Abstract
Polycystic ovary syndrome (PCOS) is a common reproductive disorder characterized by elevated androgens and antimüllerian hormone (AMH). These hormones remain elevated throughout pregnancy, and potential effects of hormone exposure on offspring from women with PCOS remain largely unexplored. Expanding on recent reports of prenatal AMH exposure in mice, we have fully characterized the reproductive consequences of prenatal AMH (pAMH) exposure throughout the lifespan of first- and second-generation offspring of both sexes. We also sought to elucidate mechanisms underlying pAMH-induced reproductive effects. There is a known reciprocal relationship between AMH and androgens, and in PCOS and PCOS-like animal models, androgen feedback is dysregulated at the level of the hypothalamus. Kisspeptin neurons express androgen receptors and play a critical role in sexual development and function. We therefore hypothesized that pAMH-induced reproductive phenotypes would be mediated by androgen signaling at the level of kisspeptin cells. We tested the pAMH model in kisspeptin-specific androgen receptor knockout (KARKO) mice and found that virtually all pAMH-induced phenotypes assayed are eliminated in KARKO offspring compared to littermate controls. By demonstrating the necessity of androgen receptor in kisspeptin cells to induce pAMH phenotypes, we have advanced understanding of the interactions between AMH and androgens in the context of prenatal exposure, which could have significant implications for children of women with PCOS.
Keywords: antimüllerian hormone, androgen receptor, kisspeptin, PCOS, prenatal hormone exposure
Polycystic ovary syndrome (PCOS) affects 1 in 10 reproductive-age women and is the most common cause of anovulatory infertility (1). PCOS classically presents with disrupted ovulation, polycystic ovaries, and androgen excess. Hyperandrogenism persists throughout any pregnancies (2-5), with potential consequences for offspring. Daughters from women with PCOS have a 5-fold increased risk for developing PCOS themselves (6), and sons have been shown to have altered hormones during puberty (7, 8). Since loci identified by PCOS genome-wide association studies account for less than 10% of disease heritability (9) and more than 20% of the variation observed in PCOS can be explained by environmental factors (10), nongenetic influences such as altered gestational hormones could confer risk to offspring.
In addition to the characteristic increase in androgens, antimüllerian hormone (AMH) levels have been found to correlate with the severity of clinical manifestations of PCOS, including signs of hyperandrogenism (11-14), and genetic studies in PCOS women have identified associations between androgen levels and polymorphisms in the genes encoding AMH and its ligand-binding receptor, AMHR2 (15-17). In PCOS patients, ovarian follicles are arrested at a stage in follicular development when AMH production is highest (18), and each individual follicle produces more AMH than follicles from healthy controls (19). AMH levels in follicular fluid and serum are at least 5- and 3-fold higher, respectively, in PCOS women (20-22). These increased AMH levels remain elevated into the second trimester of pregnancy and at term (23, 24). The effects of prenatal AMH exposure on offspring have yet to be fully examined.
Although AMH is clearly dysregulated in PCOS, only one group has explored the effects of prenatal AMH (pAMH) exposure thus far (23, 25). In mice, peripheral administration of AMH late in gestation resulted in marked reproductive disruptions in female offspring (23). pAMH females showed delayed puberty and disrupted early estrous cycling, as well as alteration at all levels of the hypothalamic-pituitary-gonadal (HPG) axis. pAMH-induced reproductive phenotypes are transgenerational, with second- and third-generation females also affected (25). However, initial investigations did not examine time points beyond puberty and early adulthood. Assessments of late adulthood are especially relevant because in utero androgen exposure results in altered reproductive senescence in mice (26). Further, possible consequences to pAMH male offspring have not been explored, and underlying pathophysiological mechanisms have yet to be fully elucidated.
One proposed pathway for pAMH-induced reproductive effects is AMH action at the level of the hypothalamus to activate the HPG axis and favor excess androgen production (23). Although peripherally administered AMH does not cross the placenta, it does alter maternal serum hormones and placental gene expression to create an androgenic environment in utero (23). Prenatal androgen excess in mice contributes to the development of PCOS-like reproductive features (26-29). pAMH phenotypes can be reversed and prevented with a gonadotropin-releasing hormone (GnRH) antagonist (23), further suggesting a central, androgen-mediated mechanism. However, the contribution of androgens to pAMH-induced phenotypes has not been directly tested.
Because GnRH neurons do not express androgen receptors (ARs), androgens would act upstream, possibly on hypothalamic kisspeptin neurons. Kisspeptin neurons in the arcuate nucleus (ARC) of the hypothalamus are considered the pacemakers of pulsatile GnRH release (30) and are critical for normal sexual maturation and function (31). Normally, androgens negatively feed back to the hypothalamus by binding to AR in ARC kisspeptin neurons, decreasing kisspeptin expression and suppressing luteinizing hormone (LH) pulses (32). In PCOS and PCOS-like mouse models, sex steroid feedback to the HPG axis is altered, as LH pulsatility remains high despite the presence of elevated androgens (23, 33, 34). Together, this evidence led us to hypothesize that the pAMH-induced reproductive phenotype would be mediated by androgen signaling at the level of kisspeptin cells.
Here, we characterize a full profile throughout the lifespan of first- and second-generation pAMH offspring of both sexes. Using Cre-LoxP technology, we generated a kisspeptin-specific AR knockout (KARKO) mouse line and assessed the reproductive consequences of pAMH in KARKO offspring to determine whether androgens were mediating this phenotype and where they might be acting. We found that nearly every pAMH-induced deficit present in controls was eliminated in their KARKO littermates. By determining the necessity of AR in kisspeptin cells to induce pAMH phenotypes, we have advanced understanding of the interactions between AMH and androgens in the context of prenatal exposure.
Materials and Methods
Mice
Mice were group-housed on a 12:12 light/dark cycle with an ad libitum standard chow diet and water in a temperature-controlled room. All experimental procedures were approved by the University of California, San Diego Institutional Animal Care and Use Committee.
Initial characterization of first (F1) and second (F2) generation pAMH offspring was completed using PER2::LUC transgenic mice (RRID::006852) IMSR_JAX:006852) (35) maintained in our local colony on a C57BL/6 background. Mice with the PER2::LUC transgene are viable and fertile with no developmental or morphological differences compared to wild-type littermates (35, 36).
To generate kisspeptin-specific AR knockout mice, we crossed together a previously established Ar-flox mouse (RRID: IMSR_NM-CKO-0010) (37) with a validated Kiss1-Cre mouse (RRID: IMSR_JAX:023426) (38) to generate a KARKO mouse. Arflox/flox;Kiss1Cre+ females were bred to Arflox/Y to produce Arflox/flox;Kiss1Cre+ (KARKO) conditional knockouts or Arflox/flox;Kiss1Cre– control littermates (Ctrl). KARKO Rosa-tdT reporter mice were generated by crossing KARKO or Kiss1-Cre mice with Ai9 Rosa-tdTomato mice (RRID: IMSR_JAX:007909) (39), to create mice in which Kiss1-Cre expressing cells were identifiable by tdTomato expression. Mice used for these experiments were either Arflox/flox;Kiss1Cre+;RosatdT+/– or Arwt/wt;Kiss1Cre+; RosatdT+/– (Ctrl).
For genotyping, genomic DNA was isolated from tail tip at postnatal day (d) 21 and polymerase chain reaction (PCR) was run using the following primers: Ar-flox: F—GTTGATACCTTAACCTCTGC; R—TTCAGCGGCTCTTTTGAAG. Cre: F—GCATTACCGGTCGTAGCAACGAGTG; R—GAACGCTAGAGCCTGTTTTGCACGTTC. Rosa-tdTomato: F1-AAGGGAGCTGCAGTGGAGTA; R1- CCGAAAATCTGTGGGAAGTC; F2—GGCATTAAAGCAGCGTATCC; R2—CTGTTCCTGTACGGCATGG. Germline recombination in the KARKO line was detected using primers: ARKO: F—GTTGATACCTTAACCTCTGC; R—CCTACATGTACTGTGAGAGG. Germline recombination in the KARKO Rosa-tdT line was apparent by a pink hue of the skin.
Generation of Prenatal Antimüllerian Hormone Offspring
To generate pAMH offspring, virgin adult females (age 3-4 months) were paired with adult males (age 3-4 months) and checked for copulatory plug as indication of embryonic day (e) 0.5. Timed-pregnant dams were randomly assigned to receive daily intraperitoneal injections from E16.5 to E18.5 of either vehicle (VEH) or 0.12 mg/kg/day AMH (recombinant human MIS/AMH protein, R&D Systems, No. 1737-MS), as previously reported (23). AMH was reconstituted in stocks of 100 μg/mL according to the manufacturer’s instructions (4-mM HCl + 0.1% bovine serum albumin) and diluted into 0.01-M phosphate-buffered saline (PBS) pH 7.4. Dams received different volume injections based on weight (100 μL/10 g). Experimenters were blinded to experimental groups. F1 offspring of both sexes were born into experimental pAMH or VEH control groups, then weaned at 21 days to begin reproductive phenotyping. Minimum sample sizes were determined based on previous publications of reductive phenotypes following prenatal hormone exposure (23, 25, 26), and 2 separate cohorts of pAMH and VEH offspring were generated to confirm the reproducibility of the results.
F2 pAMH and VEH offspring were the product of the F1 fertility assay, in which F1 pAMH and VEH females were paired with experimentally naive wild-type C57BL/6 males (Envigo). Litters from a random subset (n = 4) of pAMH and VEH pairings in the last month of the assay (when F1 dams were age ~5 months) were kept after weaning and phenotyped.
Virgin adult KARKO (Arflox/flox;Kiss1Cre+) females (age 3-4 months) were placed in timed matings with adult control (Arflox/Y;Kiss1Cre–) males (age 3-4 months), and received AMH or VEH injections as described earlier. F1 offspring of both sexes and genotypes were born into experimental pAMH or VEH control groups, then weaned at 21 days to begin phenotyping. Germline-recombined mice were identified and removed from the experiment.
Assessment of Body Weight, Pubertal Onset, and Anogenital Distance
Body weight (in grams) was measured at various ages throughout postnatal development. Beginning after weaning at 21 days old, daily checks were made for pubertal onset as marked by vaginal opening in females and preputial separation in males. Following vaginal opening, vaginal smears were sampled daily until cellular composition was consistent with first estrus. Anogenital distance was measured at 30 days old.
Estrous Cycling
For each time point assessed for estrous cyclicity (60 days, 90 days, 8 months, 10 months, 12 months), adult mice were sampled by vaginal lavage for 16 consecutive days. The slides with vaginal smears were stained with 0.1% methylene blue, examined by light microscopy, and stage of cycle was determined by the composition of cell types present (40). Diestrus (D) was characterized by the presence of predominantly leukocytes, proestrus (P) by the presence of mostly nucleated cells, estrus (E) by the predominance of cornified epithelial cells, and metestrus (M) by the presence of leukocytes with some cornified epithelial cells.
Fertility Assays
Starting at 90 days of age, VEH and pAMH females were paired with experimentally naive wild-type C57BL/6 adult males for 90 days. VEH and pAMH males were paired with experimentally naive wild-type C57BL/6 adult females, and female mates were checked daily for formation of a copulatory plug for 10 days. Following the plugging assay period, males were left in their pairing for the completion of a 90-day period. For both sexes, the dates of birth of each litter, number of pups per litter, and total number of litters produced were recorded. Litters were left with their mothers until weaning at 21 days old.
Tissue Harvest and Histology
All mice were euthanized with CO2 or isoflurane inhalation followed by rapid decapitation. A vaginal smear was collected from females and sacrificed during D. Trunk blood was collected. One ovary was dissected and rapidly frozen on dry ice, then stored at –80 °C. The other ovary and uterus were dissected and fixed in a solution of 10% acetic acid, 30% formaldehyde, and 60% ethanol.
For histological analysis, fixed ovaries or testes were paraffin embedded with the assistance of Reveal Biosciences, Inc, and then serially sectioned at 4 to 6 µm using a microtome. Following staining with hematoxylin and eosin (Sigma-Aldrich), every fifth section was scored for corpora lutea and antral follicles. To avoid double counting, only sections including the ovum nucleus were counted. Cysts and atretic follicles were also quantified. Images were acquired using an EVOS Cell Imaging System or an Olympus VS200 Slide Scanner at the UCSD Neurosciences Imaging Core.
Hormone Assays
Trunk blood collected at the time of euthanizing was allowed to clot for 90 minutes at room temperature. Samples were centrifuged at 2000 rpm for 20 minutes, and serum samples were transferred to a fresh tube and stored at –80 °C. Luteinizing hormone (LH) and follicle-stimulating hormone (FSH) levels were measured using MILLIPLEX MAP Mouse Pituitary Magnetic Bead Kit (Millipore Sigma) and read on Luminex xMAP (Millipore Sigma). Estradiol (E2), testosterone (T), and AMH levels were analyzed by radioimmunoassay at the University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core in Charlottesville, Virginia.
Quantitative Real-time Polymerase Chain Reaction
Harvested ovaries were flash frozen on dry ice and stored at –80 °C. Total RNA was isolated using TRIzol reagent (Ambion) followed by chloroform (Sigma-Aldrich) extraction. RNA was purified using a Zymo Clean & Concentrator kit according to the manufacturer’s instruction, and reverse transcribed (RT) using iScript cDNA synthesis kit (Bio-Rad Laboratories). Quantitative real-time PCR was carried out in triplicates of each sample on CFX Connect Real-Time PCR detection system (Bio-Rad Laboratories). Cyp19a1 expression was detected using validated primers: F- GAGTCTGGATCAGTGGAGAG; R- CACGCTTGCTGCCGAATC. Fold change was calculated by the ∆∆CT method, using H2afz as the control housekeeping gene: F-TCACCGCAGAGGTACTTGAG; R-GATGTGTGGGATGACACCA.
Immunohistochemistry, Microscopy, and Image Analysis
Brains from KARKO Rosa-tdT reporter mice were harvested and fixed in 4% paraformaldehyde solution overnight, then transferred to 30% sucrose. Sections 40 μM thick containing the ARC of the hypothalamus were sectioned using a cryostat and transferred to PBS. Following antigen retrieval in 1X Citra buffer (Biogenex), sections were washed, then blocked in PBS-T with 5% normal goat serum (Sigma) for 1 hour at room temperature before incubation with primary AR antibody (1:50, rabbit polyclonal, Abcam catalog No. ab74272, RRID:AB_1280747) at 4 °C overnight. Sections were washed again and incubated in Alexa Fluor 488 secondary antibody (1:100, goat antirabbit, Thermo Fisher Scientific catalog No. A-11008, RRID:AB_143165) for 30 minutes. Sections were washed and mounted, then coverslipped with ProLong with 4′,6-diamidino-2-phenylindole (DAPI; Invitrogen).
Ar and Kiss1 were analyzed in wild-type gonads using RNAScope according to our previous procedure (41). Briefly, 20-μm coronal sections were collected from fresh-frozen testis and ovary. Sections were fixed in chilled 4% paraformaldehyde, washed 2 times with 1X PBS, and dehydrated through a series of ethanol washes. RNAscope Multiplex Fluorescent v2 Assay (Advanced Cell Diagnostic, 323100) was performed according to the manufacturer’s instructions with Mm-Kiss1 (500141). Sections were coverslipped with ProLong Gold (Invitrogen). The Mm-Kiss1 probe was highly effective in detecting mouse Kiss1 messenger RNA (mRNA) (41).
Fluorescent microscopy was performed at the University of California, San Diego, Nikon Imaging Core using a Nikon Eclipse Ti2-E microscope with Plan Apo objectives. Samples were excited by the Lumencor SpectraX and acquired with a DS-Qi2 CMOS camera using NIS-Elements software. Lighting was determined by using the minimum light-emitting diode intensity and exposure time for each channel using positive and negative control slides, and then used for all subsequent acquisition. Images were imported into FIJI (National Institutes of Health ImageJ). TdTomato-marked kisspeptin-expressing cells and cells positive for AR protein expression were counted manually using the FIJI Cell Counter tool.
Statistics
Statistical analyses were performed using Prism 9 (GraphPad) as indicated in the figure legends, with P less than .05 indicated by an asterisk (*), P less than .01 by **, P less than .001 by ***, and P less than .0001 by ****. Bars in all figures represent means ± SEM. Unpaired t tests were used to compare 2 treatment groups. Data were assessed for Gaussian distribution by Shapiro-Wilk normalcy test, and when a nonnormal distribution was indicated, a nonparametric Mann-Whitney test was performed. When comparing 2 variables (eg, treatment and time, treatment and age, treatment and sex, treatment and genotype), a 2-way analysis of variance was used and significant interactions were resolved using the Sidak multiple comparisons test.
Results
To examine the effect of prenatal AMH (pAMH) exposure, pregnant dams received intraperitoneal injections of AMH or vehicle (VEH) during the late gestational period, as previously reported (23). F1 pAMH or VEH offspring were assessed for reproductive phenotypes beginning after weaning (Fig. 1A).
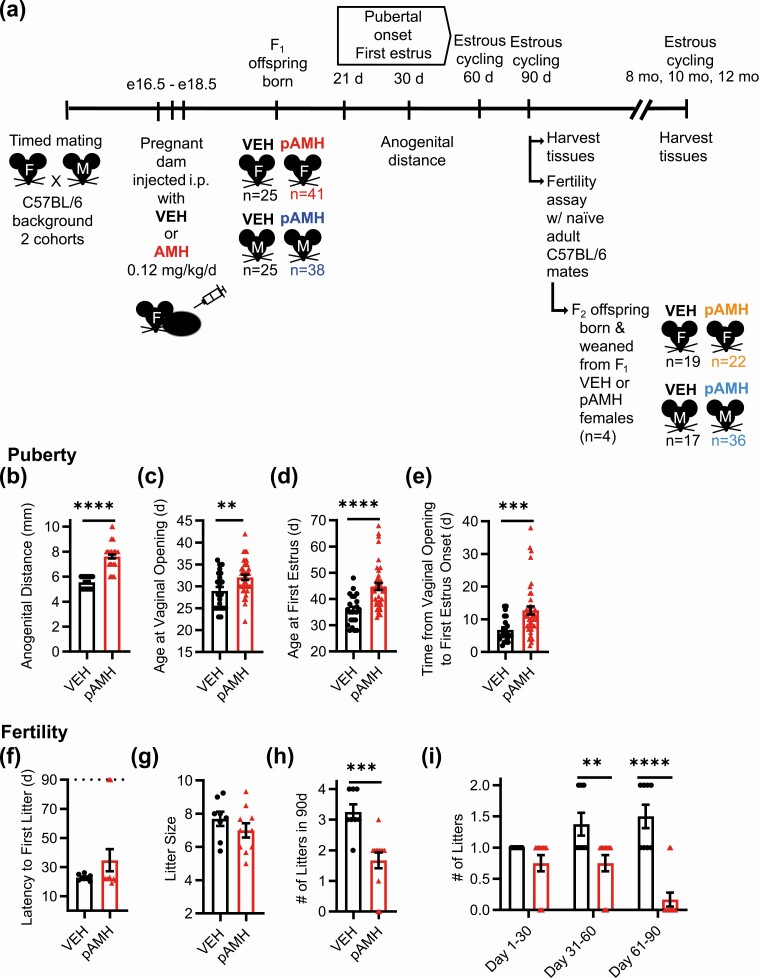
Figure 1.
Experimental design and assessment of prenatal antimüllerian hormone (pAMH)-induced phenotypes in F1 female offspring. A, Schematic of experimental design. Pregnant dams on a C57BL/6 background received injections of either AMH or vehicle (VEH) on embryonic days (e) 16.5, 17.5, and 18.5. First-generation F1 offspring were born into treatment groups (VEH or pAMH) for both sexes (F and M), weaned at postnatal day 21, and examined for reproductive phenotypes. One cohort was euthanized at age 5 months, and the other was euthanized at age 10 months. Second-generation F2 offspring of both sexes were born from F1 pAMH females paired with experimentally naive C57BL/6 males, then assessed for the same measures as first generation. B, Anogenital distance in F1 VEH (black, n = 25) and pAMH (red, n = 41) females was measured at 30 days old (P < .0001, 95% CI 5.327-5.757 vs 7.280-7.940). Assessment for pubertal onset began at 21 days old, as measured by C, time to vaginal opening (P < .01, 95% CI, 27.15-30.76 vs 30.86-33.24); D, time to first estrus (P < .0001, 95% CI, 33.31-38.32 vs 42.09-47.47); and E, time between vaginal opening and first estrus (P < .001, 95% CI, 5.190-8.447 vs 10.24-15.23). In a 90-day fertility assay beginning at age 3 months, F1 VEH (n = 8) and pAMH (n = 12) were paired with naive C57BL/6 males to be assessed for F, latency to first litter (P = .55, 95% CI, 21.03-24.47 vs 18.03-51.30); G, average litter size per female (P = .24, 95% CI, 6.677-8.698 vs 6.024-7.960); H, total number of litters produced (P = .004, 95% CI, 2.659-3.841 vs 1.103-2.231); and I, number of litters produced in each month of the assay. Treatment × time, F2,54 = 7.812, P = .001; days 1 to 30 P = .51, 95% CI of diff, –0.2350 to 0.7350; days 31 to 60 P = .007, 95% CI of diff, 0.1400-1.110; days 61 to 90 P < .0001, 95% CI of diff, 0.8483-1.818. Dotted line in F indicates the 90-day fertility assay cutoff. Data were analyzed using B-H, Mann-Whitney test, or I, 2-way analysis of variance with Sidak multiple comparisons test. *P less than .05; **P less than .01; ***P less than .001; and ****P less than .0001.
First-Generation Prenatal Antimüllerian Hormone Females Exhibit Signs of Dysregulated Reproductive Axis Throughout the Lifespan
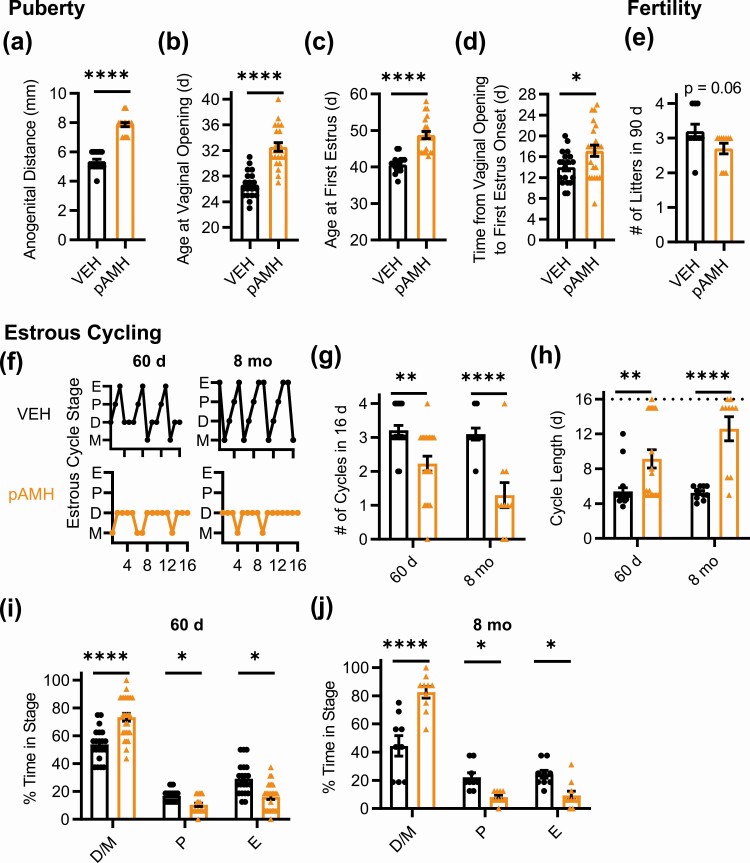
We began with phenotypes that had been previously reported to confirm the reproducibility of the model. Consistent with previous findings, F1 pAMH female offspring showed significantly increased anogenital distance at postnatal day 30 (Fig. 1b), indicative of in utero androgen exposure. pAMH females exhibited significant delays in puberty as measured by time to vaginal opening (Fig. 1C) and first E (Fig. 1D). First E was not merely shifted by the delayed pubertal onset, as there was also a prolonged period in between the onset of puberty and first E (Fig. 1E). pAMH did not induce significant body weight changes in females at any of time points assayed (Supplementary Fig. 1A) (42).
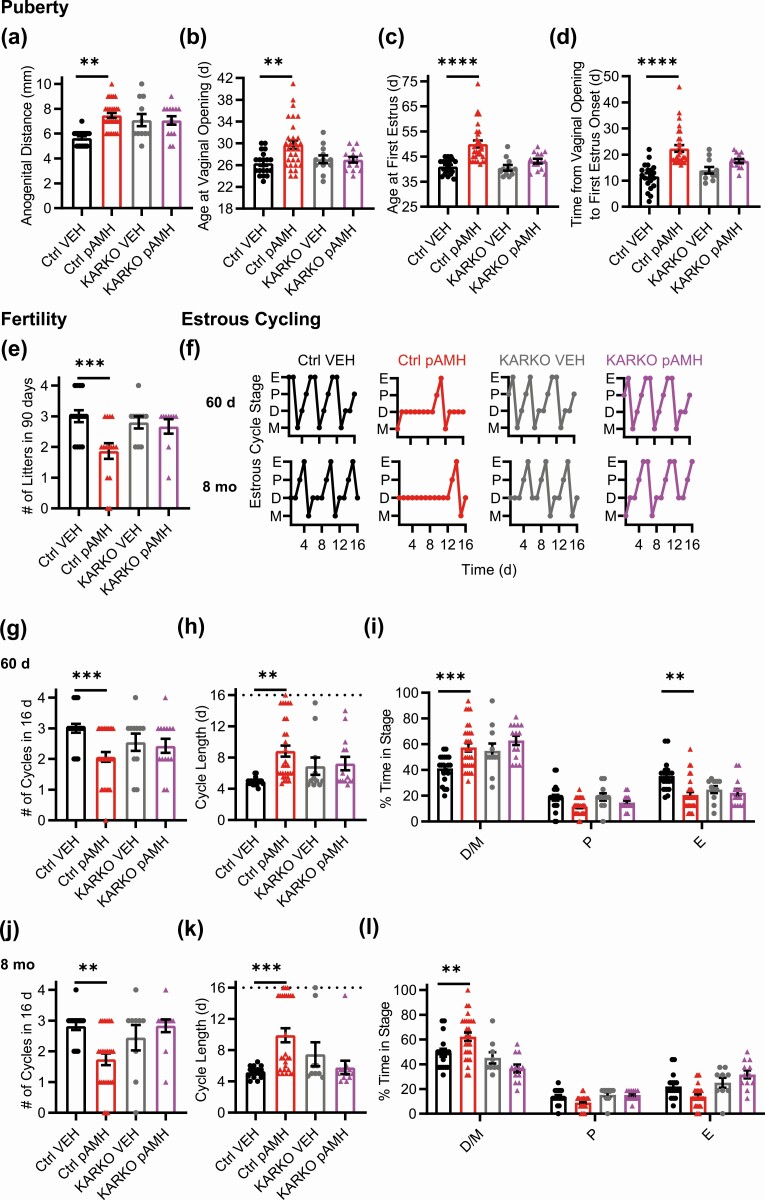
To measure fecundity, we performed a 90-day fertility assay in which pAMH females paired with experimentally naive wild-type males showed no delays in time to first litter (Fig. 1F) and normal litter sizes (Fig. 1G) compared to VEH. As previously reported, the number of litters throughout the assay was significantly decreased, including a few pAMH females that failed to produce any litters (Fig. 1H). To increase the temporal resolution of the assay, we examined each month of the assay separately. We found no deficits until after the first 30 days of the assay, when pAMH females were older and produced increasingly fewer litters over time (Fig. 1I).
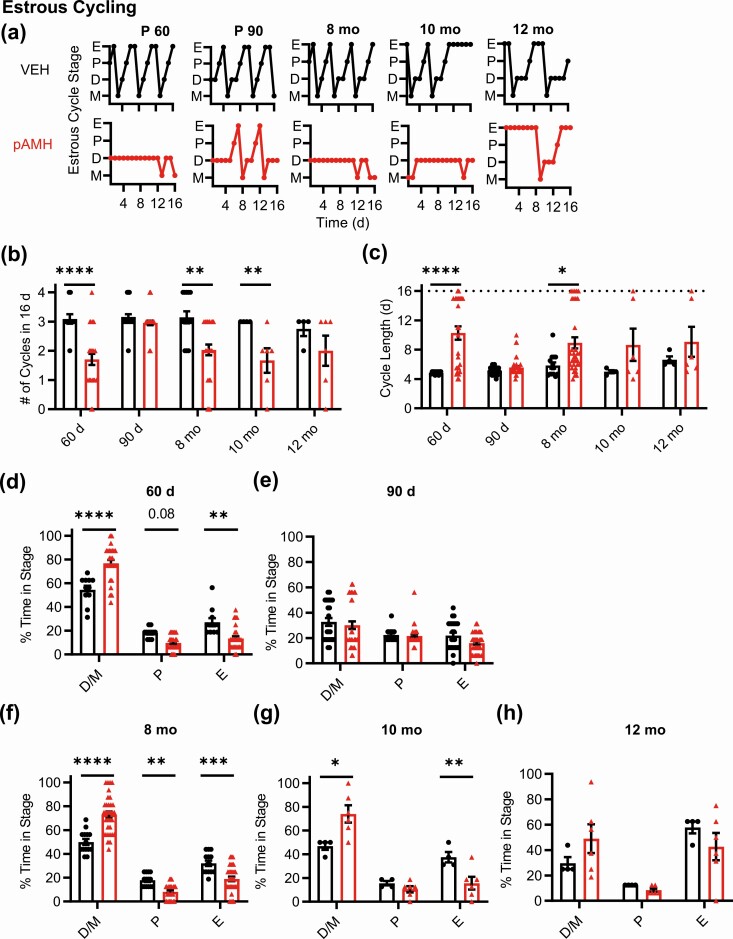
We next evaluated estrous cyclicity across the lifespan (Fig. 2A). Because some pAMH mice did not reach first estrus until 45-55 days old, we began our assessment at 60 days of age, which was the beginning of the range previously reported (23). At 60 days, pAMH females had significantly fewer cycles in a 16-day period (Fig. 2B) and longer cycle length (Fig. 2C). pAMH mice rarely entered pre-estrus/ovulatory proestrus and E stages, and spent significantly more time in D/M stages Fig. 2D). Interestingly, these cycling deficits were ameliorated at 90 days (Fig. 2A-2C and 2E), consistent with our fertility assay findings that pAMH mice have a brief window of normal reproductive capacity around age 3 months. Because prenatal androgen exposure can advance reproductive aging (26), we evaluated estrous cyclicity at age 8 to 12 months. At age 8 months, pAMH females exhibited cycling patterns identical to deficits observed at 60 days, and distinct from those expected in reproductive senescence (43), significantly fewer cycles during the evaluation period (see Fig. 2B), longer cycle length (see Fig. 2C), less time in proestrus/E, and more time in D/M (Fig. 2F). These pAMH-induced deficits persisted into age 10 months (see Fig. 2A-2C and 2G). By age 12 months, mice exhibited prolonged E patterns consistent with reproductive senescence (43) and were statistically no different from VEH controls (see Fig. 2A-2C and 2H).
Figure 2.
Prenatal antimüllerian hormone (pAMH) induces significant disruptions in estrous cycling across the lifespan of first-generation female offspring. A, Representative estrous cycles of the same vehicle (VEH; black) and pAMH (red) female offspring at various ages: postnatal day 60, 90, and age 8, 10, and 12 months. E, estrus; D, diestrus; M, metestrus; P, proestrus. B, Number of cycles and C, estrous cycle length, in the 16-day sampling period at 60 days old (VEH n = 11, pAMH n = 27), 90 d (VEH n = 25, pAMH = 41), age 8 months (VEH n = 14, pAMH n = 33), 10 months (VEH n = 4, pAMH n = 6), and 12 months (VEH n = 4, pAMH n = 6). Dotted line in C indicates the 16-day cutoff. Number of cycles: Treatment × Age, F4,161 = 3.910, P = .005; 60 d P < .0001, 95% CI of diff, 0.6523-2.122; 90 d P = .83, 95% CI of diff, –0.3125 to 0.7301; 8-month P < .0001, 95% CI of diff, 0.4573-1.768, 10-month P < .05, 95% CI of diff, 0.007199-2.659, 12-month P = .54, 95% CI of diff, –0.5761 to 2.076. Cycle length: treatment × age, F4,161 = 3.493, P = .009; 60 d P < .0001, 95% CI of diff, –8.309 to –2.537; 90 d P = .97, 95% CI of diff, –2.562 to 1.533; 8-month P = .011, 95% CI of diff, –5.656 to –0.5088, 10-month P = .3, 95% CI of diff, –8.875 to 1.541, 12-month P = .71, 95% CI of diff, –0.7.666 to 2.750. Percentage of time spent in each estrous cycle stage during the 16-day sampling period at D 60 d (treatment × stage, F2,108 = 26.75, P < .0001; D/M P < .0001, 95% CI of diff, 31.45-13.16; P P = .08, 95% CI of diff, –0.6871 to 17.61; E P = .001, 95% CI of diff, 4.700 to 22.99); E, 90 d (treatment × stage, F2,192 = 0.7096, P = .49); F, age 8 months (treatment × stage, F2,135 = 33.48, P < .0001; D/M P < .0001, 95% CI of diff, –31.24 to –14.59; P P = .016, 95% CI of diff, 1.389-18.04; E P < .001, 95% CI of diff, 4.879-21.53), G, 10 months (treatment × stage, F2,24 = 11.77, P < .001; D/M P = .003, 95% CI of diff, –45.71 to –8.471; P P = .86, 95% CI of diff, 13.40-23.84; E P = .02, 95% CI of diff, 3.255-40.50); and H, 12 months (treatment × stage, F2,24 = 2.247, P = .13). Data were analyzed using 2-way analysis of variance with Sidak multiple comparisons test.
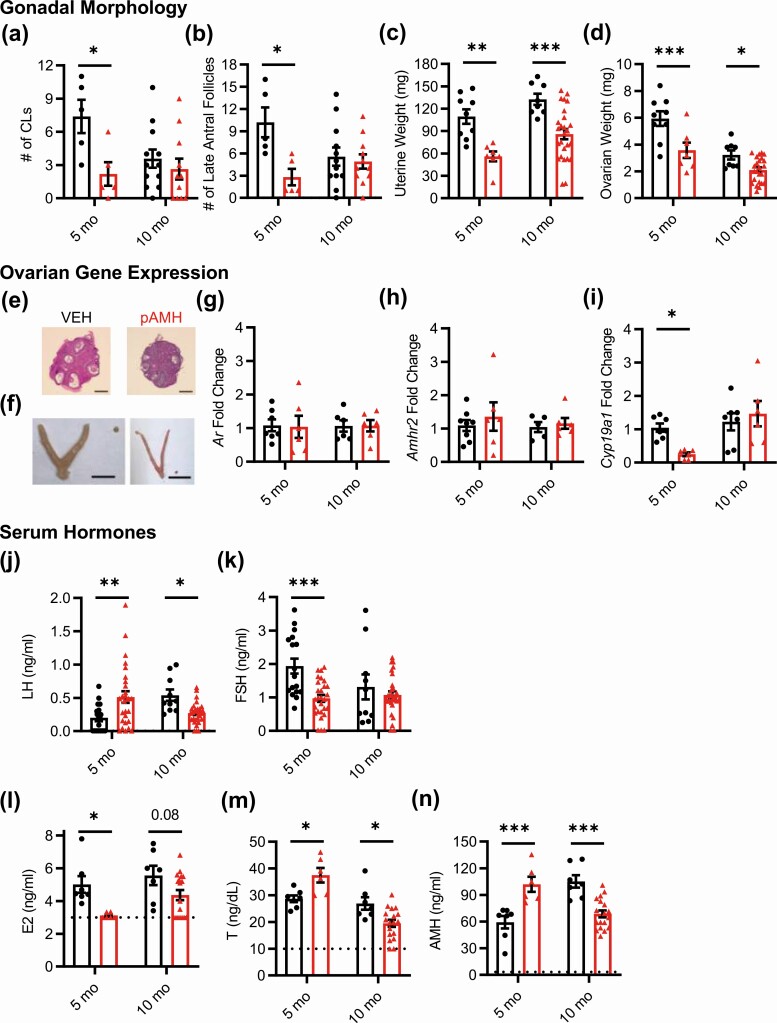
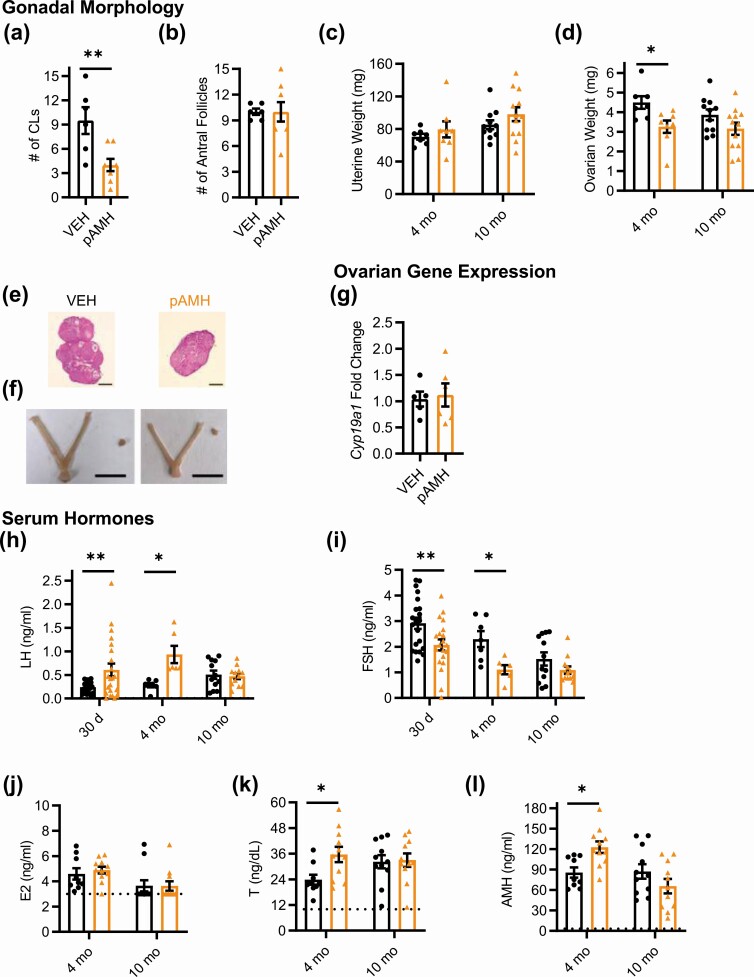
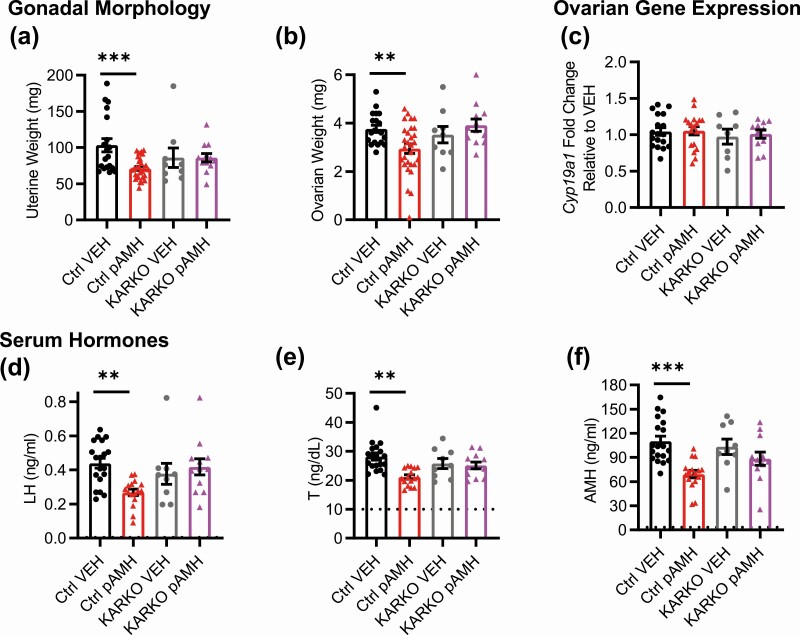
We harvested blood and reproductive tissues at 2 time points: age 5 months, when fertility had again begun to decline in pAMH females, and age 10 months to capture late adulthood effects. We assessed ovarian morphology for markers of ovulation, as well as follicular maturation. At age 5 months, pAMH ovaries showed a significantly decreased number of corpora lutea (Fig. 3A) and late antral follicles (Fig. 3B). No cystic follicles were observed, and there were no significant differences in the number of preantral/early antral follicles or atretic follicles (data not shown). Gross examination of the female reproductive tract revealed significantly smaller uteri (Fig. 3C) and ovaries (Fig. 3D and 3E) in pAMH compared to VEH. Although pAMH-induced decreased uterine and ovarian weights persisted until age 10 months, no significant differences were observed in the number of corpora lutea or late antral follicles at this time point. No changes in ovarian Ar or Amhr2 mRNA expression were observed (Fig. 3F and 3G). However, we did find decreased ovarian expression of Cyp19a1, which encodes the aromatase enzyme that converts androgens to estrogens (Fig. 3H).
Figure 3.
Prenatal antimüllerian hormone (pAMH) alters gonadal morphology, ovarian gene expression, and serum hormone levels across the lifespan of F1 females. A, Number of corpora lutea (CLs) (treatment × age, F1,29 = 3.576, P < .05; age 5 months P = .019, 95% CI of diff, 0.7734-9.627; 10 months P = .7, 95% CI of diff, 1.975-3.869); B, number of late antral follicles (treatment × age, F1,29 = 5.607, P = .02; age 5 months P = .008, 95% CI of diff, 1.810-12.99; 10 months P = .89, 95% CI of diff, –3.015 to 4.364); C, uterine weight (treatment, F1,46 = 26.32, P < .0001; age 5 months P = .002, 95% CI of diff, 18.33-88.69; 10 months P < .001, 95% CI of diff, 18.35-74.80); and D, ovarian weight (treatment, F1,46 = 20.61, P < .0001; age 5 months P < .001, 95% CI of diff, 0.9818-3.742; 10 months P < .05, 95% CI of diff, 0.002805-2.239) of female offspring at age 5 (vehicle; VEH n = 5, pAMH n = 5) and 10 (VEH n = 12, pAMH n = 11) months. Representative images from 5-month-old VEH and pAMH female offspring of E, hematoxylin and eosin-stained ovaries (scale bar, 400 µm), and F, harvested uteri and ovaries (scale bar, 1 cm). Messenger RNA expression levels of G, Ar (treatment × age, F1,22 = 0.01383, P = .91; treatment, F1,22 = 0.007455, P = .93); H, Amhr2 (treatment × age, F1,22 = 0.09367, P = .76; treatment, F1,22 = 0.5490, P = .47); and I, Cyp19a1 (treatment × age, F1,22 = 4.883, P = .04; age 5 months P < .05, 95% CI of diff, 0.0002904-1.594; 10 months P = .73, 95% CI of diff, –1.038 to 0.5562) from ovaries of VEH (n = 7) and pAMH (n = 6) female offspring at age 5 and 10 months. Serum J, luteinizing hormone (LH), and K, follicle-stimulating hormone (FSH) levels, at age 5 (VEH n = 17, pAMH n = 28) and 10 (VEH n = 10, pAMH = 29) months. LH: treatment × age, F F1,80=15.33, P < .001; 5 months P = .003, 95% CI of diff, –0.5301 to –0.09483; 10 months P = .04, 95% CI of diff, 0.009950-0.5291. FSH: treatment × age, F1,80 = 4.206, P = .04; 5 months P = .0001, 95% CI of diff, 0.4462-1.476; 10 mo P = .61, 95% CI of diff, –0.3768 to 0.8554. Serum L, estradiol (E2); M, testosterone (T); and N, AMH levels at age 5 (VEH n = 7, pAMH n = 6) and 10 (VEH n = 7, pAMH = 18) months. E2: treatment, F1,34 = 11.86, P = .002; 5 months P = .021, 95% CI of diff, 0.2587-3.544; 10 months P = .08, 95% CI of diff, –0.1190 to 2.511. T: treatment × age, F1,34 = 16.74, P < .001; 5 months P = .01, 95% CI of diff, –16.15 to –1.579; 10 months P = .01, 95% CI of diff, 1.622-13.29. AMH: treatment × age, F1,34 = 37.71, P < .0001; 5 months P < .001, 95% CI of diff, –66.10 to –19.04; 10 months P < .001, 95% CI of diff, 17.71-55.38. Dotted lines in L to N indicate sensitivity threshold of the hormone assay. Data were analyzed using 2-way analysis of variance with Sidak multiple comparisons test.
Finally, we examined serum levels of pituitary gonadotropins and sex steroid hormones at ages 5 and 10 months (Fig. 3I-3M). At age 5 months, LH was significantly increased and FSH was significantly decreased in pAMH females compared to VEH. Consistent with decreased uterine weight and aromatase expression, E2 was decreased and T was increased at this time point. AMH levels in pAMH females were also significantly elevated. Notably, this hormonal profile was not maintained at age 10 months, when we observed significant reversals of these patterns. At the late adulthood time point, pAMH females had significantly decreased LH, T, and AMH levels. There was a trend toward decreased E2, but no difference in FSH was observed between pAMH and VEH at age 10 months.
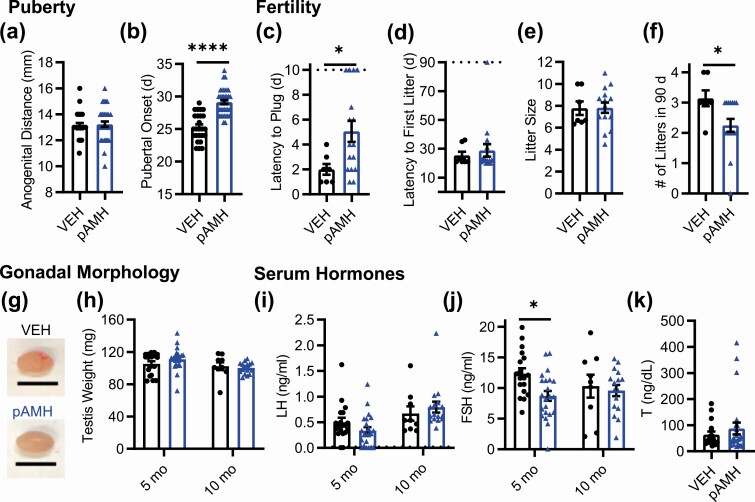
First-Generation Prenatal Antimüllerian Hormone Male Offspring Also Show Significant Reproductive Deficits
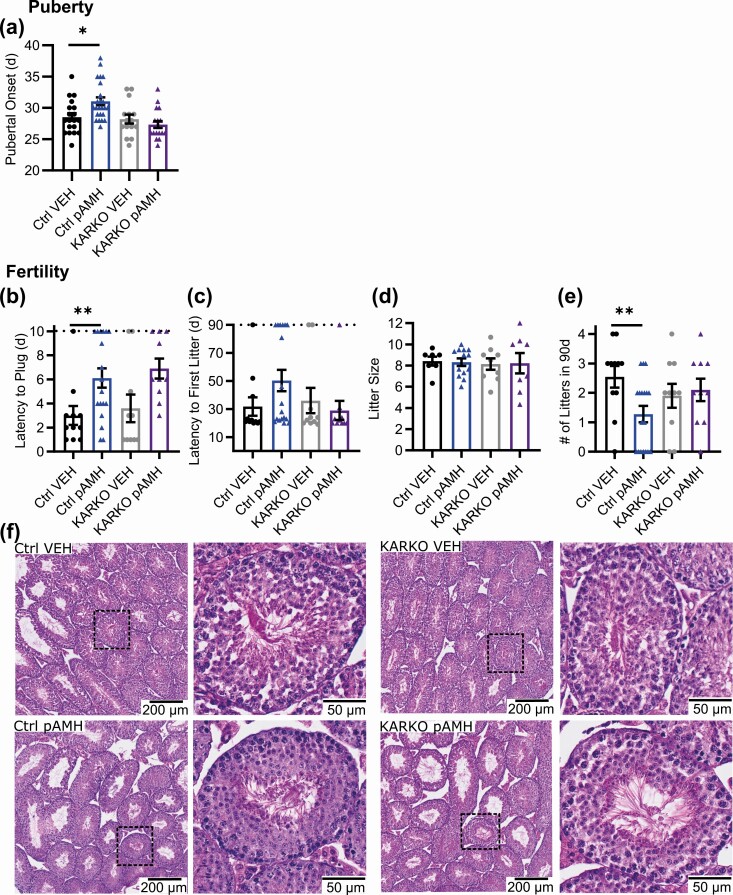
Because an androgenic environment in utero has been linked to neuroendocrine disruption in males (44, 45), F1 male offspring were generated and phenotyped concurrently with their female littermates. No differences in anogenital distance were observed between pAMH and VEH males (Fig. 4A). As with the females, pAMH induced significant delays in pubertal onset in male offspring, as measured by preputial separation (Fig. 4B). Again, pAMH did not induce significant body weight changes in males at any time point assayed (Supplementary Fig. 1B) (42). Copulation of pAMH vs VEH males was measured in a 10-day plugging assay. Whereas all VEH males plugged within 4 days, pAMH males showed significant delays and several did not plug by the 10-day cutoff (Fig. 4C). The pAMH-induced latency to plug did not result in an increased time to first litter in a subsequent 90-day fertility assay (Fig. 4D). Although litter size was also normal (Fig. 4E), pAMH did significantly decrease the number of litters produced in the duration of the assay (Fig. 4F). We found no differences in testis weights (Fig. 4G and 4H). Serum LH (Fig. 4I) and T (Fig. 4J) levels did not differ, but FSH was significantly decreased at age 5 months (Fig. 4K). FSH levels normalized by age 10 months.
Figure 4.
Prenatal antimüllerian hormone (pAMH) induces significant reproductive deficits in male offspring. A, Anogenital distance in first-generation vehicle (VEH; black, n = 25) and pAMH (blue, n = 38) males was measured at 30 days old (P = .54, 95% CI, 12.58-13.59 vs 12.81-13.67). Assessment for pubertal onset began at 21 days, as measured by B, preputial separation (P < .0001, 95% CI, 24.41-26.15 vs 28.52-29.80). In a 90-day fertility assay beginning at age 3 months, VEH (n = 7) and pAMH (n = 16) were mated with naive C57BL/6 females to be assessed for C, time to plug by a 10-day cutoff indicated by the dotted line (P = .03, 95% CI, 0.9321-3.068 vs 3.243-6.882); D, latency to first litter by the 90-day cutoff indicated by the dotted line (P = .73, 95% CI, 19.02-31.83 vs 19.53-38.09); E, average litter size per male (P = .72, 95% CI, 6.258-9.313 vs 6.834-8.832); and F, total number of litters (P = .03, 95% CI, 2.505-3.781 vs 1.794-2.706). G, Representative images of testes harvested from 10-month-old VEH and pAMH male offspring (scale bar, 1 cm). H, Testis weight; I, serum luteinizing hormone (LH); and J, serum follicle-stimulating hormone (FSH) of male offspring at age 5 (VEH n = 18, pAMH n = 22) and 10 (VEH n = 9, pAMH n = 16) months. Testis weight: treatment × age, F1,61 = 1.403, P = .24; treatment, F1,61 = 0.2694, P = .61. LH: treatment × age, F1,61 = 2.206, P = .14; treatment, F1,61 = 0.04453, P = .83. FSH: treatment, F1,61 = 4.259, P = .04; 5 months P = .01, 95% CI of diff, 0.7031-6.562; 10 months P = .89, 95% CI of diff, –.126 to 4.556. K, Serum testosterone at age 10 months (VEH n = 17, pAMH n = 14) (P = .97, 95% CI, 39.49-89.05 vs 41.24-133.5). Data were analyzed using A to F, Mann-Whitney test, or H to K, 2-way analysis of variance with Sidak multiple comparisons test.
Together, our findings in the F1 generation demonstrated that prenatal AMH exposure robustly altered the neuroendocrine reproductive axis for both sexes. Alterations observed extended beyond previously reported peripubertal and early adult time points and well into late adulthood.
Second-Generation Prenatal Antimüllerian Hormone Females Exhibit Signs of Dysregulated Reproductive Axis Throughout the Lifespan
Notably, we found that reproductive-aged F1 pAMH females had significant alterations in their hormonal profile. Specifically, elevations in AMH led us to investigate whether F2 offspring of F1 pAMH females also exhibit reproductive deficits (see Fig. 1A). As in the F1 generation, F2 pAMH females had significantly increased anogenital distance at 30 days old (Fig. 5A) and robust delays in onset of puberty as measured by vaginal opening (Fig. 5B), first E (Fig. 5C), and the time between vaginal opening and first E (Fig. 5D). Again, pAMH did not induce significant body weight changes in F2 female mice (Supplementary Fig. 1C) (42). In a fertility assay with experimentally naive wild-type males, F2 pAMH females showed a trend toward decreased number of litters produced in the 90-day duration (Fig. 5E). pAMH significantly disrupted estrous cyclicity at 60 days and age 8 months in the F2 generation (Fig. 5F). At both time points, F2 pAMH females had fewer cycles in a 16-day period (Fig. 5G), longer cycle length (Fig. 5H), and spent significantly more time in the infertile D/M stages (Fig. 5I and 5J) compared to F2 VEH controls. Ovarian morphology at age 4 months revealed a significantly decreased number of corpora lutea (Fig. 6A), and no significant differences in the number of antral follicles (Fig. 6B). There were no changes in uterine weight (Fig. 6C), but ovarian weight was decreased (Fig. 6D and 6E) at this time point. There were no changes in Cyp19a1 expression (Fig. 6F). Unlike in the F1 generation, the F2 generation pAMH females did not have smaller uteri or ovaries at age 10 months (Fig. 6C and 6E).
Figure 5.
Prenatal antimüllerian hormone (pAMH) phenotypes extend into second-generation F2 offspring. A, Anogenital distance in F2 vehicle (VEH; n = 19) and pAMH (n = 22) females was measured at 30 days old (P < .0001, 95% CI, 5.081-5.656 vs 7.580-8.147). Assessment for pubertal onset began at 21 days, as measured by B, time to vaginal opening (P < .0001, 95% CI, 25.68-27.69 vs 31.13-33.96); C, time to first estrus (P < .0001, 95% CI, 39.64-41.73 vs 46.76-50.79); and D, time between vaginal opening and first estrus (P = .03, 95% CI, 12.48-15.52 vs 14.89-19.39). E, In a fertility assay beginning at age 3 months, VEH and pAMH F2 females (n = 10) were mated with naive males and assessed for number of litters produced in 90 days (P = .06, 95% CI, 2.748-3.652 vs 2.354-3.046). F, Representative estrous cycles of the same F2 VEH and pAMH females at 60 days and 8 months. E, estrus; D, diestrus; M, metestrus; P, proestrus. G, Number of cycles in VEH and pAMH females at 60 days (n = 19-22) and 8 months (n = 10). Treatment, F1,57 = 33.65, P ≤ .0001; 60 d P = .002, 95% CI of diff, 0.3511-1.615; 8 months P < .0001, 95% CI of diff, 0.8973-2.703. H, Estrous cycle length in VEH and pAMH females at 60 days (n = 19-22) and 8 months (n = 10). Treatment, F1,57 = 3.725, P < .0001; 60 d P = .002, 95% CI of diff, –6.197 to –1.260]; 8 months P < .0001, 95% CI of diff, –10.88 to –3.817. Dotted line in H indicates the 16-day cutoff. Data were analyzed using A to E, Mann-Whitney test, or G to J, 2-way analysis of variance with Sidak multiple comparisons test.
Figure 6.
F2 prenatal antimüllerian hormone (pAMH) females have altered gonadal hormones and serum hormones. Number of A, corpora lutea (CLs) (P = .006, 95% CI, 5.263-13.74 vs 2.213-5.787), and B, antral follicles in 4- to 6-month-old F2 vehicle (VEH) and pAMH (n = 6-8). C, Uterine weight (treatment × age, F1,34 = 0.06281, P = .80; treatment, F1,34 = 1.943, P = .17), and D, ovarian weight (treatment, F1,34 = 9.523, P = .004; 4 months P = .033, 95% CI of diff, 0.09057-2.384; 10 months P = .16, 95% CI of diff, –0.2190 to 1.631) of F2 females at age 4 (n = 7-8) and 10 (n = 11-12) months. Representative images from 4-month-old F2 VEH and pAMH females of E, hematoxylin and eosin–stained ovaries (scale bar, 400 µm), and F, harvested uteri and ovaries (scale bar, 1 cm). G, Messenger RNA expression levels of Cyp19a1 from F2 ovaries at age 4 months (n = 5-6, P > .99, 95% CI, 0.6448-1.435 vs 0.5545-1.682). Serum levels of H, luteinizing hormone (LH), and I, follicle-stimulating hormone (FSH) at 30 days old (n = 19-22), and 4 (n = 6-7) and 10 (n = 12) months. LH: treatment × age, F2,72 = 3.496, P = .04; 30 days P = .01, 95% CI of diff, –0.6729 to –0.06268; 4 months P = .01, 95% CI of diff, –1.192 to –0.1082; 10 months P > .99, 95% CI of diff [-0.3638, 0.4317]. FSH: Treatment x Age, F2,72 = 0.8605, P = .43; 30 days P = .008, 95% CI of diff, 0.1793-1.510; 4 months P < .05, 95% CI of diff, 0.007765-2.378; 10 months P = .53, 95% CI of diff, –0.4343 to 1.305. Serum J, estradiol (E2); K, testosterone (T); and L, AMH levels at age 4 and 10 months (n = 9-11). E2: treatment × age, F1,38 = 0.1656, P = .69; Treatment, F1,38 = 0.1399, P = .71. T: treatment × age, F1,38 = 3.041, P < .05; 4 months P = .03, 95% CI of diff, –22.46 to –1.134; 10 months P = .98, 95% CI of diff, –10.91 to 9.330. AMH: treatment × age, F1,38 = 9.440, P = .004; 4 months P = .02, 95% CI of diff, –69.49 to –4.841; 10 months P = .21, 95% CI of diff, –9.030 to 52.27. Dotted lines in J to L indicate sensitivity threshold of the hormone assay. Data were analyzed using A, B, and G, Mann-Whitney test, or C, D, H to L, 2-way analysis of variance with Sidak multiple comparisons test.
When we measured serum hormone levels in F2 females, significant changes were found at early time points that were ameliorated by late adulthood. Peripubertal gonadotropin levels were significantly altered in F2 females (Fig. 6G and 6H), consistent with high LH and low FSH observed in daughters of PCOS women during puberty (46). Elevated LH and decreased FSH was also present at age 4 months (see Fig. 6G and 6H). No differences in E2 were detected (Fig. 6I); however, F2 pAMH females had significantly increased testosterone (Fig. 6J) and AMH (Fig. 6K) at age 4 months. There were no pAMH-induced hormonal changes at age 10 months (see Fig. 6G and 6K).
Second-Generation Prenatal Antimüllerian Hormone Male Offspring Also Show Significant Reproductive Deficits
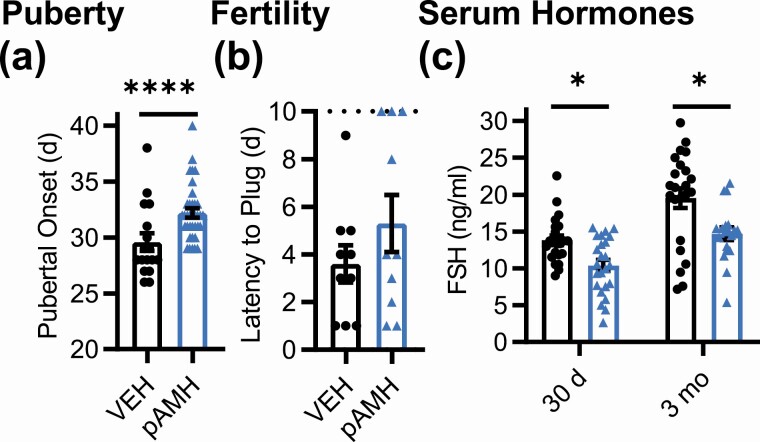
We also examined male offspring of the F2 generation, as both sexes in the F1 generation were affected. In F2 male offspring, significantly delayed pubertal onset was again found in the pAMH group compared to VEH controls (Fig. 7A) without changes in body weight (Supplementary Fig. 1D) (42). Copulation as measured by time to plug was not significantly affected in the F2 generation, although 30% of the pAMH group did not plug by the 10-day cutoff (Fig. 7B). Similar to F2 pAMH females, serum FSH in F2 pAMH males was significantly decreased at 30 days and 3 months old (Fig. 7C).
Figure 7.
Prenatal antimüllerian hormone (pAMH) phenotypes extend into second-generation F2 male offspring. A, Pubertal onset in F2 vehicle (VEH; black, n = 19) and pAMH (light blue, n = 22) males as measured by preputial separation (P = .001, 95% CI, 27.92-31.25 vs 31.35-33.04). B, Time to plug in F2 VEH and pAMH males by a 10-day cutoff, indicated by the dotted line (n = 10, P = .42, 95% CI, 1.841-5.359 vs 2.580-8.020). C, Serum follicle-stimulating hormone (FSH) levels at 30 days old (VEH n = 17, pAMH n = 28) and age 3 (VEH n = 17, pAMH n = 28) months in F2 males. FSH: treatment, F1,85 = 19.06, P < .0001; 30 days P = .02, 95% CI of diff, 0.4623-6.386; 3 months P = .001, 95% CI of diff, 1.716-8.010. Data were analyzed using A and B, Mann-Whitney test, or C, 2-way analysis of variance with Sidak multiple comparisons test.
These findings demonstrated that prenatal AMH exposure has lasting effects on offspring of both sexes and that resulting alterations could be carried on through the pAMH female F1 pregnancy into the subsequent generation. After characterizing a full profile of pAMH-induced reproductive phenotypes, we next sought to probe at a possible androgen-mediated mechanism.
Androgen Receptor in Kisspeptin Cells Is Necessary for all Prenatal Antimüllerian Hormone–Induced Phenotypes in Females
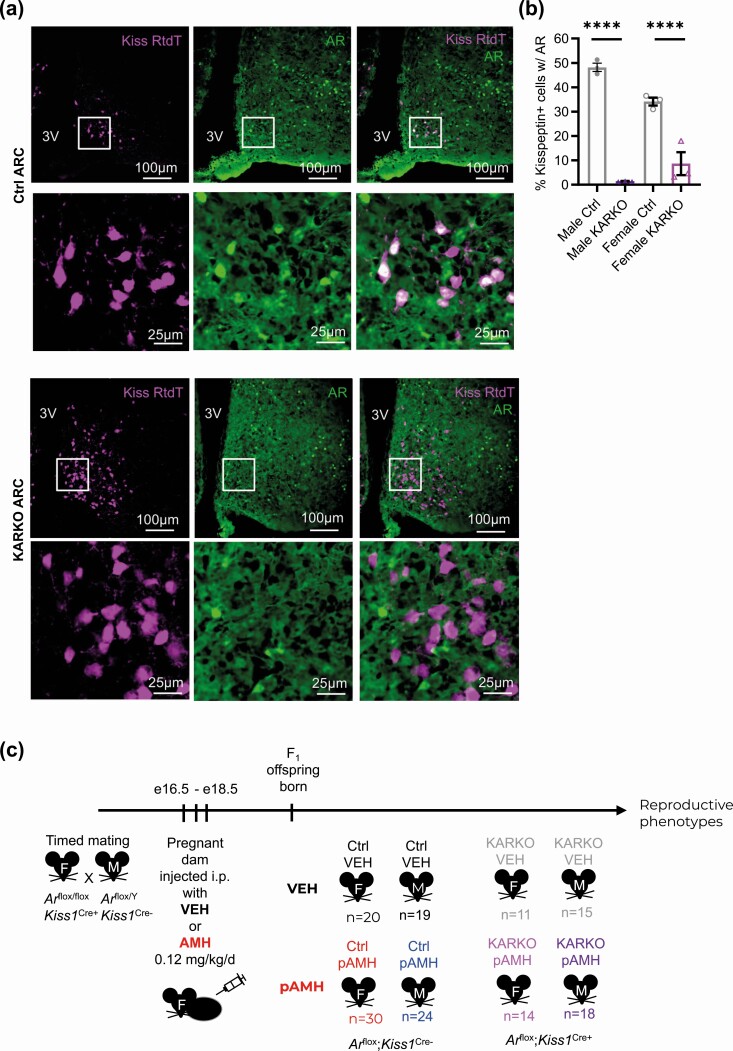
To test our hypothesis that pAMH-induced phenotypes are mediated by androgen signaling at the level of kisspeptin cells, we generated a KARKO mouse line by crossing together previously established Ar-flox (37) and Kiss1-Cre mice (38). Exon 2 of the Ar gene is flanked by LoxP sites, allowing for the functional AR protein to be present in Arflox/flox;Kiss1Cre– females and Arflox/Y;Kiss1Cre– males (Ctrl) and excised in kisspeptin-expressing cells of the Arflox/flox; Kiss1Cre+ females and Arflox/Y;Kiss1Cre+ males (KARKO). Specificity of the knockout was confirmed by crossing this KARKO mouse line with a Rosa-tdTomato reporter mouse line to visualize kisspeptin-expressing cells, followed by immunohistochemistry on sections containing the ARC region of hypothalamus and determined colocalization of AR to kisspeptin-expressing cells (Fig. 8A). Compared to littermate controls (Arflox/flox;Kiss1Cre– females and Arflox/Y; Kiss1Cre– males), KARKO mice of both sexes had significant reductions in the percentage of kisspeptin-expressing cells containing AR in the ARC (48.2 vs 1.4% and 34.1 vs 8.8% in males and females, respectively; Fig. 8B). Using fluorescent in situ hybridization to examine Ar expression in gonads of wild-type animals, we found very few Kiss1-expressing cells overall, although there was overlap with Ar in both ovaries and testes in rare cells (Supplementary Figs. 2 and 3, respectively) (42).
Figure 8.
Generation of prenatal antimüllerian hormone (pAMH) offspring in the KARKO mouse line. A, Representative fluorescent images of the arcuate nucleus (ARC) in adult males (M) from the KARKO Rosa-tdTomato reporter line. Colocalization (white) of kisspeptin Rosa-tdTomato (Kiss RtdT, magenta) and androgen receptor (AR, green) is apparent in Ctrl but not KARKO sections. Localization of AR to the nucleus is well visualized given the high circulating testosterone levels in M. B, Percentage of kisspeptin-expressing cells with AR in Ctrl and KARKO M and females (F; n = 3). Genotype × sex, F1,8 = 15.81, P = .004; M P < .0001, 95% CI of diff, 36.28-57.19; F P < .001, 95% CI of diff, 14.484-35.76. Data were analyzed using 2-way analysis of variance with Sidak multiple comparisons test. C, Schematic of experimental design. Timed matings between Arflox/flox;Kiss1Cre+ (KARKO) females and Arflox/Y;Kiss1Cre– (Ctrl) M were set up and dams received either vehicle (VEH) or AMH injections on embryonic days (e) 16.5, 17.5, and 18.5. Offspring were born into treatment groups (VEH or pAMH) for both sexes (F and M) in both genotypes (Ctrl and KARKO).
After confirming the successful recombination of the Ar-flox allele in kisspeptin-expressing neurons, we generated F1 pAMH offspring in the KARKO mouse line. Arflox/flox; Kiss1Cre+ females were mated with Arflox/Y;Kiss1Cre– males. Pregnant dams were injected with AMH or VEH late in gestation, then offspring of both genotypes (KARKO: Arflox/flox; Kiss1Cre+ females and Arflox/Y;Kiss1Cre+ males vs Ctrl: Arflox/flox; Kiss1Cre– females and Arflox/Y;Kiss1Cre– males) and sexes were born and assessed for pAMH-induced phenotypes after weaning (Fig. 8C). Importantly, we observed no statistical differences between KARKO and Ctrl within the VEH group on any measure, indicating that AR knockout in kisspeptin cells alone is insufficient to cause significant reproductive deficits in either males or females.
As in our first set of experiments, pAMH significantly increased the anogenital distance in Ctrl females (Fig. 9A). However, in KARKO females, there was no difference between the VEH and pAMH groups. Similarly, pAMH delayed the onset of puberty in Ctrl, but not KARKO females, as measured by vaginal opening (Fig. 9B), first E (Fig. 9C), and prolonged time between them (Fig. 9D) without changes in body weight in either genotype (Supplementary Fig. 1E) (42). pAMH decreased the number of litters in a fertility assay for Ctrl, but had no effect in the KARKO group (Fig. 9E). Estrous cyclicity was significantly disrupted in Ctrl pAMH females at 60 days and 8 months, but no differences were observed between KARKO pAMH and KARKO VEH (Fig. 9F-9L). At age 10 months, pAMH-induced decreases in uterine (Fig. 10A) and ovarian weight (Fig. 10B) were observed in Ctrl but not KARKO females. No differences in ovarian Cyp19a1 expression were detected (Fig. 10C), consistent with our findings at this time point in our first study. LH (Fig. 10D), T (Fig. 10E), and AMH (Fig. 10F) were all decreased in Ctrl but not KARKO females. KARKO effects on all significant pAMH-induced phenotypes in females are summarized below (Table 1).
Figure 9.
Prenatal antimüllerian hormone (pAMH) failed to induce reproductive phenotypes in KARKO female offspring. A, Anogenital distance measurements at 30 days old in Ctrl (vehicle [VEH], black, n = 20, pAMH, red, n = 30) and KARKO (VEH, gray, n = 11, pAMH, magenta, n = 14) females (treatment × genotype, F1,71 = 10.61, P = .002; Ctrl P < .0001, 95% CI of diff, –2.567 to –1.066; KARKO P > .99, 95% CI of diff, –1.028 to 1.067). Assessment for pubertal onset began at 21 days, as measured by B, time to vaginal opening (treatment × genotype, F1,71 = 5.000, P = .03; Ctrl P < .001, 95% CI of diff, –5.532 to –1.302; KARKO P > .99, 95% CI of diff, –2.799 to 2.966); C, time to first estrus (treatment × genotype, F1,71 = 5.462, P = .02; Ctrl P < .0001, 95% CI of diff, –12.57 to –5.333; KARKO P = .43, 95% CI of diff, –7.646 to 2.451); and D, time between vaginal opening and first estrus (treatment × genotype, F1,71 = 6.527, P = .012; Ctrl P < .0001, 95% CI of diff, –14.31 to –6.955; KARKO P = .22, 95% CI of diff, –8.705 to 1.562). E, In a fertility assay beginning at age 3 months, Ctrl (VEH n = 15, pAMH n = 15), and KARKO (VEH n = 10, pAMH n = 9) were assessed for number of litters produced in 90 days (treatment × genotype, F1,45 = 4.454, P = .04; Ctrl P < .001, 95% CI of diff, 0.4513-1.815; KARKO P = .92, 95% CI of diff, –0.7249 to 0.9916). F, Representative estrous cycles of the same females of each genotype and treatment at 60 d and age 8 months. D, diestrus; E, estrus; M, metestrus; P, proestrus. In a 16-day sampling period for 60 d Ctrl (VEH, black, n = 20, pAMH, red, n = 30) and KARKO (VEH, gray, n = 11, pAMH, magenta, n = 14) females; G, number of cycles (treatment × genotype, F1,71 = 4.012, P < .05; Ctrl P < .001, 95% CI of diff, 0.3909-1.476; KARKO P = .92, 95% CI of diff, –0.6402 to 0.8740); H, estrous cycle length (treatment × genotype, F1,71 = 5.103, P = .03; Ctrl P = .0001, 95% CI of diff, –6.004 to –1.760; KARKO P = .96, 95% CI of diff, –3.261 to 2.620); and I, percentage of time spent in each estrous cycle stage (treatment × genotype × stage, F2,213 = 2.677, P = .04; Ctrl D/M P < .001, 95% CI of diff, –27.65 to –4.775; Ctrl P P = .67, 95% CI of diff, –4.415 to 18.46; Ctrl E P = .002, 95% CI of diff, 3.357-26.23; KARKO D/M P = .88, 95% CI of diff, –24.02 to 7.903; KARKO P P > .99, 95% CI of diff, –11.57 to 20.36; KARKO E P > .99, 95% CI of diff, –13.51 to 18.41). In the 16-day sampling period in 8-month-old Ctrl (VEH n = 17, pAMH n = 27), and KARKO (VEH n = 9, pAMH n = 12) females, J, number of cycles (treatment × genotype, F1,61 = 5.563, P = .02; Ctrl P = .002, 95% CI of diff, 0.3329-.597; KARKO P = .9, 95% CI of diff, –1.067 to 0.7337); K, estrous cycle length (treatment × genotype, F1,61 = 10.25, P = .002; Ctrl P < .001, 95% CI of diff, –7.352 to –2.090; KARKO P = .52, 95% CI of diff, –2.076 to 5.418); and L, percentage of time spent in each estrous cycle stage (treatment × genotype × stage, F2,213 = 2.677, P = .04; Ctrl D/M P < .001, 95% CI of diff, –27.65 to –4.775; Ctrl P P = .67, 95% CI of diff, –4.415 to 18.46; Ctrl E P = .002, 95% CI of diff, 3.357-26.23; KARKO D/M P = .88, 95% CI of diff, –24.02 to 7.903; KARKO P P > .99, 95% CI of diff, –11.57 to 20.36; KARKO E P > .99, 95% CI of diff, –13.51 to 18.41). Dotted lines in H and K indicates the 16-day cutoff.
Figure 10.
Prenatal antimüllerian hormone (pAMH) failed to induce late adulthood phenotypes in KARKO female offspring. A, Uterine weight; B, ovarian weight; and C, ovarian Cyp19a1 messenger RNA expression of Ctrl (vehicle [VEH] n = 19, pAMH n = 29) and KARKO (VEH n = 9, pAMH n = 12) females at age 10 months. Serum levels of D, luteinizing hormone (LH); E, testosterone (T); and F, AMH in 10-month-old Ctrl (VEH n = 19, pAMH n = 19) and KARKO (VEH n = 9, pAMH n = 12) females. Dotted lines in E and F indicate the sensitivity threshold of the hormone assay. Data were analyzed using 2-way analysis of variance with Sidak multiple comparisons test. Uterine weight: treatment × genotype, F1,65 = 4.566, P = .04; Ctrl P = .001, 95% CI of diff, 12.95-51.16; KARKO P > .99, 95% CI of diff, –28.54 to 28.54. Ovarian weight: treatment × genotype, F1,65 = 6.071, P = .02; Ctrl P = .008, 95% CI of diff, 0.1916-1.445; KARKO P = .56, 95% CI of diff, –1.331 to 0.5419. Cyp19a1: treatment × genotype, F1,52 = 0.04301, P = .84; Treatment, F1,51 = 0.1094, P = 0.74. LH: treatment × genotype, F1,52 = 7.828, P = 0.007; Ctrl P = .001, 95% CI of diff, 0.06461-0.2771; KARKO P = .75, 95% CI of diff, –0.1789 to 0.09727. T: Treatment × genotype, F1,52 = 6.577, P = .01; Ctrl P < .0001, 95% CI of diff, 3.572-10.56; KARKO P = .93, 95% CI of diff, –3.851 to 5.225]. AMH: Treatment × genotype, F1,52 = 4.082, P < .05; Ctrl P < .0001, 95% CI of diff, 22.90-59.61; KARKO P = .29, 95% CI of diff, –9.009 to 38.70.
Table 1.
Summary of significant transgenerational prenatal antimüllerian hormone phenotypes in female control and kisspeptin-specific androgen receptor knockout mice
| Age | Phenotype | F1 generation | F1generation KARKO | F2 generation | |
|---|---|---|---|---|---|
| Peripubertal and early adulthood, 30-60 d | Anogenital distance | ↑a | NS | ↑a | |
| Pubertal onset | Vaginal opening | ↑a | NS | ↑a | |
| First estrus | ↑a | NS | ↑a | ||
| Serum hormones | LH | ↑ | |||
| FSH | ↓ | ||||
| Estrous cycling | Time in D/M | ↑a | NS | ↑a | |
| No. of cycles | ↓a | NS | ↓a | ||
| Cycle length | ↑ | NS | ↑ | ||
| Adulthood, 3-5 mo | Fertility, No. of litters | ↓a | NS | NS | |
| Uterine wt | ↓ | NS | |||
| Ovarian changes | Wt | ↓ | ↓ | ||
| No. of CLs | ↓a | ↓a | |||
| No. of late antral follicles | ↓a | ↓ | |||
| Cyp19a1 mRNA | ↓ | NS | |||
| Serum hormones | LH | ↑ | ↑a | ||
| FSH | ↓ | ↓ | |||
| E2 | ↓ | NS | |||
| T | ↑ | ↑a | |||
| AMH | ↑ | ↑ | |||
| Late adulthood, 8-10 mo | Estrous cycling | Time in D/M | ↑ | NS | ↑ |
| No. of cycles | ↓ | NS | ↓ | ||
| Cycle length | ↑ | NS | ↑ | ||
| Uterine wt | ↓ | NS | NS | ||
| Serum hormones | LH | ↑ | NS | ↑ | |
| T | ↑ | NS | ↑ | ||
| AMH | ↑ | NS | ↑ |
Abbreviations: AMH, antimüllerian hormone; CL, corpora lutea; E2, estradiol; FSH, follicle-stimulating hormone; KARKO, kisspeptin-specific androgen receptor knockout; LH, luteinizing hormone; mRNA, messenger RNA; NS, not significant; T, testosterone.
All but One of the Prenatal Antimüllerian Hormone Phenotypes Were Eliminated in Male Kisspeptin-Specific Androgen Receptor Knockout Offspring
In males, we saw similar pAMH-induced pubertal delays in Ctrl but not KARKO (Fig. 11A). Interestingly, plugging behavior was significantly delayed by pAMH both in Ctrl and KARKO (Fig. 11B). This was the only pAMH-induced phenotype assayed in either sex that was not eliminated in the KARKO group. Consistent with our prior experiment, no statistical differences were found on latency to first litter (Fig. 11C) or litter size (Fig. 11D) when paired with experimentally naive wild-type females. The number of litters produced during the duration of the assay was significantly reduced in Ctrl pAMH vs Ctrl VEH, but not in KARKO males (Fig. 11E). We found no differences in gross testicular morphology among any of the groups (Fig. 11F). Body weight was also unaffected (Supplementary Fig. 1F) (42). KARKO effects on all significant pAMH-induced phenotypes in males are summarized in Table 2.
Figure 11.
Prenatal antimüllerian hormone (pAMH) failed to induce reproductive phenotypes in KARKO male offspring. A, Pubertal onset measured by preputial separation in Ctrl (vehicle [VEH], black, n = 19, pAMH, blue, n = 24) and KARKO (VEH, gray, n = 15, pAMH, purple, n = 18) males. In a 90-day fertility assay beginning at age 3 months, Ctrl (VEH n = 7, pAMH n = 16) and KARKO (VEH n = 7, pAMH n = 16) males were assessed for B, time to plug by a 10-day cutoff indicated by the dotted line; C, latency to first litter by the 90-day cutoff indicated by the dotted line; D, average litter size per male; and E, total number of litters. Data were analyzed using 2-way analysis of variance with Sidak multiple comparisons test. Pubertal onset: treatment × genotype, F1,72 = 7.016, P = .01; Ctrl P = .008, 95% CI of diff, –4.440 to –0.5908; KARKO P = .6, 95% CI of diff, –1.324 to 3.058. Plugging: treatment × genotype, F1,45 = 0.01037, P = .92. Treatment, F1,45 = 11.94, P = .001; Ctrl P = .03, 95% CI of diff, –5.902 to –0.3204; KARKO P < .05, 95% CI of diff, –6.561 to –0.03885. First litter: treatment × genotype, F1,45 = 2.477, P = .12; Treatment, F1,45 = 0.5019, P = .48. Litter size: treatment × genotype, F1,33 = 0.02845, P = .87; treatment, F1,33 = 0.0002286, P = .99. Number of litters: treatment × genotype, F1,45 = 4.230, P < .05; Ctrl P = .02, 95% CI of diff, 0.1943-2.341; KARKO P = .92, 95% CI of diff, –1.454 to 1.054. F, Hematoxylin and eosin staining of representative testes from each of the 4 groups.
Table 2.
Summary of significant transgenerational prenatal antimüllerian hormone phenotypes in male control and kisspeptin-specific androgen receptor knockout mice
| Age | Phenotype | F1 generation | F1 generation KARKO | F2 generation | |
|---|---|---|---|---|---|
| Peripubertal and early adulthood, 30-60 d | Pubertal onset | ↑ | NS | ↑ | |
| Serum hormones | LH | NS | |||
| FSH | ↓ | ||||
| Adulthood, 3-5 mo | Fertility | Latency to plug | ↑ | ↑ | NS |
| No. of litters | ↓ | NS | |||
| Serum hormones | LH | NS | |||
| FSH | ↓ | ↓ | |||
| T | NS |
Abbreviations: KARKO, kisspeptin-specific androgen receptor knockout; FSH, follicle-stimulating hormone; LH, luteinizing hormone; NS, not significant; T, testosterone.
Discussion
Here we demonstrate that AR in kisspeptin cells is necessary for the reproductive deficits generated on pAMH exposure in both sexes. We also expand on initial reports of female reproductive deficits (see Table 1) to include those in male offspring (see Table 2).
Decreased ovarian size, androgen secretion, and AMH levels are all markers of menopause and ovarian failure in women (47). Interestingly, estrous cycling in late adulthood revealed disruptions in pAMH mice distinct from the signs of early reproductive senescence observed following prenatal androgen exposure (26). Reproductive aging in women with PCOS is complex, as the expected follicle loss over time usually correlates with relative normalization of elevated hormone levels and improved regularity of menstrual cycles (47). By these measures, PCOS women have been reported to have a slight increase in reproductive lifespan (47). Although additional research both in clinical settings and mouse models is warranted to understand the relationship between pAMH and reproductive aging, it is clear from our present data that AMH exposure in utero is sufficient to have long-lasting effects in offspring.
Sons of PCOS women have abnormal FSH, AMH, and androgen levels during the prepubertal and peripubertal period (7, 8). In ovine models, prenatal androgenization has been found to alter development and function of testicular Leydig cells (44). pAMH male mice showed significant pubertal delays and decreased number of litters in our experiments, which may be explained by decreased FSH, as loss of function mutations in the genes encoding FSH and its receptor have been shown to cause pubertal delays and infertility (48). Although FSH is also known to regulate testicular volume, Sertoli cell number, and transcriptional activation of Amh (49), we did not observe any alterations in gross testis morphology, weight, or litter size. AMH levels in adult males were below assay detection level, but LH and T levels in pAMH males were normal. Our findings are similar to those found in mice exposed to prenatal androgens, in which male offspring have normal LH pulses, T, and daily sperm production (50).
We found that adult AMH levels are increased in pAMH female offspring, which may underlie the F2-generation phenotypes and are consistent with human studies that show daughters of PCOS women have high AMH during infancy, childhood, adolescence, and adulthood (51). AMH can increase excitability of GnRH neurons in slice preparations of the hypothalamus and stimulate GnRH release from explants of the median eminence, as well as increase pituitary LH release in vivo (52). Transgenerational transmission of pAMH phenotypes in F2 and F3 females were found to occur through hypomethylation and transcriptome changes in DNA isolated from ovaries (25), although it has not been tested whether the pAMH phenotype can be transmitted through the male lineage. Of note, while prenatal administration of androgens in mice affects F1 female offspring, reproductive phenotypes do not persist into the second and third generations (51). This distinction highlights unique consequences of the pAMH exposure paradigm, and perhaps modulation of AMH levels in pregnancy could ameliorate the risk to future offspring. Additional studies are required to determine the sites of AMH action. One possible mechanism is through GnRH neurons, where AMH stimulates GnRH firing and release via AMHR2 (52). While it is unknown whether kisspeptin neurons express AMRH2, recent Ribotag pulldown of anteroventral periventricular nucleus (AVPV) kisspeptin neurons indicated no Amhr2 mRNA expression (53). These data are supported by single-cell RNA sequencing data of the whole hypothalamus that detected Kiss1 and Amrh2, but not in the same cells (54).
To test the necessity of androgen signaling at the level of kisspeptin cells, we generated a KARKO mouse line using Cre-LoxP transgenic mice. Global knockout of AR in female mice delays first E, disrupts adult ovulation, decreases fertility, and alters uterine morphology (55, 56). These females also have elevated LH that is unresponsive to negative hormonal feedback (57). Global AR knockout in males results in even greater reproductive deficits, with severely altered reproductive tract development and complete infertility (55). Hypothalamic kisspeptin neurons are known regulators of puberty and normal reproductive function, with kisspeptin neurons in the AVPV important for estrogen-positive feedback during the preovulatory LH surge and kisspeptin neurons in the ARC important for negative feedback regulation of LH pulses (58). Therefore, we expected that deleting AR in kisspeptin cells might result in reproductive disruptions. Interestingly, comparing KARKO and littermate Ctrls in the vehicle treatment group, we found that deletion of AR from kisspeptin cells had no effect on any reproductive measure assayed in either sex. This does not eliminate the possibility that kisspeptin AR regulates reproductive physiology because there are potentially redundant pathways, meaning KARKO alone is insufficient to cause significant deficits.
The loss of AR in kisspeptin cells is sufficient, however, to prevent the development of reproductive phenotypes following prenatal AMH exposure. Our findings demonstrate that pAMH action is mediated by androgenic signaling specifically at the level of kisspeptin cells in the offspring, allowing us to expand on the pathophysiological mechanism previously introduced (23). In this model, elevated AMH binds AMHR2 expressed on maternal GnRH neurons and placental cells to create an androgenic in utero environment for developing embryos. Because significant pAMH-induced effects were replicated in offspring in the KARKO study in which the AMH-injected pregnant dams are Arflox/flox; Kiss1Cre+ themselves, and thus lack AR in their kisspeptin cells during these pregnancies, AR in kisspeptin cells of the AMH-injected pregnant dams is not needed to induce the phenotype in the pAMH offspring. In pAMH offspring, it is likely altered hypothalamic signaling to the pituitary that results in decreased FSH secretion in both sexes. In females, there is also increased LH secretion. This altered gonadotropin signal to the ovary decreases aromatase expression and favors increased androgen and AMH production. Androgens then signal back to the hypothalamus to perpetuate HPG axis dysregulation and cause reproductive deficits. GnRH neurons themselves do not express AR, so androgens must modulate the HPG axis at the level of an upstream neuronal population. While GABAergic neurons have been proposed as potential conduits of reproductive disruptions following pAMH exposure (23), here we identify AR in kisspeptin cells as necessary for the development of this phenotype.
Kisspeptin expression in the ARC begins at E13 (59), before the first AMH injections, and because the production of kisspeptin drives Cre-induced recombination, kisspeptin AR in the KARKO mouse is deleted from offspring in utero as well as in adulthood. As such, the present experiments are unable to distinguish mechanistically between prenatal programming effects and continual maintenance of the phenotype by elevated androgens. Both in female and male mice, neural circuits between ARC kisspeptin neurons and GnRH neurons are established before birth (59, 60). Androgen exposure in utero has been shown to have lasting organizational effects (26), whereas peripubertal androgen excess results in transient reproductive deficits (26, 61). Novel experimental tools, such the development of an inducible Kisspeptin-Cre, would enable investigation into the timing of androgenic mechanisms.
The significant changes in gonadotropin levels and the reversal of phenotypes in both sexes point toward a mechanism upstream of the gonads, which is supported by the evidence that GnRH antagonism in pAMH offspring is sufficient to reverse reproductive phenotypes (23). AR is expressed in the majority of ARC kisspeptin neurons but in only less than 2% of AVPV kisspeptin neurons in females (62). Further, androgens have been shown to directly suppress ARC, but not AVPV, kisspeptin neurons (32). The present KARKO experiments do not eliminate the possibility of AR action in the gonads, but we provide evidence of few Kiss1 expressing neurons in the ovaries and testes (63, 64). Analysis of single-cell RNA sequencing data from 8-week-old male mouse testes shows that Kiss1 is not expressed in Sertoli or Leydig cells, and that Ar and Kiss1 only overlap in condensing sperm, which would be unable to produce the observed phenotypes (65). In the ovary, we observed that Kiss1 cells were not in the developing follicle, where Ar was most abundant. Kiss1 may colocalize with Ar in theca cells. However, studies using a theca cell-specific knockout of AR indicate that AR does not influence fertility or androgen levels in female mice (66), and our quantitative PCR experiments did not detect a significant decrease in ovarian Ar expression between Ctrl and KARKO females. While the evidence does not support a mechanistic role of AR in ovarian Kiss1 cells, future studies could clarify the tissue-specific kisspeptin cell populations in which androgens are acting to mediate pAMH phenotypes.
pAMH induced reproductive changes in Ctrl but not KARKO mice across the board, suggesting a common underlying androgenic mechanism mediating all changes. The single exception to this pattern was in the male plugging assay, in which pAMH delayed copulatory behavior both in Ctrl and KARKO mice, suggesting that the effect may be driven by a mechanism distinct from that mediating all other phenotypes assayed. A male’s propensity to plug is driven by several factors (67), including anxiety levels, which have been shown to modulate sexual behaviors (68). Prenatal androgen exposure is known to increase anxiety measures in male offspring (69); however, the effects of pAMH on behavioral measures have yet to be explored. If pAMH males have increased anxiety levels, this could explain the delayed plugging via a mechanism independent of kisspeptin AR.
Taken together, our work advances the understanding of the consequences of prenatal AMH exposure and highlights androgen signaling in kisspeptin cells as a crucial piece of the underlying mechanism. These results could have substantial implications for clinical populations in which AMH and/or androgens are elevated during pregnancy, including common disorders like PCOS. The long-lasting effects of prenatal AMH exposure on offspring highlights a potential need for screening and closer monitoring of gestational AMH. Our findings also suggest that modulation of these hormones during pregnancy could have therapeutic value. However, the use of such hormone blockers during pregnancy is generally contraindicated because of the iatrogenic risks conferred to the fetus. Further elucidation of the cellular and molecular mechanisms mediating pAMH effects may lead to novel preventive interventions and treatment options for PCOS patients and their children.
Acknowledgments
The authors thank Austin Chin, Ichiko Saotome, and Nay Chi Naing for technical assistance; Dr Erica Schoeller for advice on testis analysis; Dr Chawnshang Chang for the Ar-flox mice; and Dr Carol Elias for the Kiss1-Cre mice. We thank Drs Alexander Kauffmann and Alexandra Hudson for their assistance with RNAScope. We also thank the staff at the UCSD Nikon Imaging Core for microscope training.
Financial Support: This work was supported by the National Institutes of Health (NIH; grant No.s R01 HD072754, R01 HD100580, and R01 HD082567 to P.L.M.). It was also supported by the NIH/Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD; grant No. P50 HD012303, as part of the National Centers for Translational Research in Reproduction and Infertility, to P.L.M.). P.L.M. was also partially supported by grant numbers P30 DK063491, P30 CA023100, and P42 ES010337. E.V.H. was partially supported by grant numbers T32 GM007198 and T32 GM008666. J.C. was partially supported by grant numbers T32 HD007203 and K12 GM068524. M.Y.H. was partially supported by the Doris A. Howell Research Scholarship for Women’s Health. G.E.R. was partially supported by grant numbers P42 ES010337 and T32 GM008666. K.J.T. was partially supported by grant numbers T32 HD007203, P42 ES010337, and F32 HD090837. The University of Virginia, Center for Research in Reproduction, Ligand Assay and Analysis Core, is supported by the NIH/NICHD (grant No. P50 HD028934). The UCSD Neuroscience Microscopy Core is funded by the National Institute of Neurological Disorders and Stroke (NINDS grant No. P30 NS047101). The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Author Contributions: Emily V. Ho, conceptualization, methodology, investigation, data curation, writing—original draft preparation, writing—review and editing, visualization; Chengxian Shi, methodology, investigation, writing—original draft preparation, writing—review and editing; Jessica Cassin, methodology, investigation, writing—review and editing; Michelle Y. He, methodology, investigation, writing—review and editing; Ryan D. Nguyen, methodology, investigation, writing—original draft preparation, writing—review and editing; Genevieve E. Ryan, methodology, writing—review and editing; Karen J. Tonsfeldt, methodology, investigation, data curation, writing—review and editing, visualization; Pamela L. Mellon, conceptualization, methodology, resources, data curation, writing—review and editing, supervision, project administration, funding acquisition.
Glossary
Abbreviations
- AF
antral follicle
- AMH
antimüllerian hormone
- AMHR2
AMH receptor 2
- AR
androgen receptor
- ARC
arcuate nucleus
- AVPV
anteroventral periventricular nucleus
- Ctrl
control
- d
day
- D
diestrus
- DHT
dihydrotestosterone
- e
embryonic day
- E
estrus
- E2
estradiol
- ER
estrogen receptor
- F1
first-generation
- F2
second-generation
- FSH
follicle-stimulating hormone
- GnRH
gonadotropin-releasing hormone
- HPG
hypothalamic-pituitary-gonadal axis
- KARKO
kisspeptin-specific androgen receptor knockout
- LH
luteinizing hormone
- M
metestrus
- mRNA
messenger RNA
- P
proestrus
- pAMH
prenatal antimüllerian hormone
- PBS
phosphate-buffered saline
- PCOS
polycystic ovary syndrome
- PCR
polymerase chain reaction
- RT-PCR
reverse transcriptase–polymerase chain reaction
- ST
seminiferous tubule
- T
testosterone
- VEH
vehicle
Additional Information
Disclosures: The authors have nothing to disclose.
Data Availability
Some or all data generated or analyzed during this study are included in this published article or in the data repositories listed in “References” (https://doi.org/10.6076/D1XW21).
References
- 1. Sirmans SM, Pate KA. Epidemiology, diagnosis, and management of polycystic ovary syndrome. Clin Epidemiol. 2013;6:1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sir-Petermann T, Maliqueo M, Angel B, Lara HE, Pérez-Bravo F, Recabarren SE. Maternal serum androgens in pregnant women with polycystic ovarian syndrome: possible implications in prenatal androgenization. Hum Reprod. 2002;17(10):2573-2579. [DOI] [PubMed] [Google Scholar]
- 3. Barry JA, Kay AR, Navaratnarajah R, et al. Umbilical vein testosterone in female infants born to mothers with polycystic ovary syndrome is elevated to male levels. J Obstet Gynaecol. 2010;30(5):444-446. [DOI] [PubMed] [Google Scholar]
- 4. Anderson H, Fogel N, Grebe SK, Singh RJ, Taylor RL, Dunaif A. Infants of women with polycystic ovary syndrome have lower cord blood androstenedione and estradiol levels. J Clin Endocrinol Metab. 2010;95(5):2180-2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Maliqueo M, Lara HE, Sánchez F, Echiburú B, Crisosto N, Sir-Petermann T. Placental steroidogenesis in pregnant women with polycystic ovary syndrome. Eur J Obstet Gynecol Reprod Biol. 2013;166(2):151-155. [DOI] [PubMed] [Google Scholar]
- 6. Risal S, Pei Y, Lu H, et al. Prenatal androgen exposure and transgenerational susceptibility to polycystic ovary syndrome. Nat Med. 2019;25(12):1894-1904. [DOI] [PubMed] [Google Scholar]
- 7. Crisosto N, Echiburú B, Maliqueo M, et al. Reproductive and metabolic features during puberty in sons of women with polycystic ovary syndrome. Endocr Connect. 2017;6(8):607-613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Recabarren SE, Sir-Petermann T, Rios R, et al. Pituitary and testicular function in sons of women with polycystic ovary syndrome from infancy to adulthood. J Clin Endocrinol Metab. 2008;93(9):3318-3324. [DOI] [PubMed] [Google Scholar]
- 9. Azziz R, Carmina E, Chen Z, et al. Polycystic ovary syndrome. Nat Rev Dis Primers. 2016;2:16057. [DOI] [PubMed] [Google Scholar]
- 10. Vink JM, Sadrzadeh S, Lambalk CB, Boomsma DI. Heritability of polycystic ovary syndrome in a Dutch twin-family study. J Clin Endocrinol Metab. 2006;91(6):2100-2104. [DOI] [PubMed] [Google Scholar]
- 11. Eldar-Geva T, Margalioth EJ, Gal M, et al. Serum anti-Mullerian hormone levels during controlled ovarian hyperstimulation in women with polycystic ovaries with and without hyperandrogenism. Hum Reprod. 2005;20(7):1814-1819. [DOI] [PubMed] [Google Scholar]
- 12. Lin YH, Chiu WC, Wu CH, Tzeng CR, Hsu CS, Hsu MI. Antimüllerian hormone and polycystic ovary syndrome. Fertil Steril. 2011;96(1):230-235. [DOI] [PubMed] [Google Scholar]
- 13. Piouka A, Farmakiotis D, Katsikis I, Macut D, Gerou S, Panidis D. Anti-Mullerian hormone levels reflect severity of PCOS but are negatively influenced by obesity: relationship with increased luteinizing hormone levels. Am J Physiol Endocrinol Metab. 2009;296(2):E238-E243. [DOI] [PubMed] [Google Scholar]
- 14. Pigny P, Merlen E, Robert Y, et al. Elevated serum level of anti-mullerian hormone in patients with polycystic ovary syndrome: relationship to the ovarian follicle excess and to the follicular arrest. J Clin Endocrinol Metab. 2003;88(12):5957-5962. [DOI] [PubMed] [Google Scholar]
- 15. Kevenaar ME, Laven JS, Fong SL, et al. A functional anti-mullerian hormone gene polymorphism is associated with follicle number and androgen levels in polycystic ovary syndrome patients. J Clin Endocrinol Metab. 2008;93(4):1310-1316. [DOI] [PubMed] [Google Scholar]
- 16. Gorsic LK, Kosova G, Werstein B, et al. Pathogenic anti-Müllerian hormone variants in polycystic ovary syndrome. J Clin Endocrinol Metab. 2017;102(8):2862-2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gorsic LK, Dapas M, Legro RS, Hayes MG, Urbanek M. Functional genetic variation in the anti-Müllerian hormone pathway in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2019;104(7):2855-2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nardo LG, Yates AP, Roberts SA, Pemberton P, Laing I. The relationships between AMH, androgens, insulin resistance and basal ovarian follicular status in non-obese subfertile women with and without polycystic ovary syndrome. Hum Reprod. 2009;24(11):2917-2923. [DOI] [PubMed] [Google Scholar]
- 19. Pellatt L, Hanna L, Brincat M, et al. Granulosa cell production of anti-Müllerian hormone is increased in polycystic ovaries. J Clin Endocrinol Metab. 2007;92(1):240-245. [DOI] [PubMed] [Google Scholar]
- 20. Pigny P, Jonard S, Robert Y, Dewailly D. Serum anti-Mullerian hormone as a surrogate for antral follicle count for definition of the polycystic ovary syndrome. J Clin Endocrinol Metab. 2006;91(3):941-945. [DOI] [PubMed] [Google Scholar]
- 21. Iliodromiti S, Kelsey TW, Anderson RA, Nelson SM. Can anti-Mullerian hormone predict the diagnosis of polycystic ovary syndrome? A systematic review and meta-analysis of extracted data. J Clin Endocrinol Metab. 2013;98(8):3332-3340. [DOI] [PubMed] [Google Scholar]
- 22. Das M, Gillott DJ, Saridogan E, Djahanbakhch O. Anti-Mullerian hormone is increased in follicular fluid from unstimulated ovaries in women with polycystic ovary syndrome. Hum Reprod. 2008;23(9):2122-2126. [DOI] [PubMed] [Google Scholar]
- 23. Tata B, Mimouni NEH, Barbotin AL, et al. Elevated prenatal anti-Müllerian hormone reprograms the fetus and induces polycystic ovary syndrome in adulthood. Nat Med. 2018;24(6):834-846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Piltonen TT, Giacobini P, Edvinsson Å, et al. Circulating antimüllerian hormone and steroid hormone levels remain high in pregnant women with polycystic ovary syndrome at term. Fertil Steril. 2019;111(3):588-596.e1. [DOI] [PubMed] [Google Scholar]
- 25. Mimouni NEH, Paiva I, Barbotin AL, et al. Polycystic ovary syndrome is transmitted via a transgenerational epigenetic process. Cell Metab. 2021;33(3):513-530.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Witham EA, Meadows JD, Shojaei S, Kauffman AS, Mellon PL. Prenatal exposure to low levels of androgen accelerates female puberty onset and reproductive senescence in mice. Endocrinology. 2012;153(9):4522-4532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Moore AM, Prescott M, Campbell RE. Estradiol negative and positive feedback in a prenatal androgen-induced mouse model of polycystic ovarian syndrome. Endocrinology. 2013;154(2):796-806. [DOI] [PubMed] [Google Scholar]
- 28. Roland AV, Nunemaker CS, Keller SR, Moenter SM. Prenatal androgen exposure programs metabolic dysfunction in female mice. J Endocrinol. 2010;207(2):213-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Walters KA. Androgens in polycystic ovary syndrome: lessons from experimental models. Curr Opin Endocrinol Diabetes Obes. 2016;23(3):257-263. [DOI] [PubMed] [Google Scholar]
- 30. Tng EL. Kisspeptin signalling and its roles in humans. Singapore Med J. 2015;56(12):649-656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tena-Sempere M. Kisspeptin/GPR54 system as potential target for endocrine disruption of reproductive development and function. Int J Androl. 2010;33(2):360-368. [DOI] [PubMed] [Google Scholar]
- 32. Iwata K, Kunimura Y, Ozawa H. Hypothalamic kisspeptin expression in hyperandrogenic female rats and aging rats. Acta Histochem Cytochem. 2019;52(5):85-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Coyle C, Campbell RE. Pathological pulses in PCOS. Mol Cell Endocrinol. 2019;498:110561. [DOI] [PubMed] [Google Scholar]
- 34. Esparza LA, Terasaka T, Lawson MA, Kauffman AS. Androgen suppresses in vivo and in vitro LH pulse secretion and neural Kiss1 and Tac2 gene expression in female mice. Endocrinology. 2020;161(12):bqaa191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yoo SH, Yamazaki S, Lowrey PL, et al. PERIOD2::LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc Natl Acad Sci U S A. 2004;101(15):5339-5346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mereness AL, Murphy ZC, Sellix MT. Developmental programming by androgen affects the circadian timing system in female mice. Biol Reprod. 2015;92(4):88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yeh S, Tsai MY, Xu Q, et al. Generation and characterization of androgen receptor knockout (ARKO) mice: an in vivo model for the study of androgen functions in selective tissues. Proc Natl Acad Sci U S A. 2002;99(21):13498-13503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cravo RM, Margatho LO, Osborne-Lawrence S, et al. Characterization of Kiss1 neurons using transgenic mouse models. Neuroscience. 2011;173:37-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Madisen L, Zwingman TA, Sunkin SM, et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci. 2010;13(1):133-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cora MC, Kooistra L, Travlos G. Vaginal cytology of the laboratory rat and mouse: review and criteria for the staging of the estrous cycle using stained vaginal smears. Toxicol Pathol. 2015;43(6):776-793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lavalle SN, Chou T, Hernandez J, et al. Kiss1 is differentially regulated in male and female mice by the homeodomain transcription factor VAX1. Mol Cell Endocrinol. 2021;534:111358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tonsfeldt KJ, Ho EV, Shi C, et al. 2021 Supplementary Data for Reproductive Deficits Induced by Prenatal Anti-Mullerian Hormone Exposure RequireAndrogen Receptor in Kisspeptin Cells. In. 9/2/21 ed. Dryad. https://datadryad.org/stash/dataset/doi:10.6076/D1XW21
- 43. Koebele SV, Bimonte-Nelson HA. Modeling menopause: the utility of rodents in translational behavioral endocrinology research. Maturitas. 2016;87:5-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Connolly F, Rae MT, Bittner L, Hogg K, McNeilly AS, Duncan WC. Excess androgens in utero alters fetal testis development. Endocrinology. 2013;154(5):1921-1933. [DOI] [PubMed] [Google Scholar]
- 45. Cannarella R, Condorelli RA, Mongioì LM, La Vignera S, Calogero AE. Does a male polycystic ovarian syndrome equivalent exist? J Endocrinol Invest. 2018;41(1):49-57. [DOI] [PubMed] [Google Scholar]
- 46. Sir-Petermann T, Ladrón de Guevara A, Codner E, et al. Relationship between anti-Müllerian hormone (AMH) and insulin levels during different tanner stages in daughters of women with polycystic ovary syndrome. Reprod Sci. 2012;19(4):383-390. [DOI] [PubMed] [Google Scholar]
- 47. Hsu MI. Changes in the PCOS phenotype with age. Steroids. 2013;78(8):761-766. [DOI] [PubMed] [Google Scholar]
- 48. Layman LC. Mutations in the follicle-stimulating hormone-beta (FSH beta) and FSH receptor genes in mice and humans. Semin Reprod Med. 2000;18(1):5-10. [DOI] [PubMed] [Google Scholar]
- 49. Xu HY, Zhang HX, Xiao Z, Qiao J, Li R. Regulation of anti-Müllerian hormone (AMH) in males and the associations of serum AMH with the disorders of male fertility. Asian J Androl. 2019;21(2):109-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Holland S, Prescott M, Pankhurst M, Campbell RE. The influence of maternal androgen excess on the male reproductive axis. Sci Rep. 2019;9(1):18908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Risal S, Pei Y, Lu H, et al. Prenatal androgen exposure and transgenerational susceptibility to polycystic ovary syndrome. Nat Med. 2019;25(12):1894-1904. [DOI] [PubMed] [Google Scholar]
- 52. Cimino I, Casoni F, Liu X, et al. Novel role for anti-Müllerian hormone in the regulation of GnRH neuron excitability and hormone secretion. Nat Commun. 2016;7:10055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Stephens SBZ, Kauffman AS. Estrogen regulation of the molecular phenotype and active translatome of AVPV kisspeptin neurons. Endocrinology. 2021;162(9):bqab080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Chen R, Wu X, Jiang L, Zhang Y. Single-Cell RNA-seq reveals hypothalamic cell diversity. Cell Rep. 2017;18(13):3227-3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Zhou X. Roles of androgen receptor in male and female reproduction: lessons from global and cell-specific androgen receptor knockout (ARKO) mice. J Androl. 2010;31(3):235-243. [DOI] [PubMed] [Google Scholar]
- 56. Walters KA, McTavish KJ, Seneviratne MG, et al. Subfertile female androgen receptor knockout mice exhibit defects in neuroendocrine signaling, intraovarian function, and uterine development but not uterine function. Endocrinology. 2009;150(7):3274-3282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Cheng XB, Jimenez M, Desai R, et al. Characterizing the neuroendocrine and ovarian defects of androgen receptor-knockout female mice. Am J Physiol Endocrinol Metab. 2013;305(6):E717-E726. [DOI] [PubMed] [Google Scholar]
- 58. Smith JT. Sex steroid regulation of kisspeptin circuits. Adv Exp Med Biol. 2013;784:275-295. [DOI] [PubMed] [Google Scholar]
- 59. Kumar D, Freese M, Drexler D, Hermans-Borgmeyer I, Marquardt A, Boehm U. Murine arcuate nucleus kisspeptin neurons communicate with GnRH neurons in utero. J Neurosci. 2014;34(10):3756-3766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kumar D, Periasamy V, Freese M, Voigt A, Boehm U. In utero development of kisspeptin/GnRH neural circuitry in male mice. Endocrinology. 2015;156(9):3084-3090. [DOI] [PubMed] [Google Scholar]
- 61. Kauffman AS, Thackray VG, Ryan GE, et al. A novel letrozole model recapitulates both the reproductive and metabolic phenotypes of polycystic ovary syndrome in female mice. Biol Reprod. 2015;93(3):69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Iwata K, Kunimura Y, Matsumoto K, Ozawa H. Effect of androgen on Kiss1 expression and luteinizing hormone release in female rats. J Endocrinol. 2017;233(3):281-292. [DOI] [PubMed] [Google Scholar]
- 63. Merhi Z, Thornton K, Bonney E, Cipolla MJ, Charron MJ, Buyuk E. Ovarian kisspeptin expression is related to age and to monocyte chemoattractant protein-1. J Assist Reprod Genet. 2016;33(4):535-543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Salehi S, Adeshina I, Chen H, et al. Developmental and endocrine regulation of kisspeptin expression in mouse Leydig cells. Endocrinology. 2015;156(4):1514-1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Lukassen S, Bosch E, Ekici AB, Winterpacht A. Single-cell RNA sequencing of adult mouse testes. Sci Data. 2018;5:180192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Ma Y, Andrisse S, Chen Y, et al. Androgen receptor in the ovary theca cells plays a critical role in androgen-induced reproductive dysfunction. Endocrinology. 2017;158(1):98-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Schneider MR, Mangels R, Dean MD. The molecular basis and reproductive function(s) of copulatory plugs. Mol Reprod Dev. 2016;83(9):755-767. [DOI] [PubMed] [Google Scholar]
- 68. D’Amato FR, Pavone F. Role of anxiety in subordinate male mice sexual behavior. Pharmacol Biochem Behav. 1992;43(1):181-185. [DOI] [PubMed] [Google Scholar]
- 69. Risal S, Manti M, Lu H, et al. Prenatal androgen exposure causes a sexually dimorphic transgenerational increase in offspring susceptibility to anxiety disorders. Transl Psychiatry. 2021;11(1):45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some or all data generated or analyzed during this study are included in this published article or in the data repositories listed in “References” (https://doi.org/10.6076/D1XW21).