Abstract
The gastrointestinal tract is optimized to efficiently absorb nutrients and provide a competent barrier against a variety of lumen environmental compounds. Different regulatory mechanisms jointly collaborate to maintain intestinal homeostasis, but alterations in these mechanisms lead to a dysfunctional gastrointestinal barrier and are associated to several inflammatory conditions usually found in chronic pathologies such as inflammatory bowel disease (IBD). The gastrointestinal mucus, mostly composed of mucin glycoproteins, covers the epithelium and plays an essential role in digestive and barrier functions. However, its regulation is very dynamic and is still poorly understood. This review presents some aspects concerning the role of mucus in gut health and its alterations in IBD. In addition, the impact of gut microbiota and dietary compounds as environmental factors modulating the mucus layer is addressed. To date, studies have evidenced the impact of the three-way interplay between the microbiome, diet and the mucus layer on the gut barrier, host immune system and IBD. This review emphasizes the need to address current limitations on this topic, especially regarding the design of robust human trials and highlights the potential interest of improving our understanding of the regulation of the intestinal mucus barrier in IBD.
Keywords: dietary compounds, gastrointestinal barrier, gut microbiota, inflammatory bowel disease, mucus layer
1. Introduction to Inflammatory Bowel Disease
Inflammatory bowel disease (IBD) is a global disease associated to Western and recently westernized countries [1]. The emergence of this disease was parallel to the industrial revolution in the 1800s [2]. Being a chronic disease diagnosed early in life, the prevalence of this pathology is high and is increasing over time. Prevalence of IBD was 84 per 100,000 population in 2017 [3] and it has been estimated that it will continue increasing in the next generation, affecting tens of millions of people all over the world [4]. Therefore, the cost of this disease for health care systems is considerable and will increase steadily in the future [4,5].
The origin and causes of IBD remain unknown. It is an immune-mediated inflammatory disease and its major causative factors could be genetic, immune and environmental such as the gut microbiome and diet. Genome wide-association studies identified approximately 200 gene loci in IBD, of which more than 50% are also associated with other inflammatory and autoimmune diseases [6]. The exposure to environmental conditions influence the microbiome composition and the consequent dysbiosis (changes in the healthy microbiota) in the gastrointestinal tract can trigger inflammatory responses [7,8].
IBD is a general term encompassing ulcerative colitis (UC) and Crohn’s disease (CD). UC is limited to the colon and presents superficial mucosal inflammation that can lead to ulcerations and bleeding. CD can affect any part of the digestive tract and presents transmural inflammation and complications such as fistulas or abscesses [9]; furthermore, IBD is associated to other extra-intestinal pathologies such as arthritis and skin diseases that aggravate the quality of life of these patients. Both IBD subtypes present periods of inflammation and quiescence [10]. Regarding IBD therapeutic approaches, several drugs have been developed over the last years, including biologics that target different molecules involved in IBD pathogenesis [11,12]. However, response to treatment is highly variable [13,14] and, since there is no cure for this disease, the therapeutic goal is to maintain patients’ remission. Accordingly, a deeper understanding of the disease is needed to improve treatment of these patients.
In this review, we will focus on the gastrointestinal barrier in IBD with a particular emphasis on the role of the mucus layer in gut health and its alterations in this disease. In addition, the impact of the gut microbiota and dietary compounds as mucus modulatory factors and their complex interaction with the mucosal barrier in IBD is summarized. Data were obtained from articles published in English in journals indexed in PubMed and Web of Science from inception to August 2021 and retrieved using search terms related to (i) gastrointestinal barrier and gut homeostasis; (ii) mucus layer, mucins and IBD; (iii) modulation of immune system and mucosal inflammation; (iv) gut microbiota, probiotics and IBD; (v) dietary compounds, food bioactives and IBD.
2. Gastrointestinal Barrier
The intestinal mucosal barrier provides adequate containment of microorganisms and molecules, preserving the capacity to absorb nutrients [15]. Intestinal mucosa is covered with a monolayer of intestinal epithelial cells (IECs) that separate the external environment and sub-epithelium [16]. Alterations in this mucosal barrier may result in IBD, stressing its essential role to maintain a healthy gut environment [17]. A key regulator balancing this relationship is the gastrointestinal mucus layer, composed of a secreted mucus gel, which cover the surface of epithelium and the underlying mucosal immune system. Hence, the gut mucosa is protected by two barrier types: chemical and physical. Chemical barriers participate in the segregation of IECs and gut microbiota [18]. IECs are derived from stem cells within intestinal crypts that replicate and migrate towards villi to replenish the active turnover of epithelium [15]. Functionally, secretory IECs, as goblet and Paneth cells, are specialized in maintaining the epithelial barrier function [19]. Paneth cells are involved in the production of chemical barriers such as antimicrobial peptides in the small intestine [20], while goblet cells secrete mucins. Mucins and antimicrobial peptides are important for both physical and biochemical barriers. The different functions of IECs lead to a dynamic barrier, which protects the host from infection and inflammatory stimuli [19]. IECs act as sensors for microbial elements and can integrate signals from commensal bacteria into antimicrobial and immune regulatory responses [21]. These functions are enabled by the expression of pattern-recognition receptors that act as sensors of the microbial environment and are key regulatory elements in mucosal immune responses [19].
Mucosal homeostasis is a vital feature of the gut immune system [22]. One of the critical factors for developing IBD is the failure to maintain an adequate balance between response to pathogens and tolerance to commensal microorganisms and luminal beneficial antigens [23,24]. Under the conditions of gut barrier dysfunction, as it occurs in IBD, the homeostatic equilibrium is lost [25,26]. IBD is related with increased permeability in the gut and the associated disbalance in the immune response that leads to increased recruitment of circulating cells and secretion of pro-inflammatory mediators [15,27]. Therefore, factors as immune system, genetics and environmental ones influence the gastrointestinal barrier function and are, thus, involved in the “IBD integrome” [28].
2.1. Mucus Layer
The small intestine has a single mucus layer that facilitates the pass of nutrients, while the colon is covered by a thicker barrier. However, in the colon, the mucus layer acts as a physical barrier maintaining bacteria in symbiosis with the host and preventing bacterial infiltration into the epithelium [16,18]. The large intestine epithelium is, thus, covered by two mucus layers: an outer loose layer and an inner firm mucus attached to the epithelia [29,30]. The principal components of the gastrointestinal mucus barrier are O-linked glycoproteins called mucins. They present densely packed oligosaccharides that bind to their terminal region sialic acid and sulfate residues protecting mucins from proteases and glycosidases [31]. Mucins are produced by goblet cells present within the intestinal epithelium [32]. Mucus exocytosis from goblet cells depends on several cellular processes that modulate mucin secretion, including endocytosis and autophagy [32].
There are 18 mucin members in humans classified in two types: transmembrane and secreted mucins. Mucin central domains are composed of proline, threonine and serine (PTS) residues working as attachment sites for O-linked glycans through covalent binding of N-acetylgalactosamine to serine or threonine residues [16]. The secreted mucin MUC2 is the main glycoprotein in the intestinal mucus. MUC2 has an N-terminal domain, two PTS domains and a C-terminal domain. MUC2 N-terminal domain comprises 3 complete von Willebrand factor domains (D1-3) and the C-terminal region of D4 domain. Cysteine residues in N- and C- terminal domains facilitate inter- and intramolecular disulfide bond formation responsible for mucin polymerization [33].
MUC2 polypeptide is synthetized and dimerized in the endoplasmic reticulum of intestinal cells. Then, threonine and serine residues are glycosylated in the cis-Golgi and the trimer formation takes place in the trans-Golgi before MUC2 is packaged into secretory granules. MUC2 is composed of heterogeneous glycan chains [16], which allow MUC2 trimers to form polymers creating mucus networks in the cell surface [31,34]. MUC2 polymers undergo rapid expansion on the intestinal epithelial surface to maintain the mucus barrier during homeostasis; this expansion depends on ionic composition and water availability. Polymers can expand their volume up to 1000 times to form the framework of the mucus gel [35].
On the other hand, intestinal transmembrane mucins (MUC1, MUC3, MUC4 and MUC13) are intercalated in the apical surface of the intestinal epithelium forming the glycocalyx layer [32]. In contrast to the sterile inner layer of mucus, the outer mucus layer is rich in gut bacteria [29]. These bacteria use diet fiber as energy source; however, under a fiber-free diet they consume MUC2 polysaccharides, leading to a thinner inner mucus layer and dysbiosis [36], as well as bacteria penetration into the lamina propria contributing to IBD development [18].
2.2. Mucus Layer under Inflammatory Conditions
The stability of the mucus layer is crucial for intestinal homeostasis, in which MUC2 is secreted at a basal rate. This secretion can be influenced by mediators as cytokines, microbial products, autophagic proteins, reactive oxygen species and inflammasome components [37,38]. Commensal and pathogenic bacteria can regulate mucin production [28]. In the small intestine, a continuous basal secretion of mucus creates a flow towards the lumen that, together with antibacterial agents, keeps microorganisms away from the epithelial surface. Antibacterial agents are secreted by Paneth cells and enterocytes of the crypt bottom. On the other hand, in the colon, the inner mucus layer is the first line of defense against bacteria [39].
The mucus layer is a natural and selective habitat for the gut microbiota [40], which in turn influences mucus composition and may promote mucus secretion and increase mucus layer thickness [41]. Therefore, the gut microbiota affects mucus layer function, possibly through specific bacteria that shape the glycan profile of the mucus, although molecular details remain incompletely identified [42].
There is high number of enteropathogens that have evolved mechanisms to penetrate the mucus barrier. Most of them produce a kind of serine proteases that cleave glycoproteins such as mucins [43]. Moreover, cytokines are involved in the inflammatory response and regulate many cellular and molecular processes including mucus production. In this regard, TNF-α and IL-1β, which are implicated in inflammatory diseases, stimulate gel-forming mucins [43]. Th2 cytokines are implicated in mucin gene expression up-regulating MUC2 and MUC5AC by binding to IL-4 receptor. Endoplasmic reticulum stress in goblet cells produce immature mucins that trigger inflammation [44,45], whereas IL-10 has been found to inhibit endoplasmic reticulum stress and promote intestinal mucus production [43,46].
MUC2 knockout mice show colonization of gut epithelium by enteric pathogens [47,48]. These results suggest that the principal mucus function is to protect the gut against microbes. Binding to mucin oligosaccharide chains likely contributes to immobilize bacteria and prevents them from damaging the intestinal epithelium. MUC2 has also immune roles; small intestine goblet cells provide the passage of soluble luminal antigens by transcytosis. These low molecular weight antigens are delivered to underlying CD103+ dendritic cells and may favor IgA production and expansion of regulatory T cells, thereby driving gut homeostasis and tolerance [49]. The commensal microbiota, through its relationship with mucus, prevents colonization by pathogens. In this regard, when antibiotics perturb the gut microbiota, niches are opened facilitating disease development. The gut microbiota also breaks down short-chain fatty acids (SCFA) including acetate, propionate and butyrate [50]. Since butyrate regulates MUC2 production, the microbiota is also involved in the homeostasis of the protective mucus layer [51].
Mucin composition is altered in IBD and mucin structural changes play an important role in IBD onset [52,53]. In fact, alterations of mucus barrier and mucins are observed at IBD onset; goblet cell pathology is a hallmark of UC and CD [43]. Recently, it has been observed that the reduced mucus layer in UC is due to a reduction in the number and secretory function of goblet cells because of an inflammatory environment and due to changes in mucin secretion that persist in the absence of inflammatory cells [54].
The mucus layer is thinner in UC than in the healthy colon, while goblet cell depletion and altered MUC2 glycosylation can be also observed; in addition, MUC2 is undersulfated, weakening mucin protective function [55,56,57]. Despite these results, the expression pattern of MUC2 in UC is not clear. Conversely, MUC5AC, is consistently increased during inflammation in UC [58,59] and its reduced expression is associated with endoscopic improvement in these patients [60]. In Muc5ac−/− mice with DSS colitis, there is an increase in bacterial-epithelial contact and neutrophil recruitment to the colon, therefore, the loss of Muc5ac may exacerbate injury and inflammation in experimental murine colitis [61]. This study also showed a significant increase in MUC5AC/Muc5ac expression during colonic inflammation in biopsies from UC patients and DSS-induced mice colitis [61].
In contrast, mucus thickness is normal or greater than normal in CD, maybe due to goblet cell hyperplasia or increased MUC2 expression, although with a 50% reduction in oligosaccharide chain length [62]. Hence, several changes in the mucosal barrier underlie the complex pathology of IBD.
3. Gut Microbiota and the Mucus Layer in IBD
The microbiome plays key roles in the development of mucosal immune responses, pathogen resistance and nutrient metabolism. This fact is in part due to the interaction of the microbiota with components of the mucus layer and the IECs underneath following mucus breakdown. The outer penetrable mucus layer is, thus, the natural habitat for many commensals as they use the exposed mucin glycans for both nutritional support and as attachment sites for bacterial adhesins [63]. Bacteria produce enzymes associated with digestion of different glycans from mucus and fiber from the host diet. Although mucus digestion promotes its physiological turnover and the symbiotic dialogue between the host and commensals such as Akkermansia muciniphila, an excessive degradation may be associated to detrimental effects due to epithelial exposure to luminal pathogens [23,36].
Intestinal barrier, antimicrobial and immunomodulatory functions are influenced by several members of the gut microbiota, as recently evidenced in studies with cellular models of the epithelial and mucus layers [64,65]. Some commensals, probiotics, notably Lactobacillus and Bifidobacterium strains and probiotic mixtures have proved mucus-modulating action not only in IBD-like animal models, but also in gnotobiotic animals as well as animal models of diet-induced obesity, malnutrition and aging (Table 1). In this regard, Lactobacillus rhamnosus CNCM I-3690 induces reinforcement of the intestinal barrier against chemical-induced colitis with similar effects to those showed by the well-known beneficial human commensal Faecalibacterium prauznitzii A2-165 [66]. F. prausnitzii is a physiological sensor of gut health and exerts a complementary action with Bacteroides thetaiotaomicron as acetate consumer and butyrate producer to balance the mucus barrier by modifying goblet cell differentiation, mucin gene expression and glycosylation [67]. According to the Human Microbiome Project, Bifidobacterium dentium, as other Bifidobacterium strains, is a recognized member of the healthy infant and adult human gut microbiota [68] and its beneficial effect in rescuing mucus layer function has been proved in gnotobiotic mice [69].
Table 1.
Summary of studies evaluating in animal models the effects of gut bacterial species on the mucus layer.
| Bacterial Strain | Animal Model | Experimental Administration | Study Period | Outcomes and Mechanisms of Action | Reference |
|---|---|---|---|---|---|
| Lactobacillus rhamnosus CNCM I-3690 and L. paracasei CNCM I-3689 | DNBS-induced colitis in C57BL/6J mice | Intragastric administration with 1 × 109 CFU/mL | 10 days |
|
[66] |
| Lactobacillus rhamnosus CNCM I-3690 | DNBS-induced colitis in C57BL/6J mice | Intragastric administration with 5 × 109 CFU/mL | 10 days |
|
[73] |
| Lactobacillus reuteri R2LC and Lactobacillus reuteri 4659 | DSS-induced colitis in C57BL/6J mice | Oral gavage with 1 × 108 live bacteria | 14 days |
|
[74] |
| Bacillus subtilis JNFE0126 | DSS-induced colitis in C57BL/6J mice | B. subtilis-fermented milk oral gavage (6 × 108 CFU/mL) | 21 days |
|
[75] |
| Escherichia coli strain Nissle 1917 | DSS-induced colitis in BALB/c mice | Intragastric administration with 1 × 109 CFU/mL | 17 days |
|
[76] |
| Bifidobacterium longum NCC 2705 | Western style diet-induced obesity in C57BL/6J mice | Supplementation of the drinking water with 2 × 106 CFU/mL | 4 weeks |
|
[77] |
| Bifidobacterium dentium ATCC 27678 | Swiss Webster germfree mice | Oral gavage with 2 × 108 CFU/mL | 1–2 weeks |
|
[69] |
| Lactobacillus reuteri LR6 | Protein and energy malnutrition in Swiss mice | Diet with fermented product or bacterial suspension at 1 × 109 CFU/day | 1 week |
|
[78] |
| Akkermansia muciniphila MucT BAA-835 | Accelerated aging Ercc1-/Δ7 mice | Oral gavage with 2 × 108 CFU/200 µL | 10 weeks |
|
[71] |
| VSL#3 probiotic mixture | DSS-induced colitis in Muc2−/− mice | Oral gavage with 2.25 × 109 CFU/day | 2 weeks |
|
[79] |
| VSL#3 probiotic mixture | DSS-induced colitis in C57BL/6J mice | Oral gavage with 3 × 109 live bacteria | 60 days |
|
[80] |
| Lactobacillus johnsonii IDCC9203, Lactobacillus plantarum IDCC3501 and Bifidobacterium animalis subspecies lactis IDCC4301 (ID-JPL934 probiotic mixture) | DSS-induced colitis in BALB/c mice | Oral gavage with probiotic mixture (1 × 106–1 × 109 CFU/day) | 8 days |
|
[81] |
| Lactobacillus rhamnosus, L. acidophilus and Bifidobacterium bifidumi | High fat diet-induced obesity in Swiss mice | Oral gavage with probiotic mixture (6 × 108 CFU of each strain; final concentration of 1.8 × 109 CFU of bacteria) | 5 weeks |
|
[82] |
DNBS: dinitrobenzene sulfonic acid; DSS: dextran sulfate sodium; CFU: colony-forming units; SCFAs: short-chain fatty acids; LPS: lipopolysaccharide.
The presence of A. muciniphila within the mucus layer is another control mechanism of host mucus turnover, which is essential to gut barrier function. Despite A. muciniphila being known as a mucin-degrading bacterium, high-fat-fed mice supplemented with this bacterium show increased counts of goblet cells and secretion of antimicrobial peptides and acylglycerols involved in intestinal and glucose homeostasis [70]. A. muciniphila also restores aging-related thinness of the colonic mucus and alterations in inflammatory and immune mediators [71]. Beyond data obtained in murine models, abundance of this bacterium has been inversely associated with obesity and type 2 diabetes in humans, thereby suggesting a physiological role for this mucus colonizer in the regulation of chronic metabolic and inflammatory disorders [70,72].
In addition to bacteria themselves, some microbial components/metabolites, such as pathogen-associated molecular patterns and SCFA as well as bacterial metabolites of dietary fiber, can also act on the mucus barrier [39,83]. For example, this is the case of specific outer proteins from A. muciniphila [72], or polysaccharide A from Bacteroides fragilis [84], which are sensed by Toll-like receptors and ultimately influence host immunity. SCFA, in addition to their roles as energy source for the epithelium and inducers of immune tolerance through T-regulatory cells, are able to stimulate both the discharge of intestinal mucins and MUC2 gene expression [85]. Moreover, it has been suggested that the beneficial effects of Escherichia coli Nissle 1917 treatment on chemical-induced colitis (Table 1) may be transferable to germ free mice, but to a lower extent, via fecal microbiota transplantation after mucosal colonization and restoration of the inflammatory responsiveness [76].
The disruption of barrier function in response to IBD or mucosal stressors such as nonsteroidal anti-inflammatory drugs has been addressed in relation to the activity of microbial species on human gut permeability [86]. Diverse probiotics, in particular combination of agents such as the probiotic mixture VSL#3, which has shown beneficial effects on mice colitis (Table 1), have been evaluated in humans by placebo-controlled trials. Pouchitis is one of the intestinal diseases showing increased mucosal permeability. A Cochrane systematic review found that a specific formulation of VSL#3 was superior to placebo in maintaining pouchitis clinical remission at 9–12 months of follow-up, but neither Lactobacillus GG nor Bifidobacterium longum resulted in clinical improvements at 12 weeks and 6 months, respectively [87]. However, the evidence on this topic obtained by randomized clinical trials still presents some methodological limitations and is not supported by high-quality clinical studies [88,89]; hence, further research is warranted.
4. Dietary Compounds and the Mucus Layer in IBD
Dietary factors need to be considered when evaluating the complex relationship between the host, microbiota and the mucus layer. Dietary patterns and specific foods or nutrients may affect the gut barrier directly or indirectly by shaping microbial species known to influence mucosal protection and inflammatory processes [90]. Hence, Western diet and low-grade inflammation are interlinked factors associated with a growing number of immune-mediated inflammatory diseases such as IBD [91].
Diet is mainly composed of macronutrients including proteins, lipids and carbohydrates and micronutrients as vitamins and minerals. Some dietary factors may increase intestinal permeability and consequently contribute to barrier dysfunction in IBD, while others may reinforce the gut barrier [86]. The influence of the different food compounds in the mucus barrier has been evaluated in animal models, both in health and IBD-like models (results summarized in Table 2). Total proteins and specific protein hydrolysates and bioactive peptides from both animal and vegetable sources can affect the gastrointestinal barrier protecting against experimental IBD through modulation of the levels of mucus and IECs constituents, pro/anti-inflammatory markers, antioxidant enzymes, immune mediators and microbiota communities [92]. However, regardless of the protein sources, disruption of the intestinal crypts, number of goblet cells and protein and gene expression of Muc2 has been reported in mice fed with a high-fat diet [93].
Table 2.
Summary of studies evaluating in animal models the effects of food compounds on the mucus layer.
| Food Group/Compounds | Animal Model | Experimental Administration | Study Period | Outcomes and Mechanisms of Action | Reference |
|---|---|---|---|---|---|
| Proteins | |||||
| Total proteins | Adult finishing pigs | Three study groups (16%, normal dietary protein concentration; 13%, low dietary protein concentration; 10%, extremely low dietary protein concentration) | 50 days |
|
[94] |
| Total proteins | Growing pigs | Three study groups (18%, normal dietary protein concentration; 15%, low dietary protein concentration; 12%, extremely low dietary protein concentration) | 30 days |
|
[95] |
| Chicken and soy proteins | C57BL/6 mice | Chicken or soy protein-based diets | 4 weeks |
|
[96] |
| Milk casein | Rats | Milk casein hydrolysate | 8 days |
|
[97] |
| Milk casein | Zucker rats | Milk casein hydrolysate | 8 weeks |
|
[98] |
| Milk β-casein | Rats pups | Milk β-casein peptide f(94–123) | 9 days |
|
[99] |
| Milk β-casein | Indomethacin-induced jejunal injury in rats | Milk β-casein peptide f(94–123) | 8 days |
|
[100] |
| Goat whey | DNBS-induced colitis in CD1 mice | Goat whey proteins, fatty acids and oligosaccharides | 16 days |
|
[101] |
| Hen egg | DSS-induced colitis in piglets | Egg white lysozyme | 5 days |
|
[102] |
| Soybean protein | DSS-induced colitis in piglets | Soybean protein derived di- and tri-peptides | 5 days |
|
[103] |
| Pea protein | DSS-induced colitis in C57BL/6J mice | Pea seed protein extracts | 23 days |
|
[104] |
| Lipids | |||||
| High- and low-fat diets | C57BL/6J mice | Chicken, soy or pork protein-based administration either with low fat (12% kcal) or high fat (60% kcal) diets | 12 weeks |
|
[93] |
| High-fat diet | C57BL/6 mice | High-fat diet (56.7 Fat kcal %), in comparison with normal chow diet (12.0 Fat kcal %) | 8 weeks |
|
[105] |
| High-fat diet | Spontaneous colitis in Winnie mice | High-fat diet (46% available energy as fat), in comparison with normal chow diet (11% available energy as fat) | 9 weeks |
|
[106] |
| Flaxseed oil | LPS-induced intestinal injury in weaned piglets | Supplementation of diets with flaxseed oil in comparison with corn oil (5% weight:weight) | 3 weeks |
|
[107] |
| Fiber | |||||
| Inulin | Western style diet-induced obesity in C57BL/6J mice | 1% oligofructose-enriched inulin supplementation in the drinking water | 4 weeks |
|
[77] |
| Inulin and cellulose | Western style diet-induced obesity in C57BL/6J mice | Supplementation of high-fat diets (60 kcal% fat) with 20 % fiber | 4 weeks |
|
[108] |
| Pectin | TNBS- and DSS-induced colitis in C57BL/6J mice | Diet supplemented with characteristically high (5% orange pectin) in comparison to low (5% citrus pectin) side chain content of pectin | 10–14 days |
|
[109] |
| Microbiota-accessible carbohydrates | High-fat and fiber-deficient diet in C57BL/6J mice | Supplementation of high-fat (31.5% fat by weight) and fiber-deficient (5% fiber by weight) diet with microbiota-accessible carbohydrates | 15 weeks |
|
[110] |
DNBS: dinitrobenzene sulfonic acid; DSS: dextran sulfate sodium; LPS; lipopolysaccharide; TNBS: 2,4,6-trinitrobenzenesulfonic acid.
High-fat diet has been recently linked to the impairment of mucus layer and stimulation of epithelial oxidative stress and apoptosis, as well as induction of barrier-disrupting molecules and bacterial species [111]. Consistent with this observation, previous studies associated Western diets characterized by animal fat and proteins, sugars and processed food to higher Bacteroides and lower Prevotella populations, while the Mediterranean diet rich in fruits, vegetables, nuts and whole grains shifted toward abundance of Prevotella and fiber-degrading bacteria along with increased production of SCFA [112,113]. Likewise, as pointed by a recent analysis of the relation between dietary factors and the microbiome of healthy volunteers and IBD patients, processed and animal foods are associated with increased abundances of Firmicutes and Ruminococcus species, but plant foods and fish positively influence SCFA-producing commensals and restrain pathobionts, diet thereby influencing a characteristic microbial environment of intestinal inflammation [114]. Furthermore, high-fat diet also drives colorectal tumorigenesis in mice via intestinal dysbiosis, metabolite dysregulation and gut barrier dysfunction [115].
In addition to the high-fat content, it should be considered that other factors of the Western diet such as a low fiber content may contribute to the negative effects on inflammation. Dietary fiber enriches the gut environment and provides a rich niche for microbial growth to those species able to utilize fiber subtracts [116]. Most bacteria preferentially choose the non-digested food polysaccharides as energy source. Therefore, in fiber-deficient diets, common in the western population, gut bacteria depend to a greater extent on less favorable substrates, especially dietary and endogenous proteins and mucus glycoproteins [42,108]. Mucin glycans are catabolized through a sequential action of different microbial enzymes such as carbohydrate-active enzymes [117]. The degradation of host mucins could negatively impact on mucus homeostasis and enhance pathogen susceptibility [39,63]. This microbial activity may also lead to increased production of harmful metabolites derived from the fermentation of amino acids that contributes to mucus degradation and chronic diseases [36]. Fiber-rich diet is likely suggested to counteract protein fermentation, hence ameliorating the non-desired effects of meat and fats [116].
The preventive effect of fiber may be associated to increased production of SCFA [118], which enhance mucus and antimicrobial peptides secretion, modulate immune function and oxygen levels and reinforce epithelial tight junctions [116,119]. Indeed, some studies in mice have shown that supplementation of high-fat diets with fiber alleviate many of the adverse effects on the mucous barrier (main outcomes summarized in Table 2) in parallel to modulation of microbial composition and SCFA production. The animal models displayed intestinal alterations because of both western style diet-induced obesity and chemical-induced colitis [77,108,109,110]. Particularly, low amount of the prebiotic fiber inulin (1% supplementation in the drinking water) has been shown to correct the penetrability of the inner mucus layer and complement the favorable effects of probiotic B. longum on mucus growth [77]. Moreover, soluble inulin (20% fiber supplementation in the high-fat diet), but not insoluble cellulose, prevented microbiota encroachment and further improved gut health by resolution of metabolic alterations, adiposity and glycemic control [108]. On the contrary, the ratio between high-simple sugars/low-fiber contents in diet would predispose the activity and abundance of mucin-degrading microbiota and, in a long-term, the dysfunction of the gut barrier and subsequent inflammation [117]. Noteworthy, a recent systematic review with meta-analysis has found that the intake of dietary fiber is lower in adults with IBD in comparison to healthy individuals [120].
Beyond macronutrients, the essential role of micronutrients [121] and other dietary compounds as fatty acids [122] and phytochemicals [123] at regulating mucosal inflammation and microbiome in IBD has been recently reviewed. On the other hand, some food additives as emulsifiers, maltodextrins and carrageenan may induce increased intestinal permeability, mucus thinness and alterations in the gut microbiota associated with gut barrier dysfunction and negative effects on IBD [39,124].
Evidence from human dietary intervention studies on this topic is still limited. A few human trials have evaluated the effect of some prebiotics and symbiotics on the improvement of intestinal permeability, although most of them found only marginal or non-significant differences compared to placebo [86]. The impact of fiber on human mucus barrier is variable depending on factors such as the study population, the gastrointestinal location and the type of fiber [90]. However, a systematic review by Leech and collaborators did not identify lower fiber consumption as a risk factor for intestinal permeability [125]. On the contrary, within a Western-style diet, fat intake and either inadequate protein intake or excess animal-derived protein are suggested as independent risk factors for altered intestinal integrity [125]. A dietary intervention study found that different animal and non-meat protein sources had modest effects on the abundance of mucosa-associated microbial taxa, these effects being, however, less marked when compared to the impact of the level of saturated fats [126]. Likewise, high-fat diets negatively correlate with microbial diversity, richness and abundance of F. prausnitzii and A. muciniphila and are associated with reduced bacterial load in human fecal samples [127,128].
5. Conclusions
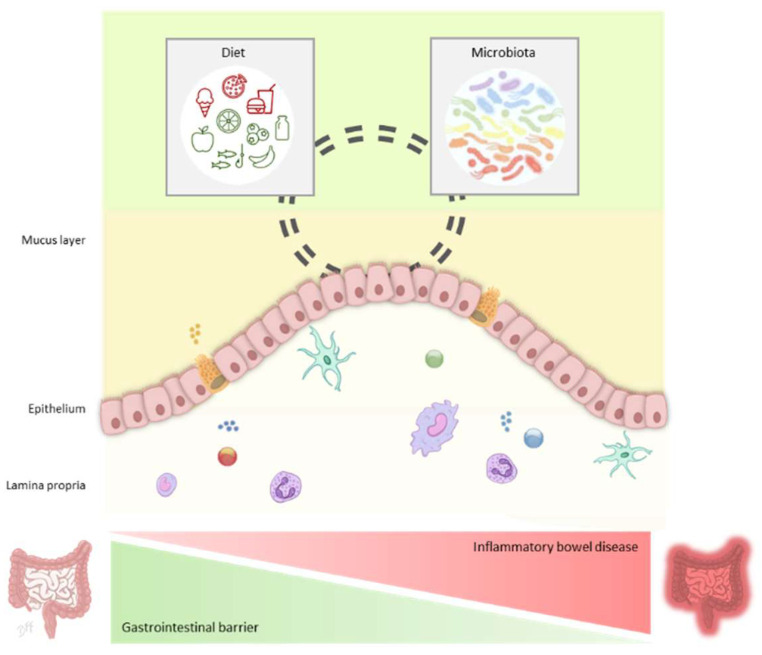
Mucosal barriers represent the first physical host defensive mechanism. They not only keep microorganisms away from the epithelium preventing microbial translocation into mucosal tissues, which would trigger exacerbated inflammatory-immune responses, but also provide a rich source of nutrients for commensals. The gastrointestinal mucus, mostly composed of mucins, plays a vital role in the proper function of the digestive tract and accordingly in human health. Hence, alterations in mucus composition, organization, secretion and degradation or its functionality are linked to a variety of diseases including IBD. A multifactorial model is proposed for IBD pathogenesis where several alterations converge and involve an intestinal barrier failure along with the dysregulation of the immune system. It still remains unclear whether mucus alterations are cause or consequence of the disease. Moreover, scientific interest on host-microbiome interactions displayed at the gastrointestinal mucus layer has increased over the last years, providing evidence that has sharply improved our knowledge on how microbiota regulates host health. Of note, gut lumen environmental factors including gut microbiota and dietary compounds and the complex three-way interaction between both elements and the mucus layer, may act on gut barrier integrity and regulate a healthy gastrointestinal homeostasis, as opposed to IBD alterations (Figure 1).
Figure 1.
Diet and the gut microbiota regulate gastrointestinal barrier in healthy gut and inflammatory bowel disease. Schematic representation of the influence of diet that may act directly on components of the gastrointestinal barrier and indirectly through shaping microbiota composition, function and its energy source. Some dietary compounds usually found in Mediterranean diet (green) may favor the gastrointestinal barrier, as opposed to the factors of Western-style diet (red). The impact of host-microbiota interactions at the gut lumen and mucus layer, epithelium and mucosal immune system is essential to balance the gastrointestinal barrier in contrast to the alterations underlying inflammatory bowel disease.
Indeed, in general terms, the studies summarized in the present review suggest that some microbiota/diet interactions play a role in maintaining gut homeostasis and mucus function. However, current research on these topics presents several limitations and some questions remain open, especially regarding the deficiencies in the design of robust clinical trials and long-term, evidence-based studies to implement findings in practice. Considering the multifactorial nature of IBD and the lack of effective therapies to cure the disease, improving our understanding on the regulation of the intestinal mucus barrier should be further considered with the goal of providing help in IBD management.
Abbreviations
IBD: inflammatory bowel disease; UC: ulcerative colitis; CD: Crohn’s disease; TNF: tumor necrosis factor; IL: interleukin; IEC: intestinal epithelial cells; PTS: proline, threonine and serine; SCFA: short-chain fatty acids
Author Contributions
Conceptualization: S.F.-T.; Writing—original draft preparation: S.F.-T., L.O.M.; Writing—review and editing: S.F.-T., L.O.M., M.C., J.P.G. All authors have read and agreed to the published version of the manuscript.
Funding
S.F.-T. was funded by the Instituto de Salud Carlos III (Sara Borrell fellowship CD17/00014). L.O.M. is funded by the Community of Madrid and Universidad Autónoma de Madrid (Ayudas Atracción de Talento modalidad 2 BMD-5800).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data described in the review are included in this published article.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ng S.C., Shi H.Y., Hamidi N., Underwood F.E., Tang W., Benchimol E.I., Panaccione R., Ghosh S., Wu J.C.Y., Chan F.K.L., et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet. 2017;390:2769–2778. doi: 10.1016/S0140-6736(17)32448-0. [DOI] [PubMed] [Google Scholar]
- 2.Mulder D.J., Noble A.J., Justinich C.J., Duffin J.M. A tale of two diseases: The history of inflammatory bowel disease. J. Crohns Colitis. 2014;8:341–348. doi: 10.1016/j.crohns.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 3.Alatab S., Sepanlou S.G., Ikuta K., Vahedi H., Bisignano C., Safiri S., Sadeghi A., Nixon M.R., Abdoli A., Abolhassani H., et al. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 2020;5:17–30. doi: 10.1016/S2468-1253(19)30333-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaplan G.G., Ng S.C. Understanding and preventing the global increase of inflammatory bowel disease. Gastroenterology. 2017;152:313–321. doi: 10.1053/j.gastro.2016.10.020. [DOI] [PubMed] [Google Scholar]
- 5.Molodecky N.A., Soon I.S., Rabi D.M., Ghali W.A., Ferris M., Chernoff G., Benchimol E.I., Panaccione R., Ghosh S., Barkema H.W., et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142:46–54.e42. doi: 10.1053/j.gastro.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 6.Khor B., Gardet A., Ramnik J.X. Genetics and Pathogenesis of Inflammatory Bowel Disease. Nature. 2011;474:307–317. doi: 10.1038/nature10209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morgan X.C., Tickle T.L., Sokol H., Gevers D., Devaney K.L., Ward D.V., Reyes J.A., Shah S.A., LeLeiko N., Snapper S.B., et al. Dysfunction of the intestinal microbiome in inflammatory bowel disease and treatment. Genome Biol. 2012;13:R79. doi: 10.1186/gb-2012-13-9-r79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aldars-García L., Chaparro M., Gisbert J.P. Systematic review: The gut microbiome and its potential clinical application in inflammatory bowel disease. Microorganisms. 2021;9:977. doi: 10.3390/microorganisms9050977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoentjen F., Dieleman L.A. Pathophysiology of inflammatory bowel diseases. Handb. Prebiotics. 2008:341–374. doi: 10.2310/gastro.5412. [DOI] [Google Scholar]
- 10.Peyrin-Biroulet L., Chamaillard M., Gonzalez F., Beclin E., Decourcelle C., Antunes L., Gay J., Neut C., Colombel J.F., Desreumaux P. Mesenteric fat in Crohn’s disease: A pathogenetic hallmark or an innocent bystander? Gut. 2007;56:577–583. doi: 10.1136/gut.2005.082925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bonovas S., Pantavou K., Evripidou D., Bastiampillai A.J., Nikolopoulos G.K., Peyrin-Biroulet L., Danese S. Safety of biological therapies in ulcerative colitis: An umbrella review of meta-analyses. Best Pract. Res. Clin. Gastroenterol. 2018;32–33:43–47. doi: 10.1016/j.bpg.2018.05.005. [DOI] [PubMed] [Google Scholar]
- 12.Weisshof R., ElJurdi K., Zmeter N., Rubin D. Emerging therapies for inflammatory bowel diseases. Dig. Dis. 2016;34:67–73. doi: 10.1007/s12325-018-0795-9. [DOI] [PubMed] [Google Scholar]
- 13.Gisbert J.P., Chaparro M. Predictors of primary response to biologic treatment [Anti-TNF, Vedolizumab, and Ustekinumab] in patients with inflammatory bowel disease: From basic science to clinical practice. J. Crohns Colitis. 2020;14:694–709. doi: 10.1093/ecco-jcc/jjz195. [DOI] [PubMed] [Google Scholar]
- 14.Digby-Bell J.L., Atreya R., Monteleone G., Powell N. Interrogating host immunity to predict treatment response in inflammatory bowel disease. Nat. Rev. Gastroenterol. Hepatol. 2020;17:9–20. doi: 10.1038/s41575-019-0228-5. [DOI] [PubMed] [Google Scholar]
- 15.De Medina F.S., Romero-Calvo I., Mascaraque C., Martínez-Augustin O. Intestinal inflammation and mucosal barrier function. Inflamm. Bowel Dis. 2014;20:2394–2404. doi: 10.1097/MIB.0000000000000204. [DOI] [PubMed] [Google Scholar]
- 16.Sharpe C., Thornton D.J., Grencis R.K. A sticky end for gastrointestinal helminths; the role of the mucus barrier. Parasite Immunol. 2018:1–10. doi: 10.1111/pim.12517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.König J., Wells J., Cani P.D., García-Ródenas C.L., MacDonald T., Mercenier A., Whyte J., Troost F., Brummer R.J. Human intestinal barrier function in health and disease. Clin. Transl. Gastroenterol. 2016;7:e196. doi: 10.1038/ctg.2016.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Okumura R., Takeda K. Maintenance of intestinal homeostasis by mucosal barriers. Inflamm. Regen. 2018;38:5. doi: 10.1186/s41232-018-0063-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peterson L.W., Artis D. Intestinal epithelial cells: Regulators of barrier function and immune homeostasis. Nat. Rev. Immunol. 2014;14:141–153. doi: 10.1038/nri3608. [DOI] [PubMed] [Google Scholar]
- 20.Salzman N.H., Underwood M.A., Bevins C.L. Paneth cells, defensins, and the commensal microbiota: A hypothesis on intimate interplay at the intestinal mucosa. Semin. Immunol. 2007;19:70–83. doi: 10.1016/j.smim.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 21.Aldars-García L., Marin A.C., Chaparro M., Gisbert J.P. The interplay between immune system and microbiota in inflammatory bowel disease: A narrative review. Int. J. Mol. Sci. 2021;22:3706. doi: 10.3390/ijms22063076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sartor R.B. Genetics and environmental interactions shape the intestinal microbiome to promote inflammatory bowel disease versus mucosal homeostasis. Gastroenterology. 2010;139:1816–1819. doi: 10.1053/j.gastro.2010.10.036. [DOI] [PubMed] [Google Scholar]
- 23.Blander J.M., Longman R.S., Iliev I.D., Sonnenberg G.F., Artis D. Regulation of inflammation by microbiota interactions with the host. Nat. Immunol. 2017;18:851–860. doi: 10.1038/ni.3780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fernández-Tomé S., Marin A.C., Moreno L.O., Baldan-Martin M., Mora-Gutiérrez I., Lanas-Gimeno A., Moreno-Monteagudo J.A., Santander C., Sánchez B., Chaparro M., et al. Immunomodulatory effect of gut microbiota-derived bioactive peptides on human immune system from healthy controls and patients with inflammatory bowel disease. Nutrients. 2019;11:2605. doi: 10.3390/nu11112605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maloy K.J., Powrie F. Intestinal homeostasis and its breakdown in inflammatory bowel disease. Nature. 2011;474:298–306. doi: 10.1038/nature10208. [DOI] [PubMed] [Google Scholar]
- 26.Bernardo D., Marin A.C., Fernández-Tomé S., Montalban-Arques A., Carrasco A., Tristán E., Ortega-Moreno L., Mora-Gutiérrez I., Díaz-Guerra A., Caminero-Fernández R., et al. Human intestinal pro-inflammatory CD11chighCCR2+CX3CR1+ macrophages, but not their tolerogenic CD11c-CCR2-CX3CR1- counterparts, are expanded in inflammatory bowel disease article. Mucosal Immunol. 2018;11:1114–1126. doi: 10.1038/s41385-018-0030-7. [DOI] [PubMed] [Google Scholar]
- 27.Isidro R.A., Appleyard C.B. Colonic macrophage polarization in homeostasis, inflammation, and cancer. Am. J. Physiol. Gastrointest. Liver Physiol. 2016;311:G59–G73. doi: 10.1152/ajpgi.00123.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dharmani P., Srivastava V., Kissoon-Singh V., Chadee K. Role of intestinal mucins in innate host defense mechanisms against pathogens. J. Innate Immun. 2009;1:123–135. doi: 10.1159/000163037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johansson M.E.V., Phillipson M., Petersson J., Velcich A., Holm L., Hansson G.C., Petersson J., Velcich A., Holm L., Hansson G.C., et al. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc. Natl. Acad. Sci. USA. 2008;105:15064–15069. doi: 10.1073/pnas.0803124105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rodriguez-Pineiro A.M., Bergstrom J.H., Ermund A., Gustafsson J.K., Schutte A., Johansson M.E.V., Hansson G.C. Studies of mucus in mouse stomach, small intestine, and colon. II. Gastrointestinal mucus proteome reveals Muc2 and Muc5ac accompanied by a set of core proteins. AJP Gastrointest. Liver Physiol. 2013;305:G348–G356. doi: 10.1152/ajpgi.00047.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ambort D., Johansson M.E.V., Gustafsson J.K., Ermund A., Hansson G.C. Perspectives on mucus properties and formation-lessons from the biochemical world. Cold Spring Harb. Perspect. Med. 2012;2:1–9. doi: 10.1101/cshperspect.a014159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Linden S.K., Sutton P., Karlsson N.G., Korolik V., Mcguckin M.A. Mucins in the mucosal barrier to infection. Mucosal Immunol. 2008;1:183–197. doi: 10.1038/mi.2008.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Klomp L.W.J., Rens L.V.A.N., Stroust G.J. Cloning and analysis of human gastric mucin cDNA reveals two types of conserved cysteine-rich domains. Biochem. J. 1995;838:831–838. doi: 10.1042/bj3080831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Corfield A.P. Mucins: A biologically relevant glycan barrier in mucosal protection. Biochim. Biophys. Acta Gen. Subj. 2015;1850:236–252. doi: 10.1016/j.bbagen.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 35.Ambort D., Johansson M.E.V., Gustafsson J.K., Nilsson H.E., Ermund A. Calcium and pH-dependent packing and release of the gel-forming MUC2 mucin. Proc. Natl. Acad. Sci. USA. 2012;109:5645–5650. doi: 10.1073/pnas.1120269109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Desai M.S., Seekatz A.M., Koropatkin N.M., Kamada N., Hickey C.A., Wolter M., Pudlo N.A., Kitamoto S., Muller A., Young V.B., et al. A Dietary Fiber-Deprived Gut Microbiota Degrades the Colonic Mucus Barrier and Enhances Pathogen Susceptibility. Cell. 2017;167:1339–1353. doi: 10.1016/j.cell.2016.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Patel K.K., Miyoshi H., Beatty W.L., Head R.D., Malvin N.P., Cadwell K., Guan J., Saitoh T., Akira S., Seglen P.O., et al. Autophagy proteins control goblet cell function by potentiating reactive oxygen species production. EMBO J. 2013;32:3130–3144. doi: 10.1038/emboj.2013.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wlodarska M., Thaiss C.A., Nowarski R., Henao-Mejia J., Zhang J.P., Brown E.M., Frankel G., Levy M., Katz M.N., Philbrick W.M., et al. NLRP6 inflammasome orchestrates the colonic host-microbial interface by regulating goblet cell mucus secretion. Cell. 2014;156:1045–1059. doi: 10.1016/j.cell.2014.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Paone P., Cani P.D. Mucus barrier, mucins and gut microbiota: The expected slimy partners? Gut. 2020;69:2232–2243. doi: 10.1136/gutjnl-2020-322260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bergstrom K.S.B., Xia L. Mucin-type O-glycans and their roles in intestinal homeostasis. Glycobiology. 2013;23:1026–1037. doi: 10.1093/glycob/cwt045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wells J.M., Brummer R.J., Derrien M., MacDonald T.T., Troost F., Cani P.D., Theodorou V., Dekker J., Méheust A., de Vos W.M., et al. Homeostasis of the gut barrier and potential biomarkers. Am. J. Physiol. Gastrointest. Liver Physiol. 2017;312:G171–G193. doi: 10.1152/ajpgi.00048.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schroeder B.O. Fight them or feed them: How the intestinal mucus layer manages the gut microbiota. Gastroenterol. Rep. 2019;7:3–12. doi: 10.1093/gastro/goy052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cornick S., Tawiah A., Chadee K. Roles and regulation of the mucus barrier in the gut. Tissue Barriers. 2015;3:1–2. doi: 10.4161/21688370.2014.982426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Heazlewood C.K., Cook M.C., Eri R., Price G.R., Tauro S.B., Taupin D., Thornton D.J., Chin W.P., Crockford T.L., Cornall R.J., et al. Aberrant mucin assembly in mice causes endoplasmic reticulum stress and spontaneous inflammation resembling ulcerative colitis. PLoS Med. 2008;5:0440–0460. doi: 10.1371/journal.pmed.0050054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shkoda A., Ruiz P.A., Daniel H., Kim S.C., Rogler G., Sartor R.B., Haller D. Interleukin-10 Blocked endoplasmic reticulum stress in intestinal epithelial cells: Impact on chronic inflammation. Gastroenterology. 2007;132:190–207. doi: 10.1053/j.gastro.2006.10.030. [DOI] [PubMed] [Google Scholar]
- 46.Hasnain S.Z., Tauro S., Das I., Tong H., Chen A.C.H., Jeffery P.L., McDonald V., Florin T.H., McGuckin M.A. IL-10 Promotes production of intestinal mucus by suppressing protein misfolding and endoplasmic reticulum stress in goblet cells. Gastroenterology. 2013;144:357–368.e9. doi: 10.1053/j.gastro.2012.10.043. [DOI] [PubMed] [Google Scholar]
- 47.Bergstrom K.S.B., Kissoon-Singh V., Gibson D.L., Ma C., Montero M., Sham H.P., Ryz N., Huang T., Velcich A., Finlay B.B., et al. Muc2 protects against lethal infectious colitis by disassociating pathogenic and commensal bacteria from the colonic mucosa. PLoS Pathog. 2010;6 doi: 10.1371/journal.ppat.1000902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hasnain S.Z., Wang H., Ghia J.E., Haq N., Deng Y., Velcich A., Grencis R.K., Thornton D.J., Khan W.I. Mucin gene deficiency in mice impairs host resistance to an enteric parasitic infection. Gastroenterology. 2010;138:1763–1771.e5. doi: 10.1053/j.gastro.2010.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McDole J.R., Wheeler L.W., McDonald K.G., Wang B., Konjufca V., Knoop K.A., Newberry R.D., Miller M.J. Goblet cells deliver luminal antigen to CD103+ dendritic cells in the small intestine. Nature. 2012;483:345–349. doi: 10.1038/nature10863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hooper L.V., Midtvedt T., Gordon J.I. How host-microbial interactions shape the nutrient environment of the mammalian intestine. Annu. Rev. Nutr. 2002;22:283–307. doi: 10.1146/annurev.nutr.22.011602.092259. [DOI] [PubMed] [Google Scholar]
- 51.Finnie I.A., Dwarakanath A.D., Taylor B.A., Rhodes J.M. Colonic mucin synthesis is increased by sodium butyrate. Gut. 1995;36:93–99. doi: 10.1136/gut.36.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Morita H., Kettlewell M.G.W., Jewell D.P., Kent P.W. Glycosylation and sulphation of colonic mucus glycoproteins in patients with ulcerative colitis and in healthy subjects. Gut. 1993;34:926–932. doi: 10.1136/gut.34.7.926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Einerhand A.W.C., Renes I.B., Makkink M.K., Van Der Sluis M., Büller H.A., Dekker J. Role of mucins in inflammatory bowel disease: Important lessons from experimental models. Eur. J. Gastroenterol. Hepatol. 2002;14:757–765. doi: 10.1097/00042737-200207000-00008. [DOI] [PubMed] [Google Scholar]
- 54.Varsha S., Kelli J., Jianyi Y., Sun L., Ruxian L., Huimin Y., Julie I., Jennifer F.-A., Nicholas Z.C., Mark D., et al. Chronic inflammation in ulcerative colitis causes long term changes in goblet cell function. Cell. Mol. Gastroenterol. Hepatol. 2021;18:1–14. doi: 10.1016/j.jcmgh.2021.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pullan R.D., Thomas G.A.O., Rhodes M., Newcombe R.G., Williams G.T., Allen A., Rhodes J. Thickness of adherent mucus gel on colonic mucosa in humans and its relevance to colitis. Gut. 1994;35:353–359. doi: 10.1136/gut.35.3.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Van Klinken B.J.W., Van Der Wal J.W.G., Einerhand A., Büller H.A., Dekker J. Sulphation and secretion of the predominant secretory human colonic mucin MUC2 in ulcerative colitis. Gut. 1999;44:387–393. doi: 10.1136/gut.44.3.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Larsson J.M.H., Karlsson H., Crespo J.G., Johansson M.E.V., Eklund L., Sjövall H., Hansson G.C. Altered O-glycosylation profile of MUC2 mucin occurs in active ulcerative colitis and is associated with increased inflammation. Inflamm. Bowel Dis. 2011;17:2299–2307. doi: 10.1002/ibd.21625. [DOI] [PubMed] [Google Scholar]
- 58.Shaoul R., Okada Y., Cutz E., Marcon M.A. Colonic Expression of MUC2, MUC5AC, and TFF1 in Inflammatory Bowel Disease in Children. J. Pediatr. Gastroenterol. Nutr. 2004;38:488–493. doi: 10.1097/00005176-200405000-00006. [DOI] [PubMed] [Google Scholar]
- 59.Forgue-Lafitte M.E., Fabiani B., Levy P.P., Maurin N., Flejou J.F., Bara J. Abnormal expression of M1/MUC5AC mucin in distal colon of patients with diverticulitis, ulcerative colitis and cancer. Int. J. Cancer. 2007;121:1543–1549. doi: 10.1002/ijc.22865. [DOI] [PubMed] [Google Scholar]
- 60.Borralho P., Vieira A., Freitas J., Chaves P., Soares J. Aberrant gastric apomucin expression in ulcerative colitis and associated neoplasia. J. Crohns Colitis. 2007;1:35–40. doi: 10.1016/j.crohns.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 61.Olli K.E., Rapp C., Connell L.O., Collins C.B., McNamee E.N., Jensen O., Jedlicka P., Allison K.C., Goldberg M.S., Gerich M.E., et al. Muc5ac expression protects the colonic barrier in experimental colitis. Inflamm. Bowel Dis. 2020;26:1353–1367. doi: 10.1093/ibd/izaa064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Derrien M., Van Passel M.W.J., Van De Bovenkamp J.H.B., Schipper R.G., De Vos W.M., Dekker J. Mucin-bacterial interactions in the human oral cavity and digestive tract. Gut Microbes. 2010;1:254–268. doi: 10.4161/gmic.1.4.12778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Johansson M.E.V., Hansson G.C. Immunological aspects of intestinal mucus and mucins. Nat. Rev. Immunol. 2016;16:639–649. doi: 10.1038/nri.2016.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sicard J., Le Bihan G., Vogeleer P., Jacques M., Harel J. Interactions of intestinal bacteria with components of the intestinal mucus. Front. Cell. Infect. Microbiol. 2017;7:387. doi: 10.3389/fcimb.2017.00387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.La Fata G., Weber P., Mohajeri M.H. Probiotics and the gut immune system: Indirect regulation. Probiotics Antimicrob. Proteins. 2018;10:11–21. doi: 10.1007/s12602-017-9322-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Laval L., Martin R., Natividad J.N., Chain F., Miquel S., De Maredsous C.D., Capronnier S., Sokol H., Verdu E., van Hylckama Vlieg J., et al. Lactobacillus rhamnosus CNCM I-3690 and the commensal bacterium Faecalibacterium prausnitzii A2-165 exhibit similar protective effects to induced barrier hyper-permeability in mice. Gut Microbes. 2015;6:1–9. doi: 10.4161/19490976.2014.990784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wrzosek L., Miquel S., Noordine M., Bouet S., Chevalier-Curt M.J., Robert V., Philippe C., Bridonneau C., Cherbuy C., Robbe-Masselot C., et al. Bacteroides thetaiotaomicron and Faecalibacterium prausnitzii influence the production of mucus glycans and the development of goblet cells in the colonic epithelium of a gnotobiotic model rodent. BMC Biol. 2013;11:61. doi: 10.1186/1741-7007-11-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Human Microbiome Project Consortium Structure, function and diversity of the healthy human microbiome. Nature. 2012;486:207–214. doi: 10.1038/nature11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Engevik M.A., Luk B., Chang-Graham A.L., Hall A., Herrmann B., Ruan W., Endres B.T., Shi Z., Garey K.W., Hyser J.M., et al. Crossm Bifidobacterium dentium fortifies the intestinal mucus layer. mBio. 2019;10:e01087-19. doi: 10.1128/mBio.01087-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Everard A., Belzer C., Geurts L., Ouwerkerk J.P., Druart C., Bindels L.B., Guiot Y., Derrien M., Muccioli G.G., Delzenne N.M., et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc. Natl. Acad. Sci. USA. 2013;110:9066–9071. doi: 10.1073/pnas.1219451110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Van Der Lugt B., Van Beek A.A., Aalvink S., Meijer B., Sovran B., Vermeij W.P., Brandt R.M.C., De Vos W.M., Savelkoul H.F.J., Steegenga W.T., et al. Akkermansia muciniphila ameliorates the age-related decline in colonic mucus thickness and attenuates immune activation in accelerated aging Ercc1 −/Δ 7 mice. Immun. Ageing. 2019;16:6. doi: 10.1186/s12979-019-0145-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Plovier H., Everard A., Druart C., Depommier C., Van Hul M., Geurts L., Chilloux J., Ottman N., Duparc T., Lichtenstein L., et al. A purified membrane protein from Akkermansia muciniphila or the pasteurized bacterium improves metabolism in obese and diabetic mice. Nat. Med. 2017;23:107–113. doi: 10.1038/nm.4236. [DOI] [PubMed] [Google Scholar]
- 73.Martín R., Chamignon C., Mhedbi-Hajri N., Chain F., Derrien M., Escribano-Vázquez U., Garault P., Cotillard A., Pham H.P. The potential probiotic Lactobacillus rhamnosus CNCM I-3690 strain protects the intestinal barrier by stimulating both mucus production and cytoprotective response. Sci. Rep. 2019;9:5398. doi: 10.1038/s41598-019-41738-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ahl D., Liu H., Schreiber O., Roos S., Phillipson M., Holm L. Lactobacillus reuteri increases mucus thickness and ameliorates dextran sulphate sodium-induced colitis in mice. Acta Physiol. 2016;317:300–310. doi: 10.1111/apha.12695. [DOI] [PubMed] [Google Scholar]
- 75.Zhang X., Tong Y., Lyu X., Wang J., Wang Y., Yang R. Prevention and alleviation of dextran sulfate sodium salt-induced inflammatory bowel disease in mice with bacillus subtilis-fermented milk via inhibition of the inflammatory responses and regulation of the intestinal flora. Front. Microbiol. 2021;11:622354. doi: 10.3389/fmicb.2020.622354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Souza L., Elian S.D., Paula M., Garcia C.C., Vieira T., Teixeira M.M., Arantes R.M., Nicoli J.R., Martins F.S. Escherichia coli strain Nissle 1917 ameliorates experimental colitis by modulating intestinal permeability, the inflammatory response and clinical signs in a faecal transplantation model. J. Med. Microbiol. 2016;65:201–210. doi: 10.1099/jmm.0.000222. [DOI] [PubMed] [Google Scholar]
- 77.Schroeder B.O., Birchenough G.M.H., Stahlman M., Arike L., Johansson M.E.V., Hansson G.C., Backhed F. Bifidobacteria or fiber protects against diet-induced microbiota-mediated colonic mucus deterioration. Cell Host Microbe. 2018;23:27–40. doi: 10.1016/j.chom.2017.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Garg S., Singh T.P., Malik R.K. In vivo implications of potential probiotic Lactobacillus reuteri lr6 on the gut and immunological parameters as an adjuvant against protein energy malnutrition. Probiotics Antimicrob. Proteins. 2020;12:517–534. doi: 10.1007/s12602-019-09563-4. [DOI] [PubMed] [Google Scholar]
- 79.Kumar M., Kissoon-Singh V., Coria A.L., Moreau F., Chadee K. Probiotic mixture VSL#3 reduces colonic inflammation and improves intestinal barrier function in Muc2 mucin-deficient mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2017;312:34–45. doi: 10.1152/ajpgi.00298.2016. [DOI] [PubMed] [Google Scholar]
- 80.Liu X., Yu R., Zou K. Probiotic mixture VSL # 3 alleviates dextran sulfate sodium-induced colitis in mice by downregulating T follicular helper cells. Curr. Med. Sci. 2019;39:371–378. doi: 10.1007/s11596-019-2045-z. [DOI] [PubMed] [Google Scholar]
- 81.Je I., Lee D., Jeong D., Hong D., Yoon J., Moon J.S., Park S. The probiotic, ID-JPL934, Attenuates dextran sulfate sodium-induced colitis in mice through inhibition of proinflammatory cytokines expression. J. Med. Food. 2018;21:1–8. doi: 10.1089/jmf.2017.4152. [DOI] [PubMed] [Google Scholar]
- 82.Bagarolli R.A., Tobar N., Oliveira A.G., Araújo T.G., Carvalho B.M., Rocha G.Z., Vecina J.F., Calisto K., Guadagnini D., Prada P.O., et al. Probiotics modulate gut microbiota and improve insulin sensitivity in DIO mice. J. Nutr. Biochem. 2017;50:16–25. doi: 10.1016/j.jnutbio.2017.08.006. [DOI] [PubMed] [Google Scholar]
- 83.Spiljar M., Merkler D., Trajkovski M. The immune system bridges the gut microbiota with systemic energy homeostasis: Focus on TLRs, mucosal barrier, and SCFAs. Front. Immunol. 2017;8:1353. doi: 10.3389/fimmu.2017.01353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Round J.L., Mazmanian S.K. Inducible Foxp3+regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proc. Natl. Acad. Sci. USA. 2010;107:12204–12209. doi: 10.1073/pnas.0909122107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bilotta A.J., Cong Y. Gut microbiota metabolite regulation of host defenses at mucosal surfaces: Implication in precision medicine. Precis. Clin. Med. 2019;2:110–119. doi: 10.1093/pcmedi/pbz008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Camilleri M. Human intestinal barrier: Effects of stressors, diet, prebiotics, and probiotics. Clin. Transl. Gastroenterol. 2021;12:e00308. doi: 10.14309/ctg.0000000000000308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nguyen N., Zhang B., Holubar S., Pardi D., Singh S. Treatment and prevention of pouchitis after ileal pouch-anal anastomosis for chronic ulcerative colitis (Review) Cochrane Database Syst. Rev. 2019;5:CD001176. doi: 10.1002/14651858.CD001176.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Derwa Y., Gracie D.J., Hamlin P.J., Ford A.C. Systematic review with meta-analysis: The efficacy of probiotics in inflammatory bowel disease. Aliment. Pharmacol. Ther. 2017;46:389–400. doi: 10.1111/apt.14203. [DOI] [PubMed] [Google Scholar]
- 89.Koretz R.L. Probiotics in gastroenterology: How pro is the evidence in adults? Am. J. Gastroenterol. 2018;113:1125–1136. doi: 10.1038/s41395-018-0138-0. [DOI] [PubMed] [Google Scholar]
- 90.Alemao C.A., Budden K.F., Gomez H.M., Rehman S.F., Marshall J.E., Shukla S.D., Donovan C., Forster S.C., Yang I.A., Keely S., et al. Impact of diet and the bacterial microbiome on the mucous barrier and immune disorders. Allergy. 2021;76:714–734. doi: 10.1111/all.14548. [DOI] [PubMed] [Google Scholar]
- 91.Rizzello F., Spisni E., Giovanardi E., Imbesi V., Salice M., Alvisi P., Valerii M.C., Gionchetti P. Implications of the westernized diet in the onset and progression of IBD. Nutrients. 2019;11:1033. doi: 10.3390/nu11051033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fernández-Tomé S., Hernández-Ledesma B., Chaparro M., Indiano-Romacho P., Bernardo D., Gisbert J.P. Role of food proteins and bioactive peptides in inflammatory bowel disease. Trends Food Sci. Technol. 2019;88:194–206. doi: 10.1016/j.tifs.2019.03.017. [DOI] [Google Scholar]
- 93.Hussain M., Ijaz M.U., Ahmad M.I., Khan I.A., Brohi S.A., Shah A.U., Shinwari K.I., Zhao D., Xu X., Zhou G., et al. Meat proteins in a high-fat diet have a substantial impact on intestinal barriers through mucus layer and tight junction protein suppression in C57BL/6J mice. Food Funct. 2019;10:6903–6914. doi: 10.1039/C9FO01760G. [DOI] [PubMed] [Google Scholar]
- 94.Fan P., Liu P., Song P., Chen X., Ma X. Moderate dietary protein restriction alters the composition of gut microbiota and improves ileal barrier function in adult pig model. Sci. Rep. 2017;7:43412. doi: 10.1038/srep43412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chen X., Song P., Fan P., He T., Jacobs D., Levesque C.L., Johnston L.J., Ji L., Ma N., Chen Y., et al. Moderate dietary protein restriction optimized gut microbiota and mucosal barrier in growing pig model. Front. Cell. Infect. Microbiol. 2018;8:246. doi: 10.3389/fcimb.2018.00246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhao F., Zhou G., Liu X., Song S., Xu X., Hooiveld G., Müller M., Liu L., Kristiansen K., Li C. Dietary protein sources differentially affect the growth of akkermansia muciniphila and maintenance of the gut mucus barrier in mice. Mol. Nutr. Food Res. 2019;63:1900589. doi: 10.1002/mnfr.201900589. [DOI] [PubMed] [Google Scholar]
- 97.Han K.-S., Deglaire A., Sengupta R., Moughan P.J. Hydrolyzed casein influences intestinal mucin gene expression in the rat. J. Agric. Food Chem. 2008;56:5572–5576. doi: 10.1021/jf800080e. [DOI] [PubMed] [Google Scholar]
- 98.Fernández-Tomé S., Martínez-Maqueda D., Tabernero M., Largo C., Recio I., Miralles B. Effect of the long-term intake of a casein hydrolysate on mucin secretion and gene expression in the rat intestine. J. Funct. Foods. 2017;33:176–180. doi: 10.1016/j.jff.2017.03.036. [DOI] [Google Scholar]
- 99.Plaisancié P., Claustre J., Estienne M., Henry G., Boutrou R., Paquet A., Léonil J. A novel bioactive peptide from yoghurts modulates expression of the gel-forming MUC2 mucin as well as population of goblet cells and Paneth cells along the small intestine. J. Nutr. Biochem. 2013;24:213–221. doi: 10.1016/j.jnutbio.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 100.Bessette C., Benoit B., Sekkal S., Bruno J., Estienne M., Léonil J., Ferrier L., Théodorou V., Plaisancié P. Protective effects of β-casofensin, a bioactive peptide from bovine β-casein, against indomethacin-induced intestinal lesions in rats. Mol. Nutr. Food Res. 2016;60:823–833. doi: 10.1002/mnfr.201500680. [DOI] [PubMed] [Google Scholar]
- 101.Araújo D.F.S., Guerra G.C.B., Pintado M.M.E., Sousa Y.R.F., Algieri F., Rodriguez-Nogales A., Araújo R.F., Gálvez J., Queiroga R.C.R.E., Rodriguez-Cabezas M.E. Intestinal anti-inflammatory effects of goat whey on DNBS-induced colitis in mice. PLoS ONE. 2017;12:e0185382. doi: 10.1371/journal.pone.0185382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lee M., Kovacs-Nolan J., Yang C., Archbold T., Fan M.Z., Mine Y. Hen egg lysozyme attenuates inflammation and modulates local gene expression in a porcine model of dextran sodium sulfate (DSS)-induced colitis. J. Agric. Food Chem. 2009;57:2233–2240. doi: 10.1021/jf803133b. [DOI] [PubMed] [Google Scholar]
- 103.Young D., Ibuki M., Nakamori T., Fan M., Mine Y. Soy-derived di- and tripeptides alleviate colon and ileum inflammation in pigs with dextran sodium sulfate-induced colitis. J. Nutr. 2012;142:363–368. doi: 10.3945/jn.111.149104. [DOI] [PubMed] [Google Scholar]
- 104.Utrilla M.P., Peinado M.J., Ruiz R., Rodriguez-Nogales A., Algieri F., Rodriguez-Cabezas M.E., Clemente A., Galvez J., Rubio L.A. Pea (Pisum sativum L.) seed albumin extracts show anti-inflammatory effect in the DSS model of mouse colitis. Mol. Nutr. Food Res. 2015;59:807–819. doi: 10.1002/mnfr.201400630. [DOI] [PubMed] [Google Scholar]
- 105.Mukai R., Handa O., Naito Y., Takayama S., Suyama Y., Ushiroda C., Majima A., Hirai Y., Mizushima K., Okayama T., et al. High-fat diet causes constipation in mice via decreasing colonic mucus. Dig. Dis. Sci. 2020;65:2246–2253. doi: 10.1007/s10620-019-05954-3. [DOI] [PubMed] [Google Scholar]
- 106.Gulhane M., Murray L., Lourie R., Tong H., Sheng Y.H., Wang R., Kang A., Schreiber V., Wong K.Y., Magor G., et al. High fat diets induce colonic epithelial cell stress and inflammation that is reversed by IL-22. Sci. Rep. 2016;6:28990. doi: 10.1038/srep28990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhu H., Wang H., Wang S., Tu Z., Zhang L., Wang X., Hou Y., Wang C., Chen J., Liu Y. Flaxseed oil attenuates intestinal damage and inflammation by regulating necroptosis and TLR4/NOD signaling pathways following lipopolysaccharide challenge in a piglet model. Mol. Nutr. Food Res. 2018;62:1700814. doi: 10.1002/mnfr.201700814. [DOI] [PubMed] [Google Scholar]
- 108.Zou J., Chassaing B., Singh V., Fythe M.D., Kumar M.V., Gewirtz A.T. Fiber-mediated nourishment of gut microbiota protects against diet-induced obesity by restoring IL-22-mediated colonic health. Cell Host Microbe. 2018;23:41–53. doi: 10.1016/j.chom.2017.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ishisono K., Mano T., Yabe T., Kitaguchi K. Dietary fiber pectin ameliorates experimental colitis in a neutral sugar side chain-dependent manner. Front. Immunol. 2019;10:2979. doi: 10.3389/fimmu.2019.02979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Shi H., Wang Q., Zheng M., Hao S., Lum J.S., Chen X., Huang X., Yu Y., Zheng K. Supplement of microbiota-accessible carbohydrates prevents neuroinflammation and cognitive decline by improving the gut microbiota-brain axis in diet-induced obese mice. J. Neuroinflammation. 2020;17:1–21. doi: 10.1186/s12974-020-01760-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Rohr M.W., Narasimhulu C.A., Rudeski-Rohr T.A., Parthasarathy S. Negative effects of a high-fat diet on intestinal permeability: A review. Adv. Nutr. 2020;11:77–91. doi: 10.1093/advances/nmz061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wu G.D., Chen J., Hoffmann C., Bittinger K., Chen Y., Sue A., Bewtra M., Knights D., Walters W.A., Knight R., et al. NIH public access. Science. 2011;334:105–108. doi: 10.1126/science.1208344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.De Filippis F., Pellegrini N., Vannini L., Jeffery I.B., La Storia A., Laghi L., Serrazanetti D.I., Di Cagno R., Ferrocino I., Lazzi C., et al. High-level adherence to a Mediterranean diet beneficially impacts the gut microbiota and associated metabolome. Gut. 2016;65:1812–1821. doi: 10.1136/gutjnl-2015-309957. [DOI] [PubMed] [Google Scholar]
- 114.Bolte L.A., Vila A.V., Imhann F., Collij V., Gacesa R., Peters V., Wijmenga C., Kurilshikov A., Fu J., Dijkstra G., et al. Long-term dietary patterns are associated with pro-inflammatory and anti-inflammatory features of the gut microbiome. Gut. 2021;70:1287–1298. doi: 10.1136/gutjnl-2020-322670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yang J., Wei H., Zhou Y., Szeto C.-H., Li C., Lin Y., Coker O.O., Lau H.C.H., Chan A.W., Sung J.J., et al. High-fat diet promotes colorectal tumorigenesis through modulating gut microbiota and metabolites. Gastroenterology. 2021;27:S0016-5085(21)03439-9. doi: 10.1053/j.gastro.2021.08.041. [DOI] [PubMed] [Google Scholar]
- 116.Makki K., Deehan E.C., Walter J., Bäckhed F. The impact of dietary fiber on gut microbiota in host health and disease. Cell Host Microbe. 2018;23:705–715. doi: 10.1016/j.chom.2018.05.012. [DOI] [PubMed] [Google Scholar]
- 117.Belzer C. Nutritional strategies for mucosal health: The interplay between microbes and mucin glycans. Trends Microbiol. 2021;30:S0966-842X(21)00135-9. doi: 10.1016/j.tim.2021.06.003. [DOI] [PubMed] [Google Scholar]
- 118.Koh A., De Vadder F., Kovatcheva-Datchary P., Bäckhed F. From dietary fiber to host physiology: Short-chain fatty acids as key bacterial metabolites. Cell. 2016;165:1332–1345. doi: 10.1016/j.cell.2016.05.041. [DOI] [PubMed] [Google Scholar]
- 119.Lewis J.D., Abreu M.T. Diet as a trigger or therapy for inflammatory bowel diseases. Gastroenterology. 2017;152:398–414.e6. doi: 10.1053/j.gastro.2016.10.019. [DOI] [PubMed] [Google Scholar]
- 120.Lambert K., Pappas D., Miglioretto C., Javadpour A., Reveley H., Frank L., Grimm M.C., Samocha-Bonet D., Hold G.L. Systematic review with meta-analysis: Dietary intake in adults with inflammatory bowel disease. Aliment. Pharmacol. Ther. 2021;54:742–754. doi: 10.1111/apt.16549. [DOI] [PubMed] [Google Scholar]
- 121.Kundra P., Rachmühl C., Lacroix C., Geirnaert A. Role of dietary micronutrients on gut microbial dysbiosis and modulation in inflammatory bowel disease. Mol. Nutr. Food Res. 2021;65:1901271. doi: 10.1002/mnfr.201901271. [DOI] [Google Scholar]
- 122.Hossen I., Hua W., Ting L., Mehmood A., Jingyi S., Duoxia X., Cao Y., Hongqing W., Zhipeng G., Kaiqi Z., et al. Phytochemicals and inflammatory bowel disease: A review. Crit. Rev. Food Sci. Nutr. 2020;60:1321–1345. doi: 10.1080/10408398.2019.1570913. [DOI] [PubMed] [Google Scholar]
- 123.Wawrzyniak P., Noureddine N., Wawrzyniak M., Lucchinetti E., Krämer S.D., Rogler G., Zaugg M., Hersberger M. Nutritional lipids and mucosal inflammation. Mol. Nutr. Food Res. 2021;65:1901269. doi: 10.1002/mnfr.201901269. [DOI] [PubMed] [Google Scholar]
- 124.Levine A., Sigall Boneh R., Wine E. Evolving role of diet in the pathogenesis and treatment of inflammatory bowel diseases. Gut. 2018;67:1726–1738. doi: 10.1136/gutjnl-2017-315866. [DOI] [PubMed] [Google Scholar]
- 125.Leech B., Mcintyre E., Steel A., Sibbritt D. Risk factors associated with intestinal permeability in an adult population: A systematic review. Int. J. Clin. Pract. 2019;73:e13385. doi: 10.1111/ijcp.13385. [DOI] [PubMed] [Google Scholar]
- 126.Lang J.M., Pan C., Cantor R.M., Tang W.H.W., Garcia-Garcia J.C., Kurtz I., Hazen S.L., Bergeron N., Krauss R.M., Lusis A.J. Impact of individual traits, saturated fat, and protein source on the gut microbiome. mBio. 2018;9:e01604–e01618. doi: 10.1128/mBio.01604-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wolters M., Ahrens J., Romaní-Pérez M., Watkins C., Sanz Y., Benítez-Páez A., Stanton C., Günther K. Dietary fat, the gut microbiota, and metabolic health—A systematic review conducted within the MyNewGut project. Clin. Nutr. 2019;38:2504–2520. doi: 10.1016/j.clnu.2018.12.024. [DOI] [PubMed] [Google Scholar]
- 128.Wan Y., Wang F., Yuan J., Li J., Jiang D., Zhang J., Li H., Wang R., Tang J., Huang T., et al. Effects of dietary fat on gut microbiota and faecal metabolites, and their relationship with cardiometabolic risk factors: A 6-month randomised. Gut. 2019;68:1417–1429. doi: 10.1136/gutjnl-2018-317609. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data described in the review are included in this published article.