Abstract
Members of the polo subfamily of protein kinases play pivotal roles in cell proliferation. In addition to the kinase domain, polo kinases have a strikingly conserved sequence in the noncatalytic C-terminal domain, termed the polo box. Here we show that the budding-yeast polo kinase Cdc5, when fused to green fluorescent protein and expressed under its endogenous promoter, localizes at spindle poles and the mother bud neck. Overexpression of Cdc5 can induce a class of cells with abnormally elongated buds in a polo box- and kinase activity-dependent manner. In addition to localizing at the spindle poles and cytokinetic neck filaments, Cdc5 induces and localizes to additional septin ring structures within the elongated buds. Without impairing kinase activity, conservative mutations in the polo box abolish the ability of Cdc5 to functionally complement the defect associated with a cdc5-1 temperature-sensitive mutation, to localize to the spindle poles and cytokinetic neck filaments, and to induce elongated cells with ectopic septin ring structures. Consistent with the polo box-dependent subcellular localization, the C-terminal domain of Cdc5, but not its polo box mutant, is sufficient for subcellular localization, and its overexpression appears to inhibit cytokinesis. These data provide evidence that the polo box is required to direct Cdc5 to specific subcellular locations and induce or organize cytokinetic structures.
Members of the polo subfamily of protein kinases have been identified in various eucaryotic organisms from budding yeast to mammals and appear to play pivotal roles in cell division and proliferation. Polo kinases include mammalian Plk (9, 16, 17, 19, 25), Snk (48), and Fnk/Prk (13, 32), Xenopus laevis Plx1 (24), Drosophila melanogaster polo (34), Schizosaccharomyces pombe Plo1 (39), and Saccharomyces cerevisiae Cdc5 (22). It is apparent from genetic and biochemical analyses that polo kinases regulate diverse cellular events at various stages of M phase (for reviews, see references 15 and 26). Analyses of the phenotypes associated with a Drosophila polo mutation or an S. pombe plo1 mutation revealed that polo kinases play an important role in bipolar spindle formation (34, 39). Microinjection of an anti-Plk antibody into cultured mammalian cells revealed a role for Plk in centrosome maturation and bipolar spindle formation (27). In addition, polo kinases appear to regulate important biochemical steps at the G2/M transition, such as activation of Cdc2 through Cdc25C phosphatase (1, 24, 40), DNA damage checkpoint adaptation (49), and activation of the anaphase-promoting complex in various eucaryotic systems (8, 10, 21, 46).
Among the important functions attributed to polo kinases are their roles in cytokinesis. In S. pombe, loss of plo1+ function results in mitotic arrest with either a monopolar spindle or a failure to form the F-actin ring and deposit septal components (39). Plo1 also appears to play a role in determining the site of cell division, since mutations in plo1 results in defects in both placement and organization of the medial ring (4). Overexpression of plo1+ in wild-type cells leads to the formation of monopolar spindles but also induces multiple septal structures at any phase of the cell cycle (39), suggesting that Plo1 is sufficient to induce actin ring and septum formation. Polo kinases play roles in cytokinesis in other eukaryotic organisms as well. Studies with a Drosophila polo mutant suggested that polo is required to form correct midzone and midbody structures during telophase (6). Furthermore, the polo mutant failed either to localize the kinesin-like protein Pavarotti (3) to the midzone or to incorporate actin and the septin Peanut (37) into a contractile ring. In S. cerevisiae, ectopic expression of an activated form of murine Plk in a wild-type genetic background induces a class of cells with unusually elongated buds (29). These cells develop multiple septal structures within the abnormally elongated buds, as indicated by the localization of Cdc10, one of the septin components constituting the septin rings at the mother bud neck (29).
Polo kinases appear to play multiple roles during M-phase progression and cytokinesis, and the roles of these kinases are likely to be conserved among evolutionarily distant eucaryotic cells. We previously reported that murine Plk is a functional homolog of S. cerevisiae Cdc5 (29). By ectopically expressing Plk, we have shown that the polo box is required for the ability of Plk to functionally complement the cdc5-1 defect by targeting the catalytic activity of the enzyme to specific subcellular locations (28). In this communication, we show that the polo box is required for the subcellular localization and function of Cdc5. Overexpression of Cdc5 induces ectopic cytokinetic structures within the abnormally elongated buds, and this phenotype is also dependent on the polo box. The data reported here suggest that Cdc5 contributes a signal to regulate cytokinesis and that the polo box plays a critical role in this event.
MATERIALS AND METHODS
Strains, growth conditions, and transformations.
The yeast strains used in this study were 1788 (isogenic diploid of EG123, MATα leu2-3,112 ura3-52 trp1-1 his4 can1r) (47), 1783 (MATa EG123), KKY921-2B (MATa cdc5-1 leu2 trp1 ura1) (22), and an Spc42-GFP strain [MATa spc42Δ::LEU2 TRP::SPC42-GFP(3X) ade2-1 trp1-1 can1-100 leu2-3,112 his3-11,15 ura3 GAL psi+ ssd1-d2] (2) (a gift of J. V. Kilmartin, Medical Research Council, Cambridge, United Kingdom). Yeast cells were cultured in YEP (1% yeast extract, 2% Bacto Peptone) supplemented with 2% glucose, 2% raffinose (Sigma, St. Louis, Mo.), or 2% galactose (J. T. Baker, Phillipsburg, N.J.) as required. Synthetic minimal medium (45) supplemented with the appropriate nutrients was used to select for plasmid maintenance. Yeast transformation was carried out by the lithium acetate method (20).
Generation of Cdc5 mutants and EGFP-Cdc5 fusion constructs.
To generate the Cdc5 polo-box mutants, PCR-based mutagenesis was carried out. All the mutant clones were sequenced to confirm the introduced mutations. YCplac111-Cdc5 was constructed by inserting a 3-kb XbaI fragment from pKK625 (YCplac22-Cdc5; a gift of A. Sugino, Osaka University, Osaka, Japan) into the YCplac111 vector digested with XbaI.
YCplac22-GAL1-HA-EGFP-Cdc5 fusion constructs were generated by inserting a DNA fragment containing the HA (hemagglutinin) epitope and two tandem copies of EGFP (enhanced green fluorescent protein) between the GAL1 promoter and the CDC5 translational initiation codon of YCplac22-GAL1-Cdc5 (pKK507; a gift of A. Sugino). The EGFP coding sequence was amplified by PCR with the pEGFP-N1 vector (Clontech Laboratories, Inc., Palo Alto, Calif.) as a template. YCplac22-GAL1-HA-EGFP-cdc5ΔDB, lacking the N-terminal residues 6 to 71, was constructed by PCR. To construct YCplac22-GAL1-HA-EGFP-cdc5 · C-term, YCplac22-GAL1-HA-EGFP-Cdc5 were digested with PpuMI and NdeI, end filled, and self-ligated. The resulting construct expresses the C terminus of Cdc5 (lacking amino acid residues 6 to 239) as a fusion protein with HA-EGFP.
To tag endogeneous Cdc5 with GFP, the C-terminal sequence of cdc5 lacking the translational termination codon was inserted into the SacI and NotI sites of pFA6a-GFP(S65T)-kanMX6 (36) containing four tandem copies of GFP at the BamHI site. The resulting construct, pSK1232, contains the C-terminal sequence of cdc5 fused in frame with five copies of GFP at its C-terminal end. Generation of a yeast strain expressing a Cdc5-GFP fusion protein under endogeneous promoter control was achieved by tagging a chromosomal copy of CDC5 with GFP. Strain 1783 (MATa EG123) was transformed with pSK1232 and then selected on YEP–2% glucose medium supplemented with G418 sulfate (Gibco-BRL) at a concentration of 300 μg/ml. The resulting G418-resistant colonies were subjected to PCR analyses to confirm integration of pSK1232 at the CDC5 locus. Cells expressing Cdc5-GFP were examined by confocal microscopy (see below). It is known that the Cdc5 protein becomes abundant in the late G2 and M phases (46). Therefore, to enhance the Cdc5-GFP signals, cells were treated with nocodazole at 15 μg/ml for 5 h prior to microscopic analysis.
The YCplac-ADE2 vector was generated by replacing the LEU2-containing AatII-BglII fragment of YCplac111 with the ADE2 marker. YCplac-ADE2-GAL1-HA–glutathione S-transferase (GST)–CDC5 constructs were generated by cloning an N-terminally tagged HA-GST-Cdc5 fragment into the YCplac-ADE2 vector digested with EcoRI and SphI.
Expression of Cdc5 proteins/cdc5 · C-term and Zymolyase treatment.
Expression of various EGFP-Cdc5 proteins and EGFP-cdc5 · C-term was carried out in YEP–2% galactose medium as described previously (29).
The chains of connected cells were treated with Zymolyase as specified in previous reports (14, 33) with modifications. Briefly, cells were fixed with 3.7% formaldehyde, washed twice with phosphate-buffered saline, and then washed once with solution A (1.2 M sorbitol, 40 mM KH2PO4-KH2PO4 [pH 6.5], 0.5 mM MgCl2). The cells were incubated with 0.1 mg of Zymolyase 20T (ICN Biomedicals, Inc.), per ml in 0.5 ml of solution A–5 μl of β-mercaptoethanol for 10 min at 37°C. Loss of the refractile appearance was evident under the microscope, indicating that cell wall removal was efficient under these conditions.
Immunoprecipitation, kinase assays, and Western analyses.
Yeast cells were lysed in TED buffer (40 mM Tris-Cl [pH 7.5], 0.25 mM EDTA, 1 mM dithiothreitol, 1 mM 4-(2-aminoethyl)-benzenesulfonyl fluoride [AEBSF; Pefabloc; Boehringer Mannheim, Indianapolis, Ind.], 10 μg of pepstatin A per ml, 10 μg of leupeptin per ml, 10 μg of aprotinin per ml) with an equal volume of glass beads (Sigma). The obtained lysates were centrifuged at 2,000 × g for 2 min to remove unbroken cells and beads. The resulting supernatants were considered to be the total cellular lysates. For immunoprecipitation, total cellular lysates were subjected to further centrifugation at 15,000 × g for 30 min to clarify heavy cellular materials. Prior to incubation with affinity-purified anti-HA antibody, the resulting supernatants (S15) were diluted to 1 ml with TBSN buffer (20 mM Tris-Cl [pH 8.0], 150 mM NaCl, 0.5% Nonidet P-40, 5 mM EGTA, 1.5 mM EDTA, 0.5 mM Na3VO4, 20 mM p-nitrophenyl phosphate) supplemented with protease inhibitors. Protein A-Sepharose 4B (Zymed, South San Francisco, Calif.) was added, and the mixture was incubated for an additional 1 h to precipitate the antibody.
Kinase assays and Western analyses were carried out as described previously (29). Briefly, the kinase activity of Cdc5 was measured in a kinase cocktail (TBMD) (50 mM Tris-Cl [pH 7.5], 10 mM MgCl2, 5 mM dithiothreitol, 2 mM EGTA, 0.5 mM Na3VO4, 20 mM p-nitrophenyl phosphate) supplemented with 4 μg of dephosphorylated casein (Sigma) and 10 μM ATP (10 μCi of [γ-32P]ATP; 1 Ci = 37 Gbq). Western analyses were carried out with affinity-purified anti-GFP antibody (Clontech) at 0.5 μg/ml. Proteins that interact with antibodies were detected by the enhanced chemiluminescence Western detection system (Amersham, Arlington Heights, Ill.).
Cell staining and immunofluorescence microscopy.
Indirect immunofluorescence was performed as described previously (28). Briefly, cells cultured under induction conditions for the indicated time were fixed with 3.7% formaldehyde. Cdc10 was localized by using affinity-purified rabbit polyclonal anti-Cdc10 antibody (a gift of J. Chant, Harvard University, Cambridge, Mass.) and rhodamine-conjugated goat anti-rabbit immunoglobulin G (IgG) (Zymed). Microtubules were visualized with YOL1/34 rat antitubulin antibody (Accurate Chemical and Scientific Corp.) and goat anti-rat CY3 antibody (Jackson ImmunoResearch Laboratories, Inc., West Grove, Pa.). Actin was localized by using rhodamine-conjugated phalloidin (Molecular Probe, Eugene, Oreg.). DNA was visualized by staining with propidium iodide at 40 μg/ml. To stain the GFP-Spc42 cells transformed with HA-GST-cdc5N209AΔDB, cells were prepared by the method of Rout and Kilmartin (42) with the following modifications. Spheroplasts were prepared from log-phase cells by digestion with 10% (vol/vol) β-glucuronidase (Sigma) and 100 μg of Zymolase 20T per ml in 1.1 M sorbitol–phosphate-buffered saline buffer at 30°C for 1.5 h. Spheroplasts were then allowed to recover by culturing in 1.1 M sorbitol–YEP–2% glucose for 30 min at 25°C. After being washed with 1.1 M sorbitol–phosphate-buffered saline buffer, the cells were mounted on a polylysine-coated slide and then fixed in methanol (−20°C) for 4 min prior to a 30-s immersion in acetone (21°C). HA-GST-cdc5N209AΔDB was localized by using affinity-purified mouse monoclonal anti-HA antibody at 2 μg/ml and tetramethylrhodamine-5-isothiocyanate (TRITC)-conjugated goat anti-mouse IgG (Zymed) at a 1:800 dilution. Confocal fluorescent images were collected with a Bio-Rad MRC 1024 confocal scan head mounted on a Nikon Optiphot microscope with a 60× Planapochromat lens. Each image is the Kalman-averaged product of approximately four scans generated by using LaserSharp software.
RESULTS
Both the polo box and the kinase activity of Cdc5 are required for functional complementation of the cdc5-1 defect.
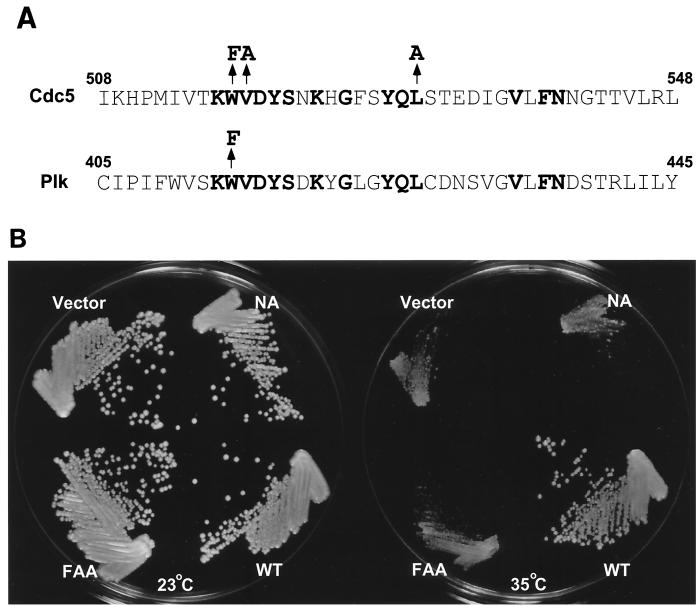
At the restrictive temperature, cells with a cdc5-1 temperature-sensitive mutation arrest late in mitosis as large-budded cells (22, 46, 49). The W414F mutation in the polo box (Fig. 1A) was previously shown to abolish the ability of Plk to functionally complement the defect associated with the cdc5-1 mutation, apparently by disrupting Plk localization at the spindle poles and cytokinetic neck filaments (28). Here, we have investigated whether the polo-box domain is also required for the function of Cdc5. A mutation analogous to the plkW414F substitution was introduced into Cdc5. Unlike Plk, the cdc5W517F mutant still possessed a significant capacity to complement the cdc5-1 defect (data not shown). We previously observed that in addition to the W414F mutation, the V415A or L427A mutations in the polo box significantly reduced the ability of Plk to complement the cdc5-1 defect (data not shown). Thus, two additional analogous mutations, V518A and L530A, were introduced into cdc5W517F. Whereas wild-type Cdc5 rescued the cdc5-1 growth defect, the resulting cdc5 mutant, cdc5W517F/V518A/L530A (for simplicity, we refer to this mutant as cdc5FAA) (Fig. 1A), was not able to restore growth to a detectable level. The N209A mutation in Cdc5 (18), which inactivates its kinase activity, also failed to complement the cdc5-1 defect (Fig. 1B). Subsequent flow cytometry analyses revealed that at the restrictive temperature, wild-type Cdc5 restored the cell division cycle defect of the cdc5-1 mutants, regenerating the 1N DNA-containing population. In contrast, cells transformed with either cdc5FAA or cdc5N209A arrested with a 2N DNA content, similar to the DNA profile observed with vector-transformed cells (data not shown). Taken together, the above data demonstrate the necessity for both the kinase activity and the polo box for Cdc5 function.
FIG. 1.
(A) Structures of the polo box in Cdc5 and Plk. Conserved amino acids in the polo box among all the members of the polo subfamily are shown in bold type. Arrows indicate amino acids changed in the point mutations in Cdc5 and Plk. (B) Either the W517F/V518A/L530A (FAA) mutations in the polo box or the N209A (NA) mutation that inactivates Cdc5 kinase activity abolishes the capacity of Cdc5 to functionally complement the cdc5-1 defect. A haploid cdc5-1 mutant strain, KKY921-2B (MATa cdc5-1 leu2 trp1 ura1) (22), was transformed with various YCplac111-Cdc5 constructs that express Cdc5 under its endogenous promoter. Transformants were selected on synthetic minimal media lacking leucine, streaked onto YEP–2% glucose, and incubated for 3 days at the indicated temperatures. Plasmids transformed are as follows (clockwise from top left): Vector, YCplac111; NA, YCplac111-cdc5N209A; WT, YCplac111-Cdc5; and FAA, YCplac111-cdc5W517F/V518A/L530A.
Cdc5-GFP localizes at spindle poles and mother bud necks when expressed under its endogenous promoter.
Previously, Shirayama et al. (46) reported that Cdc5 tagged with a myc15 epitope localizes at spindle poles, when subjected to indirect immunofluorescence studies with an anti-myc antibody. We observed that mammalian polo kinase, Plk, localizes at spindle poles and cytokinetic neck filaments when overexpressed as an EGFP-fused form in budding yeast (28). Since neck filaments are complex structures with many associating proteins, some components may not be easily accessible for immunodetection. Therefore, we have investigated Cdc5 localization in a wild-type strain, 1783, expressing Cdc5-GFP fusion at the CDC5 locus under its endogenous promoter (see Materials and Methods). These cells appear to grow normally (data not shown), suggesting that GFP-tagged Cdc5 is capable of replacing the function of the wild-type protein.
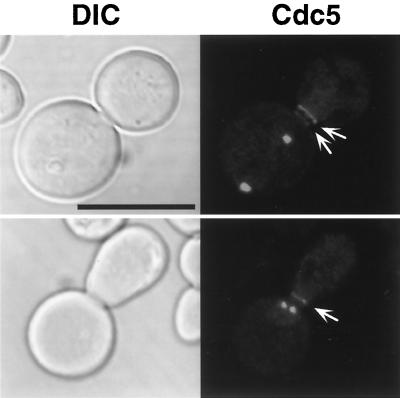
Microscopic observation of the cells expressing Cdc5-GFP revealed the presence of one or two distinct fluorescent dots, whose signals are likely to be spindle poles, as reported previously (46). In addition to these dot signals, one or occasionally two fluorescent bands at the mother bud neck were evident in the budded cells (Fig. 2). Similar results were also obtained with a wild-type W303-1A genetic background (data not shown). The additional band signals were reminiscent of Plk localization at cytokinetic neck filaments (28). However, our attempts to demonstrate colocalization of endogenous Cdc5-GFP with components at spindle poles and cytokinetic neck filaments by using indirect immunofluorescence were not successful, because Cdc5-GFP signals did not survive various fixation methods. It is noteworthy that the fluorescent signals at the bud neck were considerably weaker than those at the spindle poles, suggesting that relatively more Cdc5 proteins may localize at spindle poles than at the bud neck.
FIG. 2.
GFP tagging of endogenous Cdc5 reveals localization of Cdc5 at the mother bud neck. Endogenous Cdc5 was C-terminally tagged with five copies of GFP proteins at the CDC5 locus of a wild-type 1783 (MATa EG123) strain (see Materials and Methods). Microscopic examination revealed that in addition to one or two fluorescent dots, one or occasionally two fluorescent bands (arrows) were present at the mother bud neck in exponentially growing cells (data not shown). The dot signals are likely to be spindle poles as reported previously (46). To enhance the signals, cells were treated with nocodazole at 15 μg/ml for 5 h and examined by confocal microscopy. DIC, differential interference contrast; Cdc5, Cdc5-GFP. Bar, 5 μm.
The FAA mutations in the polo box abolish Cdc5 localization at spindle poles and cytokinetic neck filaments.
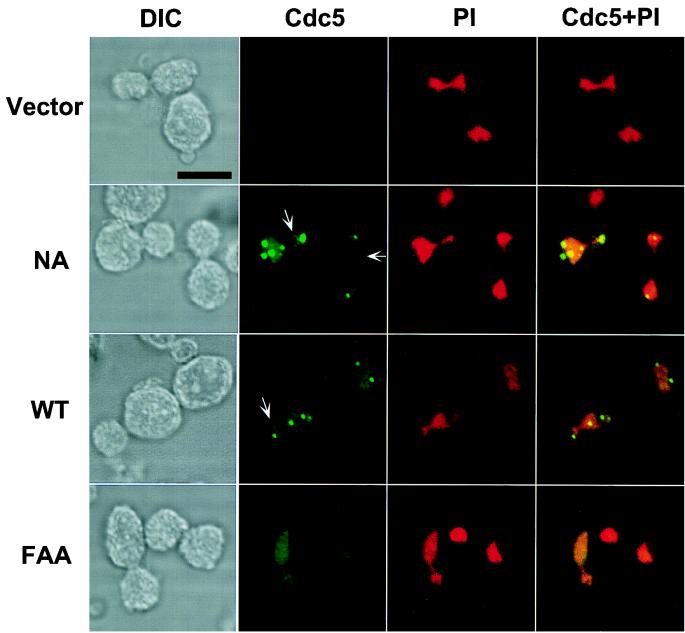
To examine whether the inability of the FAA mutant to complement the cdc5-1 defect was due to a disrupted localization, the cellular localization of Cdc5 was investigated. Constructs containing an EGFP fused at the N terminus of Cdc5 or cdc5ΔDB (Cdc5 which lacks two putative destruction boxes at the N terminus [see Materials and Methods]) were generated. When expressed under the CDC5 endogenous promoter, these constructs were able to complement the cdc5-1 defect, indicating that they are functional (data not shown). Similarly to the endogenous Cdc5-GFP localization, ectopic expression of both wild-type EGFP-Cdc5 and EGFP-cdc5N209A yielded distinct fluorescent dots in the cytoplasm as well as one bright band, or occasionally one bright and one weak band, at the mother bud neck (Fig. 3; also see Fig. 7). Subsequent immunostaining with antitubulin antibody revealed that among the multiple dots observed in some cells, two were present at each end of the mitotic spindle (Fig. 4B), indicating their localization at the spindle pole body. Occasionally, additional nascent microtubule structures were also observed with other remaining dots (data not shown), suggesting that they may associate with immature microtubule-organizing centers that possess microtubule-nucleating activity. In addition, immunostaining with anti-Cdc10 antibody showed that the bands observed with EGFP-Cdc5 colocalize with the cytokinesis-associated septin rings (see Fig. 4A and 7B). In sharp contrast, cdc5FAA failed to localize to either the spindle poles or the cytokinetic neck filaments (Fig. 3). It is noteworthy that both wild-type Cdc5 and the FAA mutant weakly stained chromosomal DNA, suggesting that Cdc5 may associate with chromatin structures independent of the polo box. Taken together, our data indicate that the polo box directs Cdc5 localization to the spindle poles and the bud neck filaments and that the FAA mutations disrupt a critical role of the polo box in targeting Cdc5 to these structures. Thus, it appears that the inability of the FAA mutant to complement the cdc5-1 defect is attributable to the loss of its specific cellular localization.
FIG. 3.
Localization of ectopically expressed wild-type and mutant forms of Cdc5 in a diploid wild-type strain, 1788. To localize Cdc5, EGFP-Cdc5 fusion constructs were generated and expressed under the control of the GAL1 promoter for 4 h. Transformants expressing EGFP fusion constructs were stained with propidium iodide to visualize chromosomal DNA and examined by confocal microscopy. Arrows indicate rings at mother bud necks. Note that EGFP-Cdc5 forms a ring structure at the mother bud neck (NA panel). Vector, YCplac22-GAL1; NA, YCplac22-GAL1-HA-EGFP-cdc5N209A; WT, YCplac22-GAL1-HA-EGFP-Cdc5; FAA, YCplac22-GAL1-HA-EGFP-cdc5W517F/V518A/L530A. DIC, differential interference contrast; Cdc5, EGFP-Cdc5 expression; PI, propidium iodide staining of nuclei; Cdc5 + PI, EGFP-Cdc5 and propidium iodide images superimposed. Bar, 5 μm.
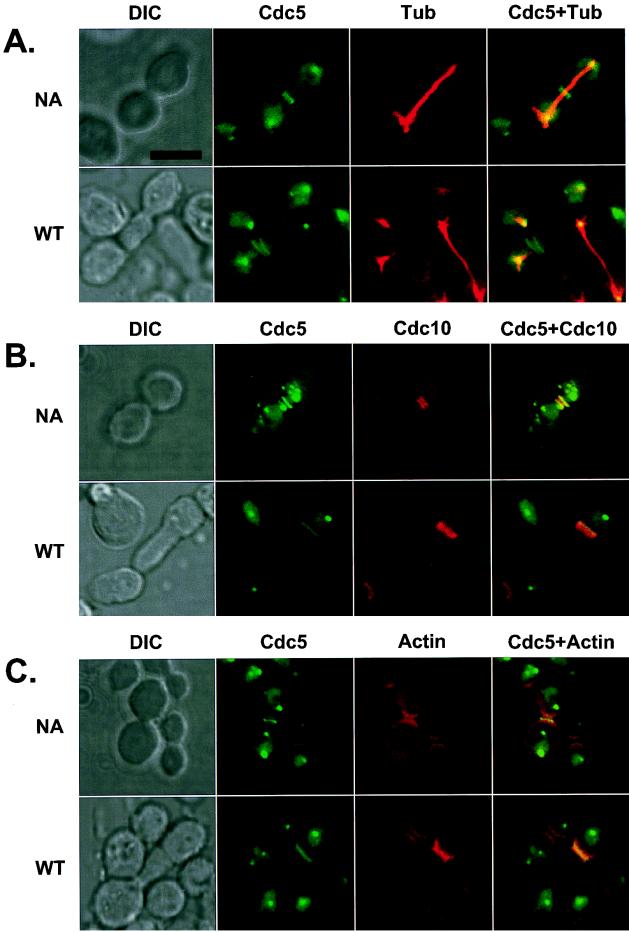
FIG. 7.
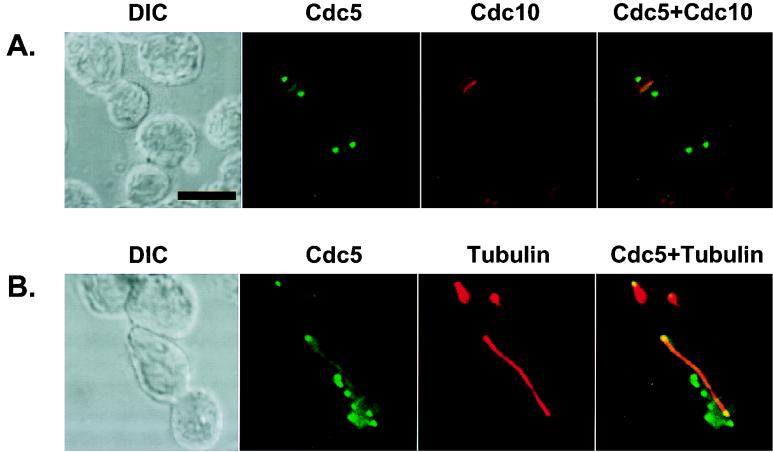
Induction of additional septal structures and actin polarization by expression of EGFP-cdc5ΔDB in a diploid wild-type strain, 1788. To enhance the signals present at the additional septal structures, the N-terminal 66 amino acid residues containing the two putative destruction boxes of Cdc5 (46) were deleted. Transformants were cultured for 12 h under inducing conditions and subjected to subsequent stainings to examine tubulin, Cdc10, and actin localization. Both normal (NA; cdc5N209AΔDB transformants) and elongated (WT; wild-type cdc5ΔDB transformants) cells are shown. (A) Cdc5 (green) localizes at the spindle poles. Spindles are visualized by microtubule staining (red). In the elongated cells, the signals at the native neck filaments were much weaker than those at the ectopic sites within the elongated buds. (B) Cdc5 (green) and Cdc10 (red) colocalize at the neck filaments and additional septal structures. (C) Cdc5 (green) can induce actin accumulation (red) at the additional septal structures. DIC, differential interference contrast; Cdc5, EGFP-cdc5ΔDB expression; Tubulin, antitubulin staining; Cdc10, anti-Cdc10 staining; Actin, rhodamine-conjugated phalloidin staining. Superimposed images are shown as Cdc5+Tubulin, Cdc5+Cdc10, and Cdc5+Actin. Bar, 5 μm.
FIG. 4.
Ectopically expressed Cdc5 localizes at the spindle poles and bud neck filaments. The EGFP-Cdc5 fusion constructs (YCplac22-GAL1-HA-EGFP-Cdc5) were expressed under the control of the GAL1 promoter in a diploid wild-type strain, 1788, for 4 h. Transformants were subjected to subsequent immunostainings to examine Cdc10 and tubulin localization. (A) EGFP-Cdc5 (green) and Cdc10 (red) localize at the neck filaments. Septin rings (red) are viewed edge-on and therefore appear as lines. Occasionally, rings of septin structures were also observed. (B) EGFP-Cdc5 (green) localizes at the spindle poles. Spindles are visualized by microtubule staining (red). The spindles appear to emanate from the structures with which Cdc5 associates. Not all the dotted EGFP-Cdc5 signals were associated with spindle poles (see the text). DIC, differential interference contrast; Cdc5, EGFP-Cdc5 expression; Cdc10, anti-Cdc10 staining; Tubulin, antitubulin staining. Superimposed images are shown as Cdc5+Cdc10 and Cdc5+Tubulin. Bar, 5 μm.
In subpopulations of cells, the presence of multiple-dot signals was evident both in wild-type Cdc5 and in cdc5N209A transformants (Fig. 3). However, induction of multiple-dot signals did not correlate with kinase activity, since transformants expressing kinase-inactive cdc5N209A showed significantly more cells with multiple dots (19% of the total population [24 of 132] when induced for 12 h) than did those expressing wild-type Cdc5 (6% of the total population [9 of 149]). Multiple-dot signals were most often present in the mother cells (Fig. 3, NA and WT panels). Among the 19% of the population with multiple dots present in cdc5N209A transformants, 17% (22 of 132) of the cells possessed multiple dots in the mother cells while only a few (2 of 132) did so in the daughter cells.
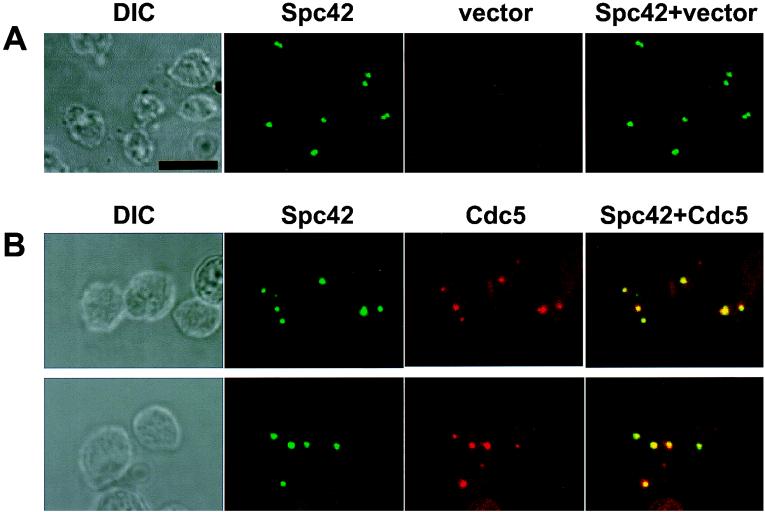
The multiple-dot signals observed with overexpression of EGFP-Cdc5 may reflect the presence of multiple microtubule-organizing centers in these cells. Spc42 is a core component of the spindle pole body (SPB), and localizes to the electron-dense central plaque of the SPB. The daughter SPB assembles on the cytoplasmic satellite structure that is attached to the mother SPB by a short segment of the nuclear membrane called the half-bridge. It has been suggested that Spc42 forms a polymeric layer at the periphery of the SPB central plaque, which has an essential function during SPB duplication (12). We overexpressed various forms of Cdc5 in a spc42 deletion strain that expresses an integrated GFP-Spc42 construct under endogenous promoter control (2). As reported previously (2), cells transformed with a vector control resulted in one or two bright dot signals in the nucleus (Fig. 5A). In contrast, when cultured under induction conditions for 12 h, cells expressing wild-type Cdc5 yielded multiple GFP-Spc42 dot signals in the cytoplasm in approximately 25% of the population. Consistent with the increased number of multiple dots observed with overexpression of EGFP-Cdc5, expression of kinase-inactive cdc5N209A resulted in a slightly larger number of dot signals than did expression of the wild type. Subsequent immunostainings revealed that multiple GFP-Spc42 dots were largely colocalized with Cdc5 (Fig. 5B). In addition, immunostaining with an antitubulin antibody revealed that multiple Spc42 dots were associated with thin nascent microtubule structures (S. Song and K. S. Lee, unpublished data). These data suggest that overexpression of Cdc5 resulted in the induction of multiple Spc42-containing subcellular structures that possess microtubule-nucleating activity.
FIG. 5.
Multiple GFP-Spc42 dot signals induced by overexpression of Cdc5 colocalize with Cdc5. A GFP-Spc42 strain transformed with either the YCplac-ADE2-GAL1-HA-GST construct or YCplac-ADE2-GAL1-HA-GST-Cdc5N209AΔDB were cultured under induction conditions for 12 h, harvested, and subjected to immunostaining with anti-HA antibody. Expression of kinase-inactive cdc5N209AΔDB resulted in slightly more cells with multiple GFP-Spc42 dots than did use of the wild type; therefore, cdc5N209AΔDB was used for this experiment. (A) GFP-Spc42 dot signals in cells transformed with vector. One or two fluorescent dot signals are evident. (B) Overexpression of Cdc5 results in induction of and colocalization with multiple GFP-Spc42-containing structures. When Cdc5 was overexpressed for 12 h, an average of four or five fluorescent dot signals were observed in approximately 25% of the population. Multiple GFP-Spc42 (green) dots observed with Cdc5 overexpression largely colocalize with multiple Cdc5 (red) signals. Immunostaining was carried out by the method of Rout and Kilmartin (42) with modifications (see Materials and Methods). HA-GST-cdc5N209AΔDB was localized by using affinity-purified monoclonal anti-HA antibody at 2 μg/ml and TRITC-conjugated goat anti-mouse IgG at a 1:800 dilution. Due to the methanol-acetone fixation, GFP-Spc42 signal intensity was diminished. It is noteworthy that not all the GFP-Spc42 and Cdc5 signals are colocalized. This is probably due to the perturbed spindle pole body organization or fragmented spindle pole bodies induced by Cdc5 overexpression (see the text). Overexpression of cdc5N209AΔDB severely reduced cell growth and resulted in an increase in cell size. DIC, differential interference contrast; Spc42, GFP-Spc42 signal; vector, YCplac-ADE2-GAL1-HA-GST construct; Cdc5, YCplac-ADE2-GAL1-HA-GST-cdc5N209AΔDB. Superimposed images are shown as Spc42+vector and Spc42+Cdc5. Bar, 5 μm.
A sharp band observed with overexpression of EGFP-Cdc5 was present mostly at the daughter side of the mother bud neck (determined by the shape of the chromosomal DNA stained with propidium iodide and the colocalization of signals from the anti-Cdc10 staining) (Fig. 4A). Closer examination revealed that a much weaker band was also present at the mother side of the mother bud neck but was not always detectable. However, the presence of two bands at the mother bud neck was evident in the transformants expressing a more stable cdc5ΔDB (see Fig. 7). At times, a ring of fluorescent signal was also observed (Fig. 3, left arrow in panel NA), indicating that Cdc5 is present as a continuous ring structure at the mother bud neck.
Induction of additional cytokinetic septal structures by ectopic expression of Cdc5.
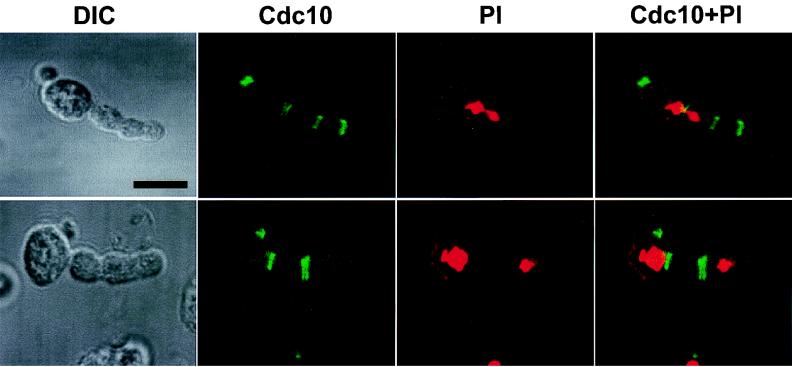
Expression of the constitutively active plkT210D in yeast resulted in the induction of a class of cells with abnormally elongated buds possessing additional septin rings (29). When Cdc5 was expressed under the control of the GAL1 promoter (pKK507; YCplac22-GAL1-Cdc5) in a wild-type genetic background for 12 h, approximately 25% of the cells displayed this abnormally elongated morphology. To investigate whether additional septin ring structures were also formed in these cells, immunostaining with an anti-Cdc10 antibody was carried out. In addition to the septin ring structures at the native cytokinetic neck filaments, an additional one to three septal structures were evident within the elongated buds (Fig. 6). Induction of these structures did not appear to correlate with the phase of the cell division cycle, since ectopic septal structures were often formed even without completion of nuclear division. In cells with two divided nuclei, these nascent septal structures were present either between or at one side of the two divided nuclei (Fig. 6). These observations indicate that the placement of these structures has occurred independently of the position of the nuclei.
FIG. 6.
Induction of additional septal structures by ectopic expression of Cdc5 in a diploid wild-type strain, 1788. Cells transformed with pKK507 (YCplac22-GAL1-Cdc5) induced cells with abnormally elongated buds. Transformants were cultured in YEP–2% galactose for 12 h prior to fixation. Subsequent immunostainings with anti-Cdc10 antibody revealed the presence of multiple septal structures within the abnormally elongated buds. Localization of Cdc10 is shown in two examples of elongated cells. DIC, differential interference contrast; Cdc10, anti-Cdc10 staining; PI, propidium iodide staining of nuclei; Cdc10+PI, superimposed images of anti-Cdc10 staining and propidium iodide staining. Bar, 5 μm.
Ectopically expressed Cdc5 localizes at additional cytokinetic septal structures.
Because Cdc5 localizes at endogenous cytokinetic neck filaments and can induce multiple septal structures within the elongated buds, the association of Cdc5 itself with the ectopic septal structures was investigated. Overexpression of EGFP-Cdc5 yielded additional distinct fluorescent bands within the abnormally elongated buds (data not shown). To enhance the localization of Cdc5 at the additional septal sites, an N-terminally truncated form of EGFP-Cdc5, EGFP-cdc5ΔDB, which has lost its N-terminal 66 amino acid residues was used. As previously reported (46), deletion of this N-terminal sequence, which contains the two putative destruction boxes, resulted in a 5- to 10-fold increase in the protein level (data not shown). At the restrictive temperature, galactose-induced expression of EGFP-cdc5ΔDB, but not of EGFP-cdc5FAAΔDB or EGFP-cdc5N209AΔDB, functionally complemented both the cell growth and the cell cycle defects associated with the cdc5-1 mutation (data not shown). The truncated cdc5 fusion proteins also gave the same localization patterns as their parental full-length Cdc5 proteins (Fig. 7), indicating that deletion of the N-terminal 66 amino acid residues did not affect the subcellular localization or functions of these Cdc5 constructs. As with full-length Cdc5 (pKK507), expression of EGFP-cdc5ΔDB, but not of the FAAΔDB or N209AΔDB mutant, induced cells with abnormally elongated buds (see below). As expected, expression of EGFP-cdc5ΔDB yielded more intense fluorescent bands within the elongated buds, reflecting the abundance of the protein in comparison to full-length EGFP-Cdc5. The additional septal structures were seen as either single or double bands (Fig. 7). Thus, we carried out immunolocalization studies with an anti-Cdc10 antibody to investigate whether the additional bands observed with EGFP-cdc5ΔDB colocalize with the ectopic septin ring structures previously observed with Cdc5 overexpression (Fig. 6). It was apparent that cdc5ΔDB colocalized with Cdc10 at both the native neck filaments and the additional septal structures (Fig. 7B); however, in the elongated cells, localization of both Cdc5 and Cdc10 at the bud neck filaments was much weaker than at the ectopic sites.
To examine whether actin was recruited to the additional septal structures, subsequent staining was carried out with rhodamine-conjugated phalloidin. We observed that actin had accumulated at both the native neck filaments and the ectopic septal structures (Fig. 7C). However, not all of the additional septal structures accumulated actin, probably because actin polarization at cytokinetic filaments occurs only during cytokinesis (for a review, see reference 31). The presence of actin at the ectopic septal structures indicates that Cdc5 expression is sufficient to recruit additional cytokinetic components to these sites. These data indicate that Cdc5 itself is targeted to ectopic, nascent septation sites and that Cdc5 may play an important role in the induction or regulation of cytokinetic events.
Both the polo box and the kinase activity of Cdc5 are required for the induction of cells with abnormally elongated buds.
The ability of galactose-driven expression of Cdc5 to induce a class of cells with unusually elongated buds indicated that polar bud growth had been deregulated. This phenotype was enhanced with expression of the more stable protein, EGFP-cdc5ΔDB. Cells with unusually elongated buds were evident as early as 6 h after induction. After 12 h of induction, 39% of the cells possessed distinctly elongated buds. In sharp contrast to EGFP-cdc5ΔDB, the introduction of either the FAA mutations in the polo box or the N209A mutation that inactivates the enzyme abolished the capacity of EGFP-cdc5ΔDB to induce this phenotype (Table 1). These data indicate that ectopic expression of Cdc5 can induce an abnormally elongated bud phenotype and that both the polo-box domain and the kinase activity of Cdc5 are required for this event.
TABLE 1.
Requirement of the polo box and kinase activity of Cdc5 for induction of an abnormally elongated bud phenotypea
| Plasmid | % of cells with abnormally elongated buds |
|---|---|
| YCplac22-GAL1 | 0.0 |
| YCplac22-GAL1-EGFP-Cdc5N209AΔDB | 0.1 |
| YCplac22-GAL1-EGFP-Cdc5ΔDB | 39.0 |
| YCplac22-GAL1-EGFP-Cdc5W517F/ V518A/L530AΔDB | 0.0 |
A diploid wild-type strain, 1788, transformed with various Cdc5 constructs was cultured in YEP–2% galactose at 30°C for 12 h. More than 1,500 cells were counted for each transformant.
The FAA mutations in the polo box do not influence the expression level or the kinase activity of Cdc5.
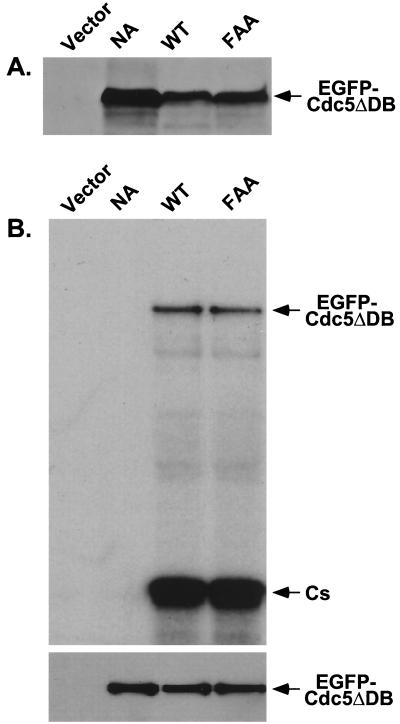
It is possible that the inability of EGFP-cdc5FAAΔDB to induce abnormally elongated buds or to localize at the cytokinetic septal structures is due to altered expression level or kinase activity. In total cellular lysates prepared after inducing for 12 h, both the wild-type and the FAA mutant proteins were present at similar levels whereas the kinase-inactive cdc5N209AΔDB was two- to threefold more abundant (Fig. 8A). In vitro kinase assays show that both EGFP-cdc5ΔDB and EGFP-cdc5FAAΔDB exhibited similar levels of both autophosphorylation and casein phosphorylation activities whereas EGFP-cdc5N209AΔDB had no detectable activity (Fig. 8B, top). Western analyses revealed that approximately equal amounts of EGFP-cdc5N209AΔDB, EGFP-cdc5ΔDB, and EGFP-cdc5FAAΔDB proteins were precipitated (Fig. 8B, bottom). These data indicate that the abrogation of the elongated bud phenotype observed with EGFP-cdc5FAAΔDB expression is specifically due to the disrupted function of the polo box by the FAA mutations and is not due to altered expression or kinase activity.
FIG. 8.
(A) The FAA mutations do not influence the level of Cdc5 expression. The wild-type 1788 cells bearing various YCplac22-GAL1-HA-EGFP-Cdc5 constructs were cultured under inducing conditions for 12 h and harvested. The same amount (400 μg each) of total cellular lysates prepared from various transformants was loaded onto each lane. After the proteins were transferred onto a polyvinylidene difluoride membrane, proteins interacting with the anti-GFP antibody were detected by immunoblotting. Vector, YCplac22-GAL1; NA, YCplac22-GAL1-HA-EGFP-cdc5N209AΔDB; WT, YCplac22-GAL1-HA-EGFP-cdc5ΔDB; FAA, YCplac22-GAL1-HA-EGFP-cdc5W517F/V518A/L530AΔDB. (B) The FAA mutations in the polo box do not impair Cdc5 kinase activity in vitro. Total cellular lysates prepared as described above were subjected to further centrifugation at 15,000 × g for 30 min to clarify heavy cellular materials. Equal amounts (1 mg each) of the resulting supernatants (S15) were diluted to 1 ml with TBSN buffer supplemented with protease inhibitors and then incubated with anti-HA antibodies. Protein A-Sepharose 4B was added, and the mixture was incubated for an additional 1 h to precipitate the antibodies. Immune complex kinase assays were carried out with casein as the substrate. Cs, casein. (Top) Reaction mixtures were electrophoresed, and the proteins were transferred onto a polyvinylidene difluoride membrane and exposed to detect kinase activities. (Bottom) The same blot was subjected to immunoblotting with anti-EGFP antibody to determine the amount of HA-EGFP-Cdc5 protein present in each immunoprecipitate. Lane designations are as defined for panel A.
Introduction of the FAA mutations in the polo box abolishes inhibition of cytokinesis by the C-terminal domain of Cdc5.
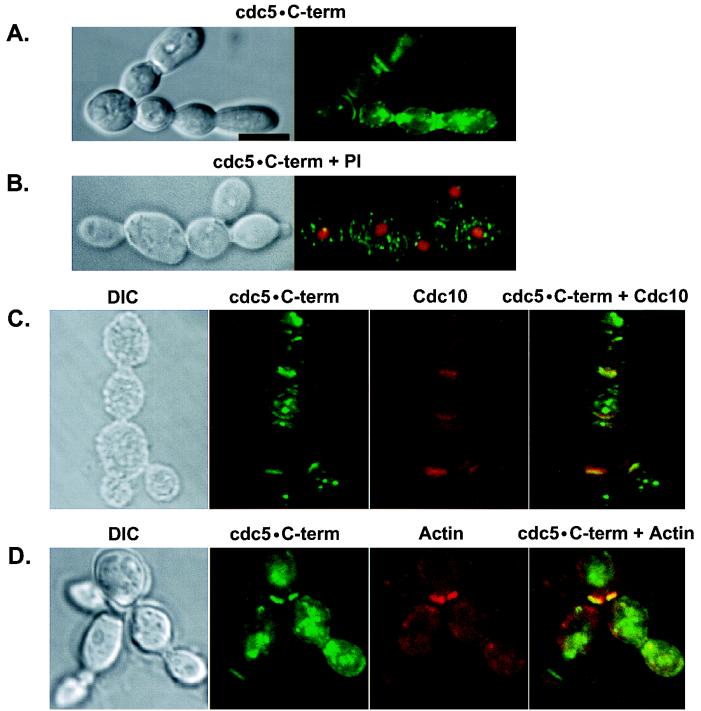
The FAA mutations in the polo-box domain abolish the localization and function of Cdc5, suggesting that the polo box functions as an essential interaction domain to target the catalytic activity of Cdc5 to specific subcellular locations. Thus, ectopic expression of the polo-box domain may lead to an inhibition of Cdc5 function by competing for an essential binding protein(s) that interacts with endogenous Cdc5. To test this possibility directly, both the wild-type and the FAA mutant forms of the C-terminal domain of Cdc5 (these constructs have amino acids 6 to 239 deleted) were expressed as HA-EGFP fusion proteins (HA-EGFP-cdc5 · C-term). Expression of the HA-EGFP-cdc5 · C-term induced about 10% of the total population to form chains of connected cells (Fig. 9A, middle; Fig. 10). These cells appear to be different from the cells with elongated buds induced by Cdc5 (Fig. 6 and 7) or Plk expression (29), in that the cells in chains have normal sizes and morphologies. Strikingly, this phenotype was completely abolished by the introduction of the FAA mutations into the polo box (Fig. 9A, bottom).
FIG. 9.
Inhibition of cytokinesis by expression of the C-terminal domain of Cdc5 is abolished by the introduction of the FAA mutations in the polo box. (A) Introduction of the FAA mutations into the polo box abolish localization of HA-EGFP-cdc5 · C-term. Wild-type 1788 cells bearing either YCplac22-GAL1-HA-EGFP-cdc5 · C-term or YCplac22-GAL1-HA-EGFP-cdc5 · C-termFAA were cultured in YEP–2% galactose medium for 12 h and then examined under the confocal microscope. HA-EGFP-cdc5 · C-term localizes as dot signals in the cytoplasm and bands at the cytokinetic neck filaments. Examples of either normal cells (top) or chains of connected cells (middle) are shown. The FAA mutant yielded only dispersed signals (bottom). (Left) Differential interference contrast; (right) HA-EGFP-cdc5 · C-term expression. cdc5 · C-term, HA-EGFP-cdc5 · C-term; cdc5 · C-termFAA, HA-EGFP-cdc5 · C-termFAA. Bar, 5 μm. (B) The FAA mutations do not influence the expression level of HA-EGFP-cdc5 · C-term. Wild-type 1788 cells bearing various constructs were cultured under inducing conditions for 12 h and harvested. The same amount (400 μg each) of total cellular lysates prepared from various transformants was loaded onto each lane. After the proteins were transferred onto a polyvinylidene difluoride membrane, proteins interacting with an anti-GFP antibody were detected by immunoblotting. cdc5 · C-term denotes the HA-GFP-cdc5 · C-term proteins expressed. Vector, YCplac22-GAL1; WT, YCplac22-GAL1-HA-EGFP-cdc5 · C-term; FAA, YCplac22-GAL1-HA-EGFP-cdc5 · C-termFAA.
FIG. 10.
Expression of HA-EGFP-cdc5 · C-term, but not its FAA mutant, results in the induction of chains of connected cells. Wild-type 1788 cells bearing YCplac22-GAL1-HA-EGFP-cdc5 · C-term was cultured in YEP–2% galactose medium for 12 h and then examined under the confocal microscope. (A) Localization of HA-EGFP-cdc5 · C-term at the multiple cytokinetic neck filaments between the connected cells. To visualize clear morphology of the connected cells, live cells were examined under the microscope without fixation. (B) Nuclei were present in most of the cell bodies present in the connected cells. To visualize nuclei in these cells, cells were fixed and then treated with propidium iodide. (C) The presence of septin ring structures between the cell bodies of connected cells. Septin rings were observed by staining cells with an anti-Cdc10 antibody. (D) Accumulation of actin was often evident between the cell bodies. DIC, differential interference contrast; cdc5 · C-term, HA-EGFP-cdc5 · C-term; PI, propidium iodide staining; Cdc10, anti-Cdc10 staining; Actin, rhodamine-conjugated phalloidin staining. Superimposed images are shown as cdc5 · C-term + PI, cdc5 · C-term + Cdc10, and cdc5 · C-term + Actin. Bar, 5 μm.
Expression of the HA-EGFP-cdc5 · C-term yielded multiple dots in the cytoplasm and strong bands at the mother bud neck, whereas its FAA mutant yielded only diffuse signals (Fig. 9A). Immunostaining with anti-Cdc10 and antitubulin antibodies revealed that, as with full-length Cdc5, the C-terminal domain localizes to the cytokinetic neck filaments and spindle poles (Fig. 10C and data not shown). Accumulated actins were often observed between the cell bodies of connected cells (Fig. 10D), suggesting that cells are in cytokinesis. A much smaller construct lacking the entire N-terminal kinase domain (amino acids 6 to 448) also localized to these sites and induced a similar phenotype, whereas its FAA mutant did not (data not shown). This observation indicates that the C-terminal domain of Cdc5 is sufficient to localize at the neck filaments and that the intact polo box is required for this event. In total cellular lysates, both HA-EGFP-cdc5 · C-term and its FAA mutant were expressed at similar levels (Fig. 9B). Thus, the inability of the FAA mutant to localize at specific subcellular locations and induce chains of connected cells is apparently due to the disruption in the function of the polo box.
The observed chains of cells may result from a failure either in cytokinesis or in cell separation after cytokinesis. To distinguish between these two possibilities, we treated these cells with Zymolyase (see Materials and Methods) and examined whether they dissociate from one another after cell wall digestion. Microscopic examination revealed that a similar percentage of the cells remained connected after this treatment or the treatment followed by sonication (data not shown). In addition, nuclei were present in most of the cell bodies of connected cells (Fig. 10B). These data strongly suggest that the chains of cells share cytoplasm as a single cell and that nuclear divisions have occurred normally without cytokinesis. Taken together, our results suggest that expression of the C-terminal domain of Cdc5, but not the FAA mutant, results in the inhibition of cytokinesis.
DISCUSSION
The conserved role of the polo box for the mitotic functions of polo kinases.
We have previously shown that in budding yeast, the W414F mutation in the polo box abolishes the ability of Plk to localize at spindle poles and cytokinetic neck filaments and to functionally complement the cdc5-1 defect, suggesting that the polo box plays a critical role in the localization and function of Plk. In the present study, we demonstrated that the role of the polo box is conserved between mammalian Plk and budding-yeast Cdc5. The analogous cdc5W517F mutation only partially impaired the ability of Cdc5 to complement the cdc5-1 defect, reflecting its partially impaired capacity to localize at the spindle poles and cytokinetic neck filaments (data not shown). However, additional mutations W517F/V518A/L530A (FAA) in the polo box domain completely abrogated the ability of Cdc5 to complement the cdc5-1 defect. This observation is consistent with the inability of the cdc5FAA mutant to localize at spindle poles and cytokinetic neck filaments. Close correlation between the Cdc5 localization at spindle poles and cytokinetic neck filaments and its ability to complement the cdc5-1 defect strongly suggest that the polo box plays a critical role in Cdc5 function by directing its subcellular localization.
Our data demonstrate that, as with Plk, both the polo-box domain and the kinase activity are required for the mitotic functions of Cdc5. The requirement of both domains extends to the capacities of Cdc5 and Plk to induce a subpopulation of cells with abnormally elongated buds and to the deposition of septins at ectopic cytokinetic structures. Ectopically expressed Cdc5 or Plk localizes at the ectopic cytokinetic sites in a polo-box-dependent manner (Fig. 7) (K. S. Lee, T. Z. Grenfell, and R. L. Erikson, unpublished data). Thus, the polo box appears to function as an interaction domain to target the catalytic activity of polo kinases to specific subcellular locations, thereby allowing efficient interaction with their physiological substrates or activators.
Polo kinases and cytokinesis.
Polo kinases in various organisms appear to localize at the subcellular structures important for cytokinesis. Both Drosophila polo and mammalian Plk localize at the centrosomes and the midzone/midbody (3, 16, 30), both of which have been implicated in directing the site of cytokinesis (11, 38, 41, 43, 50). In fission yeast, Plo1 localizes to the medial contractile ring that enforces cytokinesis, as well as to the spindle pole bodies and spindles of mitotic cells (4). We previously reported that when Plk is expressed in budding yeast, it localizes at cytokinetic neck filaments rather than at the spindle midzone, in addition to the localization at the spindle poles (28). Here we show that Cdc5 also localizes at spindle poles and cytokinetic neck filaments (Fig. 2 and 4). Thus, localization of Cdc5 or Plk at the neck filaments in budding yeast, in contrast to Plk localization at the midbody in cultured mammalian cells, may reflect the difference in the temporal and spatial arrangement of mitotic events by which cytokinetic sites are specified in these evolutionarily distant eucaryotic cells. Our observations also suggest that the cytokinetic neck filament in budding yeast is a functional counterpart of the midzone/midbody structures in higher eucaryotic cells.
It is noteworthy that indirect-immunofluorescence studies revealed that endogenous Cdc5 localizes at the spindle poles (46), but it was not detected at the bud neck filaments. We show that endogeneous Cdc5 tagged with GFP localizes at both the spindle poles and the cytokinetic neck filaments. Thus, detection of Cdc5 at the neck filaments could be attributable to the two different detection methods used.
As with induction of multiple fluorescent dot signals by overexpression of EGFP-Cdc5, overexpression of Cdc5 induces multiple GFP-Spc42 signals. In contrast, mutations in the polo box abolished this phenomenon (Song and Lee, unpublished), indicating that the polo-box domain is required for this event. Furthermore, our data show that Cdc5 localizes at multiple Spc42-containing subcellular structures (Fig. 5). Since Cdc5 induces multiple Spc42 signals in a polo-box-dependent, kinase activity-independent manner, we speculate that overexpression of an intact polo-box region may have perturbed the process of spindle pole body organization or formation, and induced primitive microtubule nucleation sites through fragmentation of spindle pole body structures. It is noteworthy that treatment of Chinese hamster ovary cells with colcemid, a microtubule-destabilizing agent, induces the dispersion of the pericentriolar materials and the subsequent production of many microtubule-nucleating sites (44).
Several lines of evidence suggest that polo kinases play an important role in inducing cytokinetic events. In fission yeast, overexpression of Plo1 induces multiple septa in any phase of the cell cycle (39). Loss of plo1+ function results in a defect in F-actin ring formation, in deposition of septal material (39), and both the placement and organization of the medial contractile ring (4). In Drosophila, a mutation in polo prevents the formation of a proper spindle midzone and disturbs the relocalization of the cytokinetic motor protein, Pav-KLP, to the midzone, preventing contractile ring formation (6). Conversely, the pav mutant shows improper localization of polo, the septin Peanut, actin, and the actin-associated protein Anillin (3). In addition, the apparent interaction between Polo and Pav appears to play an important role in organizing the cytokinetic machinery (3). Thus, it will be interesting to investigate whether the polo box is required for this interaction.
In budding yeast, ectopic expression of plkT210D, but not its polo-box mutant plkT210D/W414F, induces a class of cells with abnormally elongated buds that possess ectopic, nascent sites of cytokinesis, as indicated by the localization of the septin Cdc10 and accumulation of actin (Lee et al., unpublished). Overexpression of Cdc5 in a wild-type genetic background also induces ectopic septal structures in a polo-box-dependent manner (Fig. 7). In addition, both Cdc5 and Plk, but not the polo-box mutants, localize at the ectopic septin ring structures (Fig. 7) (Lee et al., unpublished). The polo-box-dependent induction of ectopic septin ring structures and the presence of polo kinases at the newly induced septal structures strongly suggest that polo kinases are directly involved in the regulation of cytokinetic events and that the polo box plays an important role in this process. Our data further suggest that the polo-box domain of Cdc5 or Plk plays a critical role in interacting with essential protein(s) at these distinct subcellular locations. This hypothesis is supported by our observation that overexpression of the C-terminal domain of Cdc5, but not its FAA mutant, inhibits cytokinesis, probably by competing for the common binding protein(s) that interacts with endogenous Cdc5.
To directly investigate whether an intact polo box is important for cytokinesis, a cdc5-1 strain was transformed with various cdc5 mutants and examined for their arresting phenotypes. At 35°C, the cdc5-1 cells harboring a centromeric cdc5-1 plasmid yielded only 13% of cells in cytokinesis with disassembled spindles, while 87% of the population were still arrested with long telophase spindles. In contrast, 44% of the cdc5-1 cells transformed with the corresponding cdc5FAA plasmid showed a cytokinetic arrest phenotype with disassembled spindles in large-budded cells (Song and Lee, unpublished), suggesting that cdc5FAA was able to partially relieve the mitotic exit defect of the cdc5-1 mutation.
The presence of cleavage furrow-like invaginations in the elongated buds with distinct septin rings and accumulated actin suggest that polo kinases play a direct role in providing a signal to regulate cytokinetic events. However, failure to observe a significant number of anucleate population among these cells (data not shown) suggests that the ectopic cytokinetic structures may not be fully functional, capable of pinching off daughter cells. Interestingly, among the elongated cells with additional septal structures, 23% (116 of 495) of the population were uninucleated, with an undivided spindle pole. These observations indicate that ectopic septin rings are formed in these cells without completion of both spindle pole duplication and nuclear division. Thus, it appears that induction of ectopic septal structures by Cdc5 expression is not coupled to nuclear division process.
The mechanisms regulating septin organization and ring formation may involve many components in a highly coordinated hierarchy. Our data show that overexpression of Cdc5 is sufficient to induce the formation of septin ring structures within the elongated buds and that the polo box appears to be a key element in targeting the catalytic activity of Cdc5 to regulate cortical actin and septin cytoskeleton assembly. Cdc5 genetically interacts with other late mitotic protein kinases such as Dbf2/20 and Cdc15 (21, 22). Although it is not known whether Dbf2/20 and Cdc15 play a role in cytokinesis, Mob1, a late mitotic component genetically and physically interacting with Dbf2 (23), also localizes at spindle poles and bud necks, when it is overexpressed as a GFP fusion (Song and Lee, unpublished). This observation suggests a potential role of these kinases in cytokinesis. In addition, it has been shown that Gin4 kinase plays an important role in septin organization, apparently through direct interactions with septins (7, 35). Gin4 appears to control bud growth in a distinct pathway initiated by the Clb2 cyclin (5, 7). Thus, it is possible that Cdc5 and Gin4 function in parallel pathways to coordinately regulate septin assembly and organization.
In this communication, we highlight the importance of the polo box in the mitotic functions of Cdc5 and its requirement for induction of ectopic septin ring structures. The data obtained strongly support the hypothesis that polo kinases perform crucial functions in imposing cytokinesis and therefore in regulating cell division. The identification of polo-box-interacting proteins and additional substrates will provide a better understanding of the mechanisms by which polo kinases function in regulating cytokinesis and cell division.
ACKNOWLEDGMENTS
We are grateful to Frank J. Gonzalez, Michael Bustin, and Daniel G. Lee for critical reading of this manuscript. We also thank Philip R. Lee and G.-Y. Kim for technical support, J. V. Kilmartin for the GFP-Spc42 strain, T. N. Davis for advice in immunostaining with a GFP-Spc42 strain, J. Chant for the provision of anti-Cdc10 antibody, A. Sugino for the pKK507 and pKK625 plasmids, M. S. Longtine for pFA6a-tagging plasmids, and C. Hardy for a cdc5N209A construct.
This work was supported by NIH grant CA42580 to R.L.E. R.L.E. is an American Cancer Society Research Professor.
REFERENCES
- 1.Abrieu A, Brassac T, Galas S, Fisher D, Labbe J C, Doree M. The polo-like kinase plx1 is a component of the MPF amplification loop at the G2/M-phase transition of the cell cycle in Xenopus eggs. J Cell Sci. 1998;111:1751–1757. doi: 10.1242/jcs.111.12.1751. [DOI] [PubMed] [Google Scholar]
- 2.Adams I R, Kilmartin J K. Localization of core spindle pole body (SPB) components during SPB duplication in Saccharomyces cerevisiae. J Cell Biol. 1999;145:809–823. doi: 10.1083/jcb.145.4.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adams R, Tavares A, Salzberg A, Bellen H, Glover D. pavarotti encodes a kinesin-like protein required to organize the central spindle and contractile ring for cytokinesis. Genes Dev. 1998;12:1483–1494. doi: 10.1101/gad.12.10.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bahler J, Steever A B, Wheatley S, Wang Y I, Pringle J R, Gould K L, McCollum D. Role of polo kinase and Mid1p in determining the site of cell division in fission yeast. J Cell Biol. 1998;143:1603–1616. doi: 10.1083/jcb.143.6.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benton B K, Tinkelenberg A, Gonzalez I, Cross F R. Cla4p, a Saccharomyces cerevisiae Cdc42p-activated kinase involved in cytokinesis, is activated at mitosis. Mol Cell Biol. 1997;17:5067–5076. doi: 10.1128/mcb.17.9.5067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carmena M, Riparbelli M G, Minestrini G, Tavares A M, Adams R, Callaini G, Glover D M. Drosophila polo kinase is required for cytokinesis. J Cell Biol. 1998;143:659–671. doi: 10.1083/jcb.143.3.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carroll C W, Altman R, Schieltz D, Yates J R, Kellogg D. The septins are required for the mitosis-specific activation of the Gin4 kinase. J Cell Biol. 1998;143:709–717. doi: 10.1083/jcb.143.3.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Charles J, Jaspersen S, Tinker-Kulberg R, Hwang L, Szidon A, Morgan D. The Polo-related kinase Cdc5 activates and is destroyed by the mitotic cyclin destruction machinery in S. cerevisiae. Curr Biol. 1998;8:497–507. doi: 10.1016/s0960-9822(98)70201-5. [DOI] [PubMed] [Google Scholar]
- 9.Clay F J, McEwen S J, Bertoncello I, Wilks A F, Dunn A R. Identification and cloning of a protein kinase-encoding mouse gene, Plk, related to the polo gene of Drosophila. Proc Natl Acad Sci USA. 1993;90:4882–4886. doi: 10.1073/pnas.90.11.4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Descombes P, Nigg E A. The polo-like kinase Plx1 is required for M phase exit and destruction of mitotic regulators in Xenopus egg extracts. EMBO J. 1998;17:1328–1335. doi: 10.1093/emboj/17.5.1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Devore J J, Conrad G W, Rappaport R. A model for astral stimulation of cytokinesis in animal cells. J Cell Biol. 1989;109:2225–2232. doi: 10.1083/jcb.109.5.2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Donaldson A D, Kilmartin J V. Spc42p: a phosphorylated component of the S. cerevisiae spindle pole body (SPD) with an essential function during SPB duplication. J Cell Biol. 1996;132:887–901. doi: 10.1083/jcb.132.5.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Donohue P J, Alberts G F, Guo Y, Winkles J A. Identification by targeted differential display of an immediate early gene encoding a putative serine/threonine kinase. J Biol Chem. 1995;270:10351–10357. doi: 10.1074/jbc.270.17.10351. [DOI] [PubMed] [Google Scholar]
- 14.Frazier J A, Wong M L, Longtine M S, Pringle J R, Mann M, Mitchison T J, Field C. Polymerization of purified yeast septins: evidence that organized filament arrays may not be required for septin function. J Cell Biol. 1998;143:737–749. doi: 10.1083/jcb.143.3.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glover D M, Hagan I M, Tavares A A M. Polo-like kinases: a team that plays throughout mitosis. Genes Dev. 1998;12:3777–3787. doi: 10.1101/gad.12.24.3777. [DOI] [PubMed] [Google Scholar]
- 16.Golsteyn R M, Schultz S J, Bartek J, Ziemiecki A, Ried T, Nigg E A. Cell cycle analysis and chromosomal localization of human Plk1, a putative homologue of the mitotic kinases Drosophila polo and Saccharomyces cerevisiae Cdc5. J Cell Sci. 1994;107:1509–1517. doi: 10.1242/jcs.107.6.1509. [DOI] [PubMed] [Google Scholar]
- 17.Hamanaka R, Maloid S, Smith M R, O'Connell C D, Longo D L, Ferris D K. Cloning and characterization of human and murine homologues of the Drosophila polo serine-threonine kinase. Cell Growth Differ. 1994;5:249–257. [PubMed] [Google Scholar]
- 18.Hardy C F J, Pautz A. A novel role for Cdc5p in DNA replication. Mol Cell Biol. 1996;16:6775–6782. doi: 10.1128/mcb.16.12.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holtrich U, Wolf G, Bräuninger A, Karn T, Böhme B, Rübsamen-waigmann H, Strebhardt K. Induction and down-regulation of PLK, a human serine/threonine kinase expressed in proliferating cells and tumors. Proc Natl Acad Sci USA. 1994;91:1736–1740. doi: 10.1073/pnas.91.5.1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ito H, Fukuda Y, Murata K, Kimura A. Transformation of intact yeast cells treated with alkali cations. J Bacteriol. 1983;153:163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jaspersen S L, Charles J F, Tinker-Kulberg R L, Morgan D O. A late mitotic regulatory network controlling cyclin destruction in Saccharomyces cerevisiae. Mol Biol Cell. 1998;9:2803–2817. doi: 10.1091/mbc.9.10.2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kitada K, Johnson A L, Johnston L H, Sugino A. A multicopy suppressor gene of the Saccharomyces cerevisiae G1 cell cycle mutant gene dbf4 encodes a protein kinase and is identified as CDC5. Mol Cell Biol. 1993;13:4445–4457. doi: 10.1128/mcb.13.7.4445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Komarnitsky S I, Chiang Y C, Luca F C, Chen J, Toyn J H, Winey M, Johnston L H, Denis C L. DBF2 protein kinase binds to and acts through the cell cycle-regulated MOB1 protein. Mol Cell Biol. 1998;18:2100–2107. doi: 10.1128/mcb.18.4.2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kumagai A, Dunphy W G. Purification and molecular cloning of Plx1, a Cdc25-regulatory kinase from Xenopus egg extracts. Science. 1996;273:1377–1380. doi: 10.1126/science.273.5280.1377. [DOI] [PubMed] [Google Scholar]
- 25.Lake R J, Jelinek W R. Cell cycle- and terminal differentiation-associated regulation of the mouse mRNA encoding a conserved mitotic protein kinase. Mol Cell Biol. 1993;13:7793–7801. doi: 10.1128/mcb.13.12.7793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lane H, Nigg E A. Cell-cycle control: POLO-like kinases join the outer circle. Trends Cell Biol. 1997;7:63–68. doi: 10.1016/S0962-8924(96)10051-9. [DOI] [PubMed] [Google Scholar]
- 27.Lane H A, Nigg E A. Antibody microinjection reveals an essential role for human polo-like kinase 1 (Plk1) in the functional maturation of mitotic centrosomes. J Cell Biol. 1996;135:1701–1713. doi: 10.1083/jcb.135.6.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee K S, Grenfell T, Yarm F, Erikson R. Mutation of the polo-box disrupts localization and mitotic functions of the mammalian polo kinase Plk. Proc Natl Acad Sci USA. 1998;95:9301–9306. doi: 10.1073/pnas.95.16.9301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee K S, Erikson R L. Plk is a functional homolog of Saccharomyces cerevisiae Cdc5, and elevated Plk activity induces multiple septation structures. Mol Cell Biol. 1997;17:3408–3417. doi: 10.1128/mcb.17.6.3408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee K S, Yuan Y-L, Kuriyama R, Erikson R L. Plk is an M-phase-specific protein kinase and interacts with a kinesin-like protein, CHO1/MKLP-1. Mol Cell Biol. 1995;15:7143–7151. doi: 10.1128/mcb.15.12.7143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lew D J, Reed S I. Cell cycle control of morphogenesis in budding yeast. Curr Opin Genet Dev. 1995;5:17–23. doi: 10.1016/s0959-437x(95)90048-9. [DOI] [PubMed] [Google Scholar]
- 32.Li B, Ouyang B, Pan H, Reissmann P T, Slamon D J, Arceci R, Lu L, Dai W. prk, a cytokine-inducible human protein serine/threonine kinase whose expression appears to be down-regulated in lung carcinomas. J Biol Chem. 1996;271:19402–19408. doi: 10.1074/jbc.271.32.19402. [DOI] [PubMed] [Google Scholar]
- 33.Lippincott J, Li R. Sequential assembly of myosin II, an IQGAP-like protein, and filamentous actin to a ring structure involved in budding yeast cytokinesis. J Cell Biol. 1998;140:355–366. doi: 10.1083/jcb.140.2.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Llamazares S, Moreira A, Tavares A, Girdham C, Spruce B A, Gonzalez C, Karess R E, Glover D M, Sunkel C E. polo encodes a protein kinase homolog required for mitosis in Drosophila. Genes Dev. 1991;5:2153–2165. doi: 10.1101/gad.5.12a.2153. [DOI] [PubMed] [Google Scholar]
- 35.Longtine M S, Fares H, Pringle J R. Role of the yeast Gin4p protein kinase in septin assembly and the relationship between septin assembly and septin function. J Cell Biol. 1998;143:719–736. doi: 10.1083/jcb.143.3.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Longtine M S, McKenzie III A, Demarini D J, Shah N G, Wach A, Brachat A, Philippsen P, Pringle J R. Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast. 1998;14:953–961. doi: 10.1002/(SICI)1097-0061(199807)14:10<953::AID-YEA293>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 37.Neufeld T P, Rubin G M. The Drosophila peanut gene is required for cytokinesis and encodes a protein similar to yeast putative bud neck filament proteins. Cell. 1994;77:371–379. doi: 10.1016/0092-8674(94)90152-x. [DOI] [PubMed] [Google Scholar]
- 38.Oegema K, Mitchison T J. Rappaport rules: cleavage furrow induction in animal cells. Proc Natl Acad Sci USA. 1997;94:4817–4820. doi: 10.1073/pnas.94.10.4817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ohkura H, Hagan I M, Glover D M. The conserved Schizosaccharomyces pombe kinase plo1, required to form a bipolar spindle, the actin ring, and septum, can drive septum formation in G1 and G2 cells. Genes Dev. 1995;9:1059–1073. doi: 10.1101/gad.9.9.1059. [DOI] [PubMed] [Google Scholar]
- 40.Qian Y W, Erikson E, Li C, Maller J L. Activated polo-like kinase Plx1 is required at multiple points during mitosis in Xenopus laevis. Mol Cell Biol. 1998;18:4262–4271. doi: 10.1128/mcb.18.7.4262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rappaport R. Establishment of the mechanism of cytokinesis in animal cells. Int Rev Cytol. 1986;105:245–281. doi: 10.1016/s0074-7696(08)61065-7. [DOI] [PubMed] [Google Scholar]
- 42.Rout M P, Kilmartin J V. Components of the yeast spindle and spindle pole body. J Cell Biol. 1990;111:1913–1927. doi: 10.1083/jcb.111.5.1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Satterwhite L L, Pollard T D. Cytokinesis. Curr Opin Cell Biol. 1992;4:43–52. doi: 10.1016/0955-0674(92)90057-j. [DOI] [PubMed] [Google Scholar]
- 44.Sellitto C, Kuriyama R. Distribution of pericentriolar material in multipolar spindles induced by colcemid treatment in Chinese hamster ovary cells. J Cell Sci. 1988;89:57–65. doi: 10.1242/jcs.89.1.57. [DOI] [PubMed] [Google Scholar]
- 45.Sherman F, Fink G R, Hicks J B. Methods in yeast genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1986. [Google Scholar]
- 46.Shirayama M, Zachariae W, Ciosk R, Nasmyth K. The Polo-like kinase Cdc5p and the WD-repeat protein Cdc20p/fizzy are regulators and substrates of the anaphase promoting complex in Saccharomyces cerevisiae. EMBO J. 1998;17:1336–1349. doi: 10.1093/emboj/17.5.1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Siliciano P G, Tatchell K. Transcription and regulatory signals at the mating type locus in yeast. Cell. 1984;37:969–978. doi: 10.1016/0092-8674(84)90431-8. [DOI] [PubMed] [Google Scholar]
- 48.Simmons D L, Neel B G, Stevens R, Evett G, Erikson R L. Identification of an early-growth-response gene encoding a novel putative protein kinase. Mol Cell Biol. 1992;12:4164–4169. doi: 10.1128/mcb.12.9.4164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Toczyski D P, Galgoczy D J, Hartwell L H. CDC5 and CKII control adaptation to the yeast DNA damage checkpoint. Cell. 1997;90:1097–1106. doi: 10.1016/s0092-8674(00)80375-x. [DOI] [PubMed] [Google Scholar]
- 50.White J G, Borisy G G. On the mechanisms of cytokinesis in animal cells. J Theor Biol. 1983;101:289–316. doi: 10.1016/0022-5193(83)90342-9. [DOI] [PubMed] [Google Scholar]