Abstract
Though Morusin isolated from the root of Morus alba was known to have antioxidant, anti-inflammatory, antiangiogenic, antimigratory, and apoptotic effects, the underlying antitumor effect of Morusin is not fully understood on the glycolysis of liver cancers. Hence, in the current study, the antitumor mechanism of Morusin was explored in Hep3B and Huh7 hepatocellular carcninomas (HCC) in association with glycolysis and G1 arrest. Herein, Morusin significantly reduced the viability and the number of colonies in Hep3B and Huh7 cells. Moreover, Morusin significantly increased G1 arrest, attenuated the expression of cyclin D1, cyclin D3, cyclin E, cyclin-dependent kinase 2 (CDK2), cyclin-dependent kinase 4 (CDK4), and cyclin-dependent kinase 6 (CDK6) and upregulated p21 and p27 in Hep3B and Huh7 cells. Interestingly, Morusin significantly activated phosphorylation of the adenosine 5′-monophosphate (AMP)-activated protein kinase (AMPK)/acetyl-CoA carboxylase (ACC) but attenuated the expression of the p-mammalian target of protein kinase B (AKT), rapamycin (mTOR), c-Myc, hexokinase 2(HK2), pyruvate kinases type M2 (PKM2), and lactate dehydrogenase (LDH) in Hep3B and Huh7 cells. Consistently, Morusin suppressed lactate, glucose, and adenosine triphosphate (ATP) in Hep3B and Huh7 cells. Conversely, the AMPK inhibitor compound C reduced the ability of Morusin to activate AMPK and attenuate the expression of p-mTOR, HK2, PKM2, and LDH-A and suppressed G1 arrest induced by Morusin in Hep3B cells. Overall, these findings suggest that Morusin exerts an antitumor effect in HCCs via AMPK mediated G1 arrest and antiglycolysis as a potent dietary anticancer candidate.
Keywords: Morusin, G1 arrest, glycolysis, AMPK, liver cancer
1. Introduction
Hepatocellular cancers (HCCs) are known the most common type of primary liver cancers worldwide. Though chemotherapy, radiotherapy, immunotherapy, and surgery have been used for treatment of HCCs for years [1], antiglycolytic agents including 3-bromopyruvate are of interest for the treatment of HCCs in combination with classical anticancer agents such as sorafenib or alone [2,3].
Emerging evidence reveals that metabolic intermediates of glycolysis, known as “the process of conversion of glucose into pyruvate followed by lactate production”, facilitate cancer proliferation and progression [4,5], which was defined as the Warburg effect [6,7]. Among several cancers, liver cancer progression and resistance are more associated with metabolic glycolysis [8], since aerobic glycolysis induces an acidic environment to aid proliferation, angiogenesis, invasion, metastasis, and immune evasion in HCC [9].
Accumulating evidence indicates that phosphofructokinase 1 (PFK1), hexokinase 2 (HK2), and pyruvate kinases type M2 (PKM2) are critically involved in aerobic glycolysis in HCC in association with other signaling networks such as the PI3K/Akt/mTOR pathway, HIF-1α, c-Myc, AMPK, and noncoding RNAs [10].
Recently, natural compounds are of interest as potent adjuvants to cancer therapy with lower toxicity and fewer side effects [11]. Indeed, several natural compounds such as muscone [12], oleanolic acid [13], isobavachalcone [14], baicalein [15], astrakurkurone [16], and others were found to have anticancer potential in liver cancers [17]. Likewise, though Morusin derived from the roots of Morus alba was found to have anticancer effects in several cancers, the underlying antitumor mechanisms still remain unclear. Hence, in the present study, the antitumor mechanism of Morusin was examined in Hep 3B and Huh7 HCCs in association with cell cycle arrest and glycolysis related signaling.
2. Results
2.1. Morusin Increases Cytotoxicity and Induces G1 Arrest in Huh7 and Hep3B Cells
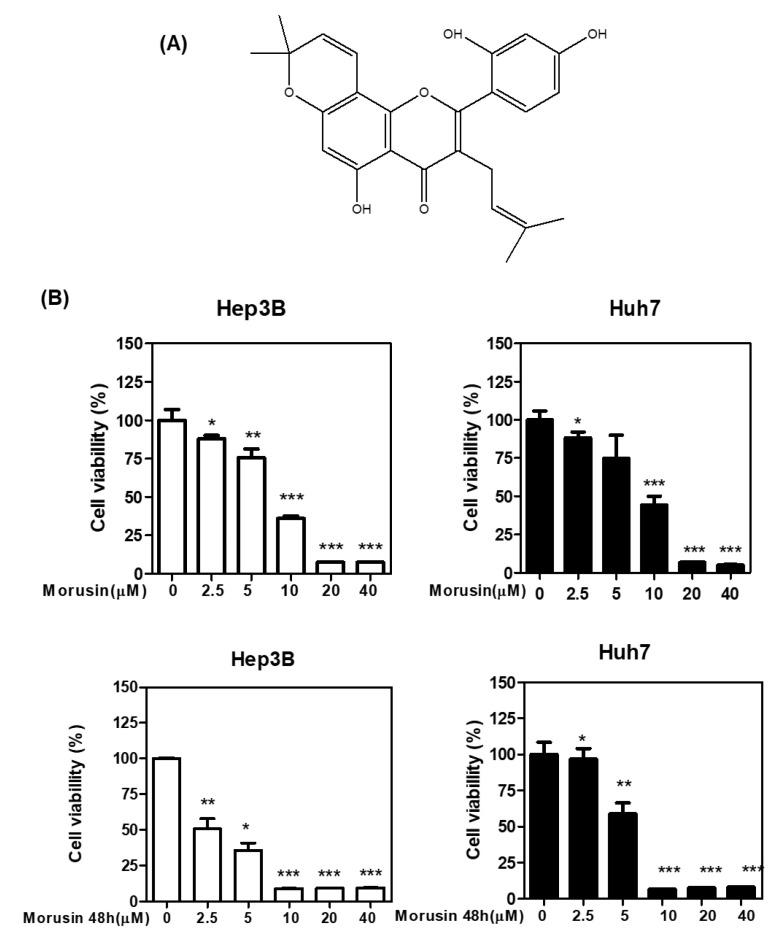
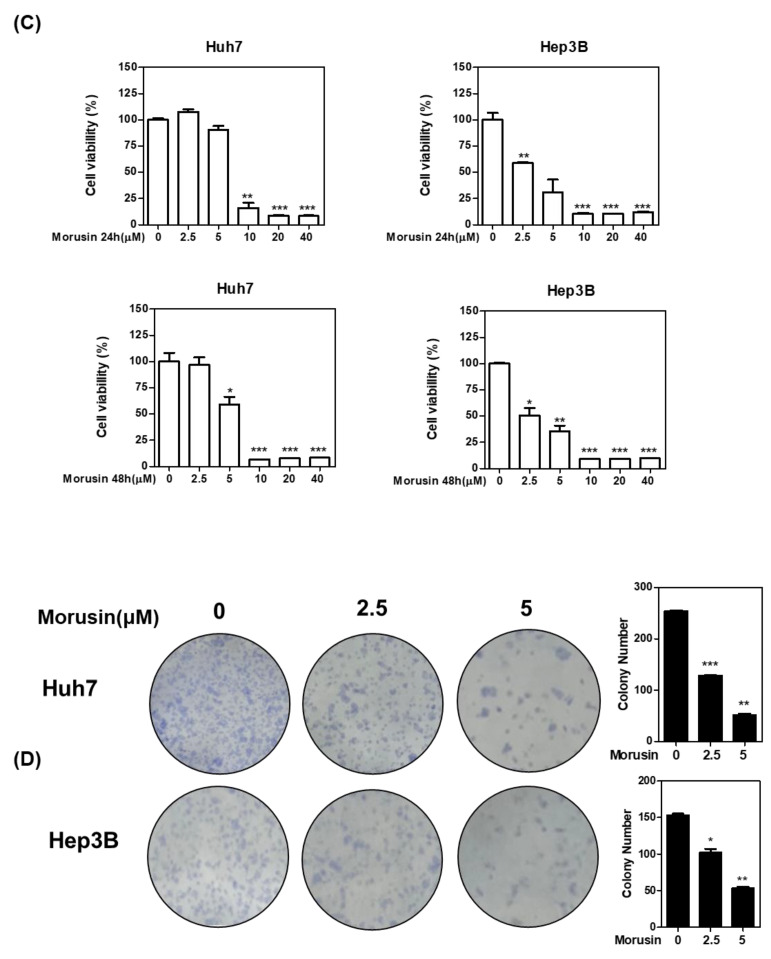
To check the cytotoxicity of Morusin (Figure 1A), MTT, CCK-8, and colony formation assays were carried out in Huh7 and Hep3B cells. As shown in Figure 1B,C, Morusin significantly reduced the viability of Huh7 and Hep3B cells in a concentration and time dependent manner by MTT and CCK-8 assay. Consistently, Morusin significantly abrogated the number of colonies in Huh7 and Hep3B cells by colony formation assay as a long-term cytotoxicity assay (Figure 1D).
Figure 1.
Chemical structure of Morusin and its effect on cytotoxicity in HCCs. (A) Chemical structure of Morusin (B) Effect of Morusin on the viability of Huh7 and Hep3B cells by MTT assay. Huh7 and Hep3B cells were seeded into 96-well microplates and treated with various concentrations (2.5, 5, 10, 20, and 40 µM) of Morusin for 24 h and 48 h. Cell viability was measured by MTT assay. * p < 0.05, **, p < 0.01, ***, p < 0.001 vs. untreated control. (C) Effect of Morusin on Huh7 and Hep3B cells. Cells were cultured with Morusin (0–40 μM) for 24 h and 48 h and then measured by CCK-8 assay (D) Effect of Morusin on the number of colonies in Hep3B cells by colony formation assay. Huh7 and Hep3B cells were seeded onto 6-well plates for a week. Then the colonies stained with crystal violet were counted. Data represent means ± SD. *, p < 0.05, **, p < 0.01, ***, p < 0.001 vs. untreated control (n = 3).
2.2. Morusin Increases G1 Arrest and Modulates G1 Related Proteins in Huh7 and Hep3B Cells
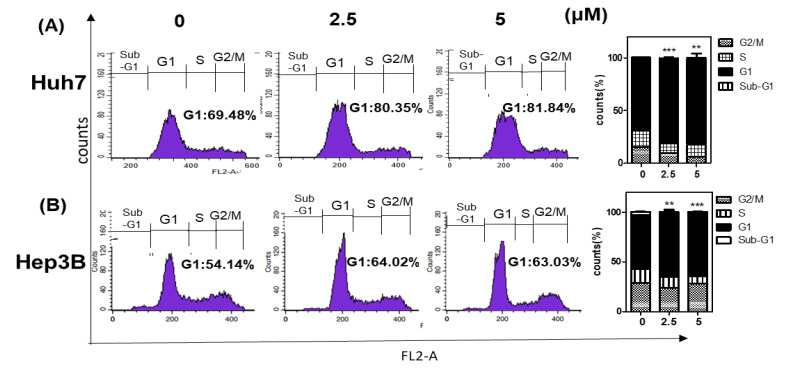
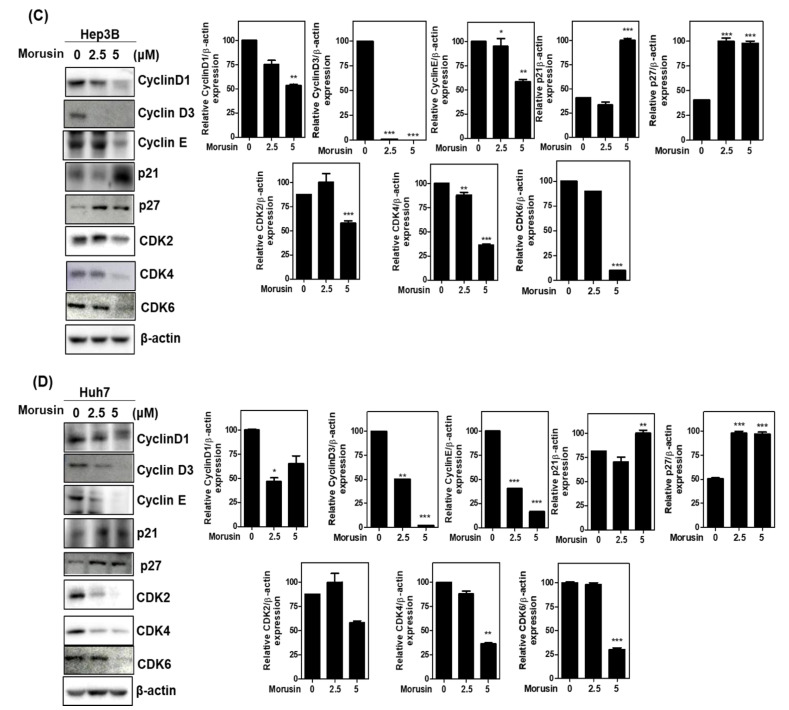
To test whether the cytotoxicity of Morusin was due to cell cycle arrest, cell cycle analysis was performed in Huh7 and Hep3B cells. As shown in Figure 2A,B, Morusin significantly increased G1 portion compared to the untreated control in Huh7 and Hep3B cells (Figure 2A,B). Hence, the effect of Morusin was evaluated in Huh7 and Hep3B cells by Western blotting. Here Morusin significantly abrogated the expression of Cyclin D1, CyclinD3, Cyclin E, CDK2, CDK4, and CDK6 and upregulated p21 and p27 in Huh7 and Hep3B cells (Figure 2C,D).
Figure 2.
Effect of Morusin on G1 arrest and its related proteins in Huh7 and Hep3B cells. (A) Effect of Morusin on G1 arrest in Hep3B cells. *, p < 0.05, ***, p < 0.001 vs. untreated control. (B) Effect of Morusin on G1 arrest in Huh7 cells. Huh7 and Hep3B cells exposed to Morusin (2.5, 5 μM) for 24 h were incubated with RNase A (10 mg/mL) for 1 h at 37 °C and stained with propidium iodide (50 μg/mL) for 30 min at room temperature in the dark. The stained cells were analyzed for DNA content by FACSCalibur. **, p <0.01, ***, p <0.001 vs. untreated control (C) Effect of Morusin on G1 related proteins in Hep3B cells *, p < 0.05, **, p < 0.01, ***, p < 0.001 vs. untreated control. (D) Effect of Morusin on G1 related proteins in Huh7 cells. Huh7 and Hep3B cells were treated with Morusin (2.5, 5 μM) for 24 h. Cell extracts were prepared and subjected to Western blotting with Cyclin D1, Cyclin D3, Cyclin E, p21, p27, CDK2, CDK4, and CDK6 antibodies. β-actin was used as an internal control. Data represent means ± SD. *, p < 0.05, **, p < 0.01, ***, p < 0.001 vs. untreated control (n = 3).
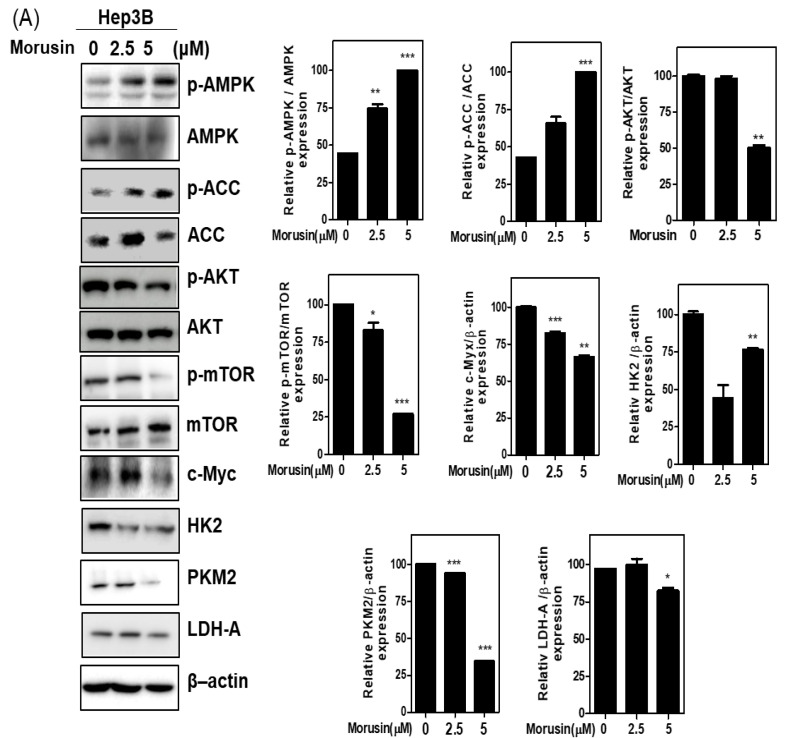
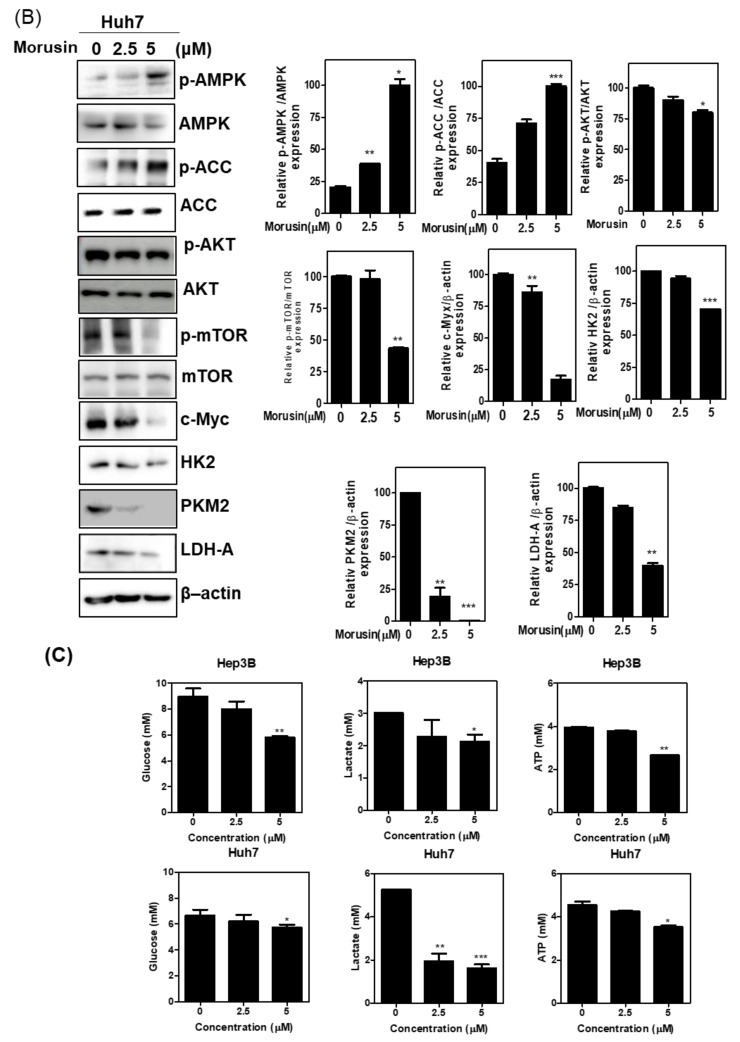
2.3. Morusin Reduces Glycolysis Related Proteins in Huh7 and Hep3B Cells
It is well documented that cancer cells are more active in glycolysis to obtain more ATP for metabolic activity than normal cells, which is named Warburg [18]. Thus, to check the effect of Morusin on glycolysis related proteins, Western blotting was performed in in Huh7 and Hep3B cells. Herein, Morusin significantly activated phosphorylation of AMPK/ACC but attenuated the expression of p-mTOR, p-AKT, cMyc, HK2, PKM2, and LDH-A in Huh7 and Hep3B cells (Figure 3A,B). Consistently, Morusin suppressed lactate, glucose, and ATP in Hep3B and Huh7 cells (Figure 3C).
Figure 3.
Effect of Morusin on glycolysis related proteins in Hep3B (A) and Huh7 (B) cells. Huh7 and Hep3B cells were treated with Morusin (2.5, 5 μM) for 24 h. Cell extracts were prepared and subjected to Western blotting with the antibodies of p-AMPK, AMPK, p-AKT, AKT ACC p-ACC, ACC, p-mTOR, mTOR, c-Myc, HK2, PKM2, LDH-A, and β-actin. (C) Glucose, lactate, and ATP in conditioned medium were measured using colorimetric assay kit. Data represent means ± SD. *, p < 0.05, **, p < 0.01, ***, p < 0.001 vs. untreated control (n = 3).
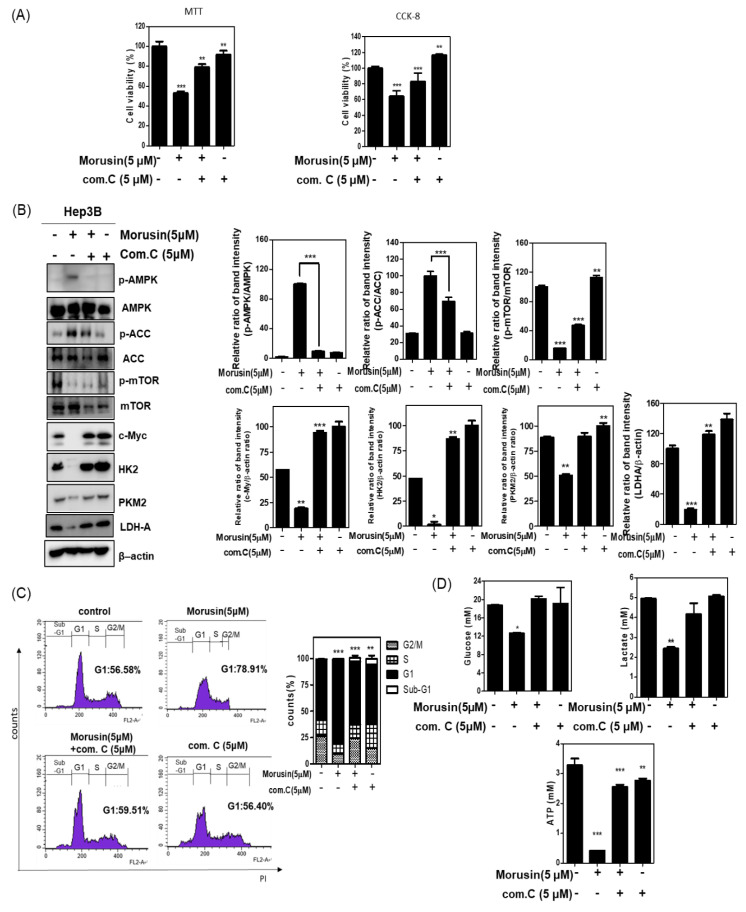
2.4. The Pivotal Role of AMPK in the Antitumor Effect of Morusin in Hep3B Cells
To confirm the role of AMPK in the antitumor effect of Morusin, an inhibitor study was conducted with AMPK inhibitor compound C in Hep3B cells. The cytotoxicity by Morusin was significantly rescued by compound C in Hep3B cells (Figure 4A).
Figure 4.
Effect of compound C on glycolysis related proteins and G1 arrest in Morusin treated Hep3B cells. (A) Hep3B cells were treated with Moursin in the absence and presence of compound C. Cell viability was evaluated using MTT and CCK-8 assays. (B) Effect of AMPK inhibitor compound C on glycolysis related proteins in Morusin treated Hep3B cells. Hep3B cells were treated with Morusin (5 μM) in the presence or absence of compound C (5 μM) for 24 h. Cell extracts were prepared and subjected to Western blotting with the antibodies of p-mTOR, mTOR, p-ACC, ACC p-AMPK, AMPK, c-Myc, HK2, PKM2, and β-actin. Data represent means ± SD. *, p < 0.05, **, p < 0.01, ***, p < 0.001 vs. untreated control. (C) Effect of compound C on G1 arrest in Morusin treated Hep3B cells. Hep3B cells exposed to Morusin (5 μM) in the presence or absence of compound C for 24 h were subjected to cell cycle analysis by staining with propidium iodide (50 μg/mL) (n = 3). (D) Glucose, lactate, and ATP in conditioned medium were measured by using colorimetric assay kit. Data represent means ± SD. *, p < 0.05, **, p < 0.01, ***, p < 0.001 vs. untreated control (n = 3).
Herein, compound C reduced the ability of Morusin to activate AMPK and attenuate the expression of p-mTOR, p-ACC, HK2, PKM2, and LDH-A in Hep3B cells (Figure 4B). Consistently, compound C suppressed G1 arrest induced by Morusin in Hep3B cells (Figure 4C). Conversely, suppressed glucose, lactate, and ATP by Morusin was significantly reversed by compound C in Hep3B cells (Figure 4D).
3. Discussion
Generally, three common phonotype enzymes, hexokinase 2 (HK2), phosphofructokinase 1 (PFK1), and pyruvate kinases type M2 (PKM2) were known in the glycolytic process of HCCs. Moreover, AMPK, PI3K/Akt pathway, HIF-1α, and c-Myc have emerged as aerobic glycolysis related proteins in hepatocellular carcinoma [10].
In the current study, the antitumor mechanism of Morusin was investigated in Huh7 and Hep3B cells in association with glycolysis and G1 arrest. Usually MTT and CCK-8 assays have been used for short term cytotoxicity [19,20], while colony formation has been applied for long term cytotoxicity [21,22]. Here, Morusin significantly showed cytotoxicity by MTT and CCK-8 assays and abrogated the number of colonies in Huh7 and Hep3B cells, implying the antitumor potency of Morusin. Likewise, Morusin induced apoptosis and inhibited angiogenesis in hepatocellular carcinoma cells by inhibiting the IL6/STAT3 signaling pathway [23]. Moreover, previous studies demonstrated that Morusin has an antitumor effect in several cancers such as lung [24], breast [25], renal [26], and pancreatic cancers [27].
It is well documented that cell proliferation depends on four distinct phases of the cell cycle including G0/G1, S, G2, and M, which is usually regulated by several cyclin-dependent kinases (CDKs) [28]. Cell cycle arrest is known as a stopping point in the cell cycle through the G1 phase, S phase (synthesis), G2 phase (interphase), and M phase (mitosis and cytokinesis) for DNA duplication or cell division [29,30]. The expression of p21Cip1/WAF1 maintains pRb and induces G1 arrest [31]. Moreover, mitogenic signals induce cyclin D1 expression for binding to CDK4/or CDK6 in the G1 phase of the cell cycle, while cyclin E and CDK2 are activated in the late G1 phase [32,33]. Here, Morusin significantly increased G1 arrest in Huh7 and Hep3B cells. Consistently, Morusin reduced the expression of cyclin D1, cyclin D3, cyclin E, CDK2, CDK4, and CDK6 and upregulated p21 and p27 in Huh7 and Hep3B cells, indicating cytotoxicity is induced partially due to G1 arrest by Morusin.
Accumulating evidence reveals that cancer cells rapidly consume glucose and convert it into lactate for uncontrolled replication and proliferation [30]. Among the metabolic intermediates of glycolysis, fructose-1, 6 bisphosphate enhances the antiapoptotic effect in cancer cells by reducing the release of cytochrome C [34], while pyruvate facilitates chemoresistance by overexpression of p-glycoprotein, leading to the export and import of lactate as the product of pyruvate oxidation [35]. Moreover, it is well documented that AMPK as a conserved and ubiquitously expressed energy sensor reduces the phosphorylation of mTOR and c-Myc [36], leading to inhibition of glycolysis and cell proliferation [36,37]. Recent studies demonstrate that AKT promotes cancer progression and the Warburg effect, since it facilitates glycolysis in cancer cells [38]. The accumulation of lactate in cancer cells promotes proliferation and growth, implying that inhibition of AKT by Morusin suppresses the growth and survival of liver cancer cells via the reduction in the Warburg effect. Herein, Morusin significantly activated phosphorylation of AMPK/ACC but attenuated the expression of p-AKT, p-mTOR, c-Myc, HK2, PKM2, and LDH-A and suppressed lactate, glucose, and ATP in Huh7 and Hep3B cells, strongly demonstrating the antiglycolytic effect of Morusin in HCCs. Notably, compound K suppressed glycolysis in hepatocellular cells [38], and Lapachol inhibited glycolysis in melanoma cancer cells [39]. Moreover, Morusin induced autophagy in lung cancer cells by activating AMPK [40].
AMPK can be a target to control glycolysis in several cancers, since AMPK is activated to inhibit biosynthetic pathways that consume ATP and enhance ATP-generating pathways involving glucose transport and glycolysis [41]. Thus, AMPK activator Metformin has been used in several cancers related to glycolysis [42]. To confirm the pivotal role of AMPK in the Morusin induced antitumor effect, AMPK inhibitor compound C was used in Morusin-treated Hep3B cells. As expected, compound C suppressed the capability of Morusin to activate AMPK, inactivate ACC, attenuate the expression of p-mTOR, HK2, PKM2, and LDH-A, and induce G1 arrest in Hep3B cells, indicating the important role of AMPK in the Morusin-exerted antitumor effect. Conversely, compound C rescued the suppression of lactate, glucose, and ATP by Morusin in Hep3B and Huh7 cells. Moreover, Gao et al. [17] reported that Morusin exerted an apoptotic effect in HepG2 and Hep3B cells and the HepG2 xenograft model via inhibition of STAT3 phosphorylation, implying the therapeutic potential of Morusin.
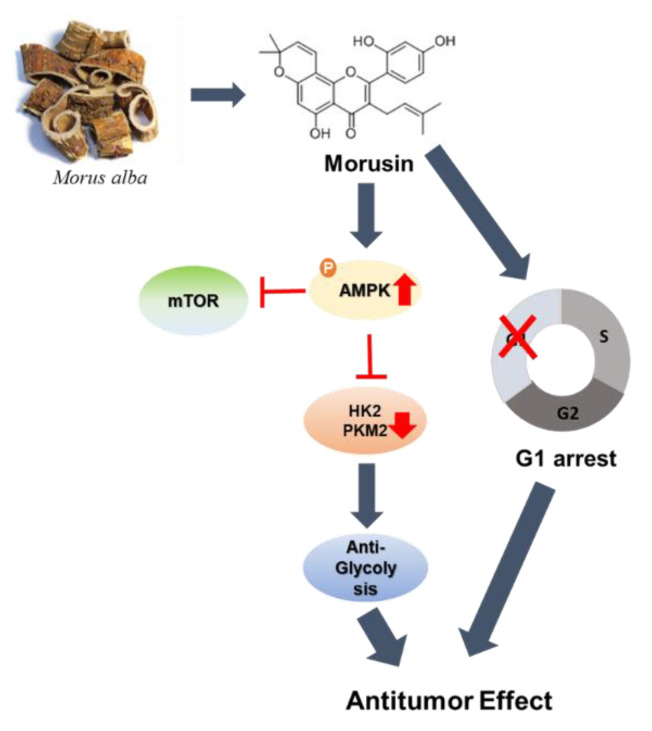
In summary, Morusin significantly abrogated the viability and the number of colonies, increased G1 arrest, attenuated the expression of cyclin D1, cyclin D3, cyclin E, CDK2, CDK4, and CDK6 and upregulated p21 and p27 in Huh7 and Hep3B cells. Moreover, Morusin significantly activated phosphorylation of AMPK/ACC and attenuated the expression of p-AKT, p-mTOR, c-Myc, HK2, PKM2, and LDH-A in Huh7 and Hep3B cells. Conversely, the AMPK inhibitor compound C reduced the ability of Morusin to activate AMPK, inactivate ACC, attenuate the expression of p-mTOR, HK2, PKM2, and LDH-A and induce G1 arrest in Morusin-treated Hep3B cells (Figure 5). Taken together, these findings provide evidence that Morusin exhibits an antitumor effect in HCCs via AMPK mediated G1 arrest and antiglycolysis as a potent dietary anticancer candidate.
Figure 5.
Schematic diagram of the antitumor mechanism of Morusin via G1 arrest and antiglycolysis by AMPK activation.
4. Materials and Methods
4.1. Morusin
Morusin (CAS # 62596-29-6) with a purity of over 99% was purchased from Sigma–Aldrich (Sigma–Aldrich, St Louis, MO, USA), diluted in 0.1% DMSO and stored for the next experiments.
4.2. Cell Culture
Human hepatocellular cancer cell lines including Hep3B (ATCC HB-8064) and Huh7 (PTA-4583) cells were bought from American Type Culture Collection (ATCC). The cells were maintained in DMEM and RPMI1640 with 10% FBS and 1% antibiotic (Welgene, South Korea).
4.3. Cytotoxicity Assay (MTT and CCK-8 Assay)
The cytotoxicity of Morusin was assessed by using a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. Briefly, Huh7 and Hep3B cells (1 × 104 cells/well) were exposed to various concentrations (0, 2.5, 5, 10, 20, 40 μM) of Morusin for 24 h, incubated with MTT (1 mg/mL) (Sigma Chemical, St Louis, MO, USA) for 2 h and then treated with MTT lysis solution overnight. Then, cell viability was calculated as a percentage of viable cells in the Morusin-treated group versus the untreated control by detecting optical density by using a microplate reader (Molecular Devices Co., San Jose, CA, USA) at 570 nm. Also, HCCs were exposed to 10 µL CCK-8 reagent and were incubated at 37 °C for 2 h. The optical density (OD) values were determined using a microplate reader at 450 nm.
4.4. Colony Formation Assay
Huh7 and Hep3B cells were seeded onto 6-well plates at a density of 1000 cells per well for a week. Then the number of colonies stained with crystal violet were counted.
4.5. Cell Cycle Analysis
Based on Gao et al.’s paper [13], Huh7 and Hep3B cells (1 × 106 cells/well) were exposed to Morusin (0, 2.5, or 5 μM) for 24 h and fixed in 75% ethanol at −20 °C. The cells were incubated with RNase A (10 mg/mL) for 1 h at 37 °C and stained with propidium iodide (50 μg/mL) for 30 min in the dark. The stained cells were analyzed for DNA content by FACSCalibur (Becton Dickinson, Franklin Lakes, NJ, USA).
4.6. Western Blotting
Based on Kim et al.’s paper [43], Huh7 and Hep3B cells (1 × 106 cells/mL) exposed to various concentrations of Morusin for 24 h were lyzed in lysis buffer on ice. The supernatants obtained by centrifugation were quantified for protein concentration and were transferred to a Hybond ECL transfer membrane for detection with antibodies of CyclinD1, CyclinD3, CyclinE, p21, CDK2, CDK4, p-AKT, AKT, p-mTOR, mTOR, p-AMPK, p-ACC, ACC, HK2, PKM2, LDH-A, and c-Myc (Cell signaling Technology, Beverly, MA, USA) and CDK6, LDH, and p27 (Santa Cruz Biotechnologies, Santa Cruz, CA), and β-actin (Sigma–Aldrich, St. Louis, MO, USA).
4.7. Lactate, Glucose and ATP Assay
Cells (1 × 106 cells/ml) were treated with Morusin for 24 h and then cell culture medium was collected. Lactate (K-607), glucose (K-606), and ATP (K-354) in media were measured using a colorimetric assay kit (BioVision Inc., Milpitas, CA, USA) according to manufacturer instructions.
4.8. Statistical Analysis
For statistical analysis of the data, all data analyzed by using GraphPad Prism software (Version 5.0, California, USA) were expressed as means ± standard deviation (SD). A Student’s t-test was used for comparison of the two groups and the statistically significant difference was determined as p values of <0.05 between the control and Morusin-treated groups.
Acknowledgments
We appreciate Namin Baek for providing some information on Morusin.
Abbreviations
| BSA | Bovine serum albumin |
| ECL | Cysteine aspartyl-specific protease |
| HCCs | Human hepatocellular carcinoma cells |
| mTOR | Mammalian target of rapamycin |
| FACS | Fluorescence-activated cell sorting |
| MTT | 3-(4:5-dimethylthiazol-2yl)-2:5-diphenyltetrazolium bromide |
| PARP | Poly (ADP-ribose) polymerase |
| FBS | Fetal bovine serum |
| OD | Optical density |
| SDS | Sodium dodecylsulfate |
| HK2 | Hexokinase 2 |
| PBS | Phosphate buffered saline |
| PKM2 | Phosphate buffered saline Pyruvate kinase isozymes M2 |
Author Contributions
A.-R.C., W.-Y.P., B.-S.S., and S.-H.K. designed and conceived the paper. J.-E.P., C.-H.A., D.-Y.S., H.-J.L., E.I. and W.-Y.P. documented the papers and summarized the data. W.-Y.P. and J.-E.P. created the figures. W.-Y.P. and E.I. drafted the manuscript. B.-S.S. and H.-J.L. edited the figures. S.-H.K. and B.-S.S. supervised and revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Korea Science and Engineering Foundation (KOSEF) grant funded by the Korea government (MEST) (No.2020R1A2C1004816) and (No.2021R1A2C2003277).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All the data and materials supporting the conclusions are included in the main paper.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Anwanwan D., Singh S.K., Singh S., Saikam V., Singh R. Challenges in liver cancer and possible treatment approaches. Biochim. Biophys. Acta Rev. Cancer. 2020;1873:188314. doi: 10.1016/j.bbcan.2019.188314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ganapathy-Kanniappan S., Vali M., Kunjithapatham R., Buijs M., Syed L.H., Rao P.P., Ota S., Kwak B.K., Loffroy R., Geschwind J.F. 3-bromopyruvate: A new targeted antiglycolytic agent and a promise for cancer therapy. Curr. Pharm. Biotechnol. 2010;11:510–517. doi: 10.2174/138920110791591427. [DOI] [PubMed] [Google Scholar]
- 3.le Roux C.W., Wilkinson S.D., Pavitt D.V., Muller B.R., Alaghband-Zadeh J. A new antiglycolytic agent. Pt 1Ann. Clin. Biochem. 2004;41:43–46. doi: 10.1258/000456304322664681. [DOI] [PubMed] [Google Scholar]
- 4.Ganapathy-Kanniappan S., Geschwind J.F. Tumor glycolysis as a target for cancer therapy: Progress and prospects. Mol. Cancer. 2013;12:152. doi: 10.1186/1476-4598-12-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Akram M. Mini-review on glycolysis and cancer. J. Cancer Educ. 2013;28:454–457. doi: 10.1007/s13187-013-0486-9. [DOI] [PubMed] [Google Scholar]
- 6.Schwartz L., Supuran C.T., Alfarouk K.O. The Warburg Effect and the Hallmarks of Cancer. Anticancer Agents Med. Chem. 2017;17:164–170. doi: 10.2174/1871520616666161031143301. [DOI] [PubMed] [Google Scholar]
- 7.Koppenol W.H., Bounds P.L., Dang C.V. Otto Warburg’s contributions to current concepts of cancer metabolism. Nat. Rev. Cancer. 2011;11:325–337. doi: 10.1038/nrc3038. [DOI] [PubMed] [Google Scholar]
- 8.Jiao L., Zhang H.L., Li D.D., Yang K.L., Tang J., Li X., Ji J., Yu Y., Wu R.Y., Ravichandran S., et al. Regulation of glycolytic metabolism by autophagy in liver cancer involves selective autophagic degradation of HK2 (hexokinase 2) Autophagy. 2018;14:671–684. doi: 10.1080/15548627.2017.1381804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ganapathy-Kanniappan S. Molecular intricacies of aerobic glycolysis in cancer: Current insights into the classic metabolic phenotype. Crit. Rev. Biochem. Mol. Biol. 2018;53:667–682. doi: 10.1080/10409238.2018.1556578. [DOI] [PubMed] [Google Scholar]
- 10.Feng J., Li J., Wu L., Yu Q., Ji J., Wu J., Dai W., Guo C. Emerging roles and the regulation of aerobic glycolysis in hepatocellular carcinoma. J. Exp. Clin. Cancer Res. 2020;39:126. doi: 10.1186/s13046-020-01629-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin S.R., Chang C.H., Hsu C.F., Tsai M.J., Cheng H., Leong M.K., Sung P.J., Chen J.C., Weng C.F. Natural compounds as potential adjuvants to cancer therapy: Preclinical evidence. Br. J. Pharmacol. 2020;177:1409–1423. doi: 10.1111/bph.14816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qi W., Li Z., Yang C., Jiangshan Dai J., Zhang Q., Wang D., Wu C., Xia L., Xu S. Inhibitory mechanism of muscone in liver cancer involves the induction of apoptosis and autophagy. Oncol. Rep. 2020;43:839–850. doi: 10.3892/or.2020.7484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gao C., Li X., Yu S., Liang L. Inhibition of cancer cell growth by oleanolic acid in multidrug resistant liver carcinoma is mediated via suppression of cancer cell migration and invasion, mitochondrial apoptosis, G2/M cell cycle arrest and deactivation of JNK/p38 signalling pathway. J. BUON. 2019;24:1964–1969. [PubMed] [Google Scholar]
- 14.Li B., Xu N., Wan Z., Ma L., Li H., Cai W., Chen X., Huang Z., He Z. Isobavachalcone exerts antiproliferative and proapoptotic effects on human liver cancer cells by targeting the ERKs/RSK2 signaling pathway. Oncol. Rep. 2019;41:3355–3366. doi: 10.3892/or.2019.7090. [DOI] [PubMed] [Google Scholar]
- 15.Bie B., Sun J., Li J., Guo Y., Jiang W., Huang C., Yang J., Li Z. Baicalein, a Natural Anti-Cancer Compound, Alters MicroRNA Expression Profiles in Bel-7402 Human Hepatocellular Carcinoma Cells. Cell. Physiol. Biochem. 2017;41:1519–1531. doi: 10.1159/000470815. [DOI] [PubMed] [Google Scholar]
- 16.Dasgupta A., Dey D., Ghosh D., Lai T.K., Bhuvanesh N., Dolui S., Velayutham R., Acharya K. Astrakurkurone, a sesquiterpenoid from wild edible mushroom, targets liver cancer cells by modulating Bcl-2 family proteins. IUBMB Life. 2019;71:992–1002. doi: 10.1002/iub.2047. [DOI] [PubMed] [Google Scholar]
- 17.Abbaszadeh Z., Cesmeli S., Biray Avci C. Crucial players in glycolysis: Cancer progress. Gene. 2020;726:144158. doi: 10.1016/j.gene.2019.144158. [DOI] [PubMed] [Google Scholar]
- 18.Kumar P., Nagarajan A., Uchil P.D. Analysis of Cell Viability by the MTT Assay. Cold Spring Harb. Protoc. 2018;2018:pdb.prot095505. doi: 10.1101/pdb.prot095505. [DOI] [PubMed] [Google Scholar]
- 19.Carmichael J., DeGraff W.G., Gazdar A.F., Minna J.D., Mitchell J.B. Evaluation of a tetrazolium-based semiautomated colorimetric assay: Assessment of radiosensitivity. Cancer Res. 1987;47:943–946. [PubMed] [Google Scholar]
- 20.Munshi A., Hobbs M., Meyn R.E. Clonogenic cell survival assay. Methods Mol. Med. 2005;110:21–28. doi: 10.1385/1-59259-869-2:021. [DOI] [PubMed] [Google Scholar]
- 21.Kajiwara Y., Panchabhai S., Levin V.A. A new preclinical 3-dimensional agarose colony formation assay. Technol. Cancer Res. Treat. 2008;7:329–334. doi: 10.1177/153303460800700407. [DOI] [PubMed] [Google Scholar]
- 22.Tartakoff A.M. Cell cycle arrest of S. cerevisiae in conjunction with labeling of the cell wall. STAR Protoc. 2021;2:100646. doi: 10.1016/j.xpro.2021.100646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pompili G. Organization of the radiodiagnostic department. Minerva Med. 1970;61:4397–4406. [PubMed] [Google Scholar]
- 24.Park H.J., Min T.R., Chi G.Y., Choi Y.H., Park S.H. Induction of apoptosis by morusin in human non-small cell lung cancer cells by suppression of EGFR/STAT3 activation. Biochem. Biophys. Res. Commun. 2018;505:194–200. doi: 10.1016/j.bbrc.2018.09.085. [DOI] [PubMed] [Google Scholar]
- 25.Kang S., Kim E.O., Kim S.H., Lee J.H., Ahn K.S., Yun M., Lee S.G. Morusin induces apoptosis by regulating expression of Bax and Survivin in human breast cancer cells. Oncol. Lett. 2017;13:4558–4562. doi: 10.3892/ol.2017.6006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang C., Luo J., Luo X., Jia W., Fang Z., Yi S., Li L. Morusin exerts anti-cancer activity in renal cell carcinoma by disturbing MAPK signaling pathways. Ann. Transl. Med. 2020;8:327. doi: 10.21037/atm.2020.02.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim C., Kim J.H., Oh E.Y., Nam D., Lee S.G., Lee J., Kim S.H., Shim B.S., Ahn K.S. Blockage of STAT3 Signaling Pathway by Morusin Induces Apoptosis and Inhibits Invasion in Human Pancreatic Tumor Cells. Pancreas. 2016;45:409–419. doi: 10.1097/MPA.0000000000000496. [DOI] [PubMed] [Google Scholar]
- 28.Ahn H., Im E., Lee D.Y., Lee H.J., Jung J.H., Kim S.H. Antitumor Effect of Pyrogallol via miR-134 Mediated S Phase Arrest and Inhibition of PI3K/AKT/Skp2/cMyc Signaling in Hepatocellular Carcinoma. Int. J. Mol. Sci. 2019;20:3985. doi: 10.3390/ijms20163985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Coffman J.A. Cell cycle development. Dev. Cell. 2004;6:321–327. doi: 10.1016/S1534-5807(04)00067-X. [DOI] [PubMed] [Google Scholar]
- 30.El-Deiry W.S. p21(WAF1) Mediates Cell-Cycle Inhibition, Relevant to Cancer Suppression and Therapy. Cancer Res. 2016;76:5189–5191. doi: 10.1158/0008-5472.CAN-16-2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Coqueret O. Linking cyclins to transcriptional control. Gene. 2002;299:35–55. doi: 10.1016/S0378-1119(02)01055-7. [DOI] [PubMed] [Google Scholar]
- 32.Nigg E.A. Cyclin-dependent protein kinases: Key regulators of the eukaryotic cell cycle. Bioessays. 1995;17:471–480. doi: 10.1002/bies.950170603. [DOI] [PubMed] [Google Scholar]
- 33.Bose S., Le A. Glucose Metabolism in Cancer. Adv. Exp. Med. Biol. 2018;1063:3–12. doi: 10.1007/978-3-319-77736-8_1. [DOI] [PubMed] [Google Scholar]
- 34.Israelsen W.J., Vander Heiden M.G. Pyruvate kinase: Function, regulation and role in cancer. Semin. Cell Dev. Biol. 2015;43:43–51. doi: 10.1016/j.semcdb.2015.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu L., Ulbrich J., Muller J., Wustefeld T., Aeberhard L., Kress T.R., Muthalagu N., Rycak L., Rudalska R., Moll R., et al. Deregulated MYC expression induces dependence upon AMPK-related kinase 5. Nature. 2012;483:608–612. doi: 10.1038/nature10927. [DOI] [PubMed] [Google Scholar]
- 36.Faubert B., Boily G., Izreig S., Griss T., Samborska B., Dong Z., Dupuy F., Chambers C., Fuerth B.J., Viollet B., et al. AMPK is a negative regulator of the Warburg effect and suppresses tumor growth in vivo. Cell Metab. 2013;17:113–124. doi: 10.1016/j.cmet.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim Y.H., Liang H., Liu X., Lee J.S., Cho J.Y., Cheong J.H., Kim H., Li M., Downey T.J., Dyer M.D., et al. AMPKalpha modulation in cancer progression: Multilayer integrative analysis of the whole transcriptome in Asian gastric cancer. Cancer Res. 2012;72:2512–2521. doi: 10.1158/0008-5472.CAN-11-3870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shin N., Lee H.J., Sim D.Y., Im E., Park J.E., Park W.Y., Cho A.R., Shim B.S., Kim S.H. Apoptotic effect of compound K in hepatocellular carcinoma cells via inhibition of glycolysis and Akt/mTOR/c-Myc signaling. Phytother. Res. 2021;35:3812–3820. doi: 10.1002/ptr.7087. [DOI] [PubMed] [Google Scholar]
- 39.Shankar Babu M., Mahanta S., Lakhter A.J., Hato T., Paul S., Naidu S.R. Lapachol inhibits glycolysis in cancer cells by targeting pyruvate kinase M2. PLoS ONE. 2018;13:e0191419. doi: 10.1371/journal.pone.0191419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Park H.J., Park S.H. Induction of cytoprotective autophagy by morusin via AMP-activated protein kinase activation in human non-small cell lung cancer cells. Nutr. Res. Pract. 2020;14:478–489. doi: 10.4162/nrp.2020.14.5.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang W.L., Perillo W., Liou D., Marambaud P., Wang P. AMPK inhibitor compound C suppresses cell proliferation by induction of apoptosis and autophagy in human colorectal cancer cells. J. Surg. Oncol. 2012;106:680–688. doi: 10.1002/jso.23184. [DOI] [PubMed] [Google Scholar]
- 42.Rocha G.Z., Dias M.M., Ropelle E.R., Osorio-Costa F., Rossato F.A., Vercesi A.E., Saad M.J., Carvalheira J.B. Metformin amplifies chemotherapy-induced AMPK activation and antitumoral growth. Clin. Cancer Res. 2011;17:3993–4005. doi: 10.1158/1078-0432.CCR-10-2243. [DOI] [PubMed] [Google Scholar]
- 43.Kim M.J., Sim D.Y., Lee H.M., Lee H.J., Kim S.H. Hypolipogenic Effect of Shikimic Acid Via Inhibition of MID1IP1 and Phosphorylation of AMPK/ACC. Int. J. Mol. Sci. 2019;20:582. doi: 10.3390/ijms20030582. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the data and materials supporting the conclusions are included in the main paper.