Abstract
DNA damage and/or hyperproliferative signals activate the wild-type p53 tumor suppressor protein, which induces a G1 cell cycle arrest or apoptosis. Although the mechanism of p53-mediated cell cycle arrest is fairly well defined, the p53-dependent pathway regulating apoptosis is poorly understood. Here we report the functional characterization of murine ei24 (also known as PIG8), a gene directly regulated by p53, whose overexpression negatively controls cell growth and induces apoptotic cell death. Ectopic ei24 expression markedly inhibits cell colony formation, induces the morphological features of apoptosis, and reduces the number of β-galactosidase-marked cells, which is efficiently blocked by coexpression of Bcl-XL. The ei24/PIG8 gene is localized on human chromosome 11q23, a region frequently altered in human cancers. These results suggest that ei24 may play an important role in negative cell growth control by functioning as an apoptotic effector of p53 tumor suppressor activities.
Inactivation of the p53 tumor suppressor is the most common genetic alteration detected in human malignancies, occurring in more than 50% of all tumors. Genetically engineered mice that lack p53 expression invariably develop lethal tumors within 3 to 6 months of age, underscoring the importance of p53 in the prevention of cancer (9, 21). Wild-type p53 (wtp53) plays a key role in tumor suppression by monitoring DNA damage and executing pathways that negatively control cell growth, either by blocking cells in the G1 phase of the cell cycle or inducing apoptosis (for review, see reference 26). wtp53 regulates these processes, at least in part, by functioning as a transactivator of gene expression, and its activity as a tumor suppressor correlates with this function (32). wtp53 induces the expression of its target genes by binding to DNA in a sequence-specific fashion and by interacting with various components of the transcription complex. Some of the better-characterized target genes transcriptionally activated by p53 include p21cipl, gadd45, cyclin G, mdm2, bax, fas/APO1, and IGF-BP3 (24, 26).
A critical target that mediates wtp53-induced cell cycle arrest is p21Cip1, which is a cyclin-dependent kinase inhibitor (11, 14, 37). Overexpression of p21Cip1 in murine fibroblasts, like that of wtp53, inhibits cell growth in the G1 phase of the cell cycle (11). Consistent with this observation, fibroblasts that lack p21Cip1 fail to efficiently arrest in G1 phase in response to wtp53 during DNA damage (8). By contrast, the mechanism by which p53 induces apoptosis is poorly understood. Several p53-regulated target genes are bona fide proapoptotic factors, such as bax and fas/APO1 (29, 30). However, elimination of bax or fas/APO1 gene expression has no consequence for p53-induced cell death in gamma-irradiated murine thymocytes, suggesting that there are alternative mediators of p53-dependent apoptosis (23, 35).
ei24 is a DNA damage response gene originally isolated from NIH 3T3 fibroblasts that were undergoing etoposide-induced cell death (25). Since etoposide-induced apoptosis in these cells is p53 dependent and requires new RNA and protein synthesis, it was speculated that ei24 could be a p53-regulated gene. Indeed, subsequent studies with cells that conditionally express wtp53 or gamma irradiation of normal and p53−/− thymocytes established a clear correlation between p53 function and ei24 gene expression. Subsequently, the human ei24 gene, PIG8 (designated ei24/PIG8) was isolated by Polyak and coworkers from colon carcinoma cells undergoing apoptosis in response to the ectopic expression of wtp53 (33). In this study, serial analysis of gene expression of more than 7,200 mRNA transcripts revealed that PIG8 was one of only 14 identified genes that were induced in a p53-dependent manner (33).
Murine ei24 mRNA is 2.4 kb in length, polyadenylated, and widely expressed in many tissues to varying degrees (25). The Ei24/PIG8 protein is highly conserved between mouse and human and shares more than 50% similarity to an open reading frame in Caenorhabditis elegans. The deduced amino acid sequence yields little or no information about the possible function of this gene. Nonetheless, the fact that ei24 gene expression is rapidly induced as part of a p53-dependent pathway in DNA-damaged cells and that this response precedes or parallels the onset of apoptosis suggest that ei24 may play an important role in negatively controlling cell growth and/or tumor suppression (25, 33).
In the present study, we demonstrate that ei24 is an immediate-early p53 response gene and that overexpression of ei24 suppresses cell growth by inducing apoptotic cell death. The ei24/PIG8 gene is located on human chromosome 11q23 in a region that is frequently altered in several human malignancies. These findings indicate that ei24 may be a newly recognized tumor suppressor that plays an important role in the prevention of certain cancers.
MATERIALS AND METHODS
Cell lines and culture methods.
All cell lines were cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum, glutamine (2 mM), and penicillin (100 IU/ml)-streptomycin (100 μg/ml) at 37°C in 5% CO2 unless otherwise indicated. 2a39pBabePuro and 2a39p53ER cells are derived from p53-deficient mouse embryo fibroblasts and express E1A and T24 H-ras oncogenes (25, 36). The 2a39pBabePuro cells serve as a vector-only negative control, whereas 2a39p53ER fibroblasts express a conditionally active human wtp53 gene fused to a modified hormone-binding domain of the murine estrogen receptor (25, 36). Functional activation of the p53ER fusion protein was achieved by treating 2a39p53 ER cells with 3.3 μM tamoxifen (Sigma, St. Louis, Mo.), resulting in the induction of apoptosis in these cells (36). COS-7 is an African green monkey kidney cell line that expresses functionally inactive wtp53 due to the simian virus 40 large-T antigen oncogene (American Type Culture Collection, Manassas, Va.). (10)1 cells are immortal murine fibroblasts containing deletions in the endogenous p53 gene and are null for p53 expression (15). Rat1 fibroblasts express endogenous wtp53 and were used for the β-galactosidase (β-Gal) marker apoptosis assays, as previously described (6). The parental murine myeloid leukemia M1 cell line, which is null for endogenous p53, and the derivative M1tsp53 cell line that expresses the temperature-sensitive mutant p53 protein (A135V) were kindly provided by Dan Liebermann (Temple University, Philadelphia, Pa.) and were cultured in RPMI 1640 supplemented with 10% horse serum at 37°C in 5% CO2. M1tsp53 cells were cultured at 32.5°C to convert the temperature-sensitive mutant p53A135V into the wild-type conformation. Where indicated, cells were treated with 12.5 ng of recombinant IL-6 per ml (R & D Systems, Minneapolis, Minn.) to inhibit p53-mediated apoptosis. Cells were transfected with plasmid DNA by the lipofectin-mediated method as suggested by the manufacturer (Gibco BRL, Gaithersburg, Md.) or by the calcium-phosphate precipitation method (22).
Plasmid DNA.
The prkMei24 plasmid, which expresses a FLAG epitope-tagged version of murine ei24, was constructed by subcloning the 0.8-kb XbaI/NdeI and 0.47-kb NdeI/NotI ei24 cDNA fragments from pKSf1-clone 11 (25) into the prk5 vector. The FLAG epitope-tagged coding sequence was fused at the 3′ end of the murine ei24 cDNA by PCR with the following pair of primers: 5′-GCTTAGCAAAGTTGTGAATGCC-3′ (forward primer) and 5′-CGGCGGCCGCCTACTTGTCATCGTCGTCCTTGTAGTCATGGCCTGCAGCAGCTTTCAGTTTGGCAGGAGA-3′ (reverse primer). The prkHei24 plasmid expressing human ei24/Pig8 was constructed by subcloning the 1.3-kb NdeI/NotI cDNA fragment from PIG8 (33) and a 0.7-kb BamHI/NdeI fragment into prk5. The 0.7-kb BamHI/NdeI DNA fragment was created by reverse transcription-PCR with the following pair of primers: 5′-AAGAATTCATGGGGCAGGGCCGGAGCCG-3′ (forward primer) and 5′-GGACATATGCAGGAGGCTAACCAGCT-3′ (reverse primer). The template for this reaction was total RNA prepared from human myeloid leukemia ML-1 cells. The ei24 promoter-reporter plasmid (pGLKH) was constructed by inserting the proximal 3.5-kb KpnI/HindIII DNA fragment from the murine genomic BACM-83-M10 clone (Genome Systems, Inc., St. Louis, Mo.), which contains the promoter region and exon 1 of the murine ei24 gene, upstream of the luciferase reporter in the pGL2 vector (Promega Corp., Madison, Wis.).
Immunoblotting and immunoprecipitation.
Transiently transfected COS-7 cells that remained attached or were nonadherent were collected either separately or pooled and lysed in radioimmunoprecipitation assay (RIPA) buffer (50 mM Tris-HCl [pH 7.6], 150 mM NaCl, 1% Triton X-100, 0.1% sodium deoxycholate, and Protease Inhibitor Cocktail [Boehringer Mannheim, Indianapolis, Ind.]). The samples were analyzed for protein content by the Bradford method (Bio-Rad Laboratories, Hercules, Calif.), separated through a sodium dodecyl sulfate (SDS)–12% polyacrylamide gel and transferred to a PVDF-PLUS transfer membrane (Micron Separations, Inc., Westboro, Mass.). The membranes were probed with rabbit antiserum (Ab22), which is specific for the N-terminal murine Ei24 peptide (PQSVERKQESEPRIVS), and subsequently incubated with a horseradish peroxidase-monkey anti-rabbit IgG secondary antibody (Amersham, Arlington Heights, Il.). The samples were washed three times in TBST (10 mM Tris [pH 8], 44 mM NaCl, 0.05% Tween 20) (17) and analyzed with the SuperSignal Chemiluminescent Western blotting kit according to the manufacturer's protocol (Pierce, Rockland, Ill.). Immunoprecipitations (IP) were performed as previously described (17). Briefly, cells were lysed in RIPA buffer without SDS and the nonspecific adsorbents were removed by preincubating the cell extract with 40 μl of 50% protein A–Sepharose. The specific antibodies were added to the cell extract with 30 μl of 50% protein A–Sepharose in a final volume of 500 μl and incubated at 4°C for 2 h. The immunocomplexes were washed three times with RIPA buffer without SDS and resuspended in sample buffer for SDS-polyacrylamide gel electrophoresis analysis.
Gel mobility shift assays.
Electromobility shift assays (EMSA) were carried out as previously described (12). The synthetic double-stranded oligonucleotides used in this study included the following sequences: p53CON, 5′-AGGCATGCCTAGGCATGCCT-3′; p53RE, 5′-GGGCTGGCAGGCCGGAGCTAGTTCCTAA-3′; and p53MRE, 5′-GGGCTGGTAGTCCGGAGTTATTTCCTAA-3′. The probes were radiolabeled with [γ-32P]ATP and T4 polynucleotide kinase and incubated with 100 ng of baculovirus-expressed human p53 protein (>95% pure) alone or together with 2 μg of PAb421 (Oncogene Research Products, Cambridge, Mass.) in binding buffer containing 20 mM HEPES (pH 7.9), 25 mM KCl, 0.1 mM EDTA, 2 mM MgCl2, 0.5 mM dithiothreitol, 0.25% Nonidet P-40, 2 mM spermidine, 10% glycerol, 0.1 ng of bovine serum albumin and 0.04 μg of poly(dG-dC) in a final reaction volume of 20 μl at 22°C for 15 min. As previously described, the monoclonal PAb421 antibody is required to activate wtp53 DNA binding (19). The protein-DNA complexes were resolved in a native 4% polyacrylamide gel and analyzed by autoradiography.
Northern blot analyses.
Total RNA was harvested with the RNeasy Mini Kit as recommended by the manufacturer (Qiagen, Valencia, Calif.). The RNA samples (10 μg) were denatured in 1 M glyoxal–10 mM NaH2PO4 (pH 7.0) for 1 h at 50°C and resolved through a 1.2% agarose gel. The RNA samples were transferred to a Zeta-Probe blotting membrane (Bio-Rad) in transfer buffer containing 10 mM NaOH. The membrane was blocked in hybridization solution (1 mM EDTA, 0.25 M Na2HPO4 [pH 7.2], and 7% SDS) for 5 min and hybridized with α-32P-radiolabeled DNA probes for 16 h in fresh hybridization solution at 65°C. The membrane was washed twice at 65°C for 30 min per wash in buffer I (1 mM EDTA, 40 mM Na2HPO4 [pH 7.2], and 5% SDS) followed by two washes in buffer II (1 mM EDTA, 40 mM Na2HPO4 [pH 7.2], and 1% SDS). The samples were quantitated by PhosphorImager analysis with Imagequant software.
Microinjection.
Cells were microinjected with a Nikon Diaphot 300 inverted microscope with an Eppendorf pressure injector (model 5246) and micromanipulator (model 5171). Cells were plated on glass coverslips 16 h prior to injection in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum. The cells were injected with a green fluorescent protein (GFP) expression plasmid alone or with 10 pg of either prk5 vector only or ei24/PIG8 expression plasmid DNA. On average, 100 to 150 cells were injected per sample. Twenty-four hours after injection, cells expressing GFP were scored for morphological features of apoptosis and photographed to document phenotypic changes.
β-Gal marker apoptosis assay.
The effect of ei24 expression on cell viability was examined in transient transfection assays as previously described (6). Rat1 fibroblasts were plated in 24-well tissue culture dishes at 3.5 × 104 cells/well and transiently transfected by the Lipofectamine procedure (Gibco BRL) with a β-Gal plasmid to biochemically mark productively transfected cells. Cells were cotransfected in triplicate with the β-Gal marker and either prk5 vector only (Vector), prk5Mei24 (Mei24), pBabeBcl-XL (Bcl-XL), or Mei24 plus Bcl-XL (Mei24 + Bcl-XL) DNA. Transfections were balanced for equal amounts of total DNA. Twenty-four hours posttransfection, the cells were fixed and stained with X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) to detect β-Gal-expressing cells. Transfected cells were quantitated by counting the number of blue-stained cells within a defined area (five randomly selected microscopic fields). The experiment was repeated three times, once with independently prepared plasmid DNAs.
Fluorescence in situ hybridization.
A human ei24/PIG8 bacterial artificial chromosome (BAC) clone was labeled with digoxigenin-11-dUTP (Boehringer Mannheim) by nick translation and hybridized to normal metaphase chromosomes in a solution containing 50% formamide, 10% dextran sulfate, and 2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate). Specific hybridization signals were detected with fluorescein-labeled digoxigenin antibody (green) (Oncor, Inc., Gaithersburg, Md.). Definitive chromosomal assignment was confirmed by cohybridization of human ei24/PIG8 BAC clone with a biotinylated chromosome 11 centromere-specific probe, D11Z1 (red) (Oncor, Inc.). The labeled probes were hybridized to metaphase cells in a solution containing 60% formamide, 10% dextran sulfate, and 2× SSC. Specific probe signals were detected by incubating the slides in fluorescein-labeled anti-digoxigenin and Texas red avidin (Oncor, Inc.). The chromosomes were then stained with 4′,6-diamidino-2-phenylindole (DAPI) and photographed.
RESULTS
ei24 gene expression is specifically induced by p53.
Previous studies demonstrated that ei24 gene expression is induced in response to elevated levels of wtp53 in a variety of different cell-based assays, suggesting that ei24 is a p53-regulated target gene. However, it should be noted that in each case studied, the cells responded to the elevated levels of p53 by undergoing apoptosis (25, 33). This fact raised the possibility that ei24 is not a p53-regulated gene per se; rather, ei24 may be a sensor of cell death which is indirectly induced during apoptosis.
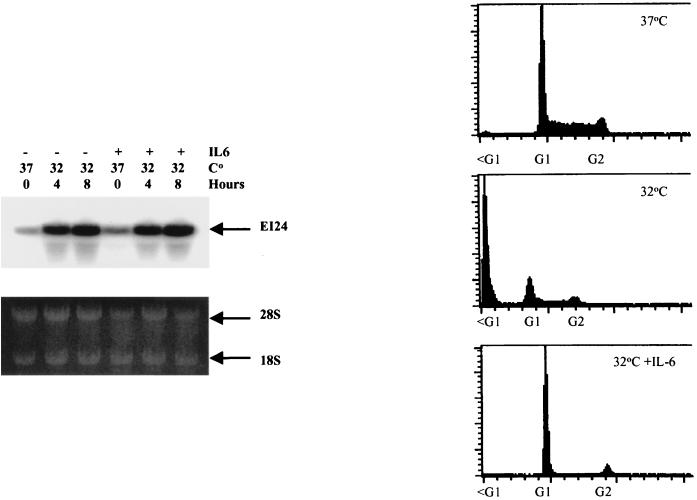
To directly address this issue, we employed the murine myeloid leukemia M1 cell line, which expresses the temperature-sensitive mutant p53-val135 (tsp53) gene (40). The M1tsp53 cells express high levels of p53 in the mutant conformation and actively proliferate when grown at 37°C. However, when the cells are shifted to 32.5°C, the tsp53 protein assumes a wild-type conformation, which rapidly induces cell death. Interestingly, the M1tsp53 cells are efficiently rescued from p53-mediated cell death at 32.5°C by the addition of interleukin-6 (IL-6) (40). We and others have recently demonstrated that certain cytokines, such as IL-6, inhibit p53-mediated cell death by blocking the p53 pathway at a point that is downstream from the regulation of its target genes (4, 34). This system therefore affords the opportunity to study the regulation of gene expression in response to wtp53 without inducing apoptosis. When M1tsp53 cells were cultured at 32.5°C, ei24 mRNA levels were markedly induced and this response was concomitant with a loss in cell viability (Fig. 1). Pretreatment of the M1tsp53 cells with IL-6 before shifting the temperature to 32.5°C completely blocked cell death but had no effect on the induction of ei24 gene expression (Fig. 1). Identical results were obtained when bax expression was examined under these conditions (data not shown). Therefore, ei24 gene expression directly responds to p53 and is not induced as a secondary response to p53-mediated cell death.
FIG. 1.
ei24 expression is selectively induced in response to wtp53. (Left) Murine M1 myeloid cells expressing a temperature-sensitive mutant p53 were grown at 37°C (mutant conformation) or shifted to 32.5°C (wtp53 conformation and function). In parallel and prior to the temperature shift, cells were pretreated with 12.5 ng of IL-6 per ml (treatment is indicated by a +), which blocks p53-mediated cell death and induces differentiation (40). At the indicated intervals, cells were harvested and analyzed for ei24 gene expression by Northern blotting. Ethidium bromide-stained RNA was photographed prior to transfer to confirm equal loading of samples (bottom). PhosphorImager analysis demonstrates the induction of steady-state levels of ei24 mRNA during activation of p53 at the permissive temperature (top). Cell viability was monitored by propidium iodide staining and fluorescence-activated cell sorter analysis (right). Cells grown at the permissive temperature undergo apoptosis in the absence of IL-6 and maintain viability while arresting in the G1 phase of the cell cycle in the presence of IL-6.
ei24 is a direct target for wild-type p53.
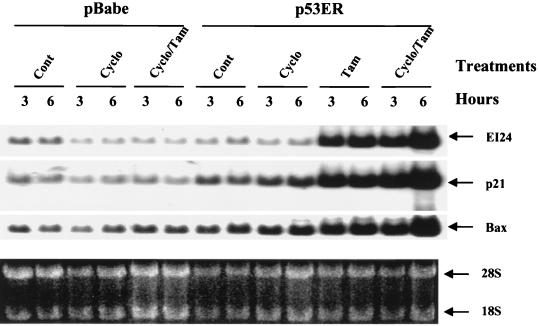
Although ei24 gene expression correlated with p53 levels and was not a by-product of cell death, it was not clear whether ei24 was an immediate- or delayed-early p53 response gene. For example, p53 could activate ei24 gene expression by inducing an intermediate factor that regulates ei24 expression. To address this mechanism, we utilized a p53−/− mouse embryo fibroblast cell line that constitutively expresses a p53 estrogen receptor (p53ER) fusion protein that is not active under normal growth conditions (25, 27). The p53ER fusion protein is functionally activated by the addition of tamoxifen (Tam), and this subsequently induces the expression of p53 target genes and apoptotic cell death (Fig. 2) (25, 36). To test the possibility that an intermediate factor may regulate ei24 expression, we pretreated cells with cycloheximide (10 μg/ml) for 45 min to inhibit de novo protein synthesis and then added 3.3 μM Tam for an additional 3 or 6 h to functionally activate p53ER. Preliminary characterization of the assay determined that protein synthesis was inhibited approximately 93% by cycloheximide within 45 min, as determined by 35S-methionine–cysteine incorporation (data not shown). We also considered the nature of the very short half-life of wtp53 (∼15 to 20 min), since protein synthesis was inhibited during the cycloheximide treatments. Stability measurements determined that the half-life of p53ER was greater than 5 h when activated by Tam (data not shown), thus ensuring the availability of p53ER at the later time points (3 and 6 h). Northern blot analysis of the vector-only control cells (pBabe) demonstrated that cycloheximide or cotreatment with cycloheximide and Tam had no significant effect on the expression of endogenous ei24 mRNA steady-state levels. By contrast, functional activation of wtp53 by Tam treatment of p53ER fibroblasts dramatically induced ei24 mRNA levels in either the presence or the absence of cycloheximide (Fig. 2). These data indicate that ei24 is an immediate-early-response gene that is directly regulated by wtp53.
FIG. 2.
ei24 is an immediate-early wtp53 response gene. Murine 2a39 fibroblast cell lines harboring either the pBabepuro-only vector (pBabe) or expressing the conditional wild-type p53ER fusion protein (p53ER) were cultured under normal growth conditions (Cont) or treated with either 10 μg of cycloheximide (Cyclo) per ml, 100 μM Tam, or cycloheximide plus Tam (Cyclo/Tam) for the indicated times. Cells that were treated with both cycloheximide and Tam were preincubated with cycloheximide for 45 min to efficiently inhibit protein synthesis prior to activation of p53ER with Tam. Total RNA was isolated and analyzed by Northern blotting for ei24, p21cip1, and bax expression (top). RNA was stained with ethidium bromide prior to transfer and photographed to document equal loading of RNA (bottom).
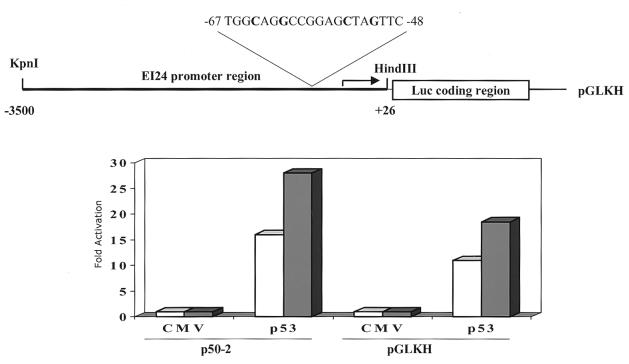
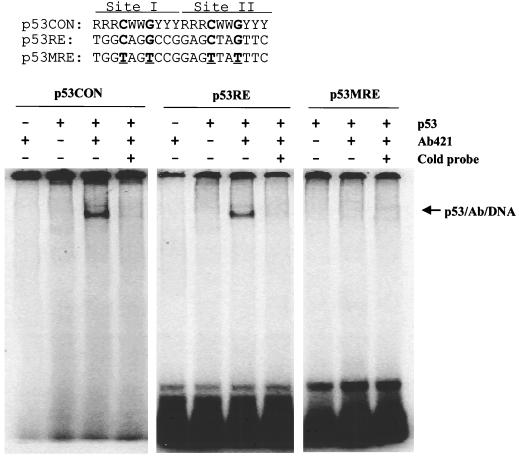
To confirm these findings, we isolated an ∼150-kb murine ei24 genomic BAC clone that contains the entire cDNA coding region. From this BAC clone, we isolated a 3.5-kb DNA fragment containing the promoter region and exon 1 and subcloned these sequences into a luciferase reporter plasmid (see Materials and Methods). Reporter activity was examined in transiently transfected (10)1 fibroblasts, which are devoid of endogenous p53 protein, thereby eliminating potential complications of trans-dominant effects (15, 41). Transient transfection of the ei24 luciferase reporter into p53-null cells resulted in a low but significant level of activity (∼30-fold higher than that of the promoterless reporter), demonstrating that the subcloned ei24 DNA sequence functions as a promoter to drive luciferase expression (Fig. 3). Cotransfection of the reporter with wtp53 resulted in a 15- to 25-fold higher induction of luciferase activity, demonstrating that the ei24 promoter fragment is inducible by p53 (Fig. 3). The promoter region was sequenced and found to contain putative p53 DNA binding consensus sites (Fig. 3 and 4). To verify this potential site as a specific p53 binding element, we synthesized complementary oligonucleotides corresponding to this element (p53RE) and mutated oligonucleotides as a negative control (p53MRE). The mutations were introduced into the fourth and seventh positions of sites I and II of the ei24 probe (p53MRE), and these nucleotides were selected and targeted on the basis of earlier studies demonstrating the strict requirement of these residues for p53 DNA binding (10). Human wtp53 protein, purified from recombinant baculovirus-infected insect cells, was used in these binding assays. Consistent with previous reports, it was also necessary to include the murine monoclonal antibody PAb421, which activates DNA binding by binding to its epitope in the C terminus of p53 (19). Electrophoretic mobility shift analysis demonstrated that purified wtp53 protein can specifically bind to the ei24 wild-type site (p53RE) but not to the mutated probe (p53MRE) (Fig. 4). These results demonstrate that the ei24 promoter contains functional p53 DNA binding sites and is efficiently transactivated by wtp53. Together with the p53ER-cycloheximide studies, these data establish ei24 as an immediate-early wtp53-inducible gene.
FIG. 3.
The ei24 promoter is transactivated by wtp53. The 3.5-kb DNA fragment containing the ei24 promoter region was inserted upstream of a luciferase reporter (pGLKH) as schematically diagrammed (top). The location of the p53 DNA binding consensus sites are identified. To test the transcriptional activity and p53 responsiveness of the ei24 promoter, murine 10(1) fibroblasts (p53-null cells) were cotransfected with pGLKH and either a vector-only (CMV) or wtp53 expression plasmid (p53). In parallel, 10(1) cells were cotransfected with p50-2, a previously characterized p53 responsive reporter (41), and either CMV or p53 to serve as a positive control for p53 function. Levels of promoter activity, which are measured in relative light units (RLU), in the absence of p53 (CMV) have been normalized. Fold activation represents the increased reporter activity during wtp53 expression (p53) as compared to that of the CMV samples (bottom). Open and filled bars represent the results obtained from two independent experiments. The average basal activity of the promoterless luciferase reporter (pGL) and ei24 promoter-containing reporter (pGLKH) is ∼7,000 RLU and 190,000 RLU, respectively.
FIG. 4.
The ei24 promoter contains wtp53 DNA binding sites. The p53 DNA binding consensus site (p53CON) (10) consists of two 10-bp repeats (top). The potential p53 consensus site located at positions −67 to −48 in the ei24 promoter (p53RE) and a corresponding mutated sequence (p53MRE) are listed below for comparison. Radiolabeled probes corresponding to these double-stranded DNA sites were incubated with 100 ng of purified human p53 protein with and without 2 μg of PAb421 as described in Materials and Methods. As previously reported, the monoclonal antibody PAb421 is required to activate purified p53 protein for DNA binding (19). The protein-DNA complexes were resolved in native 4% polyacrylamide gels and visualized by PhosphorImager analysis (bottom).
ei24/PIG8 is highly conserved throughout evolution.
It was previously reported that murine and human ei24/PIG8 cDNAs encode 317- and 318-amino-acid proteins, respectively (25, 33). A search of the GenBank database for related sequences revealed a series of human expressed sequence tag (EST) clones that matched the murine C-terminal coding region, with the exception of a single cytidine insertion that shifts the reading frame of the published sequence at codon 266. Sequencing of both the human (33) and murine (25) cDNA clones obtained from these groups, with high-temperature Taq1 polymerase, confirmed the accuracy of the EST sequence and demonstrated that the published ei24/PIG8 sequences were incorrect. Furthermore, sequence analysis of the murine genomic ei24 BAC clone within the corresponding coding region also confirmed the existence of the extra nucleotide which we had previously detected in the EST and cDNA clones. Therefore the C-terminal 50 amino acids (residues 267 to 317) of the published murine Ei24 protein sequence do not exist and are replaced by an additional 92 amino acids (Fig. 5). Similarly, the published C-terminal 50 amino acids of human PIG8 are incorrect and are replaced by an additional 92 amino acids (data not shown). The corrections in the murine and human ei24/PIG8 sequences have been deposited in GenBank. Comparison of the corrected mouse and human ei24/PIG8 sequences demonstrates 98% identity between these species and murine Ei24 is 27% identical (52% similar) to an open reading frame in C. elegans (CELF37C12.2; GenBank accession no. U00033). These results indicate that ei24 is remarkably conserved during evolution, possibly to maintain an important biological function.
FIG. 5.
Predicted amino acid sequence of murine Ei24 protein. The previously published sequences of murine and human Ei24/PIG8 proteins contained a frameshift mutation corresponding to codon 266 (25, 33). The corrected murine Ei24 primary amino acid sequence is presented and the site of divergence from the published sequence is indicated by the arrowhead. The open reading frame of murine Ei24 is 358 amino acids, which is 98% identical to human Ei24/PIG8 and more than 50% similar to sequences found in C. elegans. The predicted sequence lacks obvious functional motifs but does contain six putative membrane-spanning domains, which are underlined.
ei24 suppresses cell growth and induces cell death.
To study the function of ei24, sequences encoding murine ei24, which is FLAG tagged at the C terminus, and the full-length human ei24/PIG8 cDNA (unmodified) were cloned into the prk5 vector under the transcriptional control of a cytomegalovirus (CMV) promoter. These plasmids were transiently transfected into COS-7 cells with Lipofectamine or by calcium phosphate precipitation. Ei24 protein expression was analyzed by Western blot with a rabbit polyclonal serum that was prepared from animals immunized with an N-terminal Ei24-conjugated peptide. This antiserum cross-reacts with both mouse and human Ei24/PIG8 protein (Fig. 6 and data not shown). Murine ei24 expression was detected with Ab22 only in samples transiently transfected with full-length ei24 expression vectors (Fig. 6A). Although Ab22 is specific and can readily recognize ectopically expressed Ei24 protein, it is not yet of sufficient titer or affinity to detect endogenous Ei24. Expression of murine Ei24 was also confirmed by IP-Western blot analysis with a FLAG-specific antibody for the IP step and Ab22 for probing the nylon membrane (Fig. 6B). Interestingly, murine ei24/PIG8 gene expression in the floating cells was approximately 500-fold higher per microgram of protein than in the healthy cells that remained attached to the tissue culture dish (Fig. 6B). These results suggested that ei24/PIG8 may be antithetical to cell growth.
FIG. 6.
Expression of ei24 in transiently transfected COS-7 cells. COS-7 cells were transfected with 1 or 10 μg of prk5 vector-only or mouse ei24-FLAG expression plasmids (prkMei24-1 and -3 are identical but independently prepared DNA). At 48 h posttransfection, detached and adherent cells were harvested either separately or pooled. (A) Lysates were prepared from pooled cells and 50 μg of total protein was analyzed by direct Western blotting with Ei24-specific rabbit polyclonal Ab-22 antiserum, as described in Materials and Methods. (B) Lysates were prepared from detached (D) and adherent (A) cells and either 50 μg or 500 μg of total cell protein was immunoprecipitated with anti-FLAG antibody and analyzed by Western blotting with Ab-22 as the primary antibody. A 10-fold-smaller amount of protein from the detached cells yielded severalfold-higher levels of Ei24 than the adherent cells. The position of the 32-kDa molecular weight marker is indicated. Hemagglutinin-tagged murine Ei24 protein (HA-Mei24) isolated from recombinant baculovirus-infected Sf9 cells was used as a positive control.
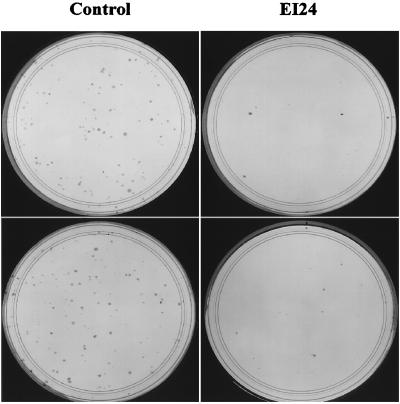
The effects of ei24 on cell growth were initially addressed in colony reduction assays by cotransfecting COS-7 cells with pcDNA3-neo and either prk5 vector-only or prkHEI24 plasmid DNA. The cells were selected in 0.7 mg of G418 per ml for 10 days and colonies that formed during this time were then stained with Wright-Giemsa dye. As shown in Fig. 7, colonies readily formed when transfected with pcDNA3-neo and the vector-only plasmid (101 ± 12 CFU). By contrast, the number of colonies that formed when cotransfected with the neomycin resistance marker and ei24/PIG8 expression vectors were dramatically reduced (8 ± 2 CFU for murine ei24 and 7 ± 2 CFU for human ei24/PIG8), representing a >90% reduction in colony formation when ei24/PIG8 is expressed. Efficient growth suppression was also observed upon transfection of human lung carcinoma H358 cells with ei24/PIG8 (data not shown). These results demonstrate that ei24/PIG8 is a negative growth regulator but do not distinguish between possible effects on cell cycle arrest or cell death.
FIG. 7.
ei24 suppresses colony growth of COS-7 cells. Cells were cotransfected with pcDNA3-neo and either prk5 vector-only DNA (Vector) or prkHei24 (EI24), which expresses human ei24/PIG8 protein. The transfected cells were selected in 0.7 mg of G418 per ml for 10 days, stained with Wright-Giemsa dye, and quantitated for colony formation.
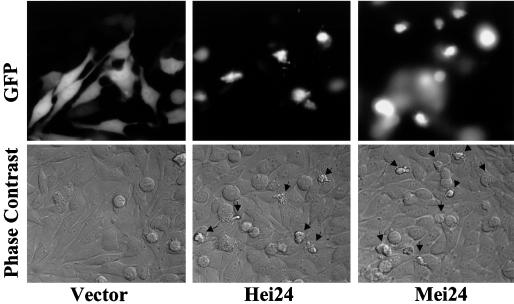
To study these biological processes in more detail, NIH 3T3 fibroblasts were coinjected with a GFP expression plasmid together with the prk5 vector-only or ei24 expression constructs. Twenty-four hours after injection, the cells were analyzed for morphological changes by phase-contrast and fluorescence microscopy and photographed. As shown in Fig. 8, the GFP-positive cells injected with the vector-only DNA appeared normal, whereas the cells that were injected with the ei24 expression plasmid displayed the typical morphological features of apoptosis (39), including significant membrane blebbing, vacuolization, and nuclear condensation. Induction of apoptosis was similarly observed in microinjection assays when using HeLa cells (data not shown). These results suggest that ei24 suppresses cell growth through the activation of an apoptotic pathway.
FIG. 8.
Ectopic expression of ei24 in immortal murine fibroblasts induces morphological features of apoptosis. NIH 3T3 cells were coinjected with a GFP expression plasmid and either prk5 vector-only (Vector), prkHei24 (Hei24), or prkMei24 (Mei24) DNA. Twenty-four hours after injection, cells were examined by phase-contrast (bottom) and fluorescence (top) microscopy to identify productively injected cells and photographed. Morphological changes consistent with an apoptotic response were only apparent in ei24-expressing cells. These results are representative of three independent experiments, including studies with HeLa cells.
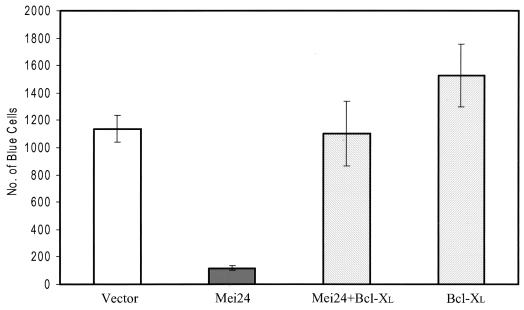
Induction of apoptosis by ei24 was demonstrated with a transient transfection β-Gal reporter assay (6). In these experiments, Rat1 cells were transfected with a β-Gal reporter, which allows productively transfected cells to be identified by staining them blue with X-Gal (6). Cotransfection of Rat1 fibroblasts with the prk5 vector-only and β-Gal reporter plasmids resulted in the formation of approximately 1,200 blue-stained cells per defined area (Fig. 9). By contrast, cotransfection of Rat1 cells with β-Gal and ei24 reduced this number by approximately 90%. Inclusion of a Bcl-XL expression plasmid, which encodes a suppressor of apoptosis (2), in the β-Gal- and ei24-cotransfected samples effectively restored the number of blue cells to the level observed in control-transfected cells. These results demonstrate that ei24 can function in an apoptotic pathway that is efficiently repressed by Bcl-XL.
FIG. 9.
Ectopic expression of ei24 induces apoptosis that is inhibited by Bcl-XL. Rat1 fibroblasts were transiently transfected in triplicate with the β-Gal reporter and prk5 vector-only plasmid (Vector) or cotransfected with the β-Gal reporter and either prk5Mei24 (Mei24), pBabeBcl-XL (Bcl-XL), or Mei24 plus Bcl-XL (Mei24 + Bcl-XL). Forty-eight hours after transfection, cells were stained for β-Gal activity and quantitated by scoring blue positive cells within a defined area. These experiments were repeated three times, once with independently prepared plasmid DNAs. Error bars, standard error.
DISCUSSION
DNA damage inhibits normal cell growth by activating p53-regulated pathways leading to either a G1 phase cell cycle arrest or apoptosis. The mechanism for p53-mediated cell cycle arrest has been well characterized, whereas its role in regulating cell death is poorly understood. We report here the characterization of ei24/PIG8, which is a highly conserved gene that is directly regulated by wtp53 and negatively controls cell growth by inducing apoptosis.
The ei24 promoter lacks a canonical TATA box but does contain multiple copies of putative SP1, AP1, and AP2 sites (data not shown). These features are characteristic of many housekeeping genes, and consistent with this observation, ei24 is expressed in many tissues, including heart, skeletal muscle, lung, and liver (25). Our data also demonstrate that the ei24 promoter contains p53 DNA binding sites and is directly regulated by wtp53. Although the human promoter has not yet been isolated, ei24/PIG8 expression is inducible in human cells by wtp53 (33), implying that the p53 response element has been conserved. Therefore, ei24 is a DNA damage-inducible gene that lies downstream in the p53 pathway. An interesting model proposed by Chen and coworkers (5) suggests that the levels of wtp53 are an important determinant in whether a cell undergoes cell cycle arrest or apoptosis. Growth arrest has been correlated with low levels of p53, whereas at higher levels of p53 cells are more prone to apoptosis. It remains to be determined whether ei24 expression is selectively regulated by p53 in a dose-dependent and/or cell context-specific manner. In addition, other signals (e.g., differentiation) that are p53 independent may also regulate ei24 gene expression and will need to be addressed.
ei24/PIG8 was mapped by fluorescence in situ hybridization analysis to human chromosome 11q23, a region frequently altered in human cancers (data not shown). Within this region is the ATM (mutated in ataxia telangiectasia) gene and the MLL gene, the latter a primary target for rearrangement in acute lymphoblastic and therapy-related myelogenous leukemias. However, there are a number of human malignancies, such as invasive cervical cancer, breast carcinoma, and malignant melanoma, that exhibit loss of heterozygosity within 11q23, and the putative tumor suppressor gene(s) located in this region has not yet been identified (1, 13, 16, 18). Since ei24 is a downstream target of wild-type p53 and overexpression of ei24 inhibits cell growth and induces apoptosis, we propose that ei24/PIG8 is a reasonable tumor suppressor candidate that should be addressed in these cancers.
Our data indicate that enforced expression of ei24 induces apoptosis independent of functional p53. Microinjection studies demonstrated that ei24 induces an apoptotic response in HeLa cells (data not shown), which encode wtp53 but fail to express functional p53 protein due to its rapid degradation directed by the human papillomavirus E6 oncogene product (26). Similarly, transfection of ei24 into COS-7 cells, which are functionally null for p53 due to inactivation by simian virus T antigen (26), inhibits cell growth and induces cell death. These results suggest that once expressed, ei24 can initiate an apoptotic response without further requirement for wtp53 activity. These results also strengthen the argument that ei24 is downstream within the p53 pathway and suggest that ei24 may play an important role as an apoptotic effector of p53 tumor suppressor activity. However, physiological levels of ei24 may not always lead to cell death and other genetic factors or signals may modify or override its function (e.g., cytokine inhibition of p53-mediated cell death; see below).
The finding that IL-6 efficiently blocked apoptosis of M1-tsp53 cells at the permissive temperature without down-regulating ei24 expression is not surprising (Fig. 1). Cytokines override p53-mediated cell death by inhibiting a downstream step of the pathway and not by directly blocking the induction of target genes (4, 34). Cytokine-signaling pathways clearly activate the expression of Bcl-XL and Bcl-2 and it is presumably through these potent survival factors that cytokines block p53-induced apoptosis (31, 34). Consistent with these observations, Bcl-XL was also found to block ei24-mediated apoptosis of Rat1 cells (Fig. 9).
The mechanism by which ei24 activates apoptosis is not yet understood. The data supporting a role of ei24 in negatively controlling cell growth and mediating cell death are fourfold. (i) ei24 is induced in cells undergoing p53-mediated apoptosis. (ii) Microinjection of an ei24 expression vector into murine NIH 3T3 fibroblasts (Fig. 8) or human HeLa cervical carcinoma cells (data not shown) induces morphological features of apoptosis. (iii) ei24 efficiently blocks cell growth in colony reduction assays. (iv) Bcl-XL overrides the reduction of β-Gal-marked cells when transiently transfected with ei24. It is important to note that ei24 is overexpressed in these assays, and therefore, the significance of ei24 in apoptosis must be addressed in a more physiological setting. To do so, we are generating an ei24-deficient mouse model to examine the contribution of endogenous ei24 to p53-mediated apoptosis and tumor suppressor pathways in more detail.
Previously identified downstream target genes of p53 that may play a role in apoptosis include bax, killer/DR5, IGF-BP3, PAG608, and fas/APO1 (3, 20, 29, 30, 38). However, mice deficient in bax or fas are completely competent for p53-mediated thymic cell death in response to irradiation, suggesting that other targets or multiple targets are required for apoptosis (7, 23, 28, 35). In light of the data presented here, we propose that ei24 may be a component of this apoptotic cascade in response to p53 and so contributes to tumor suppression.
ACKNOWLEDGMENTS
We especially thank JinLing Wang and John R. Jeffers for their technical assistance and John L. Cleveland for critically reviewing the manuscript. We also acknowledge the contributions of Sam Lucas and Richard Ashmun to cell cycle analyses, of Linda Valentine and Thomas Look to the cytogenetic analyses, and of Richard Bram to the microinjection studies. We thank Richard Kriwacki for his advice on the predicted structure of ei24/PIG8.
This work was supported in part by NIH/NCI grant CA63230 (to G.P.Z.), NIH/NCI Cancer Center Support Grant 5 P30 CA21765, and the American Lebanese Syrian Associated Charities of St. Jude Children's Research Hospital (ALSAC).
REFERENCES
- 1.Bethwaite P B, Koreth J, Herrington C S, McGee J O. Loss of heterozygosity occurs at the D11S29 locus on chromosome 11q23 in invasive cervical carcinoma. Br J Cancer. 1995;71:814–818. doi: 10.1038/bjc.1995.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boise L H, Gonzalez-Garcia M, Postema C E, Ding L, Lindsten T, Turka L A, Mao X, Nunez G, Thompson C B. bcl-x, a bcl-2-related gene that functions as a dominant regulator of apoptotic cell death. Cell. 1993;74:597–608. doi: 10.1016/0092-8674(93)90508-n. [DOI] [PubMed] [Google Scholar]
- 3.Buckbinder L, Talbott R, Velasco-Miguel S, Takenaka I, Faha B, Seizinger B R, Kley N. Induction of the growth inhibitor IGF-binding protein 3 by p53. Nature. 1995;377:646–649. doi: 10.1038/377646a0. [DOI] [PubMed] [Google Scholar]
- 4.Canman C E, Gilmer T M, Coutts S B, Kastan M B. Growth factor modulation of p53-mediated growth arrest versus apoptosis. Genes Dev. 1995;9:600–611. doi: 10.1101/gad.9.5.600. [DOI] [PubMed] [Google Scholar]
- 5.Chen X, Ko L J, Jayaraman L, Prives C. p53 levels, functional domains, and DNA damage determine the extent of the apoptotic response of tumor cells. Genes Dev. 1996;10:2438–2451. doi: 10.1101/gad.10.19.2438. [DOI] [PubMed] [Google Scholar]
- 6.Chittenden T, Flemington C, Houghton A B, Ebb R G, Gallo G J, Elangovan B, Chinnadurai G, Lutz R J. A conserved domain in Bak, distinct from BH1 and BH2, mediates cell death and protein binding functions. EMBO J. 1995;14:5589–5596. doi: 10.1002/j.1460-2075.1995.tb00246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clarke A R, Purdie C A, Harrison D J, Morris R G, Bird C C, Hooper M L, Wyllie A H. Thymocyte apoptosis induced by p53-dependent and independent pathways. Nature. 1993;362:849–852. doi: 10.1038/362849a0. [DOI] [PubMed] [Google Scholar]
- 8.Deng C, Zhang P, Harper J W, Elledge S J, Leder P. Mice lacking p21CIP1/WAF1 undergo normal development, but are defective in G1 checkpoint control. Cell. 1995;82:675–684. doi: 10.1016/0092-8674(95)90039-x. [DOI] [PubMed] [Google Scholar]
- 9.Donehower L A, Harvey M, Slagle B L, McArthur M J, Montgomery C A, Jr, Butel J S, Bradley A. Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature. 1992;356:215–221. doi: 10.1038/356215a0. [DOI] [PubMed] [Google Scholar]
- 10.el-Deiry W S, Kern S E, Pietenpol J A, Kinzler K W, Vogelstein B. Definition of a consensus binding site for p53. Nat Genet. 1992;1:45–49. doi: 10.1038/ng0492-45. [DOI] [PubMed] [Google Scholar]
- 11.el-Deiry W S, Tokino T, Velculescu V E, Levy D B, Parsons R, Trent J M, Lin D, Mercer W E, Kinzler K W, Vogelstein B. WAF1, a potential mediator of p53 tumor suppression. Cell. 1993;75:817–825. doi: 10.1016/0092-8674(93)90500-p. [DOI] [PubMed] [Google Scholar]
- 12.Friedlander P, Legros Y, Soussi T, Prives C. Regulation of mutant p53 temperature-sensitive DNA binding. J Biol Chem. 1996;271:25468–25478. doi: 10.1074/jbc.271.41.25468. [DOI] [PubMed] [Google Scholar]
- 13.Hampton G M, Mannermaa A, Winquist R, Alavaikko M, Blanco G, Taskinen P J, Kiviniemi H, Newsham I, Cavenee W K, Evans G A. Loss of heterozygosity in sporadic human breast carcinoma: a common region between 11q22 and 11q23.3. Cancer Res. 1994;54:4586–4589. [PubMed] [Google Scholar]
- 14.Harper J W, Adami G R, Wei N, Keyomarsi K, Elledge S J. The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell. 1993;75:805–816. doi: 10.1016/0092-8674(93)90499-g. [DOI] [PubMed] [Google Scholar]
- 15.Harvey D, Levine A J. P53 alteration is a common event in the spontaneous immortalization of primary BALB/C murine fibroblasts. Genes Dev. 1991;5:2375–2385. doi: 10.1101/gad.5.12b.2375. [DOI] [PubMed] [Google Scholar]
- 16.Herbst R A, Larson A, Weiss J, Cavenee W K, Hampton G M, Arden K C. A defined region of loss of heterozygosity at 11q23 in cutaneous malignant melanoma. Cancer Res. 1995;55:2494–2496. [PubMed] [Google Scholar]
- 17.Hinds P W, Finlay C A, Quartin R S, Baker S J, Fearon E R, Vogelstein B, Levine A J. Mutant p53 DNA clones from human colon carcinomas cooperate with ras in transforming primary rat cells: a comparison of the “hot spot” mutant phenotypes. Cell Growth Differ. 1990;1:571–580. [PubMed] [Google Scholar]
- 18.Huettner P C, Gerhard D S, Li L, Gersell D J, Dunnigan K, Kamarasova T, Rader J S. Loss of heterozygosity in clinical stage IB cervical carcinoma: relationship with clinical and histopathologic features. Hum Pathol. 1998;29:364–370. doi: 10.1016/s0046-8177(98)90117-4. [DOI] [PubMed] [Google Scholar]
- 19.Hupp T R, Meek D W, Midgley C A, Lane D P. Regulation of the specific DNA binding function of p53. Cell. 1992;71:875–886. doi: 10.1016/0092-8674(92)90562-q. [DOI] [PubMed] [Google Scholar]
- 20.Israeli D, Tessler E, Haupt Y, Elkeles A, Wilder S, Amson R, Telerman A, Oren M. A novel p53-inducible gene, PAG608, encodes a nuclear zinc finger protein whose overexpression promotes apoptosis. EMBO J. 1997;16:4384–4392. doi: 10.1093/emboj/16.14.4384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jacks T, Remington L, Williams B O, Schmitt E M, Halachmi S, Bronson R T, Weinberg R A. Tumor spectrum analysis in p53-mutant mice. Curr Biol. 1994;4:1–7. doi: 10.1016/s0960-9822(00)00002-6. [DOI] [PubMed] [Google Scholar]
- 22.Jordan M, Schallhorn A, Wurm F M. Transfecting mammalian cells: optimization of critical parameters affecting calcium-phosphate precipitate formation. Nucleic Acids Res. 1996;24:596–601. doi: 10.1093/nar/24.4.596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Knudson C M, Tung K S, Tourtellotte W G, Brown G A, Korsmeyer S J. Bax-deficient mice with lymphoid hyperplasia and male germ cell death. Science. 1995;270:96–99. doi: 10.1126/science.270.5233.96. [DOI] [PubMed] [Google Scholar]
- 24.Ko L J, Prives C. p53: puzzle and paradigm. Genes Dev. 1996;10:1054–1072. doi: 10.1101/gad.10.9.1054. [DOI] [PubMed] [Google Scholar]
- 25.Lehar S M, Nacht M, Jacks T, Vater C A, Chittenden T, Guild B C. Identification and cloning of EI24, a gene induced by p53 in etoposide-treated cells. Oncogene. 1996;12:1181–1187. [PubMed] [Google Scholar]
- 26.Levine A J. p53, the cellular gatekeeper for growth and division. Cell. 1997;88:323–331. doi: 10.1016/s0092-8674(00)81871-1. [DOI] [PubMed] [Google Scholar]
- 27.Littlewood T D, Hancock D C, Danielian P S, Parker M G, Evan G I. A modified oestrogen receptor ligand-binding domain as an improved switch for the regulation of heterologous proteins. Nucleic Acids Res. 1995;23:1686–1690. doi: 10.1093/nar/23.10.1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lowe S W, Schmitt E M, Smith S W, Osborne B A, Jacks T. p53 is required for radiation-induced apoptosis in mouse thymocytes. Nature. 1993;362:847–849. doi: 10.1038/362847a0. [DOI] [PubMed] [Google Scholar]
- 29.Miyashita T, Reed J C. Tumor suppressor p53 is a direct transcriptional activator of the human bax gene. Cell. 1995;80:293–299. doi: 10.1016/0092-8674(95)90412-3. [DOI] [PubMed] [Google Scholar]
- 30.Owen-Schaub L B, Zhang W, Cusack J C, Angelo L S, Santee S M, Fujiwara T, Roth J A, Deisseroth A B, Zhang W W, Kruzel E. Wild-type human p53 and a temperature-sensitive mutant induce Fas/APO-1 expression. Mol Cell Biol. 1995;15:3032–3040. doi: 10.1128/mcb.15.6.3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Packham G, White E L, Eischen C M, Yang H, Parganas E, Ihle J N, Grillot D A, Zambetti G P, Nunez G, Cleveland J L. Selective regulation of Bcl-XL by a Jak kinase-dependent pathway is bypassed in murine hematopoietic malignancies. Genes Dev. 1998;12:2475–2487. doi: 10.1101/gad.12.16.2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pietenpol J A, Tokino T, Thiagalingam S, El-Deiry W S, Kinzler K W, Vogelstein B. Sequence-specific transcriptional activation is essential for growth suppression by p53. Proc Natl Acad Sci USA. 1994;91:1998–2002. doi: 10.1073/pnas.91.6.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Polyak K, Xia Y, Zweier J L, Kinzler K W, Vogelstein B. A model for p53-induced apoptosis. Nature. 1997;389:300–305. doi: 10.1038/38525. [DOI] [PubMed] [Google Scholar]
- 34.Quelle F W, Wang J, Feng J, Wang D, Cleveland J L, Ihle J N, Zambetti G P. Cytokine rescue of p53-dependent apoptosis and cell cycle arrest is mediated by distinct Jak kinase signaling pathways. Genes Dev. 1998;12:1099–1107. doi: 10.1101/gad.12.8.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reinke V, Lozano G. The p53 targets mdm2 and Fas are not required as mediators of apoptosis in vivo. Oncogene. 1997;15:1527–1534. doi: 10.1038/sj.onc.1201316. [DOI] [PubMed] [Google Scholar]
- 36.Vater C, Bartle L, Dionne C, Littlewood T, Goldmacher V. Induction of apoptosis by tamoxifen-activation of a p53-estrogen receptor fusion protein expressed in E1A and T24 H-ras transformed p53−/− mouse embryo fibroblasts. Oncogene. 1996;13:739–748. [PubMed] [Google Scholar]
- 37.Waldman T, Kinzler K, Vogelstein B. p21 is necessary for the p53-mediated G1 arrest in human cancer cells. Cancer Res. 1995;55:5187–5190. [PubMed] [Google Scholar]
- 38.Wu G S, Burns T F, McDonald III E R, Jiang W, Meng R, Krantz I D, Kao G, Gan D D, Zhou J Y, Muschel R, Hamilton S R, Spinner N B, Markowitz S, Wu G, El-Deiry W S. KILLER/DR5 is a DNA damage-inducible p53-regulated death receptor gene. Nat Genet. 1997;17:141–143. doi: 10.1038/ng1097-141. [DOI] [PubMed] [Google Scholar]
- 39.Wyllie A. Apoptosis. Clues in the p53 murder mystery. Nature. 1997;389:237–238. doi: 10.1038/38405. [DOI] [PubMed] [Google Scholar]
- 40.Yonish-Rouach E, Resnitzky D, Lotem J, Sachs L, Kimchi A, Oren M. Wild-type p53 induces apoptosis of myeloid leukaemic cells that is inhibited by interleukin-6. Nature. 1991;352:345–347. doi: 10.1038/352345a0. [DOI] [PubMed] [Google Scholar]
- 41.Zambetti G P, Bargonetti J, Walker K, Prives C, Levine A J. Wild-type p53 mediates positive regulation of gene expression through a specific DNA sequence element. Genes Dev. 1992;6:1143–1152. doi: 10.1101/gad.6.7.1143. [DOI] [PubMed] [Google Scholar]