Abstract
Pituitary adenylate cyclase-activating peptide (PACAP) is a neuropeptide with a widespread occurrence and diverse effects. PACAP has well-documented neuro- and cytoprotective effects, proven in numerous studies. Among others, PACAP is protective in models of diabetes-associated diseases, such as diabetic nephropathy and retinopathy. As the neuropeptide has strong neurotrophic and neuroprotective actions, we aimed at investigating the effects of PACAP in a rat model of streptozotocin-induced diabetic neuropathy, another common complication of diabetes. Rats were treated with PACAP1-38 every second day for 8 weeks starting simultaneously with the streptozotocin injection. Nerve fiber morphology was examined with electron microscopy, chronic neuronal activation in pain processing centers was studied with FosB immunohistochemistry, and functionality was assessed by determining the mechanical nociceptive threshold. PACAP treatment did not alter body weight or blood glucose levels during the 8-week observation period. However, PACAP attenuated the mechanical hyperalgesia, compared to vehicle-treated diabetic animals, and it markedly reduced the morphological signs characteristic for neuropathy: axon–myelin separation, mitochondrial fission, unmyelinated fiber atrophy, and basement membrane thickening of endoneurial vessels. Furthermore, PACAP attenuated the increase in FosB immunoreactivity in the dorsal spinal horn and periaqueductal grey matter. Our results show that PACAP is a promising therapeutic agent in diabetes-associated complications, including diabetic neuropathy.
Keywords: diabetes, PACAP, neuroprotection, myelin, dorsal horn, periaqueductal grey
1. Introduction
Pituitary adenylate cyclase-activating peptide (PACAP) is a member of the vasoactive intestinal peptide (VIP)/secretin/glucagon peptide family and has two biologically active isoforms—PACAP-27 and PACAP-38, with the latter being predominant in mammals [1]. PACAP acts through G-protein-coupled receptors: its specific receptor is PAC1, while VPAC1 and VPAC2 receptors bind PACAP and VIP with the same affinity. PACAP has a widespread distribution in the body, with the highest expression levels in the nervous system and endocrine glands, where it acts as a neurotransmitter, neuromodulator, and neurohormone [2,3,4,5,6]. In addition, PACAP and its receptors are widely expressed in peripheral organs [1,7,8,9,10], and the peptide plays different roles in numerous physiological processes in the cardiovascular, respiratory, urogenital, musculoskeletal, and digestive systems [11,12,13,14,15,16]. One of the established effects of the neuropeptide is its neurotrophic/neuroprotective action [17,18,19,20,21,22]. This has been proven in numerous neuronal insults and models of neurodegenerative diseases, such as spinal atrophy [23], amyotrophic lateral sclerosis [24], Alzheimer’s disease [25,26,27], stroke [28,29,30], Parkinson’s disease [20,31,32,33], Huntington chorea [34], and several types of retinal injuries [21,35,36,37].
Several reports have proven that PACAP is also protective in diabetes-related pathological conditions, such as diabetic nephropathy [38,39] and retinopathy [40,41,42,43,44,45,46]. It has been demonstrated that PACAP is protective in the inner, neuronal retinal layers in diabetic retinopathy and also acts on pigment epithelial cells in hyperglycemic conditions [47,48,49]. Vasculopathy is in the background of several diabetic complications. PACAP has been shown to ameliorate hyperglycemia-induced vascular dysfunction in isolated vessels [50]. Diabetic neuropathy is a common microvascular complication of diabetes, affecting around 50–70% of diabetic patients [51,52]. PACAP is involved in glucose metabolism and insulin secretion [53,54], in addition to protective effects exerted on the insulin-producing pancreatic beta cells [55,56]. More importantly, from a neuropathy point of view, PACAP influences Schwann cell functions [57,58], stimulates axonal growth [59,60], and promotes regeneration of peripheral nerves [61,62,63,64]. However, it is not known whether it has protective effects in diabetic neuropathy. Therefore, in the present study, we aimed at investigating the neuroprotective effects of PACAP in an experimental model of diabetic neuropathy in rats.
The two major predictors of developing neuropathy are the duration of diabetes and the degree of metabolic instability [65]. Hyperglycemia results in excessive production of reactive oxygen species (ROS), overproduction of advanced glycation end products and glutamate, and decreased production of neuroprotective factors and hyperglycemia-activated signaling pathways, such as the polyol, hexosamine, and DAG-PKC pathways [65,66,67,68,69]. Signs and symptoms of diabetic neuropathy include loss of reflexes, dysesthesia, and paresthesia, along with neuropathic pain—hyperalgesia and allodynia [70]. Diabetic neuropathy is associated with alterations in the structure of peripheral nerves as well as in central structures of the pain processing pathway. However, the mechanism of neuropathic pain is still not understood, and it is an unmet medical need due to the ineffectiveness of the currently available therapy in a great proportion of cases. Previously, it has been shown that streptozotocin (STZ)-induced diabetes leads to alterations in the dorsal horn of the spinal cord and the mesencephalic periaqueductal grey matter (PAG) [71]. Among others, altered c-Fos and FosB expressions in these centers have been reported [72,73,74,75,76]. Therefore, in the present study, we also investigated the expression of FosB, a marker of chronic neuronal activation [77,78], in the above pain processing centers, in addition to the detailed morphological analysis of peripheral nerve fibers and functional assessment of pain sensation.
2. Results
2.1. Blood Glucose Levels and Body Weight
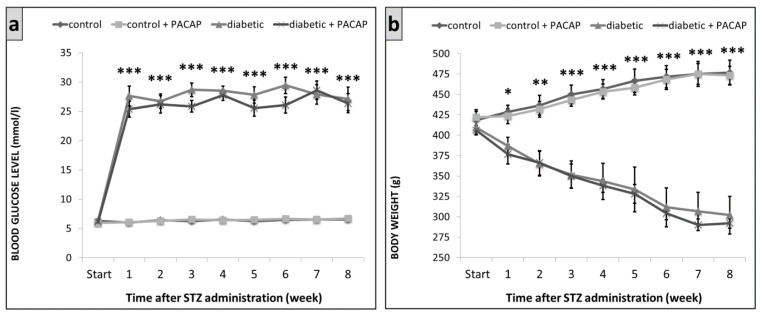
Vehicle (saline) treated diabetic and PACAP-treated diabetic groups had a significant rise in blood sugar levels after the 7th day of the experiment. Blood glucose level was 6.6 ± 0.34 mmol/L in the vehicle-treated control and 6.7 ± 0.24 mmol/L in the PACAP-treated control group on week 8. These values were 27.1 ± 2.02 mmol/L in the vehicle-treated diabetic and 26.4 ± 1.65 mmol/L in the PACAP-treated diabetic group (Figure 1a). These data show that blood glucose levels were not significantly affected by PACAP treatment.
Figure 1.
Change of blood glucose levels (a) and body weight (b) in control and diabetic groups. Data show mean ± SEM of n = 5/6 rats/group, * p < 0.05, ** p < 0.01, *** p < 0.001 vs. control groups.
Vehicle-treated diabetic and PACAP-treated diabetic groups showed a significant decrease in their body weight from the second week. On week 8, we measured 477 ± 15 g in the vehicle-treated control, 473 ± 11 g in the PACAP-treated control groups, while only 302 ± 23 g in the diabetic and 292 ± 6 g in the PACAP-treated diabetic groups (Figure 1b). PACAP-38 treatment did not influence the body weight loss.
2.2. Functional Tests
2.2.1. Randall–Selitto Test
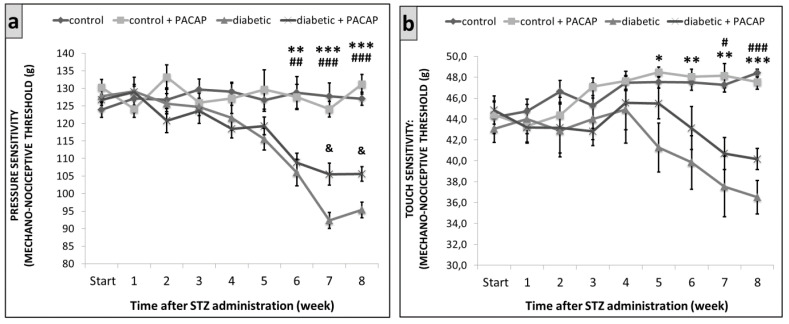
The Randal–Selitto test revealed an increased pressure sensitivity in the vehicle-treated diabetic and PACAP-treated diabetic groups after the 6th week of the experiment, compared to the vehicle-treated control and PACAP-treated control groups. Pressure sensitivity increased to a lower extent in the PACAP-treated diabetic group on the 7th and 8th weeks, compared to the vehicle-treated diabetic group (Figure 2a).
Figure 2.
Diabetes-induced mechanical hyperalgesia in Wistar rats, the mechanical nociceptive threshold of pressure sensitivity by Randall-Selitto test (a) and touch sensitivity by dynamic plantar aesthesiometer (DPA) test (b). Data are presented as means ± SEM of 5/6 rats/group. * p < 0.05, ** p < 0.01, *** p < 0.001 vs. vehicle-treated diabetic group, # p < 0.05, ## p < 0.01, ### p < 0.001 vs. PACAP-treated diabetic group, & p < 0.05 vs. vehicle-treated diabetic group.
2.2.2. Dynamic Plantar Aesthesiometer (DPA)
The DPA test showed an increased touch sensitivity in the vehicle-treated diabetic and PACAP-treated diabetic groups after the 5th week of the experiment, compared to the vehicle-treated control and PACAP-treated control groups. It was less prominent in the PACAP-treated diabetic group from the 5th week, compared to the vehicle-treated diabetic group, but the difference was not statistically significant (Figure 2b).
2.3. Immunohistochemistry
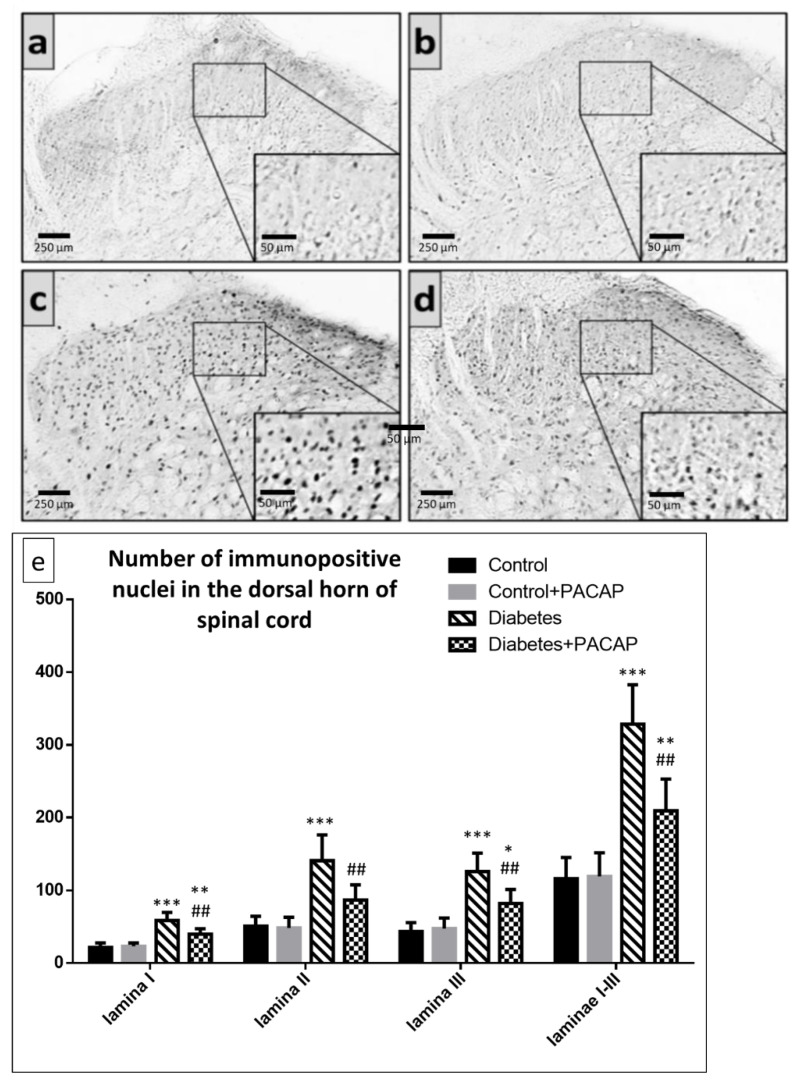
Our study showed that the number of FosB immunoreactive nuclei in laminae I-III of the spinal cord dorsal horn of segments L4–L5 was higher in the vehicle-treated diabetic and PACAP-treated diabetic groups, compared to the vehicle-treated control and PACAP-treated control groups. PACAP-treated diabetic animals had, however, significantly fewer FosB positive nuclei than vehicle-treated diabetic rats (Figure 3).
Figure 3.
Representative images of FosB immunohistochemistry in the spinal dorsal horn of segments L4–L5 in (a) vehicle-treated control, (b) PACAP-treated control, (c) vehicle-treated diabetic, and (d) PACAP-treated diabetic groups. Histograms (e) show the number of FosB immunoreactive nuclei in laminae I-III of the spinal dorsal horn of segments L4–L5. Data are means ± SEM of n = 5/6 rats/group.* p < 0.05, ** p < 0.01, *** p < 0.001 vs. vehicle-treated control, ## p < 0.01 vs. vehicle-treated diabetic group.
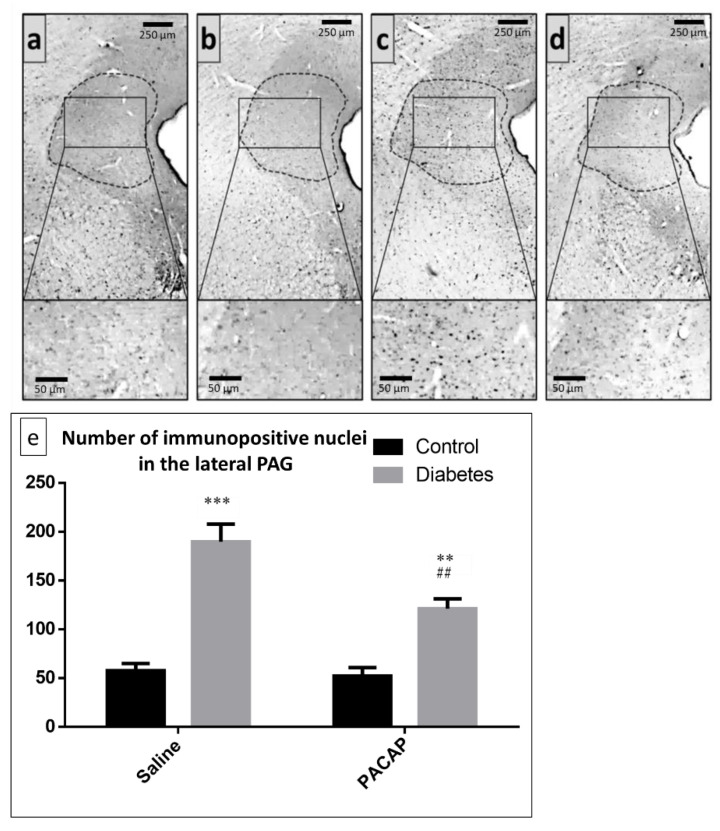
The number of FosB-positive nuclei in the lateral part of PAG was also investigated. We found that both vehicle- (saline) and PACAP-treated diabetic animals showed an elevated number of FosB immunoreactive nuclei in the lateral PAG, compared to the vehicle-treated control and PACAP-treated control groups. When comparing the PACAP-treated diabetic group to the vehicle-treated diabetic group, we found that PACAP was effective in significantly reducing the neuronal activity in the lateral PAG (Figure 4).
Figure 4.
Representative images of FosB-positive nuclei in the lateral part of PAG (marked by dotted lines) (a) in vehicle-treated control, (b) PACAP-treated control, (c) vehicle-treated diabetic, and (d) PACAP-treated diabetic groups. Histogram (e) shows the number of FosB-positive/immunoreactive nuclei in the lateral part of PAG in vehicle-treated control, vehicle-treated diabetic, PACAP-treated control, and PACAP-treated diabetic groups. Data are means ± SEM of n = 5/6 rats/group. ** p < 0.01, *** p < 0.001 vs. vehicle-treated control, ## p < 0.01 vs. vehicle-treated diabetic groups.
2.4. Electron Microscopy of the Sciatic Nerve
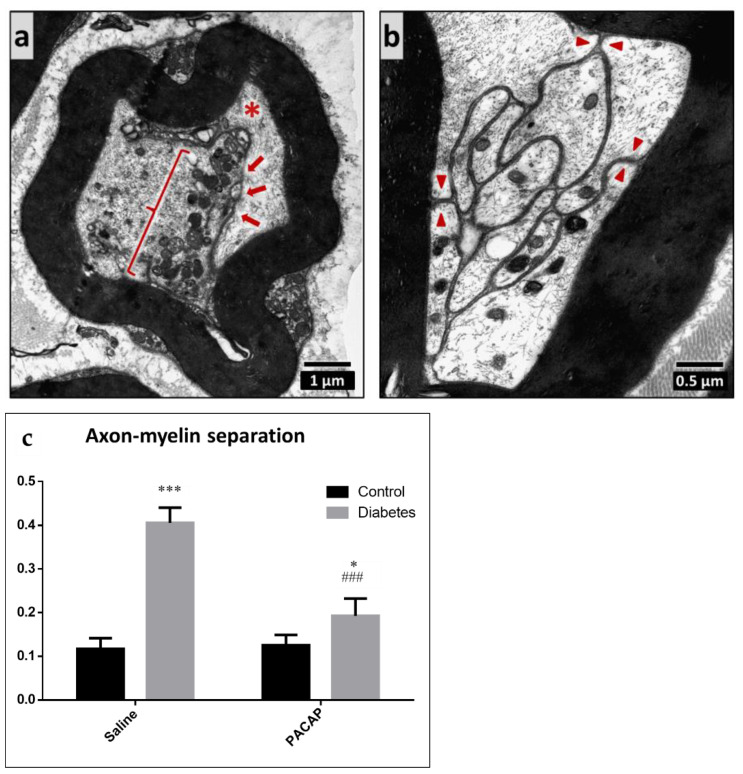
In vehicle-treated control and PACAP-treated control animals, normal peripheral nerve structure was found without any signs of myelin or axonal injury. PACAP treatment did not cause any changes under control (no diabetes) situations. However, the sciatic nerve of diabetic animals showed signs of neuropathy: axon–myelin separation, elevated average mitochondrial number in the axons, unmyelinated fiber atrophy, and basement membrane thickening. The percentage of the axon–myelin separation was significantly higher in the diabetic groups, compared to the control groups. However, it was significantly less prominent in the PACAP-treated diabetic group (Figure 5).
Figure 5.
Representative electron microscopic images of axon–myelin separation in myelinated axons. The cell membrane of the Schwann cell is detached from the myelin sheath (arrows) and the axon is dislocated (star) (a). The separated cell membrane of the Schwann cell is visible (arrowheads, (b)). Histogram (c) shows the ratio of the axon–myelin separation in vehicle-treated control, vehicle-treated diabetic, PACAP-treated control, and PACAP-treated diabetic groups. Data are shown as means ± SEM of n = 5/6 rats/group. * p < 0.05, *** p < 0.001 vs. vehicle-treated control; ### p < 0.001 vs. vehicle-treated diabetic group.
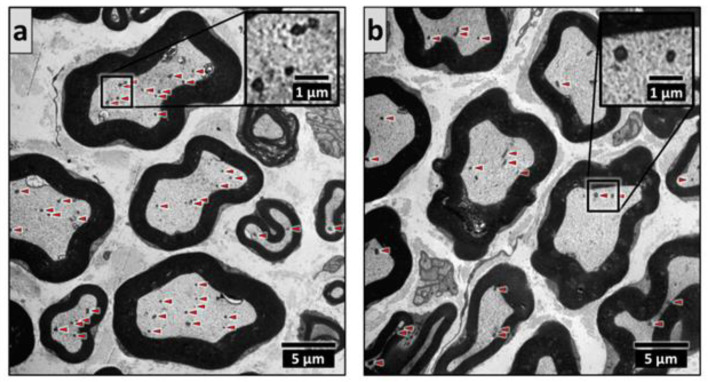
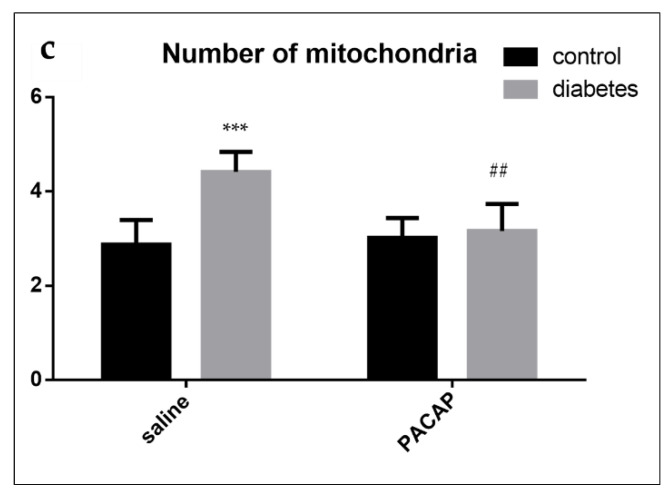
A marked elevation in the mitochondrial number in the myelinated axons was found in the vehicle-treated diabetic group. This could not be observed in the PACAP-treated diabetic group; thus, PACAP successfully prevented the rise in mitochondrial number (Figure 6).
Figure 6.
Representative electron microscopic images of diabetes-induced mitochondrial fission in vehicle-treated diabetic (a) and PACAP-treated diabetic (b) groups. Mitochondria are marked by arrowheads. Histogram (c) shows the average mitochondrial number in one myelinated axon in vehicle-treated control, vehicle-treated diabetic, PACAP-treated control, and PACAP-treated diabetic groups. Data are shown as means ± SEM, n = 5/6 rats/group. *** p < 0.001 vs. vehicle-treated control, ## p < 0.01 vs. vehicle-treated diabetic groups.
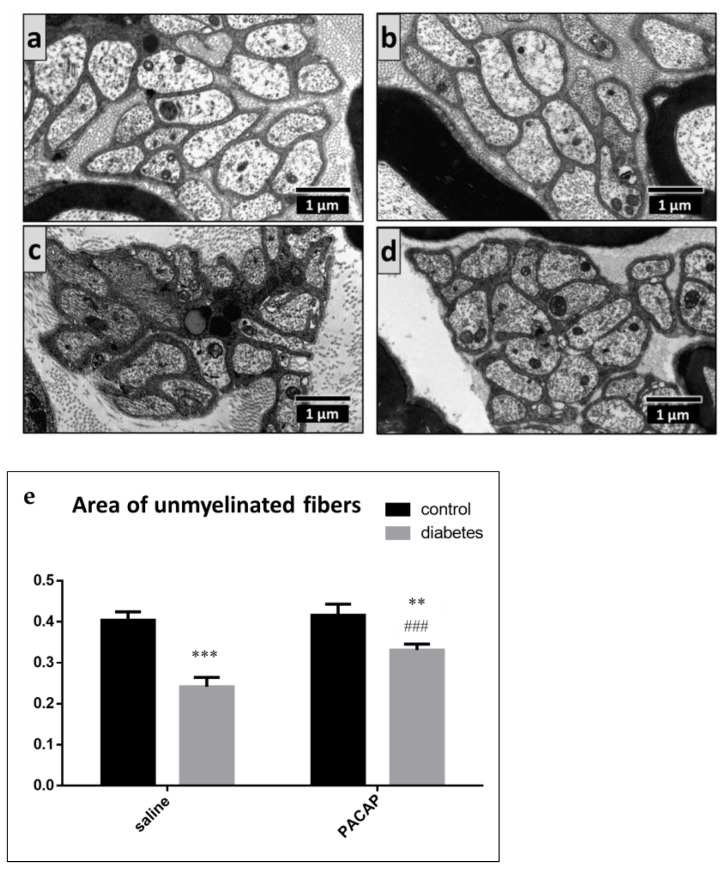
We also found unmyelinated fiber atrophy, characterized by a decrease in the unmyelinated fiber area in the vehicle-treated diabetic and PACAP-treated diabetic groups, compared to the vehicle-treated control and PACAP-treated control groups. This decrease was significantly less severe in the PACAP-treated diabetic group. No difference was observed between the vehicle-treated control and PACAP-treated control groups (Figure 7).
Figure 7.
Representative electron microscopic images of unmyelinated fibers in vehicle-treated control (a), PACAP-treated control (b), vehicle-treated diabetic (c), and PACAP-treated diabetic (d) groups. Histogram (e) shows the area of the unmyelinated fibers (e) in vehicle-treated control, PACAP-treated control, vehicle-treated diabetic, and PACAP-treated diabetic groups. Data are represented as means ± SEM of n = 5/6 rats/group. ** p < 0.01, *** p < 0.001 vs. vehicle-treated control; ### p < 0.05 vs. vehicle-treated diabetic groups.
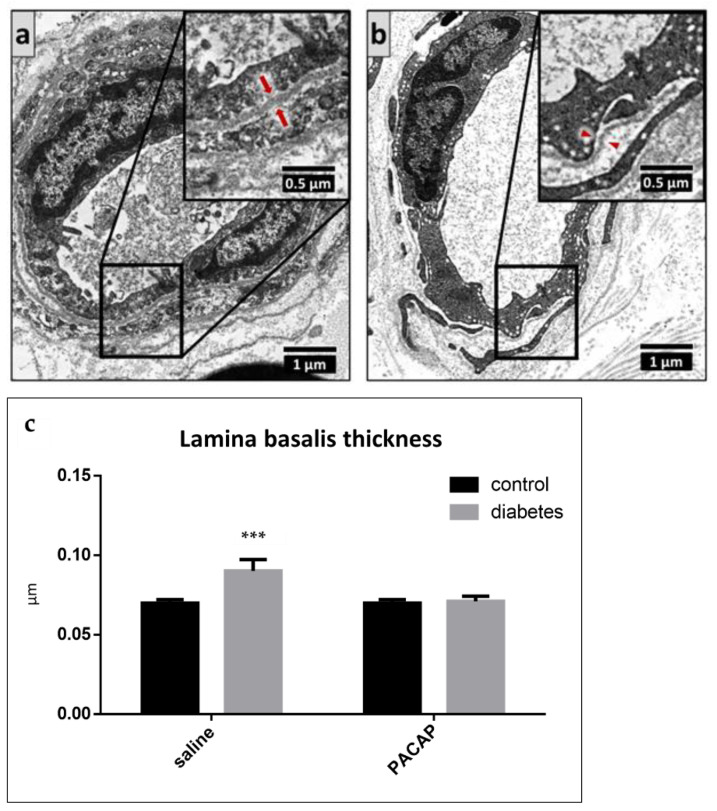
Electron microscopy also revealed thickening of the basement membrane in the endoneurial capillaries in the vehicle-treated diabetic group. PACAP-treated diabetic animals did not show any basement membrane thickening; there was no difference between the vehicle-treated control, PACAP-treated control, and PACAP-treated diabetic groups (Figure 8).
Figure 8.
Representative electron microscopic images of the basement membrane of endoneurial capillaries in vehicle-treated diabetic ((a), arrows) and PACAP-treated diabetic ((b), arrowheads) groups. Histogram (c) shows the thickening of the basement membrane of the endoneurial capillaries in vehicle-treated control, PACAP-treated control, vehicle-treated diabetic, and PACAP-treated diabetic groups. Data are shown as means ± SEM of n = 5/6 rats/group, *** p < 0.001 vs. vehicle-treated control groups.
3. Discussion
In the present study, we demonstrated that in vivo PACAP treatment is protective in diabetic neuropathy. An 8-week PACAP-38 treatment effectively counteracted the functional and morphological changes observed in diabetic rats without altering the blood glucose levels or body weight. This observation is in accordance with our previous results studying the effects of PACAP treatment in diabetic nephropathy [38]. These data suggest that PACAP, in spite of its effects on glucose homeostasis and insulin secretion [53,54], is protective in our diabetic neuropathy model, not by acting directly on glucose levels, but most probably due to its neuro- and general cytoprotective effects [20,79]. Although, as outlined above, PACAP is known to stimulate insulin secretion, it did not alter blood glucose levels in this model of type I diabetes. Whether it affects insulin levels in this model or in a model of type II diabetes awaits further investigations.
In the present study, we found that STZ-treated control diabetic rats displayed the morphological signs of diabetic neuropathy, i.e., axon–myelin separation, an increase in axonal mitochondria number, unmyelinated fiber atrophy, and basement membrane thickening of perineurial vessels. All these signs were attenuated by in vivo PACAP treatment.
Previous studies have reported that axon–myelin separation is due to hyperglycemia [80], Na+ channel dysfunction [81], and glycogen accumulation in Schwann cells [82], which led to a hyperosmolar perineurial environment, causing axon–myelin separation and demyelination [83]. Our study showed that PACAP treatment markedly attenuated this axon–myelin separation. PACAP is known to be involved in myelin maturation and synthesis by inducing the expression of myelin markers [58,84], and it has a trophic and antiapoptotic effect on Schwann cells [57,85]. PACAP receptors are upregulated in peripheral nerve injury in the Schwann cells, and the peptide promotes myelin gene expression, inhibits the release of pro-inflammatory cytokines, and stimulates anti-inflammatory cytokines [86,87].
In experimental diabetic neuropathy, the increase in mitochondrial number has been reported in myelinated axons [80,88,89]. Presumably, hyperglycemia-induced oxidative stress stimulates mitochondrial fission, which leads to the overproduction of mitochondrial ROS resulting in small aberrant, more electron-dense mitochondria with a reduced respiratory capacity [88]. It has been suggested that the inhibition of mitochondrial fission would relieve the ROS-induced oxidative stress [90]. The attenuated response in PACAP-treated animals might be due to the ability of PACAP to decrease oxidative metabolite levels, increase antioxidant potential [91], and stimulate antioxidant enzymes, such as peroxiredoxin [92], heme oxygenase-1 [93], superoxide-dismutase [94], and glutathione [39,95].
Unmyelinated fiber atrophy was found in STZ-induced diabetic rats, with a reduced cross-sectional area of the axons, similar to other reports [96]. This could also be attenuated by PACAP treatment. The protective effects of PACAP in nerve degeneration have been confirmed by dozens of studies [97,98]. Among others, PACAP promotes cell survival and neurite outgrowth [62,99], enhances axonal sprouting [100], and stimulates neuronal differentiation during development and regeneration [64,101,102,103,104]. In peripheral nerve injuries, PACAP has been shown to be upregulated and to promote regeneration partly through stimulation of other growth factors, such as glial cell line-derived neurotrophic factor [97,105,106,107]. Given the importance of endogenous PACAP in nerve regeneration, not surprisingly, mice lacking endogenous PACAP show a slower axonal regeneration with an increased pro-inflammatory environment [61]. These authors suggested that endogenous PACAP is involved in the controlled immune response that is necessary for proper nerve regeneration after injury [61]. This action of PACAP has been recently supported by human data: transcriptional profiling of the skin from patients with carpal tunnel syndrome revealed that the gene encoding PACAP was the most strongly upregulated gene and its expression was associated with recovery of intraepidermal nerve fibers [62].
Diabetes is also associated with the thickening of the basement membrane of the vasa nervosum as a consequence of the increased expression and decreased breakdown of collagen IV [108,109,110]. We found that diabetes resulted in the thickening of the basement membrane, attenuated by PACAP treatment. Similar to our present results, PACAP was found to attenuate basement membrane thickening in diabetic nephropathy [39]. Similar vascular protective effects have been observed in murine endothelial cells exposed to glucose: PACAP elicited an antiproliferative effect under chronic hyperglycemic conditions [111]. PACAP has also been demonstrated to protect endothelial cells against oxidative stress [112]. The protective effects on vessels are reflected in morphological signs, but PACAP has also been shown to reduce hyperglycemia-induced vascular dysfunction [50]. In that study, PACAP restored the disturbed relaxation of the vessels to an extent comparable to superoxide dismutase without direct scavenging of ROS. The elevated levels of fibroblast growth factor basic, matrix metalloproteinase 9, and nephroblastoma associated with endothelial dysfunction could be reduced by PACAP administration [50]. The model used in our study mimics type I diabetes, as streptozotocin leads to toxic degeneration of the insulin-producing beta cells. Based on these observations, however, it is plausible that PACAP could also be protective in neuropathies observed in type II diabetes, as the main mechanism of neuropathic induction is not directly related to the model itself but more to the increased glucose levels. However, in order to prove this point, further experiments are required.
The observed morphological signs of the protective effects of PACAP were also reflected in functional improvement in our study. Mechanical hyperalgesia is present in early diabetic neuropathy [113]. In our study, the intraperitoneally administered PACAP significantly attenuated the enhancement of pressure sensitivity by measuring mechanical hyperalgesia. In addition to the protective effects on nerve fibers, the anti-nociceptive and anti-hyperalgesic effects of PACAP might also be due to the decreased release of the pro-nociceptive neuropeptides [114]. In our present study, we also investigated the activation of pain-processing central structures. The expression of the acute neuronal activity marker c-Fos has been shown to be increased in the STZ diabetic model in the PAG and dorsal horn of the spinal cord [71]. Previous studies have found that FosB expression is significantly elevated in rats in the case of chronic pain and stress but not acute pain [115,116]. The dorsal horn of segment L4 of the spinal cord corresponds to the primary nociceptive afferent regions of the rat’s hind paw [117], while the lateral PAG is an important center of the descending anti-nociceptive system [118]. Here, we described chronic neuronal activity (i.e., FosB expression) in the spinal dorsal horn of segments L4–L5 and in the lateral part of PAG in our STZ diabetes model and found that PACAP treatment effectively prevented FosB activation in these centers. Our earlier findings in mice lacking endogenous PACAP support these findings, as PACAP knockout mice showed increased basal neuronal activity (i.e., c-Fos) in the lateral PAG [119]. The importance of endogenous PACAP in pain-processing centers has been highlighted also by several other studies [120,121,122,123].
In conclusion, here we show, for the first time, that PACAP treatment can attenuate or moderate the pathological changes of diabetic neuropathy, suggesting that PACAP could have therapeutic potential in diabetic neuropathy.
4. Materials and Methods
4.1. Animals
The experiment was carried out using adult male Wistar rats (n = 22) weighing 360–420 g. The experimental animals were housed under light/dark cycles of 12:12 h and received normal rat chow and drinking water ad libitum. Rats were randomly divided into four groups: (1) vehicle (saline)-treated control (non-diabetic) (n = 5); (2) PACAP-treated control (non-diabetic) (n = 5); (3) vehicle-treated diabetic (n = 5), and (4) PACAP-treated diabetic (n = 5) groups (in the figures, these groups are referred to as control, control + PACAP, diabetic, diabetic + PACAP groups, respectively). Diabetes was induced by a single dose of 65 mg/kg intravenous streptozotocin injection (Sigma, Budapest, Hungary). PACAP (20 µg PACAP1-38/100 μL saline solution) was injected intraperitoneally every second day for eight weeks, starting simultaneously with the streptozotocin injection to PACAP-treated control and PACAP-treated diabetic groups. The dose of PACAP was based on previous observations where this dose was effective in a rat model of diabetic nephropathy [38,39]. Vehicle-treated control and vehicle-treated diabetic groups received 100 μL saline intraperitoneally. Body weight and blood glucose levels (Accu-Check Active, Roche, Budapest, Hungary) were measured weekly, rats with glucose levels higher than 11 mmol/L were considered diabetic. Experimental procedures were carried out in accordance with approved protocols (University of Pecs; BA02/2000-24/2011). We performed in vivo behavioral tests on all experimental animals of the four groups. Following 8 weeks of survival, animals were processed for histological analysis.
4.2. Functional Tests
4.2.1. Mechanical Nociceptive Threshold—Randall–Selitto Test
The pressure sensitivity of the hind paw was measured by the Randall–Selitto test using Ugo Basile Analgesia Meter on a weekly basis. During the Randall–Selitto test, a continuously increasing pressure—at a maximum of 250 g—was applied to the hind paw of the rats. The increasing pressure caused the withdrawal of the paw, which was considered as the mechanical nociceptive threshold in grams [124,125]. Three measurements were made on both left and right hind paws, and the average of the assessments was taken. A decreased mechanical nociceptive threshold in this test can be considered as hyperalgesia [113].
4.2.2. Mechanical Nociceptive Threshold—Dynamic Plantar Aesthesiometer Test (DPA)
Touch sensitivity on the plantar surface of the hind paws was determined by a dynamic plantar aesthesiometer (DPA) (Ugo Basile, Gemonio, Italy) on a weekly basis. During the DPA test, a continuously increasing force—at a maximum of 50 g in 10 s—was applied to the hind paw by the elevation of a blunt-end needle and the aesthesiometer automatically detected the latency time and force (in grams) at the time of paw withdrawal. The decreased mechanical nociceptive threshold in the DPA test is a sign of allodynia since this mechanical stimulus is not painful to the rats [126].
4.3. Histology
4.3.1. Tissue Collection and Preparation for Histology
Animals were anesthetized with an overdose of isoflurane (Forane, Abbott Hungary, Budapest, Hungary) on week 8. Rats were transcardially perfused with 25 mL of phosphate-buffered saline (PBS), followed by 300 mL of 4% paraformaldehyde solution in Millonig buffer for 20 min. The brain and spinal cord were dissected and then placed into the same fixative solution for post-fixation for 72 h at 4 °C. The sciatic nerve was also removed from all animals and further processed for electron microscopy.
A tissue block containing the midbrain was isolated from the brains by cutting at the frontal planes of the posterior border of the median eminence and the transverse fissure. A tissue block of the L4-L5 spinal cord segments was also dissected. Blocks were sectioned by a Leica VT S 1000 (Leica, Wetzlar, Germany) vibratome. Five series of 30 µm coronal sections, interspaced by 120 µm, were collected in anti-freeze solution and stored at −20 °C until further use.
4.3.2. Immunohistochemistry for FosB
Free-floating labeling for the chronic neuronal activation marker FosB was performed on a series of the midbrain and spinal cord sections, as published earlier [127]. Briefly, sections were permeabilized with 0.5% Triton X-100 solution. Normal goat serum (2%, NGS, Jackson Immunoresearch, Europe Ltd., Suffolk, UK) was used to reduce non-specific binding. Subsequently, sections were treated with rabbit anti-FosB antibodies (1:500, Santa Cruz, sc-48 Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA) in PBS with 2% NGS overnight. After washes, sections were treated with biotinylated goat anti-rabbit serum and avidin–biotin complex (Vectastain ABC Elite Kit Vector Lbs., Burlingame, CA, USA) according to the supplier’s protocol. The labeling was developed in Tris buffer (pH 7.4) with 0.02% 3, 3′ diamino-benzidine (DAB) (Sigma) and 0.00003 v/v% H2O2. The reaction was carried out under visual control and was stopped after 7 min with PBS. Then, preparations were washed and mounted on gelatin-covered slides. After drying, sections were dehydrated with ethanol solutions (50%, 70%, 96%, absolute, absolute 5 min, respectively), cleared by xylene (2 × 20 min), and coverslipped using Depex (Fluka, Heidelberg, Germany). Specificity of the FosB antiserum (Santa Cruz, sc-48) was characterized earlier [128,129]. Western blot studies also support the specificity (http://datasheets.scbt.com/sc-48.pdf, 2017). Preabsorption experiment in the rat revealed that the blocking peptide (sc-48-P, Santa Cruz) prevented the immunolabeling. In line with this, omission and/or replacement of the primary or secondary antibodies by non-immune sera abolished the signal in all tests (images not shown).
4.3.3. Digital Imaging and Morphometry at Light Microscopic Level
The DAB-labeled FosB immunohistochemistry was studied and digitalized by a Nikon Microphot FXA microscope with a Spot RT camera (Nikon, Tokyo, Japan). For each animal, five serial sections were photographed. The number of FosB positive nuclei was determined using non-edited digital images by manual cell counting. The whole cross-sectional surface areas of the lateral PAG, as well as the dorsal horn laminae I, II, and III, individually were measured, and summed (laminae I + II + II) at L4–L5 spinal segments were evaluated, as marked in Figure 3 and Figure 4. Cell counting was carried out by a skilled neurophysiologist who was not informed about the identity of preparations. For documentation and publication purposes, the micro-photos were grey-scaled and contrasted using Adobe Photoshop 7.0.1 software.
4.3.4. Electron Microscopy
Sciatic nerve samples were placed in fixing solution (2.5% glutaraldehyde + 2% formalin + 0.1 M PBS) immediately after dissection in +4 °C for 24 h. A post-fixation procedure was performed with 1% osmium tetroxide. After dehydration in ascending alcohol (50%–70%–90%–96%) and subsequent transfer to propylene oxide, samples were embedded in Araldite resin. Semithin sections (0.5 µm) were cut by ultramicrotome (Leica Ultracut R), stained with 1% toluidine blue (Sigma), and examined with a Nikon Eclipse 80i microscope. Ultrathin sections were prepared from the area of interest and were contrasted by 2.5% uranyl–acetic acid and lead citrate. Slides were examined using a JEM-1200 EX-II electron microscope. The following parameters were analyzed: percentage of the axon-myelin separation, mitochondrial number in myelinated axons, area of the unmyelinated fibers, and thickness of the basement membrane.
4.4. Statistical Analysis
Statistical analysis was performed in GraphPad Prism 6.01 software. Two-way analysis of variance (ANOVA) was used to detect significant differences between groups. Multiple comparisons were performed by Tukey’s test. Data are presented as means ± S.E.M (standard error of the mean). Differences were considered statistically significant when p < 0.05.
Acknowledgments
The authors would like to express their acknowledgments to Dora Omboli, Veronika Antal, and Jozsef Abel for their valuable help in conducting the experiments.
Author Contributions
Conceptualization, Z.H. and D.R.; methodology, Z.H., B.G. and E.P.; project administration, P.K.; resources, Z.H., G.T., E.P. and B.G.; investigation, E.B., D.N., P.K., B.G. and E.P.; supervision, A.T.; formal analysis, data curation, validation, P.K.; visualization, P.K.; writing—original draft preparation, P.K. and G.R.; writing—review and editing, B.G., D.R., A.T. and Z.H.; funding acquisition, Z.H., A.T., D.R. and B.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Research, Development and Innovation Fund FK129190, K135457, K119759; National Brain Research Program NAP2017-1.2.1-NKP-2017-00002; MTA-TKI-14016; GINOP-2.3.2-15-2016-00050 “PEPSYS”; EFOP-3.6.2-16-2017-00008; EFOP-3.6.3-00009; EFOP-3.6.1-16-2016-00004; Higher Education Institutional Excellence Programme of the Ministry of Human Capacities in Hungary: 20765/3/2018/FEKUTSTRAT, 2020-4.1.1-TKP2020—FIKP III.
Institutional Review Board Statement
This research was approved by the Animal Welfare Committee of the University of Pécs, and the National Scientific Ethical Committee on Animal Experimentation (ÁTET) at the Ministry of Agriculture, fully complied with Decree No. 40/2013. (II. 14.) of the Hungarian Government and the EU Directive 2010/63/EU on the protection of animals used for scientific purposes (ethical permission numbers: BA02/2000-24/2011.
Data Availability Statement
The data presented in this study are available in the article, there is no supplementary data.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Vaudry D., Falluel-Morel A., Bourgault S., Basille M., Burel D., Wurtz O., Fournier A., Chow B.K.C., Hashimoto H., Galas L., et al. Pituitary adenylate cyclase-activating polypeptide and its receptors: 20 Years after the discovery. Pharmacol. Rev. 2009;61:283–357. doi: 10.1124/pr.109.001370. [DOI] [PubMed] [Google Scholar]
- 2.May V., Johnson G.C., Hammack S.E., Braas K.M., Parsons R.L. PAC1 Receptor internalization and endosomal MEK/ERK activation is essential for PACAP-mediated neuronal excitability. J. Mol. Neurosci. 2021;71:1536–1542. doi: 10.1007/s12031-021-01821-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Messlinger K., Balcziak L.K., Russo A.F. Cross-talk signaling in the trigeminal ganglion: Role of neuropeptides and other mediators. J. Neural Transm. 2020;127:431–444. doi: 10.1007/s00702-020-02161-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ciranna L., Costa L. Pituitary adenylate cyclase-activating polypeptide modulates hippocampal synaptic transmission and plasticity: New therapeutic suggestions for fragile X syndrome. Front. Cell. Neurosci. 2019;13:524. doi: 10.3389/fncel.2019.00524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gastelum C., Perez L., Hernandez J., Le N., Vahrson I., Sayers S., Wagner E.J. Adaptive changes in the central control of energy homeostasis occur in response to variations in energy status. Int. J. Mol. Sci. 2021;22:2728. doi: 10.3390/ijms22052728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vélez E.J., Unniappan S. A comparative update on the neuroendocrine regulation of growth hormone in vertebrates. Front. Endocrinol. 2021;11:614981. doi: 10.3389/fendo.2020.614981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Toth D., Szabo E., Tamas A., Juhasz T., Horvath G., Fabian E., Opper B., Szabo D., Maugeri G., D’Amico A.G., et al. Protective effects of PACAP in peripheral organs. Front. Endocrinol. 2020;11:377. doi: 10.3389/fendo.2020.00377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oride A., Kanasaki H., Mijiddorj T., Sukhbaatar U., Yamada T., Kyo S. Expression and regulation of pituitary adenylate cyclase-activating polypeptide in rat placental cells. Reprod. Sci. 2016;23:1080–1086. doi: 10.1177/1933719116630421. [DOI] [PubMed] [Google Scholar]
- 9.Costa M., Spencer N.J., Brookes S.J.H. The role of enteric inhibitory neurons in intestinal motility. Auton. Neurosci. 2021;235:102854. doi: 10.1016/j.autneu.2021.102854. [DOI] [PubMed] [Google Scholar]
- 10.Horvath G., Opper B., Reglodi D. The neuropeptide pituitary adenylate cyclase-activating polypeptide (PACAP) is protective in inflammation and oxidative stress-induced damage in the kidney. Int. J. Mol. Sci. 2019;20:4944. doi: 10.3390/ijms20194944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Szentleleky E., Szegeczki V., Karanyicz E., Hajdu T., Tamas A., Toth G., Zakany R., Reglodi D., Juhasz T. Pituitary adenylate cyclase activating polypeptide (PACAP) reduces oxidative and mechanical stress-evoked matrix degradation in chondrifying cell cultures. Int. J. Mol. Sci. 2019;20:168. doi: 10.3390/ijms20010168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Girard B.M., Campbell S.E., Beca K.I., Perkins M., Hsiang H., May V., Vizzard M.A. Intrabladder PAC1 receptor antagonist, PACAP(6-38), reduces urinary bladder frequency and pelvic sensitivity in mice exposed to repeated variate stress (RVS) J. Mol. Neurosci. 2021;71:1575–1588. doi: 10.1007/s12031-020-01649-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jozsa G., Fulop B.D., Kovacs L., Czibere B., Szegeczki V., Kiss T., Hajdu T., Tamas A., Helyes Z., Zakany R., et al. Lack of pituitary adenylate cyclase–activating polypeptide (PACAP) disturbs callus formation. J. Mol. Neurosci. 2021;71:1543–1555. doi: 10.1007/s12031-019-01448-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rytel L., Wojtkiewicz J., Snarska A., Mikołajczyk A. Changes in the neurochemical characterization of enteric neurons in the porcine duodenum after administration of low-dose salmonella enteritidis lipopolysaccharides. J. Mol. Neurosci. 2021;71:1556–1566. doi: 10.1007/s12031-019-01473-y. [DOI] [PubMed] [Google Scholar]
- 15.Szabo D., Sarszegi Z., Polgar B., Saghy E., Nemeth A., Reglodi D., Makkos A., Gorbe A., Helyes Z., Ferdinandy P., et al. Pacap-38 in acute st-segment elevation myocardial infarction in humans and pigs: A translational study. Int. J. Mol. Sci. 2021;22:2883. doi: 10.3390/ijms22062883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chiba Y., Ueda C., Kohno N., Yamashita M., Miyakawa Y., Ando Y., Suto W., Hirabayashi T., Takenoya F., Takasaki I., et al. Attenuation of relaxing response induced by pituitary adenylate cyclase-activating polypeptide in bronchial smooth muscle of experimental asthma. Am. J. Physiol. Lung. Cell. Mol. Physiol. 2020;319:L786–L793. doi: 10.1152/ajplung.00315.2020. [DOI] [PubMed] [Google Scholar]
- 17.Zheng Y., Zhang L., Xie J., Shi L. The emerging role of neuropeptides in Parkinson’s disease. Front. Aging Neurosci. 2021;13:646726. doi: 10.3389/fnagi.2021.646726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reglodi D., Kiss P., Lubics A., Tamas A. Review on the protective effects of PACAP in models of neurodegenerative diseases in vitro and in vivo. Curr. Pharm. Des. 2011;17:962–972. doi: 10.2174/138161211795589355. [DOI] [PubMed] [Google Scholar]
- 19.Reglodi D., Kiss P., Szabadfi K., Atlasz T., Gabriel R., Horvath G., Szakaly P., Sandor B., Lubics A., Laszlo E., et al. PACAP is an endogenous protective factor-insights from PACAP-deficient mice. J. Mol. Neurosci. 2012;48:482–492. doi: 10.1007/s12031-012-9762-0. [DOI] [PubMed] [Google Scholar]
- 20.Reglodi D., Tamas A., Jungling A., Vaczy A., Rivnyak A., Fulop B.D., Szabo E., Lubics A., Atlasz T. Protective effects of pituitary adenylate cyclase activating polypeptide against neurotoxic agents. Neurotoxicology. 2018;66:185–194. doi: 10.1016/j.neuro.2018.03.010. [DOI] [PubMed] [Google Scholar]
- 21.Shioda S., Takenoya F., Wada N., Hirabayashi T., Seki T., Nakamachi T. Pleiotropic and retinoprotective functions of PACAP. Anat. Sci. Int. 2016;91:313–324. doi: 10.1007/s12565-016-0351-0. [DOI] [PubMed] [Google Scholar]
- 22.Lee E.H., Seo S.R. Neuroprotective roles of pituitary adenylate cyclase-activating polypeptide in neurodegenerative diseases. BMB Rep. 2014;47:369–375. doi: 10.5483/BMBRep.2014.47.7.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martínez-Rojas V.A., Jiménez-Garduño A.M., Michelatti D., Tosatto L., Marchioretto M., Arosio D., Basso M., Pennuto M., Musio C. ClC-2-like chloride current alterations in a cell model of spinal and bulbar muscular atrophy, a polyglutamine disease. J. Mol. Neurosci. 2021;71:662–674. doi: 10.1007/s12031-020-01687-5. [DOI] [PubMed] [Google Scholar]
- 24.D'Amico A.G., Maugeri G., Saccone S., Federico C., Cavallaro S., Reglodi D., D'Agata V. PACAP modulates the autophagy process in an in vitro model of amyotrophic lateral sclerosis. Int. J. Mol. Sci. 2020;21:2943. doi: 10.3390/ijms21082943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perényi H., Szegeczki V., Horvath G., Hinnah B., Tamás A., Radak Z., Abraham D., Zakany R., Reglodi D., Juhasz T. Physical activity protects the pathological alterations of Alzheimer’s disease kidneys via the activation of PACAP and BMP signaling pathways. Front. Cell. Neurosci. 2020;14:243. doi: 10.3389/fncel.2020.00243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schaler A.W., Runyan A.M., Clelland C.L., Sydney E.J., Fowler S.L., Figueroa H.Y., Shioda S., Santa-Maria I., Duff K.E., Myeku N. PAC1 receptor-mediated clearance of tau in postsynaptic compartments attenuates tau pathology in mouse brain. Sci. Transl. Med. 2021;13:eaba7394. doi: 10.1126/scitranslmed.aba7394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen X.Y., Du Y.F., Chen L. Neuropeptides exert neuroprotective effects in Alzheimer’s disease. Front. Mol. Neurosci. 2019;11:493. doi: 10.3389/fnmol.2018.00493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nonaka N., Banks W.A., Shioda S. Pituitary adenylate cyclase-activating polypeptide: Protective effects in stroke and dementia. Peptides. 2020;130:170332. doi: 10.1016/j.peptides.2020.170332. [DOI] [PubMed] [Google Scholar]
- 29.Cherait A., Maucotel J., Lefranc B., Leprince J., Vaudry D. Intranasal administration of PACAP is an efficient delivery route to reduce infarct volume and promote functional recovery after transient and permanent middle cerebral artery occlusion. Front. Endocrinol. 2021;11:585082. doi: 10.3389/fendo.2020.585082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reglodi D., Tamas A., Somogyvari-Vigh A., Szanto Z., Kertes E., Lenard L., Arimura A., Lengvari I. Effects of pretreatment with PACAP on the infarct size and functional outcome in rat permanent focal cerebral ischemia. Peptides. 2002;23:2227–2234. doi: 10.1016/S0196-9781(02)00262-0. [DOI] [PubMed] [Google Scholar]
- 31.Jungling A., Reglodi D., Maasz G., Zrinyi Z., Schmidt J., Rivnyak A., Horvath G., Pirger Z., Tamas A. Alterations of nigral dopamine levels in Parkinson’s disease after environmental enrichment and PACAP treatment in aging rats. Life. 2021;11:35. doi: 10.3390/life11010035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Solés-Tarrés I., Cabezas-Llobet N., Vaudry D., Xifró X. Protective effects of pituitary adenylate cyclase-activating polypeptide and vasoactive intestinal peptide against cognitive decline in neurodegenerative diseases. Front. Cell. Neurosci. 2020;14:221. doi: 10.3389/fncel.2020.00221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lamine-Ajili A., Fahmy A.M., Létourneau M., Chatenet D., Labonté P., Vaudry D., Fournier A. Effect of the pituitary adenylate cyclase-activating polypeptide on the autophagic activation observed in in vitro and in vivo models of Parkinson’s disease. Biochim. Biophys. Acta Mol. Basis Dis. 2016;1862:688–695. doi: 10.1016/j.bbadis.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 34.Tamas A., Lubics A., Lengvari I., Reglodi D. Protective effects of PACAP in excitotoxic striatal lesion. Ann. N. Y. Acad. Sci. 2006;1070:570–574. doi: 10.1196/annals.1317.083. [DOI] [PubMed] [Google Scholar]
- 35.Kvarik T., Reglodi D., Werling D., Vaczy A., Kovari P., Szabo E., Kovacs K., Hashimoto H., Ertl T., Gyarmati J., et al. The protective effects of endogenous PACAP in oxygen-induced retinopathy. J. Mol. Neurosci. 2021 doi: 10.1007/s12031-021-01846-2. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kovacs A.K., Atlasz T., Werling D., Szabo E., Reglodi D., Toth G.K. Stability test of PACAP in eye drops. J. Mol. Neurosci. 2021;71:1567–1574. doi: 10.1007/s12031-020-01532-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ye D., Shi Y., Xu Y., Huang J. PACAP attenuates optic nerve crush-induced retinal ganglion cell apoptosis via activation of the CREB-Bcl-2 pathway. J. Mol. Neurosci. 2019;68:475–484. doi: 10.1007/s12031-019-01309-9. [DOI] [PubMed] [Google Scholar]
- 38.Banki E., Degrell P., Kiss P., Kovacs K., Kemeny A., Csanaky K., Duh A., Nagy D., Toth G., Tamas A., et al. Effect of PACAP treatment on kidney morphology and cytokine expression in rat diabetic nephropathy. Peptides. 2013;42:125–130. doi: 10.1016/j.peptides.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 39.Banki E., Kovacs K., Nagy D., Juhasz T., Degrell P., Csanaky K., Kiss P., Jancso G., Toth G., Tamas A., et al. Molecular mechanisms underlying the nephroprotective effects of PACAP in diabetes. J. Mol. Neurosci. 2014;54:300–309. doi: 10.1007/s12031-014-0249-z. [DOI] [PubMed] [Google Scholar]
- 40.Szabadfi K., Atlasz T., Kiss P., Danyadi B., Tamas A., Helyes Z., Hashimoto H., Shintani N., Baba A., Toth G., et al. Mice deficient in pituitary adenylate cyclase activating polypeptide (PACAP) are more susceptible to retinal ischemic injury in vivo. Neurotox. Res. 2012;21:41–48. doi: 10.1007/s12640-011-9254-y. [DOI] [PubMed] [Google Scholar]
- 41.Szabadfi K., Szabo A., Kiss P., Reglodi D., Setalo G., Jr., Kovacs K., Tamas A., Toth G., Gabriel R. PACAP promotes neuron survival in early experimental diabetic retinopathy. Neurochem. Int. 2014;64:84–91. doi: 10.1016/j.neuint.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 42.Szabadfi K., Reglodi D., Szabo A., Szalontai B., Valasek A., Setalo G., Kiss P., Tamas A., Wilhelm M., Gabriel R. Pituitary adenylate cyclase activating polypeptide, a potential therapeutic agent for diabetic retinopathy in rats: Focus on the vertical information processing pathway. Neurotox. Res. 2016;29:432–446. doi: 10.1007/s12640-015-9593-1. [DOI] [PubMed] [Google Scholar]
- 43.D’Amico A.G., Maugeri G., Reitano R., Bucolo C., Saccone S., Drago F., D’Agata V. PACAP modulates expression of hypoxia-inducible factors in streptozotocin-induced diabetic rat retina. J. Mol. Neurosci. 2015;57:501–509. doi: 10.1007/s12031-015-0621-7. [DOI] [PubMed] [Google Scholar]
- 44.D’Amico A.G., Maugeri G., Rasà D.M., Bucolo C., Saccone S., Federico C., Cavallaro S., D’Agata V. Modulation of IL-1β and VEGF expression in rat diabetic retinopathy after PACAP administration. Peptides. 2017;97:64–69. doi: 10.1016/j.peptides.2017.09.014. [DOI] [PubMed] [Google Scholar]
- 45.D’Amico A.G., Maugeri G., Musumeci G., Reglodi D., D’Agata V. PACAP and NAP: Effect of two functionally related peptides in diabetic retinopathy. J. Mol. Neurosci. 2021;71:1525–1535. doi: 10.1007/s12031-020-01769-4. [DOI] [PubMed] [Google Scholar]
- 46.Maugeri G., D’Amico A.G., Saccone S., Federico C., Cavallaro S., D’Agata V. PACAP and VIP inhibit HIF-1α-mediated VEGF expression in a model of diabetic macular edema. J. Cell. Physiol. 2017;232:1209–1215. doi: 10.1002/jcp.25616. [DOI] [PubMed] [Google Scholar]
- 47.Maugeri G., D’Amico A.G., Bucolo C., D’Agata V. Protective effect of PACAP-38 on retinal pigmented epithelium in an in vitro and in vivo model of diabetic retinopathy through EGFR-dependent mechanism. Peptides. 2019;119:170108. doi: 10.1016/j.peptides.2019.170108. [DOI] [PubMed] [Google Scholar]
- 48.Scuderi S., D’Amico A.G., Castorina A., Imbesi R., Carnazza M.L., D’Agata V. Ameliorative effect of PACAP and VIP against increased permeability in a model of outer blood retinal barrier dysfunction. Peptides. 2013;39:119–124. doi: 10.1016/j.peptides.2012.11.015. [DOI] [PubMed] [Google Scholar]
- 49.Fabian E., Horvath G., Opper B., Atlasz T., Toth G., Reglodi D. PACAP is protective against cellular stress in retinal pigment epithelial cells. Int. J. Pept. Res. Ther. 2021;27:1221–1228. doi: 10.1007/s10989-021-10162-7. [DOI] [Google Scholar]
- 50.Solymar M., Ivic I., Balasko M., Fulop B.D., Toth G., Tamas A., Reman G., Koller A., Reglodi D. Pituitary adenylate cyclase-activating polypeptide ameliorates vascular dysfunction induced by hyperglycaemia. Diabetes Vasc. Dis. Res. 2018;15:277–285. doi: 10.1177/1479164118757922. [DOI] [PubMed] [Google Scholar]
- 51.Feldman E.L., Russell J.W., Sullivan K.A., Golovoy D. New insights into the pathogenesis of diabetic neuropathy. Curr. Opin. Neurol. 1999;12:553–563. doi: 10.1097/00019052-199910000-00009. [DOI] [PubMed] [Google Scholar]
- 52.Zochodne D.W. Is early diabetic neuropathy a disorder of the dorsal root ganglion? A hypothesis and critique of some current ideas on the etiology of diabetic neuropathy. J. Periph. Nerv. Syst. 1996;1:119–130. [PubMed] [Google Scholar]
- 53.Sanlioglu A.D., Karacay B., Balci M.K., Griffith T.S., Sanlioglu S. Therapeutic potential of VIP vs PACAP in diabetes. J. Mol. Endocrinol. 2012;49:157–167. doi: 10.1530/JME-12-0156. [DOI] [PubMed] [Google Scholar]
- 54.Liu M., Yang X., Bai T., Liu Z., Liu T., Wang Y., Cui L., Liu Y., Zhang Y. PACAP stimulates insulin secretion by PAC1 receptor and ion channels in β-cells. Cell Signal. 2019;61:48–56. doi: 10.1016/j.cellsig.2019.05.006. [DOI] [PubMed] [Google Scholar]
- 55.Shao S., Yang Y., Yuan G., Zhang M., Yu X. Signaling molecules involved in lipid-induced pancreatic beta-cell dysfunction. DNA Cell Biol. 2013;32:41–49. doi: 10.1089/dna.2012.1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nakata M., Shintani N., Hashimoto H., Baba A., Yada T. Intra-islet PACAP protects pancreatic β-cells against glucotoxicity and lipotoxicity. J. Mol. Neurosci. 2010;42:404–410. doi: 10.1007/s12031-010-9383-4. [DOI] [PubMed] [Google Scholar]
- 57.Maugeri M., D’Amico A.G., Musumeci G., Reglodi D., D’Agata V. Effects of PACAP on Schwann cells: Focus on nerve injury. Int. J. Mol. Sci. 2020;21:8233. doi: 10.3390/ijms21218233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Castorina A., Scuderi S., D’Amico A.G., Drago F., D’Agata V. PACAP and VIP increase the expression of myelin-related proteins in rat schwannoma cells: Involvement of PAC1/VPAC2 receptor-mediated activation of PI3K/Akt signaling pathways. Exp. Cell Res. 2014;322:108–121. doi: 10.1016/j.yexcr.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 59.Nielsen K.M., Chaverra M., Hapner S.J., Nelson B.R., Todd V., Zigmond R.E., Lefcort F. PACAP promotes sensory neuron differentiation: Blockade by neurotrophic factors. Mol. Cell. Neurosci. 2004;25:629–641. doi: 10.1016/j.mcn.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 60.Fukiage C., Nakajima T., Takayama Y., Minagawa Y., Shearer T.R., Azuma M. PACAP induces neurite outgrowth in cultured trigeminal ganglion cells and recovery of corneal sensitivity after flap surgery in rabbits. Am. J. Ophthalmol. 2007;143:255–262. doi: 10.1016/j.ajo.2006.10.034. [DOI] [PubMed] [Google Scholar]
- 61.Armstrong B.D., Abad C., Chhith S., Cheung-Lau G., Hajji O.E., Nobuta H., Waschek J.A. Impaired nerve regeneration and enhanced neuroinflammatory response in mice lacking pituitary adenylyl cyclase activating peptide. Neuroscience. 2008;151:63–73. doi: 10.1016/j.neuroscience.2007.09.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Baskozos G., Sandy-Hindmarch O., Clark A.J., Windsor K., Karlsson P., Weir G.A., McDermott L.A., Burchall J., Wiberg A., Furniss D., et al. Molecular and cellular correlates of human nerve regeneration: ADCYAP1/PACAP enhance nerve outgrowth. Brain. 2020;143:2009–2026. doi: 10.1093/brain/awaa163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang Z., Shan W., Li H., Feng J., Lu S., Ou B., Ma M., Ma Y. The PACAP-derived peptide MPAPO facilitates corneal wound healing by promoting corneal epithelial cell proliferation and trigeminal ganglion cell axon regeneration. Int. J. Biol. Sci. 2019;15:2676–2691. doi: 10.7150/ijbs.35630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang T., Li Y., Guo M., Dong X., Liao M., Du M., Wang X., Yin H., Yan H. Exosome-mediated delivery of the neuroprotective peptide PACAP38 promotes retinal ganglion cell survival and axon regeneration in rats with traumatic optic neuropathy. Front. Cell Dev. Biol. 2021;9:659783. doi: 10.3389/fcell.2021.659783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Harati Y. Diabetic neuropathies: Unanswered questions. Neurol. Clin. 2007;25:303–317. doi: 10.1016/j.ncl.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 66.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 67.Duby J.J., Campbell R.K., Setter S.M., White J.R., Rasmussen K.A. Diabetic neuropathy: An intensive review. Am. J. Heal. Pharm. 2004;61:160–176. doi: 10.1093/ajhp/61.2.160. [DOI] [PubMed] [Google Scholar]
- 68.Conte M., Sabbatinelli J., Chiariello A., Martucci M., Santoro A., Monti D., Arcaro M., Galimberti D., Scarpini E., Bonfigli A.R., et al. Disease-specific plasma levels of mitokines FGF21, GDF15, and Humanin in type II diabetes and Alzheimer’s disease in comparison with healthy aging. GeroScience. 2021;43:985–1001. doi: 10.1007/s11357-020-00287-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yu Y., Singh H., Kwon K., Tsitrin T., Petrini J., Nelson K.E., Pieper R. Protein signatures from blood plasma and urine suggest changes in vascular function and IL-12 signaling in elderly with a history of chronic diseases compared with an age-matched healthy cohort. GeroScience. 2021;43:593–606. doi: 10.1007/s11357-020-00269-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Greene D.A., Feldman E.L., Stevens M.J., Sima A.A.F., Albers J.W., Pfeifer M.A. Diabetic Neuropathy. In: Rifkin H., Porte D., Sherwin R., editors. Ellenberg and Rifkin Diabetes Mellitus. Appleton & Lange; Stamford, CT, USA: 1997. pp. 1009–1076. [Google Scholar]
- 71.Morgado C., Terra P.P., Tavares I. Neuronal hyperactivity at the spinal cord and periaqueductal grey during painful diabetic neuropathy: Effects of gabapentin. Eur. J. Pain. 2010;14:693–699. doi: 10.1016/j.ejpain.2009.11.011. [DOI] [PubMed] [Google Scholar]
- 72.Johnson M.S., Ryals J.M., Wright D.E. Diabetes-induced chemogenic hypoalgesia is paralleled by attenuated stimulus-induced Fos expression in the spinal cord of diabetic mice. J. Pain. 2007;8:637–649. doi: 10.1016/j.jpain.2007.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Morgado C., Tavares I. Diabetes C-fos expression at the spinal dorsal horn of streptozotocin-induced diabetic rats. Diabetes Metab. Res. Rev. 2007;23:644–652. doi: 10.1002/dmrr.751. [DOI] [PubMed] [Google Scholar]
- 74.Raposo D., Morgado C., Pereira-Terra P., Tavares I. Nociceptive spinal cord neurons of laminae I-III exhibit oxidative stress damage during diabetic neuropathy which is prevented by early antioxidant treatment with epigallocatechin-gallate (EGCG) Brain Res. Bull. 2015;110:68–75. doi: 10.1016/j.brainresbull.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 75.Lantéri-Minet M., de Pommery J., Herdegen T., Weil-Fugazza J., Bravo R., Menétrey D. Differential time course and spatial expression of Fos, Jun, and Krox-24 proteins in spinal cord of rats undergoing subacute or chronic somatic inflammation. J. Comp. Neurol. 1993;333:223–235. doi: 10.1002/cne.903330208. [DOI] [PubMed] [Google Scholar]
- 76.Herdegen T., Tölle T.R., Bravo R., Zieglgänsberger W., Zimmermann M. Sequential expression of JUN B, JUN D and FOS B proteins in rat spinal neurons: Cascade of transcriptional operations during nociception. Neurosci. Lett. 1991;129:221–224. doi: 10.1016/0304-3940(91)90466-7. [DOI] [PubMed] [Google Scholar]
- 77.Madsen T.M., Bolwig T.G., Mikkelsen J.D. Differential regulation of c-fos and fosB in the rat brain after amygdala kindling. Cell. Mol. Neurobiol. 2006;26:87–100. doi: 10.1007/s10571-006-9202-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Motojima Y., Ueta Y., Sakai A. Analysis of the proportion and neuronal activity of excitatory and inhibitory neurons in the rat dorsal spinal cord after peripheral nerve injury. Neurosci. Lett. 2021;749:135707. doi: 10.1016/j.neulet.2021.135707. [DOI] [PubMed] [Google Scholar]
- 79.Aubert N., Vaudry D., Falluel-Morel A., Desfeux A., Fisch C., Ancian P., de Jouffrey S., Le Bigot J.F., Couvineau A., Laburthe M., et al. PACAP prevents toxicity induced by cisplatin in rat and primate neurons but not in proliferating ovary cells: Involvement of the mitochondrial apoptotic pathway. Neurobiol. Dis. 2008;32:66–80. doi: 10.1016/j.nbd.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 80.Lennertz R.C., Medler K.A., Bain J.L., Wright D.E., Stucky C.L. Impaired sensory nerve function and axon morphology in mice with diabetic neuropathy. J. Neurophysiol. 2011;106:905–914. doi: 10.1152/jn.01123.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Love S., Duchen L.W. Morphological Abnormalities in Myelinated nerve fibres caused by Leiurus, Centruroides and Phoneutria venoms and their prevention by tetrodotoxin. Q. J. Exp. Physiol. 1986;71:115–122. doi: 10.1113/expphysiol.1986.sp002962. [DOI] [PubMed] [Google Scholar]
- 82.Low P.A., Dyck P.J., Schmelzer J.D. Mammalian peripheral nerve sheath has unique responses to chronic elevations of endoneurial fluid pressure. Exp. Neurol. 1980;70:300–306. doi: 10.1016/0014-4886(80)90029-1. [DOI] [PubMed] [Google Scholar]
- 83.Powell H.C., Myers R.R. Axonopathy and microangiopathy in chronic alloxan diabetes. Acta Neuropathol. 1984;65:128–137. doi: 10.1007/BF00690466. [DOI] [PubMed] [Google Scholar]
- 84.Vincze A., Reglodi D., Helyes Z., Hashimoto H., Shintani N., Abrahám H. Role of endogenous pituitary adenylate cyclase activating polypeptide (PACAP) in myelination of the rodent brain: Lessons from PACAP-deficient mice. Int. J. Dev. Neurosci. 2011;29:923–935. doi: 10.1016/j.ijdevneu.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 85.Castorina A., Tiralongo A., Giunta S., Carnazza M.L., Rasi G., D’Agata V. PACAP and VIP prevent apoptosis in schwannoma cells. Brain Res. 2008;1241:29–35. doi: 10.1016/j.brainres.2008.09.035. [DOI] [PubMed] [Google Scholar]
- 86.Woodley P.K., Min Q., Li Y., Mulvey N.F., Parkinson D.B., Dun X.P. Distinct VIP and PACAP functions in the distal nerve stump during peripheral nerve regeneration. Front. Neurosci. 2019;13:1326. doi: 10.3389/fnins.2019.01326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Musumeci G., Leggio G.M., Marzagalli R., Al-Badri G., Drago F., Castorina A. Identification of dysregulated microRNA networks in schwann cell-like cultures exposed to immune challenge: Potential crosstalk with the protective VIP/PACAP neuropeptide system. Int. J. Mol. Sci. 2018;19:981. doi: 10.3390/ijms19040981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Edwards J.L., Quattrini A., Lentz S.I., Figueroa-Romero C., Cerri F., Backus C., Hong Y., Feldman E.L. Diabetes regulates mitochondrial biogenesis and fission in mouse neurons. Diabetologia. 2010;53:160–169. doi: 10.1007/s00125-009-1553-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Vincent A.M., Edwards J.L., McLean L.L., Hong Y., Cerri F., Lopez I., Quattrini A., Feldman E.L. Mitochondrial biogenesis and fission in axons in cell culture and animal models of diabetic neuropathy. Acta Neuropathol. 2010;120:477–489. doi: 10.1007/s00401-010-0697-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Galloway C.A., Lee H., Nejjar S., Jhun B.S., Yu T., Hsu W., Yoon Y. Transgenic control of mitochondrial fission induces mitochondrial uncoupling and relieves diabetic oxidative stress. Diabetes. 2012;61:2093–2104. doi: 10.2337/db11-1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ohtaki H., Satoh A., Nakamachi T., Yofu S., Dohi K., Mori H., Ohara K., Miyamoto K., Hashimoto H., Shintani N., et al. Regulation of oxidative stress by pituitary adenylate cyclase-activating polypeptide (PACAP) mediated by PACAP receptor. J. Mol. Neurosci. 2010;42:397–403. doi: 10.1007/s12031-010-9350-0. [DOI] [PubMed] [Google Scholar]
- 92.Botia B., Seyer D., Ravni A., Bénard M., Falluel-Morel A., Cosette P., Jouenne T., Fournier A., Vaudry H., Gonzalez B.J., et al. Peroxiredoxin 2 is involved in the neuroprotective effects of PACAP in cultured cerebellar granule neurons. J. Mol. Neurosci. 2008;36:61–72. doi: 10.1007/s12031-008-9075-5. [DOI] [PubMed] [Google Scholar]
- 93.Kinhult J., Uddman R., Cardell L.O. The induction of carbon monoxide-mediated airway relaxation by PACAP 38 in isolated guinea pig airways. Lung. 2001;179:1–8. doi: 10.1007/s004080000043. [DOI] [PubMed] [Google Scholar]
- 94.Laszlo E., Juhasz T., Varga A., Czibere B., Kovacs K., Degrell P., Horvath G., Jancso G., Szakaly P., Tamas A., et al. Protective Effect of PACAP on Ischemia/Reperfusion-Induced Kidney Injury of Male and Female Rats: Gender Differences. J. Mol. Neurosci. 2019;68:408–419. doi: 10.1007/s12031-018-1207-y. [DOI] [PubMed] [Google Scholar]
- 95.Masmoudi-Kouki O., Douiri S., Hamdi Y., Kaddour H., Bahdoudi S., Vaudry D., Basille M., Leprince J., Fournier A., Vaudry H., et al. Pituitary adenylate cyclase-activating polypeptide protects astroglial cells against oxidative stress-induced apoptosis. J. Neurochem. 2011;117:403–411. doi: 10.1111/j.1471-4159.2011.07185.x. [DOI] [PubMed] [Google Scholar]
- 96.Murakami T., Iwanaga T., Ogawa Y., Fujita Y., Sato E., Yoshitomi H., Sunada Y., Nakamura A. Development of sensory neuropathy in streptozotocin-induced diabetic mice. Brain Behav. 2013;3:35–41. doi: 10.1002/brb3.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tamas A., Reglodi D., Farkas O., Kovesdi E., Pal J., Povlishock J.T., Schwarcz A., Czeiter E., Szanto Z., Doczi T., et al. Effect of PACAP in central and peripheral nerve injuries. Int. J. Mol. Sci. 2012;13:8430–8448. doi: 10.3390/ijms13078430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Toth D., Tamas A., Reglodi D. The neuroprotective and biomarker potential of PACAP in human traumatic brain injury. Int. J. Mol. Sci. 2020;21:827. doi: 10.3390/ijms21030827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gonzalez B.J., Basille M., Vaudry D., Fournier A., Vaudry H. Pituitary adenylate cyclase-activating polypeptide promotes cell survival and neurite outgrowth in rat cerebellar neuroblasts. Neuroscience. 1997;78:419–430. doi: 10.1016/S0306-4522(96)00617-3. [DOI] [PubMed] [Google Scholar]
- 100.Suarez V., Guntinas-Lichius O., Streppel M., Ingorokva S., Grosheva M., Neiss W.F., Angelov D.N., Klimaschewski L. The axotomy-induced neuropeptides galanin and pituitary adenylate cyclase-activating peptide promote axonal sprouting of primary afferent and cranial motor neurones. Eur. J. Neurosci. 2006;24:1555–1564. doi: 10.1111/j.1460-9568.2006.05029.x. [DOI] [PubMed] [Google Scholar]
- 101.Waschek J.A. Multiple actions of pituitary adenylyl cyclase activating peptide in nervous system development and regeneration. Dev. Neurosci. 2002;24:14–23. doi: 10.1159/000064942. [DOI] [PubMed] [Google Scholar]
- 102.Chung J., Kubota H., Ozaki Y., Uda S., Kuroda S. Timing-dependent actions of NGF required for cell differentiation. PLoS ONE. 2010;5:e9011. doi: 10.1371/journal.pone.0009011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gey M., Wanner R., Schilling C., Pedro M.T., Sinske D., Knöll B. Atf3 mutant mice show reduced axon regeneration and impaired regeneration-associated gene induction after peripheral nerve injury. Open Biol. 2016;6:160091. doi: 10.1098/rsob.160091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tsuchida M., Nakamachi T., Sugiyama K., Tsuchikawa D., Watanabe J., Hori M., Yoshikawa A., Imai N., Kagami N., Matkovits A., et al. PACAP Stimulates Functional Recovery after Spinal Cord Injury through Axonal Regeneration. J. Mol. Neurosci. 2014;54:380–387. doi: 10.1007/s12031-014-0338-z. [DOI] [PubMed] [Google Scholar]
- 105.Armstrong B.D., Abad C., Chhith S., Cheung-Lau G., Hajji O.E., Coute A.C., Ngo D.H., Waschek J.A. Impairment of axotomy-induced pituitary adenylyl cyclase-activating peptide gene expression in T helper 2 lymphocyte-deficient mice. Neuroreport. 2006;17:309–312. doi: 10.1097/01.wnr.0000199465.54907.74. [DOI] [PubMed] [Google Scholar]
- 106.Kimura H., Kawatani M., Ito E., Ishikawa K. Effects of pituitary adenylate cyclase-activating polypeptide on facial nerve recovery in the guinea pig. Laryngoscope. 2003;113:1000–1006. doi: 10.1097/00005537-200306000-00016. [DOI] [PubMed] [Google Scholar]
- 107.Kimura H., Kawatani M., Ito E., Ishikawa K. PACAP facilitate the nerve regeneration factors in the facial nerve injury. Regul. Pept. 2004;123:135–138. doi: 10.1016/j.regpep.2004.04.020. [DOI] [PubMed] [Google Scholar]
- 108.Nishikawa T., Giardino I., Edelstein D., Brownlee M. Changes in diabetic retinal matrix protein mRNA levels in a common transgenic mouse strain. Curr. Eye Res. 2000;21:581–587. doi: 10.1076/0271-3683(200007)2111-ZFT581. [DOI] [PubMed] [Google Scholar]
- 109.Roy S., Maiello M., Lorenzi M. Increased expression of basement membrane collagen in human diabetic retinopathy. J. Clin. Invest. 1994;93:438–442. doi: 10.1172/JCI116979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Shimizu F., Sano Y., Haruki H., Kanda T. Advanced glycation end-products induce basement membrane hypertrophy in endoneurial microvessels and disrupt the blood-nerve barrier by stimulating the release of TGF-β and vascular endothelial growth factor (VEGF) by pericytes. Diabetologia. 2011;54:1517–1526. doi: 10.1007/s00125-011-2107-7. [DOI] [PubMed] [Google Scholar]
- 111.Castorina A., Giunta S., Mazzone V., Cardile V., D’Agata V. Effects of PACAP and VIP on hyperglycemia-induced proliferation in murine microvascular endothelial cells. Peptides. 2010;31:2276–2283. doi: 10.1016/j.peptides.2010.08.013. [DOI] [PubMed] [Google Scholar]
- 112.Rácz B., Gasz B., Borsiczky B., Gallyas F., Tamás A., Józsa R., Lubics A., Kiss P., Roth E., Ferencz A., et al. Protective effects of pituitary adenylate cyclase activating polypeptide in endothelial cells against oxidative stress-induced apoptosis. Gen. Comp. Endocrinol. 2007;153:115–123. doi: 10.1016/j.ygcen.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 113.Malcangio M., Tomlinson D.R. A pharmacologic analysis of mechanical hyperalgesia in streptozotocin/diabetic rats. Pain. 1998;76:151–157. doi: 10.1016/S0304-3959(98)00037-2. [DOI] [PubMed] [Google Scholar]
- 114.Németh J., Reglofdi D., Pozsgai G., Szabó Á., Elekes K., Pintér E., Szolcsányi J., Helyes Z. Effect of pituitary adenylate cyclase activating polypeptide-38 on sensory neuropeptide release and neurogenic inflammation in rats and mice. Neuroscience. 2006;143:223–230. doi: 10.1016/j.neuroscience.2006.07.028. [DOI] [PubMed] [Google Scholar]
- 115.Perrotti L.I., Hadeishi Y., Ulery P.G., Barrot M., Monteggia L., Duman R.S., Nestler E.J. Induction of deltaFosB in reward-related brain structures after chronic stress. J. Neurosci. 2004;24:10594–10602. doi: 10.1523/JNEUROSCI.2542-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wang H., Tao X., Huang S.T., Wu L., Tang H.L., Song Y., Zhang G., Zhang Y.M. Chronic stress is associated with pain precipitation and elevation in DeltaFosB expression. Front. Pharmacol. 2016;7:138. doi: 10.3389/fphar.2016.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Luis-Delgado O.E., Barrot M., Rodeau J.L., Ulery P.G., Freund-Mercier M.J., Lasbennes F. The transcription factor deltaFosB is recruited by inflammatory pain. J. Neurochem. 2006;98:1423–1431. doi: 10.1111/j.1471-4159.2006.03970.x. [DOI] [PubMed] [Google Scholar]
- 118.Heinricher M.M., Tavares I., Leith J.L., Lumb B.M. Descending control of nociception: Specificity, recruitment and plasticity. Brain Res. Rev. 2009;60:214–225. doi: 10.1016/j.brainresrev.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Gaszner B., Kormos V., Kozicz T., Hashimoto H., Reglodi D., Helyes Z. The behavioral phenotype of pituitary adenylate-cyclase activating polypeptide-deficient mice in anxiety and depression tests is accompanied by blunted c-Fos expression in the bed nucleus of the stria terminalis, central projecting Edinger-Westphal nucleus, ventral lateral septum, and dorsal raphe nucleus. Neuroscience. 2012;202:283–299. doi: 10.1016/j.neuroscience.2011.11.046. [DOI] [PubMed] [Google Scholar]
- 120.Maekawa F., Fujiwara K., Tsukahara S., Yada T. Pituitary adenylate cyclase-activating polypeptide neurons of the ventromedial hypothalamus project to the midbrain central gray. Neuroreport. 2006;17:221–224. doi: 10.1097/01.wnr.0000198945.62326.ba. [DOI] [PubMed] [Google Scholar]
- 121.Castorina A., Vogiatzis M., Kang J.W.M., Keay K.A. PACAP and VIP expression in the periaqueductal grey of the rat following sciatic nerve constriction injury. Neuropeptides. 2019;74:60–69. doi: 10.1016/j.npep.2018.12.002. [DOI] [PubMed] [Google Scholar]
- 122.Yamaoka S., Oshima Y., Horiuchi H., Morino T., Hino M., Miura H., Ogata T. Altered gene expression of RNF34 and PACAP possibly involved in mechanism of exercise-induced analgesia for neuropathic pain in rats. Int. J. Mol. Sci. 2017;18:1962. doi: 10.3390/ijms18091962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yokai M., Kurihara T., Miyata A. Spinal astrocytic activation contributes to both induction and maintenance of pituitary adenylate cyclase-activating polypeptide type 1 receptor-induced long-lasting mechanical allodynia in mice. Mol. Pain. 2016;12:1744806916646383. doi: 10.1177/1744806916646383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Fuchs D., Birklein F., Reeh P.W., Sauer S.K. Sensitized peripheral nociception in experimental diabetes of the rat. Pain. 2010;151:496–505. doi: 10.1016/j.pain.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 125.Randall L.O., Selitto J.J. A method for measurement of analgesic activity on inflamed tissue. Arch. Int. Pharmacodyn. Ther. 1957;111:409–419. [PubMed] [Google Scholar]
- 126.Bölcskei K., Helyes Z., Szabó Á., Sándor K., Elekes K., Németh J., Almási R., Pintér E., Petho G., Szolcsányi J. Investigation of the role of TRPV1 receptors in acute and chronic nociceptive processes using gene-deficient mice. Pain. 2005;117:368–376. doi: 10.1016/j.pain.2005.06.024. [DOI] [PubMed] [Google Scholar]
- 127.Kormos V., Gáspár L., Kovács L., Farkas J., Gaszner T., Csernus V., Balogh A., Hashimoto H., Reglodi D., Helyes Z., et al. Reduced response to chronic mild stress in PACAP mutant mice is associated with blunted FosB expression in limbic forebrain and brainstem centers. Neuroscience. 2016;330:335–358. doi: 10.1016/j.neuroscience.2016.06.004. [DOI] [PubMed] [Google Scholar]
- 128.Wallace D.L., Vialou V., Rios L., Carle-Florence T.L., Chakravarty S., Kumar A., Graham D.L., Green T.A., Kirk A., Iñiguez S.D., et al. The influence of ΔfosB in the nucleus accumbens on natural reward-related behavior. J. Neurosci. 2008;28:10272–10277. doi: 10.1523/JNEUROSCI.1531-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sterrenburg L., Gaszner B., Boerrigter J., Santbergen L., Bramini M., Elliott E., Chen A., Peeters B.W.M.M., Roubos E.W., Kozicz T. Chronic stress induces sex-specific alterations in methylation and expression of corticotropin-releasing factor gene in the rat. PLoS ONE. 2011;6:e28128. doi: 10.1371/journal.pone.0028128. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available in the article, there is no supplementary data.