Abstract
The functional complexity of higher organisms is not easily accounted for by the size of their genomes. Rather, complexity appears to be generated by transcriptional, translational, and post-translational mechanisms and tissue organization that produces a context-dependent response of cells to specific stimuli. One property of gene products that likely increases the ability of cells to respond to stimuli with complexity is the multifunctionality of expressed proteins. Receptor for hyaluronan-mediated motility (RHAMM) is an example of a multifunctional protein that controls differential responses of cells in response-to-injury contexts. Here, we trace its evolution into a sensor-transducer of tissue injury signals in higher organisms through the detection of hyaluronan (HA) that accumulates in injured microenvironments. Our goal is to highlight the domain and isoform structures that generate RHAMM’s function complexity and model approaches for targeting its key functions to control cancer progression.
Keywords: RHAMM, HA, signaling, domains, isoforms, evolution, cancer, multi-functions
1. Introduction
The one gene-one enzyme hypothesis proposed by Beadle and Tatum in 1941 [1], stating that genes produce protein products with a single function, helped shape our understanding of biochemical processes. However, the concept of protein function has since evolved in complexity, with many studies demonstrating that cell signaling can be highly context-dependent, allowing for the ability of single proteins to exert multiple and often opposing functions depending on cell state (e.g., proliferating, senescent, stem-like), environmental signals and protein subcellular compartmentalization [2,3]. However, classifying multifunctional proteins has been challenging. Traditionally, proteins are named for the function they were originally discovered for, but this classification can have an undesirable effect of focusing and thereby restricting perception of function. Receptor for hyaluronan-mediated motility (RHAMM) is one protein whose multifunctional properties are often overlooked, yet it is emerging as an important hub in signaling networks that regulate the interconnected processes of inflammation, interstitial fibrosis, and neoplasia. The multiple functions of RHAMM depend upon its domain and isoform structure [4,5], subcellular localization [6], as well as tissue [7] and cellular context [8,9,10].
RHAMM was originally discovered for its association with the cell surface, its ability to bind to hyaluronan (HA), and its function in promoting avian and mammalian fibroblast motility [11]. This protein was subsequently, and intensively, investigated for its critical role in HA-mediated regulation of inflammation, fibrosis, and disease progression, notably lung disorders and neoplasia [12,13,14,15,16]. Later, RHAMM was shown to also occur in intracellular compartments, and its known intracellular functions now include regulation of interphase and mitotic spindle microtubule dynamics, centrosome functions, and participation in gene transcription [6]. At first glance, this functional diversity may seem unrelated, but the aim of this review is to provide a context in which these multiple functions of RHAMM coordinate a higher-order response to tissue injury.
Proteins such as RHAMM that perform multiple functions are often referred to as “moonlighting or promiscuous proteins” [17,18], but this assumes there is one dominant or mainstream function while others are extra or subsidiary [3,6]. Often, proteins acquire new functions with evolution, which generates novelty and complexity by building upon pre-existing domain structures [19]. Therefore, we use the term multifunctional [2,18] rather than moonlighting in describing the multiple functions of RHAMM. Here, we posit that RHAMM initially functioned as a heparin-binding protein that was co-opted to also bind to HA when this glycosaminoglycan appeared relatively late in evolution.
2. Evolution of the Hyaluronome
The hyaluronan genome (“hyaluronome”), which coordinates the metabolism and functions of HA, is complex, comprising synthases, hyaluronidases, and hyaluronan receptors/binding proteins [12,14]. HA is produced by three integral plasma membrane hyaluronan synthases, HAS1-3, that are moderately tissue-specific in their expression and produce HA polymers of different sizes [20]. Knockout and knockdown strategies have revealed both overlapping and distinct functions of these enzymes. At least seven hyaluronidases (HYAL1-5, TMEM2, and KIAA1199/CEMIP/HYBID) have been described that can depolymerize HA, each differing in the sizes of the HA polymers that they produce [21,22,23]. HA receptors that sense the native and degraded HA polymers and activate intracellular signaling include CD44, RHAMM/HMMR, LYVE1, STAB2, TLR4, and LAYN [24,25,26,27,28,29,30,31]. The extracellular organization of HA, which is also critical to its functions, is achieved by multiple proteins, including aggrecan, link protein, and versican [32,33].
HA is a structurally simple linear polymer composed of repeating units of N-acetyl glucosamine and b-glucuronic acid. Like its sulfated glycosaminoglycan (GAG) cousins, HA has key roles in inflammation, but it is strikingly different from its sulfated GAG cousins in its high viscoelastic properties, its production at the cell membrane, and its polymer size-dependent functions in inflammation [12,14,34]. The viscoelastic properties of HA are considered to have been essential for the development and function of an endoskeleton [35,36,37], while the production of HA at the cell surface coupled with its polymer size-dependent functions was utilized as a rapid and sophisticated danger-sensing mechanism [12,38,39]. Both of these properties were likely critical for the emergence of vertebrates when HA first consistently appeared in evolution [40,41,42] (Figure 1).
Figure 1.
Evolution of RHAMM. The tree shows the appearance of hyaluronan binding ability of RHAMM with the appearance of vertebrates. More information about the evolutionary expression of RHAMM: https://www.ncbi.nlm.nih.gov/protein/?term=rhamm (accessed: 20 September 2021) and HAS2: https://www.ncbi.nlm.nih.gov/protein/?term=HAS2 (accessed: 20 September 2021) within various taxonomic groups.
Notably, invertebrates and lower organisms do not produce HA (Figure 1) [43], although, they synthesize sulfated GAGs (Figure 1) [44,45,46]. In the early chordate phyla, lancelets, but not tunicates, express a has2 gene with 57% identity to human HAS2 (Figure 1). HAS genes are only consistently found in vertebrates, predicting that the capability for producing HA was an important change contributing to vertebrate evolution. HA synthases likely evolved from an ancestral glycosyltransferase that also gave rise to the enzymes that produce related polysaccharides such as chondroitin sulfate, chitin, and even cellulose [40,47]. This is suggested by the following common features: the glycosyltransferases that produce HA, chitin, and cellulose B-1,4 glycan are all integral membrane proteins, have invariant amino acid sequences in their catalytic domains, and can under specific conditions synthesize several polysaccharide products [42,47,48]. For example, mammalian HAS1 can produce chitin, a poly-glucosamine polymer, if supplied with only activated glucosamine (UDP-GlcNAc) [40]. Additionally, a Xenopus glycosyltransferase, DG42, can produce both HA or chitin polymers, where the chitin polymers are used as primers for HA oligosaccharide (O-HA) synthesis [47].
Interestingly, even though they do not produce HA, many invertebrate species express genes orthologous to vertebrate HA receptors. For example, despite the inability to produce HA, tunicates express proteins with a link module (Xlink) that is homologous to the vertebrate CD44 link module [49,50]. Rather than binding HA, Xlink domains bind to heparin, which is a sulfated glycosaminoglycan synthesized by all invertebrates and lower species analyzed to date [45,51]. Xlink domains have functions in cell migration and apoptosis [49]. Thus, the CD44 link module is predicted to have evolved from heparin-binding ancestor proteins that were recruited to bind to HA as a result of domain shuffling [50]. Like HA, the secondary structure of RHAMM is largely helical (Figure 2A). RHAMM orthologues, which are also helical proteins, are found in invertebrate species, but these have low sequence identity with human RHAMM (varying between 18 and 30%, shown in ants (A. heyeri), Figure 3 and Figure 4). The conservation of RHAMM protein is predicted (predictprotein.org (accessed: 18 September 2021)) [52] to exist primarily in the carboxy-terminal region that contains clusters of basic amino acids (Figure 3A) and that binds to HA in vertebrates (yellow-boxed sequence, Figure 3B). This region of mammalian RHAMM has also been demonstrated to bind to heparin [53] and ERK1 [54] and is highly conserved (>80%) in all vertebrates. In addition to these sequences, vertebrate and invertebrate RHAMM sequences both contain regions of coiled coils, which can perform structural [55] and signaling functions [56], and di-leucine repeat motifs, which are involved in receptor endocytosis [57]. Collectively, these similarities suggest that ancient functions of RHAMM were lectin-like (heparin-binding) and associated with signaling functions that were later adapted to HA signaling in vertebrates. The heparin-binding function of RHAMM was likely adapted to bind to HA during vertebrate evolution. Other known RHAMM domains, assessed by sequence homology with human RHAMM, are not present in invertebrates and only appear later in evolution, with the emergence of vertebrates (Figure 3 and Figure 4). These include N-terminal sequences that mediate binding to microtubules, and the carboxyl-terminal leucine zipper, which binds to TPX2 [58], and likely transcription factors such as E2F1 [59] and Bach1 [60]. The binding repertoire expansion of RHAMM during vertebrate evolution predicts that the multifunctionality of RHAMM has increased over time.
Figure 2.
The predicted secondary structure of human RHAMM (A) and the known binding domains for which binding partners have been experimentally verified. (A) The secondary structure of RHAMM is predicted to be mostly helical (predictprotein.org (accessed: 18 September 2021)) and (B) the major binding domains identified to date occur in the alpha-helical carboxyl terminus. * p value < 0.05, ** p value < 0.01.
Figure 3.
Homology of human RHAMM with invertebrates. (A) The conservation of RHAMM protein is predicted to be highest in the carboxyl-terminus (predictprotein.org (accessed: 18 September 2021)) that contains the basic amino acid clusters required for binding to HA and heparin (open box). (B) Sequence identity and homology (https://blast.ncbi.nlm.nih.gov/ (accessed: 18 September 2021)) with Acromymex heyeri, an invertebrate ant, is low and, as predicted in A, occurs primarily in the C-terminus containing basic amino acid clusters required for binding to hyaluronan and heparin in human RHAMM protein. The leucine zipper in this region (double-boxed) typical of vertebrate RHAMM and predicted to bind to transcription factors, is not present.
Figure 4.
Sequence homology between human RHAMM and Danio rerio (zebrafish). The sequence homology in the N- and C-termini has increased relative to human RHAMM from that observed in invertebrates, particularly in the C-terminal hyaluronan/heparin-binding domain. The leucine zipper, which is predicted to bind to transcription factors, is present.
3. RHAMM Isoform Structure and Function
The RHAMM gene contains 18 exons that can be expressed both as a full-length protein and, like CD44, as multiple isoforms generated by alternative splicing, alternate start codon usage, and/or post-translational protein processing (Figure 5A,B). In general, RHAMM is poorly expressed in normal tissues [61], but expression of the full-length protein and isoforms are dramatically and transiently increased during tissue response to injury [14,24,62], and these are constitutively increased with diseases such as breast cancer, multiple myeloma, and bronchopulmonary dysplasia [24,62,63]. Here, we consider both human and mouse RHAMM processing, since these two species are most frequently used to parse the function of this protein. The extent and type of processing for generating RHAMM isoforms differ in humans and mice (Figure 5A,B).
Figure 5.
Isoform structures of RHAMM in (A) human and (B) mouse genomes. RHAMM isoforms are generated by alternative splicing and/or alternate start site/codon usage. (C) Full-length mouse GFP-RHAMM decorates interphase microtubules, while mouse GFP-RHAMM X3 is more diffuse and accumulates in the nucleus.
Human RHAMM (Figure 5A) isoforms are generated by alternative splicing of mRNA (uniprot.org (accessed: 18 September 2021)) [64]. The major alternatively spliced forms of RHAMM are A–D (also referred to as 1–4) (Figure 5A), but alternative splicing of exons 2–4 [65], 4, 5, and 13 have also been reported [63,66]. Alternatively spliced RHAMM isoforms are frequently expressed with human disease, particularly in cancers, including multiple myeloma [66,67] breast [68,69], pancreatic [4], and colon cancers [70]. Notably, both an increase in canonical or full-length RHAMM relative to normal tissue and the isoform balance are deregulated in multiple myeloma [67] and pancreatic [4] and colon tumors [70]. Human cancer cells (e.g., PC3MLN4 prostate cancer cells) express full-length and isoform B, although protein products are not easily resolved from one another using standard SDS-PAGE and Western blots (Figure 5). The functions of these human RHAMM isoforms have not been extensively investigated. Isoform B is commonly expressed in cancers and promotes liver metastasis in a mouse model of islet tumorigenesis as well as in tail vein assays [71]. Mechanistically, the pro-metastasis effect of isoform B is associated with sustained activation of EGFR.
Mouse RHAMM (Figure 5B) is similar to the human counterpart but encodes a 20 aa sequence that is repeated five times in the full-length protein and contains an additional sequence unique to mouse RHAMM. The protein/polysaccharide binding domains located in the N- and C-termini are conserved. Mouse RHAMM protein isoforms differ from human comparators by varying more widely in their molecular weight. Mouse isoforms are referred to as full-length (794 aa) and isoforms X1–4 (Figure 5B). Although X1 and X2 isoforms are generated by alternative mRNA splicing, it is likely that X3 results from alternative start codon usage while X4 derives from a combination of these two mechanisms. Western blot assays using antibodies specific to the N- or C-terminus confirm this pattern of isoform expression. N-terminus-specific antibodies reveal only a full-length protein, while C terminus-specific antibodies detect proteins with molecular weights corresponding to isoforms X3 (72 kDa) and 4 (70 kDa) that are expressed in Ras-transformed mouse fibroblasts in vitro [72]. Notably, X3 and X4 correspond to the molecular weights of RHAMM proteins detected on macrophage cell surfaces by surface biotinylation following tissue injury [73]. The additional smaller protein bands detected by Western blot assays may be post-translationally processed forms.
4. RHAMM Multi-Functions and Domains
The complex domain structure of RHAMM contributes to both the multifunctionality of this protein and its subcellular distribution. RHAMM binds to, indirectly associates with, and/or modifies the functions of both polysaccharides and proteins. The binding domains responsible for these functions and their partner interactions have been partially identified. The following have been shown to directly bind to RHAMM: HA [74], heparin [53], TPX2 [6], calmodulin [68], and ERK1 [54] (Figure 2B). RHAMM also indirectly associates with or functions through growth factor receptors such as EGFR [4,8,75,76] and PDGFR [77], HA receptors such as CD44 [10,78], and intracellular signaling proteins such as CTNNB1 [79], BRCA1/AURKA [80], ERK2, MEK1 [54], and E2F1 [59]. These interactions firmly establish both extracellular and intracellular RHAMM as a multifunctional signaling entity.
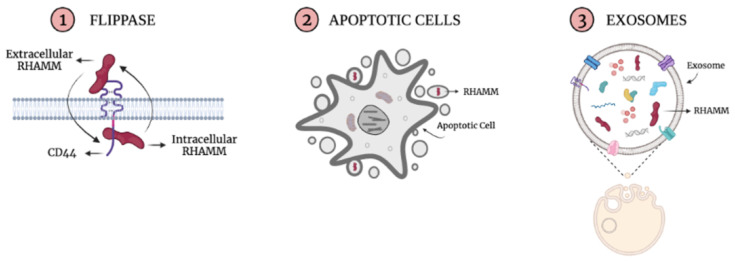
The first binding interaction described for RHAMM was its association with HA [11] and heparin [53]. Subsequent studies showed that LMW-HA and O-HA signaling during excisional wound repair requires RHAMM expression [81]. Truncation and site mutation experiments localized the HA and heparin-binding domain to an alpha-helical region in the carboxy-terminus that contains two clusters of basic amino acids (klk and klr) separated by a leucine zipper (Figure 2B, yellow) [74]. Substitution of specific basic amino acids in this region ablates HA binding, implicating an electrostatic surface as a driving force for RHAMM/HA interactions [82,83]. Indeed, peptide mimicry analyses predict that the association between HA and the carboxyl-terminal domain of RHAMM is largely ionic rather than structural [83,84]. The presence of a leucine zipper that joins the two basic, alpha-helical regions predict an ability for RHAMM to dimerize. The RHAMM/HA-heparin-binding region, therefore, more closely resembles the GAG binding regions of proteins [85], such as GRO chemokines [86], than the CD44 link module. Like RHAMM, GRO chemokines contain helical surfaces of basic amino acids that are primordial for GAG binding and include residues involved in dimerization contacts [86]. Oligomerization of these proteins can alter the exposure of basic amino acid motifs required for binding to glycosaminoglycans (e.g., BBXB and BBXXB motifs) [87] and/or create a glycosaminoglycan-binding groove [82]. Thus, a capability to dimerize can profoundly affect the glycosaminoglycan binding properties of proteins. Conversely, the binding of glycosaminoglycans to proteins, such as SDF-1, promotes dimerization [88] and heterodimerization [89], which are vital to the bioactivity of certain chemokines. These properties suggest that the leucine zipper (Figure 2B) of RHAMM may similarly affect its association with HA. In terms of cellular functions, RHAMM/HA interactions have been shown to regulate cell motility [11,12], myofibroblast activation into a pro-fibrosis state [90,91,92], macrophage chemotaxis and pro-inflammatory activation [92,93], adipogenesis [90,91,94], fibroblast neoplastic transformation [5], and cancer cell proliferation and survival [95,96]. Importantly, the ability of RHAMM to bind to HA is also required for maintenance of sarcoma transformation in experimental mouse models [5]. These functional consequences have largely been assumed to result from extracellular RHAMM/HA interactions since HA is an extracellular matrix component that is released by plasma membrane synthases, and RHAMM display at the cell surface has been documented by flow cytometry [11,73,97], surface biotinylation [73], and detection of GFP tags in non-permeabilized cells (Figure 6). However, the mechanisms responsible for RHAMM release and the domains required for its secretion are currently a matter of debate since RHAMM lacks a leader sequence for export through the Golgi/ER, a transmembrane domain, or post-translational modifications that permit a direct association with the plasma membrane (e.g., GPI-linkage). Plausible mechanisms to account for the display of RHAMM at the cell surface include unconventional export [98], release from dying cells [99], and/or export via exosomes [100] (Figure 7). Evidence to date suggests that once exported, RHAMM associates with the cell surface through interactions with integral cell surface proteins and thus functions as a co-receptor.
Figure 6.
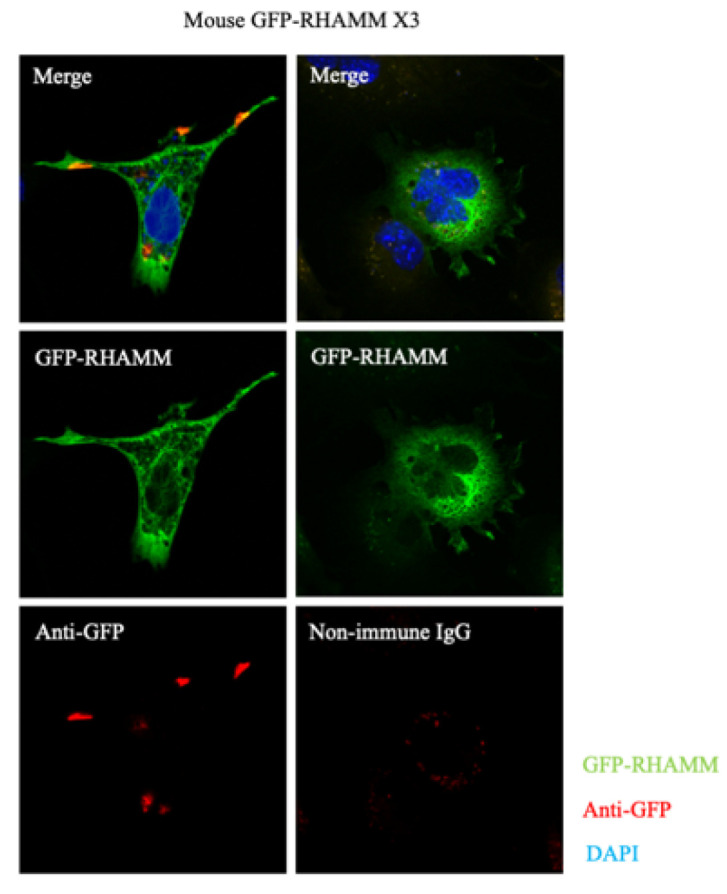
Subcellular localization of GFP-RHAMM expressed in mouse fibroblasts. Non-permeabilized cells exhibit diffuse localization for GFP-RHAMM X3. Anti-GFP antibodies were used to detect cell-surface RHAMM, which occurs in cell processes.
Figure 7.
The appearance of RHAMM at the cell surface in the absence of an encoded signal peptide, transmembrane domain, or GPI-linker predicts it is exported unconventionally by using exosomes or flippase mechanisms and/or released by apoptotic cells. Once exported via unconventional mechanisms, RHAMM can interact with cell surface receptors, such as CD44, to facilitate its extracellular signaling functions.
Although HA is largely studied for its extracellular functions, it is now acknowledged to also occur in intracellular compartments including the cytoplasm and nucleus [101], where it co-associates with intracellular RHAMM in the nucleus [97] and on mitotic spindles [102]. The phenotypic consequences of intracellular HA/RHAMM interactions are presently unclear [26], but one likely function in the nucleus is regulation of gene expression, particularly of genes linked to inflammatory pathologies [101].
Several groups have shown that RHAMM also binds to a variety of intracellular proteins. It binds to tubulin (TUBA1A, TUB2B, and TUBG2) [54,103] via two separate domains, which result in an association with both interphase and mitotic spindle microtubules (Figure 2B). The first domain occurs in exon 4 of the N-terminus, while the second requires a leucine zipper embedded in the basic region of the carboxyl-terminus [54,103]. The leucine zipper interacts dynamically with microtubules, while the N-terminal microtubule interaction is more stable (e.g., deletion of exon 4 strongly reduces the association of RHAMM with tubulin) (Figure 5C). Loss of the N-terminal interphase microtubule-binding domain in both mouse and human RHAMM (e.g., mouse isoform X3 and human RHAMM B) is transforming and promotes tumor progression to metastasis, while the full-length protein with an intact microtubule-binding ability does not [5,71,77]. One consequence of the loss of this binding domain is a marked change in the subcellular distribution of RHAMM. Thus, loss of either exon 4 [68] or truncation of N-terminal residues 1–184 shifts RHAMM distribution from interphase microtubules to more diffuse staining and, notably, a presence in the cell nucleus (Figure 5C). Loss of this domain is common in cancer and has also been reported in injured tissues [10,73,104]. Whether the subcellular shift resulting from loss of interphase microtubule binding is mechanistically connected to these processes is not clear. Thus, for both humans and mice, deletion of the N-terminal interphase microtubule-binding domain markedly changes the subcellular distribution of RHAMM and enhances neoplastic initiation and/or metastasis.
The RHAMM carboxy terminus contains a sequence that mediates an association with TPX2 and TUBG2 of the mitotic spindle [58] and with both ERK1,2/MEK1 and TUBA1A/TUB2B. The RHAMM/TPX2/TUBG2 complexes provide cues for the spatial association of AURKA with the spindle that is required for centrosome maturation, entry into mitosis, formation, and function of the bipolar spindle, and cytokinesis [58,105]. The association of RHAMM with mitotic spindles also affects RanGTP-mediated microtubule nucleation [106], spindle orientation [107], normal spindle assembly [54,108], and spindle integrity [54,58]. However, the consequences of these RHAMM/TUBG2 interactions are likely to be context-dependent. For example, less than 10% of mitotic spindles are aberrant in Rhamm−/− fibroblasts in vitro [54] and, although the carboxy-terminal mitotic binding region of RHAMM alters spindle orientation in HeLa cells in vitro [109] and granulosa cells in vivo [110], its loss does not affect spindle orientation of neuro-progenitor cells [107]. RHAMM interactions with TPX2 and other proteins also play a role in the localization of the microtubule organizing center (MTOC) and centrosomes. For example, RHAMM/TPX2 complexes are required for centrosome separation in prophase of mitosis [111], while RHAMM/SPTA1 complexes are necessary for the rear polarization of the MTOC, mediating directed smooth muscle cell migration [108]. The RHAMM/ERK1,2/MEK/TUBA1A/TUB2B complexes modulate interphase microtubule dynamics, which requires ERK1,2 activity [54]. RHAMM binds directly to ERK1 via non-canonical docking sequences [54]. Consistent with a binding specificity for this MAP kinase, RHAMM-loss shares a subset of ERK1 knockout phenotypes: neither result in embryonic lethality (although ERK2 knockout does) [112,113], and genomic loss of either gene promotes adipogenesis [91,94,113]. RHAMM binds directly to ERK1 via non-canonical docking sequences [54]. Consistent with a binding specificity for this MAP kinase, RHAMM-loss shares a subset of ERK1 knockout phenotypes: neither result in embryonic lethality (although ERK2 knockout does) [112,113], and genomic loss of either gene promotes adipogenesis [91,94,113]. Intriguingly, the RHAMM human sequence contains eight di-leucine motifs that are often present in the cytoplasmic domains of receptors and are required for protein internalization and trafficking [114,115] and which can play key roles in the spatial and temporal regulation of signaling pathways [57]. The presence of these motifs raises the possibility that cytoplasmic RHAMM may function as an adaptor protein to promote the internalization of receptors. This possibility is intriguing because RHAMM forms complexes with extracellular matrix receptors such as CD44 [10,78,96,116] and growth factor receptors such as EGFR and PDGFR [8,77]. RHAMM also associates with F-actin [117], CTNNB1 [79], and the transcription factors E2F1 [59] and Bach1 [60], although the domains required for these interactions have, to our knowledge, not been reported.
In summary, the domain structure of RHAMM, which is both novel and complex relative to other HA-binding proteins, reveals an impressive repertoire for multiple protein and polysaccharide partners that is estimated to include over 99 binding partners. The functional consequence of these domains is determined by isoform structure.
5. RHAMM Multi-Functions in Cancer
It is intriguing to note that RHAMM can, like CD44 [118], have opposing functions that impact oncogenicity. For example, Rhamm-loss reduces fibroblast motility but conversely stimulates keratinocyte migration during excisional wound repair in vivo [8]. This wound and cell context-dependent effect appears also to be manifested in diseases such as cancer. For example, either embryonic deletion of Rhamm [7] or isoform (RHAMM B) overexpression [4] similarly promote pancreatic cancer in mouse models of susceptibility and xenograft models. Correspondingly, RHAMM knockdown in breast cancer cell lines can either promote or inhibit motility depending upon the molecular subtype [9]. These in vitro results suggest that RHAMM expression status has intrinsic and tumor cell-specific effects depending upon the cell type. However, in the case of pancreatic cancer, the dominance of a pro- or anti-tumor effect of RHAMM may also be influenced by Rhamm-loss in the tumor microenvironment [7], in addition to its intrinsic consequences to tumor cell functions, which will affect cancer-associated fibroblasts and immune cell tumorigenic functions.
Most analyses linking RHAMM expression to a prognostic outcome are based upon macro-level analyses of total tumor RHAMM mRNA or protein expression. These show that elevated RHAMM expression is characteristic of most cancers and prognostic of a poor outcome in many. For example, elevated RHAMM expression is an indicator of poor prognosis in breast cancer [119,120,121], multiple myeloma [122], oral squamous cell carcinoma [123], ovarian cancer [124], lung cancer [125], prostate cancer [126], colon cancer [127], and gastric cancer [128]. However, histology analyses of these human tumors reveal that RHAMM expression is heterogeneous, and high RHAMM expression is often limited to a small subset of cancer cells [119]. The consequence of RHAMM to tumor progression may therefore depend upon both the cancer cell subtype that is expressing RHAMM and the spatial context of these RHAMM+ cells within the tumor (e.g., in invasive or metastatic niches). It is important to note that in some contexts, RHAMM is a tumor suppressor. For example, genomic reduction of RHAMM is associated with the initiation and aggression of malignant peripheral nerve sheath tumors [129]. When RHAMM is viewed as a multifunctional protein capable of interactions with a wide range of binding partners that can result in cell-specific functions, these opposing results are not necessarily surprising but highlight the critical importance of in-depth studies to provide a roadmap for targeting RHAMM in appropriate cancer types. Despite this apparent functional complexity, RHAMM is an attractive therapeutic target due to its restricted homeostatic expression predicting a good safety profile, its association with a poor outcome in most cancers, and its critical functions that promote inflammation and fibrosis [12,62], which exacerbate cancer progression [130]. Furthermore, and most importantly, experimental analyses described below using RHAMM peptide mimetics to disrupt cell surface HA-RHAMM signaling have unexpectedly revealed that this extracellular interaction may mastermind the multiple functions of RHAMM by providing the initiating and activating signal for its downstream functional complexity (Figure 8).
Figure 8.
The interaction between cell surface RHAMM and HA is proposed to activate the multiple functions of extracellular and intracellular RHAMM. Since RHAMM plays a critical role in many HA-mediated functions, using reagents to target the HA-binding region of RHAMM is expected to reduce the pathologies associated with RHAMM activation.
6. Therapeutic Strategies for Targeting RHAMM
In addition to its multifunctional nature, interest in using RHAMM as a therapeutic target in cancer has been reduced by the additional challenge of its largely coiled-coil secondary structure, which is generally difficult to target using traditional therapeutics such as small molecule inhibitors. Small molecule inhibitors have been successfully used for abrogating uncontrolled receptor activation and cell signaling in cancer. However, these function by binding, sometimes irreversibly, into the catalytic pockets of proteins to inhibit protein–ligand interactions and signal activation [131]. They are therefore not ideal reagents for binding to unstructured coiled-coil regions [131]. However, peptides that mimic the HA-binding region of RHAMM and compete with cell-surface RHAMM for HA have been shown to subsequently block the multiple functions of RHAMM in experimental models of disease [62,83]. For example, they blunt the signaling required for macrophage infiltration into injured tissues such as excisional wounds [104] and bleomycin-damaged lungs [62,132], thereby reducing tissue inflammation and destruction. These peptides also reduce both fibroblast motility and myofibroblast differentiation to consequently diminish fibrosis during excisional repair [104] and experimentally induced scleroderma [90]. Therefore, blocking this initial signal may be a reasonable approach for controlling the multifunctional complexity of aberrant RHAMM signaling during the progression of some cancers. Although RHAMM peptide mimetics have not yet been extensively tested for their ability to reduce tumorigenesis, they have been reported to reduce melanoma tumorigenesis [133] and breast and prostate cancer cell invasion [84,134] and proliferation in vitro and in vivo [135]. Since the complex processes of inflammation, fibrosis, and cancer invasion, proliferation, and metastasis require coordinated cell motility, microtubule/cytoskeleton dynamics, and gene expression, these results are consistent with a model whereby cell surface RHAMM/HA interactions activate and regulate multiple downstream intracellular and extracellular RHAMM functions. The successful use of RHAMM/HA binding peptide mimetics in controlling complex diseases in experimental models also argues for an effectiveness in blunting the most lethal aspect of some cancers, which is metastatic disease.
7. Conclusions
Targeting multifunctional proteins such as RHAMM that are only transiently expressed with tissue injury but chronically upregulated with disease are underutilized as therapeutic targets. However, relating their domain structure to function and defining domain functional hierarchy within specific tissue and disease contexts is critical for therapeutic success. This review has summarized the current knowledge on RHAMM domain structure and function and highlighted the critical importance of RHAMM/HA interactions as an activating signal for many multiple functions of this protein. Further research is required to determine if and when this activating signal promotes or suppresses progression of specific cancers.
Acknowledgments
Figure 7 and Figure 8 in this paper were created using BioRender.
Author Contributions
Conceptualization, E.A.T.; writing—original draft preparation, B.J.M. and E.A.T.; writing—review and editing, E.A.T., B.J.M., C.T., A.C.N. and J.B.M.; visualization, E.A.T. and B.J.M.; funding acquisition, B.J.M. All authors have read and agreed to the published version of the manuscript.
Funding
B.J.M. is supported by the Translational Breast Cancer Research Scholarship academic award funded by the Breast Cancer Society of Canada. E.A.T. is supported by the Breast Cancer Society of Canada and funding from the LRCP catalyst, WSS CIHR Seed Grant, and Collaborative Research Seed Grant. J.B.M. is funded by the Atwater Fund, Elsa Pardee and Chairman’s Fund Professor in Cancer Research. A.C.N. is funded by the American Cancer Society 132574-CSDG-18-139-01-CSM.
Conflicts of Interest
The authors declare no conflict of interests.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Beadle G.W., Tatum E.L. Genetic Control of Biochemical Reactions in Neurospora. Proc. Natl. Acad. Sci. USA. 1941;27:499–506. doi: 10.1073/pnas.27.11.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Espinosa-Cantu A., Cruz-Bonilla E., Noda-Garcia L., DeLuna A. Multiple Forms of Multifunctional Proteins in Health and Disease. Front. Cell Dev. Biol. 2020;8:451. doi: 10.3389/fcell.2020.00451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Radisky D.C., Stallings-Mann M., Hirai Y., Bissell M.J. Single proteins might have dual but related functions in intracellular and extracellular microenvironments. Nat. Rev. Mol. Cell Biol. 2009;10:228–234. doi: 10.1038/nrm2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choi S., Wang D., Chen X., Tang L.H., Verma A., Chen Z., Kim B.J., Selesner L., Robzyk K., Zhang G., et al. Function and clinical relevance of RHAMM isoforms in pancreatic tumor progression. Mol. Cancer. 2019;18:92. doi: 10.1186/s12943-019-1018-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hall C.L., Yang B., Yang X., Zhang S., Turley M., Samuel S., Lange L.A., Wang C., Curpen G.D., Savani R.C., et al. Overexpression of the hyaluronan receptor RHAMM is transforming and is also required for H-ras transformation. Cell. 1995;82:19–26. doi: 10.1016/0092-8674(95)90048-9. [DOI] [PubMed] [Google Scholar]
- 6.Maxwell C.A., McCarthy J., Turley E. Cell-surface and mitotic-spindle RHAMM: Moonlighting or dual oncogenic functions? Pt 7J. Cell Sci. 2008;121:925–932. doi: 10.1242/jcs.022038. [DOI] [PubMed] [Google Scholar]
- 7.Lin A., Feng J., Chen X., Wang D., Wong M., Zhang G., Na J., Zhang T., Chen Z., Chen Y.T., et al. High levels of truncated RHAMM cooperate with dysfunctional p53 to accelerate the progression of pancreatic cancer. Cancer Lett. 2021;514:79–89. doi: 10.1016/j.canlet.2021.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tolg C., Liu M., Cousteils K., Telmer P., Alam K., Ma J., Mendina L., McCarthy J.B., Morris V.L., Turley E.A. Cell-specific expression of the transcriptional regulator RHAMM provides a timing mechanism that controls appropriate wound re-epithelialization. J. Biol. Chem. 2020;295:5427–5448. doi: 10.1074/jbc.RA119.010002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang J., Li D., Shen W., Sun W., Gao R., Jiang P., Wang L., Liu Y., Chen Y., Zhou W., et al. RHAMM inhibits cell migration via the AKT/GSK3beta/Snail axis in luminal A subtype breast cancer. Anat. Rec. 2020;303:2344–2356. doi: 10.1002/ar.24321. [DOI] [PubMed] [Google Scholar]
- 10.Tolg C., Hamilton S.R., Nakrieko K.A., Kooshesh F., Walton P., McCarthy J.B., Bissell M., Turley E.A. Rhamm−/− fibroblasts are defective in CD44-mediated ERK1,2 motogenic signaling, leading to defective skin wound repair. J. Cell Biol. 2006;175:1017–1028. doi: 10.1083/jcb.200511027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hardwick C., Hoare K., Owens R., Hohn H.P., Hook M., Moore D., Cripps V., Austen L., Nance D.M., Turley E.A. Molecular cloning of a novel hyaluronan receptor that mediates tumor cell motility. J. Cell Biol. 1992;117:1343–1350. doi: 10.1083/jcb.117.6.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu M., Tolg C., Turley E. Dissecting the Dual Nature of Hyaluronan in the Tumor Microenvironment. Front. Immunol. 2019;10:947. doi: 10.3389/fimmu.2019.00947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garantziotis S., Brezina M., Castelnuovo P., Drago L. The role of hyaluronan in the pathobiology and treatment of respiratory disease. Am. J. Physiol. Lung Cell Mol. Physiol. 2016;310:L785–L795. doi: 10.1152/ajplung.00168.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garantziotis S., Savani R.C. Hyaluronan biology: A complex balancing act of structure, function, location and context. Matrix Biol. 2019;78–79:1–10. doi: 10.1016/j.matbio.2019.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tavianatou A.G., Caon I., Franchi M., Piperigkou Z., Galesso D., Karamanos N.K. Hyaluronan: Molecular size-dependent signaling and biological functions in inflammation and cancer. FEBS J. 2019;286:2883–2908. doi: 10.1111/febs.14777. [DOI] [PubMed] [Google Scholar]
- 16.Nikitovic D., Tzardi M., Berdiaki A., Tsatsakis A., Tzanakakis G.N. Cancer microenvironment and inflammation: Role of hyaluronan. Front. Immunol. 2015;6:169. doi: 10.3389/fimmu.2015.00169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adamo A., Frusteri C., Pallotta M.T., Pirali T., Sartoris S., Ugel S. Moonlighting Proteins Are Important Players in Cancer Immunology. Front. Immunol. 2020;11:613069. doi: 10.3389/fimmu.2020.613069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nuno-Cabanes C., Rodriguez-Navarro S. The promiscuity of the SAGA complex subunits: Multifunctional or moonlighting proteins? Biochim. Biophys. Acta Gene Regul. Mech. 2021;1864:194607. doi: 10.1016/j.bbagrm.2020.194607. [DOI] [PubMed] [Google Scholar]
- 19.Prud’homme B., Gompel N., Carroll S.B. Emerging principles of regulatory evolution. Proc. Natl. Acad. Sci. USA. 2007;104((Suppl. 1)):8605–8612. doi: 10.1073/pnas.0700488104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kobayashi T., Chanmee T., Itano N. Hyaluronan: Metabolism and Function. Biomolecules. 2020;10:1525. doi: 10.3390/biom10111525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Triggs-Raine B., Natowicz M.R. Biology of hyaluronan: Insights from genetic disorders of hyaluronan metabolism. World J. Biol. Chem. 2015;6:110–120. doi: 10.4331/wjbc.v6.i3.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu J., Yan W., Han P., Tian D. The emerging role of KIAA1199 in cancer development and therapy. Biomed. Pharmacother. 2021;138:111507. doi: 10.1016/j.biopha.2021.111507. [DOI] [PubMed] [Google Scholar]
- 23.Yamaguchi Y., Yamamoto H., Tobisawa Y., Irie F. TMEM2: A missing link in hyaluronan catabolism identified? Matrix Biol. 2019;78–79:139–146. doi: 10.1016/j.matbio.2018.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tolg C., McCarthy J.B., Yazdani A., Turley E.A. Hyaluronan and RHAMM in wound repair and the “cancerization” of stromal tissues. Biomed. Res. Int. 2014;2014:103923. doi: 10.1155/2014/103923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weigel P.H. Planning, evaluating and vetting receptor signaling studies to assess hyaluronan size-dependence and specificity. Glycobiology. 2017;27:796–799. doi: 10.1093/glycob/cwx056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Skandalis S.S., Karalis T., Heldin P. Intracellular hyaluronan: Importance for cellular functions. Semin. Cancer Biol. 2020;62:20–30. doi: 10.1016/j.semcancer.2019.07.002. [DOI] [PubMed] [Google Scholar]
- 27.Turley E.A., Noble P.W., Bourguignon L.Y. Signaling properties of hyaluronan receptors. J. Biol. Chem. 2002;277:4589–4592. doi: 10.1074/jbc.R100038200. [DOI] [PubMed] [Google Scholar]
- 28.Avenoso A., Bruschetta G., D’Ascola A., Scuruchi M., Mandraffino G., Saitta A., Campo S., Campo G. Hyaluronan Fragmentation During Inflammatory Pathologies: A Signal that Empowers Tissue Damage. Mini Rev. Med. Chem. 2020;20:54–65. doi: 10.2174/1389557519666190906115619. [DOI] [PubMed] [Google Scholar]
- 29.Jackson D.G. Hyaluronan in the lymphatics: The key role of the hyaluronan receptor LYVE-1 in leucocyte trafficking. Matrix Biol. 2019;78–79:219–235. doi: 10.1016/j.matbio.2018.02.001. [DOI] [PubMed] [Google Scholar]
- 30.Pratt R.L. Hyaluronan and the Fascial Frontier. Int. J. Mol. Sci. 2021;22:6845. doi: 10.3390/ijms22136845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tammi M.I., Oikari S., Pasonen-Seppanen S., Rilla K., Auvinen P., Tammi R.H. Activated hyaluronan metabolism in the tumor matrix—Causes and consequences. Matrix Biol. 2019;78–79:147–164. doi: 10.1016/j.matbio.2018.04.012. [DOI] [PubMed] [Google Scholar]
- 32.Bollyky P.L., Bogdani M., Bollyky J.B., Hull R.L., Wight T.N. The role of hyaluronan and the extracellular matrix in islet inflammation and immune regulation. Curr. Diab. Rep. 2012;12:471–480. doi: 10.1007/s11892-012-0297-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spinelli F.M., Vitale D.L., Sevic I., Alaniz L. Hyaluronan in the Tumor Microenvironment. Adv. Exp. Med. Biol. 2020;1245:67–83. doi: 10.1007/978-3-030-40146-7_3. [DOI] [PubMed] [Google Scholar]
- 34.Joy R.A., Vikkath N., Ariyannur P.S. Metabolism and mechanisms of action of hyaluronan in human biology. Drug Metab. Pers. Ther. 2018;33:15–32. doi: 10.1515/dmpt-2017-0031. [DOI] [PubMed] [Google Scholar]
- 35.Fam H., Bryant J.T., Kontopoulou M. Rheological properties of synovial fluids. Biorheology. 2007;44:59–74. [PubMed] [Google Scholar]
- 36.Gupta R.C., Lall R., Srivastava A., Sinha A. Hyaluronic Acid: Molecular Mechanisms and Therapeutic Trajectory. Front. Vet. Sci. 2019;6:192. doi: 10.3389/fvets.2019.00192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rebenda D., Vrbka M., Cipek P., Toropitsyn E., Necas D., Pravda M., Harti M. On the Dependence of Rheology of Hyaluronic Acid Solutions and Frictional Behavior of Articular Cartilage. Materials. 2020;13:2659. doi: 10.3390/ma13112659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kavasi R.M., Berdiaki A., Spyridaki I., Corsini E., Tsatsakis A., Tzanakakis G., Nikitovic D. HA metabolism in skin homeostasis and inflammatory disease. Food Chem. Toxicol. 2017;101:128–138. doi: 10.1016/j.fct.2017.01.012. [DOI] [PubMed] [Google Scholar]
- 39.Frevert C.W., Felgenhauer J., Wygrecka M., Nastase M.V., Schaefer L. Danger-Associated Molecular Patterns Derived From the Extracellular Matrix Provide Temporal Control of Innate Immunity. J. Histochem. Cytochem. 2018;66:213–227. doi: 10.1369/0022155417740880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Takeo S., Fujise M., Akiyama T., Habuchi H., Itano N., Matsuo T., Aigaki T., Kimata K., Nakato H. In vivo hyaluronan synthesis upon expression of the mammalian hyaluronan synthase gene in Drosophila. J. Biol. Chem. 2004;279:18920–18925. doi: 10.1074/jbc.M314293200. [DOI] [PubMed] [Google Scholar]
- 41.Csoka A.B., Stern R. Hypotheses on the evolution of hyaluronan: A highly ironic acid. Glycobiology. 2013;23:398–411. doi: 10.1093/glycob/cws218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.DeAngelis P.L. Evolution of glycosaminoglycans and their glycosyltransferases: Implications for the extracellular matrices of animals and the capsules of pathogenic bacteria. Anat. Rec. 2002;268:317–326. doi: 10.1002/ar.10163. [DOI] [PubMed] [Google Scholar]
- 43.He Z., Mei L., Connell M., Maxwell C.A. Hyaluronan Mediated Motility Receptor (HMMR) Encodes an Evolutionarily Conserved Homeostasis, Mitosis, and Meiosis Regulator Rather than a Hyaluronan Receptor. Cells. 2020;9:819. doi: 10.3390/cells9040819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Paschinger K., Wilson I.B.H. Anionic and zwitterionic moieties as widespread glycan modifications in non-vertebrates. Glycoconj J. 2020;37:27–40. doi: 10.1007/s10719-019-09874-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kamimura K., Maeda N. Glypicans and Heparan Sulfate in Synaptic Development, Neural Plasticity, and Neurological Disorders. Front. Neural Circuits. 2021;15:595596. doi: 10.3389/fncir.2021.595596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nishihara S. Glycosyltransferases and transporters that contribute to proteoglycan synthesis in Drosophila: Identification and functional analyses using the heritable and inducible RNAi system. Methods Enzymol. 2010;480:323–351. doi: 10.1016/S0076-6879(10)80015-1. [DOI] [PubMed] [Google Scholar]
- 47.Stern R. Go Fly a Chitin: The Mystery of Chitin and Chitinases in Vertebrate Tissues. Front. Biosci. 2017;22:580–595. doi: 10.2741/4504. [DOI] [PubMed] [Google Scholar]
- 48.Yoshida M., Itano N., Yamada Y., Kimata K. In vitro synthesis of hyaluronan by a single protein derived from mouse HAS1 gene and characterization of amino acid residues essential for the activity. J. Biol. Chem. 2000;275:497–506. doi: 10.1074/jbc.275.1.497. [DOI] [PubMed] [Google Scholar]
- 49.Yoneda M., Nakamura T., Murai M., Wada H. Evidence for the heparin-binding ability of the ascidian Xlink domain and insight into the evolution of the Xlink domain in chordates. J. Mol. Evol. 2010;71:51–59. doi: 10.1007/s00239-010-9363-x. [DOI] [PubMed] [Google Scholar]
- 50.Kawashima T., Kawashima S., Tanaka C., Murai M., Yoneda M., Putnam N.H., Rokhsar D.S., Kanehisa M., Satoh N., Wada H. Domain shuffling and the evolution of vertebrates. Genome Res. 2009;19:1393–1403. doi: 10.1101/gr.087072.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rozbesky D., Monistrol J., Jain V., Hillier J., Padilla-Parra S., Jones E.Y. Drosophila OTK Is a Glycosaminoglycan-Binding Protein with High Conformational Flexibility. Structure. 2020;28:507–515.e5. doi: 10.1016/j.str.2020.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bernhofer M., Dallago C., Karl T., Satagopam V., Heinzinger M., Littmann M., Olenyi T., Qiu J., Schutze K., Yachdav G., et al. PredictProtein-Predicting Protein Structure and Function for 29 Years. Nucleic Acids Res. 2021;49:W535–W540. doi: 10.1093/nar/gkab354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang B., Hall C.L., Yang B.L., Savani R.C., Turley E.A. Identification of a novel heparin binding domain in RHAMM and evidence that it modifies HA mediated locomotion of ras-transformed cells. J. Cell Biochem. 1994;56:455–468. doi: 10.1002/jcb.240560406. [DOI] [PubMed] [Google Scholar]
- 54.Tolg C., Hamilton S.R., Morningstar L., Zhang J., Zhang S., Esguerra K.V., Telmer P.G., Luyt L.G., Harrison R., McCarthy J.B., et al. RHAMM promotes interphase microtubule instability and mitotic spindle integrity through MEK1/ERK1/2 activity. J. Biol. Chem. 2010;285:26461–26474. doi: 10.1074/jbc.M110.121491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang N., Yao L.L., Li X.D. Regulation of class V myosin. Cell Mol. Life Sci. 2018;75:261–273. doi: 10.1007/s00018-017-2599-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Abriata L.A., Albanesi D., Dal Peraro M., de Mendoza D. Signal Sensing and Transduction by Histidine Kinases as Unveiled through Studies on a Temperature Sensor. Acc. Chem. Res. 2017;50:1359–1366. doi: 10.1021/acs.accounts.6b00593. [DOI] [PubMed] [Google Scholar]
- 57.Moore R., Vogt K., Acosta-Martin A.E., Shire P., Zeidler M., Smythe E. Integration of JAK/STAT receptor-ligand trafficking, signalling and gene expression in Drosophila melanogaster cells. J. Cell Sci. 2020;133:jcs246199. doi: 10.1242/jcs.246199. [DOI] [PubMed] [Google Scholar]
- 58.Chen H., Mohan P., Jiang J., Nemirovsky O., He D., Fleisch M.C., Niederacher D., Pilarski L.M., Lim C.J., Maxwell C.A. Spatial regulation of Aurora A activity during mitotic spindle assembly requires RHAMM to correctly localize TPX2. Cell Cycle. 2014;13:2248–2261. doi: 10.4161/cc.29270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Meier C., Spitschak A., Abshagen K., Gupta S., Mor J.M., Wolkenhauer O., Haier J., Vollmar B., Alla V., Pützer B.M. Association of RHAMM with E2F1 promotes tumour cell extravasation by transcriptional up-regulation of fibronectin. J. Pathol. 2014;234:351–364. doi: 10.1002/path.4400. [DOI] [PubMed] [Google Scholar]
- 60.Yamasaki C., Tashiro S., Nishito Y., Sueda T., Igarashi K. Dynamic cytoplasmic anchoring of the transcription factor Bach1 by intracellular hyaluronic acid binding protein IHABP. J. Biochem. 2005;137:287–296. doi: 10.1093/jb/mvi031. [DOI] [PubMed] [Google Scholar]
- 61.Chen Y.T., Chen Z., Du Y.N. Immunohistochemical analysis of RHAMM expression in normal and neoplastic human tissues: A cell cycle protein with distinctive expression in mitotic cells and testicular germ cells. Oncotarget. 2018;9:20941–20952. doi: 10.18632/oncotarget.24939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Savani R.C. Modulators of inflammation in Bronchopulmonary Dysplasia. Semin. Perinatol. 2018;42:459–470. doi: 10.1053/j.semperi.2018.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Misra S., Hascall V.C., Markwald R.R., Ghatak S. Interactions between Hyaluronan and Its Receptors (CD44, RHAMM) Regulate the Activities of Inflammation and Cancer. Front. Immunol. 2015;6:201. doi: 10.3389/fimmu.2015.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jain E., Bairoch A., Duvaud S., Phan I., Redaschi N., Suzek B.E., Martin M.J., McGarvey P., Gasteiger E. Infrastructure for the life sciences: Design and implementation of the UniProt website. BMC Bioinf. 2009;10:136. doi: 10.1186/1471-2105-10-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Villegas-Ruiz V., Salcedo M., Zentella-Dehesa A., de Oca E.V., Roman-Basaure E., Mantilla-Morales A., Dávila-Borja V.M., Juárez-Méndez S. A case of cervical cancer expressed three mRNA variant of Hyaluronan-mediated motility receptor. Int. J. Clin. Exp. Pathol. 2014;7:2256–2264. [PMC free article] [PubMed] [Google Scholar]
- 66.Crainie M., Belch A.R., Mant M.J., Pilarski L.M. Overexpression of the receptor for hyaluronan-mediated motility (RHAMM) characterizes the malignant clone in multiple myeloma: Identification of three distinct RHAMM variants. Blood. 1999;93:1684–1696. doi: 10.1182/blood.V93.5.1684. [DOI] [PubMed] [Google Scholar]
- 67.Maxwell C.A., Rasmussen E., Zhan F., Keats J.J., Adamia S., Strachan E., Crainie M., Walker R., Belch A.R., Pilarski L.M., et al. RHAMM expression and isoform balance predict aggressive disease and poor survival in multiple myeloma. Blood. 2004;104:1151–1158. doi: 10.1182/blood-2003-11-4079. [DOI] [PubMed] [Google Scholar]
- 68.Assmann V., Jenkinson D., Marshall J.F., Hart I.R. The intracellular hyaluronan receptor RHAMM/IHABP interacts with microtubules and actin filaments. Pt 22J. Cell Sci. 1999;112:3943–3954. doi: 10.1242/jcs.112.22.3943. [DOI] [PubMed] [Google Scholar]
- 69.Hamilton S.R., Fard S.F., Paiwand F.F., Tolg C., Veiseh M., Wang C., McCarthy J.B., Bissell M.J., Koropatnick J., Turley E.A. The hyaluronan receptors CD44 and Rhamm (CD168) form complexes with ERK1,2 that sustain high basal motility in breast cancer cells. J. Biol. Chem. 2007;282:16667–16680. doi: 10.1074/jbc.M702078200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Line A., Slucka Z., Stengrevics A., Silina K., Li G., Rees R.C. Characterisation of tumour-associated antigens in colon cancer. Cancer Immunol. Immunother. 2002;51:574–582. doi: 10.1007/s00262-002-0322-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Du Y.C., Chou C.K., Klimstra D.S., Varmus H. Receptor for hyaluronan-mediated motility isoform B promotes liver metastasis in a mouse model of multistep tumorigenesis and a tail vein assay for metastasis. Proc. Natl. Acad. Sci. USA. 2011;108:16753–16758. doi: 10.1073/pnas.1114022108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Entwistle J., Zhang S., Yang B., Wong C., Li Q., Hall C.L., Mowat M., Greenberg A.H., Turley E.A. Characterization of the murine gene encoding the hyaluronan receptor RHAMM. Gene. 1995;163:233–238. doi: 10.1016/0378-1119(95)00398-P. [DOI] [PubMed] [Google Scholar]
- 73.Zaman A., Cui Z., Foley J.P., Zhao H., Grimm P.C., Delisser H.M., Savani R.C. Expression and role of the hyaluronan receptor RHAMM in inflammation after bleomycin injury. Am. J. Respir. Cell Mol. Biol. 2005;33:447–454. doi: 10.1165/rcmb.2004-0333OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yang B., Yang B.L., Savani R.C., Turley E.A. Identification of a common hyaluronan binding motif in the hyaluronan binding proteins RHAMM, CD44 and link protein. EMBO J. 1994;13:286–296. doi: 10.1002/j.1460-2075.1994.tb06261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tolg C., Yuan H., Flynn S.M., Basu K., Ma J., Tse K.C.K., Kowalska B., Vullanesku D., Cowman M.K., McCarthy J.B., et al. Hyaluronan modulates growth factor induced mammary gland branching in a size dependent manner. Matrix Biol. 2017;63:117–132. doi: 10.1016/j.matbio.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 76.Hatano H., Shigeishi H., Kudo Y., Higashikawa K., Tobiume K., Takata T., Kamata N. RHAMM/ERK interaction induces proliferative activities of cementifying fibroma cells through a mechanism based on the CD44-EGFR. Lab. Investig. 2011;91:379–391. doi: 10.1038/labinvest.2010.176. [DOI] [PubMed] [Google Scholar]
- 77.Zhang S., Chang M.C., Zylka D., Turley S., Harrison R., Turley E.A. The hyaluronan receptor RHAMM regulates extracellular-regulated kinase. J. Biol. Chem. 1998;273:11342–11348. doi: 10.1074/jbc.273.18.11342. [DOI] [PubMed] [Google Scholar]
- 78.Carvalho A.M., Soares da Costa D., Paulo P.M.R., Reis R.L., Pashkuleva I. Co-localization and crosstalk between CD44 and RHAMM depend on hyaluronan presentation. Acta Biomater. 2021;119:114–124. doi: 10.1016/j.actbio.2020.10.024. [DOI] [PubMed] [Google Scholar]
- 79.Kouvidi K., Berdiaki A., Tzardi M., Karousou E., Passi A., Nikitovic D., Tzanakakis G.N. Receptor for hyaluronic acid- mediated motility (RHAMM) regulates HT1080 fibrosarcoma cell proliferation via a beta-catenin/c-myc signaling axis. Biochim. Biophys. Acta. 2016;1860:814–824. doi: 10.1016/j.bbagen.2016.01.019. [DOI] [PubMed] [Google Scholar]
- 80.Blanco I., Kuchenbaecker K., Cuadras D., Wang X., Barrowdale D., de Garibay G.R., Librado P., Sánchez-Gracia A., Rozas J., Bonifaci N., et al. Assessing associations between the AURKA-HMMR-TPX2-TUBG1 functional module and breast cancer risk in BRCA1/2 mutation carriers. PLoS ONE. 2015;10:e0120020. doi: 10.1371/journal.pone.0120020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tolg C., Telmer P., Turley E. Specific sizes of hyaluronan oligosaccharides stimulate fibroblast migration and excisional wound repair. PLoS ONE. 2014;9:e88479. doi: 10.1371/journal.pone.0088479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ziebell M.R., Prestwich G.D. Interactions of peptide mimics of hyaluronic acid with the receptor for hyaluronan mediated motility (RHAMM) J. Comput. Aided Mol. Des. 2004;18:597–614. doi: 10.1007/s10822-004-5433-8. [DOI] [PubMed] [Google Scholar]
- 83.Hauser-Kawaguchi A., Luyt L.G., Turley E. Design of peptide mimetics to block pro-inflammatory functions of HA fragments. Matrix Biol. 2019;78–79:346–356. doi: 10.1016/j.matbio.2018.01.021. [DOI] [PubMed] [Google Scholar]
- 84.Esguerra K.V., Tolg C., Akentieva N., Price M., Cho C.F., Lewis J.D., McCarthy J.B., Turley E.A., Luyt L.G. Identification, design and synthesis of tubulin-derived peptides as novel hyaluronan mimetic ligands for the receptor for hyaluronan-mediated motility (RHAMM/HMMR) Integr. Biol. 2015;7:1547–1560. doi: 10.1039/C5IB00222B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Boittier E.D., Gandhi N.S., Ferro V., Coombe D.R. Cross-Species Analysis of Glycosaminoglycan Binding Proteins Reveals Some Animal Models Are “More Equal” than Others. Molecules. 2019;24:924. doi: 10.3390/molecules24050924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gulati K., Jamsandekar M., Poluri K.M. Mechanistic insights into molecular evolution of species-specific differential glycosaminoglycan binding surfaces in growth-related oncogene chemokines. R. Soc. Open Sci. 2017;4:171059. doi: 10.1098/rsos.171059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Liang W.G., Triandafillou C.G., Huang T.Y., Zulueta M.M., Banerjee S., Dinner A.R., Hung S.H., Tang W.J. Structural basis for oligomerization and glycosaminoglycan binding of CCL5 and CCL3. Proc. Natl. Acad. Sci. USA. 2016;113:5000–5005. doi: 10.1073/pnas.1523981113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fermas S., Gonnet F., Sutton A., Charnaux N., Mulloy B., Du Y., Baleux F., Daniel R. Sulfated oligosaccharides (heparin and fucoidan) binding and dimerization of stromal cell-derived factor-1 (SDF-1/CXCL 12) are coupled as evidenced by affinity CE-MS analysis. Glycobiology. 2008;18:1054–1064. doi: 10.1093/glycob/cwn088. [DOI] [PubMed] [Google Scholar]
- 89.Crown S.E., Yu Y., Sweeney M.D., Leary J.A., Handel T.M. Heterodimerization of CCR2 chemokines and regulation by glycosaminoglycan binding. J. Biol. Chem. 2006;281:25438–25446. doi: 10.1074/jbc.M601518200. [DOI] [PubMed] [Google Scholar]
- 90.Wu K., Kim S., Liu V.M., Sabino A., Minkhorst K., Yazdani A., Turley E.A. Function-blocking RHAMM peptides attenuate fibrosis and promote anti-fibrotic adipokines in a bleomycin-induced murine model of systemic sclerosis. J. Investig. Dermatol. 2021;141:1482–1492.e4. doi: 10.1016/j.jid.2019.11.032. [DOI] [PubMed] [Google Scholar]
- 91.Truong J.L., Liu M., Tolg C., Barr M., Dai C., Raissi T.C., Wong E., DeLyzer T., Yazdani A., Turley E.A. Creating a Favorable Micro-Environment for Fat Grafting in a Novel Model of Radiation Induced Mammary Fat Pad Fibrosis. Plast Reconstr. Surg. 2020;145:116–126. doi: 10.1097/PRS.0000000000006344. [DOI] [PubMed] [Google Scholar]
- 92.Cui Z., Liao J., Cheong N., Longoria C., Cao G., DeLisser H.M., Savani R.C. The Receptor for Hyaluronan-Mediated Motility (CD168) promotes inflammation and fibrosis after acute lung injury. Matrix Biol. 2019;78–79:255–271. doi: 10.1016/j.matbio.2018.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Foley J.P., Lam D., Jiang H., Liao J., Cheong N., McDevitt T.M., Zaman A., Wright J.R., Savani R.C. Toll-like receptor 2 (TLR2), transforming growth factor-beta, hyaluronan (HA), and receptor for HA-mediated motility (RHAMM) are required for surfactant protein A-stimulated macrophage chemotaxis. J. Biol. Chem. 2012;287:37406–37419. doi: 10.1074/jbc.M112.360982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bahrami S.B., Tolg C., Peart T., Symonette C., Veiseh M., Umoh J.U., Holdsworth D.W., McCarthy J.B., Luyt M.J., Bissell M.J., et al. Receptor for hyaluronan mediated motility (RHAMM/HMMR) is a novel target for promoting subcutaneous adipogenesis. Integr. Biol. 2017;9:223–237. doi: 10.1039/C7IB00002B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shigeishi H., Higashikawa K., Takechi M. Role of receptor for hyaluronan-mediated motility (RHAMM) in human head and neck cancers. J. Cancer Res. Clin. Oncol. 2014;140:1629–1640. doi: 10.1007/s00432-014-1653-z. [DOI] [PubMed] [Google Scholar]
- 96.Song J.M., Im J., Nho R.S., Han Y.H., Upadhyaya P., Kassie F. Hyaluronan-CD44/RHAMM interaction-dependent cell proliferation and survival in lung cancer cells. Mol. Carcinog. 2019;58:321–333. doi: 10.1002/mc.22930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Veiseh M., Kwon D.H., Borowsky A.D., Tolg C., Leong H.S., Lewis J.D., Turley E.A., Bissell M.J. Cellular heterogeneity profiling by hyaluronan probes reveals an invasive but slow-growing breast tumor subset. Proc. Natl. Acad. Sci. USA. 2014;111:E1731–E1739. doi: 10.1073/pnas.1402383111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Balmer E.A., Faso C. The Road Less Traveled? Unconventional Protein Secretion at Parasite-Host Interfaces. Front. Cell Dev. Biol. 2021;9:662711. doi: 10.3389/fcell.2021.662711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhang W., Lavine K.J., Epelman S., Evans S.A., Weinheimer C.J., Barger P.M., Mann D.L. Necrotic myocardial cells release damage-associated molecular patterns that provoke fibroblast activation in vitro and trigger myocardial inflammation and fibrosis in vivo. J. Am. Heart Assoc. 2015;4:e001993. doi: 10.1161/JAHA.115.001993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kahroba H., Davatgaran-Taghipour Y. Exosomal Nrf2: From anti-oxidant and anti-inflammation response to wound healing and tissue regeneration in aged-related diseases. Biochimie. 2020;171–172:103–109. doi: 10.1016/j.biochi.2020.02.011. [DOI] [PubMed] [Google Scholar]
- 101.Hascall V.C., Majors A.K., De La Motte C.A., Evanko S.P., Wang A., Drazba J.A., Strong S.A., Wight T.N. Intracellular hyaluronan: A new frontier for inflammation? Biochim. Biophys. Acta. 2004;1673:3–12. doi: 10.1016/j.bbagen.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 102.Evanko S.P., Parks W.T., Wight T.N. Intracellular hyaluronan in arterial smooth muscle cells: Association with microtubules, RHAMM, and the mitotic spindle. J. Histochem. Cytochem. 2004;52:1525–1535. doi: 10.1369/jhc.4A6356.2004. [DOI] [PubMed] [Google Scholar]
- 103.Joukov V., Groen A.C., Prokhorova T., Gerson R., White E., Rodriguez A., Walter J.C., Livingston D.M. The BRCA1/BARD1 heterodimer modulates ran-dependent mitotic spindle assembly. Cell. 2006;127:539–552. doi: 10.1016/j.cell.2006.08.053. [DOI] [PubMed] [Google Scholar]
- 104.Tolg C., Hamilton S.R., Zalinska E., McCulloch L., Amin R., Akentieva N., Winnik F., Savani R., Bagli D.J., Luyt L.G., et al. A RHAMM mimetic peptide blocks hyaluronan signaling and reduces inflammation and fibrogenesis in excisional skin wounds. Am. J. Pathol. 2012;181:1250–1270. doi: 10.1016/j.ajpath.2012.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Nikonova A.S., Astsaturov I., Serebriiskii I.G., Dunbrack R.L., Golemis E.A. Aurora A kinase (AURKA) in normal and pathological cell division. Cell Mol. Life Sci. 2013;70:661–687. doi: 10.1007/s00018-012-1073-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Scrofani J., Sardon T., Meunier S., Vernos I. Microtubule nucleation in mitosis by a RanGTP-dependent protein complex. Curr. Biol. 2015;25:131–140. doi: 10.1016/j.cub.2014.11.025. [DOI] [PubMed] [Google Scholar]
- 107.Li H., Kroll T., Moll J., Frappart L., Herrlich P., Heuer H., Ploubidou A. Spindle Misorientation of Cerebral and Cerebellar Progenitors Is a Mechanistic Cause of Megalencephaly. Stem. Cell Rep. 2017;9:1071–1080. doi: 10.1016/j.stemcr.2017.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Silverman-Gavrila R.V., Silverman-Gavrila L.B., Bilal K.H., Bendeck M.P. Spectrin alpha is important for rear polarization of the microtubule organizing center during migration and spindle pole assembly during division of neointimal smooth muscle cells. Cytoskeleton. 2015;72:157–170. doi: 10.1002/cm.21222. [DOI] [PubMed] [Google Scholar]
- 109.Dunsch A.K., Hammond D., Lloyd J., Schermelleh L., Gruneberg U., Barr F.A. Dynein light chain 1 and a spindle-associated adaptor promote dynein asymmetry and spindle orientation. J. Cell Biol. 2012;198:1039–1054. doi: 10.1083/jcb.201202112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Li H., Moll J., Winkler A., Frappart L., Brunet S., Hamann J., Kroll T., Verlhac M.H., Heuer H., Herrlich P., et al. RHAMM deficiency disrupts folliculogenesis resulting in female hypofertility. Biol. Open. 2015;4:562–571. doi: 10.1242/bio.201410892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Eibes S., Gallisa-Sune N., Rosas-Salvans M., Martinez-Delgado P., Vernos I., Roig J. Nek9 Phosphorylation Defines a New Role for TPX2 in Eg5-Dependent Centrosome Separation before Nuclear Envelope Breakdown. Curr. Biol. 2018;28:121–129.e4. doi: 10.1016/j.cub.2017.11.046. [DOI] [PubMed] [Google Scholar]
- 112.Tolg C., Poon R., Fodde R., Turley E.A., Alman B.A. Genetic deletion of receptor for hyaluronan-mediated motility (Rhamm) attenuates the formation of aggressive fibromatosis (desmoid tumor) Oncogene. 2003;22:6873–6882. doi: 10.1038/sj.onc.1206811. [DOI] [PubMed] [Google Scholar]
- 113.Bost F., Aouadi M., Caron L., Even P., Belmonte N., Prot M., Dani C., Hofman P., Pagès G., Pouysségur J., et al. The extracellular signal-regulated kinase isoform ERK1 is specifically required for in vitro and in vivo adipogenesis. Diabetes. 2005;54:402–411. doi: 10.2337/diabetes.54.2.402. [DOI] [PubMed] [Google Scholar]
- 114.Diaz-Bello B., Rangel-Garcia C.I., Salvador C., Carrisoza-Gaytan R., Escobar L.I. The polarization of the G-protein activated potassium channel GIRK5 to the vegetal pole of Xenopus laevis oocytes is driven by a di-leucine motif. PLoS ONE. 2013;8:e64096. doi: 10.1371/journal.pone.0064096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Murrell-Lagnado R.D., Frick M. P2X4 and lysosome fusion. Curr. Opin. Pharmacol. 2019;47:126–132. doi: 10.1016/j.coph.2019.03.002. [DOI] [PubMed] [Google Scholar]
- 116.Li M., Jin S., Cao Y., Xu J., Zhu S., Li Z. Emodin regulates cell cycle of non-small lung cancer (NSCLC) cells through hyaluronan synthase 2 (HA2)-HA-CD44/receptor for hyaluronic acid-mediated motility (RHAMM) interaction-dependent signaling pathway. Cancer Cell Int. 2021;21:19. doi: 10.1186/s12935-020-01711-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Thangavel C., Boopathi E., Liu Y., Haber A., Ertel A., Bhardwaj A., Addya S., Williams N., Ciment S.J., Cotzia P., et al. RB Loss Promotes Prostate Cancer Metastasis. Cancer Res. 2017;77:982–995. doi: 10.1158/0008-5472.CAN-16-1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lopez J.I., Camenisch T.D., Stevens M.V., Sands B.J., McDonald J., Schroeder J.A. CD44 attenuates metastatic invasion during breast cancer progression. Cancer Res. 2005;65:6755–6763. doi: 10.1158/0008-5472.CAN-05-0863. [DOI] [PubMed] [Google Scholar]
- 119.Wang C., Thor A.D., Moore D.H., Zhao Y., Kerschmann R., Stern R., Watson P.H., Turley E.A. The overexpression of RHAMM, a hyaluronan-binding protein that regulates ras signaling, correlates with overexpression of mitogen-activated protein kinase and is a significant parameter in breast cancer progression. Clin. Cancer Res. 1998;4:567–576. [PubMed] [Google Scholar]
- 120.Schutze A., Vogeley C., Gorges T., Twarock S., Butschan J., Babayan A., Klein D., Knauer S.K., Metzen E., Müller V., et al. RHAMM splice variants confer radiosensitivity in human breast cancer cell lines. Oncotarget. 2016;7:21428–21440. doi: 10.18632/oncotarget.7258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zhou Q., Liu X., Lv M., Sun E., Lu X., Lu C. Genes That Predict Poor Prognosis in Breast Cancer via Bioinformatical Analysis. Biomed. Res. Int. 2021;2021:6649660. doi: 10.1155/2021/6649660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Katz B.Z. Adhesion molecules--The lifelines of multiple myeloma cells. Semin. Cancer Biol. 2010;20:186–195. doi: 10.1016/j.semcancer.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 123.Zhu S.W., Wang S., Wu Z.Z., Yang Q.C., Chen D.R., Wan S.C., Sun Z.J. Overexpression of CD168 is related to poor prognosis in oral squamous cell carcinoma. Oral Dis. 2021 doi: 10.1111/odi.13766. [DOI] [PubMed] [Google Scholar]
- 124.Buttermore S.T., Hoffman M.S., Kumar A., Champeaux A., Nicosia S.V., Kruk P.A. Increased RHAMM expression relates to ovarian cancer progression. J. Ovarian Res. 2017;10:66. doi: 10.1186/s13048-017-0360-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wang D., Narula N., Azzopardi S., Smith R.S., Nasar A., Altorki N.K., Mittal V., Somwar R., Stiles B.M., Du Y.C.N. Expression of the receptor for hyaluronic acid mediated motility (RHAMM) is associated with poor prognosis and metastasis in non-small cell lung carcinoma. Oncotarget. 2016;7:39957–39969. doi: 10.18632/oncotarget.9554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Rizzardi A.E., Vogel R.I., Koopmeiners J.S., Forster C.L., Marston L.O., Rosener N.K., Akentieva N., Price M.A., Metzger G.J., Warlick C.A., et al. Elevated hyaluronan and hyaluronan-mediated motility receptor are associated with biochemical failure in patients with intermediate-grade prostate tumors. Cancer. 2014;120:1800–1809. doi: 10.1002/cncr.28646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Chen W., Gao C., Liu Y., Wen Y., Hong X., Huang Z. Bioinformatics Analysis of Prognostic miRNA Signature and Potential Critical Genes in Colon Cancer. Front. Genet. 2020;11:478. doi: 10.3389/fgene.2020.00478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lu X.Q., Zhang J.Q., Zhang S.X., Qiao J., Qiu M.T., Liu X.R., Chen X.X., Gao C., Zhang H.H. Identification of novel hub genes associated with gastric cancer using integrated bioinformatics analysis. BMC Cancer. 2021;21:697. doi: 10.1186/s12885-021-08358-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Levy P., Vidaud D., Leroy K., Laurendeau I., Wechsler J., Bolasco G., Parfait B., Wolkenstein P., Vidaud M., Bièche I. Molecular profiling of malignant peripheral nerve sheath tumors associated with neurofibromatosis type 1, based on large-scale real-time RT-PCR. Mol. Cancer. 2004;3:20. doi: 10.1186/1476-4598-3-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Schwertfeger K.L., Cowman M.K., Telmer P.G., Turley E.A., McCarthy J.B. Hyaluronan, Inflammation, and Breast Cancer Progression. Front. Immunol. 2015;6:236. doi: 10.3389/fimmu.2015.00236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Gurevich E.V., Gurevich V.V. Beyond traditional pharmacology: New tools and approaches. Br. J. Pharmacol. 2015;172:3229–3241. doi: 10.1111/bph.13066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Savani R.C., Hou G., Liu P., Wang C., Simons E., Grimm P.C., Stern R., Greenberg A.H., DeLisser H.M., Khalil N. A role for hyaluronan in macrophage accumulation and collagen deposition after bleomycin-induced lung injury. Am. J. Respir. Cell Mol. Biol. 2000;23:475–484. doi: 10.1165/ajrcmb.23.4.3944. [DOI] [PubMed] [Google Scholar]
- 133.Xu X.M., Chen Y., Chen J., Yang S., Gao F., Underhill C.B., Creswell K., Zhang L. A peptide with three hyaluronan binding motifs inhibits tumor growth and induces apoptosis. Cancer Res. 2003;63:5685–5690. [PubMed] [Google Scholar]
- 134.Akentieva N.P., Shushanov S.S. Inhibitory effect of RHAMM-target peptides on invasion of breast cancer cells. Vopr. Onkol. 2016;62:831–838. [PubMed] [Google Scholar]
- 135.Akentieva N.P., Shushanov S.S. RHAMM (receptor hyaluronan-mediated motility)-target peptides induce apoptosis in prostate cancer cells. Vopr. Onkol. 2016;62:514–518. [PubMed] [Google Scholar]