Abstract
Periodontal disease seems to be correlated with low vitamin D serum levels, preterm birth (PTB) and low birth weight (LBW), although the literature still lacks a consensus. This study aimed to investigate this correlation in a cohort of pregnant women over 20 weeks of gestation from the University Hospital “Maggiore della Carità”, Novara, Italy. We assessed serum levels of vitamin D and oral health status through the following indexes: Oral Hygiene Index (OHI), Plaque Control Record (PCR), Gingival Bleeding Index (GBI), and Community Periodontal Index of Treatment Needs (CPTIN). Moreover, we assessed the number of PTB and LBW among the newborns. Out of 121 pregnant women recruited, 72 (mean age 29.91 ± 3.64 years) were included. There was a statistically significant correlation between preterm and OHI > 3 (p = 0.033), and between LBW and OHI > 3 (p = 0.005) and CPITN = 3 (p = 0.027). Both pregnant women with vitamin D deficiency ((25-hydroxy-vitamin D) < 30 ng/mL) and PTB plus LBW newborns were significantly correlated (p < 0.05) with poor levels of all oral health status indexes during pregnancy. Furthermore, these conditions (women with hypovitaminosis D and combination of PTB and LBW) were shown to be significantly correlated (p < 0.001). Taken together, our findings reported a high prevalence of PTB and LBW with poor oral health and vitamin D deficiency in pregnant women.
Keywords: periodontal disease, oral hygiene, vitamin D, vitamin D deficiency, preterm birth, low birth weight, pregnancy
1. Introduction
Periodontal disease is one of the more common inflammatory diseases in the adult population, with an incidence varying from 5 to 30% [1,2,3,4]. Periodontal disease, initiated by bacterial biofilms, can cause the destruction of soft and hard periodontal tissues, consequently leading to tooth loss [5,6,7]. Periodontal disease produces inflammatory mediators and microbial products that can enter the systemic circulation through the mouth and reach distant organs [8,9]. Indeed, several studies have shown a link between periodontal disease and some frequent systemic pathologies [10] such as diabetes [11], cardiovascular disease [12,13], respiratory disease [14], obesity [15], cancer [16,17], and preterm birth (PTB) [7]. Vitamin D is a secosteroid that can be ascribed to steroid hormones if considering its biological effects on several tissues [18,19,20,21,22]. Vitamin D might affect periodontal disease both through an effect on bone mineral density and through immunomodulatory effects [23,24,25]. Consequently, the effects of low vitamin D levels on periodontal health represent a matter of interest. Over the past few decades, vitamin D deficiency has been associated with gingivitis and periodontitis of different severities [26,27,28]. In vitro, studies demonstrated that vitamin D might decrease the number of Porphyromonas gingivalis (Pg) through active autophagy [29], and also the inflammatory burden of periodontitis in rodent models [30,31].
PTB (before 37 weeks of gestation) is a main cause of neonatal mortality worldwide and a major contributor to long-term disability among survivors [32]. Each year, about 15 million infants are born before the end of physiological pregnancy [33], and more than half of all permanent sequelae that infants suffer at cardiovascular, neurological, respiratory, and congenital levels could be attributed to PTB [18]. Moreover, PTB is associated with low birth weight (LBW) of less than 2500 grams, and, even with the great advances in obstetrics, the rate of LBW associated with PTB has not decreased along the last few decades [34].
Although the exact mechanisms by which periodontal disease could adversely affect pregnancy are still unclear, the presence of DNA of oral pathogens associated with periodontitis has been found in amniotic fluid [35,36], placental tissues [37], and the genital tract [38]. The anaerobic bacteria Fusobacterium nucleatum (Fn) and Pg are the most important periodontal pathogens that could induce placental inflammation and subsequent damage [39]. Particularly, Pg is the most common microorganism found in the amniotic fluid and placental tissue [40,41]. Bacteria involved in periodontal disease could spread from the oral cavity to the uterine district, causing the consequent cascade of immuno-inflammatory mediators such as PGE2, IL-6, IL-1, and TNF-alpha that might be implicated in adverse pregnancy outcomes [42,43,44,45]. Alternatively, inflammatory cytokines indirectly affect placental development and fetal growth leading to PTB due to their effects on uterine contractions [44,46,47,48].
Scientific evidence showed that due to the high heterogeneity of the investigated cohorts and due to the use of several non-clinical indices, the assessment of the prevalence of gingivitis during pregnancy is challenging [49,50,51], with wide ranges of reported values from 30 to 70% and up to 100% during the second and third trimester [44,52,53,54].
Moreover, hormonal changes related to pregnancy seem to play a role as a co-factor in periodontal disease onset [52,53,55], whereas chronic inflammation of deep periodontal tissue might negatively affect pregnancy outcomes [43,54,55,56,57,58,59,60,61,62], such as PTB and LBW.
Although a relationship between periodontal disease and PTB has been hypothesized [63,64,65], many confounding factors make it difficult to assess the relationship between these two conditions, as underlined by the lack of consensus within the scientific community [57,64,65,66,67,68].
Several studies have investigated the correlation among vitamin D deficiency, PTB, and LBW [69,70,71,72]. Recent studies have suggested that the effects of 25-hydroxy-vitamin D (25(OH)vit. D) on gestational weight gain might be explained by biologic activities of this micronutrient on adipose tissue. Indeed, vitamin D receptors are present on human adipocytes and 25(OH)vit. D levels seem to influence lipogenesis, lipolysis, adipogenesis, and reducing adipose tissue inflammation [73,74,75]. Additionally, due to the anabolic effect of 25(OH)vit. D on growth, vitamin D deficiency might be associated with impaired maternal weight gain and fetal growth among vitamin D-deficient mothers [76]. Moreover, a limited number of studies have suggested that 25(OH)vit. D concentration can be related to PTB and LBW [77,78], although the results of these studies are still debated and conflicting.
Hence, the present cross-sectional study aimed to increase the level of evidence on this controversial topic by investigating the correlation among hypovitaminosis D, periodontal disease, and PTB and LBW in a cohort of pregnant women.
2. Materials and Methods
2.1. Participants
In this cross-sectional study, we recruited all the adult pregnant women (i.e., >20 weeks of gestation) referred to the Obstetrics and Gynecology Unit of the University Hospital “Maggiore della Carità”, Novara, Italy, over a 12-month period (January 2019—December 2019). Exclusion criteria were: (1) psychiatric or neurological diseases; (2) evidence of main concurrent diseases; (3) undergoing treatment with corticosteroids, immunoglobulin, or immunosuppressive drugs; (4) fully edentulous patients; (5) suffering or having suffered in the past from major oral infections; (6) being unable to understand the informed consent; (7) patients undergoing vitamin D supplementation.
This study was approved by the local ethics committee (CE 61/10, prot.392). All participants were asked to carefully read and sign an informed consent form, and researchers protected the privacy and the study procedures according to the Declaration of Helsinki with pertinent national and international regulatory requirements. Data collection and reporting were performed in accordance with the STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) Guidelines.
2.2. Clinical Assessment and Outcome Measures
Medical history of all patients was collected considering the following data: age, ethnicity, body mass index (BMI) before pregnancy, health condition (hyperglycemia, hypertension, depressive syndrome, poor weight gain, nephropathy, broncho pneumopathies), and alcohol, smoke, and narcotic consumption. In addition, specific obstetric and gynecologic information was collected, such as: number and outcome of previous pregnancies, type of previous birth, any obstetric complication of previous pregnancies (such as gestational hypertension and preeclampsia, urinary tract infection, vaginal bleeding, miscarriage, PTB, postpartum depression), characteristics of pregnancies, and birth weight of the newborns. Lastly, we assessed serum 25(OH)vit. D (ng/mL) and the number of patients with serum levels of 25(OH)vit. D < 30 ng/mL and <20 ng/mL.
Finally, all the participants underwent an oral clinical examination at the Dental Clinic of the University Hospital “Maggiore della Carità”, Novara, Italy. The clinical examination was performed by M.M., a dentist with more than 30 years of experience in periodontal diagnosis and therapy. The examination included the recording of the following outcomes: simplified Oral Hygiene Index (OHI) [79], for the presence of debris/stain and tartar on the dental elements; Plaque Control Record (PCR) [80], to assess the presence of plaque on the dental elements; Gingival Bleeding Index (GBI) [81,82], to evaluate gingival inflammation; and Community Periodontal Index of Treatment Needs (CPTIN) [83], to assess periodontal treatment needs.
2.3. Statistical Analysis
Data management and analyses were conducted according to a pre-specified statistical analytical plan. Statistical analysis was performed using STATA v. 12 (StataCorp LP, College Station, TX, USA). The continuous variables are presented as means ± standard deviations, or median and interquartile range. The Shapiro–Wilk test was performed to assess the distribution of all continuous data; as the data did not follow a normal distribution, Wilcoxon rank sum test was used to compare continuous variables between the two groups. Fisher exact test was performed to compare categorical variables between the two groups. Pearson correlation coefficients and regression analyses assessed associations and correlations among the oral health status of study participants, analyzing a correlation with clinical and demographic features. A p-value of 0.05 was considered statistically significant.
3. Results
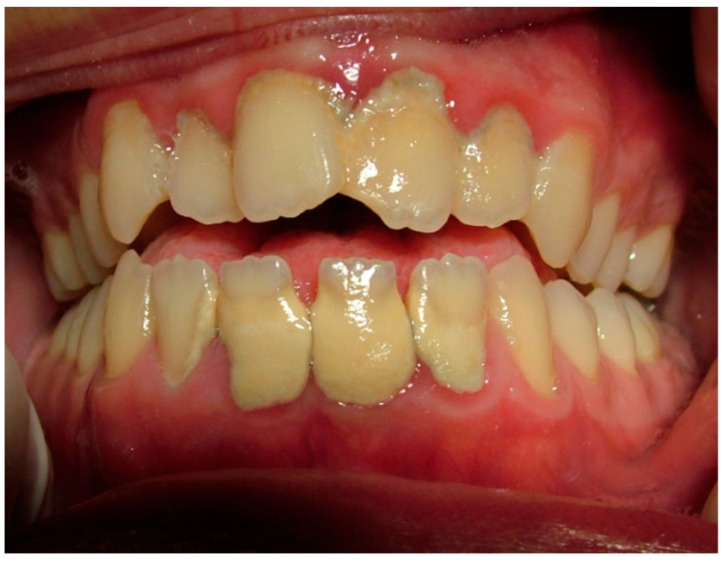
From the 121 patients recruited, 72 women, mean age 29.91 ± 3.64 years, fulfilling the inclusion criteria, underwent oral clinical examination and were enrolled in the present study. Fifty-two women were Caucasian (72.2%), fourteen African (19.4%), and six Asian (8.3%). Gestational age was between the 20th and 25th week for twenty-one women, twenty-seven were between the 25th and 30th week, twenty-two were between the 30th and 35th week, and only two were over the 35th week. We identified six underweight women (body mass index, BMI ≤ 18.49) and fifty-five had BMI > 18.49 and <24.99. Ten women were overweight (BMI ≥ 25.00) and one was obese (BMI ≥ 30) (Figure 1 depicts a pregnant woman with periodontal disease).
Figure 1.
A case of a pregnant woman with periodontal disease due to poor oral health status.
Five women referred to complications of their previous pregnancy and five had PTB. One woman treated her hyperglycemia with an oral hypoglycemic. Another one was affected by thrombophilia. Only one had a story of uterine myomas. Uro-genital inflammation was diagnosed in six women before the ongoing pregnancy. Four women underwent uterine surgery. One pregnancy was medically assisted, while seventy-one were spontaneous. Details of patients’ characteristics are reported in Table 1.
Table 1.
Anamnestic and clinical characteristics of the study participants (n = 72).
| Age (Years) | 29.91 ± 3.64 |
| Gestational age between 20th and 25th week | 21 (29.2%) |
| Gestational age between 25th and 30th week | 27 (37.5%) |
| Gestational age between 30th and 35th week | 22 (30.6%) |
| Gestational age over 35th week | 2 (2.8%) |
| Previous pregnancy | 36 (50.0%) |
| - one full-term pregnancy | 25 (69.4%) |
| - between 2 and 3 full-term pregnancies | 9 (12.5%) |
| - more than 3 full-term pregnancies | 2 (5.6%) |
| - complications | 5 (13.9%) |
| - miscarriages | 13 (36.1%) |
| - single pregnancies | 29 (80.6%) |
| - twin pregnancies | 7 (19.4%) |
| - natural birth | 30 (83.3%) |
| - caesarean section | 6 (16.7%) |
| - preterm birth | 5 (13.9%) |
| PBLW risk factors | 40 (55.6%) |
| Smokers | 7 (9.7%) |
| Drink alcohol | 0 (0%) |
| Supplementation with fluorides | 4 (15.4%) |
| (25(OH)vit. D) serum levels | 20.51 ± 6.7 |
| 25(OH)vit. D < 30 ng/mL | 65 (90.3%) |
| 25(OH)vit. D < 20 ng/mL | 32 (44.4%) |
Continuous variables are expressed as means ± standard deviations; categorical variables are expressed as counts/percentages. Abbreviations: PBLW= preterm birth plus low weight; 25(OH)vit. D = 25-hydroxy-vitamin D.
The mean serum level of 25(OH)vit. D was 20.5 ± 6.7 ng/mL; fifty-nine patients (90.3%) reported hypovitaminosis D ((25(OH)vit. D) < 30 ng/mL) and 32 (44.4%) had a serum level of (25(OH)vit. D) < 20 ng/mL. On gingival probing, GBI was >25 in forty patients and the OHI was unsatisfactory due to plaque, pigmentation, and calculus in twenty-seven women. With respect to the CPTIN score, fifty-nine women required periodontal care. Patients’ periodontal conditions are listed in Table 2.
Table 2.
Oral hygiene status in pregnant women included in this study (n = 72).
| Total (n = 72) | PTB (n = 21) | LWB (n = 14) | PBLW (n = 10) | |
|---|---|---|---|---|
| ≤1.2 | 45 (62.5%) | 7 (33.3%) | 3 (21.4%) | 2 (20.0%) |
| >1.2 ≤ 3.0 | 20 (27.8%) | 5 (23.8%) | 3 (21.4%) | 3 (30.0%) |
| >3.0 ≤ 6.0 | 7 (9.7%) | 9 (42.9%) | 8 (57.1%) | 5 (50.0%) |
| PCR | ||||
| From 0% to 25% | ||||
| >25% and ≤50% | 23 (31.9 %) | 3 (14.3%) | 2 (14.3%) | 0 (0%) |
| >50% and ≤75% | 17 (23.6%) | 10 (47.6%) | 5 (35.7%) | 1 (10.0%) |
| >75% | 14 (19.4%) | 5 (23.8%) | 5 (35.7%) | 7 (70.0%) |
| GBI | ||||
| From 0% to 25% | ||||
| >25% and ≤50% | 13 (18.1%) | 11 (52.4%) | 8 (57.1%) | 3 (30.0%) |
| >50% and ≤75% | 24 (13.3%) | 0 (0%) | 3 (21.4%) | 4 (40.0%) |
| >75% | 3 (4.2%) | 0 (0%) | 3 (21.4%) | 3 (30.0%) |
| CPTIN | ||||
| 0 | ||||
| 1 | 19 (26.4%) | 4 (19.1%) | 0 (0%) | 0 (0%) |
| 2 | 23 (31.9%) | 8 (38.1%) | 6 (42.9%) | 2 (20.0%) |
| 3 | 17 (23.6%) | 9 (42.9%) | 8 (57.1%) | 8 (80.0%) |
| ≤1.2 | 45 (62.5%) | 7 (33.3%) | 3 (21.4%) | 2 (20.0%) |
Categorical data are expressed as counts (%). Abbreviations: PTB = preterm birth; LWB = low-weight birth; PBLW = preterm birth plus low-weight birth; OHI = Oral Hygiene Index; PCR = Plaque Control and Record; GBI = Gingival Bleeding Index; CPTIN = Community Periodontal Index of Treatment Needs.
No miscarriages were recorded among all the women studied. Sixty women had a vaginal delivery while cesarean section was performed in twelve cases. Fourteen of these were twin pregnancies. Fifty-one women (70.83%) had full-term delivery (after 37th week of gestation), 20 women (27.78%) had late preterm delivery (between 32nd and 37th week), and only one patient had early preterm delivery (between 28th and 32nd week). Fifty-six neonates (77.8%) had normal weight at birth (>2500 < 4000 g), 14 neonates (19.4%) were low-weight (≥1501 ≤ 2500 g) and 2 (2.8%) were macrosomic (≥4000 g). A large part of the population (n = 62, 86.11%) gave birth at term to normal weight or macrosomic neonates, while 13.89% (n = 10) had PTB and low-weight neonates.
The statistical analysis did not show a significant correlation (p > 0.05) among preterm delivery and the value of PCR, GBI, and CPTIN. On the other hand, there was a statistically significant correlation between preterm and OHI>3 (p = 0.033). Similarly, no statistically significant correlation was detected in these groups between LBW and PCR and GBI values (p > 0.05), while there was a significant correlation in the case of OHI>3 (p = 0.005) and CPITN = 3 (p = 0.027).
Lastly, considering the combination of PTB and LBW, we found a significant correlation with OHI>3 (p = 0.001), PCR>75% (p = 0.035), GBI≥50% and <75% (p = 0.035), and CPTIN = 3 (p < 0.004).
Concerning the serum level of vitamin D, a statistically significant correlation was found between vitamin D deficiency (serum level of 25(OH)vit. D) < 30 ng/ml) and PCR>75% (p = 0.021), GBI>50% (p = 0.002), OHI>3 (p < 0.008), and CPTIN code 3 (p < 0.014). Furthermore, women with hypovitaminosis D have a significant correlation with the combination of PTB and LBW (p < 0.001).
4. Discussion
The aim of the present cross-sectional study was to investigate the correlation among hypovitaminosis D, periodontal disease, PTB, and LBW in a cohort of pregnant women.
Based on the presented data, a significant correlation among the birth of premature babies with OHI>3 as well a correlation between the birth of low-weight neonates and both OHI>3 and code 3 of the CPTIN could be detected. Interestingly, by correlating the birth of both premature and underweight neonates with the dento-periodontal indices, we obtained statistically significant correlations with them.
The prevalence of vitamin D deficiency in our study sample was very high (90.3%), and a statistically significant correlation was found between vitamin D deficiency and all the oral status indexes. Furthermore, women with hypovitaminosis D have a significant correlation with the combination of PTB and LBW (p < 0.001), although there was only a non-significant trend with the two parameters (PTB and LBW), considered as single variables.
The potential mechanisms of how periodontal disease could affect pregnancy outcomes are still not totally understood [68]. Nevertheless, it must be underlined that periodontal infection induces elevated cytokine levels in the host [84,85], and that elevated serum and/or amniotic fluid levels of proinflammatory cytokines, such as IL-1, IL-6, and TNF-α, may stimulate the production of prostaglandins in the chorion, which is associated with intra-amniotic inflammation and PTB development [86,87]. In addition, the hematogenous dissemination of periodontal pathogens could trigger metastatic infection at the feto-placental unit. Moreover, during pregnancy, the elevated levels of female sex hormones are responsible for increasing vascular permeability, which has been clinically correlated to an increased level of oral plaque and consequently gum bleeding scores [68].
Studies showed that periodontal pathogens can be detected in the maternofetal unit, where they might be involved in the development and progression of inflammation [40,88]. Moreover, there is striking evidence for an increased risk of PTB through vaginal infections, as well as for an association between the oral and the vaginal microbiome (e.g., low hygiene status is associated with increased bv risk) [89,90]. Indeed, an antenatal infection screen-and-treat program might be useful in routine pregnancy care to prevent PTB, LBW, and adverse pregnancy outcomes [89,91].
Furthermore, it was interesting to notice the strong correlation among hypovitaminosis D and periodontal disease, even if in the literature the results are controversial. In a recent metanalysis, Machado et al. [92] supported an association between serum vitamin D levels and chronic periodontitis. On other hand, a review by Miller and Pavlesen [93] investigated the relationship between vitamin D and chronic periodontal disease in older adults, affirming that, although the biologic mechanisms suggest that vitamin D deficiency could be a risk factor for the development or progression of periodontal disease, the findings from studies were overly supportive. With respect to our results, they are confirmatory of these previous findings.
Moreover, in our study, a similar positive correlation between vitamin D insufficiency and PTB with LBW was found. Wang et al. [94] affirmed that 25(OH)vit. D sufficiency in the second and third trimesters was associated with higher gestational duration. Similar results were shown in a recent metanalysis on 24 studies [95]. The authors reported that vitamin D deficiency in the second trimester is likely associated with an increased risk of PTB with an odds ratio of 1.12. These findings were confirmed by a systematic review [96], suggesting that women receiving vitamin D supplementation during pregnancy not only had improved maternal vitamin D levels at term, but also a reduced risk of LBW (average risk ratio 0.40).
Therefore, an oral hygiene assessment should always be taken into consideration in pregnant women in common clinical practice, as has also been performed for other disabling conditions [28,97,98]. In this scenario, we recommend that periodontal therapy and oral rehabilitation should be included within the clinical management of the oral health status in pregnant women.
Moreover, the elevated demand for micronutrients during pregnancy leads to women being at high risk of vitamin D deficiency and periodontal disease; thus, adequate vitamin D supplementation plays a crucial role in pregnant women to reduce the risk of periodontal disease, and even the risk of PTB and LBW in newborns [95,99,100].
This study is not free from limitations: first, the cross-sectional study design precludes it from external validity; second, even though all the patients referred to the obstetric clinic were screened and potentially eligible for the study, the dataset included a small sample. In addition, the decision to use different periodontal screening indices to evaluate oral health status was taken to increase the feasibility of the study; however, we are aware that a complete periodontal chart (with full-mouth plaque and full-mouth bleeding values), as well as radiographic and microbiological analysis, would have provided additional information for this study.
5. Conclusions
Taken together, in this cross-sectional study, we assessed the correlation among hypovitaminosis D, periodontal disease, PTB, and LBW in a cohort of pregnant women. We showed that a high prevalence of PTB and LBW is present in pregnant women affected by periodontal disease and vitamin D deficiency. In light of these results, an adequate screening of oral health and 25(OH)vit. D serum levels should be implemented in common clinical practice for pregnant women. Further prospective studies on larger cohorts are warranted to investigate the role that oral health rehabilitative programs and vitamin D supplementation might have in reducing periodontal disease in pregnant women and even the incidence of PTB and LBW.
Author Contributions
Conceptualization, M.F., M.M., and A.d.S.; methodology, M.M. and A.d.S.; formal analysis, M.F. and D.C.; investigation, M.F., A.R., and P.M.-M.; resources, M.M.; writing—original draft preparation, M.F.; writing—review and editing, M.M. and A.d.S.; visualization, A.R., G.R.U., P.M.-M., G.F., F.P., P.L.F.B., and D.C.; supervision, M.M. and A.d.S.; submission, M.F., M.M., and A.d.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was conducted according to the guidelines of the Declaration of Helsinki and approved by the local ethics committee (CE 61/10, prot.392).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The dataset is available upon request.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Pihlstrom B.L., Michalowicz B.S., Johnson N.W. Periodontal diseases. Lancet. 2005;366:1809–1820. doi: 10.1016/S0140-6736(05)67728-8. [DOI] [PubMed] [Google Scholar]
- 2.Kim J.H., Oh J.W., Lee Y., Yun J.H., Choi S.H., Lee D.W. Quantification of Bacteria in Mouth-Rinsing Solution for the Diagnosis of Periodontal Disease. J. Clin. Med. 2021;10:891. doi: 10.3390/jcm10040891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coventry J., Griffiths G., Scully C., Tonetti M. ABC of oral health: Periodontal disease. BMJ. 2000;321:36–39. doi: 10.1136/bmj.321.7252.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pihlstrom B.L. Periodontal risk assessment, diagnosis and treatment planning. Periodontology 2000. 2001;25:37–58. doi: 10.1034/j.1600-0757.2001.22250104.x. [DOI] [PubMed] [Google Scholar]
- 5.Dhamecha H.R.R., Jagwani D.S., Rao D.M., Jadhav K., Shaikh S., Jalalpure S. Local drug delivery 20 systems in the management of periodontitis: A scientific review. J. Control. Release. 2019;307:393–409. doi: 10.1016/j.jconrel.2019.06.038. [DOI] [PubMed] [Google Scholar]
- 6.Kassebaum N.J., Bernabé E., Dahiya M., Bhandari B., Murray C.J., Marcenes W. Global burden of severe periodontitis in 1990–2010: A systematic review and meta-regression. J. Dent. Res. 2014;93:1045–1053. doi: 10.1177/0022034514552491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cafiero C., Spagnuolo G., Marenzi G., Martuscelli R., Colamaio M., Leuci S. Predictive Periodontitis: The Most Promising Salivary Biomarkers for Early Diagnosis of Periodontitis. J. Clin. Med. 2021;10:1488. doi: 10.3390/jcm10071488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nomura Y., Morozumi T., Numabe Y., Ogata Y., Nakayama Y., Sugaya T., Nakamura T., Sato S., Takashiba S., Sekino S., et al. Estimation of the Periodontal Inflamed Surface Area by Simple Oral Examination. J. Clin. Med. 2021;10:723. doi: 10.3390/jcm10040723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aniko-Włodarczyk M., Jaroń A., Preuss O., Grzywacz A., Trybek G. Evaluation of the Effect of Surgical Extraction of an Impacted Mandibular Third Molar on the Periodontal Status of the Second Molar-Prospective Study. J. Clin. Med. 2021;10:2655. doi: 10.3390/jcm10122655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paquette D.W. The periodontal infection-systemic disease link: A review of the truth or myth. J. Int. Acad. Periodontol. 2002;4:101–109. [PubMed] [Google Scholar]
- 11.Borgnakke W.S., Ylöstalo P.V., Taylor G.W., Genco R.J. Effect of periodontal disease on diabetes: Systematic review of epidemiologic observational evidence. J. Periodontol. 2013;84((Suppl. S4)):S135–S152. doi: 10.1902/jop.2013.1340013. [DOI] [PubMed] [Google Scholar]
- 12.Friedewald V.E., Kornman K.S., Beck J.D., Genco R., Goldfine A., Libby P., Offenbacher S., Ridker P.M., Van Dyke T.E., Roberts W.C. The American Journal of Cardiology and Journal of Periodontology Editors’ Consensus: Periodontitis and atherosclerotic cardiovascular disease. Am. J. Cardiol. 2009;104:59–68. doi: 10.1016/j.amjcard.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 13.Carrizales-Sepùlveda E.F., Ordaz-Farìaz A., Vera-Pineda R., Flores-Ramirez R. Periodontal Disease, Systemic Inflammation and the Risk of Cardiovascular disease. Heart Lung Circ. 2018;27:1327–1334. doi: 10.1016/j.hlc.2018.05.102. [DOI] [PubMed] [Google Scholar]
- 14.Azarpazhooh A., Leake J.L. Systematic review of the association between respiratory diseases and oral health. J. Periodontol. 2006;77:1465–1482. doi: 10.1902/jop.2006.060010. [DOI] [PubMed] [Google Scholar]
- 15.Martinez-Herrera M., Silvestre-Rangil J., Silvestre F.J. Association between obesity and periodontal disease. A systematic review of epidemiological studies and controlled clinical trials. Med. Oral Patol. Oral Cir. Bucal. 2017;22:708–715. doi: 10.4317/medoral.21786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li W., Xu J., Zhang R., Li Y., Wang J., Zhang X., Lin L. Is periodontal disease a risk indicator for colorectal cancer? A systematic review and meta-analysis. J. Clin. Periodontol. 2021;48:336–347. doi: 10.1111/jcpe.13402. [DOI] [PubMed] [Google Scholar]
- 17.Dizdar O., Hayran M., Guven D.C., Yılmaz T.B., Taheri S., Akman A.C., Bilgin E., Hüseyin B., Berker E. Increased cancer risk in patients with periodontitis. Curr. Med. Res. Opin. 2017;33:2195–2200. doi: 10.1080/03007995.2017.1354829. [DOI] [PubMed] [Google Scholar]
- 18.Iolascon G., Mauro G.L., Fiore P., Cisari C., Benedetti M.G., Panella L., de Sire A., Calafiore D., Moretti A., Gimigliano F. Can vitamin D deficiency influence muscle performance in postmenopausal women? A multicenter retrospective study. Eur. J. Phys. Rehabil. Med. 2018;54:676–682. doi: 10.23736/S1973-9087.17.04533-6. [DOI] [PubMed] [Google Scholar]
- 19.Gimigliano F., Moretti A., de Sire A., Calafiore D., Iolascon G. The combination of vitamin D deficiency and overweight affects muscle mass and function in older post-menopausal women. Aging Clin. Exp. Res. 2018;30:625–631. doi: 10.1007/s40520-018-0921-1. [DOI] [PubMed] [Google Scholar]
- 20.Srikuea R., Hirunsai M., Charoenphandhu N. Regulation of vitamin D system in skeletal muscle and resident myogenic stem cell during development, maturation, and ageing. Sci. Rep. 2020;10:8239. doi: 10.1038/s41598-020-65067-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Behm C., Blufstein A., Gahn J., Kubin B., Nemec M., Moritz A., Rausch-Fan X., Andrukhov O. 1,25(OH)2D3 Differently Affects Immunomodulatory Activities of Mesenchymal Stem Cells Depending on the Presence of TNF-α, IL-1β and IFN-γ. J. Clin. Med. 2019;8:2211. doi: 10.3390/jcm8122211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Polly P., Tan T.C. The role of vitamin D in skeletal and cardiac muscle function. Front. Physiol. 2014;5:145. doi: 10.3389/fphys.2014.00145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martinon P., Fraticelli L., Giboreau A., Dussart C., Bourgeois D., Carrouel F. Nutrition as a Key Modifiable Factor for Periodontitis and Main Chronic Diseases. J. Clin. Med. 2021;10:197. doi: 10.3390/jcm10020197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anbarcioglu E., Kirtiloglu T., Öztürk A., Kolbakir F., Acıkgöz G., Colak R. Vitamin D deficiency in patients with aggressive periodontitis. Oral Dis. 2018;25:242–249. doi: 10.1111/odi.12968. [DOI] [PubMed] [Google Scholar]
- 25.Ebersole J.L., Lambert J., Bush H., Huja P.E., Basu A. Serum nutrient levels and aging effects on periodontitis. Nutrients. 2018;10:1986. doi: 10.3390/nu10121986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Antonoglou G.N., Knuuttila M., Niemelä O., Raunio T., Karttunen R., Vainio O., Hedberg P., Ylöstalo P., Tervonen T. Low serum level of 1,25(OH)2D is associated with chronic periodontitis. J. Periodontal Res. 2015;50:274–280. doi: 10.1111/jre.12207. [DOI] [PubMed] [Google Scholar]
- 27.Dietrich T., Joshipura K.J., Dawson-Hughes B., Bischoff-Ferrari H.A. Association between serum concentrations of 25-hydroxyvitamin D 3 and periodontal disease in the US population. Am. J. Clin. Nutr. 2004;80:108–113. doi: 10.1093/ajcn/80.1.108. [DOI] [PubMed] [Google Scholar]
- 28.de Sire A., Ferrillo M., Gennari A., Cisari C., Pasqua S., Foglio Bonda P.L., Invernizzi M., Migliario M. Bone health, vitamin D status and oral hygiene screening in breast cancer women before starting osteoporosis treatment: A cross-sectional study. J. Biol. Regul. Homeost. Agents. 2021;35:397–402. doi: 10.23812/20-686-L. [DOI] [PubMed] [Google Scholar]
- 29.Hu X., Niu L., Ma C., Huang Y., Yang X., Shi Y., Pan C., Liu J., Wang H., Li Q., et al. Calcitriol decreases live Porphyromonas gingivalis internalized into epithelial cells and monocytes by promoting autophagy. J. Periodontol. 2019;91:956–966. doi: 10.1002/JPER.19-0510. [DOI] [PubMed] [Google Scholar]
- 30.Oh C., Kim H.J., Kim H.M. Vitamin D maintains E-cadherin intercellular junctions by downregulating MMP-9 production in human gingival keratinocytes treated by TNF. J. Periodontal Implant Sci. 2019;49:270–286. doi: 10.5051/jpis.2019.49.5.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li H., Zhong X., Li W., Wang Q. Effects of 1,25-dihydroxyvitamin on experimental periodontitis and ahr/nf-b/nlrp3 inflammasome pathway in a mouse model. J. Appl. Oral Sci. 2019;27:1–10. doi: 10.1590/1678-7757-2018-0713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Menon R. Preterm birth: A global burden on maternal and child health. Pathog. Glob. Health. 2012;106:139–140. doi: 10.1179/204777312X13462106637729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ren H., Du M. Role of Maternal Periodontitis in Preterm Birth. Front. Immunol. 2017;8:139. doi: 10.3389/fimmu.2017.00139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Calixto N.R., Alves C.M., Abreu L.M., Thomaz E.B., Vidal F.C., Filho I.S., Lopes F.F. Detection of periodontal pathogens in mothers of preterm birth and/or low weight. Med. Oral Patol. Oral Cir. Bucal. 2019;24:e776–e781. doi: 10.4317/medoral.23135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gauthier S., Tétu A., Himaya E., Morand M., Chandad F., Rallu F., Bujold E. The origin of Fusobacterium nucleatum involved in intra-amniotic infection and preterm birth. J. Matern. Fetal Neonatal Med. 2011;24:1329–1332. doi: 10.3109/14767058.2010.550977. [DOI] [PubMed] [Google Scholar]
- 36.Ercan E., Eratalay K., Deren O., Gur D., Ozyuncu O., Altun B., Kanly C., Ozdemir P., Akincibay H. Evaluation of perio-dontal pathogens in amniotic fluid and the role of periodontal disease in pre-term birth and low birth weight. Acta Odontol. Scand. 2013;71:553–559. doi: 10.3109/00016357.2012.697576. [DOI] [PubMed] [Google Scholar]
- 37.Vanterpool S.F., Been J.V., Houben M.L., Nikkels P.G., De Krijger R.R., Zimmermann L.J., Kramer B.W., Progulske-Fox A., Reyes L. Porphyromonas gingivalis within placental villous mesenchyme and umbilical cord stroma is associated with adverse pregnancy outcome. PLoS ONE. 2016;11:e146157. doi: 10.1371/journal.pone.0146157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cassini M.A., Pilloni A., Condò S.G., Vitali L.A., Pasquantonio G., Cerroni L. Periodontal bacteria in the genital tract: Are they related to adverse pregnancy outcome? Int. J. Immuno-Pathol. Pharmacol. 2013;26:931–939. doi: 10.1177/039463201302600411. [DOI] [PubMed] [Google Scholar]
- 39.Fischer L.A., Demerath E., Bittner-Eddy P., Costalonga M. Placental colonization with periodontal pathogens: The potential missing link. Am. J. Obstet. Gynecol. 2019;221:383–392. doi: 10.1016/j.ajog.2019.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.León R., Silva N., Ovalle A., Chaparro A., Ahumada A., Gajardo M., Martinez M., Gamonal J. Detection of Porphyromonas gingivalis in the amniotic fluid in pregnant women with a diagnosis of threatened premature labor. J. Periodontol. 2007;78:1249–1255. doi: 10.1902/jop.2007.060368. [DOI] [PubMed] [Google Scholar]
- 41.Hasegawa-Nakamura K., Tateishi F., Nakamura T., Nakajima Y., Kawamata K., Douchi T., Hatae M., Noguchi K. The possible mechanism of preterm birth associated with periodontopathic Porphyromonas gingivalis. J. Periodontal Res. 2011;46:497–504. doi: 10.1111/j.1600-0765.2011.01366.x. [DOI] [PubMed] [Google Scholar]
- 42.Han Y.W., Redline R.W., Li M., Lihong Y., McCormick T.S. Fusobacterium nucleatum Induces Premature and Term Stillbirths in Pregnant Mice: Implication of Oral Bacteria in Preterm Birth. Infect. Immun. 2004;72:2272–2279. doi: 10.1128/IAI.72.4.2272-2279.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Boggess K.A. Pathogenicity of periodontal pathogens during pregnancy. Am. J. Obstet. Gynecol. 2005;193:311–312. doi: 10.1016/j.ajog.2005.04.056. [DOI] [PubMed] [Google Scholar]
- 44.Zi M.Y., Longo P.L., Bueno-Silva B., Mayer M.P. Mechanisms Involved in the Association between Periodontitis and Complications in Pregnancy. Front. Public Health. 2015;2:290. doi: 10.3389/fpubh.2014.00290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ao M., Miyauchi M., Furusho H., Inubushi T., Kitagawa M., Nagasaki A., Sakamoto S., Kozai K., Takata T. Dental Infection of Porphyromonas gingivalis Induces Preterm Birth in Mice. PLoS ONE. 2015;10:e0137249. doi: 10.1371/journal.pone.0137249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Saini R., Saini S., Saini S.R. Periodontitis: A risk for delivery of premature labor and low birth weight infants. J. Nat. Sci. Biol. Med. 2011;2:50–52. doi: 10.4103/0976-9668.82321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shanthi V., Vanka A., Bhambal A., Saxena V., Saxena S., Kumar S.S. Association of pregnant women periodontal status to preterm and low-birth weight babies: A systematic and evidence-based review. Dent. Res. J. 2012;9:368–380. [PMC free article] [PubMed] [Google Scholar]
- 48.Haerian-Ardakani A., Eslami Z., Rashidi-Meibodi F., Haerian A., Dallalnejad P., Shekari M., Moein Taghavi A., Akbari S. Relationship between maternal periodontal disease and low birth weight babies. Iran. J. Reprod. Med. 2013;11:625–630. [PMC free article] [PubMed] [Google Scholar]
- 49.Teshome A., Yitayeh A. Relationship between periodontal disease and preterm low birth weight: Systematic review. Pan Afr. Med. J. 2016;24:215. doi: 10.11604/pamj.2016.24.215.8727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.AlJehani Y.A. Risk Factors of Periodontal Disease: Review of the Literature. Int. J. Dent. 2014;2014:182513. doi: 10.1155/2014/182513. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 51.Holmstrup P., Plemons J., Meyle J. Non-plaque-induced gingival disease. J. Clin. Periodontol. 2018;45((Suppl. S20)):S28–S43. doi: 10.1111/jcpe.12938. [DOI] [PubMed] [Google Scholar]
- 52.Di Placido G., Tumini V., D’Archivio D., Di Peppe G. Gingival hyperplasia in pregnancy. II. Etiopathogenic factors and mechanisms. Minerva Stomatol. 1998;47:223–229. [PubMed] [Google Scholar]
- 53.Wu M., Chen S.W., Jiang S.Y. Relationship between gingival inflammation and pregnancy. Mediat. Inflamm. 2015;2015:623427. doi: 10.1155/2015/623427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wandera M., Åstrøm A.N., Okullo I., Tumwine J.K. Determinants of periodontal health in pregnant women and association with infants’ anthropometric status: A prospective cohort study from Eastern Uganda. BMC Pregnancy Childbirth. 2012;12:90. doi: 10.1186/1471-2393-12-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Laine M.A. Effect of pregnancy on periodontal and dental health. Acta Odontol. Scand. 2002;60:257–264. doi: 10.1080/00016350260248210. [DOI] [PubMed] [Google Scholar]
- 56.Wimmer G., Pihlstrom B.L. A critical assessment of adverse pregnancy outcome and periodontal disease. J. Clin. Periodontol. 2008;35((Suppl. S8)):380–397. doi: 10.1111/j.1600-051X.2008.01284.x. [DOI] [PubMed] [Google Scholar]
- 57.Huck O., Tenenbaum H., Davideau J.L. Relationship between Periodontal Diseases and Preterm Birth: Recent Epidemiological and Biological Data. J. Pregnancy. 2011;2011:164654. doi: 10.1155/2011/164654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lauren M., Minire A., Maldi X., Mirton M., Aferdita M. The Impact of Periodontitis in the Preterm Birth and Body Size of Newborns. Mater. Sociomed. 2012;24:44–47. doi: 10.5455/msm.2012.24.44-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Srinivas S.K., Parry S. Periodontal Disease and Pregnancy Outcomes: Time to Move On? J. Women’s Health. 2012;21:121–125. doi: 10.1089/jwh.2011.3023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sanz M., Kornman K., Working Group 3 of Joint EFP/AAP Workshop Periodontitis and adverse pregnancy outcomes: Consensus report of the Joint EFP/AAP Workshop on Periodontitis and Systemic Diseases. J. Clin. Periodontol. 2013;40((Suppl. S14)):S164–S169. doi: 10.1111/jcpe.12083. [DOI] [PubMed] [Google Scholar]
- 61.Bansal M., Khatri M., Kumar A., Bhatia G. Relationship between Maternal Periodontal Status and Preterm Low Birth Weight. Rev. Obstet. Gynecol. 2013;6:135–140. [PMC free article] [PubMed] [Google Scholar]
- 62.Iqbal P.S., Khan S.N., Haris M., Narayanan M., Laju S., Kumar S.S. Assessment of Systemic Inflammatory Markers in Patients with Aggressive Periodontitis. J. Int. Oral Health. 2015;7((Suppl. S2)):48–51. [PMC free article] [PubMed] [Google Scholar]
- 63.Mesa F., Pozo E., Blanc V., Puertas A., Bravo M., O’Valle F. Are periodontal bacterial profiles and placental inflammatory infiltrate in pregnancy related to birth outcomes? J. Periodontol. 2013;84:1327–1336. doi: 10.1902/jop.2012.120462. [DOI] [PubMed] [Google Scholar]
- 64.Offenbacher S., Katz V., Fertik G., Collins J., Boyd D., Maynor G., McKaig R., Beck J. Periodontal Infection as a Possible Risk Factor for Preterm Low Birth Weight. J. Periodontol. 1996;67((Suppl. S10)):1103–1113. doi: 10.1902/jop.1996.67.10s.1103. [DOI] [PubMed] [Google Scholar]
- 65.Manrique-Corredor E.J., Orozco-Beltran D., Lopez-Pineda A., Quesada J.A., Gil-Guillen V.F., Carratala-Munuera C. Maternal periodontitis and preterm birth: Systematic review and meta-analysis. Community Dent. Oral Epidemiol. 2019;47:243–251. doi: 10.1111/cdoe.12450. [DOI] [PubMed] [Google Scholar]
- 66.Komine-Aizawa S., Aizawa S., Hayakawa S. Periodontal diseases and adverse pregnancy outcomes. J. Obstet. Gynaecol. Res. 2019;45:5–12. doi: 10.1111/jog.13782. [DOI] [PubMed] [Google Scholar]
- 67.Daalderop L.A., Wieland B.V., Tomsin K., Reyes L., Kramer B.W., Vanterpool S.F., Been J.V. Periodontal Disease and Pregnancy Outcomes: Overview of Systematic Reviews. JDR Clin. Trans. Res. 2018;3:10–27. doi: 10.1177/2380084417731097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bobetsis Y.A., Graziani F., Gürsoy M., Madianos P.N. Periodontal disease and adverse pregnancy outcomes. Periodontol. 2000. 2020;83:154–174. doi: 10.1111/prd.12294. [DOI] [PubMed] [Google Scholar]
- 69.Amiri M., Rostami M., Bidhendi-Yarandi R., Fallahzadeh A., Simbar M., Ramezani Tehrani F. Relationship between vitamin D status in the first trimester of the pregnancy and gestational weight gain: A mediation analysis. Arch. Gynecol. Obstet. 2021:1–10. doi: 10.1007/s00404-021-06163-y. [DOI] [PubMed] [Google Scholar]
- 70.Onsonby A.L., Lucas R.M., Lewis S., Halliday J. Vitamin D status during pregnancy and aspects of offspring health. Nutrients. 2010;2:389–407. doi: 10.3390/nu2030389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pérez-López F.R. Vitamin D: The secosteroid hormone and human reproduction. Gynecol. Endocrinol. 2007;23:13–24. doi: 10.1080/09513590601045629. [DOI] [PubMed] [Google Scholar]
- 72.Horne-Lyman A., Fawzi W.W. Vitamin D during pregnancy and maternal, neonatal and infant health outcomes: A systematic review and meta-analysis. Paediatr. Perinat. Epidemiol. 2012;26((Suppl. S1)):75–90. doi: 10.1111/j.1365-3016.2012.01283.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cianferotti L., Demay M.B. VDR-mediated inhibition of DKK1 and SFRP2 suppresses adipogenic differentiation of murine bone marrow stromal cells. J. Cell. Biochem. 2007;101:80–88. doi: 10.1002/jcb.21151. [DOI] [PubMed] [Google Scholar]
- 74.Blumberg J.M., Tzameli I., Astapova I., Lam F.S., Flier J.S., Hollenberg A.N. Complex role of the vitamin D receptor and its ligand in adipogenesis in 3T3-L1 cells. J. Biol. Chem. 2006;281:11205–11213. doi: 10.1074/jbc.M510343200. [DOI] [PubMed] [Google Scholar]
- 75.Abbas M.A. Physiological functions of Vitamin D in adipose tissue. J. Steroid Biochem. Mol. Biol. 2017;165:369–381. doi: 10.1016/j.jsbmb.2016.08.004. [DOI] [PubMed] [Google Scholar]
- 76.Marya R.K., Rathee S., Dua V., Sangwan K. Effect of vitamin D supplementation during pregnancy on foetal growth. Indian J. Med. Res. 1998;88:488–492. [PubMed] [Google Scholar]
- 77.McDonnell S.L., Baggerly K.A., Baggerly C.A., Aliano J.L., French C.B., Baggerly L.L., Ebeling M.D., Rittenberg C.S., Goodier C.G., Mateus Niño J.F., et al. Maternal 25(OH)D concentrations ≥40 ng/mL associated with 60% lower preterm birth risk among general obstetrical patients at an urban medical center. PLoS ONE. 2017;12:e0180483. doi: 10.1371/journal.pone.0180483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Amegah A.K., Klevor M.K., Wagner C.L. Maternal vitamin D insufficiency and risk of adverse pregnancy and birth outcomes: A systematic review and meta-analysis of longitudinal studies. PLoS ONE. 2017;12:e0173605. doi: 10.1371/journal.pone.0173605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Greene J.C., Vermillion J.R. The simplified oral hygiene index. J. Am. Dent. Assoc. 1964;68:7–13. doi: 10.14219/jada.archive.1964.0034. [DOI] [PubMed] [Google Scholar]
- 80.O’Leary T.J., Drake R.B., Naylor J.E. The plaque control record. J. Periodontol. 1972;43:38. doi: 10.1902/jop.1972.43.1.38. [DOI] [PubMed] [Google Scholar]
- 81.Carter H.G., Barnes G.P. The Gingival Bleeding Index. J. Periodontol. 1974;45:801–805. doi: 10.1902/jop.1974.45.11.801. [DOI] [PubMed] [Google Scholar]
- 82.Ainamo J., Bay I. Problems and proposals for recording gingivitis and plaque. Int. Dent. J. 1975;25:229–235. [PubMed] [Google Scholar]
- 83.Cutress T.W., Ainamo J., Sardo-Infirri J. The community periodontal index of treatment needs (CPITN) procedure for population groups and individuals. Int. Dent. J. 1987;37:222–233. [PubMed] [Google Scholar]
- 84.Lohsoonthorn V., Qiu C., Williams M.A. Maternal serum Creactive protein concentrations in early pregnancy and subsequent risk of preterm delivery. Clin. Biochem. 2007;40:330–335. doi: 10.1016/j.clinbiochem.2006.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sugita N., Kobayashi T., Kikuchi A., Shimada Y., Hirano E., Sasahara J., Tanaka K., Yoshie H. Immunoregulatory gene polymorphisms in Japanese women with preterm births and periodontitis. J. Reprod. Immunol. 2012;93:94–101. doi: 10.1016/j.jri.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 86.Buhimschi C.S., Dulay A.T., Abdel-Razeq S., Zhao G., Lee S., Hodgson E.J., Bhandari V., Buhimschi I.A. Fetal inflammatory response in women with proteomic biomarkers characteristic of intra-amniotic inflammation and preterm birth. BJOG. 2009;116:257–267. doi: 10.1111/j.1471-0528.2008.01925.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Park C.W., Yoon B.H., Park J.S., Jun J.K. An elevated maternal serum C-reactive protein in the context of intra-amniotic inflammation is an indicator that the development of amnionitis, an intense fetal and AF inflammatory response are likely in patients with preterm labor: Clinical implications. J. Matern. Fetal Neonatal Med. 2013;26:847–853. doi: 10.3109/14767058.2013.783806. [DOI] [PubMed] [Google Scholar]
- 88.Han Y.W., Fardini Y., Chen C., Iacampo K.G., Peraino V.A., Shamonki J.M., Redline R.W. Term stillbirth caused by oral Fusobacterium nucleatum. Pt 2Obstet. Gynecol. 2010;115:442–445. doi: 10.1097/AOG.0b013e3181cb9955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Farr A., Kiss H., Hagmann M., Marschalek J., Husslein P., Petricevic L. Routine Use of an Antenatal Infection Screen-and-Treat Program to Prevent Preterm Birth: Long-Term Experience at a Tertiary Referral Center. Birth. 2015;42:173–180. doi: 10.1111/birt.12154. [DOI] [PubMed] [Google Scholar]
- 90.Kiss H., Petricevic L., Husslein P. Prospective randomised controlled trial of an infection screening programme to reduce the rate of preterm delivery. BMJ. 2004;329:371. doi: 10.1136/bmj.38169.519653.EB. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Farr A., Sustr V., Kiss H., Rosicky I., Graf A., Makristathis A., Foessleitner P., Petricevic L. Oral probiotics to reduce vaginal group B streptococcal colonization in late pregnancy. Sci Rep. 2020;10:19745. doi: 10.1038/s41598-020-76896-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Machado V., Lobo S., Proença L., Mendes J.J., Botelho J. Vitamin D and Periodontitis: A Systematic Review and Meta-Analysis. Nutrients. 2020;12:2177. doi: 10.3390/nu12082177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Millen A.E., Pavlesen S. Could Vitamin D influence risk for Periodontal Disease—To “D” or not to “D”? Curr. Oral Health Rep. 2020;7:98–111. doi: 10.1007/s40496-020-00253-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang S., Xin X., Luo W., Mo M., Si S., Shao B., Shen Y., Cheng H., Yu Y. Association of vitamin D and gene variants in the vitamin D metabolic pathway with preterm birth. Nutrition. 2021;89:111349. doi: 10.1016/j.nut.2021.111349. [DOI] [PubMed] [Google Scholar]
- 95.Lian R.H., Qi P.A., Yuan T., Yan P.J., Qiu W.W., Wei Y., Hu Y.G., Yang K.H., Yi B. Systematic review and meta-analysis of vitamin D deficiency in different pregnancy on preterm birth: Deficiency in middle pregnancy might be at risk. Medicine. 2021;100:e26303. doi: 10.1097/MD.0000000000026303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.De-Regil L.M., Palacios C., Lombardo L.K., Peña-Rosas J.P. Vitamin D supplementation for women during pregnancy. Sao Paulo Med. J. 2016;134:274–275. doi: 10.1590/1516-3180.20161343T2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.De Sire A., Baricich A., Ferrillo M., Migliario M., Cisari C., Invernizzi M. Buccal hemineglect: Is it useful to evaluate the differences between the two halves of the oral cavity for the multidisciplinary rehabilitative management of right brain stroke survivors? A cross-sectional study. Top. Stroke Rehabil. 2020;27:208–214. doi: 10.1080/10749357.2019.1673592. [DOI] [PubMed] [Google Scholar]
- 98.De Sire A., Invernizzi M., Ferrillo M., Gimigliano F., Baricich A., Cisari C., De Marchi F., Foglio Bonda P.L., Mazzini L., Migliario M. Functional status and oral health in patients with amyotrophic lateral sclerosis: A cross-sectional study. NeuroRehabilitation. 2021;48:49–57. doi: 10.3233/NRE-201537. [DOI] [PubMed] [Google Scholar]
- 99.Levy B., O’Callaghan K.M., Qamar H., Mahmud A.A.M., Gernand A.D., Islam M.M., Roth D.E. Response to Prenatal and Postpartum Cholecalciferol Supplementation. J. Nutr. 2021;24:nxab265. doi: 10.1093/jn/nxab265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kanasaki K., Kumagai A. The impact of micronutrient deficiency on pregnancy complications and development origin of health and disease. J. Obstet. Gynaecol. Res. 2021;47:1965–1972. doi: 10.1111/jog.14770. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The dataset is available upon request.