Abstract
Simple Summary
Cell-free and concentrated ascites reinfusion therapy (CART) was a safe and effective palliative therapy in malignancy-related ascites. Abdominal distension, dyspnea, and fatigue were alleviated significantly after CART. The mean time to the next paracentesis was 20.7 days. In total, 17% of patients had improved performance status after CART.
Abstract
Background: Malignancy-related ascites (MRA) is one of the symptoms causing discomfort in advanced cancer patients. Cell-free and concentrated ascites reinfusion therapy (CART) is one of the palliative treatments widely conducted in Japan only. Methods: A systematic review following a meta-analysis of CART was performed. The efficiency and adverse events were evaluated. Results: A total of 2567 patients and 6013 procedures of CART were identified in this study. The mean volume of MRA collected was 4.29 (95% confidence interval (CI) 3.47–5.11) L, and the volume reinfused after concentrating was 0.49 (95% CI 0.39–0.60) L. A total of 86.1 (95% CI 77.1–95.2) g protein and 42.9 (95% CI 36.0–50.0) g albumin was reinfused. The mean time to the next paracentesis was 20.7 (95% CI 15.6–25.8) days. The body weight was reduced by 3.38 (95% CI 1.90–4.86; p < 0.01) kg, and abdominal circumference was reduced by 7.86 (95% CI 6.58–9.14; p < 0.001) cm. Serum albumin increased an average of 0.14 (95% CI −0.01–0.28; p = 0.07) mg/dL the day after CART. Abdominal distension, dyspnea, and fatigue were alleviated by 6.0 (95% CI 5.59–6.51), 2.66 (95% CI 2.05–3.28), and 2.64 (95% CI 1.86–3.42) points using a numerical rating scale system ranging from 0 to 10. Overall, 17% (95% CI 0.03–0.31%) of patients had improved performance status after CART. Significant body temperature elevation was observed, at an average of 0.4 °C (95% CI 0.18–0.62 °C). Conclusions: CART might be a safe and effective palliative therapy in MRA and further clinical trials are necessary.
Keywords: cell-free and concentrated ascites reinfusion therapy, malignant-related ascites, chemotherapy
1. Introduction
Ascites is the pathological accumulation of fluid within the abdominal cavity. The most common causes of malignancy-related ascites (MRA) are adenocarcinomas of the ovary, which account for approximately 10% of all cases of ascites, followed by carcinomas of the breast, colon, stomach, and pancreas [1]. MRA results in impairment in quality of life (QOL) and significant symptoms, mainly due to increased intraabdominal pressure and pain, nausea, anorexia, vomiting, fatigue, and dyspnea [2]. It is also a sign of advanced cancer and a poor prognosis, averaging about 20 weeks from time of diagnosis [3].
A variety of medical, interventional, and surgical therapies are now available for the management of both complications and symptoms, but there are limited guidelines for the treatment of MRA [4,5,6]. The most common first-line options are diuretics and intermittent paracentesis. Even small-volume paracentesis can alleviate the abdominal distension of terminally ill cancer patients with malignant ascites [7]. Indwelling catheters, ports, and shunts have all been proposed to reduce morbidity and improve QOL [8]. A newer modality, hyperthermic intraperitoneal chemotherapy (HIPEC), has demonstrated a survival advantage as a prophylactic strategy in gastric and ovarian cancers [9,10].
In palliative therapies, cell-free and concentrated ascites reinfusion therapy (CART) is also used for treating refractory ascites. CART comprises three processes. After the ascites is first filtered to remove cell components, it is concentrated to reduce its volume. The fluid obtained through these processes, including useful proteins such as albumin and globulin, is finally reinfused intravenously [11]. This therapy has been widely used in Japan to reduce symptoms in patients with MRA. Hypoalbuminemia, which is prone to malignant ascites, has been reported as a risk factor for febrile neutropenia, a side effect of cancer chemotherapy [12]. With CART, the proteins included in ascites are collected and intravenously reinfused, avoiding the loss of beneficial proteins through paracentesis.
There was no controlled trial conducted between CART and puncture groups to determine the efficacy and safety of CART. To explore the efficacy and safety of CART for malignant ascites, we conducted a systematic review and meta-analysis of observational studies.
2. Materials and Methods
2.1. Study Overview
The protocol of this systematic review and meta-analysis was composed following the standard guidelines for a systematic review of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement and registered on the website of the University Hospital Medical Information Network Clinical Trials Registration (UMIN000044541) [13,14]. Institutional Review Board approval was not required because of the nature of this study.
2.2. Study Search
Four major online databases, namely PubMed, Web of Science, Cochrane, and Embase, were searched. The following formula was applied for PubMed: (ascites reinfusion therapy) OR (cell-free and concentrated ascites reinfusion therapy) OR (CART) AND (malignant ascites). Two review authors (MI and HC) independently screened the titles and abstracts and carefully evaluated the full text to select eligible articles. In cases of discrepancy, they reached a consensus through discussion. Review articles and the included original articles were hand-searched (MI and HC) for additional research papers that met the inclusion criteria.
2.3. Inclusion and Exclusion Criteria
Full articles and brief reports published in any language that provided data for the effectiveness of CART for malignant ascites were examined. To be included, a study had to include (1) patients with malignant ascites, (2) data evaluating the effectiveness of CART, and (3) detailed information of the CART procedure. The exclusion criteria were as follows: (1) data of procedure only, (2) cirrhosis ascites only, and (3) no information identifying the efficacy of CART.
2.4. Risk of Bias
Two reviewers independently assessed the methodological quality of the selected studies using the Newcastle–Ottawa quality assessment, evaluating the quality of observational studies [15]. Due to the nature of single-arm studies, “selection of the non-exposed cohort” and “comparability” domains were not applicable in this study. The final score would then be 6 stars at most in the modified Newcastle–Ottawa quality assessment. Disagreement between reviewers was discussed, and agreement was reached by consensus.
2.5. Outcomes
The general characteristics of CART, such as the volume of ascites collected and reinfused, were analyzed. The efficacy of CART was identified by reduced body weight and abdominal circumference, increased serum albumin and total protein, and improved estimated glomerular filtration rate (eGFR) and creatinine. Eastern Cooperative Oncology Group performance status (ECOG PS) and alleviation of symptoms including abdominal distension, dyspnea, fatigue, lack of appetite, abdominal pain, and nausea and vomiting were evaluated [16]. The adverse events of CART were evaluated using the common terminology criteria for adverse events (CTCAT ver. 5.0).
Laboratory findings were checked the day after each CART procedure and adverse events were appraised during the procedure of CART. The ratio of improved PS was considered as the primary outcome of the long-term effect of CART, but only four studies discussed PS as a category variable. A short-term effect of CART was analyzed by the changes in body weight, abdominal girth, and serum albumin according to the paracentesis and reinfusion procedures in CART.
2.6. Data Extraction
Two review authors, MI and HC, independently extracted data, including the name of the first author, the publication year, the publication country, the types of immunohistochemical markers, the numbers of patients with positive results, the numbers of patients evaluated, and Newcastle–Ottawa quality assessment-related information.
2.7. Statistics
All analyses were performed in Review Manager ver. 5.3 (Cochrane Collaboration, Oxford, UK). Figures prepared using Review Manager were adjusted as necessary. Mean differences and 95% confidence intervals (95% CIs) were compared before and after CART. Heterogeneity evaluated with the I2 statistic was interpreted as follows: I2 = 0% indicates no heterogeneity, 0% < I2 < 25% indicates the least heterogeneity, 25% ≤ I2 < 50% indicates mild heterogeneity, 50% ≤ I2 < 75% indicates moderate heterogeneity, and 75% ≤ I2 indicates strong heterogeneity [17]. A p-value of < 0.05 was considered significant.
3. Results
3.1. Study Search and Study Characteristics
A total of 95 articles, including 93 articles through database searching and 2 articles by hand-searching, were identified. There were 63, 45, and 15 articles left after removing duplication, screening, and full-article reading, respectively (Supplementary Figure S1). There was no consensus on the direction of filtration, that is, inside–out or outside–in, and the processing conditions, such as filtration speed, concentration speed, and driving force to filter and concentrate ascites. Keisuke-modified cell-free and concentrated ascites reinfusion therapy (KM-CART) was used in 2128 patients, with an outside–in filtration direction. All studies used the same AHF-MO ascitic filtration filter and AHF-UP ascitic concentration filter (both from Asahi Kasei Medical Co., Ltd., Tokyo, Japan) for filtering and concentrating the ascites. The outcomes of the enrolled studies are shown in Supplementary Table S1. Due to the nature of the single-arm study, the modified Newcastle–Ottawa Scale scores varied from 4 to 6 stars (Supplementary Table S2).
A total of 2567 patients with MRA and 6013 procedures of CART were identified [18,19,20,21,22,23,24,25,26,27,28,29,30,31,32] (Table 1). All studies were conducted in Japan. All but two articles were reported in English [22,27]. There were four and five studies focused on MRA due to gastric and gynecological cancers, respectively, and the remaining six studies enrolled MRA patients with different kinds of malignancies. Chemotherapy with CART was reported by seven studies. The mean volume of MRA collected was 4.29 L (95% confidence interval (CI) 3.47–5.11 L), and the volume reinfused after concentrating was 0.49 L (95% CI 0.39–0.60). A total of 86.1 g (95% CI 77.1–95.2 g) protein and 42.9 g (95% CI 36.0–50.0 g) albumin was reinfused. The mean time to the next paracentesis was 20.7 days (95% CI 15.6–25.8 days) (Supplementary Figures S2–S6).
Table 1.
Background and characteristics of studies included.
| First Author | Year | Tumors | Types | Patients | Chemotherapy | Age (y) | Procedures | Collection (mL) (SD) | Reinfusion (mL) (SD) | Protein (g) (SD) |
|---|---|---|---|---|---|---|---|---|---|---|
| Hanada | 2018 | mixed | CART | 51 | 14 | 64 | 104 | 5855 (1790) | 764 (320) | ND |
| Hanafusa | 2017 | mixed | mixed | 142 | ND | 65.7 | 350 | 3709 (1730) | 491 (320) | 66.8 (32.4) |
| Ito | 2015 | mixed | CART | 37 | ND | 59.7 | 100 | 3197 (1424) | 302 (150) | 93.1 (51.62) |
| Ito | 2020 | mixed | CART | 43 | ND | 58.7 | 123 | 3207 (1427) | 299 (152) | 91.3 (53) |
| Iwaki | 2018 | mixed | KM-CART | 19 | ND | 62.8 | 39 | 7000 (2600) | ND | ND |
| Kawata | 2019 | Gyn | CART | 29 | 2 | 56.6 | 47 | 2937 (820) | 272 (84) | 85 (33.2) |
| Maeda | 2014 | Gas | CART | 5 | ND | 63.6 | 51 | 4007 (1304) | 561 (205) | 75 (29.8) |
| Matsusaki | 2020 | mixed | KM-CART | 2109 | ND | 60.7 | 2224 † | 6200 (2600) | 610 (300) | 67.3 (44.5) |
| Nagata | 2020 | Gas | CART | 30 | 30 | 59.5 | 100 | 4000 (200) | ND | ND |
| Ohta | 2017 | Gas | CART | 6 | ND | 73.8 | 12 | 3850 | 485 | ND |
| Togami | 2014 | Gyn | NA | 4 | ND | ND | 15 | 3190 (1086) | 538 (249) | ND |
| Ueda | 2012 | Gyn | CART | 22 | 14 | ND | 57 | 3290 (1200) | NA | ND |
| Wang | 2015 | Gyn | CART | 9 | 6 | 67.7 | 58 | 7730 (3390) | 920 (470) | 161.2 (89.1) |
| Yamaguchi | 2015 | Gas | CART | 30 | 30 | 58 | 127 | 3056 (1250) | 334 (162) | 85.5 (46.9) |
| Yamamoto | 2021 | Gyn | CART | 31 | 11 | 66.4 | 49 | 3009 (1253) | 392 (190) | ND |
†: 4781 procedures conducted and data analyzed in 2224 procedures; Mixed: mixed kinds of malignancies; Gyn: gynecological malignancies; Gas: gastrointestinal malignancies; SD: standardized difference; CART: cell-free and concentrated ascites reinfusion therapy; KM-CART: Keisuke modified CART; ND: not described.
3.2. Efficiency of CART
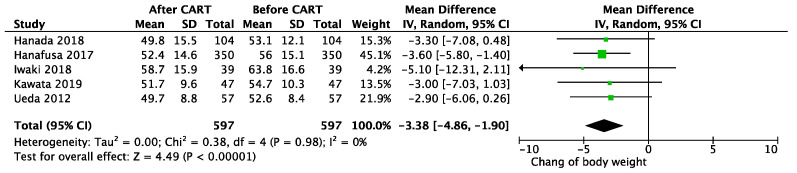
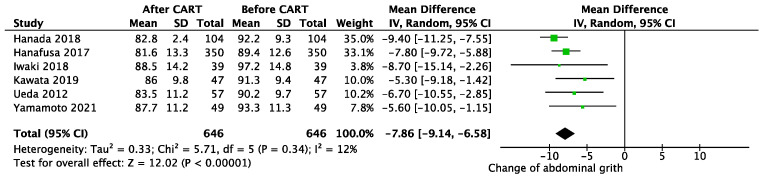
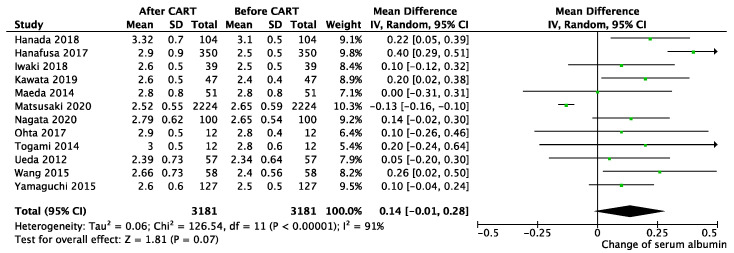
After CART, mean body weight was reduced by 3.38 kg (95% CI 1.90–4.86 kg; p < 0.01; I2 = 0%, p for heterogeneity = 0.98) (Figure 1), and abdominal circumference was reduced 7.86 cm (95% CI 6.58–9.14 cm; p < 0.01; I2 = 12%, p for heterogeneity = 0.34) (Figure 2). The day after CART, serum albumin increased an average of 0.14 mg/dL (95% CI −0.01–0.28 mg/dL; p = 0.07; I2 = 91%, p for heterogeneity <0.01) (Figure 3), and serum total protein increased 0.18 mg/dL (95% CI −0.23–0.59 mg/dL; p = 0.39; I2 = 98%, p for heterogeneity <0.01) (Supplementary Figure S7). Only one study reported decreased concentrations of protein and albumin due to the infusion of 500 mL to 1500 mL during the procedure of paracentesis to prevent hypotension [25]. Creatinine was decreased 0.1 g/dL (95% CI 0.07–0.13 g/dl; p < 0.01; I2 = 0%, p for heterogeneity = 0.94) (Supplementary Figure S8), and eGFR improved 6.95 mL/min/1.73 m2 (95% CI 5.37–8.54 mL/min/1.73 m2; p < 0.01; I2 = 98%, p for heterogeneity= 0.68) (Supplementary Figure S9).
Figure 1.
Change in body weight before and after CART.
Figure 2.
Change in abdominal circumference before and after CART.
Figure 3.
Change in serum albumin before and after CART.
An improvement in PS was reported by 10 studies. Due to different evaluation methods, only four studies were included in the meta-analysis, showing that 17% (95% CI 3–31%) of the patients had improved PS after CART [24,26,27,29] (Supplementary Figure S10). Most studies also described the alleviation of symptoms after CART, with abdominal distension, dyspnea, and fatigue improved 6.0 (95% CI 5.59–6.51), 2.66 (95% CI 2.05–3.28), and 2.64 (95% CI 1.86–3.42) points using a numerical rating scale system ranging from 0 to 10 (Table 2) [18,25].
Table 2.
Effect of CART on the alleviation of symptoms using a numerical rating scale system (0–10).
| Symptoms | Before (95% CI) |
After (95% CI) |
Mean Difference (95% CI) |
p Value |
|---|---|---|---|---|
| Abdominal distension | 8.10 (7.78, 8.42) | 2.12 (1.80, 2.44) | 6.00 (5.49–6.51) | <0.01 |
| Dyspnea | 4.40 (3.03, 5.77) | 1.67 (0.89, 2.45) | 2.66 (2.05–3.28) | <0.01 |
| Fatigue | 6.17 (4.11, 8.23) | 3.54 (2.27, 4.82) | 2.64 (1.86–3.42) | <0.01 |
| Lack of appetite | 6.15 (4.56, 7.73) | 3.62 (3.04, 4.20) | 2.58 (1.53–3.63) | <0.01 |
| Abdominal pain | 3.90 (2.53, 5.27) | 2.15 (1.26, 3.03) | 1.74 (1.14–2.35) | <0.01 |
| Nausea and vomiting | 3.21 (1.05, 5.36) | 1.79 (0.01, 4.14) | 1.40 (0.86–1.95) | <0.01 |
3.3. Adverse Events in CART
Only two studies reported hypotension during ascites drainage [19,25]. Nine studies used 100 or 200 mg of hydrocortisone before reinfusion to prevent fever during reinfusion, but an increase in body temperature was observed, at an average of 0.4 °C (95% CI 0.18–0.62 °C) (Supplementary Figure S11). There were only four episodes of Grade 2 fever according to the common terminology criteria for adverse events (CTCAE). A decrease in platelets was also observed after CART 4.39 × 104/μL (95% CI 2.22–6.57 × 104/μL; p < 0.01; I2 = 60%, p for heterogeneity = 0.01) (Supplementary Figure S12).
4. Discussion
This study evaluated the effects of CART in 2567 patients and 6013 procedures, with a mean time to next paracentesis of 20.7 days, showing that CART reduced body weight and abdominal circumference, increased serum albumin and total protein, improved PS, and alleviated symptoms. Significant body temperature elevations, by 0.4 °C on average, were observed among the patients, although these were not clinically important. This was reported by previous studies [19]. In addition, decreased platelets were observed after CART, but there was no report on the necessity of platelet transfusion. Only limited adverse events were observed during the procedures, except for four episodes of Grade 2 fever (CTCAE Ver5.0). CART was identified as a safe and effective palliative therapy in the treatment of MRA.
Fever was a notable adverse event upon the reinfusion of concentrated ascites. Concomitant steroid and/or non-steroidal anti-inflammatory drug (NSAID) use before reinfusion was significantly and negatively associated with increases in body temperature [19]. The concentration of inflammatory cytokines in ascites was not related to body temperature change, and the presence of IL-10 in ascites was related to longer survival after CART [21]. Of special note was that albumin, total protein, and eGFR were significantly increased after the reinfusion of concentrated ascitic fluid. It is considered that symptom relief itself can be achieved by paracentesis alone without the reinfusion of collected ascites. The effects of reinfusion of the concentrated ascitic fluid may be maintained for 20.7 days, this potentially being longer than the effects of total paracentesis alone (10 to 14 days) [33,34,35].
In total, 17% of patients showed increased ECOG PS after CART, and continued chemotherapies were frequently conducted in these patients. The combination of CART and antineoplastic agents was proven to be as safe as CART alone in cases of MRA [36]. CART may contribute to improved survival in patients with advanced gynecological and gastrointestinal cancers [26,29]. The combination of CART followed by chemotherapy could be a treatment option for cancer patients with MRA.
Several limitations to this study must be considered when interpreting the results. First, there were no controlled trials, and all studies included in this review were single-arm studies. Second, there was no consensus about puncture volume, concentrate ratio, etc., in the procedure of CART, resulting in high heterogeneity in most subgroup analyses. Third, the background characteristics of patients enrolled in the study might be different due to the nature of an observational study, which posed a substantial risk for selection bias. Fourth, the limited study investigated the long-term effect of CART. Although various outcomes were compared before and just after the procedure, they seemed to lack reliability for the long-term efficacy of CART. Fifth, the effect of CART was analyzing per procedure, which posed a substantial risk of selection bias. Sixth, CART was used in Japan only, and whether the conclusion can be generalized to different countries needs to be verified.
5. Conclusions
CART might be a safe and effective palliative therapy for MRA. There were no serious adverse events during the procedures. Further clinical trials are needed to confirm the efficacy and safety of CART for malignant ascites.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/cancers13194873/s1, Table S1: Outcomes of each study enrolled; Table S2: Evaluation of the studies by modified-Newcastle-Ottawa Scale; Figure S1. PRISMA flow chart for study selection; Figure S2. The mean volume of MRA collected; Figure S3. Mean volume of MRA reinfused; Figure S4. Mean amount of total protein reinfused; Figure S5. Mean amount of albumin reinfused; Figure S6. Median time to next paracentesis; Figure S7. Change of total protein after CART; Figure S8. Change of creatine after CART; Figure S9. Change of eGFR after CART; Figure S10. Improved performance status after CART; Figure S11. Change of body temperature after CART; Figure S12. Change of platelets after CART.
Author Contributions
H.C. and M.I. were involved in data acquisition and drafting of the manuscript. H.C. contributed to study conception, data acquisition, analysis, interpretation, and drafting. H.C. and N.H. performed data acquisition, interpretation, and critical revision. H.K., R.O., T.S., S.T., T.H., Y.I., K.W. and N.S. were involved in the interpretation and critical revision. All authors provided final approval and took accountability. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
Nobuhiko Seki has received research grants and a speaker honorarium from Ono Pharmaceutical Company, Bristol-Myers Company, MSD, AstraZeneca, Chugai Pharmaceutical, Boehringer Ingelheim, Pfizer Japan, Eli Lilly Japan, Taiho Pharmaceutical, Daiichi Sankyo Company, and Merck Biopharma Company.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Becker G. Acites. In: Schwab M., editor. Encyclopedia of Cancer. Springer; Berlin/Heidelberg, Germany: 2009. pp. 16–18. [Google Scholar]
- 2.Stukan M. Drainage of malignant ascites: Patient selection and perspectives. Cancer Manag. Res. 2017;9:115–130. doi: 10.2147/CMAR.S100210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sangisetty S.L., Miner T.J. Malignant ascites: A review of prognostic factors, pathophysiology and therapeutic measures. World J. Gastrointest Surg. 2012;4:87–95. doi: 10.4240/wjgs.v4.i4.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saif M.W., Siddiqui I.A., Sohail M.A. Management of ascites due to gastrointestinal malignancy. Ann. Saudi Med. 2009;29:369–377. doi: 10.4103/0256-4947.55167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Becker G., Galandi D., Blum H.E. Malignant ascites: Systematic review and guideline for treatment. Eur. J. Cancer. 2006;42:589–597. doi: 10.1016/j.ejca.2005.11.018. [DOI] [PubMed] [Google Scholar]
- 6.Kietpeerakool C., Rattanakanokchai S., Jampathong N., Srisomboon J., Lumbiganon P. Management of drainage for malignant ascites in gynaecological cancer. Cochrane Database Syst. Rev. 2019;12:CD007794. doi: 10.1002/14651858.CD007794.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ito T., Yokomichi N., Ishiki H., Kawaguchi T., Masuda K., Tsukuura H., Funaki H., Suzuki K., Oya K., Nakagawa J., et al. Optimal Paracentesis Volume for Terminally Ill Cancer Patients With Ascites. J. Pain Symptom Manag. 2021;6:202. doi: 10.1016/j.jpainsymman.2021.04.010. [DOI] [PubMed] [Google Scholar]
- 8.Hodge C., Badgwell B.D. Palliation of malignant ascites. J. Surg. Oncol. 2019;120:67–73. doi: 10.1002/jso.25453. [DOI] [PubMed] [Google Scholar]
- 9.Desiderio J., Chao J., Melstrom L., Warner S., Tozzi F., Fong Y., Parisi A., Woo Y. The 30-year experience—A meta-analysis of randomised and high-quality non-randomised studies of hyperthermic intraperitoneal chemotherapy in the treatment of gastric cancer. Eur. J. Cancer. 2017;79:1–14. doi: 10.1016/j.ejca.2017.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cianci S., Riemma G., Ronsini C., De Franciscis P., Torella M., Schiattarella A., La Verde M., Colacurci N. Hyperthermic intraperitoneal chemotherapy (HIPEC) for ovarian cancer recurrence: Systematic review and meta-analysis. Gland. Surg. 2020;9:1140–1148. doi: 10.21037/gs-20-335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ito T., Hanafusa N. CART: Cell-free and Concentrated Ascites Reinfusion Therapy against malignancy-related ascites. Transfus. Apher. Sci. 2017;56:703–707. doi: 10.1016/j.transci.2017.08.018. [DOI] [PubMed] [Google Scholar]
- 12.Kloft C., Wallin J., Henningsson A., Chatelut E., Karlsson M.O. Population Pharmacokinetic-Pharmacodynamic Model for Neutropenia with Patient Subgroup Identification: Comparison across Anticancer Drugs. Clin. Cancer Res. 2006;12:5481–5490. doi: 10.1158/1078-0432.CCR-06-0815. [DOI] [PubMed] [Google Scholar]
- 13.Urrútia G., Bonfill X. PRISMA declaration: A proposal to improve the publication of systematic reviews and meta-analyses. Med. Clin. 2010;135:507–511. doi: 10.1016/j.medcli.2010.01.015. [DOI] [PubMed] [Google Scholar]
- 14.University Hospital Medical Information Network. [(accessed on 30 June 2021)]. Available online: https://uploaduminacjp/cgi-open-bin/ctr_e/ctr_viewcgi?recptno=R000050886.
- 15.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010;25:603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 16.Oken M.M., Creech R.H., Tormey D.C., Horton J., Davis T.E., McFadden E.T., Carbone P.P. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am. J. Clin. Oncol. 1982;5:649–655. doi: 10.1097/00000421-198212000-00014. [DOI] [PubMed] [Google Scholar]
- 17.Higgins J.P.T., Thompson S.G., Deeks J., Altman D.G. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hanada R., Yokomichi N., Kato C., Miki K., Oyama S., Morita T., Kawahara R. Efficacy and safety of reinfusion of concentrated ascitic fluid for malignant ascites: A concept-proof study. Support. Care Cancer. 2017;26:1489–1497. doi: 10.1007/s00520-017-3980-5. [DOI] [PubMed] [Google Scholar]
- 19.Hanafusa N., Isoai A., Ishihara T., Inoue T., Ishitani K., Utsugisawa T., Yamaka T., Ito T., Sugiyama H., Arakawa A., et al. Safety and efficacy of cell-free and concentrated ascites reinfusion therapy (CART) in refractory ascites: Post-marketing surveillance results. PLoS ONE. 2017;12:e0177303. doi: 10.1371/journal.pone.0177303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ito T., Hanafusa N., Iwase S., Noiri E., Nangaku M., Nakagawa K., Miyagawa K. Effects of cell-free and concentrated ascites reinfusion therapy (CART) on symptom relief of malignancy-related ascites. Int. J. Clin. Oncol. 2014;20:623–628. doi: 10.1007/s10147-014-0750-y. [DOI] [PubMed] [Google Scholar]
- 21.Ito T., Hanafusa N., Iwase S., Noiri E., Nangaku M., Nakagawa K., Miyagawa K. Ascitic IL-10 Concentration Predicts Prognosis of Patients Undergoing Cell-Free and Concentrated Ascites Reinfusion Therapy. Ther. Apher. Dial. 2019;24:90–95. doi: 10.1111/1744-9987.12863. [DOI] [PubMed] [Google Scholar]
- 22.Iwaki R., Shindo K., Kawahara H., Nanba N., Kuramoto Y., Beppu T., Uesugi T., Tanaka Y., Uesugi Y. Cell-Free Ascites Reinfusion Therapy(CART)Modified by Keisuke Matsusaki(KM-CART)at Our Hospital. Gan Kagaku Ryoho. Cancer Chemother. 2018;45:2165–2167. [PubMed] [Google Scholar]
- 23.Kawata Y., Nagasaka K., Matsumoto Y., Oda K., Tanikawa M., Sone K., Mori-Uchino M., Tsuruga T., Arimoto T., Osuga Y., et al. Usefulness of cell-free and concentrated ascites reinfusion therapy in the therapeutic management of advanced ovarian cancer patients with massive ascites. Int. J. Clin. Oncol. 2018;24:420–427. doi: 10.1007/s10147-018-1371-7. [DOI] [PubMed] [Google Scholar]
- 24.Maeda O., Ando T., Ishiguro K., Watanabe O., Miyahara R., Nakamura M., Funasaka K., Kazuhiro F., Ando Y., Goto H. Safety of repeated cell-free and concentrated ascites reinfusion therapy for malignant ascites from gastrointestinal cancer. Mol. Clin. Oncol. 2014;2:1103–1106. doi: 10.3892/mco.2014.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matsusaki K., Orihashi K. Feasibility, efficacy, and safety of cell-free and concentrated ascites reinfusion therapy (KM-CART) for malignant ascites. Artif. Organs. 2020;44:1090–1097. doi: 10.1111/aor.13691. [DOI] [PubMed] [Google Scholar]
- 26.Nagata Y., Kato K., Miyamoto T., Hirano H., Shoji H., Iwasa S., Honma Y., Takashima A., Hamaguchi T., Matsushita H., et al. Safety and efficacy of cell-free and concentrated ascites reinfusion therapy (CART) in gastrointestinal cancer patients with massive ascites treated with systemic chemotherapy. Support. Care Cancer. 2020;28:5861–5869. doi: 10.1007/s00520-020-05401-4. [DOI] [PubMed] [Google Scholar]
- 27.Ohta K., Ikenaga M., Ueda M., Iwaki R., Kinoshita T., Mishima K., Shindo K., Chinen Y., Itakura H., Takayama T., et al. Cell-Free and Concentrated Ascites Reinfusion Therapy for Malignant Intractable Ascites from Colorectal Cancer. Gan Kagaku Ryoho. Cancer Chemother. 2017;44:1556–1558. [PubMed] [Google Scholar]
- 28.Togami S., Hori S., Kamio M., Matsuo T., Yoshinaga M., Douchi T. Clinical usefulness of concentrated ascites reinfusion therapy (CART) for gynecological cancer patients with refractory massive ascites due to cancerous peritonitis. Eur. J. Gynaecol. Oncol. 2014;35:301–303. [PubMed] [Google Scholar]
- 29.Ueda T., Maehara M., Takahashi Y., Nakayama N., Kondo H., Shirota K., Yoshizato T., Miyamoto S. Clinical significance of cell-free and concentrated ascites re-infusion therapy for advanced and recurrent gynecological cancer. Anticancer. Res. 2012;32:2353–2357. [PubMed] [Google Scholar]
- 30.Wang L., Okubo T., Shinsaka M., Kobayashi A., Ogasawara M., Sakaguchi R., Nagai T., Seki H. Efficacy and safety of cell-free and concentrated ascites reinfusion therapy (CART) in gynecologic cancer patients with a large volume of ascites. J. Obstet. Gynaecol. Res. 2015;41:1614–1620. doi: 10.1111/jog.12763. [DOI] [PubMed] [Google Scholar]
- 31.Yamaguchi H., Kitayama J., Emoto S., Ishigami H., Ito T., Hanafusa N., Watanabe T. Cell-free and concentrated ascites reinfusion therapy (CART) for management of massive malignant ascites in gastric cancer patients with peritoneal metastasis treated with intravenous and intraperitoneal paclitaxel with oral S. Eur. J. Surg. Oncol. 2015;41:875–880. doi: 10.1016/j.ejso.2015.04.013. [DOI] [PubMed] [Google Scholar]
- 32.Yamamoto K., Nagao S., Tsu T., Matsushima T., Ishido Y., Narita M., Suzuki K., Nakazawa H., Shibutani T., Jimi T., et al. Quality of life assessment of cell-free and concentrated ascites reinfusion therapy during initial treatment for advanced ovarian cancer: A prospective cohort study. J. Obstet. Gynaecol. Res. 2021;47:1536–1543. doi: 10.1111/jog.14670. [DOI] [PubMed] [Google Scholar]
- 33.Ross G., Kessler H., Gatenby R., Hartz W., Ross L. Sonographically guided paracentesis for palliation of symptomatic malignant ascites. Am. J. Roentgenol. 1989;153:1309–1311. doi: 10.2214/ajr.153.6.1309. [DOI] [PubMed] [Google Scholar]
- 34.Heiss M.M., Murawa P., Koralewski P., Kutarska E., Kolesnik O.O., Ivanchenko V.V., Dudnichenko A.S., Aleknaviciene B., Razbadauskas A., Gore M., et al. The trifunctional antibody catumaxomab for the treatment of malignant ascites due to epithelial cancer: Results of a prospective randomized phase II/III trial. Int. J. Cancer. 2010;127:2209–2221. doi: 10.1002/ijc.25423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jatoi A., Nieva J.J., Qin R., Loprinzi C.L., Wos E.J., Novotny P.J., Moore J.D.F., Mowat R.B., Bechar N., Pajon J.E.R., et al. A Pilot Study of Long-Acting Octreotide for Symptomatic Malignant Ascites. Oncology. 2012;82:315–320. doi: 10.1159/000337246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ishitani K., Isoai A., Ito T., Sugiyama H., Arakawa A., Yamada Y., Onodera H., Kobayashi R., Torii N., Soneda N., et al. Clinical usefulness of cell-free and concentrated ascites reinfusion therapy (CART) in combination with chemotherapy for malignant ascites: A post-marketing surveillance study. Int. J. Clin. Oncol. 2021;26:1130–1138. doi: 10.1007/s10147-021-01883-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.