Abstract
As sessile organisms, the precise development phase transitions are very important for the success of plant adaptability, survival and reproduction. The transition from juvenile to the adult phase—referred to as the vegetative phase change—is significantly influenced by numbers of endogenous and environmental signals. Here, we showed that brassinosteroid (BR), a major growth-promoting steroid hormone, positively regulates the vegetative phase change in Arabidopsis thaliana. The BR-deficient mutant det2-1 and BR-insensitive mutant bri1-301 displayed the increased ratio of leaf width to length and reduced blade base angle. The plant specific transcription factors SQUAMOSA PROMOTER BINDING PROTEIN-LIKE (SPL) are key masters for the vegetative phase transition in plants. The expression levels of SPL9, SPL10 and SPL15 were significantly induced by BR treatment, but reduced in bri1-116 mutant compared to wild-type plants. The gain-of-function pSPL9:rSPL9 transgenic plants displayed the BR hypersensitivity on hypocotyl elongation and partially suppressed the delayed vegetative phase change of det2-1 and bri1-301. Furthermore, we showed that BRASSINAZOLE-RESISTANT 1 (BZR1), the master transcription factor of BR signaling pathway, interacted with SPL9 to cooperatively regulate the expression of downstream genes. Our findings reveal an important role for BRs in promoting vegetative phase transition through regulating the activity of SPL9 at transcriptional and post-transcriptional levels.
Keywords: vegetative phase change, cell elongation, SPL9, BZR1
1. Introduction
The transition from the juvenile to adult stage is referred to as the vegetative phase change and is crucial for the reproductive success and survival of higher plants [1]. In Arabidopsis, the change of vegetative stage can be characterized by the appearance of abaxial trichomes, increasing the complexity of leaf shape and the ratio of leaf length to width [2,3]. The microRNA 156 (miR156) and its target transcription factors SQUAMOSA PROMOTER BINDING PROTEIN-LIKE (SPL) are the main regulatory factors of the vegetative phase transition in plants [4,5]. A wide range of signals, including sugar, gibberellin acid (GA), abscisic acid (ABA), auxin and endogenous epigenetic factors, regulate the activity of the miR156-SPL module to modulate the vegetative phase change [3,6,7,8]. These interconnected signal transduction networks integrate multiple developmental, environmental and hormonal signals to precisely optimize the vegetative developmental phase transition in the changing environmental conditions.
MicroRNA156 (miR156) is an evolutionarily conserved miRNA, which determines the juvenile-to-adult transition and regulates diverse aspects of plant growth and development [9,10,11,12]. MiR156 is enriched in the juvenile-stage leaves and gradually declines as the shoot develops, while the expression levels of SPLs are reversed, which are abundant at adult stages [9,10,13,14]. MiR156 is encoded by eight homologous genes, miR156A-miR156H in the Arabidopsis genome [5]. The single mir156a and mir156c mutant displayed the slightly early vegetative phase change, but the mir156a/c double mutant and mir156a/b/c triple mutant exhibited much early juvenile-to-adult transition [15,16]. Constitutive overexpression of miR156 in Arabidopsis, as well as in maize, rice, tomato, and tobacco, all led to a delay in the phase transition from juvenile to adult [2,10,14,16,17,18]. However, overexpression of miR156 target mimic, MIM156, which produces an mRNA specifically binding to miR156, inhibits the activity of miR156 and increases the transcript levels of SPLs (such as SPL3, SPL4, SPL5, SPL9, and SPL15), thus shortening the juvenile phase [9,10,19,20]. SPLs encode a family of plant specific transcription factors, which contain a highly conserved DNA-binding domain, the SBP domain [21,22,23]. In Arabidopsis, 10 of the 16 SPL genes have a sequence complementary to miR156, and they differently respond to the changes of miR156 levels [5,22,23,24,25,26]. MiR156 blocks the expression of all 10 targeted SPLs in the first two rosette leaves, which is due to the high levels of miR156 in these leaves. Because the transcript levels of miR156 decrease in the subsequent leaves, the transcripts of SPL3/SPL9/SPL15 quickly increase [5,23,26]. SPL9 and SPL15 are the most well-understood members of SPL family in Arabidopsis thaliana. The loss-of-function mutants spl9 and spl15 results in the increase of shoot branching, shortening plastochron during vegetative growth and delay of the phase transition from juvenile to adult. Whereas the transgenic plants proSPL9:rSPL9, which expressed a miR156-resistant form of SPL9 driven by its native promoter, displayed the reduced shoot branch and much early vegetative phase change [10,18,27,28]. SPL9 directly binds to the promoter of LAS to repress axillary bud formation [29]. Our recent study further showed that gibberellin inhibited the formation of axillary buds in Arabidopsis by promoting the degradation of DELLA proteins to release their inhibiting effects on the transcriptional activity of SPL9 [30].
Brassinosteroids (BRs), as a classic of plant steroid hormone, play important roles in plant growth and development [31,32]. BRs directly bind to the extracellular domain of the receptor kinase BRASSINOSTEROID INSENSITIVE1 (BRI1) which interacts with coreceptor BRI1-ASSOCIATED RECEPTOR KINASE1 (BAK1), and as a result rapidly activates the receptor’s intracellular kinase domain through homology dimerization and trans-phosphorylation [33,34,35,36]. Activated BRI1 phosphorylates activate a series of kinases, such as BRASSINOSTEROID-SIGNALING KINASE1 (BSK1) and CONSTITUTIVE DIFFERENTIAL GROWTH1 (CDG1), as well as the Ser/Thr phosphatase BRI1-SUPPRESSOR1 (BSU1) which inactivates the kinase BRASSINOSTEROID INSENSITIVE2 (BIN2) by dephosphorylation, then BIN2 was degraded by the F-box protein KINKSUPPRESSED (KIB1) [37,38,39,40]. Inactivation of BIN2 makes the unphosphorylated transcription factors BRASSINAZOLE RESISTANT 1 (BZR1) and BRI1-EMS-SUPPRESSOR 1 (BES1) by PROTEIN PHOSPHATASE 2A (PP2A) enter and accumulate in the nucleus, which directly regulates the expression of BR responsive genes [41,42,43,44,45,46]. BZR1 and BES1 interact with other growth promoting transcription factors, such as PIF4 and ARF6, to regulate cell elongation by cooperatively regulating their downstream gene expression [47,48]. Hydrogen peroxide (H2O2) induces the oxidation of BZR1 to increase the transcription activity of BZR1 by enhancing the interaction between BZR1 with PIF4 and ARF6 [49]. BZR1 and BES1 also have been reported to interact with diverse proteins to integrate with BR and numbers of hormonal and environmental signals to regulate plant growth and development.
Genetic analysis showed that the BR-deficient and BR-insensitive mutants displayed the round leaves and the significant decreased ratio of leaf length to width compared to wild-type plants, suggesting BR maybe involved in the vegetative phase change. Here, in this study, we showed that BR treatment promoted the expression of SPL9, SPL10, and SPL15, while mutation of BRI1 led to the decreased expression levels of these genes. The pSPL9:rSPL9 displayed the BR hypersensitivity and suppressed the delayed vegetative phase change of det2-1 and bri1-301. BZR1 interacts with SPL9 to coordinately regulate the downstream gene expression. These results demonstrate that BR promotes the vegetative growth of plants by increasing the activity of SPL9 at both transcriptional and post-transcription levels.
2. Results
2.1. BZR1 Interacts with SPL9 In Vitro and In Vivo
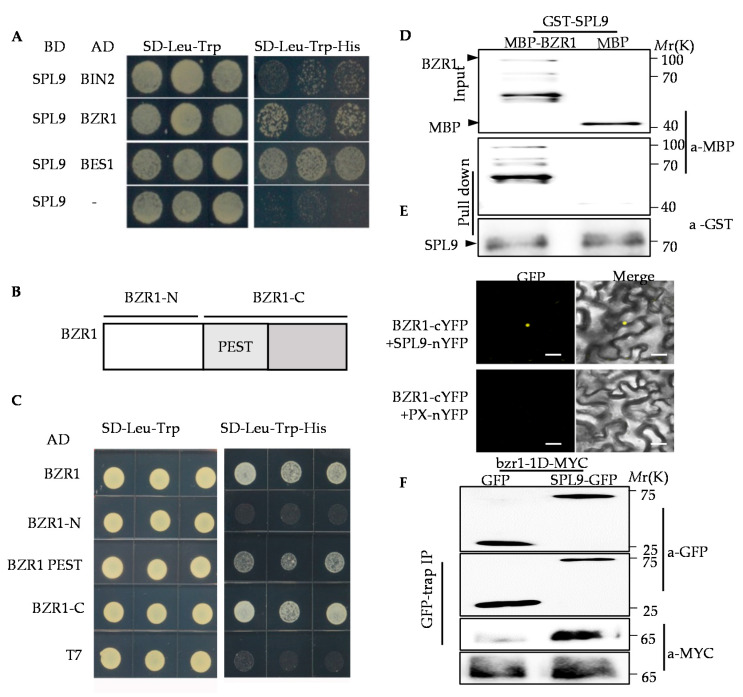
To further investigate the function of BZR1 in plant growth and development, we screened the proteins interacting with BZR1 by Yeast two-hybrid (Y2H) system. SPL9 was identified from the putative BZR1-interacting candidates. Additional Y2H assays showed that SPL9 interacted with BZR1 and BES1 in yeast (Figure 1A). BZR1 contains the amino-terminal DNA binding domain, PEST domain, which is responsible for the interaction with PP2A, and the carboxy-terminal domain, which itself is critical for the association with BIN2. The Y2H assays showed that SPL9 had the high binding ability with BZR1-C, weak binding ability with PEST domain, and did not interact with BZR1-N (Figure 1B,C). To confirm the interaction between BZR1 and SPL9, protein-protein pull down assay was performed using purified proteins fused glutathione S transferase (GST) or maltose binding protein (MBP) expressed from Escherichia coli. The results showed that GST-SPL9 protein pulled down MBP-fused BZR1, but not MBP alone (Figure 1D). To determine whether BZR1 interacts with SPL9 in plants, transient bimolecular fluorescence complementation (BiFC) assays were carried out in the tobacco leaves. The results showed that the strong fluorescent signals were detected in the nucleus of the epidermal cells of tobacco leaves when BZR1-cYFP was co-transformed with SPL9-nYFP, but not with control x-nYFP (Figure 1E). Furthermore, we performed Co-IP assay using YFP-trap beads and Arabidopsis protoplast, in which co-expressing 35S:GFP and 35S:bzr1-1D-MYC or co-expressing 35S:SPL9-GFP and 35S:bzr1-1D-MYC. The results showed that BZR1 interacted with SPL9 in plants (Figure 1F). All these results proved that BZR1 interacts with SPL9 in vitro and in vivo.
Figure 1.
BZR1 interacts with SPL9 in vitro and in vivo. (A) SPL9 interacted with BZR1, BES1, and BIN2 in yeast. AD means activation domain; BD means DNA binding domain; SD means Synthetic dextrose minimal medium. (B) A diagram of the structure of BZR1. (C) The C-terminal of BZR1 was required for the interaction between BZR1 and SPL9 in yeast. PEST means the PEST domain. (D) Maltose binding protein (MBP) or MBP-BRASSINAZOLE RESISTANT 1 (BZR1) protein were incubated with glutathione-agarose beads combining glutathione S transferase (GST)-SQUAMOSA PROMOTER BINDING PROTEIN-LIKE9 (SPL9) and then eluted and analyzed by anti-MBP and anti-GST immunoblotting. (E) BiFC assays showed the interaction between BZR1 and SPL9 in plants. GFP means the green fluorescent protein. (F) BZR1 interacts with SPL9 in vivo. Immunoprecipitation (IP) was performed using Arabidopsis protoplast co-expressing 35S:GFP and 35S:bzr1-1D-MYC or co-expressing 35S:SPL9-GFP and 35S:bzr1-1D-MYC. The coimmunoprecipitation experiments were performed using GFP-Trap agarose beads, and the immunoblots were probed with anti-Myc or anti-YFP antibodies.
2.2. Enhancing the Activity of SPL9 Led to the BR Hypersensitivity
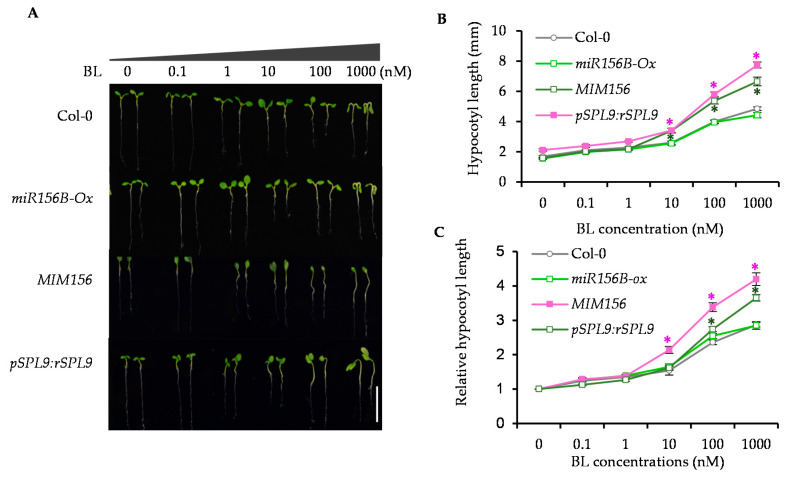
In Arabidopsis, several members of the SPL family are regulated post-transcriptionally by miR156. To determine whether the miR156 and SPL9 are involved in the BR signaling pathway, we studied the BR response of wild type, miR156B-Ox, in which miR156B was expressly driven by 35S promoter, the miR156 target mimics line (MIM156) that reduces miR156 activity, and pSPL9:rSPL9 plants where the resistant SPL9 (rSPL9) nontargeted by miR156 was expressed under its native promoter. The results showed that BR promoted the hypocotyl elongation of wild-type plants in a dose-dependent manner under the consistent light condition. The miR156B-Ox and spl9/spl15 displayed the BR response similar to that of wild-type plants, but the MIM156 and pSPL9:rSPL9 displayed hypersensitivity to BR compared to wild-type plants (Figure 2A–C and Figure S1A–C). To determine the BR response of miR156/SPL9 module, we grew wild type, miR156B-Ox and pSPL9:rSPL9 on the ½ MS medium containing different concentrations of BR biosynthesis inhibitor propiconazole (PPZ). We found miR156B-Ox displayed much smaller rosette leaves than wild-type plants, and pSPL9:rSPL9 showed significantly increased rosette leaf sizes (Figure S2). Furthermore, the bzr1-1D gain-of-function mutant displayed the PPZ-resistant phenotypes, but such PPZ resistance of bzr1-1D were significantly reduced by miR156 overexpression (Figure S3). These results indicated that the plants with high activity of SPL9 are hypersensitive to BR.
Figure 2.
The MIM156 and pSPL9:SPL9 transgenic plants displayed the increased BR sensitivity on hypocotyl elongation. (A) Representative images of seven-day-old seedlings of wild-type and miR156B-Ox, MIM156 and pSPL9:rSPL9 grown on the ½ MS medium with different concentrations of brassinolide (BL). (B,C) Activated SPL9 by expressing the mimic miR156 or the miR156-resistant form of SPL9 increased the BR response. The hypocotyl lengths were measured from at least 30 plants. Relative hypocotyl length were average of 30 plants and normalized to the untreated plants. Error bars represent standard deviation. * p < 0.05.
2.3. rSPL9 Partially Suppressed the Dwarf Phenotypes of det2-1 and bri1-301
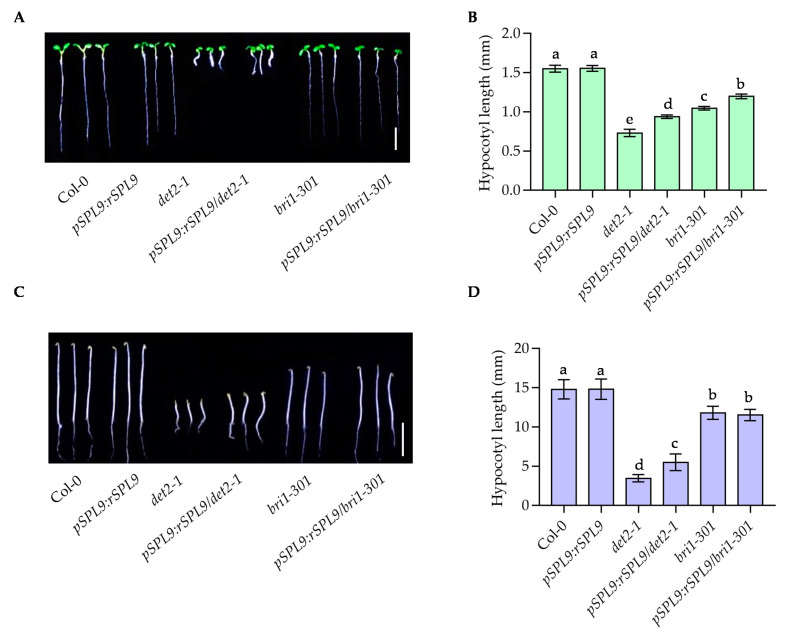
To further investigate the functions of SPL9 in BR signaling pathway, we crossed pSPL9:rSPL9 with BR-deficient mutant det2-1 and BR-insensitive mutant bri1-301 to generate pSPL9:rSPL9/det2-1 and pSPL9:rSPL9/bri1-301. When growing in light, det2-1, and bri1-301 seedlings had shorter hypocotyl length and the hypocotyl length of pSPL9:rSPL9 is similar to that of Col-0, but pSPL9:rSPL9 can partially suppress the short hypocotyl phenotypes of det2-1 and bri1-301 (Figure 3A,B). When growing in the dark, pSPL9:rSPL9 plants still can partially suppress the short hypocotyl phenotype of det2-1 (Figure 3C,D). The results indicated that SPL9 is a positive regulator of BR signaling pathway.
Figure 3.
The dwarf phenotypes of det2-1 and bri1-301 were partially suppressed by the pSPL9:rSPL9 transgenic plants. (A,B) Seedlings of wild type and indicated plants were grown under constant light for 7 days. Hypocotyl lengths were measured from at least 30 plants. Error bars represent standard deviation. Different letters above the bars indicated statistically significant differences between the samples (ANOVA analysis followed by Uncorrected Fisher’s LSD (Least-significant difference) multiple comparisons test, p < 0.05). (C,D) Seedlings of wild type and indicated plants were grown in the dark for 7 days. Hypocotyl lengths were measured from at least 30 plants. Error bars represent standard deviation. Different letters above the bars indicated statistically significant differences between the samples (ANOVA analysis followed by Uncorrected Fisher’s LSD multiple comparisons test, p < 0.05).
2.4. BR Ppromotes the Change of Vegetative Phase in Arabidopsis
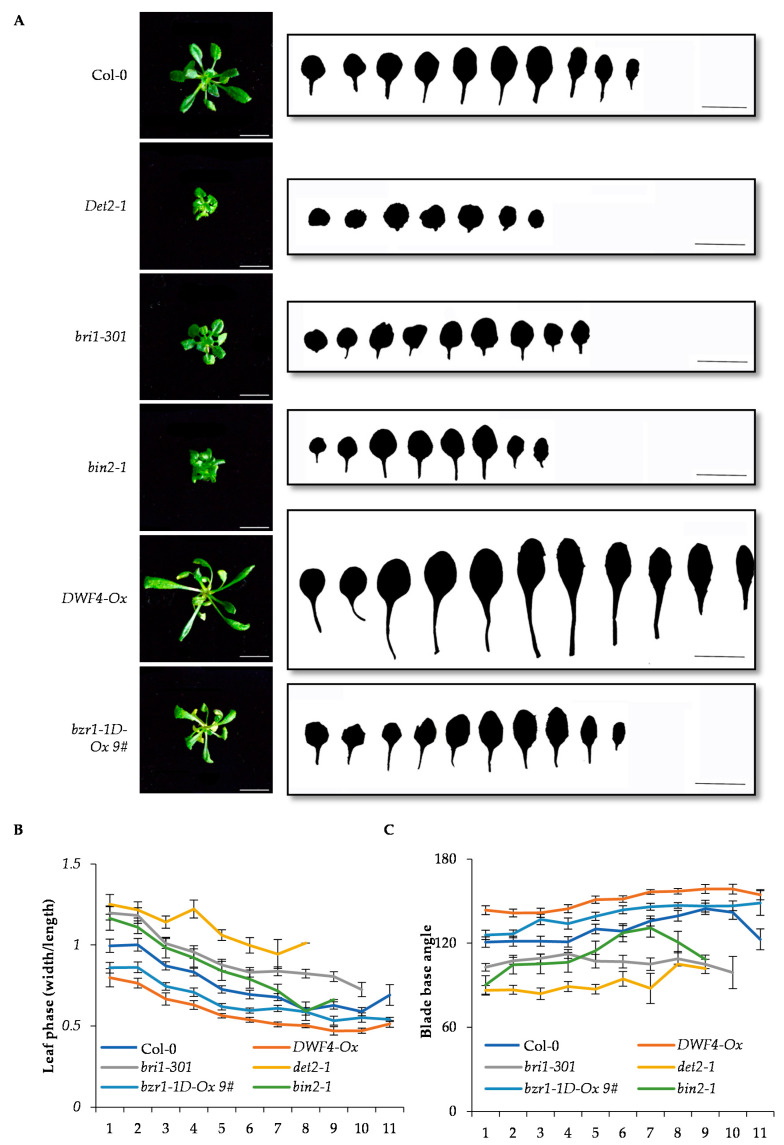
The miR156-SPL9 module has been reported to play a critical role in the progression from the juvenile phase to the adult phase in plants. Considering that BZR1 interacts with SPL9 to regulate cell elongation, we want to determine whether BR and BZR1 participate in the vegetative phase change in plants. The BR-deficient mutants det2-1, and BR-insensitive mutants bri1-301, and the constitutively active forms bin2-1 all displayed the increased ratio of leaf width to length, and the decreased blade base angle (Figure 4A–C). Meanwhile the BR signal enhanced materials such as BRI1 overexpression (BRI1-Ox), DWF4 overexpression (DWF4-Ox), and bzr1-1D overexpression (bzr1-1D-Ox), showed the decreased ratio of leaf width to length and the increased blade base angle (Figure 4A–C). These results indicated that BR promotes the vegetative phase change.
Figure 4.
Brassinosteroid (BR) promotes vegetative phase change of plants. (A) The shape phenotypes of full expanded rosette leaves of wild type and different BR-related mutants. (B) The ratio of leaf width to leaf length of full expanded rosette leaves of wild type and different BR-related mutants. The leaf width and leaf length were measure from at least 30 plants. Error bars represent standard deviation. The x axis indicates the number of leaves. (C) The base angle of full expanded rosette leaves of wild type and different BR-related mutants. The leaf base angle were measure from at least 30 plants. Error bars represent standard deviation. The x axis indicates the number of leaves.
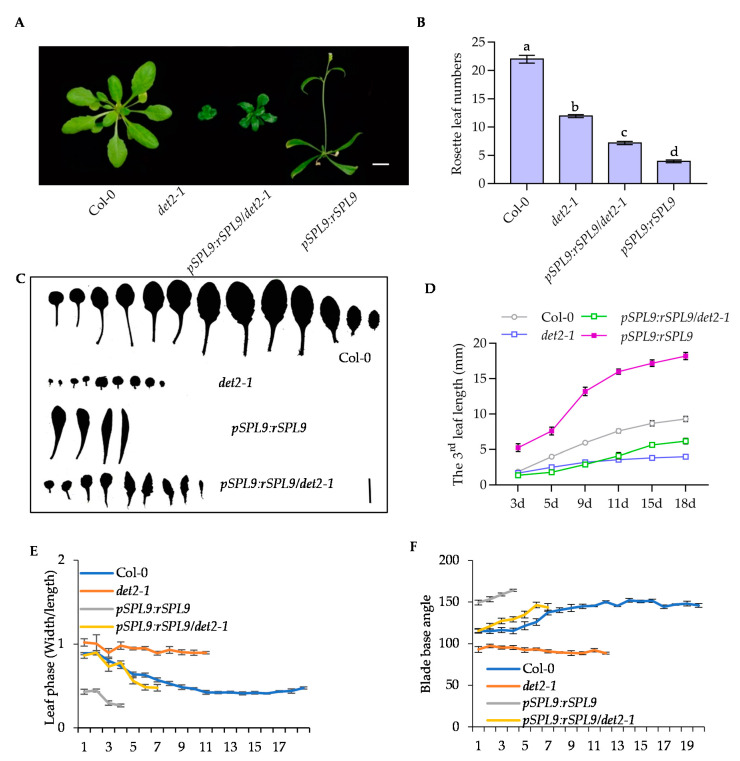
To determine the role of SPL9 in BR-mediated vegetative phase change, we described the growth phenotypes of wild type, det2-1, bri1-301, pSPL9:rSPL9, pSPL9:rSPL9/det2-1 and pSPL9:rSPL9/bri1-301. The results showed of det2-1 and bri1-301 have a bigger ratio of leaf width to length and smaller blade base angle, while pSPL9:rSPL9 have a smaller ratio of leaf width to length and bigger blade base angle (Figure 5A–F, Figure S4A–F). What’s more, the rounder leaves of det2-1 and bri1-301 were suppressed by pSPL9:rSPL9 (Figure 5C, Figure S4C). In addition, the pSPL9:rSPL9 can partially affect the rosette leaf numbers and leaf growth rate of det2-1 and bri1-301 (Figure 5B,D, Figure S4B,D). Furthermore, we found that Col-0 appeared the abaxial trichome in the 7th rosette leaf, while in bri1-301 there appeared the abaxial trichome in the 9th leaf, significantly delaying the appearance of abaxial trichomes in the pSPL9:rSPL9 plant (Figure S5). These results indicated that SPL9 promotes vegetative phase change downstream of BR.
Figure 5.
The pSPL9:rSPL9 suppressed the delayed vegetative phase change of det2-1 mutant. (A) The growth phenotype of 5-week-old wild type, det2-1, pSPL9:rSPL9 and pSPL9:rSPL9/det2-1 plants. (B) The rosette leaf numbers of wild type, det2-1, pSPL9:rSPL9 and pSPL9:rSPL9/det2-1 after flowering. Error bars represent standard deviation (n = 10). Different letters above the bars indicated statistically significant differences between the samples (ANOVA analysis followed by Uncorrected Fisher’s LSD multiple comparisons test, p < 0.05). (C) The shape phenotypes of full expanded rosette leaves of wild type, det2-1, pSPL9:rSPL9 and pSPL9:rSPL9/det2-1. (D) The 3rd leaf length of wild type, det2-1, pSPL9:rSPL9 and pSPL9:rSPL9/det2-1 after initiation different days. The leaf lengths were measure from at least 30 plants. Error bars represent standard deviation. (E) The ratio of leaf width to leaf length of full expanded rosette leaves of wild type and indicated plants. The leaf width and leaf length were measure from at least 30 plants. Error bars represent standard deviation. (F) The base angle of full expanded rosette leaves of wild type and indicated plants. The leaf base angle were measure from at least 30 plants. Error bars represent standard deviation.
2.5. BR Induces the Expression of Several SPL Genes
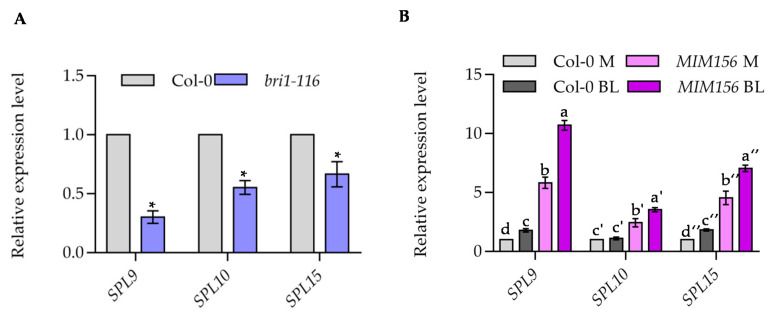
Given the important roles of BR on the progression from the juvenile phase to the adult phase, we speculated that BR regulate the expression of miR156 and SPL genes. To test this hypothesis, we analyzed the expression levels of SPL genes in wild type and BR-insensitive mutant bri1-116. Quantitative RT-PCR analysis showed that the expression levels of SPL9, SPL10 and SPL15 were significantly reduced in bri1-116 mutant (Figure 6A). To further analyze the effects of BR on the expression of SPL genes, we analyzed the transcript levels of SPL genes in wild type with or without BR treatment. The results showed that BR treatment induced the expression of SPL9, but had no significant effects on the expression of SPL10 and SPL15 in wild type plants (Figure 6B). In order to eliminate the effects of miR156 on the expression of SPL genes, we analyzed the regulation of BR on the expression of SPLs in MIM156 plants. The expression levels of SPL9, SPL10 and SPL15 were significantly increased in MIM156 compared to that in wild-type plants (Figure 6B). These results revealed that BR induces the expression of SPL9, SPL10 and SPL15.
Figure 6.
BR induces the expression of SPL9, SPL10 and SPL15. (A) Quantitative RT-PCR analysis the expression of SPL9, SPL10 and SPL15 in wild type and bri1-116 mutants. Seedlings of Col-0 and bri1-116 were grown on ½ MS medium under constant light for 7 days. PP2A gene was analyzed as an internal control. Error bars represent standard deviation of three independent experiments. Asterisk between bars indicated statistically significant differences between the samples (Student t test, * p < 0.05). (B) BR increased the transcript of SPL9, SPL10 and SPL15. Seedlings of wild type and MIM156 were grown on ½ MS medium for 7 days, and then treated with 100 nM BL for 3 h. PP2A gene was analyzed as an internal control. Error bars represent standard deviation of three independent experiments. Different letters above the bars indicated statistically significant differences between the samples (ANOVA analysis followed by Uncorrected Fisher’s LSD multiple comparisons test, p < 0.05).
2.6. BZR1 and SPL9 Coordinately Regulate the Expression of Downstream Genes
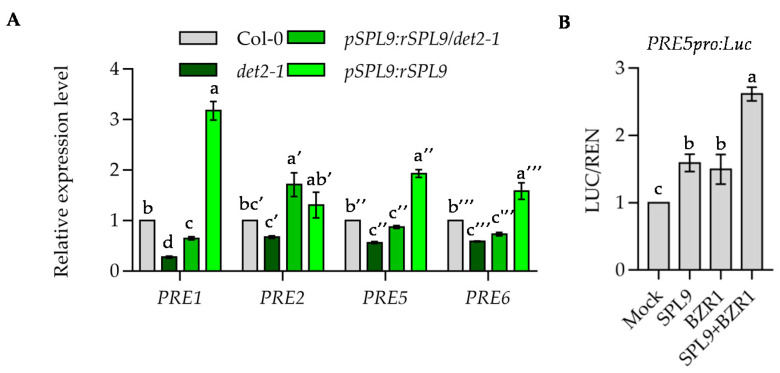
The HLH transcription factors PRE promote cell elongation participating in various hormones and environmental signals. Our previous study showed that BZR1 directly binds to the promoters of PREs to induce their expression. To examine whether SPL9 promotes cell elongation by regulating the expression of PREs, we performed the quantitative RT-PCR analysis in wild type, det2-1, spl9/spl15, pSPL9:rSPL9 and pSPL9:rSPL9/det2-1. The results showed the expression levels of PRE1, PRE5 and PRE6 were similar in wild type and spl9/spl15, but much higher in pSPL9:rSPL9 lines than that in wild-type plants, suggesting SPL9 induces the expression of PRE1, PRE5 and PRE6 (Figure 7A and Figure S1D). Consistent with previous results, the transcript levels of PRE1, PRE5 and PRE6 were decreased in det2-1 mutants, but these decreased expression levels of these genes were partially suppressed by pSPL9:rSPL9 (Figure 7A). To test whether BZR1 and SPL9 cooperatively regulate the expression of PREs, we performed transient gene expression analysis by generating promoter-luciferase (LUC) reporter construct with PRE5 promoter in mesophyll protoplasts of Arabidopsis leaves. We observed that the luciferase activity derived from the pPRE5:LUC increased when BZR1 and SPL9 were transfected alone, and significantly induced when BZR1 and SPL9 were co-expressed (Figure 7B). These results indicated that BZR1 and SPL9 cooperatively induce the expression of PRE5.
Figure 7.
BZR1 and SPL9 cooperatively regulate the downstream gene expression. (A) Quantitative RT-PCR analysis the expression of members of PRE family in wild type and indicated plants. Seedlings of wild type, det2-1, pSPL9:rSPL9 and pSPL9:rSPL9/det2-1 were grown on ½ MS medium under constant light for 7 days. PP2A gene was analyzed as an internal control. Error bars represent standard deviation of three independent experiments. Different letters above the bars indicated statistically significant differences between the samples (ANOVA analysis followed by Uncorrected Fisher’s LSD multiple comparisons test, p < 0.05). (B) Transient assays show BZR1 and SPL9 cooperative activation of the pPRE5:LUC reporter gene. The construct containing pPRE5:LUC (luciferase) and 35S:REN (renilla luciferase) and constructs overexpressing the indicated effecters were transfected to Arabidopsis protoplasts simultaneously. The LUC activity was normalized to REN. Error bars indicate SD of three biological repeats. Different letters above the bars indicated statistically significant differences between the samples (ANOVA analysis followed by Uncorrected Fisher’s LSD multiple comparisons test, p < 0.05).
3. Discussion
Plant vegetative phase change is regulated by various of environmental and endogenous signals largely through influencing the activity of miR156-SPL module. Here, we showed that BR play critical roles for the transition from the juvenile to adult stage. BR-deficient mutant det2-1 and BR-insensitive mutant bri1-301 both displayed the round leaves and the increased ratio of leaf width to length, while overexpression of BRI1 or DWF4 exhibited the decreased ratio of leaf width to length. BR treatment significantly increased the expression of SPL9. Activated SPL9 by overexpression of MIM156 or miR156-resistant form rSPL9 resulted in the hypersensitivity to BR and partially suppressed the dwarf phenotypes of det2-1 and bri1-301. Furthermore, we showed that BZR1 interacts with SPL9 to coordinately regulate the expression of downstream genes. Our work reveals that age and BR pathways are integrated to regulate plant growth and development through the direct physical interaction between SPL9 and BZR1.
BRs function as one type of growth-promoting hormones and are biosynthesized in the young tissues to promote plant growth and development [32]. BR biosynthesis defect resulted in delayed plant growth, rounded rosette leaves and the increased the blade base angle, suggesting BR is involved in the vegetative phase change of plants [34,36,50]. However, the molecular mechanism by which BR regulates the vegetative phase change remains unclear. In this study, we showed that BZR1 interacted with SPL9 to integrate the age and BR signaling pathways to regulate the vegetative phase change. Mutation of BR receptor BRI1 resulted in the significant reduced expression levels of SPL9, SPL10 and SPL15, which prolonged the vegetative phase. The round rosette leaves of BR-deficient mutant det2-1 was suppressed by the pSPL9:rSPL9, suggesting SPL9 regulate the vegetative growth of plants downstream of BR signaling. In addition, SPL9 directly interacted with BZR1 to promote the downstream gene expression. These results indicated that BR promotes the vegetative phase change through dual regulation of activity of SPL9 at the transcriptional and post-transcriptional levels.
MiR156 and its target gene SPL transcription factors are reported to regulate a wide range of plant growth and development by modulating the biosynthesis and signal transduction of plant hormones [9,10]. DELLA proteins, the key repressors of Gibberellin acid (GA) signaling pathway, interact with SPL9 to interfere SPL9 transcriptional activity and consequently delay floral transition and inhibit axillary meristem initiation [30,51]. In common wheat (Triticum aestivum), TaSPL8 knock-out mutants displayed the erect leaves and increased spike number in high planting density. TaSPL8 directly binds to the promoters of BR biosynthesis gene CYP90D2 and AUXIN RESPONSE FACTOR to induce their expression, suggesting TaSPL8 might increase lamina joint through auxin signaling and BR biosynthesis [52]. Here, in this study, we showed that the dwarf phenotypes of det2-1 and bri1-301 were partially suppressed by the pSPL9:rSPL9 transgenic plants. The activation by expression rSPL9 or MIM156 resulted in the increased BR sensitivity and overexpression of miR156B partially suppressed the PPZ resistance of bzr1-1D. The spl9/spl15 mutants displayed the BR response similar to that of wild type, which may be due to the functional redundancy of SPL gene family. BR induces the expression of several SPL genes. BZR1, the master regulator of BR signaling pathway, interacts with SPL9 to coordinately regulate the downstream gene expression. These results indicated that SPL9 is a positive regulator of BR signaling pathway, and work together with BZR1 to promote cell elongation and vegetative phase change in Arabidopsis.
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
Arabidopsis thaliana Col-0 accession was the wild-type used as control for all phenotypic comparisons. Plants for general growth and seed harvesting were grown in the green house with 16-h light/8-h dark photoperiod at 22–24 °C. Arabidopsis lines used in this research include: miR156B-Ox, MIM156, pSPL9:rSPL9 [9,28]; det2-1, bri1-116, bir1-301, DWF4-Ox, bin2-1 [43,48,53,54,55]; pSPL9:rSPL9/det2-1, pSPL9:rSPL9/bri1-301 (generated by pSPL9:rSPL9 crossing with det2-1 or bri1-301). For hypocotyl length, leaf phase (width/length), blade base angle measurement, the seedlings were scanned by aK708 scanner (BenQ, Shanghai, China), and measured using ImageJ software (National Institute of Mental Health, Bethesda, MD, USA). For rosette leaf numbers measurement, plants were grown under short-day conditions (8 h light/16 h dark) until flowering.
4.2. Plasmid and Transgenic Plants
Full-length cDNA of SPL9 without stop codon were amplified by PCR and cloned into pENTR TM /SD/D-TOPO TM vectors (Thermo Fisher, Waltham, MA, USA) and then recombined with destination vector pGAL4BDGW (GAL4BD-X), pDEST15 (N-GST), and pX-nYFP (p35S::X-nYFP). For bzr1-1D-Ox transgenic plants, the constructs of bzr1-1D were performed using the quick-change site-directed mutagenesis kit (Strategene), and then recombined with destination vector pX-YFP (p35S::bzr1-1D-YFP), finally, introduced into Agrobacterium tumefaciens (strain GV3101), and transformed into Col-0 plants by the floral dipping method.
4.3. In Vitro Pull-Down Assays
Glutathione beads containing 1 µg of GST-SPL9 were incubated with 1 µg MBP or MBP- BZR1, which were purified from bacteria, in incubation buffer (20 mM Tris-HCl pH 7.5, 100 mM NaCl, 1 mM EDTA) at 4 °C for 1 h, and the beads were washed 10 times with wash buffer (20 mM Tris-HCl pH 7.5, 300 mM NaCl, 0.5% TritonX-100, 1 mM EDTA). The beads binding proteins complex were eluted with 2 × SDS sample buffer, and separated on 8% SDS-PAGE gels, then analyzed with anti-MBP (NEB, 1:5000 dilution) and anti-GST (Santa Cruz Biotechnology, Dallas, TX, USA, 1:5000 dilution) antibodies.
4.4. Bimolecular Fluorescence Complementation Assays
Full-length coding sequences of SPL9 and BZR1 were fused in-frame with the N-terminal of YFP and C-terminal of YFP, respectively. Agrobacterial suspensions containing SPL9-nYFP or BZR1-cYFP constructs were injected into tobacco (Nicotiana tabacum) leaves simultaneously. The transfected plants were cultivated in the greenhouse for at least 36 h at 22 °C, and then were used to analyze fluorescent signals using an LSM700 laser scanning confocal microscope (Zeiss, Oberkochen, Germany).
4.5. RNA Extraction, Reverse Transcription and Real-Time PCR
Total RNA was extracted from wild type and various mutants grown on ½ MS medium with 1% sucrose under constant light for 7 days using Trizol RNA extraction kit (TransGen Biotech, Beijing, China). First-strand cDNA were synthesized using RevertAid reverse transcriptase (Thermo Fisher, Waltham, MA, USA). Quantitative PCR analyses were performed on a CFX connect real-time PCR detection system (Bio-Rad, Hercules, CA, USA) using a SYBR green reagent (Roche, Basel, Switzerland) with gene-specific primers.
4.6. Transient Gene Expression Assays
Protoplast isolation and PEG transformation was carried out as described previously [56,57]. Protoplasts were harvested by centrifugation and lysed in 100 µL of passive lysis buffer (Promega, Madison, WI, USA). Firefly and Renilla (as internal standard) luciferase activities were measured using a dual-luciferase reporter kit (Promega, Madison, WI, USA).
5. Conclusions
In summary, we found that BR promotes the vegetative phase change by regulating SPL9 activity at both transcriptional and post-transcriptional levels. BR-deficient or -insensitive mutants displayed the round leaf and reduced leaf blade base angle, whereas BRI1-Ox and DWF4-Ox showed the slender leaf and increased leaf base angle. BR induces the expression of SPLs. SPL9 interacted with BZR1 to coordinately regulate the downstream gene expression. These results demonstrated that SPL9 regulates cell elongation and vegetative phase change downstream of developmental and hormonal signals.
Acknowledgments
We thank Haiyan Yu and Xiaomin Zhao from the Analysis and Testing Center of SKLMT (State Key Laboratory of Microbial Technology, Shandong University) for assistance with the laser scanning confocal microscopy. We thank Professor Jiawei Wang for providing the seeds of p35S:miR156B, p35S:MIM156, pSPL9:rSPL9, pRGA:RGA∆17, pSPL9:rSPL9/pRGA:RGA∆17 and pSPL9:rSPL9-GR.
Abbreviations
| BR | Brassinosteroid |
| PPZ | propiconazole |
| BZR1 | BRASSINAZOLE-RESISTANT 1 |
| SPL | SQUAMOSA PROMOTER BINDING PROTEIN-LIKE |
| miR156B-Ox | miR156 overexpression mutant |
| MIM156 | miR156 repression mutant |
| PRE | Paclobutrazol-resistance |
| GST | Glutathione S-transferase |
| YFP | Yellow fluorescence protein |
| MBP | Maltose-binding protein |
| 3-AT | 3-amino-1,2,4-triazole |
| BiFC | Bimolecular fluorescence complementation |
| BD | Binding domain |
| AD | Acting domain |
| RT-PCR | Quantitative real-time PCR |
| Co-IP | Co-immunoprecipitation |
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/ijms221910415/s1.
Author Contributions
L.W., P.Y. and M.F. designed the experiments; L.W., P.Y., J.L. and Y.H. conducted the experiments; C.H. and M.-Y.B. provided the critical discussion on the work; and L.W. and M.F. wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
Please add: This research was funded by the National Natural Science Foundation of China (grants no. 31970306, 32070210, and 31870262), by the Shandong Province Natural Science Foundation (grants no. 2019LZGC015 and ZR2019ZD16) and by China Postdoctoral Science Foundation (grant no. 2019M662333).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Baurle I., Dean C. The timing of developmental transitions in plants. Cell. 2006;125:655–664. doi: 10.1016/j.cell.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 2.Poethig R.S. Phase change and the regulation of developmental timing in plants. Science. 2003;301:334–336. doi: 10.1126/science.1085328. [DOI] [PubMed] [Google Scholar]
- 3.Yu S., Lian H., Wang J.W. Plant developmental transitions: The role of microRNAs and sugars. Curr. Opin. Plant Biol. 2015;27:1–7. doi: 10.1016/j.pbi.2015.05.009. [DOI] [PubMed] [Google Scholar]
- 4.Chuck G., Cigan A.M., Saeteurn K., Hake S. The heterochronic maize mutant Corngrass1 results from overexpression of a tandem microRNA. Nat. Genet. 2007;39:544–549. doi: 10.1038/ng2001. [DOI] [PubMed] [Google Scholar]
- 5.Wu G., Poethig R.S. Temporal regulation of shoot development in Arabidopsis thaliana by miR156 and its target SPL3. Development. 2006;133:3539–3547. doi: 10.1242/dev.02521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xie Y., Liu Y., Wang H., Ma X., Wang B., Wu G., Wang H. Phytochrome-interacting factors directly suppress MIR156 expression to enhance shade-avoidance syndrome in Arabidopsis. Nat. Commun. 2017;8:348. doi: 10.1038/s41467-017-00404-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu M., Leichty A.R., Hu T., Poethig R.S. H2A.Z promotes the transcription of MIR156A and MIR156C in Arabidopsis by facilitating the deposition of H3K4me3. Development. 2018;145:dev152868. doi: 10.1242/dev.152868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu Y., Zhang L., Wu G. Epigenetic regulation of juvenile-to-adult transition in plants. Front. Plant Sci. 2018;9:1048. doi: 10.3389/fpls.2018.01048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang J.W., Czech B., Weigel D. miR156-regulated SPL transcription factors define an endogenous flowering pathway in Arabidopsis thaliana. Cell. 2009;138:738–749. doi: 10.1016/j.cell.2009.06.014. [DOI] [PubMed] [Google Scholar]
- 10.Wu G., Park M.Y., Conway S.R., Wang J.W., Weigel D., Poethig R.S. The sequential action of miR156 and miR172 regulates developmental timing in Arabidopsis. Cell. 2009;138:750–759. doi: 10.1016/j.cell.2009.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu Y., Qian Z., Zhou B., Wu G. Age-dependent heteroblastic development of leaf hairs in Arabidopsis. New Phytol. 2019;224:741–748. doi: 10.1111/nph.16054. [DOI] [PubMed] [Google Scholar]
- 12.Ye B.B., Zhang K., Wang J.W. The role of miR156 in rejuvenation in Arabidopsis thaliana. J. Integr. Plant Biol. 2020;62:550–555. doi: 10.1111/jipb.12855. [DOI] [PubMed] [Google Scholar]
- 13.Gou J., Tang C., Chen N., Wang H., Debnath S., Sun L., Flanagan A., Tang Y., Jiang Q., Allen R.D., et al. SPL7 and SPL8 represent a novel flowering regulation mechanism in switchgrass. New Phytol. 2019;222:1610–1623. doi: 10.1111/nph.15712. [DOI] [PubMed] [Google Scholar]
- 14.Wang J.W. Regulation of flowering time by the miR156-mediated age pathway. J. Exp. Bot. 2014;65:4723–4730. doi: 10.1093/jxb/eru246. [DOI] [PubMed] [Google Scholar]
- 15.He J., Xu M., Willmann M.R., McCormick K., Hu T., Yang L., Starker C.G., Voytas D.F., Meyers B.C., Poethig R.S. Threshold-dependent repression of SPL gene expression by miR156/miR157 controls vegetative phase change in Arabidopsis thaliana. PLoS Genet. 2018;14:e1007337. doi: 10.1371/journal.pgen.1007337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu S., Cao L., Zhou C.M., Zhang T.Q., Lian H., Sun Y., Wu J., Huang J., Wang G., Wang J.W. Sugar is an endogenous cue for juvenile-to-adult phase transition in plants. Elife. 2013;2:e00269. doi: 10.7554/eLife.00269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feng S., Xu Y., Guo C., Zheng J., Zhou B., Zhang Y., Ding Y., Zhang L., Zhu Z., Wang H., et al. Modulation of miR156 to identify traits associated with vegetative phase change in tobacco (Nicotiana tabacum) J. Exp. Bot. 2016;67:1493–1504. doi: 10.1093/jxb/erv551. [DOI] [PubMed] [Google Scholar]
- 18.Schwarz S., Grande A.V., Bujdoso N., Saedler H., Huijser P. The microRNA regulated SBP-box genes SPL9 and SPL15 control shoot maturation in Arabidopsis. Plant Mol. Biol. 2008;67:183–195. doi: 10.1007/s11103-008-9310-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Franco-Zorrilla J.M., Valli A., Todesco M., Mateos I., Puga M.I., Rubio-Somoza I., Leyva A., Weigel D., Garcia J.A., Paz-Ares J. Target mimicry provides a new mechanism for regulation of microRNA activity. Nat. Genet. 2007;39:1033–1037. doi: 10.1038/ng2079. [DOI] [PubMed] [Google Scholar]
- 20.Todesco M., Rubio-Somoza I., Paz-Ares J., Weigel D. A collection of target mimics for comprehensive analysis of microRNA function in Arabidopsis thaliana. PLoS Genet. 2010;6:e1001031. doi: 10.1371/journal.pgen.1001031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Preston J.C., Hileman L.C. Functional evolution in the plant SQUAMOSA-PROMOTER BINDING PROTEIN-LIKE (SPL) gene family. Front. Plant Sci. 2013;4:80. doi: 10.3389/fpls.2013.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Riese M., Hohmann S., Saedler H., Munster T., Huijser P. Comparative analysis of the SBP-box gene families in P. patens and seed plants. Gene. 2007;401:28–37. doi: 10.1016/j.gene.2007.06.018. [DOI] [PubMed] [Google Scholar]
- 23.Xie K., Wu C., Xiong L. Genomic organization, differential expression, and interaction of SQUAMOSA promoter-binding-like transcription factors and microRNA156 in rice. Plant Physiol. 2006;142:280–293. doi: 10.1104/pp.106.084475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cardon G., Hohmann S., Klein J., Nettesheim K., Saedler H., Huijser P. Molecular characterisation of the Arabidopsis SBP-box genes. Gene. 1999;237:91–104. doi: 10.1016/S0378-1119(99)00308-X. [DOI] [PubMed] [Google Scholar]
- 25.Rhoades M.W., Reinhart B.J., Lim L.P., Burge C.B., Bartel B., Bartel D.P. Prediction of plant microRNA targets. Cell. 2002;110:513–520. doi: 10.1016/S0092-8674(02)00863-2. [DOI] [PubMed] [Google Scholar]
- 26.Schwab R., Ossowski S., Riester M., Warthmann N., Weigel D. Highly specific gene silencing by artificial microRNAs in Arabidopsis. Plant Cell. 2006;18:1121–1133. doi: 10.1105/tpc.105.039834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Usami T., Horiguchi G., Yano S., Tsukaya H. The more and smaller cells mutants of Arabidopsis thaliana identify novel roles for SQUAMOSA PROMOTER BINDING PROTEIN-LIKE genes in the control of heteroblasty. Development. 2009;136:955–964. doi: 10.1242/dev.028613. [DOI] [PubMed] [Google Scholar]
- 28.Wang J.W., Schwab R., Czech B., Mica E., Weigel D. Dual effects of miR156-targeted SPL genes and CYP78A5/KLUH on plastochron length and organ size in Arabidopsis thaliana. Plant Cell. 2008;20:1231–1243. doi: 10.1105/tpc.108.058180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tian C., Zhang X., He J., Yu H., Wang Y., Shi B., Han Y., Wang G., Feng X., Zhang C., et al. An organ boundary-enriched gene regulatory network uncovers regulatory hierarchies underlying axillary meristem initiation. Mol. Syst. Biol. 2014;10:755. doi: 10.15252/msb.20145470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang Q.Q., Wang J.G., Wang L.Y., Wang J.F., Wang Q., Yu P., Bai M.Y., Fan M. Gibberellin repression of axillary bud formation in Arabidopsis by modulation of DELLA-SPL9 complex activity. J. Integr. Plant Biol. 2020;62:421–432. doi: 10.1111/jipb.12818. [DOI] [PubMed] [Google Scholar]
- 31.Chaiwanon J., Wang W., Zhu J.Y., Oh E., Wang Z.Y. Information integration and communication in plant growth regulation. Cell. 2016;164:1257–1268. doi: 10.1016/j.cell.2016.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Clouse S.D., Sasse J.M. BRASSINOSTEROIDS: Essential regulators of plant growth and development. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1998;49:427–451. doi: 10.1146/annurev.arplant.49.1.427. [DOI] [PubMed] [Google Scholar]
- 33.Li J., Wen J., Lease K.A., Doke J.T., Tax F.E., Walker J.C. BAK1, an Arabidopsis LRR receptor-like protein kinase, interacts with BRI1 and modulates brassinosteroid signaling. Cell. 2002;110:213–222. doi: 10.1016/S0092-8674(02)00812-7. [DOI] [PubMed] [Google Scholar]
- 34.Nam K.H., Li J. BRI1/BAK1, a receptor kinase pair mediating brassinosteroid signaling. Cell. 2002;110:203–212. doi: 10.1016/S0092-8674(02)00814-0. [DOI] [PubMed] [Google Scholar]
- 35.Santiago J., Henzler C., Hothorn M. Molecular mechanism for plant steroid receptor activation by somatic embryogenesis co-receptor kinases. Science. 2013;341:889–892. doi: 10.1126/science.1242468. [DOI] [PubMed] [Google Scholar]
- 36.Wang X., Kota U., He K., Blackburn K., Li J., Goshe M.B., Huber S.C., Clouse S.D. Sequential transphosphorylation of the BRI1/BAK1 receptor kinase complex impacts early events in brassinosteroid signaling. Dev. Cell. 2008;15:220–235. doi: 10.1016/j.devcel.2008.06.011. [DOI] [PubMed] [Google Scholar]
- 37.Kim T.W., Guan S., Burlingame A.L., Wang Z.Y. The CDG1 kinase mediates brassinosteroid signal transduction from BRI1 receptor kinase to BSU1 phosphatase and GSK3-like kinase BIN2. Mol. Cell. 2011;43:561–571. doi: 10.1016/j.molcel.2011.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim T.W., Guan S., Sun Y., Deng Z., Tang W., Shang J.X., Sun Y., Burlingame A.L., Wang Z.Y. Brassinosteroid signal transduction from cell-surface receptor kinases to nuclear transcription factors. Nat. Cell Biol. 2009;11:1254–1260. doi: 10.1038/ncb1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tang W., Kim T.W., Oses-Prieto J.A., Sun Y., Deng Z., Zhu S., Wang R., Burlingame A.L., Wang Z.Y. BSKs mediate signal transduction from the receptor kinase BRI1 in Arabidopsis. Science. 2008;321:557–560. doi: 10.1126/science.1156973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhu J.Y., Li Y., Cao D.M., Yang H., Oh E., Bi Y., Zhu S., Wang Z.Y. The F-box protein KIB1 mediates brassinosteroid-induced inactivation and degradation of GSK3-like kinases in Arabidopsis. Mol. Cell. 2017;66:648–657. doi: 10.1016/j.molcel.2017.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.He J.X., Gendron J.M., Yang Y., Li J., Wang Z.Y. The GSK3-like kinase BIN2 phosphorylates and destabilizes BZR1, a positive regulator of the brassinosteroid signaling pathway in Arabidopsis. Proc. Natl. Acad. Sci. USA. 2002;99:10185–10190. doi: 10.1073/pnas.152342599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tang W., Yuan M., Wang R., Yang Y., Wang C., Oses-Prieto J.A., Kim T.W., Zhou H.W., Deng Z., Gampala S.S., et al. PP2A activates brassinosteroid-responsive gene expression and plant growth by dephosphorylating BZR1. Nat. Cell Biol. 2011;13:124–131. doi: 10.1038/ncb2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang Z.Y., Nakano T., Gendron J., He J., Chen M., Vafeados D., Yang Y., Fujioka S., Yoshida S., Asami T., et al. Nuclear-localized BZR1 mediates brassinosteroid-induced growth and feedback suppression of brassinosteroid biosynthesis. Dev. Cell. 2002;2:505–513. doi: 10.1016/S1534-5807(02)00153-3. [DOI] [PubMed] [Google Scholar]
- 44.Yin Y., Wang Z.Y., Mora-Garcia S., Li J., Yoshida S., Asami T., Chory J. BES1 accumulates in the nucleus in response to brassinosteroids to regulate gene expression and promote stem elongation. Cell. 2002;109:181–191. doi: 10.1016/S0092-8674(02)00721-3. [DOI] [PubMed] [Google Scholar]
- 45.Sun Y., Fan X.Y., Cao D.M., Tang W., He K., Zhu J.Y., He J.X., Bai M.Y., Zhu S., Oh E., et al. Integration of brassinosteroid signal transduction with the transcription network for plant growth regulation in Arabidopsis. Dev. Cell. 2010;19:765–777. doi: 10.1016/j.devcel.2010.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yu X., Li L., Zola J., Aluru M., Ye H., Foudree A., Guo H., Anderson S., Aluru S., Liu P., et al. A brassinosteroid transcriptional network revealed by genome-wide identification of BESI target genes in Arabidopsis thaliana. Plant J. 2011;65:634–646. doi: 10.1111/j.1365-313X.2010.04449.x. [DOI] [PubMed] [Google Scholar]
- 47.Oh E., Zhu J.Y., Bai M.Y., Arenhart R.A., Sun Y., Wang Z.Y. Cell elongation is regulated through a central circuit of interacting transcription factors in the Arabidopsis hypocotyl. Elife. 2014;3:e03031. doi: 10.7554/eLife.03031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Oh E., Zhu J.Y., Wang Z.Y. Interaction between BZR1 and PIF4 integrates brassinosteroid and environmental responses. Nat. Cell Biol. 2012;14:802–809. doi: 10.1038/ncb2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tian Y., Fan M., Qin Z., Lv H., Wang M., Zhang Z., Zhou W., Zhao N., Li X., Han C., et al. Hydrogen peroxide positively regulates brassinosteroid signaling through oxidation of the BRASSINAZOLE-RESISTANT1 transcription factor. Nat. Commun. 2018;9:1063. doi: 10.1038/s41467-018-03463-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li J., Nagpal P., Vitart V., McMorris T.C., Chory J. A role for brassinosteroids in light-dependent development of Arabidopsis. Science. 1996;272:398–401. doi: 10.1126/science.272.5260.398. [DOI] [PubMed] [Google Scholar]
- 51.Yu S., Galvao V.C., Zhang Y.C., Horrer D., Zhang T.Q., Hao Y.H., Feng Y.Q., Wang S., Schmid M., Wang J.W. Gibberellin regulates the Arabidopsis floral transition through miR156-targeted SQUAMOSA promoter binding-like transcription factors. Plant Cell. 2012;24:3320–3332. doi: 10.1105/tpc.112.101014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu K., Cao J., Yu K., Liu X., Gao Y., Chen Q., Zhang W., Peng H., Du J., Xin M., et al. Wheat TaSPL8 modulates leaf angle through auxin and brassinosteroid signaling. Plant Physiol. 2019;181:179–194. doi: 10.1104/pp.19.00248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bai M.Y., Shang J.X., Oh E., Fan M., Bai Y., Zentella R., Sun T.P., Wang Z.Y. Brassinosteroid, gibberellin and phytochrome impinge on a common transcription module in Arabidopsis. Nat. Cell Biol. 2012;14:810–817. doi: 10.1038/ncb2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li J.G., Fan M., Hua W., Tian Y., Chen L.G., Sun Y., Bai M.Y. Brassinosteroid and Hydrogen Peroxide Interdependently Induce Stomatal Opening by Promoting Guard Cell Starch Degradation. Plant Cell. 2020;32:984–999. doi: 10.1105/tpc.19.00587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang L., Tian Y., Shi W., Yu P., Hu Y., Lv J., Fu C., Fan M., Bai M.Y. The miR396-GRFs Module Mediates the Prevention of Photo-oxidative Damage by Brassinosteroids during Seedling De-Etiolation in Arabidopsis. Plant Cell. 2020;32:2525–2542. doi: 10.1105/tpc.20.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wu F.H., Shen S.C., Lee L.Y., Lee S.H., Chan M.T., Lin C.S. Tape-Arabidopsis Sandwich—A simpler Arabidopsis protoplast isolation method. Plant Methods. 2009;5:16. doi: 10.1186/1746-4811-5-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yoo S.D., Cho Y.H., Sheen J. Arabidopsis mesophyll protoplasts: A versatile cell system for transient gene expression analysis. Nat. Protoc. 2007;2:1565–1572. doi: 10.1038/nprot.2007.199. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.