Abstract
The transmissible respiratory disease COVID-19, caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has affected millions of people worldwide since its first reported outbreak in December of 2019 in Wuhan, China. Since then, multiple studies have shown an inverse correlation between the levels of high-density lipoprotein (HDL) particles and the severity of COVID-19, with low HDL levels being associated with an increased risk of severe outcomes. Some studies revealed that HDL binds to SARS-CoV-2 particles via the virus’s spike protein and, under certain conditions, such as low HDL particle concentrations, it facilitates SARS-CoV-2 binding to angiotensin-converting enzyme 2 (ACE2) and infection of host cells. Other studies, however, reported that HDL suppressed SARS-CoV-2 infection. In both cases, the ability of HDL to enhance or suppress virus infection appears to be dependent on the expression of the HDL receptor, namely, the Scavenger Receptor Class B type 1 (SR-B1), in the target cells. SR-B1 and HDL represent crucial mediators of cholesterol metabolism. Herein, we review the complex role of HDL and SR-B1 in SARS-CoV-2-induced disease. We also review recent advances in our understanding of HDL structure, properties, and function during SARS-CoV-2 infection and the resulting COVID-19 disease.
Keywords: SARS-CoV-2, COVID-19, HDL, SR-B1
1. Introduction
The novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the etiologic agent of the severe respiratory illness called coronavirus disease 2019 (COVID-19) [1,2], which is a transmissible respiratory disease affecting 223 countries and, as of August 2021, accountable for more than 200 million confirmed cases and around 4.5 million confirmed deaths worldwide [3]. SARS-CoV-2 is a positive-sense single-stranded, non-segmented, enveloped RNA virus that utilizes endocytosis or membrane fusion to enter host cells [4]. The first step in viral entry into the host cell is the binding of the viral transmembrane spike (S) glycoprotein to a host cell transmembrane angiotensin-converting enzyme 2 (ACE2) protein [5]. Subsequently, membrane proteases, such as furin and the transmembrane protease serine 2 (TMPRSS2), sequentially cleave the spike protein, thereby priming it for entry [6]. However, other cellular proteases, such as cathepsins B and L, have also been implicated in SARS-CoV-2 spike priming [7]. Following host cell infection, ACE2 is downregulated and its enzymatic activity on the membrane is reduced [8]. Some studies showed that the mucosa of the oral cavity [9] and lung epithelial cells [10] highly express ACE2, which determines the initial tissue entry site of the virus. Endothelial cells [11], as well as human heart pericytes [12], also have high expression of ACE2, which may explain the pathophysiological effects related to the cardio-pulmonary system [13,14,15,16].
Studies of human clinical samples showed that SARS-CoV-2 viral infection is associated with changes in the host plasma lipid profile, which were proposed as a potential biomarker to support diagnostics and clinical management [17,18,19,20]. Multiple studies showed reduced high-density lipoprotein-cholesterol (HDL-C) levels, reflecting reduced levels of HDL particles, in the plasma of COVID-19 patients compared to healthy subjects [17,18,21,22]. These studies demonstrated an inverse correlation between HDL levels and the severity of COVID-19, with low HDL levels being associated with an increased risk of severe outcomes [17,18,21,22]. Furthermore, low levels of HDL were associated with decreased SARS-CoV-2 clearance [23], while patient recovery was accompanied by a correction in HDL-C and other lipid parameters [18,21,24]. It was reported that reduced levels of HDL in COVID-19 patients were associated with increased levels of the inflammatory marker C-reactive protein (CRP) [18,21,22,24] and possibly development of the “cytokine storm” that was observed in certain COVID-19 patients [19,23,24,25]. The above observations are consistent with HDL exerting a protective effect in COVID-19 disease, although the alternative, namely, that low HDL may be an epiphenomenon, cannot be ruled out. However, it was reported that SARS-CoV-2 binds to HDL particles via the virus’s spike protein and in vitro studies using human embryonic kidney 293 (HEK293) and human hepatoma HepG2 cell lines have shown, at least under certain conditions (such as low HDL particle concentrations), that this facilitates SARS-CoV-2 binding to ACE2 and the infection of cells [26,27]. On the other hand, others have reported findings with cultured human hepatoma Huh-7 and African green monkey kidney Vero-E6 cell lines, demonstrating that, while low concentrations of HDL promote SARS-CoV-2-mediated infection, higher HDL particle concentrations suppress SARS-CoV-2 infection (compared to the absence of HDL) [27,28]. Furthermore, both the enhancement of SARS-CoV-2 infection by low concentrations and the suppression of SARS-CoV-2 infection by high concentrations of HDL particles appears to be dependent on the expression of the HDL receptor, namely, the scavenger receptor class B type I (SR-B1, encoded by the SCARB1 gene) [29,30], in the target cells [26,28]. These findings suggest that HDL particles may exert complex effects on COVID-19 disease. To try to shed light on the complex role of HDL in SARS-CoV-2-induced disease, we reviewed recent advances in our understanding of HDL structure, properties, and functions during SARS-CoV-2 infection and the resulting COVID-19 disease.
2. Virus Infection and Cholesterol
Cholesterol is an essential lipid component of biomembranes of animal cells and enveloped animal viruses (reviewed in [31,32]), including coronaviruses [33,34,35,36,37,38]. Cholesterol-rich microdomains, called lipid rafts, on host cell plasma membranes have an important role in viral entry and budding [39]. Cholesterol-depleting molecules, such as methyl-β-cyclodextrin, inhibit the cellular entry of several viruses, such as human immunodeficiency virus type 1 (HIV-1), rotaviruses, and coronaviruses [38,40,41,42]. Mechanistic studies of HIV infection showed that cholesterol promotes proper spatial organization and interaction of host receptors and co-receptors within lipid rafts facilitating viral entry [37]. A recent study demonstrated that plasma membrane cholesterol increased the trafficking of ACE2 and the furin protease to lipid rafts, thereby increasing SARS-CoV-2 infection [22]. Specifically, the removal of plasma membrane cholesterol (by treatment with methyl-β cyclodextrin) reduced the levels of ACE2 and the furin protease in lipid rafts, thereby reducing SARS-CoV-2 infection; on the other hand, loading cells with cholesterol via treatment with apolipoprotein (Apo) E and serum increased the trafficking of ACE2 and the furin protease to lipid rafts, resulting in increased SARS-CoV-2 infection [22]. This suggests that plasma membrane cholesterol is required for proper trafficking and localization of receptors that facilitate SARS-CoV-2 infection. This study shows the importance of cholesterol and cholesterol-rich microdomains of the host plasma membrane for the proper assembly of SARS-CoV-2 entrance factors.
Some viruses were shown to use cellular receptors that are involved in cholesterol homeostasis to infect target cells. For example, the hepatitis C virus co-opts the low-density lipoprotein receptor (LDLR) and the HDL receptor SR-B1 to infect hepatocytes. As mentioned above, it was recently demonstrated that SR-B1 may affect the ability of SARS-CoV-2 to infect cells by mediating the effects of HDL on virus infection [26,27,28].
3. The HDL Receptor SR-B1
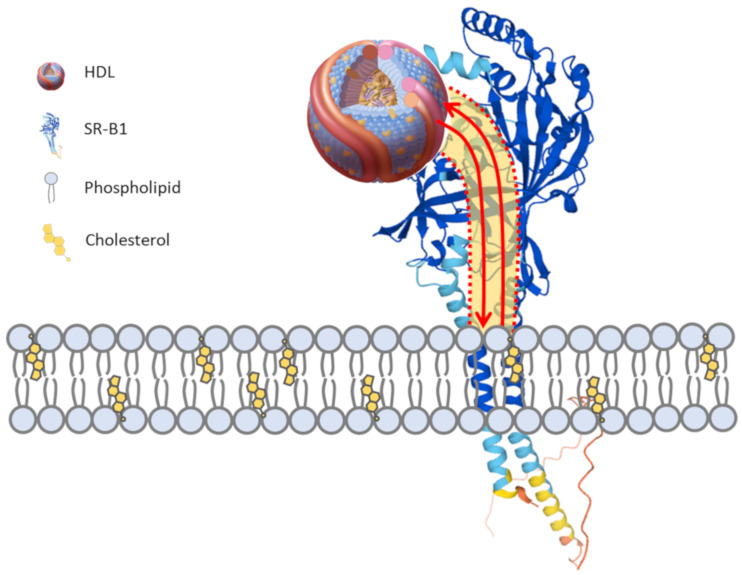
SR-B1 (encoded by the SCARB1 gene) is a multi-ligand scavenger receptor with a high affinity for HDL and mediates the selective exchange of lipids between cells and bound HDL particles [43,44] (Figure 1). SR-B1 is a member of a family of structurally related receptors, including SR-B2 (CD36) and SR-B3 (LIMP2), in mammals. Each of these contains two transmembrane domains, namely, a relatively short cytoplasmic amino and carboxy termini and a relatively large and heavily glycosylated extracellular (lysosomal in the case of LIMP2) domain through which a hydrophobic channel extends to the membrane-proximal region [44]. It is apparently through this channel that SR-B1 mediates the exchange of lipids between the cell membrane and the HDL bound to its extracellular domain (Figure 1).
Figure 1.
HDL and SR-B1 selective lipid exchange. The high-density lipoprotein (HDL) receptor, scavenger receptor class B type I (SR-B1), contains two transmembrane domains and a large extracellular domain that contains a hydrophobic channel (depicted in yellow shading bound by dashed red lines) leading from the HDL binding site to the juxtamembrane region. The binding of HDL to SR-B1 on the surface of cells promotes the bidirectional flow of lipids (red arrows) through the hydrophobic channel of SR-B1 to the membrane. Lipids are exchanged between HDL and the cell without the net internalization or degradation of the HDL particle. The concentration gradient of cholesterol between HDL and the cell plasma membrane is thought to dictate whether there is a net cellular uptake or efflux of lipids. The predicted structure of HDL was from [45]. The predicted structure of human SR-B1 was generated using Alphafold and based on [46]. The SR-B1 structure was first reported by [47].
By virtue of its ability to mediate the lipid exchange between cells and bound HDL, SR-B1 plays a key role in the accumulation of cholesterol in steroidogenic cells, such as those of the adrenal cortex, which store HDL-derived cholesterol in cytoplasmic lipid droplets for use in steroid hormone biosynthesis [44,48] (Figure 2). Likewise, SR-B1 in hepatocytes plays a key role in mediating the cellular uptake of HDL-derived cholesterol, which is either secreted (as cholesterol or after conversion to bile acids) into the bile for elimination or repackaged into nascent lipoproteins and secreted into the blood as part of the cholesterol cycle [44,48]. In this way, SR-B1 drives a process called reverse cholesterol transport (RCT) through which HDL clears excess cholesterol from the artery wall and carries it to the liver [44,48] (Figure 2). RCT is believed to be a major pathway by which HDL protects against the development of atherosclerotic cardiovascular disease [49,50,51]. Studies in mice showed that the absence of functional SR-B1 leads to increased plasma cholesterol concentrations, which is associated with enlarged HDL particles with increased unesterified cholesterol content and altered apolipoprotein composition [52,53,54,55,56]. Although inactivating mutations in the SCARB1 gene in humans are rare, similar increases in HDL cholesterol as well as early-onset coronary artery atherosclerotic disease were reported in human compound heterozygotes carrying two inactivating mutations in SCARB1 [57]. This is similar to findings in mice in which SR-B1 is knocked out in the context of other atherogenic mutations [54,58,59]. This highlights the important atheroprotective role that is played by SR-B1-mediated HDL-dependent RCT.
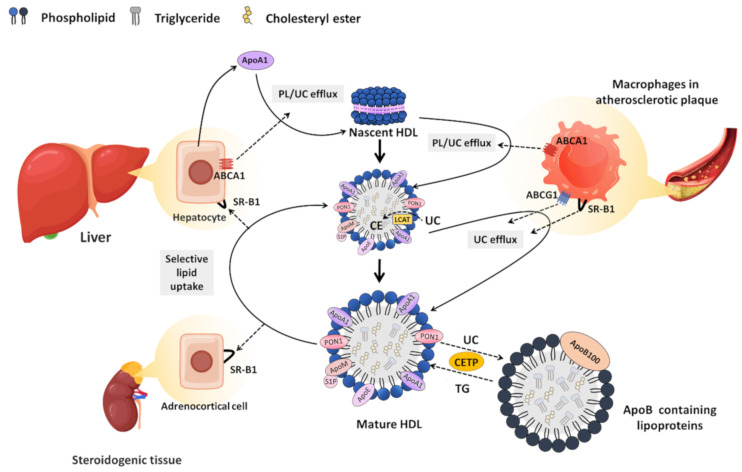
Figure 2.
Biosynthesis and metabolism of HDL. The biosynthesis of HDL starts with the synthesis and secretion of apolipoprotein (Apo) A-1 as a lipid-free protein from hepatocytes. Through the efflux mediated by the transporter ATP-binding cassette transporter (ABC)-A1, ApoA1 obtains phospholipids (PL) and unesterified cholesterol (UC) from cells to form a disc-shaped nascent HDL particle. These particles take up additional PL and UC through ABCA1-mediated efflux. Lecithin:cholesterol acyltransferase (LCAT) converts UC into the more hydrophobic cholesteryl ester (CE), which is taken to the center of the HDL, shaping HDL into a spherical particle. Spherical HDL serves as an acceptor for UC efflux mediated by ABCG1 and SR-B1. Cholesteryl ester transfer protein (CETP) mediates the exchange of cholesteryl esters in HDL with triglycerides in ApoB-containing lipoproteins, reducing the HDL cholesterol levels. HDL cholesterol (UC and CE) is directly cleared in the liver via SR-B1, which mediates a process called selective lipid uptake in which the lipid moiety of HDL is taken up by cells and the lipid-depleted particle returns to the circulation. Cholesterol taken up in the liver can either be recycled into newly assembled ApoB-containing lipoproteins or can undergo net excretion into the bile. A similar SR-B1-mediated pathway of selective lipid uptake from HDL occurs in steroidogenic cells of the adrenal gland, testes, and ovary to support steroid hormone biosynthesis.Black dotted arrows represent the movement or conversion of cholesterol. Solid black arrows represent the movement or conversion of HDL particles.
4. SR-B1 and Viral Infection
SR-B1 was previously reported to mediate hepatitis C virus (HCV) entry into hepatocytes [60,61] and to enhance dengue virus infection [62]. Wei and colleagues recently demonstrated that, in the presence of low concentrations of HDL, SR-B1 facilitates SARS-CoV-2 infection of cultured human hepatoma Huh-7 cells and African green monkey Vero E6 cells. They reported that in the presence of 6 µg HDL protein/mL, SARS-CoV-2 infection was enhanced by SR-B1 overexpression and inhibited by SR-B1 silencing or pharmacological inhibition [26]. They showed that while SR-B1 cannot bind to the virus spike protein directly, the virus spike protein mediated the SARS-CoV-2 binding to HDL particles, and it appears that SR-B1 binding to those HDL particles enhanced SARS-CoV-2 infection of cells via ACE2. Finally, an antibody against the S1 subunit of the spike protein that reportedly blocks the putative cholesterol/HDL-binding site strongly inhibited the entry of pseudoviral particles into the cell [26]. In the case of HCV infection of hepatocytes, different ligands of SR-B1 were shown to differentially affect infection of cultured cells: HDL was also shown to enhance, while oxidized LDL (another SR-B1 ligand which may bind to a different ligand-binding site from that engaged by HDL) inhibited HCV infection [63,64]. Furthermore, PDZK1, which is an adaptor protein that binds to SR-B1’s carboxy-terminal cytoplasmic tail and stabilizes it against degradation in liver hepatocytes [65], was found to be essential for the efficient entry of HCV into cultured hepatocyte-like cells [66]. This was most likely because the deletion of PDZK1 in liver hepatocytes resulted in a dramatic loss of SR-B1 protein [67]. Furthermore, small-molecule inhibitors of SR-B1-mediated lipid transfer (blockers of lipid transfer BLT-3 and BLT-4) were reported to abrogate the stimulation of HCV infectivity by human serum or HDL, suggesting that the enhancement of viral infection might be dependent on SR-B1’s lipid exchange activity [68,69]. Similarly, Wei and colleagues reported that BLT-1, which is a related small-molecule inhibitor of SR-B1-mediated lipid transfer between bound HDL and cells, inhibited the SARS-CoV-2 infection of Huh-7 hepatocyte-like cells [26]. This suggests that SR-B1-mediated lipid transfer activity may be necessary for the HDL stimulation of SARS-CoV-2 infection.
Cho and co-workers also reported data showing that low concentrations of HDL (15 µg protein/mL) enhanced the SARS-CoV-2 infection of cultured Vero E6 cells, consistent with the findings of Wei et al. [26,27]. These low concentrations (6–15 µg HDL protein/mL) are much lower than those normally found in plasma (approximately 1000–1500 µg HDL protein/mL [70]) or expected for interstitial fluids (typically 10–25% of plasma concentrations [71]). However, Cho and co-workers also reported that higher HDL concentrations (in the order of 60 µg protein/mL) suppressed viral infection [27], although it was suggested that differences in the HDL preparations may have contributed to the differences between their findings and those reported by Wei and co-workers [72]. Similarly, Thaxton and co-workers reported (in a preprint) that a synthetic HDL-like nanoparticle also suppressed SARS-CoV-2 infection of cultured HEK293 and Vero E6 cells in a manner that was suppressed by antisense-mediated SR-B1 knockdown [28]. Together, these results may suggest that while low (sub-physiological) concentrations of HDL appear to enhance SARS-CoV-2 infection of cultured cells, higher HDL concentrations appear to suppress SARS-CoV-2 infection of cultured cells. In both cases, this involved pathways that are dependent on the HDL receptor SR-B1. Whether the suppression of SARS-CoV-2 infection of cells, mediated by higher concentrations of HDL, is dependent on SR-B1’s lipid transfer activity and whether these processes occur in vivo in animal models or human patients remains to be determined.
As mentioned above, both ACE2 and SR-B1 were reported to localize (at least in some cell types and under certain conditions) to cholesterol-rich domains (lipid rafts/caveolae) [22,73]. Interestingly, SR-B1 is known to modify the cholesterol content of the plasma membrane. For example, when overexpressed in cultured Chinese hamster ovary (CHO) and COS-7 fibroblast-like cells, SR-B1 increases the accessibility of cholesterol on the outer leaflet of the plasma membrane, as measured by the cholesterol’s sensitivity to modification by cholesterol oxidase [74,75,76]. This may be the consequence of the SR-B1-mediated transfer of cholesterol from HDL particles into the plasma membrane, increasing local cholesterol concentrations, or SR-B1-mediated transfer of cholesterol from the membrane to HDL particles, reducing the local concentration, since SR-B1 can mediate the exchange of cholesterol between cells and bound HDL particles in both directions [76,77] (Figure 1). In either case, this is dependent on SR-B1’s hydrophobic lipid channel, which is blocked by the BLT class of inhibitors [47,78]. On the other hand, it was reported that SR-B1-mediated redistribution of plasma membrane cholesterol may be independent of its ability to bind HDL [74,75].
Nevertheless, this suggests a possible mechanism by which SARS-CoV-2 binding to HDL may enhance its infection of cells: due to the virus binding to HDL, the HDL binding to SR-B1, and the virus binding to ACE2, this may bring SR-B1 into proximity to ACE2 on the cellular plasma membrane, resulting in an SR-B1-mediated redistribution of cholesterol in the local environment of ACE2 in such a way as to favor viral entry. Circumstantial evidence supports this model. For example, SR-B1, ACE2, and furin were all reported to be present in cholesterol-rich microdomains on the plasma membrane [22,73]. Cholesterol-rich microdomains were suggested to function as entrance gates for viruses [34,41,79], including SARS-CoV-2 [80]. The depletion of membrane cholesterol from lipid rafts with cyclodextrin interfered with the localization of ACE2 and furin in lipid rafts and reduced SARS-CoV-2 infection [22]. The ability of SR-B1 to modulate plasma membrane cholesterol distribution/content suggests that it may influence the cholesterol content of lipid rafts and the localization of lipid-raft-associated proteins, such as ACE2 and furin. Therefore, it is possible that through spike protein binding to cholesterol, SARS-CoV-2 association with HDL may position it to exploit the physiological function of SR-B1 to optimize conditions for its fusion and entry processes within host cells.
However, this explanation does not easily explain the apparent HDL concentration dependence of HDL’s effect on SARS-CoV-2 infection of cells [28]. The ability of SARS-CoV-2 to bind to HDL (via the spike protein binding to cholesterol), as well as the relative sizes of spherical HDL particles (up to 14 nm in diameter [81]) and SARS-CoV-2 particles (100 nm in diameter [82]) suggests that low HDL concentrations may favor a single virus particle binding to a single HDL particle via the virus particle spike proteins binding to cholesterol on the HDL particle, but leaving abundant virus spike proteins accessible for interaction with the cell surface ACE2 (see Figure 3). This would also allow for bridging between SR-B1 (via HDL binding) and ACE2 (via SARS-CoV-2 binding), which is consistent with enhanced SARS-CoV-2 infection mediated by low HDL concentrations [26]. On the other hand, higher, more physiological HDL concentrations may favor multiple HDL particles binding to individual SARS-CoV-2 particles (Figure 1). Since this binding occurs via SARS-CoV-2 spike proteins binding to HDL cholesterol [26,27,28], the decoration of virus particles with HDL particles may prevent the virus spike proteins from interacting with ACE2 receptors on the cell’s surface. Alternatively, the concentration of many HDL particles in the vicinity of ACE2 receptor may alter plasma membrane cholesterol in such a way as to make viral entry, or other steps in virus infection, less favorable.
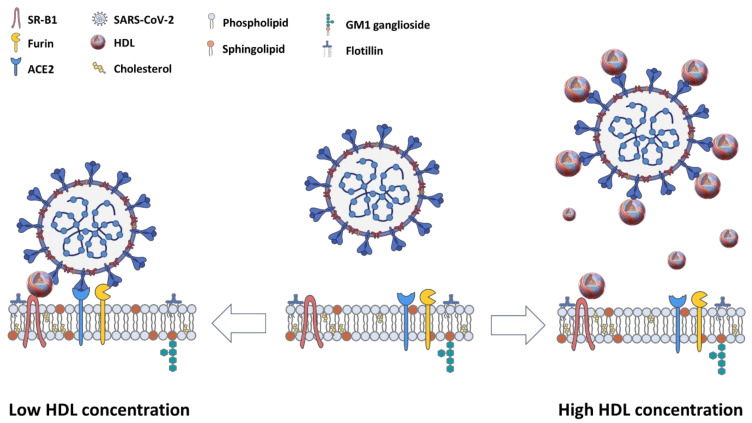
Figure 3.
Hypothetical HDL concentration-dependent effects on SARS-CoV-2 infection. In the absence of HDL (center), SARS-CoV-2 binds to ACE2 via the virus’s spike protein, but without the participation of the HDL receptor, SR-B1. When HDL is present at a low particle concentration (left), HDL particles bind to SARS-CoV-2 via the virus’s spike protein but leave other molecules of spike protein accessible for binding to ACE2 on cells, allowing for bridging of ACE2 and SR-B1 [26]. This may allow SR-B1 to modify the local membrane environment of the ACE2 receptor in a way that favors viral entry, although other mechanisms are possible. When HDL is present at high particle concentrations (right), virus particles may bind to multiple HDL particles. This may result in HDL competing with ACE2 for binding to viral spike proteins, preventing SARS-CoV-2 from entry into host cells. Alternatively, the binding of the complex of one virus particle with multiple HDL particles to SR-B1 may result in alterations in the membrane lipid composition that disrupt viral entry into cells. Another possibility is that at higher HDL particle concentrations, HDL that is not bound to SARS-CoV-2 particles may compete with the HDL bound to SARS-CoV-2 for binding to SR-B1, preventing bridging of SR-B1 and the ACE2 receptor. SARS-CoV-2 virus picture was captured from BioRender.com.
Cell lysis is often used as a surrogate for virus infection in cell culture studies [27]. Therefore, possible effects of HDL on other steps in the virus life cycle, such as viral replication or budding from cells, should be considered. For example, in addition to viral entry, lipid rafts also play a role in the budding of some viruses from cells. HIV and rotavirus proteins were shown to sort into lipid rafts [83,84,85,86]. Furthermore, cholesterol found on viral envelopes is derived from the cell membrane from which the virus buds and plays an important role in viral stability and infectivity. The depletion of cholesterol from viral envelopes of some viruses reduces infectivity and cholesterol replenishment restores infectivity [33,79,87]. Analogous to its role on host cell membranes, cholesterol on viral envelope membranes was shown to play a role in organizing the receptors required for viral fusion, as seen in the assembly of hemagglutinin trimers on the Influenza viral envelope [88]. Therefore, HDL and/or SR-B1 may also impact viral infection indirectly by regulating plasma membrane cholesterol distribution and the cholesterol content of viral envelopes. Whether this is relevant for SARS-CoV-2 is not yet known. Given the complex effects of HDL on SARS-CoV-2 infection of cells revealed from in vitro cell culture studies, it is important to understand the nature of HDL particles in both healthy individuals and those infected with SARS-CoV-2.
5. HDL Structural Complexity and Function
HDL is characterized by a small particle size (7–14 nm diameter), high density (1.063–1.21 g/mL), and unique apolipoprotein composition [89,90], distinguishing it from other lipoproteins. Proteomic analysis of HDL revealed over 200 associated proteins involved in multiple processes, including lipid transport, inflammation, immunity, hemostasis, and proteolysis [91,92,93,94,95]. This large number of proteins, together with the small particle size, emphasizes that HDL represents a class of diverse lipoprotein particles with diverse functions despite similar physical characteristics [91,92,93,94]. Proteomic analysis led to the identification of distinctive proteome profiles that are associated with distinct HDL sub-species, suggesting that distinct HDL functions may be attributed to particular HDL sub-species. However, the relationship between HDL function and HDL sub-species is not fully understood [89]. HDL lipids consist mainly of phospholipids, sphingomyelin, and unesterified cholesterol at the particle surface with cholesteryl esters and small amounts of triglycerides comprising a hydrophobic core [96,97]. In addition, lipidomic analyses revealed the presence of a range of minor lipid species that may contribute to the functional diversity of HDL particles. These include lysophospholipids, such as sphingosine-1-phosphate (S1P); ceramide; plasmalogens; bioactive steroids, such as oxysterols and estrogen; and minor amounts of di- and mono-acylglycerol and free fatty acids [98]. HDL particles were also reported to carry lipid-soluble vitamins, such as vitamin E [99], and other bioactive macromolecules, including microRNAs [100]. Furthermore, the HDL particle composition is dynamic, changing with different physiological and pathophysiological states [94,98,101]. Together, this diversity in composition gives rise to substantial variability and complexity in the HDL particle’s function.
Despite the variety of HDL particles, their biosynthesis begins with the synthesis and secretion of the major apolipoprotein (ApoA1) as a lipid-free protein, which acquires phospholipids and cholesterol in the circulation through efflux mediated by ATP-binding cassette transporter (ABC)-A1 to form disc-shaped premature HDL particles [51,102,103,104,105] (Figure 2). These particles continue to accumulate unesterified cholesterol, which, through the action of lecithin:cholesterol acyltransferase (LCAT), is converted into the more hydrophobic cholesteryl ester that is sequestered in the center of HDL, forming a hydrophobic core, ultimately shaping HDL into a spherical particle [106,107]. Over time, HDL particles increase in size as they circulate through the bloodstream and incorporate more cholesterol from cells via ABCG1-mediated cholesterol efflux, and exchange lipids with other lipoproteins through the actions of phospholipid transport protein (PLTP) and cholesteryl ester transfer protein (CETP) (reviewed by [49]) (Figure 2).
HDL cholesterol (both free and esterified) is taken up by hepatocytes in the liver via a process called selective lipid uptake in which the lipid components of the HDL particle are taken up by the cell without the net internalization and lysosomal degradation of the lipoprotein particle itself [44,108,109], distinguishing the mechanism of HDL cholesterol delivery to cells from the better known endocytic mechanisms involved in the delivery of cholesterol by other lipoproteins, such as low-density lipoprotein. A similar pathway of selective lipid uptake from HDL occurs in steroidogenic cells of the adrenal gland, testes, and ovary [44,108,109]. The identification of SR-B1 as an HDL receptor led to the discovery that it mediates selective HDL lipid uptake and is responsible for the pathway of cholesterol delivery to the liver and steroidogenic tissues [43,44]. HDL-mediated selective cholesterol uptake by hepatocytes in the liver is the final step in the athero- and cardio-protective RCT pathway in which HDL picks up excess cholesterol from the artery wall and delivers it, as described above, to the liver for recycling or biliary excretion [44,48] (Figure 2).
6. Anti-Oxidative and Anti-Inflammatory Properties of HDL
HDL is recognized to have pleiotropic cellular effects, such as anti-oxidant [110,111], anti-inflammatory [110,112,113], and anti-cytotoxic effects [114,115,116,117,118,119,120]. Moreover, it is involved in the cholesterol efflux of cells [50,97], which protects macrophages from LDL-induced apoptosis and enhances endothelial function by activating nitric oxide synthase to promote endothelial repair and induce angiogenesis [121,122,123]. Cholesterol efflux capacity mainly depends on HDL’s size, HDL acting as an extracellular acceptor, macrophage cholesterol content, and the expression of macrophage cholesterol transporter proteins [124,125]. In vivo studies indicated that ABCA1 and ABCG1 play a key role in facilitating cholesterol efflux and reverse cholesterol transport [126,127]. Moreover, mice that are deficient in these transporters have marked increases in foam cell accumulation and display enlarged cholesterol-rich lipid rafts with high toll-like receptor 4 expression and an elevated response to stimulation by LPS [128,129,130].
HDL also reduces the expression of cytokines, such as tumor necrosis factor α (TNF-α) and interleukin-1 that mediate the upregulation of leukocyte–endothelial adhesion molecules. This process is believed to be mediated by ApoA1, as well as HDL-associated lipids, including S1P [131,132]. The majority of S1P in plasma was reported to be carried by HDL, bound to HDL-associated ApoM [133]. ApoM was reported to be present on only 5% of HDL particles [134], suggesting that only a subset of HDL particles carry S1P and may represent a sub-species that is responsible for at least some of HDL’s protective functions. It was shown that the S1P receptor 1 (S1P1) is responsible for the anti-inflammatory effects of HDL-associated S1P and induces a shift in macrophage polarization from the pro-inflammatory M1 to the anti-inflammatory M2 phenotype [135]. HDL-associated S1P and S1P1 also stimulate phosphatidylinositol-3-kinase (PI3K)/Akt and signal transducer and activator of transcription-3 (STAT3) survival signaling pathways, leading to macrophage chemotaxis and protection against apoptosis [114,120,135,136]. Furthermore, HDL-associated S1P acts as a biased ligand for S1P1, suppressing inflammatory activation of endothelial cells [137].
The genetic manipulation of ApoA1 expression in septic mice showed that its deficiency strongly increased inflammatory cytokine production, while ApoA1 overexpression in septic mice, or their treatment with HDL or with ApoA1 mimetic peptides, reduced inflammation and protected them from sepsis, at least in part, through neutralization of bacterial endotoxins [131,132,138,139,140]. These properties appear to be dependent on the abundance of HDL particles containing ApoA1. Similarly, in a mouse model that was engineered to express CETP, the pharmacological inhibition of CETP, leading to increased HDL-cholesterol levels, suppressed sepsis-induced inflammation and improved survival [141]. In agreement with this, CETP gain-of-function mutations in humans were associated with increased mortality due to sepsis, while reduced CETP function was associated with increased survival [141]. CETP exchanges cholesteryl esters on HDL for triglycerides from other lipoproteins (Figure 2); therefore, CETP inhibition or reduced CETP activity results in increased HDL cholesterol levels, whereas increased CETP function is associated with lower HDL cholesterol. This demonstrates, at least in the setting of sepsis, that HDL-targeted therapy may have beneficial outcomes.
The protein and lipid compositions of HDL particles also influence their anti-oxidative properties [142]. HDL’s ability to decrease LDL oxidation is inversely correlated to its free cholesterol and sphingomyelin content and positively correlated to its S1P content [143]. Furthermore, several HDL apolipoprotein components, such as ApoA1 [143,144], ApoE [144], and ApoM [145], were correlated with HDL anti-oxidative properties [89]. ApoA1 is essential both for the structure of HDL and the maintenance of the lipid environment in which enzymes such as paraoxonase 1 (PON1) and LCAT operate [96]. Therefore, it is likely that ApoA1 plays an important role (albeit indirect) in the anti-oxidative properties of HDL.
HDL-associated hydrolases, including PON1, LCAT, and platelet-activating factor acetylhydrolase (PAF-AH), contribute strongly to the anti-oxidative activity of HDL [146,147]. There are three members of the paraoxonase family: PON1, PON2, and PON3. PON1 and PON3 are synthesized by the liver and secreted into the blood, where they assemble with HDL particles [148,149]. PON1 can hydrolyze a wide variety of substrates, such as lactones, glucuronide drugs, thiolactones, aryl esters, cyclic carbonates, organophosphorus pesticides, and nerve gases [150]. Knocking out PON1 in mice results in HDL with impaired anti-oxidative activity [151,152]. An interesting recent work by Huang et al. [153] proposed that PON1 can form a tertiary complex with HDL and myeloperoxidase, which can modulate the activity of the latter. LCAT can hydrolyze oxidized acyl chains of oxidized phospholipids, generating lysophosphatidylcholine and oxidized free fatty acids [154]. Furthermore, LCAT can work as a chain-breaking anti-oxidant via its cysteine residues [155]. There is evidence that mutations in LCAT may reduce anti-oxidative capacity of HDL [156]. However, the inhibition of LCAT activity does not affect HDL’s capacity to neutralize lipid hydroperoxides derived from LDL [157].
7. Effects of Chronic Disease on HDL Structure and Function and Implications for COVID-19
Abundant research over the past decade has revealed that HDL’s composition and function change dramatically with various chronic diseases, including infectious diseases, such as sepsis; metabolic diseases, such as obesity and diabetes; and cardiovascular disease [91,158,159,160,161,162,163]. This includes changes in the levels of HDL-associated proteins and lipid components and/or modification of those components, such as by non-enzymatic glycation and oxidation of both protein and lipid species, leading to the accumulation of dysfunctional HDL particles [158] (Figure 4).
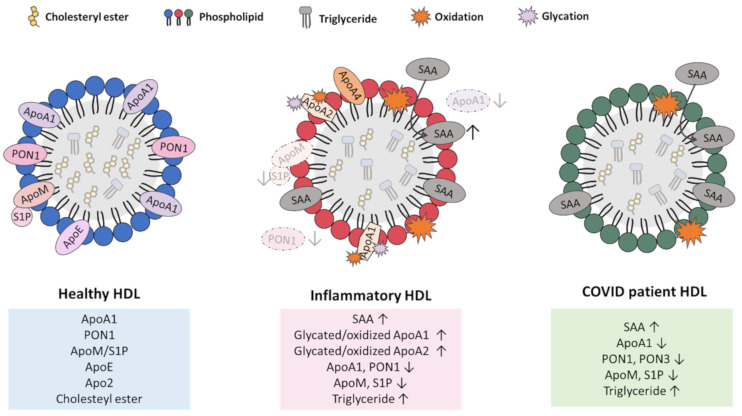
Figure 4.
HDL remodeling in inflammatory/cardiometabolic disease and COVID-19. HDL is extensively remodeled during inflammatory and cardiometabolic disease (center) and in COVID-19 patients (right). Healthy HDL is shown on the left. Similar changes in HDL in inflammatory and cardiometabolic disease include oxidation and glycation of HDL-associated proteins, including ApoA1; replacement of ApoA1 with serum amyloid A-1 (SAA1); reductions in paraoxonase 1 (PON1), ApoM/sphingosine-1-phosphate (S1P), and ApoE; and increases in core triglycerides. These changes are associated with reductions in the beneficial functions of HDL (anti-oxidant, anti-inflammatory, and anti-cytotoxic properties, as well as cholesterol efflux) and gain of detrimental functions (pro-oxidant, pro-inflammatory, and pro-cytotoxic properties). Similar changes were reported in HDL during COVID-19 disease, suggesting similar changes in HDL particle functions may contribute to disease development. Arrows pointing up represents increase and arrows pointing down represents decrease for respective compound.
In general, cardiovascular and inflammatory diseases are accompanied by decreases in some HDL-associated apolipoproteins, including ApoA4, and increases in other proteins, such as the acute phase protein serum amyloid A-1 (SAA1) [91,159]. Inflammatory HDL is characterized by a replacement of the major protein constituent of HDL, namely, ApoA1, with the acute phase protein SAA1 [159,160,161,164]. SAA1-enriched HDL particles lead to higher HDL binding to macrophages, a reduction in cholesterol efflux by macrophages, and increased selective uptake of CE by macrophages [161,165]. This suggests that the alterations in HDL properties as a result of changes that are brought about by chronic inflammation may shift HDL from protecting to promoting disease development (Figure 4).
Chronic metabolic disease was also reported to lead to the modification of HDL-associated ApoA1. For example, HDL from type 2 diabetes (T2D) patients contains ApoA1 and ApoA2 proteins that are modified by non-enzymatic glycation [162,163,166,167]. Glycation leads to multimerization of ApoA1 and ApoA2, forming high-molecular-weight aggregates [168,169]. The glycation of ApoA1 may impair its ability to stimulate cholesterol efflux from cells, as well as its ability to activate LCAT, which plays an important role in HDL maturation and its anti-oxidative properties [170]. Glycated ApoA1 has a three-fold shorter half-life than native ApoA1, leading to accelerated turnover/degradation [171,172]. Finally, the glycation of HDL was reported to compromise its anti-inflammatory properties [173]. Similar modifications were reported in HDL from elderly subjects and HDL from smokers [169,171,174,175,176,177]. Oxidation of HDL-associated ApoA1 was also reported in those with metabolic disease, the elderly, and in smokers, and dramatically reduces HDL’s ability to mediate cholesterol efflux [178,179,180]. HDL from diabetic patients and smokers also exhibits elevated triglyceride levels compared to non-diabetics and non-smokers, respectively [181,182,183,184]. Similarly, SAA-enriched HDL from myocardial infarction patients contained increased triglyceride content [160] (Figure 4). At least one report suggests that chronic disease may impact HDL properties in such a way as to influence its effects on SARS-CoV-2 infection of cells. Specifically, Cho and co-workers reported that while native HDL (60 µg protein/mL) could suppress SARS-CoV-2 infection of cultured Vero E6 cells, the glycation of HDL (as seen in patients with type 2 diabetes) impaired its ability to suppress SARS-CoV-2 infection [27]. However, the effect of HDL glycation on the ability of low concentrations of HDL to enhance SARS-CoV-2 infection of target cells has not been described. Furthermore, several studies reported interactions between obesity, diabetes, smoking, and other cardiometabolic risk factors with disease severity in COVID-19 patients [185,186,187,188,189]. However, these relationships were reported to be no longer significant when HDL was controlled for, suggesting that the effects of these conditions on COVID-19 may be mediated through HDL. In fact, one study (unpublished at the time of this writing) suggests that baseline HDL levels appear to be associated with the risk of hospitalization for COVID-19 [190]. Therefore, the quality and function of HDL in individuals may influence the outcomes of subsequent SARS-CoV-2 infection.
8. HDL Alterations in COVID-19
Several reports showed that, consistent with other chronic and acute inflammatory conditions, dramatic changes in the abundance and composition of HDL particles occur during COVID-19 disease development [191] (Figure 4). These changes include substantial reductions in overall HDL-cholesterol levels, and in HDL-associated apolipoproteins including ApoA1; ApoA2; ApoA4; ApoC-1, 2, 3; ApoJ; ApoE; ApoD; ApoF; PON1; and PON3 [191,192]. A recent study showed that the serum levels of S1P and its HDL-associated carrier ApoM are also significantly decreased in COVID-19 patients compared to healthy volunteers [191,193]. On the other hand, SAA1 is increased on the HDL of COVID-19 patients in a manner that was associated with COVID-19 severity [191,194]. The increased association of SAA1 with HDL was also accompanied by a blunting of the anti-apoptotic and anti-inflammatory effects of HDL examined in cultured human umbilical vein endothelial cells (HUVEC) that were challenged with TNF-α [191]. It was suggested that HDL-bound SAA may be useful as a biomarker for COVID-19 severity and prognosis [194]. Although HDL was reported to carry a variety of microRNA species and to act to shuttle microRNAs between different cell types and tissues, the effects of COVID-19 on HDL-associated microRNAs have not yet been reported. Whether/how the observed changes in HDL structure, composition, and function during COVID-19 disease affect the ability of HDL to modify SARS-CoV-2 infection of target cells has not been explored. As mentioned above, it was reported that native HDL is able to affect SARS-CoV-2 infection of cultured Vero E6 cells in a dose-dependent manner [27]. It would be of interest to determine whether HDL enriched in SAA1 and depleted of ApoA1, as seen in patients with COVID-19 and other inflammatory diseases, may exhibit an altered ability to modify SARS-CoV-2 infection of target cells. If so, this may suggest a mechanism whereby ongoing disease promotes virus infection by impairing the protective effects of HDL. Furthermore, the observed reductions in HDL (particularly ApoA1-containing HDL) during COVID-19 disease may favor conditions in which low HDL levels actually promote virus infection of target cells, as was reported in cell culture systems [26,27]. Further experiments are required to determine whether this may be the case. For example, if the reductions in HDL levels that occur during the course of COVID-19 disease impact on the severity of disease, then interventions that are aimed at raising HDL levels may be beneficial. In this light, the recent reports that CETP inhibition is beneficial in sepsis, at least in mouse models, suggests similar interventions may have beneficial impacts on COVID-19 disease [141].
9. Spike Protein, HDL, and Endothelial Cell Function
Emerging evidence in the literature suggests that SARS-CoV-2 and its spike protein promote endothelial cell toxicity [195,196,197]. Indeed, it is possible for SARS-CoV-2 to enter the circulation and affect multiple tissues and cell types expressing ACE2 [10], including endothelial cells [198]. Endothelial dysfunction is increasingly becoming a concern as it is believed to be at the core of several life-threatening complications associated with COVID-19, such as venous thromboembolic disease and multi-organ involvement [199].
Histological analysis of tissue samples from deceased COVID-19 patients showed systemic vascular disease associated with thrombi in microvessels and endothelial damage/necrosis [196]. Histological analysis of lung tissue showed an accumulation of the virus and evidence of apoptosis in capillary endothelial cells [196]. Moreover, histological examination of lung capillaries showed marked microangiopathy, as displayed by the fragmented appearance of the capillary walls and disruption in capillary endothelial cell adhesion. The extrapulmonary tissues examined displayed a significantly lower SARS-CoV-2 viral RNA signal compared to that observed in the lung tissue but did show strong staining of SARS-CoV-2 pseudovirions (SARS-CoV-2 spike/capsid proteins lacking RNA), which co-localized with ACE2-positive endothelial cells of microvascular beds. These pseudovirion-positive endothelial cells displayed an upregulation of complement proteins, pro-inflammatory cytokines, and caspase-3 staining. In response to these findings, Magro and colleagues proposed a model for explaining SARS-CoV-2 disease sequelae, where (1) initial microangiopathy in infected lung capillaries causes endothelial cell death releasing pseudovirions into the circulation, and (2) the pseudovirions dock on ACE2-positive endothelial cells of extrapulmonary microvascular beds, leading to endothelial toxicity, activation of the complement pathway resulting in a pro-coagulant state, and cytokine release culminating in a cytokine storm. These findings were further supported in a follow-up study that examined the colocalization of spike proteins, cytokines, complement proteins, and caspase-3 in HUVEC cells in vitro following incubation with spike proteins, and in endothelial cells of microvascular beds in vivo following the injection of spike protein in mice [200]. Other groups showed that the SARS-CoV-2 spike protein alone can cause endothelial damage/dysfunction manifested via impairments in endothelial nitric oxide synthase activity and mitochondrial function [201]. Finally, another study conducted a histological analysis of COVID-19 tissue specimens and showed viral inclusions in endothelial cells, endothelial inflammation, and endothelial apoptosis, as indicated by caspase-3 staining [195]. Altogether, there is compelling evidence of the vasculopathy associated with SARS-CoV-2 infection.
The SARS-CoV-2 spike protein was also implicated as a mediator of “long COVID,” which is a term that is used to describe the long-term persistence of symptoms in COVID-19 patients following recovery from acute virus infection and discharge from the hospital [202]. The symptoms of long COVID vary but include shortness of breath, cough, fatigue, and depression, and reflect the persistent effects on multiple organs [202]. Much remains unknown regarding the causes of long COVID; however, there was some suggestion that the SARS-CoV-2 spike protein alone can trigger persistent alterations in cellular inflammatory gene expression in cultured human primary bronchial epithelial cells [203], suggesting a potential mechanism that may be involved. However, whether and how HDL and SR-B1 might affect this is not known.
It is well known that HDL enhances endothelial function, promotes endothelial cell integrity, and suppresses inflammatory gene activation in endothelial and other cell types [137,204,205,206]. This stems from HDL’s anti-inflammatory, anti-oxidative, and anti-apoptotic effects, possibly reflecting the roles of specific sub-species of HDL particles, resulting in the activation of signaling pathways stemming from their interaction with SR-B1 and HDL-bound S1P-activating S1PRs [137,204,205,206]. The SARS-CoV-2 spike protein is known to interact with HDL, at least in vitro, by virtue of its ability to bind cholesterol [26,207]. If it similarly interacts with HDL in vivo, then this may further contribute to the alterations in HDL structure and function, impacting HDL’s ability to elicit protective signaling in endothelial and other cell types, preventing normal HDL-mediated protective pathways, and rendering cells susceptible to processes triggering inflammation and dysfunction. Furthermore, the association of SARS-CoV-2 spike protein with HDL may target the spike protein to cell types that are normally protected by HDL, such as endothelial cells, which express the HDL receptor SR-B1. Thus, HDL may serve to facilitate spike-protein-induced cellular toxicity. On the other hand, it is possible that the binding of a spike protein by HDL may serve to sequester and/or clear the spike protein in a fashion that is similar to the HDL-mediated clearance of bacterial endotoxin [140,208,209,210]. Further research is required to understand the consequences of spike protein interactions with HDL and the role of HDL in spike-protein-induced cellular toxicity.
10. Conclusions
HDL has the potential to influence disease development resulting from SARS-CoV-2 infection by virtue of its ability to directly modulate infection of cells by the virus, as well as by virtue of its ability to modulate protective functions, such as anti-inflammatory, anti-cytotoxic, and anti-oxidative effects. These protective functions are mediated by HDL sub-species with particular compositions of proteins, lipids, and other macromolecules, of which, ApoA1, ApoM, and S1P are notable examples. The mechanism by which HDL can, under different conditions, either enhance or suppress SARS-CoV-2 infection of cells is not currently known. We have suggested that HDL concentration may be a factor; however, the role of HDL sub-species has not been examined in detail. However, it is important to point out that while associations of HDL levels with COVID-19 disease severity, as well as in vitro cell biological data, may be consistent with a potential for HDL to influence SARS-CoV-2-mediated disease, they do not conclusively show in vivo causality. This awaits in vivo pre-clinical and, if warranted, clinical intervention studies. While HDL may have the potential to influence SARS-CoV-2-mediated disease, the evidence clearly demonstrates that SARS-CoV-2-mediated disease also dramatically influences HDL abundance, composition, and undoubtedly function, similar to the effects of other inflammatory and cardiometabolic diseases. How these two processes intersect during SARS-CoV-2-mediated disease development remains to be further elucidated. Understanding the particular properties and sub-species of HDL that protect against versus promote SARS-CoV-2 infection and resulting disease development may provide opportunities for beneficial therapeutic interventions.
Author Contributions
Conceptualization, G.E.G.K. and B.L.T.; Writing—Original Draft Preparation, G.E.G.K., J.-A.Y. and E.H.S.; Writing—Review and Editing, G.E.G.K., J.-A.Y., E.H.S. and B.L.T.; Supervision, B.L.T.; Project Administration, B.L.T. All authors have read and agreed to the published version of the manuscript.
Funding
Research in the Trigatti Lab is supported by grants from the Canadian Institutes of Health Research (MOP-74753 and PJT-162272) to B.L.T., E.H.S was the recipient of an Ontario Graduate Scholarship.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ou X., Liu Y., Lei X., Li P., Mi D., Ren L., Guo L., Guo R., Chen T., Hu J., et al. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat. Commun. 2020;11:1620. doi: 10.1038/s41467-020-15562-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bourgonje A.R., Abdulle A.E., Timens W., Hillebrands J.L., Navis G.J., Gordijn S.J., Bolling M.C., Dijkstra G., Voors A.A., Osterhaus A.D.M.E., et al. Angiotensin-converting enzyme 2 (ACE2), SARS-CoV-2 and the pathophysiology of coronavirus disease 2019 (COVID-19) J. Pathol. 2020;251:228–248. doi: 10.1002/path.5471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coronavirus Disease (COVID-19) [(accessed on 27 August 2021)]. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019.
- 4.Glebov O.O. Understanding SARS-CoV-2 endocytosis for COVID-19 drug repurposing. FEBS J. 2020;287:3664–3671. doi: 10.1111/febs.15369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Walls A.C., Park Y.J., Tortorici M.A., Wall A., McGuire A.T., Veesler D. Structure, Function, and Antigenicity of the SARS-CoV-2 Spike Glycoprotein. Cell. 2020;181:281–292.e5. doi: 10.1016/j.cell.2020.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoffmann M., Kleine-Weber H., Pöhlmann S. A Multibasic Cleavage Site in the Spike Protein of SARS-CoV-2 Is Essential for Infection of Human Lung Cells. Mol. Cell. 2020;78:779–784.e5. doi: 10.1016/j.molcel.2020.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.-H., Nitsche A., et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020;181:271–280. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Glowacka I., Bertram S., Herzog P., Pfefferle S., Steffen I., Muench M.O., Simmons G., Hofmann H., Kuri T., Weber F., et al. Differential Downregulation of ACE2 by the Spike Proteins of Severe Acute Respiratory Syndrome Coronavirus and Human Coronavirus NL63. J. Virol. 2010;84:1198–1205. doi: 10.1128/JVI.01248-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu H., Zhong L., Deng J., Peng J., Dan H., Zeng X., Li T., Chen Q. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int. J. Oral Sci. 2020;12:8. doi: 10.1038/s41368-020-0074-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zou X., Chen K., Zou J., Han P., Hao J., Han Z. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front. Med. 2020;14:185–192. doi: 10.1007/s11684-020-0754-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sluimer J.C., Gasc J.M., Hamming I., Van Goor H., Michaud A., Van Den Akker L.H., Jütten B., Cleutjens J., Bijnens A.P.J.J., Corvol P., et al. Angiotensin-converting enzyme 2 (ACE2) expression and activity in human carotid atherosclerotic lesions. J. Pathol. 2008;215:273–279. doi: 10.1002/path.2357. [DOI] [PubMed] [Google Scholar]
- 12.Chen L., Li X., Chen M., Feng Y., Xiong C. The ACE2 expression in human heart indicates new potential mechanism of heart injury among patients infected with SARS-CoV-2. Cardiovasc. Res. 2020;116:1097–1100. doi: 10.1093/cvr/cvaa078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zheng Y.Y., Ma Y.T., Zhang J.Y., Xie X. COVID-19 and the cardiovascular system. Nat. Rev. Cardiol. 2020;17:259–260. doi: 10.1038/s41569-020-0360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kochi A.N., Tagliari A.P., Forleo G.B., Fassini G.M., Tondo C. Cardiac and arrhythmic complications in patients with COVID-19. J. Cardiovasc. Electrophysiol. 2020;31:1003–1008. doi: 10.1111/jce.14479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nicin L., Abplanalp W.T., Mellentin H., Kattih B., Tombor L., John D., Schmitto J.D., Heineke J., Emrich F., Arsalan M., et al. Cell type-specific expression of the putative SARS-CoV-2 receptor ACE2 in human hearts. Eur. Heart J. 2020;41:1804–1806. doi: 10.1093/eurheartj/ehaa311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thum T. SARS-CoV-2 receptor ACE2 expression in the human heart: Cause of a post-pandemic wave of heart failure? Eur. Heart J. 2020;41:1807–1809. doi: 10.1093/eurheartj/ehaa410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hu X., Chen D., Wu L., He G., Ye W. Low Serum Cholesterol Level Among Patients with COVID-19 Infection in Wenzhou, China. SSRN Electron. J. 2020 doi: 10.2139/ssrn.3544826. [DOI] [Google Scholar]
- 18.Wei X., Zeng W., Su J., Wan H., Yu X., Cao X., Tan W., Wang H. Hypolipidemia is associated with the severity of COVID-19. J. Clin. Lipidol. 2020;14:297–304. doi: 10.1016/j.jacl.2020.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tanaka S., De Tymowski C., Assadi M., Zappella N., Jean-Baptiste S., Robert T., Peoch K., Lortat-Jacob B., Fontaine L., Bouzid D., et al. Lipoprotein concentrations over time in the intensive care unit COVID-19 patients: Results from the ApoCOVID study. PLoS ONE. 2020;15:e0239573. doi: 10.1371/journal.pone.0239573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang G., Zhang Q., Zhao X., Dong H., Wu C., Wu F., Yu B., Lv J., Zhang S., Wu G., et al. Low high-density lipoprotein level is correlated with the severity of COVID-19 patients: An observational study. Lipids Health Dis. 2020;19 doi: 10.1186/s12944-020-01382-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hu X., Chen D., Wu L., He G., Ye W. Declined serum high density lipoprotein cholesterol is associated with the severity of COVID-19 infection. Clin. Chim. Acta. 2020;510:105–110. doi: 10.1016/j.cca.2020.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang H., Yuan Z., Pavel M.A., Hobson R., Hansen S. The role of high cholesterol in age-related COVID19 lethality. bioRxiv Prepr. Serv. Biol. 2020 doi: 10.1101/2020.05.09.086249. [DOI] [Google Scholar]
- 23.Ding X., Zhang J., Liu L., Yuan X., Zang X., Lu F., He P., Wang Q., Zhang X., Xu Y., et al. High-density lipoprotein cholesterol as a factor affecting virus clearance in covid-19 patients. Respir. Med. 2020;175:106218. doi: 10.1016/j.rmed.2020.106218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roccaforte V., Daves M., Lippi G., Spreafico M., Bonato C. Altered lipid profile in patients with COVID-19 infection. J. Lab. Precis. Med. 2021;6 doi: 10.21037/jlpm-20-98. [DOI] [Google Scholar]
- 25.Caricchio R., Gallucci M., Dass C., Zhang X., Gallucci S., Fleece D., Bromberg M., Criner G.J. Preliminary predictive criteria for COVID-19 cytokine storm. Ann. Rheum. Dis. 2021;80:88–95. doi: 10.1136/annrheumdis-2020-218323. [DOI] [PubMed] [Google Scholar]
- 26.Wei C., Wan L., Yan Q., Wang X., Zhang J., Yang X., Zhang Y., Fan C., Li D., Deng Y., et al. HDL-scavenger receptor B type 1 facilitates SARS-CoV-2 entry. Nat. Metab. 2020;2:1391–1400. doi: 10.1038/s42255-020-00324-0. [DOI] [PubMed] [Google Scholar]
- 27.Cho K.-H., Kim J.-R., Lee I.-C., Kwon H.-J. Native High-Density Lipoproteins (HDL) with Higher Paraoxonase Exerts a Potent Antiviral Effect against SARS-CoV-2 (COVID-19), While Glycated HDL Lost the Antiviral Activity. Antioxidants. 2021;10:209. doi: 10.3390/antiox10020209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Henrich S.E., McMahon K.M., Palacio N., Bhalla P., Penaloza-MacMaster P., Thaxton C.S. Targeting Scavenger Receptor Type B-1 (SR-B1) and Cholesterol Inhibits Entry of SARS-CoV-2 Pseudovirus in Cell Culture. bioRxiv. 2020 doi: 10.1101/2020.12.14.420133. [DOI] [Google Scholar]
- 29.PrabhuDas M., Bowdish D., Drickamer K., Febbraio M., Herz J., Kobzik L., Krieger M., Loike J., Means T.K., Moestrup S.K., et al. Standardizing Scavenger Receptor Nomenclature. J. Immunol. 2014;192:1997–2006. doi: 10.4049/jimmunol.1490003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.PrabhuDas M.R., Baldwin C.L., Bollyky P.L., Bowdish D.M.E., Drickamer K., Febbraio M., Herz J., Kobzik L., Krieger M., Loike J., et al. A Consensus Definitive Classification of Scavenger Receptors and Their Roles in Health and Disease. J. Immunol. 2017;198:3775–3789. doi: 10.4049/jimmunol.1700373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heaton N.S., Randall G. Multifaceted roles for lipids in viral infection. Trends Microbiol. 2011;19:368–375. doi: 10.1016/j.tim.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mazzon M., Mercer J. Lipid interactions during virus entry and infection. Cell. Microbiol. 2014;16:1493–1502. doi: 10.1111/cmi.12340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang H., Li Y., Sadaoka T., Tang H., Yamamoto T., Yamanishi K., Mori Y. Human herpesvirus 6 envelope cholesterol is required for virus entry. J. Gen. Virol. 2006;87:277–285. doi: 10.1099/vir.0.81551-0. [DOI] [PubMed] [Google Scholar]
- 34.Osuna-Ramos J.F., Reyes-Ruiz J.M., Del Ángel R.M. The Role of Host Cholesterol During Flavivirus Infection. Front. Cell. Infect. Microbiol. 2018;8:388. doi: 10.3389/fcimb.2018.00388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sousa I.P., Carvalho C.A.M., Gomes A.M.O. Current Understanding of the Role of Cholesterol in the Life Cycle of Alphaviruses. Viruses. 2020;13:35. doi: 10.3390/v13010035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Iša P., Realpe M., Romero P., López S., Arias C.F. Rotavirus RRV associates with lipid membrane microdomains during cell entry. Virology. 2004;322:370–381. doi: 10.1016/j.virol.2004.02.018. [DOI] [PubMed] [Google Scholar]
- 37.Yi L., Fang J., Isik N., Chim J., Jin T. HIV gp120-induced interaction between CD4 and CCR5 requires cholesterol-rich microenvironments revealed by live cell fluorescence resonance energy transfer imaging. J. Biol. Chem. 2006;281:35446–35453. doi: 10.1074/jbc.M607302200. [DOI] [PubMed] [Google Scholar]
- 38.Li G.M., Li Y.G., Yamate M., Li S.M., Ikuta K. Lipid rafts play an important role in the early stage of severe acute respiratory syndrome-coronavirus life cycle. Microbes Infect. 2007;9:96–102. doi: 10.1016/j.micinf.2006.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ripa I., Andreu S., López-Guerrero J.A., Bello-Morales R. Membrane Rafts: Portals for Viral Entry. Front. Microbiol. 2021;12:1274. doi: 10.3389/fmicb.2021.631274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liao Z., Cimakasky L.M., Hampton R., Nguyen D.H., Hildreth J.E.K. Lipid Rafts and HIV Pathogenesis: Host Membrane Cholesterol Is Required for Infection by HIV Type 1. Volume 17. Mary Ann Liebert, Inc.; Larchmont, NY, USA: 2001. [DOI] [PubMed] [Google Scholar]
- 41.Dou X., Li Y., Han J., Zarlenga D.S., Zhu W., Ren X., Dong N., Li X., Li G. Cholesterol of lipid rafts is a key determinant for entry and post-entry control of porcine rotavirus infection. BMC Vet. Res. 2018;14:45. doi: 10.1186/s12917-018-1366-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Glende J., Schwegmann-Wessels C., Al-Falah M., Pfefferle S., Qu X., Deng H., Drosten C., Naim H.Y., Herrler G. Importance of cholesterol-rich membrane microdomains in the interaction of the S protein of SARS-coronavirus with the cellular receptor angiotensin-converting enzyme 2. Virology. 2008;381:215–221. doi: 10.1016/j.virol.2008.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Acton S., Rigotti A., Landschulz K.T., Xu S., Hobbs H.H., Krieger M. Identification of Scavenger Receptor SR-BI as a High Density Lipoprotein Receptor. Science. 1996;271:518–520. doi: 10.1126/science.271.5248.518. [DOI] [PubMed] [Google Scholar]
- 44.Krieger M. Scavenger receptor class B type I is a multiligand HDL receptor that influences diverse physiologic systems. J. Clin. Investig. 2001;108:793–797. doi: 10.1172/JCI14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huang R., Silva R.A.G.D., Jerome W.G., Kontush A., Chapman M.J., Curtiss L.K., Hodges T.J., Davidson W.S. Apolipoprotein A-I structural organization in high-density lipoproteins isolated from human plasma. Nat. Struct. Mol. Biol. 2011;18:416–422. doi: 10.1038/nsmb.2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jumper J., Evans R., Pritzel A., Green T., Figurnov M., Ronneberger O., Tunyasuvunakool K., Bates R., Žídek A., Potapenko A., et al. Highly accurate protein structure prediction with AlphaFold. Nature. 2021;596:583–589. doi: 10.1038/s41586-021-03819-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Neculai D., Schwake M., Ravichandran M., Zunke F., Collins R.F., Peters J., Neculai M., Plumb J., Loppnau P., Pizarro J.C., et al. Structure of LIMP-2 provides functional insights with implications for SR-BI and CD36. Nature. 2013;504:172–176. doi: 10.1038/nature12684. [DOI] [PubMed] [Google Scholar]
- 48.Zannis V.I., Chroni A., Krieger M. Role of apoA-I, ABCA1, LCAT, and SR-BI in the biogenesis of HDL. J. Mol. Med. 2006;84:276–294. doi: 10.1007/s00109-005-0030-4. [DOI] [PubMed] [Google Scholar]
- 49.Rothblat G.H., Phillips M.C. High-density lipoprotein heterogeneity and function in reverse cholesterol transport. Curr. Opin. Lipidol. 2010;21:229–238. doi: 10.1097/MOL.0b013e328338472d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Favari E., Chroni A., Tietge U.J.F., Zanotti I., Escolà-Gil J.C., Bernini F. Cholesterol efflux and reverse cholesterol transport. Handb. Exp. Pharmacol. 2015;224:181–206. doi: 10.1007/978-3-319-09665-0_4. [DOI] [PubMed] [Google Scholar]
- 51.Lewis G.F., Rader D.J. New insights into the regulation of HDL metabolism and reverse cholesterol transport. Circ. Res. 2005;96:1221–1232. doi: 10.1161/01.RES.0000170946.56981.5c. [DOI] [PubMed] [Google Scholar]
- 52.Trigatti B., Rayburn H., Viñals M., Braun A., Miettinen H., Penman M., Hertz M., Schrenzel M., Amigo L., Rigotti A., et al. Influence of the high density lipoprotein receptor SR-BI on reproductive and cardiovascular pathophysiology. Proc. Natl. Acad. Sci. USA. 1999;96:9322–9327. doi: 10.1073/pnas.96.16.9322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Miettinen H.E., Rayburn H., Krieger M. Abnormal lipoprotein metabolism and reversible female infertility in HDL receptor (SR-BI)-deficient mice. J. Clin. Investig. 2001;108:1717–1722. doi: 10.1172/JCI13288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Braun A., Zhang S., Miettinen H.E., Ebrahim S., Holm T.M., Vasile E., Post M.J., Yoerger D.M., Picard M.H., Krieger J.L., et al. Probucol prevents early coronary heart disease and death in the high-density lipoprotein receptor SR-BI/apolipoprotein E double knockout mouse. Proc. Natl. Acad. Sci. USA. 2003;100:7283–7288. doi: 10.1073/pnas.1237725100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Van Eck M., Bos I.S.T., Hildebrand R.B., Van Rij B.T., Van Berkel T.J.C. Dual role for scavenger receptor class B, type I on bone marrow-derived cells in atherosclerotic lesion development. Am. J. Pathol. 2004;165:785–794. doi: 10.1016/S0002-9440(10)63341-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rigotti A., Trigatti B., Babitt J., Penman M., Xu S., Krieger M. Scavenger receptor BI-A cell surface receptor for high density lipoprotein. Curr. Opin. Lipidol. 1997;8:181–188. doi: 10.1097/00041433-199706000-00009. [DOI] [PubMed] [Google Scholar]
- 57.Koenig S.N., Sucharski H.C., Jose E., Dudley E.K., Madiai F., Cavus O., Argall A.D., Williams J., Murphy N.P., Keith C.B., et al. Inherited Variants in SCARB1 Cause Severe Early-Onset Coronary Artery Disease. Circ. Res. 2021;129:296–307. doi: 10.1161/CIRCRESAHA.120.318793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang S., Picard M.H., Vasile E., Zhu Y., Raffai R.L., Weisgraber K.H., Krieger M. Diet-induced occlusive coronary atherosclerosis, myocardial infarction, cardiac dysfunction, and premature death in scavenger receptor class B type I-deficient, hypomorphic apolipoprotein ER61 mice. Circulation. 2005;111:3457–3464. doi: 10.1161/CIRCULATIONAHA.104.523563. [DOI] [PubMed] [Google Scholar]
- 59.Fuller M., Dadoo O., Serkis V., Abutouk D., MacDonald M., Dhingani N., Macri J., Igdoura S.A., Trigatti B.L. The effects of diet on occlusive coronary artery atherosclerosis and myocardial infarction in scavenger receptor class B, type 1/low-density lipoprotein receptor double knockout mice. Arterioscler. Thromb. Vasc. Biol. 2014;34:2394–2403. doi: 10.1161/ATVBAHA.114.304200. [DOI] [PubMed] [Google Scholar]
- 60.Tong Y., Zhu Y., Xia X., Liu Y., Feng Y., Hua X., Chen Z., Ding H., Gao L., Wang Y., et al. Tupaia CD81, SR-BI, Claudin-1, and Occludin Support Hepatitis C Virus Infection. J. Virol. 2011;85:2793–2802. doi: 10.1128/JVI.01818-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Burlone M.E., Budkowska A. Hepatitis C virus cell entry: Role of lipoproteins and cellular receptors. J. Gen. Virol. 2009;90:1055–1070. doi: 10.1099/vir.0.008300-0. [DOI] [PubMed] [Google Scholar]
- 62.Li Y., Kakinami C., Li Q., Yang B., Li H. Human Apolipoprotein A-I Is Associated with Dengue Virus and Enhances Virus Infection through SR-BI. PLoS ONE. 2013;8:e0070390. doi: 10.1371/journal.pone.0070390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Voisset C., Callens N., Blanchard E., Op De Beeck A., Dubuisson J., Vu-Dac N. High density lipoproteins facilitate hepatitis C virus entry through the scavenger receptor class B type I. J. Biol. Chem. 2005;280:7793–7799. doi: 10.1074/jbc.M411600200. [DOI] [PubMed] [Google Scholar]
- 64.Von Hahn T., Lindenbach B.D., Boullier A., Quehenberger O., Paulson M., Rice C.M., McKeating J.A. Oxidized low-density lipoprotein inhibits hepatitis C virus cell entry in human hepatoma cells. Hepatology. 2006;43:932–942. doi: 10.1002/hep.21139. [DOI] [PubMed] [Google Scholar]
- 65.Kocher O., Birrane G., Tsukamoto K., Fenske S., Yesilaltay A., Pal R., Daniels K., Ladias J.A.A., Krieger M. In vitro and in vivo analysis of the binding of the C terminus of the HDL receptor scavenger receptor class B, type I (SR-BI), to the PDZ1 domain of its adaptor protein PDZK1. J. Biol. Chem. 2010;285:34999–35010. doi: 10.1074/jbc.M110.164418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Eyre N.S., Drummer H.E., Beard M.R. The SR-BI Partner PDZK1 Facilitates Hepatitis C Virus Entry. PLoS Pathog. 2010;6:e1001130. doi: 10.1371/journal.ppat.1001130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kocher O., Yesilaltay A., Cirovic C., Pal R., Rigotti A., Krieger M. Targeted Disruption of the PDZK1 Gene in Mice Causes Tissue-specific Depletion of the High Density Lipoprotein Receptor Scavenger Receptor Class B Type I and Altered Lipoprotein Metabolism. J. Biol. Chem. 2003;278:52820–52825. doi: 10.1074/jbc.M310482200. [DOI] [PubMed] [Google Scholar]
- 68.Dreux M., Pietschmann T., Granier C., Voisset C., Ricard-Blum S., Mangeot P.E., Keck Z., Foung S., Vu-Dac N., Dubuisson J., et al. High density lipoprotein inhibits hepatitis C virus-neutralizing antibodies by stimulating cell entry via activation of the scavenger receptor BI. J. Biol. Chem. 2006;281:18285–18295. doi: 10.1074/jbc.M602706200. [DOI] [PubMed] [Google Scholar]
- 69.Voisset C., Op de Beeck A., Horellou P., Dreux M., Gustot T., Duverlie G., Cosset F.L., Vu-Dac N., Dubuisson J. High-density lipoproteins reduce the neutralizing effect of hepatitis C virus (HCV)-infected patient antibodies by promoting HCV entry. J. Gen. Virol. 2006;87:2577–2581. doi: 10.1099/vir.0.81932-0. [DOI] [PubMed] [Google Scholar]
- 70.Eieenberg S. High density lipoprotein metabolism. J. Lipids Res. 1984;10:1017–1058. doi: 10.1016/S0022-2275(20)37713-0. [DOI] [PubMed] [Google Scholar]
- 71.Lundberg J., Rudling M., Angelin B. Interstitial fluid lipoproteins. Curr. Opin. Lipidol. 2013;24:327–331. doi: 10.1097/MOL.0b013e3283630846. [DOI] [PubMed] [Google Scholar]
- 72.Cho K.-H. Importance of Apolipoprotein A-I and A-II Composition in HDL and Its Potential for Studying COVID-19 and SARS-CoV-2. Medicines. 2021;8:38. doi: 10.3390/medicines8070038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Babitt J., Trigatti B., Rigotti A., Smart E.J., Anderson R.G.W., Xu S., Krieger M. Murine SR-BI, a high density lipoprotein receptor that mediates selective lipid uptake, is N-glycosylated and fatty acylated and colocalizes with plasma membrane caveolae. J. Biol. Chem. 1997;272:13242–13249. doi: 10.1074/jbc.272.20.13242. [DOI] [PubMed] [Google Scholar]
- 74.De La Llera-Moya M., Rothblat G.H., Connelly M.A., Kellner-Weibel G., Sakr S.W., Phillips M.C., Williams D.L. Scavenger receptor BI (SR-BI) mediates free cholesterol flux independently of HDL tethering to the cell surface. J. Lipid Res. 1999;40:575–580. doi: 10.1016/S0022-2275(20)32462-7. [DOI] [PubMed] [Google Scholar]
- 75.Papale G.A., Nicholson K., Hanson P.J., Pavlovic M., Drover V.A., Sahoo D. Extracellular hydrophobic regions in scavenger receptor BI play a key role in mediating HDL-cholesterol transport. Arch. Biochem. Biophys. 2010;496:132–139. doi: 10.1016/j.abb.2010.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Papale G.A., Hanson P.J., Sahoo D. Extracellular disulfide bonds support scavenger receptor-BI-mediated cholesterol transport. Biochemistry. 2011;50:6245. doi: 10.1021/bi2005625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ji Y., Jian B., Wang N., Sun Y., De La Llera Moya M., Phillips M.C., Rothblat G.H., Swaney J.B., Tall A.R. Scavenger receptor BI promotes high density lipoprotein-mediated cellular cholesterol efflux. J. Biol. Chem. 1997;272:20982–20985. doi: 10.1074/jbc.272.34.20982. [DOI] [PubMed] [Google Scholar]
- 78.Nieland T.J.F., Penman M., Dori L., Krieger M., Kirchhausen T. Discovery of chemical inhibitors of the selective transfer of lipids mediated by the HDL receptor SR-BI. Proc. Natl. Acad. Sci. USA. 2002;99:15422–15427. doi: 10.1073/pnas.222421399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bajimaya S., Frankl T., Hayashi T., Takimoto T. Cholesterol is required for stability and infectivity of influenza A and respiratory syncytial viruses. Virology. 2017;510:234–241. doi: 10.1016/j.virol.2017.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Li X., Zhu W., Fan M., Zhang J., Peng Y., Huang F., Wang N., He L., Zhang L., Holmdahl R., et al. Dependence of SARS-CoV-2 infection on cholesterol-rich lipid raft and endosomal acidification. Comput. Struct. Biotechnol. J. 2021;19:1933–1943. doi: 10.1016/j.csbj.2021.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kontush A. HDL particle number and size as predictors of cardiovascular disease. Front. Pharmacol. 2015;6:218. doi: 10.3389/fphar.2015.00218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Laue M., Kauter A., Hoffmann T., Möller L., Michel J., Nitsche A. Morphometry of SARS-CoV and SARS-CoV-2 particles in ultrathin plastic sections of infected Vero cell cultures. Sci. Rep. 2021;11:3515. doi: 10.1038/s41598-021-82852-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zheng Y.H., Plemenitas A., Fielding C.J., Peterlin B.M. Nef increases the synthesis of and transports cholesterol to lipid rafts and HIV-1 progeny virions. Proc. Natl. Acad. Sci. USA. 2003;100:8460–8465. doi: 10.1073/pnas.1437453100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nguyen D.H., K Hildreth J.E. Evidence for Budding of Human Immunodeficiency Virus Type 1 Selectively from Glycolipid-Enriched Membrane Lipid Rafts. Journal of Virology. 2000;74:3264–3272. doi: 10.1128/JVI.74.7.3264-3272.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ono A., Freed E.O. Plasma membrane rafts play a critical role in HIV-1 assembly and release. Proc. Natl. Acad. Sci. USA. 2001;98:13925–13930. doi: 10.1073/pnas.241320298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cuadras M.A., Greenberg H.B. Rotavirus infectious particles use lipid rafts during replication for transport to the cell surface in vitro and in vivo. Virology. 2003;313:308–321. doi: 10.1016/S0042-6822(03)00326-X. [DOI] [PubMed] [Google Scholar]
- 87.Guyader M., Kiyokawa E., Abrami L., Turelli P., Trono D. Role for Human Immunodeficiency Virus Type 1 Membrane Cholesterol in Viral Internalization. J. Virol. 2002;76:10356–10364. doi: 10.1128/JVI.76.20.10356-10364.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Domanska M.K., Dunning R.A., Dryden K.A., Zawada K.E., Yeager M., Kasson P.M. Hemagglutinin Spatial Distribution Shifts in Response to Cholesterol in the Influenza Viral Envelope. Biophys. J. 2015;109:1917–1924. doi: 10.1016/j.bpj.2015.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Davidson W.S., Silva R.A.G.D., Chantepie S., Lagor W.R., Chapman M.J., Kontush A. Proteomic analysis of defined hdl subpopulations reveals particle-specific protein clusters: Relevance to antioxidative function. Arterioscler. Thromb. Vasc. Biol. 2009;29:870–876. doi: 10.1161/ATVBAHA.109.186031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Havel R.J., Eder H.A., Bragdon J.H. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J. Clin. Investig. 1955;34:1345–1353. doi: 10.1172/JCI103182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Vaisar T., Pennathur S., Green P.S., Gharib S.A., Hoofnagle A.N., Cheung M.C., Byun J., Vuletic S., Kassim S., Singh P., et al. Shotgun proteomics implicates protease inhibition and complement activation in the antiinflammatory properties of HDL. J. Clin. Investig. 2007;117:746–756. doi: 10.1172/JCI26206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gordon S.M., Deng J., Tomann A.B., Shah A.S., Lu L.J., Davidson W.S. Multi-dimensional co-separation analysis reveals protein-protein interactions defining plasma lipoprotein subspecies. Mol. Cell. Proteom. 2013;12:3123–3134. doi: 10.1074/mcp.M113.028134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Riwanto M., Rohrer L., Roschitzki B., Besler C., Mocharla P., Mueller M., Perisa D., Heinrich K., Altwegg L., Von Eckardstein A., et al. Altered activation of endothelial anti-and proapoptotic pathways by high-density lipoprotein from patients with coronary artery disease: Role of high-density lipoprotein-proteome remodeling. Circulation. 2013;127:891–904. doi: 10.1161/CIRCULATIONAHA.112.108753. [DOI] [PubMed] [Google Scholar]
- 94.Heinecke J.W. HDL’s protein cargo: Friend or foe in cardioprotection? Circulation. 2013;127:868–869. doi: 10.1161/CIRCULATIONAHA.112.000889. [DOI] [PubMed] [Google Scholar]
- 95.HDL Proteome Watch Page. [(accessed on 11 August 2021)]. Available online: https://homepages.uc.edu/~davidswm/HDLproteome.html.
- 96.Rye K.A., Barter P.J. Thematic review series: High density lipoprotein structure, function, and metabolism cardioprotective functions of HDLs 1. J. Lipid Res. 2014;55:168–179. doi: 10.1194/jlr.R039297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ohashi R., Mu H., Wang X., Yao Q., Chen C. Reverse cholesterol transport and cholesterol efflux in atherosclerosis. QJM-Mon. J. Assoc. Phys. 2005;98:845–856. doi: 10.1093/qjmed/hci136. [DOI] [PubMed] [Google Scholar]
- 98.Kontush A., Lhomme M., Chapman M.J. Thematic review series: High density lipoprotein structure, function, and metabolism: Unraveling the complexities of the HDL lipidome. J. Lipid Res. 2013;54:2950–2963. doi: 10.1194/jlr.R036095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mardones P., Strobel P., Miranda S., Leighton F., Quiñones V., Amigo L., Rozowski J., Krieger M., Rigotti A. α-tocopherol metabolism is abnormal in scavenger receptor class B type I (SR-BI)-deficient mice. J. Nutr. 2002;132:443–449. doi: 10.1093/jn/132.3.443. [DOI] [PubMed] [Google Scholar]
- 100.Michell D.L., Vickers K.C. Lipoprotein carriers of microRNAs. Biochim. Biophys. Acta. 2016;1861:2069. doi: 10.1016/j.bbalip.2016.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Shah A.S., Tan L., Long J.L., Davidson W.S. Proteomic diversity of high density lipoproteins: Our emerging understanding of its importance in lipid transport and beyond. J. Lipid Res. 2013;54:2575–2585. doi: 10.1194/jlr.R035725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rader D.J. Molecular regulation of HDL metabolism and function: Implications for novel therapies. J. Clin. Investig. 2006;116:3090–3100. doi: 10.1172/JCI30163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Brooks-Wilson A., Marcil M., Clee S.M., Zhang L.H., Roomp K., Van Dam M., Yu L., Brewer C., Collins J.A., Molhuizen H.O.F., et al. Mutations in ABC1 in Tangier disease and familial high-density lipoprotein deficiency. Nat. Genet. 1999;22:336–345. doi: 10.1038/11905. [DOI] [PubMed] [Google Scholar]
- 104.Bodzioch M., Orsó E., Klucken J., Langmann T., Böttcher A., Diederich W., Drobnik W., Barlage S., Büchler C., Porsch-Özcürümez M., et al. The gene encoding ATP-binding cassette transporter I is mutated in Tangier disease. Nat. Genet. 1999;22:347–351. doi: 10.1038/11914. [DOI] [PubMed] [Google Scholar]
- 105.Zannis V.I., Fotakis P., Koukos G., Kardassis D., Ehnholm C., Jauhiainen M., Chroni A. Proceedings of the Handbook of Experimental Pharmacology. Volume 224. Springer; New York, NY, USA: 2015. Hdl biogenesis, remodeling, and catabolism; pp. 53–111. [DOI] [PubMed] [Google Scholar]
- 106.Ahsan L., Ossoli A.F., Freeman L., Vaisman B., Amar M.J., Shamburek R.D., Remaley A.T. The HDL Handbook: Biological Functions and Clinical Implications. 2nd ed. Elsevier Inc.; Amsterdam, The Netherlands: 2013. Role of Lecithin: Cholesterol Acyltransferase in HDL Metabolism and Atherosclerosis; pp. 159–194. [Google Scholar]
- 107.Thacker S.G., Rousset X., Esmail S., Zarzour A., Jin X., Collins H.L., Sampson M., Stonik J., Demosky S., Malide D.A., et al. Increased plasma cholesterol esterification by LCAT reduces diet-induced atherosclerosis in SR-BI knockout mice. J. Lipid Res. 2015;56:1282–1295. doi: 10.1194/jlr.M048629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Desforges J.F., Gordon D.J., Rifkind B.M. High-Density Lipoprotein—The Clinical Implications of Recent Studies. N. Engl. J. Med. 1989;321:1311–1316. doi: 10.1056/NEJM198911093211907. [DOI] [PubMed] [Google Scholar]
- 109.Glass C., Pittman R.C., Weinstein D.B., Steinberg D. Dissociation of tissue uptake of cholesterol ester from that of apoprotein A-I of rat plasma high density lipoprotein: Selective delivery of cholesterol ester to liver, adrenal, and gonad. Proc. Natl. Acad. Sci. USA. 1983;80:5435–5439. doi: 10.1073/pnas.80.17.5435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Barter P.J., Nicholls S., Rye K.A., Anantharamaiah G.M., Navab M., Fogelman A.M. Antiinflammatory properties of HDL. Circ. Res. 2004;95:764–772. doi: 10.1161/01.RES.0000146094.59640.13. [DOI] [PubMed] [Google Scholar]
- 111.Navab M., Hama S.Y., Cooke C.J., Anantharamaiah G.M., Chaddha M., Jin L., Subbanagounder G., Faull K.F., Reddy S.T., Miller N.E., et al. Normal high density lipoprotein inhibits three steps in the formation of mildly oxidized low density lipoprotein: Step 1. J. Lipid Res. 2000;41:1481–1494. doi: 10.1016/S0022-2275(20)33461-1. [DOI] [PubMed] [Google Scholar]
- 112.Murphy A.J., Woollard K.J. High-density lipoprotein: A potent inhibitor of inflammation: Frontiers in research review: Physiological and pathological functions of high-density lipoprotein. Clin. Exp. Pharmacol. Physiol. 2010;37:710–718. doi: 10.1111/j.1440-1681.2009.05338.x. [DOI] [PubMed] [Google Scholar]
- 113.Urundhati A., Huang Y., Lupica J.A., Smith J.D., DiDonato J.A., Hazen S.L. Modification of high density lipoprotein by myeloperoxidase generates a pro-inflammatory particle. J. Biol. Chem. 2009;284:30825–30835. doi: 10.1074/jbc.M109.047605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gonzalez L., Qian A.S., Tahir U., Yu P., Trigatti B.L. Sphingosine-1-phosphate receptor 1, expressed in myeloid cells, slows diet-induced atherosclerosis and protects against macrophage apoptosis in ldlr KO mice. Int. J. Mol. Sci. 2017;18:2721. doi: 10.3390/ijms18122721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yu P., Qian A.S., Chathely K.M., Trigatti B.L. PDZK1 in leukocytes protects against cellular apoptosis and necrotic core development in atherosclerotic plaques in high fat diet fed ldl receptor deficient mice. Atherosclerosis. 2018;276:171–181. doi: 10.1016/j.atherosclerosis.2018.05.009. [DOI] [PubMed] [Google Scholar]
- 116.Durham K.K., Chathely K.M., Mak K.C., Momen A., Thomas C.T., Zhao Y.-Y., MacDonald M.E., Curtis J.M., Husain M., Trigatti B.L. HDL protects against doxorubicin-induced cardiotoxicity in a scavenger receptor class B type 1-, PI3K-, and Akt-dependent manner. Am. J. Physiol. Circ. Physiol. 2018;314:H31–H44. doi: 10.1152/ajpheart.00521.2016. [DOI] [PubMed] [Google Scholar]
- 117.Durham K.K., Kluck G., Mak K.C., Deng Y.D., Trigatti B.L. Treatment with apolipoprotein A1 protects mice against doxorubicin-induced cardiotoxicity in a scavenger receptor class B, type I-dependent manner. Am. J. Physiol. Circ. Physiol. 2019;316:H1447–H1457. doi: 10.1152/ajpheart.00432.2018. [DOI] [PubMed] [Google Scholar]
- 118.Ruiz M., Okada H., Dahlbäck B. HDL-associated ApoM is anti-apoptotic by delivering sphingosine 1-phosphate to S1P1 & S1P3 receptors on vascular endothelium. Lipids Health Dis. 2017;16 doi: 10.1186/S12944-017-0429-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Nofer J.-R., Levkau B., Wolinska I., Junker R., Fobker M., von Eckardstein A., Seedorf U., Assmann G. Suppression of Endothelial Cell Apoptosis by High Density Lipoproteins (HDL) and HDL-associated Lysosphingolipids*. J. Biol. Chem. 2001;276:34480–34485. doi: 10.1074/jbc.M103782200. [DOI] [PubMed] [Google Scholar]
- 120.Feuerborn R., Becker S., Potì F., Nagel P., Brodde M., Schmidt H., Christoffersen C., Ceglarek U., Burkhardt R., Nofer J.R. High density lipoprotein (HDL)-associated sphingosine 1-phosphate (S1P) inhibits macrophage apoptosis by stimulating STAT3 activity and survivin expression. Atherosclerosis. 2017;257:29–37. doi: 10.1016/j.atherosclerosis.2016.12.009. [DOI] [PubMed] [Google Scholar]
- 121.Terasaka N., Yu S., Yvan-Charvet L., Wang N., Mzhavia N., Langlois R., Pagler T., Li R., Welch C.L., Goldberg I.J., et al. ABCG1 and HDL protect against endothelial dysfunction in mice fed a high-cholesterol diet. J. Clin. Investig. 2008;118:3701–3713. doi: 10.1172/JCI35470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Terasaka N., Wang N., Yvan-Charvet L., Tall A.R. High-density lipoprotein protects macrophages from oxidized low-density lipoprotein-induced apoptosis by promoting efflux of 7-ketocholesterol via ABCG1. Proc. Natl. Acad. Sci. USA. 2007;104:15093–15098. doi: 10.1073/pnas.0704602104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Cui D., Thorp E., Li Y., Wang N., Yvan-Charvet L., Tall A.R., Tabas I. Pivotal Advance: Macrophages become resistant to cholesterol-induced death after phagocytosis of apoptotic cells. J. Leukoc. Biol. 2007;82:1040–1050. doi: 10.1189/jlb.0307192. [DOI] [PubMed] [Google Scholar]
- 124.Talbot C.P.J., Plat J., Ritsch A., Mensink R.P. Determinants of cholesterol efflux capacity in humans. Prog. Lipid Res. 2018;69:21–32. doi: 10.1016/j.plipres.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 125.Niisuke K., Kuklenyik Z., Horvath K.V., Gardner M.S., Toth C.A., Asztalos B.F. Composition-function analysis of HDL subpopulations: Influence of lipid composition on particle functionality. J. Lipid Res. 2020;61:306–315. doi: 10.1194/jlr.RA119000258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wang N., Silver D.L., Thiele C., Tall A.R. ATP-binding Cassette Transporter A1 (ABCA1) Functions as a Cholesterol Efflux Regulatory Protein*. J. Biol. Chem. 2001;276:23742–23747. doi: 10.1074/jbc.M102348200. [DOI] [PubMed] [Google Scholar]
- 127.Kennedy M.A., Barrera G.C., Nakamura K., Baldán Á., Tarr P., Fishbein M.C., Frank J., Francone O.L., Edwards P.A. ABCG1 has a critical role in mediating cholesterol efflux to HDL and preventing cellular lipid accumulation. Cell Metab. 2005;1:121–131. doi: 10.1016/j.cmet.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 128.Zhu X., Owen J.S., Wilson M.D., Li H., Griffiths G.L., Thomas M.J., Hiltbold E.M., Fessler M.B., Parks J.S. Macrophage ABCA1 reduces MyD88-dependent Toll-like receptor trafficking to lipid rafts by reduction of lipid raft cholesterol. J. Lipid Res. 2010;51:3196. doi: 10.1194/jlr.M006486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zhu X., Lee J.Y., Timmins J.M., Brown J.M., Boudyguina E., Mulya A., Gebre A.K., Willingham M.C., Hiltbold E.M., Mishra N., et al. Increased cellular free cholesterol in macrophage-specific Abca1 knock-out mice enhances pro-inflammatory response of macrophages. J. Biol. Chem. 2008;283:22930–22941. doi: 10.1074/jbc.M801408200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Koseki M., Hirano K.I., Masuda D., Ikegami C., Tanaka M., Ota A., Sandoval J.C., Nakagawa-Toyama Y., Sato S.B., Kobayashi T., et al. Increased lipid rafts and accelerated lipopolysaccharide-induced tumor necrosis factor-α secretion in Abca1-deficient macrophages. J. Lipid Res. 2007;48:299–306. doi: 10.1194/jlr.M600428-JLR200. [DOI] [PubMed] [Google Scholar]
- 131.Baker P.W., Rye K.A., Gamble J.R., Vadas M.A., Barter P.J. Ability of reconstituted high density lipoproteins to inhibit cytokine- induced expression of vascular cell adhesion molecule-1 in human umbilical vein endothelial cells. J. Lipid Res. 1999;40:345–353. doi: 10.1016/S0022-2275(20)33375-7. [DOI] [PubMed] [Google Scholar]
- 132.Recalde D., Ostos M.A., Badell E., Garcia-Otin A.L., Pidoux J., Castro G., Zakin M.M., Scott-Algara D. Human Apolipoprotein A-IV Reduces Secretion of Proinflammatory Cytokines and Atherosclerotic Effects of a Chronic Infection Mimicked by Lipopolysaccharide. Arterioscler. Thromb. Vasc. Biol. 2004;24:756–761. doi: 10.1161/01.ATV.0000119353.03690.22. [DOI] [PubMed] [Google Scholar]
- 133.Christoffersen C., Obinata H., Kumaraswamy S.B., Galvani S., Ahnström J., Sevvana M., Egerer-Sieber C., Muller Y.A., Hla T., Nielsen L.B., et al. Endothelium-protective sphingosine-1-phosphate provided by HDL-associated apolipoprotein M. Proc. Natl. Acad. Sci. USA. 2011;108:9613–9618. doi: 10.1073/pnas.1103187108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Liu M., Allegood J., Zhu X., Seo J., Gebre A.K., Boudyguina E., Cheng D., Chuang C.C., Shelness G.S., Spiegel S., et al. Uncleaved ApoM Signal Peptide Is Required for Formation of Large ApoM/Sphingosine 1-Phosphate (S1P)-enriched HDL Particles. J. Biol. Chem. 2015;290:7861–7870. doi: 10.1074/jbc.M114.631101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hughes J.E., Srinivasan S., Lynch K.R., Proia R.L., Ferdek P., Hedrick C.C. Sphingosine-1-phosphate induces an antiinflammatory phenotype in macrophages. Circ. Res. 2008;102:950–958. doi: 10.1161/CIRCRESAHA.107.170779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Al-Jarallah A., Chen X., González L., Trigatti B.L. High Density Lipoprotein Stimulated Migration of Macrophages Depends on the Scavenger Receptor Class B, Type I, PDZK1 and Akt1 and Is Blocked by Sphingosine 1 Phosphate Receptor Antagonists. PLoS ONE. 2014;9:e106487. doi: 10.1371/journal.pone.0106487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Galvani S., Sanson M., Blaho V.A., Swendeman S.L., Conger H., Dahlbäck B., Kono M., Proia R.L., Smith J.D., Hla T. HDL-bound sphingosine 1-phosphate acts as a biased agonist for the endothelial cell receptor S1P1 to limit vascular inflammation. Sci. Signal. 2015;8:ra79. doi: 10.1126/scisignal.aaa2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Guo L., Ai J., Zheng Z., Howatt D.A., Daugherty A., Huang B., Li X.A. High density lipoprotein protects against polymicrobe-induced sepsis in mice. J. Biol. Chem. 2013;288:17947–17953. doi: 10.1074/jbc.M112.442699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Pajkrt D., Doran J.E., Koster F., Lerch P.G., Arnet B., Van Der Poll T., Ten Cate J.W., Van Deventer S.J.H. Antiinflammatory effects of reconstituted high-density lipoprotein during human endotoxemia. J. Exp. Med. 1996;184:1601–1608. doi: 10.1084/jem.184.5.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Gupta H., Dai L., Datta G., Garber D.W., Grenett H., Li Y., Mishra V., Palgunachari M.N., Handattu S., Gianturco S.H., et al. Inhibition of lipopolysaccharide-induced inflammatory responses by an apolipoprotein AI mimetic peptide. Circ. Res. 2005;97:236–243. doi: 10.1161/01.RES.0000176530.66400.48. [DOI] [PubMed] [Google Scholar]
- 141.Trinder M., Wang Y., Madsen C.M., Ponomarev T., Bohunek L., Daisely B.A., Kong H.J., Blauw L.L., Nordestgaard B.G., Tybjærg-Hansen A., et al. Inhibition of Cholesteryl Ester Transfer Protein Preserves High-Density Lipoprotein Cholesterol and Improves Survival in Sepsis. Circulation. 2021;143:921–934. doi: 10.1161/CIRCULATIONAHA.120.048568. [DOI] [PubMed] [Google Scholar]
- 142.Jomard A., Osto E. High Density Lipoproteins: Metabolism, Function, and Therapeutic Potential. Front. Cardiovasc. Med. 2020;7:39. doi: 10.3389/fcvm.2020.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kontush A., Therond P., Zerrad A., Couturier M., Négre-Salvayre A., De Souza J.A., Chantepie S., Chapman M.J. Preferential sphingosine-1-phosphate enrichment and sphingomyelin depletion are key features of small dense HDL3 particles: Relevance to antiapoptotic and antioxidative activities. Arterioscler. Thromb. Vasc. Biol. 2007;27:1843–1849. doi: 10.1161/ATVBAHA.107.145672. [DOI] [PubMed] [Google Scholar]
- 144.Panzenböck U., Stocker R. Formation of methionine sulfoxide-containing specific forms of oxidized high-density lipoproteins. Biochim. Biophys. Acta-Proteins Proteom. 2005;1703:171–181. doi: 10.1016/j.bbapap.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 145.Elsøe S., Ahnström J., Christoffersen C., Hoofnagle A.N., Plomgaard P., Heinecke J.W., Binder C.J., Björkbacka H., Dahlbäck B., Nielsen L.B. Apolipoprotein M binds oxidized phospholipids and increases the antioxidant effect of HDL. Atherosclerosis. 2012;221:91–97. doi: 10.1016/j.atherosclerosis.2011.11.031. [DOI] [PubMed] [Google Scholar]
- 146.Kontush A., Chapman M.J. Functionally defective high-density lipoprotein: A new therapeutic target at the crossroads of dyslipidemia, inflammation, and atherosclerosis. Pharmacol. Rev. 2006;58:342–374. doi: 10.1124/pr.58.3.1. [DOI] [PubMed] [Google Scholar]
- 147.Kontush A., Chapman M.J. Antiatherogenic small, dense HDL-Guardian angel of the arterial wall? Nat. Clin. Pract. Cardiovasc. Med. 2006;3:144–153. doi: 10.1038/ncpcardio0500. [DOI] [PubMed] [Google Scholar]
- 148.Farid A.S., Horii Y. Modulation of paraoxonases during infectious diseases and its potential impact on atherosclerosis. Lipids Health Dis. 2012;11:92. doi: 10.1186/1476-511X-11-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Manolescu B.N., Busu C., Badita D., Stanculescu R., Berteanu M. Paraoxonase 1-an Update of the Antioxidant Properties of High- Density Lipoproteins. Maedica. 2015;10:173–177. [PMC free article] [PubMed] [Google Scholar]
- 150.Ahmed Z., Ravandi A., Maguire G.F., Emili A., Draganov D., La Du B.N., Kuksis A., Connelly P.W. Multiple substrates for paraoxonase-1 during oxidation of phosphatidylcholine by peroxynitrite. Biochem. Biophys. Res. Commun. 2002;290:391–396. doi: 10.1006/bbrc.2001.6150. [DOI] [PubMed] [Google Scholar]
- 151.Shih D.M., Xia Y.R., Wang X.P., Miller E., Castellani L.W., Subbanagounder G., Cheroutre H., Faull K.F., Berliner J.A., Witztum J.L., et al. Combined serum paraoxonase knockout/apolipoprotein E knockout mice exhibit increased lipoprotein oxidation and atherosclerosis. J. Biol. Chem. 2000;275:17527–17535. doi: 10.1074/jbc.M910376199. [DOI] [PubMed] [Google Scholar]
- 152.Ng D.S., Chu T., Esposito B., Hui P., Connelly P.W., Gross P.L. Paraoxonase-1 deficiency in mice predisposes to vascular inflammation, oxidative stress, and thrombogenicity in the absence of hyperlipidemia. Cardiovasc. Pathol. 2008;17:226–232. doi: 10.1016/j.carpath.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 153.Huang Y., Wu Z., Riwanto M., Gao S., Levison B.S., Gu X., Fu X., Wagner M.A., Besler C., Gerstenecker G., et al. Myeloperoxidase, paraoxonase-1, and HDL form a functional ternary complex. J. Clin. Investig. 2013;123:3815–3828. doi: 10.1172/JCI67478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Goyal J., Wang K., Liu M., Subbaiah P.V. Novel function of lecithin-cholesterol acyltransferase: Hydrolysis of oxidized polar phospholipids generated during lipoprotein oxidation. J. Biol. Chem. 1997;272:16231–16239. doi: 10.1074/jbc.272.26.16231. [DOI] [PubMed] [Google Scholar]
- 155.McPherson P.A.C., Young I.S., McEneny J. A dual role for lecithin:cholesterol acyltransferase (EC 2.3.1.43) in lipoprotein oxidation. Free Radic. Biol. Med. 2007;43:1484–1493. doi: 10.1016/j.freeradbiomed.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 156.Holleboom A.G., Daniil G., Fu X., Zhang R., Hovingh G.K., Schimmel A.W., Kastelein J.J.P., Stroes E.S.G., Witztum J.L., Hutten B.A., et al. Lipid oxidation in carriers of lecithin: Cholesterol acyltransferase gene mutations. Arterioscler. Thromb. Vasc. Biol. 2012;32:3066–3075. doi: 10.1161/ATVBAHA.112.255711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Zerrad-Saadi A., Therond P., Chantepie S., Couturier M., Rye K.A., Chapman M.J., Kontush A. HDL3-mediated inactivation of LDL-associated phospholipid hydroperoxides is determined by the redox status of apolipoprotein A-I and HDL particle surface lipid rigidity: Relevance to inflammation and atherogenesis. Arterioscler. Thromb. Vasc. Biol. 2009;29:2169–2175. doi: 10.1161/ATVBAHA.109.194555. [DOI] [PubMed] [Google Scholar]
- 158.Goulinet S., Chapman M.J. Plasma LDL and HDL subspecies are heterogenous in particle content of tocopherols and oxygenated and hydrocarbon carotenoids: Relevance to oxidative resistance and atherogenesis. Arterioscler. Thromb. Vasc. Biol. 1997;17:786–796. doi: 10.1161/01.ATV.17.4.786. [DOI] [PubMed] [Google Scholar]
- 159.Alwaili K., Bailey D., Awan Z., Bailey S.D., Ruel I., Hafiane A., Krimbou L., Laboissiere S., Genest J. The HDL proteome in acute coronary syndromes shifts to an inflammatory profile. Biochim. Biophys. Acta-Mol. Cell Biol. Lipids. 2012;1821:405–415. doi: 10.1016/j.bbalip.2011.07.013. [DOI] [PubMed] [Google Scholar]
- 160.Clifton P.M., Mackinnon A.M., Barter P.J. Effects of serum amyloid A protein (SAA) on composition, size, and density of high density lipoproteins in subjects with myocardial infarction. J. Lipid Res. 1985;26:1389–1398. doi: 10.1016/S0022-2275(20)34244-9. [DOI] [PubMed] [Google Scholar]
- 161.Han C.Y., Tang C., Guevara M.E., Wei H., Wietecha T., Shao B., Subramanian S., Omer M., Wang S., O’Brien K.D., et al. Serum amyloid A impairs the antiinflammatory properties of HDL. J. Clin. Investig. 2016;126:266–281. doi: 10.1172/JCI83475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Stadler J.T., Marsche G. Obesity-Related Changes in High-Density Lipoprotein Metabolism and Function. Int. J. Mol. Sci. 2020;21:8985. doi: 10.3390/ijms21238985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Azmi S., Ferdousi M., Liu Y., Adam S., Siahmansur T., Ponirakis G., Marshall A., Petropoulos I.N., Ho J.H., Syed A.A., et al. The role of abnormalities of lipoproteins and HDL functionality in small fibre dysfunction in people with severe obesity. Sci. Rep. 2021;11:12573. doi: 10.1038/s41598-021-90346-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Han C.Y., Chiba T., Campbell J.S., Fausto N., Chaisson M., Orasanu G., Plutzky J., Chait A. Reciprocal and coordinate regulation of serum amyloid A versus apolipoprotein A-I and paraoxonase-1 by inflammation in murine hepatocytes. Arterioscler. Thromb. Vasc. Biol. 2006;26:1806–1813. doi: 10.1161/01.ATV.0000227472.70734.ad. [DOI] [PubMed] [Google Scholar]
- 165.Camarota L.M., Woollett L.A., Howles P.N. Reverse cholesterol transport is elevated in carboxyl ester lipase-knockout mice. FASEB J. 2011;25:1370–1377. doi: 10.1096/fj.10-169680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Srivastava R.A.K. Dysfunctional HDL in diabetes mellitus and its role in the pathogenesis of cardiovascular disease. Mol. Cell. Biochem. 2018;440:167–187. doi: 10.1007/s11010-017-3165-z. [DOI] [PubMed] [Google Scholar]
- 167.Farbstein D., Levy A.P. HDL dysfunction in diabetes: Causes and possible treatments. Expert Rev. Cardiovasc. Ther. 2012;10:353–361. doi: 10.1586/erc.11.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Curtiss L.K., Witztum J.L. Plasma apolipoproteins AI, AII, B, CI, and E are glucosylated in hyperglycemic diabetic subjects. Diabetes. 1985;34:452–461. doi: 10.2337/diab.34.5.452. [DOI] [PubMed] [Google Scholar]
- 169.Park K.H., Shin D.G., Kim J.R., Cho K.H. Senescence-Related Truncation and Multimerization of Apolipoprotein A-I in High-Density Lipoprotein With an Elevated Level of Advanced Glycated End Products and Cholesteryl Ester Transfer Activity. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2010;65A:600–610. doi: 10.1093/gerona/glq034. [DOI] [PubMed] [Google Scholar]
- 170.Nobecourt E., Davies M.J., Brown B.E., Curtiss L.K., Bonnet D.J., Charlton F., Januszewski A.S., Jenkins A.J., Barter P.J., Rye K.-A. The impact of glycation on apolipoprotein A-I structure and its ability to activate lecithin:cholesterol acyltransferase. Diabetologia. 2007;50:643–653. doi: 10.1007/s00125-006-0574-z. [DOI] [PubMed] [Google Scholar]
- 171.Kashyap S.R., Osme A., Ilchenko S., Golizeh M., Lee K., Wang S., Bena J., Previs S.F., Smith J.D., Kasumov T. Glycation Reduces the Stability of ApoAI and Increases HDL Dysfunction in Diet-Controlled Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2018;103:388–396. doi: 10.1210/jc.2017-01551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Pietzsch J., Julius U., Nitzsche S., Hanefeld M. In vivo evidence for increased apolipoprotein A-I catabolism in subjects with impaired glucose tolerance. Diabetes. 1998;47:1928–1934. doi: 10.2337/diabetes.47.12.1928. [DOI] [PubMed] [Google Scholar]
- 173.Liu D., Ji L., Zhang D., Tong X., Pan B., Liu P., Zhang Y., Huang Y., Su J., Willard B., et al. Nonenzymatic glycation of high-density lipoprotein impairs its anti-inflammatory effects in innate immunity. Diabetes. Metab. Res. Rev. 2012;28:186–195. doi: 10.1002/dmrr.1297. [DOI] [PubMed] [Google Scholar]
- 174.Kasumov T., Golizeh M., Kashyap S. Glycation and Deamidation Result in HDL Dysfunction in Patients with Type 2 Diabetes. Diabetes. 2018;67:330-OR. doi: 10.2337/db18-330-OR. [DOI] [Google Scholar]
- 175.Seres I., Paragh G., Deschene E., Fulop T., Khalil A. Study of factors influencing the decreased HDL associated PON1 activity with aging. Exp. Gerontol. 2004;39:59–66. doi: 10.1016/j.exger.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 176.Berrougui H., Isabelle M., Cloutier M., Grenier G., Khalil A. Age-related impairment of HDL-mediated cholesterol efflux. J. Lipid Res. 2007;48:328–336. doi: 10.1194/jlr.M600167-JLR200. [DOI] [PubMed] [Google Scholar]
- 177.Tracy R.P., Psaty B.M., Macy E., Bovill E.G., Cushman M., Cornell E.S., Kuller L.H. Lifetime smoking exposure affects the association of C-reactive protein with cardiovascular disease risk factors and subclinical disease in healthy elderly subjects. Arterioscler. Thromb. Vasc. Biol. 1997;17:2167–2176. doi: 10.1161/01.ATV.17.10.2167. [DOI] [PubMed] [Google Scholar]
- 178.Bergt C., Oram J.F., Heinecke J.W. Oxidized HDL: The paradox-idation of lipoproteins. Arterioscler. Thromb. Vasc. Biol. 2003;23:1488–1490. doi: 10.1161/01.ATV.0000090570.99836.9C. [DOI] [PubMed] [Google Scholar]
- 179.Li J., Zhou C., Xu H., Brook R.D., Liu S., Yi T., Wang Y., Feng B., Zhao M., Wang X., et al. Ambient air pollution is associated with HDL (High-Density Lipoprotein) dysfunction in healthy adults. Arterioscler. Thromb. Vasc. Biol. 2019;39:513–522. doi: 10.1161/ATVBAHA.118.311749. [DOI] [PubMed] [Google Scholar]
- 180.Shao B., Oda M.N., Oram J.F., Heinecke J.W. Myeloperoxidase: An inflammatory enzyme for generating dysfunctional high density lipoprotein. Curr. Opin. Cardiol. 2006;21:322–328. doi: 10.1097/01.hco.0000231402.87232.aa. [DOI] [PubMed] [Google Scholar]
- 181.Park K.-H., Shin D.-G., Cho K.-H. Dysfunctional Lipoproteins from Young Smokers Exacerbate Cellular Senescence and Atherogenesis with Smaller Particle Size and Severe Oxidation and Glycation. Toxicol. Sci. 2014;140:16–25. doi: 10.1093/toxsci/kfu076. [DOI] [PubMed] [Google Scholar]
- 182.Kim S.-M., Lim S.-M., Yoo J.-A., Woo M.-J., Cho K.-H. Consumption of high-dose vitamin C (1250 mg per day) enhances functional and structural properties of serum lipoprotein to improve anti-oxidant, anti-atherosclerotic, and anti-aging effects via regulation of anti-inflammatory microRNA. Food Funct. 2015;6:3604–3612. doi: 10.1039/C5FO00738K. [DOI] [PubMed] [Google Scholar]
- 183.Schofield J.D., Liu Y., Rao-Balakrishna P., Malik R.A., Soran H. Diabetes Dyslipidemia. Diabetes Ther. 2016;7:203. doi: 10.1007/s13300-016-0167-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.MR T. Diabetic dyslipidaemia: From basic research to clinical practice. Diabetologia. 2003;46:733–749. doi: 10.1007/S00125-003-1111-Y. [DOI] [PubMed] [Google Scholar]
- 185.Zhang Y., Yang H., Li S., Li W.-D., Wang J., Wang Y. Association analysis framework of genetic and exposure risks for COVID-19 in middle-aged and elderly adults. Mech. Ageing Dev. 2021;194:111433. doi: 10.1016/j.mad.2021.111433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Williamson E.J., Walker A.J., Bhaskaran K., Bacon S., Bates C., Morton C.E., Curtis H.J., Mehrkar A., Evans D., Inglesby P., et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584:430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Ho F.K., Celis-Morales C.A., Gray S.R., Katikireddi S.V., Niedzwiedz C.L., Hastie C., Ferguson L.D., Berry C., Mackay D.F., Gill J.M.R., et al. Modifiable and non-modifiable risk factors for COVID-19, and comparison to risk factors for influenza and pneumonia: Results from a UK Biobank prospective cohort study. BMJ Open. 2020;10:e040402. doi: 10.1136/bmjopen-2020-040402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Aung N., Khanji M.Y., Munroe P.B., Petersen S.E. Causal Inference for Genetic Obesity, Cardiometabolic Profile and COVID-19 Susceptibility: A Mendelian Randomization Study. Front. Genet. 2020;11:586308. doi: 10.3389/fgene.2020.586308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Scalsky R.J., Chen Y.-J., Desai K., O’Connell J.R., Perry J.A., Hong C.C. Baseline cardiometabolic profiles and SARS-CoV-2 infection in the UK Biobank. PLoS ONE. 2021;16:e0248602. doi: 10.1371/journal.pone.0248602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Lassale C., Hamer M., Hernáez Á., Gale C.R., Batty G.D. High density lipoprotein cholesterol and risk of subsequent COVID-19 hospitalisation: The UK Biobank study. medRxiv Prepr. Serv. Heal. Sci. 2021 doi: 10.1101/2021.01.20.21250152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Begue F., Tanaka S., Mouktadi Z., Rondeau P., Veeren B., Diotel N., Tran-Dinh A., Robert T., Vélia E., Mavingui P., et al. Altered high-density lipoprotein composition and functions during severe COVID-19. Sci. Rep. 2021;11:2291. doi: 10.1038/s41598-021-81638-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Shen B., Yi X., Sun Y., Bi X., Du J., Zhang C., Quan S., Zhang F., Sun R., Qian L., et al. Proteomic and Metabolomic Characterization of COVID-19 Patient Sera. Cell. 2020;182:59. doi: 10.1016/j.cell.2020.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Marfia G., Navone S., Guarnaccia L., Campanella R., Mondoni M., Locatelli M., Barassi A., Fontana L., Palumbo F., Garzia E., et al. Decreased serum level of sphingosine-1-phosphate: A novel predictor of clinical severity in COVID-19. EMBO Mol. Med. 2021;13:e13424. doi: 10.15252/emmm.202013424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Li H., Xiang X., Ren H., Xu L., Zhao L., Chen X., Long H., Wang Q., Wu Q. Serum Amyloid A is a biomarker of severe Coronavirus Disease and poor prognosis. J. Infect. 2020;80:646–655. doi: 10.1016/j.jinf.2020.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Varga Z., Flammer A.J., Steiger P., Haberecker M., Andermatt R., Zinkernagel A.S., Mehra M.R., Schuepbach R.A., Ruschitzka F., Moch H. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Magro C.M., Mulvey J., Kubiak J., Mikhail S., Suster D., Crowson A.N., Laurence J., Nuovo G. Severe COVID-19: A multifaceted viral vasculopathy syndrome. Ann. Diagn. Pathol. 2021;50:151645. doi: 10.1016/j.anndiagpath.2020.151645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Teuwen L.-A., Geldhof V., Pasut A., Carmeliet P. COVID-19: The vasculature unleashed. Nat. Rev. Immunol. 2020;20:389–391. doi: 10.1038/s41577-020-0343-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Hamming I., Timens W., Bulthuis M.L.C., Lely A.T., Navis G.J., van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 2004;203:631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Huertas A., Montani D., Savale L., Pichon J., Tu L., Parent F., Guignabert C., Humbert M. Endothelial cell dysfunction: A major player in SARS-CoV-2 infection (COVID-19)? Eur. Respir. J. 2020;56:2001634. doi: 10.1183/13993003.01634-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Nuovo G.J., Magro C., Shaffer T., Awad H., Suster D., Mikhail S., He B., Michaille J.-J., Liechty B., Tili E. Endothelial cell damage is the central part of COVID-19 and a mouse model induced by injection of the S1 subunit of the spike protein. Ann. Diagn. Pathol. 2021;51:151682. doi: 10.1016/j.anndiagpath.2020.151682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Lei Y., Zhang J., Schiavon C.R., He M., Chen L., Shen H., Zhang Y., Yin Q., Cho Y., Andrade L., et al. SARS-CoV-2 Spike Protein Impairs Endothelial Function via Downregulation of ACE 2. Circ. Res. 2021;128:1323–1326. doi: 10.1161/CIRCRESAHA.121.318902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Mandal S., Barnett J., Brill S.E., Brown J.S., Denneny E.K., Hare S.S., Heightman M., Hillman T.E., Jacob J., Jarvis H.C., et al. Long-COVID’: A cross-sectional study of persisting symptoms, biomarker and imaging abnormalities following hospitalisation for COVID-19. Thorax. 2021;76:396–398. doi: 10.1136/thoraxjnl-2020-215818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Evans N., Nichols J., Pruitt K., Almodovar S. “Lung Time No See”: SARS-Cov-2 Spike Protein Changes Genetic Expression in Human Primary Bronchial Epithelial Cells After Recovery. FASEB J. 2021;35 doi: 10.1096/fasebj.2021.35.S1.03097. [DOI] [Google Scholar]
- 204.Al-Jarallah A., Trigatti B.L. A role for the scavenger receptor, class B type I in high density lipoprotein dependent activation of cellular signaling pathways. Biochim. Biophys. Acta-Mol. Cell Biol. Lipids. 2010;1801:1239–1248. doi: 10.1016/j.bbalip.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 205.Mineo C., Shaul P.W. Novel biological functions of high-density lipoprotein cholesterol. Circ. Res. 2012;111:1079–1090. doi: 10.1161/CIRCRESAHA.111.258673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Robert J., Osto E., von Eckardstein A. The Endothelium Is Both a Target and a Barrier of HDL’s Protective Functions. Cells. 2021;10:1041. doi: 10.3390/cells10051041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Correa Y., Waldie S., Thépaut M., Micciula S., Moulin M., Fieschi F., Pichler H., Trevor Forsyth V., Haertlein M., Cárdenas M. SARS-CoV-2 spike protein removes lipids from model membranes and interferes with the capacity of high density lipoprotein to exchange lipids. J. Colloid Interface Sci. 2021;602:732–739. doi: 10.1016/j.jcis.2021.06.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Hardardóttir I., Moser A.H., Fuller J., Fielding C., Feingold K., Grünfeld C. Endotoxin and cytokines decrease serum levels and extra hepatic protein and mRNA levels of cholesteryl ester transfer protein in syrian hamsters. J. Clin. Investig. 1996;97:2585–2592. doi: 10.1172/JCI118707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Wurfel M.M., Kunitake S.T., Lichenstein H., Kane J.P., Wright S.D. Lipopolysaccharide (LPS)-binding protein is carried on lipoproteins and acts as a cofactor in the neutralization of LPS. J. Exp. Med. 1994;180:1025–1035. doi: 10.1084/jem.180.3.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Levels J.H.M., Marquart J.A., Abraham P.R., van den Ende A.E., Molhuizen H.O.F., van Deventer S.J.H., Meijers J.C.M. Lipopolysaccharide Is Transferred from High-Density to Low-Density Lipoproteins by Lipopolysaccharide-Binding Protein and Phospholipid Transfer Protein. Infect. Immun. 2005;73:2321–2326. doi: 10.1128/IAI.73.4.2321-2326.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]