Abstract
Preoperative identification of unresectable pleural mesothelioma could spare unnecessary surgical intervention and accelerate the initiation of medical treatments. The aim of this study is to determine predictors of unresectability, testing our impression that the contraction of the ipsilateral hemithorax is often associated with exploratory thoracotomy. Between 1994 and 2020, 291 patients undergoing intended macroscopic complete resection for mesothelioma after chemotherapy were retrospectively investigated. Eligible patients (n = 58) presented a preoperative 3 mm slice-thickness chest computed tomography without pleural effusion or hydropneumothorax. Lung volumes (segmented using a semi-automated method), modified-Response Evaluation Criteria in Solid Tumors (RECIST) measurements, and spirometries were collected after chemotherapy. Multivariable analysis was performed to determine the predictors of unresectability. An unresectable disease was found at the time of operation in 25.9% cases. By multivariable analysis, the total lung capacity (p = 0.03) and the disease burden (p = 0.02) were found to be predictors of unresectability; cut-off values were <77.5% and >120.5 mm, respectively. Lung volumes were not confirmed to be associated with unresectability at multivariable analysis, probably due to the correlation with the disease burden (p < 0.001; r = −0.4). Our study suggests that disease burden and total lung capacity could predict MPM unresectability, helping surgeons in recommending surgery or not in a multimodality setting.
Keywords: mesothelioma, thoracic surgery, RECIST
1. Introduction
Malignant pleural mesothelioma (MPM) is an aggressive asbestos-related tumor with a poor prognosis. To date, multimodality treatment including chemotherapy and surgery, with or without radiotherapy, is the gold standard therapy for selected patients with epithelial and early stage MPM [1]. In this setting, the goal of surgery is to achieve the macroscopic complete resection (MCR) [2], obtained by either extrapleural pneumonectomy (EPP) or pleurectomy/decortication (PD). The average rate of MCR reported in the literature is 70% [3]; thus, 30% of patients underwent aborted resection due to a disease technically unresectable found at the time of surgery. The preoperative identification of an unresectable MPM could avoid futile explorative thoracotomy (ET) with R2 resection, accelerate the initiation of medical therapies, and prevent unnecessary costs to the National Health System.
In our experience, the most common factor precluding MCR is the diffuse chest wall invasion (DCWI), frequently associated with the contraction of the ipsilateral hemithorax, that has higher pleural thickness and lower aerated lung volumes. The aim of this study is to determine preoperative predictors of unresectability, testing our anecdotal impression that the contraction of the ipsilateral hemithorax is often associated with ET.
2. Material and Methods
2.1. Patient Selection
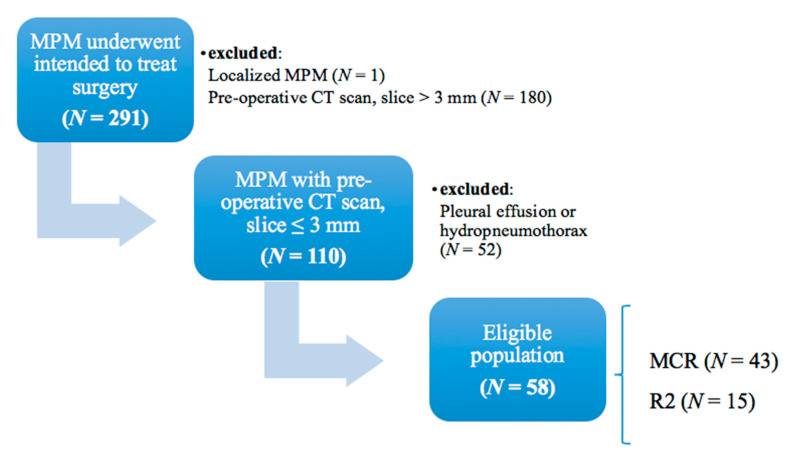
Between July 1994 and August 2020, 291 patients undergoing intended MCR for MPM after iCT at Padova University Hospital were retrospectively investigated. The data collection and study protocol have both been approved by the local ethics committee (n.pd732-2220T), and participating patients were asked to sign a written consent. Eligible patients (n = 58) were those with a preoperative 3 mm slice-thickness chest computed tomography (CT) scan without pleural effusion or hydropneumothorax (Figure 1).
Figure 1.
Flow diagram detailing study cohort. Final eligible population was composed by 43 patients underwent macroscopic complete resection (MCR), and 15 patients with an unresectable disease underwent explorative thoracotomy with a R2 resection (R2). MPM: malignant pleural mesothelioma; CT: computed tomography.
Demographics (age at surgery and sex) and all relevant clinical and radiological variables, i.e., histology, side, talc pleurodesis, pathological stage, preoperative pulmonary function tests (PFTs), preoperative lung ventilation/perfusion scan, standardized uptake volume (SUV) max and metabolic response at post-iCT 18F-fluorodeoxyglucose positron emission tomography/CT (18F-FDG PET/CT) scan, lung volume measurements, and pleural thickness (disease burden and maximum pleural thickness at each level) according to Response Evaluation Criteria in Solid Tumors (RECIST) modified criteria, were collected in order to identify possible predictors of unresectability.
2.2. Preoperative Evaluation and Surgical Approach
At our institution, eligibility criteria for multimodality treatment included biopsy-proven MPM (of any histological subtype) at clinical stage T1-3 N0-1 M0 and anticipated complete resectability by EPP or PD, as estimated by an experienced thoracic surgeon in a multidisciplinary setting.
We prefer to perform chemotherapy in preparation for surgery for hopeful MCR. In fact, according to our experience, induction chemotherapy (iCT) (a) can be administered with high dosage in patients no longer debilitated by surgery; (b) can lead to a down-staging of the disease, allowing obtaining a satisfactory MCR; and (c) allows for a better surgical selection based on the response to chemotherapy (a poor response may avoid an unnecessary surgical treatment in a more aggressive disease). For the iCT, a platinum-based regimen with gemcitabine or pemetrexed was used for two to six cycles. Subsequently, patients were preoperatively evaluated with a chest CT scan, a 18F-FDG PET/CT scan (since 2010), an echocardiogram, PFTs, cardiopulmonary exercise testing, and a lung ventilation/perfusion scan.
In particular, the percentage to predicted levels of current volume (CV), forced vital capacity (FVC), forced expiratory volume in 1s (FEV1), total lung capacity (TLC), and diffusion lung capacity for carbon monoxide (DLCO) were collected with a spirometer (Biomedin, Padova, Italy) using a standardized method [4,5,6]. Interpretation of the spirometric data was performed following European Respiratory Society/American Thoracic Society guidelines [7].
Surgery was performed within 4–6 weeks of completing the final cycle of chemotherapy in patients whose pathology had at least been stabilized at the CT scan and PET/CT scan.
The eighth edition of the lung cancer tumor, node, and metastasis (TNM) staging system was used to define the extent of the disease [8]. The PD was based on total visceral and parietal pleura removal, while the EPP was employed in case of macroscopic pulmonary parenchyma invasion, both through an extended posterolateral thoracotomy (including sixth rib resection and/or multilevel thoracotomies). The resection and reconstruction of the pericardium and/or diaphragm were performed only in the case of macroscopic involvement. MCR was defined as the removal of all grossly visible and palpable tumors. ET for unresectable MPM, secondary either to DCWI or to the invasion of intrathoracic organs, was labeled as R2 resection. DCWI was defined as an intraoperative finding of tumor invasion through the endothoracic fascia into more than two intercostal muscles or ribs and/or multifocal invasion of the chest wall precluding MCR [3].
2.3. Radiological Evaluation
Two thoracic radiologists measured aerated lung volumes and pleural thickness using an Open Source Software (3D Slicer, Brigham and Women’s Hospital, Harvard University, NIH, www.slicer.org; downloaded on 11 June 2020) using contrast-enhanced CT images with 3 mm slice thickness. Pulmonary segmentation was performed via a semi-automated method [9]. First, a gray level threshold between −1020 and −250 Hounsfield units was applied. Subsequently, the airways (main bronchi and trachea) were cropped out and the volume (cm3) of each lung separately computed. The volumetric difference between the lung affected by MPM and the contralateral one was calculated.
The pleural thickness was evaluated according to RECIST modified criteria [10], subdividing the hemithorax divided into three levels: the upper level extended from the apex of the lung to the inferior margin of the aortic arch, the middle level included the pleura between the upper and lower levels, and the lower level below the left atrium. In the case of lesions too small to measure and in the case of the absence of lesions, default values of 5 and 0 mm were assigned, respectively. The two maximum tumor thicknesses perpendicular to the chest wall or mediastinum were measured at each level, and the sum of the six measurements was reported as the disease burden (mm).
2.4. Statistical Analysis
The data were reported as absolute numbers, percentages, or median values with interquartile range (IQR). The Fisher exact test and the Mann–Whitney test were performed to analyze categorical and continuous variables, respectively. The Spearman test was employed to evaluate any correlation between variables. Optimal cut-off values were determined by maximizing the sum of sensitivity and specificity. Variables with a p-value < 0.05 at univariable regression analysis were entered into multivariable analysis in order to evaluate independent predictors of unresectability. A p-value < 0.05 was considered statistically significant. All tests were two-tailed. All the statistical analyses were performed using SPSS 27 version for windows (SPSS, Inc., Chicago, IL, USA) and GraphPad Prism 8 Version 8.4.3 for macOS.
3. Results
3.1. Patient Characteristics
Patient characteristics are summarized in Table 1.
Table 1.
Patient characteristics.
| Characteristics | MCR, N = 43 | R2, N = 15 | p-Value |
|---|---|---|---|
| Age (years), median (IQR) | 64 (56–68) | 63 (59–69) | 0.6 |
| Sex, n (%) | 0.01 | ||
| Male | 25 (58) | 14 (93) | |
| Female | 18 (42) | 1 (7) | |
| Side, n (%) | 0.4 | ||
| Right | 22 (51) | 10 (67) | |
| Left | 21 (49) | 5 (33) | |
| iCT regimen, n (%) | Platinum + pemetrexed, 42 (97.7) gemcitabine, 1 (2.3) |
Platinum + pemetrexed, 13 (87) Platinum + gemcitabine, 2 (13) |
0.2 |
| iCT cycles, median (IQR) | 4 (4–5.25) | 4 (4–4) | 0.5 |
| CV (%), median (IQR) | 92.5 (78–106.8) | 73 (65–77) | <0.001 |
| FVC (%), median (IQR) | 92 (78–105.3) | 73 (60–75) | <0.001 |
| FEV1 (%), median (IQR) | 89 (77–102) | 77 (67–87) | 0.007 |
| TLC (%), median (IQR) | 89 (79–97) | 75 (71–80) | 0.003 |
| DLCO (%), median (IQR) | 76 (63–87) | 66 (51–77) | 0.04 |
| Scintigraphy scan, n (%) | 0.3 | ||
| No | 10 (23) | 6 (40) | |
| Ventilation/Perfusion | 30 (70) | 9 (60) | |
| Perfusion only | 3 (7) | 0 (0) | |
| Ipsilateral lung perfusion (%), median (IQR) | 37.76 (32.57–45.88) | 34.05 (19.62–38.86) | 0.08 |
| Ipsilateral lung ventilation (%), median (IQR) | 33.31 (25–44.88) | 28.4 (9.92–35.34) | 0.07 |
| Post-induction PET/CT, n (%) | 0.2 | ||
| No | 11 (26) | 3 (20) | |
| Negative/reduced | 16 (37) | 3 (20) | |
| Stable/augmented | 16 (37) | 9 (60) | |
| Preoperative SUV max, median (IQR) | 7.58 (2.53–11.23) | 7.95 (5.99–11.52) | 0.3 |
| Talc pleurodesis, n (%) | 0.1 | ||
| No | 12 (28) | 8 (53) | |
| Yes | 31 (72) | 7 (47) | |
| Histology, n (%) | >0.99 | ||
| Epithelial | 37 (86) | 13 (87) | |
| Non-epithelial | 6 (14) | 2 (13) | |
| cTNM8, n (%) | >0.99 | ||
| I | 34 (79) | 12 (80) | |
| II | 9 (21) | 3 (20) | |
| pTNM8, n (%) | <0.001 | ||
| Complete remission-I–II | 29 (67) | 0 (0) | |
| III–IV | 14 (33) | 15 (100%) | |
| Ipsilateral pathological lung volume (cm3), median (IQR) | 1944 (1528–2352) | 1545 (1322–1782) | 0.03 |
| Difference in contralateral and ipsilateral lung volume (cm3), median (IQR) | 677.2 (217.4–1252) | 1371 (667.4–2164) | 0.02 |
| Difference in contralateral and ipsilateral lung volume (%), median (IQR) | 25.28 (7.4–41.73) | 47.01 (28.39–59.01) | 0.01 |
| Max pleural thickness at upper level (mm), median (IQR) | 10 (5–20) | 23 (9–32) | 0.002 |
| Max pleural thickness at medium level (mm), median (IQR) | 12 (5–21) | 22 (13–35) | 0.007 |
| Max pleural thickness at inferior level (mm), median (IQR) | 13 (5–21) | 28 (17–43) | 0.005 |
| Disease burden (mm), median (IQR) | 57 (36–99) | 133 (70–181) | 0.001 |
MCR = macroscopic complete resection; iCT = induction chemotherapy; CV = current volume; FVC = forced vital capacity; FEV1 = forced expiratory volume in 1 s; TLC = total lung capacity; DLCO = diffusion lung capacity for carbon monoxide; PET/CT = positron emission tomography/computed tomography; cTNM8 = clinical tumor, node and metastasis Eight Edition; pTNM8 = pathological tumor, node and metastasis Eight Edition.
Forty-three patients attained MCR, 33 (77%) with PD and 10 (23%) with EPP, while 15 patients were found to be R2 resections, due to DCWI (n = 13), aortic adventitia infiltration (n = 1), and diaphragmatic pillar infiltration (n = 1). The two groups (MCR and R2) were homogeneous for age, side, iCT (regimen and number of cycles administered), SUV max, and metabolic response at 18F-FDG PET/CT scan after iCT, talc pleurodesis, histology (epithelial vs non-epithelial), and clinical stage. Conversely, the R2 group included more males (p = 0.01), lower PFTs (VC% p < 0.001; FVC% p < 0.001; FEV1% p = 0.007; TLC% p = 0.003; DLCO p = 0.04), lower ipsilateral pathological lung volume (p = 0.03), higher volumetric difference between the contralateral and the ipsilateral pathological lung (p = 0.01), and higher disease burden (p = 0.001). Moreover, the R2 group presented a trend toward lower ventilation (p = 0.07) and perfusion (p = 0.08) measurements at the preoperative ventilation/perfusion scan. Clinical and radiological characteristics of the 15 patients who underwent ET are reported in Table 2.
Table 2.
Characteristic of 15 patients who underwent exploratory thoracotomy.
| Patient | Sex | Side | Cause of R2 | TLC (%) | Disease Burden (mm) | Ipsilateral Lung Volume (cm3) | Difference Contralateral-Ipsilateral Lung Volume (%) |
|---|---|---|---|---|---|---|---|
| 1 | M | Right | DCWI | 88 | 241 | 1337.17 | 49.25 |
| 2 | M | Left | DCWI | 71 | 201 | 989.9 | 76.68 |
| 3 | M | Right | DCWI | 75 | 99 | 1512.07 | 59.01 |
| 4 | M | Right | DCWI | 73 | 58 | 2130.43 | 1.88 |
| 5 | M | Right | DCWI | 77 | 35 | 1584.56 | 52.92 |
| 6 | M | Left | Aortic adventitia infiltration | 86 | 183 | 2009.94 | 33.98 |
| 7 | M | Left | DCWI | 93 | 133 | 1683.51 | 28.39 |
| 8 | M | Right | DCWI | 72 | 181 | 991.43 | 75.10 |
| 9 | M | Right | DCWI | 74 | 70 | 1776.78 | 46.35 |
| 10 | M | Right | Diaphragmatic pillar infiltration | 71 | 134 | 2964.71 | −28.35 |
| 11 | M | Right | DCWI | 80 | 140 | 1545.46 | 47.01 |
| 12 | M | Left | DCWI | 56 | 162 | 711.33 | 75.26 |
| 13 | M | Right | DCWI | 75 | 89 | 1471.99 | 34.46 |
| 14 | M | Left | DCWI | 60 | 123 | 1321.67 | 55.41 |
| 15 | F | Right | DCWI | 77 | 34 | 1782.33 | 0.74 |
TLC = total lung capacity; M = male; F = female; DCWI = diffuse chest wall invasion.
3.2. Preoperative Predictors of Unresectability in MPM
Table 3 displays the results of univariable and multivariable regression analysis performed to determine the association of preoperative factors with unresectability.
Table 3.
Univariable and multivariable regression analysis.
| Univariable | Multivariable | ||
|---|---|---|---|
| p-Value | OR (95%CI) | p-Value | |
| TLC (%) | 0.005 | 0.920 (0.853–0.992) | 0.03 |
| Ipsilateral pathological lung volume (cm3) | 0.04 | 1.000 (0.998–1.002) | 0.9 |
| Difference in contralateral and ipsilateral lung volume (%) | 0.03 | 0.995 (0.955–1.037) | 0.8 |
| Disease burden (mm) | 0.002 | 1.020 (1.003–1.038) | 0.02 |
TLC = total lung capacity.
In univariable analysis, the TLC% (p = 0.005), the ipsilateral pathological lung volume (p = 0.04), the volumetric difference between the contralateral and the ipsilateral pathological lung (p = 0.03), and the disease burden (p = 0.002) were associated with ET, while only the TLC% (p = 0.03, OR 0.920 95%CI 0.853–0.992) and the disease burden (p = 0.02, OR 1.020 95%IC 1.003–1.038) were confirmed as independent predictors of unresectability in multivariable analysis. The optimal cut-off value for the TLC% and the disease burden as predictors of unresectability were <77.5% (AUC = 0.81, p < 0.001, sensitivity = 73.3%, specificity = 80%) and >120.5 mm (AUC = 0.77, p = 0.002, sensitivity = 60%, specificity = 90.7%), respectively.
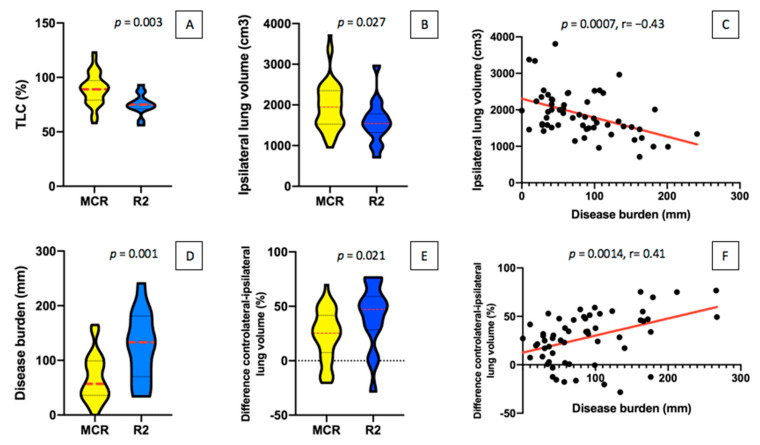
Linear regression analysis demonstrated a correlation between the variation of the disease burden and the variation in ipsilateral pathological lung volume (p < 0.001; r = −0.4) and in the difference between the contralateral and the ipsilateral pathological lung (p = 0.001; r = 0.4). The optimal cut-off value for the ipsilateral pathological lung volume and the volumetric difference between the contralateral and the ipsilateral pathological lung were <1794 cm3 (AUC = 0.69, p = 0.03, sensitivity = 80%, specificity = 58.2%) and >1298 cm3 (AUC = 0.70, p = 0.02, sensitivity = 60%, specificity = 81.4%) or >46.22% (AUC = 0.71, p = 0.02; sensitivity = 60%, specificity = 81.4%), respectively (Figure 2 and Figure 3).
Figure 2.
Comparison of between resectable (MCR) and unresectable (R2) cohorts in TLC% (A), ipsilateral lung volume (B), disease burden (D), and difference in contralateral and ipsilateral lung volume (E). Correlation between the increase of disease burden with both the decrease of the ipsilateral pathological lung volume (C) and the increase of the difference between the contralateral and the ipsilateral pathological lung (F). TLC: total lung capacity.
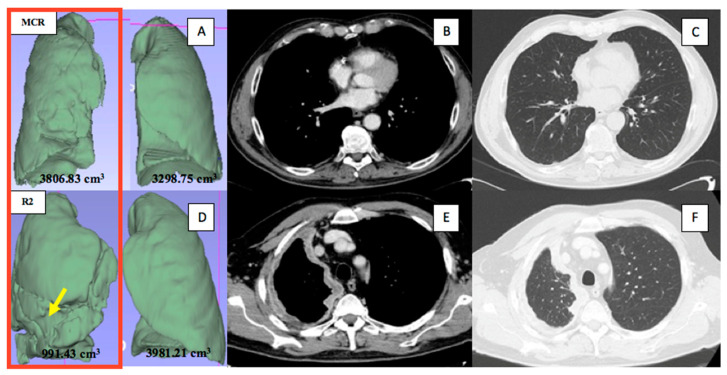
Figure 3.
Representative radiological measurements. Upper panels, a patient with a right-sided MPM affected by the lowest disease burden, who underwent macroscopic complete resection: the aerated ipsilateral and contralateral lung volumes (A) and the pleural thickness (too small to measure) in the axial CT images on the portal venous phase (B) and the lung window (C), at the medium level. Lower panels, a patient with a right-sided MPM affected by the highest disease burden (the arrow indicated the furrows on the three-dimensional lung reconstruction caused by the pleural disease), who underwent R2 resection: the aerated ipsilateral and contralateral lung volumes (D) and the pleural thickness in the axial CT images on the portal venous phase (E) and the lung window (F), at the upper level.
In the MCR group, 36 (84%) and 39 (91%) patients presented the TLC ≥ 77.5% and the disease burden ≤ 120.5 mm, respectively, whereas 34 (79%) had both. In the R2 group, 11 (73%) and 9 (60%) patients had the TLC < 77.5% and the disease burden > 120.5 mm, respectively, while 5 (33%) presented both.
4. Discussion
4.1. MCR Is the Central Principle of Surgery for MPM
The MPM represents a challenge for surgeons in defining the oncological principles of resection, due to its peculiar growth along the pleural surface with the predilection for local invasion. Compared to most solid tumors in which the anatomical resection can provide a microscopic free margin (R0 resection), often avoiding direct manipulation of the tumor itself, in MPM this is technically very difficult to obtain; thus, the optimal result of most surgical interventions is an MCR with microscopic positive margins (R1 resection) [3,11,12,13].
Hence, MCR has become the central principle of surgery for MPM, supported by retrospective evidence highlighting advantages in survival when compared to R2 resection [3,14,15,16]. According to a recent literature review, 30% of patients underwent aborted resection due to a disease technically found unresectable at the time of surgery [3]. Therefore, improving the preoperative identification of unresectable MPM could avoid futile ET, accelerate the initiation of medical therapies, and prevent unnecessary costs to the National Health System [17]. In our experience, the most common factor precluding MCR is DCWI, which is frequently associated with the contraction of the ipsilateral hemithorax.
4.2. The Role of CT and Spirometry
Consequently, we analyzed the radiological parameters that correlated with the contracted hemithorax (that is aerated lung volumes and pleural thickness) and PFTs (particularly TLC% as an indicator of restrictive syndrome) as possible preoperative predictors of unresectability. Recently, Burt and collaborators created a novel three-dimensional radiographic metric of the thoracic cage volume (TCV) and demonstrated that a 5% decrease in TCV compared with the contralateral side was significantly associated with unresectability due to DCWI [3]. Nevertheless, the aforementioned novel method was based on a fully manual segmentation and, when we tried to reproduce the same measurements, it required almost two hours per patient. For this reason, in order to test our hypothesis, we used two methods already codified in the literature, which are faster and easier to reproduce: the semi-automated segmentation of the aerated lung volumes (thirty minutes per patient) [9] and the RECIST modified criteria measuring pleural thickness (ten minutes per patient) [10]. Both lung volumes and pleural thickness according to RECIST modified criteria play an important and consolidated prognostic role in MPM survival [9,10,18,19,20,21], but to the best of our knowledge, they have not ever been tested as predictors of unresectability. The TLC% (p = 0.03, OR 0.920 95%CI 0.853–0.992) and the disease burden (p = 0.02, OR 1.020 95%IC 1.003–1.038) were found to be independent predictors of unresectability in multivariable analysis, with an optimal cut-off value of <77.5% (AUC = 0.81, p < 0.001, sensitivity = 73.3%, specificity = 80%) and >120.5 mm (AUC = 0.77, p = 0.002, sensitivity = 60%, specificity = 90.7%), respectively; whereas aerated lung volumes were significantly associated with ET only in univariable analysis, probably due to the strong correlation with the disease burden. In fact, the linear regression analysis highlighted a correlation between the increase of disease burden with both the decrease of the ipsilateral pathological lung volume (p < 0.001; r = −0.4) and the increase of the difference between the contralateral and the ipsilateral pathological lung (p = 0.001; r = 0.4). Over time, MPM inevitably leads to a restrictive syndrome (TLC < 81%); in fact, the pleural thickness squeezes the lung parenchyma and makes it become stiffer. Moreover, it reduces thoracic cage expansion and diaphragmatic mobility, causing an impairment to respiratory mechanics with ventilator pump failure [22,23]. We previously reported the role of PFTs as indicators of cytoreductive efficacy of iCT [22,23]. With this study, we add an important role to PFTs: they could be an additional tool to better improve the preoperative identification of MPM disease not amenable to MCR, mostly if a higher disease burden is present. Pleural thickness has been recently reported as a useful prognostic indicator of MPM: the International Association for the Study of Lung Cancer recently revised the definition of MPM staging (Eighth Edition) and mentioned that pleural thickness might be useful in T-component evaluation [20,21,24]. The two patients in our study with aortic adventitia infiltration and diaphragmatic pillar infiltration presented disease burden values of 183 and 134 mm, respectively; both were identified at the cut-off level. Therefore, compared to Burt et al. who considered only unresectability due to DCWI [3], the use of the disease burden according to RECIST modified criteria also could individuate the unresectable disease secondary to the invasion of the intrathoracic organs.
4.3. Limitations of the Study
This study presents some limitations including, firstly, its retrospective nature. Secondly, PTFs results are strongly dependent on the compliance of the patient and could be impaired in the event of inadequate pain control. Thirdly, most patients received talc pleurodesis: we were unable to evaluate the influence of this procedure on PFTs and on radiological assessment. Fourthly, it should be addressed that we have excluded patients with pleural effusion or hydropneumothorax because the effusion may have hampered the measurement of pleural thickness and, given that the first step of the applied semi-automatic segmentation of the lungs was based on a threshold, the air component of the hydropneumothorax may have caused a bias in the computation of pulmonary volumes. Lastly, we acknowledge that the magnetic resonance imaging (MRI) is superior to CT for prediction of the chest wall invasion, but we did not perform it routinely in the pre-operative evaluation, representing a limitation of our study.
5. Conclusions
Our study suggests that disease burden, in addition to the prognostic role already known in the literature, and TLC% could predict MPM unresectability. Simple, non-invasive, and inexpensive tests (computed tomography and spirometry) can help surgeons in recommending surgery or not in a multimodality setting. This data should be validated further by prospective studies with larger samples.
Author Contributions
Conceptualization: A.B., A.D. and C.G.; methodology: A.B., A.D., C.G. and F.R.; software: A.M., S.T. and G.Z.; validation: F.R. and A.D.; formal analysis: A.B., N.B. and A.D.; investigation: A.Z. and G.P.; resources: G.P. and F.R.; data curation, A.B., A.M., S.T. and G.Z.; writing—original draft preparation: A.B., A.D. and C.G., review and editing: A.Z. and F.R.; visualization: G.P. and F.R.; supervision: A.D., C.G., A.Z. and F.R.; project administration: A.B. and A.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Ethics Committee of Padua Hospital (n.pd732-2220T).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.National Comprehensive Cancer Network. 2021. Malignant Pleural Mesothelioma (Version 1.2021) [(accessed on 4 December 2020)]. Available online: https://www.nccn.org/professionals/physician_gls/pdf/mpm_blocks.pdf.
- 2.Rusch V., Baldini E.H., Bueno R., De Perrot M., Flores R., Hasegawa S., Klepetko W., Krug L., Lang-Lazdunski L., Pass H., et al. The role of surgical cytoreduction in the treatment of malignant pleural mesothelioma: Meeting summary of the International Mesothelioma Interest Group Congress, 11–14 September 2012, Boston, MA, USA. J. Thorac. Cardiovasc. Surg. 2013;145:909–910. doi: 10.1016/j.jtcvs.2013.01.039. [DOI] [PubMed] [Google Scholar]
- 3.Burt M.B., Lee H.S., Raghuram A.C., Strange C., Mason J., Strange T., Delago J., Sugarbaker D.J. Preoperative prediction of unresectability in malignant pleural mesothelioma. J. Thorac. Cardiovasc. Surg. 2020;159:2512–2520. doi: 10.1016/j.jtcvs.2019.11.035. [DOI] [PubMed] [Google Scholar]
- 4.Miller M.R., Hankinson J., Brusasco V. Standardization of spirometry. Eur. Respir. J. 2005;26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 5.Wanger J., Clausen J.L., Coates A., Pedersen O.F., Brysasco V., Burgos F., Casaburi R., Crapo R., Enright P., van der Grinten C.P.M., et al. Standardization of the measurement of lung volumes. Eur. Respir. J. 2005;26:511–522. doi: 10.1183/09031936.05.00035005. [DOI] [PubMed] [Google Scholar]
- 6.MacIntyre N., Crapo R.O., Viegi G., Johnson D.C., van der Grinten C.P.M., Brusasco V., Burgos F., Casaburi R., Coates A., Enright P., et al. Standardization of the single-breath determination of carbon monoxide uptake in the lung. Eur. Respir. J. 2005;26:720–735. doi: 10.1183/09031936.05.00034905. [DOI] [PubMed] [Google Scholar]
- 7.Pellegrino R., Viegi G., Brusasco V., Crapo R.O., Burgos F., Casaburi R.E.A., Wanger J. Interpretative strategies for lung function tests. Eur. Respir. J. 2005;26:948–968. doi: 10.1183/09031936.05.00035205. [DOI] [PubMed] [Google Scholar]
- 8.Bezenji L., Van Schil P.E., Carp L. The eight TNM classification for malignant pleural mesothelioma. Transl. Lung Cancer Res. 2018;7:543–549. doi: 10.21037/tlcr.2018.07.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sensakovic W.F., Armato S.G., III, Straus C., Roberts R.Y., Caligiuri P., Starkey A., Kindler H.L. Computerized segmentation and measurement of malignant pleural mesothelioma. Med. Phys. 2011;38:238–244. doi: 10.1118/1.3525836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Armato S.G., III, Nowak A.K. Revised modified RECIST criteria for the assessment of response in malignant pleural mesothelioma (version 1.1) J. Thorac. Oncol. 2018;13:1012–1021. doi: 10.1016/j.jtho.2018.04.034. [DOI] [PubMed] [Google Scholar]
- 11.Marulli G., Breda C., Fontana P., Ratto G.B., Leoncini G., Alloisio M., Rea F. Pleurectomy-decortication in malignant pleural mesothelioma: Are different surgical techniques associated with different outcomes? Results from a multicentre study. Eur. J. Cardiothorac. Surg. 2017;52:63–69. doi: 10.1093/ejcts/ezx079. [DOI] [PubMed] [Google Scholar]
- 12.Sugarbaker D.J. Macroscopic complete resection: The goal of primary surgery in multimodality therapy for pleural mesothelioma. J. Thorac. Oncol. 2006;1:175–176. doi: 10.1097/01243894-200602000-00014. [DOI] [PubMed] [Google Scholar]
- 13.Treasure T. What is the best approach for surgery of malignant pleural mesothelioma? It is to put our efforts into obtaining trustworthy evidence for practice. J. Thorac. Cardiovasc. Surg. 2016;151:307–309. doi: 10.1016/j.jtcvs.2015.09.086. [DOI] [PubMed] [Google Scholar]
- 14.Sugarbaker D.J., Flores R.M., Jaklitsch M.T., Richards W.G., Strauss G.M., Corson J.M., Mentzer S.J. Resection margins, extrapleural nodal status and cell type determine post-operative long term survival in trimodality therapy of malignant pleural mesothelioma: Results in 183 patients. J. Thorac. Cardiovasc. Surg. 1999;171:54–63. doi: 10.1016/S0022-5223(99)70469-1. [DOI] [PubMed] [Google Scholar]
- 15.Rusch V.W., Giroux D., Kennedy C. Initial analysis of the international association for the study of lung cancer mesothelioma database. J. Thorac. Oncol. 2012;7:1631–1639. doi: 10.1097/JTO.0b013e31826915f1. [DOI] [PubMed] [Google Scholar]
- 16.Lang-Lazdunski L., Bille A., Papa S., Ruffini E., Cangir A.K., Rice D., Van Meerbeeck J.P. Pleurectomy/decortication, hyperthermic pleural lavage with povidone-iodine, prophylactic radiotherapy and systemic chemotherapy in patients with malignant pleural mesothelioma: A 10-year experience. J. Thorac. Cardiovasc. Surg. 2015;149:558–566. doi: 10.1016/j.jtcvs.2014.10.041. [DOI] [PubMed] [Google Scholar]
- 17.Giles A.E., Kidane B. Commentary: Know your enemy–understanding futility in the battle against malignant pleural mesothelioma. J. Thorac. Cardiovasc. Surg. 2020;159:2523–2524. doi: 10.1016/j.jtcvs.2020.01.034. [DOI] [PubMed] [Google Scholar]
- 18.Armato S.G., III, Sensakovic W.F. Automated lung segmentation for thoracic CT: Impact on computer-aided diagnosis. Acad. Radiol. 2004;11:1011–1021. doi: 10.1016/j.acra.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 19.Labby E.Z., ArmaTo S.G., III, Dignam J.J., Straus C., Kindler H.L., Nowak A.K. Lung volume measurements as surrogate marker for patient response in malingant pleural mesothelioa. J. Thorac. Oncol. 2013;8:478–486. doi: 10.1097/JTO.0b013e31828354c8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hashimoto M., Takeuchi J., Teruhisa T., Kuroda A., Nakamura A., Nakamichi T., Hasegawa S. Pleural thickness after neoadjuvant chemotherapy is a prognostic factor in malignant pleural mesothelioma. J. Thorac. Cardiovasc. Surg. 2019;157:404–413. doi: 10.1016/j.jtcvs.2018.09.106. [DOI] [PubMed] [Google Scholar]
- 21.De Perrot M. Commentary: Is thoracic cage volume a new parameter for clinical staging in mesothelioma? J. Thorac. Cardiovasc. Surg. 2020;159:2520–2521. doi: 10.1016/j.jtcvs.2019.12.048. [DOI] [PubMed] [Google Scholar]
- 22.Marulli G., Rea F., Nicotra S., Favaretto A.G., Perissinotto E., Chizzolini M., Braccioni F. Effect of induction chemotherapy on lung function and exercise capacity in patients affected by malignant pleural mesothelioma. Eur. J. Cardioth. Surg. 2010;37:1464–1469. doi: 10.1016/j.ejcts.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 23.Marulli G., Di Chiara F., Braccioni F., Perissinotto E., Pasello G., Favaretto A.G., Rea F. Changes in pulmonary function tests predict radiological response to chemotherapy in malignant pleural mesothelioma. Eur. J. Cardiothorac. Surg. 2013;44:104–110. doi: 10.1093/ejcts/ezs624. [DOI] [PubMed] [Google Scholar]
- 24.Nowak A.K., Chansky K., Rice D.C., Pass H.I., Kindler H.L., Shemanski L., Yoshimura M. The IASLC Mesothelioma Staging Project: Proposals for revisions of the T descriptors in the forthcoming eighth editions of the TNM classification for mesothelioma. J. Thorac. Oncol. 2016;11:2089–2099. doi: 10.1016/j.jtho.2016.08.147. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.