relation to pigmentation, comparison of current pain with pain at the time of previous treatments, treatment‐related cough, migraine, oedema, early (day 7) and late (day 60) phlebitis, telangiectatic matting, and lipothymia |
|
| Funding sources | No details provided | |
| Declarations of interest | None declared | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants randomly assigned in a 1:1 ratio using open‐source, web‐based randomisation software (Stat Trek, http://stattrek.com/statistics/random‐number‐generator.aspx) |
| Allocation concealment (selection bias) | Low risk | Quote: "The computer generated allocation sequence was kept by an independent nurse,who prepared opaque, sealed envelopes for each group. The nurse prepared the medications in a room separate from the treatment room." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Both medications were in liquid form and identical in appearance within the syringes (colourless, odourless, and with similar viscosity) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Each image was analysed by 2 independent observers who were blinded to the medication used. Still blindly, the safety outcomes were analysed at each post treatment visit for the occurrence of serious adverse events." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Of 115 eligible participants, 58 were randomised to receive 0.2% polidocanol diluted in 70% hypertonic glucose; in this group 1 did not receive allocated intervention and 7 lost follow‐up. 57 were allocated to receive 75% hypertonic glucose alone, in this group 1 did not receive allocated intervention and 10 lost to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | No details given |
| Other bias | Unclear risk | No details given |
Carlin 1987.
| Study characteristics | ||
| Methods |
Study design: a double‐blind, double‐paired comparison, placebo‐controlled study Method of randomisation: not mentioned Blinding: participant ‐ yes, treating doctor ‐ yes Power calculation: not mentioned Total number of participants: 20 Total number of procedures: 80 Treatment localisation: 4 quadrants of legs on each participant Number of exclusions post‐randomisation: none Number of withdrawals and reasons: not mentioned |
|
| Participants |
Setting: outpatient site Country: USA Gender: women Age: range 29 ‐ 55 Inclusion criteria: bilaterally symmetrical telangiectasias of the lower extremities Exclusion criteria: women taking hormonal therapy, anticoagulants or disulfiram |
|
| Interventions |
Technique: each participant's legs were divided into 4 quadrants Treatment 1. sodium tetradecyl sulfate (STS) 0.5% Treatment 2. polidocanol 0.5% Treatment 3. heparsal (saline 20% with heparin 100 units/mL) Control: placebo ‐ normal saline 0.9% Duration of follow‐up: 24 weeks Use of compression: no details provided |
|
| Outcomes | Visual improvement: clinical assessment of improvement: scale of 1 to 5 (poor, fair, moderate, good, excellent) Adverse events:
Participant satisfaction (dissatisfied, neither, satisfied, very satisfied) Pain: scale of 1 to 4 (none, mild, moderate, severe) Number of treatments required for clearance (from 1 to > 6) |
|
| Funding sources | No details provided | |
| Declarations of interest | No details provided | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details given |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participaents and doctor were blinded for sclerosant agent |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details given |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | High risk | Some of the adverse reactions were not statistically analysed by sclerosing agent used. Patient satisfaction data were not separated based on sclerosing agent used |
| Other bias | High risk | Participants received separate simultaneous treatments and analysis, and thereby the risks of bias by carry‐over effect exist |
Christiansen 2015.
| Study characteristics | ||
| Methods |
Study design: randomised, paired study Method of randomisation: not mentioned Blinding: participant ‐ yes, treating doctor ‐ no, outcomes assessors ‐ yes Power calculation: not mentioned Total number of participants: 14 Total number of procedures: 28 Treatment localisation: 28 vessel pairs were treated; 13 blue and 15 red vessels; the vessels were divided into 4 groups according to size; 0.3 mm, 0.3 ‐ 0.5 mm, 0.5 ‐ 1 mm, and 1 ‐ 2.5 mm Total vessel treated: 28 vessel pairs were treated, 13 blue and 15 red vessels. Participants with skin types ranging from I to V. The vessels were divided into 4 groups according to size: 0.3 mm, 0.3 – 0.5 mm, 0.5 – 1 mm, and 1 – 2.5 mm Number of exclusions post‐randomisation: none Number of withdrawals and reasons: none |
|
| Participants |
Setting: outpatient site Country: Denmark Gender: women Age: mean: 36.8, SD: 9.3, range: 25 – 60 years Inclusion criteria: only participants having a pair of close‐to‐identical telangiectasias with identical diameters (less than 2.5 mm) located on lower extremities and with the same colour were included in the study In each group, specific parameters for pulse duration and fluence were selected according to the vessel colour and diameter Exclusion criteria: "participants with more than moderately suntanned skin or participants who had been exposed to sunlight within one week prior to the date of treatment were excluded from the study. Pre‐cancers or with ski malignancies and with Koebner phenomena or light, sensitive skin diseases were excluded." |
|
| Interventions |
Treatment 1: manually‐adjusted laser fluences, guided by clinical endpoints of visual coagulation Treatment 2: theoretically optimised and clinically verified fixed fluence settings Technique: 1 of the paired vessels was randomly chosen and treated with a theoretically optimised and clinically verified. These settings were used as the starting point when treating the corresponding vessel on the other side, but in this case the laser fluence was manually adjusted to the lowest level that could produce a visual coagulation or visual obstruction of the blood flow (the clinically judged fluence) 1 or 2 treatment sessions were performed with a single pass. Prior to treatment, a thin layer of transparent optical index‐matching gel was applied to the skin surface Duration of follow up: 4 months Use of compression: not mentioned |
|
| Outcomes | Pain associated with treatment ‐ the participants were asked to rate the pain according to a VAS (0 – 10) Degree of erythema and oedema ‐ the clinical investigator registered the degree of erythema and oedema based on 3 categories: 0 none, 1 medium, and 2 heavy at 5 min post‐treatment The first follow‐up took place 2 months after the first treatment and a second treatment was then performed if necessary. The second follow‐up visit took place 4 months after the first treatment |
|
| Funding sources | No details provided | |
| Declarations of interest | None declared | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States "randomised", no further details given |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The vessel clearances were evaluated, using close‐up photographs taken prior to the first treatment and at the 2‐month and 4‐month follow‐up visits, by both the clinical investigator and the participants |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | None absent |
| Selective reporting (reporting bias) | Unclear risk | No details given |
| Other bias | Unclear risk | No details given |
Goldman 2002.
| Study characteristics | ||
| Methods |
Study design: double‐blinded, randomised study Method of randomisation: coin toss (information obtained through study author Mitchell P Goldman, MD) Blinding: participant ‐ yes; treating doctor ‐ yes; outcome assessors ‐ yes Power calculation: not mentioned Total number of participants: 129 Total number of procedures: 129 Treatment localisation: leg veins Categories by size of veins: < 1 mm in diameter = 42 patients; 1 ‐ 3 mm = 41 participants and 3 ‐ 6 mm = 46 participants Number of exclusions post‐randomisation: none Number of withdrawals and reasons: not mentioned |
|
| Participants |
Setting: outpatient site Country: USA Gender: not mentioned Age: not mentioned Inclusion criteria: varicose, reticular and/or telangiectatic leg veins Exclusion criteria: incompetence at the saphenofemoral or saphenopopliteal junctions |
|
| Interventions |
Treatment 1. Vein less than 1 mm in diameter were randomised to be treated with either polidocanol 0.5% or STS 0.25% Treatment 2. Veins 1 ‐ 3 mm in diameter with polidocanol 1% or STS 0.5% Treatment 3. Veins 3 ‐ 6 mm in diameter to polidocanol 3% or STS 1.5% Treatment was performed in a standard technique Photographs were taken and questionnaires were administered before treatment and at 1, 4 and 16 weeks after treatment Duration of follow‐up: 16 weeks Use of compression: not mentioned |
|
| Outcomes | Efficacy: 3 vascular surgeons blinded to intervention agent assessed photographs from baseline, week 1, week 4, and week 16 on a scale of 1 to 5 Adverse sequelae: data were not separated for veins < 1 mm Participant satisfaction: data were not separated for veins < 1 mm | |
| Funding sources | Kreussler & Co GmbH (manufacturer of sclerosant), Beisdorf‐Jobst (manufacturer of compression hosiery) | |
| Declarations of interest | None declared | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Coin toss (information provided by study author) |
| Allocation concealment (selection bias) | Low risk | Physician was given the solution to inject by nurse (information provided by study author) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The treating physician was blinded as to the agent being injected |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blind assessment by 3 vascular surgeons |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No details given |
| Selective reporting (reporting bias) | Unclear risk | No details given |
| Other bias | Unclear risk | No details given |
Hamel‐Desnos 2009.
| Study characteristics | ||
| Methods |
Study design: multicentre, prospective controlled randomised trial Method of randomisation: not mentioned Blinding: participant ‐ no; treating doctor ‐ no; outcome assessors ‐ not reported Power calculation: not mentioned Total number of participants: 105 Total number of procedures: 199 Treatment localisation: leg veins Number of exclusions post‐randomisation: none Number of withdrawals and reasons: not mentioned |
|
| Participants |
Setting: outpatient site Country: France Gender: male and female Age: range 20 ‐ 82 years Inclusion criteria: varicose, reticular or telangiectatic leg veins or both, documented thrombophilia Exclusion criteria: mental or psychiatric disturbance, pregnancy, renal failure, uncontrolled arterial hypertension, coagulopathy |
|
| Interventions |
Treatment 1: warfarin plus sclerotherapy Treatment 2: nadroparin plus sclerotherapy |
|
| Outcomes | Occurrence of a symptomatic or asymptomatic DVT or PE | |
| Funding sources | No details provided | |
| Declarations of interest | No details provided | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported in the study |
| Allocation concealment (selection bias) | Unclear risk | Not reported in the study |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Impossible to blind participants due to interventions |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported in the study |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported in the study |
| Selective reporting (reporting bias) | Unclear risk | Not reported in the study |
| Other bias | Unclear risk | Not reported in the study |
Hoss 2020.
| Study characteristics | ||
| Methods |
Study design: prospective, randomised, double‐blind, split‐leg, single‐centre clinical trial Method of randomisation: not mentioned Blinding: participant ‐ yes; treating doctor ‐ yes; outcome assessors ‐ yes Power calculation: not mentioned Total number of participants: 30 Total number of procedures: 60 Treatment localisation: leg veins Number of exclusions post‐randomisation: none Number of withdrawals and reasons: none |
|
| Participants |
Setting: outpatient site Country: USA Gender: women Age: mean 53.6 (32 ‐ 70 years) Inclusion criteria: varicose, reticular or telangiectatic leg veins, or both Exclusion criteria: CEAP class C2 or greater, history of DVT, pregnancy, allergy |
|
| Interventions |
Treatment 1: sclerotherapy with POL:air ratio 1:2 Treatment 2: sclerotherapy with POL:air ratio 1:4 |
|
| Outcomes | Efficacy was rated on a quartile percentage scale (1 = 0% – 25%, 2 = 26% – 50%, 3 = 51% – 75%, and 4 = 76% – 100%) Adverse events: erythema, pigmentation,urtication/swelling, ankle/pedal oedema, ecchymosis, ulceration, matting, hyperpigmentation |
|
| Funding sources | No details provided | |
| Declarations of interest | None declared | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details given |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Both participants and evaluating investigators were blinded to the randomisation schedule |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Both participants and evaluating investigators were blinded to the randomisation schedule |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No details given |
| Selective reporting (reporting bias) | Unclear risk | No details given |
| Other bias | Unclear risk | No details given |
Ianosi 2019.
| Study characteristics | ||
| Methods |
Study design: single‐centre, split‐body, randomised comparative trial comparing hypertonic 20% saline + 2% lignocaine 2% versus POL 0.5% versus long‐pulsed neodymium‐yttrium aluminium garnet (Nd:YAG) Method of randomisation: no details provided Blinding: participant ‐ no, treating doctor ‐ no Power calculation: no Total number of participants randomised: 285 Total number of participants analysed: 244 Total number of procedures: 288 Treatment localisation: lower limb Number of exclusions post‐randomisation: no details provided Number of withdrawals and reasons: 41 lost to follow‐up |
|
| Participants |
Setting: outpatient site Country: Romania Gender: women Age: mean 37.7 (range 18 ‐ 65) Inclusion criteria: "patients over 18 with primary leg telangiectasias up to 2 mm in diameter (C1A) as single objective sign and those that accepted to enter the study signed the informed consent form." Exclusion criteria: ".. i) Patients with symptoms associated with telangiectasias (C1S); ii) patients with superficial venous reflux assessed with Doppler ultrasound of the lower limbs; iii) patients with deep venous thrombosis or post‐thrombotic syndrome (ES); iv) pregnant or breastfeeding patients; and v) patients with neoplasms or other systemic conditions or under chronic treatment for any other disease." |
|
| Interventions |
Treatment 1: HS group ‐ treated with hypertonic: 20% saline/2% lignocaine Treatment 2: POL group with polidocanol: 0.5% Treatment 3: LAS group with Nd:YAG laser with a wavelength of 1064 nm Duration of follow‐up: "There were two identical sessions at 60‐day interval. Assessment of vessel clearing and complications was conducted at 60 and 120 days." Use of compression: not reported |
|
| Outcomes | Efficacy: 6‐point scale: 0: no change; 1: 1% ‐ 20% cleared; 2: 21% ‐ 40% cleared; 3: 41% ‐ 60% cleared; 4: 61% ‐ 80% cleared; and 5: 81% ‐ 100% cleared Adverse effects: burns, hypo‐ and hyperpigmentation, and thrombosis |
|
| Funding sources | No funding received | |
| Declarations of interest | None declared | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States "randomised" but no other details given |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding was not possible due to different techniques |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details given |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 41/285 participants lost to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | No details given |
| Other bias | Unclear risk | No details given |
Kahle 2006.
| Study characteristics | ||
| Methods |
Study design: prospective, randomised, double‐blind, placebo‐controlled study Method of randomisation: Microsoft Excel program Blinding: participants ‐ yes; outcomes assessors ‐ yes Power calculation: no details provided Total number of participants: 48 people with telangiectasias Total number of procedures: 48 Treatment localisation: lower limbs Number of exclusions post‐randomisation: none Number of withdrawals and reasons: no details provided |
|
| Participants |
Setting: outpatient site Country: Germany Gender: no details provided Age: no details provided Inclusion criteria: telangiectasias in lower limbs Exclusion criteria: no details provided |
|
| Interventions |
Treatment: POL Control: placebo (normal saline solution) Duration of follow‐up: 4 weeks Use of compression: yes |
|
| Outcomes | Improvement scale 0 to 100 and questionnaire of satisfaction of participants VAS (0 ‐ 10) | |
| Funding sources | No details provided | |
| Declarations of interest | No details provided | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Microsoft Excel program |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Double‐blind", no further details given |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded examiner for analysing photographs |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No details given |
| Selective reporting (reporting bias) | Unclear risk | No details given |
| Other bias | Unclear risk | No details given |
Kern 2004.
| Study characteristics | ||
| Methods |
Study design: A single‐blind, randomised, comparative study Method of randomisation: this study was a randomised, single‐blinded trial. Area treated and type of veins: only the lateral face of the thigh was considered for the study. A single leg per person was treated. If both thighs were affected, only the left one was treated and evaluated for the study, as defined by the study protocol. Each participant was randomly assigned either to chromated glycerin, POL solution, or POL foam Blinding: participants were unaware of the choice of the sclerosing method. The treating physician (P.K.) was not blinded. A doubled‐blinded study is not realizable with the different sclerosing aware of the sclerosing agent used, because of the great difference in viscosity between glycerin and POL Power calculation: not mentioned Total number of participants: 150 Total number of procedures: 150 Treatment localisation: lateral face of the thigh Number of exclusions post‐randomisation: none Number of withdrawals and reasons: 3 lost to follow‐up |
|
| Participants |
Setting: outpatient site Country: Switzerland Gender: women Age: range 17 ‐ 80 years, mean: 46.5. Inclusion criteria: consecutive, informed, consenting women who were presenting with primary telangiectasias and reticular leg veins on the lateral face of the thigh (C1 A or S) Exclusion criteria: reflux in the deep veins, saphenous trunk, saphenous junctions or saphenous collateral, incompetent perforators, non‐compressible deep or superficial veins, recanalised thrombus, allergy against chrome or polidocanol, and previous sclerotherapy |
|
| Interventions |
Treatment 1: chromated glycerin Treatment 2: POL solution Treatment 3: POL foam Technique: 2 mL syringes were used for chromated glycerin and POL solution, whereas 2‐mL sterilised glass syringes were used for the foam technique. Injections were performed with a 30.5‐gauge needle. All treatments were performed with the participant in the supine position. Approximately 60 to 100 injections were performed during the session. Reticular feedings veins were injected before the telangiectasias. Participants remained supine for 5 minutes after the last injection before a compressive bandage was applied with either an elastic band or a graduated compressive stocking (26 ‐ 33 mm Hg), which was worn for 1 week during the day Number of sessions: A single sclerosing session was performed in each participant. Treatment sites were photographed immediately before and after the treatment in supine position. Immediately after the treatment, the participants were asked to assess the pain score on a visual scale Duration of follow up: 5 weeks Use of compression: graduated compressive stocking |
|
| Outcomes | Visual improvement: participants were questioned about their satisfaction using a visual scale ranging from negative results to great satisfaction. The physician had the possibility of assessing satisfaction on a scale from 0 to 100, with 100 representing the maximal satisfaction rate. A control photograph was performed at 5 weeks after treatment in similar conditions as those set for the pretreatment picture. Two independent experts who were not involved in the treatment phase and who were blinded to the sclerosant used were asked to analyse the photographs and to give an efficacy score or a vessel clearance score from 0 to 10 and to report side effects. To assess the intra‐observer reproducibility of photograph analysis, the expert, still blinded, made a second analysis of the same pictures 1 month later Side effects: the treating physician, not blinded to the agent, checked for immediate side effects or those reported by the participants. He also identified microthrombi, pigmentations or matting, which may be difficult to evaluate on the photographs | |
| Funding sources | No details provided | |
| Declarations of interest | No details provided | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States "randomised", no further details given |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The treating physician, not blinded to the agent |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Two independent experts who were not involved in the treatment phase and who were blinded to the sclerosant used were asked to analyse the photographs and to give an efficacy score or a vessel clearance score from 0 to 10 and to report side effects. Both experts are phlebologist physicians skilled with sclerotherapy. To assess the intraobserver reproducibility of photograph analysis, the expert, still blinded, made a second analysis of the same pictures 1 month later |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 3 participants lost to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | No details given |
| Other bias | Unclear risk | No details given |
Kern 2007.
| Study characteristics | ||
| Methods |
Study design: prospective, randomised open‐label study Method of randomisation: computer‐generated random table Blinding: participant ‐ no; treating doctor ‐ no; outcome assessors ‐ yes Power calculation: precalculation of the sample required size showed that to detect a true difference of half a standard deviation at a 1‐sided 5% significance level with a power of 80% Total number of participants: 100 consecutive participants Total number of procedures: 100 Treatment localisation: lateral aspect of the thigh Number of exclusions post‐randomisation: none Number of withdrawals and reasons: 4 lost to follow‐up |
|
| Participants |
Setting: outpatient site Country: Switzerland Gender: women Age: median 47 years (range 20 ‐ 72) Inclusion criteria: primary telangiectasias and reticular veins on the lateral aspect of the thigh (C1A or SEPAS1PN), women with aesthetic problems and normal duplex who sought treatment for the first time Exclusion criteria: previous sclerotherapy and allergy to chrome |
|
| Interventions |
Treatment 1: sclerotherapy (chromated glycerin) plus compression Treatment 2: sclerotherapy (chromated glycerin) without compression Technique: "The patients had comparable types and sizes of telangiectasias and reticular veins: the diameter of reticular veins ranged between 1 to 2.9 mm, that of telangiectasias between 0.2 and 1 mm. These vessels could be identified from a distance of 2 meters by the human eye and the camera. One lower limb per patient was treated in a single session. If both thighs were affected, only the left one was treated and evaluated for the study as defined by the protocol. The whole treated thigh area was photographed immediately before sclerotherapy in supine position in order to avoid arbitrary selection of a limited skin surface. The same digital camera (Coolpix 990, Nikon, Tokyo, Japan), lighting conditions (flash), and focal distance were used. Chromated glycerin (Scleremo, Laboratoires Bailleul, Paris, France) was used in all patients as the sclerosing agent. Injections were performed with a 2 mL silicone syringe and a 30 G ½‐inch needle with the patient in supine position. Sixty to 100 injections were made on the lower limb during the session. The maximum amount of sclerosing solution injected was 10 mL per patient. Reticular feeding veins were injected prior to injection of the telangiectasias. Patients remained supine for 5 minutes after the last injection. In the compression group, a thigh‐length stocking Sigvaris 702 Top Fine (23 ‐ 32 mm Hg) was applied respecting the individual lower limb dimensions. Patients were asked to wear the compression stockings daily for 3 weeks and to remove it during night time. Patients filled in a quality of life questionnaire (SF‐36) prior to treatment." Duration of follow‐up: 3 weeks Use of compression: compression stocking |
|
| Outcomes | Outcomes assessed by participant satisfaction analysis and quantitative evaluation of photographs taken from the lateral aspect of the thigh before and again at 52 days on the average after sclerotherapy by 2 blinded expert reviewers Quality of life: participants completed a quality‐of‐life questionnaire (SF‐36) before treatment and again at the control |
|
| Funding sources | No details provided | |
| Declarations of interest | None declared | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Prior to treatment, each participant was randomly assigned (computer‐generated random table) either to no compression or to compression for 3 weeks following sclerotherapy |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Impossible to blind participants and personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Analysis of the photographs using the vessel disappearance score revealed a very good agreement between the 2 independently‐working blinded reviewers (intraclass correlation coefficient 0.93) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3 withdrawals from the without‐compression group and 1 withdrawal from the chromated glycerin group |
| Selective reporting (reporting bias) | Unclear risk | No details given |
| Other bias | Unclear risk | No details given |
Kern 2011.
| Study characteristics | ||
| Methods |
Study design: prospective randomised double‐blind trial Method of randomisation: computer‐generated random table Blinding: participants ‐ yes, doctor ‐ yes, outcome assessor ‐ yes Power calculation: calculations showed that 100 participants, 50 in each group, were required to detect a true difference of half a SD at a 1‐sided 5% significance level with a power of 80% Total number of patients: 110 Total number of procedures: 110 Treatment localisation: lateral thigh Number of exclusions post‐randomisation: none Number of withdrawals and reasons: 8 participants; 5 lost to follow‐up and 3 for poor quality of the accompanying photos |
|
| Participants |
Setting: outpatient site Country: Switzerland Gender: women Age: not mentioned Inclusion criteria: women were eligible if presenting for cosmetic reasons for first‐time sclerotherapy of lateral thigh reticular or telangiectatic veins (C1A or S). Participants had no evidence of superficial or deep vein insufficiency as assessed by duplex ultrasound Exclusion criteria: allergy to chrome No patient declined participation, and no patient reported chrome allergy, so no patient was excluded from the study |
|
| Interventions |
Treatment 1: pure chromated glycerin (glycerin 72% and chromium alum 0.8%, Scleremo, Laboratoires Bailleul, Paris, France) Treatment 2: chromated glycerin mixed with ⅓ lidocaine‐epinephrine 1% The same physician treated all included participants. The whole treated thigh area was photographed immediately before sclerotherapy in a supine position to avoid arbitrary selection of a limited skin surface. The same digital camera (Coolpix 990, Nikon), lighting conditions (flash), and focal distance were used. Injections were performed using 2‐mL silicone syringes and a 30G 0.5‐in. needle with the participant in a supine position. The maximum amount of injected sclerosing solution was 10 mL per participant. 60 to 80 injections of very small volumes of sclerosing agent were given during treatment session. Reticular feeding veins were injected before telangiectasias. Telangiectasias were treated one after the other without re‐injecting the same area. Immediately after treatment, participants were asked to score their pain using a visual 100‐point scales (0 = no pain, 100 = maximum pain). Participants remained supine for 5 minutes after their last injection. Subsequently, a thigh‐length compression stocking (23 – 32 mmHg) was applied Duration of follow‐up: 3 weeks Use of compression: compression stocking |
|
| Outcomes | Participant pain scores (participants graded their pain by drawing a line on the scale) Rate of clinical vessel disappearance |
|
| Funding sources | No details provided | |
| Declarations of interest | None declared | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Before treatment, each participant was randomly assigned (computer‐generated random table) to pure chromated glycerin (glycerin 72% and chromium alum 0.8%, Scleremo, Laboratoires Bailleul, Paris, France) or to CGX |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and the treating physician were blinded to the treating agent. To maintain double‐blind conditions, a dedicated non‐blinded nurse prepared syringes for injection after randomisation in an adjacent room. The visual aspect of syringes and viscosity were similar |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Two independent blinded experts with extensive sclerotherapy experience analysed the photographs using a visual a priori score of vessel disappearance |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 8 participants; 5 lost to follow‐up and 3 for accompanying photos of poor quality |
| Selective reporting (reporting bias) | Unclear risk | No details given |
| Other bias | Unclear risk | No details given |
Kern 2012.
| Study characteristics | ||
| Methods |
Study design: prospective randomised trial Method of randomisation: computer‐generated random table Blinding: participants ‐ yes, doctor ‐ no, outcome assessor ‐ no Power calculation: were required to detect a true difference of half of a standard deviation at a 1‐sided 5% significance level with a power of 80% Total number of participants: 53 Total number of procedures: 106 Treatment localisation: leg veins Number of exclusions post‐randomisation: none Number of withdrawals and reasons: none |
|
| Participants |
Setting: outpatient site Country: Switzerland Gender: women Age: 49 mean age (range 29 ‐ 74) Inclusion criteria: women were eligible if presenting for cosmetic reasons for sclerotherapy of reticular and telangiectatic leg veins (C1A or S). Distribution of telangiectasias and reticular leg veins had to be similar in both legs. Participants had no signs of superficial or deep vein insufficiency as assessed using duplex ultrasound Exclusion criteria: allergies to chrome and to lidocaine‐epinephrine No patient declined participation, and there were no patient‐reported allergies, so no patient was excluded from the study |
|
| Interventions |
Treatment 1: standard treatment technique (successive injections of chromated glycerin mixed with one‐third lidocaine‐epinephrine 1%) Treatment 2: new 2‐step technique (first treating only reticular veins with a single injection at the base of each cluster of telangiectasias and then successively injecting all remaining telangiectasias a few minutes later) Both legs were treated during the same sclerotherapy session. One lower limb underwent standard treatment technique, the other one underwent the new 2‐step technique. Reticular vein diameter ranged between 1 and 2 mm and that of telangiectasias between 0.2 and 1 mm Duration of follow‐up: 3 weeks Use of compression: compression stocking |
|
| Outcomes | The main study outcome was participant pain score. Immediately after each leg treatment, participants were asked to score their pain using a 100‐point visual analogue scale (0 = no pain, 100 = maximal pain). Participants graded their pain by drawing a line on the scale. At the end of the session, participants were asked to indicate on which leg injections were more comfortable | |
| Funding sources | No details provided | |
| Declarations of interest | None declared | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Before treatment, each participant was randomly assigned (computer‐generated random table) to receive the standard technique or the 2‐step technique first |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants were blinded to the randomisation and did not know which technique was going to be performed first |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Immediately after each leg treatment, participants were asked to score their pain using a 100‐point VAS (0 = no pain, 100 = maximal pain). Participants graded their pain by drawing a line on the scale. At the end of the session, participants were asked to indicate on which leg injections were more comfortable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 53 participants were randomised, so there were 53 legs in the standard technique group and 53 legs in the 2‐steps technique group |
| Selective reporting (reporting bias) | Unclear risk | No details given |
| Other bias | Unclear risk | No details given |
Klein 2013.
| Study characteristics | ||
| Methods |
Study design: randomised, controlled pilot study Method of randomisation: randomisation list with software PASS 2008 Blinding: participants ‐ no, doctor ‐ no, outcome assessors ‐ yes Power calculation: not mentioned Total number of patients: 15 Total number of procedures: 30 Treatment localisation: leg veins Number of exclusions post‐randomisation: none Number of withdrawals and reasons: 1 lost at last visit |
|
| Participants |
Setting: outpatient site Country: Germany Gender: women Age: range 28 ‐ 62 years (mean: 44 years) Inclusion criteria: written informed consent, White ethnicity, and Fitzpatrick skin type I–IV Exclusion criteria: age younger than 18 years, pregnancy, breast‐feeding, suntan, and a risk of non‐compliance 5 out of 15 women were post‐menopausal, and the others used contraception. Before laser therapy was applied, underlying chronic venous insufficiency was excluded by duplex ultrasonography. The vessel diameter was measured by means of a dermatoscope with an integrated scale |
|
| Interventions |
Groups: "Patients were assigned to Groups A, B, or C. For the first five patients (Group A), we used a mean radiant exposure of 64.07 J/cm2, a mean pulse duration of 26.65 ms, and a spot size of 4 mm. Because we neither observed any side effects nor any visible response, the radiant exposure was increased. For the next five patients (Group B), we applied mean radiant exposures of 96 J/cm2 and mean pulse durations of 54.8 ms with the 4 and 6 mm handpiece. After 6 weeks, slight vessel clearance was seen without any adverse events so that we further increased the radiant exposure. For the last five patients (Group C), we applied mean radiant exposures of 107 J/cm2, mean pulse durations of 85.8 ms, and a spot size of 6 mm as well as double pulses for the last two patients." Technique: "All patients had similarly sized telangiectatic leg veins on both legs and were randomly allocated to either PDL therapy (Sclerolaser plusTM, Candela Corp., Wayland, MA) or diode laser therapy (MeDioStar, Asclepion, Jena, Germany) on either leg. The treatment allocation was deposited in a closed envelope. The envelopes were numbered consecutively from 1 to 15, and the investigator always opened the envelope with the lowest available parameters: Wavelength 595 nm, radiant exposure 16 J/cm2, elliptical spot of 2 7 mm, and pulse duration 1.5 ms. The PDL had an integrated cryogen spray cooling system. Before the application of ICG, 11 patients received test spots with the diode laser alone (808 nm, spot size 4 or 6 mm, radiant exposure 75–110 J/cm2, pulse duration 26–87 ms) for safety reasons. The same laser parameters were applied for diode alone and ICG þ DL besides the application of double pulses. Afterwards, patients obtained an intravenous access in the median cubital vein. ICG (Pulsion Medical Systems AG, Munich, Germany) was dissolved in an aqueous solvent (50 mg of ICG in 10 mL of solvent) at a concentration of 5 mg/mL and immediately intravenously injected as a bolus. The administered ICG‐concentration was 2 mg/kg body weight ICG fluorescence was visualized with a commercial fluorescence imaging system (IC‐View1; Pulsion Medical Systems, Munich, Germany), consisting of a digital video recorder with an integrated near‐infrared (NIR) light source (energy 0.16 W, wavelength 780 nm) and recorded on video tape (BASF; Ludwigshafen, Germany). The object lens of the camera was covered with a filter (835 nm) to collect NIR radiation and reject visible light. Approximately 2 min after the ICG injection, diode laser therapy was started because of the short half‐life time of ICG in plasma (5 min). The handpiece of the diode laser had an integrated cooling device and a cold transparent ultrasound gel was applied onto the treatment area. Because ICG‐augmented diode laser therapy was used for the treatment of telangiectatic leg veins for the first time, we started with low radiant exposures. The treatment objective was to achieve blanching of vessels without inducing unspecific thermal damage, such as skin burns or scars. Radiant exposure and pulse duration were gradually increased according to the clinical reaction and the first clinical results (dose escalation). Furthermore, two different spot sizes were applied (4 and 6 mm)." Duration of follow‐up: 6 weeks Use of compression: not mentioned |
|
| Outcomes | Pain: during each treatment session with PDL and ICG þ DL, pain was documented on a VAS ranging from 0 to 10; 0 represented no pain and 10 equalled extremely severe pain. Photo documentation was done at each visit (Canon EOS D30, 50 mm lens)
Side effects: crusts, blistering, oedema, erythema (mild, moderate, and severe), hypopigmentation or hyperpigmentation, telangiectatic matting, and scarring
Vessel clearance well as by a blinded observer as follows: no clearance (0%), slight clearance (< 25%), moderate clearance (25% – 50%), good clearance (51% – 75%), or excellent clearance (> 75%)
The cosmetic appearance of the treatment area was rated on a scale from 0 (very bad cosmetic appearance) to 10 (very good cosmetic appearance) by the participant and by the blinded observer by means of colour digital photography At each visit, the treatment area was rated by the participants as well as by a blinded observer as follows: no clearance (0%), slight clearance (< 25%), moderate clearance (25% – 50%), good clearance (51% –75%), or excellent clearance (> 75%) |
|
| Funding sources | "None" | |
| Declarations of interest | Quote "The Diode laser MeDioStar was provided as a loan by Asclepion, Jena, Germany." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The Center for Clinical Studies at the University Hospital Regensburg generated the randomisation list with the software PASS 2008 (NCSS). The allocation of each participant to the respective treatment modality was labelled right/left for ICG‐augmented diode laser therapy or FPDL treatment |
| Allocation concealment (selection bias) | Low risk | The treatment allocation was deposited in a closed envelope |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Furthermore, the cosmetic appearance of the treatment area was rated on a scale from 0 (very bad cosmetic appearance) to 10 (very good cosmetic appearance) by the patient and by the blinded observer by means of colour digital photography at each visit." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 14/15 participants completed each step of the study protocol. 1 participant missed the last visit |
| Selective reporting (reporting bias) | Unclear risk | No details given |
| Other bias | Unclear risk | No details given |
Leach 2003.
| Study characteristics | ||
| Methods |
Study design: split‐body, randomised study Method of randomisation: coin toss to determine which leg was assigned each treatment Blinding: participant ‐ no, doctor ‐ no Power calculation: not mentioned Total number of participants randomised: 13 participants had each lower limb randomised to receive 1 of 2 sclerosing agents Total number of procedures: 26 Treatment localisation: leg veins Exclusions post‐randomisation: none Number of withdrawals and reasons: none |
|
| Participants |
Setting: outpatient site Country: USA Gender: women Age: mean 57 years (range 41 ‐ 70) Inclusion criteria: presence of leg veins 0.2 to 0.4 mm in diameter without evidence of feeding reticular veins Exclusion criteria: incompetence at the saphenofemoral or saphenopopliteal junctions |
|
| Interventions |
Treatment 1: STS 0.25% Treatment 2: chromated glycerin 72% 1 leg of each participant was randomised to receive either STS 0.25% or chromated glycerin 72%, and the other leg received the other agent Duration of follow‐up: ranged from 2 to 6 months Use of compression: not mentioned |
|
| Outcomes | Efficacy: vessel clearance (yes, no) assessed by treating physician at follow‐up Adverse events: bruising, swelling, hyperpigmentation (yes, no) assessed by treating physician at follow‐up Pain: assessed by participant at time of injection (yes, no) |
|
| Funding sources | No funding source stated | |
| Declarations of interest | No details provided | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A coin toss |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding mentioned. STS and CG have different viscosities that are visible at time of injection |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | 2 independent experts who were not involved in the treatment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no incomplete data |
| Selective reporting (reporting bias) | Low risk | All specified outcomes were reported |
| Other bias | Unclear risk | No details given |
Lupton 2002.
| Study characteristics | ||
| Methods |
Study design: randomised trial Method of randomisation: not mentioned Blinding: participants ‐ no, doctor ‐ no, outcome assessors ‐ yes Power calculation: not mentioned Total number of participants: 20 Total number of procedures: 40 Treatment localisation: lower extremities Number of exclusions post‐randomisation: none Number of withdrawals and reasons: none |
|
| Participants |
Setting: outpatient site Country: USA Gender: women Age: range 27 – 68 years (mean 45 years) Inclusion criteria: skin photo types I – III, with size‐matched superficial leg telangiectases (diameter range 0.1 – 1.5 mm; mean 0.5 mm) Exclusion criteria: prior history of lower extremity telangiectasia treatment, clinical evidence of severe vascular incompetence, on anticoagulant treatment, or those currently pregnant or breastfeeding |
|
| Interventions |
Treatment 1: 2 treatments with a long‐pulsed 1064 nm Nd:YAG laser (Varia, Cool Touch Laser Corp., Auburn, LA) Treatment 2: 0.25% STS (Sotradecol, Elkins‐Sinn Inc., Cherry Hill, NJ) sclerotherapy Participants were randomised to receive 2 treatments with a long‐pulsed 1064 nm Nd:YAG laser to telangiectases on 1 leg and 0.25% STS sclerotherapy to those on the other. Size‐matched vessels on the thighs, knees, calves, ankles, and popliteal fossae received treatment by a single operator. Laser treatments were delivered through a 5.5 mm collimated spot size at 1 Hz using fluences of 125 – 150 J/cm2 (mean 135 J/cm2). A pulse duration of 25 msec was used for smaller vessels and a 50 msec pulse width was applied for vessels larger than 0.5 mm in diameter. Epidermal cooling was achieved with a cryogen spray of varying pre‐ and post‐treatment durations depending upon the skin photo type of the participant (i.e. longer pre‐cooling with darker skin photo types) and the size of the vessel (i.e. increased post‐cooling delay for larger vessels in order to effect full‐thickness mural denaturation). Precooling durations ranged from 0 to 5 msec, post‐cooling durations ranged from 20 to 50 msec, and post‐cooling delays ranged from 5 to 20 msec Duration of follow‐up: 1 ‐ 3 months Use of compression: compression stocking |
|
| Outcomes | Photographic documentation and clinical improvement scores were determined 1 month after the first treatment session, and 1 and 3 months after the second treatment session by 2 masked independent assessors using a quartile grading scale of 0: less than 25% improvement, 1: 26% – 50% improvement, 2: 51% – 75% improvement, and 3: greater than 75% improvement Side effects of treatment were also recorded at each treatment and follow‐up visit |
|
| Funding sources | Quote "No significant interest with commercial supporters" | |
| Declarations of interest | None declared | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Participants were randomised to receive two treatments with a long‐pulsed 1064 nm Nd:YAG laser" Comment: states "randomised", no further details given |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not possible to blind participants and personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Photographic documentation and clinical improvement scores were determined 1 month after the first treatment session, and 1 and 3 months after the second treatment session by 2 masked independent assessors using a quartile grading scale of 0: less than 25% improvement, 1: 26% – 50% improvement, 2: 51% – 75% improvement, and 3: greater than 75% improvement. Side effects of treatment were also recorded at each treatment and follow‐up visit |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no incomplete data |
| Selective reporting (reporting bias) | Unclear risk | No details given |
| Other bias | Unclear risk | No details given |
McCoy 1999.
| Study characteristics | ||
| Methods |
Study design: randomised, controlled, blinded trial Method of randomisation: not mentioned Blinding: participants ‐ yes, physician ‐ yes and outcomes assessment ‐ yes Power calculation: not mentioned Total number of participants: 81 Total number of procedures: 162 Treatment localisation: leg veins Number of exclusions post‐randomisation: none Number of withdrawals and reasons: none |
|
| Participants |
Setting: outpatient site Country: Australia Gender: women Age: range: 21 ‐ 76 years (mean 44.3 years) Inclusion criteria: women with primary idiopathic telangiectasia on the legs were eligible to participate in the trial if they demonstrated symmetrical areas of vessels on both legs and identifiable reticular feeding veins. Gave informed consent Exclusion criteria: telangiectasia around the ankles or clusters of microvessels arising secondary to surgical scars were excluded because they are rarely symmetrical, in the authors' experience results of treatment are unpredictable in these instances. Other exclusion criteria included previous sclerotherapy (although patients who had undergone previous venous surgery were included), clinical or duplex doppler evidence of major saphenous or large perforator incompetence, history of ischaemic heart disease, vasculitis of any aetiology, diabetes mellitus, current pregnancy or the regular use of anticoagulants |
|
| Interventions |
Treatment 1: POL (POL 1% was derived by diluting POL 3% with normal saline) Treatment 2: hypertonic saline (hypertonic saline 20% was derived by combing 2 mL of 30% saline with 1 mL of 2% lignocaine hydrochloride to constitute 3 mL of sclerosant) For each participant, 1 leg was randomly assigned POL and the other hypertonic saline as the sclerosant. Participants were unaware of the choice of sclerosant for each leg until after the 2‐month assessment. However, the treating physician was aware at the time of treatment. All treatments were undertaken with the participant in the supine position. Becton‐Dickison needles #30 gauge and 3 mL disposable syringes were used. Sufficient solution to completely blanch a reticular feeding system and all of its visible blanches and telangiectasia was infused. 1 treatment only was performed per leg. Compression was not employed, and no specific restrictions were placed on participants by activities or exercise following the treatment Duration of follow‐up: 2 months Use of compression: not used |
|
| Outcomes | Clinical assessment: the treating physician: Rated improvement on a scale of 0 (no improvement) to 10 (complete disappearance). Photographic assessment rated improvement based on before‐and‐after photographs using the same 0 to 10 scale method. Side effects of treatment were also assessed. Participants rated pain of injection for each leg from 0 (not painful) to 10 (extremely painful) | |
| Funding sources | No details provided | |
| Declarations of interest | No details provided | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "For each participant, one leg was randomly assigned polidocanol and the other hypertonic saline as the sclerosant" Comment: states "randomised", no further details given |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants were unaware of the choice of sclerosant for each leg until after the 2‐month assessment. However, the treating physician was aware at the time of treatment |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | A non‐treating physician, blinded to sclerosants used for each leg, rated improvement based on before‐and‐after photographs using the same 0 to 10 scale method |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No details given |
| Selective reporting (reporting bias) | Unclear risk | No details given |
| Other bias | Unclear risk | No details given |
Moreno‐Moraga 2013.
| Study characteristics | ||
| Methods |
Study design: prospective comparative study Method of randomisation: day of scheduled appointments Blinding: participants ‐ no, doctor ‐ no, outcome assessors ‐ yes Power calculation: not mentioned Total number of participants: 90 participants; 30 group A treatment POL, 30 group B Laser, 30 group C POL + Laser. For allocation the author used scheduled appointments. All participants explored on Mondays were assigned to group A, Wednesdays to group B, and Thursdays were allocated to group C Total number of procedures: 180 Treatment localisation: leg veins Number of exclusions post‐randomisation: none Number of withdrawals and reasons: none |
|
| Participants |
Setting: outpatient site Country: Spain Gender: women Age: range 19 ‐ 46 Inclusion criteria: women, varicules (1.5 mm) Exclusion criteria: less than 18 years of age, pregnancy, breastfeeding, scarring or infection at the treatment site, use of iron supplements or anti‐coagulants, history of photosensitivity, or scarring |
|
| Interventions |
Treatment 1: POL in micro‐foam form, prepared following the Tessari technique using POL concentration of 0.3% with CO2 Treatment 2: laser ‐ 1064 nm long pulse Nd:YAG Treatment 3: POL in micro‐foam form plus laser 1064 nm long pulse Nd:YAG The procedure was repeated after 8 weeks Duration of follow‐up: 16 weeks Use of compression: compression stocking |
|
| Outcomes | Improvement or resolution with blind evaluators, unfamiliar with the study and the participants, using scale: very good > 90% improvement, good 70% to 89%, fair 50% to 69% and poor results < 50% The satisfaction of participants with VAS Adverse events such as burns, hyperpigmentations or matting was related |
|
| Funding sources | No details provided | |
| Declarations of interest | No details provided | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | For allocation the author used scheduled appointments. All participants explored on Mondays were assigned to group A, Wednesdays to group B, and Thursdays were allocated to group C |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not applicable |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Improvement or resolution with blinded evaluators, unfamiliar with the study and the participants, used the scale: very good > 90% improvement, good 70% to 89%, fair 50 to 69% and poor results < 50% |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no incomplete data |
| Selective reporting (reporting bias) | Unclear risk | No details given |
| Other bias | Unclear risk | No details given |
Moreno‐Moraga 2014.
| Study characteristics | ||
| Methods |
Study design: prospective randomised study Method of randomisation: no details given Blinding: participant ‐ no, doctor ‐ no, outcome assessors ‐ yes Power calculation: not mentioned Total number of participants: 320; 79 legs treated with POL and 517 treated with laser + POL Total number of procedures: 640: 79 legs treated with POL and 517 treated with laser + POL Treatment localisation: leg veins Number of exclusions post‐randomisation: none Number of withdrawals and reasons: 22 did not complete the study |
|
| Participants |
Setting: outpatient site Country: Spain Gender: women Age: range 19 ‐ 72 years (mean: 46.3 years) Inclusion criteria: age from 18 to 74 years, Fitzpatrick skin types II to IV, not having received any previous treatment for varicose veins Exclusion criteria: confirmation reflux in the deep system by Doppler, organic or psychological conditions which advised against the treatment or which could alter the assessment of results |
|
| Interventions |
Treatment 1: POL Treatment 2: laser plus POL The microfoam was obtained using 2 x 10 mL omnifix syringes with a Luer‐Lock connection, a 3‐way stopcock to connect the syringes and a 15G load needle with an air microfilter. Two millilitres of POL (Aethoxysclerol) were used at 0.3% and 8 mL of air. Pumping and switching the solution from one syringe to the other 15 times produces a stable microfoam following the Tessari technique The laser used was the 1064‐nm‐long pulse Nd:YAG (Laserscope, San Jose, CA, USA). The diameter of the spot used was equal to or slightly larger than the size of the vessel, ranging between 2 mm and 5 mm. The energy per pulse was similar, regardless of the spot size. For 2 mm spots, the energy per pulse was 9.42 J, whereas, for 5 mm spots, it was 11.77J/pulse. Pulse width was 30 ms for class I and II, and 50 ms class III Duration of follow‐up: 3 months, 3 years Use of compression: compression stocking |
|
| Outcomes | Vessel clearance: the treated areas were analysed by macro‐photography. A subjective clearing scale was established as follows: 0 points (20% clearing), 2 points (40% clearing), 3 points (60% clearing) 4 points (80% clearing) and 5 points (full clearing of veins). Three blinded physician investigators, familiar with leg vein treatment, rated the degree of clearing on the basis of all photographed images Any possible complications and side effects were recorded during the procedure, at the immediate post‐treatment stage and at the long‐term follow‐up Enquiries were made as to the pain experienced during the procedure for each treatment, rated as light, moderate, severe or very severe |
|
| Funding sources | No details provided | |
| Declarations of interest | No details provided | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details given |
| Allocation concealment (selection bias) | Low risk | Sealed envelope |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Impossible to blind participants |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Three blinded physician investigators, familiar with leg vein treatment, rated the degree of clearing on the basis of all photographed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 22 of the 320 participants did not complete the study |
| Selective reporting (reporting bias) | Unclear risk | No details given |
| Other bias | Unclear risk | No details given |
Munia 2012.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Method of randomisation: not mentioned Blinding: participants ‐ no, doctor ‐ no, outcome assessors ‐ yes Power calculation: not mentioned Total number of participants: 30 Total number of procedures: 60 Treatment localisation: lower extremity Number of exclusions post‐randomisation: none Number of withdrawals and reasons: none Source of funding: no significant interest with commercial supporters |
|
| Participants |
Setting: outpatient site Country: Brazil Gender: women Age:range 25 ‐ 65 years (mean 43 years) Inclusion criteria: skin photo types I to VI and lower extremity telangiectases 0.5 to 1.5 mm in diameter; only lower extremity telangiectasia (CEAP C1) Exclusion criteria: women with evident signs of chronic venous insufficiency or varices (CEAP grades C2 and up) and those who were pregnant, breastfeeding, or undergoing anticoagulant therapy |
|
| Interventions |
Treatment 1: Nd:YAG laser (Xeo, Cutera) Treatment 2: sclerotherapy with 75% glucose solution "For each patient, we selected comparable treatment areas on the lower limbs; one leg was randomly assigned laser and the other sclerotherapy as the choice of treatment. The same physician applied both modalities on selected sites of the ankle, calf, popliteal cavum, and thigh of each leg in three sessions at 1‐month intervals. Laser was applied to one leg with a cooled spot size of 3 mm at 1 Hz, fluence of 100 to 120 J/cm2, and a pulse width of 15 ms (on smaller vessels) or 30 ms (on larger vessels). The skin was cooled before and after pulses, more carefully in patients with higher skin photo types and large or blue vessels. The other leg was treated with 75% glucose solution low‐flow injections, performed using a 3 mL syringe and a 30 G needle until complete clearing of the targeted vessel was achieved while the surrounding area was preserved. Immediately upon withdrawal of the needle, puncture sites were covered with small dry cotton balls and taped with Micropore. The total volume of injected sclerosant solution was 1 to 2 mL per session. and patients were advised to avoid sun exposure for 7 days." Duration of follow‐up: 1 week Use of compression: no compression was employed after treatment sessions |
|
| Outcomes | Participants were photographed before and 1 week after treatment. This follow‐up visit was standardised for all participants The applying physician and 2 independent observers rated photographic improvement of the treated areas on a scale of 0 (no improvement) to 10 (complete clearing of the vessels) Complications and adverse effects were noted during follow‐up Also at this 1‐week after‐treatment visit, all participants were asked to respond to a questionnaire rating pain (none, little, very painful, or extremely painful); clearing of the vessels (poor, fair, good, or excellent); and satisfaction with the results (not at all, little, or completely satisfied) |
|
| Funding sources | Quote: "No significant interest with commercial supporters" | |
| Declarations of interest | None declared | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States "randomised" but no details given |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Impossible to blind participants |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The applying physician and 2 independent observers rated photographic improvement of the treated areas on a scale of 0 (no improvement) to 10 (complete clearing of the vessels). Complications and adverse effects were noted during follow‐up |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed the study |
| Selective reporting (reporting bias) | Unclear risk | No details given |
| Other bias | Unclear risk | No details given |
Nguyen 2020.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Method of randomisation: randomisation by right or left limb Blinding: participants ‐ no, doctor ‐ no, outcome assessors ‐ yes Power calculation: not mentioned Total number of participants: 22 Total number of procedures: 44 Treatment localisation: lower extremity Number of exclusions post‐randomisation: none Number of withdrawals and reasons: none |
|
| Participants |
Setting: outpatient site Country: Vietnam Gender: both genders Age: mean 32 years Inclusion criteria: skin photo type IV and lower extremity telangiectases or reticular veins (CEAP C1) Exclusion criteria: pregnancy; lactation; anticoagulation agent use; active skin infection; or history of herpes simplex infection, thrombosis, hypercoagulability, diabetes, hypertrophic/keloid scar, and cardiovascular, renal, and liver disease |
|
| Interventions | The Laser 1064 was used in right leg, a single pass with 5 mm fixed spot size, pulse width of 20 ms for telangiectasias, and 30 ms for reticular veins and fluences of 120 to 220J/cm2 was used The Laser 755 was used in left side, a single pass with 5 mm fixed spot size, pulse width of 12 ms for telangiectasia, and 20 ms for reticular veins and fluences of 55 to 140J/cm2 was used In all cases, fluence levels were adjusted to achieve clinical endpoints of vessel blanching or greyish colour within the vessel |
|
| Outcomes | Pain level Efficacy was evaluated by clinical examination and comparison of pre‐ and post‐treatment standardised photos |
|
| Funding sources | No funding received | |
| Declarations of interest | None declared | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quasi‐randomised, randomisation by right or left limb |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All results were independently evaluated by 2 authors |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No details given |
| Selective reporting (reporting bias) | Unclear risk | No details given |
| Other bias | Unclear risk | No details given |
Norris 1989.
| Study characteristics | ||
| Methods |
Study design: a double‐blind, double‐paired comparison study Method of randomisation: not mentioned Blinding: participants ‐ yes, physician ‐ yes and outcomes assessment ‐ yes Power calculation: the study population size was determined as being large enough to disclose statistically significant differences should they exist Total number of participants: 20 participants with bilaterally symmetric telangiectasias of the legs and thighs Total number of procedures: 80 Treatment localisation: leg veins Number of exclusions post‐randomisation: none Number of withdrawals and reasons: none |
|
| Participants |
Setting: outpatient site Country: USA Gender: women Age: range 30 ‐ 57 years Inclusion criteria: women with bilaterally symmetric telangiectasias of the legs and thighs Exclusion criteria: patients with a history of phlebitis and those taking hormones and anticoagulants. Women taking disulfiram were specifically excluded because ethanol is present in the POL preparation and may interact with disulfiram |
|
| Interventions |
Treatment 1: POL (Aetoxisclerol; Laberatoires Pharmaceufiques Dexo S.A., Nanterre, France) in concentrations of 0.25% Treatment 2: POL (Aetoxisclerol; Laberatoires Pharmaceufiques Dexo S.A., Nanterre, France) in concentrations of 0.50% Treatment 3: POL (Aetoxisclerol; Laberatoires Pharmaceufiques Dexo S.A., Nanterre, France) in concentrations of 0.75% Treatment 4: POL (Aetoxisclerol; Laberatoires Pharmaceufiques Dexo S.A., Nanterre, France) in concentrations of 1.0% Quote "The lower extremities were divided into four quadrants with roughly equivalent numbers and sizes of vessels, ranging in size from approximately 0.2 to 1 mm. Thus each patient served as her own control with respect to vessel size. Each area was treated with a maximum of 2 mL of the same solution at each visit. Each patient received injections with polidocanol in concentrations of 0.25%, 0.50%, 0.75%, and 1.0%, each labelled and coded as an unknown and randomly assigned to one of the leg quadrants. Patients received injections approximately every 4 weeks until all the involved vessels had disappeared, or for a maximum of six visits. Patients were asked to avoid strenuous exercise for 48 hours after treatment and to wear antiembolism elastic stockings with gradient pressure (Jobst Anti‐EM; Jobst, Toledo, Ohio) for at least the first 48 hours after treatment and as much as possible for 2 weeks thereafter." Duration of follow‐up: 2 weeks Use of compression: support hose were used to make external pressure constant for each participant so that pressure was not a variable in the study |
|
| Outcomes | Quote "Patients were evaluated both subjectively and objectively. Permanent data were maintained in the form of colour photographs at baseline before the beginning of the study and before each treatment. By comparing photographs for each concentration used, improvement was rated by the investigators (M. J. N, M. C. C.) as poor‐to‐moderate, good, or excellent, with poor response being little or no visible effect and excellent being complete clearing of involved veins. Itching, hyperpigmentation, and neovascularization were rated as none and present (mild to severe). Itching was judged by each patient, and the other two side effects were determined by the investigators. The number of treatments needed to clear each quadrant was noted from one to greater than six; the decision was based on a precipitous drop in the volume of sclerosant used compared with the preceding treatment. Patient satisfaction was rated as dissatisfied, neither satisfied nor dissatisfied, satisfied, or very satisfied. Patients were asked to select their sclerosant of choice at the completion of the study." | |
| Funding sources | No details provided | |
| Declarations of interest | No details provided | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details given |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Each participant received injections with POL in concentrations of 0.25%, 0.50%, 0.75% and 1.0%, each labelled and coded as an unknown and randomly assigned to one of the leg quadrants |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details given |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed the study |
| Selective reporting (reporting bias) | Unclear risk | No details given |
| Other bias | Unclear risk | No details given |
Ochoa 2021.
| Study characteristics | ||
| Methods |
Study design: randomised clinical trial Method of randomisation: not mentioned Blinding: participant ‐ no; treating doctor ‐ yes; outcome assessors ‐ yes Power calculation: sample size was calculated to give a statistical power of 80% Total number of participants: 720 Total number of procedures: 720 Treatment localisation: leg veins Number of exclusions post‐randomisation: 111 Number of withdrawals and reasons: 111 lost to follow‐up |
|
| Participants |
Setting: outpatient site Country: Mexico Gender: men and women Age: mean 41 years group A and 42 years group B Inclusion criteria: varicose, reticular or telangiectatic leg veins, or both Exclusion criteria: incompetence at the saphenofemoral or saphenopopliteal junctions; history of DVT; general poor health |
|
| Interventions |
Group A: received 250 lipoprotein‐lipase release unit of sulodexide twice a day for 7 days before scheduled sclerotherapy and this continued for 3 months after the initial procedure Group B: control group, underwent sclerotherapy without sulodexide treatment Duration of follow‐up: 16 weeks Use of compression: not mentioned |
|
| Outcomes | Incidence of hyperpigmentation Degree of hyperpigmentation Vein disappearance rate Major bleeding |
|
| Funding sources | Union Internationale de Phlébologie 2018 Research Fellowship Kreussler Young Scientists' Sclerotherapy Grant award | |
| Declarations of interest | Quote: "A.J.G.O. has previously been paid consulting fees and for speaking in a meeting organized by Alfasigma." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details given |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The photographs were evaluated by 2 independent physicians blinded to group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No details given |
| Selective reporting (reporting bias) | Unclear risk | No details given |
| Other bias | Unclear risk | No details given |
Ozden 2011.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Method of randomisation: randomisation list Blinding: participants ‐ no, doctor ‐ no, outcome assessors ‐ yes Power calculation: not mentioned Total number of participants: 21 Total number of procedures: 64 Treatment localisation: leg veins Number of exclusions post‐randomisation: none Number of withdrawals and reasons: 5 patients, 3 due to lack of time and 2 intolerability to pain |
|
| Participants |
Setting: outpatient site Country: Turkey Gender: women Age: mean 42.28 years (range 32 ‐ 60) Inclusion criteria: White women aged between 20 and 60 years with spider leg veins Exclusion criteria: prior history of DVT, previous treatment of leg veins, pregnancy, poor wound‐healing diseases, hypercoagulability and large varicose veins A series of 16 patients with size‐matched superficial telangiectases of the lower extremities were randomly assigned to receive 3 consecutive monthly treatments with the long‐pulsed 1064 nm Nd:YAG on 1 leg and 532‐nm KTP laser irradiation on the other |
|
| Interventions |
Treatment 1: Nd:YAG laser Treatment 2: KTP laser Participants received treatments with the Nd:YAG laser to vessels on 1 leg and with the KTP laser to the other leg, for up to 3 sessions every 4 weeks Duration of follow‐up: 1 month Use of compression: no compression stocking |
|
| Outcomes | The endpoint for laser sessions was determined to be when either visual elimination or darkening of the vessel was observed. Response to treatment was rated on a quartile system: 0 = no clearing; 1 = 1% ‐ 24% clearing; 2 = 25% ‐ 49% clearing; 3 = 50% ‐ 74% clearing; 4 = 75% ‐ 94% clearing; 5 = 95% ‐ 100% Side effects of treatment were also recorded at each treatment and follow‐up visit |
|
| Funding sources | No details provided | |
| Declarations of interest | None declared | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was done by a person not involved in this study, who sent the patients on hand of a randomisation list" |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Results were judged visually by 2 experienced (1 independent) physicians on patient examination |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 5 participants: 3 lost to follow‐up and 2 intolerability to pain |
| Selective reporting (reporting bias) | Unclear risk | No details given |
| Other bias | Unclear risk | No details given |
Parlar 2015.
| Study characteristics | ||
| Methods |
Study design: prospective, randomised, open‐label trial Method of randomisation: not mentioned Blinding: participants ‐ no, doctor ‐ no, outcome assessors ‐ yes Power calculation: not mentioned Total number of participants: 56 Total number of procedures: 112 Treatment localisation: leg veins Number of exclusions post‐randomisation: none Number of withdrawals and reasons: 5 lost to follow‐up |
|
| Participants |
Setting: outpatient site Country: Switzerland Gender: women Age: mean 46 years (range 23 ‐ 66) Inclusion criteria: female volunteers of at least 18 years old and present typical primary leg telangiectasias and reticular veins and Fitzpatrick skin types 1 – 3 Exclusion criteria: women with varicose veins greater than C1 as confirmed clinically and by duplex investigation, arterial occlusive disease and known pregnancy were excluded from the study. If the veins were not distributed symmetrically on the lower limb, women were excluded. One leg was randomly assigned to get treatment with the multiple synchronized long‐pulsed Nd:YAG laser, while the other received foam sclerotherapy with POL 0.5% All participants underwent clinical examination of the legs focused on the skin, venous system, joints, arteries and colour‐flow duplex imaging. Duplex ultrasound was done with a Siemens D34. Included participants did not exhibit a reflux lasting more than 1 second in the deep veins, saphenous junctions, trunk and tributaries or perforating veins. All had fully compressible superficial and deep veins. All measurements were documented in a case record form. Room temperature was documented |
|
| Interventions |
Treatment 1: sclerotherapy Treatment 2: Nd:YAG laser Quote "The participants were randomly assigned to get treatment with sclerotherapy or Nd:YAG laser either on the right or left lower extremity in form of a side‐by‐side comparison. The patients had comparable types and sizes of telangiectasias (0.2 – 1 mm) and reticular veins (1– 2.9 mm). An area of 10 ‐ 15 cm was marked on each side and photographical documentation was carried out (Canon Power Shot G9, digital camera, Tokyo, Japan). If the veins were not distributed symmetrically on the lower limb, subjects were excluded. We used for documentation the same digital camera, lighting conditions (flash) and focal distance. One leg was treated with the multiple synchronized long‐pulsed Nd:YAG laser (Cynergy MultiplexTM; Cynosure©, Inc., Westford, MA, USA) using fluences between 100 and 200 J/cm² with a spot size of 3 – 7 mm and pulse width of 10 – 50 ms. To minimize pain and thermal injury we used the skin cooling system Cryo 6 Cold Air Device (Zimmer MedizinSystems©, Irvine, CA, USA). The endpoint of the laser treatment was the clinically observable closure of the vessels during irradiation as well as the blanching and darkening for the goal of a complete photocoagulation. The sclerotherapy on the other leg was performed using polidocanol 0.5% foam (Aethoxysklerol©, Kreussler Pharma, Wiesbaden, Germany with the device Sclerivein© from Help Medical, Paris, France for producing sterile sclerosing foam) and injections were performed with a 2 mL silicone syringe and a 30‐ gauge needle with the patient in supine position. By using a Transillumination Vein Access (Veinlite©; Translite LL, Sugarland, TX, USA) we were also capable to treat the feeder reticular varicose and their telangiectasias web (maritime pine tree pattern) with polidocanol foam 0.5%. During daytime, the participants were advised to wear compression stockings Swiss class II (26 – 32 mmHg) for 3 weeks." Duration of follow‐up: 6 months Use of compression: compression stocking |
|
| Outcomes | Quote "Digital photographs were taken at each follow‐up visit under the conditions set for the pre‐treatment assessment. The participants had to reply to a questionnaire rating the pain respectively their satisfaction as a secondary outcome using the visual analogue scale ranging from 1 (no pain) to 6 (very strong pain) respectively 1 (not satisfied) to 6 (very satisfied). The applying physician evaluated at each visit and two blinded experts at the end of the study the photographs for clearing of the vessels using a six‐point scale of 1 = no improvement, 2 = minimal improvement (10% – 20%), 3 = fair improvement (30% – 40%), 4 = good improvement (50% – 70%), 5 = very good improvement (> 70%), 6 = extremely good improvement (100% cleared). The blinded experts dermatologists) were not included in the clinical phase of the study. Side‐effects of treatment including pigmentation, necrosis and matting were also assessed. The treating physician, not blinded to the treatment, checked for side‐effects, as well." | |
| Funding sources | No funding received | |
| Declarations of interest | None declared | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States "randomised", no details given |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Impossible to blind participants |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The blinded experts (dermatologists) were not included in the clinical phase of the study. Side‐effects of treatment including pigmentation, necrosis and matting were also assessed. The treating physician, not blinded to the treatment, checked for side effects as well |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 5 participants lost to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | No details given |
| Other bias | Unclear risk | No details given |
Peterson 2012a.
| Study characteristics | ||
| Methods |
Design: prospective, randomised, blind trial Method of randomisation: not mentioned Blinding: participants ‐ not mentioned, doctor ‐ not mentioned, outcome assessors ‐ yes Power calculation: not mentioned Total number of participants: 20 Total number of procedures: 40 Treatment localisation: leg veins Number of exclusions post‐randomisation: none Number of withdrawals and reasons: none |
|
| Participants |
Setting: outpatient site Country: USA Gender: women Age: range 31 ‐ 65 years Inclusion criteria: women or men in good general health aged 18 ‐ 65 years with reticular leg veins, planning to undergo sclerotherapy of their reticular and telangiectatic leg veins Exclusion criteria: people suffered from any uncontrolled systemic disease, significant history or current evidence of a medical, psychological, or other disorder, history of or the presence of any skin condition/disease that might interfere with the diagnosis or evaluation of study parameters; history of acute DVT; history of thrombophilia; hypersensitivity |
|
| Interventions | Telangiectatic leg veins on both legs, measuring less than 1 mm, were treated with glycerin 72%. The patients were randomised to receive treatment to one of their legs with foam sclerotherapy prepared from STS and CO2 and the other leg received treatment with foam sclerotherapy prepared with STS and room air Duration of follow‐up: 3 ‐ 4 weeks Use of compression: compression stocking |
|
| Outcomes | 3 ‐ 4 weeks post‐treatment, participants were evaluated by a blinded investigator for the presence of clot, telangiectatic matting, ulceration, hyperpigmentation and reticular veins | |
| Funding sources | No details provided | |
| Declarations of interest | No details provided | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States "randomised", no details given |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | At 3 ‐ 4 weeks post‐treatment, participants were evaluated by a blinded investigator |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed the study |
| Selective reporting (reporting bias) | Unclear risk | No details given |
| Other bias | Unclear risk | No details given |
Peterson 2012b.
| Study characteristics | ||
| Methods |
Study design: double‐blind prospective comparative trial Method of randomisation: not mentioned Blinding: participants ‐ yes, doctor ‐ yes, outcome assessors ‐ yes Power calculation: not mentioned Total number of participants: 63 Total number of procedures: 126 Treatment localisation: leg veins Number of exclusions post‐randomisation: none Number of withdrawals and reasons: none |
|
| Participants |
Setting: outpatient site Country: USA Gender: men and women Age: range 25 ‐ 75 years Inclusion criteria: men and women, skin type I to IV, aged 25 to 75, only people with appropriate telangiectasias and reticular veins on both legs unassociated with great saphenous veins or small saphenous veins reflux were enrolled Exclusion criteria: pregnant, history of DVT, underlying hypercoagulable states, neurological disease, CEAP classification of 3 or greater, or participation in a clinical trial over the previous 6 months |
|
| Interventions |
Treatment 1: POL Treatment 2: hypertonic saline Each leg was then randomised to receive treatment with POL or hypertonic saline. telangiectasias smaller than 1 mm in diameter were treated with POL 0.5% or 11.7% hypertonic saline. Larger reticular veins measuring 1 mm to 3 mm in diameter were treated with higher concentrations of POL 1% or 23.4% hypertonic saline Duration of follow‐up: 12 weeks Use of compression: compression stocking |
|
| Outcomes | Quote: "An independent blinded physician assessed patients at each post‐treatment visit both for disappearance of veins and occurrence of adverse events (phlebitis, oedema, hyperpigmentation, telangiectatic matting, and ulcerations). The response of telangiectasias and reticular leg veins to sclerosant was evaluated using photographs taken at a baseline visits and based on a quartile scale of improvement: 1 (0% ‐ 25%), 2 (26% ‐ 50%), 3 (51% ‐ 75%), 4 (76% ‐ 100%). A subject self‐satisfaction questionnaire in which improvement was rated from 1 to 4, was completed at the final visit at week 12." | |
| Funding sources | Quote: "This study was sponsored through an educational grant by Merz." | |
| Declarations of interest | Quote: "Drs. Goldman, Duffy, and R. Weiss are consultants for and have received honoraria from Merz. Dr. Goldman is also a consultant for Mylan/Bioniche and STD Pharmaceuticals. Dr. Goldman and Dr. M. and R. Weiss are also consultants for and perform research for BTG Pharmaceuticals, Ltd." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details given |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The participant and treating physician were blinded to the agent being injected |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | An independent blinded physician assessed participants at each post‐treatment visit both for disappearance of veins and occurrence of adverse events (phlebitis, oedema, hyperpigmentation, telangiectatic matting, and ulcerations). The response of telangiectasias and reticular leg veins to sclerosant was evaluated using photographs taken at a baseline visits and based on a quartile scale of improvement: 1 (0% ‐ 25%), 2 (26% ‐ 50%), 3 (51% ‐ 75%), 4 (76% ‐ 100%) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All 63 participants completed the study |
| Selective reporting (reporting bias) | Unclear risk | No details given |
| Other bias | Unclear risk | No details given |
Prescott 1992.
| Study characteristics | ||
| Methods |
Study design: randomised trial Method of randomisation: not mentioned Blinding: not mentioned Power calculation: no Total number of participants: 60 Total number of procedures: 120 Treatment localisation: leg veins Number of exclusions post‐randomisation: none Number of withdrawals and reasons: none |
|
| Participants |
Setting: outpatient site Country: Canada Gender; women Age: < 60 years old Inclusion criteria: patients with telangiectasias who had already been treated for underlying varices Exclusion criteria: not mentioned |
|
| Interventions |
Treatment 1: hypertonic dextrose solution 10% Treatment 2: STS 0.15% Duration: until participant discontinued or resolution of telangiectasias Duration of follow‐up: timeframe not provided Use of compression: compression stocking |
|
| Outcomes | Efficacy:
Adverse events: pain, ecchymosis, matting, post‐sclerotic pigmentation |
|
| Funding sources | No details provided | |
| Declarations of interest | No details provided | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States "randomised", no details given |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details given |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | All participants completed the study |
| Selective reporting (reporting bias) | High risk | Descriptive data are not provided |
| Other bias | Unclear risk | No details given |
Rabe 2010.
| Study characteristics | ||
| Methods |
Study design: a double‐blind, randomised, comparative clinical trial Method of randomisation: computerised randomisation procedure Blinding: participants ‐ yes, doctor ‐ yes, outcome assessors ‐ yes Power calculation: not mentioned Total number of participants: 316 randomised patients, 160 with telangiectases were randomly assigned to 0.5% POL, 1% STS or placebo, and 156 with reticular veins received 1% polidocanol, 1% STS or placebo Total number of procedures: 316 Treatment localisation: leg veins Number of exclusions post‐randomisation: none Number of withdrawals and reasons: 9 stopped prematurely |
|
| Participants |
Setting: outpatient site Country: Germany Gender: women and men Age: range 18 ‐ 70 years Inclusion criteria: patients with C1 veins; veins selected for injection were clearly visible telangiectases or reticular veins in a predefined treatment area (10 x 10 cm) Exclusion criteria: C2 – C6 venous insufficiency, CEAP classifications Es and Ec, AD and PO, and those who had undergone sclerotherapy within 12 weeks on the ipsilateral leg or within four weeks on the contralateral leg, laser treatment or surgery were excluded. Patients with a history of acute or major superficial or DVT, coagulopathies, major leg oedema, arterial occlusive disease, or had clinically relevant electrocardiogram (ECG) abnormalities, acute severe systemic disease or poor general health, severe generalised infection, acute febrile states, symptoms of microangiopathy or neuropathy or inflammatory skin disease in the treatment area were also excluded |
|
| Interventions |
Treatment 1: POL Treatment 2: STS Control: placebo Quote "Eligible patients were allocated by a computerized randomisation procedure to receive treatment with polidocanol, STS or placebo (3:2:1). A disproportional distribution was chosen to increase the number of patients receiving the authorized active medication and to decrease the amount of patients getting a placebo treatment. In the polidocanol group, the C1 spider veins were injected with 0.5% polidocanol (Aethoxysklerol 0.5%) and the reticular veins with 1% polidocanol (Aethoxysklerol 1%), as authorized in Germany and many other countries, whereas in the STS group both vein types were treated with 1% STS according to the US‐marketing authorization. In the placebo group, both vein types were injected with isotonic saline." Duration of follow‐up: 26 weeks Use of compression: compression stocking |
|
| Outcomes | Quote "Digital photographs of the treatment area and the leg were taken at screening, immediately before the first injection and 12 and 26 weeks after the last injection. The images taken at weeks 12 and 26 were compared with the images taken before first treatment. The treatment area was rated by the investigator and two independent blinded medical experts using a 5‐grade scale: 1 ‘worse than before’, 2 ‘same as before’, 3 ‘moderate improvement’, 4 ‘good improvement’ and 5 ‘complete treatment success’. A teaching manual with schematic drawings of different examples for each category was prepared and handed out at the beginning of the study. Treatment success was grade 4 or 5 on the 5‐grade scale and treatment failure was grade 1, 2 or 3. The patients rated their satisfaction with the current treatment at weeks 12 and 26 using a verbal rating scale of 1 ¼ ‘very unsatisfied’, 2 ¼ ‘somewhat unsatisfied’, 3 ¼ ‘slightly satisfied’, 4 ¼ ‘satisfied’ and 5 ¼ ‘very satisfied’. The patients were given the digital images taken at the first visit to remind them what the veins looked like before treatment." | |
| Funding sources | No details provided | |
| Declarations of interest | None declared | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Eligible patients were allocated by a computerised randomisation procedure to receive treatment with POL, STS or placebo (3:2:1) |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | A double‐blind, randomised, comparative clinical |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The treatment area was rated by the investigator and 2 independent blinded medical experts using a 5‐grade scale: 1 ‘worse than before’, 2 ‘same as before’, 3 ‘moderate improvement’, 4 ‘good improvement’, and 5 ‘complete treatment success’ |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 9/316 participants stopped the study prematurely. Quote: "The most frequent reasons for stopping were AEs, noncompliance with study protocol, personal reasons or lost to follow‐up. The most frequent protocol deviations relevant for exclusion from the per protocol (PP) data‐set were violations against time windows between visits, positive thrombophilia tests and incomplete treatment." |
| Selective reporting (reporting bias) | Unclear risk | No details given |
| Other bias | Unclear risk | No details given |
Rao 2005.
| Study characteristics | ||
| Methods |
Study design: double‐blind prospective comparative trial Method of randomisation: not mentioned Blinding: participants ‐ yes, doctor ‐ yes, outcome assessors ‐ yes Power calculation: not mentioned Total number of participants: 20 Total number of procedure: 40 Treatment localisation: leg veins Number of exclusions post‐randomisation: none Number of withdrawals and reasons: none |
|
| Participants |
Setting: outpatient site Country: USA Gender: 19 women, 1 man Age: not mentioned Inclusion criteria: varicose, reticular or telangiectatic leg veins or both, without incompetence at the saphenofemoral or saphenopopliteal junctions Exclusion criteria: each person’s leg veins were evaluated for extent, quality, and size and were placed into one of the following study categories: veins < 1 mm in diameter, veins 1 to 3 mm in diameter, and veins 3 to 6 mm in diameter |
|
| Interventions |
Treatment 1: STS Treatment 2: POL Sclerotherapy was performed by standard technique on only 1 leg in a single treatment session, always by the same physician for a given participant Duration of follow‐up: 12 weeks Use of compression: compression stocking |
|
| Outcomes | 4 independent physicians, who were blinded to the sclerosing agents administered, assessed these images (photographs) for overall clearance of vessels based on vessel size. Participants completed questionnaires to assess the tolerability and satisfaction of treatment with each sclerosing agent | |
| Funding sources | No details provided | |
| Declarations of interest | No details provided | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details given |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The treating physician was blinded to the agent being injected |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | 4 independent physicians blinded assessed the photographs for overall clearance of vessels |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All 20 participants completed the study |
| Selective reporting (reporting bias) | Unclear risk | No details given |
| Other bias | Unclear risk | No details given |
Schul 2011.
| Study characteristics | ||
| Methods |
Study design: prospective randomised trial Method of randomisation: not mentioned Blinding: participants ‐ no, doctor ‐ no, outcome assessors ‐ yes Power calculation: not mentioned Total number of participants: 58 Total number of procedure: 58 Treatment localisation: leg veins Number of exclusions post‐randomisation: none Number of withdrawals and reasons: 6 lost to follow‐up |
|
| Participants |
Setting: outpatient site Country: USA Gender: 58 women Age: range 18 ‐ 71 years Inclusion criteria: female, with a VDS ≥ 4, isolated dilated and ectatic reticular veins and telangiectasias. Varicose, reticular, and/or telangiectatic leg veins without incompetence at the saphenous tributary or saphenopopliteal junctions Exclusion criteria: age less than 18, allergy to sotradecol, presence of overlapping pain syndromes and prospective pregnancy during the study, sclerotherapy in the preceding 2 years and recent therapeutic compression therapy |
|
| Interventions | Compression stocking (20 ‐ 30 mmHg) or sclerotherapy with POL | |
| Outcomes | QoL parameters with Aberdeen Score | |
| Funding sources | Quote: "This study additionally received support through the American College of Phlebology BNS Jobst Award of 2007." | |
| Declarations of interest | Quote: "Conflicts of interest: Bioniche Pharma USA (Lake Forest, IL, USA) provided all of the Sotradecol™ stock 3% solution; BSN Medical (Rutherford College, NC, USA) provided compression stocking supplies and financial support for independent statistical analysis." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random‐number series developed by Microsoft Excel for Windows |
| Allocation concealment (selection bias) | Unclear risk | Not reported in the study |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Impossible blinding of participants |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No reference to it |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4 participants in compression arms and 2 in sclerotherapy |
| Selective reporting (reporting bias) | Unclear risk | Not reported |
| Other bias | Unclear risk | Not reported |
Zhang 2012.
| Study characteristics | ||
| Methods |
Study design: prospective, multicentre, randomised, double‐blind study Method of randomisation: not mentioned Blinding: participants ‐ yes, doctor‐ yes, outcome assessors ‐ yes Power calculation: not mentioned Total number of participants: 288, 216 to receive POL (72 patients in each group) and 72 patients to receive placebo Total number of procedure: 288 Treatment localisation: leg veins Number of exclusions post‐randomisation: none Number of withdrawals and reasons: "Two patients assigned to polidocanol 1% and one patient assigned to placebo did not receive any study medication and were therefore excluded from the safety population (n = 285)." |
|
| Participants |
Setting: outpatient site Country: China Gender: men and women Age: range 18 ‐ 75 years Inclusion criteria: C1 or C2 non‐saphenous varicose veins of the lower legs and a normal deep venous system; given informed consent Exclusion criteria: history of DVT or high risk of thrombosis; arterial occlusive disease; thromboembolic diseases; acute severe systemic diseases or very poor general health; known hypercoagulability or current anticoagulation therapy; major leg oedema; febrile states; symptoms of microangiopathy or neuropathy or inflammatory skin disease in the treatment area; or predisposition to allergies |
|
| Interventions |
Treatment Group A: C1 spider veins of < 1 mm; treatment: POL 0.5% or corresponding placebo (same solution but without the active ingredient) Treatment Group B: C1 reticular veins and/or small‐sized veins of 1 mm – 5 mm; treatment: POL 1% or corresponding placebo Treatment Group C: C2 medium‐sized and/or large non‐saphenous subcutaneous varicose veins of > 5 mm with reflux of 0.5 second; treatment: POL 3% or corresponding placebo Duration of follow‐up: 3 months Use of compression: compression stocking |
|
| Outcomes | Primary efficacy variable: efficacy of the study treatment at 3 months after the last injection The efficacy of the treatment was rated according to the following 5‐grade scale: 1 ‘worse than before’ (more veins in the treatment area are observable than before or veins are more dilated or look worse than before), 0% disappearance but worsening; 2 ‘same as before’ (no improvement but also no worsening observable), from 0% up to 25% disappearance; 3 ‘moderate improvement (improvement observable but not yet satisfactory, needs to be treated again, from 26% up to 50% disappearance; 4 ‘good improvement (satisfactory treatment success, only slight improvement still possible), from 51% up to 75% disappearance; 5 ‘complete treatment success' (no improvement necessary), ≥ 75% disappearance. Patients who had Grade 4 or 5 were counted as responders Secondary efficacy variables: investigators' and patients' assessments of treatment satisfaction at the final examination according to a 5‐point verbal rating scale: 1 very unsatisfied; 2 somewhat unsatisfied; 3 slightly satisfied; 4 satisfied; 5 very satisfied. Safety variable: Overall safety was rated by a physician on a 5‐point scale: 1 ‘one or more serious adverse drug reactions’; 2 ‘treatment caused problems, more than three non‐serious adverse drug reactions per treatment, daily life activity was impaired’; 3 ‘treatment slightly tolerated, less than three non‐serious adverse drug reactions per treatment, slight impairment of daily life activity’; 4 ‘treatment well tolerated, less than three non‐serious adverse drug reactions per treatment, no impairment of daily life activity’; 5 ‘treatment very well tolerated, no adverse drug reactions’. Other safety variables were changes in vital signs, physical examination, ECG and clinical laboratory measures |
|
| Funding sources | Quote "The study was funded by Kreussler." Quote "All investigators appreciate the kind donation by Sigvaris, Switzerland, in providing each patient with compression stockings class II and the necessary support. We also thank Excel Pharma Studies (Beijing, People’s Republic of China) for helping to conduct the study. Trilogy Writing and Consulting GmbH (Frankfurt, Germany) provided medical writing support on behalf of Kreussler." | |
| Declarations of interest | Quote "Drs Schliephake and Otto were employees of Kreussler. Dr Mark Malouf has served as a consultant for Kreussler. Prof. Zhang Jiwei, Prof. Jing Zaiping and Prof. Yong‐quan Gu received research support as investigators in this study sponsored by Kreussler." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States "randomised", no details given |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | They suggest blinding, confirmed by study author |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | They suggest blinding, confirmed by author |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Two patients assigned to polidocanol 1% and one patient assigned to placebo did not receive any study medication and were therefore excluded from the safety population (n = 285)." |
| Selective reporting (reporting bias) | Unclear risk | No details given |
| Other bias | Unclear risk | No details given |
BMI: body mass index CEAP: Clinical, Etiological, Anatomical and Pathophysiological (CEAP) classification system for chronic venous disorders CG: chromated glycerin CGX:chromated glycerin mixed DVT: deep vein thrombosis ECG: electrocardiogram FPDL: following pulsed dye laser ICG:indocyanine green KTP: potassium titanyl phosphate Nd:YAG: neodymium‐doped yttrium aluminium garnet NIR: near‐infrared PAD: peripheral arterial disease PE: pulmonary embolism POL: polidocanol QoL: quality of life SD: standard deviation SF‐36: short form‐36 (QoL questionnaire) STS: sodium tetradecyl sulfate VAS: visual analogue scale VDS: venous dysfunction score
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Alora 1999 | Not randomised |
| Conrad 1995 | Not randomised |
| Dinsdale 2014 | The study included face and legs telangiectasias and did not separate the results |
| Gillet 2017 | Not randomised and CEAP ‐ C4, C5 and C6 |
| McDaniel 1999 | Not randomised |
| Omura 2003 | Not randomised |
| Sadick 2003 | Not randomised |
| Spendel 2002 | Only KTP laser (532 nm) in different vessel diameters |
| Weiss 1990 | Not randomised |
| Woo 2003 | Not randomised |
CEAP: Clinical, Etiological, Anatomical and Pathophysiological (CEAP) classification system for chronic venous disorders KTP: potassium titanyl phosphate
Characteristics of ongoing studies [ordered by study ID]
NCT04132323.
| Study name | Clinical efficacy of low concentrate detergents versus hypertonic glucose for the treatment of telangiectasia: a prospective randomized clinical trial |
| Methods |
Study design: prospective, double‐blind randomised trial Method of randomisation: no details provided Blinding: participant ‐ yes evaluator ‐ yes Power calculation: no details mentioned Total number of participants: 172 Total number of procedures: 172 Treatment localisation: lower extremities Source of funding: not mentioned |
| Participants |
Setting: outpatient centre Country: Russia Total number: 172 Gender: women Age: over 18 years Inclusion criteria: single primary or secondary telangiectasias unrelated to the reticular veins Exclusion criteria:
|
| Interventions |
Treatment 1. Inject 75% glucose, with a 2 ml luer‐lock syringe through 30 G needle in telangiectasia until the vessel disappears Treatment 2. Inject 0.05% STS with a 2 ml luer‐lock syringe through 30 G needle in telangiectasia until the vessel disappears Treatment 3. Inject 0.1% STS with a 2 ml luer‐lock syringe through 30 G needle in telangiectasia until the vessel disappears Treatment 4. Inject 0.15% STS with a 2 ml luer‐lock syringe through 30 G needle in telangiectasia until the vessel disappears |
| Outcomes | Disappearance of the telangiectasia Pain during the procedure (VAS) |
| Starting date | October 2019 |
| Contact information | Derzhavin Tambov, State University |
| Notes |
NCT04690803.
| Study name | The effect of cooling on sclerotherapy efficacy |
| Methods |
Study design: prospective, randomised clinical trial Method of randomisation: no details provided Blinding: participant ‐ no; evaluator ‐ yes Power calculation: no details mentioned Total number of participants: 20 Total number of procedures: 40 Treatment localisation: lower extremities Source of funding: not mentioned |
| Participants |
Setting: outpatient centre Country: USA Total number: 20 Gender: both genders Age: 25 to 75 years Inclusion criteria:
Exclusion criteria:
|
| Interventions |
Active comparator: treatment with forced‐air cooling No intervention: treatment without forced‐air cooling |
| Outcomes | To evaluate the effect of forced‐air cooling on sclerotherapy To compare reticular vein and telangiectasia diameter measurements |
| Starting date | January 2020 |
| Contact information | West Dermatology Research Center/Cosmetic Laser Dermatology |
| Notes |
Zaleski‐Larsen 2017.
| Study name | A comparison of the effect of non‐chromated glycerin solution with 1% lidocaine formulated (2:1) with and without epinephrine used in combination with foam POL on adverse events and efficacy following sclerotherapy for reticular veins and telangiectasias of the lower extremities |
| Methods |
Study design: prospective, double‐blind randomised split‐leg comparison clinical trial Method of randomisation: no details provided Blinding: participant ‐ yes; evaluator ‐ yes Power calculation: no details mentioned Total number of participants: 30 Total number of procedures: 60 Treatment localisation: lower extremities Number of exclusions post‐randomisation: no details provided Number of withdrawals and reasons: no details provided Source of funding: not mentioned |
| Participants |
Setting: outpatient centre Country: USA Total number: 30 Gender: no details provided Age: no details provided Inclusion criteria: patients with CEAP C1 Exclusion criteria: no details provided. |
| Interventions |
Treatment 1: non‐chromated glycerin solution with epinephrine Treatment 2: non‐chromated glycerin solution without epinephrine POL foam (0.5% ‐ 1.0%) was first used to treat the reticular veins in both groups Duration of follow‐up: 3 months Use of compression: no details provided |
| Outcomes | Improvement and adverse events (pain, pruritus, oedema, erythema, ecchymosis, ulceration and pigmentations) |
| Starting date | 2015 |
| Contact information | No details provided |
| Notes |
CEAP: Clinical, Etiological, Anatomical and Pathophysiological (CEAP) classification system for chronic venous disorders POL: polidocanol STS: sodium tetradecyl sulfate VAS: visual analogue scale
Differences between protocol and review
Matting was included because it is an important adverse effect observed after the treatment of sclerotherapy and is clinically important to both vascular surgeon and patients.
Contributions of authors
LCUN: protocol drafting, acquiring trial reports, trial selection, data extraction, data analysis, data interpretation, review drafting, guarantor of the review. DGC: protocol drafting, trial selection, data extraction, data analysis, data interpretation, review drafting. JCCB: protocol drafting, trial selection, data interpretation, review drafting. RLGF: protocol drafting, trial selection, data extraction, data analysis, data interpretation, review drafting.
Sources of support
Internal sources
No sources of support provided
External sources
-
Chief Scientist Office, Scottish Government Health Directorates, The Scottish Government, UK
The Cochrane Vascular editorial base is supported by the Chief Scientist Office.
Declarations of interest
LCUN: none known. DGC: none known. JCCB: none known. RLGF: none known.
New
References
References to studies included in this review
Alos 2006 {published data only}
- Alos J, Carreno P, Lopez JA, Estadella B, Serra-Prat M, Marinel-Lo J. Efficacy and safety of sclerotherapy using polidocanol foam: a controlled clinical trial. European Journal of Vascular and Endovascular Surgery 2006;31(1):101-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Bayer 2021 {published data only}
- Bayer A, Kuznik N, Langan EA, Recke A, Recke AL, Faerber G, et al. Clinical outcome of short-term compression after sclerotherapy for telangiectatic varicose veins. Journal of Vascular Surgery 2021;9(2):435-43. [PMID: ] [DOI] [PubMed] [Google Scholar]
Benigni 1999 {published data only}
- Benigni JP, Sadoun S, Thirion V, Sica M, Demagny A, Chahim M. Telangiectases and reticular veins treatment with a 0.25% aetoxisclerol foam. Presentation of a pilot study. Phlébologie 1999;52(3):283-90. [Google Scholar]
Bertanha 2017 {published data only}
- Bertanha M, Jaldin RG, Moura R, Pimenta RE, Mariuba JV, Lucio Filho CE, et al. Sclerotherapy for reticular veins in the lower limbs: a triple-blind randomized clinical trial. JAMA Dermatology 2017;153(12):1249-55. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bertanha 2021 {published data only}
- Bertanha M, Yoshida WB, Bueno de Camargo PA, Moura R, Reis de Paula D, Padovani CR, et al. Polidocanol plus glucose versus glucose alone for the treatment of telangiectasias: triple blind, randomised controlled trial (PG3T). European Journal of Vascular and Endovascular Surgery 2021;61(1):128-35. [PMID: ] [DOI] [PubMed] [Google Scholar]
Carlin 1987 {published data only}
- Carlin MC, Ratz JL. Treatment of telangiectasia: comparison of sclerosing agents. Journal of Dermatologic Surgery and Oncology 1987;13(11):1181-4. [PMID: ] [DOI] [PubMed] [Google Scholar]
Christiansen 2015 {published data only}
- Christiansen K, Drosner M, Bjerring P. Optimized settings for Nd:YAG laser treatments of leg telangiectasias. Journal of Cosmetic and Laser Therapy 2015;17(2):69-76. [PMID: ] [DOI] [PubMed] [Google Scholar]
Goldman 2002 {published data only}
- Goldman MP. Treatment of varicose and telangiectatic leg veins: double-blind prospective comparative trial between aethoxyskerol and sotradecol. Dermatologic Surgery 2002;28(1):52-5. [PMID: ] [DOI] [PubMed] [Google Scholar]
Hamel‐Desnos 2009 {published data only}
- Hameo-Desnos CM, Gillet JL, Desnos PR, Allaert FA. Sclerotherapy of varicose veins in patients with documented thrombophilia: a prospective controlled randomized study of 105 cases. Phebology 2009;24(4):176-82. [DOI] [PubMed] [Google Scholar]
Hoss 2020 {published data only}
- Hoss E, Kollipara R, Boen M, Alhaddad M, Goldman MP. Comparison of the safety and efficacy of foam sclerotherapy with 1: 2 polidocanol to air ratio versus 1: 4 ratio for the treatment of reticular veins of the lower extremities. Dermatologic Surgery 2020;46(12):1715-20. [PMID: ] [DOI] [PubMed] [Google Scholar]
Ianosi 2019 {published data only}
- Ianosi G, Ianosi S, Calbureanu-Popescu MX, Tutunaru C, Calina D, Neagoe D. Comparative study in leg telangiectasias treatment with Nd:YAG laser and sclerotherapy. Experimental and Therapeutic Medicine 2019;17(2):1106-12. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kahle 2006 {published data only}
- Kahle B, Leng K. Efficiency of sclerotherapy of spider leg veins. A prospective, randomized, double-blind, placebo-controlled study. Vasomed 2006;18:148. [Google Scholar]
Kern 2004 {published data only}
- Kern P, Ramelet AA, Wutschert R, Bounameaux H, Hayoz D. Single-blind, randomized study comparing chromated glycerin, polidocanol solution, and polidocanol foam for treatment of telangiectatic leg veins. Dermatologic Surgery 2004;30(3):367-72. [PMID: ] [DOI] [PubMed] [Google Scholar]
Kern 2007 {published data only}
- Kern P, Ramelet AA, Wutschert R, Hayoz D. Compression after sclerotherapy for telangiectasias and reticular leg veins: a randomized controlled study. Journal of Vascular Surgery 2007;45(6):1212-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Kern 2011 {published data only}
- Kern P, Ramelet AA, Wutschert R, Mazzolai L. A double-blind, randomized study comparing pure chromated glycerin with chromated glycerin with 1% lidocaine and epinephrine for sclerotherapy of telangiectasias and reticular veins. Dermatologic Surgery 2011;37(11):1590-4. [PMID: ] [DOI] [PubMed] [Google Scholar]
Kern 2012 {published data only}
- Kern P. Sclerotherapy of telangiectasias: a painless two-step technique. Dermatologic Surgery 2012;38(6):860-4. [PMID: ] [DOI] [PubMed] [Google Scholar]
Klein 2013 {published data only}
- Klein A, Buschmann M, Babilas P, Landthaler M, Baumler W. Indocyanine green-augmented diode laser therapy vs. long-pulsed Nd:YAG (1064 nm) laser treatment of telangiectatic leg veins: a randomized controlled trial. British Journal of Dermatology 2013;169(2):365-73. [PMID: ] [DOI] [PubMed] [Google Scholar]
Leach 2003 {published data only}
- Leach BC, Goldman MP. Comparative trial between sodium tetradecyl sulfate and glycerin in the treatment of telangiectatic leg veins. Dermatologic Surgery 2003;29(6):612-5. [PMID: ] [DOI] [PubMed] [Google Scholar]
Lupton 2002 {published data only}
- Lupton JR, Alster TS, Romero P. Clinical comparison of sclerotherapy versus long-pulsed Nd:YAG laser treatment for lower extremity telangiectases. Dermatologic Surgery 2002;28(8):694-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
McCoy 1999 {published data only}
- McCoy S, Evans A, Spurrier N. Sclerotherapy for leg telangiectasia - a blinded comparative trial of polidocanol and hypertonic saline. Dermatologic Surgery 1999;25(5):381-5; discussion 385-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Moreno‐Moraga 2013 {published data only}
- Moreno-Moraga J, Hernandez E, Royo J, Alcolea J, Isarria MJ, Pascu ML, et al. Optimal and safe treatment of spider leg veins measuring less than 1.5 mm on skin type IV patients, using repeated low-fluence Nd:YAG laser pulses after polidocanol injection. Laser in Medical Science 2013;28(3):925-33. [DOI] [PubMed] [Google Scholar]
Moreno‐Moraga 2014 {published data only}
- Moreno-Moraga J, Smarandache A, Pascu ML, Royo J, Trelles MA. 1064 nm Nd:YAG long pulse laser after polidocanol microfoam injection dramatically improves the result of leg vein treatment: a randomized controlled trial on 517 legs with a three-year follow-up. Phlebology 2014;29(10):658-66. [PMID: ] [DOI] [PubMed] [Google Scholar]
Munia 2012 {published data only}
- Munia MA, Wolosker N, Munia CG, Chao WS, Puech-Leao P. Comparison of laser versus sclerotherapy in the treatment of lower extremity telangiectases: a prospective study. Dermatologic Surgery 2012;38(4):635-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Nguyen 2020 {published data only}
- Nguyen HT, Firas AN, Van TT. Long-pulsed 1064-nm and 755-nm lasers for C1 leg veins on skin type IV patients: a side-by-side comparison. Lasers in Medical Science 2020;36(4):829-35. [PMID: ] [DOI] [PubMed] [Google Scholar]
Norris 1989 {published data only}
- Norris MJ, Carlin MC, Ratz JL. Treatment of essential telangiectasia: effects of increasing concentrations of polidocanol. Journal of the American Academy of Dermatology 1989;20(4):643-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Ochoa 2021 {published data only}
- Ochoa AJ, Carrillo J, Manríquez D, Manrique F, Vazquez AN. Reducing hyperpigmentation after sclerotherapy: a randomized clinical trial. Journal of Vascular Surgery 2021;9(1):154-62. [PMID: ] [DOI] [PubMed] [Google Scholar]
Ozden 2011 {published data only}
- Ozden MG, Bahcivan M, Aydin F, Senturk N, Bek Y, Canturk T, et al. Clinical comparison of potassium-titanyl-phosphate (KTP) versus neodymium:YAG (Nd:YAG) laser treatment for lower extremity telangiectases. Journal of Dermatological Treatment 2011;22(3):162-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Parlar 2015 {published data only}
- Parlar B, Blazek C, Cazzaniga S, Naldi L, Kloetgen HW, Borradori L, et al. Treatment of lower extremity telangiectasias in women by foam sclerotherapy vs. Nd:YAG laser: a prospective, comparative, randomized, open-label trial. Journal of the European Academy of Dermatology and Venereology 2015;29(3):549-54. [PMID: ] [DOI] [PubMed] [Google Scholar]
Peterson 2012a {published data only}
- Peterson JD, Goldman MP. An investigation of side-effects and efficacy of foam-based sclerotherapy with carbon dioxide or room air in the treatment of reticular leg veins: a pilot study. Phlebology 2012;27(2):73-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Peterson 2012b {published data only}
- Peterson JD, Goldman MP, Weiss RA, Duffy DM, Fabi SG, Weiss MA, et al. Treatment of reticular and telangiectatic leg veins: double-blind, prospective comparative trial of polidocanol and hypertonic saline. Dermatologic Surgery 2012;38(8):1322-30. [PMID: ] [DOI] [PubMed] [Google Scholar]
Prescott 1992 {published data only}
- Prescott RJ. A comparative study of two sclerosing agents in the treatment of telangiectasias. In: Phlebology '92. Vol. 2. Paris: John Libbey Eurotext, 1992:803-4. [Google Scholar]
Rabe 2010 {published data only}
- Rabe E, Schliephake D, Otto J, Breu FX, Pannier F. Sclerotherapy of telangiectases and reticular veins: a double-blind, randomized, comparative clinical trial of polidocanol, sodium tetradecyl sulphate and isotonic saline (EASI study). Phlebology 2010;25(3):124-31. [PMID: ] [DOI] [PubMed] [Google Scholar]
Rao 2005 {published data only}
- Rao J, Wildemore JK, Goldman MP. Double-blind prospective comparative trial between foamed and liquid polidocanol and sodium tetradecyl sulfate in the treatment of varicose and telangiectatic leg veins. Dermatologic Surgery 2005;31(6):631-5; discussion 635. [PMID: ] [DOI] [PubMed] [Google Scholar]
Schul 2011 {published data only}
- Schul MW, Eaton T, Edman B. Compression versus sclerotherapy for patients with isolated refluxing reticular veins and telangiectasia: a randomized trial comparing quality-of-life outcomes. Phebology 2011;26(4):148-156. [DOI] [PubMed] [Google Scholar]
Zhang 2012 {published data only}
- Zhang J, Jing Z, Schliephake DE, Otto J, Malouf GM, Gu YQ. Efficacy and safety of Aethoxysklerol (R) (polidocanol) 0.5%, 1% and 3% in comparison with placebo solution for the treatment of varicose veins of the lower extremities in Chinese patients (ESA-China Study). Phlebology 2012;27(4):184-90. [PMID: ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Alora 1999 {published data only}
- Alora MB, Stern RS, Arndt KA, Dover JS. Comparison of the 595 nm long-pulse (1.5 msec) and ultralong-pulse (4 msec) lasers in the treatment of leg veins. Dermatologic Surgery 1999;25(6):445-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Conrad 1995 {published data only}
- Conrad P, Malouf GM, Stacey MC. The Australian polidocanol (aethoxysklerol) study. Results at 2 years. Dermatologic Surgery 1995;21(4):334-6; discussion 337-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Dinsdale 2014 {published data only}
- Dinsdale G, Murray A, Moore T, Ferguson J, Wilkinson J, Richards H, et al. A comparison of intense pulsed light and laser treatment of telangiectases in patients with systemic sclerosis: a within-subject randomized trial. Rheumatology (Oxford, England) 2014;53(8):1422-30. [PMID: ] [DOI] [PubMed] [Google Scholar]
Gillet 2017 {published data only}
- Gillet JL, Desnos CH, Lausecker M, Daniel C, Guex JJ, Allaert FA. Sclerotherapy is a safe method of treatment of chronic venous disorders in older patients: a prospective and comparative study of consecutive patients. Phlebology 2017;32(4):234-40. [PMID: ] [DOI] [PubMed] [Google Scholar]
McDaniel 1999 {published data only}
- McDaniel DH, Ash K, Lord J, Newman J, Adrian RM, Zukowski M. Laser therapy of spider leg veins: clinical evaluation of a new long pulsed alexandrite laser. Dermatologic Surgery 1999;25(1):52-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Omura 2003 {published data only}
- Omura NE, Dover JS, Arndt KA, Kauvar AN. Treatment of reticular leg veins with a 1064 nm long-pulsed Nd:YAG laser. Journal of the American Academy of Dermatology 2003;48(1):76-81. [PMID: ] [DOI] [PubMed] [Google Scholar]
Sadick 2003 {published data only}
- Sadick NS. Laser treatment with a 1064-nm laser for lower extremity class I-III veins employing variable spots and pulse width parameters. Dermatologic Surgery 2003;29(9):916-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Spendel 2002 {published data only}
- Spendel S, Prandl EC, Schintler MV, Siegl A, Wittgruber G, Hellbom B, et al. Treatment of spider leg veins with the KTP (532 nm) laser - a prospective study. Lasers in Surgery and Medicine 2002;31(3):194-201. [PMID: ] [DOI] [PubMed] [Google Scholar]
Weiss 1990 {published data only}
- Weiss RA, Weiss MA. Incidence of side effects in the treatment of telangiectasias by compression sclerotherapy: hypertonic saline vs. polidocanol. Journal of Dermatologic Surgery and Oncology 1990;16(9):800-4. [PMID: ] [DOI] [PubMed] [Google Scholar]
Woo 2003 {published data only}
- Woo WK, Jasim ZF, Handley JM. 532-nm Nd:YAG and 595-nm pulsed dye laser treatment of leg telangiectasia using ultralong pulse duration. Dermatologic Surgery 2003;29(12):1176-80; discussion 1180. [PMID: ] [DOI] [PubMed] [Google Scholar]
References to ongoing studies
NCT04132323 {published data only}
- NCT04132323. Clinical efficacy of low concentrate detergents versus hypertonic glucose for treatment of telangiectasia: a prospective randomized clinical trial. clinicaltrials.gov/ct2/show/NCT04132323 (first received 16 October 2019).
NCT04690803 {published data only}
- NCT04690803. The effect of cooling on sclerotherapy efficacy. clinicaltrials.gov/ct2/show/NCT04690803 (first received 31 December 2020).
Zaleski‐Larsen 2017 {published data only}
- Zaleski-Larsen L, Bolton J, Goldman M. A comparative of the effect of non-chromated glycerin solution with 1% lidocaine formulated (2:1) with and without epinephrine used in combination with foam polidocanol on adverse events and efficacy following sclerotherapy for reticular veins and telangiectasias of the lower extremities. Phlebology 2017;32(2 (Suppl 1)):53-4. [Google Scholar]
Additional references
Andrade 2009
- Andrade AR, Pitta GB, Castro AA, Miranda-Júnior F. Evaluation of venous reflux by color duplex scanning in patients with varicose veins of the lower limbs: correlation with clinical severity by CEAP classification [Avaliação do refluxo venoso superficial ao mapeamento dúplex em portadores de varizes primárias de membros inferiores: correlação com a gravidade clínica da classificação CEAP]. Jornal Vascular Brasileiro 2009;8(1):14-20. [Google Scholar]
Babilas 2010
- Babilas P, Schreml S, Szeimies RM, Landthaler M. Intense pulsed light (IPL): a review. Lasers in Surgery and Medicine 2010;42(2):93-104. [PMID: ] [DOI] [PubMed] [Google Scholar]
Chadornneau 2012
- Chadornneau JM. Treatment of the télangiectasies by the technique of thermocoagulation. Study on 50 patients. fcaresystems.com/wp-content/uploads/2012/11/Clinical-study-on-50-patients.pdf (accessed prior to 26 June 2017).
Deeks 2019
- Deeks JJ, Higgins JP, Altman DG (editors). Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins J, Thomas J, Chandler J, Cumpston M, Li T, Page M, et al. editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.
Eklof 2004
- Eklof B, Rutherford RB, Bergan JJ, Carpentier PH, Gloviczki P, Kistner RL, et al. Revision of the CEAP classification for chronic venous disorders: consensus statement. Journal of Vascular Surgery 2004;40(6):1248-52. [PMID: ] [DOI] [PubMed] [Google Scholar]
Goldberg 2012
- Goldberg DJ. Current trends in intense pulsed light. Journal of Clinical and Aesthetic Dermatology 2012;5(6):45-53. [PMID: ] [PMC free article] [PubMed] [Google Scholar]
Goldman 2004
- Goldman MP. Optimal management of facial telangiectasia. American Journal of Clinical Dermatology 2004;5(6):423-34. [PMID: ] [DOI] [PubMed] [Google Scholar]
Grade 2004
- Atkins D, Best D, Briss PA, Eccles M, Falck-Ytter Y, Flottorp S, et al, GRADE Working Group. Grading quality of evidence and strength of recommendations. BMJ 2004;328(7454):1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hercogova 2002
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Higgins 2021a
- Higgins JP, Eldridge S, Li T (editors). Chapter 23: Including variants on randomized trials. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). The Cochrane Collaboration, 2021. Available from www.training.cochrane.org/handbook.
Higgins 2021b
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). The Cochrane Collaboration, 2021. Available from www.training.cochrane.org/handbook.
Karch 2002
- Karch A, Kasperczyk J. Somatic risk factors for chronic venous insufficiency in women: preliminary report [Somatyczne uwarunkowania ryzyka przewleklej niewydolnosci zylnej u kobiet: doniesienie wstepne]. Wiadomosci Lekarskie (Warsaw, Poland) 2002;55 (Suppl 1):212-6. [PMID: ] [PubMed] [Google Scholar]
Kozarev 2011
- Kozarev J. Use of long pulse Nd:YAG 1064nm laser for treatment of rosacea telangiectatica. Journal of the Laser and Health Academy 2011;1:33-6. [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Lurie 2020
- Lurie F, Passman M, Meisner M, Dalsing M, Masuda E, Welch H, et al. The 2020 update of the CEAP classification system and reporting standards. Journal of Vascular Surgery 2020;8(3):342-52. [DOI: 10.1016/j.jvsv.2019.12.075] [DOI] [PubMed] [Google Scholar]
McCoppin 2011
- McCoppin HH, Hovenic WW, Wheeland RG. Laser treatment of superficial leg veins: a review. Dermatologic Surgery 2011;37(6):729-41. [PMID: ] [DOI] [PubMed] [Google Scholar]
Meesters 2014
- Meesters AA, Pitassi LH, Campos V, Wolkerstorfer A, Dierickx CC. Transcutaneous laser treatment of leg veins. Lasers in Medical Science 2014;29(2):481-92. [PMID: ] [DOI] [PubMed] [Google Scholar]
Micali 2016
- Micali G, Gerber PA, Lacarrubba F, Schafer G. Improving treatment of erythematotelangiectatic rosacea with laser and/or topical therapy through enhanced discrimination of its clinical features. Journal of Clinical and Aesthetic Dermatology 2016;9(7):30-9. [PMID: ] [PMC free article] [PubMed] [Google Scholar]
Mujadzic 2015
- Mujadzic M, Ritter EF, Given KS. A novel approach for the treatment of spider veins. Aesthetic Surgery Journal 2015;35(7):NP221-9. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Porter 1995
- Porter JM, Moneta GL. Reporting standards in venous disease: an update. International Consensus Committee on Chronic Venous Disease. Journal of Vascular Surgery 1995;21(4):635-45. [PMID: ] [DOI] [PubMed] [Google Scholar]
Rabe 2014
- Rabe E, Breu FX, Cavezzi A, Coleridge Smith P, Frullini A, Gillet JL, et al. European guidelines for sclerotherapy in chronic venous disorders. Phlebology 2014;29(6):338-54. [PMID: ] [DOI] [PubMed] [Google Scholar]
Ramelet 2002
- Ramelet AA. Phlebectomy. Technique, indications and complications. International Angiology 2002;21(2-1):46-51. [PubMed] [Google Scholar]
Raulin 2003
- Raulin C, Greve B, Grema H. IPL technology: a review. Lasers in Surgery and Medicine 2003;32(2):78-87. [PMID: ] [DOI] [PubMed] [Google Scholar]
Review Manager 2020 [Computer program]
- Review Manager 5 (RevMan 5). Version 5.4. Copenhagen: The Cochrane Collaboration, 2020.
Ruckley 2008
- Ruckley CV, Evans CJ, Allan PL, Lee AJ, Fowkes FG. Telangiectasia in the Edinburgh Vein Study: epidemiology and association with trunk varices and symptoms. European Journal of Vascular and Endovascular Surgery 2008;36(6):719-24. [DOI] [PubMed] [Google Scholar]
Schwartz 2011
- Schwartz L, Maxwell H. Sclerotherapy for lower limb telangiectasias. Cochrane Database of Systematic Reviews 2011, Issue 12. Art. No: CD008826. [DOI: 10.1002/14651858.CD008826.pub2] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Scuderi 2002
- Scuderi A, Raskin B, Al Assal F, Scuderi P, Scuderi MA, Rivas CE, et al. The incidence of venous disease in Brazil based on the CEAP classification. International Angiology 2002;21(4):316-21. [PMID: ] [PubMed] [Google Scholar]
Shamma 2005
- Shamma AR, Ramadan FM, Vonderlieth K. Transdermal laser treatment of facial telangiectasia - comparison of the 532 nm KTP to the 940 nm diode wavelength. In: International Vein Congress. Miami, FL, April 2004.
Smith 1999
- Smith JJ, Garratt AM, Guest M, Greenhalgh RM, Davies AH. Evaluating and improving health-related quality of life in patients with varicose veins. Journal of Vascular Surgery 1999;30(4):710-9. [DOI] [PubMed] [Google Scholar]
Smith 2015
- Smith PC. Management of reticular veins and telangiectases. Phlebology 2015;30(2 Suppl):46-52. [PMID: ] [DOI] [PubMed] [Google Scholar]
Stangl 2008
- Stangl S, Hadshiew I, Kimmig WO. Side effects and complications using intense pulsed light (IPL) sources. Medical Laser Application 2008;23(1):15-20. [Google Scholar]
Tessari 2001
- Tessari L, Cavezzi A, Frullini A. Preliminary experience with a new sclerosing foam in the treatment of varicose veins. Dermatologic Surgery 2001;27(1):58-60. [PMID: ] [PubMed] [Google Scholar]
Thomson 2016
- Thomson L. Sclerotherapy of telangiectasias or spider veins in the lower limb: a review. Journal of Vascular Nursing 2016;34(2):61-2. [PMID: ] [DOI] [PubMed] [Google Scholar]
Tierney 2009
- Tierney E, Hanke CW. Randomized controlled trial: comparative efficacy for the treatment of facial telangiectasias with 532 nm versus 940 nm diode laser. Lasers in Surgery and Medicine 2009;41(8):555-62. [PMID: ] [DOI] [PubMed] [Google Scholar]
Vitale‐Lewis 2008
- Vitale-Lewis VA. Aesthetic treatment of leg veins. Aesthetic Surgery Journal 2008;28(5):573-83. [PMID: ] [DOI] [PubMed] [Google Scholar]
Wall 2007
- Wall TL. Current concepts: laser treatment of adult vascular lesions. Seminars in Plastic Surgery 2007;21(3):147-58. [PMID: ] [DOI] [PMC free article] [PubMed]
Wittens 2015
- Wittens C, Davies AH, Baekgaard N, Broholm R, Cavezzi A, Chastanet S, et al. Editor's Choice - Management of Chronic Venous Disease: Clinical Practice Guidelines of the European Society for Vascular Surgery (ESVS). European Journal of Vascular and Endovascular Surgery 2015;49(6):678-737. [PMID: ] [DOI] [PubMed] [Google Scholar]
Worthington‐Kirsch 2005
- Worthington-Kirsch RL. Injection sclerotherapy. Seminars in Interventional Radiology 2005;22(3):209-17. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Yiannakopoulou 2016
- Yiannakopoulou E. Safety concerns for sclerotherapy of telangiectases, reticular and varicose veins. Pharmacology 2016;98(1-2):62-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Nakano 2017
- Nakano LC, Cacione DG, Baptista-Silva JC, Flumignan RL. Treatment for telangiectasias and reticular veins. Cochrane Database of Systematic Reviews 2017, Issue 7. Art. No: CD012723. [DOI: 10.1002/14651858.CD012723] [DOI] [PMC free article] [PubMed] [Google Scholar]


