Abstract
Rheumatoid arthritis (RA) is caused by prolonged periodic interactions between genetic, environmental, and immunologic factors. Posttranslational modifications (PTMs) such as citrullination, carbamylation, and acetylation are correlated with the pathogenesis of RA. PTM and cell death mechanisms such as apoptosis, autophagy, NETosis, leukotoxic hypercitrullination (LTH), and necrosis are related to each other and induce autoantigenicity. Certain microbial infections, such as those caused by Porphyromonas gingivalis, Aggregatibacter actinomycetemcomitans, and Prevotella copri, can induce autoantigens in RA. Anti-modified protein antibodies (AMPA) containing anti-citrullinated protein/peptide antibodies (ACPAs), anti-carbamylated protein (anti-CarP) antibodies, and anti-acetylated protein antibodies (AAPAs) play a role in pathogenesis as well as in prediction, diagnosis, and prognosis. Interestingly, smoking is correlated with both PTMs and AMPAs in the development of RA. However, there is lack of evidence that smoking induces the generation of AMPAs.
Keywords: rheumatoid arthritis, pathogenesis, posttranslational modification (PTM), citrullination, carbamylation, acetylation, anti-modified protein antibodies (AMPAs), anti-citrullinated protein/peptide antibodies (ACPAs), anti-carbamylated protein (anti-CarP) antibodies, anti-acetylated protein antibodies (AAPAs)
1. Introduction
Rheumatoid arthritis (RA), the most common form of chronic inflammatory arthritis, is mainly targeting synovial joints [1,2,3]. However, RA is also a systemic autoimmune disease that involves not only joints but other organs such as the lungs, pericardium, sclera, peripheral nerves, skin, and vessels [4,5,6]. Untreated RA destroys the articular cartilage and nearby bones, resulting in functional disability [7,8]. The current strategy for RA treatment focuses on early and aggressive management before irreversible articular damage [7,9]. Thus, recent research has focused on events occurring before the presentation of RA; specifically, the pathogenesis and preclinical stage.
The 2010 American College of Rheumatology (ACR)–European League Against Rheumatism (EULAR) criteria are often used as the basis for a diagnosis of RA (Table 1). The new scoring system results in a score of 0–10, and a score ≥ 6 is considered satisfactory for the diagnosis of RA. The 2010 ACR–EULAR criteria include anti-citrullinated protein/peptide antibodies (ACPAs) and rheumatoid factor (RF). The diagnostic criteria for ACPAs are the presentation of an early disease course and the prediction of an aggressive disease course [10].
Table 1.
Scoring for rheumatoid arthritis (RA) diagnosis.
| Domain | Category | Score |
|---|---|---|
| Joint involvement | 1 large joint (Shoulder, elbow, hip, knee, ankle) | 0 |
| 2–10 large joints | 1 | |
| 1–3 small joints (MCP, PIP, thumb IP, MTP, wrists) | 2 | |
| 4–10 small joints | 3 | |
| More than 10 joints (Including at least 1 small joint) | 5 | |
| Serologic study | Negative RF and negative ACPAs (Under ULN) | 0 |
| Low-positive 1 RF or low-positive ACPAs | 2 | |
| High-positive 2 RF or high positive ACPAs | 3 | |
| Acute phase reactants | Normal CRP and normal ESR | 0 |
| Abnormal CRP or abnormal ESR | 1 | |
| Duration of symptoms | <6 weeks | 0 |
| ≥6 weeks | 1 |
Revised 2010 ACR–EULAR criteria consist of four domains: joint involvement, serologic study including RF and ACPAs, acute phase reactants (CRP and ESR), and duration of symptoms [10]. 1 Low-positive, ≤3 × ULN 2 High-positive, ≥3 × ULN. Abbreviations: MCP, metacarpophalangeal joint; PIP, proximal interphalangeal joint; IP, interphalangeal joint; MTP, metatarsophalangeal joint; RF, rheumatoid factor; ACPAs, anti-citrullinated protein/peptide antibodies; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; ULN, upper limit of normal.
The pathogenesis of RA has not yet been fully identified, as the characteristic pathological features make it difficult to identify the causative factors [7,11,12,13]. First, RA is the result of the interaction between numerous genetic, environmental, and immunological factors. Second, the various backgrounds of races and ethnicities each have different trigger factors, which further complicate diagnosis. In addition, all the causative factors have usually been interacting for a prolonged duration before the onset of RA.
2. Pathogenesis
The average prevalence rate of RA is 0.1% to 1.0%, and the condition, which is more common in women than it is in men, has the highest rate among rheumatologic diseases [14]. However, the prevalence and occurrence rate vary according to race and ethnicity [15,16,17]. The differences exist not only in prevalence but also in disease activity and clinical outcomes [16,18]. There are also differences in the frequency of human leukocyte antigen (HLA) alleles, single-nucleotide polymorphisms (SNPs), and disease manifestation [19,20]. The most important genetic factor is shared epitope (SE) of HLA-DRB1 of major histocompatibility complex (MHC) [21,22,23]. The HLA-DRB1 allele associated with MHC is the most popular genetic factor in RA, and it increases the risk (HLA-DRB1*0401, *0404/*0408, *0405, *0101, *1001, and *1402), whereas HLA-DRB1*13 has a protective effect and decreases the risk [3,20,23,24,25,26].
However, other genetic factors also exist in non-HLA regions, such as peptidylarginine deiminase (PAD), signal transducers and activators of transcription 4 (STAT4), protein tyrosine phosphatase N22 (PTPN22), tumor necrosis factor (TNF) receptor-associated factor 1-C5 (TRAF1-C5), and interleukin (IL)-1 receptor-associated kinase 1 (IRAK1) genes [20,27,28,29,30,31]. Genes involved in T cell activation or the nuclear factor (NF)-κB pathway and SNPs are linked in RA [1,32].
Genome-wide association studies (GWAS) are widely used to identify gene candidates that correlate with RA [1,2,3,33,34]. Recently, a large Korean cohort study reported that the SLAMF6, CXCL13, SWAP70, NFKBIA, ZF-P36L1, and LINC00158 loci may be new genetic factors [35]. In a Chinese cohort, the potential involvement of the IL12RB2, BOLL-PLCL1, CCR2, TCF, and IQGAP1 loci were also identified through a GWAS study [36]. When environmental factors such as smoking, microorganisms, race, and periodontitis are combined with genetic factors, the immune tolerance breaks down [7,24,37,38]. For example, commencement of smoking by a person with the HLA-DRB1 SE gene in-creases the potential to develop RA [25,39].
Certain infectious microorganisms (e.g., Epstein–Barr virus (EBV), parvovirus B19, Proteus sp., and Escherichia coli) may have cross-reactivity for sensitizing autoantigens by molecular mimicry [40,41,42]. Porphyromonas gingivalis, the major pathogen of periodontal disease, expresses the bacterial PAD gene and leads to citrullination [43,44]. The gut microbe Prevotella copri accumulates in the feces and has homologous epitopes of N-acetylglucosamine-6-sulfatase (GNS) and filamin A (FLNA), which suggests that it is a potential RA trigger [45]. PAD changes the characteristics of proteins by citrullination and induces autoantigenicity [46,47,48]. Citrullination is followed by the production of ACPAs, the key molecule involved in RA pathology, in genetically susceptible individuals [49].
The normal diarthrodial joint space is filled with a small amount of synovial fluid (SF) [50], and the innermost part of the joint capsule and tendon sheath is covered with one to three layers of specialized cells, called the synovial membrane [51]. Synovial cells connect to each other through cadherin 11 [52]. The two types of synoviocytes are type A macrophage-like synoviocytes and type B fibroblast-like synoviocytes (FLS) [52,53]. RA progression with synovitis leads to edema of the synovium and an increase in the extracellular matrix. Abnormal proliferation structure of synovial tissue, called pannus, then forms [3,54].
The pannus infiltrates nearby structures, including the normal synovium, cartilage, and bony structure [55,56]. Synoviocytes in RA patients have abnormal proliferation traits similar to those of cancer cells [57], and mutations in p53, a tumor suppressor gene, have been reported in the synovium of these patients [58,59,60]. This phenomenon correlates with hyperactivated synoviocytes, which are apoptosis-resistant [61]. The variable mutation patterns of p53 may correlate with the heterogeneity of RA [59].
Dysregulation of the cell death mechanism is a causative factor at any step of RA pathogenesis, and in RA, synoviocytes exhibit altered apoptotic responses [62]. Autophagy may be dysregulated in severe conditions, such as cell senescence or growth factor starvation, inducing self-cannibalism [46]. Any dysregulation of the cell death mechanism could be a source of autoantigens by introducing an epitope of intracellular molecules to the naïve immune system.
Neutrophil extracellular traps (NETs) are formed by inflammatory stimuli in a process called NETosis, which captures invading microorganisms [63]. NETosis is another form of cell death that is distinguishable from apoptosis and necrosis [64,65]. NETs are composed of extruded intracellular components such as DNA, histones, granular proteins (myeloperoxidase (MPO), elastase, and lactoferrin), and cytoplasmic proteins (such as calprotectin and catalase.) [46,63]. This suggests that NETosis leads to exposure of intracellular and intranuclear molecules, which can induce autoimmunogenicity [66].
Recent studies claim that citrullination is not a common pathway associated with NETosis [67]. Therefore, the concept of leukotoxic hypercitrullination (LTH) and defective mitophagy has emerged as a more precise description [67,68]. Although LTH is the main source of de novo pathogenic citrullination in RA, NETosis may act as a redistributor of steady-state citrullinome in neutrophils, also inducing autoimmunogenicity [68].
Any event causing the breakdown of self-tolerance triggers the antigen presenting cell (APC) to sense the autoantigen, such as fibrinogen, vimentin, enolase, and so on, which then expresses the antigen with the MHC II [69]. The antigen presented by the MHC II stimulates the T cell receptor (TCR), which is expressed on the surface of cluster of differentiation 4 positive (CD4+) T cells [69,70]. However, this process is not sufficient to activate T cells. The surface molecule CD80/86 of the APC also binds to CD28 on the T cell surface [71], and binding between CD80/86 and CD28 acts as a “co-signal” for T cell activation [72].
Activated T cells differentiate into helper T1 (TH1) and T17 (TH17) cells, which express CD40 ligand (CD40L) [73]. CD40L of CD4+ TH cells binds to CD40 of B cells, inducing B cell differentiation into plasma cells, which secrete autoantibodies such as RF and ACPAs [74]. Autoantibodies bind to autoantigens to form immune complexes, which activate the complement system. Furthermore, TH cells produce numerous cytokines such as interferon (IFN)-γ, TNF-α, lymphotoxin-β, ILs (especially IL-6 and IL-17), and granulocyte-macrophage colony-stimulating factor (GM-CSF). Rituximab targets CD20, sometimes called “medical splenectomy” and is used for RA treatment because it depletes B cells [75].
Several groups of T cells, called effector T cells (TEff cells), rapidly respond to these stimuli and activate macrophage-like synoviocytes and FLS [76]. Activated macrophage-like synoviocytes release proinflammatory cytokines including TNF-α and ILs (IL-1, IL-6, IL-12, IL-15, IL-18, and IL-23), whereas activated FLS produce IL-1, IL-6, and TNF-α [77]. Recent theory suggests that TH17 cells are more important than TH1 cells are in RA pathophysiology [78,79]. TH17 cells secrete IL-17, which enhances the upregulation of inflammatory cytokine production in synoviocytes, including TNF-α, IL-1, and IL-6. It also mediates neutrophil, granulopoiesis, and osteoclast differentiation. IL-23 from TH17 cells affects the activity and glycosylation of autoantibodies [80]. The TH1/TH17 ratio in patients with RA, is inversely proportional to disease activity [81].
The Janus kinase (JAK)/STAT signaling pathway in these inflammatory cells amplifies the immune response [82]. Other intracellular signaling pathways are also involved, such as spleen tyrosine kinase (Syk), mitogen-activated protein kinases (MAPKs), and NF-κB [83]. Inhibition of these pathway is one of the mechanisms of action of small-molecule drugs. JAK inhibitors such as tofacitinib, baricitinib, and upadacitinib have been approved for the treatment of RA by the US Food and Drug Administration (FDA) [84]. Fostamatinib, which blocks Syk, usually used in chronic immune thrombocytopenia (ITP), has been in trials for RA treatment [85,86,87].
Regulatory T (TReg) cells expressing CD25 and the transcription factor forkhead box P3 (FOXP3) regulate other immune cells to maintain tolerance [88]. Some studies suggest that the loss of cytotoxic T lymphocyte antigen 4 (CTLA-4) expression interferes with the suppressive role of TReg cells [89]. Abatacept, a fusion protein including the constant fragment (Fc) region of immunoglobulin (Ig) G1 and the extracellular part of CTLA-4, competes with CD28 of T cells such as TReg cells. Because the binding affinity of abatacept to CD80/86 of APC is higher than that to CD28, abatacept modulates excessive immune responses in RA [72].
TNF-α stimulates the proliferation of T cells and B cells and activates FLS to produce matrix metalloproteinases (MMPs) [1,90]. Autophagy is upregulated in FLS after TNF-α exposure, and RA patients show higher levels of autophagy than normal persons do [91]. TNF-α also upregulates adhesion molecules on endothelial cells and their pathological neovascularization [1]. Production of IL-1, IL-6, and GM-CSF is accelerated by TNF-α [92], whereas IL-6 interacts with TNF-α to promote the cell cycle for the proliferation of FLS, leading to RA induction [93,94]. TNF-α induces the expression of dickkopf-1 (DKK-1), which downregulates the Wnt receptors of osteoblast precursors [94]. TNF-α also interacts with osteoclast precursors and osteoblasts (OBs), leading to pathological bone destruction in RA by fusing osteoclast precursors to form activated osteoclasts (OCs), stabilizing OB, inducing osteocyte apoptosis, and enhancing bone absorption [95,96,97]. Figure 1 shows a schematic illustration of RA pathogenesis.
Figure 1.
Schema of rheumatoid arthritis (RA) pathogenesis. Black, blue, and red arrows indicate differentiation, secretion of proinflammatory molecules, and stimulation, respectively. Broken red arrow indicates stimuli mediated by specific molecules. Abbreviations: TLR, Toll-like receptor; CD, cluster of differentiation; CTLA-4, cytotoxic T lymphocyte antigen-4; MHC, major histocompatibility complex; TCR, T cell receptor; TH cell, helper T cell; TH1 cell, helper TH1 cell; TH17 cell, helper TH17 cell; TReg cell, regulatory T cell; RF, rheumatoid factor; ACPAs, anticitrullinated protein/peptide antibodies; IC, immune complex; AutoAg, autoantigen; EGF, epidermal growth factor; TGF-β, transforming growth factor-β; IFN-γ, interferon-γ; IL, interleukin; MMP, matrix metalloproteinase; FLS, fibroblast-like synoviocytes; OC, osteoclast; OB, osteoblasts [3]. Created with Biorender.com.
Ai, R. et al. [98] found that there are epigenetically similar regions in the FLS of RA patients, including a study that found that Huntingtin-interacting protein-1 (HIP-1) in the Huntington’s disease signaling pathway is correlated with FLS in RA patients. The action of HIP-1 is related to FLS invasion of the matrix, which regulates the severity of RA [98,99].
Air pollutants appear to play a role in RA pathogenesis, and the ACPAs titer could be predicted by exposure to particulate matter with a diameter ≤2.5 μm (PM 2.5) [11]. In addition, ozone exposure and living near high-traffic roads were recognized as risk factors for RA in a meta-analysis [100]. A body mass index (BMI) indicating adiposity is linked to the risk of RA development, and this is more significant in women [13]. Some studies have shown that the gut microbiome and its metabolites may induce RA by stimulating TH17 cells of mucosal immune tissue that control the production of autoantibodies [101,102,103,104].
Disease-modifying antirheumatic drugs (DMARDs) are a group of drugs that regulate the activity of RA. In recent decades, biological DMARDs have been developed based on the pathophysiology of RA, particularly targeting certain molecules or pathways. Table 2 shows a list of biologic DMARDs targeting cytokines and cell-surface molecules approved by the FDA [84].
Table 2.
Biologic and targeted synthetic disease-modifying antirheumatic drugs (DMARDs) for rheumatoid arthritis (RA).
| DMARDs | Mechanism | Route of Administration |
|---|---|---|
| Abatacept | Fusion protein consists of extracellular domain of CTLA-4 and Fc region of IgG1, binding to CD80/86 | IV, SC |
| Adalimumab | TNF-α inhibitor | SC |
| Infliximab | IV | |
| Certolizumab pegol | SC | |
| Golimumab | SC | |
| Etanercept | SC | |
| Rituximab | Monoclonal antibody against CD20 | IV |
| Tocilizumab | Humanized monoclonal antibody against IL-6 receptor | IV, SC |
| Sarilumab | SC | |
| Tofacitinib | JAK1/JAK3 inhibitor | PO |
| Baricitinib | JAK1/JAK2 inhibitor | PO |
| Upadacitinib | JAK1 inhibitor | PO |
| Filgotinib | PO | |
| Peficitinib | Pan-JAK(JAK1/JAK2/JAK3/Tyk2) inhibitor | PO |
Abbreviations: DMARDs, disease-modifying antirheumatic drugs; CTLA-4, cytotoxic T lymphocyte-associated protein 4; Fc, constant fragment; IgG, immunoglobulin G; CD, cluster of differentiation; TNF-α, tumor necrosis factor-α; IL, interleukin; JAK, Janus kinase; Tyk, tyrosine kinase; SC, subcutaneous; IV, intravenous; PO, per os (orally).
3. Citrullination in RA
Citrullination is a process that converts the amino acid arginine to citrulline [46], and it is catalyzed by a Ca2+-dependent enzyme, PAD [105,106]. Every event where citrulline is converted to arginine increases the mass by 0.984 Da and the loss of one positive charge [105]. This posttranslational modification (PTM) alters acidity, which affects the isoelectric point (pI), ability to form hydrogen bonds, interaction with other amino acid residues, and protein unfolding [107,108]. Furthermore, these changes can influence the function and half-life of the associated proteins [109], suggesting that citrullination may create a new protein [106,110]. The formation of a citrullinated protein also suggests the possibility of generating new epitopes that could act as a new autoantigen that escapes self-immune tolerance [111]. Citrullinated peptides have a higher binding affinity to the HLA-DRB1 (DRB1*0401 or *0404) antigen-binding groove than to the corresponding arginine-containing peptide [112].
The Ca2+ dependency of PAD, which catalyzes citrullination, may be the main switch for the regulation of citrullination in the body [68]. PAD requires not only Ca2+ but also a reducing environment to maintain free thiol cystine activity [113]. The oxidative environment of the extracellular space inhibits citrullination [114]. Furthermore, the body regulates the concentration of Ca2+ using numerous channels and hormones by investing energy.
Citrulline-specific CD4+ T cells have been found in both human and mouse models [115,116] and citrulline-specific TH1 and TH17 cells are increased in RA patients [117,118]. The sequence of human citrullinated enolase peptide-1 (CEP-1) is similar to that of the α-enolase of P. gingivalis; the anti-CEP-1 antibodies and the enolase of P. gingivalis also have cross-reactivity with equivalent epitopes [119]. Administration of glucocorticoids as an RA treatment may reduce the level of citrullination [120,121]. A significantly higher proportion of citrullinated protein has been detected in synovial biopsy samples from RA patients than in the synovium of healthy individuals [120].
In chronic obstructive pulmonary disease (COPD) patients and smokers, vimentin levels are increased [122]. COPD patients without RA sometimes show positive test results for anti-mutated citrullinated vimentin antibodies (anti-MCV), one of the ACPA tests, and anti-MCV positivity is correlated with the manifestation of a severe form of extra-articular RA [123]. Additionally, PAD2 expression accelerates citrullination in the lungs, which is enhanced by smoking [124,125]. In a rat model of autoimmune encephalitis, Odoardi et al. [126] demonstrated that the autoimmune cells acquire the capacity to enter the CNS only after residing within the lung tissue; first the autoimmune cells drained to lymphatics of airways, then entered to blood circulation to reach for the CNS. In conclusion, Valesini et al. [46] hypothesized that citrullination in the lung could be an extra-articular factor in the origin of RA autoimmunogenicity that generates lung-resident autoreactive T cells, which migrate to other target organs by the upregulation of chemokine receptors—as in case of a rat model of autoimmune encephalitis [126].
3.1. PAD Family
PAD, which hydrolyzes guanidinium side chains in peptidylarginine to peptidylcitrulline and ammonia [30], belongs to another larger group of enzymes called the amidinotransferase superfamily. Isoforms of PAD share approximately 50% sequence similarity [113]. PAD5, a designation not currently used, was once considered to be a human homolog of rodent PAD4; however, it was proved that the PAD5 is identical to PAD4, as indicated by expression, sequence data, and genomic organization [30]. Another notable type of PAD is found in eukaryotes. The PAD of P. gingivalis (PPAD), the major pathogen of periodontitis, is independent of the Ca2+ concentration [127,128], which makes it active at higher pH, and it has a preference for C-terminal arginine citrullination, regardless of whether it is the peptide-bound or free form [128,129]. When arginine gingipains get activated, bacterial enzymes similar to human trypsin, they cut polypeptides into short peptide fragments with a C-terminal arginine [46]. Then, PPAD rapidly citrullinates C-terminal arginine in the fragment [130]. Table 3 shows the site of expression and substrate of the five isoforms of PAD in addition to those of PPAD.
Table 3.
Isoforms of peptidylarginine deiminase (PAD) compared with PAD of Porphyromonas gingivalis (PPAD).
| Type | Site of Expression | Substrate | Reference |
|---|---|---|---|
| PAD1 | Epidermis Uterus Hair follicle, sweat gland Stomach |
Filaggrin Keratin-associated protein MEK1 |
[131,132] |
| PAD2 | CNS, PNS Skeletal muscle Immune cells, spleen Skin, secretory gland Uterus, pancreas, kidney, inner ear |
Myelin basic protein in CNS Histones Actin Vimentin RNAP2 β-catenin Enolase Fibrinogen |
[133,134,135,136] |
| PAD3 | Hair follicle Keratinocyte Peripheral nerve |
Vimentin Filaggrin Trichohyalin Apoptosis-inducing factors |
[137] |
| PAD4 | Immune cells, spleen Secretory gland Brain Uterus Joints |
Histones and nucleophosmin/B23 Type I collagen ING4 p300, p21, p53 Lamin C GSK3β PAD4 |
[138,139] |
| PAD6 | Egg, ovary, testis, early embryo | Uncertain | [30,140] |
| PPAD | Enzyme of P. gingivalis | Fibrinogen α-enolase Collagen type II PPAD |
[44,119,141,142] |
There are five PAD isoenzymes. PAD2 stabilized by the NTZ, substrates β-catenin. Trichohyalin is a major structural protein in hair follicles. Note that PAD4 has a homodimer structure, the only type of PAD localized to the cell nucleus. PAD4 and PPAD can autocitrulate to generate antibodies. PAD6 is uniquely expressed in germ cells, and its precise role is unclear; it is thought to be essential for germ cell-specific structures in zygote/embryo development. PPAD is independent of Ca2+ concentration and active at higher pH; it prefers C-terminal arginine citrullination. Abbreviations: PAD, peptidylarginine deiminase; CNS, central nervous system; PNS, peripheral nervous system; PPAD, PAD of Porphyromonas gingivalis; NTZ, nitazoxanide [30,46,68,105,143,144,145].
Autocitrullination is occasionally considered to mediate the inactivation and regulation of enzymes, but its definite role is still controversial [48,146,147]. PAD4 has multiple citrullination sites including the Arg-372 and Arg-374 of PAD, which are considered potential autocitrullination targets. Autocitrullination modifies the structure of PAD4 and may increase its recognition by autoantibodies [48]. Activity of PAD and PPAD is elevated in patients with both RA and periodontitis [147]. Citrullination by PPAD may induce an immunologic response against citrullinated proteins in RA patients with periodontitis and SE [46]. A higher IgG anti-P. gingivalis antibody titer is associated with HLA-DRB1 neutral alleles [148].
The roles of PAD2 and PAD4 have been identified in RA, and they have been detected in macrophages of the SF of RA patients (RA-SF) and granulocytes isolated from the synovium of a mouse arthritis model, respectively [149,150]. Autocitrullination of PAD4 enhances the chance of its recognition by autoantibodies, and anti-PAD4 antibodies have predictive and prognostic value in RA [48,151]. PPAD also undergoes autocitrullination and generates antibodies that are cross-reactive with other citrullinated proteins of the human body [128]. However, in contrast with anti-PAD4 antibodies, anti-PPAD antibodies have no correlation with disease activity or ACPAs levels [142].
3.2. ACPAs, a Diagnostic and Prognostic Tool in RA
ACPAs are a group of antibodies that sense citrulline-containing proteins/peptides and share partial cross-reactivity [152,153]. An in vitro study showed that 66% of ACPAs showed cross-reactivity with different epitopes, whereas 33% were mono-active [154]. ACPA is a well-established diagnostic serology test molecule for RA, with a specificity of 85–95% and a sensitivity of 67% [155,156,157,158]. The specificity and sensitivity of the ACPA test has improved gradually from the first generation (anti-CCP1 test) to the third generation (anti-CCP3 test) [46,159]. ACPA is useful for the prediction of RA because it already exists before the onset of RA [160], and its positivity indicates a more erosive disease course and severity [161,162,163].
Although there are various isotypes of ACPAs, such as IgG, IgA, and IgM, the IgG isotype is the most dominant form in RA patients [164]. The Fc region of ACPA undergoes remodeling, which causes its Fc fragment to show two characteristics: decreased galactosylation and sialylation and increased core fucosylation, which differs from those of other serum antibodies [165,166,167,168,169]. Sialylation has a protective effect against the autoimmunogenicity of anti-type II collagen antibodies [165].
Therefore, remodeling of the Fc region could indicate the alteration of the functional activities of ACPA [165,168], and this remodeling of ACPA occurs before the change in total serum Ig [167,170]. In vitro, ACPA binds to the Fc receptors of myeloid lineage immune cells, activating the component system through both classical and alternative pathways [171,172]. Citrullinated fibrinogen–ACPA complexes in RA patients could activate macrophages to release TNF-α [173]. IgM–RF enhances this cascade and extends the spectrum by inducing the secretion of other cytokines (IL-1β, IL-6, and IL-8) [171].
The Fc-glycan profile showed different Fc receptor and complement binding affinities [168]. The agalatosylated profile of the Fc of ACPA facilitates the production of high-affinity RF for agalactosylated IgG [166]. ACPA also undergoes extensive variable domain glycosylation associated with the SE allele, except for IgM ACPA [174,175]. The incorporation of N-linked glycosylation sites modulates the affinity of ACPA [166,168,176]. Because ACPAs are a collection of heterogeneous antibodies, the specificity of ACPAs differs even in individual patients [177,178]. Candidate citrullinated autoantigens are listed in Table 4.
Table 4.
Candidate proteins and peptides as targets of anti-citrullinated protein/peptide antibodies (ACPAs).
| Origin | Candidate Protein/Peptide | Site of Expression in Human | Reference |
|---|---|---|---|
| Endogenous | Fibrinogen | Inflamed joint | [179,180,181] |
| Vimentin | Inflamed joint | [180,182,183,184] | |
| α-enolase | Joint tissue, inflammatory cells ¶ | [180,181,185,186] | |
| Fibronectin | Plasma, synovium, SF | [187,188,189] | |
| Type II collagen | Joint tissue | [181] | |
| Histone § | Nucleus | [190,191] | |
| BIP § | ER | [170,192] | |
| Tenascin-C | ECM of joint | [193,194] | |
| Filaggrin | Epithelium | [195,196] | |
| Apo E | Plasma, CNS, RA-SF | [197,198] | |
| MNDA | Inflammatory cells ¶ | [197,199] | |
| β-actin | Inflammatory cells ¶ | [197,199] | |
| FUSE-BP § | Nucleus | [200] | |
| hnRNP § | Nucleus, RA-SF | [201] | |
| Exogenous | Viral citrullinated peptides from EBV antigen | — | [190] |
| α-enolase from P. gingivalis | [190] | ||
| GNS sequence homologue of surface proteins of the P. copri | [45] |
Fibrinogen, vimentin, and α-enolase are well-known targets of ACPAs. Numerous proteins and peptides are targets of ACPAs. Target proteins or peptides could originate from both endogenous and exogenous proteins. The site of expression did not consider the circulating autoantigen. § Ubiquitous expression pattern. ¶ Not limited to mentioned tissue only (usually nonspecific). Abbreviations: BIP, immunoglobulin binding protein; Apo E, apolipoprotein E; MNDA, myeloid nuclear differentiation antigen; FUSE-BP, far upstream element-binding proteins; EBV, Epstein–Barr virus; GNS, N-acetylglucosamine-6-sulfatase; SF, synovial fluid; ER, endoplasmic reticulum; ECM, extracellular matrix; RA-SF, synovial fluid of rheumatoid arthritis patient; CNS, central nervous system.
The concentration of IgM–ACPA in the RA-SF, corrected for the total amount of IgG, was higher than that in the serum of the same patient, suggesting that there is local production of ACPAs [202]. Following binding to peripheral blood mononuclear cells, ACPA activates extracellular signal-regulated kinase (ERK) 1/2 and c-Jun N-terminal kinase (JNK) signaling pathways. This effect further enhances inhibitor of NF-κB kinase (IKK)-α phosphorylation, followed by activation of NF-κB and production of TNF-α [203]. Certain ACPAs, such as anti-citrullinated vimentin antibodies, induce NET formation, and NETosis provides the citrullinated autoantigen and PAD enzymes, which provides a positive feedback loop, further enhancing the formation of ACPAs [183].
In summary, ACPA has a pathogenic effect on RA, both in vitro and in vivo. First, ACPA induces NETosis, creating positive feedback. Second, ACPA stimulates macrophages to induce TNF-α and activate the complement of both classical and alternative pathways. Third, ACPA interacts with other autoantibodies associated with RA to initiate disease cascades. Fourth, these substances pre-existed before the onset of RA, called the pre-clinical stage, and their titer increases with a widening spectrum and stronger affinity during the development of RA. Fifth, the characteristics of ACPA could be altered through remodeling by PTM. Finally, these mechanisms mediate the applicability and usefulness of ACPA not only in the diagnosis of RA but also as an indicator of a more severe clinical course and structural damage.
A recent study by Chirivi et al. [204] showed that therapeutically inhibiting NET formation with ACPA, especially targeting the N-termini of histones 2A and 4, suppressed NET release or uptake by macrophages in various mouse models. Won et al. [205] detected circulating citrullinated antigens, such as type II collagen and filaggrin, in the sera of RA patients, including seronegative RA, using a monoclonal 12G1 antibody and proposed the possibility of it serving as a diagnostic tool for seronegative RA.
3.3. Citrullination and Cell Death Mechanism
Citrullination is the process of preparing for apoptosis, and it involves the induction of a functional loss of filament proteins [106]. In apoptosis, unlimited Ca2+ influx into the intracellular space activates the PAD, leading to citrullination [46]. In the normal apoptosis process, phagocytosis rapidly eliminates apoptotic bodies [206]. However, once dysregulation occurs during apoptosis or phagocytosis to eliminate apoptotic bodies, citrullinated intracellular antigens are exposed [207]. Similarly, the necrosis of neutrophils releases PAD into the extracellular space and leads to citrullination [208]. Compared with the SF of osteoarthritis (OA) patients, that of RA patients showed an elevation in the concentration of intranuclear materials and the activity of PAD4 [151]. PAD4 activity in vitro, released to the extracellular space, is higher in necrosis than it is in NETosis [209].
The names of newly identified cell death mechanisms, LTH and defective mitophagy, were formulated to distinguish them from other forms of neutrophil death that do not involve hypercitrullination, even if they form NET-like structures [68]. LTH releases citrullinated intranuclear molecules [210]. In LTH, perforin and the membrane attack complex (MAC), the pore-forming cytolytic proteins, induce the influx of Ca2+ and other ions, leading to osmotic lysis [211,212,213]. LTH is independent of ERK and nicotinamide adenine dinucleotide phosphate (NADPH) oxidase activity, a hallmark of NETosis [214,215].
Initiation of LTH originates not only endogenously but also exogenously. Streptomyces sp. have bacterial calcium ionophores, such as ionomycin and calcimycin [67]. Other bacterial pore-forming toxins also trigger LTH. The periodontal pathogen Aggregatibacter actinomycetemcomitans secretes the pore-forming protein leukotoxin A (LtxA) to activate citrullination in neutrophils [216,217,218].
3.4. Microorganisms Inducing Citrullination
P. gingivalis induces citrullination using PPAD independent of Ca2+ and pH level [43]. As described above, A. actinomycetemcomitans and Streptomyces sp. induce LTH that involves citrullination; P. copri and EBV have homologous epitopes that could trigger the production of ACPAs and autoimmunogenicity. Some other bacterial pathogens also induce membranolytic damage, which may induce citrullination in neutrophils and ACPA production via cell death mechanisms. Certain microbiomes in the lung, gut, and urothelium also produce pore-forming toxins that target neutrophils [219]. Staphylococcus aureus and Streptococcus pyogenes are microorganisms that colonize the extra-articular mucosa and produce Panton-Valentine leukocidin (PVL) and streptolysin O (SLO), respectively, which could induce the citrullination of neutrophils [67,220,221,222].
4. Carbamylation in RA
Carbamylation occurs in the human body under uremic or inflammatory conditions [223,224]. Carbamylation is both an enzymatic and nonenzymatic process of adding the “carbamoyl” part (−CONH2−), which is related to cyanate (−N=C=O), to proteins, or to peptides [225,226,227]. This PTM process leads to the production of homocitrulline or α-carbamyl-protein [226]. Carbamylation is thought to be an enzyme-independent process that typically occurs under uremic conditions [228,229]. Urea dissolved in water spontaneously generates cyanate, which is a carbamylate protein or peptide [230]. However, any inflammation causes oxidation of thiocyanate to produce cyanate, catalyzed by MPO and peroxide, regardless of whether the conditions are uremic or not [231].
Smoking may also induce carbamylation [232], and mononuclear cells of treatment-naïve RA patients show a correlation between autophagy and the level of carbamylation [233]. The carbamylated form of hemoglobin and low-density lipoprotein (LDL), detectable by laboratory tests, is correlated with acute or chronic renal failure and atherosclerosis, respectively [234,235,236,237,238]. Despite these findings, the precise role and effect of carbamylation in RA have not yet been definitively established.
4.1. Anti-Carbamylated Protein Antibodies, a Novel Hallmark for RA
Homocitrulline, which is generated by carbamylation, shows immunogenicity in RA, producing anti-carbamylated protein (anti-CarP) antibodies [239]. Anti-CarP antibodies are detected in RA patients, regardless of ACPA-positivity or ACPA-negativity [240]. Interestingly, anti-CarP antibodies and ACPA demonstrated definitive cross-reactivity in vitro [240]. The sensitivity and specificity of anti-CarP antibodies for RA diagnosis are 44% and 89%, respectively [241].
HLA-DR3 alleles, found at higher levels in ACPA-negative RA patients than in controls, are related to anti-CarP antibody-positive RA without ACPAs [242,243,244,245]. Although IgG levels of anti-CarP antibodies were shown to increase under uremic conditions and smoking in a mouse model, the levels did not increase in heavy smokers or show any association with smoking, or the association became insignificant after correcting for ACPAs in humans [244,246,247,248]. In ACPA-negative RA patients, positivity of anti-CarP antibodies is associated with more erosive manifestation of RA than negativity, independent of RF or ACPA [249,250,251]. Anti-CarP antibodies are also used to screen for those at risk, and the odds ratio is highest when all three autoantibodies (RF, ACPAs, and anti-CarP antibodies) are co-analyzed [252]. The combined presence of ACPAs and anti-CarP antibodies could strongly indicate RA [253].
4.2. Similarity between Anti-CarP Antibodies and ACPAs in RA
Studies have shown that carbamylation plays a role that is like citrullination in RA [249,254,255]. Similar to RF and ACPAs, anti-CarP antibodies show an association with risk when combined with smoking [256]. The presence of RF is associated with positivity for both ACPAs and anti-CarP antibodies [257]. Smoking induces both citrullination and carbamylation. However, there is a lack of evidence that smoking induces the production of autoantibodies; conversely, it correlates with the initiation of intolerance to multiple autoantigens, which may correlate with the overlapping of RF, ACPAs, and anti-CarP antibodies [258]. Specifically, cigarette smoking only induces autoimmunity, which is affected by HLA-DRB1 SE, and evidence does not support the notion that it induces de novo intolerance to citrullinated or carbamylated proteins or peptides [39].
Like ACPAs, anti-CarP antibodies could exist for several years before the onset of RA, and they could increase gradually in quantity just before disease presentation [254,259]. Notably, the presence of both ACPAs and anti-CarP antibodies strongly suggests RA, even if they are not only specific for RA [248]. The presence of each autoantibody is a marker of aggressive joint destruction in RA patients, making it possible to predict progression to RA in arthralgia patients [49,260]. Avidity of ACPAs and anti-CarP antibodies are lower than other antibodies to usual recall antigen; nevertheless, ACPAs and anti-CarP antibodies, composed of broad isotypes and subclasses (such as IgM, IgA, and IgG subclasses), undergo isotype-switching to diminish their avidity more [261].
5. Citrullination and Carbamylation in Other Disease
Published data suggest that smoking and periodontitis due to P. gingivalis are risk factors for cardiovascular disease [262]. Citrullinated proteins and PAD4 were detected in the atherosclerotic plaques in individuals without RA [263], and the ACPAs in RA patients can target these proteins from atherosclerotic plaques [264]. According to Hermans et al. [265], ST-segment elevation myocardial infarction (STEMI) is associated with ACPA. Citrullination is also associated with neurodegenerative diseases, cancers [105,144], and type 1 diabetes [266]. Citrullination of MBP in the CNS is correlated with the onset of MS [267,268], and it causes other autoimmune diseases, such as systemic lupus erythematosus (SLE) and autoimmune encephalomyelitis [268,269]. Because PAD and citrullination are involved in transcription, NET formation, and cell signaling, the connection between malignancy and citrullination has also been suggested [134,144].
Carbamylation is related to cardiovascular diseases [264] and plays a role as a pro-atherosclerotic activator by accumulating cholesterol in macrophages, inducing endothelial apoptosis, and increasing scavenger receptor recognition [231]. Cataracts are induced by carbamylation of α-crystallin [227]. Usually, the carbamylation of protein hormones downregulates the function of hormones; however, carbamylation of uncommon residues occasionally exhibits different effects on the intensity of downregulation, such as the A-chain of insulin, oxytocin, and arginine-vasopressin [270,271,272].
In the uremic status of end-stage renal disease (ESRD), the lysine residue of erythropoietin (EPO) is carbamylated and decreases in activity, resulting in the production of non-functional EPO, which leads to hypoxemia [273,274]. Patients with inflammatory bowel disease (IBD) showed significant differences in the proportion that presented with serum anti-CarP antibodies compared with normal controls [248]. Carbamylated LDL (c-LDL) shows low affinity for its receptor, which decreases the clearance rate in rabbits [275,276]. c-LDL induces the accumulation of cholesterol and the formation of foam cells and signals for inflammation [277], and it facilitates monocyte adhesion to the endothelium and vascular smooth muscle to enhance their proliferation [278].
6. Acetylation, Another Autoantibody-Inducing PTM Process
Acetylation, which normally occurs in the human body through both co-translation and post-translation [279,280], has two distinct pathways through which an acetyl group donated by acetyl-coenzyme A is attached to either the N-terminus of proteins or lysine residues [281]. Acetylation is a reversible enzymatic process catalyzed by various N-terminal and lysine acetyltransferases [281,282]. Similar to carbamylation, acetylation can modify lysine residues, change the molecular features of proteins or peptides, and induce the production of modified epitopes [160].
In addition, acetylated proteins can induce the production of anti-acetylated protein antibodies (AAPAs) [160]. In seronegative RA patients, acetylation of histones, a PAD-independent process, showed cross-reactivity with ACPA [283]. Kampstra et al. [284] showed that autoantibodies to PTM molecules, called anti-modified protein antibodies (AMPAs), which include ACPAs, anti-CarP antibodies, and AAPAs, could be part of a single concept because they share partial cross-reactivity with each post-translated autoantigen. Su et al. [285] showed that the dysregulation of acetylation attenuates the development of RA by downregulating FOXP3 expression.
7. Conclusions
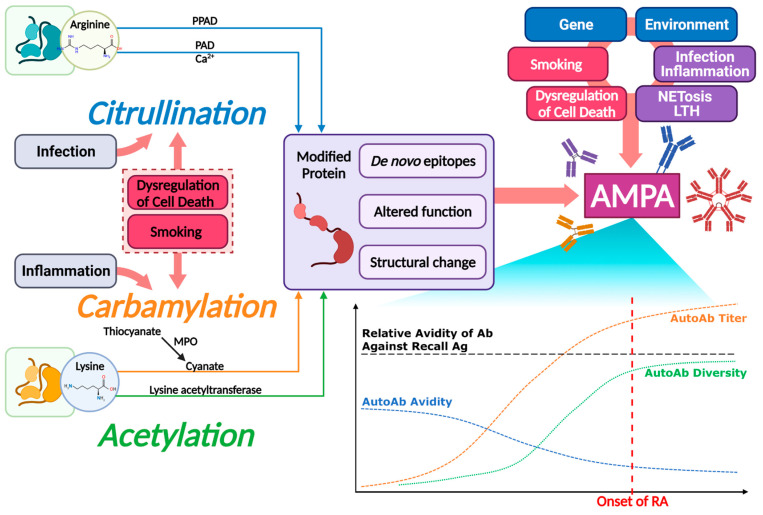
Figure 2 shows a schema that illustrates posttranslational modifications (PTMs) and anti-modified protein antibodies (AMPAs) in RA pathogenesis. Citrullination and carbamylation are reported in a wide range of inflammatory tissues and, therefore, are considered as markers of inflammation rather than specific disease-dependent processes. PAD, which mediates citrullination, has five isoenzymes, including some with the potential to autocitrullinate. PPAD is an exogenous PAD independent of Ca2+ and pH. Acetylation is also involved in autoimmunogenicity and the production of autoantibodies in RA. Both carbamylation and acetylation have a common target of lysine residues, but the results of each PTM have distinguishable characteristics.
Figure 2.
Schematic illustration of roles of posttranslational modifications (PTMs) and anti-modified protein antibodies (AMPAs) in rheumatoid arthritis (RA) pathogenesis. Citrullination, carbamylation, and acetylation self-modify proteins to generate autoantigenicity. Autoantigens induce the production of AMPAs. In pre-clinical stage of RA, the production of AMPAs is accompanied by the remodeling of antibodies. Simultaneously, AMPAs interact with other pathogenic factors of RA and undergoes isotype switching. Remodeling of autoantibodies lower the avidity of AMPAs, paradoxically heightening autoimmunogenicity. Titer and diversity of AMPAs gradually increase, driving the development of RA. All values in the graph are qualitative. Abbreviations: PAD, peptidylarginine deiminase; PPAD, PAD of Porphyromonas gingivalis; MPO, myeloperoxidase; LTH, leukotoxic hypercitrullination; Ab, antibody; Ag, antigen; AutoAb, autoantibody. Created with Biorender.com.
PTMs, including citrullination, carbamylation, and acetylation alone, are not sufficient to induce intolerance and autoimmunity, but this does not negate their importance. Because RA is caused by complex interactions between multiple pathogenic factors, the role of these PTMs is as significant as that of other pathogenic factors. PTMs are also correlated with one or more pulmonary, cardiovascular, neurodegenerative, malignant, or other autoimmune diseases, other than RA.
AMPA is closely related to the pathogenesis, prediction, diagnosis, and prognosis of RA, whereas smoking induces PTMs and interacts with AMPA to enhance the risk of RA development. Cell death mechanisms, including apoptosis, autophagy, NETosis, LTH, and necrosis induce or present PTM autoantigens, generating AMPAs. Some microbes such as P. gingivalis can cause self-citrullination of molecules to produce de novo epitopes, and A. actinomycetemcomitans uses LtxA to induce LTH in citrullinated neutrophils.
Abbreviations
| AAPAs | Anti-acetylated protein antibodies |
| Ab | Antibody |
| ACPAs | Anti-citrullinated protein/peptide antibodies |
| ACR | American College of Rheumatology |
| Ab | Antibody |
| Ag | Antigen |
| AMPAs | Anti-modified protein antibodies |
| Anti-CarP | Anti-carbamylated protein |
| Anti-MCV | Anti-mutated citrullinated vimentin antibodies |
| APC | Antigen presenting cell |
| Apo E | Apolipoprotein E |
| AutoAb | Autoantibody |
| AutoAg | Autoantigen |
| BIP | Immunoglobulin binding protein |
| BMI | Body mass index |
| CD | Cluster of differentiation |
| CD4+ T cell | Cluster of differentiation 4 positive T cell |
| CD40L | Cluster of differentiation 4 ligand |
| CEP-1 | Citrullinated enolase peptide-1 |
| c-LDL | Carbamylated LDL |
| CNS | Central nervous system |
| COPD | Chronic obstructive pulmonary disease |
| CRP | C-reactive protein |
| CTLA-4 | Cytotoxic T lymphocyte antigen 4 |
| DKK-1 | Dickkopf-1 |
| DMARDs | Disease-modifying antirheumatic drugs |
| EBV | Epstein–Barr virus |
| ECM | Extracellular matrix |
| EGF | Epidermal growth factor |
| EPO | Erythropoietin |
| ER | Endoplasmic reticulum |
| ERK | Extracellular signal-regulated kinase |
| ESR | Erythrocyte sedimentation rate |
| ESRD | End stage renal disease |
| EULAR | European League Against Rheumatism |
| Fc | Constant fragment |
| FLNA | Filamin A |
| FLS | Fibroblast-like synoviocyte |
| FOXP3 | Forkhead box P3 |
| FUSE-BP | Far upstream element-binding proteins |
| GM-CSF | Granulocyte-macrophage colony-stimulating factor |
| GNS | N-acetylglucosamine-6-sulfatase |
| GWAS | Genome-wide association studies |
| HIP-1 | Huntingtin-interacting protein-1 |
| HLA | Human leukocyte antigen |
| IBD | Inflammatory bowel disease |
| IC | Immune complex |
| IFN-γ | Interferon-γ |
| Ig | Immunoglobulin |
| IKK | Inhibitor of NF-κB kinase |
| IL | Interleukin |
| IP | Interphalangeal joint |
| IRAK1 | Interleukin-1 receptor-associated kinase 1 |
| ITP | Immune thrombocytopenia |
| IV | Intravenous |
| JAK | Janus kinase |
| JNK | c-Jun N-terminal kinase |
| LDL | Low-density lipoprotein |
| LTH | Leukotoxic hypercitrullination |
| LtxA | Leukotoxin A |
| MAC | Membrane attack complex |
| MAPKs | Mitogen-activated protein kinases |
| MCP | Metacarpophalangeal joint |
| MHC | Major histocompatibility complex |
| MMP | Matrix metalloproteinase |
| MNDA | Myeloid nuclear differentiation antigen |
| MPO | Myeloperoxidase |
| MS | Multiple sclerosis |
| MTP | Metatarsophalangeal joint |
| NADPH | Nicotinamide adenine dinucleotide phosphate |
| NET | Neutrophil extracellular trap |
| NF | Nuclear factor |
| NTZ | Nitazoxanide |
| OA | Osteoarthritis |
| OB | Osteoblast |
| OC | Osteoclast |
| PAD | Peptidylarginine deiminase |
| pI | Iso-electric point |
| PIP | Proximal interphalangeal joint |
| PM | Particulate matter |
| PNS | Peripheral nervous system |
| PO | per os (orally) |
| PPAD | PAD of Porphyromonas gingivalis |
| PTM | Posttranslational modification |
| PTPN22 | Protein tyrosine phosphatase N22 |
| PVL | Panton–Valentine leukocidin |
| RA | Rheumatoid arthritis |
| RA-SF | Synovial fluid of rheumatoid arthritis patients |
| RF | Rheumatoid factor |
| SC | Subcutaneous |
| SE | Shared epitope |
| SF | Synovial fluid |
| SLE | Systemic lupus erythematosus |
| SLO | Streptolysin O |
| SNP | Single-nucleotide polymorphism |
| STAT | Signal transducers and activators of transcription |
| STEMI | ST-segment elevation myocardial infarction |
| Syk | Spleen tyrosine kinase |
| TCR | T cell receptor |
| Teff cell | Effector T cells |
| TGF-β | Transforming growth factor-β |
| TH1 cell | Helper T1 cell |
| TH17 cell | Helper T17 cell |
| TLR | Toll-like receptor |
| TNF | Tumor necrosis factor |
| TRAF1-C5 | TNF receptor associated factor 1-C5 |
| TReg cell | Regulatory T cell |
| Tyk | Tyrosine kinase |
| ULN | Upper limit of normal |
| FDA | US Food and Drug Administration |
Author Contributions
E.-J.K. wrote the original draft and edited and reviewed the final version of the manuscript. J.H.J. edited and reviewed the original draft and final version of the manuscript. Both authors have read and agreed to the version of the manuscript to be published.
Funding
This research was supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (Grant number: HI20C0495, HI16C2177), and by a National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT, Nos. NRF-2020R1A2C3004123 and NRF-2019R1A5A2027588).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.McInnes I.B., Schett G. The pathogenesis of rheumatoid arthritis. N. Engl. J. Med. 2011;365:2205–2219. doi: 10.1056/NEJMra1004965. [DOI] [PubMed] [Google Scholar]
- 2.Testa D., Calvacchi S., Petrelli F., Giannini D., Bilia S., Alunno A., Puxeddu I. One year in review 2021: Pathogenesis of rheumatoid arthritis. Clin. Exp. Rheumatol. 2021;39:445–452. [PubMed] [Google Scholar]
- 3.Shah A., Clair E.W. Rheumatoid Arthritis. In: Jameson J.L., Fauci A.S., Kasper D.L., Hauser S.L., Longo D.L., Loscalzo J., editors. Harrison’s Principles of Internal Medicine, 20e. McGraw-Hill Education; New York, NY, USA: 2018. [Google Scholar]
- 4.England B.R., Hershberger D. Management issues in rheumatoid arthritis-associated interstitial lung disease. Curr. Opin. Rheumatol. 2020;32:255–263. doi: 10.1097/BOR.0000000000000703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Conforti A., Di Cola I., Pavlych V., Ruscitti P., Berardicurti O., Ursini F., Giacomelli R., Cipriani P. Beyond the joints, the extra-articular manifestations in rheumatoid arthritis. Autoimmun. Rev. 2020;20:102735. doi: 10.1016/j.autrev.2020.102735. [DOI] [PubMed] [Google Scholar]
- 6.Grassi W., De Angelis R., Lamanna G., Cervini C. The clinical features of rheumatoid arthritis. Eur. J. Radiol. 1998;27:S18–S24. doi: 10.1016/S0720-048X(98)00038-2. [DOI] [PubMed] [Google Scholar]
- 7.Lin Y.-J., Anzaghe M., Schülke S. Update on the Pathomechanism, Diagnosis, and Treatment Options for Rheumatoid Arthritis. Cells. 2020;9:880. doi: 10.3390/cells9040880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Imagama T., Tokushige A., Seki K., Taguchi T. Weight Bearing Joints Destruction In Rheumatoid Arthritis. Curr. Rheumatol. Rev. 2017;13:37–42. doi: 10.2174/1573397112666160331142548. [DOI] [PubMed] [Google Scholar]
- 9.Nell V.P.K., Machold K., Eberl G., Stamm T.A., Uffmann M., Smolen J.S. Benefit of very early referral and very early therapy with disease-modifying anti-rheumatic drugs in patients with early rheumatoid arthritis. Rheumatology. 2004;43:906–914. doi: 10.1093/rheumatology/keh199. [DOI] [PubMed] [Google Scholar]
- 10.Kay J., Upchurch K.S. ACR/EULAR 2010 rheumatoid arthritis classification criteria. Rheumatology. 2012;51:vi5–vi9. doi: 10.1093/rheumatology/kes279. [DOI] [PubMed] [Google Scholar]
- 11.Alex A.M., Kunkel G., Sayles H., Arcos J.D.F., Mikuls T.R., Kerr G.S. Exposure to ambient air pollution and autoantibody status in rheumatoid arthritis. Clin. Rheumatol. 2019;39:761–768. doi: 10.1007/s10067-019-04813-w. [DOI] [PubMed] [Google Scholar]
- 12.Nemtsova M.V., Zaletaev D., Bure I.V., Mikhaylenko D., Kuznetsova E.B., Alekseeva E.A., Beloukhova M., Deviatkin A.A., Lukashev A.N., Zamyatnin A. Epigenetic Changes in the Pathogenesis of Rheumatoid Arthritis. Front. Genet. 2019;10:570. doi: 10.3389/fgene.2019.00570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ohno T., Aune D., Heath A.K. Adiposity and the risk of rheumatoid arthritis: A systematic review and meta-analysis of cohort studies. Sci. Rep. 2020;10:1–12. doi: 10.1038/s41598-020-71676-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scott D.L., Wolfe F., Huizinga T.W. Rheumatoid arthritis. Lancet. 2010;376:1094–1108. doi: 10.1016/S0140-6736(10)60826-4. [DOI] [PubMed] [Google Scholar]
- 15.Alamanos Y., Voulgari P.V., Drosos A.A. Incidence and Prevalence of Rheumatoid Arthritis, Based on the 1987 American College of Rheumatology Criteria: A Systematic Review. Semin. Arthritis Rheum. 2006;36:182–188. doi: 10.1016/j.semarthrit.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 16.Greenberg J.D., Spruill T.M., Shan Y., Reed G., Kremer J.M., Potter J., Yazici Y., Ogedegbe G., Harrold L.R. Racial and Ethnic Disparities in Disease Activity in Patients with Rheumatoid Arthritis. Am. J. Med. 2013;126:1089–1098. doi: 10.1016/j.amjmed.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tobon G., Youinou P., Saraux A. The environment, geo-epidemiology, and autoimmune disease: Rheumatoid arthritis. J. Autoimmun. 2010;35:10–14. doi: 10.1016/j.jaut.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 18.Hughes L.B., Beasley T.M., Patel H., Tiwari H.K., Morgan S.L., Baggott J.E., Saag K.G., McNicholl J., Moreland L.W., Alarcon G.S., et al. Racial or ethnic differences in allele frequencies of single-nucleotide polymorphisms in the methylenetetrahydrofolate reductase gene and their influence on response to methotrexate in rheumatoid arthritis. Ann. Rheum. Dis. 2006;65:1213–1218. doi: 10.1136/ard.2005.046797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.del Rincón I., Battafarano D.F., Arroyo R.A., Murphy F.T., Fischbach M., Escalante A. Ethnic variation in the clinical manifestations of rheumatoid arthritis: Role of HLA–DRB1 alleles. Arthritis Rheum. 2003;49:200–208. doi: 10.1002/art.11000. [DOI] [PubMed] [Google Scholar]
- 20.Viatte S., Plant D., Raychaudhuri S. Genetics and epigenetics of rheumatoid arthritis. Nat. Rev. Rheumatol. 2013;9:141–153. doi: 10.1038/nrrheum.2012.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Diaz-Gallo L.-M., Ramsköld D., Shchetynsky K., Folkersen L., Chemin K., Brynedal B., Uebe S., Okada Y., Alfredsson L., Klareskog L., et al. Systematic approach demonstrates enrichment of multiple interactions between non-HLA risk variants and HLA-DRB1 risk alleles in rheumatoid arthritis. Ann. Rheum. Dis. 2018;77:1454–1462. doi: 10.1136/annrheumdis-2018-213412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Raychaudhuri S., Sandor C., Stahl E.A., Freudenberg J., Lee H.-S., Jia X., Alfredsson L., Padyukov L., Klareskog L., Worthington J., et al. Five amino acids in three HLA proteins explain most of the association between MHC and seropositive rheumatoid arthritis. Nat. Genet. 2012;44:291–296. doi: 10.1038/ng.1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao M., Mauer L., Sayles H., Cannon G.W., Reimold A., Kerr G.S., Baker J.F., Thiele G.M., England B.R., Mikuls T.R. HLA-DRB1 Haplotypes, Shared Epitope, and Disease Outcomes in US Veterans with Rheumatoid Arthritis. J. Rheumatol. 2019;46:685–693. doi: 10.3899/jrheum.180724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dedmon L.E. The genetics of rheumatoid arthritis. Rheumatology. 2020;59:2661–2670. doi: 10.1093/rheumatology/keaa232. [DOI] [PubMed] [Google Scholar]
- 25.Hiwa R., Ikari K., Ohmura K., Nakabo S., Matsuo K., Saji H., Yurugi K., Miura Y., Maekawa T., Taniguchi A., et al. HLA-DRB1 Analysis Identified a Genetically Unique Subset within Rheumatoid Arthritis and Distinct Genetic Background of Rheumatoid Factor Levels from Anticyclic Citrullinated Peptide Antibodies. J. Rheumatol. 2018;45:470–480. doi: 10.3899/jrheum.170363. [DOI] [PubMed] [Google Scholar]
- 26.O’Dell J.R., Nepom B.S., Haire C., Gersuk V.H., Gaur L., Moore G.F., Drymalski W., Palmer W., Eckhoff P.J., Klassen L.W., et al. HLA-DRB1 typing in rheumatoid arthritis: Predicting response to specific treatments. Ann. Rheum. Dis. 1998;57:209–213. doi: 10.1136/ard.57.4.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abbasifard M., Imani D., Bagheri-Hosseinabadi Z. PTPN22 gene polymorphism and susceptibility to rheumatoid arthritis (RA): Updated systematic review and meta-analysis. J. Gene Med. 2020;22:e3204. doi: 10.1002/jgm.3204. [DOI] [PubMed] [Google Scholar]
- 28.Rizvi S.T.F., Arif A., Azhar A. TNF gene promoter region polymorphisms and association with young-onset rheumatoid arthritis. Pak. J. Pharm. Sci. 2019;32:2295–2297. [PubMed] [Google Scholar]
- 29.Potter C., Eyre S., Cope A., Worthington J., Barton A. Investigation of association between the TRAF family genes and RA susceptibility. Ann. Rheum. Dis. 2007;66:1322–1326. doi: 10.1136/ard.2006.065706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vossenaar E.R., Zendman A.J., van Venrooij W.J., Pruijn G.J. PAD, a growing family of citrullinating enzymes: Genes, features and involvement in disease. BioEssays. 2003;25:1106–1118. doi: 10.1002/bies.10357. [DOI] [PubMed] [Google Scholar]
- 31.Feitsma A.L., Toes R.E., Begovich A.B., Chokkalingam A.P., De Vries R.R.P., Huizinga T.W.J., van der Helm-van Mil A.H. Risk of progression from undifferentiated arthritis to rheumatoid arthritis: The effect of the PTPN22 1858T-allele in anti-citrullinated peptide antibody positive patients. Rheumatology. 2007;46:1092–1095. doi: 10.1093/rheumatology/kem006. [DOI] [PubMed] [Google Scholar]
- 32.Lin C.H., Cho C.L., Tsai W.C., Ou T.T., Wu C.C., Yen J.H., Liu H.W. Inhibitors of kB-like gene polymorphisms in rheumatoid arthritis. Immunol. Lett. 2006;105:193–197. doi: 10.1016/j.imlet.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 33.Croia C., Bursi R., Sutera D., Petrelli F., Alunno A., Puxeddu I. One year in review 2019: Pathogenesis of rheumatoid arthritis. Clin. Exp. Rheumatol. 2019;37:347–357. [PubMed] [Google Scholar]
- 34.Giannini D., Antonucci M., Petrelli F., Bilia S., Alunno A., Puxeddu I. One year in review 2020: Pathogenesis of rheumatoid arthritis. Clin. Exp. Rheumatol. 2020;38:387–397. [PubMed] [Google Scholar]
- 35.Kwon Y.-C., Lim J., Bang S.-Y., Ha E., Hwang M.Y., Yoon K., Choe J.-Y., Yoo D.-H., Lee S.-S., Lee J., et al. Genome-wide association study in a Korean population identifies six novel susceptibility loci for rheumatoid arthritis. Ann. Rheum. Dis. 2020;79:1438–1445. doi: 10.1136/annrheumdis-2020-217663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leng R.-X., Di D.-S., Ni J., Wu X.-X., Zhang L.-L., Wang X.-F., Liu R.-S., Huang Q., Fan Y.-G., Pan H.-F., et al. Identification of new susceptibility loci associated with rheumatoid arthritis. Ann. Rheum. Dis. 2020;79:1565–1571. doi: 10.1136/annrheumdis-2020-217351. [DOI] [PubMed] [Google Scholar]
- 37.Alsalahy M.M., Nasser H.S., Hashem M.M., Elsayed S.M. Effect of tobacco smoking on tissue protein citrullination and disease progression in patients with rheumatoid arthritis. Saudi Pharm. J. 2010;18:75–80. doi: 10.1016/j.jsps.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Klareskog L., Stolt P., Lundberg K., Källberg H., Bengtsson C., Grunewald J., Rönnelid J., Harris H.E., Ulfgren A.-K., Rantapää-Dahlqvist S., et al. A new model for an etiology of rheumatoid arthritis: Smoking may trigger HLA–DR (shared epitope)–restricted immune reactions to autoantigens modified by citrullination. Arthritis Rheum. 2005;54:38–46. doi: 10.1002/art.21575. [DOI] [PubMed] [Google Scholar]
- 39.Hedström A.K., Rönnelid J., Klareskog L., Alfredsson L. Complex Relationships of Smoking, HLA-DRB1 Genes, and Serologic Profiles in Patients With Early Rheumatoid Arthritis: Update From a Swedish Population-Based Case-Control Study. Arthritis Rheumatol. 2019;71:1504–1511. doi: 10.1002/art.40852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Balandraud N., Roudier J. Epstein-Barr virus and rheumatoid arthritis. Jt. Bone Spine. 2018;85:165–170. doi: 10.1016/j.jbspin.2017.04.011. [DOI] [PubMed] [Google Scholar]
- 41.Silman A.J., Pearson J.E. Epidemiology and genetics of rheumatoid arthritis. Arthritis Res. 2002;4((Suppl. 3)):S265–S272. doi: 10.1186/ar578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Newkirk M.M., Goldbach-Mansky R., Senior B.W., Klippel J., Schumacher H.R., El-Gabalawy H.S. Elevated levels of IgM and IgA antibodies to Proteus mirabilis and IgM antibodies to Escherichia coli are associated with early rheumatoid factor (RF)-positive rheumatoid arthritis. Rheumatology. 2005;44:1433–1441. doi: 10.1093/rheumatology/kei036. [DOI] [PubMed] [Google Scholar]
- 43.de Smit M.J., Brouwer E., Vissink A., van Winkelhoff A.J. Rheumatoid arthritis and periodontitis; a possible link via citrullination. Anaerobe. 2011;17:196–200. doi: 10.1016/j.anaerobe.2011.03.019. [DOI] [PubMed] [Google Scholar]
- 44.Maresz K.J., Hellvard A., Sroka A., Adamowicz K., Bielecka E., Koziel J., Gawron K., Mizgalska D., Marcinska K.A., Benedyk M., et al. Porphyromonas gingivalis Facilitates the Development and Progression of Destructive Arthritis through Its Unique Bacterial Peptidylarginine Deiminase (PAD) PLOS Pathog. 2013;9:e1003627. doi: 10.1371/journal.ppat.1003627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pianta A., Arvikar S.L., Strle K., Drouin E.E., Wang Q., Costello C.E., Steere A.C. Two rheumatoid arthritis–specific autoantigens correlate microbial immunity with autoimmune responses in joints. J. Clin. Investig. 2017;127:2946–2956. doi: 10.1172/JCI93450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Valesini G., Gerardi M.C., Iannuccelli C., Pacucci V.A., Pendolino M., Shoenfeld Y. Citrullination and autoimmunity. Autoimmun. Rev. 2015;14:490–497. doi: 10.1016/j.autrev.2015.01.013. [DOI] [PubMed] [Google Scholar]
- 47.Shoda H. Citrullination and rheumatoid arthritis. Nihon Rinsho. Jpn. J. Clin. Med. 2016;74:902–906. [PubMed] [Google Scholar]
- 48.Andrade F., Darrah E., Gucek M., Cole R.N., Rosen A., Zhu X. Autocitrullination of human peptidyl arginine deiminase type 4 regulates protein citrullination during cell activation. Arthritis Rheum. 2010;62:1630–1640. doi: 10.1002/art.27439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kurowska W., Kuca-Warnawin E.H., Radzikowska A., Maśliński W. The role of anti-citrullinated protein antibodies (ACPA) in the pathogenesis of rheumatoid arthritis. Cent. Eur. J. Immunol. 2017;42:390–398. doi: 10.5114/ceji.2017.72807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cascone P., Vetrano S., Nicolai G., Fabiani F. Temporomandibular joint biomechanical restrictions: The fluid and synovial membrane. J. Craniofac. Surg. 1999;10:301–307. doi: 10.1097/00001665-199907000-00003. [DOI] [PubMed] [Google Scholar]
- 51.Barland P., Novikoff A.B., Hamerman D. Electron microscopy of the human synovial membrane. J. Cell Biol. 1962;14:207–220. doi: 10.1083/jcb.14.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li F., Tang Y., Song B., Yu M., Li Q., Zhang C., Hou J., Yang R. Nomenclature clarification: Synovial fibroblasts and synovial mesenchymal stem cells. Stem Cell Res. Ther. 2019;10:1–7. doi: 10.1186/s13287-019-1359-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Falconer J., Murphy A.N., Young S.P., Clark A.R., Tiziani S., Guma M., Buckley C.D. Review: Synovial Cell Metabolism and Chronic Inflammation in Rheumatoid Arthritis. Arthritis Rheumatol. 2018;70:984–999. doi: 10.1002/art.40504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bustamante M.F., Garcia-Carbonell R., Whisenant K.D., Guma M. Fibroblast-like synoviocyte metabolism in the pathogenesis of rheumatoid arthritis. Arthritis Res. 2017;19:1–12. doi: 10.1186/s13075-017-1303-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu H., Zhu Y., Gao Y., Qi D., Zhao L., Zhao L., Liu C., Tao T., Zhou C., Sun X., et al. NR1D1 modulates synovial inflammation and bone destruction in rheumatoid arthritis. Cell Death Dis. 2020;11:1–18. doi: 10.1038/s41419-020-2314-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rana A.K., Li Y., Dang Q., Yang F. Monocytes in rheumatoid arthritis: Circulating precursors of macrophages and osteoclasts and, their heterogeneity and plasticity role in RA pathogenesis. Int. Immunopharmacol. 2018;65:348–359. doi: 10.1016/j.intimp.2018.10.016. [DOI] [PubMed] [Google Scholar]
- 57.Park Y.-J., Yoo S.-A., Kim W.-U. Role of Endoplasmic Reticulum Stress in Rheumatoid Arthritis Pathogenesis. J. Korean Med Sci. 2014;29:2–11. doi: 10.3346/jkms.2014.29.1.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Firestein G.S., Echeverri F., Yeo M., Zvaifler N.J., Green D. Somatic mutations in the p53 tumor suppressor gene in rheumatoid arthritis synovium. Proc. Natl. Acad. Sci. USA. 1997;94:10895–10900. doi: 10.1073/pnas.94.20.10895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kullmann F., Judex M., Neudecker I., Lechner S., Jüsten H.P., Green D., Wessinghage D., Firestein G.S., Gay S., Schölmerich J., et al. Analysis of the p53 tumor suppressor gene in rheumatoid arthritis synovial fibroblasts. Arthritis Rheum. 1999;42:1594–1600. doi: 10.1002/1529-0131(199908)42:8<1594::AID-ANR5>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 60.Yamanishi Y., Boyle D.L., Green D.R., Keystone E.C., Connor A., Zollman S., Firestein G.S. p53 tumor suppressor gene mutations in fibroblast-like synoviocytes from erosion synovium and non-erosion synovium in rheumatoid arthritis. Arthritis Res. Ther. 2005;7:R12–R18. doi: 10.1186/ar1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Baier A., Meineckel I., Gay S., Pap T. Apoptosis in rheumatoid arthritis. Curr. Opin. Rheumatol. 2003;15:274–279. doi: 10.1097/00002281-200305000-00015. [DOI] [PubMed] [Google Scholar]
- 62.Korb A., Pavenstädt H., Pap T. Cell death in rheumatoid arthritis. Apoptosis. 2009;14:447–454. doi: 10.1007/s10495-009-0317-y. [DOI] [PubMed] [Google Scholar]
- 63.Vorobjeva N.V., Chernyak B.V. NETosis: Molecular Mechanisms, Role in Physiology and Pathology. Biochemistry. 2020;85:1178–1190. doi: 10.1134/S0006297920100065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Galluzzi L., Vitale I., Abrams J.M., Alnemri E.S., Baehrecke E.H., Blagosklonny M.V., Dawson T.M., Dawson V., El-Deiry W.S., Fulda S., et al. Molecular definitions of cell death subroutines: Recommendations of the Nomenclature Committee on Cell Death 2012. Cell Death Differ. 2011;19:107–120. doi: 10.1038/cdd.2011.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Brinkmann V., Reichard U., Goosmann C., Fauler B., Uhlemann Y., Weiss D.S., Weinrauch Y., Zychlinsky A. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 66.Cahilog Z., Zhao H., Wu L., Alam A., Eguchi S., Weng H., Ma D. The Role of Neutrophil NETosis in Organ Injury: Novel Inflammatory Cell Death Mechanisms. Inflammation. 2020;43:2021–2032. doi: 10.1007/s10753-020-01294-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Konig M.F., Andrade F. A Critical Reappraisal of Neutrophil Extracellular Traps and NETosis Mimics Based on Differential Requirements for Protein Citrullination. Front. Immunol. 2016;7:461. doi: 10.3389/fimmu.2016.00461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Darrah E., Andrade F. Rheumatoid arthritis and citrullination. Curr. Opin. Rheumatol. 2018;30:72–78. doi: 10.1097/BOR.0000000000000452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Roche P.A., Furuta K. The ins and outs of MHC class II-mediated antigen processing and presentation. Nat. Rev. Immunol. 2015;15:203–216. doi: 10.1038/nri3818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yang W., Chen X., Hu H. CD4+ T-Cell Differentiation In Vitro. Methods Mol. Biol. 2020;2111:91–99. doi: 10.1007/978-1-0716-0266-9_8. [DOI] [PubMed] [Google Scholar]
- 71.Ville S., Poirier N., Blancho G., Vanhove B. Co-Stimulatory Blockade of the CD28/CD80-86/CTLA-4 Balance in Transplantation: Impact on Memory T Cells? Front. Immunol. 2015;6:411. doi: 10.3389/fimmu.2015.00411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Korhonen R., Moilanen E. Abatacept, a novel CD80/86-CD28 T cell co-stimulation modulator, in the treatment of rheumatoid arthritis. Basic Clin. Pharmacol. Toxicol. 2009;104:276–284. doi: 10.1111/j.1742-7843.2009.00375.x. [DOI] [PubMed] [Google Scholar]
- 73.Kamali A.N., Noorbakhsh S.M., Hamedifar H., Jadidi-Niaragh F., Yazdani R., Bautista J.M., Azizi G. A role for Th1-like Th17 cells in the pathogenesis of inflammatory and autoimmune disorders. Mol. Immunol. 2018;105:107–115. doi: 10.1016/j.molimm.2018.11.015. [DOI] [PubMed] [Google Scholar]
- 74.Samuels J., Ng Y.-S., Coupillaud C., Paget D., Meffre E. Human B Cell Tolerance and Its Failure in Rheumatoid Arthritis. Ann. N. Y. Acad. Sci. 2005;1062:116–126. doi: 10.1196/annals.1358.014. [DOI] [PubMed] [Google Scholar]
- 75.Calero I., Nieto J.A., Sanz I. B Cell Therapies for Rheumatoid Arthritis: Beyond B cell Depletion. Rheum. Dis. Clin. N. Am. 2010;36:325–343. doi: 10.1016/j.rdc.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 76.Weyand C.M., Goronzy J.J. The immunology of rheumatoid arthritis. Nat. Immunol. 2020;22:10–18. doi: 10.1038/s41590-020-00816-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bartok B., Firestein G.S. Fibroblast-like synoviocytes: Key effector cells in rheumatoid arthritis. Immunol. Rev. 2009;233:233–255. doi: 10.1111/j.0105-2896.2009.00859.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kim E.K., Kwon J.-E., Lee S.-Y., Lee E.-J., Kim D.S., Moon S.-J., Lee J., Kwok S.-K., Park S.-H., Cho M.-L. IL-17-mediated mitochondrial dysfunction impairs apoptosis in rheumatoid arthritis synovial fibroblasts through activation of autophagy. Cell Death Dis. 2017;8:e2565. doi: 10.1038/cddis.2016.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lubberts E. The IL-23–IL-17 axis in inflammatory arthritis. Nat. Rev. Rheumatol. 2015;11:415–429. doi: 10.1038/nrrheum.2015.53. [DOI] [PubMed] [Google Scholar]
- 80.Pfeifle R., Rothe T., Ipseiz N., Scherer H.U., Culemann S., Harre U., Ackermann J.A., Seefried M., Kleyer A., Uderhardt S., et al. Regulation of autoantibody activity by the IL-23-T(H)17 axis determines the onset of autoimmune disease. Nat. Immunol. 2017;18:104–113. doi: 10.1038/ni.3579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bazzazi H., Aghaei M., Memarian A., Asgarian-Omran H., Behnampour N., Yazdani Y. Th1-Th17 Ratio as a New Insight in Rheumatoid Arthritis Disease. Iran. J. Allergy Asthma Immunol. 2018;17:68–77. [PubMed] [Google Scholar]
- 82.Malemud C.J. The role of the JAK/STAT signal pathway in rheumatoid arthritis. Ther. Adv. Musculoskelet. Dis. 2018;10:117–127. doi: 10.1177/1759720X18776224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Massalska M., Maslinski W., Ciechomska M. Small Molecule Inhibitors in the Treatment of Rheumatoid Arthritis and Beyond: Latest Updates and Potential Strategy for Fighting COVID-19. Cells. 2020;9:1876. doi: 10.3390/cells9081876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fraenkel L., Bathon J.M., England B.R., St. Clair E.W., Arayssi T., Carandang K., Deane K.D., Genovese M., Huston K.K., Kerr G., et al. 2021 American College of Rheumatology Guideline for the Treatment of Rheumatoid Arthritis. Arthritis Rheumatol. 2021;73:1108–1123. doi: 10.1002/art.41752. [DOI] [PubMed] [Google Scholar]
- 85.Kunwar S., Devkota A.R., Ghimire D.K.C. Fostamatinib, an oral spleen tyrosine kinase inhibitor, in the treatment of rheumatoid arthritis: A meta-analysis of randomized controlled trials. Rheumatol. Int. 2016;36:1077–1087. doi: 10.1007/s00296-016-3482-7. [DOI] [PubMed] [Google Scholar]
- 86.Kang Y., Jiang X., Qin D., Wang L., Yang J., Wu A., Huang F., Ye Y., Wu J. Efficacy and Safety of Multiple Dosages of Fostamatinib in Adult Patients With Rheumatoid Arthritis: A Systematic Review and Meta-Analysis. Front. Pharmacol. 2019;10:897. doi: 10.3389/fphar.2019.00897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tanaka Y., Millson D., Iwata S., Nakayamada S. Safety and efficacy of fostamatinib in rheumatoid arthritis patients with an inadequate response to methotrexate in phase II OSKIRA-ASIA-1 and OSKIRA-ASIA-1X study. Rheumatology. 2020;60:2884–2895. doi: 10.1093/rheumatology/keaa732. [DOI] [PubMed] [Google Scholar]
- 88.Malemud C.J. Defective T-Cell Apoptosis and T-Regulatory Cell Dysfunction in Rheumatoid Arthritis. Cells. 2018;7:223. doi: 10.3390/cells7120223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Flores-Borja F., Jury E.C., Mauri C., Ehrenstein M.R. Defects in CTLA-4 are associated with abnormal regulatory T cell function in rheumatoid arthritis. Proc. Natl. Acad. Sci. USA. 2008;105:19396–19401. doi: 10.1073/pnas.0806855105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Radner H., Aletaha D. Anti-TNF in rheumatoid arthritis: An overview. Wien. Med. Wochenschr. 2015;165:3–9. doi: 10.1007/s10354-015-0344-y. [DOI] [PubMed] [Google Scholar]
- 91.Dai Y., Ding J., Yin W., He Y., Yu F., Ye C., Hu S., Yu Y. Increased Autophagy Enhances the Resistance to Tumor Necrosis Factor-Alpha Treatment in Rheumatoid Arthritis Human Fibroblast-Like Synovial Cell. BioMed Res. Int. 2018;2018:4941027. doi: 10.1155/2018/4941027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Noack M., Miossec P. Selected cytokine pathways in rheumatoid arthritis. Semin. Immunopathol. 2017;39:365–383. doi: 10.1007/s00281-017-0619-z. [DOI] [PubMed] [Google Scholar]
- 93.Kaneshiro K., Sakai Y., Suzuki K., Uchida K., Tateishi K., Terashima Y., Kawasaki Y., Shibanuma N., Yoshida K., Hashiramoto A. Interleukin-6 and tumour necrosis factor-α cooperatively promote cell cycle regulators and proliferate rheumatoid arthritis fibroblast-like synovial cells. Scand. J. Rheumatol. 2019;48:353–361. doi: 10.1080/03009742.2019.1602164. [DOI] [PubMed] [Google Scholar]
- 94.Cici D., Corrado A., Rotondo C., Cantatore F.P. Wnt Signaling and Biological Therapy in Rheumatoid Arthritis and Spondyloarthritis. Int. J. Mol. Sci. 2019;20:5552. doi: 10.3390/ijms20225552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Marahleh A., Kitaura H., Ohori F., Kishikawa A., Ogawa S., Shen W.-R., Qi J., Noguchi T., Nara Y., Mizoguchi I. TNF-α Directly Enhances Osteocyte RANKL Expression and Promotes Osteoclast Formation. Front. Immunol. 2019;10:2925. doi: 10.3389/fimmu.2019.02925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kitaura H., Marahleh A., Ohori F., Noguchi T., Shen W.-R., Qi J., Nara Y., Pramusita A., Kinjo R., Mizoguchi I. Osteocyte-Related Cytokines Regulate Osteoclast Formation and Bone Resorption. Int. J. Mol. Sci. 2020;21:5169. doi: 10.3390/ijms21145169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wang T., He C. TNF-α and IL-6: The Link between Immune and Bone System. Curr. Drug Targets. 2020;21:213–227. doi: 10.2174/1389450120666190821161259. [DOI] [PubMed] [Google Scholar]
- 98.Ai R., Laragione T., Hammaker D., Boyle D.L., Wildberg A., Maeshima K., Palescandolo E., Krishna V., Pocalyko D., Whitaker J.W., et al. Comprehensive epigenetic landscape of rheumatoid arthritis fibroblast-like synoviocytes. Nat. Commun. 2018;9:1921. doi: 10.1038/s41467-018-04310-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Laragione T., Brenner M., Lahiri A., Gao E., Harris C., Gulko P.S. Huntingtin-interacting protein 1 (HIP1) regulates arthritis severity and synovial fibroblast invasiveness by altering PDGFR and Rac1 signalling. Ann. Rheum. Dis. 2018;77:1627–1635. doi: 10.1136/annrheumdis-2018-213498. [DOI] [PubMed] [Google Scholar]
- 100.Di D., Zhang L., Wu X., Leng R. Long-term exposure to outdoor air pollution and the risk of development of rheumatoid arthritis: A systematic review and meta-analysis. Semin. Arthritis Rheum. 2019;50:266–275. doi: 10.1016/j.semarthrit.2019.10.005. [DOI] [PubMed] [Google Scholar]
- 101.Chu X.-J., Cao N.-W., Zhou H.-Y., Meng X., Guo B., Zhang H.-Y., Li B.-Z. The oral and gut microbiome in rheumatoid arthritis patients: A systematic review. Rheumatology. 2020;60:1054–1066. doi: 10.1093/rheumatology/keaa835. [DOI] [PubMed] [Google Scholar]
- 102.Ferro M., Charneca S., Dourado E., Guerreiro C.S., Fonseca J.E. Probiotic Supplementation for Rheumatoid Arthritis: A Promising Adjuvant Therapy in the Gut Microbiome Era. Front. Pharmacol. 2021;12 doi: 10.3389/fphar.2021.711788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kishikawa T., Maeda Y., Nii T., Motooka D., Matsumoto Y., Matsushita M., Matsuoka H., Yoshimura M., Kawada S., Teshigawara S., et al. Metagenome-wide association study of gut microbiome revealed novel aetiology of rheumatoid arthritis in the Japanese population. Ann. Rheum. Dis. 2019;79:103–111. doi: 10.1136/annrheumdis-2019-215743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yao Y., Cai X., Fei W., Ren F., Wang F., Luan X., Chen F., Zheng C. Regulating Gut Microbiome: Therapeutic Strategy for Rheumatoid Arthritis During Pregnancy and Lactation. Front. Pharmacol. 2020;11:594042. doi: 10.3389/fphar.2020.594042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.György B., Tóth E., Tarcsa E., Falus A., Buzás E.I. Citrullination: A posttranslational modification in health and disease. Int. J. Biochem. Cell Biol. 2006;38:1662–1677. doi: 10.1016/j.biocel.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 106.Inagaki M., Takahara H., Nishi Y., Sugawara K., Sato C. Ca2+-dependent deimination-induced disassembly of intermediate filaments involves specific modification of the amino-terminal head domain. J. Biol. Chem. 1989;264:18119–18127. doi: 10.1016/S0021-9258(19)84685-9. [DOI] [PubMed] [Google Scholar]
- 107.Orgován G., Noszál B. The complete microspeciation of arginine and citrulline. J. Pharm. Biomed. Anal. 2011;54:965–971. doi: 10.1016/j.jpba.2010.11.023. [DOI] [PubMed] [Google Scholar]
- 108.Carrasco-Marín E., Paz-Miguel J.E., López-Mato P., Alvarez-Domínguez C., Leyva-Cobián F. Oxidation of defined antigens allows protein unfolding and increases both proteolytic processing and exposes peptide epitopes which are recognized by specific T cells. Immunology. 1998;95:314–321. doi: 10.1046/j.1365-2567.1998.00618.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Proost P., Loos T., Mortier A., Schutyser E., Gouwy M., Noppen S., Dillen C., Ronsse I., Conings R., Struyf S., et al. Citrullination of CXCL8 by peptidylarginine deiminase alters receptor usage, prevents proteolysis, and dampens tissue inflammation. J. Exp. Med. 2008;205:2085–2097. doi: 10.1084/jem.20080305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tarcsa E., Marekov L.N., Mei G., Melino G., Lee S.C., Steinert P.M. Protein unfolding by peptidylarginine deiminase. Substrate specificity and structural relationships of the natural substrates trichohyalin and filaggrin. J. Biol. Chem. 1996;271:30709–30716. doi: 10.1074/jbc.271.48.30709. [DOI] [PubMed] [Google Scholar]
- 111.Romero V., Fert-Bober J., Nigrovic P.A., Darrah E., Haque U.J., Lee D.M., Van Eyk J., Rosen A., Andrade F. Immune-Mediated Pore-Forming Pathways Induce Cellular Hypercitrullination and Generate Citrullinated Autoantigens in Rheumatoid Arthritis. Sci. Transl. Med. 2013;5:209ra150. doi: 10.1126/scitranslmed.3006869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ting Y.T., Petersen J., Ramarathinam S.H., Scally S.W., Loh K.L., Thomas R., Suri A., Baker D.G., Purcell A.W., Reid H.H., et al. The interplay between citrullination and HLA-DRB1 polymorphism in shaping peptide binding hierarchies in rheumatoid arthritis. J. Biol. Chem. 2018;293:3236–3251. doi: 10.1074/jbc.RA117.001013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bicker K.L., Thompson P.R. The protein arginine deiminases: Structure, function, inhibition, and disease. Biopolymers. 2012;99:155–163. doi: 10.1002/bip.22127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ottaviano F.G., Handy D.E., Loscalzo J. Redox Regulation in the Extracellular Environment. Circ. J. 2008;72:1–16. doi: 10.1253/circj.72.1. [DOI] [PubMed] [Google Scholar]
- 115.Cordova K.N., Willis V.C., Haskins K., Holers V.M. A Citrullinated Fibrinogen-Specific T Cell Line Enhances Autoimmune Arthritis in a Mouse Model of Rheumatoid Arthritis. J. Immunol. 2013;190:1457–1465. doi: 10.4049/jimmunol.1201517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Brentville V.A., Vankemmelbeke M., Metheringham R.L., Durrant L.G. Post-translational modifications such as citrullination are excellent targets for cancer therapy. Semin. Immunol. 2020;47:101393. doi: 10.1016/j.smim.2020.101393. [DOI] [PubMed] [Google Scholar]
- 117.James E.A., Rieck M., Pieper J., Gebe J.A., Yue B.B., Tatum M., Peda M., Sandin C., Klareskog L., Malmström V., et al. Citrulline-specific Th1 cells are increased in rheumatoid arthritis and their frequency is influenced by disease duration and therapy. Arthritis Rheumatol. 2014;66:1712–1722. doi: 10.1002/art.38637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Cianciotti B.C., Ruggiero E., Campochiaro C., Oliveira G., Magnani Z.I., Baldini M., Doglio M., Tassara M., Manfredi A.A., Baldissera E., et al. CD4+ Memory Stem T Cells Recognizing Citrullinated Epitopes Are Expanded in Patients With Rheumatoid Arthritis and Sensitive to Tumor Necrosis Factor Blockade. Arthritis Rheumatol. 2019;72:565–575. doi: 10.1002/art.41157. [DOI] [PubMed] [Google Scholar]
- 119.Lundberg K., Kinloch A., Fisher B.A., Wegner N., Wait R., Charles P., Mikuls T.R., Venables P.J. Antibodies to citrullinated α-enolase peptide 1 are specific for rheumatoid arthritis and cross-react with bacterial enolase. Arthritis Rheum. 2008;58:3009–3019. doi: 10.1002/art.23936. [DOI] [PubMed] [Google Scholar]
- 120.Makrygiannakis D., Revu S., Engström M., Klint E.A., Nicholas A.P., Pruijn G.J., Catrina A.I. Local administration of glucocorticoids decreases synovial citrullination in rheumatoid arthritis. Arthritis Res. Ther. 2012;14:R20. doi: 10.1186/ar3702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Fisher B.A., Venables P.J. Inhibiting citrullination in rheumatoid arthritis: Taking fuel from the fire. Arthritis Res. Ther. 2012;14:108. doi: 10.1186/ar3740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Milara J., Peiró T., Serrano A., Cortijo J. Epithelial to mesenchymal transition is increased in patients with COPD and induced by cigarette smoke. Thorax. 2013;68:410–420. doi: 10.1136/thoraxjnl-2012-201761. [DOI] [PubMed] [Google Scholar]
- 123.Gerardi M.C., De Luca N., Alessandri C., Iannuccelli C., Valesini G., Di Franco M. Frequency of Antibodies to Mutated Citrullinated Vimentin in Chronic Obstructive Pulmonary Disease: Comment on the Article by Demoruelle et al. Arthritis Rheum. 2013;65:1672–1673. doi: 10.1002/art.37905. [DOI] [PubMed] [Google Scholar]
- 124.Makrygiannakis D., Hermansson M., Ulfgren A.-K., Nicholas A.P., Zendman A.J.W., Eklund A., Grunewald J., Skold C.M., Klareskog L., Catrina A.I. Smoking increases peptidylarginine deiminase 2 enzyme expression in human lungs and increases citrullination in BAL cells. Ann. Rheum. Dis. 2008;67:1488–1492. doi: 10.1136/ard.2007.075192. [DOI] [PubMed] [Google Scholar]
- 125.Damgaard D., Nielsen M.F.B., Gaunsbaek M.Q., Palarasah Y., Svane-Knudsen V., Nielsen C.H. Smoking is associated with increased levels of extracellular peptidylarginine deiminase 2 (PAD2) in the lungs. Clin. Exp. Rheumatol. 2015;33:405–408. [PubMed] [Google Scholar]
- 126.Odoardi F., Sie C., Streyl K., Ulaganathan V.K., Schläger C., Lodygin D., Heckelsmiller K., Nietfeld W., Ellwart J., Klinkert W.E.F., et al. T cells become licensed in the lung to enter the central nervous system. Nature. 2012;488:675–679. doi: 10.1038/nature11337. [DOI] [PubMed] [Google Scholar]
- 127.McGraw W.T., Potempa J., Farley D., Travis J. Purification, Characterization, and Sequence Analysis of a Potential Virulence Factor from Porphyromonas gingivalis, Peptidylarginine Deiminase. Infect. Immun. 1999;67:3248–3256. doi: 10.1128/IAI.67.7.3248-3256.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Quirke A.M., Lugli E.B., Wegner N., Hamilton B.C., Charles P., Chowdhury M., Ytterberg A.J., Zubarev R.A., Potempa J., Culshaw S., et al. Heightened immune response to autocitrullinated Porphyromonas gingivalis peptidylarginine deiminase: A potential mechanism for breaching immunologic tolerance in rheumatoid arthritis. Ann. Rheum. Dis. 2014;73:263–269. doi: 10.1136/annrheumdis-2012-202726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Abdullah S.-N., Farmer E.-A., Spargo L., Logan R., Gully N. Porphyromonas gingivalis peptidylarginine deiminase substrate specificity. Anaerobe. 2013;23:102–108. doi: 10.1016/j.anaerobe.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 130.Olsen I., Singhrao S.K., Potempa J. Citrullination as a plausible link to periodontitis, rheumatoid arthritis, atherosclerosis and Alzheimer’s disease. J. Oral Microbiol. 2018;10:1487742. doi: 10.1080/20002297.2018.1487742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ishida-Yamamoto A., Senshu T., Eady R.A., Takahashi H., Shimizu H., Akiyama M., Iizuka H. Sequential Reorganization of Cornified Cell Keratin Filaments Involving Filaggrin-Mediated Compaction and Keratin 1 Deimination. J. Investig. Dermatol. 2002;118:282–287. doi: 10.1046/j.0022-202x.2001.01671.x. [DOI] [PubMed] [Google Scholar]
- 132.Qin H., Liu X., Li F., Miao L., Li T., Xu B., An X., Muth A., Thompson P.R., Coonrod S.A., et al. PAD1 promotes epithelial-mesenchymal transition and metastasis in triple-negative breast cancer cells by regulating MEK1-ERK1/2-MMP2 signaling. Cancer Lett. 2017;409:30–41. doi: 10.1016/j.canlet.2017.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Young R.A. RNA polymerase II. Annu. Rev. Biochem. 1991;60:689–715. doi: 10.1146/annurev.bi.60.070191.003353. [DOI] [PubMed] [Google Scholar]
- 134.Qu Y., Olsen J.R., Yuan X., Cheng P.F., Levesque M.P., Brokstad K.A., Hoffman P.S., Oyan A.M., Zhang W., Kalland K.-H., et al. Small molecule promotes β-catenin citrullination and inhibits Wnt signaling in cancer. Nat. Chem. Biol. 2017;14:94–101. doi: 10.1038/nchembio.2510. [DOI] [PubMed] [Google Scholar]
- 135.Lee C.-Y., Wang D., Wilhelm M., Zolg D.P., Schmidt T., Schnatbaum K., Reimer U., Pontén F., Uhlén M., Hahne H., et al. Mining the Human Tissue Proteome for Protein Citrullination. Mol. Cell. Proteom. 2018;17:1378–1391. doi: 10.1074/mcp.RA118.000696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Damgaard D., Bawadekar M., Senolt L., Stensballe A., Shelef M.A., Nielsen C.H. Relative efficiencies of peptidylarginine deiminase 2 and 4 in generating target sites for anti-citrullinated protein antibodies in fibrinogen, alpha-enolase and histone H3. PLoS ONE. 2018;13:e0203214. doi: 10.1371/journal.pone.0203214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Rogers G., Winter B., McLaughlan C., Powell B., Nesci T. Peptidylarginine Deiminase of the Hair Follicle: Characterization, Localization, and Function in Keratinizing Tissues. J. Investig. Dermatol. 1997;108:700–707. doi: 10.1111/1523-1747.ep12292083. [DOI] [PubMed] [Google Scholar]
- 138.Guo Q., Fast W. Citrullination of Inhibitor of Growth 4 (ING4) by Peptidylarginine Deminase 4 (PAD4) Disrupts the Interaction between ING4 and p53. J. Biol. Chem. 2011;286:17069–17078. doi: 10.1074/jbc.M111.230961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Stadler S.C., Vincent C.T., Fedorov V.D., Patsialou A., Cherrington B.D., Wakshlag J.J., Mohanan S., Zee B.M., Zhang X., Garcia B.A., et al. Dysregulation of PAD4-mediated citrullination of nuclear GSK3β activates TGF-β signaling and induces epithelial-to-mesenchymal transition in breast cancer cells. Proc. Natl. Acad. Sci. USA. 2013;110:11851–11856. doi: 10.1073/pnas.1308362110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Esposito G., Vitale A.M., Leijten F.P.J., Strik A.M., Koonen-Reemst A.M.C.B., Yurttas P., Robben T.J.A.A., Coonrod S., Gossen J.A. Peptidylarginine deiminase (PAD) 6 is essential for oocyte cytoskeletal sheet formation and female fertility. Mol. Cell. Endocrinol. 2007;273:25–31. doi: 10.1016/j.mce.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 141.Wegner N., Wait R., Sroka A., Eick S., Nguyen K.-A., Lundberg K., Kinloch A., Culshaw S., Potempa J., Venables P.J. Peptidylarginine deiminase from Porphyromonas gingivalis citrullinates human fibrinogen and α-enolase: Implications for autoimmunity in rheumatoid arthritis. Arthritis Rheum. 2010;62:2662–2672. doi: 10.1002/art.27552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Konig M.F., Paracha A.S., Moni M., Bingham C.O., 3rd, Andrade F. Defining the role of Porphyromonas gingivalis peptidylarginine deiminase (PPAD) in rheumatoid arthritis through the study of PPAD biology. Ann. Rheum. Dis. 2015;74:2054–2061. doi: 10.1136/annrheumdis-2014-205385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Witalison E.E., Thompson P.R., Hofseth L.J. Protein Arginine Deiminases and Associated Citrullination: Physiological Functions and Diseases Associated with Dysregulation. Curr. Drug Targets. 2015;16:700–710. doi: 10.2174/1389450116666150202160954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wang S., Wang Y. Peptidylarginine deiminases in citrullination, gene regulation, health and pathogenesis. Biochim. Biophys. Acta Bioenerg. 2013;1829:1126–1135. doi: 10.1016/j.bbagrm.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Slack J.L., Causey C.P., Thompson P.R. Protein arginine deiminase 4: A target for an epigenetic cancer therapy. Experientia. 2010;68:709–720. doi: 10.1007/s00018-010-0480-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Liu X., Wichapong K., Lamers S., Reutelingsperger C.P.M., Nicolaes G.A.F. Autocitrullination of PAD4 does not alter its enzymatic activity: In vitro and in silico studies. Int. J. Biochem. Cell Biol. 2021;134:105938. doi: 10.1016/j.biocel.2021.105938. [DOI] [PubMed] [Google Scholar]
- 147.Laugisch O., Wong A., Sroka A., Kantyka T., Koziel J., Neuhaus K., Sculean A., Venables P.J., Potempa J., Möller B., et al. Citrullination in the periodontium—a possible link between periodontitis and rheumatoid arthritis. Clin. Oral Investig. 2015;20:675–683. doi: 10.1007/s00784-015-1556-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Arévalo-Caro C., Romero-Sánchez C., Garavito-Rodríguez E. Relation between anti-Porphyromonas gingivalis antibody titers and HLA-DRB1 neutral alleles in individuals with rheumatoid arthritis. Acta Odontol. Scand. 2021:1–9. doi: 10.1080/00016357.2021.1959053. [DOI] [PubMed] [Google Scholar]
- 149.Vossenaar E.R., Radstake T.R.D., van der Heijden A., van Mansum M.A.M., Dieteren C., De Rooij D.-J., Barrera P., Zendman A.J.W., van Venrooij W.J. Expression and activity of citrullinating peptidylarginine deiminase enzymes in monocytes and macrophages. Ann. Rheum. Dis. 2004;63:373–381. doi: 10.1136/ard.2003.012211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Vossenaar E.R., Nijenhuis S., Helsen M.M.A., Van Der Heijden A., Senshu T., van den Berg W.B., Van Venrooij W.J., Joosten L.A.B. Citrullination of synovial proteins in murine models of rheumatoid arthritis. Arthritis Rheum. 2003;48:2489–2500. doi: 10.1002/art.11229. [DOI] [PubMed] [Google Scholar]
- 151.Zhao J., Zhao Y., He J., Jia R., Li Z. Prevalence and significance of anti-peptidylarginine deiminase 4 antibodies in rheumatoid arthritis. J. Rheumatol. 2008;35:969–974. [PubMed] [Google Scholar]
- 152.Trouw L.A., Huizinga T.W.J., Toes R.E. Autoimmunity in rheumatoid arthritis: Different antigens—common principles. Ann. Rheum. Dis. 2012;72:ii132–ii136. doi: 10.1136/annrheumdis-2012-202349. [DOI] [PubMed] [Google Scholar]
- 153.Ge C., Xu B., Liang B., Lönnblom E., Lundström S.L., Zubarev R.A., Ayoglu B., Nilsson P., Skogh T., Kastbom A., et al. Structural Basis of Cross-Reactivity of Anti-Citrullinated Protein Antibodies. Arthritis Rheumatol. 2019;71:210–221. doi: 10.1002/art.40698. [DOI] [PubMed] [Google Scholar]
- 154.Li S., Yu Y., Yue Y., Liao H., Xie W., Thai J., Mikuls T.R., Thiele G.M., Duryee M.J., Sayles H., et al. Autoantibodies From Single Circulating Plasmablasts React With Citrullinated Antigens and Porphyromonas gingivalis in Rheumatoid Arthritis. Arthritis Rheumatol. 2015;68:614–626. doi: 10.1002/art.39455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Aggarwal R., Liao K., Nair R., Ringold S., Costenbander K.H. Anti-citrullinated peptide antibody assays and their role in the diagnosis of rheumatoid arthritis. Arthritis Rheum. 2009;61:1472–1483. doi: 10.1002/art.24827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Chatfield S.M., Wicks I.P., Sturgess A.D., Roberts L.J. Anti-citrullinated peptide antibody: Death of the rheumatoid factor? Med. J. Aust. 2009;190:693–695. doi: 10.5694/j.1326-5377.2009.tb02640.x. [DOI] [PubMed] [Google Scholar]
- 157.Rönnelid J., Hansson M., Mathsson-Alm L., Cornillet M., Reed E., Jakobsson P.-J., Alfredsson L., Holmdahl R., Skriner K., Serre G., et al. Anticitrullinated protein/peptide antibody multiplexing defines an extended group of ACPA-positive rheumatoid arthritis patients with distinct genetic and environmental determinants. Ann. Rheum. Dis. 2017;77:203–211. doi: 10.1136/annrheumdis-2017-211782. [DOI] [PubMed] [Google Scholar]
- 158.Nishimura K., Sugiyama D., Kogata Y., Tsuji G., Nakazawa T., Kawano S., Saigo K., Morinobu A., Koshiba M., Kuntz K.M., et al. Meta-analysis: Diagnostic Accuracy of Anti–Cyclic Citrullinated Peptide Antibody and Rheumatoid Factor for Rheumatoid Arthritis. Ann. Intern. Med. 2007;146:797–808. doi: 10.7326/0003-4819-146-11-200706050-00008. [DOI] [PubMed] [Google Scholar]
- 159.Szekanecz Z., Szabo Z., Zeher M., Soós L., Dankó K., Horváth I., Lakos G. Superior performance of the CCP3.1 test compared to CCP2 and MCV in the rheumatoid factor-negative RA population. Immunol. Res. 2013;56:439–443. doi: 10.1007/s12026-013-8425-8. [DOI] [PubMed] [Google Scholar]
- 160.van Delft M.A.M., Huizinga T.W.J. An overview of autoantibodies in rheumatoid arthritis. J. Autoimmun. 2020;110:102392. doi: 10.1016/j.jaut.2019.102392. [DOI] [PubMed] [Google Scholar]
- 161.Kurowska W., Slowinska I., Krogulec Z., Syrowka P., Maslinski W. Antibodies to Citrullinated Proteins (ACPA) Associate with Markers of Osteoclast Activation and Bone Destruction in the Bone Marrow of Patients with Rheumatoid Arthritis. J. Clin. Med. 2021;10:1778. doi: 10.3390/jcm10081778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Van Der Woude D., Syversen S.W., Van der Voort E.I., Verpoort K.N., Goll G.L., Van Der Linden M.P.M., van der Helm-van Mil A.H., Van Der Heijde D.M., Huizinga T.W.J., Kvien T.K., et al. The ACPA isotype profile reflects long-term radiographic progression in rheumatoid arthritis. Ann. Rheum. Dis. 2010;69:1110–1116. doi: 10.1136/ard.2009.116384. [DOI] [PubMed] [Google Scholar]
- 163.Koga T., Okada A., Fukuda T., Hidaka T., Ishii T., Ueki Y., Kodera T., Nakashima M., Takahashi Y., Honda S., et al. Anti-citrullinated peptide antibodies are the strongest predictor of clinically relevant radiographic progression in rheumatoid arthritis patients achieving remission or low disease activity: A post hoc analysis of a nationwide cohort in Japan. PLoS ONE. 2017;12:e0175281. doi: 10.1371/journal.pone.0175281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Arlestig L., Mullazehi M., Kokkonen H., Rocklöv J., Rönnelid J., Dahlqvist S.R. Antibodies against cyclic citrullinated peptides of IgG, IgA and IgM isotype and rheumatoid factor of IgM and IgA isotype are increased in unaffected members of multicase rheumatoid arthritis families from northern Sweden. Ann. Rheum. Dis. 2011;71:825–829. doi: 10.1136/annrheumdis-2011-200668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Ohmi Y., Ise W., Harazono A., Takakura D., Fukuyama H., Baba Y., Narazaki M., Shoda H., Takahashi N., Ohkawa Y., et al. Sialylation converts arthritogenic IgG into inhibitors of collagen-induced arthritis. Nat. Commun. 2016;7:11205. doi: 10.1038/ncomms11205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Scherer H.U., van der Woude D., Ioan-Facsinay A., El Bannoudi H., Trouw L.A., Wang J., Häupl T., Burmester G.-R., Deelder A.M., Huizinga T.W.J., et al. Glycan profiling of anti-citrullinated protein antibodies isolated from human serum and synovial fluid. Arthritis Rheum. 2010;62:1620–1629. doi: 10.1002/art.27414. [DOI] [PubMed] [Google Scholar]
- 167.Rombouts Y., Ewing E., Van De Stadt L.A., Selman M.H.J., Trouw L.A., Deelder A.M., Huizinga T.W.J., Wuhrer M., Van Schaardenburg D., Toes R., et al. Anti-citrullinated protein antibodies acquire a pro-inflammatory Fc glycosylation phenotype prior to the onset of rheumatoid arthritis. Ann. Rheum. Dis. 2013;74:234–241. doi: 10.1136/annrheumdis-2013-203565. [DOI] [PubMed] [Google Scholar]
- 168.Lundström S.L., Fernandes-Cerqueira C., Ytterberg A.J., Ossipova E., Hensvold A.H., Jakobsson P.J., Malmström V., Catrina A.I., Klareskog L., Lundberg K., et al. IgG antibodies to cyclic citrullinated peptides exhibit profiles specific in terms of IgG subclasses, Fc-glycans and a fab-Peptide sequence. PLoS ONE. 2014;9:e113924. doi: 10.1371/journal.pone.0113924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Hafkenscheid L., Bondt A., Scherer H.U., Huizinga T.W.J., Wuhrer M., Toes R., Rombouts Y. Structural Analysis of Variable Domain Glycosylation of Anti-Citrullinated Protein Antibodies in Rheumatoid Arthritis Reveals the Presence of Highly Sialylated Glycans. Mol. Cell. Proteom. 2017;16:278–287. doi: 10.1074/mcp.M116.062919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Shoda H., Fujio K., Shibuya M., Okamura T., Sumitomo S., Okamoto A., Sawada T., Yamamoto K. Detection of autoantibodies to citrullinated BiP in rheumatoid arthritis patients and pro-inflammatory role of citrullinated BiP in collagen-induced arthritis. Arthritis Res. Ther. 2011;13:R191. doi: 10.1186/ar3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Anquetil F., Clavel C., Offer G., Serre G., Sebbag M. IgM and IgA Rheumatoid Factors Purified from Rheumatoid Arthritis Sera Boost the Fc Receptor– and Complement-Dependent Effector Functions of the Disease-Specific Anti–Citrullinated Protein Autoantibodies. J. Immunol. 2015;194:3664–3674. doi: 10.4049/jimmunol.1402334. [DOI] [PubMed] [Google Scholar]
- 172.Kempers A.C., Nejadnik M.R., Rombouts Y., Ioan-Facsinay A., Van Oosterhout M., Jiskoot W., Huizinga T.W.J., Toes R.E.M., Scherer H.U. Fc gamma receptor binding profile of anti-citrullinated protein antibodies in immune complexes suggests a role for FcγRI in the pathogenesis of synovial inflammation. Clin. Exp. Rheumatol. 2018;36:284–293. [PubMed] [Google Scholar]
- 173.Sokolove J., Zhao X., Chandra P.E., Robinson W.H. Immune complexes containing citrullinated fibrinogen costimulate macrophages via Toll-like receptor 4 and Fcγ receptor. Arthritis Rheum. 2010;63:53–62. doi: 10.1002/art.30081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Kempers A.C., Hafkenscheid L., Dorjée A.L., Moutousidou E., van de Bovenkamp F.S., Rispens T., Trouw L.A., van Oosterhout M., Huizinga T.W., Toes R., et al. The extensive glycosylation of the ACPA variable domain observed for ACPA-IgG is absent from ACPA-IgM. Ann. Rheum. Dis. 2018;77:1087–1088. doi: 10.1136/annrheumdis-2017-211533. [DOI] [PubMed] [Google Scholar]
- 175.Rombouts Y., Willemze A., van Beers J.J., Shi J., Kerkman P.F., van Toorn L., Janssen G.M., Zaldumbide A., Hoeben R.C., Pruijn G.J., et al. Extensive glycosylation of ACPA-IgG variable domains modulates binding to citrullinated antigens in rheumatoid arthritis. Ann. Rheum. Dis. 2016;75:578–585. doi: 10.1136/annrheumdis-2014-206598. [DOI] [PubMed] [Google Scholar]
- 176.Hafkenscheid L., De Moel E., Smolik I., Tanner S., Meng X., Jansen B.C., Bondt A., Wuhrer M., Huizinga T.W.J., Toes R.E.M., et al. N-Linked Glycans in the Variable Domain of IgG Anti–Citrullinated Protein Antibodies Predict the Development of Rheumatoid Arthritis. Arthritis Rheumatol. 2019;71:1626–1633. doi: 10.1002/art.40920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Too C.L., Murad S., Hansson M., Alm L.M., Dhaliwal J.S., Holmdahl R., Jakobsson P.-J., Alfredsson L., Klareskog L., Rönnelid J., et al. Differences in the Spectrum of Anti–Citrullinated Protein Antibody Fine Specificities Between Malaysian and Swedish Patients With Rheumatoid Arthritis: Implications for Disease Pathogenesis. Arthritis Rheumatol. 2016;69:58–69. doi: 10.1002/art.39827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Van Beers J.J., Willemze A., Jansen J.J., Engbers G.H., Salden M., Raats J., Drijfhout J.W., van der Helm-van Mil A.H., Toes R.E., Pruijn G.J. ACPA fine-specificity profiles in early rheumatoid arthritis patients do not correlate with clinical features at baseline or with disease progression. Arthritis Res. Ther. 2013;15:R140. doi: 10.1186/ar4322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Masson-Bessiere C., Sebbag M., Girbal-Neuhauser E., Nogueira L., Vincent C., Senshu T., Serre G. The major synovial targets of the rheumatoid arthritis-specific antifilaggrin autoantibodies are deiminated forms of the alpha- and beta-chains of fibrin. J. Immunol. 2001;166:4177–4184. doi: 10.4049/jimmunol.166.6.4177. [DOI] [PubMed] [Google Scholar]
- 180.Ioan-Facsinay A., Willemze A., Robinson D.B., Peschken C.A., Markland J., van der Woude D., Elias B., Ménard H.A., Newkirk M., Fritzler M.J., et al. Marked differences in fine specificity and isotype usage of the anti-citrullinated protein antibody in health and disease. Arthritis Rheum. 2008;58:3000–3008. doi: 10.1002/art.23763. [DOI] [PubMed] [Google Scholar]
- 181.Snir O., Widhe M., Von Spee C., Lindberg J., Padyukov L., Lundberg K., Engström A., Venables P.J., Lundeberg J., Holmdahl R., et al. Multiple antibody reactivities to citrullinated antigens in sera from patients with rheumatoid arthritis: Association with HLA-DRB1 alleles. Ann. Rheum. Dis. 2008;68:736–743. doi: 10.1136/ard.2008.091355. [DOI] [PubMed] [Google Scholar]
- 182.Vossenaar E.R., Després N., Lapointe E., Van Der Heijden A., Lora M., Senshu T., Van Venrooij W.J., Ménard H.A. Rheumatoid arthritis specific anti-Sa antibodies target citrullinated vimentin. Arthritis Res. 2004;6:R142–R150. doi: 10.1186/ar1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Khandpur R., Carmona-Rivera C., Vivekanandan-Giri A., Gizinski A., Yalavarthi S., Knight J.S., Friday S., Li S., Patel R., Subramanian V., et al. NETs Are a Source of Citrullinated Autoantigens and Stimulate Inflammatory Responses in Rheumatoid Arthritis. Sci. Transl. Med. 2013;5:178ra40. doi: 10.1126/scitranslmed.3005580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Roland P.N., Mignot S.G., Bruns A., Hurtado M., Palazzo E., Hayem G., Dieudé P., Meyer O., Martin S.C. Antibodies to mutated citrullinated vimentin for diagnosing rheumatoid arthritis in anti-CCP-negative patients and for monitoring infliximab therapy. Arthritis Res. Ther. 2008;10:R142. doi: 10.1186/ar2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Kinloch A., Tatzer V., Wait R., Peston D., Lundberg K., Donatien P., Moyes D., Taylor P.C., Venables P.J. Identification of citrullinated α-enolase as a candidate autoantigen in rheumatoid arthritis. Arthritis Res. 2005;7:R1421–R1429. doi: 10.1186/ar1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Lee J.Y., Kang M.J., Choi J.Y., Park J.S., Park J.K., Lee E.Y., Lee E.B., Pap T., Yi E.C., Song Y.W. Apolipoprotein B binds to enolase-1 and aggravates inflammation in rheumatoid arthritis. Ann. Rheum. Dis. 2018;77:1480–1489. doi: 10.1136/annrheumdis-2018-213444. [DOI] [PubMed] [Google Scholar]
- 187.Sofat N., Wait R., Robertson S.D., Baines D.L., Baker E.H. Interaction between extracellular matrix molecules and microbial pathogens: Evidence for the missing link in autoimmunity with rheumatoid arthritis as a disease model. Front. Microbiol. 2015;5:783. doi: 10.3389/fmicb.2014.00783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Van Beers J.J., Willemze A., Stammen-Vogelzangs J., Drijfhout J.W., Toes R.E., Pruijn G.J.M. Anti-citrullinated fibronectin antibodies in rheumatoid arthritis are associated with human leukocyte antigen-DRB1 shared epitope alleles. Arthritis Res. Ther. 2012;14:R35. doi: 10.1186/ar3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Kuusela P., Ruoslahti E., Engvall E., Vaheri A. Immunological interspecies cross-reactions of fibroblast surface antigen (fibronectin) Immunochemistry. 1976;13:639–642. doi: 10.1016/0019-2791(76)90203-2. [DOI] [PubMed] [Google Scholar]
- 190.Johansson L., Pratesi F., Brink M., Arlestig L., D’Amato C., Bartaloni D., Migliorini P., Rantapa-Dahlqvist S. Antibodies directed against endogenous and exogenous citrullinated antigens pre-date the onset of rheumatoid arthritis. Arthritis Res. 2016;18:1–11. doi: 10.1186/s13075-016-1031-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Meng X., Ezzati P., Smolik I., Bernstein C.N., Hitchon C.A., El-Gabalawy H.S. Characterization of Autoantigens Targeted by Anti-Citrullinated Protein Antibodies In Vivo: Prominent Role for Epitopes Derived from Histone 4 Proteins. PLoS ONE. 2016;11:e0165501. doi: 10.1371/journal.pone.0165501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Panayi G.S., Corrigall V.M. Immunoglobulin heavy-chain-binding protein (BiP): A stress protein that has the potential to be a novel therapy for rheumatoid arthritis. Biochem. Soc. Trans. 2014;42:1752–1755. doi: 10.1042/BST20140230. [DOI] [PubMed] [Google Scholar]
- 193.Schwenzer A., Jiang X., Mikuls T.R., Payne J., Sayles H.R., Quirke A.-M., Kessler B., Fischer R., Venables P.J., Lundberg K., et al. Identification of an immunodominant peptide from citrullinated tenascin-C as a major target for autoantibodies in rheumatoid arthritis. Ann. Rheum. Dis. 2015;75:1876–1883. doi: 10.1136/annrheumdis-2015-208495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Cutolo M., Soldano S., Paolino S. Potential roles for tenascin in (very) early diagnosis and treatment of rheumatoid arthritis. Ann. Rheum. Dis. 2019;79:e42. doi: 10.1136/annrheumdis-2019-215063. [DOI] [PubMed] [Google Scholar]
- 195.Union A., Meheus L., Humbel R.L., Conrad K., Steiner G., Moereels H., Pottel H., Serre G., De Keyser F. Identification of citrullinated rheumatoid arthritis-specific epitopes in natural filaggrin relevant for antifilaggrin autoantibody detection by line immunoassay. Arthritis Rheum. 2002;46:1185–1195. doi: 10.1002/art.10229. [DOI] [PubMed] [Google Scholar]
- 196.Schellekens G.A., De Jong B.A., van den Hoogen F.H., Van De Putte L.B., Van Venrooij W.J. Citrulline is an essential constituent of antigenic determinants recognized by rheumatoid arthritis-specific autoantibodies. J. Clin. Investig. 1998;101:273–281. doi: 10.1172/JCI1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Van Beers J.J.B.C., Schwarte C.M., Stammen-Vogelzangs J., Oosterink E., Božič B., Pruijn G.J.M. The rheumatoid arthritis synovial fluid citrullinome reveals novel citrullinated epitopes in apolipoprotein E, myeloid nuclear differentiation antigen, and β-actin. Arthritis Rheum. 2012;65:69–80. doi: 10.1002/art.37720. [DOI] [PubMed] [Google Scholar]
- 198.Vogt L.M., Kwasniewicz E., Talens S., Scavenius C., Bielecka E., Ekdahl K.N., Enghild J.J., Mörgelin M., Saxne T., Potempa J., et al. Apolipoprotein E Triggers Complement Activation in Joint Synovial Fluid of Rheumatoid Arthritis Patients by Binding C1q. J. Immunol. 2020;204:2779–2790. doi: 10.4049/jimmunol.1900372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.De Bont C.M., Eerden N., Boelens W.C., Pruijn G.J.M., Bont C.M. Neutrophil proteases degrade autoepitopes of NET-associated proteins. Clin. Exp. Immunol. 2019;199:1–8. doi: 10.1111/cei.13392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Goëb V., Thomas-L’Otellier M., Daveau R., Charlionet R., Fardellone P., Le Loët X., Tron F., Gilbert D., Vittecoq O. Candidate autoantigens identified by mass spectrometry in early rheumatoid arthritis are chaperones and citrullinated glycolytic enzymes. Arthritis Res. Ther. 2009;11:R38. doi: 10.1186/ar2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Konig M.F., Giles J.T., Nigrovic P.A., Andrade F. Antibodies to native and citrullinated RA33 (hnRNP A2/B1) challenge citrullination as the inciting principle underlying loss of tolerance in rheumatoid arthritis. Ann. Rheum. Dis. 2016;75:2022–2028. doi: 10.1136/annrheumdis-2015-208529. [DOI] [PubMed] [Google Scholar]
- 202.Willemze A., Shi J., Mulder M., Stoeken-Rijsbergen G., Drijfhout J.W., Huizinga T.W.J., Trouw L.A., Toes R.E. The concentration of anticitrullinated protein antibodies in serum and synovial fluid in relation to total immunoglobulin concentrations. Ann. Rheum. Dis. 2013;72:1059–1063. doi: 10.1136/annrheumdis-2012-202747. [DOI] [PubMed] [Google Scholar]
- 203.Lu M.C., Lai N.S., Yin W.Y., Yu H.C., Huang H.B., Tung C.H., Huang K.Y., Yu C.L. Anti-citrullinated protein antibodies activated ERK1/2 and JNK mitogen-activated protein kinases via binding to surface-expressed citrullinated GRP78 on mononuclear cells. J. Clin. Immunol. 2013;33:558–566. doi: 10.1007/s10875-012-9841-6. [DOI] [PubMed] [Google Scholar]
- 204.Chirivi R.G.S., van Rosmalen J.W.G., van der Linden M., Euler M., Schmets G., Bogatkevich G., Kambas K., Hahn J., Braster Q., Soehnlein O., et al. Therapeutic ACPA inhibits NET formation: A potential therapy for neutrophil-mediated inflammatory diseases. Cell. Mol. Immunol. 2020;18:1528–1544. doi: 10.1038/s41423-020-0381-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Won P., Kim Y., Jung H., Rim Y.A., Sohn D.H., Robinson W.H., Moon S.-J., Ju J.H. Pathogenic Role of Circulating Citrullinated Antigens and Anti-Cyclic Monoclonal Citrullinated Peptide Antibodies in Rheumatoid Arthritis. Front. Immunol. 2021;12:2389. doi: 10.3389/fimmu.2021.692242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Taylor R.C., Cullen S.P., Martin S.J. Apoptosis: Controlled demolition at the cellular level. Nat. Rev. Mol. Cell Biol. 2008;9:231–241. doi: 10.1038/nrm2312. [DOI] [PubMed] [Google Scholar]
- 207.Alghamdi M., Alasmari D., Assiri A., Mattar E., AlJaddawi A.A., Alattas S.G., Redwan E.M. An Overview of the Intrinsic Role of Citrullination in Autoimmune Disorders. J. Immunol. Res. 2019;2019:1–39. doi: 10.1155/2019/7592851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Blachère N.E., Parveen S., Fak J., Frank M.O., Orange D.E. Inflammatory but not apoptotic death of granulocytes citrullinates fibrinogen. Arthritis Res. Ther. 2015;17:1–8. doi: 10.1186/s13075-015-0890-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Spengler J., Lugonja B.A., Ytterberg A.J., Zubarev R., Creese A.J., Pearson M.J., Grant M.M., Milward M., Lundberg K., Buckley C.D., et al. Release of Active Peptidyl Arginine Deiminases by Neutrophils Can Explain Production of Extracellular Citrullinated Autoantigens in Rheumatoid Arthritis Synovial Fluid. Arthritis Rheumatol. 2015;67:3135–3145. doi: 10.1002/art.39313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Pratesi F., Dioni I., Tommasi C., Alcaro M.C., Paolini I., Barbetti F., Boscaro F., Panza F., Puxeddu I., Rovero P., et al. Antibodies from patients with rheumatoid arthritis target citrullinated histone 4 contained in neutrophils extracellular traps. Ann. Rheum. Dis. 2013;73:1414–1422. doi: 10.1136/annrheumdis-2012-202765. [DOI] [PubMed] [Google Scholar]
- 211.Tegla C.A., Cudrici C., Patel S., Trippe R., 3rd, Rus V., Niculescu F., Rus H. Membrane attack by complement: The assembly and biology of terminal complement complexes. Immunol. Res. 2011;51:45–60. doi: 10.1007/s12026-011-8239-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Podack E.R., Hengartner H., Lichtenheld M.G. A central role of perforin in cytolysis? Annu. Rev. Immunol. 1991;9:129–157. doi: 10.1146/annurev.iy.09.040191.001021. [DOI] [PubMed] [Google Scholar]
- 213.Morgan P., Luzio J.P., Campbell A.K. Intracellular Ca2+ and cell injury: A paradoxical role of Ca2+ in complement membrane attack. Cell Calcium. 1986;7:399–411. doi: 10.1016/0143-4160(86)90042-4. [DOI] [PubMed] [Google Scholar]
- 214.Douda D.N., Khan M.A., Grasemann H., Palaniyar N. SK3 channel and mitochondrial ROS mediate NADPH oxidase-independent NETosis induced by calcium influx. Proc. Natl. Acad. Sci. USA. 2015;112:2817–2822. doi: 10.1073/pnas.1414055112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Parker H., Dragunow M., Hampton M.B., Kettle T., Winterbourn C.C. Requirements for NADPH oxidase and myeloperoxidase in neutrophil extracellular trap formation differ depending on the stimulus. J. Leukoc. Biol. 2012;92:841–849. doi: 10.1189/jlb.1211601. [DOI] [PubMed] [Google Scholar]
- 216.Konig M.F., Abusleme L., Reinholdt J., Palmer R.J., Teles R.P., Sampson K., Rosen A., Nigrovic P.A., Sokolove J., Giles J.T., et al. Aggregatibacter actinomycetemcomitans-induced hypercitrullination links periodontal infection to autoimmunity in rheumatoid arthritis. Sci. Transl. Med. 2016;8:369ra176. doi: 10.1126/scitranslmed.aaj1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Nagasawa T., Kato S., Furuichi Y. Evaluation of the Virulence of Aggregatibacter actinomycetemcomitans Through the Analysis of Leukotoxin. Methods Mol. Biol. 2020;2210:185–193. doi: 10.1007/978-1-0716-0939-2_18. [DOI] [PubMed] [Google Scholar]
- 218.Vega B.A., Schober L.T., Kim T., Belinka B.A., Kachlany S.C. Aggregatibacter actinomycetemcomitans Leukotoxin (LtxA) Requires Death Receptor Fas, in Addition to LFA-1, To Trigger Cell Death in T Lymphocytes. Infect. Immun. 2019;87:e00309-19. doi: 10.1128/IAI.00309-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Brusca S.B., Abramson S.B., Scher J.U. Microbiome and mucosal inflammation as extra-articular triggers for rheumatoid arthritis and autoimmunity. Curr. Opin. Rheumatol. 2014;26:101–107. doi: 10.1097/BOR.0000000000000008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Dal Peraro M., van der Goot F.G. Pore-forming toxins: Ancient, but never really out of fashion. Nat. Rev. Microbiol. 2016;14:77–92. doi: 10.1038/nrmicro.2015.3. [DOI] [PubMed] [Google Scholar]
- 221.Los F.C.O., Randis T.M., Aroian R.V., Ratner A. Role of Pore-Forming Toxins in Bacterial Infectious Diseases. Microbiol. Mol. Biol. Rev. 2013;77:173–207. doi: 10.1128/MMBR.00052-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Grace L.E., Bukhari M., Lauder R.M., Bishop L.A., Taylor A.M. AB0097 The Presence of Staphylococcal Toxins in The Urine of Patients with Rheumatoid Arthritis. Ann. Rheum. Dis. 2016;75:930. doi: 10.1136/annrheumdis-2016-eular.1663. [DOI] [Google Scholar]
- 223.Pavone B., Sirolli V., Giardinelli A., Bucci S., Forlì F., Di Cesare M., Sacchetta P., Di Pietro N., Pandolfi A., Urbani A., et al. Plasma protein carbonylation in chronic uremia. J. Nephrol. 2011;24:453–464. doi: 10.5301/JN.2011.8342. [DOI] [PubMed] [Google Scholar]
- 224.Kalim S., Karumanchi S.A., Thadhani R.I., Berg A.H. Protein Carbamylation in Kidney Disease: Pathogenesis and Clinical Implications. Am. J. Kidney Dis. 2014;64:793–803. doi: 10.1053/j.ajkd.2014.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Long J., Parada X.V., Kalim S. Protein Carbamylation in Chronic Kidney Disease and Dialysis. Adv. Clin. Chem. 2018;87:37–67. doi: 10.1016/bs.acc.2018.07.002. [DOI] [PubMed] [Google Scholar]
- 226.Jaisson S., Pietrement C., Gillery P. Carbamylation-Derived Products: Bioactive Compounds and Potential Biomarkers in Chronic Renal Failure and Atherosclerosis. Clin. Chem. 2011;57:1499–1505. doi: 10.1373/clinchem.2011.163188. [DOI] [PubMed] [Google Scholar]
- 227.Verbrugge F.H., Tang W.H.W., Hazen S.L. Protein carbamylation and cardiovascular disease. Kidney Int. 2015;88:474–478. doi: 10.1038/ki.2015.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Flückiger R., Harmon W., Meier W., Loo S., Gabbay K.H. Hemoglobin Carbamylation in Uremia. N. Engl. J. Med. 1981;304:823–827. doi: 10.1056/NEJM198104023041406. [DOI] [PubMed] [Google Scholar]
- 229.Hawkins C.L. Role of cyanate in the induction of vascular dysfunction during uremia: More than protein carbamylation? Kidney Int. 2014;86:875–877. doi: 10.1038/ki.2014.256. [DOI] [PubMed] [Google Scholar]
- 230.Stark G.R., Stein W.H., Moore S. Reactions of the Cyanate Present in Aqueous Urea with Amino Acids and Proteins. J. Biol. Chem. 1960;235:3177–3181. doi: 10.1016/S0021-9258(20)81332-5. [DOI] [Google Scholar]
- 231.Wang Z., Nicholls S., Rodriguez E.R., Kummu O., Hörkkö S., Barnard J.W., Reynolds W.F., Topol E., A DiDonato J., Hazen S.L. Protein carbamylation links inflammation, smoking, uremia and atherogenesis. Nat. Med. 2007;13:1176–1184. doi: 10.1038/nm1637. [DOI] [PubMed] [Google Scholar]
- 232.Ospelt C., Bang H., Feist E., Camici G., Keller S., Detert J., Krämer A., Gay S., Ghannam K., Burmester G.R. Carbamylation of vimentin is inducible by smoking and represents an independent autoantigen in rheumatoid arthritis. Ann. Rheum. Dis. 2017;76:1176–1183. doi: 10.1136/annrheumdis-2016-210059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Manganelli V., Recalchi S., Capozzi A., Riitano G., Mattei V., Longo A., Di Franco M., Alessandri C., Bombardieri M., Valesini G., et al. Autophagy induces protein carbamylation in fibroblast-like synoviocytes from patients with rheumatoid arthritis. Rheumatology. 2018;57:2032–2041. doi: 10.1093/rheumatology/key174. [DOI] [PubMed] [Google Scholar]
- 234.Apostolov E.O., Shah S.V., Ok E., Basnakian A.G. Quantification of Carbamylated LDL in Human Sera by a New Sandwich ELISA. Clin. Chem. 2005;51:719–728. doi: 10.1373/clinchem.2004.044032. [DOI] [PubMed] [Google Scholar]
- 235.Stim J., Shaykh M., Anwar F., Ansari A., Arruda J.A., Dunea G. Factors determining hemoglobin carbamylation in renal failure. Kidney Int. 1995;48:1605–1610. doi: 10.1038/ki.1995.454. [DOI] [PubMed] [Google Scholar]
- 236.Davenport A., Jones S.R., Goel S., Astley J.P., Hartog M. Differentiation of acute from chronic renal impairment by detection of carbamylated haemoglobin. Lancet. 1993;341:1614–1617. doi: 10.1016/0140-6736(93)90757-8. [DOI] [PubMed] [Google Scholar]
- 237.Wynckel A., Randoux C., Millart H., Desroches C., Gillery P., Canivet E., Chanard J. Kinetics of carbamylated haemoglobin in acute renal failure. Nephrol. Dial. Transplant. 2000;15:1183–1188. doi: 10.1093/ndt/15.8.1183. [DOI] [PubMed] [Google Scholar]
- 238.Ok E., Basnakian A.G., Apostolov E.O., Barri Y.M., Shah S.V. Carbamylated low-density lipoprotein induces death of endothelial cells: A link to atherosclerosis in patients with kidney disease. Kidney Int. 2005;68:173–178. doi: 10.1111/j.1523-1755.2005.00391.x. [DOI] [PubMed] [Google Scholar]
- 239.Bright R., Proudman S.M., Rosenstein E.D., Bartold P.M. Is there a link between carbamylation and citrullination in periodontal disease and rheumatoid arthritis? Med. Hypotheses. 2015;84:570–576. doi: 10.1016/j.mehy.2015.03.006. [DOI] [PubMed] [Google Scholar]
- 240.Reed E., Jiang X., Kharlamova N., Ytterberg A.J., Catrina A.I., Israelsson L., Mathsson-Alm L., Hansson M., Alfredsson L., Rönnelid J., et al. Antibodies to carbamylated α-enolase epitopes in rheumatoid arthritis also bind citrullinated epitopes and are largely indistinct from anti-citrullinated protein antibodies. Arthritis Res. 2016;18:1–9. doi: 10.1186/s13075-016-1001-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Shi J., Van Steenbergen H.W., Van Nies J.A.B., Levarht E.W.N., Huizinga T.W.J., van der Helm-van Mil A.H., Toes R.E.M., Trouw L.A. The specificity of anti-carbamylated protein antibodies for rheumatoid arthritis in a setting of early arthritis. Arthritis Res. Ther. 2015;17:1–6. doi: 10.1186/s13075-015-0860-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Verpoort K.N., van Gaalen F.A., van der Helm-van Mil A.H., Schreuder G.M.T., Breedveld F.C., Huizinga T.W.J., de Vries R.R.P., Toes R.E.M. Association of HLA-DR3 with anti-cyclic citrullinated peptide antibody-negative rheumatoid arthritis. Arthritis Rheum. 2005;52:3058–3062. doi: 10.1002/art.21302. [DOI] [PubMed] [Google Scholar]
- 243.Irigoyen P., Lee A.T., Wener M.H., Li W., Kern M., Batliwalla F., Lum R.F., Massarotti E., Weisman M., Bombardier C., et al. Regulation of anti-cyclic citrullinated peptide antibodies in rheumatoid arthritis: Contrasting effects of HLA-DR3 and the shared epitope alleles. Arthritis Rheum. 2005;52:3813–3818. doi: 10.1002/art.21419. [DOI] [PubMed] [Google Scholar]
- 244.Jiang X., Trouw L.A., van Wesemael T.J., Shi J., Bengtsson C., Källberg H., Malmström V., Israelsson L., Hreggvidsdottir H., Verduijn W., et al. Anti-CarP antibodies in two large cohorts of patients with rheumatoid arthritis and their relationship to genetic risk factors, cigarette smoking and other autoantibodies. Ann. Rheum. Dis. 2014;73:1761–1768. doi: 10.1136/annrheumdis-2013-205109. [DOI] [PubMed] [Google Scholar]
- 245.Regueiro C., Rodriguez-Rodriguez L., Triguero-Martinez A., Nuño L., Castaño-Nuñez A.L., Villalva A., Perez-Pampin E., Lopez-Golan Y., Abasolo L., Ortiz A.M., et al. Specific Association of HLA—DRB 1*03 With Anti–Carbamylated Protein Antibodies in Patients With Rheumatoid Arthritis. Arthritis Rheumatol. 2019;71:331–339. doi: 10.1002/art.40738. [DOI] [PubMed] [Google Scholar]
- 246.Kummu O., Turunen S.P., Wang C., Lehtimäki J., Veneskoski M., Kastarinen H., Koivula M.K., Risteli J., Kesäniemi Y.A., Hörkkö S. Carbamyl adducts on low-density lipoprotein induce IgG response in LDLR-/- mice and bind plasma autoantibodies in humans under enhanced carbamylation. Antioxid. Redox. Signal. 2013;19:1047–1062. doi: 10.1089/ars.2012.4535. [DOI] [PubMed] [Google Scholar]
- 247.van Delft M.A.M., Verheul M.K., Burgers L.E., Derksen V.F.A.M., van der Helm-van Mil A.H., van der Woude D., Huizinga T.W.J., Toes R.E.M., Trouw L.A. The isotype and IgG subclass distribution of anti-carbamylated protein antibodies in rheumatoid arthritis patients. Arthritis Res. 2017;19:1–12. doi: 10.1186/s13075-017-1392-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Verheul M.K., Van Erp S.J.H., Van Der Woude D., Levarht E.W.N., Mallat M.J.K., Verspaget H.W., Stolk J., Toes R.E., van der Meulen-de A.E., Hiemstra P., et al. Anti-carbamylated protein antibodies: A specific hallmark for rheumatoid arthritis. Comparison to conditions known for enhanced carbamylation; renal failure, smoking and chronic inflammation. Ann. Rheum. Dis. 2016;75:1575–1576. doi: 10.1136/annrheumdis-2016-209248. [DOI] [PubMed] [Google Scholar]
- 249.Shi J., Knevel R., Suwannalai P., van der Linden M.P., Janssen G.M.C., van Veelen P., Levarht N.E.W., van der Helm-van Mil A.H., Cerami A., Huizinga T.W.J., et al. Autoantibodies recognizing carbamylated proteins are present in sera of patients with rheumatoid arthritis and predict joint damage. Proc. Natl. Acad. Sci. USA. 2011;108:17372–17377. doi: 10.1073/pnas.1114465108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Ajeganova S., van Steenbergen H.W., Verheul M.K., Forslind K., Hafström I., Toes R.E., Huizinga T.W., Svensson B., Trouw L.A., van der Helm-van Mil A.H. The association between anti-carbamylated protein (anti-CarP) antibodies and radiographic progression in early rheumatoid arthritis: A study exploring replication and the added value to ACPA and rheumatoid factor. Ann. Rheum. Dis. 2017;76:112–118. doi: 10.1136/annrheumdis-2015-208870. [DOI] [PubMed] [Google Scholar]
- 251.Truchetet M.E., Dublanc S., Barnetche T., Vittecoq O., Mariette X., Richez C., Blanco P., Mahler M., Contin-Bordes C., Schaeverbeke T. Association of the Presence of Anti-Carbamylated Protein Antibodies in Early Arthritis With a Poorer Clinical and Radiologic Outcome: Data From the French ESPOIR Cohort. Arthritis Rheumatol. 2017;69:2292–2302. doi: 10.1002/art.40237. [DOI] [PubMed] [Google Scholar]
- 252.Verheul M.K., Böhringer S., van Delft M.A.M., Jones J.D., Rigby W.F.C., Gan R.W., Holers V.M., Edison J.D., Deane K.D., Janssen K.M.J., et al. Triple Positivity for Anti-Citrullinated Protein Autoantibodies, Rheumatoid Factor, and Anti-Carbamylated Protein Antibodies Conferring High Specificity for Rheumatoid Arthritis: Implications for Very Early Identification of At-Risk Individuals. Arthritis Rheumatol. 2018;70:1721–1731. doi: 10.1002/art.40562. [DOI] [PubMed] [Google Scholar]
- 253.Verheul M., Böhringer S., van Delft M., Jones J., Rigby W., Gan R., Holers V., Edison J., Deane K., Janssen K., et al. The combination of three autoantibodies, ACPA, RF and anti-CarP antibodies is highly specific for rheumatoid arthritis: Implications for very early identification of individuals at risk to develop rheumatoid arthritis. Arthritis Rheumatol. 2018;70:1721–1731. doi: 10.1002/art.40562. [DOI] [PubMed] [Google Scholar]
- 254.Gan R.W., Trouw L.A., Shi J., Toes R.E., Huizinga T.W., Demoruelle M.K., Kolfenbach J.R., Zerbe G.O., Deane K.D., Edison J.D., et al. Anti-carbamylated Protein Antibodies Are Present Prior to Rheumatoid Arthritis and Are Associated with Its Future Diagnosis. J. Rheumatol. 2015;42:572–579. doi: 10.3899/jrheum.140767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Pruijn G.J.M. Citrullination and Carbamylation in the Pathophysiology of Rheumatoid Arthritis. Front. Immunol. 2015;6:192. doi: 10.3389/fimmu.2015.00192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Regueiro C., Rodriguez-Rodriguez L., Lopez-Mejias R., Nuno L., Triguero-Martinez A., Perez-Pampin E., Corrales A., Villalba A., Lopez-Golan Y., Abasolo L., et al. A predominant involvement of the triple seropositive patients and others with rheumatoid factor in the association of smoking with rheumatoid arthritis. Sci. Rep. 2020;10:3355. doi: 10.1038/s41598-020-60305-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Brink M., Hansson M., Mathsson-Alm L., Wijayatunga P., Verheul M.K., Trouw L.A., Holmdahl R., Rönnelid J., Klareskog L., Rantapää-Dahlqvist S. Rheumatoid factor isotypes in relation to antibodies against citrullinated peptides and carbamylated proteins before the onset of rheumatoid arthritis. Arthritis Res. 2016;18:1–11. doi: 10.1186/s13075-016-0940-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.van Wesemael T., Ajeganova S., Humphreys J., Terao C., Muhammad A., Symmons D.P.M., MacGregor A.J., Hafström I., Trouw L.A., Mil A.H.M.V.D.H.-V., et al. Smoking is associated with the concurrent presence of multiple autoantibodies in rheumatoid arthritis rather than with anti-citrullinated protein antibodies per se: A multicenter cohort study. Arthritis Res. 2016;18:285. doi: 10.1186/s13075-016-1177-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Brink M., Verheul M.K., Rönnelid J., Berglin E., Holmdahl R., Toes R.E., Klareskog L., Trouw L.A., Rantapää-Dahlqvist S. Anti-carbamylated protein antibodies in the pre-symptomatic phase of rheumatoid arthritis, their relationship with multiple anti-citrulline peptide antibodies and association with radiological damage. Arthritis Res. Ther. 2015;17:25–28. doi: 10.1186/s13075-015-0536-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Bos W.H., Wolbink G.J., Boers M., Tijhuis G.J., de Vries N., Van Der Horst-Bruinsma I.E., Tak P.P., Van De Stadt R.J., Van Der Laken C.J., Dijkmans B.A.C., et al. Arthritis development in patients with arthralgia is strongly associated with anti-citrullinated protein antibody status: A prospective cohort study. Ann. Rheum. Dis. 2009;69:490–494. doi: 10.1136/ard.2008.105759. [DOI] [PubMed] [Google Scholar]
- 261.van Delft M.A.M., Verheul M.K., E Burgers L., Rantapää-Dahlqvist S., van der Helm-van Mil A.H., Huizinga T.W.J., Toes R.E.M., A Trouw L. The anti-carbamylated protein antibody response is of overall low avidity despite extensive isotype switching. Rheumatology. 2018;57:1583–1591. doi: 10.1093/rheumatology/key135. [DOI] [PubMed] [Google Scholar]
- 262.Khumaedi A.I., Purnamasari D., Wijaya I.P., Soeroso Y. The relationship of diabetes, periodontitis and cardiovascular disease. Diabetes Metab. Syndr. 2019;13:1675–1678. doi: 10.1016/j.dsx.2019.03.023. [DOI] [PubMed] [Google Scholar]
- 263.Sokolove J., Brennan M.J., Sharpe O., Lahey L.J., Kao A.H., Krishnan E., Edmundowicz D., Lepus C.M., Wasko M.C., Robinson W.H. Brief Report: Citrullination Within the Atherosclerotic Plaque: A Potential Target for the Anti-Citrullinated Protein Antibody Response in Rheumatoid Arthritis. Arthritis Rheum. 2013;65:1719–1724. doi: 10.1002/art.37961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Spinelli F.R., Pecani A., Conti F., Mancini R., Alessandri C., Valesini G. Post-translational modifications in rheumatoid arthritis and atherosclerosis: Focus on citrullination and carbamylation. J. Int. Med Res. 2016;44:81–84. doi: 10.1177/0300060515593258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Hermans M.P.J., van der Velden D., Cabezas J.M.M., Putter H., Huizinga T.W.J., Kuiper J., Toes R.E.M., Schalij M.J., Jukema J.W., van der Woude D. Long-term mortality in patients with ST-segment elevation myocardial infarction is associated with anti-citrullinated protein antibodies. Int. J. Cardiol. 2017;240:20–24. doi: 10.1016/j.ijcard.2017.04.046. [DOI] [PubMed] [Google Scholar]
- 266.Yang M.L., Sodre F.M.C., Mamula M.J., Overbergh L. Citrullination and PAD Enzyme Biology in Type 1 Diabetes—Regulators of Inflammation, Autoimmunity, and Pathology. Front. Immunol. 2021;12:678953. doi: 10.3389/fimmu.2021.678953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Yang L., Tan D., Piao H. Myelin Basic Protein Citrullination in Multiple Sclerosis: A Potential Therapeutic Target for the Pathology. Neurochem. Res. 2016;41:1845–1856. doi: 10.1007/s11064-016-1920-2. [DOI] [PubMed] [Google Scholar]
- 268.Mondal S., Thompson P.R. Protein Arginine Deiminases (PADs): Biochemistry and Chemical Biology of Protein Citrullination. Accounts Chem. Res. 2019;52:818–832. doi: 10.1021/acs.accounts.9b00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Caprariello A.V., Rogers J.A., Morgan M.L., Hoghooghi V., Plemel J., Koebel A., Tsutsui S., Dunn J.F., Kotra L.P., Ousman S.S., et al. Biochemically altered myelin triggers autoimmune demyelination. Proc. Natl. Acad. Sci. USA. 2018;115:5528–5533. doi: 10.1073/pnas.1721115115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Bisset G.W., Poisner A.M., Smyth D.G. Carbamylation of Oxytocin and Arginine-Vasopressin. Nature. 1963;199:69–70. doi: 10.1038/199069a0. [DOI] [PubMed] [Google Scholar]
- 271.Oimomi M., Hatanaka H., Yoshimura Y., Yokono K., Baba S., Taketomi Y. Carbamylation of Insulin and Its Biological Activity. Nephron. 1987;46:63–66. doi: 10.1159/000184303. [DOI] [PubMed] [Google Scholar]
- 272.Kraus L.M., Traxinger R., Kraus A.P., Kraus R.T.L.M. Uremia and insulin resistance: N-carbamoyl-asparagine decreases insulin-sensitive glucose uptake in rat adipocytes. Kidney Int. 2004;65:881–887. doi: 10.1111/j.1523-1755.2004.00456.x. [DOI] [PubMed] [Google Scholar]
- 273.Park K.-D., Mun K.-C., Chang E.-J., Park S.-B., Kim H.-C. Inhibition of erythropoietin activity by cyanate. Scand. J. Urol. Nephrol. 2004;38:69–72. doi: 10.1080/00365590310006291. [DOI] [PubMed] [Google Scholar]
- 274.Satake R., Kozutsumi H., Takeuchi M., Asano K. Chemical modification of erythropoietin: An increase in in vitro activity by guanidination. Biochim. Biophys. Acta Protein Struct. Mol. Enzym. 1990;1038:125–129. doi: 10.1016/0167-4838(90)90020-G. [DOI] [PubMed] [Google Scholar]
- 275.Hörkkö S., Huttunen K., Kervinen K., Kesäniemi Y.A. Decreased clearance of uraemic and mildly carbamylated low-density lipoprotein. Eur. J. Clin. Investig. 1994;24:105–113. doi: 10.1111/j.1365-2362.1994.tb00974.x. [DOI] [PubMed] [Google Scholar]
- 276.Weisgraber K.H., Innerarity T.L., Mahley R.W. Role of lysine residues of plasma lipoproteins in high affinity binding to cell surface receptors on human fibroblasts. J. Biol. Chem. 1978;253:9053–9062. doi: 10.1016/S0021-9258(17)34284-9. [DOI] [PubMed] [Google Scholar]
- 277.Chistiakov D.A., Melnichenko A.A., Orekhov A.N., Bobryshev Y.V. How do macrophages sense modified low-density lipoproteins? Int. J. Cardiol. 2017;230:232–240. doi: 10.1016/j.ijcard.2016.12.164. [DOI] [PubMed] [Google Scholar]
- 278.Asci G., Basci A., Shah S.V., Basnakian A., Töz H., Ozkahya M., Duman S., Ok E. Carbamylated low-density lipoprotein induces proliferation and increases adhesion molecule expression of human coronary artery smooth muscle cells. Nephrology. 2008;13:480–486. doi: 10.1111/j.1440-1797.2008.00948.x. [DOI] [PubMed] [Google Scholar]
- 279.Verdin E., Ott M. 50 years of protein acetylation: From gene regulation to epigenetics, metabolism and beyond. Nat. Rev. Mol. Cell Biol. 2014;16:258–264. doi: 10.1038/nrm3931. [DOI] [PubMed] [Google Scholar]
- 280.Narita T., Weinert B., Choudhary C. Functions and mechanisms of non-histone protein acetylation. Nat. Rev. Mol. Cell Biol. 2018;20:156–174. doi: 10.1038/s41580-018-0081-3. [DOI] [PubMed] [Google Scholar]
- 281.Silva R.D., Martinho R.G. Developmental roles of protein N-terminal acetylation. Proteomics. 2015;15:2402–2409. doi: 10.1002/pmic.201400631. [DOI] [PubMed] [Google Scholar]
- 282.Arnesen T., Van Damme P., Polevoda B., Helsens K., Evjenth R., Colaert N., Varhaug J.E., Vandekerckhove J., Lillehaug J.R., Sherman F., et al. Proteomics analyses reveal the evolutionary conservation and divergence of N-terminal acetyltransferases from yeast and humans. Proc. Natl. Acad. Sci. USA. 2009;106:8157–8162. doi: 10.1073/pnas.0901931106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Lloyd K.A., Wigerblad G., Sahlström P., Garimella M.G., Chemin K., Steen J., Titcombe P.J., Marklein B., Zhou D., Stålesen R., et al. Differential ACPA Binding to Nuclear Antigens Reveals a PAD-Independent Pathway and a Distinct Subset of Acetylation Cross-Reactive Autoantibodies in Rheumatoid Arthritis. Front. Immunol. 2018;9:3033. doi: 10.3389/fimmu.2018.03033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Kampstra A.S.B., Dekkers J.S., Volkov M., Dorjée A.L., Hafkenscheid L., Kempers A.C., Van Delft M., Kissel T., Reijm S., Janssen G.M.C., et al. Different classes of anti-modified protein antibodies are induced on exposure to antigens expressing only one type of modification. Ann. Rheum. Dis. 2019;78:908–916. doi: 10.1136/annrheumdis-2018-214950. [DOI] [PubMed] [Google Scholar]
- 285.Su Q., Jing J., Li W., Ma J., Zhang X., Wang Z., Zhou Z., Dai L., Shao L. Impaired Tip60-mediated Foxp3 acetylation attenuates regulatory T cell development in rheumatoid arthritis. J. Autoimmun. 2019;100:27–39. doi: 10.1016/j.jaut.2019.02.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.