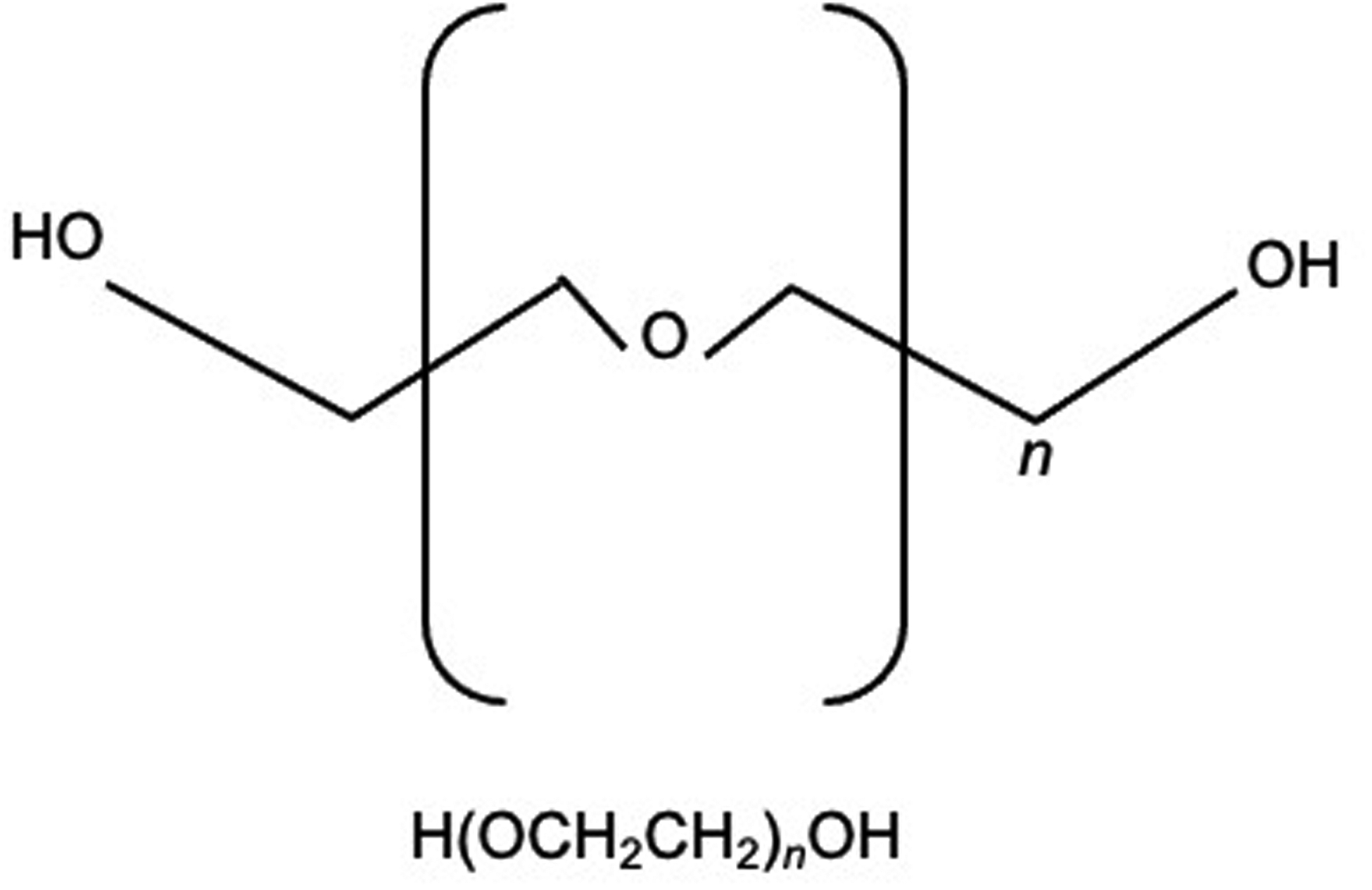
Polyethylene glycol (PEG) is a widely used laxative that has been anecdotally linked to adverse neurobehavioral effects in some children. PEG is a synthetic, neutral, hydrophilic polymer that can be linear or branched, and is composed of varying units of ethylene oxide joined by ether linkages. The molecular structure of PEG is depicted in figure 1 where n represents the number of ethylene oxide units. The molecular weight of PEG polymers depends on the number of ethylene oxide units incorporated during the polymerization process, and PEG 3350 is the molecular weight of the laxative formulation. PEG has a long history dating back to the mid-1800’s and was historically known as a reliable marker for studies of water, solute and nutrient absorption due to its high solubility (1). PEG has numerous applications in clinical medicine and has been utilized as a laxative for more than 70 years (1) because it exerts a high osmotic pressure within the intestinal lumen (2), making it useful both for bowel preparation prior to colonoscopy (3) and treatment of chronic constipation (4).
Figure 1.
In pediatrics, PEG 3350 has been extensively studied in children with functional constipation in whom it improves bowel movement frequency, consistency and straining when compared to placebo (5) and other laxatives (6). Safety studies following the biochemical and clinical profiles of children older than 2 years of age taking PEG 3350 for more than 3 months revealed no major adverse events or laboratory serum abnormalities (7). Similarly, other studies reported mostly gastrointestinal side effects that typically responded to dose adjustment. In 2009, however, the Drug Safety Oversight Board of the United States Food and Drug Administration published a summary of neuropsychiatric side effects that had been reported in children taking PEG for the treatment of constipation including seizures, tics, lethargy, aggression, tremors and obsessive-compulsive behaviors (8). This report raised concern that PEG, or possibly small amounts of more absorbable contaminants found in some preparations of PEG, might have direct neurobehavioral effects. Causative and correlative effects, however, are challenging to distinguish in patient cohorts.
In this issue, Salman et al. (9) take a translational approach to this problem, testing the behavioral effects of administering PEG 3350 once daily for 14 days to young adult, outbred CD-1 mice. They found no difference in three different measures of anxiety-like behaviors in mice that received low dose or high dose PEG 3350 when compared to mice treated with a different laxative (magnesium citrate) or vehicle. This was despite expected changes in stool consistency in laxative-treated mice. Interestingly, the authors did find significant differences in the alpha and beta diversity of fecal microbiota as well as the relative abundance of bacterial taxa, mostly in the mice given high dose PEG or magnesium citrate, suggestive of high dose laxative effects rather than PEG-specific effects. Although these microbiota changes were still evident 14 days after laxative administration was discontinued, they were not associated with any deficits in behavioral testing.
Although this study was short-term in length, likely because it required daily orogastric gavage to administer laxatives to rodents, it provides important evidence that PEG does not directly cause anxiety-like behaviors, at least in mice. In fact, the biggest effect seen on anxiety behaviors in this study was due to repeated testing, with the effect observed in control as well as experimental groups. This prominent testing effect as well as the weight loss observed in all of the mice during the 14-day period of intensive handling, highlights the challenges of rigorously studying cognitive and behavioral phenotypes in rodents. Nevertheless, the results from this work add important evidence that PEG 3350 does not directly cause neurobehavioral problems. This study, moreover, illustrates the power of animal model studies to disassociate cause and effect in probing the etiology of neurobehavioral changes linked with therapies for digestive disease.
In summary, Salman and colleagues pave the way for future studies aiming to answer the question: is treatment with PEG 3350 the cause of the neurobehavioral changes reported in children with constipation? Longer-term translational and human clinical studies are needed to better answer this question. Nonetheless, even if data from such work does not support a direct association between PEG3350 and behavioral changes, caregiver concern for potential side effects will likely persist in clinical practice. Fortunately, the arsenal for the medical treatment of constipation continues to expand in pediatrics and provides many different options to individualize patient care based on symptoms.
Funding:
NIH K08DK110532 and R03DK125636 (M.R.)
Footnotes
Conflict of interest: The authors have nothing to disclose.
References:
- 1.Fordtran JS, Hofmann AF. Seventy Years of Polyethylene Glycols in Gastroenterology: The Journey of PEG 4000 and 3350 From Nonabsorbable Marker to Colonoscopy Preparation to Osmotic Laxative. Gastroenterology. 2017;152(4):675–80. [DOI] [PubMed] [Google Scholar]
- 2.Davis GR, Santa Ana CA, Morawski SG, Fordtran JS. Inhibition of water and electrolyte absorption by polyethylene glycol (PEG). Gastroenterology. 1980;79(1):35–9. [PubMed] [Google Scholar]
- 3.Goldman J, Reichelderfer M. Evaluation of rapid colonoscopy preparation using a new gut lavage solution. Gastrointestinal endoscopy. 1982;28(1):9–11. [DOI] [PubMed] [Google Scholar]
- 4.Puxty JA, Fox RA. Golytely: a new approach to faecal impaction in old age. Age Ageing. 1986;15(3):182–4. [DOI] [PubMed] [Google Scholar]
- 5.Nurko S, Youssef NN, Sabri M, Langseder A, McGowan J, Cleveland M, et al. PEG3350 in the treatment of childhood constipation: a multicenter, double-blinded, placebo-controlled trial. The Journal of pediatrics. 2008;153(2):254–61, 61 e1. [DOI] [PubMed] [Google Scholar]
- 6.Gordon M, MacDonald JK, Parker CE, Akobeng AK, Thomas AG. Osmotic and stimulant laxatives for the management of childhood constipation. The Cochrane database of systematic reviews. 2016(8):CD009118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pashankar DS, Loening-Baucke V, Bishop WP. Safety of polyethylene glycol 3350 for the treatment of chronic constipation in children. Arch Pediatr Adolesc Med. 2003;157(7):661–4. [DOI] [PubMed] [Google Scholar]
- 8.US Food and Drug Administration : Drug Safety Oversight Board. [Available from: https://www.fda.gov/AboutFDA/CentersOffices/OfficeofMedicalProductsandTobacco/CDER/ucm082129.htm.
- 9.Salman SWK, Marte-Ortiz P, Rumpf W, Mashburn-Warren L, Lauber C, Bailey M, Maltz R. Polyethylene glycol 3350 changes stool consistency and the microbiome but not behavior of CD1 mice. Journal of pediatric gastroenterology and nutrition. 2021. [DOI] [PubMed] [Google Scholar]


