Abstract
Gliomas are the most common central nervous system tumors. New technologies, including genetic research and advanced statistical methods, revolutionize the therapeutic approach to the patient and reveal new points of treatment options. Moreover, the 2021 World Health Organization Classification of Tumors of the Central Nervous System has fundamentally changed the classification of gliomas and incorporated many molecular biomarkers. Given the rapid progress in neuro-oncology, here we compile the latest research on prognostic and predictive biomarkers in gliomas. In adult patients, IDH mutations are positive prognostic markers and have the greatest prognostic significance. However, CDKN2A deletion, in IDH-mutant astrocytomas, is a marker of the highest malignancy grade. Moreover, the presence of TERT promoter mutations, EGFR alterations, or a combination of chromosome 7 gain and 10 loss upgrade IDH-wildtype astrocytoma to glioblastoma. In pediatric patients, H3F3A alterations are the most important markers which predict the worse outcome. MGMT promoter methylation has the greatest clinical significance in predicting responses to temozolomide (TMZ). Conversely, mismatch repair defects cause hypermutation phenotype predicting poor response to TMZ. Finally, we discussed liquid biopsies, which are promising diagnostic, prognostic, and predictive techniques, but further work is needed to implement these novel technologies in clinical practice.
Keywords: biomarker, brain neoplasms, gliomas, liquid biopsy, predictive value, prognosis, WHO CNS5
1. Introduction
Gliomas, broadly categorized by their cell of origin, are the most common central nervous system (CNS) tumors [1]. Gliomas account for ~30% of all primary brain tumors, 80% of all malignant ones, and the vast majority of deaths caused by primary brain tumors [2]. The incidence of gliomas in adults varies from 1.9 to 9.6 per 100,000 depending on age, sex, ethnicity, and geographic location [3,4]. The median age at diagnosis varies by histological subtype, with pilocytic astrocytomas occurring more frequently in children and adolescents, low-grade oligodendrogliomas peaking in the third and fourth decades, and glioblastomas mainly presenting in patients over 50 years of age [5,6]. Although most gliomas occur in the four lobes of the brain [frontal (23.6%), temporal (17.4%), parietal (10.6%), and occipital (2.8%)], a small proportion can appear in the brain stem, cerebellum, and spinal cord [3]. Survival outcomes are largely dependent on grade, with CNS World Health Organization (WHO) grade 1 having the best relative survival [7,8], and CNS WHO grade 4 having the worst overall survival (OS) rate, with just 6.8% of patients living for five years after diagnosis [7].
The initial management of gliomas usually consists of maximally safe surgical resection, which in addition to reducing tumor volume allows for tissue acquisition for an accurate histological diagnosis and tumor genotyping [9]. This is often followed by radiotherapy (RT) and temozolomide (TMZ) chemotherapy [10]. The emergence of tumor-treating fields (TTFields)—low-intensity alternating electric fields that disrupt mitosis at the metaphase to anaphase transition—has also recently emerged as a promising modality to improve standard of care [11,12]. Furthermore, TTFields alter cellular membranes, rendering cells more permeable to chemotherapeutics [13], so when added to standard of care in randomized phase III clinical studies, TTFields increase life expectancy by four months with minimal side-effects [14].
Prognostic and predictive markers play an important role in clinical practice for the assessment of prognosis and the selection of appropriate therapy. This is especially important in gliomas due to the possible occurrence of so-called pseudoprogression in MRI [15]. Pseudoprogression is chemo- and radiation-induced brain tissue reaction that resembles true tumor progression in 30% of patients receiving standard of care for glioblastoma (GBM), which could be distinguished based on biomarker analysis [16]. Moreover, given advances in sequencing technologies and resulting knowledge about genetic changes occurring in tumors, new biomarkers are expected to significantly improve patient management. Due to the rapid progress in neuro-oncology, here we compile the latest research on prognostic and predictive biomarkers in gliomas.
2. The 2021 WHO Classification of Tumors of the Central Nervous System
The fifth edition of the WHO Classification of Tumors of the Central Nervous System (WHO CNS5) is the current international standard for glioma nomenclature and diagnosis. Traditionally, CNS tumor grading has been based exclusively on histological features, but certain biomarkers can now provide powerful prognostic information. Therefore, molecular parameters have been added to more precisely grade gliomas and for further estimating prognosis within multiple tumor types. In 2016, the WHO CNS classification for the first time used molecular markers to classify gliomas, and in 2021, placed even more emphasis on them [1,17]. Numerous molecular alterations with clinicopathologic usefulness are included in WHO CNS5 [1]. In this classification system, the primary genetic markers for gliomas are IDH mutation status, codeletion of chromosomal arms 1p and 19q (1p/19q codeletion), H3F3A alterations, nuclear alpha-thalassemia/mental retardation X-linked syndrome (ATRX) gene mutations, O6-methylguanine-DNA methyltransferase (MGMT) promoter methylation status, loss of cyclin-dependent kinase inhibitor 2A (CDKN2A), epidermal growth factor receptor (EGFR) amplification, combined gain of chromosome 7 and loss of chromosome 10 (7+/10−), and telomerase reverse transcriptase (TERT) promoter mutations [18]. The great number of biomarkers not only indicates a paradigm change in classification, but it also has implications for how patients with these malignancies are managed clinically.
Moreover, several major changes have been implemented in the novel classification. WHO CNS5 has developed a new method to classify gliomas and grouped them into six different families (see Table 1). Importantly, WHO CNS5 distinguishes between diffuse gliomas that predominantly affect adults (referred to as “adult-type”) and those that primarily affect children (referred to as “pediatric-type”). Neoplasms are graded within types using Arabic numerals rather than across different tumor types using Roman numerals [19]. The terms diffuse or anaplastic are no longer used to describe the grade of malignancy. This simplifies the classification, for example astrocytoma, IDH-mutant covers grades 2–4 and eliminates the terms “glioblastoma, IDH-mutant”, “diffuse astrocytoma”, “anaplastic astrocytoma”. Moreover, in the context of a pediatric-type tumor, the term “glioblastoma” is no longer used. Furthermore, previously, certain tumor names included anatomic site modifiers, while others did not, despite the fact that they occurred in specified areas. Names have therefore been simplified as much as possible, and only location, age, or genetic modifiers with clinical utility have been used [1].
Table 1.
Families and types of gliomas according to the 2021 World Health Organization Classification of Tumors of the Central Nervous System [1].
| Families | Types |
|---|---|
| Adult-type diffuse gliomas | Astrocytoma, IDH-mutant |
| Oligodendroglioma, IDH-mutant, and 1p/19q-codeleted | |
| Glioblastoma, IDH-wildtype | |
| Pediatric-type diffuse low-grade gliomas | Diffuse astrocytoma, MYB- or MYBL1-altered |
| Angiocentric glioma | |
| Polymorphous low-grade neuroepithelial tumor of the young | |
| Diffuse low-grade glioma, MAPK pathway-altered | |
| Pediatric-type diffuse high-grade gliomas | Diffuse midline glioma, H3 K27-altered |
| Diffuse hemispheric glioma, H3 G34-mutant | |
| Diffuse pediatric-type high-grade glioma, H3-wildtype and IDH-wildtype | |
| Infant-type hemispheric glioma | |
| Circumscribed astrocytic gliomas | Pilocytic astrocytoma |
| High-grade astrocytoma with piloid features | |
| Pleomorphic xanthoastrocytoma | |
| Subependymal giant cell astrocytoma | |
| Chordoid glioma | |
| Astroblastoma, MN1-altered |
3. IDH1 and IDH2
Recurrent mutations in the active site of IDH1, occurring in 12% of malignant gliomas, were first reported in 2008 [20]. Although IDH1 and IDH2 are highly similar and catalyze identical reactions, their expression differs in different cancers and their subtypes. IDH1 mutations predominate in gliomas [21,22] and are nearly all caused by a single amino acid substitution at codon 132 (Figure 1A) [23]. Cancer-associated IDH1 mutations produce R(−)-2-hydroxyglutarate (2HG) instead of α-ketoglutarate [24], the latter altering cancer metabolism and creating oxidative stress [25]. Alpha-ketoglutarate levels influence the hypoxia-inducible factor subunit HIF-1α, a transcription factor that promotes tumor growth when oxygen levels are low [26] and also inhibit histone demethylation, which is essential for the terminal differentiation of lineage-specific progenitor cells [27]. NADPH production is impaired in gliomas with IDH1 mutations, which may sensitize the tumors to radiation and chemotherapy, explaining why patients with IDH mutant neoplasms live longer [28]. Moreover, IDH1 mutations occurred in a substantial proportion of patients, who were on average 17 years younger than patients who did not have this abnormality [20,28,29].
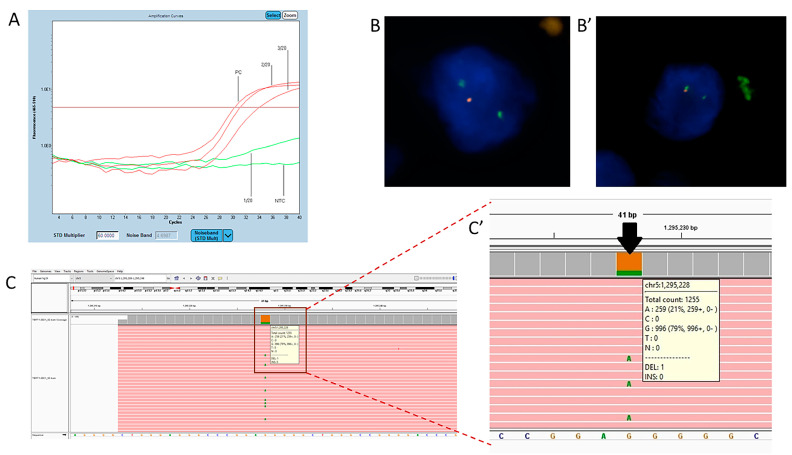
Figure 1.
Application of fluorescence in situ hybridization (FISH), next-generation sequencing (NGS), and quantitative polymerase chain reaction (qPCR) in the genetic diagnosis of glioma. (A) Representative screenshot of an isocitrate dehydrogenase 1 (IDH1) mutation. Red amplification curves represent positive signals for the mutation in exon 4, codon 132 of the IDH1 gene detected in positive control (PC) and two patients’ samples (2/20 and 3/20). The test does not distinguish between IDH1 changes: R132H (c.395G>A), R132C (c.394C>T), R132S (c.394C>A), R132G (c.394C>G), R132L (c.395G>T), R132P (c.395G>C). Sample 1/20 negative for the presence of mutations in IDH1 codons 132 and 100 and the IDH2 gene at codons 140 and 172 (IDH-RT38, Entrogen). (B) Applications of FISH in genetic diagnostics in glioma on biological material collected by stereotactic biopsy. Deletion of 1p32 (cell on the left). (B’) Deletion of 19q13 (cell on the right) (Abbott, Molecular). (C) Representative screenshot of a telomerase reverse transcriptase (TERT) variant using Integrative Genomics Viewer (IGV). Aligned NGS data produced with Entrogen’s Targeted Hotspot Panel 16 kit. (C’) Close-up of TERT promoter variant NC_00005.9: g.1295228G>A; (commonly called C228T mutation) Reported as pathogenic.
IDH mutations play a crucial role in glioma classification. Especially, in adult-type diffuse gliomas where all types require IDH assessment (see Table 1). Currently, to classify a tumor as oligodendroglioma both the IDH-mutation and 1p/19q codeletion should be identified [1]. Moreover, in IDH-wildtype diffuse astrocytic gliomas the presence of one or more of three genetic parameters (EGFR gene amplification, TERT promoter mutation, 7+/10−) appears to be adequate to assign the highest CNS WHO grade and classify them as glioblastoma-wildtype. Such an approach avoids confusion and makes it easier to include these patients in clinical trials [30]. Besides, the Cancer Genome Atlas (TCGA) research network indicated that IDH, 1p/19q, and TP53 status captured lower-grade glioma subtypes more precisely than histological classification [31].
Positive Prognostic Factors
Mutations in IDH1 or IDH2 are positive prognostic factors (see Table 1). In comparison to IDH-wildtype gliomas, patients with IDH-mutant gliomas have a much better prognosis [16,26,27,32,33,34,35,36,37]. Large meta-analyses have shown that IDH mutations are associated with longer overall survival (OS) and progression-free survival (PFS) [38,39], regardless of grade [40]. The most favorable clinical outcomes were observed in lower-grade gliomas with IDH mutations and 1p/19q codeletion [31]. In malignant gliomas, the combination of IDH1 mutations and MGMT methylation status is more predictive of survival than either IDH1 or MGMT alone [40]. The multigenic mechanism behind its prognostic value is very much in line with the current observations done in the mechanistic pan-cancer studies. The activity of specific metabolic modules shows a stronger association with survival than any of its gene components alone [41].
In adult patients, IDH mutation has the greatest prognostic significance and clinical utility. Therefore, it should be assessed first and foremost.
4. H3F3A
H3.3 is a universal, replication-independent histone mostly found at transcription start sites and in telomeric regions, and it is associated with active and open chromatin [42,43]. The H3.3 histone is encoded by two different genes, H3F3A and H3F3B [44]. H3F3A alterations define an epigenetic subgroup of high-grade gliomas (HGG) with distinct clinical features and a global methylation pattern [30,44,45,46,47]. H3F3A mutations affect two amino acids, K27 and G34 of H3.3, in one-third of pediatric malignant gliomas. There appear to be strong links between H3F3A alterations and the age of onset of HGG, with the H3F3A K27 mutation occurring in children and H3F3A G34 in adolescents and young adults [44]. Moreover, H3.3 K27M mutations mainly occur in thalamic malignant neoplasms, whereas H3.3 G34R or G34V mutations tend to occur in cerebral hemisphere tumors [44,46]. As a result, WHO CNS5 classified tumors with alterations in H3F3A to the pediatric-type diffuse high grade gliomas family (see Table 1) [1]. Diffuse midline glioma, H3 K27-mutant, was previously described in the 2016 WHO classification. However, the nomenclature has been modified as H3 K27-alterted to reflect the fact that other molecular alterations can explain this type in addition to the previously known H3 K27 mutation [1,17].
Negative Prognostic Factor
Brain tumors are the leading cause of cancer-related death in children [47]. Most childhood brain tumors are CNS WHO grade 1, but the H3F3A alterations are associated with significantly worse outcomes (see Table 1) [45]. The H3 K27-altered diffuse midline glioma is an aggressive tumor corresponding to CNS WHO grade 4. Similarly, detection of an H3 G34 mutation in a diffuse glioma, irrespective of histological grade, indicates high-grade biology [30]. The K27M mutation represents a particularly unfavorable group, with a three-year OS of only 5% [48]. Overall, in pediatric patients, the determination of protein K27 and G34 defects is of the greatest clinical importance.
5. ATRX
ATRX is a key component of a multiprotein complex that also contains death-associated protein 6 (DAXX). ATRX regulates chromatin remodeling, nucleosome assembly, telomere maintenance, and histone H3.3 deposition in transcriptionally silent genomic regions [49]. ATRX protein loss and ATRX gene mutations are hallmarks of genomic instability and ALT-immortalized cell lines [50,51]. ATRX is frequently mutated in astrocytomas, IDH-mutant (about 60–70%) [52]. Diffuse astrocytic tumors harboring IDH mutations can be diagnosed as astrocytoma, IDH-mutant if there is loss of ATRX nuclear expression and/or diffuse p53 immunopositivity without the need for 1p/19q testing [30]. Moreover, loss of nuclear ATRX expression is one of the criteria to diagnose high-grade astrocytoma with piloid features [19].
Prognostic Factor
Pekmezci et al. reported that ATRX alterations were not associated with survival in astrocytomas IDH-mutant (see Table 2). On the other hand, in glioblastomas IDH-wildtype, ATRX alterations were associated with favorable outcomes [53]. Therefore, further research is required to assess its prognostic value.
Table 2.
Prognostic biomarkers in gliomas.
| Biomarker | Prognostic Value | OS (HR) 95% CI | PFS (HR) 95% CI | n | CNS WHO Grade |
Reference |
|---|---|---|---|---|---|---|
| IDH mutation | Positive | 0.241 (0.107–0.544) | NA | 98 | 4 | [40] |
| IDH mutation | Positive | 0.33 (0.25–0.42) | 0.38 (0.21–0.68) | 2190 | AGG | [38] |
| IDH mutation | Positive | 0.20 (0.06–0.58) | 0.14 (0.05–0.38) | 108 | LGG | [34] |
| 1p/19q codeletion | Positive | 0.33 | 0.34 | 79 | 4 | [33] |
| 1p/19q codeletion | Positive | 0.30 (0.12–0.75) | 0.34 (0.17–0.69) | 203 | 2 | [54] |
| 1p/19q codeletion | Positive | 0.43 (0.35–0.53) | 0.63 (0.52–0.76) | 3408 | AGG | [55] |
| MAPK pathway alterations | Positive | 0.19 (0.07–0.50) | NA | 64 | AGG | [56] |
| ATRX | Positive | 0.36 (0.17–0.81) | NA | 1206 | AGG | [53] |
| mMGMT (TMZ-treated PT) | Positive | 0.59 (0.37–0.94) | NA | 274 | 4 | [36] |
| mMGMT | Positive | 0.42 (0.38–0.45) | 0.43 (0.38–0.48) | 5103 | 4 | [57] |
| mMGMT (TMZ-treated PT) | Positive | 0.46 (0.41–0.52) | 0.48 (0.40–0.57) | 7888 | 4 | [58] |
| mMGMT (TMZ-free) | No effect | 0.97 (0.91–1.03) | 0.76 (0.57–1.02) | 7888 | 4 | [58] |
| TERT-mut (mMGMT PT) | Positive | 0.73 (0.55–0.98) | NA | 2819 | AGG | [59] |
| TERT-mut (MGMT-free) | Negative | 1.86 (1.54–2.26) | NA | 2819 | AGG | [59] |
| TERT-mut | Negative | 1.37 (1.08–1.76) | 1.37 (1.08–1.72) | 785 | AGG | [60] |
| TERT-mut | Negative | 1.38 (1.15–1.67) | 1.31 (1.06–1.63) | 11519 | LGG | [61] |
| High cfDNA | Negative | 1.82 (0.61–5.42) | NA | 42 | 4 | [62] |
| High ctDNA | Negative | 2.43 (1.19–4.95) | 2.19 (1.26–3.81) | 62 | 4 | [63] |
| High ctDNA | Negative | NA | NA | 85 | AGG | [64] |
| H3F3A | Negative | 4.27 (1.3–14.5) | NA | 42 | 4 | [45] |
| CDKN2A | Negative | 2.2 | 2.1 | 2193 | AGG | [65] |
| EGFR amplification | Negative | 0.43 (0.24–0.77) | NA | 718 | LGG | [66] |
| lncRNA | Negative | 2.09 (1.68–2.58) | NA | 1415 | AGG | [67] |
| Elevated serum YKL-40 | Negative | 1.4 (1.2–2.0) | NA | 343 | HGG | [68] |
| miRNA-221 upreg. | Negative | 1.66 (1.34–2.04) | 1.14 (1.02–1.26) | 1069 | AGG | [69] |
| miRNA-221 upreg. | Negative | 2.13 (1.05–4.31) | NA | 50 | 4 | [70] |
| miRNA-221 upreg. | Negative | 1.269 (1.054–1.527) | NA | 4708 | AGG | [71] |
| miRNA-155 upreg. | Negative | 1.4 (1.19–1.63) | NA | 1259 | AGG | [72] |
| miRNA-21 upreg. | Negative | 1.91 (1.34–2.73) | 1.23 (0.41–3.72) | 1059 | AGG | [73] |
| miRNA-21 upreg. | Negative | 1.681 (1.265–2.097) | NA | 1681 | AGG | [74] |
| miRNA-21 upreg. | Negative | 1.591 (1.278–1.981) | NA | 4708 | AGG | [71] |
| miRNA-222 upreg. | Negative | 2.09 (1.00–4.37) | NA | 50 | 4 | [70] |
| miRNA-222 upreg. | Negative | 1.72 (1.31–2.26) | 1.02 (0.86–1.22) | 1546 | AGG | [75] |
| miRNA-15b upreg. | Negative | 1.584 (1.199–2.092) | NA | 4708 | AGG | [71] |
| miRNA-148a upreg. | Negative | 1.122 (1.023–1.231) | NA | 4708 | AGG | [71] |
| miRNA-196 upreg. | Negative | 1.877 (1.033–3.411) | NA | 4708 | AGG | [71] |
| miRNA-210 upreg. | Negative | 1.251 (1.010–1.550) | NA | 4708 | AGG | [71] |
Abbreviations: AGG—all grade gliomas; ATRX—a-thalassemia/mental retardation X-linked syndrome; CDKN2A—Cyclin-dependent kinase inhibitor 2A; ctDNA—circulating tumor DNA; EGFR—epidermal growth factor receptor; HR—hazard ratio; IDH—isocitrate dehydrogenase; LGG—lower-grade glioma; mMGMT—MGMT methylated; MGMT—O 6-methylguanine-DNA methyltransferase; miRNA—microRNA; n—number of patients; NA—non-accessible; OS—overall survival; PFS—progression-free survival; PT—patients; TERT—human telomerase reverse transcriptase; TERT-mut—TERT promoter mutated glioma; TMZ—temozolomide; upreg.—upregulated; CNS WHO grade—Central Nervous System World Health Organization Grade; lncRNA—long non-coding RNA.
6. TERT
The telomerase reverse transcriptase gene encodes the catalytic reverse transcriptase subunit of telomerase that maintains telomere length. Somatic TERT mutations involving regulatory regions in addition to coding sequences may represent important driver events in cancer [76]. Mutations in the coding region of TERT are uncommon, but mutations in the promoter region have been detected in a high percentage of human melanomas and metastatic cancers [77]. Two well-established TERT promoter mutations result from cytidine-to-thymidine transitions in a dipyrimidine motif (C228T and C250T) (Figure 1C). The mutations create an alternative binding motif for ETS transcription factors and ternary complex factors (TCFs) near the transcription start site, resulting in up to a two-fold increase in transcription [76]. TERT promoter mutations reactivate telomerase, allowing for indefinite telomere maintenance and enabling cellular immortalization [78,79]. The C228T mutation accounts for 75% and C250T for 25% of all TERT promoter mutations [80] (Figure 1C). Approximately 70% of all adult primary glioblastomas harbor TERT promoter mutations [81,82]. Moreover, TERT promoter mutations were observed in almost all gliomas with concurrent total 1p/19q loss and IDH1/2 mutations (98%) [79].
Negative Prognostic Factor
Various studies have reported that telomerase activation or increased TERT expression are associated with shorter survival in gliomas [83,84,85]. In their meta-analysis, Vuong et al. reported that TERT promoter mutations are significantly associated with worse OS and PFS (see Table 1). Moreover, they noted that the prognostic significance of TERT mutations depends upon IDH status and tumor histology [61].
TERT promoter mutation is a negative prognostic factor, but mainly in IDH-wildtype gliomas. Pekmezci et al. reported that in astrocytomas IDH-wildtype, the TERT-wildtype group had a significantly better OS than the TERT-mutated group. In contrast, TERT-mutated tumors were not associated with survival in astrocytomas IDH-mutant. Moreover, in oligodendrogliomas, patients in the TERT-wildtype group had significantly worse OS than those in the TERT-mutated group [53]. Finally, TERT promoter mutation is one of the three genetic parameters that WHO CNS5 uses to upgrade astrocytoma, IDH-wildtype to glioblastoma, IDH-wildtype [1]. As a result, additional research is necessary to determine its multigenic relationship.
7. CDKN2A
Cyclin-dependent kinase inhibitor 2A (CDKN2A) is located on the short arm of chromosome 9 (p21.3) [86]. In several tumor subtypes, homozygous deletion of CDKN2A is associated with increased carcinogenesis and a poor prognosis [65]. The presence of a homozygous deletion of cyclin-dependent kinase inhibitor 2A/B (CDKN2A/B) is now playing an important role in glioma classification as a negative prognostic biomarker.
Negative Prognostic Factor
Many studies have reported that CDKN2A deletion is associated with significantly shorter PFS and OS in both lower-grade glioma (LGG) and HGG (see Table 1) [48,65,86,87,88,89,90,91,92]. The CDKN2A homozygous deletion is a significant prognostic factor in IDH-mutant glioma patients across multiple histologic WHO grades [65]. Ghasimi et al. reported that CDKN2A/B risk genotypes are also related to glioblastoma IDH-wildtype [93]. Allelic loss of 9p21.3, which contains CDKN2A, is a prognostic factor in 1p/19q-codeleted grade 3 gliomas. Allelic loss of 9p21.3, detected in 41.7% of tumors, was associated with shorter PFS and OS in univariate analysis [94]. Based on the robust literature and the cIMPACT-NOW update 5 recommendations, astrocytoma, IDH-mutant that harbors homozygous CDKN2A/B deletion is graded as CNS WHO grade 4. In other words, the presence of homozygous CDKN2A/B deletion is a marker of the highest malignancy grade in the group of diffuse, IDH-mutant astrocytomas [1,95].
Moreover, CDKN2A/B is one of the criteria to diagnose High-grade astrocytoma with piloid features alongside a piloid cytology, frequent MAPK pathway gene alterations, loss of ATRX nuclear expression, and a distinct DNA methylation pattern [19].
8. 1p/19q Codeletion
Chromosomal deletion of both 1p and 19q, so called codeletion, represents an unbalanced translocation t(1;19)(q10;p10) [96,97] (Figure 1B). This alteration is associated with oligodendrogliomas and sensitivity to alkylating agent chemotherapy [98].
8.1. Classifying Marker
1p/19q is a key mutation that allows more accurate classification of tumors than histological evaluation [31]. Due to its prognostic significance, oligodendrogliomas with classic histological features remain a molecularly heterogeneous type that should be stratified according to 1p/19q status [54]. 1p/19q codeletion rates were 70.8% in oligodendroglioma grade 3 and 23.1% in astrocytoma grade 3 [99]. 1p/19q codeletion is closely linked to IDH mutations [38,100] and is nearly mutually exclusive with ATRX mutations [101]. According to the new classification, all oligodendrogliomas are 1p/19q codeleted [1].
8.2. Prognostic Factor
1p/19q codeletion is a favorable prognostic factor (see Table 1) associated with a better PFS and OS [55,102,103] regardless of the detection method used [55,96,104]. The mutation indicates a relatively homogenous disease subtype [34,102]. Furthermore, isodeleted chromosome 1p predicted a favorable OS and PFS equivalent to codeleted 1p/19q, particularly in low-grade gliomas, but isodeletion of 19q only predicted prolonged PFS [55].
8.3. Predictive Factor
Loss of chromosome 1p/19q predicts both a persistent chemosensitivity and a favorable prognosis in LGGs that respond to TMZ [103]. Weller et al. reported that 1p/19q codeletion is a predictor of prolonged survival in patients receiving PCV (procarbazine, lomustine (CCNU), and vincristine) in addition to radiotherapy vs. radiotherapy alone [105]. Furthermore, PCV treatment is particularly effective in tumors with a high percentage of 1p/19q loss of heterozygosity and IDH1 mutations [106,107].
9. Chromosome 7 Gain and Chromosome 10 Loss
Gliomas frequently have chromosomal alterations, however, chromosome 7 gain and chromosome 10 loss are of particular interest. There are nine possible combinations exhibiting both, gain of at least one arm on 7 and loss of at least one arm on 10. The most frequent constellation is complete chromosome 7 gain and complete chromosome 10 loss (79%), followed by 7+/10q−(5%), by 7p+/10−(5%), and by 7q+/10−(4%) [108].
9.1. Negative Prognostic Factor
7+/10− is a negative prognostic factor in gliomas. Particularly, in IDH-wildtype GBM, 7+/10− represents a hallmark molecular change. Astrocytic tumors not fulfilling the morphological criteria for GBM but carrying 7+/10− exhibit a clinical course similar to that of morphologically unequivocal GBM [108]. Therefore, in WHO CNS5, 7+/10− is one of the three genetic parameters to upgrade astrocytoma (grade 2 or 3), IDH-wildtype to glioblastoma, IDH-wildtype [1]. Three combinations, 7+/10−, 7q+/10−, and 7+/10q−, were linked to a poor prognosis, similar to that of GBM patients. As a result, all patients with 7+/10−, 7+/10q−, and 7q+/10− should be classified as having the prognostic 7+/10− signature [108].
Regardless of whether it is a complete or partial loss of chromosome 10, patients had significantly shorter survival compared to those with no chromosome 10 abnormalities [109]. Moreover, gain of whole chromosome 7 was associated with a 4.7-fold greater risk of tumor recurrence, even after correcting for surgical status and other genetic changes [110].
9.2. Positive Predictive Marker
MGMT is located on chromosome 10q26 and the loss of chromosome 10q is a form of MGMT inactivation. Richard et al. proved GBM patients with dual inactivation of MGMT, by hypermethylation of the MGMT promoter and by loss of the long arm of chromosome 10, have longer OS and PFS and receive greater benefit from TMZ treatment. Individuals with dual MGMT inactivation had a median OS of 21.5 months, compared to 12 months and 8.1 months, respectively, for groups with single MGMT inactivation by hypermethylation or 10q deletion. Furthermore, all long-term survivors (OS > 30 months) with a sustained response to TMZ treatment had dual MGMT inactivation [111].
10. MYB
MYB transcription factors are proto-oncogenes. Myb proto-oncogene like 1 (MYBL1) is a member of the MYB family. MYB alteration influences proliferation and differentiation of progenitor cells [112]. In gliomas, MYB gene alterations are detected more frequently in young children and typically affect the cerebral hemispheres [113]. cIMPACT-Now Update 4 reviewed the status of WHO grade 2 IDH-wildtype/H3-wildtype diffuse gliomas, including those with MYB or MYBL1 rearrangements, and recommended the use of integrated diagnostics to link their histological and genetic characteristics [114]. Therefore, WHO CNS5 introduced a new type of glioma called diffuse astrocytoma, MYB- or MYBL1-altered belonging to the family of pediatric-type diffuse low-grade gliomas. Moreover, WHO CNS5 classifies diffuse astrocytomas, MYB- or MYBL1-altered as CNS WHO grade 1. Lastly, MYB gene is altered in nearly all Angiocentric gliomas [1].
Positive Prognostic Factor
In glioma patients with MYB and MYBL1 mutations, the clinical course is generally indolent. Patients with these tumors have a prolonged disease course and good overall survival [114]. Chiang et al. reported that the 10-year OS is 90%, and 10-year PFS is 95%. Therefore, mutations in the MYB or MYBL1 genes appear to be a positive predictor of prognosis [113].
11. MN1
Meningioma 1 (MN1) gene is a transcriptional co-regulator located on chromosome 22q [115,116]. Alterations in MN1 frequently occur in astroblastoma, a type of gliomas. Astroblastoma is a neoplasm that primarily affects children, teenagers, and young adults, and it mainly involves the cerebral hemispheres. The term “astroblastoma” is thought to be misleading because these tumors are neither astrocytic nor blastic [117]. Therefore, WHO CNS5 has identified astroblastoma as “MN1-altered” to improve diagnostic clarity for this type. Even so, more research is needed to establish distinct histopathological and molecular characteristics that can identify MN1-mutated astroblastomas from morphologically identical neuroepithelial tumors with similar genetic changes [1].
Positive Prognostic Factor
MN1 is a positive prognostic factor [115,116]. Gliomas with upregulated MN1 have better OS and PFS [116]. According to Lehman et al. MN1 rearranged astroblastomas were associated with a favorable prognosis which was mainly in comparison to BRAF V600E-mutated pleomorphic xanthoastrocytoma [118].
12. MAPK Pathway
The mitogen-activated protein kinase (MAPK) pathway comprises several key signaling components and phosphorylation events that play a role in tumorigenesis. These activated kinases transmit extracellular signals that regulate cell growth, differentiation, proliferation, apoptosis, and migration functions [119]. The MAPK pathway gene alterations most frequently affect NF1, followed by BRAF and FGFR1 [120]. Alterations affecting genes encoding members of the MAPK pathway have previously been found to occur in up to 100% of CNS WHO grade 1 Pilocytic astrocytomas [121,122].
A tumor that has mutations in FGFRs and/or BRAF and morphologically resembles a diffuse glioma, according to the new WHO CNS5 classification, qualifies as diffuse low grade glioma, MAPK pathway-altered. On the other hand, a tumor with these mutations and neuroepithelial features can be classified as polymorphous low-grade neuroepithelial tumor of the young. Moreover, a KIAA1549–BRAF fusion is almost pathognomonic of pilocytic astrocytoma and high-grade astrocytoma with piloid features [114]. In both of these tumors NF1 occurs frequently. Lastly, BRAF mutations are also found in pleomorphic xanthoastrocytoma [1].
Positive Prognostic Factor
The MAPK pathway activation is a predictor of a favorable patient outcome. Overall, patients with the MAPK pathway activation in the absence of H3K27M had a better prognosis (91% 5-year survival), whereas patients with H3K27M had a worse prognosis across all histological grades, suggesting that H3K27M is the dominant prognostic indicator [56]. A KIAA1549:BRAF fusion was associated with longer OS and PFS. This prognostic significance was regardless of the FGFR1 status and the FGFR1 immunohistochemical expression. On the other hand, tumors negative for a KIAA1549:BRAF fusion, the FGFR1 pK656E point mutation resulted in a significantly worse outcome, whereas the overexpression of FGFR1 was related to a better prognosis [123].
13. EGFR
Epidermal growth factor receptor, also known as HER1 or ERBB1, is a transmembrane receptor tyrosine kinase in the ERBB family [124]. EGFR overexpression and/or mutations play a central role in cell division, migration, adhesion, differentiation, and apoptosis [125]. Genetic alterations in EGFR—including mutations, rearrangements, alternative splicing, and focal amplifications—are the dominant receptor tyrosine kinase lesions in GBM. EGFR deletions and point mutations that keep EGFR in an active conformation are found in 57% of GBMs [126].
Among EGFR mutants, the most common is EGFR(△2–7), also called EGFRvIII [127,128,129]. EGFR(△2–7) is characterized by a 267 amino acid deletion in the extracellular domain, which results in a receptor that cannot bind ligand and is constitutively active [128]. EGFR(△2–7) is thought to represent a late event, occurring after EGFR-wildtype amplification. EGFR amplification and EGFR(△2–7) mutations might represent concerted evolutionary events that drive the aggressive nature of GBM by promoting invasion and angiogenesis via distinct signaling pathways [129].
13.1. Negative Prognostic Factor
EGFR amplifications have been reported to indicate a much more aggressive tumor subpopulation [130]. Aibaidula et al. reported patients with EGFR-amplified LGGs had significantly shorter OS than those with EGFR non-amplified tumors (median OS 1.03 y vs. 2.67 y, p = 0.003) [66] (see Table 1). Labussière et al. suggested that the influence of EGFR status on prognosis could be more complicated; patients with EGFR amplifications had a better prognosis in the TERT-mutated context than patients with TERT-wildtype tumors. On the other hand, EGFR-wildtype GBM patients had longer survival with TERT-wildtype than patients with EGFR-wildtype and TERT-mutated [131]. Zou et al. showed that EGFR amplification and IDH mutations are mutually exclusive [38]. Therefore, further research is required to determine their multi-genic interaction.
In LGG, EGFR mutations indicate increased lesion infiltration of specific immune cell types and a poor prognosis [132]. EGFR(△2–7) confers a growth advantage to GBM, and patients with EGFR(△2–7) mutations have significantly shorter survival. EGFR(△2–7) overexpression in the presence of EGFR amplification is the strongest indicator of a poor prognosis [133].
On top of that, in WHO CNS5 EGFR gene amplification is one of the criteria to upgrade IDH-wildtype diffuse astrocytic tumor in adults to glioblastoma, IDH-wildtype [1].
13.2. Promising Predictive Factor
Currently, intensive research is being done to develop drugs targeting EGFR. Monoclonal antibodies such as cetuximab and nimotuzumab have not been effective due to BBB (blood brain barrier) and their molecular weight. However, GC1118 is a novel anti-EGFR monoclonal antibody and has shown promising results. A phase II trial of GC1118 for recurrent GBM patients with EGFR amplification is underway (NCT03618667) [134]. Tyrosine kinase inhibitors (e.g., gefitinib and erlotinib) have failed to show remarkable improvement in patients with non-progressive or recurrent glioblastoma in various phase II clinical trials [135]. However, a new covalent-binding EGFR-TKI, CM93 showed better efficacy in pre-clinical than other EGFR-TKIs, which is promising [136]. Moreover, PI3K inhibitors, such as XL765, a dual mTOR and PI3K oral inhibitor, are currently examined in glioblastoma patients and a phase I trial is underway [135]. The recent study conducted by Zając et al. proved that inhibition of the PI3K/Akt/mTOR pathway sensitizes glioma cells to apoptosis upon temozolomide treatment [137]. Furthermore, epigenetic alterations also play a role in the development of the resistance to EGFR inhibitors. Treatment using epigenetic regulators, alone or in conjunction with EGFR inhibitors, offers a new hope for glioblastoma patients. For example, the combination of histone deacetylase (HDAC) inhibitor + EGFR inhibitor can prevent the development of the resistance in glioblastoma cells [138]. Finally, the development of hybrid compounds is another active area in glioblastoma therapeutics. For instance, Sahaquine contains hydroxamic acid and primaquine linked by dicarboxylic acid [139]. This hybrid molecule can selectively inhibit HDAC6 protein at nanomolar concentration. Moreover, Sahaquine suppresses P-glycoprotein activity, which contributes to glioblastoma drug resistance [140].
In summary, currently EGFR-targeting drugs do not prolong OS and PFS, but intensive research for new types of drugs is ongoing.
14. MGMT
O6-methylguanine-DNA methyltransferase is a DNA-repair protein that inhibits the cross-linking of double-stranded DNA by alkylating agents [141]. MGMT expression levels in gliomas may influence responses to alkylating agents. Promoter methylation regulates MGMT expression by epigenetic silencing of the MGMT gene [142]. The MGMT promoter is methylated in ~45% of glioma patients [143,144].
14.1. Positive Predictive Factor
MGMT promoter methylation has the most impact on clinical practice for patients with glioblastoma [145]. Patients with methylated MGMT benefit from TMZ, while patients without methylation do not [33,36,142,144,146,147,148,149], a phenomenon present in all age groups [59]. Moreover, double inactivation of MGMT by promoter methylation and loss of 10q result in greater sensitivity to TMZ than promoter methylation or absence of 10q alone [113]. A combination of both IDH mutations and MGMT promoter methylation was associated with the best response rates to TMZ [33,36]. Moreover, Roszkowski et al. found that patients with both MGMT promoter methylation and IDH1 mutations receiving radiotherapy had a better prognosis than those with MGMT methylation alone [150]. However, Vuong et al. reported that not all GBM patients with methylated MGMT may benefit from TMZ, postulating that it is possible that only GBM patients mutated by TERT with MGMT methylation are sensitive [59].
14.2. Positive Prognostic Factor
Not only MGMT is a positive predictive factor for TMZ therapy (see Table 1), but also MGMT is a positive prognostic marker. Many studies have reported that MGMT methylation predicts longer OS [33,81,151,152,153]. Zhang et al. conducted a meta-analysis of 15 studies reporting the effect of MGMT promoter methylation on OS by univariate analysis and 14 studies by multivariate analysis. The combined hazard ratios (HR) were 0.67 (95% CI 0.58–0.78) and 0.49 (95% CI 0.38–0.64), respectively. The pooled HR estimates for PFS were 0.72 (95% CI 0.55–0.95) by univariate analysis and 0.51 (95% CI 0.38–0.69) by multivariate analysis [143]. The survival time of glioblastoma patients with only an IDH1 mutation was shorter than for patients with both IDH1 mutations and MGMT methylation [40]. PFS was longer in patients with MGMT promoter methylation who received TMZ [33,36,57,154,155,156]. Furthermore, Shah et al. reported that methylation at different sites of the MGMT promoter results in various PFS [157].
However, there is evidence to suggest that MGMT is not a favorable prognostic factor in a selected group of patients. Boots-Sprenger et al. failed to identify a favorable prognostic association for IDH1 mutations and MGMT promoter methylation in patients over 50 years of age [158]. Moreover, Nguyen et al. reported that MGMT-methylated patients showed improved survival only in the presence of TERT promoter mutations (TERT-mt) [159]. Finally, Dahlrot et al. discovered an association between MGMT methylation and OS starting at nine months following diagnosis but no association before that [160].
15. Mismatch Repair
Although the effectiveness of TMZ is largely dependent on the methylation status of the promoter of the MGMT gene, the integrity of the mismatch repair (MMR) system also plays a very important role. The MMR system is a protein complex including MSH2, MSH6, MLH1, and PMS2 proteins. MMR attempts to repair the O6-meG:T mismatch caused by TMZ by removing a patch of the newly synthesized strand containing thymine [161]. The MMR system recognizes mispaired O6-MeG:T and excises the newly synthesized strand, leaving the parental strand with O6-MeG intact. These futile cycles repeat, leading to cell cycle arrest and apoptosis. On the other hand, if the MMR system is defective then mutations accumulate leading to hypermutated phenotypes. Thus, the tumor is resistant to temozolomide, by not responding to TMZ-induced mispairing [162].
The predictive value of the MMR system is so important that Suwala et al. even proposed a new glioma type—primary mismatch repair-deficient IDH-mutant astrocytomas (PMMRDIA). PMMRDIA were histologically high-grade and were mainly found in younger patients (median age 14 years) and all of them had a defective MMR system. They also reported that compared to reference cohorts of other IDH-mutant gliomas, PMMRDIA had by far the worst clinical outcome with a median survival of only 15 months irrespective of histological or molecular features [163].
On the other hand, Caccese et al. in a multicenter study reported that MMR protein expression status did not affect survival in HGG patients. They also showed that alteration of MMR protein expression was statistically more frequent in grade 3 gliomas, in recurrent disease, in patients treated with temozolomide, and in IDH-mutant gliomas [164].
15.1. Hypermutation Phenotype
The hypermutation phenotype is defined by a dramatic increase in the mutation rate. This phenomenon occurs rarely in newly-diagnosed gliomas, but common in recurrent tumors after the use of alkylating agents [165]. There are two main pathways to hypermutation. First, a de novo pathway associated with constitutional defects in DNA polymerase and MMR genes. Second, a more common post-treatment mechanism is related to acquired resistance in chemotherapy-sensitive gliomas that relapse following temozolomide treatment [166].
15.2. Questionable Predictive Value for Immune Checkpoint Inhibitors (ICI)
Immune checkpoint inhibition is an attractive therapeutic avenue for hypermutated tumors. Pembrolizumab, a programmed cell death protein 1 (PD-1) inhibitor, was recently approved for the treatment of microsatellite instability–high, or MMR-deficient solid cancers in adults and children, for all tumor locations and histological types [167]. The most pronounced responses to ICI have been among tumors known to have high mutational burdens [168] such as subsets of non-small cell lung cancers [169], malignant melanomas [170] renal cell carcinoma [171], and MMR-deficient tumors [172]. However, in gliomas, the outcomes are less favorable. Touat et al. showed that MMR-deficient gliomas were characterized by a lack of substantial T-cell infiltrates, extensive intratumoral heterogeneity, and a low rate of response to ICIs. They stated that patients with hypermutated gliomas had worse median OS when treated with PD-1 inhibitors than patients treated with other systematic agents (16.10 months (95% CI 3.98–22.21) versus 8.07 (95% CI 2.79–15.08.21)) [166]. Moreover, the disappointing results of the Checkmate 143 trial (NCT02017717), which evaluated nivolumab and ipilimumab, has found no improvement in survival in patients with recurrent GBM [173]. Therefore, the usefulness of ICI in hypermutated glioma patients is questionable.
16. Liquid Biopsies
There has been a renaissance in molecular techniques over the last 20 years, including in real-time quantitative PCR (qPCR), FISH analysis, and next-generation sequencing (NGS) (Figure 1). On the other hand, small amount of material encourages for biological analyses from body fluids such as blood, cerebrospinal fluid (CSF), and urine, with CTCs, cell-free nucleic acids (cfNAs), ctDNA, and extracellular vesicles (EVs) all extracted for downstream analyses (Figure 2). This approach may also be beneficial for tracking dynamic changes in the tumor throughout therapy, given the minimally or non-invasive nature of the test. Moreover, most of these biomarkers have a short half-life (up to 3 h) so disintegrate quickly when present freely in plasma [174]. The multitude of available diagnostic options is at the stage of scientific research, which makes it difficult to compare methods. Furthermore, there is no widespread agreement among scientists regarding which nucleic acids (RNA or DNA), which biological fluids (serum, CSF, or urine), or which analytical technique (targeted or whole genome sequencing, PCR, or microarray) should be studied the most [175]. Therefore, it is hard to compare sensitivity and specificity of different methods. On top of that, not all patients provide their consent to particular diagnostic methods.
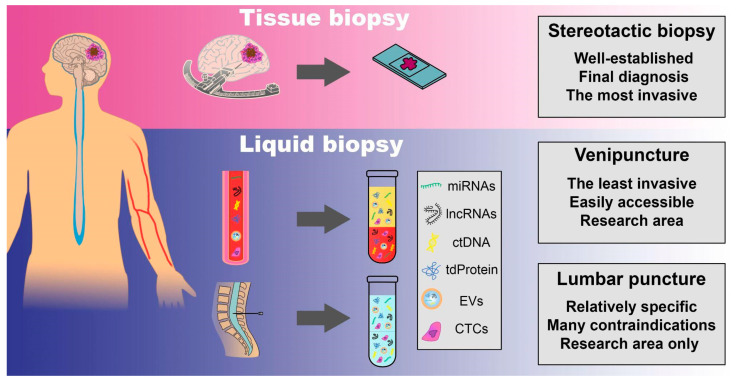
Figure 2.
Obtaining the material to analyze biomarkers in glioma patients. The material can be obtained using tissue biopsy or liquid biopsies. A stereotactic biopsy is a well-established surgical procedure used to acquire tissue samples. Venipuncture and lumbar puncture are methods to obtain liquid biopsies. Circulating tumor cells (CTCs), extracellular vesicles (EVs), tumor-derived proteins (dtProteins), circulating tumor DNA (ctDNA), long non-coding RNAs (lncRNAs) and microRNA (miRNAs) are released into the bloodstream and cerebrospinal fluid (CSF) from primary tumors and metastatic deposits. Material collected by venipuncture, the least invasive technique, can be extracted, and plasma or serum can then be analyzed. The evaluation of circulating biomarkers in CSF is a relatively specific method, however it has many contraindications and currently remains only in the research area.
In gliomas, material to the analysis can be acquired primarily from blood or CSF. While blood drawing is a relatively simple procedure, a lumbar puncture (LP) has several contraindications. The most important is an intracranial space–occupying lesion with mass effect as well as a posterior fossa mass because it can lead to herniation of the cerebellar tonsils, regardless of the volume of CSF that is sampled [176]. Hence, patients should be carefully selected for LP.
Despite numerous limitations to overcome, liquid biopsies can be the future of personalized medicine due to their major advantages over tissue biopsies. Below we summarize various types of liquid biopsies.
17. Circulating Tumor DNA
Circulating tumor DNA (ctDNA) comprises small fragments of DNA (180–200 base pairs) released by tumor cells into the bloodstream, predominantly by cell death and apoptotic cells [177,178,179]. ctDNA has the potential to carry a wide spectrum of specific primary brain tumor mutations, and thus if detected in body fluids provides a valuable non-invasive or minimally invasive way to sample cancerous tissues (Figure 2) [174,180]. Moreover, the European Medicines Agency and the US Food and Drug Administration (FDA) have approved ctDNA tests for specific indications in the absence of evaluable tumor tissue [179].
Piccioni et al. found that half of primary brain tumor patients had detectable ctDNA [181], and Liang et al. proved that it is possible to distinguish a primary brain tumor from a metastasis based on ctDNA [182]. ctDNA concentrations vary depending on the cancer type, and GBMs produce extremely low plasma concentrations [183]. Nevertheless, ctDNA can be detected in body fluids such as CSF and blood, and the concordance rate between CSF ctDNA mutations and tumor DNA is quite high [184]. Li et al.’s analysis showed that the sensitivity of ctDNA detection increases when CSF is used instead of blood [185]. Compared to plasma ctDNAs, CSF ctDNAs more clearly represented the progressive mutational alterations of driver genes [185,186]. The existence of the blood-brain barrier (BBB) explains the limited concentration of ctDNA in glioma patients’ blood and the reduced sensitivity compared to CSF [174]. On the other hand, CSF cannot be collected easily and noninvasively for the diagnosis of glioma which limits the application of the CSF DNA analysis.
In a multivariable analysis, CSF ctDNA positivity remained a statistically relevant prognostic factor. CSF-positive subjects had a four-fold increased risk of death compared to CSF-negative subjects [64]. Bagley et al. reported that in patients with newly diagnosed GBM, high baseline plasma ctDNA concentrations were associated with worse OS and PFS regardless of other prognostic factors [62]. Moreover, Nørøxe et at. found that ctDNA levels changed during glioblastoma therapy, peaking before diagnostic surgery and declining as the cancer progressed [187].
However, there are still many challenges in detecting ctDNA which can limit the prognostic and predictive value. The most important is the detection method. CtDNA sequencing techniques must be extremely sensitive and specific to overcome the low concentration of ctDNA and the presence of DNA from normal cells, which can result in false positives. While qPCR is quick and inexpensive, it can only detect mutant allele fractions (MAF) greater than 10% [188]. Digital PCR (dPCR) tests using microfluidic platforms are highly sensitive and quantitative, and are widely used to measure ctDNA levels and it can detect MAF below 0.1% [189]. These methods are generally suited to investigating a small number of mutations and are often applied to analysis of cancer hot-spot mutations [179]. NGS-based approaches allow for high throughput analysis, can screen for undiscovered variants, and can also find structural variants and copy number variations, but they have a lower sensitivity (about 1%) than dPCR and higher cost [189]. Generally, there are three ways to increase the sensitivity and specificity, and consequently prognostic and predictive value of ctDNA. First, is to have a more precise machine. Second, is to increase the amount of collected blood. Lastly, we can search for many mutations in one genome molecule [190].
Another challenge is to reduce the background noise which can originate from white blood cells (WBC). Clonal hematopoiesis, a process that leads to expansion of mutations in peripheral blood cells, is an additional source of DNA that adds a layer of complexity when interpreting results. One way to overcome this problem is to sequence DNA profiles of WBC and compare them to analyzed ctDNA [191].
Because of the growing acceptance of ctDNA, the field is moving away from exploratory ctDNA studies and toward clinical trials, where ctDNA is used to influence decision-making [179].
18. MicroRNAs
MicroRNAs (miRNAs) are noncoding RNA molecules about 22 nucleotides long that influence gene expression by interacting with messenger RNAs (mRNAs) [192,193]. They are under sophisticated control. miRNAs are thought to influence up to 60% of protein-coding genes [193]. Many studies over the past few years have documented the control of miRNA metabolism and function by various mechanisms [194]. miRNAs can be assessed in body fluids such as blood, CSF, or urine as well as tissues, but the latter analysis may be hampered when only small amounts of neoplastic tissue are present: the assessment of somatic changes such as point mutations or 1p/19q codeletions requires an appropriate amount of material for genetic testing. Thus, peripheral blood miRNA expression is a potential source of material obtainable via a relatively non-invasive procedure (Figure 2) that could provide an innovative solution for assessing diagnosis, prognosis, and predicting responses to therapy with so-called “liquid biopsies” (see below).
18.1. Potential Classification Marker
Due to their participation in carcinogenesis and stability, miRNAs can serve as unique biomarkers for the minimally invasive diagnosis of glioblastoma [195,196]. Roth et al. investigated whether a specific blood-derived miRNA fingerprint could be defined in glioblastoma patients, and in doing so showed that miRNAs can be considered as biomarkers and their detection in the blood justifies the need for further testing [197]. In 2019, Wang et al. conducted a meta-analysis which showed that cell-free miRNA-21 is the most promising diagnostic miRNA for glioma detection, followed by miRNA-125 and miRNA-222 [198]. Moreover, ParvizHamidi et al. reported that both miRNA-21 and miRNA-26 were significantly upregulated in pre- and postoperative serum samples from glioblastoma patients [199]. In contrast, Wei et al. found that blood miRNA-125b levels were considerably lower in glioma patients than in the general population, with a clear downward trend in miRNA-125b levels as tumor stage increased [200]. Akers et al. reported that miRNAs from cisternal cerebrospinal fluid had sensitivity of 80% and specificity of 76% for glioblastoma detection whereas lumbar CSF had sensitivity of 28% and specificity of 95% [201]. According to Teplyuk et al., miRNA-200 family members are significantly elevated in the CSF of patients with brain metastases but not in individuals with any other pathological conditions, allowing differentiation between glioblastoma and metastatic brain tumors [202].
Nilsson et al. reported that platelets can absorb RNA-containing membrane vesicles in vitro and in vivo. Platelets from glioma patients absorbed vesicles containing mutant EGFR(△2–7), a well-known GBM biomarker (see above). Eighty percent of patients with EGFR(△2–7)-mutated GBM tumors also harbored the mutation in platelets compared to none in healthy controls. This conclusion is likely to apply to other tumor-related RNAs, as a glioma-associated signature was discovered after RNA profiling of glioma and healthy patients. Further research on upregulated RNAs could lead to the identification of new circulating biomarkers [203].
18.2. Potential Prognostic and Predictive Markers
Over the past decade, there has been significant interest in the functional relevance of miRNAs as prognostic and predictive biomarkers. A number of meta-analyses have been conducted to investigate their prognostic significance. Upregulation of plasma miRNA-222, miRNA-155, miRNA-221, and miRNA-21 is associated with a worse prognosis [69,75]. Furthermore, there is a strong negative correlation between elevated miRNA-21 in serum and OS and PFS [71,73,195] (see Table 1). Glioblastoma patients with high levels of miRNA-10 family members in tissue had a much poorer survival rate than those with low levels of miRNA-10 [204].
In parallel, miRNA expression is often associated with responses to therapy, so miRNAs may also have a potential role as predictive biomarkers. Upregulated plasma levels of miRNA-223 and miRNA-125b-2 improved cell survival when treated with TMZ [205]. miRNA-125b-2 is overexpressed in GBM tissues and the corresponding stem cells (GBMSCs). Downregulation of miRNA-125b-2 expression in GBMSCs may allow TMZ to trigger apoptosis in these cells [206]. Moreover, Siegal et al. reported that miRNA-10b and miRNA-21 are predictive factors for bevacizumab (a vascular endothelial growth factor (VEGF) inhibitor) responses, discovering that the serum expression of these miRNAs was inversely linked to tumor size in patients receiving bevacizumab [207]. miRNA-181d could be a useful biomarker to determine which patients will benefit the most from TMZ therapy. Zhang et al. reported that MGMT expression inversely correlated with miRNA-181d expression in independent GBM samples [208]. While elevation of miRNA-181d may indicate a better response to TMZ, upregulation of miRNA-21 in a tumor sample, however, may indicate a poorer response [209]. Moreover, miRNA-21 expression is also closely associated with radio-resistance in diverse malignant glioma cell lines [210].
There have been many reports of miRNA up- or downregulation in glioma patients; however, definitive miRNA signatures for glioma classification or as prognostic or predictive biomarkers are still under evaluation. The patterns of miRNA expression in patients with gliomas are presented in Table 3.
Table 3.
Upregulated and downregulated miRNAs in gliomas.
| miRNAs Upregulated in Gliomas | miRNAs Downregulated in Gliomas | ||||
|---|---|---|---|---|---|
| miRNA | Sample | References | miRNA | Sample | References |
| miRNA-21 | tissue, blood, CSF | [71,74,199,211,212,213,214,215,216,217,218,219] | miRNA-137 | tissue | [216,219,220] |
| miRNA-221 | tissue, blood | [71,213,214,221,222] | miRNA-342-3p | tissue, blood | [197,219] |
| miRNA-10 | tissue, blood, CSF | [204,216,220,223] | miRNA-124 | tissue | [71,217] |
| miRNA-222 | tissue, blood, CSF | [70,75,198] | miRNA-181a, miRNA-181b, and miRNA-181c | tissue | [221] |
| miRNA-210 | tissue, blood | [71,220] | miRNA-31 | tissue | [216] |
| miRNA-17 | tissue | [214] | miRNA-101 | tissue | [216] |
| miRNA-155 | tissue | [72,215] | miRNA-222 | tissue | [216] |
| miRNA-576-5p | blood | [224] | miRNA-330 | tissue | [216] |
| miRNA-340 | blood | [224] | miRNA-7 | tissue | [216] |
| miRNA-626 | blood | [224] | miRNA-7-5P | blood | [224] |
| miRNA-630 | tissue | [215] | let-7g-5p | blood | [224] |
| miRNA-1260 | tissue | [215] | miRNA-320 | blood | [224] |
| miRNA-542-5p | tissue | [215] | miRNA-125b | blood | [200] |
| miRNA-142-5p | tissue | [215] | miRNA-29 | blood | [220] |
| miRNA-106a-5p | blood | [220] | miRNA-197 | blood | [220] |
| miRNA-185 | blood | [220] | miRNA-205 | blood | [220] |
| miRNA-125 | CSF, blood | [198] | miRNA-485 | blood | [220] |
| miRNA-15b | tissue | [71] | miRNA-106 | tissue | [71] |
| miRNA-148a | tissue | [71] | |||
| miRNA-196 | tissue | [71] | |||
| miRNA-15a | tissue | [217] | |||
| miRNA-16 | tissue | [217] | |||
| miRNA-23a | tissue | [217] | |||
| miRNA-9 | tissue | [217] | |||
19. Long Non-Coding RNA
Long non-coding RNAs (lncRNAs) are a new class of non-protein-coding transcripts that have been associated with cancer progression [225]. They comprise a wide variety of RNA transcripts with a length of more than 200 nucleotides but no significant protein-coding capacity [226]. One of the distinguishing characteristics of lncRNAs is their tissue- and cell type-specific expression pattern, which could be used to precisely categorize glioma subtypes and predict treatment responses [227] (Figure 2).
19.1. Prognostic Markers
In 2020, Li et al. conducted a meta-analysis to evaluate the prognostic value of lncRNA expression in glioma patients. They showed a significant association between high lncRNA expression level and shorter OS (HR 2.09, 95% CI 1.68–2.58, p < 0.001). Moreover, lncRNA expression was significantly associated with a tumor diameter and malignancy [67]. Several other studies found that levels of lncRNAs are associated with prognosis. For example, LINC00152, LINC00319, and FAM225B were closely associated with unfavorable prognosis in glioma patients [228,229,230]. Moreover, Aslan et al. showed that lncRNA H19, AC091932.1, AC064875.1, AC010273.2, and AC131097.4 were negatively correlated with OS, whereas lncRNA FLG-AS1, AL138767.3, and ISX-AS1 were positively correlated with OS, indicating they play a protective role in LGG [231].
Recently, many studies have been published to find a set of lncRNAs with the best prognostic value. For this purpose, advanced statistical methods were applied to improve prognostic accuracy. For example, a set of six lncRNAs (AC005013.5, UBE2R2-AS1, ENTPD1-AS1, RP11-89C21.2, AC073115.6, and XLOC_004803) was an independent prognostic factor after adjusting for other clinical covariates. The set was able to stratify patients into high- and low-risk groups with significantly different survival (median 0.899 vs. 1.611 years, p = 3.87e−09, log-rank test) in the training cohort [232]. Moreover, Luan et al. identified 10 autophagy-associated lncRNAs with prognostic value (PCBP1-AS1, TP53TG1, DHRS4-AS1, ZNF674-AS1, GABPB1-AS1, DDX11-AS1, SBF2-AS1, MIR4453HG, MAPKAPK5-AS1, and COX10-AS1) in glioma patients using multivariate Cox regression analyses. The OS was shorter in the high-risk group than that in the low-risk group [HR = 5.307, 95% CI: 4.195–8.305; p < 0.0001] [233].
19.2. Predictive Markers
LncRNAs also have predictive value. It is proven that they affect chemotherapeutic drug resistance by regulating miRNA expression. For example, LINC00470 is reported to promote cell proliferation, invasion, and TMZ resistance through sponging miRNA-134 [234]. Furthermore, lncRNAs can interact with miRNA-21, which is related to processes regulating radio- and/or chemosensitivity [210] or miRNA-301a, which promotes radiation resistance. Thus, lncRNAs play important roles in the formation of tumor microenvironments and the acquisition of therapeutic resistance [235,236]. Recently, lncRNAs are receiving a large amount of attention as immunotherapy targets. Due to the fact that in gliomas the expression of immune-related lncRNAs is disrupted, and the clinical characteristics of glioma patients receiving immunotherapy are dependent on lncRNA expression [237].
20. Tumor-Derived Proteins
Tumor-derived proteins may be detected in the bloodstream, making them suitable for noninvasive diagnostic verification (Figure 2). Several serum-based biomarkers have been studied in glioma patients to see whether they have substantial prognostic or predictive significance. So far, tumor derived proteins are one of the most extensively studied biomarkers in glioma patients [238].
20.1. Prognostic Markers
Iwamoto et al. found serum YKL-40 level was significantly lower in patients with no radiographic disease compared with patients with radiographic disease in both the grade 3 gliomas and the glioblastoma cohorts. In these patients, longitudinal increases in serum YKL-40 were linked to an increased risk of mortality. Moreover, increases in YKL-40 were linked to a lower survival rate in grade 3 gliomas (HR = 1.4, p = 0.0001) and glioblastomas (HR = 1.4, p < 0.0001). On the other hand, serum levels of YKL-40 in patients with LGG were not associated with radiographic disease status [68]. Hormigo et al. showed YKL-40 and MMP-9 can be monitored in patients’ serum and help confirm the absence of active disease in GBM and YKL-40 in grade 3 glioma patients. Additionally, they reported YKL-40 can be used as a predictor of survival in patients with HGG [239]. Vaitkiene et al., using the decision tree analysis, indicated that serums ANGPT1, TIMP1, IP10, and TGFβ1 are promising combinations of targets for glioma diagnosis. The serum protein profiles of ANGPT1, TIMP1, IP10, and TGFβ1 were linked with the presence of an astrocytoma irrespective of its malignancy grade, while OPN and IP10 were associated with GBM patient survival [240]. Furthermore, many angiogenic proteins are associated with survival in glioma patients. The plasma levels of IGFBP-2 and VEGF, the serum level of plasminogen activator inhibitor-1 (PAI-1) were inversely associated with PFS and OS [238].
20.2. Predictive Markers
So far, serum proteins have been predictive markers mainly for anti-angiogenic treatments. For example, Tabouret et al. indicated that high MMP2 plasma levels are related to longer survival in patients with recurrent HGG treated with bevacizumab but not with cytotoxic agents [241]. Moreover, Chinnaiyan et al. found IGFBP-5 as a possible predictive protein marker for combined treatment with bevacizumab, HDAC inhibitor vorinostat, and irinotecan in a limited study (n = 10 recurrent glioblastoma patients) [242].
21. Extracellular Vesicles
Extracellular vesicles (EVs) are made up of an aqueous core containing soluble proteins and nucleic acids that are encased in a lipid bilayer [243]. EVs can be divided into two categories: in first, exosomes are released via exocytosis when multivesicular bodies fuse with the plasma membrane, and the second consists of microvesicles that shed directly from the cell membrane via budding [180]. Moreover, EVs can be classified according to their size, cargo, and density [244].
EVs are secreted by donor cells from the tumor niche and received by acceptor cells that may be located away from the tumor. EVs acquisition influences several signaling pathways through the internalization of various molecules (miRNAs, proteins, receptors, ligands, DNAs, and RNAs) (Figure 2) promoting processes such as invasion, angiogenesis, viability, migration, chemo-, and immunoresistance [244]. EVs are a reflection of the tumor’s extensive heterogeneity as well as its treatment adaptations [245]. Moreover, Gao et al. reported that the tumor microenvironment is altered by EV-mediated contact between glioma cells and non-glioma brain cells, which promotes tumor growth [246]. Recently, EVs emerged as a promising source of biomarkers for prognostic and predictive purposes. For example, EGFRvIII, an oncogenic glioma-specific growth factor receptor, has been shown to be present in EVs secreted by glioma cells [243,247]. Moreover, Wang et al. found that EGFR+ EVs can be used as glioma diagnostic and prognostic indicators. Using flow cytometry analysis, they demonstrated that EGFR expression in serum EVs can accurately distinguish high-grade glioma patients from low-grade glioma patients. Furthermore, EGFR in EVs correlates with the ki-67 labeling index in tumor tissue [175]. On the other hand, André-Grégoire et al. showed the TMZ treatment modulates glioma stem cells-released EVs. The release of extracellular vesicles was increased in temozolomide-resistant tumor cells. Moreover, TMZ increased the levels of cell adhesion proteins in extracellular vesicles [248]. Although EVs can be found in a variety of biofluids, it is still unclear which source is best for their isolation and prognostic value [249].
Similar to other liquid biopsy molecules, the isolation methods influence the sensitivity and specificity. It is especially observed in EVs where the density-based separation method is the most commonly used [188]. Density gradient centrifugation separates EVs and non-EV structures based on differences in buoyancy, making it currently the only isolation method that eliminates the majority of contaminants. However, the density gradient centrifugation is lacking a standardized protocol. EVs can be isolated through differential ultracentrifugation, rotor types, applied g-forces, and duration of centrifugation steps. Therefore, it results in different sensitivity and specificity, and consequently in various prognostic values [250].
22. Circulating Tumor Cells
Circulating tumor cells (CTCs) are cells that are released into the bloodstream from primary tumors and metastatic deposits [251] (Figure 2). In many recent studies, CTCs have been found in the blood of GBM patients [252,253,254,255,256,257,258,259,260,261]. GBMs discharge CTCs with invasive mesenchymal properties into the bloodstream, revealing mutations that were not observed in the primary tumor [256,260]. Sullivan et al. reported that acquired mutations in EGFR, RB1, and SETD2 were absent in the primary tumor but were present at all metastatic sites [260]. Liu et al. reported that GBM-derived CTCs possess a cancer stem cell-like phenotype with resistance to radiation, chemotherapy, and stress-induced apoptosis [255]. Moreover, CTCs are valuable for tumor characterization in cases where tissue biopsies are difficult to obtain or the acquired tissue is of poor quality [255].
CTCs are present at as few as one cell per 109 in the blood of patients with metastatic cancer. Detecting CTCs with high specificity and sensitivity is technically challenging [262]. The isolation methodologies of CTCs can have an impact on quality and quantity of the specimen, and consequently on sensitivity and specificity. Therefore, choosing the isolation method and source of CTCs can influence their prognostic and predictive value. There are several methods to isolate CTCs from the bloodstream. Immunomagnetic positive enrichment, immunomagnetic negative enrichment, microfluidic positive enrichment, size selection methods, and density centrifugation are the most popular. In the immunomagnetic positive enrichment, specific antibodies with ferritic properties attach to CTCs which are then separated from the blood using magnetic force. In immunomagnetic negative enrichment, ferritic antibodies target leukocytes, primarily using CD-45, and then they are wash out living CTCs, which in theory, should be the only nucleated cells.
CellSearch® (Menarini Silicon Biosystems Inc., Huntington Valley, PA, USA) is the only FDA-approved platform for CTCs isolation. It uses a combination of positive and negative immunomagnetic enrichment [263]. CellSearch is a validated method for CTCs enumeration in breast cancer [264], colon cancer [265], and prostate cancer [266]. However, CellSearch cannot be used for non-epithelial tumors, as it only targets EpCAM. Therefore, its usefulness in gliomas is questionable. Moreover, CellSearch does not provide opportunities to develop custom assays and CTCs can have different antigens, for example EGFR. As a result, this technology could not detect all CTCs and have reduced sensitivity or false negative results.
Due to the lack of regularly expressed tumor markers and high inter- and intra-tumor heterogeneity, detecting glioma CTCs has been difficult [252]. Despite challenges in isolating, detecting, and diagnosing CTCs, a number of studies have now been published that outline new and effective detection strategies, but none has yet received widespread recognition and validation that would progress clinical acceptance [257,259,267]. The presence of CTCs correlates with the risk of metastasis and the frequency of relapse after surgery [253].
CTCs can serve as a stratification of patients with metastatic disease. In metastatic breast cancer the prognostic efficacy of CTCs has been proven. No study has yet been conducted in glioblastomas to assess how the presence and level of CTCs in the blood would influence survival in metastatic disease.
CTCs are promising prognostic and predictive factors; however, they are in the area of research and more studies are required to implement this novel technology in clinical practice.
23. Conclusions
Many biomarkers have been reported and are now clinically used in the management of neuro-oncology patients. They now play a crucial role in improving diagnostic accuracy, determining prognosis, and predicting treatment responses. Moreover, the 2021 WHO CNS classification put the highest emphasis on molecular markers than ever before.
23.1. Prognostic Markers
In adult patients, IDH mutations have the greatest prognostic significance, and a number of robust meta-analyses have shown that IDH mutations are associated with longer OS and PFS. The most favorable clinical outcomes are in patients with a combination of IDH mutation and 1p/19q codeletion. On the other hand, CDKN2A mutation indicates the highest malignancy grade in the group of diffuse, IDH-mutant astrocytomas. Additionally, TERT promoter mutations, EGFR alterations, and 7+/10− upgrade IDH-wildtype astrocytomas to glioblastomas. In pediatric patients, H3F3A alterations are the most important markers which predict the worse outcome. MYB, MN1, and MAPK pathway alterations, however, are positive prognostic factors.
23.2. Predictive Markers
MGMT promoter methylation has the greatest clinical significance in predicting responses to TMZ. 1p/19q codeletion and loss of chromosome 10 are also positive predictive markers for the TMZ response. On the other hand, MMR defects lead to hypermutation phenotype and predict poor response to TMZ. Surprisingly, gliomas with hypermutation phenotype, have shown no improvement in outcomes after immune checkpoint inhibitors treatment. So far, EGFR alterations are promising predictive factors for novel targeted therapies and more clinical trials are required.
23.3. Liquid Biopsies
miRNAs, lncRNA, ctDNA, extracellular vesicles, tumor-derived proteins, and CTCs are promising diagnostic, prognostic, and predictive biomarkers, but further work is needed to implement this novel technology in clinical practice. There is a need for minimally invasive methods to detect genetic biomarkers for the molecular characterization of brain tumors. A complete and comprehensive understanding of the genomic alterations that trigger gliomas continues to be essential for diagnostics, prognostics, and targeted therapies.
Author Contributions
Conceptualization P.Ś. and M.A.L., resources P.Ś. and M.A.L., writing—original draft preparation P.Ś. and M.G.B., writing—review and editing P.Ś., M.A.L. and J.F.; visualization P.Ś. and M.G.B., supervision M.A.L., funding acquisition J.K. and J.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by funds for statutory research from the Ludwik Rydygier Collegium Medicum Nicolaus Copernicus University (UMK CM 2018 WL103).
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Louis D.N., Perry A., Wesseling P., Brat D.J., Cree I.A., Figarella-Branger D., Hawkins C., Ng H.K., Pfister S.M., Reifenberger G., et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro-Oncology. 2021;23:1231–1251. doi: 10.1093/neuonc/noab106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schwartzbaum J.A., Fisher J.L., Aldape K.D., Wrensch M. Epidemiology and molecular pathology of glioma. Nat. Clin. Pract. Neurol. 2006;2:494–503. doi: 10.1038/ncpneuro0289. [DOI] [PubMed] [Google Scholar]
- 3.Ostrom Q.T., Bauchet L., Davis F.G., Deltour I., Fisher J.L., Langer C.E., Pekmezci M., Schwartzbaum J.A., Turner M.C., Walsh K.M., et al. The epidemiology of glioma in adults: A “state of the science” review. Neuro-Oncology. 2014;16:896–913. doi: 10.1093/neuonc/nou087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Molinaro A.M., Taylor J.W., Wiencke J.K., Wrensch M.R. Genetic and molecular epidemiology of adult diffuse glioma. Nat. Rev. Neurol. 2019;15:405–417. doi: 10.1038/s41582-019-0220-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McNeill K.A. Epidemiology of Brain Tumors. Neurol. Clin. 2016;34:981–998. doi: 10.1016/j.ncl.2016.06.014. [DOI] [PubMed] [Google Scholar]
- 6.Hansen S., Rasmussen B.K., Laursen R.J., Kosteljanetz M., Schultz H., Nørgård B.M., Guldberg R., Gradel K.O. Treatment and survival of glioblastoma patients in Denmark: The Danish Neuro-Oncology Registry 2009–2014. J. Neuro-Oncol. 2018;139:479–489. doi: 10.1007/s11060-018-2892-7. [DOI] [PubMed] [Google Scholar]
- 7.Ostrom Q.T., Cioffi G., Gittleman H., Patil N., Waite K., Kruchko C., Barnholtz-Sloan J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2012–2016. Neuro-Oncology. 2019;21:v1–v100. doi: 10.1093/neuonc/noz150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sant M., Minicozzi P., Lagorio S., Johannesen T.B., Marcos-Gragera R., Francisci S. The EUROCARE Working Group Survival of European patients with central nervous system tumors. Int. J. Cancer. 2011;131:173–185. doi: 10.1002/ijc.26335. [DOI] [PubMed] [Google Scholar]
- 9.Tan A.C., Ashley D.M., López G.Y., Malinzak M., Friedman H.S., Khasraw M. Management of glioblastoma: State of the art and future directions. CA Cancer J. Clin. 2020;70:299–312. doi: 10.3322/caac.21613. [DOI] [PubMed] [Google Scholar]
- 10.De Vleeschouwer S., Bergers G. Glioblastoma: To Target the Tumor Cell or the Microenvironment? In: de Vleeschouwer S., editor. Glioblastoma. Codon Publications; Brisbane, QLD, Australia: 2017. [PubMed] [Google Scholar]
- 11.Zhu P., Zhu J.-J. Tumor treating fields: A novel and effective therapy for glioblastoma: Mechanism, efficacy, safety and future perspectives. Chin. Clin. Oncol. 2017;6:41. doi: 10.21037/cco.2017.06.29. [DOI] [PubMed] [Google Scholar]
- 12.Gera N., Yang A., Holtzman T.S., Lee S.X., Wong E.T., Swanson K.D. Tumor Treating Fields Perturb the Localization of Septins and Cause Aberrant Mitotic Exit. PLoS ONE. 2015;10:e0125269. doi: 10.1371/journal.pone.0125269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chang E., Patel C.B., Pohling C., Young C., Song J., Flores T.A., Zeng Y., Joubert L.-M., Arami H., Natarajan A., et al. Tumor treating fields increases membrane permeability in glioblastoma cells. Cell Death Discov. 2018;4:1–13. doi: 10.1038/s41420-018-0130-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stupp R., Taillibert S., Kanner A.A., Read W., Steinberg D.M., Lhermitte B., Toms S., Idbaih A., Ahluwalia M.S., Fink K., et al. Effect of Tumor-Treating Fields Plus Maintenance Temozolomide vs. Maintenance Temozolomide Alone on Survival in Patients with Glioblastoma. JAMA. 2017;318:2306–2316. doi: 10.1001/jama.2017.18718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brandsma D., Stalpers L., Taal W., Sminia P., Bent M.J.V.D. Clinical features, mechanisms, and management of pseudoprogression in malignant gliomas. Lancet Oncol. 2008;9:453–461. doi: 10.1016/S1470-2045(08)70125-6. [DOI] [PubMed] [Google Scholar]
- 16.De Wit M.C., De Bruin H.G., Eijkenboom W., Smitt P.A.S., Bent M.V.D. Immediate post-radiotherapy changes in malignant glioma can mimic tumor progression. Neurology. 2004;63:535–537. doi: 10.1212/01.WNL.0000133398.11870.9A. [DOI] [PubMed] [Google Scholar]
- 17.Louis D.N., Perry A., Reifenberger G., von Deimling A., Figarella-Branger D., Cavenee W.K., Ohgaki H., Wiestler O.D., Kleihues P., Ellison D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016;131:803–820. doi: 10.1007/s00401-016-1545-1. [DOI] [PubMed] [Google Scholar]
- 18.Bruford E.A., Braschi B., Denny P., Jones T.E.M., Seal R.L., Tweedie S. Guidelines for human gene nomenclature. Nat. Genet. 2020;52:754–758. doi: 10.1038/s41588-020-0669-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Louis D.N., Wesseling P., Aldape K., Brat D.J., Capper D., Cree I.A., Eberhart C., Figarella-Branger D., Fouladi M., Fuller G.N., et al. cIMPACT-NOW update 6: New entity and diagnostic principle recommendations of the cIMPACT-Utrecht meeting on future CNS tumor classification and grading. Brain Pathol. 2020;30:844–856. doi: 10.1111/bpa.12832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parsons D.W., Jones S., Zhang X., Lin J.C.-H., Leary R.J., Angenendt P., Mankoo P., Carter H., Siu I.-M., Gallia G.L., et al. An Integrated Genomic Analysis of Human Glioblastoma Multiforme. Science. 2008;321:1807–1812. doi: 10.1126/science.1164382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cohen A.L., Holmen S.L., Colman H. IDH1 and IDH2 Mutations in Gliomas. Curr. Neurol. Neurosci. Rep. 2013;13:1–7. doi: 10.1007/s11910-013-0345-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang H.-Y., Tang K., Liang T.-Y., Zhang W.-Z., Li J.-Y., Wang W., Hu H.-M., Li M.-Y., Wang H.-Q., He X.-Z., et al. The comparison of clinical and biological characteristics between IDH1 and IDH2 mutations in gliomas. J. Exp. Clin. Cancer Res. 2016;35:1–9. doi: 10.1186/s13046-016-0362-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang H., Ye D., Guan K.-L., Xiong Y. IDH1 and IDH2 Mutations in Tumorigenesis: Mechanistic Insights and Clinical Perspectives. Clin. Cancer Res. 2012;18:5562–5571. doi: 10.1158/1078-0432.CCR-12-1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dang L., White D.W., Gross S., Bennett B.D., Bittinger M.A., Driggers E.M., Fantin V.R., Jang H.G., Jin S., Keenan M.C., et al. Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature. 2009;462:739–744. doi: 10.1038/nature08617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ježek P. 2-Hydroxyglutarate in Cancer Cells. Antioxid. Redox Signal. 2020;33:903–926. doi: 10.1089/ars.2019.7902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao S., Lin Y., Xu W., Jiang W., Zha Z., Wang P., Yu W., Li Z., Gong L., Peng Y., et al. Glioma-Derived Mutations in IDH1 Dominantly Inhibit IDH1 Catalytic Activity and Induce HIF-1. Science. 2009;324:261–265. doi: 10.1126/science.1170944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lu C., Ward P., Kapoor G.S., Rohle D., Turcan S., Abdel-Wahab O., Edwards C.R., Khanin R., Figueroa M.E., Melnick A., et al. IDH mutation impairs histone demethylation and results in a block to cell differentiation. Nature. 2012;483:474–478. doi: 10.1038/nature10860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bleeker F.E., Atai N.A., Lamba S.E., Jonker A., Rijkeboer D., Bosch K.S., Tigchelaar W., Troost D., Vandertop W.P., Bardelli A., et al. The prognostic IDH1 R132 mutation is associated with reduced NADP+-dependent IDH activity in glioblastoma. Acta Neuropathol. 2010;119:487–494. doi: 10.1007/s00401-010-0645-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Metellus P., Coulibaly B., Colin C., De Paula A.M., Vasiljevic A., Taieb D., Barlier A., Boisselier B., Mokhtari K., Wang X.W., et al. Absence of IDH mutation identifies a novel radiologic and molecular subtype of WHO grade II gliomas with dismal prognosis. Acta Neuropathol. 2010;120:719–729. doi: 10.1007/s00401-010-0777-8. [DOI] [PubMed] [Google Scholar]
- 30.Brat D.J., Aldape K., Colman H., Holland E.C., Louis D.N., Jenkins R.B., Kleinschmidt-DeMasters B.K., Perry A., Reifenberger G., Stupp R., et al. cIMPACT-NOW update 3: Recommended diagnostic criteria for “Diffuse astrocytic glioma, IDH-wildtype, with molecular features of glioblastoma, WHO grade IV”. Acta Neuropathol. 2018;136:805–810. doi: 10.1007/s00401-018-1913-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cancer Genome Atlas Research Network. Brat D.J., Verhaak R.G.W., Aldape K.D., Yung W.K.A., Salama S.R., Cooper L.A.D., Rheinbay E., Miller C.R., Vitucci M., et al. Integrative Genomic Analysis of Diffuse Lower-Grade Gliomas. N. Engl. J. Med. 2015;372:2481–2498. doi: 10.1056/nejmoa1402121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ichimura K., Pearson D.M., Kocialkowski S., Baäcklund L.M., Chan R., Jones D.T.W., Collins V.P. IDH1 mutations are present in the majority of common adult gliomas but rare in primary glioblastomas. Neuro-Oncology. 2009;11:341–347. doi: 10.1215/15228517-2009-025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Songtao Q., Lei Y., Si G., Yanqing D., Huixia H., Xuelin Z., Lanxiao W., Fei Y. IDH mutations predict longer survival and response to temozolomide in secondary glioblastoma. Cancer Sci. 2011;103:269–273. doi: 10.1111/j.1349-7006.2011.02134.x. [DOI] [PubMed] [Google Scholar]
- 34.Sabha N., Knobbe C.B., Maganti M., Al Omar S., Bernstein M., Cairns R., Cako B., von Deimling A., Capper D., Mak T.W., et al. Analysis of IDH mutation, 1p/19q deletion, and PTEN loss delineates prognosis in clinical low-grade diffuse gliomas. Neuro-Oncology. 2014;16:914–923. doi: 10.1093/neuonc/not299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sun H., Yin L., Li S., Han S., Song G., Liu N., Yan C. Prognostic significance of IDH mutation in adult low-grade gliomas: A meta-analysis. J. Neuro-Oncology. 2013;113:277–284. doi: 10.1007/s11060-013-1107-5. [DOI] [PubMed] [Google Scholar]
- 36.Yang P., Zhang W., Wang Y., Peng X., Chen B., Qiu X., Li G., Li S., Wu C., Yao K., et al. IDH mutation and MGMT promoter methylation in glioblastoma: Results of a prospective registry. Oncotarget. 2015;6:40896–40906. doi: 10.18632/oncotarget.5683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lewandowska M.A., Furtak J., Szylberg T., Roszkowski K., Windorbska W., Rytlewska J., Jozwicki W. An analysis of the prognostic value of IDH1 (isocitrate dehydrogenase 1) mutation in Polish glioma patients. Mol. Diagn. Ther. 2013;18:45–53. doi: 10.1007/s40291-013-0050-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zou P., Xu H., Chen P., Yan Q., Zhao L., Zhao P., Gu A. IDH1/IDH2 Mutations Define the Prognosis and Molecular Profiles of Patients with Gliomas: A Meta-Analysis. PLoS ONE. 2013;8:e68782. doi: 10.1371/journal.pone.0068782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dai Y., Ning X., Han G., Li W. Assessment of the Association between Isocitrate Dehydrogenase 1 Mutation and Mortality Risk of Glioblastoma Patients. Mol. Neurobiol. 2015;53:1501–1508. doi: 10.1007/s12035-015-9104-7. [DOI] [PubMed] [Google Scholar]
- 40.Molenaar R.J., Verbaan D., Lamba S.E., Zanon C., Jeuken J.W., Boots-Sprenger S.H., Wesseling P., Hulsebos T.J., Troost D., Van Tilborg A.A., et al. The combination of IDH1 mutations and MGMT methylation status predicts survival in glioblastoma better than either IDH1 or MGMT alone. Neuro-Oncology. 2014;16:1263–1273. doi: 10.1093/neuonc/nou005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cubuk C., Hidalgo M.R., Amadoz A., Pujana M.A., Mateo F., Herranz C., Carbonell-Caballero J., Dopazo J. Gene Ex-pression Integration into Pathway Modules Reveals a Pan-Cancer Metabolic Landscape. Cancer Res. 2018;78:6059–6072. doi: 10.1158/0008-5472.CAN-17-2705. [DOI] [PubMed] [Google Scholar]
- 42.Talbert P.B., Henikoff S. Histone variants—Ancient wrap artists of the epigenome. Nat. Rev. Mol. Cell Biol. 2010;11:264–275. doi: 10.1038/nrm2861. [DOI] [PubMed] [Google Scholar]
- 43.Wu G., Broniscer A., McEachron T.A., Lu C., Paugh B.S., Becksfort J., Qu C., Ding L., Huether R., Parker M., et al. Somatic histone H3 alterations in pediatric diffuse intrinsic pontine gliomas and non-brainstem glioblastomas. Nat. Genet. 2012;44:251–253. doi: 10.1038/ng.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sturm D., Witt H., Hovestadt V., Khuong-Quang D.-A., Jones D.T.W., Konermann C., Pfaff E., Tönjes M., Sill M., Bender S., et al. Hotspot Mutations in H3F3A and IDH1 Define Distinct Epigenetic and Biological Subgroups of Glioblastoma. Cancer Cell. 2012;22:425–437. doi: 10.1016/j.ccr.2012.08.024. [DOI] [PubMed] [Google Scholar]
- 45.Khuong-Quang D.-A., Buczkowicz P., Rakopoulos P., Liu X.-Y., Fontebasso A.M., Bouffet E., Bartels U., Albrecht S., Schwartzentruber J., Letourneau L., et al. K27M mutation in histone H3.3 defines clinically and biologically distinct subgroups of pediatric diffuse intrinsic pontine gliomas. Acta Neuropathol. 2012;124:439–447. doi: 10.1007/s00401-012-0998-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schwartzentruber J., Korshunov A., Liu X.-Y., Jones D.T.W., Pfaff E., Jacob K., Sturm D., Fontebasso A.M., Quang D.-A.K., Tönjes M., et al. Driver mutations in histone H3.3 and chromatin remodelling genes in paediatric glioblastoma. Nature. 2012;482:226–231. doi: 10.1038/nature10833. [DOI] [PubMed] [Google Scholar]
- 47.Hamisch C., Kickingereder P., Fischer M., Simon T., Ruge M.I. Update on the diagnostic value and safety of stereotactic biopsy for pediatric brainstem tumors: A systematic review and meta-analysis of 735 cases. J. Neurosurg. Pediatr. 2017;20:261–268. doi: 10.3171/2017.2.PEDS1665. [DOI] [PubMed] [Google Scholar]
- 48.Korshunov A., Ryzhova M., Hovestadt V., Bender S., Sturm D., Capper D., Meyer J., Schrimpf D., Kool M., Northcott P.A., et al. Integrated analysis of pediatric glioblastoma reveals a subset of biologically favorable tumors with associated molecular prognostic markers. Acta Neuropathol. 2015;129:669–678. doi: 10.1007/s00401-015-1405-4. [DOI] [PubMed] [Google Scholar]
- 49.Liu X.-Y., Gerges N., Korshunov A., Sabha N., Khuong-Quang D.-A., Fontebasso A.M., Fleming A., Hadjadj D., Schwartzentruber J., Majewski J., et al. Frequent ATRX mutations and loss of expression in adult diffuse astrocytic tumors carrying IDH1/IDH2 and TP53 mutations. Acta Neuropathol. 2012;124:615–625. doi: 10.1007/s00401-012-1031-3. [DOI] [PubMed] [Google Scholar]
- 50.Lovejoy C.A., Li W., Reisenweber S., Thongthip S., Bruno J., De Lange T., De S., Petrini J., Sung P.A., Jasin M., et al. Loss of ATRX, Genome Instability, and an Altered DNA Damage Response Are Hallmarks of the Alternative Lengthening of Telomeres Pathway. PLoS Genet. 2012;8:e1002772. doi: 10.1371/journal.pgen.1002772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kannan K., Inagaki A., Silber J., Gorovets D., Zhang J., Kastenhuber E.R., Heguy A., Petrini J.H., Chan T.A., Huse J.T. Whole exome sequencing identifies ATRX mutation as a key molecular determinant in lower-grade glioma. Oncotarget. 2012;3:1194–1203. doi: 10.18632/oncotarget.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jiao Y., Killela P.J., Reitman Z., Rasheed B.A., Heaphy C.M., De Wilde R.F., Rodriguez F.J., Rosemberg S., Shinjo S., Marie S., et al. Frequent ATRX, CIC, FUBP1 and IDH1 mutations refine the classification of malignant gliomas. Oncotarget. 2012;3:709–722. doi: 10.18632/oncotarget.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pekmezci M., Rice T., Molinaro A.M., Walsh K., Decker P.A., Hansen H., Sicotte H., Kollmeyer T.M., McCoy L.S., Sarkar G., et al. Adult infiltrating gliomas with WHO 2016 integrated diagnosis: Additional prognostic roles of ATRX and TERT. Acta Neuropathol. 2017;133:1001–1016. doi: 10.1007/s00401-017-1690-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Figarella-Branger D., Mokhtari K., Dehais C., Jouvet A., Uro-Coste E., Colin C., Carpentier C., Forest F., Maurage C.-A., Vignaud J.-M., et al. Mitotic index, microvascular proliferation, and necrosis define 3 groups of 1p/19q codeleted anaplastic oligodendrogliomas associated with different genomic alterations. Neuro-Oncology. 2014;16:1244–1254. doi: 10.1093/neuonc/nou047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhao J., Ma W., Zhao H. Loss of heterozygosity 1p/19q and survival in glioma: A meta-analysis. Neuro-Oncology. 2013;16:103–112. doi: 10.1093/neuonc/not145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ryall S., Krishnatry R., Arnoldo A., Buczkowicz P., Mistry M., Siddaway R., Ling C., Pajovic S., Yu M., Rubin J.B., et al. Targeted detection of genetic alterations reveal the prognostic impact of H3K27M and MAPK pathway aberrations in paediatric thalamic glioma. Acta Neuropathol. Commun. 2016;4:1–10. doi: 10.1186/s40478-016-0353-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Meng W., Jiang Y., Ma J. Is the prognostic significance of O6-methylguanine-DNA methyltransferase promoter methylation equally important in glioblastomas of patients from different continents? A systematic review with meta-analysis. Cancer Manag. Res. 2017;9:411–425. doi: 10.2147/CMAR.S140447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhao Y.-H., Wang X., Cao C.-J., Weng H., Xu C.-S., Li K., Li J.-L., Lan J., Zeng X.-T., Li Z.-Q. The Clinical Significance of O6-Methylguanine-DNA Methyltransferase Promoter Methylation Status in Adult Patients with Glioblastoma: A Meta-analysis. Front. Neurol. 2018;9:127. doi: 10.3389/fneur.2018.00127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vuong H.G., Nguyen T.Q., Ngo T.N.M., Nguyen H.C., Fung K.-M., Dunn I.F. The interaction between TERT promoter mutation and MGMT promoter methylation on overall survival of glioma patients: A meta-analysis. BMC Cancer. 2020;20:1–9. doi: 10.1186/s12885-020-07364-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Arita H., Yamasaki K., Matsushita Y., Nakamura T., Shimokawa A., Takami H., Tanaka S., Mukasa A., Shirahata M., Shimizu S., et al. A combination of TERT promoter mutation and MGMT methylation status predicts clinically relevant subgroups of newly diagnosed glioblastomas. Acta Neuropathol. Commun. 2016;4:1–14. doi: 10.1186/s40478-016-0351-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vuong H.G., Altibi A.M., Duong U.N., Ngo H.T., Pham T.Q., Chan A.K.-Y., Park C.-K., Fung K.-M., Hassell L. TERT promoter mutation and its interaction with IDH mutations in glioma: Combined TERT promoter and IDH mutations stratifies lower-grade glioma into distinct survival subgroups—A meta-analysis of aggregate data. Crit. Rev. Oncol. 2017;120:1–9. doi: 10.1016/j.critrevonc.2017.09.013. [DOI] [PubMed] [Google Scholar]
- 62.Bagley S.J., Nabavizadeh S.A., Till J., Abdalla A., Sanga H., Mays J., Prior T., Jurgielewicz A., Guiry S., Davtyan K. A Prospective Validation Cohort Study of Baseline Plasma Cell-Free DNA (CfDNA) as a Prognostic Biomarker in Newly Diagnosed Gli-oblastoma (GBM) Am. Soc. Clin. Oncol. 2020;38:2508. doi: 10.1200/JCO.2020.38.15_suppl.2508. [DOI] [Google Scholar]
- 63.Bagley S.J., Till J., Abdalla A., Sangha H.K., Yee S.S., Freedman J., Black T.A., Hussain J., Binder Z.A., Brem S. Associa-tion of Plasma Cell-Free DNA with Survival in Patients with IDH Wild-Type Glioblastoma. Neuro-Oncol. Adv. 2021;3:vdab011. doi: 10.1093/noajnl/vdab011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Miller A., Shah R., Pentsova E.I., Pourmaleki M., Briggs S., Distefano N., Zheng Y., Skakodub A., Mehta S.A., Campos C., et al. Tracking tumour evolution in glioma through liquid biopsies of cerebrospinal fluid. Nat. Cell Biol. 2019;565:654–658. doi: 10.1038/s41586-019-0882-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lu V.M., O’Connor K.P., Shah A.H., Eichberg D.G., Luther E.M., Komotar R.J., Ivan M.E. The prognostic significance of CDKN2A homozygous deletion in IDH-mutant lower-grade glioma and glioblastoma: A systematic review of the contemporary literature. J. Neuro-Oncol. 2020;148:221–229. doi: 10.1007/s11060-020-03528-2. [DOI] [PubMed] [Google Scholar]
- 66.Aibaidula A., Chan A.K.-Y., Shi Z., Li Y., Zhang R., Yang R., Li K.K.-W., Chung N.Y.-F., Yao Y., Zhou L., et al. Adult IDH wild-type lower-grade gliomas should be further stratified. Neuro-Oncology. 2017;19:1327–1337. doi: 10.1093/neuonc/nox078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li J., Liang R., Song C., Xiang Y., Liu Y. Prognostic and clinicopathological significance of long non-coding RNA in glioma. Neurosurg. Rev. 2018;43:1–8. doi: 10.1007/s10143-018-0965-x. [DOI] [PubMed] [Google Scholar]
- 68.Iwamoto F.M., Hottinger A.F., Karimi S., Riedel E., Dantis J., Jahdi M., Panageas K.S., Lassman A.B., Abrey L.E., Fleisher M., et al. Serum YKL-40 is a marker of prognosis and disease status in high-grade gliomas. Neuro-Oncology. 2011;13:1244–1251. doi: 10.1093/neuonc/nor117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Song Y., He M., Zhang J., Xu J. High expression of microRNA 221 is a poor predictor for glioma. Medicine. 2020;99:e23163. doi: 10.1097/MD.0000000000023163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang R., Pang B., Xin T., Guo H., Xing Y., Xu S., Feng B., Liu B., Pang Q. Plasma miR-221/222 Family as Novel Descriptive and Prognostic Biomarkers for Glioma. Mol. Neurobiol. 2015;53:1452–1460. doi: 10.1007/s12035-014-9079-9. [DOI] [PubMed] [Google Scholar]
- 71.Zhang Y., Chen J., Xue Q., Wang J., Zhao L., Han K., Zhang D., Hou L. Prognostic Significance of MicroRNAs in Glioma: A Systematic Review and Meta-Analysis. BioMed Res. Int. 2019;2019:4015969. doi: 10.1155/2019/4015969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhou Y., Wang X., Liu Z., Huang X., Li X., Cheng K., Jiang X. Prognostic role of microRNA-155 expression in gliomas: A meta-analysis. Clin. Neurol. Neurosurg. 2018;176:103–109. doi: 10.1016/j.clineuro.2018.12.005. [DOI] [PubMed] [Google Scholar]
- 73.Jiang G., Mu J., Liu X., Peng X., Zhong F., Yuan W., Deng F., Peng X., Peng S., Zeng X. Prognostic value of miR-21 in gliomas: Comprehensive study based on meta-analysis and TCGA dataset validation. Sci. Rep. 2020;10:1–10. doi: 10.1038/s41598-020-61155-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li C., Sun J., Xiang Q., Liang Y., Zhao N., Zhang Z., Liu Q., Cui Y. Prognostic role of microRNA-21 expression in gliomas: A meta-analysis. J. Neuro-Oncol. 2016;130:11–17. doi: 10.1007/s11060-016-2233-7. [DOI] [PubMed] [Google Scholar]
- 75.Song Y., Zhang J., He M., Xu J. Prognostic Role of MicroRNA 222 in Patients with Glioma: A Meta-analysis. BioMed Res. Int. 2020;2020:1–7. doi: 10.1155/2020/4689689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Huang F.W., Hodis E., Xu M.J., Kryukov G., Chin L., Garraway L.A. Highly Recurrent TERT Promoter Mutations in Human Melanoma. Science. 2013;339:957–959. doi: 10.1126/science.1229259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Horn S., Figl A., Rachakonda P.S., Fischer C., Sucker A., Gast A., Kadel S., Moll I., Nagore E., Hemminki K., et al. TERT Promoter Mutations in Familial and Sporadic Melanoma. Science. 2013;339:959–961. doi: 10.1126/science.1230062. [DOI] [PubMed] [Google Scholar]
- 78.Mancini A., Xavier-Magalhães A., Woods W.S., Nguyen K.-T., Amen A.M., Hayes J.L., Fellmann C., Gapinske M., McKinney A.M., Hong C., et al. Disruption of the β1L Isoform of GABP Reverses Glioblastoma Replicative Immortality in a TERT Promoter Mutation-Dependent Manner. Cancer Cell. 2018;34:513–528. doi: 10.1016/j.ccell.2018.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Arita H., Narita Y., Fukushima S., Tateishi K., Matsushita Y., Yoshida A., Miyakita Y., Ohno M., Collins V.P., Kawahara N., et al. Upregulating mutations in the TERT promoter commonly occur in adult malignant gliomas and are strongly associated with total 1p19q loss. Acta Neuropathol. 2013;126:267–276. doi: 10.1007/s00401-013-1141-6. [DOI] [PubMed] [Google Scholar]
- 80.Mosrati M.A., Malmström A., Lysiak M., Krysztofiak A., Hallbeck M., Milos P., Hallbeck A.-L., Bratthäll C., Strandéus M., Stenmark-Askmalm M., et al. TERT Promoter Mutations and Polymorphisms as Prognostic Factors in Primary Glioblastoma. Oncotarget. 2015;6:16663–16673. doi: 10.18632/oncotarget.4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shu C., Wang Q., Yan X., Wang J. The TERT promoter mutation status and MGMT promoter methylation status, combined with dichotomized MRI-derived and clinical features, predict adult primary glioblastoma survival. Cancer Med. 2018;7:3704–3712. doi: 10.1002/cam4.1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Simon M., Hosen I., Gousias K., Rachakonda S., Heidenreich B., Gessi M., Schramm J., Hemminki K., Waha A., Kumar R. TERT promoter mutations: A novel independent prognostic factor in primary glioblastomas. Neuro-Oncology. 2014;17:45–52. doi: 10.1093/neuonc/nou158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Labussiere M., Idbaih A., Wang X.W., Marie Y., Boisselier B., Falet C., Paris S., Laffaire J., Carpentier C., Criniere E., et al. All the 1p19q codeleted gliomas are mutated on IDH1 or IDH2. Neurology. 2010;74:1886–1890. doi: 10.1212/WNL.0b013e3181e1cf3a. [DOI] [PubMed] [Google Scholar]
- 84.Spiegl-Kreinecker S., Lötsch D., Ghanim B., Pirker C., Mohr T., Laaber M., Weis S., Olschowski A., Webersinke G., Pichler J., et al. Prognostic quality of activating TERT promoter mutations in glioblastoma: Interaction with the rs2853669 polymorphism and patient age at diagnosis. Neuro-Oncology. 2015;17:1231–1240. doi: 10.1093/neuonc/nov010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yuan Y., Qi C., Maling G., Xiang W., Yanhui L., Ruofei L., Yunhe M., Jiewen L., Qing M. TERT mutation in glioma: Frequency, prognosis and risk. J. Clin. Neurosci. 2016;26:57–62. doi: 10.1016/j.jocn.2015.05.066. [DOI] [PubMed] [Google Scholar]
- 86.Appay R., Dehais C., Maurage C.-A., Alentorn A., Carpentier C., Colin C., Ducray F., Escande F., Idbaih A., Kamoun A., et al. CDKN2A homozygous deletion is a strong adverse prognosis factor in diffuse malignant IDH-mutant gliomas. Neuro-Oncology. 2019;21:1519–1528. doi: 10.1093/neuonc/noz126.000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ma S., Rudra S., Campian J.L., Dahiya S., Dunn G.P., Johanns T., Goldstein M., Kim A.H., Huang J. Prognostic impact of CDKN2A/B deletion, TERT mutation, and EGFR amplification on histological and molecular IDH-wildtype glioblastoma. Neuro-Oncol. Adv. 2020;2:vdaa126. doi: 10.1093/noajnl/vdaa126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Manuel J.M., Ghosh D., Narasinga Rao K.V.L., Sibin M.K., Venkatesh H.N., Ch L., Chetan G.K. Role of Concurrent Methylation Pattern of MGMT, TP53 and CDKN2A Genes in the Prognosis of High Grade Glioma. J. Carcinog. Mutagen. 2016;7:1. doi: 10.4172/2157-2518.1000250. [DOI] [Google Scholar]
- 89.Shirahata M., Ono T., Stichel D., Schrimpf D., Reuss D.E., Sahm F., Koelsche C., Wefers A., Reinhardt A., Huang K., et al. Novel, improved grading system(s) for IDH-mutant astrocytic gliomas. Acta Neuropathol. 2018;136:153–166. doi: 10.1007/s00401-018-1849-4. [DOI] [PubMed] [Google Scholar]
- 90.Wang H., Wang X., Xu L., Zhang J., Cao H. Analysis of the EGFR Amplification and CDKN2A Deletion Regulated Transcriptomic Signatures Reveals the Prognostic Significance of SPATS2L in Patients with Glioma. Front. Oncol. 2021;11:713. doi: 10.3389/fonc.2021.551160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wu F., Li G., Liu H., Zhao Z., Chai R., Liu Y., Jiang H., Zhai Y., Feng Y., Li R., et al. Molecular subtyping reveals immune alterations in IDH wild-type lower-grade diffuse glioma. J. Pathol. 2020;251:272–283. doi: 10.1002/path.5468. [DOI] [PubMed] [Google Scholar]
- 92.Yang R.R., Shi Z., Zhang Z., Chan A.K., Aibaidula A., Wang W., Kwan J.S.H., Poon W.S., Chen H., Li W., et al. IDH mutant lower grade (WHO Grades II/III) astrocytomas can be stratified for risk by CDKN2A, CDK4 and PDGFRA copy number alterations. Brain Pathol. 2019;30:541–553. doi: 10.1111/bpa.12801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ghasimi S., Wibom C., Dahlin A., Brännström T., Golovleva I., Andersson U., Melin B. Genetic risk variants in the CDKN2A/B, RTEL1 and EGFR genes are associated with somatic biomarkers in glioma. J. Neuro-Oncol. 2016;127:483–492. doi: 10.1007/s11060-016-2066-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Alentorn A., Dehais C., Ducray F., Carpentier C., Mokhtari K., Figarella-Branger D., Chinot O., Cohen-Moyal E., Ramirez C., Loiseau H., et al. Allelic loss of 9p21.3 is a prognostic factor in 1p/19q codeleted anaplastic gliomas. Neurology. 2015;85:1325–1331. doi: 10.1212/WNL.0000000000002014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Brat D.J., Aldape K., Colman H., Figrarella-Branger D., Fuller G.N., Giannini C., Holland E.C., Jenkins R.B., Kleinschmidt-DeMasters B., Komori T., et al. cIMPACT-NOW update 5: Recommended grading criteria and terminologies for IDH-mutant astrocytomas. Acta Neuropathol. 2020;139:603–608. doi: 10.1007/s00401-020-02127-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chamberlain M.C., Born N. Prognostic significance of relative 1p/19q codeletion in oligodendroglial tumors. J. Neuro-Oncol. 2015;125:249–251. doi: 10.1007/s11060-015-1906-y. [DOI] [PubMed] [Google Scholar]
- 97.Jenkins R.B., Blair H., Ballman K.V., Giannini C., Arusell R.M., Law M., Flynn H., Passe S., Felten S., Brown P.D., et al. A t(1;19)(q10;p10) Mediates the Combined Deletions of 1p and 19q and Predicts a Better Prognosis of Patients with Oligodendroglioma. Cancer Res. 2006;66:9852–9861. doi: 10.1158/0008-5472.CAN-06-1796. [DOI] [PubMed] [Google Scholar]
- 98.Eckel-Passow J.E., Lachance D.H., Molinaro A.M., Walsh K., Decker P.A., Sicotte H., Pekmezci M., Rice T.W., Kosel M.L., Smirnov I.V., et al. Glioma Groups Based on 1p/19q, IDH, and TERTPromoter Mutations in Tumors. N. Engl. J. Med. 2015;372:2499–2508. doi: 10.1056/NEJMoa1407279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jiang H., Ren X., Cui X., Wang J., Jia W., Zhou Z., Lin S. 1p/19q codeletion and IDH1/2 mutation identified a subtype of anaplastic oligoastrocytomas with prognosis as favorable as anaplastic oligodendrogliomas. Neuro-Oncology. 2013;15:775–782. doi: 10.1093/neuonc/not027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Reuss D.E., Sahm F., Schrimpf D., Wiestler B., Capper D., Koelsche C., Schweizer L., Korshunov A., Jones D.T.W., Hovestadt V., et al. ATRX and IDH1-R132H immunohistochemistry with subsequent copy number analysis and IDH sequencing as a basis for an “integrated” diagnostic approach for adult astrocytoma, oligodendroglioma and glioblastoma. Acta Neuropathol. 2014;129:133–146. doi: 10.1007/s00401-014-1370-3. [DOI] [PubMed] [Google Scholar]
- 101.Huse J.T., Diamond E., Wang L., Rosenblum M.K. Mixed glioma with molecular features of composite oligodendroglioma and astrocytoma: A true “oligoastrocytoma”? Acta Neuropathol. 2014;129:151–153. doi: 10.1007/s00401-014-1359-y. [DOI] [PubMed] [Google Scholar]
- 102.Hu X., Martinez-Ledesma E., Zheng S., Kim H., Barthel F., Jiang T., Hess K.R., Verhaak R.G. Multigene signature for predicting prognosis of patients with 1p19q co-deletion diffuse glioma. Neuro-Oncology. 2017;19:786–795. doi: 10.1093/neuonc/now285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kaloshi G., Benouaich-Amiel A., Diakite F., Taillibert S., Lejeune J., Laigle-Donadey F., Renard M.-A., Iraqi W., Idbaih A., Paris S., et al. Temozolomide for low-grade gliomas: Predictive impact of 1p/19q loss on response and outcome. Neurology. 2007;68:1831–1836. doi: 10.1212/01.wnl.0000262034.26310.a2. [DOI] [PubMed] [Google Scholar]
- 104.Vogazianou A.P., Chan R., Bäcklund L.M., Pearson D.M., Liu L., Langford C.F., Gregory S.G., Collins V.P., Ichimura K. Distinct patterns of 1p and 19q alterations identify subtypes of human gliomas that have different prognoses. Neuro-Oncology. 2010;12:664–678. doi: 10.1093/neuonc/nop075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Weller M., Stupp R., Hegi M., Bent M.V.D., Tonn J.C., Sanson M., Wick W., Reifenberger G. Personalized care in neuro-oncology coming of age: Why we need MGMT and 1p/19q testing for malignant glioma patients in clinical practice. Neuro-Oncology. 2012;14:iv100–iv108. doi: 10.1093/neuonc/nos206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cairncross G., Berkey B., Shaw E., Jenkins R., Scheithauer B., Brachman D., Buckner J., Fink K., Souhami L., Laperierre N., et al. Phase III Trial of Chemotherapy Plus Radiotherapy Compared with Radiotherapy Alone for Pure and Mixed Anaplastic Oligodendroglioma: Intergroup Radiation Therapy Oncology Group Trial 9402. J. Clin. Oncol. 2006;24:2707–2714. doi: 10.1200/JCO.2005.04.3414. [DOI] [PubMed] [Google Scholar]
- 107.Erdem-Eraslan L., Gravendeel L.A., de Rooi J., Eilers P.H., Idbaih A., Spliet W.G., Dunnen W.F.D., Teepen J.L., Wesseling P., Smitt P.A.S., et al. Intrinsic Molecular Subtypes of Glioma Are Prognostic and Predict Benefit from Adjuvant Procarbazine, Lomustine, and Vincristine Chemotherapy in Combination with Other Prognostic Factors in Anaplastic Oligodendroglial Brain Tumors: A Report From EORTC Study 26951. J. Clin. Oncol. 2013;31:328–336. doi: 10.1200/jco.2012.44.1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Stichel D., Ebrahimi A., Reuss D., Schrimpf D., Ono T., Shirahata M., Reifenberger G., Weller M., Hänggi D., Wick W., et al. Distribution of EGFR amplification, combined chromosome 7 gain and chromosome 10 loss, and TERT promoter mutation in brain tumors and their potential for the reclassification of IDHwt astrocytoma to glioblastoma. Acta Neuropathol. 2018;136:793–803. doi: 10.1007/s00401-018-1905-0. [DOI] [PubMed] [Google Scholar]
- 109.Balesaria S., Brock C., Bower M., Clark J., Nicholson S.K., Lewis P., De Sanctis S., Evans H., Peterson D., Mendoza N., et al. Loss of chromosome 10 is an independent prognostic factor in high-grade gliomas. Br. J. Cancer. 1999;81:1371–1377. doi: 10.1038/sj.bjc.6693403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Roth J.J., Fierst T.M., Waanders A.J., Yimei L., Biegel J.A., Santi M. Whole Chromosome 7 Gain Predicts Higher Risk of Recurrence in Pediatric Pilocytic Astrocytomas Independently from KIAA1549-BRAF Fusion Status. J. Neuropathol. Exp. Neurol. 2016;75:306–315. doi: 10.1093/jnen/nlw001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Richard S., Tachon G., Milin S., Wager M., Karayan-Tapon L. Dual MGMT inactivation by promoter hypermethylation and loss of the long arm of chromosome 10 in glioblastoma. Cancer Med. 2020;9:6344–6353. doi: 10.1002/cam4.3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhou Y., Ness S.A. Myb Proteins: Angels and Demons in Normal and Transformed Cells. Front. Biosci. J. Virtual Libr. 2011;16:1109–1131. doi: 10.2741/3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chiang C.-H., Harreld J.H., Tinkle C.L., Moreira D.C., Li X., Acharya S., Qaddoumi I., Ellison D.W. A single-center study of the clinicopathologic correlates of gliomas with a MYB or MYBL1 alteration. Acta Neuropathol. 2019;138:1091–1092. doi: 10.1007/s00401-019-02081-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ellison D.W., Hawkins C., Jones D.T.W., Onar-Thomas A., Pfister S.M., Reifenberger G., Louis D.N. cIMPACT-NOW update 4: Diffuse gliomas characterized by MYB, MYBL1, or FGFR1 alterations or BRAFV600E mutation. Acta Neuropathol. 2019;137:683–687. doi: 10.1007/s00401-019-01987-0. [DOI] [PubMed] [Google Scholar]
- 115.Wood M.D., Tihan T., Perry A., Chacko G., Turner C., Pu C., Payne C., Yu A., Bannykh S.I., Solomon D.A. Multimodal molecular analysis of astroblastoma enables reclassification of most cases into more specific molecular entities. Brain Pathol. 2017;28:192–202. doi: 10.1111/bpa.12561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Saini M., Jha A.N., Tangri R., Qudratullah M., Ali S. MN1 overexpression with varying tumor grade is a promising predictor of survival of glioma patients. Hum. Mol. Genet. 2020;29:3532–3545. doi: 10.1093/hmg/ddaa231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Mhatre R., Sugur H.S., Nandeesh B.N., Chickabasaviah Y., Saini J., Santosh V. MN1 rearrangement in astroblastoma: Study of eight cases and review of literature. Brain Tumor Pathol. 2019;36:112–120. doi: 10.1007/s10014-019-00346-x. [DOI] [PubMed] [Google Scholar]
- 118.Lehman N.L., Usubalieva A., Lin T., Allen S.J., Tran Q.T., Mobley B.C., McLendon R.E., Schniederjan M.J., Georgescu M.-M., Couce M., et al. Genomic analysis demonstrates that histologically-defined astroblastomas are molecularly heterogeneous and that tumors with MN1 rearrangement exhibit the most favorable prognosis. Acta Neuropathol. Commun. 2019;7:1–11. doi: 10.1186/s40478-019-0689-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Santarpia L., Lippman S.L., El-Naggar A.K. Targeting the MAPK–RAS–RAF signaling pathway in cancer therapy. Expert Opin. Ther. Targets. 2012;16:103–119. doi: 10.1517/14728222.2011.645805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Reinhardt A., Stichel D., Schrimpf D., Sahm F., Korshunov A., Reuss D.E., Koelsche C., Huang K., Wefers A.K., Hovestadt V., et al. Anaplastic astrocytoma with piloid features, a novel molecular class of IDH wildtype glioma with recurrent MAPK pathway, CDKN2A/B and ATRX alterations. Acta Neuropathol. 2018;136:273–291. doi: 10.1007/s00401-018-1837-8. [DOI] [PubMed] [Google Scholar]
- 121.Jones D.T.W., The International Cancer Genome Consortium PedBrain Tumor Project. Hutter B., Jaeger N., Korshunov A., Kool M., Warnatz H.-J., Zichner T., Lambert S.R., Ryzhova M., et al. Recurrent somatic alterations of FGFR1 and NTRK2 in pilocytic astrocytoma. Nat. Genet. 2013;45:927–932. doi: 10.1038/ng.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Jones D.T.W., Gronych J., Lichter P., Witt O., Pfister S.M. MAPK pathway activation in pilocytic astrocytoma. Experientia. 2011;69:1799–1811. doi: 10.1007/s00018-011-0898-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Becker A.P., Scapulatempo-Neto C., Carloni A.C., Paulino A., Sheren J., Aisner D.L., Musselwhite E., Clara C., Machado H.R., Oliveira R.S., et al. KIAA1549: BRAF Gene Fusion and FGFR1 Hotspot Mutations Are Prognostic Factors in Pilo-cytic Astrocytomas. J. Neuropathol. Exp. Neurol. 2015;74:743–754. doi: 10.1097/NEN.0000000000000213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Arteaga C.L., Engelman J.A. ERBB Receptors: From Oncogene Discovery to Basic Science to Mechanism-Based Cancer Therapeutics. Cancer Cell. 2014;25:282–303. doi: 10.1016/j.ccr.2014.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Li X., Wu C., Chen N., Gu H., Yen A., Cao L., Wang E., Wang L. PI3K/Akt/mTOR signaling pathway and targeted therapy for glioblastoma. Oncotarget. 2016;7:33440–33450. doi: 10.18632/oncotarget.7961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Brennan C.W., Verhaak R.G., McKenna A., Campos B., Noushmehr H., Salama S., Zheng S., Chakravarty D., Sanborn J.Z., Berman S.H., et al. The Somatic Genomic Landscape of Glioblastoma. Cell. 2013;155:462–477. doi: 10.1016/j.cell.2013.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Keller S., Schmidt M.H.H. EGFR and EGFRvIII Promote Angiogenesis and Cell Invasion in Glioblastoma: Combination Therapies for an Effective Treatment. Int. J. Mol. Sci. 2017;18:1295. doi: 10.3390/ijms18061295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Gan H.K., Kaye A.H., Luwor R.B. The EGFRvIII variant in glioblastoma multiforme. J. Clin. Neurosci. 2009;16:748–754. doi: 10.1016/j.jocn.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 129.Eskilsson E., Rosland G.V., Talasila K.M., Knappskog S., Keunen O., Sottoriva A., Foerster S., Solecki G., Taxt T., Jirik R., et al. EGFRvIII mutations can emerge as late and heterogenous events in glioblastoma development and promote angiogenesis through Src activation. Neuro-Oncology. 2016;18:1644–1655. doi: 10.1093/neuonc/now113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Bale T.A., Jordan J.T., Rapalino O., Ramamurthy N., Jessop N., DeWitt J.C., Nardi V., Alvarez M.M.-L., Frosch M., Batchelor T.T., et al. Financially effective test algorithm to identify an aggressive, EGFR-amplified variant of IDH-wildtype, lower-grade diffuse glioma. Neuro-Oncology. 2018;21:596–605. doi: 10.1093/neuonc/noy201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Labussière M., Boisselier B., Mokhtari K., Di Stefano A.-L., Rahimian A., Rossetto M., Ciccarino P., Saulnier O., Paterra R., Marie Y., et al. Combined analysis of TERT, EGFR, and IDH status defines distinct prognostic glioblastoma classes. Neurology. 2014;83:1200–1206. doi: 10.1212/WNL.0000000000000814. [DOI] [PubMed] [Google Scholar]
- 132.Hao Z., Guo D. EGFR mutation: Novel prognostic factor associated with immune infiltration in lower-grade glioma; an exploratory study. BMC Cancer. 2019;19:1–13. doi: 10.1186/s12885-019-6384-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Shinojima N., Tada K., Shiraishi S., Kamiryo T., Kochi M., Nakamura H., Makino K., Saya H., Hirano H., Kuratsu J.-I., et al. Prognostic value of epidermal growth factor receptor in patients with glioblastoma multiforme. Cancer Res. 2003;63:6962–6970. [PubMed] [Google Scholar]
- 134.Lee K., Koo H., Kim Y., Kim D., Son E., Yang H., Lim Y., Hur M., Lee H., Choi S., et al. Therapeutic Efficacy of GC1118, a Novel Anti-Egfr Antibody, against Glioblastoma with High Egfr Amplification in Patient-Derived Xenografts. Cancers. 2020;12:3210. doi: 10.3390/cancers12113210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Nadeem Abbas M., Kausar S., Wang F., Zhao Y., Cui H. Cui Advances in Targeting the Epidermal Growth Factor Receptor Pathway by Synthetic Products and Its Regulation by Epigenetic Modulators as a Therapy for Glioblastoma. Cells. 2019;8:350. doi: 10.3390/cells8040350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ni J., Yang Y., Wang Q., Bergholz J.S., Jiang T., Roberts T.M., Gray N.S., Zhao J.J. Targeting EGFR in Glioblastoma with a Novel Brain-Penetrant Small Molecule EGFR-TKI. BioRxiv. 2021;2021:426030. doi: 10.1101/2021.01.09.426030. [DOI] [Google Scholar]
- 137.Zając A., Sumorek-Wiadro J., Langner E., Wertel I., Maciejczyk A., Pawlikowska-Pawlęga B., Pawelec J., Wasiak M., Hułas-Stasiak M., Bądziul D., et al. Involvement of PI3K Pathway in Glioma Cell Resistance to Temozolomide Treatment. Int. J. Mol. Sci. 2021;22:5155. doi: 10.3390/ijms22105155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Duque M.B., Pinheiro K.D.V., Thomaz A., Da Silva C.A., Freire N.H., Brunetto A.T., Schwartsmann G., Jaeger M., de Farias C., Roesler R. Combined Inhibition of HDAC and EGFR Reduces Viability and Proliferation and Enhances STAT3 mRNA Expression in Glioblastoma Cells. J. Mol. Neurosci. 2019;68:49–57. doi: 10.1007/s12031-019-01280-5. [DOI] [PubMed] [Google Scholar]
- 139.Zhang X., Zhang J., Tong L., Luo Y., Su M., Zang Y., Li J., Lu W., Chen Y. The discovery of colchicine-SAHA hybrids as a new class of antitumor agents. Bioorgan. Med. Chem. 2013;21:3240–3244. doi: 10.1016/j.bmc.2013.03.049. [DOI] [PubMed] [Google Scholar]
- 140.Zhang I., Beus M., Stochaj U., Le P.U., Zorc B., Rajić Z., Petrecca K., Maysinger D. Inhibition of glioblastoma cell proliferation, invasion, and mechanism of action of a novel hydroxamic acid hybrid molecule. Cell Death Discov. 2018;4:1–14. doi: 10.1038/s41420-018-0103-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Pegg A.E., Dolan M.E., Moschel R.C. Structure, Function, and Inhibition of O6-Alkylguanine-DNA Alkyltransferase. In: Cohn W.E., Moldave K., editors. Progress in Nucleic Acid Research and Molecular Biology. Volume 51. Academic Press; Cambridge, MA, USA: 1995. pp. 167–223. [DOI] [PubMed] [Google Scholar]
- 142.Hegi M.E., Liu L., Herman J.G., Stupp R., Wick W., Weller M., Mehta M., Gilbert M.R. Correlation of O6-Methylguanine Methyltransferase (MGMT) Promoter Methylation with Clinical Outcomes in Glioblastoma and Clinical Strategies to Modulate MGMT Activity. J. Clin. Oncol. 2008;26:4189–4199. doi: 10.1200/JCO.2007.11.5964. [DOI] [PubMed] [Google Scholar]
- 143.Zhang K., Wang X.-Q., Zhou B., Zhang L. The prognostic value of MGMT promoter methylation in Glioblastoma multiforme: A meta-analysis. Fam. Cancer. 2013;12:449–458. doi: 10.1007/s10689-013-9607-1. [DOI] [PubMed] [Google Scholar]
- 144.Yin A.-A., Zhang L.-H., Cheng J.-X., Dong Y., Liu B.-L., Han N., Zhang X. The Predictive but Not Prognostic Value of MGMT Promoter Methylation Status in Elderly Glioblastoma Patients: A Meta-Analysis. PLoS ONE. 2014;9:e85102. doi: 10.1371/journal.pone.0085102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Thon N., Kreth S., Kreth F.W. Personalized treatment strategies in glioblastoma: MGMT promoter methylation status. OncoTargets Ther. 2013;6:1363–1372. doi: 10.2147/OTT.S50208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Esteller M., Goodman S.N., Herman J.G. Inactivation of the DNA-Repair Gene MGMT and the Clinical Response of Gliomas to Alkylating Agents. N. Engl. J. Med. 2000;343:1350–1354. doi: 10.1056/NEJM200011093431901. [DOI] [PubMed] [Google Scholar]
- 147.Hegi M.E., Hamou M.-F., de Tribolet N., Kros J.M., Mariani L., Mirimanoff R.O., Stupp R. MGMT Gene Silencing and Benefit from Temozolomide in Glioblastoma. N. Engl. J. Med. 2005;52:997–1003. doi: 10.1056/NEJMoa043331. [DOI] [PubMed] [Google Scholar]
- 148.Malmström A., Grønberg B.H., Marosi C., Stupp R., Frappaz D., Schultz H., Abacioglu U., Tavelin B., Lhermitte B., Hegi M., et al. Temozolomide versus standard 6-week radiotherapy versus hypofractionated radiotherapy in patients older than 60 years with glioblastoma: The Nordic randomised, phase 3 trial. Lancet Oncol. 2012;13:916–926. doi: 10.1016/S1470-2045(12)70265-6. [DOI] [PubMed] [Google Scholar]
- 149.Vaubel R.A., Tian S., Remonde D.A., Schroeder M.A., Mladek A.C., Kitange G.J., Caron A., Kollmeyer T.M., Grove R., Peng S., et al. Genomic and Phenotypic Characterization of a Broad Panel of Patient-Derived Xenografts Reflects the Diversity of Glioblastoma. Clin. Cancer Res. 2019;26:1094–1104. doi: 10.1158/1078-0432.CCR-19-0909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Roszkowski K., Furtak J., Zurawski B., Szylberg T., Lewandowska M.A. Potential Role of Methylation Marker in Glioma Supporting Clinical Decisions. Int. J. Mol. Sci. 2016;17:1876. doi: 10.3390/ijms17111876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Reis R., Costa B., Caeiro C., Guimarães I., Martinho O., Jaraquemada T., Augusto I., Castro L., Osório L., Linhares P., et al. Prognostic value of MGMT promoter methylation in glioblastoma patients treated with temozolomide-based chemoradiation: A Portuguese multicentre study. Oncol. Rep. 2010;23:1655–1662. doi: 10.3892/or_00000808. [DOI] [PubMed] [Google Scholar]
- 152.Mansouri A., Hachem L.D., Mansouri S., Nassiri F., Laperriere N.J., Xia D., Lindeman N.I., Wen P.Y., Chakravarti A., Mehta M.P., et al. MGMT promoter methylation status testing to guide therapy for glioblastoma: Refining the approach based on emerging evidence and current challenges. Neuro-Oncology. 2018;21:167–178. doi: 10.1093/neuonc/noy132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Radke J., Koch A., Pritsch F., Schumann E., Misch M., Hempt C., Lenz K., Löbel F., Paschereit F., Heppner F., et al. Predictive MGMT status in a homogeneous cohort of IDH wildtype glioblastoma patients. Acta Neuropathol. Commun. 2019;7:1–9. doi: 10.1186/s40478-019-0745-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Chai E.A.R., Li G., Liu Y., Zhang K., Zhao Z., Wu F., Chang Y., Pang B., Li J., Li Y., et al. Predictive value of MGMT promoter methylation on the survival of TMZ treated IDH-mutant glioblastoma. Cancer Biol. Med. 2021;18:271–282. doi: 10.20892/j.issn.2095-3941.2020.0179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Wick W., Platten M., Meisner C., Felsberg J., Tabatabai G., Simon M., Nikkhah G., Papsdorf K., Steinbach J.P., Sabel M., et al. Temozolomide chemotherapy alone versus radiotherapy alone for malignant astrocytoma in the elderly: The NOA-08 randomised, phase 3 trial. Lancet Oncol. 2012;13:707–715. doi: 10.1016/S1470-2045(12)70164-X. [DOI] [PubMed] [Google Scholar]
- 156.Reifenberger G., Hentschel B., Felsberg J., Schackert G., Simon M., Schnell O., Westphal M., Wick W., Pietsch T., Loeffler M., et al. Predictive impact of MGMT promoter methylation in glioblastoma of the elderly. Int. J. Cancer. 2011;131:1342–1350. doi: 10.1002/ijc.27385. [DOI] [PubMed] [Google Scholar]
- 157.Shah N., Lin B., Sibenaller Z., Ryken T., Lee H., Yoon J.-G., Rostad S., Foltz G. Comprehensive Analysis of MGMT Promoter Methylation: Correlation with MGMT Expression and Clinical Response in GBM. PLoS ONE. 2011;6:e16146. doi: 10.1371/journal.pone.0016146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Boots-Sprenger S.H., Sijben A., Rijntjes J., Tops B.B., Idema A.J., Rivera A.L., Bleeker F.E., Gijtenbeek A.M., Diefes K., Heathcock L., et al. Significance of Complete 1p/19q Co-Deletion, IDH1 Mutation and MGMT Promoter Methylation in Gli-omas: Use with Caution. Mod. Pathol. 2013;26:922–929. doi: 10.1038/modpathol.2012.166. [DOI] [PubMed] [Google Scholar]
- 159.Nguyen H.N., Lie A., Li T., Chowdhury R., Liu F., Ozer B., Wei B., Green R.M., Ellingson B.M., Wang H.-J., et al. HumanTERTpromoter mutation enables survival advantage from MGMT promoter methylation inIDH1wild-type primary glioblastoma treated by standard chemoradiotherapy. Neuro-Oncol. 2016;19:394–404. doi: 10.1093/neuonc/now189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Dahlrot R., Larsen P., Boldt H.B., Kreutzfeldt M.S., Hansen S., Hjelmborg J.B., Kristensen B.W. Posttreatment Effect of MGMT Methylation Level on Glioblastoma Survival. J. Neuropathol. Exp. Neurol. 2019;78:633–640. doi: 10.1093/jnen/nlz032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Von Bueren A.O., Bacolod M.D., Hagel C., Heinimann K., Fedier A., Kordes U., Pietsch T., Koster J., Grotzer M., Friedman H.S., et al. Mismatch repair deficiency: A temozolomide resistance factor in medulloblastoma cell lines that is uncommon in primary medulloblastoma tumours. Br. J. Cancer. 2012;107:1399–1408. doi: 10.1038/bjc.2012.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Jiapaer S., Furuta T., Tanaka S., Kitabayashi T., Nakada M. Potential Strategies Overcoming the Temozolomide Resistance for Glioblastoma. Neurol. Med. Chir. 2018;58:405–421. doi: 10.2176/nmc.ra.2018-0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Suwala A.K., Stichel D., Schrimpf D., Kloor M., Wefers A.K., Reinhardt A., Maas S.L.N., Kratz C.P., Schweizer L., Hasselblatt M., et al. Primary mismatch repair deficient IDH-mutant astrocytoma (PMMRDIA) is a distinct type with a poor prognosis. Acta Neuropathol. 2020;141:85–100. doi: 10.1007/s00401-020-02243-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Caccese M., Ius T., Simonelli M., Fassan M., Cesselli D., DiPasquale A., Cavallin F., Padovan M., Salvalaggio A., Gardiman M.P., et al. Mismatch-Repair Protein Expression in High-Grade Gliomas: A Large Retrospective Multicenter Study. Int. J. Mol. Sci. 2020;21:6716. doi: 10.3390/ijms21186716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Choi S., Yu Y., Grimmer M.R., Wahl M., Chang S.M., Costello J.F. Temozolomide-associated hypermutation in gliomas. Neuro-Oncology. 2018;20:1300–1309. doi: 10.1093/neuonc/noy016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Touat M., Li Y.Y., Boynton A.N., Spurr L.F., Iorgulescu J.B., Bohrson C.L., Cortes-Ciriano I., Birzu C., Geduldig J.E., Pelton K., et al. Mechanisms and therapeutic implications of hypermutation in gliomas. Nature. 2020;580:517–523. doi: 10.1038/s41586-020-2209-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Lemery S., Keegan P., Pazdur R. First FDA Approval Agnostic of Cancer Site—When a Biomarker Defines the Indication. [(accessed on 20 July 2021)]. Available online: https://www.nejm.org/doi/10.1056/NEJMp1709968. [DOI] [PubMed]
- 168.McGranahan N., Furness A.J.S., Rosenthal R., Ramskov S., Lyngaa R., Saini S.K., Jamal-Hanjani M., Wilson G.A., Birkbak N., Hiley C., et al. Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science. 2016;351:1463–1469. doi: 10.1126/science.aaf1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Borghaei H., Paz-Ares L., Horn L., Spigel D.R., Steins M., Ready N.E., Chow L.Q., Vokes E.E., Felip E., Holgado E., et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2015;373:1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Hodi F.S., O’Day S.J., McDermott D.F., Weber R.W., Sosman J.A., Haanen J.B., Gonzalez R., Robert C., Schadendorf D., Hassel J.C., et al. Improved Survival with Ipilimumab in Patients with Metastatic Melanoma. N. Engl. J. Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Motzer R.J., Escudier B., McDermott D.F., George S., Hammers H.J., Srinivas S., Tykodi S.S., Sosman J.A., Procopio G., Plimack E.R., et al. Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2015;373:1803–1813. doi: 10.1056/NEJMoa1510665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Le D.T., Uram J.N., Wang H., Bartlett B., Kemberling H., Eyring A.D., Skora A.D., Luber B.S., Azad N.S., Laheru D., et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N. Engl. J. Med. 2015;372:2509–2520. doi: 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Daniel P., Sabri S., Chaddad A., Meehan B., Jean-Claude B., Rak J., Abdulkarim B.S. Temozolomide Induced Hypermutation in Glioma: Evolutionary Mechanisms and Therapeutic Opportunities. Front. Oncol. 2019;9:41. doi: 10.3389/fonc.2019.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Bark J.M., Kulasinghe A., Chua B., Day B.W., Punyadeera C. Circulating biomarkers in patients with glioblastoma. Br. J. Cancer. 2019;122:295–305. doi: 10.1038/s41416-019-0603-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Wang H., Jiang D., Li W., Xiang X., Zhao J., Yu B., Wang C., He Z., Zhu L., Yang Y. Evaluation of serum extracellular vesicles as noninvasive diagnostic markers of glioma. Theranostics. 2019;9:5347–5358. doi: 10.7150/thno.33114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Engelborghs S., Niemantsverdriet E., Struyfs H., Blennow K., Brouns R., Comabella M., Dujmovic I., van der Flier W., Froelich L., Galimberti D., et al. Consensus guidelines for lumbar puncture in patients with neurological diseases. Alzheimer’s Dement. Diagn. Assess. Dis. Monit. 2017;8:111–126. doi: 10.1016/j.dadm.2017.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Siravegna G., Marsoni S., Siena S., Bardelli A. Integrating liquid biopsies into the management of cancer. Nat. Rev. Clin. Oncol. 2017;14:531–548. doi: 10.1038/nrclinonc.2017.14. [DOI] [PubMed] [Google Scholar]
- 178.Mair R., Mouliere F., Smith C.G., Chandrananda D., Gale D., Marass F., Tsui D.W., Massie C.E., Wright A.J., Watts C. Measurement of Plasma Cell-Free Mitochondrial Tumor DNA Improves Detection of Glioblastoma in Patient-Derived Ortho-topic Xenograft Models. Cancer Res. 2019;79:220–230. doi: 10.1158/0008-5472.CAN-18-0074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Wan J., Massie C., Garcia-Corbacho J., Mouliere F., Brenton J.D., Caldas C., Pacey S.C., Baird R., Rosenfeld N. Liquid biopsies come of age: Towards implementation of circulating tumour DNA. Nat. Rev. Cancer. 2017;17:223–238. doi: 10.1038/nrc.2017.7. [DOI] [PubMed] [Google Scholar]
- 180.Cordova C., Syeda M.M., Corless B., Wiggins J.M., Patel A., Kurz S.C., Delara M., Sawaged Z., Utate M., Placantonakis D. Abstract A65: Longitudinal Detection of TERT-Mutant Plasma Cell-Free Circulating Tumor DNA in Newly Diagnosed Glioblastoma Patients. AACR; Philadelphia, PA, USA: 2020. [Google Scholar]
- 181.Piccioni D.E., Achrol A.S., Kiedrowski L.A., Banks K., Boucher N., Barkhoudarian G., Kelly D.F., Juarez T., Lanman R.B., Raymond V.M., et al. Analysis of cell-free circulating tumor DNA in 419 patients with glioblastoma and other primary brain tumors. CNS Oncol. 2019;8:CNS34. doi: 10.2217/cns-2018-0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Liang J., Zhao W., Lu C., Liu D., Li P., Ye X., Zhao Y., Zhang J., Yang D. Next-Generation Sequencing Analysis of ctDNA for the Detection of Glioma and Metastatic Brain Tumors in Adults. Front. Neurol. 2020;11:544. doi: 10.3389/fneur.2020.00544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Bettegowda C., Sausen M., Leary R.J., Kinde I., Wang Y., Agrawal N., Bartlett B., Wang H., Luber B., Alani R.M., et al. Detection of Circulating Tumor DNA in Early- and Late-Stage Human Malignancies. Sci. Transl. Med. 2014;6:224ra24. doi: 10.1126/scitranslmed.3007094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Zhao Z., Zhang C., Li M., Shen Y., Feng S., Liu J., Li F., Hou L., Chen Z., Jiang J., et al. Applications of cerebrospinal fluid circulating tumor DNA in the diagnosis of gliomas. Jpn. J. Clin. Oncol. 2020;50:325–332. doi: 10.1093/jjco/hyz156. [DOI] [PubMed] [Google Scholar]
- 185.Li J.-H., He Z.-Q., Lin F.-H., Chen Z.-H., Yang S.-Y., Duan H., Jiang X.-B., Al-Nahari F., Zhang X.-H., Wang J.-H., et al. Assessment of ctDNA in CSF may be a more rapid means of assessing surgical outcomes than plasma ctDNA in glioblastoma. Mol. Cell. Probes. 2019;46:101411. doi: 10.1016/j.mcp.2019.06.001. [DOI] [PubMed] [Google Scholar]
- 186.Wang L., Yu J., Xu J., Zheng C., Li X., Du J. The analysis of microRNA-34 family expression in human cancer studies comparing cancer tissues with corresponding pericarcinous tissues. Gene. 2015;554:1–8. doi: 10.1016/j.gene.2014.10.032. [DOI] [PubMed] [Google Scholar]
- 187.Nørøxe D.S., Østrup O., Yde C.W., Ahlborn L.B., Nielsen F.C., Michaelsen S.R., Larsen V.A., Skjøth-Rasmussen J., Bren-num J., Hamerlik P. Cell-Free DNA in Newly Diagnosed Patients with Glioblastoma—A Clinical Prospective Feasibility Study. Oncotarget. 2019;10:4397. doi: 10.18632/oncotarget.27030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Chen M., Zhao H. Next-generation sequencing in liquid biopsy: Cancer screening and early detection. Hum. Genom. 2019;13:1–10. doi: 10.1186/s40246-019-0220-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Heredia-Soto V., Rodríguez-Salas N., Feliu J. Liquid Biopsy in Pancreatic Cancer: Are We Ready to Apply It in the Clinical Practice? Cancers. 2021;13:1986. doi: 10.3390/cancers13081986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Wan J.C.M., Heider K., Gale D., Murphy S., Fisher E., Mouliere F., Ruiz-Valdepenas A., Santonja A., Morris J., Chandrananda D., et al. ctDNA monitoring using patient-specific sequencing and integration of variant reads. Sci. Transl. Med. 2020;12:eaaz8084. doi: 10.1126/scitranslmed.aaz8084. [DOI] [PubMed] [Google Scholar]
- 191.Bauml J., Levy B. Clonal Hematopoiesis: A New Layer in the Liquid Biopsy Story in Lung Cancer. Clin. Cancer Res. 2018;24:4352–4354. doi: 10.1158/1078-0432.CCR-18-0969. [DOI] [PubMed] [Google Scholar]
- 192.Bartel D.P. MicroRNAs: Genomics, Biogenesis, Mechanism, and Function. Cell. 2004;116:281–297. doi: 10.1016/S0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 193.Kamińska K., Nalejska E., Kubiak M., Wojtysiak J., Żołna Ł., Kowalewski J., Lewandowska M.A. Prognostic and Predictive Epigenetic Biomarkers in Oncology. Mol. Diagn. Ther. 2018;23:83–95. doi: 10.1007/s40291-018-0371-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Krol J., Loedige I., Filipowicz W. The widespread regulation of microRNA biogenesis, function and decay. Nat. Rev. Genet. 2010;11:597–610. doi: 10.1038/nrg2843. [DOI] [PubMed] [Google Scholar]
- 195.Baraniskin A., Kuhnhenn J., Schlegel U., Maghnouj A., Zöllner H., Schmiegel W., Hahn S., Schroers R. Identification of microRNAs in the cerebrospinal fluid as biomarker for the diagnosis of glioma. Neuro-Oncology. 2011;14:29–33. doi: 10.1093/neuonc/nor169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Zhou Q., Liu J., Quan J., Liu W., Tan H., Li W. MicroRNAs as potential biomarkers for the diagnosis of glioma: A systematic review and meta-analysis. Cancer Sci. 2018;109:2651–2659. doi: 10.1111/cas.13714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Roth P., Wischhusen J., Happold C., Chandran P.A., Hofer S., Eisele G., Weller M., Keller A. A specific miRNA signature in the peripheral blood of glioblastoma patients: Glioblastoma-Associated MiRNA Profile in Peripheral Blood. J. Neurochem. 2011;118:449–457. doi: 10.1111/j.1471-4159.2011.07307.x. [DOI] [PubMed] [Google Scholar]
- 198.Wang J., Che F., Zhang J. Cell-free microRNAs as non-invasive biomarkers in glioma: A diagnostic meta-analysis. Int. J. Biol. Markers. 2019;34:232–242. doi: 10.1177/1724600819840033. [DOI] [PubMed] [Google Scholar]
- 199.ParvizHamidi M., Haddad G., Ostadrahimi S., Ostadrahimi N., Sadeghi S., Fayaz S., Fard-Esfahani P. Circulating MiR-26a and MiR-21 as Biomarkers for Glioblastoma Multiform. Biotechnol. Appl. Biochem. 2019;66:261–265. doi: 10.1002/bab.1707. [DOI] [PubMed] [Google Scholar]
- 200.Wei X., Chen D., Lv T., Li G., Qu S. Serum MicroRNA-125b as a Potential Biomarker for Glioma Diagnosis. Mol. Neurobiol. 2014;53:163–170. doi: 10.1007/s12035-014-8993-1. [DOI] [PubMed] [Google Scholar]
- 201.Akers J.C., Ramakrishnan V., Kim R., Phillips S., Kaimal V., Mao Y., Hua W., Yang I., Fu C.-C., Nolan J., et al. miRNA contents of cerebrospinal fluid extracellular vesicles in glioblastoma patients. J. Neuro-Oncol. 2015;123:205–216. doi: 10.1007/s11060-015-1784-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Teplyuk N.M., Mollenhauer B., Gabriely G., Giese A., Kim E., Smolsky M., Kim R.Y., Saria M.G., Pastorino S., Kesari S., et al. MicroRNAs in cerebrospinal fluid identify glioblastoma and metastatic brain cancers and reflect disease activity. Neuro-Oncology. 2012;14:689–700. doi: 10.1093/neuonc/nos074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Nilsson R.J.A., Balaj L., Hulleman E., Van Rijn S., Pegtel D.M., Walraven M., Widmark A., Gerritsen W.R., Verheul H., Vandertop W.P., et al. Blood platelets contain tumor-derived RNA biomarkers. Blood. 2011;118:3680–3683. doi: 10.1182/blood-2011-03-344408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Gabriely G., Yi M., Narayan R.S., Niers J.M., Wurdinger T., Imitola J., Ligon K.L., Kesari S., Esau C., Stephens R.M., et al. Human Glioma Growth Is Controlled by MicroRNA-10b. Cancer Res. 2011;71:3563–3572. doi: 10.1158/0008-5472.CAN-10-3568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Huang B., Luo Q., Han Y., Huang D., Tang Q., Wu L. MiR-223/PAX6 Axis Regulates Glioblastoma Stem Cell Proliferation and the Chemo Resistance to TMZ via Regulating PI3K/Akt Pathway. J. Cell. Biochem. 2017;118:3452–3461. doi: 10.1002/jcb.26003. [DOI] [PubMed] [Google Scholar]
- 206.Shi L., Zhang S., Feng K., Wu F., Wan Y., Wang Z., Zhang J., Wang Y., Yan W., Fu Z., et al. MicroRNA-125b-2 confers human glioblastoma stem cells resistance to temozolomide through the mitochondrial pathway of apoptosis. Int. J. Oncol. 2011;40:119–129. doi: 10.3892/ijo.2011.1179. [DOI] [PubMed] [Google Scholar]
- 207.Siegal T., Charbit H., Paldor I., Zelikovitch B., Canello T., Benis A., Wong M.L., Morokoff A., Kaye A.H., Lavon I. Dynamics of circulating hypoxia-mediated miRNAs and tumor response in patients with high-grade glioma treated with bevacizumab. J. Neurosurg. 2016;125:1008–1015. doi: 10.3171/2015.8.JNS15437. [DOI] [PubMed] [Google Scholar]
- 208.Zhang W., Zhang J., Hoadley K., Kushwaha D., Ramakrishnan V., Li S., Kang C., You Y., Jiang C., Song S.W., et al. miR-181d: A predictive glioblastoma biomarker that downregulates MGMT expression. Neuro-Oncology. 2012;14:712–719. doi: 10.1093/neuonc/nos089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Shi L., Chen J., Yang J., Pan T., Zhang S., Wang Z. MiR-21 protected human glioblastoma U87MG cells from chemotherapeutic drug temozolomide induced apoptosis by decreasing Bax/Bcl-2 ratio and caspase-3 activity. Brain Res. 2010;1352:255–264. doi: 10.1016/j.brainres.2010.07.009. [DOI] [PubMed] [Google Scholar]
- 210.Gwak H.-S., Kim T.H., Jo G.H., Kim Y.-J., Kwak H.-J., Kim J.H., Yin J., Yoo H., Lee S.H., Park J.B. Silencing of MicroRNA-21 Confers Radio-Sensitivity through Inhibition of the PI3K/AKT Pathway and Enhancing Autophagy in Malignant Glioma Cell Lines. PLoS ONE. 2012;7:e47449. doi: 10.1371/journal.pone.0047449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Akers J.C., Hua W., Li H., Ramakrishnan V., Yang Z., Quan K., Zhu W., Li J., Figueroa J., Hirshman B.R. A Cerebrospinal Fluid MicroRNA Signature as Biomarker for Glioblastoma. Oncotarget. 2017;8:68769. doi: 10.18632/oncotarget.18332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Buruiană A., Florian Ș.I., Florian A.I., Timiș T.-L., Mihu C.M., Miclăuș M., Oșan S., Hrapșa I., Cataniciu R.C., Far-caș M. The Roles of MiRNA in Glioblastoma Tumor Cell Communication: Diplomatic and Aggressive Negotiations. Int. J. Mol. Sci. 2020;21:1950. doi: 10.3390/ijms21061950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Conti A., Aguennouz M., La Torre D., Tomasello C., Cardali S.M., Angileri F., Maio F., Cama A., Germanò A., Vita G., et al. miR-21 and 221 upregulation and miR-181b downregulation in human grade II–IV astrocytic tumors. J. Neuro-Oncol. 2009;93:325–332. doi: 10.1007/s11060-009-9797-4. [DOI] [PubMed] [Google Scholar]
- 214.Møller H.G., Rasmussen A.P., Andersen H.H., Johnsen K.B., Henriksen M., Duroux M. A Systematic Review of Mi-croRNA in Glioblastoma Multiforme: Micro-Modulators in the Mesenchymal Mode of Migration and Invasion. Mol. Neurobiol. 2013;47:131–144. doi: 10.1007/s12035-012-8349-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Piwecka M., Rolle K., Belter A., Barciszewska A.-M., Żywicki M., Michalak M., Nowak S., Naskręt-Barciszewska M.Z., Barciszewski J. Comprehensive analysis of microRNA expression profile in malignant glioma tissues. Mol. Oncol. 2015;9:1324–1340. doi: 10.1016/j.molonc.2015.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Visani M., De Biase D., Marucci G., Cerasoli S., Nigrisoli E., Reggiani M.L.B., Albani F., Baruzzi A., Pession A. The PERNO study group Expression of 19 microRNAs in glioblastoma and comparison with other brain neoplasia of grades I–III. Mol. Oncol. 2013;8:417–430. doi: 10.1016/j.molonc.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Ye X., Wei W., Zhang Z., He C., Yang R., Zhang J., Wu Z., Huang Q., Jiang Q. Identification of microRNAs associated with glioma diagnosis and prognosis. Oncotarget. 2017;8:26394–26403. doi: 10.18632/oncotarget.14445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Wang Q., Li P., Li A., Jiang W., Wang H., Wang J., Xie K. Plasma specific miRNAs as predictive biomarkers for diagnosis and prognosis of glioma. J. Exp. Clin. Cancer Res. 2012;31:97. doi: 10.1186/1756-9966-31-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Li H.-Y., Li Y.-M., Shi X.-W., Chen H. Circulating microRNA-137 is a potential biomarker for human glioblastoma. Eur. Rev. Med. Pharmacol. Sci. 2016;20:3599–3604. [PubMed] [Google Scholar]
- 220.Garcia C.M., Toms S.A. The Role of Circulating MicroRNA in Glioblastoma Liquid Biopsy. World Neurosurg. 2020;138:425–435. doi: 10.1016/j.wneu.2020.03.128. [DOI] [PubMed] [Google Scholar]
- 221.Ciafrè S., Galardi S., Mangiola A., Ferracin M., Liu C.-G., Sabatino G., Negrini M., Maira G., Croce C., Farace M. Extensive modulation of a set of microRNAs in primary glioblastoma. Biochem. Biophys. Res. Commun. 2005;334:1351–1358. doi: 10.1016/j.bbrc.2005.07.030. [DOI] [PubMed] [Google Scholar]
- 222.Srinivasan S., Patric I.R.P., Somasundaram K. A Ten-MicroRNA Expression Signature Predicts Survival in Glioblastoma. PLoS ONE. 2011;6:e17438. doi: 10.1371/journal.pone.0017438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Møllersen K., Zortea M., Schopf T.R.G., Kirchesch H.M., Godtliebsen F. Comparison of computer systems and ranking criteria for automatic melanoma detection in dermoscopic images. PLoS ONE. 2017;12:e0190112. doi: 10.1371/journal.pone.0190112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Dong L., Li Y., Han C., Wang X., She L., Zhang H. miRNA microarray reveals specific expression in the peripheral blood of glioblastoma patients. Int. J. Oncol. 2014;45:746–756. doi: 10.3892/ijo.2014.2459. [DOI] [PubMed] [Google Scholar]
- 225.Zhang X., Sun S., Pu J.K.S., Tsang A.C.O., Lee D., Man V.O.Y., Lui W.M., Wong T.S., Leung G.K.K. Long non-coding RNA expression profiles predict clinical phenotypes in glioma. Neurobiol. Dis. 2012;48:1–8. doi: 10.1016/j.nbd.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 226.Ulitsky I., Bartel D.P. lincRNAs: Genomics, evolution, and mechanisms. Cell. 2013;154:26–46. doi: 10.1016/j.cell.2013.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Peng Z., Liu C., Wu M. New insights into long noncoding RNAs and their roles in glioma. Mol. Cancer. 2018;17:1–10. doi: 10.1186/s12943-018-0812-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Cai J., Zhang J., Wu P., Yang W., Ye Q., Chen Q., Jiang C. Blocking LINC00152 suppresses glioblastoma malignancy by impairing mesenchymal phenotype through the miR-612/AKT2/NF-κB pathway. J. Neuro-Oncol. 2018;140:225–236. doi: 10.1007/s11060-018-2951-0. [DOI] [PubMed] [Google Scholar]
- 229.Li Q., Wu Q., Li Z., Hu Y., Zhou F., Zhai Z., Yue S., Tian H. LncRNA LINC00319 is associated with tumorigenesis and poor prognosis in glioma. Eur. J. Pharmacol. 2019;861:172556. doi: 10.1016/j.ejphar.2019.172556. [DOI] [PubMed] [Google Scholar]
- 230.Li J., Zhang Q., Ge P., Zeng C., Lin F., Wang W., Zhao J. FAM225B Is a Prognostic lncRNA for Patients with Recurrent Glioblastoma. Dis. Markers. 2020;2020:1–7. doi: 10.1155/2020/8888085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Aslan K., Turco V., Blobner J., Sonner J.K., Liuzzi A.R., Núñez N.G., De Feo D., Kickingereder P.V., Fischer M., Green E., et al. Heterogeneity of response to immune checkpoint blockade in hypermutated experimental gliomas. Nat. Commun. 2020;11:1–14. doi: 10.1038/s41467-020-14642-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Zhou M., Zhang Z., Zhao H., Bao S., Cheng L., Sun J. An Immune-Related Six-lncRNA Signature to Improve Prognosis Prediction of Glioblastoma Multiforme. Mol. Neurobiol. 2017;55:1–14. doi: 10.1007/s12035-017-0572-9. [DOI] [PubMed] [Google Scholar]
- 233.Luan F., Chen W., Chen M., Yan J., Chen H., Yu H., Liu T., Mo L. An autophagy-related long non-coding RNA signature for glioma. FEBS Open Bio. 2019;9:653–667. doi: 10.1002/2211-5463.12601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Wu C., Su J., Long W., Qin C., Wang X., Xiao K., Li Y., Xiao Q., Wang J., Pan Y., et al. LINC00470 promotes tumour proliferation and invasion, and attenuates chemosensitivity through the LINC00470/miR-134/Myc/ABCC1 axis in glioma. J. Cell. Mol. Med. 2020;24:12094–12106. doi: 10.1111/jcmm.15846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Yang J.-K., Yang J.-P., Tong J., Jing S.-Y., Fan B., Wang F., Sun G.-Z., Jiao B.-H. Exosomal miR-221 targets DNM3 to induce tumor progression and temozolomide resistance in glioma. J. Neuro-Oncol. 2016;131:255–265. doi: 10.1007/s11060-016-2308-5. [DOI] [PubMed] [Google Scholar]
- 236.Fan Q., Yang L., Zhang X., Peng X., Wei S., Su D., Zhai Z., Hua X., Li H. The emerging role of exosome-derived non-coding RNAs in cancer biology. Cancer Lett. 2017;414:107–115. doi: 10.1016/j.canlet.2017.10.040. [DOI] [PubMed] [Google Scholar]
- 237.Chae Y., Roh J., Kim W. The Roles Played by Long Non-Coding RNAs in Glioma Resistance. Int. J. Mol. Sci. 2021;22:6834. doi: 10.3390/ijms22136834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Best M.G., Sol N., Zijl S., Reijneveld J.C., Wesseling P., Wurdinger T. Liquid biopsies in patients with diffuse glioma. Acta Neuropathol. 2015;129:849–865. doi: 10.1007/s00401-015-1399-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Hormigo A., Gu B., Karimi S., Riedel E., Panageas K.S., Edgar M.A., Tanwar M.K., Rao J.S., Fleisher M., DeAngelis L., et al. YKL-40 and Matrix Metalloproteinase-9 as Potential Serum Biomarkers for Patients with High-Grade Gliomas. Clin. Cancer Res. 2006;12:5698–5704. doi: 10.1158/1078-0432.CCR-06-0181. [DOI] [PubMed] [Google Scholar]
- 240.Vaitkiene P., Urbanaviciute R., Grigas P., Steponaitis G., Tamasauskas A., Skiriutė D. Identification of Astrocytoma Blood Serum Protein Profile. Cells. 2019;9:16. doi: 10.3390/cells9010016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Tabouret E., Boudouresque F., Barrie M., Matta M., Boucard C., Loundou A., Carpentier A., Sanson M., Metellus P., Figarella-Branger M., et al. Association of matrix metalloproteinase 2 plasma level with response and survival in patients treated with bevacizumab for recurrent high-grade glioma. Neuro-Oncology. 2013;16:392–399. doi: 10.1093/neuonc/not226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Chinnaiyan P., Chowdhary S., Potthast L., Prabhu A., Tsai Y.-Y., Sarcar B., Kahali S., Brem S., Yu H.M., Rojiani A., et al. Phase I trial of vorinostat combined with bevacizumab and CPT-11 in recurrent glioblastoma. Neuro-Oncology. 2011;14:93–100. doi: 10.1093/neuonc/nor187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Basu B., Ghosh M.K. Extracellular Vesicles in Glioma: From Diagnosis to Therapy. BioEssays. 2019;41:e1800245. doi: 10.1002/bies.201800245. [DOI] [PubMed] [Google Scholar]
- 244.Quezada C., Torres Á., Niechi I., Uribe D., Contreras-Duarte S., Toledo F., Martín R.S., Gutiérrez J., Sobrevia L. Role of extracellular vesicles in glioma progression. Mol. Asp. Med. 2018;60:38–51. doi: 10.1016/j.mam.2017.12.003. [DOI] [PubMed] [Google Scholar]
- 245.Westphal M., Lamszus K. Circulating biomarkers for gliomas. Nat. Rev. Neurol. 2015;11:556–566. doi: 10.1038/nrneurol.2015.171. [DOI] [PubMed] [Google Scholar]
- 246.Gao X., Zhang Z., Mashimo T., Shen B., Nyagilo J., Wang H., Wang Y., Liu Z., Mulgaonkar A., Hu X.-L., et al. Gliomas Interact with Non-glioma Brain Cells via Extracellular Vesicles. Cell Rep. 2020;30:2489–2500. doi: 10.1016/j.celrep.2020.01.089. [DOI] [PubMed] [Google Scholar]
- 247.Tom M.C., Cahill D.P., Buckner J.C., Dietrich J., Parsons M.W., Yu J.S. Management for Different Glioma Subtypes: Are All Low-Grade Gliomas Created Equal? Am. Soc. Clin. Oncol. Educ. Book. 2019;39:133–145. doi: 10.1200/EDBK_238353. [DOI] [PubMed] [Google Scholar]
- 248.André-Grégoire G., Bidère N., Gavard J. Temozolomide affects Extracellular Vesicles Released by Glioblastoma Cells. Biochimie. 2018;155:11–15. doi: 10.1016/j.biochi.2018.02.007. [DOI] [PubMed] [Google Scholar]
- 249.Azam Z., Quillien V., Wang G., To S.-S.T. The potential diagnostic and prognostic role of extracellular vesicles in glioma: Current status and future perspectives. Acta Oncol. 2019;58:353–362. doi: 10.1080/0284186X.2018.1551621. [DOI] [PubMed] [Google Scholar]
- 250.EV-TRACK Consortium. Van Deun J., Mestdagh P., Agostinis P., Akay Ö., Anand S., Anckaert J., Martinez Z.A., Baetens T., Beghein E., et al. EV-TRACK: Transparent reporting and centralizing knowledge in extracellular vesicle research. Nat. Methods. 2017;14:228–232. doi: 10.1038/nmeth.4185. [DOI] [PubMed] [Google Scholar]
- 251.Pantel K., Speicher M. The biology of circulating tumor cells. Oncogene. 2015;35:1216–1224. doi: 10.1038/onc.2015.192. [DOI] [PubMed] [Google Scholar]
- 252.Bang-Christensen S.R., Pedersen R.S., Pereira M.A., Clausen T.M., Løppke C., Sand N.T., Ahrens T.D., Jørgensen A.M., Lim Y.C., Goksøyr L., et al. Capture and Detection of Circulating Glioma Cells Using the Recombinant VAR2CSA Malaria Protein. Cells. 2019;8:998. doi: 10.3390/cells8090998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Chistiakov D.A., Chekhonin V.P. Circulating tumor cells and their advances to promote cancer metastasis and relapse, with focus on glioblastoma multiforme. Exp. Mol. Pathol. 2018;105:166–174. doi: 10.1016/j.yexmp.2018.07.007. [DOI] [PubMed] [Google Scholar]
- 254.Li M.X., Ren X.H., Jiang H., Yang K.Y., Lin S., Cui Y. Identification of circulating tumor cells in peripheral blood for gliomas by detection of aneuploid cells. Zhonghua Yi Xue Za Zhi. 2019;99:1184–1188. doi: 10.3760/cma.j.issn.0376-2491.2019.15.013. [DOI] [PubMed] [Google Scholar]
- 255.Liu T., Xu H., Huang M., Ma W., Saxena D., Lustig R.A., Alonso-Basanta M., Zhang Z., Rourke D.M.O., Zhang L., et al. Circulating glioma cells exhibit stem cell-like properties. Cancer Res. 2018;78:6632–6642. doi: 10.1158/0008-5472.CAN-18-0650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Lombard A., Goffart N., Rogister B. Glioblastoma Circulating Cells: Reality, Trap or Illusion? Stem Cells Int. 2015;2015:1–11. doi: 10.1155/2015/182985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Lynch D., Powter B., Po J.W., Cooper A., Garrett C., Koh E.-S., Sheridan M., Van Gelder J., Darwish B., McKechnie S., et al. Isolation of Circulating Tumor Cells from Glioblastoma Patients by Direct Immunomagnetic Targeting. Appl. Sci. 2020;10:3338. doi: 10.3390/app10093338. [DOI] [Google Scholar]
- 258.Müller C., Holtschmidt J., Auer M., Heitzer E., Lamszus K., Schulte A., Matschke J., Langer-Freitag S., Gasch C., Stoupiec M., et al. Hematogenous dissemination of glioblastoma multiforme. Sci. Transl. Med. 2014;6:247ra101. doi: 10.1126/scitranslmed.3009095. [DOI] [PubMed] [Google Scholar]
- 259.Qi Y., Sun Q., Deng G., Zhang H., Xu Y., Li Y., Huang S., Li Y., Ye Z., Wang Y., et al. Identifying circulating glioma cells and their clusters as diagnostic markers by a novel detection platform. Clin. Transl. Med. 2021;11:e318. doi: 10.1002/ctm2.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Sullivan J.P., Nahed B., Madden M.W., Oliveira S.M., Springer S., Bhere D., Chi A.S., Wakimoto H., Rothenberg S.M., Sequist L.V., et al. Brain Tumor Cells in Circulation Are Enriched for Mesenchymal Gene Expression. Cancer Discov. 2014;4:1299–1309. doi: 10.1158/2159-8290.CD-14-0471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Zachariah M.A., Oliveira-Costa J.P., Carter B.S., Stott S.L., Nahed B.V. Blood-based biomarkers for the diagnosis and monitoring of gliomas. Neuro-Oncology. 2018;20:1155–1161. doi: 10.1093/neuonc/noy074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Nagrath S., Sequist L.V., Maheswaran S., Bell D.W., Irimia D., Ulkus L., Smith M.R., Kwak E.L., Digumarthy S., Muzikansky A., et al. Isolation of rare circulating tumour cells in cancer patients by microchip technology. Nature. 2007;450:1235–1239. doi: 10.1038/nature06385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Allard W.J., Matera J., Miller M.C., Repollet M., Connelly M.C., Rao C., Tibbe A.G.J., Uhr J.W., Terstappen L. Tumor Cells Circulate in the Peripheral Blood of All Major Carcinomas but not in Healthy Subjects or Patients with Nonmalignant Diseases. Clin. Cancer Res. 2004;10:6897–6904. doi: 10.1158/1078-0432.CCR-04-0378. [DOI] [PubMed] [Google Scholar]
- 264.Cristofanilli M., Budd G.T., Ellis M.J., Stopeck A., Matera J., Miller M.C., Reuben J.M., Doyle G.V., Allard W.J., Terstappen L., et al. Circulating Tumor Cells, Disease Progression, and Survival in Metastatic Breast Cancer. N. Engl. J. Med. 2004;351:781–791. doi: 10.1056/NEJMoa040766. [DOI] [PubMed] [Google Scholar]
- 265.Cohen S.J., Punt C.J.A., Iannotti N., Saidman B.H., Sabbath K.D., Gabrail N.Y., Picus J., Morse M., Mitchell E., Miller M.C., et al. Relationship of Circulating Tumor Cells to Tumor Response, Progression-Free Survival, and Overall Survival in Patients with Metastatic Colorectal Cancer. J. Clin. Oncol. 2008;26:3213–3221. doi: 10.1200/JCO.2007.15.8923. [DOI] [PubMed] [Google Scholar]
- 266.De Bono J.S., Scher H.I., Montgomery R.B., Parker C., Miller M.C., Tissing H., Doyle G.V., Terstappen L., Pienta K., Raghavan D. Circulating Tumor Cells Predict Survival Benefit from Treatment in Metastatic Castration-Resistant Prostate Cancer. Clin. Cancer Res. 2008;14:6302–6309. doi: 10.1158/1078-0432.CCR-08-0872. [DOI] [PubMed] [Google Scholar]
- 267.Touat M., Duran-Peña A., Alentorn A., Lacroix L., Massard C., Idbaih A. Emerging Circulating Biomarkers in Glioblas-toma: Promises and Challenges. Expert Rev. Mol. Diagn. 2015;15:1311–1323. doi: 10.1586/14737159.2015.1087315. [DOI] [PubMed] [Google Scholar]