Abstract
Disease outbreaks often highlight existing inequalities and injustices within society. The COVID-19 pandemic has underscored long-existing health inequalities, both within countries and between the Global North and South. These disparities have been observed throughout the pandemic, from disparities in the severity and impact of the initial waves of cases to disparities in who was most protected during the roll-out of vaccination (1–3). As the Delta variant surges in many countries, structural inequalities shape the trajectory of the pandemic and exacerbate existing health disparities. In the age of vaccination, the “double burden” of disparities in both exposure to infection and vaccination coverage intersect to determine the current and future patterns of infection, immunity, and mortality. It is important to consider the ways in which these disparities, with overlapping but distinct drivers, interact to determine population-level immunity and the burden of COVID-19 in different communities. Individuals or communities can experience different pathways to immunity, whether through infection, vaccination, or both. Using San Francisco as a case study, we show how a sero-epidemiological approach can illuminate disparities in the “pathway to immunity”.
Structural disparities in SARS-CoV-2 infection rates and vaccine coverage
During the initial waves of the COVID-19 pandemic, disparities in disease burden were largely driven by differences in infection rates, as a result of factors including occupation, ability to shelter in place or to take sick leave, access to testing, housing status and crowding, and neighborhood exposure. In addition to driving disparities in infection rates with this novel virus, existing structural inequalities are associated with disparities in the risk of comorbidities such as diabetes and heart disease (as a result of factors such as, but not limited to, nutrition, access to exercise and increased stress), which increase the likelihood of hospitalization and death from COVID-19, and with disparities in access to healthcare both in managing comorbidities and in accessing care for COVID-19.
As vaccine roll-outs advance in the United States and globally, there are disparities in both vaccine access and uptake. These disparities are multifactorial and complex, including reduced technology access and literacy (4), barriers in access to healthcare, concern about the safety of the vaccines (5), mistrust as a result of a history of medical racism and discrimination, and poor access to reliable information about the vaccine. In the age of vaccination, policymakers must understand the way in which societal structures affect disparities in both infection and vaccination. These disparities may interact to affect both population level immunity and the burden of COVID-19 in different communities. This is relevant both in the present and in the future, as policymakers consider the impact of new variants and the distribution of booster vaccinations, as well as preparing for and responding to other diseases.
Serology can disentangle the double burden of disparities in infection and vaccination
Given the high levels of disease under-ascertainment, serology (i.e., the measurement of antibodies) has been particularly useful for understanding SARS-CoV-2 infection levels in the population. When there is variability in testing rates and healthcare seeking behavior, serology is an even more useful tool. Serology provides a unique opportunity to measure biomarkers of infection and vaccination simultaneously, and to relate these metrics to demographic and geographic factors. In settings where vaccines based on the SARS-CoV-2 spike protein (e.g., currently available mRNA or adenovirus vector vaccines) are used, measuring long-lived antibody responses to both spike and non-spike proteins can be used to disentangle immune responses elicited by infection from vaccination. While structural inequalities are by no means limited to the United States, here we focus on a case example leveraging serology to understand inequalities from a domestic perspective.
San Francisco as a case study
Even in San Francisco, a city which has had a relatively successful early and sustained COVID-19 response, and has achieved high vaccination coverage over the past few months, reported case counts of COVID-19 and hospitalization rates have been higher in socioeconomically deprived areas, amongst homeless individuals, and within the city’s Latinx and Black communities (6, 7). Disparities in vaccination coverage have also been reported, particularly in the early months of vaccine roll-out, creating a double burden for some vulnerable communities. In San Francisco, whilst some disparities have now been addressed, vaccination remains much lower in homeless individuals and in Black/African American individuals(8).
To measure disparities in both infection rates and vaccination, we leveraged a SARS-CoV-2 serosurveillance platform launched in March 2020 that utilizes residual blood samples taken from two hospital networks in San Francisco. Estimates derived from this platform during the first wave of the pandemic showed seroprevalence in Latinx individuals to be nearly two times higher than in white individuals, and nearly two times higher in homeless individuals than the population average (9). We collected samples from 1,014 individuals undergoing routine blood draws between February 4 and February 17, 2021, capturing transmission during the first 11 months of the epidemic and the early roll-out of vaccination. These samples were tested using two serologic assays: one detecting antibodies to SARS-CoV-2 elicited by infection and not by vaccines currently used in the US, and one detecting antibodies to SARS-CoV-2 elicited by both infection and vaccination. We used Bayesian statistical models to estimate the proportion of the population that was seropositive due to natural infection and the proportion seropositive due to vaccination, stratified by age, race and ZIP code of residence. We found that 28.2% of the tested population had any antibodies to SARS-CoV-2, and 8.9% had been previously infected.
We identified striking differences in prior infection rates and vaccination rates across the city (Figure 1). Across all age and demographic groups, ZIP codes in the southeastern region of the city, comprising medically underserved neighborhoods, had demonstrably higher rates of prior infection and lower rates of vaccination. We identified stark differences in prior infection rates by race/ethnicity: we estimated that the risk of prior infection of Latinx residents was 5.4 times greater than the risk of white residents (Figure 2). These trends were echoed in older individuals (aged 65+) as well (see Supplement). We identified disparities in vaccination coverage among the 65+ year old population, who were eligible to receive the vaccine during this time period. We estimated that despite being approximately half as likely to have been previously infected, white San Francisco residents over the age of 65 were twice as likely to be vaccinated as Black residents (see Supplement).
Figure 1:
Map of geographic disparities in SARS-CoV-2 showing the estimated probability of (a) prior infection and (b) vaccination by ZIP code in San Francisco, as of February 2021.
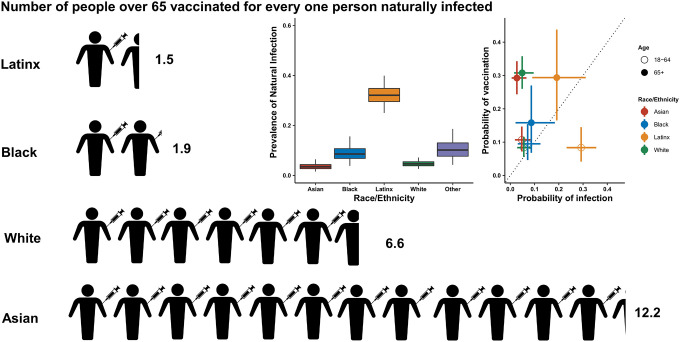
Figure 2: Relationship between probability of vaccination and probability of prior infection by race/ethnicity.
Infographic showing the number of estimated people vaccinated for every one person previously naturally infected in San Francisco within each racial/demographic group. The two graphs (inserts) show the estimated prevalence of natural infection by race/ethnicity and the probability of past infection plotted against the probability of vaccination by age and race ethnicity. Latinx includes all residents who identify as Hispanic/Latinx regardless of racial identity. Due to small sample sizes, individuals who identify as ‘other’ are not shown here.
Taken together, these findings imply that there is an imbalance between the risk of infection and the rate of vaccination in certain populations. Among the 65+ year old population, we found greatly increased ratios of vaccination compared to infection risk among Asian and white individuals, while this was much lower among Black and Latinx individuals (Figure 2). For every naturally infected Asian resident of this age group, there were 12 vaccinated Asian residents, whereas for every naturally infected Latinx resident of this age group, there were only 1.5 vaccinated Latinx residents. For both Latinx and Black individuals over 65 years old, the risk of having immunity acquired through vaccination, relative to natural infection, was up to four times lower than for white individuals (see Supplement).
Looking beyond San Francisco
The “double burden” we observed in San Francisco during the early vaccine roll-out echoes broader patterns that have been observed in San Francisco and elsewhere. Even though San Francisco was hailed as the first major US city to reach the milestone of 80% vaccination coverage in adults (10), recent increases in infection have been found to be concentrated in the neighborhoods which were hardest hit by initial infections and where we found vaccination-related immunity was lowest (11). A report from the University of Texas found striking geographic and racial stratification of cases of COVID-19 and vaccination rates in Austin, Texas, which also closely mapped with indices of deprivation and social vulnerability over ZIP codes (12). Like in San Francisco, the neighborhoods which were predominantly Latinx communities and had higher indices of deprivation also had higher incidence of SARS-CoV-2 infection and lower vaccination coverage. Disparities in SARS-CoV-2 vaccination coverage among socially vulnerable populations have been documented across the United States (13) and in other parts of the world (14).
A call to action: immediate, downstream solutions and structural, upstream solutions
While inequalities revealed during COVID-19 are not new, the pandemic has highlighted the ways in which even a city such as San Francisco which invests deeply in public health and social safety nets still has deep structural inequalities, through a combination of higher infection rates, incompatibility of living or work conditions with risk reduction, and lower or delayed access to vaccines as they were rolled out.
Various initiatives are underway around the country to pinpoint geographic and other disparities in the context of COVID-19 (e.g., (15, 16)). However, as well as highlighting disparities, it is important to consider what successful testing and vaccination initiatives may look like and which may be learnt from in future measures. For example, within San Francisco, robust community-academic partnerships have been key for effectively responding to the pandemic in vulnerable communities (17) and for narrowing gaps in vaccination coverage, such as low-barrier neighborhood vaccination sites (18). Prospectively, as we gain a better understanding of waning immunity and the potential need for vaccine booster doses, considerations of equity will remain an important consideration for allocating resources. In the context of the United States, where vaccination and infection elicit different immune responses, serology provides a powerful lens through which we can quantify these disparities directly. In addition, it is important to consider the desired metric before conducting a serosurvey, as assays measure different pathways to immunity and any disparities in infection rates may be masked by using assays that measure overall antibody prevalence.
Since the early days of the pandemic, many policy recommendations have been made for ways to reduce health disparities in infection (19) and vaccination (20). Policymakers must invest in addressing both the upstream, structural drivers of health disparities, such as providing workers with a living wage, affordable housing, and access to quality healthcare and also downstream drivers such as improved community engagement, targeted testing and vaccination provision, and assistance with common barriers to accessing healthcare such as technology access/literacy, transport, and providing accessible health information in multiple languages. While the arrival of the SARS-CoV-2 vaccine has created a ‘light at the end of the tunnel’ for this pandemic, ongoing challenges that long predate COVID-19 in achieving and maintaining equity must also be considered.
Supplementary Material
Acknowledgements
We acknowledge sources of funding support, including from the Schmidt Science Fellows, in partnership with the Rhodes Trust (ST); Chan Zuckerberg Biohub Investigator program (BG); the ZSFG Department of Medicine and Division of HIV, ID, and Global Medicine; the MIDAS Coordination Center COVID-19 Urgent Grant Program (MIDASNI2020-5) by a grant from the National Institute of General Medical Science (3U24GM132013-02S2) (IR, ST, IRB); and the National Institutes of Health/National Institute of General Medical Sciences R35GM138361-02 (IRB). We acknowledge Dr. Carina Marquez for helpful comments on the manuscript. We acknowledge Valerie Green and Phillip Williamson at Creative Testing Solutions for performing the Roche testing. We acknowledge the groups of Dr. Kara Lynch and Dr. Alan Wu for facilitating the collection of samples at ZSFG. We acknowledge the groups of Dr. Lee Besana and Dr. Marcelina Coh for facilitating the collection of samples at UCSF.
References
- 1.Perry B. L., Aronson B., Pescosolido B. A., Pandemic precarity: COVID-19 is exposing and exacerbating inequalities in the American heartland. Proc. Natl. Acad. Sci. U. S. A. 118 (2021), doi: 10.1073/pnas.2020685118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mercado-Reyes M., Malagón-Rojas J. N., Zapata S., Rodriguez-Barraquer I., Toloza-Pérez Y. G., Wiesner M., Cucunubá Z. M., Hernández-Ortíz J. P., Acosta-Reyes J., Estupiñan M. I., Miranda M. C., Galindo M., Rubio V. V., Muñoz-Galindo L., Osorio-Velázquez E. G., Ibáñez-Pinilla E. A., Parra Barrera E. L., Bermúdez A. del P., Quinche G. G., Puerto-Castro G. M., Villar L. A., Franco-Muñoz C., Castellanos J., Navarro-Lechuga E., Valle E. M., Gore-Saravia N., Oviedo Arango J. D., Ospina-Martínez M. L., Seroprevalence of Anti-Sars-Cov-2 Antibodies in Colombia, 2020: A Population-Based Study (2021), (available at https://papers.ssrn.com/abstract=3890833). [DOI] [PMC free article] [PubMed]
- 3.Murthy B. P., Sterrett N., Weller D., Zell E., Reynolds L., Toblin R. L., Murthy N., Kriss J., Rose C., Cadwell B., Wang A., Ritchey M. D., Gibbs-Scharf L., Qualters J. R., Shaw L., Brookmeyer K. A., Clayton H., Eke P., Adams L., Zajac J., Patel A., Fox K., Williams C., Stokley S., Flores S., Barbour K. E., Harris L. Q., Disparities in COVID-19 Vaccination Coverage Between Urban and Rural Counties - United States, December 14, 2020-April 10, 2021. MMWR Morb. Mortal. Wkly. Rep. 70, 759–764 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Press Valerie G., Huisingh-Scheetz Megan, and Arora Vineet M., Inequities in Technology Contribute to Disparities in COVID-19 Vaccine Distribution. JAMA Health Forum. 2, e210264 (2021). [DOI] [PubMed] [Google Scholar]
- 5.Callaghan T., Moghtaderi A., Lueck J. A., Hotez P., Strych U., Dor A., Fowler E. F., Motta M., Correlates and disparities of intention to vaccinate against COVID-19. Soc. Sci. Med. 272, 113638 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chamie G., Marquez C., Crawford E., Peng J., Petersen M., Schwab D., Schwab J., Martinez J., Es D. J., Black D., Gandhi M., Kerkhoff A. D., Jain V., Sergi F., Jacobo J., Rojas S., Tulier-Laiwa V., Gallardo-Brown T., Appa A., Chiu C., Rodgers M., Hackett J., Kistler A., Hao S., Kamm J., Dynerman D., Batson J., Greenhouse B., DeRisi J., Havlir D. V., CLIAhub Consortium, SARS-CoV-2 Community Transmission disproportionately affects Latinx population during Shelter-in-Place in San Francisco. Clin. Infect. Dis. (2020), doi: 10.1093/cid/ciaa1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Imbert E., Kinley P. M., Scarborough A., Cawley C., Sankaran M., Cox S. N., Kushel M., Stoltey J., Cohen S., Fuchs J. D., Coronavirus Disease 2019 Outbreak in a San Francisco Homeless Shelter. Clinical Infectious Diseases. 73 (2021), pp. 324–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.San Francisco Department of Public Health, COVID-19 Vaccine Doses Given to San Franciscans by Demographics (2021), (available at https://data.sfgov.org/COVID-19/COVID-19-Vaccine-Doses-Given-to-San-Franciscans-by/wv2h-rqwk).
- 9.Routledge I., Epstein A., Takahashi S., Janson O., Hakim J., Duarte E., Turcios K., Vinden J., Sujishi K., Rangel J., Coh M., Besana L., Ho W.-K., Oon C.-Y., Ong C. M., Yun C., Lynch K., Wu A. H. B., Wu W., Karlon W., Thornborrow E., Peluso M. J., Henrich T. J., Pak J. E., Briggs J., Greenhouse B., Rodriguez-Barraquer I., Citywide serosurveillance of the initial SARS-CoV-2 outbreak in San Francisco using electronic health records. Nat. Commun. 12, 3566 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.City and County of San Francisco, San Francisco Reaches Milestone: 80% of Eligible Residents Have Received at Least One Vaccine Dose. (2021), (available at https://sfmayor.org/article/san-francisco-reaches-milestone-80-eligible-residents-have-received-least-one-vaccine-dose).
- 11.Neilson S., Who is the coronavirus surge impacting in San Francisco? (2021), (available at https://www.sfchronicle.com/health/article/Who-is-the-Covid-19-surge-impacting-in-San-16321854.php).
- 12.Woody Spencer, Javan Emily, Johnson Kaitlyn, Pasco Remy, JohnsonLeón Maureen, Lachmann Michael, Fox Spencer J., Meyers Lauren Ancel, “Spatial distribution of COVID-19 infections and vaccinations in Austin, Texas” (2021), doi: 10.15781/m60y-tc90. [DOI] [Google Scholar]
- 13.Hughes M. M., Wang A., Grossman M. K., Pun E., Whiteman A., Deng L., Hallisey E., Sharpe J. D., Ussery E. N., Stokley S., Musial T., Weller D. L., Murthy B. P., Reynolds L., Gibbs-Scharf L., Harris L., Ritchey M. D., Toblin R. L., County-Level COVID-19 Vaccination Coverage and Social Vulnerability - United States, December 14, 2020-March 1, 2021. MMWR Morb. Mortal. Wkly. Rep. 70, 431–436 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paton J., U.K. Vaccination Rates Struggle in Places Worst-Hit by Covid-19. Bloomberg News (2021), (available at https://www.bloomberg.com).
- 15.Advance OC, Orange County Equity Map (2020) (available at https://www.advanceoc.com/orange-county-equity-map/).
- 16.Dottle R., Tartar A., Greenfield R., Qiu Y., Tracking Covid Vaccines by Race: Black and Hispanic Rates Lower Than White and Asian (2021), Report (available at https://www.bloomberg.com/graphics/covid-vaccine-tracker-global-distribution/us-vaccine-demographics.html).
- 17.Jessica Fields, Raul Gutierrez J, Carina Marquez, Kim Rhoads, Margot Kushel, Alicia Fernández, Gabriel Chamie, Francine Rios-Fetchko, Elizabeth Imbert, Cynthia Nagendra, Eva Pardo, Ghilamichael Andemeskel, Susana Rojas, Jon Jacobo, Valerie Tulier-Laiwa, Monique LeSarre, Gerald Green, Michael Shaw, Chris Iglesias, Jane Garcia, Aaron Ortiz, Giuliana Martinez, Andrés Aranda-Díaz, Gwendolyn Westbrook, Michelle Pierce J., Calder Lorenz, Donna Hilliard, Isela Ford, Naveena Bobba, Kimi Watkins-Tartt, Diane Havlir, Kirsten Bibbins-Domingo, Community-Academic Partnerships to Address Covid-19 Inequities: Lessons from the San Francisco Bay Area. Catalyst non-issue content. 2, doi: 10.1056/CAT.21.0135. (2021). [DOI] [Google Scholar]
- 18.Marquez C., Kerkhoff A. D., Naso J., Contreras M. G., Castellanos E., Rojas S., Peng J., Rubio L., Jones D., Jacobo J., Rojas S., Gonzalez R., Fuchs J. D., Black D., Ribeiro S., Nossokoff J., Tulier-Laiwa V., Martinez J., Chamie G., Pilarowski G., DeRisi J., Petersen M., Havlir D. V., A multi-component, community-based strategy to facilitate COVID-19 vaccine uptake among Latinx populations: from theory to practice. medRxiv, 2021.06.07.21258230 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang M. L., Behrman P., Dulin A., Baskin M. L., Buscemi J., Alcaraz K. I., Goldstein C. M., Carson T. L., Shen M., Fitzgibbon M., Addressing inequities in COVID-19 morbidity and mortality: research and policy recommendations. Transl. Behav. Med. 10, 516–519 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fernández L., Shorett P., Lessons in Equity From the Front Lines of COVID-19 Vaccination. JAMA Health Forum. 2, e210612–e210612 (2021). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.