Abstract
Oncolytic adenovirus therapy is gaining importance as a novel treatment option for the management of various cancers. Different concepts of modification within the adenovirus vector have been identified that define the mode of action against and the interaction with the tumour. Adenoviral vectors allow for genetic manipulations that restrict tumour specificity and also the expression of specific transgenes in order to support the anti-tumour effect. Additionally, replication of the virus and reinfection of neighbouring tumour cells amplify the therapeutic effect. Another important aspect in oncolytic adenovirus therapy is the virus induced cell death which is a process that activates the immune system against the tumour. This review describes which elements in adenovirus vectors have been identified for modification not only to utilize oncolytic adenovirus vectors into conditionally replicating adenoviruses (CRAds) that allow replication specifically in tumour cells but also to confer specific characteristics to these viruses. These advances in development resulted in clinical trials that are summarized based on the conceptual design.
Keywords: adenovirus, cancer, immunotherapy, oncolytic virus, CRAd, vector design, arming, tropism
1. Introduction
In recent years, the development of oncolytic viruses and their implementation in clinical trials have gained increased attention. Adenoviruses are among the best-studied viruses in the field of oncolytic virotherapy and several molecular biological discoveries can be traced back to adenovirus research [1,2]. They belong to the class of non-enveloped viruses and contain double-stranded linear DNA with a 26–48 kb genome in an icosahedral capsid of ~950 Å in diameter [3], excluding the fibres, which are between 110 and 370 Å long, depending on the serotype [4]. Human adenoviruses are of high genetic stability and low pathogenicity. They are comparatively easy to produce in high titres and purity. This makes them particularly suitable for various applications, from gene and cancer therapy to vaccine development [5].
The life cycle of adenoviruses is extrachromosomal and they are therefore considered as non-integrating vectors that can infect both replicating and non-replicating cells [6]. According to their antigenic properties, over 50 serologically distinguishable subtypes of human adenoviruses have been described so far [7,8] but recent efforts have revealed 104 genotypes that have been registered (http://hadvwg.gmu.edu/ (accessed on 23 September 2021)). Serotypes 2 and 5 are most commonly used in research.
Gene therapy with replication-deficient adenoviruses is limited to the expression of a transgene and, in the case of suicide gene therapy, to the killing of the infected cell. In the oncolytic approach, on the other hand, the viruses replicate, followed by lysis of the infected cell and release of new infectious units into the immediate tumour environment. An important aspect of adenoviral oncolysis is that it is also effective in tumour stem cells, which often cause tumour recurrences due to intrinsic resistance to therapy [9].
Since the early 1990s, sophisticated methods and advances in molecular biology and virology led to a resumption of virus vector development [10,11]. In particular, the establishment of human embryonic kidney cells (HEK293) that express the adenoviral E1 region was important for the development of first-generation adenoviral vectors that were made replication-defective by deleting the early E1 gene and making room for a transgene sequence of up to 4.5 kb [12]. The E1 region in the HEK293 cell line provides trans-complementation and enables replication of the adenoviral vector, making it to a feasible production cell line.
This review article focuses primarily on strategies of genetic engineering of oncolytic adenovirus vectors for tumour-specific replication, the main prerequisite for oncolysis. It also describes aspects of transgene expression and stimulation of the immune system. These concepts are currently being tested in clinical trials that are summarized based on adenovirus vector design which will determine as a decisive aspect for further improvements in vector design and combination with other treatment options in the future.
2. The Four Main Concepts for Conditionally Replicating Adenoviruses Used in Actual Clinical Trials
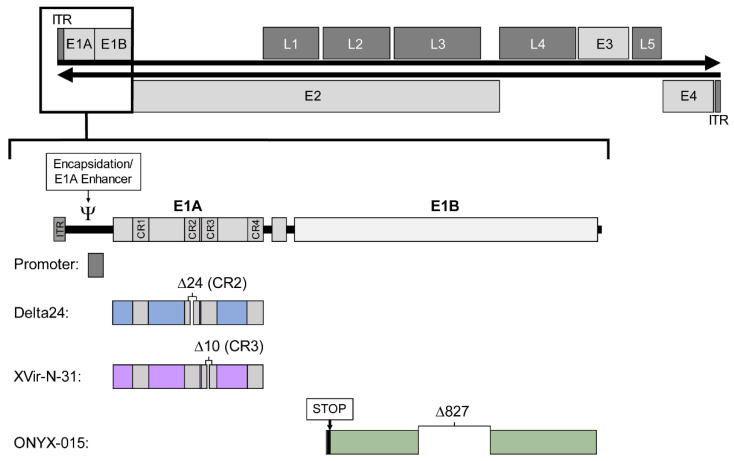
The replication cycle of adenoviruses can be divided into two phases. Accordingly, the adenoviral genome is organised into early (E1–E4) and late (L1–L5) transcription units, the latter encoding structural proteins (Figure 1). The early region 1A (E1A) encodes five different proteins [13,14], the two main isoforms 12S and 13S are required for early replication. The E1A 13S protein consists of 289 amino acids with four conserved regions (CR1–CR4) and can transactivate the other early transcription units E1B, E2, E3, and E4 [15], while E1A 12S lacks CR3.
Figure 1.
Structure of the wild-type adenovirus serotype 5 genome and the four concepts for generating conditionally replicating adenoviruses applied in vectors of current clinical trials. The affected E1 region is shown enlarged to illustrate the changes. The double-stranded DNA of about 36 kb in length (black horizontal arrows), flanked by two inverted terminal repeats (ITR) comprises four early transcription units (E1–E4, light grey boxes) and five late transcription units (L1–L5, dark grey boxes). The E1 region (enlarged below) consists of the encapsidation signal/E1A enhancer, the E1A promoter, E1A with four conserved regions (CR1–CR4) and E1B. The four concepts are listed below: Changes in the promoter (grey), Delta24 (blue) with a deletion in CR2, XVir-N-31 (purple) with a deletion in CR3, and ONYX-015 (green) with a stop codon and a deletion in E1B55K.
E1 deleted adenoviruses are considered as replication-deficient and are used as shuttle vectors in gene therapy or for vaccination. Replication-competent adenoviruses that are restricted in their replication to cancer cells are therefore referred to as CRAd (Conditionally replicating Adenoviruses) [16] and are used clinically in oncolytic adenovirus therapy.
There are four general strategies in current clinical adenoviral vectors to achieve conditional replication: one concept is the replacement or overlay of the native E1 promoter with cancer cell-specific promoters, the other three concepts involve modification of the early transcription units E1A and E1B. In a number of constructs, an E1A modification is combined with a tumour specific promoter. However, there are several other promising approaches that have not yet been tested in clinical trials.
2.1. Specific Cellular Promoters Control E1A and Adenoviral Replication
Tumour-specific promoters are characterized by their ability to cause high expression of specific genes that are crucial for a malignant phenotype. Control of E1A-mediated viral replication by a tumour- or tissue-specific promoter restricts replication to the appropriate cells. Deletions in the E1A regulatory region should not interfere with either the inverted terminal repeat (ITR; 1–103 bp) or the packaging signal (194–358 bp) that overlaps with the E1A enhancer region.
Therefore, exogenous promoters are mostly inserted without any or with small deletions in the E1A regulatory region, leaving the E1A enhancer/encapsidation intact as in CRAd-Survivin-pk7 [17]. For further deletions, the packaging signal has to be rescued and cloned elsewhere as in the case of Ar6pAE2fE3F and CG0070. [18]. This allows tighter control of replication. The parallel regulation of the E4 region by a specific promoter also increases specificity, as the E4 gene products are necessary for productive viral replication [19]. To control E1A transcription, E2F-responsive elements [12], promoters for survivin [10], human telomerase reverse transcriptase (hTERT) [13] or chromogranin A (CgA) [20] were employed.
Restriction of adenoviral replication to specific tumour entities and tissues was also successfully achieved. For prostate tissue, the probasin promoter (to drive E1A) and the enhancer element of prostate-specific antigen (PSA) (to drive E1B) were used [21] and for hepatocellular carcinoma (HCC) the alpha-feto protein (AFP) promoter [22].
A synthetic fusion construct of core promoter and enhancers of the human tyrosinase was engineered for Ad2xTyr, an oncolytic adenovirus in which parts of the E1A regulatory region as well as the E4 promoter were replaced. Ad2xTyr showed tumour selectivity against melanoma cells versus normal fibroblasts and keratinocytes [19].
2.2. Modifications in E1A Control Specificity of Replication
Another approach to achieve tumour-specific adenoviral replication is to alter the protein-coding genes in the E1 region by introducing loss-of-function mutations. A cancer cell with specific molecular features can compensate for this defect, thus enabling replication.
2.2.1. E1A Deletions: Delta 24
In a non-replicating cell, the retinoblastoma protein (pRB) interacts as a negative regulator with proteins of the E2F transcription factor family and thus control cell cycle progression [23]. This pathway is often genetically disrupted in tumour cells [24]. The CR2 region of the adenoviral E1A protein also interacts with pRB that results in release of E2F and enables viral replication [25,26]. In Delta-24, a deletion of 24 base pairs in the adenovirus E1A CR2 prevents the binding of E1A to pRB [27]. This virus cannot release E2F in normal cells and does not replicate. In tumour cells with mutated or dysregulated pRB, E2F is no longer negatively regulated by pRB and can activate viral gene transcription and thus replication [28].
In various further developments of Ad5-Delta-24, the modified E1A region is regulated via a tumour-specific promoter. In AdCN103, the 24 bp deletion of E1A is combined with a modified hTERT promoter [29] and in SynOV1.1 (Ad5-Delta-24-RGD-hGMCSF) with an enhanced cancer-specific alpha-fetoprotein (AFP) promoter. In other Ad5-Delta-24 derivatives, E1A transcription is controlled by one or more E2F-responsive elements such as in LOAd703 (Ad5/35-E2F-Delta-24) [30], ICOVIR-5 [31] and -7 (Ad5-E2F-Delta-24-RGD) [32], and VCN-01 (Ad E2F-Delta-24-RGD-PH20) [33].
2.2.2. E1A 13S Deletion: Dl520, Ad-Delo3-RGD (XVir-N-31)
The adenoviral E2 promoter can be divided into an early and a late promoter for the regulation of the E2 transcription unit, which includes the DNA polymerase. The late E2 promoter region contains Y-boxes, binding sites for the transcription factor YB-1 (Y-box binding protein-1) [34,35]. A selective viral replication has been shown to be related to YB-1 expression [35]. Elevated YB-1 protein levels and subcellular localisation in the nucleus were demonstrated in malignant tissue and are associated with poor prognosis and multidrug resistance [36,37]. In normal adult cells, YB-1 is either not expressed or only localized in the cytoplasm [38,39]. Since E1A13S is critical for the translocalisation of YB-1 from the cytoplasm into the nucleus, adenoviruses lacking E1A13S expression are replication deficient in normal cells [40]. Dl520 is an oncolytic adenovirus in which E1A13S is not produced due to a deletion of 10 bp in the splice acceptor site of E1A CR3 [41]. A further development represents Ad-Delo3 RGD (XVir-N-31) that has additional deletions in E1B19K and E3 and an RGD motif in the fibre protein and is currently registered as a phase I clinical trial for glioblastoma [42,43] (EudraCT number: 2016-000292-25).
2.2.3. E1B55K Deletion: Dl1520 (ONYX-015), Oncorine (H101)
The E1B region of the adenovirus genome encodes a 55-kD protein (E1B55K) that binds and inactivates p53. This binding is thought to be essential for virus replication, supposedly because E1A triggers p53-dependent apoptosis [44]. Dl1520 is a chimeric adenovirus (Ad5/2) with a deletion in E1B55K and a stop codon that terminates translation of the protein, and is identical to ONYX-015 [45,46]. This virus has limited tumour selectivity, thus challenging the concept [47,48]. However, based on this vector, the variant Oncorine (H101) was developed with an adenovirus 5 genome and partial deletion in E3 that has been approved for clinical application in China [49].
3. Oncolytic Adenoviruses and the Immune System
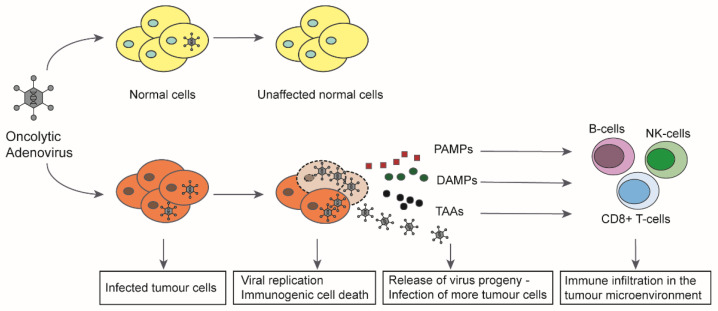
The induction of immunogenic cell death has proved to be a key aspect in virotherapy [50] (Figure 2). Based on this mechanism, oncolytic viruses can revert the tumour immune escape. It was shown that virus induced oncolysis results in apoptosis, necrosis, necroptosis, pyroptosis, or autophagy that releases signal molecules with a pathogen-associated molecular pattern (PAMP). Infected cells also release damage-associated molecules (DAMP), including HMGB1, heat shock proteins (HSP), intracellular matrix proteins, DNA, ATP, uric acid, or heparin sulphate [51,52]. These molecules stimulate the innate immune response and attract antigen-presenting cells (APCs). Tumour-associated antigens (TAA) and neoantigens in the tumour microenvironment can be taken up by APCs and trigger a systemic immune response against the tumour, so that a “cold” tumour can be transformed into a “hot” tumour that is recognised by the immune system [53]. The terms ‘oncolytic virotherapy’ and ‘oncolytic immunotherapy’ are therefore used synonymously. Oncolytic adenoviruses thus act multimodally against tumours by causing local inflammation, viral spread, destruction of the tumour microenvironment and release of tumour antigens, leading to a systemic anti-tumour response resulting in complete tumour eradication.
Figure 2.
Mechanism of action of oncolytic adenoviruses in cancer cells. In normal, non-cancerous cells, oncolytic adenoviruses are unable to replicate upon infection leaving the cells unaffected. In tumour cells, the viruses can successfully replicate upon infection, leading to production of more virus progeny and eventual induction of immunogenic cell death (ICD). The cell lysis leads to release of virus progeny, pathogen-associated molecular patterns (PAMP), damage-associated molecular patterns (DAMP), and tumour associated antigens (TAA) into the tumour microenvironment (TME). The released virus progeny further infects the uninfected tumour cells and continues the viral spread. The immunostimulatory molecules released into the TME attract immune cells to the tumour and lead to the activation of both innate and adaptive immunity against the tumour (modified with permission from [52,54]).
4. Arming of Adenoviral Vectors to Improve Their Therapeutic Effect
Besides tissue specific replication, vector systems have been developed that improve oncolytic and cytotoxic properties of adenoviruses. Thus, deletion of the E1B19K protein—a Bcl-2 homologue that blocks apoptosis induction—has been shown to increase viral spread and enhance TNF-alpha-mediated cell death [55,56].
4.1. Strategies to Include Transgenes in Adenoviral Vectors
Apart from strategies that warrant cancer-specific replication, genes that promote oncolysis or stimulate of the immune system have also been introduced.
Derived from early studies for the development of non-replicating vector systems, most oncolytic adenoviruses use deletions in the E3 region, as this region is non-essential for viral replication that allows inserts of up to 8.3 kb [10]. Expression cassettes are usually designed with exogenous promoters such as the human cytomegalovirus promoter or the heat shock protein 70 promoter [30,57]. In addition, ribosome entry sites, splice acceptor sites, or 2A self-cleaving peptide linkers can be used to regulate transcription of the transgene [58,59]. Another approach is to regulate inserted transgenes via the adenoviral E3 promoter and E3 polyadenylation site [60]. Here, the presence of the E3 adenoviral death protein (ADP) increases the oncolytic efficacy [61]. An alternative to the E3 region is the positioning of a transgene within the late genes which seems to have advantages in terms of specificity, expression level, and timing of expression. The insertion of hGM-CSF into the late gene L3 led to a high expression level of this transgene, which was moreover dependent on adenoviral DNA replication. In contrast, when the transgene was inserted into the E3 region, the expression was weak and independent of replication [62]. Similar observations were made when p53 was coupled with a late gene (L5, fibre) [63].
4.2. Transgenes That Confer Improved Therapeutic Effect
To enhance the therapeutic effect of oncolytic adenoviruses, various approaches have been employed such as gene-directed enzyme prodrug therapy (GDEPT), enhancement of virus-induced apoptosis and viral spread, expression of specific genes and attraction of the immune system.
The advantage of GDEPT is the local conversion of non-toxic compounds into cytotoxic drugs, thus minimizing systemic toxicity [64]. Some examples of enzymes used in oncolytic virotherapy are herpes simplex thymidine kinase in combination with ganciclovir or cytosine deaminase in combination with 5-fluorocytosine [65]. Other possibilities include the downregulation of cellular genes by RNA interference (RNAi) technologies [66]. The spread of oncolytic adenovirus particles within the tissue is mostly limited by the connective tissue. Therefore, viruses have been engineered to express enzymes that digest the extracellular matrix—e.g., human hyaluronidase to degrade hyaluronic acid or to express fusogenic membrane glycoproteins to induce cell fusion [67].
Bioselection of Ad5 mutants with enhanced anti-tumour activity in an in vivo tumour environment as selection pressure resulted in the variant AdT1 with a single adenine insertion within the retention domain of the endoplasmic reticulum (ER) of E3 gp19K that causes an enhanced release of the virus from the infected cell [68]. As described above, adenoviruses are generally immunogenic, but the effect can be enhanced by arming the oncolytic adenoviruses with transgenes encoding for immune stimulatory factors like TNF-α, IFNα, interleukin (IL)-2, IL-12, CD40L, the OX40 ligand (OX40L) [69] or the human granulocyte-macrophage colony-stimulating factor (GM-CSF) [70]. The 4-1BB ligand (4-1BBL, CD137L) was incorporated in LOAd703 [30] because it is known to enhance immunologic memory and expand natural killer (NK) cells [71]. For the treatment of HER2-positive cancer, the human trastuzumab heavy and light chain antibody encoding genes were inserted into an adenoviral vector and linked through an internal ribosome entry site. In the infected cell, the polypeptides were assembled into functional antibodies [72]. Immune checkpoint inhibitors (ICI), already successfully used in cancer therapy, have also been incorporated into adenoviral vectors in the form of transgenes encoding antibodies against the T lymphocyte-associated antigen-4 (CTLA-4) [73] or mini-antibodies against PD-L1 in a coinfected helper-dependent adenovirus (HDAd) [74].
5. Delivery and Tropism of Oncolytic Adenoviruses
Systemic application of adenoviral vectors leads to viral uptake in all organs and especially in the liver [75]. After intravenous administration, 90% of the virus is eliminated within the first 24 h by elements of the innate immune system [76]. Liver-resident macrophages (Kupffer cells) in particular efficiently take up and inactivate adenoviruses circulating in the blood [77], resulting in an estimated half-life in the blood of about two minutes [78]. A retention of the adenovirus in certain organs [79] can be achieved by direct injection into specific organs. However, within 14 days of Ad5 administration, a virus-specific humoral and cellular adaptive immune response occurs, eliminating virions and adenovirus-infected cells [80].
To ensure a potent anti-tumour response of oncolytic adenoviruses, modifications and delivery methods must be considered to achieve sufficient viral uptake into tumour cells.
5.1. Genetic Modifications to Alter the Tropism of Adenoviruses for Clinical Use
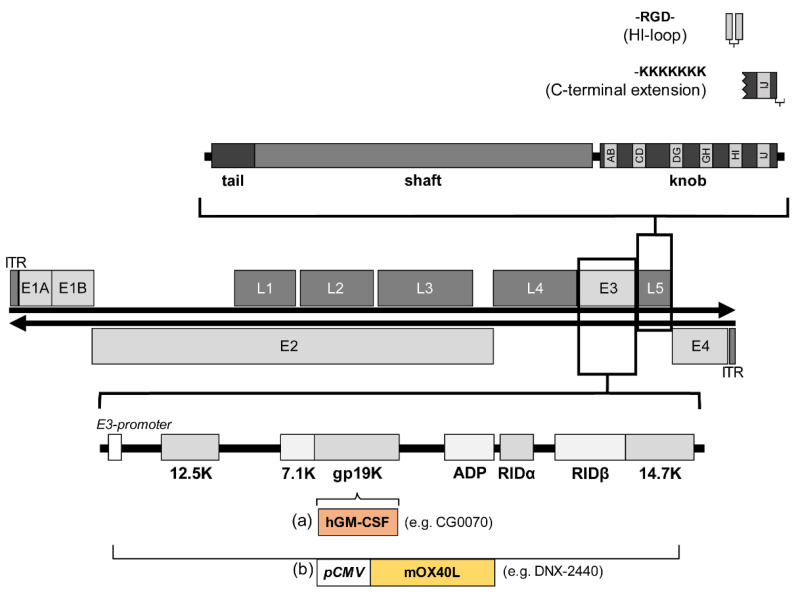
Cellular entry of adenoviruses is a two-step process consisting of binding of the viral fibre knob to the cell via the coxsackievirus and adenovirus receptor (CAR) [81] and interaction of Arg-Gly-Asp motifs (RGD) in the penton base with cellular integrins [82], leading to the internalisation of Ad5 vectors via clathrin-coated pits [83]. While integrins are homogeneously expressed in different tissues, CAR expression varies which largely affects the infectious capacity of andenoviruses [84]. For example, melanoma cells without CAR derived from metastases of patients were found to be resistant to WTAD infection and cell killing [85]. By integrating the RGD motif into the HI loop of the fibre knob domain [86] or into the hexon protein, enhanced infection of cells with low CAR expression was achieved [87]. A different strategy was used with the adenovirus CRAd-Survivin-pk7 in which a polylysine modification (Figure 3) with a heparan sulphate binding domain was incorporated into the fibre protein (pk7), resulting in high affinity for tumour-specific heparan sulphate proteoglycans [88].
Figure 3.
Structure of the wild-type adenovirus serotype 5 genome and the concepts for altering natural tropism by fibre modification (upper section) and incorporating transgenes into the adenoviral E3 region (lower section). The affected L5 region encodes for the fibre protein; fibre monomer with tail, shaft, and knob domains is enlarged in the upper section to illustrate the modifications. Incorporation of an Arg-Gly-Asp motif (RGD) into the HI-loop enables attachment to the host cell via integrins, and C-terminal extension of lysin residues at the fibre head via heparansulfate-containing receptors. The lower section illustrates the two concepts of incorporating transgenes into the E3 region: (a) Most of the E3 region is retained while a transgene like the human granulocyte-macrophage colony-stimulating factor (GM-CSF) in the adenoviral vector CG0070 is inserted, here GM-CSF expression is regulated by the E3 promoter. (b) Replacement of the E3 transcription unit with, for example, the pCMV-mOX40L-expression cassette in the adenoviral vector DNX-2440 where the expression of the mouse OX40L is regulated by the cytomegalovirus (CMV) promoter.
5.2. Modification of Natural Tropism
Since adenoviral cell entry receptors are ubiquitously expressed in various tissues of mammals, one strategy to ensure tumour-exclusive therapy effects aims to ablate the natural viral tropism by altering the RGD motif in the penton base [89], incorporate mutations or deletions in the fibre protein, or by forming chimeras between two different adenovirus types [90]. A very commonly used adenovirus fibre chimera is a combination of the adenovirus serotype 3 fibre knob with an adenovirus serotype 5 shaft, thus recognizing CD46 instead of CAR [91,92].
To introduce a new tropism, Douglas et al. chemically conjugated an antibody fragment against the fibre protein with a folate molecule and obtained a targeted adenovirus [93]. The folate receptor is overexpressed on the surface of a variety of malignant cells. Similarly, tissue-specific targeting via the epidermal growth factor receptor (EGFR) [94], or via a tumour marker like melanoma-associated high molecular weight antigen [95], have been developed with the assistance of dimeric antibody fragments (diabodies) [96]. Another strategy includes the exchange of most of the fibre protein with an exogenous protein binding domain (affibody) [97] that has been specifically screened for high affinity binding to a tissue or tumour marker and for instance enables cellular attachment via HER2/neu rather than CAR [98].
5.3. Delivery Strategies
Besides local administration of oncolytic virus into the lesion, cell-mediated delivery is a novel and promising approach to improve the clinical usage of oncolytic adenoviruses. Here, neural [99] or mesenchymal [100] stem cells were infected with oncolytic adenoviruses in vitro and then delivered intratumourally or systemically into the patient. Tissue specific stem cells migrate to their appropriate tissue niche and would then distribute the virus, thus masking the viral vector to the host immune system and improve viral spread within the tumour [101].
6. Clinical Trials with Oncolytic Adenoviruses
Adenoviruses are the most commonly used oncolytic viruses in clinical trials [102]. At the time of writing this review, we conducted a search on clinicaltrials.org using the term “oncolytic adenovirus”, and subsequently also all individual names of the different adenoviral vectors mentioned, and identified 59 trials in various phases from 2005 to present. Of the 59 trials, 17 have been completed and 38 are ongoing. These trials are testing 18 different oncolytic adenoviruses administered intratumourally or intravenously. The majority were in phase I-II, three trials were in phase III and involved two oncolytic adenoviruses, Oncorine (H101) and CG0070. Table 1, Table 2, Table 3, Table 4 and Table 5 provides a detailed overview of the clinical trials collected on the website. We will only summarise the most advanced studies in this overview.
Table 1.
Clinical trials using oncolytic adenoviruses whose replication is controlled by cellular promoters.
| Concept: Promoter | Indication | Clinical Trials.gov No. | Vector Name | Construct | Additional Features | Pre-Clinical | I | II | III | Combination | Application of AdV | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| E2F1- promoter |
Bladder Cancer | NCT00109655 | CG0070 | Ad5-E2F-E1A-GMCSF | GM-CSF | 2005 | Intravesical | |||||
| NCT02365818 | 2015 completed | Intravesical | ||||||||||
| NCT04452591 | 2020 recruiting | Intravesical | ||||||||||
| NCT04610671 | 2020 recruiting |
Nivolumab | Intravesical | |||||||||
| NCT04387461 | 2020 recruiting | Pembrolizumab | Intravesical | |||||||||
| hTERT- promoter |
Hepatocellular Carcinoma | NCT02293850 | OBP-301 | Ad5-hTERT-E1A-IRES-E1B | 2014 unknown | Intratumoural | ||||||
| Melanoma | NCT03190824 | 2017 active | Intratumoural | |||||||||
| Solid Tumours | NCT03172819 | 2017 active | Pembrolizumab | Intratumoural | ||||||||
| Esophageal Cancer | NCT03213054 | 2017 recruiting |
Radiation | Intratumoural | ||||||||
| Esophagogastric Adenocarcinoma |
NCT03921021 | 2019 recruiting | Pembrolizumab | Intratumoural | ||||||||
| Head and Neck Squamous Cell Carcinoma | NCT04685499 | 2020 recruiting | Pembrolizumab | Intratumoural | ||||||||
| Esophageal and Gastroesophageal Junction Adenocarcinoma | NCT04391049 | 2020 not recruiting |
Paclitaxel, Carboplatin, Radiation | Intratumoural | ||||||||
| Survivin- promoter |
Glioma | NCT03072134 | CRAd-S-pk7 | NSC-CRAd-Survivin-pk7 | pk7 | 2017 active | Intratumoural | |||||
| CgA- promoter |
Neuroendocrine Tumours | NCT02749331 | AdVince | CgA -PTD-miR122 | PTD-peptide, miRNA 122 | 2016 recruiting | Intraarterial | |||||
Table 2.
Clinical trials using oncolytic adenoviruses in which a delta 24 variant of E1A is controlled by cellular promoters.
| Concept: Promoter & Δ24 | Indication | Clinical Trials.gov No. | Vector Name | Construct | Additional Features | Preclinical | I | II | III | Combination | Application of AdV | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AFP promoter |
Hepatocellular Carcinoma | NCT04612504 | SynOV1.1 | Ad5-AFP-delta24-RGD | GM-CSF | 2020 recruiting | Atezolizumab | Intratumoural | ||||
| E2F1 promoter |
Melanoma | NCT01864759 | ICOVIR-5 | Ad5-E2F-delta24-RGD | 2013 completed |
Intravenous | ||||||
| Solid Tumours | NCT01844661 | Mesenchymal allogenic cells | 2013 completed | Intravenous | ||||||||
| Glioma | NCT04758533 | 2021 not recruiting |
Intravenous | |||||||||
| Advanced Solid Tumours Pancreatic Adenocarcinoma | NCT02045602 | VCN-01 | Ad5-E2F-delta24-RGD -PH20 | Hyaluronidase (PH20) | 2014 completed |
Gemcitabine, Abraxane | Intravenous | |||||
| Pancreatic Adenocarcinoma | NCT02045589 | 2014 completed |
Gemcitabine, Abraxane | Intravenous | ||||||||
| Refractory Retinoblastoma | NCT03284268 | 2017 recruiting |
Intravitreal | |||||||||
| Squamous cell Carcinoma of Head and Neck | NCT03799744 | 2019 recruiting |
Durvalumab | Intravenous | ||||||||
| Pancreatic Cancer | NCT02705196 | LOAd-703 | Ad5/35-E2F-delta24 | CD40L, 4-1BBL | 2016 recruiting |
Gemcitabine, Nab-Paclitaxel, Atezolizumab |
Intratumoural | |||||
| Pancreatic Cancer/Ovarian, biliary, colorectal Cancer | NCT03225989 | 2017 completed |
Standard chemotherapy or Gemcitabine | Intratumoural | ||||||||
| Colorectal Cancer | NCT03555149 | 2018 recruiting |
Standard chemotherapy | Intratumoural | ||||||||
| Melanoma | NCT04123470 | 2019 recruiting |
Atezolizumab | Intratumoural | ||||||||
Table 3.
Clinical trials using oncolytic adenoviruses with a 24 base pair deletion in E1A.
| Concept: | Indication | Clinical Trials.gov No | Vector Name | Construct | Additional Features | Preclinical | I | II | III | Combination | Application of AdV | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Δ24 | Ovarian Cancer, Primary Peritoneal Cancer | NCT00562003 | Ad5-Delta 24RGD | 2017 completed |
Intraperitoneal | |||||||
| Brain Cancer | NCT00805376 | DNX-2401 | Ad5-Delta 24RGD | 2018 completed |
Intratumoural | |||||||
| Glioblastoma | NCT01582516 | 2012 completed | Intratumoural | |||||||||
| Glioblastoma or Gliosarcoma | NCT01956734 | 2013 completed |
Temozolomide | Intratumoural | ||||||||
| Brain Cancer | NCT02798406 | 2016 completed | Pembrolizumab | Intratumoural | ||||||||
| Glioblastoma or Gliosarcoma | NCT02197169 | 2014 completed |
Interferon-gamma | Intratumoural | ||||||||
| Gliomas | NCT03178032 | 2017 active, not recruiting | Radiotherapy, chemotherapy | Intratumoural | ||||||||
| Gliomas | NCT03896568 | Ad5-Delta 24RGD | Mesenchymal stem cells as carriers | 2019 recruiting |
Intraarterial | |||||||
| Gliomas | NCT03714334 | DNX-2440 | Ad5-delta24-RGD-OX40L | CMV-OX40L-BGHpA repl. E3 | 2018 recruiting |
Intratumoural | ||||||
| Glioblastoma and multiple Solid Tumours | NCT04714983 | 2021 recruiting |
Intratumoural | |||||||||
| Solid Tumours | NCT01598129 | ONCOS-102 | Ad5/3-delta24-GMCSF | GM-CSF | 2012 completed |
Cyclophosphamide | Intratumoural and intravenous | |||||
| Melanoma | NCT03003676 | 2016 active, not recruiting | Cyclophosphamide, Pembrolizumab |
Intratumoural | ||||||||
| Colorectal, Ovarian, Appendiceal Cancer | NCT02963831 | 2017 active, not recruiting |
Durvalumab | Intraperitoneal | ||||||||
| Colorectal, Ovarian, Appendiceal Cancer | NCT03514836 | 2018 active, not recruiting |
Durvalumab | Intratumoural | ||||||||
| Prostate Cancer | NCT04097002 | ORCA-010 | Ad5-delta24-RGD | E3/19K-T1 protein | 2019 recruiting | Intratumoural | ||||||
| Melanoma | NCT04217473 | TILT 123 | Ad5/3-delta24-TNFα-IRES-IL2 | TNFα-IRES-IL2 in E3 |
2020 recruiting |
Intratumoural | ||||||
| Melanoma, Solid Tumours | NCT04695327 | 2021 recruiting |
Intratumoural | |||||||||
| Diverse HER2 positive Solid Tumours | NCT03740256 | CAdVec | Ad5-Delta 24 | HER2-specific autol. CAR T cells | 2018 recruiting |
Helper dependent Ad expressing PD-1 minibody | Intratumoural | |||||
Table 4.
Clinical trials using oncolytic adenoviruses with E1A13S or E1B55K deletions.
| Concept: | Indication | Clinical Trials.gov No. | Vector Name | Construct | Additional Features | Preclinical | I | II | III | Combination | Application of AdV |
|---|---|---|---|---|---|---|---|---|---|---|---|
| ΔE1A 13S |
Glioblastoma | 2016-000292-25 (EudraCT no.) | XVir-N-31 | Ad5 E1A13S/E1B19K/E3 deletions, Fibre RGD | 2021 active, not recruiting | Intratumoural | |||||
| ΔE1B 55K |
Nasopharyngeal Carcinoma | no number | Ad5 E1B55K/E3 deletions | 2005 approved by the State Food and Drug Administration of China |
Cisplatin, 5-Fluorouracil, Adriamycin |
Intratumoural | |||||
| Refractory Malignant Ascites | NCT04771676 | Oncorine (H101) | 2021 recruiting | Intraperitoneal | |||||||
| Lung Cancer | NCT02579564 | 2015 active, not recruiting |
Gemcitabine, Vinorelbine, Paclitaxel, Pemetrexed, Endostar, Cisplatin | Intrathoracic | |||||||
| Hepatocellular Carcinoma | NCT03780049 | 2018 recruiting | Oxaliplatin, 5-Fluorouracil, Leucovorin |
Intraarterial | |||||||
| Prostate Cancer | NCT02555397 | Ad5-yCD/ mutTKSR39rep-hIL12 |
E1B55K deletion | yCD, mutTk, IL-12 * |
2015 unknown |
Intraprostatic | |||||
| Metastatic Pancreatic Cancer | NCT03281382 | 2017 unknown |
5-Fluorocytosine (5-FC), chemotherapy |
Intratumoural | |||||||
* yCD = yeast cytosine deaminase, HSV-1 TKSR39/mutTk = mutant form of herpes simplex virus type 1 thymidine kinase.
Table 5.
Clinical trials using the vector enadenotucirev (Colo-Ad1), a vector whose specificity is based on selection in colon carcinoma.
| Other Approaches | Indication | Clinical Trials.gov No. | Vector Name | Construct | Additional Features | Preclinical | I | II | III | Combination | Application of AdV | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Evolution | Solid Tumours | NCT02053220 | Enadenotucirev (Colo-Ad1) |
Ad3/11 Chimera | 2014 completed | Intravenous | ||||||
| Solid Tumours of Epithelial Origin | NCT02028442 | 2014 completed |
Intravenous | |||||||||
| Ovarian Cancer | NCT02028117 | 2014 completed | Intravenous | |||||||||
| Colorectal Cancer, Squamous Cell Carcinoma of the Head and Neck, Epithelial Tumours | NCT02636036 | 2015, active not recruiting | Nivolumab | Intravenous | ||||||||
| Rectal Cancer | NCT03916510 | 2019 recruiting |
Capecitabine, radiation | Intravenous | ||||||||
| Epithelial Tumours | NCT03852511 | NG-350A | Anti-CD40 Ab | 2019 recruiting |
Intratumoural, intravenous | |||||||
| Epithelial Tumours | NCT04053283 | NG-641 | FAP/CD3, CXCL9, CXCL10, IFNa | 2019 recruiting |
chemotherapy, checkpoint inhibitors |
Intratumoural, intravenous | ||||||
| Squamous Cell Carcinoma of the Head and Neck | NCT04830592 | NG-641 | 2021 active, not recruiting | Pembrolizumab | Intravenous | |||||||
6.1. Clinical Vectors with Specific Cellular Promoters That Control E1A
Telomelysin (OBP-301) is an oncolytic adenovirus in which the hTERT promoter regulates both E1A and E1B, which are linked by an IRES element [103]. In an initial phase I dose escalation study in 16 patients with solid tumours, a single intratumoural injection resulted in a partial response in 56.7% of patients. Only grade 1/2 side effects were observed [104]. Phase II trials are currently ongoing for the treatment of patients with metastatic melanoma (NCT03190824) or adenocarcinoma of the oesophagus (NCT03921021). Among the first vectors using the tumour-selective E2F-1 promoter for E1A transcription are Ar6pAE2fE3F and Ar6pAE2fF (with and without the viral E3 region, respectively) [105]. These vectors were further enhanced, resulting in CG0070 (Ad5-E2F-E1A-E3-GMCSF) that expresses GMCSF in the E3 region and is currently tested in several clinical trials. In a 2018 phase II study published by [106], 45 patients with BCG-naïve non-muscle invasive bladder cancer were included. The overall response rate was 47% after 6 months and treatment was well tolerated. Interestingly, CIS may be the pathological subgroup that responds best to CG0070. The six-month complete response (CR) rates in patients with CIS and pure CIS were 50% and 58%, respectively. At six months, no patients with CIS-containing tumours developed muscle-invasive disease. The result is encouraging and promising, as progression occurred in approximately 9.8% to 40% of patients with intravesical BCG treatment. A phase III trial of CG0070 as monotherapy started in 2020 (NCT02365818), and two phase I-II trials in combination with chemotherapy are ongoing.
6.2. Clinical Vectors with E1A Deletion: Ad5-Delta-24
The first AdDelta-24 derivative being tested in clinical trials was DNX-2401 (Delta-24-RGD) [16] mainly for the treatment of glioma and has already completed four phase I trials (NCT01582516, NCT01956734, NCT02798406, NCT02197169) with reported improved long-term survival and evidence of effective immune activation. Five of 25 patients survived more than three years after treatment, and three patients experienced a dramatic decrease in tumour size, resulting in a progression-free survival of three years [107]. This was followed by six phase I/II studies and confirmed the safety profile. In further studies, the original oncolytic construct was combined with other drugs or/and further developments of AdDelta-24 were used. A phase II trial of DNX-2401 in combination with pembrolizumab, an anti-PD-1 antibody, included 49 patients with recurrent glioblastoma (NCT02798406). Median overall survival was 12.5 months and survival at 18 months was 20.2% compared to the median overall survival of 7.2 months with monotherapies of iomustine and temozolomide, the results with the oncolytic adenovirus were encouraging [108].
Table 1, Table 2, Table 3, Table 4 and Table 5 Clinical trials with oncolytic adenoviruses up to the time of writing, according to searches on clinicaltrials.org from 2005 to August 2021. 59 trials were found in various phases from 2005 to date. Of these 59 trials, 17 have been completed and 38 are ongoing. These trials are testing 18 different oncolytic adenoviruses. In Table 1, Table 2, Table 3 and Table 4, the clinical trials are grouped according to the concepts for conditionally replicating adenoviruses explained in Figure 1, while Table 5 lists the clinical trials with enadenotuvirev (Colo-Ad1).
6.3. Clinical Vectors with Specific Cellular Promoters That Control E1A-Delta-24
ICOVIR-5 (Ad5-E2F-Delta-24-RGD) was administered systemically intravenously in a phase I trial in melanoma patients (NCT01864759) and was well tolerated [109]. Although there was no tumour regression in a total of 12 patients, viral DNA was detected in metastatic skin or liver lesions in four patients, suggesting that ICOVIR-5 can target and detect metastatic tumour cells when administered intravenously.
VCN-01 (Ad5-E2F-Delta-24-RGD-PH20) expresses hyaluronidase (PH20), which enhances intratumoural spread of the virus [33]. It is currently being tested in several clinical trials in combination with chemotherapy or immune checkpoint inhibitor in advanced pancreatic cancer (NCT02045589 and NCT02045602) and squamous cell carcinoma of the head and neck (NCT03799744). In a trial for retinoblastoma (NCT03284268), administration of VCN-01 was well tolerated and exhibited anti-tumour activity in retinoblastoma vitreous seeds [110].
6.4. Clinical Vectors with E1B55K Deletions
ONYX-015 (dl1520) was the first oncolytic adenovirus in clinical trials for the treatment of head and neck cancer [111]. Although no objective response was observed, tumour necrosis at the injection site was detected in 5 of 22 patients. Phase I clinical trials for Oncorine (H101) were initiated in China in 2000. In addition to a tolerable safety profile, 3 of 15 patients reported remarkable tumour shrinkage. The phase III trial showed that Oncorine achieved a better positive response rate (79%) in patients with squamous cell carcinoma of the head and neck (SCCHN) in combination with chemotherapy than with chemotherapy alone (40%) [112]. Based on this study, it was approved by the State Food and Drug Administration (SDFA) of China in 2005 as the world’s first commercialised oncolytic virus for the treatment of SCCHN in combination with chemotherapy.
6.5. Clinical Vectors Based on Direct Evolution
A novel adenoviral vector, enadenotucirev (Colo-Ad1), is derived from a pool of different serotypes of species B to F by directed evolution and was selected to replicate only in colon cancer cells [113]. Three phase I trials were conducted. One of them included 17 patients with solid tumours and examined the efficacy of intratumoural versus intravenous application. Viral DNA was detected in tumour samples in 11 of 12 patients after intravenous infusion and in 2 of 5 after intratumoural injection. Both methods were well tolerated and no treatment-related serious adverse events were reported. Two other phase I trials with enadenotucirev are currently recruiting patients with colon cancer, head and neck cancer, or other epithelial tumours for combination therapy with enadenotucirev and nivolumab (PD-1 inhibitor) (NCT02636036) or patients with rectal cancer (NCT03916510) to be treated in combination with radiotherapy and chemotherapy (capecitabine). To elicit further immune responses against the tumour, two variants of enadenotucirev are currently being investigated in phase I clinical trials: NG-350A (NCT03852511), which expresses the CD40 antibody and NG-641 (NCT04053283), which expresses the bispecific T-cell engager (BiTE) FAP/CD3 chemokine ligands 9 and 10 (CXCL9 and CXCL10) and interferon alpha (IFNα).
7. Conclusions and Future Perspectives
The clinical application of therapies based on oncolytic adenoviruses has proven their enormous potential, but is still in its infancy. In particular, the wide-ranging compatibility with already established therapies (without increased toxicity), the multiple cell-killing effects, and the induction of an immunogenic cell death makes it highly attractive in the clinical arena.
Three aspects are of crucial relevance in the generation of novel viral vectors: (a) tissue specificity, (b) replication and lysis capability; and (c) triggering of the immune system in order to achieve a systemic therapy response. There are several sophisticated methods to increase the selectivity of infection of tumour cells to obtain a higher level of safety and efficacy—e.g., by adding certain transgenes. However, selectively replicating oncolytic adenoviruses are often attenuated in their replicative capacity compared to wild type [114]. This not only affects lysis of the tumour but also activation of the immune system. Combination with small molecules, interacting with signalling pathway, such as the JAK/STAT pathway [115], radiation [116], or chemotherapy, are possible strategies to address this issue [117].
The most promising aspect of the development of new adenoviral vectors is the activation of the immune system by the virus, which would represent a vaccination strategy against the tumour. A better understanding of mutually supporting effects of introducing certain transgenes or combining them with immune checkpoint inhibitors will allow novel directions in treatment strategies to increase oncolytic or immune stimulatory efficacy. Several clinical trials are currently underway, and it will gradually become clear which strategies and modifications will provide the best results. An important aspect in these trials will be a thorough patient stratification that addresses the characteristics of the used oncolytic adenovirus. As we have only recently begun to translate the knowledge gained mainly in the laboratory into the clinic, new findings will automatically generate a great deal of interest in the enormous possibilities offered by the great versatility in developing new treatment strategies for cancer with adenoviruses.
Author Contributions
Conceptualization, K.M. and R.N.; Writing—original draft and visualization, K.M., F.G.K., D.W., S.V.H., and M.E.; Writing—review and editing, K.M., R.N., and P.S.H.; Supervision R.N. All authors have read and agreed to the published version of the manuscript.
Funding
F.G.K. is receiving a Ph.D. scholarship from the Stiftung der Deutschen Wirtschaft.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
P.S.H. is co-founder of XVir Therapeutics GmbH. All other authors declare no competing interests.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Chow L.T., Gelinas R.E., Broker T.R., Roberts R.J. An amazing sequence arrangement at the 5′ ends of adenovirus 2 messenger RNA. Cell. 1977;12:1–8. doi: 10.1016/0092-8674(77)90180-5. [DOI] [PubMed] [Google Scholar]
- 2.Kovesdi I., Reichel R., Nevins J.R. Identification of a cellular transcription factor involved in E1A trans-activation. Cell. 1986;45:219–228. doi: 10.1016/0092-8674(86)90386-7. [DOI] [PubMed] [Google Scholar]
- 3.Gallardo J., Perez-Illana M., Martin-Gonzalez N., San Martin C. Adenovirus Structure: What Is New? Int. J. Mol. Sci. 2021;22:5240. doi: 10.3390/ijms22105240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roelvink P.W., Kovesdi I., Wickham T.J. Comparative analysis of adenovirus fiber-cell interaction: Adenovirus type 2 (Ad2) and Ad9 utilize the same cellular fiber receptor but use different binding strategies for attachment. J. Virol. 1996;70:7614–7621. doi: 10.1128/jvi.70.11.7614-7621.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McDonald I., Murray S.M., Reynolds C.J., Altmann D.M., Boyton R.J. Comparative systematic review and meta-analysis of reactogenicity, immunogenicity and efficacy of vaccines against SARS-CoV-2. NPJ Vaccines. 2021;6:74. doi: 10.1038/s41541-021-00336-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ehrhardt A., Haase R., Schepers A., Deutsch M.J., Lipps H.J., Baiker A. Episomal vectors for gene therapy. Curr. Gene Ther. 2008;8:147–161. doi: 10.2174/156652308784746440. [DOI] [PubMed] [Google Scholar]
- 7.Robinson C.M., Singh G., Lee J.Y., Dehghan S., Rajaiya J., Liu E.B., Yousuf M.A., Betensky R.A., Jones M.S., Dyer D.W., et al. Molecular evolution of human adenoviruses. Sci. Rep. 2013;3:1812. doi: 10.1038/srep01812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jones M.S., 2nd, Harrach B., Ganac R.D., Gozum M.M., Dela Cruz W.P., Riedel B., Pan C., Delwart E.L., Schnurr D.P. New adenovirus species found in a patient presenting with gastroenteritis. J. Virol. 2007;81:5978–5984. doi: 10.1128/JVI.02650-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mantwill K., Naumann U., Seznec J., Girbinger V., Lage H., Surowiak P., Beier D., Mittelbronn M., Schlegel J., Holm P.S. YB-1 dependent oncolytic adenovirus efficiently inhibits tumor growth of glioma cancer stem like cells. J. Transl. Med. 2013;11:216. doi: 10.1186/1479-5876-11-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bett A.J., Haddara W., Prevec L., Graham F.L. An efficient and flexible system for construction of adenovirus vectors with insertions or deletions in early regions 1 and 3. Proc. Natl. Acad. Sci. USA. 1994;91:8802–8806. doi: 10.1073/pnas.91.19.8802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gao J., Mese K., Bunz O., Ehrhardt A. State-of-the-art human adenovirus vectorology for therapeutic approaches. FEBS Lett. 2019;593:3609–3622. doi: 10.1002/1873-3468.13691. [DOI] [PubMed] [Google Scholar]
- 12.Graham F.L., Smiley J., Russell W.C., Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J. Gen. Virol. 1977;36:59–74. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- 13.Stephens C., Harlow E. Differential splicing yields novel adenovirus 5 E1A mRNAs that encode 30 kd and 35 kd proteins. EMBO J. 1987;6:2027–2035. doi: 10.1002/j.1460-2075.1987.tb02467.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perricaudet M., Akusjarvi G., Virtanen A., Pettersson U. Structure of two spliced mRNAs from the transforming region of human subgroup C adenoviruses. Nature. 1979;281:694–696. doi: 10.1038/281694a0. [DOI] [PubMed] [Google Scholar]
- 15.King C.R., Zhang A., Tessier T.M., Gameiro S.F., Mymryk J.S. Hacking the Cell: Network Intrusion and Exploitation by Adenovirus E1A. mBio. 2018;9:e00390-18. doi: 10.1128/mBio.00390-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suzuki K., Fueyo J., Krasnykh V., Reynolds P.N., Curiel D.T., Alemany R. A conditionally replicative adenovirus with enhanced infectivity shows improved oncolytic potency. Clin. Cancer Res. 2001;7:120–126. [PubMed] [Google Scholar]
- 17.Zhu Z.B., Makhija S.K., Lu B., Wang M., Rivera A.A., Kim-Park S., Ulasov I.V., Zhou F., Alvarez R.D., Siegal G.P., et al. Incorporating the survivin promoter in an infectivity enhanced CRAd-analysis of oncolysis and anti-tumor effects in vitro and in vivo. Int. J. Oncol. 2005;27:237–246. doi: 10.3892/ijo.27.1.237. [DOI] [PubMed] [Google Scholar]
- 18.Johnson L., Shen A., Boyle L., Kunich J., Pandey K., Lemmon M., Hermiston T., Giedlin M., McCormick F., Fattaey A. Selectively replicating adenoviruses targeting deregulated E2F activity are potent, systemic antitumor agents. Cancer Cell. 2002;1:325–337. doi: 10.1016/S1535-6108(02)00060-0. [DOI] [PubMed] [Google Scholar]
- 19.Banerjee N.S., Rivera A.A., Wang M., Chow L.T., Broker T.R., Curiel D.T., Nettelbeck D.M. Analyses of melanoma-targeted oncolytic adenoviruses with tyrosinase enhancer/promoter-driven E1A, E4, or both in submerged cells and organotypic cultures. Mol. Cancer Ther. 2004;3:437–449. [PubMed] [Google Scholar]
- 20.Leja J., Dzojic H., Gustafson E., Oberg K., Giandomenico V., Essand M. A novel chromogranin-A promoter-driven oncolytic adenovirus for midgut carcinoid therapy. Clin. Cancer Res. 2007;13:2455–2462. doi: 10.1158/1078-0432.CCR-06-2532. [DOI] [PubMed] [Google Scholar]
- 21.Yu D.C., Chen Y., Seng M., Dilley J., Henderson D.R. The addition of adenovirus type 5 region E3 enables calydon virus 787 to eliminate distant prostate tumor xenografts. Cancer Res. 1999;59:4200–4203. [PubMed] [Google Scholar]
- 22.Hallenbeck P.L., Chang Y.N., Hay C., Golightly D., Stewart D., Lin J., Phipps S., Chiang Y.L. A novel tumor-specific replication-restricted adenoviral vector for gene therapy of hepatocellular carcinoma. Hum. Gene Ther. 1999;10:1721–1733. doi: 10.1089/10430349950017725. [DOI] [PubMed] [Google Scholar]
- 23.Weintraub S.J., Prater C.A., Dean D.C. Retinoblastoma protein switches the E2F site from positive to negative element. Nature. 1992;358:259–261. doi: 10.1038/358259a0. [DOI] [PubMed] [Google Scholar]
- 24.Knudsen E.S., Nambiar R., Rosario S.R., Smiraglia D.J., Goodrich D.W., Witkiewicz A.K. Pan-cancer molecular analysis of the RB tumor suppressor pathway. Commun. Biol. 2020;3:158. doi: 10.1038/s42003-020-0873-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fattaey A.R., Harlow E., Helin K. Independent regions of adenovirus E1A are required for binding to and dissociation of E2F-protein complexes. Mol. Cell Biol. 1993;13:7267–7277. doi: 10.1128/MCB.13.12.7267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nevins J.R. Transcriptional regulation. A closer look at E2F. Nature. 1992;358:375–376. doi: 10.1038/358375a0. [DOI] [PubMed] [Google Scholar]
- 27.Whyte P., Ruley H.E., Harlow E. Two regions of the adenovirus early region 1A proteins are required for transformation. J. Virol. 1988;62:257–265. doi: 10.1128/jvi.62.1.257-265.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fueyo J., Gomez-Manzano C., Alemany R., Lee P.S., McDonnell T.J., Mitlianga P., Shi Y.X., Levin V.A., Yung W.K., Kyritsis A.P. A mutant oncolytic adenovirus targeting the Rb pathway produces anti-glioma effect in vivo. Oncogene. 2000;19:2–12. doi: 10.1038/sj.onc.1203251. [DOI] [PubMed] [Google Scholar]
- 29.Zhang W., Cai R., Luo J., Wang Y., Cui Q., Wei X., Zhang H., Qian C. The oncolytic adenovirus targeting to TERT and RB pathway induced specific and potent anti-tumor efficacy in vitro and in vivo for hepatocellular carcinoma. Cancer Biol. Ther. 2007;6:1726–1732. doi: 10.4161/cbt.6.11.4831. [DOI] [PubMed] [Google Scholar]
- 30.Eriksson E., Milenova I., Wenthe J., Stahle M., Leja-Jarblad J., Ullenhag G., Dimberg A., Moreno R., Alemany R., Loskog A. Shaping the Tumor Stroma and Sparking Immune Activation by CD40 and 4-1BB Signaling Induced by an Armed Oncolytic Virus. Clin. Cancer Res. 2017;23:5846–5857. doi: 10.1158/1078-0432.CCR-17-0285. [DOI] [PubMed] [Google Scholar]
- 31.Cascallo M., Alonso M.M., Rojas J.J., Perez-Gimenez A., Fueyo J., Alemany R. Systemic toxicity-efficacy profile of ICOVIR-5, a potent and selective oncolytic adenovirus based on the pRB pathway. Mol. Ther. 2007;15:1607–1615. doi: 10.1038/sj.mt.6300239. [DOI] [PubMed] [Google Scholar]
- 32.Rojas J.J., Cascallo M., Guedan S., Gros A., Martinez-Quintanilla J., Hemminki A., Alemany R. A modified E2F-1 promoter improves the efficacy to toxicity ratio of oncolytic adenoviruses. Gene Ther. 2009;16:1441–1451. doi: 10.1038/gt.2009.103. [DOI] [PubMed] [Google Scholar]
- 33.Rodriguez-Garcia A., Gimenez-Alejandre M., Rojas J.J., Moreno R., Bazan-Peregrino M., Cascallo M., Alemany R. Safety and efficacy of VCN-01, an oncolytic adenovirus combining fiber HSG-binding domain replacement with RGD and hyaluronidase expression. Clin. Cancer Res. 2015;21:1406–1418. doi: 10.1158/1078-0432.CCR-14-2213. [DOI] [PubMed] [Google Scholar]
- 34.Bhat G., SivaRaman L., Murthy S., Domer P., Thimmappaya B. In vivo identification of multiple promoter domains of adenovirus EIIA-late promoter. EMBO J. 1987;6:2045–2052. doi: 10.1002/j.1460-2075.1987.tb02469.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Holm P.S., Bergmann S., Jurchott K., Lage H., Brand K., Ladhoff A., Mantwill K., Curiel D.T., Dobbelstein M., Dietel M., et al. YB-1 relocates to the nucleus in adenovirus-infected cells and facilitates viral replication by inducing E2 gene expression through the E2 late promoter. J. Biol. Chem. 2002;277:10427–10434. doi: 10.1074/jbc.M106955200. [DOI] [PubMed] [Google Scholar]
- 36.Lasham A., Print C.G., Woolley A.G., Dunn S.E., Braithwaite A.W. YB-1: Oncoprotein, prognostic marker and therapeutic target? Biochem. J. 2013;449:11–23. doi: 10.1042/BJ20121323. [DOI] [PubMed] [Google Scholar]
- 37.Bargou R.C., Jurchott K., Wagener C., Bergmann S., Metzner S., Bommert K., Mapara M.Y., Winzer K.J., Dietel M., Dorken B., et al. Nuclear localization and increased levels of transcription factor YB-1 in primary human breast cancers are associated with intrinsic MDR1 gene expression. Nat. Med. 1997;3:447–450. doi: 10.1038/nm0497-447. [DOI] [PubMed] [Google Scholar]
- 38.Dahl E., En-Nia A., Wiesmann F., Krings R., Djudjaj S., Breuer E., Fuchs T., Wild P.J., Hartmann A., Dunn S.E., et al. Nuclear detection of Y-box protein-1 (YB-1) closely associates with progesterone receptor negativity and is a strong adverse survival factor in human breast cancer. BMC Cancer. 2009;9:410. doi: 10.1186/1471-2407-9-410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Heumann A., Kaya O., Burdelski C., Hube-Magg C., Kluth M., Lang D.S., Simon R., Beyer B., Thederan I., Sauter G., et al. Up regulation and nuclear translocation of Y-box binding protein 1 (YB-1) is linked to poor prognosis in ERG-negative prostate cancer. Sci. Rep. 2017;7:2056. doi: 10.1038/s41598-017-02279-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bieler A., Mantwill K., Dravits T., Bernshausen A., Glockzin G., Kohler-Vargas N., Lage H., Gansbacher B., Holm P.S. Novel three-pronged strategy to enhance cancer cell killing in glioblastoma cell lines: Histone deacetylase inhibitor, chemotherapy, and oncolytic adenovirus dl520. Hum. Gene Ther. 2006;17:55–70. doi: 10.1089/hum.2006.17.55. [DOI] [PubMed] [Google Scholar]
- 41.Haley K.P., Overhauser J., Babiss L.E., Ginsberg H.S., Jones N.C. Transformation properties of type 5 adenovirus mutants that differentially express the E1A gene products. Proc. Natl. Acad. Sci. USA. 1984;81:5734–5738. doi: 10.1073/pnas.81.18.5734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rognoni E., Widmaier M., Haczek C., Mantwill K., Holzmuller R., Gansbacher B., Kolk A., Schuster T., Schmid R.M., Saur D., et al. Adenovirus-based virotherapy enabled by cellular YB-1 expression in vitro and in vivo. Cancer Gene Ther. 2009;16:753–763. doi: 10.1038/cgt.2009.20. [DOI] [PubMed] [Google Scholar]
- 43.Czolk R., Schwarz N., Koch H., Schotterl S., Wuttke T.V., Holm P.S., Huber S.M., Naumann U. Irradiation enhances the therapeutic effect of the oncolytic adenovirus XVir-N-31 in brain tumor initiating cells. Int. J. Mol. Med. 2019;44:1484–1494. doi: 10.3892/ijmm.2019.4296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Debbas M., White E. Wild-type p53 mediates apoptosis by E1A, which is inhibited by E1B. Genes Dev. 1993;7:546–554. doi: 10.1101/gad.7.4.546. [DOI] [PubMed] [Google Scholar]
- 45.Barker D.D., Berk A.J. Adenovirus proteins from both E1B reading frames are required for transformation of rodent cells by viral infection and DNA transfection. Virology. 1987;156:107–121. doi: 10.1016/0042-6822(87)90441-7. [DOI] [PubMed] [Google Scholar]
- 46.Bischoff J.R., Kirn D.H., Williams A., Heise C., Horn S., Muna M., Ng L., Nye J.A., Sampson-Johannes A., Fattaey A., et al. An adenovirus mutant that replicates selectively in p53-deficient human tumor cells. Science. 1996;274:373–376. doi: 10.1126/science.274.5286.373. [DOI] [PubMed] [Google Scholar]
- 47.Harada J.N., Berk A.J. p53-Independent and -dependent requirements for E1B-55K in adenovirus type 5 replication. J. Virol. 1999;73:5333–5344. doi: 10.1128/JVI.73.7.5333-5344.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.O’Shea C.C., Johnson L., Bagus B., Choi S., Nicholas C., Shen A., Boyle L., Pandey K., Soria C., Kunich J., et al. Late viral RNA export, rather than p53 inactivation, determines ONYX-015 tumor selectivity. Cancer Cell. 2004;6:611–623. doi: 10.1016/j.ccr.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 49.Yu W., Fang H. Clinical trials with oncolytic adenovirus in China. Curr. Cancer Drug Targets. 2007;7:141–148. doi: 10.2174/156800907780058817. [DOI] [PubMed] [Google Scholar]
- 50.Prestwich R.J., Errington F., Diaz R.M., Pandha H.S., Harrington K.J., Melcher A.A., Vile R.G. The case of oncolytic viruses versus the immune system: Waiting on the judgment of Solomon. Hum. Gene Ther. 2009;20:1119–1132. doi: 10.1089/hum.2009.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cervera-Carrascon V., Havunen R., Hemminki A. Oncolytic adenoviruses: A game changer approach in the battle between cancer and the immune system. Expert Opin. Biol. Ther. 2019;19:443–455. doi: 10.1080/14712598.2019.1595582. [DOI] [PubMed] [Google Scholar]
- 52.Cunliffe T.G., Bates E.A., Parker A.L. Hitting the Target but Missing the Point: Recent Progress towards Adenovirus-Based Precision Virotherapies. Cancers. 2020;12:3327. doi: 10.3390/cancers12113327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Galon J., Bruni D. Approaches to treat immune hot, altered and cold tumours with combination immunotherapies. Nat. Rev. Drug Discov. 2019;18:197–218. doi: 10.1038/s41573-018-0007-y. [DOI] [PubMed] [Google Scholar]
- 54.Davola M.E., Mossman K.L. Oncolytic viruses: How “lytic” must they be for therapeutic efficacy? Oncoimmunology. 2019;8:e1581528. doi: 10.1080/2162402X.2019.1596006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu T.C., Hallden G., Wang Y., Brooks G., Francis J., Lemoine N., Kirn D. An E1B-19 kDa gene deletion mutant adenovirus demonstrates tumor necrosis factor-enhanced cancer selectivity and enhanced oncolytic potency. Mol. Ther. 2004;9:786–803. doi: 10.1016/j.ymthe.2004.03.017. [DOI] [PubMed] [Google Scholar]
- 56.Sauthoff H., Heitner S., Rom W.N., Hay J.G. Deletion of the adenoviral E1b-19kD gene enhances tumor cell killing of a replicating adenoviral vector. Hum. Gene Ther. 2000;11:379–388. doi: 10.1089/10430340050015851. [DOI] [PubMed] [Google Scholar]
- 57.Blackburn R.V., Galoforo S.S., Corry P.M., Lee Y.J. Adenoviral-mediated transfer of a heat-inducible double suicide gene into prostate carcinoma cells. Cancer Res. 1998;58:1358–1362. [PubMed] [Google Scholar]
- 58.Pelletier J., Sonenberg N. Internal initiation of translation of eukaryotic mRNA directed by a sequence derived from poliovirus RNA. Nature. 1988;334:320–325. doi: 10.1038/334320a0. [DOI] [PubMed] [Google Scholar]
- 59.Ryan M.D., King A.M., Thomas G.P. Cleavage of foot-and-mouth disease virus polyprotein is mediated by residues located within a 19 amino acid sequence. J. Gen. Virol. 1991;72:2727–2732. doi: 10.1099/0022-1317-72-11-2727. [DOI] [PubMed] [Google Scholar]
- 60.Hawkins L.K., Hermiston T. Gene delivery from the E3 region of replicating human adenovirus: Evaluation of the E3B region. Gene Ther. 2001;8:1142–1148. doi: 10.1038/sj.gt.3301509. [DOI] [PubMed] [Google Scholar]
- 61.Suzuki K., Alemany R., Yamamoto M., Curiel D.T. The presence of the adenovirus E3 region improves the oncolytic potency of conditionally replicative adenoviruses. Clin. Cancer Res. 2002;8:3348–3359. [PubMed] [Google Scholar]
- 62.Robinson M., Ge Y., Ko D., Yendluri S., Laflamme G., Hawkins L., Jooss K. Comparison of the E3 and L3 regions for arming oncolytic adenoviruses to achieve a high level of tumor-specific transgene expression. Cancer Gene Ther. 2008;15:9–17. doi: 10.1038/sj.cgt.7701093. [DOI] [PubMed] [Google Scholar]
- 63.Sauthoff H., Pipiya T., Heitner S., Chen S., Norman R.G., Rom W.N., Hay J.G. Late expression of p53 from a replicating adenovirus improves tumor cell killing and is more tumor cell specific than expression of the adenoviral death protein. Hum. Gene Ther. 2002;13:1859–1871. doi: 10.1089/104303402760372954. [DOI] [PubMed] [Google Scholar]
- 64.Zhang J., Kale V., Chen M. Gene-directed enzyme prodrug therapy. AAPS J. 2015;17:102–110. doi: 10.1208/s12248-014-9675-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Freytag S.O., Stricker H., Pegg J., Paielli D., Pradhan D.G., Peabody J., DePeralta-Venturina M., Xia X., Brown S., Lu M., et al. Phase I study of replication-competent adenovirus-mediated double-suicide gene therapy in combination with conventional-dose three-dimensional conformal radiation therapy for the treatment of newly diagnosed, intermediate- to high-risk prostate cancer. Cancer Res. 2003;63:7497–7506. [PubMed] [Google Scholar]
- 66.Brachtlova T., van Ginkel J.-W., Luinenburg M.J., de Menezes R.X., Koppers-Lalic D., Pegtel D.M., Dong W., de Gruijl T.D., van Beusechem V.W. Expression of Oncolytic Adenovirus-encoded RNAi Molecules is most effective in a pri-miRNA precursor format. Mol. Ther. Oncolytics. 2020;19:332–343. doi: 10.1016/j.omto.2020.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Guedan S., Gros A., Cascallo M., Vile R., Mercade E., Alemany R. Syncytia formation affects the yield and cytotoxicity of an adenovirus expressing a fusogenic glycoprotein at a late stage of replication. Gene Ther. 2008;15:1240–1245. doi: 10.1038/gt.2008.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gros A., Martinez-Quintanilla J., Puig C., Guedan S., Mollevi D.G., Alemany R., Cascallo M. Bioselection of a gain of function mutation that enhances adenovirus 5 release and improves its antitumoral potency. Cancer Res. 2008;68:8928–8937. doi: 10.1158/0008-5472.CAN-08-1145. [DOI] [PubMed] [Google Scholar]
- 69.Jiang H., Rivera-Molina Y., Gomez-Manzano C., Clise-Dwyer K., Bover L., Vence L.M., Yuan Y., Lang F.F., Toniatti C., Hossain M.B., et al. Oncolytic Adenovirus and Tumor-Targeting Immune Modulatory Therapy Improve Autologous Cancer Vaccination. Cancer Res. 2017;77:3894–3907. doi: 10.1158/0008-5472.CAN-17-0468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bristol J.A., Zhu M., Ji H., Mina M., Xie Y., Clarke L., Forry-Schaudies S., Ennist D.L. In vitro and in vivo activities of an oncolytic adenoviral vector designed to express GM-CSF. Mol. Ther. 2003;7:755–764. doi: 10.1016/S1525-0016(03)00103-5. [DOI] [PubMed] [Google Scholar]
- 71.Lynch D.H. The promise of 4-1BB (CD137)-mediated immunomodulation and the immunotherapy of cancer. Immunol. Rev. 2008;222:277–286. doi: 10.1111/j.1600-065X.2008.00621.x. [DOI] [PubMed] [Google Scholar]
- 72.Liikanen I., Tahtinen S., Guse K., Gutmann T., Savola P., Oksanen M., Kanerva A., Hemminki A. Oncolytic Adenovirus Expressing Monoclonal Antibody Trastuzumab for Treatment of HER2-Positive Cancer. Mol. Cancer Ther. 2016;15:2259–2269. doi: 10.1158/1535-7163.MCT-15-0819. [DOI] [PubMed] [Google Scholar]
- 73.Dias J.D., Hemminki O., Diaconu I., Hirvinen M., Bonetti A., Guse K., Escutenaire S., Kanerva A., Pesonen S., Loskog A., et al. Targeted cancer immunotherapy with oncolytic adenovirus coding for a fully human monoclonal antibody specific for CTLA-4. Gene Ther. 2012;19:988–998. doi: 10.1038/gt.2011.176. [DOI] [PubMed] [Google Scholar]
- 74.Tanoue K., Rosewell Shaw A., Watanabe N., Porter C., Rana B., Gottschalk S., Brenner M., Suzuki M. Armed Oncolytic Adenovirus-Expressing PD-L1 Mini-Body Enhances Antitumor Effects of Chimeric Antigen Receptor T Cells in Solid Tumors. Cancer Res. 2017;77:2040–2051. doi: 10.1158/0008-5472.CAN-16-1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Suzuki M., Singh R., Moore M.A., Song W.R., Crystal R.G. Similarity of strain- and route-dependent murine responses to an adenovirus vector using the homologous thrombopoietin cDNA as the reporter genes. Hum. Gene Ther. 1998;9:1223–1231. doi: 10.1089/hum.1998.9.8-1223. [DOI] [PubMed] [Google Scholar]
- 76.Worgall S., Wolff G., Falck-Pedersen E., Crystal R.G. Innate immune mechanisms dominate elimination of adenoviral vectors following in vivo administration. Hum. Gene Ther. 1997;8:37–44. doi: 10.1089/hum.1997.8.1-37. [DOI] [PubMed] [Google Scholar]
- 77.Tao N., Gao G.P., Parr M., Johnston J., Baradet T., Wilson J.M., Barsoum J., Fawell S.E. Sequestration of adenoviral vector by Kupffer cells leads to a nonlinear dose response of transduction in liver. Mol. Ther. 2001;3:28–35. doi: 10.1006/mthe.2000.0227. [DOI] [PubMed] [Google Scholar]
- 78.Alemany R., Suzuki K., Curiel D.T. Blood clearance rates of adenovirus type 5 in mice. J. Gen. Virol. 2000;81:2605–2609. doi: 10.1099/0022-1317-81-11-2605. [DOI] [PubMed] [Google Scholar]
- 79.Wood M., Perrotte P., Onishi E., Harper M.E., Dinney C., Pagliaro L., Wilson D.R. Biodistribution of an adenoviral vector carrying the luciferase reporter gene following intravesical or intravenous administration to a mouse. Cancer Gene Ther. 1999;6:367–372. doi: 10.1038/sj.cgt.7700090. [DOI] [PubMed] [Google Scholar]
- 80.Yang Y., Nunes F.A., Berencsi K., Furth E.E., Gonczol E., Wilson J.M. Cellular immunity to viral antigens limits E1-deleted adenoviruses for gene therapy. Proc. Natl. Acad. Sci. USA. 1994;91:4407–4411. doi: 10.1073/pnas.91.10.4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tomko R.P., Xu R., Philipson L. HCAR and MCAR: The human and mouse cellular receptors for subgroup C adenoviruses and group B coxsackieviruses. Proc. Natl. Acad. Sci. USA. 1997;94:3352–3356. doi: 10.1073/pnas.94.7.3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wickham T.J., Mathias P., Cheresh D.A., Nemerow G.R. Integrins alpha v beta 3 and alpha v beta 5 promote adenovirus internalization but not virus attachment. Cell. 1993;73:309–319. doi: 10.1016/0092-8674(93)90231-E. [DOI] [PubMed] [Google Scholar]
- 83.Medina-Kauwe L.K. Endocytosis of adenovirus and adenovirus capsid proteins. Adv. Drug Deliv. Rev. 2003;55:1485–1496. doi: 10.1016/j.addr.2003.07.010. [DOI] [PubMed] [Google Scholar]
- 84.Fechner H., Haack A., Wang H., Wang X., Eizema K., Pauschinger M., Schoemaker R., Veghel R., Houtsmuller A., Schultheiss H.P., et al. Expression of coxsackie adenovirus receptor and alphav-integrin does not correlate with adenovector targeting in vivo indicating anatomical vector barriers. Gene Ther. 1999;6:1520–1535. doi: 10.1038/sj.gt.3301030. [DOI] [PubMed] [Google Scholar]
- 85.Rivera A.A., Davydova J., Schierer S., Wang M., Krasnykh V., Yamamoto M., Curiel D.T., Nettelbeck D.M. Combining high selectivity of replication with fiber chimerism for effective adenoviral oncolysis of CAR-negative melanoma cells. Gene Ther. 2004;11:1694–1702. doi: 10.1038/sj.gt.3302346. [DOI] [PubMed] [Google Scholar]
- 86.Dmitriev I., Krasnykh V., Miller C.R., Wang M., Kashentseva E., Mikheeva G., Belousova N., Curiel D.T. An adenovirus vector with genetically modified fibers demonstrates expanded tropism via utilization of a coxsackievirus and adenovirus receptor-independent cell entry mechanism. J. Virol. 1998;72:9706–9713. doi: 10.1128/JVI.72.12.9706-9713.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Krasnykh V., Dmitriev I., Mikheeva G., Miller C.R., Belousova N., Curiel D.T. Characterization of an adenovirus vector containing a heterologous peptide epitope in the HI loop of the fiber knob. J. Virol. 1998;72:1844–1852. doi: 10.1128/JVI.72.3.1844-1852.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Blackhall F.H., Merry C.L., Davies E.J., Jayson G.C. Heparan sulfate proteoglycans and cancer. Br. J. Cancer. 2001;85:1094–1098. doi: 10.1054/bjoc.2001.2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Einfeld D.A., Schroeder R., Roelvink P.W., Lizonova A., King C.R., Kovesdi I., Wickham T.J. Reducing the native tropism of adenovirus vectors requires removal of both CAR and integrin interactions. J. Virol. 2001;75:11284–11291. doi: 10.1128/JVI.75.23.11284-11291.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Koizumi N., Mizuguchi H., Sakurai F., Yamaguchi T., Watanabe Y., Hayakawa T. Reduction of natural adenovirus tropism to mouse liver by fiber-shaft exchange in combination with both CAR- and alphav integrin-binding ablation. J. Virol. 2003;77:13062–13072. doi: 10.1128/JVI.77.24.13062-13072.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Krasnykh V.N., Mikheeva G.V., Douglas J.T., Curiel D.T. Generation of recombinant adenovirus vectors with modified fibers for altering viral tropism. J. Virol. 1996;70:6839–6846. doi: 10.1128/jvi.70.10.6839-6846.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sirena D., Lilienfeld B., Eisenhut M., Kalin S., Boucke K., Beerli R.R., Vogt L., Ruedl C., Bachmann M.F., Greber U.F., et al. The human membrane cofactor CD46 is a receptor for species B adenovirus serotype 3. J. Virol. 2004;78:4454–4462. doi: 10.1128/JVI.78.9.4454-4462.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Douglas J.T., Rogers B.E., Rosenfeld M.E., Michael S.I., Feng M., Curiel D.T. Targeted gene delivery by tropism-modified adenoviral vectors. Nat. Biotechnol. 1996;14:1574–1578. doi: 10.1038/nbt1196-1574. [DOI] [PubMed] [Google Scholar]
- 94.Haisma H.J., Grill J., Curiel D.T., Hoogeland S., van Beusechem V.W., Pinedo H.M., Gerritsen W.R. Targeting of adenoviral vectors through a bispecific single-chain antibody. Cancer Gene Ther. 2000;7:901–904. doi: 10.1038/sj.cgt.7700198. [DOI] [PubMed] [Google Scholar]
- 95.Nettelbeck D.M., Rivera A.A., Kupsch J., Dieckmann D., Douglas J.T., Kontermann R.E., Alemany R., Curiel D.T. Retargeting of adenoviral infection to melanoma: Combining genetic ablation of native tropism with a recombinant bispecific single-chain diabody (scDb) adapter that binds to fiber knob and HMWMAA. Int. J. Cancer. 2004;108:136–145. doi: 10.1002/ijc.11563. [DOI] [PubMed] [Google Scholar]
- 96.Holliger P., Prospero T., Winter G. “Diabodies”: Small bivalent and bispecific antibody fragments. Proc. Natl. Acad. Sci. USA. 1993;90:6444–6448. doi: 10.1073/pnas.90.14.6444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nord K., Gunneriusson E., Ringdahl J., Stahl S., Uhlen M., Nygren P.A. Binding proteins selected from combinatorial libraries of an alpha-helical bacterial receptor domain. Nat. Biotechnol. 1997;15:772–777. doi: 10.1038/nbt0897-772. [DOI] [PubMed] [Google Scholar]
- 98.Henning P., Magnusson M.K., Gunneriusson E., Hong S.S., Boulanger P., Nygren P.A., Lindholm L. Genetic modification of adenovirus 5 tropism by a novel class of ligands based on a three-helix bundle scaffold derived from staphylococcal protein A. Hum. Gene Ther. 2002;13:1427–1439. doi: 10.1089/10430340260185067. [DOI] [PubMed] [Google Scholar]
- 99.Ahmed A.U., Thaci B., Tobias A.L., Auffinger B., Zhang L., Cheng Y., Kim C.K., Yunis C., Han Y., Alexiades N.G., et al. A preclinical evaluation of neural stem cell-based cell carrier for targeted antiglioma oncolytic virotherapy. J. Natl. Cancer Inst. 2013;105:968–977. doi: 10.1093/jnci/djt141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yoon A.R., Hong J., Li Y., Shin H.C., Lee H., Kim H.S., Yun C.O. Mesenchymal Stem Cell-Mediated Delivery of an Oncolytic Adenovirus Enhances Antitumor Efficacy in Hepatocellular Carcinoma. Cancer Res. 2019;79:4503–4514. doi: 10.1158/0008-5472.CAN-18-3900. [DOI] [PubMed] [Google Scholar]
- 101.Ahmed A.U., Thaci B., Alexiades N.G., Han Y., Qian S., Liu F., Balyasnikova I.V., Ulasov I.Y., Aboody K.S., Lesniak M.S. Neural stem cell-based cell carriers enhance therapeutic efficacy of an oncolytic adenovirus in an orthotopic mouse model of human glioblastoma. Mol. Ther. 2011;19:1714–1726. doi: 10.1038/mt.2011.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Goradel N.H., Mohajel N., Malekshahi Z.V., Jahangiri S., Najafi M., Farhood B., Mortezaee K., Negahdari B., Arashkia A. Oncolytic adenovirus: A tool for cancer therapy in combination with other therapeutic approaches. J. Cell Physiol. 2019;234:8636–8646. doi: 10.1002/jcp.27850. [DOI] [PubMed] [Google Scholar]
- 103.Kawashima T., Kagawa S., Kobayashi N., Shirakiya Y., Umeoka T., Teraishi F., Taki M., Kyo S., Tanaka N., Fujiwara T. Telomerase-specific replication-selective virotherapy for human cancer. Clin. Cancer Res. 2004;10:285–292. doi: 10.1158/1078-0432.CCR-1075-3. [DOI] [PubMed] [Google Scholar]
- 104.Nemunaitis J., Tong A.W., Nemunaitis M., Senzer N., Phadke A.P., Bedell C., Adams N., Zhang Y.A., Maples P.B., Chen S., et al. A phase I study of telomerase-specific replication competent oncolytic adenovirus (telomelysin) for various solid tumors. Mol. Ther. 2010;18:429–434. doi: 10.1038/mt.2009.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Jakubczak J.L., Ryan P., Gorziglia M., Clarke L., Hawkins L.K., Hay C., Huang Y., Kaloss M., Marinov A., Phipps S., et al. An oncolytic adenovirus selective for retinoblastoma tumor suppressor protein pathway-defective tumors: Dependence on E1A, the E2F-1 promoter, and viral replication for selectivity and efficacy. Cancer Res. 2003;63:1490–1499. [PubMed] [Google Scholar]
- 106.Packiam V.T., Lamm D.L., Barocas D.A., Trainer A., Fand B., Davis R.L., III, Clark W., Kroeger M., Dumbadze I., Chamie K. An open label, single-arm, phase II multicenter study of the safety and efficacy of CG0070 oncolytic vector regimen in patients with BCG-unresponsive non-muscle-invasive bladder cancer: Interim results. Urol. Oncol. Semin. Orig. Investig. 2018;36:440–447. doi: 10.1016/j.urolonc.2017.07.005. [DOI] [PubMed] [Google Scholar]
- 107.Lang F.F., Conrad C., Gomez-Manzano C., Yung W.K.A., Sawaya R., Weinberg J.S., Prabhu S.S., Rao G., Fuller G.N., Aldape K.D., et al. Phase I Study of DNX-2401 (Delta-24-RGD) Oncolytic Adenovirus: Replication and Immunotherapeutic Effects in Recurrent Malignant Glioma. J. Clin. Oncol. 2018;36:1419–1427. doi: 10.1200/JCO.2017.75.8219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ranki T., Pesonen S., Hemminki A., Partanen K., Kairemo K., Alanko T., Lundin J., Linder N., Turkki R., Ristimaki A., et al. Phase I study with ONCOS-102 for the treatment of solid tumors—An evaluation of clinical response and exploratory analyses of immune markers. J. Immunother. Cancer. 2016;4:17. doi: 10.1186/s40425-016-0121-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Garcia M., Moreno R., Gil-Martin M., Cascallo M., de Olza M.O., Cuadra C., Piulats J.M., Navarro V., Domenech M., Alemany R., et al. A Phase 1 Trial of Oncolytic Adenovirus ICOVIR-5 Administered Intravenously to Cutaneous and Uveal Melanoma Patients. Hum. Gene Ther. 2019;30:352–364. doi: 10.1089/hum.2018.107. [DOI] [PubMed] [Google Scholar]
- 110.Pascual-Pasto G., Bazan-Peregrino M., Olaciregui N.G., Restrepo-Perdomo C.A., Mato-Berciano A., Ottaviani D., Weber K., Correa G., Paco S., Vila-Ubach M., et al. Therapeutic targeting of the RB1 pathway in retinoblastoma with the oncolytic adenovirus VCN-01. Sci. Transl. Med. 2019;11:eaat9321. doi: 10.1126/scitranslmed.aat9321. [DOI] [PubMed] [Google Scholar]
- 111.Ganly I., Kirn D., Eckhardt G., Rodriguez G.I., Soutar D.S., Otto R., Robertson A.G., Park O., Gulley M.L., Heise C., et al. A phase I study of Onyx-015, an E1B attenuated adenovirus, administered intratumorally to patients with recurrent head and neck cancer. Clin. Cancer Res. 2000;6:798–806. [PubMed] [Google Scholar]
- 112.Liang M. Oncorine, the World First Oncolytic Virus Medicine and its Update in China. Curr. Cancer Drug Targets. 2018;18:171–176. doi: 10.2174/1568009618666171129221503. [DOI] [PubMed] [Google Scholar]
- 113.Kuhn I., Harden P., Bauzon M., Chartier C., Nye J., Thorne S., Reid T., Ni S., Lieber A., Fisher K., et al. Directed evolution generates a novel oncolytic virus for the treatment of colon cancer. PLoS ONE. 2008;3:e2409. doi: 10.1371/journal.pone.0002409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Heise C., Sampson-Johannes A., Williams A., McCormick F., Von Hoff D.D., Kirn D.H. ONYX-015, an E1B gene-attenuated adenovirus, causes tumor-specific cytolysis and antitumoral efficacy that can be augmented by standard chemotherapeutic agents. Nat. Med. 1997;3:639–645. doi: 10.1038/nm0697-639. [DOI] [PubMed] [Google Scholar]
- 115.Hindupur S.V., Schmid S.C., Koch J.A., Youssef A., Baur E.M., Wang D., Horn T., Slotta-Huspenina J., Gschwend J.E., Holm P.S., et al. STAT3/5 Inhibitors Suppress Proliferation in Bladder Cancer and Enhance Oncolytic Adenovirus Therapy. Int. J. Mol. Sci. 2020;21:1106. doi: 10.3390/ijms21031106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Nandi S., Ulasov I.V., Tyler M.A., Sugihara A.Q., Molinero L., Han Y., Zhu Z.B., Lesniak M.S. Low-dose radiation enhances survivin-mediated virotherapy against malignant glioma stem cells. Cancer Res. 2008;68:5778–5784. doi: 10.1158/0008-5472.CAN-07-6441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kumar S., Gao L., Yeagy B., Reid T. Virus combinations and chemotherapy for the treatment of human cancers. Curr. Opin. Mol. Ther. 2008;10:371–379. [PubMed] [Google Scholar]