Abstract
Introduction:
Retrospective studies have shown improved diagnostic yield of combined cytology and cell blocks specimens from endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) with variable additional yields in cell blocks. In this prospective study, we assessed the diagnostic performance of cytology and cell blocks in patients undergoing EBUS-TBNA.
Methods:
This was a single-center, cross-sectional study conducted between December 2017 and November 2019 including patients aged ≥18 years with mediastinal lymphadenopathy. EBUS-TBNA was performed under conscious sedation using 22G needles. Both cytology smears and cell blocks by the tissue coagulum clot technique were prepared for each patient without rapid on-site evaluation.
Results:
Data were analyzed for 93 patients (mean age 54.25 ± 13.7 years, 73 males) where both cytology and cell blocks were available. Sample adequacy was 100%. Overall diagnostic yield either by cytology or cell block was 83%. Cytology yield was 79.6%, whereas cell block was diagnostic in 73% of patients (P < 0.001). The overall additional yield of cell blocks was 3.2%. Cell blocks had additional yields of 1.8%, 0%, and 14.3% in malignancy, tuberculosis, and sarcoidosis, respectively. Tumor histology was better identified in 76% of positive cell blocks, and accurate histological subtyping was possible in 32.6% cases. Immunohistochemistry was feasible in 82.5% of all positive cell blocks, and these were judged to be adequate for the mutational analysis.
Conclusions:
Compared to cytology, EBUS-TBNA cell blocks did not significantly increase the overall diagnostic yield in unselected patients. However, cell blocks are beneficial in the characterization of tumor morphology and histological subtyping of lung cancer.
KEY WORDS: Bronchoscopy, endobronchial ultrasound, lung cancer, pathology, tuberculosis, sarcoidosis
INTRODUCTION
Recent guidelines recommend an endobronchial ultrasound (EBUS) or endoscopic ultrasound-guided fine-needle aspiration alone or in combination as the modality of choice in the staging of lung cancer.[1,2] Conventionally, EBUS-guided transbronchial needle aspiration (EBUS-TBNA) specimens are processed for cytological examination, and preparation of cell blocks is not a universal practice. Cell blocks prepared from EBUS-TBNA specimens have been useful for the immunohistochemistry study and molecular testing in lung cancer, thus enabling clinicians for personalized therapy.[3,4] In addition, EBUS-TBNA cell blocks are crucial for the better identification of tumor morphology and specific histological sub-typing, particularly in non-small cell lung cancer (NSCLC).[3,4,5] Further, cell blocks examination is reported to increase the diagnostic yield in benign mediastinal lymphadenopathy related to granulomatous diseases.[6,7,8] Therefore, routine processing of EBUS-TBNA samples for both cytology and cell blocks could be an ideal approach in the evaluation of undiagnosed mediastinal adenopathy.
Usually, there are two ways of preparing cytology smears from EBUS-TBNA specimens such as Diff Quick staining of an air-dried slide for rapid on-site evaluation (ROSE) and by alcohol fixed slides using 95% alcohol.[5] There are different methods described for the preparation of cell blocks from EBUS-TBNA specimen. Commonly described methods are collecting the material into a small piece of filter paper to form a tissue coagulum, directly injecting the material to 10% formalin, and by using special methods such as Hank's solution.[4,5] Overall, cell blocks lead to an additional diagnostic yield of 8%–10% in malignancy and 16%–33% in the diagnosis of sarcoidosis.[4,5,6,7,8,9,10] Tissue coagulum clot (TCC) method is a simple and cheap technique for cell block preparation. Briefly, in this method, the specimen in the TBNA needle is expelled into a piece of filter paper, allowed to dry till the formation of a tissue coagulum, and then the filter paper is placed in the formalin.[5] Despite the additional yield and benefits such as immunohistochemistry and molecular testing, the role of cell blocks is under-appreciated. The World Association for Bronchology and Interventional Pulmonology task force guideline on specimen acquisition and preparation of EBUS-TBNA in the diagnosis of malignancy described either cell block or core tissue facilitates immunohistochemistry examination and better histological subtyping. However, the guideline did not clearly state whether the cell block technique performs better than cytology.[11] Several retrospective studies showed added benefits of cell blocks.[7,8,10,12,13,14] Therefore, we performed a prospective study comparing EBUS-TBNA cytology and TCC cell block in a heterogeneous population presenting with undiagnosed mediastinal adenopathy. The main objectives were to find out the overall yield of cytology and cell blocks and additional yield of cell blocks in different diseases.
METHODS
Procedural details
This was a single-center, prospective, cross-sectional study conducted in the pulmonary clinic of authors institute from December 2017 to November 2019. All adults aged 18 years or greater with undiagnosed mediastinal lymphadenopathy or mediastinal mass and patients with lung cancer for staging were included. Patients with hemodynamic instability, severe dyspnea with hypoxia, and coagulopathy were excluded. Prior to EBUS, white light bronchoscopy was performed in each case, and endobronchial biopsy, bronchoalveolar lavage, and transbronchial lung biopsy were done as indicated. Baseline demographic data, indication for the procedure, clinical diagnosis, and EBUS procedure details of all the patients were collected. EBUS-TBNA was performed in the bronchoscopy suite using the EBUS bronchoscope (PENTAX EB-1970UK) introduced either through oral or nasal routes. Lignocaine 2% solution was used for topical anesthesia. Bronchoscopist-directed conscious sedation was practiced using either intravenous midazolam with fentanyl or promethazine (25 mg) with pentazocine (15 mg) (used in one patient). All but one patient initially received 2 mg midazolam (1 mg if age >60 years) and 50 μg fentanyl followed by incremental doses of 1 mg and 30–50 mcg as decided by the bronchoscopist. EBUS-TBNA was performed by three consultant pulmonologists, and a single pathologist (SP) reported the slides. Mediastinal lymph nodes were systematically examined, and nodes with short axis ≥10 mm or ≥5 mm in case of the mediastinal staging of lung cancer were aspirated with 22G (echo–HD 22 COOK medical) needle. A minimum of three passes made per nodal station. Suction using a 10 ml vacuum syringe was applied in the second pass. After each pass, cytology smears were prepared and fixed with 95% ethanol and air-dried in equal numbers. ROSE facility was not available for this study. Mostly, cell blocks were prepared from the same specimen as that of cytology. If the sample was considered inadequate, a dedicated pass made for cell block on case to case basis. Cell blocks were prepared using the TCC method, as described previously. Briefly, as the material streamed out from the needle tip, it was collected onto a precut piece of filter paper as a cone-shaped coagulum of tissue and blood mixture.[5,10] The clot was slightly air-dried on the filter paper, gently slid into a formalin container and processed in the histology laboratory. After histological processing, staining with hematoxylin and eosin was used to assess cellularity and morphology. Immunohistochemistry was performed for the identification or phenotyping of malignant cells as required. The study was approved by the institutional ethics committee and all participants provided written informed consent.
Definitions
Sample adequacy was defined as the presence of lymphoid tissue or specimen was diagnostic of pathology. Reactive lymphadenitis was diagnosed when only lymphocytes were seen, but no definite pathological diagnosis possible. A diagnostic procedure was one that yielded a specific diagnosis such as malignancy, tuberculosis, and sarcoidosis. The additional yield of cell blocks is defined as the confirmation of a specific diagnosis in cell blocks where cytology was noncontributory or reactive lymphadenitis. The presence of noncaseating granuloma diagnosed sarcoidosis whereas caseating granuloma or positive acid-fast bacilli stain or XpertMTB/Rif assay confirmed the diagnosis of tuberculosis.
Statistical analysis
The continuous variable is described in mean ± standard deviation (SD) or when required, as medians and interquartile range and the categorical variables as proportions. McNemar's test was used to assess the statistical significance of the difference in yield between the cytology and cell blocks specimens. Data were analysed using the SPSS software version 22 (IBM Corporation, Armonk, N.Y., USA).
RESULTS
Out of 122 EBUS-TBNA performed during the study period, both cytology and cell block specimens were available in 93 cases and that constitutes our study population. The mean age (SD) was 54.25 ± 13.7 years, and the majority (73 patients [78.5%]) were male. The final diagnoses in 93 patients were as follows: malignancy 56 (60%) (50 lung cancer and six extra-thoracic primaries) patients, tuberculosis 16 (17%) patients, sarcoidosis 14 (15%) patients, melioidosis two patients, nonspecific interstitial pneumonitis two patients, and posttubercular lung fibrosis, vasculitis, and silicosis in one patient each. The extra-thoracic primaries included breast carcinoma in two cases, renal cell carcinoma, germ cell tumor, buccal carcinoma, and myelodysplastic syndrome in one patient each. EBUS-TBNA was performed for mediastinal nodes in 81 (87%) cases and peri-bronchial mass in 12 (13%) cases. A total of 416 passes were made in 131 lymph nodes averaging 3.17 per lymph node station [Table 1]. The sample adequacy was 100%. A definitive diagnosis either by cytology or cell block was achieved in 77 patients resulting in an overall yield of 83%. If we include the reactive lymphadenitis as a positive yield in five patients with nongranulomatous benign disorders (where reactive lymphadenitis is an expected change), the overall diagnostic yield increases to 88% (82/93). Overall, cytology yielded a diagnosis in 74 (79.6%) cases, whereas cell blocks were diagnostic in 68 (73%) patients (P = 0.145). There were only three patients where cell blocks were diagnostic, and cytology was noncontributory, resulting in 3.2% additional yield. The different diagnoses made on EBUS-TBNA were as follows: malignancy 51 cases, reactive lymphadenitis 16 cases, tuberculosis 13 cases, sarcoidosis 11 cases, melioidosis 2 cases (aspirate was positive for capsular polysaccharide antigen of Burkholderia pseudomallei in both patients, the aspirate culture grew B. pseudomallei in one patient, and blood culture grew B. pseudomallei in the other patient). The diagnostic yield of cytology and cell block according to the specific pathology is summarized in Table 2. All reactive lymphadenitis specimens were negative for tuberculosis by XpertMTB/Rif assay.
Table 1.
Endobronchial ultrasound-guided transbronchial needle aspiration characteristics in the study population
| Characteristics | n (%) |
|---|---|
| EBUS-TBNA only | 92 (99) |
| EUS-B-FNA only | 1 (1) |
| EBUS-TBNA plus EUS-B-FNA | 0 |
| Lymph node puncture | 81 (87) |
| Peri-bronchial mass puncture | 12 (13) |
| Oral route | 75 (81) |
| Nasal route | 18 (19) |
| Diagnostic EBUS | 91 (98) |
| Staging EBUS | 2 (2) |
| Sedation used | |
| Midazolam and fentanyl | 92 (99) |
| Pentazocine and promethazine | 1 (1) |
| Doses of drugs used, mean±SD | |
| Midazolam (mg) | 2.62±0.83 |
| Fentanyl (mcg) | 66.90±23.5 |
| Number of lymph nodes punctured | 131 |
| Average number of lymph node | 1.4 |
| punctured per patient | |
| Total number of passes made | 416 |
| Average number of passes per station | 3.17 |
| Number of passes with suction | 191 |
| Lymph nodes sampled as per stations (n=131) | |
| 7 | 57 (43.5) |
| 4R | 47 (35.8) |
| 4L | 8 (6.1) |
| 10R | 6 (4.5) |
| 10L | 6 (4.5) |
| 11L | 4 (3.05) |
| 10L | 3 (2.3) |
| Procedure time in minutes, mean±SD | 57.63±10.12 |
| Sample adequacy | 93 (100) |
| Overall diagnostic yield | 77 (83) |
| Different diagnoses made of the procedure | |
| Malignancy | 51 (55) |
| Metastatic lung cancer | 46 (49) |
| Metastatic adenocarcinoma (breast) | 2 (2) |
| Metastatic renal cell carcinoma | 1 (1) |
| Metastatic buccal carcinoma | 1 (1) |
| Metastatic germ cell tumor | 1 (1) |
| Benign diseases | 42 (45) |
| Reactive lymphadenitis | 16 (17) |
| Tuberculosis | 13 (14) |
| Sarcoidosis | 11 (12) |
| Melioidosis | 2 (2) |
| Complications | |
| Minor bleeding (self-controlled) | 5 (5) |
| Dyspnea requiring overnight | 2 (2) |
| Hospitalization | |
| Mediastinitis | 0 |
| Death | 0 |
EBUS-TBNA: Endobronchial ultrasound-guided transbronchial needle aspiration, EUS-B-FNA: Endoscopic ultrasound with a bronchoscope-guided fine-needle aspiration, SD: Standard deviation
Table 2.
Overall and disease specific diagnostic yield of endobronchial ultrasound-guided transbronchial needle aspiration cytology
| Final diagnosis in all patients (n=93) | Number of cases, n (%) | Overall yield, n (%) | Cyt (+) CB (−), n (%) | Cyt (−)* CB (+), n (%) | Cyt (+) CB (+), n (%) | Cyt (−) CB (−), n (%) |
|---|---|---|---|---|---|---|
| Malignancy | 56 (60) | 51 (91) | 5 (9) | 1 (2) | 45 (80) | 5 (9) |
| Lung cancer | 50 (54) | 46 (92) | 5 (10) | 1 (2) | 40 (80) | 4 (8) |
| Breast cancer | 2 (2) | 0 | 0 | 0 | 2 (100) | 0 |
| RCC | 1 (1) | 0 | 0 | 0 | 1 (100) | 0 |
| GCT | 1 (1) | 0 | 0 | 0 | 1 (100) | 0 |
| MDS | 1 (1) | 0 | 0 | 0 | 0 | 1 (100) |
| BC | 1 (1) | 0 | 0 | 0 | 1 (100) | 0 |
| Benign diseases | 37 (40) | 26 (70) | 4 (11) | 2 (5) | 20 (54) | 11 (30) |
| Tuberculosis | 16 (17) | 13 (81) | 4 (25) | 0 | 9 (64) | 3 (22) |
| Sarcoidosis | 14 (15) | 11 (79) | 0 | 2 (14) | 2 (100) | - |
| Melioidosis† | 2 (2) | 2 (100) | - | - | 0 | 2 (100) |
| NSIP | 2 (2) | 0 | 0 | 0 | 0 | 1 (100) |
| Vasculitis | 1 (1) | 0 | 0 | 0 | 0 | 1 (100) |
| Silicosis | 1 (1) | 0 | 0 | 0 | 0 | 1 (100) |
| Post-TB fibrosis | 1 (1) | 0 | 0 | 0 | ||
| Total | 93 (100) | 77 (83) | 9 (10) | 3 (3) | 65 (70) | 16 (17) |
*All cases those are cytology negative but cell block positive constitutes additional yield, †Melioidosis was diagnosed based on microbiological evidence in the aspirate and both cytology and cell block specimen showed necrotic material. N: Total number of study participants, n (%)-number percentage. Cyt (+): Cytology positive, CB (−): Cell block negative, RCC: Renal cell carcinoma, GCT: Germ cell tumor, MDS: Myelodysplastic syndrome, BC: Buccal carcinoma, NSIP: Nonspecific interstitial pneumonitis, TB: Tuberculosis
Subgroup analysis of malignancy cases
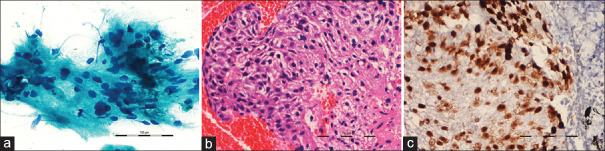
Of the 56 patients with malignancy, EBUS-TBNA was diagnostic in 51 patients (46 lung cancer and five extra-thoracic primaries), and reactive lymphadenitis was found in the remaining five patients. Subsequently, transthoracic lung biopsy confirmed primary lung cancer in four patients with reactive lymphadenitis, and one patient had established myelodysplastic syndrome. Cytology and cell blocks were positive for malignancy in 50 (89%) and 46 (82%) patients, respectively. Only one patient had cytology negative cell block positive result, constituting an additional yield of 1.8% in malignancy. Better tumor histology was identified in 76% (35/46) of positive cell blocks, and definite histological subtyping of NSCLC was possible in 32.6% (15/46) cases. Immunohistochemistry was feasible in 82.5% of all positive cell blocks, and these were judged to be adequate for the mutational analysis [Figure 1]. In 27 (48%) patients, EBUS-TBNA was the only diagnostic modality, whereas additional diagnostic procedures were performed in 29 (52%) cases. In one patient, there was a discrepancy in the diagnosis where EBUS-TBNA cytology was reported as NSCLC favoring adenocarcinoma and endobronchial biopsy confirmed squamous cell carcinoma.
Figure 1.
(a) Representative smear showing tumor cells with hyperchromatic pleomorphic nucleus and moderate cytoplasm (Papanicolaou × 400). (b) Section from the cell block showing fragments from a squamous cell carcinoma (H and E, ×200). (c) p40 immunopositivity in a section from cell block
Subgroup analysis of benign diseases
Out of 37 patients with benign disorders, EBUS-TBNA diagnosed tuberculosis in 13 patients, sarcoidosis in 11 patients, reactive lymphadenitis in 11 patients, and melioidosis in two patients. Among reactive lymphadenitis group, tuberculosis and sarcoidosis were diagnosed in three patients each based on clinical, radiological features, and response to therapy on follow-up. Thus, a final diagnosis of tuberculosis and sarcoidosis was made in 16 and 14 patients, respectively. In the remaining five patients with reactive lymphadenitis, the final diagnoses were nonspecific interstitial pneumonitis in two patients, vasculitis, silicosis, and posttubercular lung fibrosis in one patient each. The yield of cytology and cell blocks was 81% (13/16) and 56% (9/16), respectively, in tuberculosis, with a diagnostic accuracy of 81%. Cell blocks had no additional yield in tuberculosis. XpertMTB/Rif assay of EBUS-TBNA specimen was positive in seven patients. Among patients with sarcoidosis, cytology was diagnostic in 64.3% (9/14), whereas cell block diagnosed in 78.6% (11/14) cases resulting in an additional yield of 14.3% in sarcoidosis. All the 11 cell blocks stained positive in reticulin stain [Figure 2].
Figure 2.
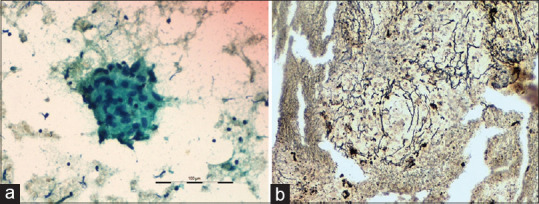
Smear showing epithelioid cell granuloma without any necrosis (Papanicolaou Stain ×400). (b) Reticulin stain on cell block section showing a reticulin rich granuloma (×200)
DISCUSSION
In this study, we found the overall yield of EBUS-TBNA cytology was nonsignificantly higher than the cell blocks in unselected patients with mediastinal lymphadenopathy. However, cell blocks were of additional value in malignancy by the better delineation of tumor morphology, and more confident histological subtyping of NSCLC was possible in immunohistochemistry. In the case of granulomatous lymphadenitis, cell blocks had no additional yield in tuberculosis, whereas these had a better yield in sarcoidosis.
The overall yield in our study is comparable to the published series.[15,16,17] The possible reasons for such a finding are the prospective nature of the study, a preponderance of malignancy cases, procedures performed by consultants, and reporting by a SP. Contrary to the previously published studies, the yield of cell blocks was lower in our study.[6,7,8,15] This may be because an adequate number of cytology smears were initially prepared from the material obtained in different passes, and the remaining material was utilized for cell blocks preparation. Further, a dedicated pass was not made for cell blocks in all cases.
In the subgroup of patients diagnosed with malignancy, the overall diagnostic yield was similar to previously published reports.[16,17] Rotolo et al. reported a combined cytology and cell blocks yield of 86%, whereas cell blocks alone were positive in 48% only.[18] Compared to their study, both the individual and combined yields of cytology and cell blocks are better in our study. In a previous study of 101 malignancy patients, EBUS-TBNA cytology and cell block yields were 95% and 93.5%, respectively.[4] Our findings are similar to this study except for a lower yield in cell blocks.
Previously, the diagnostic yield of TCC cell block technique was found significantly superior to normal saline rinsed cell block.[5] Similarly, Sanz-Santos et al. reported a 7% additional yield of TCC cell block method over cytology in patients with malignancy.[10] In 60% of cases, cell blocks were sufficient for the mutational analysis. Unlike their study, cell blocks had additional yield only in one patient in our study. However, the morphological characterization and histological subtyping of NSCLC were better in our study. The improved yield of cell block in the study by Sanz-Santos et al. is probably due to the preparation of cell blocks from the first pass or from second or third pass if clotted tissue was absent in first passes, whereas we prepared smears initially from the material expelled and remaining material was utilized for cell blocks. This might have led to less representative material in cell blocks contributing to lower yield.
Previously, EBUS-TBNA has been found to be an invaluable tool in the evaluation of granulomatous lymphadenopathy with a yield superior to conventional bronchoscopic techniques.[15,19,20] In addition, EBUS-TBNA cell blocks had increased diagnostic sensitivity for granulomatous lymphadenitis in the previous series.[6,13,14] In a retrospective study, cell blocks had an additional diagnostic yield of 45% among 84 patients with sarcoidosis.[6] Another retrospective analysis reported sensitivity and specificity of 100% for combined cytology and cell blocks examination in diagnosing granulomatous lymphadenitis.[13] Combining cytology and cell blocks, we found a sensitivity of 78.6% and an additional yield of 14.3% in sarcoidosis. The possible reason for a lower yield of cell blocks in our study is the smaller number of sarcoidosis patients. Furthermore, positive reticulin staining of all cell blocks in this study supplemented our diagnostic confidence.
The strength of the study is its prospective nature, collection of cytology, and cell block specimens from the same individual and not parallel groups and reporting by a SP to avoid interobserver bias. Finally, our study is not without limitations. First, three different bronchoscopists performed the procedure, and varying operator skills in specimen acquisition might have an impact on the overall diagnostic yield. Second, the lack of dedicated pass for cell blocks in all patients could have contributed to its lower yield. Third, the mutational analysis was not performed, and we assume that cell blocks subjected to immunohistochemistry are suitable for molecular testing.
CONCLUSIONS
In summary, TCC is a simple and convenient method of cell block preparation. The diagnostic yield of tissue coagulum cell blocks was not superior to cytology in patients with malignancy and tuberculosis, whereas it had a higher yield in sarcoidosis. In addition, cell blocks were very useful in characterizing tumor morphology, histological subtyping of lung cancer, and better identification of granuloma. Therefore, cell blocks should routinely be prepared in patients undergoing endobronchial ultrasound guided-transbronchial needle aspiration of mediastinal lymphadenopathy secondary to malignancy and granulomatous disease.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We would like to thank Dr. Anand Srinivasan, Department of Pharmacology, All India Institute of Medical Science, Bhubaneswar, India, for his guidance in sample size calculation and statistical analysis. We express our sincere gratitude to all patients who volunteered to participate in this study.
REFERENCES
- 1.Wahidi MM, Herth F, Yasufuku K, Shepherd RW, Yarmus L, Chawla M, et al. Technical Aspects of Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration: CHEST Guideline and Expert Panel Report. Chest. 2016;149:816–35. doi: 10.1378/chest.15-1216. [DOI] [PubMed] [Google Scholar]
- 2.Vilmann P, Clementsen PF, Colella S, Siemsen M, De Leyn P, Dumonceau JM, et al. Combined endobronchial and esophageal endosonography for the diagnosis and staging of lung cancer: European Society of Gastrointestinal Endoscopy (ESGE) Guideline, in cooperation with the European Respiratory Society (ERS) and the European Society of Thoracic Surgeons (ESTS) Endoscopy. 2015;47:545–59. doi: 10.1055/s-0034-1392040. [DOI] [PubMed] [Google Scholar]
- 3.Nakajima T, Yasufuku K, Suzuki M, Hiroshima K, Kubo R, Mohammed S, et al. Assessment of epidermal growth factor receptor mutation by endobronchial ultrasound-guided transbronchial needle aspiration. Chest. 2007;132:597–602. doi: 10.1378/chest.07-0095. [DOI] [PubMed] [Google Scholar]
- 4.Hopkins E, Moffat D, Parkinson I, Robinson P, Jersmann H, Dougherty B, et al. Cell block samples from endobronchial ultrasound transbronchial needle aspiration provide sufficient material for ancillary testing in lung cancer-a quaternary referral centre experience. J Thorac Dis. 2016;8:2544–50. doi: 10.21037/jtd.2016.08.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yung RC, Otell S, Illei P, Clark DP, Feller-Kopman D, Yarmus L, et al. Improvement of cellularity on cell block preparations using the so-called tissue coagulum clot method during endobronchial ultrasound-guided transbronchial fine-needle aspiration. Cancer Cytopathol. 2012;120:185–95. doi: 10.1002/cncy.20199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Erer OF, Erol S, Anar C, Aydoğdu Z, Özkan SA. Contribution of cell block obtained by endobronchial ultrasound-guided transbronchial needle aspiration in the diagnosis of malignant diseases and sarcoidosis. Endosc Ultrasound. 2017;6:265–8. doi: 10.4103/2303-9027.180763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amin EN, Russell CD, Shilo K, Islam S, Wood KL. Diagnostic value of blood clot core during endobronchial ultrasound-guided transbronchial needle aspirate. Lung. 2013;191:305–9. doi: 10.1007/s00408-013-9457-5. [DOI] [PubMed] [Google Scholar]
- 8.Iwashita T, Yasuda I, Doi S, Kato T, Sano K, Yasuda S, et al. The yield of endoscopic ultrasound-guided fine needle aspiration for histological diagnosis in patients suspected of stage I sarcoidosis. Endoscopy. 2008;40:400–5. doi: 10.1055/s-2007-995593. [DOI] [PubMed] [Google Scholar]
- 9.Nakajima T, Yasufuku K. How I do it—optimal methodology for multidirectional analysis of endobronchial ultrasound-guided transbronchial needle aspiration samples. J Thorac Oncol. 2011;6:203–6. doi: 10.1097/JTO.0b013e318200f496. [DOI] [PubMed] [Google Scholar]
- 10.Sanz-Santos J, Serra P, Andreo F, Llatjós M, Castellà E, Monsó E. Contribution of cell blocks obtained through endobronchial ultrasound-guided transbronchial needle aspiration to the diagnosis of lung cancer. BMC Cancer. 2012;12:34. doi: 10.1186/1471-2407-12-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van der Heijden EH, Casal RF, Trisolini R, Steinfort DP, Hwangbo B, Nakajima T, et al. Guideline for the acquisition and preparation of conventional and endobronchial ultrasound-guided transbronchial needle aspiration specimens for the diagnosis and molecular testing of patients with known or suspected lung cancer. Respiration. 2014;88:500–17. doi: 10.1159/000368857. [DOI] [PubMed] [Google Scholar]
- 12.Navani N, Brown JM, Nankivell M, Woolhouse I, Harrison RN, Jeebun V, et al. Suitability of endobronchial ultrasound-guided transbronchial needle aspiration specimens for subtyping and genotyping of non-small cell lung cancer: A multicenter study of 774 patients. Am J Respir Crit Care Med. 2012;185:1316–22. doi: 10.1164/rccm.201202-0294OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alici IO, Demirci NY, Yılmaz A, Demirag F, Karakaya J. The combination of cytological smears and cell blocks on endobronchial ultrasound-guided transbronchial needle aspirates allows a higher diagnostic yield. Virchows Arch. 2013;462:323–7. doi: 10.1007/s00428-013-1374-8. [DOI] [PubMed] [Google Scholar]
- 14.Gauchotte G, Vignaud JM, Ménard O, Wissler MP, Martinet Y, Siat J, et al. A combination of smears and cell block preparations provides high diagnostic accuracy for endobronchial ultrasound-guided transbronchial needle aspiration. Virchows Arch. 2012;461:505–12. doi: 10.1007/s00428-012-1296-x. [DOI] [PubMed] [Google Scholar]
- 15.von Bartheld MB, Dekkers OM, Szlubowski A, Eberhardt R, Herth FJ, in 't Veen JC, et al. Endosonography vs conventional bronchoscopy for the diagnosis of sarcoidosis: The GRANULOMA randomized clinical trial. JAMA. 2013;309:2457–64. doi: 10.1001/jama.2013.5823. [DOI] [PubMed] [Google Scholar]
- 16.Yasufuku K, Chiyo M, Koh E, Moriya Y, Iyoda A, Sekine Y, et al. Endobronchial ultrasound guided transbronchial needle aspiration for staging of lung cancer. Lung Cancer. 2005;50:347–54. doi: 10.1016/j.lungcan.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 17.Gu P, Zhao YZ, Jiang LY, Zhang W, Xin Y, Han BH. Endobronchial ultrasound-guided transbronchial needle aspiration for staging of lung cancer: A systematic review and meta-analysis. Eur J Cancer. 2009;45:1389–96. doi: 10.1016/j.ejca.2008.11.043. [DOI] [PubMed] [Google Scholar]
- 18.Rotolo N, Cattoni M, Crosta G, Nardecchia E, Poli A, Moretti F, et al. Comparison of multiple techniques for endobronchial ultrasound-transbronchial needle aspiration specimen preparation in a single institution experience. J Thorac Dis. 2017;9:S381–5. doi: 10.21037/jtd.2017.04.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Navani N, Molyneaux PL, Breen RA, Connell DW, Jepson A, Nankivell M, et al. Utility of endobronchial ultrasound-guided transbronchial needle aspiration in patients with tuberculous intrathoracic lymphadenopathy: A multicentre study. Thora×2011. 66:889–93. doi: 10.1136/thoraxjnl-2011-200063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Navani N, Booth HL, Kocjan G, Falzon M, Capitanio A, Brown JM, et al. Combination of endobronchial ultrasound-guided transbronchial needle aspiration with standard bronchoscopic techniques for the diagnosis of stage I and stage II pulmonary sarcoidosis. Respirology. 2011;16:467–72. doi: 10.1111/j.1440-1843.2011.01933.x. [DOI] [PMC free article] [PubMed] [Google Scholar]