Abstract
Breast cancer is the most common malignancy diagnosed in women. Traditionally, radical surgical resection was the cornerstone of breast cancer management, with limited exceptions. In recent times, our enhanced appreciation of the biomolecular characteristics of breast cancer has transformed the treatment paradigm to include prescription of chemotherapeutical agents, radiotherapies, targeted therapies, as well as more refined surgical approaches. While treatments with such modalities have enhanced clinico-oncological outcomes for breast cancer patients, the efforts of oncological and translational research have concentrated on the identification of novel biomarkers which may successfully inform prognosis and response to therapies, improve current therapeutic strategies, and enhance prognostication. Mi(cro)RNAs are small, non-coding molecules which are known to play regulatory roles in governing gene expression and cellular activity. Measurement of miRNA expression profiles have been illustrated to inform the response to therapies, such as conventional chemotherapy, and are currently undergoing assessment as means of enhancing treatment strategies with these cytotoxic agents. Herein, this review outlines how chemotherapy prescription has revolutionised breast cancer treatment and illustrates the novel role of miRNAs as biomarkers capable of enhancing current therapeutic strategies using chemotherapy in patients being treated with curative intent for breast cancer.
Keywords: breast cancer, chemotherapy, precision oncology, personalised medicine, miRNA
1. Introduction
Breast cancer remains the most common malignancy in women, with estimations suggesting almost 1.7 million women are diagnosed and treated for breast cancer each year, contributing 11.9% to the global cancer burden [1]. Moreover, breast cancer accounts for 30% of all female cancers and 15–20% of all female cancer deaths [2]. Although there is an increasing incidence in breast cancer diagnoses in recent years [3], the prognosis of the disease has improved dramatically, with anticipated 5-year survival outcomes improving from 40% to almost 90% over the past 50 years [4]. Traditionally, en-bloc radical resections in the form of Halstead mastectomy and axillary clearance were believed to be fundamental in controlling breast cancer, with limited exceptions [5]. In more recent times, enhanced clinical outcomes have evolved in accordance with our increased appreciation of the molecular mechanisms underpinning the heterogeneity of breast tumours, which has facilitated more conservative surgery and the personalisation of treatment strategies to increase toxicity to the tumour while minimising unnecessary morbidity to the patient. This encompasses the era of precision oncology, which has identified breast cancer as a heterogeneous disease, leading to routine substratification of these cancers into four biological distinct, intrinsic molecular subtypes, all of which have varying clinical behaviour, prognoses, treatment strategies, as well as response rates to such treatments (i.e., luminal A breast cancer (LABC), luminal B breast cancer (LBBC), human epidermal growth factor receptor-2 enriched breast cancer (HER2+) and triple-negative breast cancer (TNBC) [6]. Currently, the St. Gallen expert consensus statements highlight gene expression profile (GEP) assays (e.g., Prosogna©—PAM50 messenger RNA (mRNA) expression signature, NanoString Technologies, Seattle, WA, USA; MammaPrint©, Agilent Technologies, CA, USA; or OncotypeDX Recurrence Score© (RS), Genomic Health Inc., Redwood City, CA, USA) as the gold standard for the substratification of luminal breast tumours into their distinct intrinsic biological subtypes, while routine appraisal of estrogen (ER), progesterone (PgR) and human epidermal growth factor receptor-2 (HER2) receptors, as well as Ki-67 proliferation indices using immunohistochemistry staining, remain critical in identifying the molecular subtypes in common clinical practice [7,8,9,10]. Routine assessment of such biomarkers remains fundamental in guiding therapeutic decision making regarding adjuvant chemoendocrine agents and targeted therapies. Nonetheless, the paradigm appears to be shifting towards the adoption of the aforementioned GEP signatures to modify treatment strategies suitable to each patient while providing sensitive prognostication and predicting response to therapies, therefore validating their inclusion in oncological guidelines (such as the American Society of Clinical Oncology (ASCO), European Society of Medical Oncology (ESMO), and National Institute for Health and Clinical Excellent (NICE) treatment guidelines) [11,12,13]. However, small, non-coding ribonucleic acids (RNA) have also been acknowledged to have value in personalised medicine.
Micro ribonucleic acids (microRNA or miRNA) are small, non-coding ribonucleic acids (RNA) which are key in regulating gene expression [14]. First described by Lee et al. in 1993 [15], miRNAs have a key role in cancer proliferation, with the clinical utility of prognostic, diagnostic, and therapeutic avenues being explored through measuring miRNA expression profiles [16]. As miRNAs are modulators of oncogenesis in breast carcinoma, assessing their utility in enhancing current chemotherapeutic strategies may be useful in improving the current treatment paradigm. Accordingly, the aim of the current review is to outline how breast cancer patient management has evolved such that robust chemotherapy prescriptions have enhanced clinico-oncological outcomes and to determine the potential role of microRNA in enhancing treatment with curative intent using chemotherapy in patients diagnosed with breast cancer.
2. Breast Cancer Chemotherapy
2.1. Adjuvant Chemotherapy
Complete surgical resection has traditionally provided effective breast cancer disease control [17]. William Halstead’s radical mastectomy (which involved extensive resection of all the breast parenchyma, locoregional lymph nodes, and pectoralis major muscle) was once considered the mainstay of breast cancer management, irrespective of disease burden [5,18]. The first chemotherapeutical regimen prescribed with curative intent in breast cancer was cyclophosphamide, methotrexate, and 5-fluorouracil (CMF) prescribed by Bonadanno et al. in 1976, which significantly reduced breast cancer recurrence (94.7% of 207 patients treated with chemotherapy versus 76.0% of 179 patients spared chemotherapy) [19]. Since the late 1950s, Bernard Fisher and his National Surgical Adjuvant Breast and Bowel Project (NSABP) hypothesised the limited scientific and biomolecular rationale for radical surgery in breast cancer, as this alone was often insufficient to establish total disease control [20]. The NSABP is a clinical trial cooperative group funded by the National Cancer Institute which is responsible for several landmark studies in the fields of breast and colonic oncology, including data supporting the added value of chemotherapy in cases of breast carcinoma [21]. Within the context of ER-/lymph-node-negative (LN-) disease, the NSABP-B13, B-19, and B-23 trials highlighted the non-inferiority of prescribing four cycles of doxorubicin and cyclophosphamide (AC) versus six cycles of CMF chemotherapeutical agents or methotrexate and 5-fluorouracil (MF), while also highlighting no benefit of adding tamoxifen (a selective estrogen receptor modulator) for this disease subtype [22,23,24]. Additionally, in their NSABP B-14 and B-20 trials, Fisher et al. established that within ER+ early disease, tamoxifen combined with chemotherapy (6-cycles of CMF and tamoxifen (CMFT) or methotrexate and 5-fluorouracil and leucovorin (MTF)) provided survival advantaged over tamoxifen alone (5-year disease-free survival (DFS): MTF 90%, CMFT 89%, tamoxifen alone 85%) while also demonstrating that tamoxifen provides enhanced 5-year, 10-year, and 15-year survival over placebo (5-year: 69% vs. 57%, p < 0.0001) [25,26,27]. Following the success of these clinical trials, the hypothesis evolved to identify those with ER+/LN− disease who derive benefit from combined adjuvant chemoendocrine therapy versus those who may be spared chemotherapy and treated with tamoxifen alone; through assessment of the resected specimen paraffin-embedded blocks from the NSABP B-14 and B-20 trials, Genomic Health Inc. (Redwood City, CA, USA) designed and validated a reverse transcriptase-polymerase chain reaction (RT-PCR) 21-gene assay (OncotypeDX Recurrence Score©) capable of predicting recurrence risk and estimating benefit from cytotoxic chemotherapy prescription in these patients [9]. The subsequent results of the TAILORx trial illustrated no survival advantage for post-menopausal patients, with RS < 25, implicating indication for endocrine therapy alone for these patients [28]. In more recent times, the expansion of indications into the locally advanced and neoadjuvant settings is likely based on preliminary data from the RxPONDER trial (recruiting patients with 1–3 positive nodes) and several meta-analyses [29,30,31,32]. The landmark clinical studies assessing the role of multigene expression assays for guiding adjuvant chemotherapy prescription in ER+ breast cancer are outlined in Supplementary Table S1. In summary, Fisher’s hypothesis that all breast cancer patients required systemic treatment (particularly with chemotherapy) has been successfully challenged. The molecular era allows us to personalise approaches to optimise outcomes for patients, minimise toxicity, and achieve disease control with less aggressive and more targeted therapies. The future will allow us to address specific markers of response to facilitate tumour eradication and limit the need for prolonged and excessive therapies. The scientific community is now addressing the value of measuring mi(cro)RNA expression (both tumour and circulating) which can potentially allow prescription of appropriate targeted treatments, address early relapse, and even allow specific miRNA directed therapies.
In the 1990s, the cytotoxic effect of taxane-based chemotherapy provided the rationale for their addition for evaluation in clinical trials in the adjuvant setting; the NSABP B-28 tial randomised patients to receive four cycles of paclitaxel following four cycles of conventional adriamycin and cyclophosphamide (AC) in patients with LN+ breast cancer, reducing the relative risk of DFS events by 17% (5-year DFS: 76% vs. 72%, p = 0.006) [33]. The NSABP B-30 trial then illustrated the enhanced survival in patients with LN+ disease who received four cycles of adriamycin and cyclophosphamide, followed by four cycles of docetaxel (ACT), versus upfront ACT or doxorubicin and docetaxel (8-year DFS: 74% vs. 69% vs. 69%, respectively). At this time, cancer researcher Axel Ullrich and medical oncologist Denis Slamon highlighted the substantial information accumulating in relation to the role of the HER2/neu oncogene in 20–25% of breast tumours and recognised targeting this receptor on the cell surface by a monoclonal antibody, trastuzumab, which could inhibit cell signaling, impair oncogenesis, and improve clinical outcomes for patients [34,35,36]. While trastuzumab was initially validated for use in metastatic HER2+ disease [37,38,39], results from landmark trials such as the HERA, NSABP B-31, and PHARE have seen the expansion of clinical indications for the prescription of trastuzumab into the adjuvant setting [40,41,42]. Randomised clinical trials outlining the role of trastuzumab in HER2+ disease are outlined in Table 1.
Table 1.
Table outlining the landmark randomised clinical trials outlining the role of trastuzumab in human epidermal growth factor receptor-2 positive breast cancer.
| Author | Year | Trial | N | Patients | Arms | Findings | Journal |
|---|---|---|---|---|---|---|---|
| Pegram [39] | 1998 | Phase II Clinical Trial | 39 | Refractory metastatic HER2+ breast cancer | IV trastuzumab combined with Cisplatin | Of 37 patients followed up, 24.3% (9/37) achieved a clinical PR and had SD, respectively, while 51.3% (19/37) suffered PD. Grade III/IV toxicity was observed in 56.4% (22/39) | Journal of Clinical Oncology |
| Cobleigh [38] | 1999 | Phase II Clinical Trial | 213 | Refractory metastatic HER2+ breast cancer | IV trastuzumab | 8 patients achieved a CR (3.8%), 26 achieved a PR (12.2%), while 16 achieved an objective response (7.6%) | Journal of Clinical Oncology |
| Baselga [43] | 1999 | Phase II Clinical Trial | 46 | Metastatic HER2+ | IV trastuzumab | Of 42 followed patients, 5 patients achieved an OR (11.6%, 5/42), specifically, 1 CR and 4 PR. | Seminars in Oncology |
| Slamon [37] | 2001 | Phase III RCT, (PIVOTAL Trial) | 469 | Metastatic HER2+ breast cancer | AC vs. trastuzumab combined with AC | Combined trastuzumab and chemotherapy were associated with longer PFS (7.4 months vs. 4.6 months), a higher OR rate (50% vs. 32%), a longer duration of response (9.1 months vs. 6.1 months), a lower mortality rate at 12 months (22% vs. 33%) and prolonged survival (25.1 months vs. 20.3 months) (all p < 0.05). | New England Journal of Medicine |
| Piccart-Gebhart [40] | 2005 | HERceptin Adjuvant (HERA) Phase III RCT (NCT00045032) |
5081 | Resected HER2+ breast cancer | trastuzumab alone for 2 years, trastuzumab combined with (neo)adjuvant chemotherapy for 1-year, 2 years. | Overall, there were 347 events (i.e.: recurrence, contralateral cancer, new primary, or death) of which 220 were in the observational group, compared with 127 in the trastuzumab group (HR:0.54). Cardiotoxicity was reported in 0.5% of patients treated with trastuzumab | New England Journal of Medicine |
| Romond [44] | 2005 | Phase III RCT, NSABP B-031 & N9831 (NCT00004067 & NCT00005970) |
3351 | Operable HER2+ breast cancer | AC and Paclitaxel vs. trastuzumab combined with AC and Paclitaxel | Overall, there were 394 events (i.e.: recurrence, new primary, or death) of which 261 were in the observational group, compared with 133 in the trastuzumab group. At 3 years, the trastuzumab group had a 12% absolute improvement in DFS and a 33% reduction in mortality. | New England Journal of Medicine |
| Joensuu [45] | 2006 | Phase III RCT—FinHer trial (ISRCTN76560285) |
232 | Locally advanced HER2+ breast cancer | Docetaxel or Vinorelbine, followed by FEC randomised to receive 9 trastuzumab infusions | In those treated with trastuzumab, they had enhanced 3-year RFS (HR: 0.42, 89% vs. 78%). | New England Journal of Medicine |
| Untch [46] | 2010 | GeparQuattro Phase III RCT (NCT00288002) |
1509 | Operable or locally advanced HER2+ breast cancer | Neoadjuvant EC-T(X) with trastuzumab | pCR rates were 31.7% in those treated for HER2+ cancers, compared with 15.7% in other subtypes. Patients with no response following EC showed an unexpectedly high pCR rate following trastuzumab (16.6% vs. 3.3% in the reference group). Cardiac toxicity was comparable for both groups. | Journal of Clinical Oncology |
| Slamon [34] | 2011 | Phase III RCT (NCT00021255) |
3222 | Early stage HER2+ breast cancer | AC-T vs. AC-T with trastuzumab, vs. TCH | 5-year DFS rates were 75%, 84%, and 81%, with respective estimated survival rates of 87%, 92%, and 91%. The rates of cardiac dysfunction were significantly higher in the AC-T and trastuzumab group vs. TCH (p < 0.001). | New England Journal of Medicine |
| Baselga [47] | 2012 | NeoALLTO Phase III RCT (NCT00553358) |
455 | Early stage HER2+ breast cancer | Neoadjuvant lapatinib, trastuzumab, or combined lapatinib and trastuzumab | pCR rates were highest in the lapatinib and trastuzumab group (51.3%) vs. 29.5% and 24.7% in the trastuzumab and lapatinib groups, respectively. There were no major cardiac dysfunctions suffered. | Lancet |
| Perez [48] | 2014 | Phase III RCT, NSABP B-031 & N9831 (NCT00004067 & NCT00005970) |
4046 | Operable HER2+ breast cancer | AC and Paclitaxel vs. trastuzumab combined with AC and Paclitaxel | Adding trastuzumab to chemotherapy enhanced survival (HR: 0.63), increasing the 10-year survival from 75.2% to 84.0%. Moreover, this enhanced DFS by 40% (HR: 0.40) and improved the estimated 10-year DFS from 62.2% to 73.7%. | Journal of Clinical Oncology |
| Gianni [49] | 2014 | NeOAdjuvant Herceptin (NOAH) Phase III RCT (ISRCTN86043495) |
235 | Operable HER2+ breast cancer | NAC vs. NAC and trastuzumab, both received adjuvant trastuzumab | After 5 years of follow-up, patients treated with NAC and trastuzumab had an EFS of 58% vs. 43% in the NAC group (HR: 0.64). Of patients achieving a pCR (N = 67), 44 had received NAC and trastuzumab (66%) vs. 23 in those treated with NAC alone (34%). | Lancet Oncology |
| Cameron [50] | 2017 | HERceptin Adjuvant (HERA) Phase III RCT (NCT00045032) |
5102 | Early stage HER2+ breast cancer | Post-treatment (i.e.: surgery, (neo)adjuvant chemotherapy): trastuzumab alone for 1-year vs. trastuzumab alone for 2 years, vs. observation group | Following 11 years follow up, 1-year of trastuzumab enhanced DFS (HR: 0.76) and death (HR: 0.74) vs. observation. Receiving trastuzumab for 2 years did not improve survival vs. 1-year of treatment (HR: 1.02). Estimations of survival were 69% for 1-year, 69% for 2 years, and 63% for observations. There were increased cardiac toxicities in those treated with trastuzumab (1-year rate 4.4% and 2-year rate of 7.3%) vs. observations (0.9%) | New England Journal of Medicine |
| Earl [51] | 2019 | PERSEPHONE Phase III RCT (NCT00712140) | 4089 | Early stage HER2+ breast cancer | Post-treatment (i.e.: surgery, (neo)adjuvant chemotherapy): Adjuvant trastuzumab for 1-year vs. trastuzumab for 6 months | At 5 years follow up, treatment with 6-month of trastuzumab in the adjuvant setting is non-inferior to 12-month treatment after conventional treatment. Events were comparable for both groups (1-year: 12% vs. 6 months: 13%), with 4-year DFS rates of 89.4% and 89.8%, respectively (HR: 1.07). There were fewer toxicities reported in the 6-month group (19% vs. 24%) | New England Journal of Medicine |
N; number, HER2+; human epidermal growth factor receptor-2 positive, IV; intravenous, PR; partial response, SD; stable disease, PD; progressive disease, OR; objective response, CR; complete response, RCT; randomised controlled trial, PFS; progression-free survival, RFS; recurrence-free survival, EFS; event-free survival, pCR: pathological complete response, NAC; neoadjuvant chemotherapy, AC; Doxorubicin and Cyclophosphamide, EC-T(X); Doxorubicin and Cyclophosphamide followed by Docetaxel with or without Capecitabine, TTP; time-to-progression, FEC: 5-flurouracil, epirubicin and cyclophosphamide, AC-T; doxorubicin and cyclophosphamide, followed by docetaxel, TCH; trastuzumab, docetaxel, and carboplatin.
2.2. Neoadjuvant Chemotherapy
Oncological practice has evolved recognising the inherent value of treating patients with chemotherapy in the neoadjuvant setting. Advantages of neoadjuvant chemotherapy (NAC) included tumour downstaging, increasing patient eligibility for breast conservation surgery (BCS), as well as the generation of in vivo data in relation to tumour sensitivity, which has been illustrated to carry prognostic significance for disease recurrence and overall survival (OS) [52,53,54]. While DFS and OS outcomes are similar to those treated in the adjuvant setting, recent data from a meta-analysis of randomised trials conducted by the Early Breast Cancer Triallist’s Collaborative Group (EBCTCG) indicate that there are increased rates of locoregional recurrence (LRR) following neoadjuvant therapy (21.4% vs. 15.9%) [55]. Furthermore, there is increasing evidence outlining a survival advantage for those who achieve a pathological complete response (pCR) following NAC, compared with their contemporaries with residual disease [54,56].
In 1998, the seminal NSABP B-18 trial identified the benefit of NAC in their analysis of 1523 randomised patients with early breast cancer; 13% achieved a pCR (defined as absence of invasive tumour in the breast specimen following NAC), 36% achieved a complete clinical response, 43% achieved a partial clinical response, 37% of patients underwent axillary downstaging having presented with palpable LNs, and patients in receipt of NAC were more likely to undergo successful BCS (67% vs. 60%, p = 0.002) [57,58]. Moreover, there was no significant difference in DFS, distant DFS, and OS observed between both groups, although a non-significant difference in LRR was observed in those treated with NAC (10.7% vs. 7.6%, p = 0.120) [59]. In 2003, the NSABP B-27 randomised trial evaluated the role of adding four cycles of docetaxel to four cycles of neoadjuvant AC [60] and demonstrated increased pCR rates and axillary downstaging with added neoadjuvant docetaxel while failing to increase BCS rates, DFS, and OS outcomes [61].
Traditionally, histopathological features such as tumour size and degree of nodal involvement were the predominant selection criteria for NAC. However, the paradigm has evolved such that intrinsic tumour biology informs response rates to neoadjuvant therapies and predicts those likely to achieve pCR [62]. While molecular subtyping from diagnostic core biopsy remains critical in contemporary breast cancer management in relation to the indication for NAC, multigene expression assays (such as the 21-gene expression signature) are likely to indicate response to neoadjuvant therapies in early stage ER+ disease [29,30] (Supplementary Figure S1). With respect to HER2+ and TNBC, the clinical utility of NAC has become embedded into best-practice guidelines: A recent update from ASCO recommends the use of NAC and trastuzumab for HER2+ cancers (with the exception of T1a-T1b N0 disease), with anthracycline and taxane-based chemotherapy and trastuzumab to be utilised in high-risk LN- cases and those with LN positivity [63]. Furthermore, patients with TNBC should be offered an anthracycline and taxane-based regimen in all cases, unless diagnosed with cancer staged T1a-T1b N0 [63]. Based on the work of a recent meta-analysis, ASCO also endorse the addition of platinum-based chemotherapy in TNBC due to an increased propensity to achieve pCR (52.1% versus 37.0%) [64]. Preliminary results of the KEYNOTE522 trial supports anti-programmed death ligand-1 (PD-L1) inhibition through immune-checkpoint modulation (pembrolizumab) into practice to further enhance pCR rates (pembrolizumab and NAC: 64.8% versus placebo and NAC: 51.2%) [65]; however, ASCO report insufficient evidence at present for their inclusion in conventional neoadjuvant treatment for TNBC. Future directions for translational research efforts are focused on the extrapolation of enhancing pCR rates, facilitating the de-escalation of adjuvant treatment following pCR and reducing treatment-related toxicities for patients in receipt of these neoadjuvant therapies [66,67]. There is a vogue in recent times to suggest manipulation of treatment using miRNA replacement therapies may be useful in augmenting pCR rates to NAC in breast cancer, which is outlined in detail in this review. Table 2 outlines seminal studies validating NAC prescription in early breast cancer.
Table 2.
The outline of seminal studies validating neoadjuvant chemotherapy prescription in breast cancer patients.
| Author | Year | Study | N | Patients | Arms | Findings | Journal |
|---|---|---|---|---|---|---|---|
| Fisher [58] | 1998 | NSABP B-018 phase III, RCT | 1523 | Locally advanced breast cancer | Neoadjuvant vs. adjuvant chemotherapy prescription | Overall, 13% achieved a pCR to NAC, 36% achieved a CCR, 43% achieved a PCR, and 37% of patients downstaged their axilla previously palpable LNs. Overall, patients after NAC were more likely to undergo successful BCS (67% vs. 60%, p = 0.002) | Journal of Clinical Oncology |
| Mauri [68] | 2005 | Meta-analysis of RCTs | 3946 | Early breast cancer | NAC vs. adjuvant chemotherapy | There was no difference in DP (RR: 0.99), DR (RR: 0.94), or OS (RR: 1.00) outcomes for NAC vs. adjuvant therapy. However, there were increased LRR rates following NAC (RR: 1.22) | Journal of the National Cancer Institute |
| Bear [61] | 2006 | NSABP B-027 phase III, RCT | 2411 | Early breast cancer | NAC (AC) and Docetaxel vs. AC alone | There were increased pCR rates and axillary downstaging with added neoadjuvant docetaxel, however failed to increase BCS rates, DFS, and OS outcomes overall. The addition of neoadjuvant Docetaxel increased pCR rates | Journal of Clinical Oncology |
| Van Nes [69] | 2009 | Preoperative chemotherapy in Primary Operable Breast Cancer (POCOB) | 698 | Early breast cancer | NAC vs. adjuvant chemotherapy | At 10 years of follow-up, there was no observed difference in OS, DFS, or LRR (all P>0.05); however, NAC was associated with increased BCS rates | Breast Cancer Research and Treatment |
| EBCTCG [55] | 2018 | Meta-analysis of RCTs | 4756 | Early breast cancer | NAC vs. adjuvant chemotherapy | At 15 years follow-up, NAC was associated with increased LRR rates (21.4% vs. 15.9%), however there was no difference in DR (38.2% vs. 38.0%), BCM (34.4% vs. 33.7%) and OS (40.9% vs. 41.2%) | Lancet Oncology |
N; number, RCT; randomised controlled trial, pCR: pathological complete response, NAC; CCR; complete clinical response, PCR; partial clinical response, LN; lymph nodes, BCS; breast conservation surgery, DP; disease progression, RR; rate ratio, DR; distant recurrence, OS; overall survival, DFS; disease-free survival, LRR; locoregional recurrence, AC; Doxorubicin and Cyclophosphamide, EBCTCG; early breast cancer triallist collaborative group, BCM; breast cancer mortality.
3. MicroRNAs
MiRNAs are a contemporary class of small (19–25 nucleotides in length) non-coding endogenous RNAs which are known to play key modulatory roles in gene expression and cellular processes [14]. They were first described by Lee et al. in 1993 when studying developmental timing of Caenorhabditis elegans [15]; scientific understanding of the role of miRNA has exponentially grown in recent years, with aberrant miRNA expression profiles now understood to correlate with several diverse pathological processes, including oncogenesis [70,71]. MiRNAs regulate gene expression at a post-transcriptional level by binding to the 3′ or 5′untranslated regions of target mRNA, hindering mRNA expression through degradation or translation inhibition [72]. Overall, miRNAs can be oncogenic (oncomirs) or tumour suppressors (tumour suppressor miRNA) and influence cancer development through each of these means.
The biogenesis of miRNA is a complex, multi-step process occurring initially in the cellular nucleus, before completing the production process in the cytoplasm: MiRNA genes are transcribed by RNA polymerase II/III in the nucleus to form large, capped, and polyadenylated primary miRNA transcripts (pri-miRNA) [73]. Next, pri-miRNAs are cleaved into pre-miRNA (which are 70–90 nucleotides in length) by the coupled RNase III enzyme Drosha and its complementary binding partner DCGR8. These pre-miRNAs are the precursor molecules to miRNAs and then exported out of the nucleus in their imperfect hairpin structure by the export protein (Exportin 5) [74]. These pre-miRNAs then undergo cleavage into double-stranded miRNAs in the cytoplasm by RNase type III Dicer with either the trans-activating RNA-binding protein (TRBP) or the protein activator of the interferon-induced protein kinase (PACT) [75], with one of these strands representing the mature miRNA which forms the RNA silencing complex in conjunction with several other proteins [76]. This mature strand is then incorporated into the miRNA-associated RNA-induced silencing complex (miRISC), which guides the RISC to target mRNA due to its complementary sequences to the mature mRNA, consequentially impacting on and altering gene expression. Several studies correlate miRNA expression profiles to important biopathologic and molecular subtyping data [77,78]; using a stepwise artificial neural network model in 95 tumours, Lowery et al. identified miRNA signatures capable of predicting ER, PgR, and HER2 receptors, indicating the crucial role of individual miRNA in deriving intrinsic biological breast cancer subtypes. Furthermore, Sokilde et al. validated the hypothesis that miRNA profiles largely recapitulate molecular subtypes [77,78]. Although we are now well acquainted with the various tumour suppressor/oncogenic roles of miRNA in cancer development, the aforementioned studies underpin the critical role of various miRNA such processes, with variations even observed among differing intrinsic biological subtypes of the disease. While these studies provide promise for the identification of novel molecular subtypes capable of being targeted with future therapeutic strategies to enhance oncological outcome, other authors focus on the current breast cancer treatment paradigm. These authors highlight the potential for miRNA signatures as predictive and prognostic biomarkers that could personalise breast cancer therapeutics and improve patient selection strategies for current therapies, such as conventional cytotoxic chemotherapies [79,80].
4. MicroRNAs in Predicting Response to Neoadjuvant Chemotherapies
As previously outlined, breast oncology has evolved in recent years to recognise it is strategic and advantageous to treat patients with chemotherapy in the neoadjuvant setting [57,58]. While conventional clinicopathological characteristics have been reported to correlate with response to NAC [53,81,82,83], deciphering those likely to achieve such responses remains challenging to the oncologists, with response rates often difficult to predict. Several recent studies correlate miRNA expression profiles with response to NAC for breast cancer: Xing et al. reported that increased expression of miR-23a-3p, miR-200c-3p, miR-214-3p and reduced expression of miR-451a and miR-638 correlated to chemoresistance (Miller–Payne grade 1) [84]. In the Clinical Trials Ireland All-Ireland Cooperative Oncology Research Group (CTRIAL-IE ICORG) 10/11 prospective, multicentre translational trial, McGuire et al. highlight the inherent value of miR-21 expression as a correlate to response to standard NAC in their analysis of 114 breast cancer patients [79]. Moreover, Liu et al. illustrate reduced miR-21 expression levels after cycle 2 of NAC in responders (versus non-responders), supporting the work of CTRIAL-IE ICORG 10/11 trial [85]. In the translation research arm of the NeoALTTO prospective study, Di Cosimo et al. outlined the clinical utility of venous sampling for miR-140a-5p, miR-148a-3p, and 374a-5p, and their predictive value in determining response to following neoadjuvant therapies [86], with an increased combined predictive capability of 54% in determining pCR to trastuzumab in HER2+ disease, compared with 0% in cases of poor expression. In the GeparSixto trial, Stevic et al. described how aberrant expression of miR-199a in patient plasma was predictive of pCR to NAC in their series of 435 patients diagnosed with either early stage HER2+ or TNBC disease [87]. Kassem et al. provided promising data supporting miR-34a expression levels to accurately discriminate responders from non-responders in 39 patients being treated for locally advanced breast cancer (area under the curve (AUC): 0.995, sensitivity: 97.4%, specificity: 100.0%) [88]. Garcia-Garcia reported reduced miR-145-5p expression levels in patients successfully achieving a pCR to NAC (AUC: 0.790, p = 0.003) [89]. Table 3 illustrates prospective trials evaluating the role of miRNAs in predicting response to neoadjuvant therapies and describing the miRNAs that are relevant in this settling. With respect to adjuvant chemotherapy, using miRNA expression profiles to measure response is significantly more challenging. Factors such as timing of miRNA sampling, crude assessment of response rates to treatment and quantifying whether therapy enhanced oncological outcomes for those likely to succumb to recurrence is extremely difficult. Thus, it is unsurprising that most studies measure miRNA expression profiles with metrics indication response (i.e.: RECIST, Miller–Payne grade, Sataloff score, etc.) to NAC and not adjuvant chemotherapy.
Table 3.
Table illustrating prospective trials evaluating the role of miRNAs in predicting response to neoadjuvant therapies.
| Author | Year | Study | N | Patients | Treatment Arms | Findings | Journal |
|---|---|---|---|---|---|---|---|
| Muller [90] | 2014 | Prospective phase II Geparquinto Trial (NCT00567554) | 127 | Early stage HER2+ breast cancer | NAC with trastuzumab or lapatinib | Increased miR-21, miR-210, and miR-373 in patient’s serum following treatment with NAC correlated to response to treatment. | Breast Cancer Research and Treatment |
| Xue [91] | 2015 | Prospective phase II clinical trial | 50 | Early stage breast cancer | Carboplatin and Paclitaxel | Increased miR-621 expression profiles predicted pCR to NAC | Oncogene |
| Stevic [87] | 2018 | Prospective phase II clinical trial GeparSixto Trial (NCT01426880) | 211 | Early stage breast cancer | Docetaxel or Paclitaxel +/− Carboplatin | Aberrant miR-199a expression correlates to pCR following neoadjuvant therapies | BMC Cancer |
| Zhu [92] | 2018 | Prospective phase II clinical trial (NCT02041338) | 24 | Operable breast cancer | Epirubicin & Docetaxel | After the second cycle of NAC, reduced miR-34a expression was correlated with patients who did not respond to treatment | Cancer Medicine |
| Kahraman [93] | 2018 | Prospective, case–control study (MODE-B study) | 42 | Early stage TNBC breast cancer | Carboplatin and Paclitaxel | Identification of 74 miRNAs which predicted pCR based on changes in expression profiles pre- and post-NAC. | Scientific Reports |
| Di Cosimo [80] | 2019 | NeoALLTO Phase III RCT (NCT00553358) |
455 | Early stage HER2+ breast cancer | Neoadjuvant lapatinib, trastuzumab, or combined lapatinib and trastuzumab | Increased circulating plasma levels of miR-140a-5p, miR-148a-3p and 374a-5p were associated with pCR and miR-140a-5p predicted enhanced EFS | Clinical Cancer Research |
| Lindholm [94] | 2019 | Randomised, phase II clinical trial (NCT00773695) |
132 | Early stage HER2- breast cancer | FEC-T or FEC-P, +/− Bevacizumab | Hierarchical clustering of 627 miRNAs with response at 12 and 25 weeks to neoadjuvant treatment with NAC or NAC combined with Bevacizumab; of these, 217 had differential expression profiles (71 upregulated and 146 downregulated) between responders and non-responders. | Molecular Oncology |
| Rodriguez-Martinez [95] | 2019 | Prospective clinical trial | 53 | Locally advanced and advanced breast cancer | AC | Exosomal expression of miR-21 correlated in a stepwise fashion with patients achieving a CR having significantly reduced miR-21 vs. patients with PR and SD, respectively. | Breast Cancer Research |
| Di Cosimo [86] | 2020 | NeoALLTO Phase III RCT (NCT00553358) |
455 | Early stage HER2+ breast cancer | Neoadjuvant lapatinib, trastuzumab, or combined lapatinib and trastuzumab | After 2 weeks of neoadjuvant treatment, increased expression of miR-15a-5p, miR-140-3p, miR-320a, miR-320b, miR-363-3p, miR-378a-3p, miR-486-5p & miR-660-5p and decreased miR-30d-5p correlated with pCR to lapatinib. At 2 weeks of therapy, increased expression of miR-26a-5p & miR-374b-5p correlated with pCR to trastuzumab. Increased let-7g-5p & miR-191-5p and reduced miR-195-5p correlated with pCR to combined trastuzumab and lapatinib. | International Journal of Molecular Sciences |
| McGuire [79] | 2020 | Prospective phase II clinical trial [CTRIAL-IE ICORG] 10/11 (NCT00553358 | 114 | Early stage breast cancer | Various NAC regimens | Responders had reduced miR-21 and miR-195 vs. non-responders in all breast cancer subtypes. MiR-21 independent predicted response (OR 0.538, 95% CI 0.308–0.943). In luminal cancers, reduced expression of miR-145 and miR-21 correlated with response to NAC. | Cancers (Basel) |
| Zhang [96] | 2020 | Prospective phase II trials; SHPD001 (NCT02199418) & SHPH02 (NCT02221999) | 65 | Early stage HER2+ breast cancer | Paclitaxel, Cisplatin & trastuzumab | Low miR-222-3p expression was predictive of achieving pCR (OR: 0.258, 95% confidence interval: 0.070–0.958, p = 0.043) and favourable DFS and survival | Frontiers in Oncology |
N; number, HER2-+ human epidermal growth factor receptor-2 positive NAC; neoadjuvant chemotherapy, pCR; pathological complete response, TNBC; triple-negative breast cancer, EFS; event-free survival, HER2-; human epidermal growth factor receptor-2 negative, FEC-T; 5-fluorouracil, epirubicin, and cyclophosphamide followed by docetaxel, FEC-P; 5-fluorouracil, epirubicin, and cyclophosphamide followed by paclitaxel, AC; doxorubicin and cyclophosphamide, CR; complete response, PR: partial response, SD; stable disease, OR; odds ratio, DFS; disease-free survival.
5. MicroRNAs and Chemoresistance
It has been well established that miRNAs are capable of increasing the resistance of cancer cells to conventional chemotherapeutic drugs, endocrine hormonal agents, and radiotherapies [97,98,99,100]. Regarding chemotherapeutic resistance, several reports have revealed scientific mechanisms and rationale for resistance, including alterations of drug-target interactions, reduced active drug concentrations, and enhanced tumour cell survival [101]. Investigations of the regulatory role for miRNAs in impacting chemoresistance to chemotherapy agents are abundant, with several miRNA expression profiles implicated in predicting chemoresistance: Within TNBC, translational research studies have recently correlated decreased expression of miR-18a, miR-1207-5p and miR-5195-3p are predictors of resistance to paclitaxel or docetaxel in TNBC [102,103,104]. Furthermore, Wu et al. identified that overexpression of miR-620 facilitates tumour resistance to gemcitabine-based chemotherapies in TNBC through downregulating dCMP deaminase (DCTD) expression [105]. In the circulation, detection of increased miR-125b expression levels correlated with chemoresistance in 56 patients with invasive ductal carcinoma being treated with curative intent (p = 0.008) [106]. MiR-24 has been shown to induce chemoresistance in early breast cancer through hampering the chemotherapy-induced apoptosis and increasing cell resistance to hypoxia via the hypoxia-inducible factor-1 (HIF-1) pathway [107]. Furthermore, miR-155 has been implicated in several studies as a player in drug resistance and cancer promotion through regulation of FOXO3a signaling, interrupting TGF-beta facilitating epithelial–mesenchymal transition and inducing drug resistance through RhoA signaling [108]. Additionally, miR-221 has been illustrated to promote breast cancer resistance to adriamycin via modulation of the PTEN/Akt/mTOR signaling pathway in 25 breast cancer samples [109].
6. MicroRNAs for Therapeutic Use in Breast Cancer
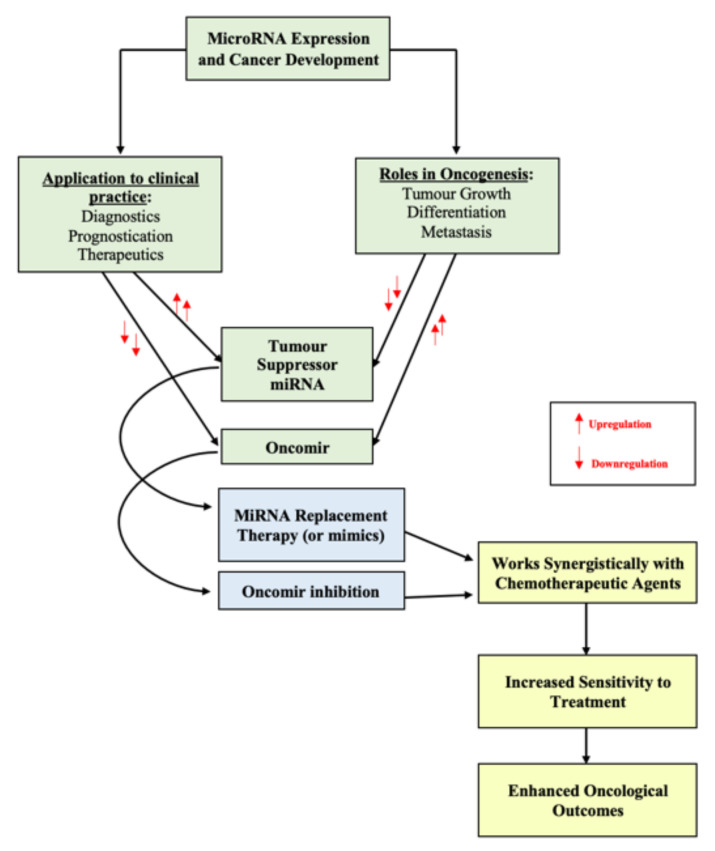
The molecular era has facilitated the use of miRNAs for the development of novel therapeutic strategies. These involve the introduction of pre-selected miRNAs into the tumour microenvironment for use as a treatment or to enhance the effect of current treatment modalities used in routine clinical practice, such as systemic chemotherapy [101,110]. MiRNAs have the capacity to function as either oncomirs or tumour suppressors, indicating there are two potential approaches for using miRNA as therapeutics—(1) oncomir inhibition which involves reducing targeted miRNA expression profiles (i.e., miRNA silencing) through introducing inhibitory miRNA to reduce the anticipated protein expression levels or (2) miRNA replacement therapy which involves inducing and overexpressing of select miRNA to reduce oncogenesis or increase sensitivity to systemic treatment (Figure 1).
Figure 1.
Figure outlining the manipulation of miRNA expression profiles for cancer therapeutics.
6.1. Oncomir Inhibition
Oncomirs are classically upregulated in malignancy [111]. The inhibition of oncogenic miRNA activity may be achieved through the use of miRNA antagonist oligonucleotides (anti-miRs), targeted miRNA silencing (antagomirs), or locked nucleic acid (LNA) [112]. Such inhibitor mechanisms can augment the sensitivity of breast cancer cells to chemotherapeutic agents in several pre-clinical studies: Li et al. report the successful transfection of miR-3609 into MCF-7/ADR cell lines to increase tumour cell sensitivity to adriamycin-based chemotherapy [113]. Furthermore, Lin et al. induced miR-133 into cisplatin-resistant TNBC cells from 65 breast cancer patients and successfully increased cell sensitivity to chemotherapy for these patients [114]. Similarly, Li et al. transfected miR-155-5p into tumour cells and successfully overcame paclitaxel resistance in previously resistant breast cancer cells [115]. Finally, Mei et al. indicate that downregulation of miR-21 increased MCF-7 breast cancer cells lines to docetaxel chemotherapy [116], while Ru et al. outline how miR-203 knockdown can successfully increase cisplatin sensitivity to chemotherapy.
6.2. MiRNA Replacement Therapy
Tumour suppressor miRNAs have the capacity to inhibit oncogenesis through regulating oncogenes and controlling genes responsible for controlling cell proliferation and apoptosis [117]. MiRNA replacement therapy involves the reintroduction of tumour suppressing miRNA (or mimics) into the tumour microenvironment to reduce oncogenesis and control cancer proliferation [118]. Zhang et al. described the potential to use the miR-34 family as tumour suppressor modulators in the setting of several epithelial cancers, including breast [119]. The works of Yu et al. and Cochrane et al. provide data illustrating the value of reintroducing and increasing expression levels of let-7a, miR-30, and miR-200c to reduce tumourigenesis and increase chemosensitivity in studies involving animals and MDA-MB-231 and MDA-MB-549 chemoresistant breast cancer cells lines [120,121,122]. Furthermore, Kalinowski reviewed the strong therapeutic potential of miR-7 replacement therapy to enhance current treatment with conventional breast cancer chemotherapy [123].
The great challenge in current and future strategies for improved outcome in breast cancer is to successfully implement an evidence-based approach which fundamentally can allow (1) using miRNAs to address treatment rationalisation—selection of appropriate length and constituents for enhancing chemotherapeutic effect, (2) enhancing liquid biopsies selection of appropriate systemic miRNA profiling to reduce the need for cytotoxic chemotherapy/address recurrence risk, (3) augmenting current molecular subtyping with subtype-specific rational miRNA profiling, and (4) using miRNAs to enhance/select chemotherapeutic and other tumour-targeting strategies.
7. Future Directions for miRNA
Despite considerable investment into the discovery, development, and augmentation of miRNAs as novel therapeutics for breast cancer patient management, this subcategory of translational research remains in its infancy. Furthermore, the efforts to use miRNA to personalise cancer therapeutics have been plentiful, with minimal advancements towards enhancing clinico-oncological outcomes through miRNA targeting. Currently, the evolution of miRNA therapeutics faces several developmental challenges. This review is limited in that most studies conducted to date provide data in relation to in vitro studies, with few stemming beyond breast cancer cell lines or animal studies. In conjunction with the accepted scientific method, clinical trials evaluating the clinical efficacy, safety profiles, and cost-effective benefit are required to support the preliminary data presented by these current studies. As has been thoroughly outlined in the current review, research in the clinical trial setting has revolutionised breast cancer patient care over the past four decades, leading to novel, personalised therapeutic strategies, minimally invasive surgical approaches to the breast and axilla, and enhanced clinico-oncological outcomes for patients who in previous eras may have succumbed to their disease. With the ongoing trials evaluating novel targeted therapies such as immune checkpoint modulation [65,124] and the adoption of poly(adenosine diphosphate–ribose) polymerase inhibitors (or PARP inhibitors) into the treatment of early stage breast cancer in BRCA mutation carriers [125], the personalisation of breast cancer patient care seems even closer than ever. Thus, this review highlights the critical emphasis which must be placed on clinical trial and translational research in order to further strive towards ‘curing’ breast cancer, through the mantra of precision oncology.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/ijms221910812/s1.
Author Contributions
Conceptualization, M.G.D. and M.J.K.; investigation, M.G.D.; resources, M.G.D.; data curation, M.G.D.; writing—original draft preparation, M.G.D.;writing, review and editing, M.G.D., N.M. and M.J.K.; visualization, M.G.D. and M.J.K.;supervision, A.J.L., N.M. and M.J.K.; project administration, M.G.D.; funding acquisition, M.G.D.,A.J.L. and M.J.K. All authors have read and agreed to the published version of the manuscript.
Funding
MGD received funding from the National Breast Cancer Research Institute, Ireland.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ferlay J., Soerjomataram I., Dikshit R., Eser S., Mathers C., Rebelo M., Parkin D.M., Forman D., Bray F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R.L., Miller K.D., Fuchs H.E., Jemal A. Cancer statistics, 2021. CA Cancer J. Clin. 2021;71:7–33. doi: 10.3322/caac.21654. [DOI] [PubMed] [Google Scholar]
- 3.DeSantis C.E., Ma J., Gaudet M.M., Newman L.A., Miller K.D., Sauer A.G., Jemal A., Siegel R.L. Breast cancer statistics, 2019. CA Cancer J. Clin. 2019;69:438–451. doi: 10.3322/caac.21583. [DOI] [PubMed] [Google Scholar]
- 4.Cancer Research UK Breast Cancer Statistics. 2021. [(accessed on 20 July 2021)]. Available online: https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/breast-cancer.
- 5.Sakorafas G., Safioleas M. Breast cancer surgery: An historical narrative. Part II. 18th and 19th centuries. Eur. J. Cancer Care. 2010;19:6–29. doi: 10.1111/j.1365-2354.2008.01060.x. [DOI] [PubMed] [Google Scholar]
- 6.Goldhirsch A., Winer E.P., Coates A.S., Gelber R.D., Piccart-Gebhart M., Thürlimann B., Senn H.J., Albain K.S., André F., Bergh J., et al. Personalizing the treatment of women with early breast cancer: Highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann. Oncol. 2013;24:2206–2223. doi: 10.1093/annonc/mdt303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morigi C. Highlights of the 16th St Gallen International Breast Cancer Conference, Vienna, Austria, 20–23 March 2019: Personalised treatments for patients with early breast cancer. Ecancermedicalscience. 2019;13:924. doi: 10.3332/ecancer.2019.924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pu M., Messer K., Davies S.R., Vickery T.L., Pittman E., Parker B.A., Ellis M.J., Flatt S.W., Marinac C.R., Nelson S.H., et al. Research-based PAM50 signature and long-term breast cancer survival. Breast Cancer Res. Treat. 2019;179:197–206. doi: 10.1007/s10549-019-05446-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paik S., Shak S., Tang G., Kim C., Baker J., Cronin M., Baehner F.L., Walker M.G., Watson D., Park T., et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N. Engl. J. Med. 2004;351:2817–2826. doi: 10.1056/NEJMoa041588. [DOI] [PubMed] [Google Scholar]
- 10.Dubsky P., Brase J.C., Jakesz R., Rudas M., Singer C.F., Greil R., Dietze O., Luisser I., Klug E., Sedivy R., et al. The EndoPredict score provides prognostic information on late distant metas-tases in ER+/HER2− breast cancer patients. Br. J. Cancer. 2013;109:2959–2964. doi: 10.1038/bjc.2013.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andre F., Ismaila N., Henry N.L., Somerfield M.R., Bast R.C., Barlow W., Collyar D.E., Hammond M.E., Kuderer N.M., Liu M.C., et al. Use of biomarkers to guide decisions on adjuvant systemic therapy for women with early-stage invasive breast cancer: ASCO clinical practice guideline update—Integration of results from TAILORx. J. Clin. Oncol. 2019;37:1956–1964. doi: 10.1200/JCO.19.00945. [DOI] [PubMed] [Google Scholar]
- 12.Senkus E., Kyriakides S., Ohno S., Penault-Llorca F., Poortmans P., Rutgers E., Zackrisson S., Cardoso F., ESMO Guidelines Committee Primary breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2015;26((Suppl. S5)):v8–v30. doi: 10.1093/annonc/mdv298. [DOI] [PubMed] [Google Scholar]
- 13.Gene Expression Profiling and Expanded Immunohistochemistry Tests to Guide the Use of Adjuvant Chemotherapy in Breast Cancer Management: MammaPrint, Oncotype DX, IHC4 and Mammostrat. 2011. [(accessed on 20 July 2020)]. Available online: https://www.nice.org.uk/guidance/dg10/documents/gene-expression-profiling-and-expanded-immunohistochemistry-tests-to-guide-selection-of-chemotherapy-regimes-in-breast-cancer-management-mammaprint-oncotype-dx-ihc4-and-mammostrat-overview2.
- 14.Davey M., Davies M., Lowery A., Miller N., Kerin M. The role of microRNA as clinical biomarkers for breast cancer surgery and treatment. Int. J. Mol. Sci. 2021;22:8290. doi: 10.3390/ijms22158290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee R.C., Feinbaum R.L., Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75:843–854. doi: 10.1016/0092-8674(93)90529-Y. [DOI] [PubMed] [Google Scholar]
- 16.Rupaimoole R., Slack F.J. MicroRNA therapeutics: Towards a new era for the management of cancer and other diseases. Nat. Rev. Drug Discov. 2017;16:203–222. doi: 10.1038/nrd.2016.246. [DOI] [PubMed] [Google Scholar]
- 17.McVeigh T., Boland M., Lowery A. The impact of the biomolecular era on breast cancer surgery. Surgeon. 2017;15:169–181. doi: 10.1016/j.surge.2016.09.007. [DOI] [PubMed] [Google Scholar]
- 18.Halsted W.S. The results of operations for the cure of cancer of the breast performed at the Johns Hopkins Hospital from June, 1889, to January, 1894. Ann. Surg. 1894;20:497–555. doi: 10.1097/00000658-189407000-00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bonadonna G., Brusamolino E., Valagussa P., Rossi A., Brugnatelli L., Brambilla C., de Lena M., Tancini G., Bajetta E., Musumeci R., et al. Combination chemotherapy as an adjuvant treatment in operable breast cancer. N. Engl. J. Med. 1976;294:405–410. doi: 10.1056/NEJM197602192940801. [DOI] [PubMed] [Google Scholar]
- 20.Fisher B. Biological research in the evolution of cancer surgery: A personal perspective. Cancer Res. 2008;68:10007–10020. doi: 10.1158/0008-5472.CAN-08-0186. [DOI] [PubMed] [Google Scholar]
- 21.Newman L.A., Mamounas E.P. Review of breast cancer clinical trials conducted by the national surgical adjuvant breast project. Surg. Clin. N. Am. 2007;87:279–305. doi: 10.1016/j.suc.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 22.Fisher B., Dignam J., Mamounas E.P., Costantino J.P., Wickerham D.L., Redmond C., Wolmark N., Dimitrov N.V., Bowman D.M., Glass A.G., et al. Sequential methotrexate and fluorouracil for the treatment of node-negative breast cancer patients with estrogen receptor-negative tumors: Eight-year results from National Surgical Adjuvant Breast and Bowel Project (NSABP) B-13 and first report of findings from NSABP B-19 comparing methotrexate and fluorouracil with conventional cyclophosphamide, methotrexate, and fluorouracil. J. Clin. Oncol. 1996;14:1982–1992. doi: 10.1200/JCO.1996.14.7.1982. [DOI] [PubMed] [Google Scholar]
- 23.Fisher B., Jeong J.H., Dignam J., Anderson S., Mamounas E., Wickerham D.L., Wolmark N. Findings from recent National Surgical Adjuvant Breast and Bowel Project adjuvant studies in stage I breast cancer. JNCI Monographs. 2001;30:62–66. doi: 10.1093/oxfordjournals.jncimonographs.a003463. [DOI] [PubMed] [Google Scholar]
- 24.Fisher B., Redmond C., Dimitrov N.V., Bowman D., Legault-Poisson S., Wigkerham D.L., Wolmark N., Fisher E.R., Margolese R., Sutherland C., et al. A randomized clinical trial evaluating sequential methotrexate and fluorouracil in the treatment of patients with node-negative breast cancer who have estrogen-receptor-negative tumors. N. Engl. J. Med. 1989;320:473–478. doi: 10.1056/NEJM198902233200801. [DOI] [PubMed] [Google Scholar]
- 25.Fisher B., Dignam J., Bryant J., Wolmark N. Five versus more than five years of tamoxifen for lymph node-negative breast cancer: Updated findings from the national surgical adjuvant breast and bowel project B-14 randomized trial. J. Natl. Cancer Inst. 2001;93:684–690. doi: 10.1093/jnci/93.9.684. [DOI] [PubMed] [Google Scholar]
- 26.Fisher B., Jeong J., Bryant J., Anderson S., Dignam J., Fisher E.R., Wolmark N. Treatment of lymph-node-negative, oestrogen-receptor-positive breast cancer: Long-term findings from National Surgical Adjuvant Breast and Bowel Project randomised clinical trials. Lancet. 2004;364:858–868. doi: 10.1016/S0140-6736(04)16981-X. [DOI] [PubMed] [Google Scholar]
- 27.Fisher B., Dignam J., Emir B., Bryant J., DeCillis A., Wolmark N., Wickerham D.L., Dimitrov N.V., Abramson N., Atkins J.N., et al. Tamoxifen and chemotherapy for lymph node-negative, estrogen receptor-positive breast cancer. J. Natl. Cancer Inst. 1997;89:1673–1682. doi: 10.1093/jnci/89.22.1673. [DOI] [PubMed] [Google Scholar]
- 28.Sparano J.A., Gray R.J., Makower D.F., Pritchard K.I., Albain K.S., Hayes D.F., Geyer C.E., Jr., Dees E.C., Goetz M.P., Olson J.A., et al. Adjuvant chemotherapy guided by a 21-gene expression assay in breast cancer. N. Engl. J. Med. 2018;379:111–121. doi: 10.1056/NEJMoa1804710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boland M.R., Al-Maksoud A., Ryan É.J., Balasubramanian I., Geraghty J., Evoy D., McCartan D., Prichard R.S., McDermott E.W. Value of a 21-gene expression assay on core biopsy to predict neoadjuvant chemotherapy response in breast cancer: Systematic review and meta-analysis. BJS. 2021;108:24–31. doi: 10.1093/bjs/znaa048. [DOI] [PubMed] [Google Scholar]
- 30.Davey M., Ryan É.J., Boland M., Barry M., Lowery A., Kerin M. Clinical utility of the 21-gene assay in predicting response to neoadjuvant endocrine therapy in breast cancer: A systematic review and meta-analysis. Breast. 2021;58:113–120. doi: 10.1016/j.breast.2021.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gonzalez-Angulo A.M., Barlow W.E., Gralow J., Meric-Bernstam F., Hayes D.F., Moinpour C., Ramsey S.D., Schott A.F., Sparks D.B., Albain K.S., et al. SWOG S1007: A phase III, randomized clinical trial of standard adjuvant endocrine therapy with or without chemotherapy in patients with one to three positive nodes, hormone receptor (HR)-positive, and HER2-negative breast cancer with recurrence score (RS) of 25 or less. J. Clin. Oncol. 2011;29:TPS104. doi: 10.1200/jco.2011.29.15_suppl.tps104. [DOI] [Google Scholar]
- 32.Kalinsky K., Barlow W.E., Meric-Bernstam F. First results from a phase III randomized clinical trial of standard adjuvant endocrine therapy (ET) +/− chemotherapy (CT) in patients (pts) with 1–3 positive nodes, hormone receptor-positive (HR+) and HER2-negative (HER2−) breast cancer (BC) with recurrence score (RS) <25: SWOG S1007 (RxPonder) Cancer Res. 2021;81 [Google Scholar]
- 33.Mamounas E.P., Bryant J., Lembersky B., Fehrenbacher L., Sedlacek S.M., Fisher B., Wickerham D.L., Yothers G., Soran A., Wolmark N. Paclitaxel after doxorubicin plus cyclophosphamide as adjuvant chemotherapy for node-positive breast cancer: Results from NSABP B-28. J. Clin. Oncol. 2005;23:3686–3696. doi: 10.1200/JCO.2005.10.517. [DOI] [PubMed] [Google Scholar]
- 34.Slamon D., Eiermann W., Robert N., Pienkowski T., Martin M., Press M., Mackey J., Glaspy J., Chan A., Pawlicki M.D., et al. Adjuvant trastuzumab in HER2-positive breast cancer. N. Engl. J. Med. 2011;365:1273–1283. doi: 10.1056/NEJMoa0910383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Slamon D.J., Clark G.M., Wong S.G., Levin W.J., Ullrich A., McGuire W.L. Human breast cancer: Correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 36.Slamon D.J., Godolphin W., Jones L.A., Holt J.A., Wong S.G., Keith D.E., Levin W.J., Stuart S.G., Udove J., Ullrich A., et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989;244:707–712. doi: 10.1126/science.2470152. [DOI] [PubMed] [Google Scholar]
- 37.Slamon D.J., Leyland-Jones B., Shak S., Fuchs H., Paton V., Bajamonde A., Fleming T., Eiermann W., Wolter J., Pegram M., et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N. Engl. J. Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 38.Cobleigh M.A., Vogel C.L., Tripathy D., Robert N.J., Scholl S., Fehrenbacher L., Wolter J.M., Paton V., Shak S., Lieberman G., et al. Multinational study of the efficacy and safety of humanized anti-HER2 monoclonal antibody in women who have HER2-overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease. J. Clin. Oncol. 1999;17:2639. doi: 10.1200/JCO.1999.17.9.2639. [DOI] [PubMed] [Google Scholar]
- 39.Pegram M.D., Lipton A., Hayes D.F., Weber B.L., Baselga J.M., Tripathy D., Baly D., Baughman S.A., Twaddell T., Glaspy J.A., et al. Phase II study of receptor-enhanced chemosensitivity using recombinant humanized anti-p185HER2/neu monoclonal antibody plus cisplatin in patients with HER2/neu-overexpressing metastatic breast cancer refractory to chemotherapy treatment. J. Clin. Oncol. 1998;16:2659–2671. doi: 10.1200/JCO.1998.16.8.2659. [DOI] [PubMed] [Google Scholar]
- 40.Piccart-Gebhart M.J., Procter M., Leyland-Jones B., Goldhirsch A., Untch M., Smith I., Gianni L., Baselga J., Bell R.H., Jackisch C., et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N. Engl. J. Med. 2005;353:1659–1672. doi: 10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- 41.Pivot X., Romieu G., Debled M., Pierga J.-Y., Kerbrat P., Bachelot T., Lortholary A., Espié M., Fumoleau P., Serin D., et al. 6 months versus 12 months of adjuvant trastuzumab for patients with HER2-positive early breast cancer (PHARE): A randomised phase 3 trial. Lancet Oncol. 2013;14:741–748. doi: 10.1016/S1470-2045(13)70225-0. [DOI] [PubMed] [Google Scholar]
- 42.Tan-Chiu E., Yothers G., Romond E., Geyer C.E., Jr., Ewer M., Keefe D., Shannon R.P., Swain S.M., Brown A., Fehrenbacher L. Assessment of cardiac dysfunction in a randomized trial comparing doxorubicin and cyclophosphamide followed by paclitaxel, with or without trastuzumab as adjuvant therapy in node-positive, human epidermal growth factor receptor 2-overexpressing breast cancer: NSABP B-31. J. Clin. Oncol. 2005;23:7811–7819. doi: 10.1200/JCO.2005.02.4091. [DOI] [PubMed] [Google Scholar]
- 43.Baselga J., Tripathy D., Mendelsohn J., Baughman S., Benz C.C., Dantis L., Sklarin N.T., Seidman A.D., Hudis C.A., Moore J., et al. Phase II study of weekly intravenous trastuzumab (Herceptin) in patients with HER2/neu-overexpressing metastatic breast cancer. Semin. Oncol. 1999;26:78–83. [PubMed] [Google Scholar]
- 44.Romond E.H., Perez E.A., Bryant J., Suman V.J., Geyer C.E., Davidson N.E., Tan-Chiu E., Martino S., Paik S., Kaufman P.A., et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N. Engl. J. Med. 2005;353:1673–1684. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 45.Joensuu H., Kellokumpu-Lehtinen P.-L., Bono P., Alanko T., Kataja V., Asola R., Utriainen T., Kokko R., Hemminki A., Tarkkanen M., et al. Adjuvant docetaxel or vinorelbine with or without trastuzumab for breast cancer. N. Engl. J. Med. 2006;354:809–820. doi: 10.1056/NEJMoa053028. [DOI] [PubMed] [Google Scholar]
- 46.Untch M., Rezai M., Loibl S., Fasching P.A., Huober J., Tesch H., Bauerfeind I., Hilfrich J., Eidtmann H., Gerber B., et al. Neoadjuvant treatment with trastuzumab in HER2-positive breast cancer: Results from the GeparQuattro study. J. Clin. Oncol. 2010;28:2024–2031. doi: 10.1200/JCO.2009.23.8451. [DOI] [PubMed] [Google Scholar]
- 47.Baselga J., Bradbury I., Eidtmann H., di Cosimo S., de Azambuja E., Aura C., Gomez H., Dinh P., Fauria K., van Dooren V., et al. Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): A randomised, open-label, multicentre, phase 3 trial. Lancet. 2012;379:633–640. doi: 10.1016/S0140-6736(11)61847-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Perez E.A., Romond E.H., Suman V.J., Jeong J.H., Sledge G., Geyer C.E., Jr., Martino S., Rastogi P., Gralow J., Swain S.M., et al. Trastuzumab plus adjuvant chemotherapy for human epidermal growth factor receptor 2–positive breast cancer: Planned joint analysis of overall survival from NSABP B-31 and NCCTG N9831. J. Clin. Oncol. 2014;32:3744–3752. doi: 10.1200/JCO.2014.55.5730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gianni L., Eiermann W., Semiglazov V., Lluch A., Tjulandin S., Zambetti M., Moliterni A., Vazquez F., Byakhov M.J., Lichinitser M. Neoadjuvant and adjuvant trastuzumab in patients with HER2-positive locally advanced breast cancer (NOAH): Follow-up of a randomised controlled superiority trial with a par-allel HER2-negative cohort. Lancet Oncol. 2014;15:640–647. doi: 10.1016/S1470-2045(14)70080-4. [DOI] [PubMed] [Google Scholar]
- 50.Cameron D., Piccart-Gebhart M.J., Gelber R.D., Procter M., Goldhirsch A., de Azambuja E., Castro G., Untch M., Smith I., Gianni L., et al. 11 years’ follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive early breast cancer: Final analysis of the HERceptin adjuvant (HERA) trial. Lancet. 2017;389:1195–1205. doi: 10.1016/S0140-6736(16)32616-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Earl H.M., Hiller L., Vallier A.-L., Loi S., McAdam K., Hughes-Davies L., Harnett A.N., Ah-See M.-L., Simcock R., Rea D., et al. 6 versus 12 months of adjuvant trastuzumab for HER2-positive early breast cancer (PERSEPHONE): 4-year disease-free survival results of a randomised phase 3 non-inferiority trial. Lancet. 2019;393:2599–2612. doi: 10.1016/S0140-6736(19)30650-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cho J.H., Park J.M., Park H.S., Park S., Kim S.I., Park B.-W. Oncologic safety of breast-conserving surgery compared to mastectomy in patients receiving neoadjuvant chemotherapy for locally advanced breast cancer. J. Surg. Oncol. 2013;108:531–536. doi: 10.1002/jso.23439. [DOI] [PubMed] [Google Scholar]
- 53.Davey M.G., Kerin E., O’Flaherty C., Maher E., Richard V., McAnena P., McLaughlin R.P., Sweeney K.J., Barry M.K., Malone C.M., et al. Clinicopathological response to neoadjuvant therapies and pathological complete response as a biomarker of survival in human epidermal growth factor receptor-2 enriched breast cancer—A retrospective cohort study. Breast. 2021;59:67–75. doi: 10.1016/j.breast.2021.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Spring L.M., Fell G., Arfe A., Sharma C., Greenup R.A., Reynolds K.L., Smith B.L., Alexander B.M., Moy B., Isakoff S.J., et al. Pathologic complete response after neoadjuvant chemotherapy and impact on breast cancer recurrence and survival: A comprehensive meta-analysis. Clin. Cancer Res. 2020;26:2838–2848. doi: 10.1158/1078-0432.CCR-19-3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Asselain B., Barlow W., Bartlett J., Bergh J., Bergsten-Nordström E., Bliss J., Boccardo F., Boddington C., Bogaerts J., Bonadonna G., et al. Long-term outcomes for neoadjuvant versus adjuvant chemotherapy in early breast cancer: Meta-analysis of individual patient data from ten randomised trials. Lancet Oncol. 2018;19:27–39. doi: 10.1016/S1470-2045(17)30777-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Boughey J.C., Ballman K.V., McCall L.M., Mittendorf E.A., Symmans W.F., Julian T.B., Byrd D., Hunt K.K. Tumor biology and response to chemotherapy impact breast cancer-specific survival in node-positive breast cancer patients treated with neoadjuvant chemotherapy: Long-term follow-up from ACOSOG Z1071 (Alliance) Ann. Surg. 2017;266:667–676. doi: 10.1097/SLA.0000000000002373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fisher B., Brown A., Mamounas E., Wieand S., Robidoux A., Margolese R.G., Cruz A.B., Jr., Fisher E.R., Wickerham D.L., Wolmark N., et al. Effect of preoperative chemotherapy on local-regional disease in women with operable breast cancer: Findings from National Surgical Adjuvant Breast and Bowel Project B-18. J. Clin. Oncol. 1997;15:2483–2493. doi: 10.1200/JCO.1997.15.7.2483. [DOI] [PubMed] [Google Scholar]
- 58.Fisher B., Bryant J., Wolmark N., Mamounas E., Brown A., Fisher E.R., Wickerham D.L., Begovic M., DeCillis A., Robidoux A., et al. Effect of preoperative chemotherapy on the outcome of women with operable breast cancer. J. Clin. Oncol. 1998;16:2672–2685. doi: 10.1200/JCO.1998.16.8.2672. [DOI] [PubMed] [Google Scholar]
- 59.Wolmark N., Wang J., Mamounas E., Bryant J., Fisher B. Preoperative Chemotherapy in patients with operable breast cancer: Nine-year results from National Surgical Adjuvant Breast and Bowel Project B-18. J. Natl. Cancer Inst. Monogr. 2001;2001:96–102. doi: 10.1093/oxfordjournals.jncimonographs.a003469. [DOI] [PubMed] [Google Scholar]
- 60.Bear H.D., Anderson S., Brown A., Smith R., Mamounas E.P., Fisher B., Margolese R., Theoret H., Soran A., Wickerham D.L., et al. The effect on tumor response of adding sequential preoperative docetaxel to preoperative doxorubicin and cyclophosphamide: Preliminary results from National Surgical Adjuvant Breast and Bowel Project Protocol B-27. J. Clin. Oncol. 2003;21:4165–4174. doi: 10.1200/JCO.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 61.Bear H.D., Anderson S., Smith R.E., Geyer C.E., Jr., Mamounas E.P., Fisher B., Brown A.M., Robidoux A., Margolese R., Kahlenberg M.S., et al. Sequential preoperative or postoperative docetaxel added to preoperative doxorubicin plus cyclophosphamide for operable breast cancer: National Surgical Adjuvant Breast and Bowel Project Protocol B-27. J. Clin. Oncol. 2006;24:2019–2027. doi: 10.1200/JCO.2005.04.1665. [DOI] [PubMed] [Google Scholar]
- 62.Boughey J.C., McCall L.M., Ballman K.V., Mittendorf E.A., Ahrendt G.M., Wilke L.G., Taback B., Leitch A.M., Flippo-Morton T., Hunt K.K. Tumor biology correlates with rates of breast-conserving surgery and pathologic complete response after neoadjuvant chemotherapy for breast cancer: Findings from the ACOSOG Z1071 (Alliance) Prospective Multicenter Clinical Trial. Ann. Surg. 2014;260:608–614. doi: 10.1097/SLA.0000000000000924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Korde L.A., Somerfield M.R., Carey L.A., Crews J.R., Denduluri N., Hwang E.S., Khan S.A., Loibl S., Morris E.A., Perez A., et al. Neoadjuvant chemotherapy, endocrine therapy, and targeted therapy for breast cancer: ASCO guideline. J. Clin. Oncol. 2021;39:1485–1505. doi: 10.1200/JCO.20.03399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Poggio F., Bruzzone M., Ceppi M., Pondé N., la Valle G., del Mastro L., de Azambuja E., Lambertini M. Platinum-based neoadjuvant chemotherapy in triple-negative breast cancer: A systematic review and meta-analysis. Ann. Oncol. 2018;29:1497–1508. doi: 10.1093/annonc/mdy127. [DOI] [PubMed] [Google Scholar]
- 65.Schmid P., Cortes J., Pusztai L., McArthur H., Kümmel S., Bergh J., Denkert C., Park Y.H., Hui R., Harbeck N., et al. Pembrolizumab for early triple-negative breast cancer. N. Engl. J. Med. 2020;382:810–821. doi: 10.1056/NEJMoa1910549. [DOI] [PubMed] [Google Scholar]
- 66.Bartsch R., Bergen E., Galid A. Current concepts and future directions in neoadjuvant chemotherapy of breast cancer. Memo-Mag. Eur. Med. Oncol. 2018;11:199–203. doi: 10.1007/s12254-018-0421-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Leon-Ferre R.A., Hieken T.J., Boughey J.C. The landmark series: Neoadjuvant chemotherapy for triple-negative and HER2-positive breast cancer. Ann. Surg. Oncol. 2021;28:2111–2119. doi: 10.1245/s10434-020-09480-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mauri D., Pavlidis N., Ioannidis J.P.A. Neoadjuvant versus adjuvant systemic treatment in breast cancer: A meta-analysis. J. Natl. Cancer Inst. 2005;97:188–194. doi: 10.1093/jnci/dji021. [DOI] [PubMed] [Google Scholar]
- 69.Van Nes J.G., Putter H., Julien J.P., Tubiana-Hulin M., van de Vijver M., Bogaerts J., De Vos M., van de Velde C.J. Preoperative chemotherapy is safe in early breast cancer, even after 10 years of follow-up; clinical and translational results from the EORTC trial 10902. Breast Cancer Res. Treat. 2009;115:101–113. doi: 10.1007/s10549-008-0050-1. [DOI] [PubMed] [Google Scholar]
- 70.Heneghan H.M., Miller N., Lowery A., Sweeney K.J., Kerin M.J. MicroRNAs as novel biomarkers for breast cancer. J. Oncol. 2009;2010:1–7. doi: 10.1155/2010/950201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Casey M.-C., Kerin M., Brown J.A., Sweeney K.J. Evolution of a research field—A micro (RNA) example. PeerJ. 2015;3:829. doi: 10.7717/peerj.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Place R.F., Li L.-C., Pookot D., Noonan E.J., Dahiya R. MicroRNA-373 induces expression of genes with complementary promoter sequences. Proc. Natl. Acad. Sci. USA. 2008;105:1608–1613. doi: 10.1073/pnas.0707594105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lee Y., Kim M., Han J., Yeom K.-H., Lee S., Baek S.H., Kim V.N. MicroRNA genes are transcribed by RNA polymerase II. EMBO J. 2004;23:4051–4060. doi: 10.1038/sj.emboj.7600385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bohnsack M.T., Czaplinski K., Gorlich D. Exportin 5 is a RanGTP-dependent dsRNA-binding protein that mediates nuclear export of pre-miRNAs. RNA. 2004;10:185–191. doi: 10.1261/rna.5167604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yi R., Qin Y., Macara I.G., Cullen B.R. Exportin-5 mediates the nuclear export of pre-microRNAs and short hairpin RNAs. Genes Dev. 2003;17:3011–3016. doi: 10.1101/gad.1158803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ha M., Kim V.N. Regulation of microRNA biogenesis. Nat. Rev. Mol. Cell Biol. 2014;15:509–524. doi: 10.1038/nrm3838. [DOI] [PubMed] [Google Scholar]
- 77.Lowery A.J., Miller N., Devaney A., McNeill R.E., Davoren P.A., Lemetre C., Benes V., Schmidt S., Blake J., Ball G., et al. MicroRNA signatures predict oestrogen receptor, progesterone receptor and HER2/neureceptor status in breast cancer. Breast Cancer Res. 2009;11:R27. doi: 10.1186/bcr2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Søkilde R., Persson H., Ehinger A., Pirona A.C., Fernö M., Hegardt C., Larsson C., Loman N., Malmberg M., Rydén L., et al. Refinement of breast cancer molecular classification by miRNA expression profiles. BMC Genom. 2019;20:503. doi: 10.1186/s12864-019-5887-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.McGuire A., Casey M.-C., Waldron R.M., Heneghan H., Kalinina O., Holian E., McDermott A., Lowery A.J., Newell J., Dwyer R.M., et al. Prospective assessment of systemic microRNAs as markers of response to neoadjuvant chemotherapy in breast cancer. Cancers. 2020;12:1820. doi: 10.3390/cancers12071820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Di Cosimo S., Appierto V., Pizzamiglio S., Tiberio P., Iorio M., Hilbers F., de Azambuja E., de la Peña L., Izquierdo M., Huober J., et al. Plasma miRNA levels for predicting therapeutic response to neoadjuvant treatment in HER2-positive breast cancer: Results from the NeoALTTO trial. Clin. Cancer Res. 2019;25:3887–3895. doi: 10.1158/1078-0432.CCR-18-2507. [DOI] [PubMed] [Google Scholar]
- 81.Davey M.G., Ryan É.J., Lowery A.J., Miller N., Kerin M.J. Clinicopathological and prognostic significance of programmed cell death ligand 1 expression in patients diagnosed with breast cancer: Meta-analysis. BJS. 2021;108:622–631. doi: 10.1093/bjs/znab103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Davey M.G., Ryan É.J., Folan P.J., O’Halloran N., Boland M.R., Barry M.K., Sweeney K.J., Malone C.M., McLaughlin R.J., Kerin M.J., et al. The impact of progesterone receptor negativity on oncological outcomes in oestrogen-receptor-positive breast cancer. BJS Open. 2021;5:zrab040. doi: 10.1093/bjsopen/zrab040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Denkert C., Loibl S., Müller B.M., Eidtmann H., Schmitt W.D., Eiermann W., Gerber B., Tesch H., Hilfrich J., Huober J., et al. Ki67 levels as predictive and prognostic parameters in pretherapeutic breast cancer core biopsies: A translational investigation in the neoadjuvant GeparTrio trial. Ann. Oncol. 2013;24:2786–2793. doi: 10.1093/annonc/mdt350. [DOI] [PubMed] [Google Scholar]
- 84.Xing A.-Y., Wang B., Li Y.-H., Chen X., Wang Y.-W., Liu H.-T., Gao P. Identification of miRNA signature in breast cancer to predict neoadjuvant chemotherapy response. Pathol. Oncol. Res. 2021;27:1609753. doi: 10.3389/pore.2021.1609753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Liu B., Su F., Lv X., Zhang W., Shang X., Zhang Y., Zhang J. Serum microRNA-21 predicted treatment outcome and survival in HER2-positive breast cancer patients receiving neoadjuvant chemotherapy combined with trastuzumab. Cancer Chemother. Pharmacol. 2019;84:1039–1049. doi: 10.1007/s00280-019-03937-9. [DOI] [PubMed] [Google Scholar]
- 86.Di Cosimo S., Appierto V., Pizzamiglio S., Silvestri M., Baselga J., Piccart M., Huober J., Izquierdo M., de la Pena L., Hilbers F.S., et al. Early modulation of circulating microRNAs levels in HER2-positive breast cancer patients treated with trastuzumab-based neoadjuvant therapy. Int. J. Mol. Sci. 2020;21:1386. doi: 10.3390/ijms21041386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Stevic I., Müller V., Weber K., Fasching P.A., Karn T., Marmé F., Schem C., Stickeler E., Denkert C., van Mackelenbergh M., et al. Specific microRNA signatures in exosomes of triple-negative and HER2-positive breast cancer patients undergoing neoadjuvant therapy within the GeparSixto trial. BMC Med. 2018;16:1–16. doi: 10.1186/s12916-018-1163-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kassem N.M., Makar W.S., Kassem H.A., Talima S., Tarek M., Hesham H., El-Desouky M.A. Circulating miR-34a and miR-125b as promising non invasive biomarkers in egyptian locally advanced breast cancer patients. Asian Pac. J. Cancer Prev. 2019;20:2749–2755. doi: 10.31557/APJCP.2019.20.9.2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.García-García F., Salinas-Vera Y.M., García-Vázquez R., Marchat L.A., Rodríguez-Cuevas S., López-González J.S., Carlos-Reyes Á., Ramos-Payán R., Aguilar-Medina M., Pérez-Plasencia C., et al. MiR-145-5p is associated with pathological complete response to neoadjuvant chemotherapy and impairs cell proliferation by targeting TGFβR2 in breast cancer. Oncol. Rep. 2019;41:3527–3534. doi: 10.3892/or.2019.7102. [DOI] [PubMed] [Google Scholar]
- 90.Müller V., Gade S., Steinbach B., Loibl S., von Minckwitz G., Untch M., Schwedler K., Lübbe K., Schem C., Fasching P.A., et al. Changes in serum levels of miR-21, miR-210, and miR-373 in HER2-positive breast cancer patients undergoing neoadjuvant therapy: A translational research project within the Geparquinto trial. Breast Cancer Res. Treat. 2014;147:61–68. doi: 10.1007/s10549-014-3079-3. [DOI] [PubMed] [Google Scholar]
- 91.Xue J., Chi Y., Chen Y., Huang S., Ye X., Niu J., Wang W., Pfeffer L.M., Shao Z.-M., Wu Z.-H., et al. MiRNA-621 sensitizes breast cancer to chemotherapy by suppressing FBXO11 and enhancing p53 activity. Oncogene. 2015;35:448–458. doi: 10.1038/onc.2015.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhu W., Liu M., Fan Y., Ma F., Xu N., Xu B. Dynamics of circulating microRNAs as a novel indicator of clinical response to neoadjuvant chemotherapy in breast cancer. Cancer Med. 2018;7:4420–4433. doi: 10.1002/cam4.1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kahraman M., Röske A., Laufer T., Fehlmann T., Backes C., Kern F., Kohlhaas J., Schrörs H., Saiz A., Zabler C., et al. MicroRNA in diagnosis and therapy monitoring of early-stage triple-negative breast cancer. Sci. Rep. 2018;8:11584. doi: 10.1038/s41598-018-29917-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lindholm E.M., Aure M.R., Haugen M.H., Sahlberg K.K., Kristensen V.N., Nebdal D., Børresen-Dale A., Lingjærde O.C., Engebraaten O. MiRNA expression changes during the course of neoadjuvant bevacizumab and chemotherapy treatment in breast cancer. Mol. Oncol. 2019;13:2278–2296. doi: 10.1002/1878-0261.12561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rodríguez-Martínez A., de Miguel-Pérez D., Ortega F.G., García-Puche J.L., Robles-Fernández I., Exposito J., Martorell-Marugan J., Carmona-Sáez P., Garrido-Navas M.D.C., Rolfo C., et al. Exosomal miRNA profile as complementary tool in the diagnostic and prediction of treatment response in localized breast cancer under neoadjuvant chemotherapy. Breast Cancer Res. 2019;21:1–9. doi: 10.1186/s13058-019-1109-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhang S., Wang Y., Wang Y., Peng J., Yuan C., Zhou L., Xu S., Lin Y., Du Y., Yang F., et al. Serum miR-222-3p as a double-edged sword in predicting efficacy and trastuzumab-induced cardiotoxicity for HER2-positive breast cancer patients receiving neoadjuvant target therapy. Front. Oncol. 2020;10:631. doi: 10.3389/fonc.2020.00631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Li H., Yang B.B. Friend or foe: The role of microRNA in chemotherapy resistance. Acta Pharmacol. Sin. 2013;34:870–879. doi: 10.1038/aps.2013.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Magee P., Shi L., Garofalo M. Role of microRNAs in chemoresistance. Ann. Transl. Med. 2015;3:332. doi: 10.3978/j.issn.2305-5839.2015.11.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Griñán-Lisón C., Olivares-Urbano M.A., Jiménez G., Ruiz E.L., del Val C., Morata-Tarifa C., Entrena J.M., González G.J., Boulaiz H., Herrera M.Z., et al. MiRNAs as radio-response biomarkers for breast cancer stem cells. Mol. Oncol. 2020;14:556–570. doi: 10.1002/1878-0261.12635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Muluhngwi P., Klinge C.M. Roles for miRNAs in endocrine resistance in breast cancer. Endocr.-Relat. Cancer. 2015;22:R279–R300. doi: 10.1530/ERC-15-0355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Casey M., Sweeney K.J., Brown J.A.L., Kerin M.J. Exploring circulating micro-RNA in the neoadjuvant treatment of breast cancer. Int. J. Cancer. 2016;139:12–22. doi: 10.1002/ijc.29985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Liu M., Gong C., Xu R., Chen Y., Wang X. MicroRNA-5195-3p enhances the chemosensitivity of triple-negative breast cancer to paclitaxel by downregulating EIF4A2. Cell. Mol. Biol. Lett. 2019;24:1–11. doi: 10.1186/s11658-019-0168-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sha L.-Y., Zhang Y., Wang W., Sui X., Liu S.-K., Wang T., Zhang H. MiR-18a upregulation decreases dicer expression and confers paclitaxel resistance in triple negative breast cancer. Eur. Rev. Med. Pharmacol. Sci. 2016;20:2201–2208. [PubMed] [Google Scholar]
- 104.Hou X., Niu Z., Liu L., Guo Q., Li H., Yang X., Zhang X. MiR-1207-5p regulates the sensitivity of triple-negative breast cancer cells to taxol treatment via the suppression of LZTS1 expression. Oncol. Lett. 2019;17:990–998. doi: 10.3892/ol.2018.9687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wu C., Zhao A., Tan T., Wang Y., Shen Z. Overexpression of microRNA-620 facilitates the resistance of triple negative breast cancer cells to gemcitabine treatment by targeting DCTD. Exp. Ther. Med. 2019;18:550–558. doi: 10.3892/etm.2019.7601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wang H., Tan G., Dong L., Cheng L., Li K., Wang Z., Luo H. Circulating MiR-125b as a marker predicting chemoresistance in breast cancer. PLoS ONE. 2012;7:e34210. doi: 10.1371/journal.pone.0034210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Roscigno G., Puoti I., Giordano I., Donnarumma E., Russo V., Affinito A., Adamo A., Quintavalle C., Todaro M., Vivanco M., et al. MiR-24 induces chemotherapy resistance and hypoxic advantage in breast cancer. Oncotarget. 2017;8:19507–19521. doi: 10.18632/oncotarget.14470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yu D.-D., Lv M.-M., Chen W.-X., Zhong S., Zhang X.-H., Chen L., Ma T.-F., Tang J.-H., Zhao J.-H. Role of miR-155 in drug resistance of breast cancer. Tumor Biol. 2015;36:1395–1401. doi: 10.1007/s13277-015-3263-z. [DOI] [PubMed] [Google Scholar]
- 109.Yin Y., Wang X., Li T., Ren Q., Li L., Sun X., Zhang B., Wang X., Han H., He Y., et al. MicroRNA-221 promotes breast cancer resistance to adriamycin via modulation of PTEN/Akt/mTOR signaling. Cancer Med. 2020;9:1544–1552. doi: 10.1002/cam4.2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bertoli G., Cava C., Castiglioni I. MicroRNAs: New biomarkers for diagnosis, prognosis, therapy prediction and therapeutic tools for breast cancer. Theranostics. 2015;5:1122–1143. doi: 10.7150/thno.11543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Svoronos A., Engelman D.M., Slack F.J. OncomiR or tumor suppressor? The duplicity of microRNAs in cancer. Cancer Res. 2016;76:3666–3670. doi: 10.1158/0008-5472.CAN-16-0359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Langer C., Rücker F.G., Buske C., Döhner H., Kuchenbauer F. Targeted therapies through microRNAs: Pulp or fiction? Ther. Adv. Hematol. 2011;3:97–104. doi: 10.1177/2040620711432582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Li D., Wang X., Yang M., Kan Q., Duan Z. MiR3609 sensitizes breast cancer cells to adriamycin by blocking the programmed death-ligand 1 immune checkpoint. Exp. Cell Res. 2019;380:20–28. doi: 10.1016/j.yexcr.2019.03.025. [DOI] [PubMed] [Google Scholar]
- 114.Lin Y., Lin F., Anuchapreeda S., Chaiwongsa R., Duangmano S., Ran B., Pornprasert S. Effect of miR-133b on progression and cisplatin resistance of triple-negative breast cancer through FGFR1-wnt-β-catenin axis. Am. J. Transl. Res. 2021;13:5969–5984. [PMC free article] [PubMed] [Google Scholar]
- 115.Li Y., Zhang L., Dong Z., Xu H., Yan L., Wang W., Yang Q., Chen C. MicroRNA-155-5p promotes tumor progression and contributes to paclitaxel resistance via TP53INP1 in human breast cancer. Pathol. Res. Pract. 2021;220:153405. doi: 10.1016/j.prp.2021.153405. [DOI] [PubMed] [Google Scholar]
- 116.Mei M., Ren Y., Zhou X., Yuan X.B., Han L., Wang G.X., Jia Z., Pu P.Y., Kang C.S., Yao Z. Downregulation of miR-21 enhances chemotherapeutic effect of taxol in breast carcinoma cells. Technol. Cancer Res. Treat. 2010;9:77–86. doi: 10.1177/153303461000900109. [DOI] [PubMed] [Google Scholar]
- 117.Zhang B., Pan X., Cobb G., Anderson T. MicroRNAs as oncogenes and tumor suppressors. Dev. Biol. 2007;302:1–12. doi: 10.1016/j.ydbio.2006.08.028. [DOI] [PubMed] [Google Scholar]
- 118.Mollaei H., Safaralizadeh R., Rostami Z. MicroRNA replacement therapy in cancer. J. Cell. Physiol. 2019;234:12369–12384. doi: 10.1002/jcp.28058. [DOI] [PubMed] [Google Scholar]
- 119.Zhang L., Liao Y., Tang L. MicroRNA-34 family: A potential tumor suppressor and therapeutic candidate in cancer. J. Exp. Clin. Cancer Res. 2019;38:53. doi: 10.1186/s13046-019-1059-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Cochrane D.R., Spoelstra N.S., Howe E.N., Nordeen S.K., Richer J.K. MicroRNA-200c mitigates invasiveness and restores sensitivity to microtubule-targeting chemotherapeutic agents. Mol. Cancer Ther. 2009;8:1055–1066. doi: 10.1158/1535-7163.MCT-08-1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Yu F., Deng H., Yao H., Liu Q., Su F., Song E. Mir-30 reduction maintains self-renewal and inhibits apoptosis in breast tumor-initiating cells. Oncogene. 2010;29:4194–4204. doi: 10.1038/onc.2010.167. [DOI] [PubMed] [Google Scholar]
- 122.Yu F., Yao H., Zhu P., Zhang X., Pan Q., Gong C., Huang Y., Hu X., Su F., Lieberman J., et al. Let-7 regulates self renewal and tumorigenicity of breast cancer cells. Cell. 2007;131:1109–1123. doi: 10.1016/j.cell.2007.10.054. [DOI] [PubMed] [Google Scholar]
- 123.Kalinowski F., Brown R.A., Ganda C., Giles K.M., Epis M.R., Horsham J., Leedman P.J. MicroRNA-7: A tumor suppressor miRNA with therapeutic potential. Int. J. Biochem. Cell Biol. 2014;54:312–317. doi: 10.1016/j.biocel.2014.05.040. [DOI] [PubMed] [Google Scholar]
- 124.Schmid P., Adams S., Rugo H.S., Schneeweiss A., Barrios C.H., Iwata H., Diéras V., Hegg R., Im S.-A., Wright G.S., et al. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N. Engl. J. Med. 2018;379:2108–2121. doi: 10.1056/NEJMoa1809615. [DOI] [PubMed] [Google Scholar]
- 125.Tutt A.N., Garber J.E., Kaufman B., Viale G., Fumagalli D., Rastogi P., Gelber R.D., de Azambuja E., Fielding A., Balmaña J., et al. Adjuvant olaparib for patients with BRCA1- or BRCA2-mutated breast cancer. N. Engl. J. Med. 2021;384:2394–2405. doi: 10.1056/NEJMoa2105215. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.