Abstract
Terminal respiratory oxidases are highly efficient molecular machines. These most important bioenergetic membrane enzymes transform the energy of chemical bonds released during the transfer of electrons along the respiratory chains of eukaryotes and prokaryotes from cytochromes or quinols to molecular oxygen into a transmembrane proton gradient. They participate in regulatory cascades and physiological anti-stress reactions in multicellular organisms. They also allow microorganisms to adapt to low-oxygen conditions, survive in chemically aggressive environments and acquire antibiotic resistance. To date, three-dimensional structures with atomic resolution of members of all major groups of terminal respiratory oxidases, heme-copper oxidases, and bd-type cytochromes, have been obtained. These groups of enzymes have different origins and a wide range of functional significance in cells. At the same time, all of them are united by a catalytic reaction of four-electron reduction in oxygen into water which proceeds without the formation and release of potentially dangerous ROS from active sites. The review analyzes recent structural and functional studies of oxygen reduction intermediates in the active sites of terminal respiratory oxidases, the features of catalytic cycles, and the properties of the active sites of these enzymes.
Keywords: membrane proteins, terminal oxidases, cytochrome oxidase, cytochromes, proton pump, electrogenic mechanisms, reactive oxygen species
1. Introduction: General Properties of Terminal Respiratory Oxidases
The membrane-embedded terminal respiratory oxidases include two main groups of functionally similar, but structurally and evolutionarily strikingly different superfamilies: heme-copper oxidases and bd-type cytochromes. The superfamily of heme-copper oxidases (HCOs) includes cytochrome oxidases (COXs) of mitochondria from higher and lower eukaryotes, HCOs of most aerobic prokaryotes, and structurally related NO reductases [1]. A distinctive feature of these enzymes is the presence of catalytic binuclear center (BNC) consisting of closely located iron ion of the heme group and copper ion (non-heme iron ion in NO reductases) [2]. The bd-type cytochrome superfamily members have to date been found only in Bacteria and Archaea [3,4,5,6].
HCOs catalyze the transfer of electrons from quinols or cytochromes to oxygen with the formation of water coupled to the generation of proton motive force [2,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22]. In contrast to bd-type oxidases, HCOs generate the proton motive force not only by the transfer of electrons and protons to the catalytic center from different sides of the membrane but also due to the unique ability for redox-coupled directed proton pumping through the membrane [23]. NO reductases, which are structurally similar to HCOs, reduce NO to N2O and are utilized by a number of pathogenic bacteria for denitrification under microaerobic and anaerobic conditions that occur in many host tissues [24].
The stoichiometric reactions for a number of members of HCO and bd oxidases are given below. They differ in the electron donor in the oxygen-reductase reaction, the presence of protons pumped through the membrane, and their number per 1 electron.
(1) aa3-type heme-copper oxidase from Rhodobacter sphaeroides (A family)
4 cyt c2+ + 8H+in + O2 → 4 cyt c3+ + 2H2O + 4H+out
(2) ba3-type heme-copper oxidase from Thermus thermophilus (B family)
4 cyt c2+ + (4 + n) H+in + O2 → 4 cyt c3+ + 2H2O + (n) H+out
(3) bo-type heme-copper oxidase from Escherichia coli (A family)
2QH2 + 8H+in + O2 → 2Q + 2H2O + 8H+out
(4) bd-type oxidase
2QH2 + 4H+in + O2 → 2Q + 2H2O + 4H+out
Where cyt c is cytochrome c; QH2 and Q are two-electron-reduced and oxidized forms of quinone (ubiquinone or menaquinone), respectively [12,25]; n~2–4 is the amount of the pumped protons in the B family HCOs [26,27]; H+in and H+out are the protons taken up from the N phase and released to the P phase, correspondingly.
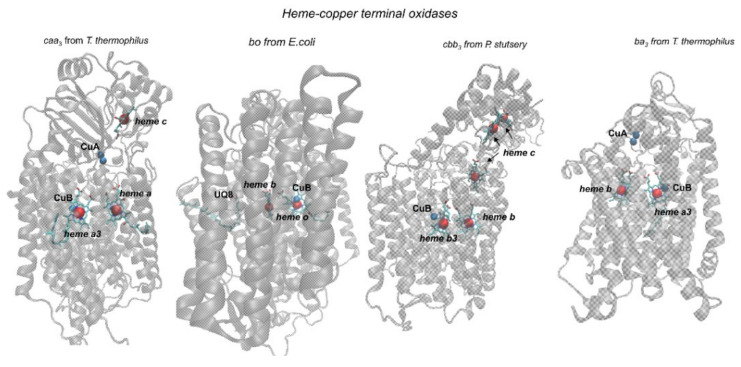
Mammalian mitochondrial cytochrome oxidase contains 13 subunits; the three largest subunits are encoded by the mitochondrial genome. They form the catalytic core of the enzyme and are homologous to the three main subunits found in most typical prokaryotic cytochrome oxidases of the A family. The first subunit contains three redox centers: low-spin heme a and oxygen reductase center (BNC) consisting of a closely located iron atom of the high-spin heme a3 and copper ion (CuB). In prokaryotic HCOs, hemes a and a3 can be replaced with b-, o-, and c-type hemes. The covalently bound c-type hemes can also serve as additional redox centers, for example, in caa3 or cbb3 oxidases (Figure 1).
Figure 1.
Overall structures of heme-copper cytochrome and quinol oxidoreductases from different families: caa3 from T. thermophilus ([28], PDB ID: 2YEV, A2 family), bo from E. coli ([29], PDB ID: 6WTI, A family), cbb3 from P. stutzeri ([30], PDB ID: 5DJQ, C family) and ba3 from T. thermophilus ([31], PDB ID: 5NDC, B family). The figure was carried out using Visual Molecular Dynamics software (v1.9.3) [32].
A bd-type cytochrome consists of two to four subunits [3,33,34,35]. The first subunit of the bd oxidase carries a low-spin heme b558 that directly accepts electrons from quinol, and a di-heme active site (DHAS) that further receives the electrons from b558 [36,37,38,39,40,41,42,43,44,45,46,47,48,49,50]. In contrast to heme-copper oxidases, bd-type cytochromes do not contain copper ions. Despite this, they catalyze the oxygen-reductase reaction with very high efficiency. The DHAS of the bd enzyme is formed by two high-spin hemes, d and b595 which are in van der Waals contact [33,34,35]. There are cases when heme d is replaced by a b-type heme [4,51]. In the case of cytochrome oxidases, the introduction of electrons into the enzyme occurs from the water-soluble cytochrome c through the second copper center CuA, consisting of two copper atoms. In the case of heme-copper quinol oxidases, electrons are introduced in the same way as in bd oxidases, through a special site of binding and oxidation of quinol (Figure 1). In the case of caa3 and cbb3 oxidases, the reduction in CuA by water-soluble cytochrome c is preceded by an intermediate reduction in an additional c-type heme redox center(s) (Figure 1). The feature which is used for the classification of HCOs and NO reductases is organization of the intraprotein proton transfer pathways (“channels”) that connect the catalytic center with the membrane cytoplasmic side and ensure the transfer of substrate protons required for water formation, as well as the transfer of protons pumped through the membrane. Based on the structure of these pathways, HCOs are divided into the three main families A, B, and C [52,53,54].
The three-dimensional structure of typical members of the A family has been established by X-ray analysis with a resolution reaching in some cases 1.5 Å. These are COX from bovine heart muscle mitochondria [55,56,57,58], bacterial aa3-type COX enzymes from Paracoccus denitrificans [59] and R. sphaeroides [60,61], bo3-type quinol oxidase from E. coli [29,62], cytochrome caa3 from T. thermophilus [28] (Figure 1). Despite the high resolution, the nature of the ligand in the active site of the crystallized enzyme (peroxide, superoxide, chlorine ion, or hydroxyl anion) is still a matter of debate [63]. For some enzymes, the structures of the oxidized and reduced enzyme form, enzyme complex with external ligands CO, CN– [64] and N3–, the several mutant forms and P and F intermediates [57] of the catalytic cycle have been resolved. Using electron microscopy, the mitochondrial respiratory supercomplex (respirasome) composed of the complexes I, III, and IV (COX) with the CICIII2CIV stoichiometry have been resolved [65]. At the same time, two-dimensional electron crystallography has shown that the monomeric form of mammalian COX is functionally active and not an artifact of enzyme purification from the mitochondrial membrane [66]. Despite clarification of the general enzyme topography, a detailed picture of the elementary processes of the transformation of oxygen atoms in the BNC and coupled charge transfer at the molecular level is only beginning to emerge [16,21,67].
A characteristic feature of the A family COX enzymes is the presence of two proton-conducting pathways (D and K channels) originating at the cytoplasmic side of the membrane and formed by highly conserved proton-exchanging groups [68]. According to the data presented in [7,68,69,70,71,72], these pathways are used to ensure the coupled transfer of protons from the cytoplasmic side of the membrane to the catalytic center (the so-called substrate protons, or protons of the substrate) and to the periplasmic space (the so-called pumped protons, or protons transferred through the membrane). Based on the model formulated in the course of studies of aa3 oxidases from P. denitrificans and R. sphaeroides, the K channel serves to transfer some of the substrate protons in the catalytic cycle, while protons pumped through the membrane and the remaining substrate protons are transferred through the D channel.
The three-dimensional structure of representatives of families B and C is also established (Figure 1) [28,29,30,31]. In the oxidases of the B and C families, only the homolog of the K channel is detected, which presumably serves the transfer of both substrate and pumped protons. The mechanism of proton transfer through this channel in these oxidases remains poorly understood ([73] and references therein). In experiments of different types with family B oxidase, variability in the stoichiometry of proton pumping (0.5–0.85 H+/e−) is observed. It is assumed that the membrane potential resulting from the operation of the enzyme can lead to a slip of the proton pump at certain stages of the catalytic cycle [27,73]. Theoretically, there is reason to believe that there is a similar decrease in the stoichiometry of proton pumping in the C family oxidase [74]. In addition to the D and K channels, another proposed proton pathway (H channel) was found in heme-copper oxidases of eukaryotes. Based on the structural data on the COX from mitochondria, it is assumed that it can serve for the transfer of protons pumped through the membrane, controlled by redox transformations of low-spin heme (heme a) ([58] and references therein). However, the role of the H channel in proton pumping is questioned in experiments with mutant forms on residues in the D, K, and H channels of cytochrome oxidase from lower eukaryotes (yeast) [75]. Thus, in fact, the functional role of the H channel remains unclear.
The classification of the bd-type cytochromes adopted until now is based on the size of the hydrophilic region between transmembrane helices 6 and 7 in subunit I, a binding domain for quinol oxidation designated as the Q-loop. Accordingly, the enzymes are divided into two subfamilies: L (long Q-loop) and S (short Q-loop) [76,77]. Very recently, Murali et al. [4] using phylogenomics identified three families and several subfamilies within the cytochrome bd superfamily. According to this classification, all the superfamily members share four transmembrane helices that bind two hemes comprising the catalytic active site. Only one of the three families possesses a conserved quinol binding site. Members of the other two families use cytochrome c and possibly another electron donor different than quinol [4]. Notwithstanding, to date all the biochemically characterized bd-type cytochromes appeared to be quinol oxidases [14,78,79,80]. Further research is required to obtain and characterize a bd enzyme that would use an electron donor other than quinol.
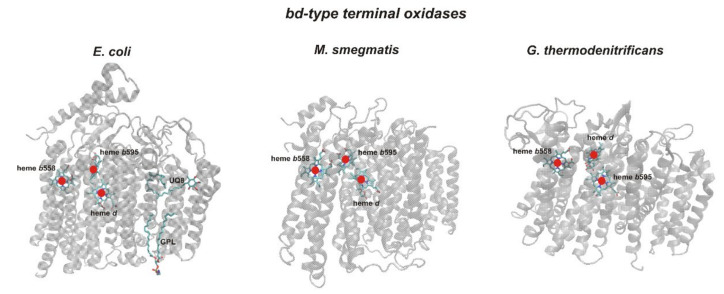
The structures of bd-type cytochromes from three different bacterial species determined at 2.7–3.3 Å resolution have been published by now. These are the crystal structure of the Geobacillus thermodenitrificans enzyme [33], and the single-particle cryoelectron microscopy (cryo-EM) structures of cytochromes bd from E. coli [34,35] and Mycobacterium smegmatis [81]. The structures show that the enzymes have a similar overall architecture. All structures are characterized by a triangular arrangement of the hemes b558, b595, and d. However, in the oxidases from E. coli and M. smegmatis, hemes d and b595 forming the DHAS are found in a ‘switched’ position with respect to the G. thermodenitrificans enzyme (Figure 2). Furthermore, the bd enzymes from the three bacteria differ in oxygen pathways that they may use. In the E. coli oxidase, it is a small hydrophobic channel that allows O2 to diffuse from the membrane interior to heme d positioned at the center of subunit I (O2 channel 1) [34,35]. In the G. thermodenitrificans enzyme, heme d located closer to the extracellular site is directly connected to the protein surface by an accessible oxygen pathway (O2 channel 2) [33]. The M. smegmatis cytochrome bd may utilize both oxygen pathways, channels 1 and 2, indicating that heme b595 located closer to the periplasmic side could be the second O2-reduction site [81]. It was also reported that in the respiratory chain of E. coli, the bd oxidases can be part of supercomplexes [9]. Cytochrome bd-I together with formate dehydrogenase and cytochrome bo forms a formate:oxygen oxidoreductase supercomplex in a 1:1:1 stoichiometry. In turn, a succinate:oxygen oxidoreductase supercomplex is composed by cytochrome bd-II and succinate:ubiquinone oxidoreductase of unknown stoichiometry [9].
Figure 2.
Overall structures of bd-type oxidases from E. coli ([35], PDB ID: 6RX4), M. smegmatis ([81], PDB ID: 7D5I), and G. thermodenitrificans K1041 ([33], PDB ID: 5DOQ). The three hemes in all structures are in a triangular arrangement but positions of heme b595 and heme d in the enzymes from E. coli and M. smegmatis are interchanged as compared to those in the G. thermodenitrificans oxidase. The figure was carried out using Visual Molecular Dynamics software (v1.9.3) [32].
Cytochrome bd has at least one proton-conducting pathway that connects the cytoplasm to the oxygen reducing center in subunit I [33,34,35,81,82,83]. The structures of cytochromes bd from G. thermodenitrificans and M. smegmatis indicate a second putative proton transfer pathway in subunit II [33,81]. The pathway leads from the cytoplasm to the DHAS. Whether this pathway is functional is not known yet.
Based on general considerations, the organization of the proton-conducting input pathways in bd-type oxidases should be simpler since the major function of the A family HCOs is a generation of the proton motive force whereas the bd enzymes do not pump additional protons across the membrane. Instead, cytochromes bd play essential roles in bacterial physiology and pathogenesis [14,80,84,85,86,87,88]. The mechanism of transfer of pumped protons along the same pathway (at least a significant part of it) as is used for the transfer of substrate protons (D channel in the A family oxidases; K channel in oxidases of the B and C families) requires a special device to prevent access of pumped protons to the oxygen-reductase center. Although there is no such requirement in the case of bd-type oxidases, the pathway for the release of protons leaving the quinol oxidation site must certainly be isolated from the pathways for proton transfer to the oxygen-reductase center of cytochrome bd. Only in this case, the bd enzyme is able to generate a proton motive force during catalysis, in accordance with the experimental data [82,83,89,90,91]. Indeed, this is confirmed by structural data [33,34,35,81]. It should also be noted that this requirement must also be met in the case of the quinol-binding site of the bo oxidase and the pathways for the pumped proton to exit to the outer side of the membrane in heme-copper cytochrome c oxidases [29,92].
In accordance with the important physiological role of the bd-type oxidases, there is accumulating evidence that cytochromes bd make bacteria resistant to nitric oxide (NO) [93,94,95,96,97,98,99,100,101,102], peroxynitrite [85,103], H2O2 [14,104,105,106,107], cyanide [108,109,110], sulfide [110,111,112,113], and ammonia [114]. The ability of the bd oxidases to degrade these hazardous compounds is provided not only by the efficiency of their binding to the reaction center and the transfer of electrons to it, but also by the supply of protons through the proton-conducting input pathways. The features of these side reactions, despite their importance, remain largely unexplored. Since the bd oxidases are absent in humans and animals, it seems promising to use them as protein targets for new antibiotics [115,116,117,118,119,120].
2. States of Active Sites as Intermediates of the Catalytic Cycle of Oxygen Reduction
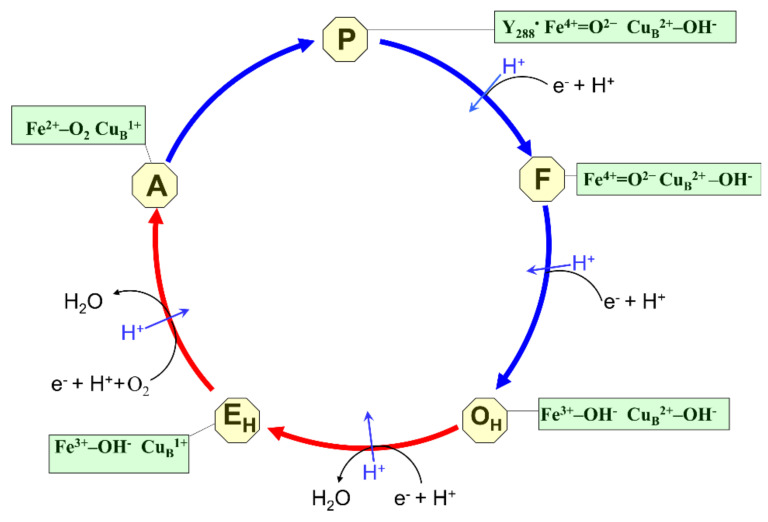
The catalytic cycle of COX consists of two half-reactions (reductive and oxidative), each including two single-electron transitions (Figure 3). The reductive phase in the newly oxidized “unrelaxed” enzyme (OH state) involves the sequential transfer of two electrons through the input redox centers to the catalytic center, towards heme a3 and CuB (OH→EH and EH→R transitions, respectively). As a result, the BNC is reduced from the fully oxidized state (OH) to the reduced state (R, not shown in Figure 3) and acquires the ability to bind molecular oxygen (see [2,121] and references therein).
Figure 3.
Catalytic cycle of the A family heme-copper oxidases. Shown are catalytic intermediates (OH, EH, A, P, F), and the structure of the binuclear heme-copper active site for each intermediate. The cycle can be divided into two parts, reductive and oxidative. The reductive part comprises transitions from OH to A. The oxidative part comprises transitions from A to OH. Pumped protons are shown by the blue arrows.
The oxidative phase begins with the oxygen molecule binding to the central iron atom of the high-spin heme a3 in the reduced BNC. As a result, the primary diatomic oxygen adduct is formed (state A), which is a mixture of Fe2+–O2 and Fe3+–O2− states. At the next stage, the O–O bond is cleaved, and compound P is formed, for which four electrons and a proton are simultaneously transferred from the active center to the O2 molecule. Three electrons result from the oxidation of the Fe2+ ion of heme a3 to the oxoferryl state (Fe4+= O2−) and CuB+ oxidation to CuB2+. The fourth electron and the proton come from the closely located conserved tyrosine residue (Y288 in the aa3 oxidase from R. sphaeroides) that forms a covalent bond with one of the histidine ligands of CuB during protein post-translational modification [122,123,124].
Two electron vacancies arising in the COX catalytic center are filled by the transfer of the third and fourth electrons from cytochrome c molecules. The transfer of the third electron from cytochrome c to Y288 leads to the formation of the F state of the BNC in the COX catalytic cycle. The transfer of the fourth electron from cytochrome c to heme a3 reduces the oxoferryl state of heme a3 into the OH oxidized state.
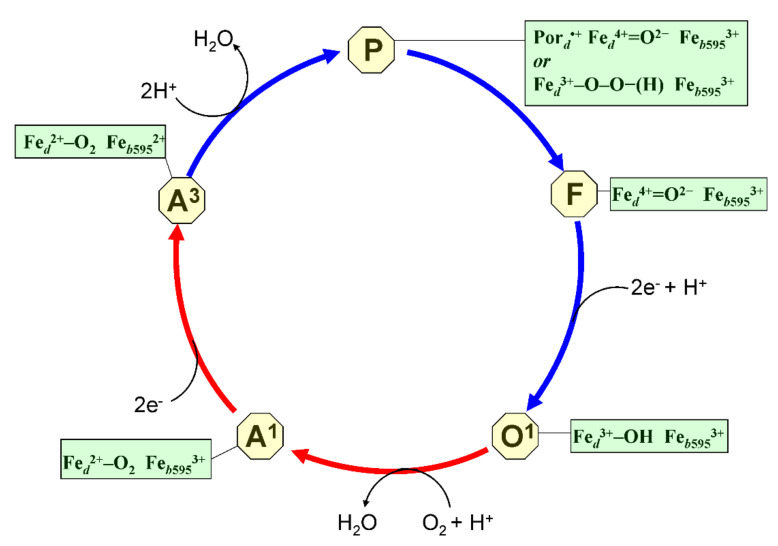
The proposed catalytic cycle of cytochrome bd is shown in Figure 4. It can also be divided into two half-reactions, reductive and oxidative. Quinol is involved in the cycle as a two-electron-donating substrate [125]. The reductive part comprises two successive transitions. The first, O1→A1 transition includes the intramolecular transfer of an electron from the input redox site (heme b558) to heme d with concomitant binding of O2 to the latter heme. Presumably, in this transition, a proton is also transferred to the hydroxide ligand of heme d to release a water molecule. The second, A1→A3 transition involves the transfer of two electrons from the first quinol molecule to hemes b558 and b595. As a consequence, the fully oxidized DHAS becomes fully reduced with O2 bound to heme d.
Figure 4.
Catalytic cycle of bd-type oxidase. Shown are catalytic intermediates (O1, A1, A3, P, F), and the proposed structure of a di-heme active site for each intermediate. The cycle can be divided into two parts, reductive and oxidative. The reductive part comprises transitions from O1 to A3. The oxidative part comprises transitions from A3 to O1.
The first reaction of the oxidative part of the cycle is A3→P transition [89]. The structure of compound P is controversial. The O–O bond splitting at this stage has either already happened or not yet. In the latter case, P is a true peroxy complex of heme d (Fed3+–O−–O−– (H+)). In the former case, the concerted transfer of four electrons from the DHAS to the bound O2 molecule is required. Three electrons come from the ferrous heme d. This converts heme d (Fed2+–O2) into the oxoferryl state (Fed4+ = O2−) with a porphyrin π-cation radical. The fourth electron is provided by the ferrous heme b595 [89,126]. Two protons could also be transferred to the oxygen molecule to produce one more water molecule. If P is a compound I intermediate, the next stage involves the radical quenching by the transfer of an electron from the ferrous heme b558 (P→F transition). The oxidative part of the reaction ends with the transfer of two electrons from the second quinol molecule and probably a proton to the DHAS (F→O1 transition). This reduces the oxoferryl state of heme d into the ferric state with a bound hydroxide ligand. The DHAS becomes completely oxidized whereas the input redox site, heme b558, gets reduced. The P→F and F→O1 transitions are coupled to membrane potential generation [82,83,91]. The proposed formation of the bound hydroxide ligand and water molecules in the above-mentioned transitions still awaits experimental support.
2.1. O/OH
In the absence of electron donors, the oxidized “unrelaxed” OH state is spontaneously converted into the oxidized stable state (O) within the second time scale. This process is accelerated at acidic pH and it is believed that the oxidized fast state of COX can only exist at alkaline pH. The O and OH states of the A family cytochrome oxidase differ in their affinity for electrons of the corresponding redox centers and in the ability to perform transmembrane proton transfer [127]. In contrast to the O resting state, injection of the electron into the just oxidized state (OH) of the A family aa3 cytochrome oxidase from P. denitrificans which is generated upon oxidation of the fully reduced enzyme by O2, results in the rapid electron transfer into the BNC, to CuB. Besides, the electron injection into the OH intermediate of the cytochrome oxidase is coupled to pumping of a proton through the membrane (Figure 3) [127,128].
Several experimental investigations on different A family cytochrome c oxidases, designed to prepare the OH state, could not find an increase in the CuB(II) midpoint potential as compared to the resting O state, due most likely to the extreme reactivity and short lifetime of the OH state [129,130,131]. At the same time, for the B family cytochrome oxidase ba3 from T. thermophilus, the existence of high and low-energy forms of the oxidized enzyme, which differ in the redox properties of heme centers, is also shown [27,121,127,132,133,134,135].
The 3D structure of the oxidized COX of the A family was solved for the mitochondrial and bacterial enzyme, for the crystals obtained at pH below 7 [59,136]. This structure corresponds to the acidic ‘‘slow’’ form of the enzyme. In this form, Tyr288 is proposed to be protonated. Meanwhile, the ‘‘fast’’ form of the enzyme requires pH 8 or higher. The structural differences between these two states remain to be investigated and are believed to be related to the ligand sphere of CuB [137]. Recently the structure at pH 7.3 was solved [138]. No structural differences between crystals obtained at the neutral and acidic pH were detected within the molecules. Structure at alkaline pH has not yet been obtained. Recently, the redox sensitivities of the new Raman marker band were observed which suggest that a radical Tyr288 is present in the fast form and a protonated Tyr288 in the slow form [63]. This is consistent with theoretical works [139,140] that in the OH state, the electron is shifted from Tyr to CuB, and the tyrosine is deprotonated in the form of a radical.
2.2. O/O1
The O1 state in cytochrome bd was first detected at steady-state in the presence of oxygen and ubiquinol by using stopped-flow spectrophotometry [125]. It was shown that under steady-state conditions, the O1 catalytic intermediate is populated up to 20%. Importantly, the O species was reported not to be an intermediate of the bd catalytic cycle [141]. The O species can be generated in vitro by incubating the “as isolated” cytochrome bd with excess lipophilic oxidant [38,97,114,142]. An apparent difference between O1 and O is the presence of an electron on the input redox site, heme b558 in O1, whereas the DHAS in both states is fully oxidized. Whether there is any difference in redox potentials of the hemes constituting the DHAS between O1 and O is not yet known. It is worth mentioning that there is significant redox interaction between heme b558 and heme b595 [44]. The O1 and O states in cytochrome bd could be analogs of OH and O in COX.
2.3. E/EH
The EH state, in contrast to the E state, is high-energy and occurs only during single-electron reduction from OH. The single-electron reduction in EH is presumably accompanied by the pumping of a proton through the membrane. Time-resolved experiments showed that there is a difference in the electron redistribution between E and EH states. In the E state, the electron equivalent is distributed among heme a, heme a3 and CuB, whereas in the EH state formed by electron injection into the OH state the electron is located exclusively on CuB ([134] and references therein).
The redox potential of heme a3 (the electron acceptor in the E state) is too low (~0.3 V) to provide proton pumping [143]. It is suggested that the E intermediate is formed in an ‘‘activated’’ form EH in which the proton is not on tyrosine but in the center of the BNC forming the second water molecule [144]. Thus, the EH state mixes the low Fea3(III) reduction potential with the higher potential of the tyrosyl radical. The transition from EH to E is presumably accompanied by the transfer of an electron to the tyrosine radical and the protonation of tyrosine. In contrast to OH, in the E state, the tyrosine residue is protonated and is no longer in the form of a radical, but in a reduced state [139,140]. In the EH state, the tyrosine residue is either in the form of a radical or it is a mixture of a tyrosine anion and a radical.
In cytochrome bd, E/EH (one-electron-reduced) is not a catalytic intermediate. The E state can be produced in vitro by deoxygenation of the “as isolated” cytochrome bd under anaerobic conditions, purging the sample repeatedly with argon gas with the aid of the vacuum/gas line [82,145,146]. This turned out to be possible because in the bd oxidase isolated under aerobic conditions, the one-electron-reduced A1 species predominates [142]. In the oxygen-free E state of cytochrome bd, the electron is located predominantly on heme d.
2.4. R/R3
In contrast to the structure of the oxidized cytochrome oxidase, the structure of the reduced enzyme lacks the oxygen ligands of heme a3 and CuB [61]. In addition, the hydrogen bond between Tyr288 and the hydroxyethylfarnesyl side chain in the porphyrin ring of heme a3 disappears, which is believed to open the possibility of proton transfer to the active center through the K channel to break the O–O bond.
R3 is not an intermediate of the cytochrome bd catalytic cycle. The R3 (three-electron-reduced) state can be obtained in vitro by incubating the bd enzyme with excess reductant under anaerobic conditions.
2.5. A and Ip
Using the CO flow-flash method it was shown that in the A family bo3 oxidase from E. coli, the 42 μs lifetime of the first step (R→A) at 625 μM O2 corresponds to a second-order rate constant of ∼3.8 × 107 M−1 s−1 for O2 binding. This rate is ∼2.5 times slower than that observed in the bovine and R. sphaeroides aa3 enzymes which also belong to the A family [147]. Reduced CuB is likely to form a transient O2 binding site on the way of the oxygen molecule from the membrane via the oxygen channel and into the a3/CuB cavity. Transient binding of oxygen to CuB in the A family oxidases is likely to be weak. It is stronger in the B family oxidases like ba3 from T. thermophiles [26,148]. In contrast to COX, heme b595, somewhat analogous to CuB, seems not to be a transient O2 binding site on the way of the ligand from outside to heme d [41].
The 3D structures of the reduced COX with CO, NO, or CN- respiratory inhibitors, which are competitive inhibitors of O2, were used to elucidate characteristics of the A state (the oxy-complex) that is too unstable to be crystallized [21]. The X-ray structures of NO and CO derivatives provided a structural basis for the bend end-on binding of O2 to Fe of heme a3 and the structure of the A state as a mixture of Fe2+–O2 and Fe3+–O2– states. This strongly suggests the remarkable stability of the A intermediate and extremely low availability of the second electron equivalent to the bound O2 from CuB. On the opposite, the X-ray structure of the fully reduced cyanide anion bound form indicates that cyanide binding significantly influences the CuB coordination structure. This structure suggests that Fe3+–O2− induces coordination in the BNC site to form three possible electron-transfer pathways, each transferring one electron equivalent, for the nonsequential three electron reduction in the bound O2. These pathways are as follows: from Fe of heme a3; from CuB; and from Y288-OH via a water molecule (W510). W510 is not a product of the enzymatic reaction but a cofactor stored in a water storage site near the O2-reduction site [21,149].
This X-ray structural analysis confirmed earlier theoretical work [150] which suggested that the O–O bond cleavage proceeds through the two-step mechanism. It is initiated by proton transfer from the cross-linked tyrosine, via one or two water molecules (W510 in the structure) to the superoxide, to form the type of peroxide intermediate (Ip), Fea3(III)OOH– CuB(II). The proton transfer is coupled to an electron transfer from the CuB-tyrosine complex. In the second step, the O–O bond is cleaved and the PM intermediate is formed, with Fea3(IV) = O2− plus CuB(II)OH– TyrO* [151,152,153]. Since both an electron and a proton move from the tyrosine in the same direction and distance to the oxygen atom, the A→PM transition does not generate a transmembrane voltage and virtually no phase of potential generation during R→A→PM steps was obtained using the electrometric technique [154] (Figure 3).
The Ip intermediate was experimentally resolved in a recent study on the B family cytochrome oxidase (ba3 from T. thermophilus) at low temperature and pH. Electron transfer from the low-spin heme b to the catalytic site was shown to be faster by a factor of ~10 (τ ~ 11 μs) than the formation of the PR ferryl (τ ~ 110 μs). This indicates that O2 is reduced before the splitting of the O–O bond [152].
The significantly faster O2 binding and the O–O bond cleavage in the T. thermophilus ba3 as compared to analogous steps in the aa3 oxidases could reflect evolutionary adaptation of the ba3 enzyme to the microaerobic conditions of the T. thermophilus HB8 species [155]. In evolutionarily distant bd oxidases, nevertheless, the same problem is solved-survival at low oxygen levels.
2.6. A1 and A3
Unlike COX, the presence of a single electron in the DHAS of cytochrome bd is sufficient to form a stable globin-like oxy-complex. The likely reason for this is the high oxygen affinity of ferrous heme d, at least in E. coli and Azotobacter vinelandii [145,146]. Significant amounts of A1 are observed in the bd-containing bacterial membranes and the isolated enzyme, as evidenced by an absorption band near 650 nm typical of the heme d oxy-complex [142,156,157]. Stopped-flow experiments showed that at steady-state about 40% of cytochrome bd is in the A1 form [125].
Transient formation of A3 was registered by the flow-flash method with a microsecond time resolution [89,146,158]. The fully reduced CO-bound cytochrome bd was photolyzed in the presence of O2. The flash-induced absorption changes associated with the reaction of oxygen with the oxidase were detected. The rate of the A3 production depends linearly on the O2 concentration giving a second-order rate constant kon of ~2 × 109 M−1s−1. The formation of A3 from the fully reduced enzyme is not electrogenic [82,83,89,91].
The available structures show that the bd enzymes from G. thermodenitrificans and E. coli use different pathways for oxygen delivery [33,34,35]. This is likely due to the fact that the O2-binding site, heme d, is located closer to the periplasmic side in the G. thermodenitrificans structure, whereas in the E. coli structure it is positioned approximately in the middle of the membrane. As a result, in the former structure, O2 can get access to heme d via an oxygen entry site located laterally at a short distance from the heme. There is no need for a functionally conserved protein cavity for the gaseous substrate to bind to heme d [33]. In the latter structure, the oxygen pathway is parallel to the membrane plane and connects the lipid interface to heme d [34,35]. It is provided by a specific hydrophobic channel that starts near Trp63 of CydB and extends towards heme d on CydA.
2.7. P and F
Similar to peroxidases, the reduction of the state P (analog of compound I) by two electrons through the intermediate state F (analog of compound II) converts the enzyme into the oxidized state OH (third and fourth electrons in the catalytic cycle). The P and F states of cytochrome oxidase contain heme iron a3 in the ferryl-oxo state. An essentially identical A 1.70 Å Fe–O distance of the ferryl center of heme a3 which was resolved in the X-ray structures of catalytic intermediates (P and F) could best be described as Fea34+ = O2− rather than Fea34+–OH− [57]. In that work they found an interstitial water molecule that could trigger a rapid proton-coupled electron transfer from tyrosine-OH to the slowly forming Fea33+–O−–O−–CuB2+ state preventing its detection, consistent with the unexpected Raman results.
The state P differs from F by an additional oxidative vacancy located on the aromatic residue near the binuclear center, covalently bound to one of the His ligands of CuB. This aromatic residue, tyrosine Y288, is suggested to provide the fourth electron during the cleavage of the O–O bond. The tyrosine radical Y288 is able to exchange with other aromatic residues (for example, W236), but at what rate this occurs and which radical is the primary one is not fully understood [152].
Flow-flash method in combination with electrometric or spectroscopy technics allows us to follow the charge transfer steps during the catalytic cycle of the proton-motive force generators [159,160,161,162,163]. In the case of the caa3 oxidase from T. thermophilus which has five electron equivalents in the reduced state, the electrogenic component with τ ~ 50 μs is associated with the PR→F transition. Together with the previous reaction step (A→PR) this is coupled to the translocation of about two charges across the membrane. The three subsequent electrogenic phases, with time constants of ~0.25 ms, ~1.4 ms, and ~4 ms, are linked to the conversion of the binuclear center through the F→OH→EH transitions, and result in the additional transfer of four charges through the membrane dielectric [134,135].
Earlier, using electrometric techniques and direct pH measurements of the A family aa3 oxidases, it was also shown that the formation of the F state from the P state and the F to OH transition are coupled to the pumping of protons across the membrane [164,165,166,167,168,169,170] (Figure 3). Each of these transitions is associated with the absorption of two protons from the internal solution of proteoliposomes with incorporated cytochrome c oxidase and the release of one proton into the external bulk phase. The EH→R transition is the least explored with a time resolution and consequently less understood step of the catalytic cycle due to technical difficulties with stabilization of cytochrome c oxidase in this state. Assuming that the OH→EH, F→O, and PM→F transitions are coupled to the pumping of approximately one proton, the resting forth pumped proton should be associated with the EH→R transition [171]. Thus, the experiments performed with a microsecond time resolution directly indicate that each of the four one-electron transitions in the catalytic cycle of the A family cytochrome c oxidase (highly effective generator of a proton motive force) is coupled to the pumping of one proton through the membrane (Figure 3) [69,70,134,135,166,171,172,173,174]. At the same time, in the B family oxidases, some time-resolved one-electron transitions (OH→EH [27] and P→F [26] or F→O, depending on the interpretation [19]) are not associated with the transfer of the pumped proton that explains the decrease in pumping stoichiometry in these oxidases (~0.5 H+/e vs. 1 H+/e) observed under certain conditions in stationary measurements.
In cytochrome bd, P was discovered as a short-lived catalytic intermediate in-between A3 and F in the flow-flash kinetic experiments [89]. It is formed from A3 nonelectrogenically with τ of 4–5 μs at 21 °C [89,90]. The chemical structure of P is still a matter of debate. In the original paper, Belevich et al. [89] suggested that P is either a true peroxy complex (analog of compound 0) or oxoferryl species with a radical on the porphyrin ring or an amino acid residue (analog of compound I). The authors leaned towards the former possibility because the radical formation would be energetically unfavorable in the presence of the reduced heme b558 in the proximity of the DHAS. Later, Paulus et al. using ultra-fast freeze-quench trapping followed by EPR and absorption spectroscopy reported that P is a heme d oxoferryl porphyrin π-cation radical intermediate that magnetically interacts with heme b595 [126]. It has to be noted that Paulus et al. performed the experiments at non-physiological, lower temperatures. It cannot be excluded that “P” observed by Belevich et al. is a mixture of the peroxy and oxoferryl cation radical species provided that they have similar spectral characteristics. Whatever the P intermediate is, at the next catalytic step it decays into a nonradical F species (analog of compound II), concomitant with the oxidation of heme b558. This transition occurs with τ of 47 μs (at 21 °C) and is coupled to the membrane potential generation [89,90]. The F species absorbing maximally at 680 nm is persistently seen in the static spectrum of the “as-prepared” cytochrome bd and was first identified as oxoferryl (Fed4+=O2−) by resonance Raman spectroscopy [175]. At steady-state the F species is populated up to 40% [125].
3. Noncatalytic States of Active Sites That Occur When External Ligands Are Added: Heme-Copper, bd
Due to the fact that the active centers of terminal oxidases are able to interact with a wide variety of external ligands, we consider only a few examples illustrating this variety.
The states of the binuclear center, similar to the states P and F, can also be obtained in the reaction of an oxidized cytochrome oxidase of the A family with H2O2. It is possible to solve the kinetics of the formation of intermediates P and F in the reaction with peroxide using the fast mixing method. For example, the first intermediate of caa3 cytochrome oxidase from T. thermophilus is formed with a bimolecular constant of 1540 M−1s−1 and has characteristics in the visible region and Soret band similar to the P intermediate resolved in the kinetics of enzyme oxidation in the single-turnover mode [134]. The second intermediate is formed with a constant of 21.5 M−1s−1 and has spectral characteristics similar to the intermediate F. The addition of the second molecule of H2O2 converts P to the F state. It is suggested that in presence of peroxide, the P-to-F transition is due to the binding of H2O2 to CuB triggering a structural change together with the uptake of H+ at the catalytic center, complemented with the annihilation of Y288 radical by the intrinsic oxidation of the enzyme [176].
In contrast to the A family oxidases, the binding of peroxide to the B family oxidases in a fully oxidized state is not observed in similar experiments. It is assumed that the BNC is in a closed state and peroxide begins to bind only when the enzyme is reduced by a single electron [132].
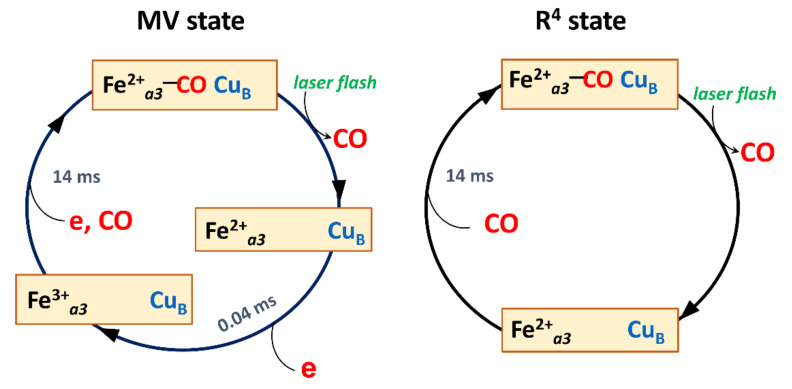
The interaction of the reduced BNC with CO in heme-copper oxidases allows us to study the kinetics of the ligand migration to the binuclear center (CO recombination) and the stages of the reverse electron transfer caused by photolysis of the bond of CO with the iron atom of a high-spin heme by a short laser flash. As typical for the heme-copper oxidases, upon photodissociation of CO from the mixed-valence caa3 cytochrome oxidase from T. thermophilus in the absence of oxygen the reverse electron transfer from heme a3 to heme a and further to the cytochrome c/CuA pair is resolved as a single transition with τ ~ 40 μs. Unlike the mitochondrial cytochrome oxidase and typical prokaryotic oxidases with four redox centers, this enzyme has an additional redox center, cytochrome c. Electron exchange between CuA and the additional cytochrome c in this enzyme proceeds much faster, therefore it is not isolated as a separate kinetic stage. The electron back-flow is followed by the return of an electron to the BNC and rebinding of CO with a rate constant of ~14 ms, a process that is only observed in the fully reduced state (Figure 5). Unlike the A family oxidases in which there is a significant reverse electron transfer from the BNC to the input redox centers, in the B family oxidases, this transfer is much smaller and does not exceed a few percentage points [26,155]. This is likely due to the lower redox potential of the low-spin heme of the B family oxidases as compared to the BNC under these conditions. Similarly, in the bd oxidases, the reverse electron transfer is also not significant under these conditions [45,47,48,82,177].
Figure 5.
Proposed scheme for a cycle of photolysis and recombination of CO to the heme-copper cytochrome oxidase caa3 from T. thermophilus (A2 family) in the two-electron-reduced mixed-valence (MV) and fully reduced (R4) states. In the R4 state, photodissociation of CO from the ferrous heme a3 iron (Fe2+a3) is followed by its rebinding to the heme with τ of 14 ms. In the MV state, in addition to the CO rebinding, there is a transient back-flow of an electron from the binuclear center to the input redox center (heme a, CuA, and cytochrome c) with τ of 0.04 ms. Intermediate binding of CO to CuB is not shown. The scheme is based on time-resolved spectroscopic studies [26,135].
When ammonia is added at pH 9 to the F state, classically generated by reaction with excess H2O2, a new PN state (in contradiction to the conventional direction of the catalytic cycle) is shown to appear. It is suggested that ammonia coordinates directly to CuB in the binuclear active center in this P state [178]. The structural and functional properties of this artificial intermediate compound require further research.
A species with an absorption maximum at 680 nm can also be produced by the addition of excess H2O2 to the bd oxidase being in the O or “as-prepared” state [82,94,179,180]. As its spectral features are similar to those observed for the F intermediate in the flow-flash and stopped-flow experiments [82,89,90,125], it can be suggested that these species also have similar or even identical chemical structures. In other words, both are likely a heme d oxoferryl intermediate.
The oxygen-binding site of cytochrome bd, heme d, can also bind external ligands. Usually, a ferrous heme binds electroneutral molecules whereas a ferric heme binds ligands in the anionic form. In the case of heme d, it is true for cyanide and CO but not for NO.
MCD and absorption spectroscopy shows that cyanide binds to ferric heme d in the O state of cytochrome bd [38]. At a high concentration, the ligand can also react with part of ferric heme b558 in the isolated enzyme [38,181]. However, if the enzyme is then reconstituted into phospholipid-based liposomes, cyanide no longer interacts with heme b558 and binds predominantly to the heme d site [181]. Interestingly, the MCD study does not reveal any substantial cyanide binding to the high-spin pentacoordinate ferric heme b595, even at the highest concentration of the ligand used, 50 mM [38]. According to the EPR study, conversely, the addition of the ligand to cytochrome bd results in its binding to the DHAS as a bridging ligand (Fed3+–C = N–Feb5953+) [37]. As discussed below, it is quite unlikely. The Fe–Fe distance between hemes d and b595 is too big to allow a bridging structure in the DHAS. Resonance Raman studies report that in the cyanide complex heme d is high-spin pentacoordinate [182,183]. This suggests that the binding of cyanide to heme d is accompanied by dissociation of the heme axial ligand.
The addition of CO to cytochrome bd in the fully reduced state (R3) leads to the ligand binding to ferrous heme d [38,40,49,77,184,185] with a high affinity [43,186]. The formation of heme d-CO adduct is accompanied by a small spectral perturbation of heme b595 [39,41,45]. The binding of the ligand with ferrous heme b595 as such is usually minor. In the case of the bd oxidases from E. coli and A. vinelandii, the CO-reactive fraction of heme b595 does not exceed 5–15% under different experimental conditions [36,40,46]. However, in the bd enzyme from G. thermodenitrificans, this fraction is larger, 20–25% [77].
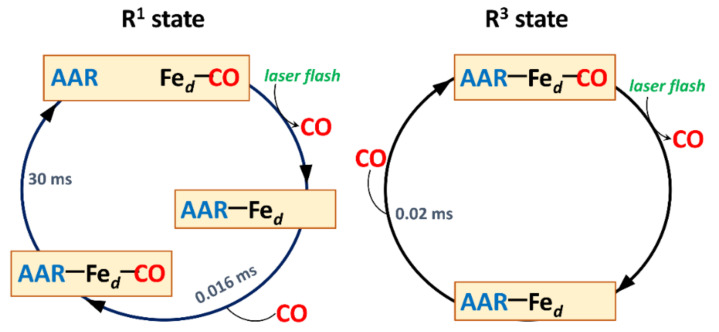
O2 in the A1 state can be replaced with CO that results in CO binding to ferrous heme d and the formation of the one-electron-reduced CO-bound species (R1-CO). Studies of photolysis and subsequent recombination of CO with heme d showed that the flash-induced dynamics of the heme iron coordination sphere between the R1 and R3 states of the enzyme is different (Figure 6) [47,48,49]. In the R3 cytochrome bd, the heme d-CO complex is hexacoordinate and the protein-derived axial ligand to the heme iron (amino acid residue, AAR) remains permanently indissociable during photolysis and recombination processes. In the R1 cytochrome bd, on the contrary, the heme d-CO complex is pentacoordinate (i.e., AAR is not bound to Fed2+) but photodissociation of CO from Fed2+ is accompanied by transient binding of AAR at the opposite side of heme d. AAR is either H19 [34] or E99 [35]. This flexibility of the heme d coordination sphere may have functional significance during the catalytic cycle.
Figure 6.
Proposed scheme for a cycle of photolysis and recombination of CO to bd-type oxidase in one-electron-reduced (R1) and fully reduced (R3) states. In the R1 state, photodissociation of CO from the ferrous heme d iron (Fed) is accompanied by binding of the axial ligand, an amino acid residue (AAR), at the opposite side of the heme. CO rebinds to Fed with τ of 0.016 ms producing a transient hexacoordinate state (AAR–Fed–CO). Then AAR is dissociated from Fed with τ of 30 ms. In the R3 state, AAR is a permanent indissociable ligand to Fed. Photodissociation of CO from Fed is followed by its rebinding to the heme with τ of 0.02 ms. AAR is either H19 [34] or E99 [35]. The scheme is based on time-resolved spectroscopic studies [47,48,49]. The electron transfer in the opposite direction (back-flow) observed in the R1 state occurs to a much lesser extent than in heme-copper oxidases and therefore is not shown in the scheme.
Unlike cyanide and CO, NO can react to different states of heme d. The reaction of cytochrome bd in the A1 (Fed2+–O2), R1 or R3 (Fed2+) states yields a nitrosyl ferrous adduct (Fed2+–NO) [38,93,95]. The O enzyme (Fed3+) reacts with NO producing a nitrosyl ferric species (Fed3+–NO or Fed2+–NO+) [97]. Reaction between cytochrome bd in the F state (Fed4+ = O2−) and NO gives a nitrite-ferric (Fed3+–NO2−) derivative [94].
Cytochrome bd in the O state also interacts with ammonia. Surprisingly, in contrast to cyanide, CO, and NO, NH3 does not inhibit but activates the bd oxidase at alkaline pH [114]. It can be hypothesized that NH3 promotes the conversion of O into P thereby accelerating the enzyme activity. It is also possible that ammonia reacts with O1 yielding F. In these two reactions, NH3 may be oxidized to NH2OH.
4. BNC vs. DHAS
A very short distance between Fe of heme a3 and CuB (4.5–5.2 Å, see references in [7]) allows the BNC of COX to bind a single diatomic molecule or ion, e.g., a peroxo group, as a bridging ligand. The Fe–Fe distance between heme d and heme b595 is much larger (11.1–11.6 Å [33,34,35]) that excludes such a possibility for the DHAS of cytochrome bd. Therefore, in this sense, heme b595 is not a functional analog of CuB. Nonetheless, the distance between the edges of heme d and heme b595 is rather small (3.5–3.8 Å [33,34,35]) suggesting they are in van der Waals contacts. The van der Waals interactions enable fast electron transfer between the hemes. The first evidence of this was the observation that the 4.5-μs transition of A3 to P is accompanied by the oxidation of heme b595 [89]. Later, a CO photolysis/recombination study of the one-electron reduced cytochrome bd provided evidence for electron transfer between hemes d and b595 within 0.2–1.5 μs [47,48]. Thus, heme b595 rapidly donates an electron and possibly a proton to heme d for the oxygen chemistry occurring in the DHAS. This function of heme b595 in cytochrome bd is similar to that of CuB in COX.
Major recent advances in the heme-copper oxidases include the role of the unique tyrosine residue which is now, in addition to the usual CuB and the iron atom of heme a3, accepted to be included in the active center. Thus, the active site of cytochrome c oxidase comprises an oxygen-binding heme, a nearby copper ion (CuB), and a tyrosine residue that is covalently linked to one of the histidine ligands of CuB and conserved throughout the superfamily of the respiratory heme-copper oxidases. The most recent results suggest that the catalytic importance of this residue considerably exceeds the original idea of assistance in the breaking of the O−O bond and that it is of key importance in modulating the redox potentials of the catalytic site intermediates and gating of the proton transfer through the K-channel. These redox potentials are now established with reasonable accuracy from both experiments and calculations, and the remarkable “leveling effect” by the active site, relative to the potentials of O2 reduction in solution, is more dramatic than originally estimated [16,144]. Hence, the neutral radical form of Tyr288 is suggested to have a more general role in the cytochrome c oxidase mechanism than thought previously.
The fact is that in the organization of the active center of heme-copper oxidases, the most important task of thermodynamic alignment (“leveling”) of all four single-electron transitions is solved, providing the necessary amount of free energy for pumping a proton in each single-electron transition when oxygen is reduced to water. At the same time, there is no such tough task for the active center of the bd oxidases. There are no indications of the presence of an aromatic radical in the active center or its participation in the catalytic cycle of a bd oxidase; when the O–O bond is broken, an additional electron is taken from the heme porphyrin. However, the organization of the bd active center can and should meet other requirements that ensure the performance of special physiological and adaptive functions of this unique cytochrome in prokaryotes. More specifically, fine-tuning of the thermodynamics of redox transitions in the active center of the bd oxidase can provide the solution of such problems as a high rate of partial stages of oxygen reduction which ensure the adaptive value of these oxidases under oxygen-limited conditions; acceleration or, vice versa, prohibition of side reactions with stress-induced metabolites (inhibitors) ensuring adaptive benefits. The complex structural and functional aspects of these issues for the bd oxidases are still under development, for which the necessary prerequisites have now appeared due to the deciphering of the three-dimensional structure of all representatives of the terminal oxidases, including the bd-type cytochromes. This seems to be a promising direction and requires further research, both experimental and theoretical.
5. Concluding Remarks
Terminal respiratory oxidases are a broad group of membrane enzymes characterized by a variety of organizations of the main catalytic nodes and evolutionary origins. Despite the similarity of the main catalytic function, the reduction in oxygen to water, these enzymes have important functional differences. On the one hand, heme-copper terminal oxidases have a mechanism of transmembrane proton pumping and are therefore more important as effective generators of a proton motive force. The bd-type cytochromes and some heme-copper oxidases of minor families, although being less effective energy converters, at the same time provide microorganisms with adaptation to low-oxygen conditions and survival in chemically aggressive environments and, as a result, resistance to antibiotics. Currently, three-dimensional structures of members of all major groups of terminal oxidases, the main families of heme-copper oxidases and bd-type cytochromes, have been solved. This provides a unique opportunity for a comprehensive structural and functional study of these important enzymes. Elucidation of the general principles and specific features of the organization and mechanisms of functioning of terminal oxidases of different types will help to decipher the mechanisms of energy conversion in biological membranes and create artificial and hybrid nanoscale objects with desired properties, establish mechanisms for the regulation and adaptation of cellular respiration, the resistance of pathogenic microorganisms, and search for new drugs.
Funding
This work was funded by the Russian Foundation for Basic Research—research projects 18-04-00503 and 19-04-00094.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hemp J., Gennis R.B. Diversity of the Heme–Copper Superfamily in Archaea: Insights from Genomics and Structural Modeling. Results Probl. Cell Differ. 2008;45:1–31. doi: 10.1007/400_2007_046. [DOI] [PubMed] [Google Scholar]
- 2.Siletsky S.A. Steps of the coupled charge translocation in the catalytic cycle of cytochrome c oxidase. Front. Biosci. 2013;18:36–57. doi: 10.2741/4086. [DOI] [PubMed] [Google Scholar]
- 3.Borisov V.B., Siletsky S.A., Paiardini A., Hoogewijs D., Forte E., Giuffrè A., Poole R.K. Bacterial Oxidases of the Cytochrome bd Family: Redox Enzymes of Unique Structure, Function, and Utility As Drug Targets. Antioxid. Redox Signal. 2021;34:1280–1318. doi: 10.1089/ars.2020.8039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murali R., Gennis R.B., Hemp J. Evolution of the cytochrome bd oxygen reductase superfamily and the function of CydAA’ in Archaea. ISME J. 2021:1–15. doi: 10.1038/s41396-021-01019-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Poole R.K., Cook G.M. Redundancy of aerobic respiratory chains in bacteria? Routes, reasons and regulation. Adv. Microb. Physiol. 2000;43:165–224. doi: 10.1016/s0065-2911(00)43005-5. [DOI] [PubMed] [Google Scholar]
- 6.Ingledew W.J., Poole R.K. The respiratory chains of Escherichia coli. Microbiol. Rev. 1984;48:222–271. doi: 10.1128/mr.48.3.222-271.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Siletsky S.A., Borisov V.B., Mamedov M.D. Photosystem II and terminal respiratory oxidases molecular machines operating in opposite directions. Front. Biosci. 2017;22:1379–1426. doi: 10.2741/4550. [DOI] [PubMed] [Google Scholar]
- 8.Malatesta F., Antonini G., Sarti P., Brunori M. Structure and function of a molecular machine: Cytochrome c oxidase. Biophys. Chem. 1995;54:1–33. doi: 10.1016/0301-4622(94)00117-3. [DOI] [PubMed] [Google Scholar]
- 9.Melo A.M., Teixeira M. Supramolecular organization of bacterial aerobic respiratory chains: From cells and back. Biochim. Biophys. Acta Bioenerg. 2016;1857:190–197. doi: 10.1016/j.bbabio.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 10.Pereira M.M., Sousa F.L., Veríssimo A.F., Teixeira M. Looking for the minimum common denominator in haem–copper oxygen reductases: Towards a unified catalytic mechanism. Biochim. Biophys. Acta Bioenerg. 2008;1777:929–934. doi: 10.1016/j.bbabio.2008.05.441. [DOI] [PubMed] [Google Scholar]
- 11.Papa S., Capitanio N., Capitanio G., Palese L.L. Protonmotive cooperativity in cytochrome c oxidase. Biochim. Biophys. Acta Bioenerg. 2004;1658:95–105. doi: 10.1016/j.bbabio.2004.04.014. [DOI] [PubMed] [Google Scholar]
- 12.Borisov V.B., Siletsky S.A. Features of Organization and Mechanism of Catalysis of Two Families of Terminal Oxidases: Heme-Copper and bd-Type. Biochemistry (Moscow) 2019;84:1390–1402. doi: 10.1134/S0006297919110130. [DOI] [PubMed] [Google Scholar]
- 13.Hederstedt L. Molecular Biology of Bacillus subtilis Cytochromes anno 2020. Biochemistry (Moscow) 2021;86:8–21. doi: 10.1134/S0006297921010028. [DOI] [PubMed] [Google Scholar]
- 14.Borisov V.B., Siletsky S.A., Nastasi M.R., Forte E. ROS Defense Systems and Terminal Oxidases in Bacteria. Antioxidants. 2021;10:839. doi: 10.3390/antiox10060839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sousa F.L., Alves R.J., Ribeiro M.A., Pereira-Leal J.B., Teixeira M., Pereira M.M. The superfamily of heme–copper oxygen reductases: Types and evolutionary considerations. Biochim. Biophys. Acta Bioenerg. 2011;1817:629–637. doi: 10.1016/j.bbabio.2011.09.020. [DOI] [PubMed] [Google Scholar]
- 16.Wikström M., Krab K., Sharma V. Oxygen Activation and Energy Conservation by Cytochrome c Oxidase. Chem. Rev. 2018;118:2469–2490. doi: 10.1021/acs.chemrev.7b00664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Capitanio N., Palese L.L., Capitanio G., Martino P.L., Richter O.-M.H., Ludwig B., Papa S. Allosteric interactions and proton conducting pathways in proton pumping aa3 oxidases: Heme a as a key coupling element. Biochim. Biophys. Acta Bioenerg. 2011;1817:558–566. doi: 10.1016/j.bbabio.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 18.Maneg O., Malatesta F., Ludwig B., Drosou V. Interaction of cytochrome c with cytochrome oxidase: Two different docking scenarios. Biochim. Biophys. Acta Bioenerg. 2004;1655:274–281. doi: 10.1016/j.bbabio.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 19.Von Ballmoos C., Ädelroth P., Gennis R.B., Brzezinski P. Proton transfer in ba3 cytochrome c oxidase from Thermus thermophilus. Biochim. Biophys. Acta Bioenerg. 2012;1817:650–657. doi: 10.1016/j.bbabio.2011.11.015. [DOI] [PubMed] [Google Scholar]
- 20.Rich P.R. Mitochondrial cytochrome c oxidase: Catalysis, coupling and controversies. Biochem. Soc. Trans. 2017;45:813–829. doi: 10.1042/BST20160139. [DOI] [PubMed] [Google Scholar]
- 21.Yoshikawa S., Shimada A. Reaction Mechanism of Cytochrome c Oxidase. Chem. Rev. 2015;115:1936–1989. doi: 10.1021/cr500266a. [DOI] [PubMed] [Google Scholar]
- 22.Forte E., Giuffrè A., Huang L.S., Berry E.A., Borisov V.B. Nitric Oxide Does Not Inhibit but Is Metabolized by the Cytochrome bcc-aa3 Supercomplex. Int. J. Mol. Sci. 2020;21:8521. doi: 10.3390/ijms21228521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wikstrom M.K.F. Proton pump coupled to cytochrome c oxidase in mitochondria. Nature. 1977;266:271–273. doi: 10.1038/266271a0. [DOI] [PubMed] [Google Scholar]
- 24.Ter Beek J., Krause N., Ädelroth P. Investigating the Proton Donor in the NO Reductase from Paracoccus denitrificans. PLoS ONE. 2016;11:e0152745. doi: 10.1371/journal.pone.0152745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Borisov V.B., Verkhovsky M.I. Oxygen as Acceptor. EcoSal Plus. 2015;6 doi: 10.1128/ecosalplus.ESP-0012-2015. [DOI] [PubMed] [Google Scholar]
- 26.Siletsky S.A., Belevich I., Jasaitis A., Konstantinov A.A., Wikström M., Soulimane T., Verkhovsky M.I. Time-resolved single-turnover of ba3 oxidase from Thermus thermophilus. Biochim. Biophys. Acta Bioenerg. 2007;1767:1383–1392. doi: 10.1016/j.bbabio.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 27.Siletsky S.A., Belevich I., Belevich N.P., Soulimane T., Wikström M. Time-resolved generation of membrane potential by ba3 cytochrome c oxidase from Thermus thermophilus coupled to single electron injection into the O and OH states. Biochim. Biophys. Acta Bioenerg. 2017;1858:915–926. doi: 10.1016/j.bbabio.2017.08.007. [DOI] [PubMed] [Google Scholar]
- 28.Lyons J., Aragao D., Slattery O., Pisliakov A., Soulimane T., Caffrey M. Structural insights into electron transfer in caa3-type cytochrome oxidase. Nature. 2012;487:514–518. doi: 10.1038/nature11182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li J., Han L., Vallese F., Ding Z., Choi S.K., Hong S., Luo Y., Liu B., Chan C.K., Tajkhorshid E., et al. Cryo-EM structures of Escherichia coli cytochrome bo3 reveal bound phospholipids and ubiquinone-8 in a dynamic substrate binding site. Proc. Natl. Acad. Sci. USA. 2021;118 doi: 10.1073/pnas.2106750118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Buschmann S., Warkentin E., Xie H., Langer J.D., Ermler U., Michel H. The Structure of cbb3 Cytochrome Oxidase Provides Insights into Proton Pumping. Science. 2010;329:327–330. doi: 10.1126/science.1187303. [DOI] [PubMed] [Google Scholar]
- 31.Andersson R., Safari C., Dods R., Nango E., Tanaka R., Yamashita A., Nakane T., Tono K., Joti Y., Båth P., et al. Serial femtosecond crystallography structure of cytochrome c oxidase at room temperature. Sci. Rep. 2017;7:1–9. doi: 10.1038/s41598-017-04817-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Humphrey W., Dalke A., Schulten K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996;14:33–38. doi: 10.1016/0263-7855(96)00018-5. [DOI] [PubMed] [Google Scholar]
- 33.Safarian S., Rajendran C., Müller H., Preu J., Langer J.D., Ovchinnikov S., Hirose T., Kusumoto T., Sakamoto J., Michel H. Structure of a bd oxidase indicates similar mechanisms for membrane-integrated oxygen reductases. Science. 2016;352:583–586. doi: 10.1126/science.aaf2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Safarian S., Hahn A., Mills D.J., Radloff M., Eisinger M.L., Nikolaev A., Meier-Credo J., Melin F., Miyoshi H., Gennis R.B., et al. Active site rearrangement and structural divergence in prokaryotic respiratory oxidases. Science. 2019;366:100–104. doi: 10.1126/science.aay0967. [DOI] [PubMed] [Google Scholar]
- 35.Theßeling A., Rasmussen T., Burschel S., Wohlwend D., Kägi J., Müller R., Böttcher B., Friedrich T. Homologous bd oxidases share the same architecture but differ in mechanism. Nat. Commun. 2019;10:5138. doi: 10.1038/s41467-019-13122-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hill J.J., Alben J.O., Gennis R.B. Spectroscopic evidence for a heme-heme binuclear center in the cytochrome bd ubiquinol oxidase from Escherichia coli. Proc. Natl. Acad. Sci. USA. 1993;90:5863–5867. doi: 10.1073/pnas.90.12.5863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tsubaki M., Hori H., Mogi T., Anraku Y. Cyanide-binding Site of bd-type Ubiquinol Oxidase from Escherichia coli. J. Biol. Chem. 1995;270:28565–28569. doi: 10.1074/jbc.270.48.28565. [DOI] [PubMed] [Google Scholar]
- 38.Borisov V., Arutyunyan A.M., Osborne J.P., Gennis R.B., Konstantinov A.A. Magnetic Circular Dichroism Used To Examine the Interaction of Escherichia coli Cytochrome bd with Ligands. Biochemistry. 1998;38:740–750. doi: 10.1021/bi981908t. [DOI] [PubMed] [Google Scholar]
- 39.Vos M.H., Borisov V.B., Liebl U., Martin J.L., Konstantinov A.A. Femtosecond resolution of ligand-heme interactions in the high-affinity quinol oxidase bd: A di-heme active site? Proc. Natl. Acad. Sci. USA. 2000;97:1554–1559. doi: 10.1073/pnas.030528197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Borisov V.B., Sedelnikova S.E., Poole R.K., Konstantinov A.A. Interaction of Cytochrome bd with Carbon Monoxide at Low and Room Temperatures. J. Biol. Chem. 2001;276:22095–22099. doi: 10.1074/jbc.M011542200. [DOI] [PubMed] [Google Scholar]
- 41.Borisov V.B., Liebl U., Rappaport F., Martin J.-L., Zhang J., Gennis R.B., Konstantinov A.A., Vos M. Interactions between Heme d and Heme b595 in Quinol Oxidase bd from Escherichia coli: A Photoselection Study Using Femtosecond Spectroscopy. Biochemistry. 2002;41:1654–1662. doi: 10.1021/bi0158019. [DOI] [PubMed] [Google Scholar]
- 42.Arutyunyan A.M., Borisov V.B., Novoderezhkin V., Ghaim J., Zhang J., Gennis R.B., Konstantinov A.A. Strong Excitonic Interactions in the Oxygen-Reducing Site of bd-Type Oxidase: The Fe-to-Fe Distance between Hemes d and b595 is 10 Å. Biochemistry. 2008;47:1752–1759. doi: 10.1021/bi701884g. [DOI] [PubMed] [Google Scholar]
- 43.Borisov V.B. Interaction of bd-type quinol oxidase from Escherichia coli and carbon monoxide: Heme d binds CO with high affinity. Biochemistry (Moscow) 2008;73:14–22. doi: 10.1134/S0006297908010021. [DOI] [PubMed] [Google Scholar]
- 44.Bloch D.A., Borisov V.B., Mogi T., Verkhovsky M.I. Heme/heme redox interaction and resolution of individual optical absorption spectra of the hemes in cytochrome bd from Escherichia coli. Biochim. Biophys. Acta Bioenerg. 2009;1787:1246–1253. doi: 10.1016/j.bbabio.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 45.Rappaport F., Zhang J., Vos M.H., Gennis R.B., Borisov V.B. Heme–heme and heme–ligand interactions in the di-heme oxygen-reducing site of cytochrome bd from Escherichia coli revealed by nanosecond absorption spectroscopy. Biochim. Biophys. Acta Bioenerg. 2010;1797:1657–1664. doi: 10.1016/j.bbabio.2010.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Borisov V.B., Verkhovsky M.I. Accommodation of CO in the di-heme active site of cytochrome bd terminal oxidase from Escherichia coli. J. Inorg. Biochem. 2013;118:65–67. doi: 10.1016/j.jinorgbio.2012.09.016. [DOI] [PubMed] [Google Scholar]
- 47.Siletsky S.A., Zaspa A.A., Poole R.K., Borisov V.B. Microsecond Time-Resolved Absorption Spectroscopy Used to Study CO Compounds of Cytochrome bd from Escherichia coli. PLoS ONE. 2014;9:e95617. doi: 10.1371/journal.pone.0095617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Siletsky S.A., Rappaport F., Poole R.K., Borisov V.B. Evidence for Fast Electron Transfer between the High-Spin Haems in Cytochrome bd-I from Escherichia coli. PLoS ONE. 2016;11:e0155186. doi: 10.1371/journal.pone.0155186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Siletsky S.A., Dyuba A.V., Elkina D.A., Monakhova M.V., Borisov V.B. Spectral-kinetic analysis of recombination reaction of heme centers of bd-type quinol oxidase from Escherichia coli with carbon monoxide. Biochemistry (Moscow) 2017;82:1354–1366. doi: 10.1134/S000629791711013X. [DOI] [PubMed] [Google Scholar]
- 50.Azarkina N., Borisov V., Konstantinov A.A. Spontaneous spectral changes of the reduced cytochrome bd. FEBS Lett. 1997;416:171–174. doi: 10.1016/S0014-5793(97)01196-4. [DOI] [PubMed] [Google Scholar]
- 51.Azarkina N., Siletsky S., Borisov V., von Wachenfeldt C., Hederstedt L., Konstantinov A.A. A Cytochrome bb′-type Quinol Oxidase in Bacillus subtilis Strain 168. J. Biol. Chem. 1999;274:32810–32817. doi: 10.1074/jbc.274.46.32810. [DOI] [PubMed] [Google Scholar]
- 52.Pereira M.M., Santana M., Teixeira M. A novel scenario for the evolution of haem–copper oxygen reductases. Biochim. Biophys. Acta Bioenerg. 2001;1505:185–208. doi: 10.1016/S0005-2728(01)00169-4. [DOI] [PubMed] [Google Scholar]
- 53.Pereira M.M., Gomes C.M., Teixeira M. Plasticity of proton pathways in haem-copper oxygen reductases. FEBS Lett. 2002;522:14–18. doi: 10.1016/S0014-5793(02)02920-4. [DOI] [PubMed] [Google Scholar]
- 54.Pereira M.M., Teixeira M. Proton pathways, ligand binding and dynamics of the catalytic site in haem-copper oxygen reductases: A comparison between the three families. Biochim. Biophys. Acta Bioenerg. 2004;1655:340–346. doi: 10.1016/j.bbabio.2003.06.003. [DOI] [PubMed] [Google Scholar]
- 55.Tsukihara T., Aoyama H., Yamashita E., Tomizaki T., Yamaguchi H., Shinzawa-Itoh K., Nakashima R., Yaono R., Yoshikawa S. The Whole Structure of the 13-Subunit Oxidized Cytochrome c Oxidase at 2.8 A. Science. 1996;272:1136–1144. doi: 10.1126/science.272.5265.1136. [DOI] [PubMed] [Google Scholar]
- 56.Yano N., Muramoto K., Shimada A., Takemura S., Baba J., Fujisawa H., Mochizuki M., Shinzawa-Itoh K., Yamashita E., Tsukihara T., et al. The Mg2+-containing Water Cluster of Mammalian Cytochrome c Oxidase Collects Four Pumping Proton Equivalents in Each Catalytic Cycle. J. Biol. Chem. 2016;291:23882–23894. doi: 10.1074/jbc.M115.711770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shimada A., Etoh Y., Kitoh-Fujisawa R., Sasaki A., Shinzawa-Itoh K., Hiromoto T., Yamashita E., Muramoto K., Tsukihara T., Yoshikawa S. X-ray structures of catalytic intermediates of cytochrome c oxidase provide insights into its O2 activation and unidirectional proton-pump mechanisms. J. Biol. Chem. 2020;295:5818–5833. doi: 10.1074/jbc.RA119.009596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shimada A., Hara F., Shinzawa-Itoh K., Kanehisa N., Yamashita E., Muramoto K., Tsukihara T., Yoshikawa S. Critical roles of the CuB site in efficient proton pumping as revealed by crystal structures of mammalian cytochrome c oxidase catalytic intermediates. J. Biol. Chem. 2021;297:100967. doi: 10.1016/j.jbc.2021.100967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Koepke J., Olkhova E., Angerer H., Müller H., Peng G., Michel H. High resolution crystal structure of Paracoccus denitrificans cytochrome c oxidase: New insights into the active site and the proton transfer pathways. Biochim. Biophys. Acta Bioenerg. 2009;1787:635–645. doi: 10.1016/j.bbabio.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 60.Svensson-Ek M., Abramson J., Larsson G., Törnroth-Horsefield S., Brzezinski P., Iwata S. The X-ray Crystal Structures of Wild-type and EQ(I-286) Mutant Cytochrome c Oxidases from Rhodobacter sphaeroides. J. Mol. Biol. 2002;321:329–339. doi: 10.1016/S0022-2836(02)00619-8. [DOI] [PubMed] [Google Scholar]
- 61.Qin L., Liu J., Mills D.A., Proshlyakov D.A., Hiser C., Ferguson-Miller S. Redox-Dependent Conformational Changes in Cytochrome c Oxidase Suggest a Gating Mechanism for Proton Uptake. Biochemistry. 2009;48:5121–5130. doi: 10.1021/bi9001387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Abramson J., Riistama S., Larsson G., Jasaitis A., Svensson-Ek M., Laakkonen L., Puustinen A., Iwata S., Wikström M. The structure of the ubiquinol oxidase from Escherichia coli and its ubiquinone binding site. Nat. Genet. 2000;7:910–917. doi: 10.1038/82824. [DOI] [PubMed] [Google Scholar]
- 63.Kruse F., Nguyen A.D., Dragelj J., Heberle J., Hildebrandt P., Mroginski M.A., Weidinger I.M. A Resonance Raman Marker Band Characterizes the Slow and Fast Form of Cytochrome c Oxidase. J. Am. Chem. Soc. 2021;143:2769–2776. doi: 10.1021/jacs.0c10767. [DOI] [PubMed] [Google Scholar]
- 64.Yano N., Muramoto K., Mochizuki M., Shinzawa-Itoh K., Yamashita E., Yoshikawa S., Tsukihara T. X-ray structure of cyanide-bound bovine heart cytochrome c oxidase in the fully oxidized state at 2.0 Å resolution. Acta Crystallogr. Sect. F Struct. Biol. Commun. 2015;71:726–730. doi: 10.1107/S2053230X15007025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Letts J., Fiedorczuk K., Sazanov L. The architecture of respiratory supercomplexes. Nature. 2016;537:644–648. doi: 10.1038/nature19774. [DOI] [PubMed] [Google Scholar]
- 66.Shinzawa-Itoh K., Sugimura T., Misaki T., Tadehara Y., Yamamoto S., Hanada M., Yano N., Nakagawa T., Uene S., Yamada T., et al. Monomeric structure of an active form of bovine cytochrome c oxidase. Proc. Natl. Acad. Sci. USA. 2019;116:19945–19951. doi: 10.1073/pnas.1907183116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wikström M., Sharma V. Proton pumping by cytochrome c oxidase—A 40 year anniversary. Biochim. Biophys. Acta Bioenerg. 2018;1859:692–698. doi: 10.1016/j.bbabio.2018.03.009. [DOI] [PubMed] [Google Scholar]
- 68.Konstantinov A.A., Siletsky S., Mitchell D., Kaulen A., Gennis R.B. The roles of the two proton input channels in cytochrome c oxidase from Rhodobacter sphaeroides probed by the effects of site-directed mutations on time-resolved electrogenic intraprotein proton transfer. Proc. Natl. Acad. Sci. USA. 1997;94:9085–9090. doi: 10.1073/pnas.94.17.9085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Siletsky S.A., Pawate A.S., Weiss K., Gennis R.B., Konstantinov A.A. Transmembrane Charge Separation during the Ferryl-oxo → Oxidized Transition in a Nonpumping Mutant of Cytochrome c Oxidase. J. Biol. Chem. 2004;279:52558–52565. doi: 10.1074/jbc.M407549200. [DOI] [PubMed] [Google Scholar]
- 70.Siletsky S.A., Zhu J., Gennis R.B., Konstantinov A.A. Partial Steps of Charge Translocation in the Nonpumping N139L Mutant of Rhodobacter sphaeroides Cytochrome c Oxidase with a Blocked D-Channel. Biochemistry. 2010;49:3060–3073. doi: 10.1021/bi901719e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vilhjálmsdóttir J., Albertsson I., Blomberg M.R.A., Ädelroth P., Brzezinski P. Proton transfer in uncoupled variants of cytochrome c oxidase. FEBS Lett. 2019;594:813–822. doi: 10.1002/1873-3468.13679. [DOI] [PubMed] [Google Scholar]
- 72.Berg J., Liu J., Svahn E., Ferguson-Miller S., Brzezinski P. Structural changes at the surface of cytochrome c oxidase alter the proton-pumping stoichiometry. Biochim. Biophys. Acta Bioenerg. 2020;1861:148116. doi: 10.1016/j.bbabio.2019.148116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Siletsky S.A., Soulimane T., Belevich I., Gennis R.B., Wikström M. Specific inhibition of proton pumping by the T315V mutation in the K channel of cytochrome ba3 from Thermus thermophilus. Biochim. Biophys. Acta Bioenerg. 2021;1862:148450. doi: 10.1016/j.bbabio.2021.148450. [DOI] [PubMed] [Google Scholar]
- 74.Blomberg M.R.A. Role of the Two Metals in the Active Sites of Heme Copper Oxidases–A Study of NO Reduction in cbb3 Cytochrome c Oxidase. Inorg. Chem. 2020;59:11542–11553. doi: 10.1021/acs.inorgchem.0c01351. [DOI] [PubMed] [Google Scholar]
- 75.Maréchal A., Xu J.-Y., Genko N., Hartley A.M., Haraux F., Meunier B., Rich P.R. A common coupling mechanism for A-type heme-copper oxidases from bacteria to mitochondria. Proc. Natl. Acad. Sci. USA. 2020;117:9349–9355. doi: 10.1073/pnas.2001572117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Borisov V.B., Gennis R.B., Hemp J., Verkhovsky M.I. The cytochrome bd respiratory oxygen reductases. Biochim. Biophys. Acta Bioenerg. 2011;1807:1398–1413. doi: 10.1016/j.bbabio.2011.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Arutyunyan A.M., Sakamoto J., Inadome M., Kabashima Y., Borisov V.B. Optical and magneto-optical activity of cytochrome bd from Geobacillus thermodenitrificans. Biochim. Biophys. Acta Bioenerg. 2012;1817:2087–2094. doi: 10.1016/j.bbabio.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 78.Borisov V.B. Cytochrome bd: Structure and properties. Biochemistry (Moscow) 1996;61:565–574. [PubMed] [Google Scholar]
- 79.Jünemann S. Cytochrome bd terminal oxidase. Biochim. Biophys. Acta Bioenerg. 1997;1321:107–127. doi: 10.1016/S0005-2728(97)00046-7. [DOI] [PubMed] [Google Scholar]
- 80.Forte E., Borisov V.B., Vicente J.B., Giuffrè A. Cytochrome bd and Gaseous Ligands in Bacterial Physiology. Adv. Microb. Physiol. 2017;71:171–234. doi: 10.1016/bs.ampbs.2017.05.002. [DOI] [PubMed] [Google Scholar]
- 81.Wang W., Gao Y., Tang Y., Zhou X., Lai Y., Zhou S., Zhang Y., Yang X., Liu F., Guddat L.W., et al. Cryo-EM structure of mycobacterial cytochrome bd reveals two oxygen access channels. Nat. Commun. 2021;12:1–8. doi: 10.1038/s41467-021-24924-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jasaitis A., Borisov V.B., Belevich N.P., Morgan J.E., Konstantinov A.A., Verkhovsky M.I. Electrogenic Reactions of Cytochrome bd. Biochemistry. 2000;39:13800–13809. doi: 10.1021/bi001165n. [DOI] [PubMed] [Google Scholar]
- 83.Belevich I., Borisov V.B., Zhang J., Yang K., Konstantinov A.A., Gennis R.B., Verkhovsky M.I. Time-resolved electrometric and optical studies on cytochrome bd suggest a mechanism of electron-proton coupling in the di-heme active site. Proc. Natl. Acad. Sci. USA. 2005;102:3657–3662. doi: 10.1073/pnas.0405683102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Forte E., Borisov V.B., Konstantinov A.A., Brunori M., Giuffrè A., Sarti P. Cytochrome bd, a key oxidase in bacterial survival and tolerance to nitrosative stress. Ital. J. Biochem. 2007;56 [PubMed] [Google Scholar]
- 85.Borisov V.B., Forte E., Siletsky S.A., Arese M., Davletshin A.I., Sarti P., Giuffrè A. Cytochrome bd protects bacteria against oxidative and nitrosative stress: A potential target for next-generation antimicrobial agents. Biochemistry (Moscow) 2015;80:565–575. doi: 10.1134/S0006297915050077. [DOI] [PubMed] [Google Scholar]
- 86.Giuffrè A., Borisov V.B., Mastronicola D., Sarti P., Forte E. Cytochrome bd oxidase and nitric oxide: From reaction mechanisms to bacterial physiology. FEBS Lett. 2011;586:622–629. doi: 10.1016/j.febslet.2011.07.035. [DOI] [PubMed] [Google Scholar]
- 87.Giuffrè A., Borisov V.B., Arese M., Sarti P., Forte E. Cytochrome bd oxidase and bacterial tolerance to oxidative and nitrosative stress. Biochim. Biophys. Acta Bioenerg. 2014;1837:1178–1187. doi: 10.1016/j.bbabio.2014.01.016. [DOI] [PubMed] [Google Scholar]
- 88.Gavrikova E.V., Grivennikova V.G., Borisov V.B., Cecchini G., Vinogradov A.D. Assembly of a chimeric respiratory chain from bovine heart submitochondrial particles and cytochrome bd terminal oxidase of Escherichia coli. FEBS Lett. 2009;583:1287–1291. doi: 10.1016/j.febslet.2009.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Belevich I., Borisov V.B., Verkhovsky M.I. Discovery of the True Peroxy Intermediate in the Catalytic Cycle of Terminal Oxidases by Real-time Measurement. J. Biol. Chem. 2007;282:28514–28519. doi: 10.1074/jbc.M705562200. [DOI] [PubMed] [Google Scholar]
- 90.Borisov V.B., Belevich I., Bloch D.A., Mogi T., Verkhovsky M.I. Glutamate 107 in Subunit I of Cytochrome bd from Escherichia coli Is Part of a Transmembrane Intraprotein Pathway Conducting Protons from the Cytoplasm to the Heme b595/Heme d Active Site. Biochemistry. 2008;47:7907–7914. doi: 10.1021/bi800435a. [DOI] [PubMed] [Google Scholar]
- 91.Borisov V.B., Murali R., Verkhovskaya M., Bloch D.A., Han H., Gennis R.B., Verkhovsky M.I. Aerobic respiratory chain of Escherichia coli is not allowed to work in fully uncoupled mode. Proc. Natl. Acad. Sci. USA. 2011;108:17320–17324. doi: 10.1073/pnas.1108217108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Siletsky S.A., Gennis R.B. Time-Resolved Electrometric Study of the F→O Transition in Cytochrome c Oxidase. The Effect of Zn2+ Ions on the Positive Side of the Membrane. Biochemistry (Moscow) 2021;86:105–122. doi: 10.1134/S0006297921010107. [DOI] [PubMed] [Google Scholar]
- 93.Borisov V.B., Forte E., Konstantinov A.A., Poole R.K., Sarti P., Giuffrè A. Interaction of the bacterial terminal oxidase cytochrome bd with nitric oxide. FEBS Lett. 2004;576:201–204. doi: 10.1016/j.febslet.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 94.Borisov V.B., Forte E., Sarti P., Brunori M., Konstantinov A.A., Giuffrè A. Nitric oxide reacts with the ferryl-oxo catalytic intermediate of the CuB-lacking cytochrome bd terminal oxidase. FEBS Lett. 2006;580:4823–4826. doi: 10.1016/j.febslet.2006.07.072. [DOI] [PubMed] [Google Scholar]
- 95.Borisov V.B., Forte E., Sarti P., Brunori M., Konstantinov A.A., Giuffrè A. Redox control of fast ligand dissociation from Escherichia coli cytochrome bd. Biochem. Biophys. Res. Commun. 2007;355:97–102. doi: 10.1016/j.bbrc.2007.01.118. [DOI] [PubMed] [Google Scholar]
- 96.Mason M.G., Shepherd M., Nicholls P.J., Dobbin P.S., Dodsworth K.S., Poole R.K., Cooper C. Cytochrome bd confers nitric oxide resistance to Escherichia coli. Nat. Chem. Biol. 2008;5:94–96. doi: 10.1038/nchembio.135. [DOI] [PubMed] [Google Scholar]
- 97.Borisov V.B., Forte E., Giuffrè A., Konstantinov A., Sarti P. Reaction of nitric oxide with the oxidized di-heme and heme–copper oxygen-reducing centers of terminal oxidases: Different reaction pathways and end-products. J. Inorg. Biochem. 2009;103:1185–1187. doi: 10.1016/j.jinorgbio.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 98.Shepherd M., Achard M.E.S., Idris A., Totsika M., Phan M.-D., Peters K.M., Sarkar S., Ribeiro C., Holyoake L.V., Ladakis D., et al. The cytochrome bd-I respiratory oxidase augments survival of multidrug-resistant Escherichia coli during infection. Sci. Rep. 2016;6:35285. doi: 10.1038/srep35285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Holyoake L.V., Hunt S., Sanguinetti G., Cook G.M., Howard M., Rowe M.L., Poole R.K., Shepherd M. CydDC-mediated reductant export in Escherichia coli controls the transcriptional wiring of energy metabolism and combats nitrosative stress. Biochem. J. 2016;473:693–701. doi: 10.1042/BJ20150536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jones-Carson J., HUSA in M., Liu L., Orlicky D.J., Vázquez-Torres A. Cytochrome bd-Dependent Bioenergetics and Antinitrosative Defenses in Salmonella Pathogenesis. mBio. 2016;7:e02052-16. doi: 10.1128/mBio.02052-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Meng Q., Yin J., Jin M., Gao H. Distinct Nitrite and Nitric Oxide Physiologies in Escherichia coli and Shewanella oneidensis. Appl. Environ. Microbiol. 2018;84 doi: 10.1128/AEM.00559-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Beebout C., Eberly A.R., Werby S.H., Reasoner S., Brannon J., De S., Fitzgerald M., Huggins M.M., Clayton D.B., Cegelski L., et al. Respiratory Heterogeneity Shapes Biofilm Formation and Host Colonization in Uropathogenic Escherichia coli. mBio. 2019;10:e02400-18. doi: 10.1128/mBio.02400-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Borisov V.B., Forte E., Siletsky S.A., Sarti P., Giuffrè A. Cytochrome bd from Escherichia coli catalyzes peroxynitrite decomposition. Biochim. Biophys. Acta Bioenerg. 2015;1847:182–188. doi: 10.1016/j.bbabio.2014.10.006. [DOI] [PubMed] [Google Scholar]
- 104.Borisov V.B., Davletshin A.I., Konstantinov A.A. Peroxidase activity of cytochrome bd from Escherichia coli. Biochemistry (Moscow) 2010;75:428–436. doi: 10.1134/S000629791004005X. [DOI] [PubMed] [Google Scholar]
- 105.Borisov V.B., Forte E., Davletshin A., Mastronicola D., Sarti P., Giuffrè A. Cytochrome bd oxidase from Escherichia coli displays high catalase activity: An additional defense against oxidative stress. FEBS Lett. 2013;587:2214–2218. doi: 10.1016/j.febslet.2013.05.047. [DOI] [PubMed] [Google Scholar]
- 106.Forte E., Borisov V.B., Davletshin A., Mastronicola D., Sarti P., Giuffrè A., Planet P.J., LaRussa S.J., Dana A., Smith H., et al. Cytochrome bd Oxidase and Hydrogen Peroxide Resistance in Mycobacterium tuberculosis. mBio. 2013;4:e01006-13. doi: 10.1128/mBio.01006-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Al-Attar S., Yu Y., Pinkse M., Hoeser J., Friedrich T., Bald D., De Vries S. Cytochrome bd Displays Significant Quinol Peroxidase Activity. Sci. Rep. 2016;6:27631. doi: 10.1038/srep27631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kita K., Konishi K., Anraku Y. Terminal oxidases of Escherichia coli aerobic respiratory chain. II. Purification and properties of cytochrome b558-d complex from cells grown with limited oxygen and evidence of branched electron-carrying systems. J. Biol. Chem. 1984;259:3375–3381. doi: 10.1016/S0021-9258(17)43305-9. [DOI] [PubMed] [Google Scholar]
- 109.Sakamoto J., Koga E., Mizuta T., Sato C., Noguchi S., Sone N. Gene structure and quinol oxidase activity of a cytochrome bd-type oxidase from Bacillus stearothermophilus. Biochim. Biophys. Acta Bioenerg. 1999;1411:147–158. doi: 10.1016/S0005-2728(99)00012-2. [DOI] [PubMed] [Google Scholar]
- 110.Forte E., Borisov V.B., Falabella M., Colaço H., Tinajero-Trejo M., Poole R.K., Vicente J., Sarti P., Giuffrè A. The Terminal Oxidase Cytochrome bd Promotes Sulfide-resistant Bacterial Respiration and Growth. Sci. Rep. 2016;6:23788. doi: 10.1038/srep23788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Korshunov S., Imlay K.R.C., Imlay J.A. The cytochrome bd oxidase of Escherichia coli prevents respiratory inhibition by endogenous and exogenous hydrogen sulfide. Mol. Microbiol. 2016;101:62–77. doi: 10.1111/mmi.13372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Forte E., Giuffrè A. How bacteria breathe in hydrogen sulfide-rich environments. Biochemist. 2016;38:8–11. doi: 10.1042/BIO03805008. [DOI] [Google Scholar]
- 113.Borisov V.B., Forte E. Terminal Oxidase Cytochrome bd Protects Bacteria Against Hydrogen Sulfide Toxicity. Biochemistry (Moscow) 2021;86:22–32. doi: 10.1134/S000629792101003X. [DOI] [PubMed] [Google Scholar]
- 114.Forte E., Siletsky S.A., Borisov V.B. In Escherichia coli Ammonia Inhibits Cytochrome bo3 But Activates Cytochrome bd-I. Antioxidants. 2020;10:13. doi: 10.3390/antiox10010013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Mascolo L., Bald D. Cytochrome bd in Mycobacterium tuberculosis: A respiratory chain protein involved in the defense against antibacterials. Prog. Biophys. Mol. Biol. 2019;152:55–63. doi: 10.1016/j.pbiomolbio.2019.11.002. [DOI] [PubMed] [Google Scholar]
- 116.Lee B.S., Sviriaeva E., Pethe K. Targeting the cytochrome oxidases for drug development in mycobacteria. Prog. Biophys. Mol. Biol. 2020;152:45–54. doi: 10.1016/j.pbiomolbio.2020.02.001. [DOI] [PubMed] [Google Scholar]
- 117.Cook G.M., Hards K., Dunn E., Heikal A., Nakatani Y., Greening C., Crick D.C., Fontes F.L., Pethe K., Hasenoehrl E., et al. Oxidative Phosphorylation as a Target Space for Tuberculosis: Success, Caution, and Future Directions. Microbiol. Spectr. 2017;5 doi: 10.1128/microbiolspec.TBTB2-0014-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bald D., Villellas C., Lu P., Koul A. Targeting Energy Metabolism in Mycobacterium tuberculosis, a New Paradigm in Antimycobacterial Drug Discovery. mBio. 2017;8:e00272-17. doi: 10.1128/mBio.00272-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hards K., Cook G.M. Targeting bacterial energetics to produce new antimicrobials. Drug Resist. Updat. 2018;36:1–12. doi: 10.1016/j.drup.2017.11.001. [DOI] [PubMed] [Google Scholar]
- 120.Winstedt L., Frankenberg L., Hederstedt L., von Wachenfeldt C. Enterococcus faecalis V583 Contains a Cytochrome bd-Type Respiratory Oxidase. J. Bacteriol. 2000;182:3863–3866. doi: 10.1128/JB.182.13.3863-3866.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Siletsky S.A., Konstantinov A.A. Cytochrome c oxidase: Charge translocation coupled to single-electron partial steps of the catalytic cycle. Biochim. Biophys. Acta Bioenerg. 2012;1817:476–488. doi: 10.1016/j.bbabio.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 122.Yoshikawa S., Shinzawa-Itoh K., Nakashima R., Yaono R., Yamashita E., Inoue N., Yao M., Fei M.J., Libeu C.P., Mizushima T., et al. Redox-Coupled Crystal Structural Changes in Bovine Heart Cytochrome c Oxidase. Science. 1998;280:1723–1729. doi: 10.1126/science.280.5370.1723. [DOI] [PubMed] [Google Scholar]
- 123.Buse G., Soulimane T., Dewor M., Meyer H.E., Blüggel M. Evidence for a copper-coordinated histidine-tyrosine cross-link in the active site of cytochrome oxidase. Protein Sci. 1999;8:985–990. doi: 10.1110/ps.8.5.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Rauhamaki V., Baumann M., Soliymani R., Puustinen A., Wikstrom M. Identification of a histidine-tyrosine cross-link in the active site of the cbb3-type cytochrome c oxidase from Rhodobacter sphaeroides. Proc. Natl. Acad. Sci. USA. 2006;103:16135–16140. doi: 10.1073/pnas.0606254103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Borisov V.B., Forte E., Sarti P., Giuffrè A. Catalytic intermediates of cytochrome bd terminal oxidase at steady-state: Ferryl and oxy-ferrous species dominate. Biochim. Biophys. Acta Bioenerg. 2011;1807:503–509. doi: 10.1016/j.bbabio.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 126.Paulus A., Rossius S.G.H., Dijk M., de Vries S. Oxoferryl-Porphyrin Radical Catalytic Intermediate in Cytochrome bd Oxidases Protects Cells from Formation of Reactive Oxygen Species. J. Biol. Chem. 2012;287:8830–8838. doi: 10.1074/jbc.M111.333542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Belevich I., Bloch D.A., Wikstrom M., Verkhovsky M.I. Exploring the proton pump mechanism of cytochrome c oxidase in real time. Proc. Natl. Acad. Sci. USA. 2007;104:2685–2690. doi: 10.1073/pnas.0608794104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bloch D., Belevich I., Jasaitis A., Ribacka C., Puustinen A., Verkhovsky M.I., Wikstrom M. The catalytic cycle of cytochrome c oxidase is not the sum of its two halves. Proc. Natl. Acad. Sci. USA. 2003;101:529–533. doi: 10.1073/pnas.0306036101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Jancura D., Berka V., Antalik M., Bagelova J., Gennis R.B., Palmer G., Fabian M. Spectral and Kinetic Equivalence of Oxidized Cytochrome c Oxidase as Isolated and “Activated” by Reoxidation. J. Biol. Chem. 2006;281:30319–30325. doi: 10.1074/jbc.M605955200. [DOI] [PubMed] [Google Scholar]
- 130.Brand S.E., Rajagukguk S., Ganesan K., Geren L., Fabian M., Han D., Gennis R.B., Durham B., Millett F. A New Ruthenium Complex To Study Single-Electron Reduction of the Pulsed OH State of Detergent-Solubilized Cytochrome Oxidase. Biochemistry. 2007;46:14610–14618. doi: 10.1021/bi701424d. [DOI] [PubMed] [Google Scholar]
- 131.Vilhjálmsdóttir J., Gennis R.B., Brzezinski P. The electron distribution in the “activated” state of cytochrome c oxidase. Sci. Rep. 2018;8:1–11. doi: 10.1038/s41598-018-25779-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Siletskiy S., Soulimane T., Azarkina N., Vygodina T., Buse G., Kaulen A., Konstantinov A. Time-resolved generation of a membrane potential by ba3 cytochrome coxidase from Thermus thermophilus. FEBS Lett. 1999;457:98–102. doi: 10.1016/S0014-5793(99)01019-4. [DOI] [PubMed] [Google Scholar]
- 133.Siletsky S.A., Belevich I., Wikström M., Soulimane T., Verkhovsky M.I. Time-resolved OH→EH transition of the aberrant ba3 oxidase from Thermus thermophilus. Biochim. Biophys. Acta Bioenerg. 2009;1787:201–205. doi: 10.1016/j.bbabio.2008.12.020. [DOI] [PubMed] [Google Scholar]
- 134.Siletsky S.A., Belevich I., Belevich N.P., Soulimane T., Verkhovsky M.I. Time-resolved single-turnover of caa3 oxidase from Thermus thermophilus. Fifth electron of the fully reduced enzyme converts OH into EH state. Biochim. Biophys. Acta Bioenerg. 2011;1807:1162–1169. doi: 10.1016/j.bbabio.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 135.Siletsky S.A., Belevich I., Soulimane T., Verkhovsky M.I., Wikström M. The fifth electron in the fully reduced caa3 from Thermus thermophilus is competent in proton pumping. Biochim. Biophys. Acta Bioenerg. 2013;1827:1–9. doi: 10.1016/j.bbabio.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 136.Yoshikawa S., Muramoto K., Shinzawa-Itoh K. Proton-Pumping Mechanism of Cytochrome c Oxidase. Annu. Rev. Biophys. 2011;40:205–223. doi: 10.1146/annurev-biophys-042910-155341. [DOI] [PubMed] [Google Scholar]
- 137.Sharma V., Karlin K.D., Wikström M. Computational study of the activated OH state in the catalytic mechanism of cytochrome c oxidase. Proc. Natl. Acad. Sci. USA. 2013;110:16844–16849. doi: 10.1073/pnas.1220379110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Luo F., Shinzawa-Itoh K., Hagimoto K., Shimada A., Shimada S., Yamashita E., Yoshikawa S., Tsukihara T. Structure of bovine cytochrome c oxidase crystallized at a neutral pH using a fluorinated detergent. Acta Crystallogr. Sect. F Struct. Biol. Commun. 2017;73:416–422. doi: 10.1107/S2053230X17008834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Sharma V., Wikström M. The role of the K-channel and the active-site tyrosine in the catalytic mechanism of cytochrome c oxidase. Biochim. Biophys. Acta Bioenerg. 2016;1857:1111–1115. doi: 10.1016/j.bbabio.2016.02.008. [DOI] [PubMed] [Google Scholar]
- 140.Blomberg M.R. Active Site Midpoint Potentials in Different Cytochrome c Oxidase Families: A Computational Comparison. Biochemistry. 2019;58:2028–2038. doi: 10.1021/acs.biochem.9b00093. [DOI] [PubMed] [Google Scholar]
- 141.Yang K., Borisov V.B., Konstantinov A.A., Gennis R.B. The fully oxidized form of the cytochrome bd quinol oxidase from E. coli does not participate in the catalytic cycle: Direct evidence from rapid kinetics studies. FEBS Lett. 2008;582:3705–3709. doi: 10.1016/j.febslet.2008.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Borisov V.B., Smirnova I.A., Krasnosel’skaya I.A., Konstantinov A.A. Oxygenated cytochrome bd from Escherichia coli can be converted into the oxidized form by lipophilic electron acceptors. Biochemistry (Moscow) 1994;59:437–443. [PubMed] [Google Scholar]
- 143.Blomberg M.R. The structure of the oxidized state of cytochrome c oxidase-experiments and theory compared. J. Inorg. Biochem. 2020;206:111020. doi: 10.1016/j.jinorgbio.2020.111020. [DOI] [PubMed] [Google Scholar]
- 144.Blomberg M.R.A. Mechanism of Oxygen Reduction in Cytochrome c Oxidase and the Role of the Active Site Tyrosine. Biochemistry. 2016;55:489–500. doi: 10.1021/acs.biochem.5b01205. [DOI] [PubMed] [Google Scholar]
- 145.Belevich I., Borisov V.B., Konstantinov A.A., Verkhovsky M.I. Oxygenated complex of cytochrome bd from Escherichia coli: Stability and photolability. FEBS Lett. 2005;579:4567–4570. doi: 10.1016/j.febslet.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 146.Belevich I., Borisov V.B., Bloch D.A., Konstantinov A.A., Verkhovsky M.I. Cytochrome bd from Azotobacter vinelandii: Evidence for High-Affinity Oxygen Binding. Biochemistry. 2007;46:11177–11184. doi: 10.1021/bi700862u. [DOI] [PubMed] [Google Scholar]
- 147.Szundi I., Kittredge C., Choi S.K., McDonald W., Ray J., Gennis R.B., Einarsdóttir Ó. Kinetics and Intermediates of the Reaction of Fully Reduced Escherichia coli bo3 Ubiquinol Oxidase with O2. Biochemistry. 2014;53:5393–5404. doi: 10.1021/bi500567m. [DOI] [PubMed] [Google Scholar]
- 148.Koutsoupakis C., Soulimane T., Varotsis C. Spectroscopic and Kinetic Investigation of the Fully Reduced and Mixed Valence States of ba3-Cytochrome c Oxidase from Thermus thermophilus. J. Biol. Chem. 2012;287:37495–37507. doi: 10.1074/jbc.M112.403600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Muramoto K., Ohta K., Shinzawa-Itoh K., Kanda K., Taniguchi M., Nabekura H., Yamashita E., Tsukihara T., Yoshikawa S. Bovine cytochrome c oxidase structures enable O2 reduction with minimization of reactive oxygens and provide a proton-pumping gate. Proc. Natl. Acad. Sci. USA. 2010;107:7740–7745. doi: 10.1073/pnas.0910410107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Blomberg M.R.A., Siegbahn P.E.M., Wikström M. Metal-Bridging Mechanism for O−O Bond Cleavage in Cytochrome c Oxidase. Inorg. Chem. 2003;42:5231–5243. doi: 10.1021/ic034060s. [DOI] [PubMed] [Google Scholar]
- 151.Du W.-G.H., Götz A.W., Yang L., Walker R.C., Noodleman L. A broken-symmetry density functional study of structures, energies, and protonation states along the catalytic O–O bond cleavage pathway in ba3 cytochrome c oxidase from Thermus thermophilus. Phys. Chem. Chem. Phys. 2016;18:21162–21171. doi: 10.1039/c6cp00349d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Poiana F., von Ballmoos C., Gonska N., Blomberg M.R.A., Ädelroth P., Brzezinski P. Splitting of the O–O bond at the heme-copper catalytic site of respiratory oxidases. Sci. Adv. 2017;3:e1700279. doi: 10.1126/sciadv.1700279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Blomberg M.R.A. Activation of O2 and NO in heme-copper oxidases–mechanistic insights from computational modelling. Chem. Soc. Rev. 2020;49:7301–7330. doi: 10.1039/D0CS00877J. [DOI] [PubMed] [Google Scholar]
- 154.Jasaitis A., Verkhovsky M.I., Morgan J.E., Verkhovskaya M., Wikström M. Assignment and Charge Translocation Stoichiometries of the Major Electrogenic Phases in the Reaction of Cytochrome c Oxidase with Dioxygen. Biochemistry. 1999;38:2697–2706. doi: 10.1021/bi982275l. [DOI] [PubMed] [Google Scholar]
- 155.Szundi I., Funatogawa C., Soulimane T., Einarsdóttir Ó. The Reactions of O2 and NO with Mixed-Valence ba3 Cytochrome c Oxidase from Thermus thermophilus. Biophys. J. 2019;118:386–395. doi: 10.1016/j.bpj.2019.11.3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Poole R.K., Kumar C., Salmon I., Chance B. The 650 nm Chromophore in Escherichia coli is an ‘Oxy-’ or Oxygenated Compound, Not the Oxidized Form of Cytochrome Oxidase d: An Hypothesis. Microbiology. 1983;129:1335–1344. doi: 10.1099/00221287-129-5-1335. [DOI] [PubMed] [Google Scholar]
- 157.Kahlow M.A., Loehr T.M., Zuberi T.M., Gennis R.B. The oxygenated complex of cytochrome d terminal oxidase: Direct evidence for iron-oxygen coordination in a chlorin-containing enzyme by resonance Raman spectroscopy. J. Am. Chem. Soc. 1993;115:5845–5846. doi: 10.1021/ja00066a071. [DOI] [Google Scholar]
- 158.Hill B.C., Hill J.J., Gennis R.B. The room temperature reaction of carbon monoxide and oxygen with the cytochrome bd quinol oxidase from Escherichia coli. Biochemistry. 1994;33:15110–15115. doi: 10.1021/bi00254a021. [DOI] [PubMed] [Google Scholar]
- 159.Mamedov M.D., Tyunyatkina A.A., Siletsky S.A., Semenov A.Y. Voltage changes involving photosystem II quinone–iron complex turnover. Eur. Biophys. J. 2006;35:647–654. doi: 10.1007/s00249-006-0069-3. [DOI] [PubMed] [Google Scholar]
- 160.Siletsky S.A., Mamedov M.D., Lukashev E.P., Balashov S.P., Dolgikh D.A., Rubin A.B., Kirpichnikov M.P., Petrovskaya L.E. Electrogenic steps of light-driven proton transport in ESR, a retinal protein from Exiguobacterium sibiricum. Biochim. Biophys. Acta Bioenerg. 2016;1857:1741–1750. doi: 10.1016/j.bbabio.2016.08.004. [DOI] [PubMed] [Google Scholar]
- 161.Siletsky S.A., Mamedov M.D., Lukashev E.P., Balashov S.P., Dolgikh D.A., Rubin A.B., Kirpichnikov M., Petrovskaya L.E. Elimination of proton donor strongly affects directionality and efficiency of proton transport in ESR, a light-driven proton pump from Exiguobacterium sibiricum. Biochim. Biophys. Acta Bioenerg. 2019;1860:1–11. doi: 10.1016/j.bbabio.2018.09.365. [DOI] [PubMed] [Google Scholar]
- 162.Siletsky S.A., Lukashev E.P., Mamedov M.D., Borisov V.B., Balashov S.P., Dolgikh D.A., Rubin A.B., Kirpichnikov M.P., Petrovskaya L.E. His57 controls the efficiency of ESR, a light-driven proton pump from Exiguobacterium sibiricum at low and high pH. Biochim. Biophys. Acta Bioenerg. 2021;1862:148328. doi: 10.1016/j.bbabio.2020.148328. [DOI] [PubMed] [Google Scholar]
- 163.Kovalev K., Volkov D., Astashkin R., Alekseev A., Gushchin I., Haro-Moreno J.M., Chizhov I., Siletsky S., Mamedov M., Rogachev A., et al. High-resolution structural insights into the heliorhodopsin family. Proc. Natl. Acad. Sci. USA. 2020;117:4131–4141. doi: 10.1073/pnas.1915888117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Siletsky S.A., Han D., Brand S., Morgan J.E., Fabian M., Geren L., Millett F., Durham B., Konstantinov A.A., Gennis R.B. Single-electron photoreduction of the PM intermediate of cytochrome c oxidase. Biochim. Biophys. Acta Bioenerg. 2006;1757:1122–1132. doi: 10.1016/j.bbabio.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 165.Ribacka C., Verkhovsky M.I., Belevich I., Bloch D.A., Puustinen A.A., Wikström M. An Elementary Reaction Step of the Proton Pump Is Revealed by Mutation of Tryptophan-164 to Phenylalanine in Cytochrome c Oxidase from Paracoccus denitrificans. Biochemistry. 2005;44:16502–16512. doi: 10.1021/bi0511336. [DOI] [PubMed] [Google Scholar]
- 166.Siletsky S., Kaulen A.D., Konstantinov A.A. Resolution of Electrogenic Steps Coupled to Conversion of Cytochrome c Oxidase from the Peroxy to the Ferryl−Oxo State. Biochemistry. 1999;38:4853–4861. doi: 10.1021/bi982614a. [DOI] [PubMed] [Google Scholar]
- 167.Belevich I., Verkhovsky M.I., Wikström M. Proton-coupled electron transfer drives the proton pump of cytochrome c oxidase. Nature. 2006;440:829–832. doi: 10.1038/nature04619. [DOI] [PubMed] [Google Scholar]
- 168.Belevich I., Gorbikova E., Belevich N.P., Rauhamaki V., Wikstrom M., Verkhovsky M.I. Initiation of the proton pump of cytochrome c oxidase. Proc. Natl. Acad. Sci. USA. 2010;107:18469–18474. doi: 10.1073/pnas.1010974107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Faxén K., Gilderson G., Ädelroth P., Brzezinski P. A mechanistic principle for proton pumping by cytochrome c oxidase. Nature. 2005;437:286–289. doi: 10.1038/nature03921. [DOI] [PubMed] [Google Scholar]
- 170.Kuznetsova S.S., Azarkina N.V., Vygodina T.V., Siletsky S.A., Konstantinov A.A. Zinc ions as cytochrome c oxidase inhibitors: Two sites of action. Biochemistry (Moscow) 2005;70:128–136. doi: 10.1007/s10541-005-0091-6. [DOI] [PubMed] [Google Scholar]
- 171.Verkhovsky M.I., Jasaitis A., Verkhovskaya M., Morgan J.E., Wikström M. Proton translocation by cytochrome c oxidase. Nature. 1999;400:480–483. doi: 10.1038/22813. [DOI] [PubMed] [Google Scholar]
- 172.Zaslavsky D., Smirnova I., Siletsky S., Kaulen A., Millett F., Konstantinov A. Rapid kinetics of membrane potential generation by cytochrome c oxidase with the photoactive Ru(II)-tris-bipyridyl derivative of cytochrome c as electron donor. FEBS Lett. 1995;359:27–30. doi: 10.1016/0014-5793(94)01443-5. [DOI] [PubMed] [Google Scholar]
- 173.Ostendorff H., Peirano R.I., Peters M.A., Schlüter A., Bossenz M., Scheffner M., Bach I. Ubiquitination-dependent cofactor exchange on LIM homeodomain transcription factors. Nature. 2002;416:99–103. doi: 10.1038/416099a. [DOI] [PubMed] [Google Scholar]
- 174.Svahn E., Faxén K., Gennis R.B., Brzezinski P. Proton pumping by an inactive structural variant of cytochrome c oxidase. J. Inorg. Biochem. 2014;140:6–11. doi: 10.1016/j.jinorgbio.2014.06.016. [DOI] [PubMed] [Google Scholar]
- 175.Kahlow M.A., Zuberi T.M., Gennis R.B., Loehr T.M. Identification of a ferryl intermediate of Escherichia coli cytochrome d terminal oxidase by resonance Raman spectroscopy. Biochemistry. 1991;30:11485–11489. doi: 10.1021/bi00113a001. [DOI] [PubMed] [Google Scholar]
- 176.Sztachova T., Pechova I., Mikulova L., Stupak M., Jancura D., Fabian M. Peroxide stimulated transition between the ferryl intermediates of bovine cytochrome c oxidase. Biochim. Biophys. Acta Bioenerg. 2021;1862:148447. doi: 10.1016/j.bbabio.2021.148447. [DOI] [PubMed] [Google Scholar]
- 177.Jünemann S., Wrigglesworth A.J.M., Rich P.R. Effects of Decyl-aurachin D and Reversed Electron Transfer in Cytochrome bd. Biochemistry. 1997;36:9323–9331. doi: 10.1021/bi970055m. [DOI] [PubMed] [Google Scholar]
- 178.Von der Hocht I., van Wonderen J.H., Hilbers F., Angerer H., MacMillan F., Michel H. Interconversions of P and F intermediates of cytochrome c oxidase from Paracoccus denitrificans. Proc. Natl. Acad. Sci. USA. 2011;108:3964–3969. doi: 10.1073/pnas.1100950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Borisov V.B., Gennis R.B., Konstantinov A.A. Interaction of cytochrome bd from Escherichia coli with hydrogen peroxide. Biochemistry (Moscow) 1995;60:231–239. [PubMed] [Google Scholar]
- 180.Borisov V., Gennis R., Konstantinov A.A. Peroxide complex of cytochrome bd: Kinetics of generation and stability. Biochem. Mol. Boil. Int. 1995;37:975–982. [PubMed] [Google Scholar]
- 181.Borisov V.B. Effect of Membrane Environment on the Ligand-Binding Properties of the Terminal Oxidase Cytochrome bd-I from Escherichia coli. Biochemistry (Moscow) 2020;85:1603–1612. doi: 10.1134/S0006297920120123. [DOI] [PubMed] [Google Scholar]
- 182.Sun J., Osborne J.P., Kahlow M.A., Kaysser T.M., Gennis R.B., Loehr T.M. Resonance Raman studies of Escherichia coli cytochrome bd oxidase. Selective enhancement of the three heme chromophores of the “as-isolated” enzyme and characterization of the cyanide adduct. Biochemistry. 1995;34:12144–12151. doi: 10.1021/bi00038a007. [DOI] [PubMed] [Google Scholar]
- 183.Sun J., Kahlow M.A., Kaysser T.M., Osborne J.P., Hill J.J., Rohlfs R.J., Hille R., Gennis R.B., Loehr T.M. Resonance Raman Spectroscopic Identification of a Histidine Ligand of b595 and the Nature of the Ligation of Chlorin d in the Fully Reduced Escherichia coli Cytochrome bd Oxidase. Biochemistry. 1996;35:2403–2412. doi: 10.1021/bi9518252. [DOI] [PubMed] [Google Scholar]
- 184.Lorence R.M., Koland J., Gennis R.B. Coulometric and spectroscopic analysis of the purified cytochrome d complex of Escherichia coli: Evidence for the identification of “cytochrome a1” as cytochrome b595. Biochemistry. 1986;25:2314–2321. doi: 10.1021/bi00357a003. [DOI] [PubMed] [Google Scholar]
- 185.Jɒnemann S., Wrigglesworth J.M. Cytochrome bd Oxidase from Azotobacter vinelandii. J. Biol. Chem. 1995;270:16213–16220. doi: 10.1074/jbc.270.27.16213. [DOI] [PubMed] [Google Scholar]
- 186.Forte E., Borisov V.B., Siletsky S.A., Petrosino M., Giuffrè A. In the respiratory chain of Escherichia coli cytochromes bd-I and bd-II are more sensitive to carbon monoxide inhibition than cytochrome bo3. Biochim. Biophys. Acta Bioenerg. 2019;1860:148088. doi: 10.1016/j.bbabio.2019.148088. [DOI] [PubMed] [Google Scholar]