Abstract
Wheat powdery mildew, caused by the obligate parasite Blumeria graminis f. sp. tritici, severely reduces wheat yields. Identifying durable and effective genes against wheat powdery mildew and further transferring them into wheat cultivars is important for finally controlling this disease in wheat production. Pm40 has been widely used in wheat breeding programs in Southwest China due to the spectrum and potentially durable resistance to powdery mildew. In the present study, a resistance test demonstrated that Pm40 is still effective against the Bgt race E20. We identified and cloned the TraesCS7B01G164000 with a total length of 4883 bp, including three exons and two introns, and encoded a protein carrying the CC-NBS-NBS-LRR domain in the Pm40-linked region flanked by two EST markers, BF478514 and BF291338, by integrating analysis of gene annotation in wheat reference genome and both sequence and expression difference in available transcriptome data. Two missense mutations were detected at positions 68 and 83 in the CC domain. The results of both cosegregation linkage analysis and qRT-PCR also suggested that TraesCS7B01G164000 was a potential candidate gene of Pm40. This study allowed us to move toward the final successfully clone and apply Pm40 in wheat resistance improvement by gene engineering.
Keywords: wheat, powdery mildew, express sequence, variation annotation, linkage region
1. Introduction
Powdery mildew, caused by Blumeria graminis f. sp. tritici (Bgt), is a widespread fungal disease that leads to a serious decrease in wheat production worldwide [1,2,3]. In Southwest China, powdery mildew is becoming increasingly devastating and could soon be the most damaging disease in wheat production because the conditions favor pathogen growth [4], increased nitrogen fertilizer and irrigation input, and this disease receives less attention compared to that of stripe rust caused by Puccinia striiformis f. sp. tritici [5]. Chemical control measures are widely used in various regions, but developing and applying resistant cultivars are the more effective, economical, and environmentally friendly means of controlling this disease [6,7]. Therefore, improving cultivar resistance by identifying and transferring broad spectrum and durable resistance Pm genes in wheat breeding programs is urgently needed.
Various scientists have been committed to discovering new loci or alleles conferring resistance to powdery mildew, and, to date, 99 Pm genes at 60 loci (Pm1–Pm68) that confer resistance to powdery mildew have been publicly reported [7,8,9,10]; these genes have been assigned to almost all chromosomes except 3D and 4D. Unfortunately, only a few Pm genes, such as Pm21, have been successfully used in the development of resistant cultivars because of the loss of resistance resulted from the rapid variation in Bgt races [11], linkage drag [12,13], and the shortage of information on both the sequence and function of Pm genes [14].
In the past, some Pm genes, such as Pm2, Pm5, Pm6, and Pm8, have been successfully applied in resistant wheat breeding programs in China [15]. At present, although Pm21 is still effective and the most widely applied Pm gene in the country [16], some newly emerged and virulent isolates have been reported to overcome Pm21 resistance in various regions [17,18]. We found that Pm40 still confers strong resistance to powdery mildew in various regions [2,19,20]. It was also effective against nearly all isolates collected from the main Chinese wheat growing regions, which indicated that Pm40 could have a large potential role in the improvement in resistance against powdery mildew in China in the future, especially in the post-Pm21 era [7]. Therefore, identifying and cloning the candidate gene of Pm40 is a valuable endeavor.
Due to its large genome, fragmented genomic information, and high proportion of repetitive sequences, it is difficult to clone genes from hexaploid wheat. To date, among the known Pm genes, only Pm1 [21], Pm2 [22], Pm3 [23], Pm5 [24], Pm8 [25], Pm21 [26], Pm24 [27], Pm38/Lr34/Yr18/Sr57 [28], Pm41 [29], Pm46/Yr46/Lr67/Sr55 [30], and Pm60 [31] have been cloned. Fast progress in high-quality assembly and annotation of the Chinese Spring wheat reference genome (IWGSC RefSeq v1.0) has provided available information and resources to identify candidate genes in a given chromosomal region by gene annotation [32]. In addition, various available transcriptome expression sequences during the interaction between the pathogen and the host have also been helpful for the identification of candidate resistance genes [33,34,35]. Both cosegregation and qRT-PCR analyses are common and useful methods to validate the function of candidate genes [4,36].
Some studies have shown that these cloned resistance genes from different species usually have similar structural domains, such as Coiled-coil (CC), Toll and interleukin-1 receptors (TIR), nucleotide binding sites (NBS), leucine-rich repeats (NLR), and receptor-like proteins/kinases (RLPs/RLKs) [15,37]. Sequence analysis further found that most of the reported powdery mildew resistance genes in wheat usually carry CC-NBS-LRR (CNL) or RLK domains [4,29]. Therefore, the genes carrying CNL and RLK domains should have a higher consideration during the process of screening and identifying candidate genes for powdery mildew resistance in wheat.
In 2007, we first identified PmE controlling resistance to powdery mildew in the wheat line YU25 by genetic analysis [38], which was consequently mapped to chromosome 7B and formally named Pm40 [19]. In a later study, Pm40 was further mapped to a short chromosomal region of 7BS flanked by Xwmc335 (with distances of 0.58 cm) and BF291338 (with distances of 0.26 cm) [20]. With the release of the Chinese spring reference genome sequence, it was feasible and workable to identify the effective candidate gene of Pm40 because the high-quality reference genome information was available.
We knew through experience that it was difficult to further narrow the chromosomal region, which was possibly related to the origin of Pm40. Originally we thought Pm40 was derived from Th. intermedium because it had Line Yu25 conferring Pm40 resistance in its pedigree [19]. Subsequent evidence, including the uniformity of agronomic traits and the good genetic stability of powdery mildew resistance in many years [2,7], the genetic behavior as a normal Mendelian factor [19,20,38], the wheat-specific amplicons produced by polymerase chain reaction (PCR) amplification, and the similar marker order with the similar total length of the linkage mapping of Pm40 [19,20], supported the view that Pm40 may result from DNA sequence change or chromosomal rearrangement of wheat self-genome during the wild cross process rather than direct transfer from Th. intermedium. Recently, many studies clearly found that the wide cross between wheat and rye (Secale cereal) caused wheat DNA sequence change or chromosomal rearrangement [39,40], and a similar result was also found when the wide cross between Arabidopsis thaliana and A. lyrata was executed [41]. In addition, a copper-binding protein gene (WCBP1) produced from the wheat genomic DNA change was possibly involved in the resistance to wheat stripe rust in YU25 [42]. Therefore, these results indirectly reinforce the view that Pm40 could be derived from the wheat-self genome changes.
In addition, regarding the Pm40 resistance mechanism to powdery mildew, some physiological and biochemical characteristics were also compared between L693 carrying Pm40 and L1034 without Pm40 [2]. Recently, transcriptome analysis showed that ATPase, PSEPS, and the HSPs of Bgt possibly played the crucial roles in establishing the pathogenesis of compatible interactions in hosts without Pm40 [6]. In contrast, in wheat line carrying Pm40, the decline in photosynthesis caused by the downregulation of photosynthesis-related genes and the subsequent accumulation of H2O2 could be an important signal mechanism of incompatible pathogenesis [43]. The analysis did not directly identify the candidate genes of Pm40 [6,43], but the available expression sequences are very important for identifying those candidate genes.
In this study, we had the following objectives: to further test the resistance of Pm40 by inoculation with Bgt race E20; to identify candidate resistance genes by comprehensive analysis using gene annotation data from the IWGSC RefSeq v1.0 in the physical region flanked by two EST markers, BF478514 and BF291338 [20], on chromosome 7B and both sequence and expression differences in the available transcriptome data; to clone the TraesCS7B01G164000 sequence and further confirm the sequence differences between L658 carrying Pm40 and L958 lacking Pm40; and to validate the candidate gene TraesCS7B01G164000 by cosegregation and qRT-PCR analyses. Comprehensively, all results supported TraesCS7B01G164000 as a potential candidate gene of Pm40.
2. Results
2.1. Identification of Pm40 Effectiveness against Bgt Infection
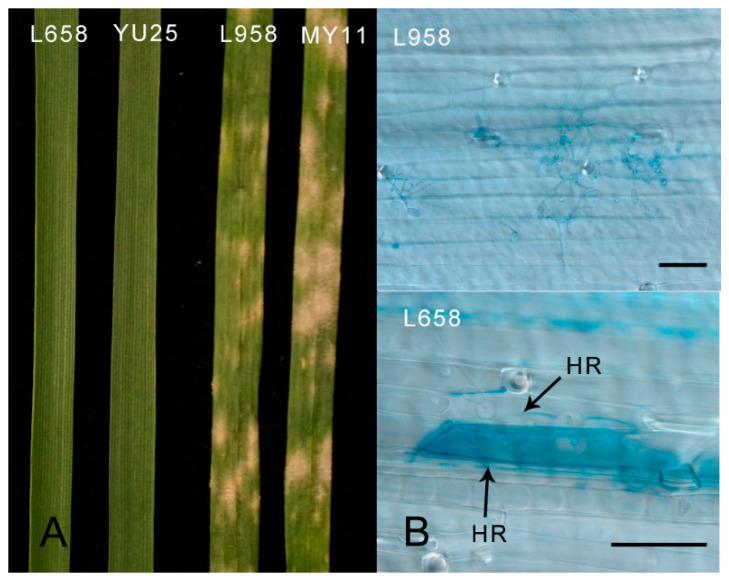
The wheat line L658 and its resistant parent YU25 exhibited high resistance (with IT = 0) to powdery mildew, while line L958 and its susceptible parent cultivar MY11 were susceptible (Figure 1A). Cytological observations further found a significant hypersensitive response in the leaf cells of L658 at 72 hpi, while this phenomenon was not observed in L958 leaf cells (Figure 1B). The results suggested that Pm40 was effective against the E20 race of Bgt.
Figure 1.
Resistance evaluations and cytological observations of cell death. (A) Resistance evaluations after 14 days of inoculation with Bgt at the seedling stage in wheat lines L658, YU25, L958, and MY11. (B) Hypersensitive response (HR) observed in wheat line L658 carrying Pm40 and wheat line L958 lacking Pm40 after 72 hpi. The dark bar indicates 50 μm.
2.2. Functional Annotation of the Genes within the Region Flanked by BF478514 and BF291338
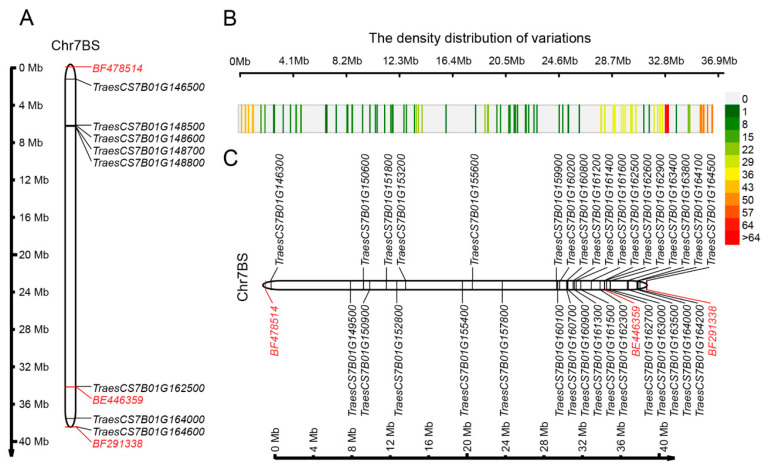
A total of 185 genes were annotated in the 37 Mb physical region flanked by BF478514 and BF291338 on chromosome 7BS, among which only one CNL gene and seven RLK genes were putative functional candidate genes (Table 1). The eight genes were clustered into two narrow subregions: one included five genes close to marker BF478514, and the other included three genes close to marker BF291338 (Figure 2A). Based on the genetic distance and expression profile, we paid more attention to the three genes (TraesCS7B01G162500 and TraesCS7B01G164600 encoding RLK proteins, and TraesCS7B01G164000 encoding a CNL protein) because the cluster was close to the marker BF291338 and the genetic distance was closer to Pm40. Furthermore, the EST marker BE446359, with a genetic distance of 0.28 cm from Pm40 [20], coincided with TraesCS7B01G162500, so TraesCS7B01G162500 was ruled out as a candidate gene of Pm40. Therefore, we focused on the two genes: TraesCS7B01G164000 encoding a CNL protein and TraesCS7B01G164600 encoding an RLK protein could be candidate genes of Pm40.
Table 1.
NBS-LRR and RLK genes within the Pm40 region were annotated in Pfam, SwissPort, Eggnog, and NR Database.
| Gene ID | Pfam Annotation | Swissprot Annotation | Eggnog Annotation | NR Annotation |
|---|---|---|---|---|
| TraesCS7B01G146500 | Protein tyrosine kinase | Serine/threonine-protein kinase | Signal transduction mechanisms | Serine/threonine-protein kinase |
| TraesCS7B01G148500 | Protein tyrosine kinase | Wall-associated receptor kinase | Signal transduction mechanisms | Wall-associated receptor kinase |
| TraesCS7B01G148600 | Protein tyrosine kinase | Wall-associated receptor kinase | Signal transduction mechanisms | Wall-associated receptor kinase |
| TraesCS7B01G148700 | Protein tyrosine kinase | Wall-associated receptor kinase | Signal transduction mechanisms | Wall-associated receptor kinase |
| TraesCS7B01G148800 | Protein tyrosine kinase | Wall-associated receptor kinase | Signal transduction mechanisms | Wall-associated receptor kinase |
| TraesCS7B01G162500 | Protein tyrosine kinase | Chitin elicitor receptor kinase | Signal transduction mechanisms | predicted protein |
| TraesCS7B01G164000 | NB-ARC domain | Putative disease resistance protein | Signal transduction mechanisms | resistance protein RGA2 |
| TraesCS7B01G164600 | Protein tyrosine kinase | PTI1-like tyrosine-protein kinase | Signal transduction mechanisms | PTI1-like tyrosine-protein kinase |
Figure 2.
Distributions of NLR and RLK genes, identified variants, and the genes containing variations. (A) The physical locations of 7 RLK genes and 1 NLR gene in the Pm40 mapping region. (B) Variants were distributed in the mapping interval of Pm40. The distribution of genes containing variants that may have an effect on the gene. (C) The distribution of genes that have variants that may affect the genes.
2.3. Polymorphic EST Sequences in the Pm40 Region
Transcriptomic data showed that 100 out of 185 genes in the Pm40 region expressed after inoculation with Bgt. The 100 expressed genes harbor 458 high-quality variants mainly close to the marker BF291338 (Figure 2B). SnpEff analysis discovered that the 458 variants resulted in 768 genetic effects, including 19 high-impact, 55 moderate-impact, 627 modifier-impact, and 67 low-impact effects. Among these effects, 19 high-impact effects were contained in nine genes, and 55 moderate-impact effects were contained in 25 genes; a total of 34 genes harbored high or moderate-impact effects. Interestingly, 24 out of the 34 genes were clustered in an approximately 8 Mb region close to BF291338 (Figure 2C). In addition, among the 34 genes, functional analysis only identified TraesCS7B01G162500 and TraesCS7B01G164000 as typical plant disease resistance genes (Table 1), and three variants in TraesCS7B01G162500 and two variants in TraesCS7B01G164000 showed moderate-impact effects (Table 2).
Table 2.
The effects of genetic variations in the Pm40 region predicted by mutation annotation.
| Gene ID | Variants_Impact_HIGH | Variants_Impact_MODERATE | |||
|---|---|---|---|---|---|
| Frameshift | Splice Acceptor | Splice Donor | Stop Gained | Missense Variant | |
| TraesCS7B01G146300 | 0 | 0 | 0 | 0 | 3 |
| TraesCS7B01G149500 | 0 | 3 | 0 | 0 | 2 |
| TraesCS7B01G150600 | 0 | 0 | 6 | 0 | 2 |
| TraesCS7B01G150900 | 2 | 0 | 0 | 0 | 0 |
| TraesCS7B01G151800 | 0 | 0 | 0 | 0 | 3 |
| TraesCS7B01G152800 | 0 | 0 | 0 | 2 | 0 |
| TraesCS7B01G153200 | 0 | 0 | 0 | 0 | 1 |
| TraesCS7B01G155400 | 0 | 0 | 0 | 0 | 3 |
| TraesCS7B01G155600 | 0 | 0 | 0 | 0 | 1 |
| TraesCS7B01G157800 | 0 | 0 | 0 | 0 | 2 |
| TraesCS7B01G159900 | 0 | 0 | 0 | 0 | 1 |
| TraesCS7B01G160100 | 0 | 0 | 0 | 0 | 2 |
| TraesCS7B01G160200 | 0 | 0 | 0 | 0 | 2 |
| TraesCS7B01G160700 | 0 | 0 | 0 | 0 | 1 |
| TraesCS7B01G160800 | 1 | 0 | 0 | 0 | 3 |
| TraesCS7B01G160900 | 0 | 0 | 0 | 0 | 1 |
| TraesCS7B01G161200 | 0 | 0 | 0 | 0 | 5 |
| TraesCS7B01G161300 | 0 | 0 | 0 | 0 | 4 |
| TraesCS7B01G161400 | 1 | 0 | 0 | 0 | 2 |
| TraesCS7B01G161500 | 0 | 0 | 0 | 0 | 2 |
| TraesCS7B01G161600 | 0 | 0 | 0 | 0 | 2 |
| TraesCS7B01G162300 | 2 | 0 | 0 | 0 | 1 |
| TraesCS7B01G162500 | 0 | 0 | 0 | 0 | 3 |
| TraesCS7B01G162600 | 0 | 0 | 0 | 0 | 1 |
| TraesCS7B01G162700 | 0 | 0 | 0 | 0 | 5 |
| TraesCS7B01G162900 | 0 | 0 | 0 | 0 | 1 |
| TraesCS7B01G163000 | 0 | 0 | 0 | 0 | 1 |
| TraesCS7B01G163400 | 0 | 0 | 0 | 1 | 0 |
| TraesCS7B01G163500 | 0 | 0 | 0 | 0 | 1 |
| TraesCS7B01G163800 | 0 | 0 | 0 | 0 | 2 |
| TraesCS7B01G164000 | 0 | 0 | 0 | 0 | 2 |
| TraesCS7B01G164100 | 0 | 1 | 0 | 0 | 0 |
| TraesCS7B01G164200 | 0 | 0 | 0 | 0 | 1 |
| TraesCS7B01G164500 | 0 | 0 | 0 | 0 | 7 |
Frameshift: A sequence variant that causes a disruption of the translational reading frame; Splice acceptor: A splice variant that changes the 2 base pair region at the 3’ end of an intron; Splice donor: A splice variant that changes the 2 base pair region at the 5’ end of an intron; Stop gained: A sequence variant whereby at least one base of a codon is changed, resulting in a premature stop codon; Missense variant: A sequence variant that changes one or more bases, resulting in a different amino acid sequence but where the length is preserved. The influence of variation on genes was classified into Variants_impact_HIGH and Variants_impact_MODERATE, proposed by SnpEff.
2.4. Differential Expression Level of Genes within Pm40 Candidate Region
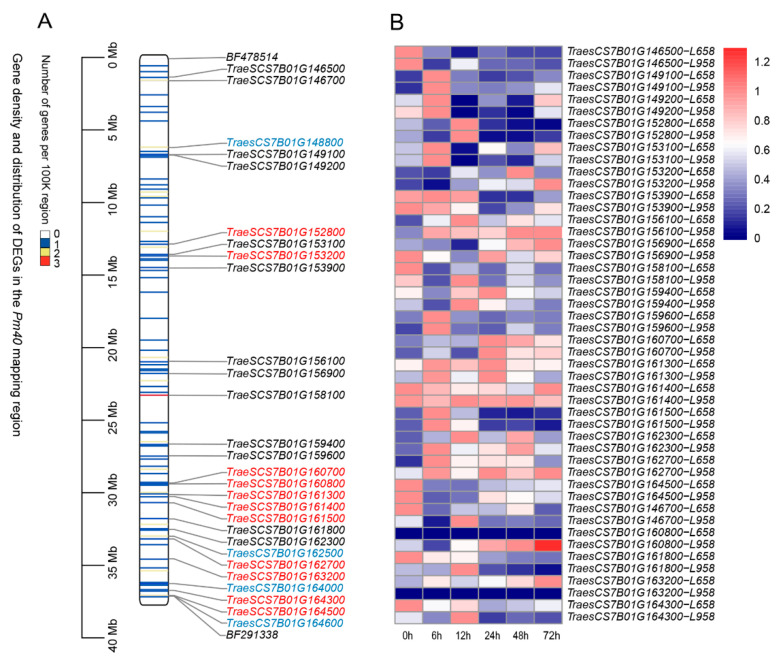
Only 24 out of the 100 expressed genes within the Pm40 mapped region were differentially expressed between the resistant line L658 and susceptible sister line L958 after inoculation with Bgt (Figure 3A), and five putative functional candidate genes screened out in the above analysis were detected in L658 and L958 (Figure 3A). These DEGs were mainly involved in lipid transport and metabolism, structural ribosome constituents, DNA binding, sodium ion transport, microtubule binding, RNA methylation, and unknown functions (Table S1); they exhibited similar expression trends in the two lines (Figure 3B). Nearly half of the DEGs were also present in the 8 Mb region close to BF291338 (Figure 3A). Moreover, most of the DEGs in this 8 Mb region also had variants that probably affected genes (Figure 3A).
Figure 3.
Distribution of differentially expressed genes and expression trends after inoculation with powdery mildew fungus. (A) Distribution of differentially expressed genes in the mapping interval of Pm40 (the genes in red indicate those with mutations that probably affect genes). (B) The expression trends of DEGs in the resistant line L658 and susceptible sister line L958 at 0 (without inoculation), 6, 12, 24, 48, and 72 h post-inoculation. Heat map drawn based on log2 RPKM values +1 converted to percentages.
2.5. Screening and Cloning of TraesCS7B01G164000 and TraesCS7B01G164600
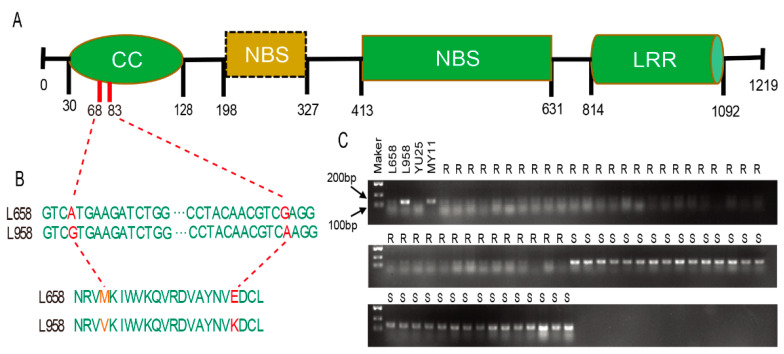
A 4883 bp sequence covering the whole coding region of TraesCS7B01G164000 was obtained from the wheat lines L658 and L958 by PCR product sequencing; this sequence included three exons and two introns and encoded a CNL protein carrying two adjacent repeated NBS domains (Figure 4A). Two SNPs were found by cloning sequence alignment between L658 and L958 (Figure 4B), and both SNPs occurred in the region encoding the CC domain. These SNPs resulted in a transition of Met to Val at amino acid 68 and Glu to Lys at amino acid 83 in L658 compared with L958 (Figure 4B). The prediction of secondary structure showed that the mutation of 83th might cause the change in the secondary structure of the amino acid sequence (Table S2), and the missense mutation might further cause the function of TraesCS7B01G164000 to be deleterious by PROVEAN analysis (Table S3).
Figure 4.
Sequence analysis of TraesCS7B01G164000 and SNP identification in the L658 sister line. (A) The TraesCS7B01G164000 domain composition and mutation sites (mutations in red indicate changes in amino acids, and vertical lines indicate positions of corresponding domains). (B) Two SNPs located in the coding region of TraesCS7B01G164000 were found in the Pm40-carrying wheat line L658 and susceptible sister line L958. (C) SNP validation of TraesCS7B01G164000 in 39 resistant lines and 30 susceptible sister lines of L658 by AS-PCR.
We also obtained a 1882 bp sequence covering the whole coding region of TraesCS7B01G164600 by sequencing PCR products amplified in wheat lines L658 and L958. Sequence alignment showed that the TraesCS7B01G164600 sequences were identical between wheat lines L658 and L958. Therefore, TraesCS7B01G164000 was the only candidate gene due to no difference in expression and sequence of TraesCS7B01G164600 in L658 and L958 after inoculation with Bgt.
2.6. Validation of the Candidate Gene by Cosegregation with Powdery Mildew Resistance
Two AS-PCR markers were developed based on the SNPs within TraesCS7B01G164000: one marker for the A/G single nucleotide variant responsible for the transition from Met to Val was effectively genotyped, while the other marker for the G/A single nucleotide variant did not amplify clear bands. Cosegregation analysis showed that all 39 resistant lines produced PCR products matching those from L658, while all 30 susceptible lines produced products matching those from L958 (Figure 4C), which indicated that the candidate gene cosegregated with powdery mildew resistance.
2.7. Expression Analysis of TraesCS7B01G164000 after Bgt Inoculation by qRT-PCR
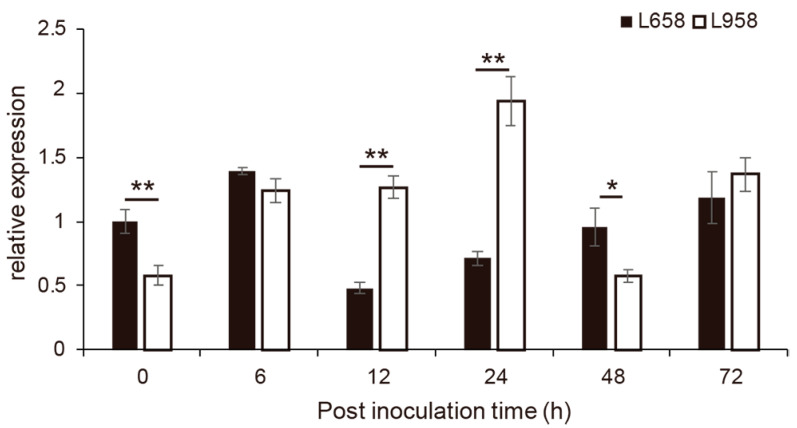
One set of qRT-PCR primers was designed for gene expression analysis according to the sequence of the third exon of TraesCS7B01G164000 (Table S4). TraesCS7B01G164000 exhibited obvious differential expression at different stages of inoculation with Bgt (Figure 5). First, the expression level of TraesCS7B01G164000 in L658 was significantly higher than that in L958 at 0 hpi without inoculation. Second, the highest expression level in L658 occurred at 6 hpi, while that in L958 occurred at 24 hpi; interestingly, the lowest expression level in L658 was detected at 12 hpi, while that in L958 occurred at 48 hpi. Finally, the trend for the expression level changes of TraesCS7B01G164000 from 48 hpi to 72 hpi was similar between L658 and L958.
Figure 5.
Relative expression changes of TraesCS7B01G164000 in the resistant line L658 and susceptible sister line L958 inoculated with powdery mildew. Statistical significance was determined using an independent sample t-test. The asterisks represent significant differences as follows: ** p < 0.01 and * p < 0.05. The asterisk at the top of the bars represents the difference between L658 and L958 interactions at each same time point.
3. Discussion
Identification and cloning of the candidate Pm40 gene are valuable for breeding applications regarding wheat resistance improvement as well as for elucidating the molecular mechanism that wheat resists the infection of the pathogen Bgt. However, limited and fragmented information on the wheat genome has resulted in little progress in wheat resistance gene cloning in the past [44]. Although transcriptome analysis has been indicated to be an effective strategy for identifying candidate genes, some important candidate genes were possibly omitted due to the unfit analysis idea of transcriptomic data and the identification of too many candidate genes [45], which made cloning candidate genes difficult. In the present study, we comprehensively used the available gene annotation data in the high-quality assembled wheat genome, the previously published linkage genetic map of Pm40 [19,20] and the recently reported transcriptomic data of both L658 carrying Pm40 and L958 lacking Pm40 to identify the candidate gene of Pm40 [43].
3.1. The Broad Spectrum and Putatively Durable Resistance of Pm40
The powdery mildew resistance gene Pm40 was first reported in 2007 and tentatively named PmE, and it was found to confer strong resistance to powdery mildew in wheat [38]. The Pm40 has been known to confer strong resistance to toxic and prevalent Bgt 15 for more than ten years in Southwest China [2,6,19,20]. Moreover, the wheat line L658 carrying Pm40 was also resistant to all 28 Bgt isolates collected from various regions of China [7]. In the present research, the resistance assessment indicated that Pm40 still provides effective resistance to the currently prevalent Bgt race E20 in Wenjiang, Sichuan Province. Moreover, the Pm40 gene has been widely used in wheat breeding for many years, and to date, we have not found a loss of resistance from Pm40 in the field of Southwest China, in which Bgt rapidly accumulates variations [46]. Both the persistence and large resistance spectrum of Pm40 indicated that it could be a durable resistance gene against powdery mildew in wheat.
3.2. The Possible Chromosomal Regions Containing the Candidate Pm40 Gene
Two similar high-density genetic linkage maps of Pm40 with total lengths of 6.18 and 6.38 cm were produced by using 579 individuals from the F2:3 population derived from the cross L693/L1034 and 3420 individuals from the F2:3 population derived from the cross L658/L958; in both maps, Pm40 was flanked by Xwmc335 and BF291338 [7,20].
It is well known that some SSRs, especially those in intergenic regions, cannot be accurately assigned to the physical map [47]; therefore, the EST marker BF478514 next to Xwmc335 was treated as the corresponding flanking marker on the side of Xwmc335. The physical interval between the two markers BF478514 and BF291338 in the Chinese Spring wheat reference genome was approximately 37 Mb, in which we identified 185 genes.
One CNL and seven RLK genes were identified from the 185 genes based on functional annotation (Table 1), and their distribution formed two obvious clusters. One cluster, including five genes, was on the side of BF478514, and the other, including three genes, was on the side of BF291338 (Figure 2A). We further focused on the region close to BF291338 because of the smaller genetic distance between Pm40 and BF291338 on the linkage map, and more of both the variants (Figure 2B) and differentially expressed genes (Figure 3A) were found in the region.
Further analysis showed that three out of the five disease resistance genes in the cluster on the side of BF478514 were not expressed, while there were also no variants in the other two expressed genes. In contrast, all three genes in the cluster on the side of BF291338 were expressed to various degrees, and there were detectable variants in the two genes (Table 2). Together, the candidate genes of Pm40 could be in the chromosomal region close to the marker BF291338.
3.3. TraesCS7B01G164000 as the Putative Candidate Gene of Pm40
In wheat, these cloned resistance genes were mainly classified into CNL- and RLK-encoding genes [4,24,29]. Among the three plant disease resistance candidate genes in the chromosomal region on the side of BF291338, TraesCS7B01G164000 encodes a CNL protein carrying two NBS domains (Figure 4A), while the other two candidates, TraesCS7B01G162500 and TraesCS7B01G164600, are RLK genes (Table 1).
Sequence variation analysis did not detect any variants in the expressed sequence of TraesCS7B01G164600 between L658 and L958 (Figure 2C). In addition, PCR product sequencing also demonstrated that the sequence of TraesCS7B01G164600 in L658 was the same as that in L958. Therefore, TraesCS7B01G164600 should be excluded from the candidate genes of Pm40. Although expression of TraesCS7B01G162500 was observed during pathogenesis after Bgt inoculation, and there were also three variants in TraesCS7B01G162500 (Table 2), we ruled out the possibility that this gene was a candidate gene of Pm40 because the marker BE446359, as one part of TraesCS7B01G162500, exhibited incomplete linkage with Pm40 in two previously reported high-density genetic maps [20].
The TraesCS7B01G164000 was expressed after Bgt inoculation [43]. Additionally, two SNPs were found in TraesCS7B01G164000 between L658 and L958 (Table 2), and silicon analysis further indicated that both SNPs could result in the change of the amino acid sequence (Table S2). In addition, the two SNPs were also demonstrated by PCR product sequencing (Figure 4B). Comprehensively, it is reasonable that TraesCS7B01G164000 is the putative candidate gene of Pm40.
3.4. Validation of TraesCS7B01G164000 as the Candidate Gene of Pm40
Most R genes that mediate effector-triggered immunity (ETI) associated with HR encode NLR proteins [48,49,50]. To date, almost all cloned Pm genes in wheat belong to CNL types, suggesting that CNL proteins play major roles in the innate immune defense of wheat against powdery mildew [29]. In the present study, the leaf cells of L658 carrying Pm40 showed HR against powdery mildew, and the gene TraesCS7B01G164000 encoded a CNL-type protein, which indicated that it was possibly the candidate gene of Pm40.
A previous study showed that TraesCS7B01G164000 had different expression trends in L658 and L958 after inoculation with Bgt, although it was not a DEG [43]. In fact, the expression of the same gene exhibited slight differences in both expression level and trend in different races [51], and different rank position of this gene in cascade signaling pathway [52]. Therefore, the proportion of genes low abundance expression cannot be directly compared because of both low sequencing depth and stage-specific expression [53,54]. In this study, qRT-PCR analysis, which is usually treated as a powerful tool for the study of low abundance expression [55], shows that the expression of TraesCS7B01G164000 in L658 cells was upregulated after inoculation with Bgt and expression peaked at 6 hpi, which agreed with previous work showed that the genes expression influencing the infection of Bgt could occur within 12 hpi [6]. Moreover, most of the DEGs encoding pathogen-related 1 (PR1) and pathogen-related 5 (PR5) were expressed at greater levels in L658 at 24 and 48 hpi, following the peak expression time of TraesCS7B01G164000, indicating that TraesCS7B01G164000 may provide the defense response against wheat powdery mildew.
In this study, missense mutations resulting in amino acid changes located at the 68th and 83th positions in the CC domain of TraesCS7B01G164000 were identified. The amino acids at these two positions have great differences in physical properties (Table S2), which may lead to changes in the secondary structure of the CC domain and further cause the function of TraesCS7B01G164000 to be damaged (Table S3), as determined by silicon analysis. The molecular mechanism of the CC domain combined with an NBS-LRR domain in mediating plant disease resistance is still controversial, but the CC domain surely plays a key role in the plant resistance response, and some CC domains can independently induce HR [31,56,57,58]. Therefore, the loss of resistance in the sister line L958 could result from two missense mutations in the CC domain. It is generally believed that the NBS-ARC domain can activate the conformation of the NBS-LRR protein and hydrolyze ATP, and studies have shown that the NBS-ARC domain can participate in the recognition of pathogenic effector proteins [59,60]. By domain analysis of the coding sequence of the TraesCS7B01G164000 gene, it was found that this gene has a special structure different from the typical CNL gene. TraesCS7B01G164000 contains two NBS domains in the middle of the CC domain and leucine-rich repeats. This unique gene structure could be associated with broad-spectrum and durable powdery mildew resistance.
Allele-specific polymerase chain reaction (AS-PCR) is an effective method for the identification of SNPs based on PCR amplification [61]. We detected an A/T mutation that caused a change in amino acid 68th by AS-PCR in the high-generation homozygous sister line of L658. This result implies that TraesCS7B01G164000 cosegregated with powdery mildew resistance despite the limited sample size of sister wheat lines.
Comprehensively, the resistance test showed that Pm40 was effective against the current prevalent Bgt race E20. TraesCS7B01G164000, encoding a CC-NBS-NBS-LRR protein, was identified as a potential candidate gene of Pm40 by integrating analysis of the genetic map of Pm40, gene annotation and transcriptome data, which was demonstrated as the valid candidate gene of Pm40 by both cosegregation analysis and qRT-PCR confirmation. Pm40 has great application potential in wheat breeding, while the AS-PCR cosegregation marker developed with Pm40 will accelerate the application of Pm40 in molecular breeding. In addition, cloning Pm40 is the objective of our next study.
4. Materials and Methods
4.1. Plant Materials, the Resistance Evaluations, and the Cytological Observations of Cell Death
Wheat line L658 carrying Pm40 and the susceptible sister line L958 derived from the F7 populations of a cross between the susceptible line MY11 and the resistant line YU25 [62] were employed for qRT-PCR analysis. The 69 homozygous inbred lines selected from the F10 populations derived from the cross YU25/MY11 were employed for cosegregation analysis. The race E20 of Bgt was used for resistance evaluations and was isolated from a mixture collected in Wen Jiang, Chengdu, Sichuan Province (latitude N 30°40′ and longitude E 103°51′) in 2020. Wheat seedlings at the three-leaf stage were inoculated by dusting conidia from sporulating seedlings. The infection types were classified using a rating scale of 0 to 4 approximately 14 days after inoculation [63]. The leaves harvested at 72 h post-inoculation (hpi) were stained by the trypan blue (TPB) staining method [64], and the leaf cell death induced by the HR response was observed and photographed using bright-field microscopy (Nikon ECLIPSE80i, Nikon Corporation, Tokyo, Japan).
4.2. Annotation of the Genes within the Physical Region of Pm40
Three molecular markers—BF478514, Xwmc335, and BF291338—were genetically linked with the Pm40 gene with genetic distance of 1.46 cm, 0.58 cm, and 0.26 cm, and Pm40 in the middle of Xwmc335 and BF291338, respectively, and both BF478154 and Xwmc335 were placed on the same side of the chromosome as Pm40 [20]. Although Xwmc335 is closer to Pm40 than BF478514 [20], we used BF478514 as the corresponding flanking marker when the chromosomal region of Pm40 was concerned because the SSR marker of Xwmc335 was not accurately mapped to the reference genome of wheat. The physical positions of these markers were determined through the Triticeae Multi-omics Center (http://202.194.139.32/PrimerServer/, accessed on 8 October 2020). Therefore, the physical interval length between the two markers BF478514 and BF291338 in the Chinese Spring reference genome was approximately 37 Mb.
4.3. Acquisition of RNA Sequencing Data and Detection of Deferentially Expressed Gene
RNA sequencing data (accession number SRP117269) of sister wheat lines carrying and lacking Pm40 after inoculation with Bgt were downloaded from the NCBI Sequence Read Archive (SRA) [6]. Reads obtained by RNA sequencing were then mapped to the IWGSC RefSeq v1.0 Wheat Genome Reference (https://wheat-urgi.versailles.inra.fr/, accessed on 9 October 2020) by HISAT2 [65]. To determine the gene expression level, the normalized expression level FPKM was calculated by StringTie [66]. To filter low-expression genes, the following criteria were used: expression level of genes CPM (count per million) value > 1 and sum of CPM > 3 in all samples. The paired comparison test of EdgeR was employed to detect deferentially expressed genes (DEGs) with the threshold of a false discovery rate (FDR) 0.001 or less and |log2 (a fold change)| ≥ 2 or higher [67]. Transcribed genes were annotated using the Non-Redundant Database, Swiss-Prot Protein Database, and Pfam database.
4.4. Variation Extraction, Filtering and Annotation
The variations were detected by comparing the expression sequences of the sister lines carrying and lacking Pm40 from the original transcriptome sequencing files. SamTools, mpileup, and bcftools were used to extract the variants located on the physical interval between the two markers BF478514 and BF291338, and then they were outputted in VCF format. The filtration conditions were set as follows: Qual ≥ 30; DP ≥ 10. Finally, combined with the gene annotation data from the Wheat Chinese Spring Reference Genome 1.0, SnpEff software was used to annotate and analyze the obtained high-quality variations [68]. The genetic effects of the variations are divided into the following four types: high-impact (e.g., nonsense mutations and frameshift mutations), moderate-impact (e.g., missense mutations), modifier (e.g., intron and intergenic mutations), and low-impact mutations (e.g., synonymous mutations) (see http://snpeff.sourceforge.net for details, accessed on 15 October 2020).
4.5. Gene Cloning and Sequence Analysis
The Chinese Spring wheat reference genome sequence was used as a template, and specific primers (Table S4) were designed to amplify the TraesCS7B01G164000 and TraesCS7B01G164600 sequences in the resistant wheat line L658 and susceptible sister line L958. Phanta Max Super-Fidelity DNA Polymerase (Vazyme, Chengdu, China) was used to amplify the TraesCS7B01G164000 and TraesCS7B01G164600 sequences. PCR was performed in a PTC-200 thermal cycler (MJ Research, Watertown, MA, USA) under the following conditions: 3 min at 95 °C for predenaturation; 34 cycles of 15 s at 95 °C, 15 s at the annealing temperature (Table S4), and 2 min 30 s at 72 °C; and a final step of 5 min at 72 °C. The amplified TraesCS7B01G164000 and TraesCS7B01G164600 gene fragments were attached to the pClone007 Versatile simple vector (TSINGKE, Chengdu, China) for sequencing at Tsingke (Chengdu, China). After sequencing and splicing, DNAMAN (version 9) software was used to detect nucleotide variation. The functional module of CD—Search in NCBI (https://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi, accessed on 17 December 2020) was used to analyze the domain structures of the cloned genes. The online server PROVEAN was used to predict the effect of gene function by amino acid variation (http://provean.jcvi.org/, accessed on 5 March 2021). The cloned genes’ sequences were submitted to NCBI and the assigned GenBank Accession numbers were MZ779114 and MZ779115.
4.6. SNP Genotyping by AS-PCR
In AS-PCR, Taq polymerase can effectively carry out DNA synthesis reactions only when the base at the 3′ end of the primer is correctly matched with the template DNA [61,69]. The primers were designed based on the SNP of TraesCS7B01G164000 detected in wheat L658 and L958, as shown in Table S4. The specificity of the primers was tested in the IWGSC RefSeq v1.0. PCR was performed in a PTC-200 thermal cycler (MJ Research, Watertown, MA, USA) under the following conditions: 4 min at 94 °C for predenaturation; 32 cycles of 45 s at 94 °C, 45 s at the annealing temperature (Table S4), and 45 s at 72 °C; and a final step of 7 min at 72 °C. After amplification, a 1.5% agarose gel was used to detect the target bands by agarose gel electrophoresis.
4.7. Gene Expression Analysis by qRT-PCR
The seedlings of L658 and L958 were cultured in a light culture chamber under 14 h light/10 h dark conditions at 18 °C and 80% humidity (Microclima MC1750E, Snijders Scientific, Tilburg, Holland). Ten-day-old seedlings of L658 and L958 were inoculated with Bgt at densities of approximately 100–200 conidia per mm2. The first leaf was randomly clipped from wheat lines L658 and L958 at 0, 6, 12, 24, 48, and 72 hpi. To minimize experimental errors, we performed three biological replicates. Total RNA was extracted from 36 leaf samples using the TRIzol reagent method (Invitrogen Life Technologies, Carlsbad, CA, USA) and repeated 3 times. The integrity and purity of the extracted RNA were measured using an Agilent 2100 BioAnalyzer (Agilent Technologies, Santa Clara, CA, USA) and a Nanodrop ND-1000 spectrophotometer (Thermo Scientific, Wilmington, DE, USA).
The specific primers for qRT-PCR analysis were designed in NCBI Primer-BLAST according to the TraesCS7B01G164000 exon sequence (Table S4). qRT-PCR was performed using a CFX96 Real-Time System (Bio-Rad Laboratories, Hercules, CA, USA) with SYBR Green qRT-PCR Master Mix (Omega, Beijing, China). The qRT-PCR execution procedure was as follows: 40 cycles of 30 s at 94 °C, 5 s at 94 °C and 30 s at 60 °C. The melting curve was set at 60 °C to 95 °C with a 0.5 °C increase per step. Then, the 2−ΔΔCt method was used to calculate the relative expression of TraesCS7B01G164000 [70], with tubulin [71] used as a reference gene. The expression levels of the target genes were analyzed using three biological replicates, in which each contained three technical replicates.
Acknowledgments
We are grateful to Tsingke Biotechnology Co., Ltd. (Beijing, China) for the technical assistance.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/ijms221910239/s1.
Author Contributions
P.L. and H.Y. (Huai Yang) conceived the experiments; S.Z., C.C. and H.Y. (Huai Yang) completed transcriptome data analysis; W.C. (Wei Chen) and H.Y. (Hao Yang) undertook phenotypic identification and cytological observation; H.Y. (Hao Yang) and W.C. (Wei Chen) performed the qRT-PCR and AS-PCR experimental procedures; F.T., P.L. and W.C. (Wanquan Chen) collected the wheat material in this study; M.Z. isolated and provided Bgt; P.L. and H.Y. (Huai Yang) wrote the manuscript; T.R. and Z.L. gave valuable suggestions in the data analysis and assisted in revising the manuscript; and all authors edited and approved the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was partially supported by the National Agricultural Park Innovation Project from the Science and Technology Department of Sichuan Province, China (2019YFN0032), Applied Basic Research Project from the Science and Technology Department of Sichuan Province, China (2020YJ0331), and the National Natural Science Foundation of China (3210150473).
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Juroszek P., Von Tiedemann A. Climate change and potential future risks through wheat diseases: A review. Eur. J. Plant. Pathol. 2013;136:21–33. doi: 10.1007/s10658-012-0144-9. [DOI] [Google Scholar]
- 2.Ma L.X., Zhong S.F., Liu N., Chen W.Q., Liu T.G., Li X., Zhang M., Ren Z.L., Yang J.Z., Luo P.G. Gene expression profile and physiological and biochemical characterization of hexaploid wheat inoculated with Blumeria graminis f. sp. tritici. Physiol. Mol. Plant. Pathol. 2015;90:39–48. doi: 10.1016/j.pmpp.2015.02.005. [DOI] [Google Scholar]
- 3.Liang Y., Xia Y., Chang X., Gong G., Yang J., Hu Y., Cahill M., Luo L., Li T., He L. Comparative proteomic analysis of wheat carrying Pm40 response to Blumeria graminis f. sp. tritici using two-dimensional electrophoresis. Int. J. Mol. Sci. 2019;20:933. doi: 10.3390/ijms20040933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen T., Xiao J., Xu J., Wan W., Qin B., Cao A., Chen W., Xing L., Du C., Gao X. Two members of TaRLK family confer powdery mildew resistance in common wheat. BMC Plant. Biol. 2016;16:1–17. doi: 10.1186/s12870-016-0713-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luo P.G., Hu X.Y., Chang Z.J., Zhang M., Zhang H.Q., Ren Z.L. A new stripe rust resistance gene transferred from Thinopyrum intermedium to hexaploid wheat (Triticum aestivum) Phytoprotection. 2009;90:57–63. doi: 10.7202/044023ar. [DOI] [Google Scholar]
- 6.Hu Y., Liang Y., Zhang M., Tan F., Zhong S., Li X., Gong G., Chang X., Shang J., Tang S. Comparative transcriptome profiling of Blumeria graminis f. sp. tritici during compatible and incompatible interactions with sister wheat lines carrying and lacking Pm40. PLoS ONE. 2018;13:e0198891. doi: 10.1371/journal.pone.0198891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tang S., Hu Y., Zhong S., Luo P. The potential role of powdery mildew-resistance gene Pm40 in Chinese wheat-breeding programs in the post-Pm21 Era. Engineering. 2018;4:500–506. doi: 10.1016/j.eng.2018.06.004. [DOI] [Google Scholar]
- 8.McIntosh R.A., Dubcovsky J., Rogers W.J., Morris C., Xia X.C. Catalogue of Gene Symbols for Wheat: 2017 Supplement.KOMUGI Wheat Genetic Resource Database. [(accessed on 5 March 2021)]. Available online: http://shigen.nig.ac.jp/wheat/komugi/genes/macgene/supplement2017.pdf.
- 9.Li H., Dong Z., Ma C., Xia Q., Tian X., Sehgal S., Koo D.H., Friebe B., Ma P., Liu W. A spontaneous wheat-Aegilops longissima translocation carrying Pm66 confers resistance to powdery mildew. Theor. Appl. Genet. 2020;133:1149–1159. doi: 10.1007/s00122-020-03538-8. [DOI] [PubMed] [Google Scholar]
- 10.He H., Liu R., Ma P., Du H., Zhang H., Wu Q., Yang L., Gong S., Liu T., Huo N., et al. Characterization of Pm68, a new powdery mildew resistance gene on chromosome 2BS of Greek durum wheat TRI 1796. Theor. Appl. Genet. 2021;134:53–62. doi: 10.1007/s00122-020-03681-2. [DOI] [PubMed] [Google Scholar]
- 11.Green A.J., Berger G., Griffey C.A., Pitman R., Thomason W., Balota M. Genetic resistance to and effect of leaf rust and powdery mildew on yield and its components in 50 soft red winter wheat cultivars. Crop. Prot. 2014;64:177–186. doi: 10.1016/j.cropro.2014.06.023. [DOI] [Google Scholar]
- 12.Summers R.W., Brown J.K.M. Constraints on breeding for disease resistance in commercially competitive wheat cultivars. Plant. Pathol. 2013;62:115–121. doi: 10.1111/ppa.12165. [DOI] [Google Scholar]
- 13.Xu H., Yi Y., Ma P., Qie Y., Fu X., Xu Y., Zhang X., An D. Molecular tagging of a new broad-spectrum powdery mildew resistance allele Pm2c in Chinese wheat landrace Niaomai. Theor. Appl. Genet. 2015;128:2077–2084. doi: 10.1007/s00122-015-2568-z. [DOI] [PubMed] [Google Scholar]
- 14.He R., Chang Z., Yang Z., Yuan Z., Zhan H., Zhang X., Liu J. Inheritance and mapping of powdery mildew resistance gene Pm43 introgressed from Thinopyrum intermedium into wheat. Theor. Appl. Genet. 2009;118:1173–1180. doi: 10.1007/s00122-009-0971-z. [DOI] [PubMed] [Google Scholar]
- 15.Yang S., Li J., Zhang X., Zhang Q., Huang J., Chen J., Hartl D.L., Tian D. Rapidly evolving R genes in diverse grass species confer resistance to rice blast disease. Proc. Natl. Acad. Sci. USA. 2013;110:18572–18577. doi: 10.1073/pnas.1318211110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cao A., Xing L., Wang X., Yang X., Wang W., Sun Y., Qian C., Ni J., Chen Y., Liu D. Serine/threonine kinase gene Stpk-V, a key member of powdery mildew resistance gene Pm21, confers powdery mildew resistance in wheat. Proc. Natl. Acad. Sci. USA. 2011;108:7727–7732. doi: 10.1073/pnas.1016981108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shi Y., Wang B., Li Q., Wu X., Wang F., Liu H., Liu Q. Analysis on the virulent genes of Erysiphe graminis f. sp. tritici and the resistance genes of wheat commercial cultivars in Shaanxi Province. J. Triticeae Crops. 2009;29:706–711. [Google Scholar]
- 18.Yang L., Xiang L., Zeng F., Wang H., Shi W., Yu D. Virulence gene structure analysis of Blumeria graminis f. sp. tritici in Hubei. Plant. Prot. 2009;35:76–79. [Google Scholar]
- 19.Luo P.G., Luo H.Y., Chang Z.J., Zhang H.Y., Zhang M., Ren Z.L. Characterization and chromosomal location of Pm40 in common wheat: A new gene for resistance to powdery mildew derived from Elytrigia intermedium. Theor. Appl. Genet. 2009;118:1059–1064. doi: 10.1007/s00122-009-0962-0. [DOI] [PubMed] [Google Scholar]
- 20.Zhong S., Ma L., Fatima S.A., Yang J., Chen W., Liu T., Hu Y., Li Q., Guo J., Zhang M. Collinearity analysis and high-density genetic mapping of the wheat powdery mildew resistance gene Pm40 in PI 672538. PLoS ONE. 2016;11:e0164815. doi: 10.1371/journal.pone.0164815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hewitt T., Müller M.C., Molnár I., Mascher M., Holušová K., Šimková H., Kunz L., Zhang J., Li J., Bhatt D. A highly differentiated region of wheat chromosome 7AL encodes a Pm1a immune receptor that recognizes its corresponding AvrPm1a effector from Blumeria graminis. New Phytol. 2021;229:2812–2826. doi: 10.1111/nph.17075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sánchez-Martín J., Steuernagel B., Ghosh S., Herren G., Hurni S., Adamski N., Vrána J., Kubaláková M., Krattinger S.G., Wicker T. Rapid gene isolation in barley and wheat by mutant chromosome sequencing. Genome Biol. 2016;17:1–7. doi: 10.1186/s13059-016-1082-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yahiaoui N., Srichumpa P., Dudler R., Keller B. Genome analysis at different ploidy levels allows cloning of the powdery mildew resistance gene Pm3b from hexaploid wheat. Plant J. 2004;37:528–538. doi: 10.1046/j.1365-313X.2003.01977.x. [DOI] [PubMed] [Google Scholar]
- 24.Xie J., Guo G., Wang Y., Hu T., Wang L., Li J., Qiu D., Li Y., Wu Q., Lu P. A rare single nucleotide variant in Pm5e confers powdery mildew resistance in common wheat. New Phytol. 2020;228:1011–1026. doi: 10.1111/nph.16762. [DOI] [PubMed] [Google Scholar]
- 25.Hurni S., Brunner S., Buchmann G., Herren G., Jordan T., Krukowski P., Wicker T., Yahiaoui N., Mago R., Keller B. Rye Pm8 and wheat Pm3 are orthologous genes and show evolutionary conservation of resistance function against powdery mildew. Plant J. 2013;76:957–969. doi: 10.1111/tpj.12345. [DOI] [PubMed] [Google Scholar]
- 26.He H., Zhu S., Zhao R., Jiang Z., Ji Y., Ji J., Qiu D., Li H., Bie T. Pm21, encoding a typical CC-NBS-LRR protein, confers broad-spectrum resistance to wheat powdery mildew disease. Mol. Plant. 2018;11:879–882. doi: 10.1016/j.molp.2018.03.004. [DOI] [PubMed] [Google Scholar]
- 27.Lu P., Guo L., Wang Z., Li B., Li J., Li Y., Qiu D., Shi W., Yang L., Wang N. A rare gain of function mutation in a wheat tandem kinase confers resistance to powdery mildew. Nat. Commun. 2020;11:1–11. doi: 10.1038/s41467-020-14294-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krattinger S.G., Lagudah E.S., Spielmeyer W., Singh R.P., Huerta-Espino J., McFadden H., Bossolini E., Selter L.L., Keller B. A putative ABC transporter confers durable resistance to multiple fungal pathogens in wheat. Science. 2009;323:1360–1363. doi: 10.1126/science.1166453. [DOI] [PubMed] [Google Scholar]
- 29.Li M., Dong L., Li B., Wang Z., Xie J., Qiu D., Li Y., Shi W., Yang L., Wu Q. A CNL protein in wild emmer wheat confers powdery mildew resistance. New Phytol. 2020;228:1027–1037. doi: 10.1111/nph.16761. [DOI] [PubMed] [Google Scholar]
- 30.Moore J.W., Herrera-Foessel S., Lan C., Schnippenkoetter W., Ayliffe M., Huerta-Espino J., Lillemo M., Viccars L., Milne R., Periyannan S. A recently evolved hexose transporter variant confers resistance to multiple pathogens in wheat. Nat. Genet. 2015;47:1494–1498. doi: 10.1038/ng.3439. [DOI] [PubMed] [Google Scholar]
- 31.Zou S., Wang H., Li Y., Kong Z., Tang D. The NB-LRR gene Pm60 confers powdery mildew resistance in wheat. New Phytol. 2018;218:298–309. doi: 10.1111/nph.14964. [DOI] [PubMed] [Google Scholar]
- 32.Appels R., Eversole K., Stein N., Feuillet C., Keller B., Rogers J., Pozniak C.J., Choulet F., Distelfeld A., Poland J. Shifting the limits in wheat research and breeding using a fully annotated reference genome. Science. 2018;361:eaar7191. doi: 10.1126/science.aar7191. [DOI] [PubMed] [Google Scholar]
- 33.Kebede A.Z., Johnston A., Schneiderman D., Bosnich W., Harris L.J. Transcriptome profiling of two maize inbreds with distinct responses to Gibberella ear rot disease to identify candidate resistance genes. BMC Genom. 2018;19:131. doi: 10.1186/s12864-018-4513-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chittem K., Yajima W.R., Goswami R.S., Del Río Mendoza L.E. Transcriptome analysis of the plant pathogen Sclerotinia sclerotiorum interaction with resistant and susceptible canola (Brassica napus) lines. PLoS ONE. 2020;15:e0229844. doi: 10.1371/journal.pone.0229844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yao L., Li Y., Ma C., Tong L., Du F., Xu M. Combined genome-wide association study and transcriptome analysis reveal candidate genes for resistance to Fusarium ear rot in maize. J. Integr. Plant Biol. 2020;62:1535–1551. doi: 10.1111/jipb.12911. [DOI] [PubMed] [Google Scholar]
- 36.Klymiuk V., Yaniv E., Huang L., Raats D., Fatiukha A., Chen S., Feng L., Frenkel Z., Krugman T., Lidzbarsky G. Cloning of the wheat Yr15 resistance gene sheds light on the plant tandem kinase-pseudokinase family. Nat. Commun. 2018;9:1–12. doi: 10.1038/s41467-018-06138-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kourelis J., Van Der Hoorn R.A. Defended to the nines: 25 years of resistance gene cloning identifies nine mechanisms for R protein function. Plant Cell. 2018;30:285–299. doi: 10.1105/tpc.17.00579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ma Q., Luo P.G., Reng T.H., Jiang H.R., Yang Z.J. Genetic analysis and chromosomal location of two new genes for resistance to powdery mildew in wheat (Triticum aestivum L.) Acta. Agron. Sin. 2007;33:1–8. [Google Scholar]
- 39.Tang Z., Li M., Chen L., Wang Y., Ren Z., Fu S. New types of wheat chromosomal structural variations in derivatives of wheat-rye hybrids. PLoS ONE. 2014;9:e110282. doi: 10.1371/journal.pone.0110282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bento M., Gustafson P., Viegas W., Silva M. Genome merger: From sequence rearrangements in triticale to their elimination in wheat–rye addition lines. Theor. Appl. Genet. 2010;121:489–497. doi: 10.1007/s00122-010-1325-6. [DOI] [PubMed] [Google Scholar]
- 41.Beaulieu J., Jean M., Belzile F. The allotetraploid Arabidopsis thaliana–Arabidopsis lyrata subsp. petraea as an alternative model system for the study of polyploidy in plants. Mol. Genet. Genom. 2009;281:421–435. doi: 10.1007/s00438-008-0421-7. [DOI] [PubMed] [Google Scholar]
- 42.Li X., Liu T., Chen W., Zhong S., Zhang H., Tang Z., Chang Z., Wang L., Zhang M., Li L. Wheat WCBP1 encodes a putative copper-binding protein involved in stripe rust resistance and inhibition of leaf senescence. BMC Plant. Biol. 2015;15:1–15. doi: 10.1186/s12870-015-0612-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hu Y., Zhong S., Zhang M., Liang Y., Gong G., Chang X., Tan F., Yang H., Qiu X., Luo L. Potential Role of Photosynthesis in the Regulation of Reactive Oxygen Species and Defence Responses to Blumeria graminis f. sp. tritici in Wheat. Int. J. Mol. Sci. 2020;21:5767. doi: 10.3390/ijms21165767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Keller B., Wicker T., Krattinger S.G. Advances in wheat and pathogen genomics: Implications for disease control. Annu. Rev. Phytopathol. 2018;56:67–87. doi: 10.1146/annurev-phyto-080516-035419. [DOI] [PubMed] [Google Scholar]
- 45.Dhokane D., Karre S., Kushalappa A.C., McCartney C. Integrated metabolo-transcriptomics reveals Fusarium head blight candidate resistance genes in wheat QTL-Fhb2. PLoS ONE. 2016;11:e0155851. doi: 10.1371/journal.pone.0155851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cheng B., Ding Y.Q., Gao X., Cao N., Xin Z.H., Zhang L.Y. The diversity of powdery mildew resistance gene loci among wheat germplasm in Southwest China. Cereal. Res. Commun. 2020;48:65–70. doi: 10.1007/s42976-020-00015-2. [DOI] [Google Scholar]
- 47.Song Q.J., Shi J.R., Singh S., Fickus E.W., Costa J.M., Lewis J., Gill B.S., Ward R., Cregan P.B. Development and mapping of microsatellite (SSR) markers in wheat. Theor. Appl. Genet. 2005;110:550–560. doi: 10.1007/s00122-004-1871-x. [DOI] [PubMed] [Google Scholar]
- 48.Jones J.D., Dangl J.L. The plant immune system. Nature. 2006;444:323–329. doi: 10.1038/nature05286. [DOI] [PubMed] [Google Scholar]
- 49.Periyannan S., Moore J., Ayliffe M., Bansal U., Wang X., Huang L., Deal K., Luo M., Kong X., Bariana H. The gene Sr33, an ortholog of barley Mla genes, encodes resistance to wheat stem rust race Ug99. Science. 2013;341:786–788. doi: 10.1126/science.1239028. [DOI] [PubMed] [Google Scholar]
- 50.Lee H., Yeom S. Plant NB-LRR proteins: Tightly regulated sensors in a complex manner. Brief Funct. Genom. 2015;14:233–242. doi: 10.1093/bfgp/elv012. [DOI] [PubMed] [Google Scholar]
- 51.Jach G., Görnhardt B., Mundy J., Logemann J., Pinsdorf E., Leah R., Schell J., Maas C. Enhanced quantitative resistance against fungal disease by combinatorial expression of different barley antifungal proteins in transgenic tobacco. Plant J. 1995;8:97–109. doi: 10.1046/j.1365-313X.1995.08010097.x. [DOI] [PubMed] [Google Scholar]
- 52.Xiao Y., Heu S., Yi J., Lu Y., Hutcheson S.W. Identification of a putative alternate sigma factor and characterization of a multicomponent regulatory cascade controlling the expression of Pseudomonas syringae pv. syringae Pss61 hrp and hrmA genes. J. Bacteriol. 1994;176:1025–1036. doi: 10.1128/jb.176.4.1025-1036.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cockrum C., Kaneshiro K.R., Rechtsteiner A., Tabuchi T.M., Strome S. A primer for generating and using transcriptome data and gene sets. Development. 2020;147:dev193854. doi: 10.1242/dev.193854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhao S., Ye Z., Stanton R. Misuse of RPKM or TPM normalization when comparing across samples and sequencing protocols. RNA. 2020;26:903–909. doi: 10.1261/rna.074922.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Devonshire A.S., Elaswarapu R., Foy C.A. Applicability of RNA standards for evaluating RT-qPCR assays and platforms. BMC Genom. 2011;12:1–10. doi: 10.1186/1471-2164-12-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bai S., Liu J., Chang C., Zhang L., Maekawa T., Wang Q., Xiao W., Liu Y., Chai J., Takken F.L. Structure-function analysis of barley NLR immune receptor MLA10 reveals its cell compartment specific activity in cell death and disease resistance. PLoS Pathog. 2012;8:e1002752. doi: 10.1371/journal.ppat.1002752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Césari S., Kanzaki H., Fujiwara T., Bernoux M., Chalvon V., Kawano Y., Shimamoto K., Dodds P., Terauchi R., Kroj T. The NB-LRR proteins RGA 4 and RGA 5 interact functionally and physically to confer disease resistance. EMBO J. 2014;33:1941–1959. doi: 10.15252/embj.201487923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Casey L.W., Lavrencic P., Bentham A.R., Cesari S., Ericsson D.J., Croll T., Turk D., Anderson P.A., Mark A.E., Dodds P.N. The CC domain structure from the wheat stem rust resistance protein Sr33 challenges paradigms for dimerization in plant NLR proteins. Proc. Natl. Acad. Sci. USA. 2016;113:12856–12861. doi: 10.1073/pnas.1609922113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Takken F.L., Goverse A. How to build a pathogen detector: Structural basis of NB-LRR function. Curr. Opin. Plant. Biol. 2012;15:375–384. doi: 10.1016/j.pbi.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 60.Burch-Smith T.M., Schiff M., Caplan J.L., Tsao J., Czymmek K., Dinesh-Kumar S.P. Correction: A Novel Role for the TIR Domain in Association with Pathogen-Derived Elicitors. PLoS Biol. 2016;14:e1002374. doi: 10.1371/journal.pbio.1002374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kwok P., Chen X. Detection of single nucleotide polymorphisms. Curr. Issues Mol. Biol. 2003;5:43–60. [PubMed] [Google Scholar]
- 62.Liu Z., Xu M., Xiang Z., Li X., Chen W., Luo P. Registration of the novel wheat lines L658, L693, L696, and L699, with resistance to Fusarium Head blight, stripe rust, and powdery mildew. J. Plant. Regist. 2015;9:121–124. doi: 10.3198/jpr2014.01.0003crg. [DOI] [Google Scholar]
- 63.Zhao Z., Sun H., Song W., Lu M., Huang J., Wu L., Wang X., Li H. Genetic analysis and detection of the gene MlLX99 on chromosome 2BL conferring resistance to powdery mildew in the wheat cultivar Liangxing 99. Theor. Appl. Genet. 2013;126:3081–3089. doi: 10.1007/s00122-013-2194-6. [DOI] [PubMed] [Google Scholar]
- 64.Chang X.L., Luo L.Y., Liang Y.P., Hu Y.T., Luo P.G., Gong G.S., Chen H.B., Khaskheli M.I., Liu T.G., Chen W.Q. Papilla formation, defense gene expression and HR contribute to the powdery mildew resistance of the novel wheat line L699 carrying Pm40 gene. Physiol. Mol. Plant. Pathol. 2019;106:208–216. doi: 10.1016/j.pmpp.2019.02.009. [DOI] [Google Scholar]
- 65.Kim D., Langmead B., Salzberg S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods. 2015;12:357–360. doi: 10.1038/nmeth.3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pertea M., Pertea G.M., Antonescu C.M., Chang T., Mendell J.T., Salzberg S.L. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat. Biotechnol. 2015;33:290–295. doi: 10.1038/nbt.3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Robinson M.D., McCarthy D.J., Smyth G.K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26:139–140. doi: 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cingolani P., Platts A., Wang L.L., Coon M., Nguyen T., Wang L., Land S.J., Lu X., Ruden D.M. A program for annotating and predicting the effects of single nucleotide polymorphisms, SnpEf. Fly. 2012;6:80–92. doi: 10.4161/fly.19695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kala N., Babu S., Manjeu J., Aadivalavan A., Khan R. Allele-specific polymerase chain reaction for the detection of single nucleotide polymorphism in amlodipine-induced gingival enlargement. J. Clin. Pharm. Ther. 2017;43:110–113. doi: 10.1111/jcpt.12587. [DOI] [PubMed] [Google Scholar]
- 70.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 71.Faheem M., Li Y., Arshad M., Jiangyue C., Jia Z., Wang Z., Xiao J., Wang H., Cao A., Xing L. A disulphide isomerase gene (PDI-V) from Haynaldia villosa contributes to powdery mildew resistance in common wheat. Sci. Rep. 2016;6:1–14. doi: 10.1038/srep24227. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.