Abstract
The clinical characteristics of patients with post-tuberculosis (TB) bronchiectasis have not been well evaluated. We enrolled 598 patients with bronchiectasis who participated in the Korean prospective bronchiectasis registry and compared the characteristics of post-TB bronchiectasis (19.7%) with post-infectious (19.6%), idiopathic (40.8%), and other (19.9%) bronchiectasis. The patients with post-TB bronchiectasis had a lower body mass index, higher rate of chronic obstructive pulmonary disease, and lower rate of asthma than those in the other groups. The patients with post-TB bronchiectasis had more upper lobe involvement, more severe radiological extent, and worse lung function than those in the other groups. Long-acting muscarinic antagonist/long-acting ß agonist use and mucolytics were more commonly used in the patients with post-TB bronchiectasis than those in the other groups, while inhaled corticosteroid/long-acting ß agonist was less commonly used. There were no significant intergroup differences in bronchiectasis severity scores except for FACED, the number of exacerbations, and quality of life. Post-TB bronchiectasis is characterised by reduced lung function and higher rates of mucolytic use when compared with other bronchiectasis; thus, adequate bronchodilator use and airway clearance techniques may alleviate symptom burden in this population.
Keywords: bronchiectasis, tuberculosis, treatment
1. Introduction
The diagnosis of non-cystic fibrosis bronchiectasis (hereafter referred to as bronchiectasis) is increasing worldwide [1,2]. The increased prevalence of bronchiectasis has led to the establishment of several prospective multicentre bronchiectasis registries [3,4,5]. Research from the international registries revealed that the aetiologies of bronchiectasis varied according to region, and tuberculosis (TB) was most common in Asian countries, including India and South Korea [6,7]. Therefore, elucidating specific clinical characteristics of patients with post-TB bronchiectasis is essential to successfully manage Asian patients with bronchiectasis.
Most studies on post-TB bronchiectasis have been performed on patients who survived after TB treatment. Bronchiectasis is a form of TB-associated lung damage that results from the destruction of elastic and muscular components of the bronchial walls [8]. Patients with post-TB bronchiectasis typically show airflow obstruction in addition to recurrent episodes of purulent sputum production and haemoptysis [9,10]. Although two studies assessed the performance of bronchiectasis severity scoring systems in post-TB bronchiectasis [11,12], there is limited information on the clinical characteristics of post-TB bronchiectasis in the context of a bronchiectasis cohort.
This study aimed to assess the clinical characteristics, respiratory treatment, exacerbations, and quality of life of patients with post-TB bronchiectasis compared with those with other bronchiectasis using a prospective multicentre bronchiectasis cohort.
2. Materials and Methods
2.1. Study Design and Participants
This study included participants enrolled in the Korean Multicentre Bronchiectasis Audit and Research Collaboration (KMBARC) registry between August 2018 and December 2019 [3]. The study population comprised adult patients (age ≥ 18 years) with stable bronchiectasis confirmed with chest computed tomography (CT). Exclusion criteria were as follows: (1) bronchiectasis due to cystic fibrosis; (2) traction bronchiectasis associated with interstitial lung disease; (3) patients being actively treated for pneumonia, pulmonary TB, or non-tuberculous mycobacteria (NTM) infection; (4) patients who were unable or unwilling to provide informed consent; and (5) pregnant patients. The detailed registry protocol and baseline characteristics of the patients were described previously [3,6].
This study protocol was approved by the institutional review board of all institutions participating in the KMBARC registry, including Hallym University Kangnam Sacred Heart Hospital (application no. 2018-07-16), and written informed consent was obtained from all patients.
2.2. Bronchiectasis Aetiology and Definition of Post- TB Bronchiectasis
The attending physicians determined the aetiology of bronchiectasis based on the test results and questionnaires according to published international guidelines [13,14]. Post-TB bronchiectasis was assigned when history or clinical evidence of TB was evident, and radiological findings of bronchiectasis were suggestive of TB, including scarring of the upper lobes, granuloma, and cavitation.
The post-infectious aetiology was defined as bronchiectasis caused by a prior infection other than TB. Idiopathic bronchiectasis was defined when no specific aetiology was determined after diagnostic workup according to the guidelines [13,14].
2.3. Assessments
The KMBARC registry collected baseline data at baseline (recruitment) and follow-up visits once a year. The collected data included demographics, comorbidities, laboratory test results (including microbiology), radiology, pulmonary function test results, and treatment [3].
Dyspnoea was assessed using the modified Medical Council Dyspnoea (mMRC) scale. Pulmonary function testing was performed according to the European Respiratory Society and American Thoracic Society technical standards and percentage of predicted forced expiratory volume in 1 s (FEV1) calculated using reference values for Korean patients [15,16]. Airflow obstruction was defined as an FEV1/forced vital capacity (FVC) ratio less than 0.7 [17]. Regarding radiologic findings, the number of involved lobes and the presence of bronchiectasis at each lobe were assessed using chest CT. Additionally, a modified Reiff score was used to evaluate radiologic severity [18]. Data on respiratory medications (inhalers, mucolytics, and long-term antibiotics), home oxygen, and physiotherapy were obtained.
The severity of bronchiectasis was determined using the bronchiectasis severity index (BSI), FACED, and E-FACED [19,20]. A consensus definition was used to determine bronchiectasis exacerbation and defined as deterioration in three of the following symptoms for at least 48 h: (1) cough; (2) sputum volume increase and/or consistent change; (3) sputum purulence; (4) dyspnoea and/or exercise intolerance; (5) fatigue and/or malaise; or (6) haemoptysis [21]. This study also used the following measurement tools related to health-related quality of life: the validated Korean version of the Bronchiectasis Health Questionnaire (BHQ) for quality of life, Patient Health Questionnaire 9 for depression, and Fatigue Severity Score for fatigue [3,22].
2.4. Statistical Analyses
Continuous data are presented as medians with interquartile ranges (IQRs), and results were compared using the Kruskal–Wallis test. Categorical data are presented as numbers (%), and results were compared using Pearson’s chi-squared test or Fisher’s exact test, as appropriate. Statistical significance was set at p < 0.05. To account for multiple comparisons, post hoc Bonferroni correction was applied if the Kruskal–Wallis test or Pearson’s chi-squared test indicated significance, in which a p value of 0.05 corresponds to 0.008 (0.05/6). All statistical analyses were performed using Stata (Release 16; StataCorp LP, College Station, TX, USA), and graphs were compiled with the use of GraphPad Prism version 9.0.2 (GraphPad Software, San Diego, CA, USA).
3. Results
3.1. Baseline Characteristics
A total of 598 patients were enrolled in this study, comprising 118 (19.7%) patients with post-TB, 117 (19.6%) with post-infectious, 244 (40.8%) with idiopathic, and 119 (19.9%) with other bronchiectasis. Common aetiologies among the other bronchiectasis included asthma (n = 32/119), NTM pulmonary disease (n = 24/119), chronic obstructive pulmonary disease (COPD) (n = 21/119), and rheumatoid arthritis (n = 15/119). The median patient age was 66 years (IQR, 60–72 years) and 56% were females. The patients with post-TB bronchiectasis showed a lower body mass index than the other three bronchiectasis groups (p = 0.002). There were no significant intergroup differences in smoking history, sputum volume, or rate of having severe dyspnoea ≥ 2 on the mMRC scale. Regarding pulmonary comorbidities, the patients with post-TB bronchiectasis had a significantly higher rate of COPD (p < 0.001) and lower rate of asthma (p < 0.001) than those in the other groups. Regarding extrapulmonary comorbidities, the patients with post-TB bronchiectasis had lower rates of rheumatoid arthritis (p = 0.008) and depression (p = 0.024) than those in the other groups (Table 1).
Table 1.
Baseline characteristics of the study population.
| Post-TB (n = 118) |
Post-Infectious (n = 117) |
Idiopathic (n = 244) |
Others (n = 119) |
p Value | |
|---|---|---|---|---|---|
| Age, years | 66 (60–72) | 67 (60–71) | 65 (60–71) | 66 (60–73) | 0.408 |
| Males | 62 (52.5) | 50 (42.7) | 100 (41.0) | 52 (43.7) | 0.215 |
| BMI, kg/m2 | 22.1 (19.5–24.6) | 22.6 (20.4–24.6) | 23.2 (21.0–25.8) * | 23.2 (20.7–25.1) | 0.002 |
| Smoking history | 0.349 | ||||
| Never-smoker | 68 (57.6) | 77 (65.8) | 162 (66.4) | 80 (67.2) | |
| Current- or ex-smoker | 50 (42.4) | 40 (34.2) | 82 (33.6) | 39 (32.8) | |
| Sputum volume, mL | 10 (5–25) | 20 (5–50) | 11 (5–32) | 10 (5–25) | 0.701 |
| mMRC dyspnoea scale | 0.107 | ||||
| <2 | 83 (70.3) | 91 (77.8) | 199 (81.6) | 95 (79.8) | |
| ≥2 | 35 (29.7) | 26 (22.2) | 45 (18.4) | 24 (20.2) | |
| Comorbidities | |||||
| Pulmonary comorbidities | |||||
| COPD | 53 (44.9) | 59 (50.4) | 69 (28.3) * | 45 (37.8) | <0.001 |
| Asthma | 17 (14.4) | 31 (26.5) | 37 (15.2) | 49 (41.2) * | <0.001 |
| Cardiovascular diseases | |||||
| Myocardial infarction | 2 (1.7) | 2 (1.7) | 2 (0.8) | 2 (1.7) | 0.759 |
| Angina | 4 (3.4) | 3 (2.6) | 6 (2.5) | 6 (5.0) | 0.592 |
| Stroke or TIA | 2 (1.7) | 2 (1.7) | 3 (1.2) | 4 (3.4) | 0.524 |
| CHF | 2 (1.7) | 5 (4.3) | 4 (1.6) | 2 (1.7) | 0.450 |
| Rheumatoid arthritis | 5 (4.2) | 6 (5.1) | 10 (4.1) | 16 (13.6) | 0.008 |
| Liver cirrhosis | 4 (3.4) | 3 (2.6) | 1 (0.4) | 0 | 0.024 |
| Chronic kidney disease | 2 (2.6) | 3 (2.6) | 4 (1.7) | 2 (1.7) | 0.891 |
| Malignancy | 16 (13.7) | 13 (11.1) | 16 (6.6) | 9 (7.8) | 0.127 |
| Diabetes mellitus | 13 (11.1) | 11 (9.4) | 34 (13.9) | 15 (12.6) | 0.640 |
| Osteoporosis | 13 (11.1) | 22 (19.0) | 22 (9.0) | 13 (10.9) | 0.052 |
| Depression | 0 | 5 (4.3) | 11 (4.5) | 4 (6.7) | 0.024 |
Data are presented as number (percentage) or median (interquartile range). * Indicates statistical significance for the comparison of the post-TB group with each other. To account for multiple comparisons, post hoc Bonferroni correction was applied, in which a p value of 0.05 corresponded to 0.008 (0.05/6). TB, tuberculosis; BMI, body mass index; mMRC, modified Medical Research Council; COPD, chronic obstructive pulmonary disease; TIA, transient ischemic attack; CHF, congestive heart failure.
3.2. Radiologic, Pulmonary Function, Microbiologic, and Laboratory Test Results and Respiratory Treatment
The patients with post-TB bronchiectasis had significantly more involvement in the right upper lobes (p < 0.001) and upper divisions of the left upper lobes (p < 0.001) than those in the other groups. In contrast, the patients with post-TB bronchiectasis had less involvement in the right lower lobes (p = 0.047) and left lower lobes (p = 0.010) than those in the other groups. The patients with post-TB and post-infectious bronchiectasis had higher modified Reiff scores than those with other bronchiectasis (p = 0.002). Regarding pulmonary function tests, the patients with post-TB bronchiectasis had a lower FEV1/FVC ratio (p = 0.025) than those in the other groups; the rate of patients with FEV1/FVC < 0.7 tended to be higher in the patients with post-TB and post-infectious bronchiectasis than those with idiopathic and other bronchiectasis, although the difference was not statistically significant (p = 0.075). Furthermore, the patients with post-TB bronchiectasis had worse lung functions, including FEV1, %predicted (p < 0.001) and FVC, %predicted (p = 0.027) than the patients in the other groups. Concerning microbiologic test results, the patients with post-TB and other bronchiectasis showed higher rates of NTM than those with post-infectious and idiopathic bronchiectasis (Table 2).
Table 2.
Radiologic, pulmonary function, microbiologic, and laboratory test results in patients with bronchiectasis according to their aetiologies.
| Post-TB (n = 118) |
Post-Infectious (n = 117) |
Idiopathic (n = 244) |
Others (n = 119) |
p Value | |
|---|---|---|---|---|---|
| Radiology | |||||
| No of involved lobes | 3 (2–5) | 3 (2–5) | 3 (2–4) | 3 (2–4) | 0.052 |
| Involved lobe | |||||
| RUL | 74 (63.3) | 49 (41.9) * | 88 (37.0) * | 36 (32.7) * | <0.001 |
| RML | 66 (56.4) | 76 (65.0) | 144 (60.5) | 75 (68.2) | 0.262 |
| RLL | 59 (50.4) | 80 (68.4) | 143 (60.1) | 64 (58.2) | 0.047 |
| LUL upper division | 63 (53.9) | 46 (39.3) | 79 (33.2) * | 26 (23.6) * | <0.001 |
| LUL lingular division | 58 (49.6) | 65 (55.6) | 137 (57.6) | 51 (46.4) | 0.191 |
| LLL | 79 (67.5) | 100 (85.5) | 180 (75.6) | 78 (70.9) | 0.010 |
| Modified Reiff score | 6.4 ± 3.8 | 6.8 ± 4.2 | 6.0 ± 4.2 | 4.9 ± 3.3 * | 0.002 |
| Pulmonary function | |||||
| FVC, L | 2.3 (1.9–3.1) | 2.5 (2.0–3.0) | 2.5 (2.0–3.0) | 2.5 (2.0–3.2) | 0.887 |
| FVC, % predicted | 70.1 (54.4–84.4) | 72.6 (60.5–79.8) | 74.4 (64.1–85.6) * | 74.7 (63.2–82.9) | 0.027 |
| FEV1, L | 1.5 (1.1–1.9) | 1.5 (1.1–1.9) | 1.7 (1.3–2.1) | 1.7 (1.2–2.1) | 0.163 |
| FEV1, % predicted | 57.6 (43.0–74.2) | 58.7 (46.5–71.3) | 65.8 (53.2–81.2) * | 64.4 (51.6–77.6) | 0.001 |
| FEV1/FVC ratio | 0.6 (0.5–0.7) | 0.6 (0.5–0.7) | 0.7 (0.6–0.8) | 0.7 (0.5–0.7) | 0.025 |
| FEV1/FVC < 0.7 | 71 (65.7) | 71 (68.9) | 119 (55.1) | 64 (61.0) | 0.075 |
| Microbiology | |||||
| NTM | 16 (13.6) | 9 (7.7) | 15 (6.2) | 22 (18.6) | 0.001 |
| Pseudomonas aeruginosa | 23 (19.5) | 23 (19.7) | 50 (20.5) | 10 (8.4) | 0.030 |
| Haemophilus influenzae | 2 (1.7) | 4 (3.4) | 2 (0.8) | 1 (0.8) | 0.243 |
| Staphylococcus aureus | 0 | 1 (0.9) | 2 (0.8) | 1 (0.8) | 0.921 |
| Moraxella catarrhalis | 0 | 0 | 2 (0.8) | 1 (0.8) | 1.000 |
| Enterobacteriaceae | 5 (4.2) | 4 (3.4) | 13 (5.3) | 1 (0.8) | 0.192 |
| Laboratory | |||||
| WBC | 6700 (5300–8300) | 7200 (6100–9100) | 7100 (5800–9000) | 6500 (5800–9000) | 0.149 |
| Haemoglobin | 13.3 (12.0–14.0) | 13.3 (12.1–14.2) | 12.9 (12.3–14.2) | 13.4 (12.3–14.2) | 0.689 |
| ESR | 23 (13–49) | 29 (15–39) | 26 (14–47) | 25 (14–56) | 0.925 |
| CRP | 0.6 (0.2–2.1) | 0.4 (0.2–1.5) | 0.4 (0.1–1.5) | 0.5 (0.2–1.3) | 0.274 |
Data are presented as number (percentage) or median (interquartile range), except for modified Reiff score, which is presented as mean ± standard deviation. * Indicates statistical significance for the comparison of the post-TB group with each other. To account for multiple comparisons, post hoc Bonferroni correction was applied, in which a p value of 0.05 corresponded to 0.008 (0.05/6). TB, tuberculosis; RUL, right upper lobe; RML, right middle lobe; RLL, right lower lobe; LUL, left upper lobe; LLL, left lower lobe; FVC, forced vital capacity; FEV1, forced expiratory volume in 1 s; NTM, non-tuberculous mycobacteria; WBC, white blood cell; ESR, erythrocyte sedimentation rate; CRP, C-reactive protein.
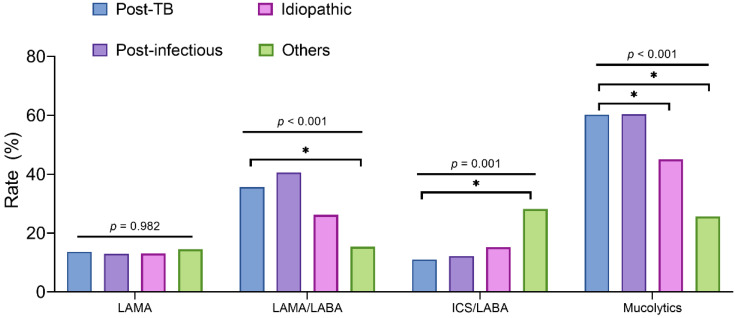
The patients with post-TB bronchiectasis used long-acting muscarinic antagonist (LAMA)/long-acting ß agonist (LABA) more frequently than those with other bronchiectasis (p < 0.001); however, inhaled corticosteroid (ICS)/LABA was much less frequently used in the patients with post-TB bronchiectasis than in those with other bronchiectasis (p = 0.001). Mucolytics were prescribed significantly more frequently in the patients with post-TB and post-infectious bronchiectasis than in those with idiopathic and other bronchiectasis (p < 0.001) (Figure 1).
Figure 1.
Comparison of respiratory medication use in patients with bronchiectasis according to their aetiologies. * Indicates statistical significance for the comparison of the post-TB group with each other. To account for multiple comparisons, post hoc Bonferroni correction was applied, in which a p value of 0.05 corresponded to 0.008 (0.05/6). TB, tuberculosis; LAMA, long-acting muscarinic antagonist; LABA, long-acting ß agonist; ICS, inhaled corticosteroid; OCS, oral corticosteroid; anti, oral antibiotics.
3.3. Bronchiectasis Severity, Exacerbations, and Quality of Life
Among the disease severity scores, only FACED was higher among the patients with post-TB bronchiectasis compared with those in the other groups (p = 0.042). Additionally, there was no significant intergroup differences in exacerbations, history of haemoptysis requiring hospitalisation, and quality of life between the four patient groups (Table 3).
Table 3.
Comparison of bronchiectasis severity, exacerbations, and quality of life of patients with bronchiectasis according to their aetiologies.
| Post-TB (n = 118) |
Post-Infectious (n = 117) |
Idiopathic (n = 244) |
Others (n = 119) |
p Value |
|
|---|---|---|---|---|---|
| Disease severity | |||||
| BSI | 7 (5–9) | 6 (5–9) | 6 (4–9) | 5 (4–8) | 0.082 |
| FACED | 2.4 ± 1.6 | 2.2 ± 1.7 | 1.9 ± 1.6 | 1.9 ± 1.4 | 0.042 |
| E-FACED | 3 (1–4) | 3 (1–3) | 2 (1–3) | 2 (1–3) | 0.101 |
| Exacerbations | |||||
| Any exacerbation | 1 (0–3) | 1 (0–2) | 1 (0–2) | 0 (0–2) | 0.892 |
| Requiring admission | 22 (18.6) | 22 (18.8) | 45 (18.4) | 20 (16.8) | 0.976 |
| Requiring ER visits | 7 (5.9) | 9 (7.7) | 21 (8.6) | 8 (6.7) | 0.812 |
| Severe exacerbation ≥ 2/year | 6 (5.1) | 8 (6.8) | 12 (4.9) | 6 (5.0) | 0.887 |
| Haemoptysis requiring admission | 7 (6.1) | 7 (6.2) | 16 (7.0) | 2 (2.4) | 0.501 |
| Bronchial artery embolization | 3 (2.6) | 1 (0.9) | 3 (1.3) | 0 | 0.422 |
| Quality of life | |||||
| BHQ | 53 (49–58) | 50 (45–56) | 53 (46–59) | 53 (45–59) | 0.111 |
| PHQ-9 | 3 (1–9) | 3 (1–9) | 3 (1–8) | 4 (2–10) | 0.495 |
| FSS | 19 (12–33) | 18 (12–32) | 19 (12–32) | 23 (14–37) | 0.219 |
Data are presented as number (percentage) or median (interquartile range), except for FACED, which is presented as mean ± standard deviation. BSI, bronchiectasis severity index; ER, emergency room; BHQ, Bronchiectasis Health Questionnaire; PHQ-9, Patient Health Questionnaire 9; FSS, Fatigue Severity Score.
4. Discussion
This Korean prospective bronchiectasis registry study revealed that approximately one-fifth of all the patients with bronchiectasis had post-TB bronchiectasis. The patients with post-TB bronchiectasis had more severe radiological extent and worse lung function than those with other bronchiectasis, which was reflected in the more frequent use of inhaled bronchodilators among the patients with post-TB bronchiectasis than those in the other groups. However, there were no significant intergroup differences in bronchiectasis severity scores (except for FACED), number of exacerbations, or quality of life between the four patient groups.
In this study, approximately two-thirds of the patients with post-TB bronchiectasis had airflow limitation, and these patients had a significantly lower FEV1 compared with those with idiopathic and other bronchiectasis. Considering the importance of identifying treatable traits in the patients with bronchiectasis to improve treatment outcome [23], the high proportion of subjects with an advanced degree of airflow limitations among the patients with post-TB bronchiectasis indicates that bronchodilators might be an attractive treatment strategy to improve lung function in these patients. Previous studies showing that bronchodilators are effective to improve lung function in bronchiectasis patients with airflow limitations further support our view [24,25]. However, as only a few retrospective studies have evaluated the effect of bronchodilators on lung function change in bronchiectasis patients [24,25], future studies with comprehensive study outcomes, including not only lung function but also quality of life and acute exacerbations, are needed. In addition, as smoking can enhance the development of airflow limitations in patients with post-TB bronchiectasis [26], strategies for enhancing smoking cessation would be important for patients with post-TB bronchiectasis.
The patients with post-TB bronchiectasis had more severe radiologic extent, measured using the modified Reiff score, than the patients with other bronchiectasis. In addition to the chest CT findings, a much higher rate of mucolytic use may suggest a higher symptom burden in the post-TB bronchiectasis group. Consequently, airway clearance techniques need to be emphasized more in patients with post-TB bronchiectasis. Such airway clearance techniques and chest physiotherapy may ultimately improve long-term outcomes such as the frequency and severity of exacerbations, hospitalisations, and quality of life in this population [27,28]. Although our study results showed no intergroup differences in exacerbations or bronchiectasis severity scores, this might be due to its cross-sectional design, which measured outcomes at one-time point. Future longitudinal study results using KMBARC follow-up data will prove whether post-TB aetiology is related to any difference in long-term outcomes among patients with bronchiectasis.
Another notable finding was that the patients with post-TB bronchiectasis showed higher NTM colonization than those with post-infectious and idiopathic bronchiectasis. TB has been suggested as a risk factor for incident NTM infection [29,30], as was shown in our study results. Considering that long-term macrolide monotherapy increases the risk of macrolide-resistant NTM pulmonary disease, a difficult-to-treat disease, it is imperative to collect sputum samples for NTM culture, especially in patients with post-TB bronchiectasis, before starting long-term macrolide treatment [30,31]. Regarding ICS use, the patients with post-TB, post-infectious, and idiopathic bronchiectasis were less likely to use ICS than those with other bronchiectasis where asthma or COPD comprised a large proportion. As prolonged use of ICS can lead to mycobacterial infection, a limited use of ICS is recommended in patients with bronchiectasis [13,14]. Thus, our study results suggest that most of our study population adheres to the current recommendation [13,14].
The major strength of our study is that it is the first to use a prospective bronchiectasis registry for patients with post-TB bronchiectasis. However, this study has limitations that should be acknowledged. First, this study only analysed baseline data from the KMBARC registry. Given the nature of a cross-sectional study, future research using longitudinal KMBARC registry data is warranted to evaluate the clinical characteristics of patients with post-TB bronchiectasis, including exacerbations, lung function decline, and long-term prognosis. Second, this study was performed in Korea, which limits the generalisation of our findings to other institutions in geographically different regions. Third, physicians’ judgments regarding the aetiology of bronchiectasis could be subjective. Although all the patients enrolled in the KMBARC were evaluated using the same clinical report form (same tests and questionnaire), the intensity of the diagnostic workup might differ between institutions and physicians. Since a diagnostic bundle for bronchiectasis in South Korea has been developed (unpublished data), this issue should be addressed in future studies.
5. Conclusions
Our study showed that approximately one-fifth of all the patients with bronchiectasis had post-TB bronchiectasis. The patients with post-TB bronchiectasis had more severe radiological extent, reduced lung function, and more frequent use of inhaled bronchodilators and mucolytics compared with the patients with other aetiologies. There were no significant intergroup differences in the bronchiectasis severity scores except for FACED, the number of exacerbations, and quality of life. Adequate bronchodilator use and airway clearance techniques may alleviate the symptom burden in patients with post-TB bronchiectasis.
Acknowledgments
The authors thank all members of the Korean Multicentre Bronchiectasis Audit and Research Collaboration (KMBARC) registry: An-Soo Jang, Bumhee Yang, Byoung Soo Kwon, Chang Youl Lee, Chang-Hoon Lee, Changhwan Kim, Chin Kook Rhee, Deog Kyeom Kim, Eun Hye Lee, Eun Kyung Kim, Hayoung Choi, Hye Yun Park, Hyun Kuk Kim, Hyun Lee, Hyung Koo Kang, Hyun-Kyung Lee, Ina Jeong, Jae Ha Lee, Jae Seung Lee, Jae Sung Choi, Ji Soo Choi, Ji Ye Jung, Ji-Ho Lee, Ji-Hyun Lee, Junghyun Kim, Jung-Kyu Lee, Juok Na, Ki Uk Kim, Kwang Ha Yoo, Sang Ha Kim, Sang Haak Lee, Sang-Heon Kim, Se Hee Lee, Sei Won Lee, Seung Jun Lee, Seung Won Ra, Shinhee Park, Sooim Sin, Soo-Jung Um, So-Young Park, Sung-Yoon Kang, Tae-Hyung Kim, Woo Jin Kim, Yee Hyung Kim, Yeon-Mok Oh, Yong Bum Park, Yong-Soo Kwon, Yoonki Hong, Youlim Kim, Young-Jae Cho, Young-Soon Yoon, Yu-Il Kim, Yun-Jeong Jeong and Yun Su Sim. Part of this article was presented in the form of an abstract at the Korean Association of Tuberculosis and Respiratory Diseases International Conference 2019, Seoul, Korea.
Author Contributions
Conceptualization, H.C., H.L. and Y.-S.K.; methodology, Y.-M.O. and Y.-S.K.; software, S.W.R. and H.K.K.; validation, J.S.L., S.-J.U. and S.-H.K.; formal analysis, H.C. and H.L.; investigation, H.C., H.L. and Y.-S.K.; resources, Y.-M.O. and Y.-S.K.; data curation, H.C., H.L. and KMBARC; writing—original draft preparation, H.C., H.L. and Y.-S.K.; writing—review and editing, H.C., H.L., S.W.R., H.K.K., J.S.L., S.-J.U., S.-H.K., Y.-M.O. and Y.-S.K.; visualization, H.C., S.W.R. and J.S.L.; supervision, Y.-M.O. and Y.-S.K.; project administration, Y.-M.O. and Y.-S.K.; funding acquisition, H.C. and Y.-S.K. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. 2020R1F1A1076570 to YSK) and the Korean Ministry of Education (No. 2021R1I1A3052416 to HC).
Institutional Review Board Statement
This study protocol was approved by the institutional review board of all institutions, including Hallym University Kangnam Sacred Heart Hospital (application no. 2018-07-16), participating in the KMBARC registry.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available upon reasonable request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Choi H., Yang B., Nam H., Kyoung D.-S., Sim Y.S., Park H.Y., Lee J.S., Lee S.W., Oh Y.-M., Ra S.W., et al. Population-based prevalence of bronchiectasis and associated comorbidities in South Korea. Eur. Respir. J. 2019;54:1900194. doi: 10.1183/13993003.00194-2019. [DOI] [PubMed] [Google Scholar]
- 2.Quint J.K., Millett E., Joshi M., Navaratnam V., Thomas S.L., Hurst J.R., Smeeth L., Brown J.S. Changes in the incidence, prevalence and mortality of bronchiectasis in the UK from 2004 to 2013: A population-based cohort study. Eur. Respir. J. 2016;47:186–193. doi: 10.1183/13993003.01033-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee H., Choi H., Sim Y.S., Park S., Kim W.J., Yoo K.H., Lee S.J., Kim T.-H., Yang B., Jeong I., et al. KMBARC registry: Protocol for a multicentre observational cohort study on non-cystic fibrosis bronchiectasis in Korea. BMJ Open. 2020;10:e034090. doi: 10.1136/bmjopen-2019-034090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Visser S.K., Bye P.T., Fox G.J., Burr L.D., Chang A.B., Holmes-Liew C.-L., King P., Middleton P.G., Maguire G.P., Smith D., et al. Australian adults with bronchiectasis: The first report from the Australian Bronchiectasis Registry. Respir. Med. 2019;155:97–103. doi: 10.1016/j.rmed.2019.07.016. [DOI] [PubMed] [Google Scholar]
- 5.Aksamit T.R., O’Donnell A.E., Barker A., Olivier K.N., Winthrop K.L., Daniels M.L.A., Johnson M., Eden E., Griffith D., Knowles M., et al. Adult Patients With Bronchiectasis: A First Look at the US Bronchiectasis Research Registry. Chest. 2017;151:982–992. doi: 10.1016/j.chest.2016.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee H., Choi H., Chalmers J.D., Dhar R., Nguyen T.Q., Visser S.K., Morgan L.C., Oh Y. Characteristics of bronchiectasis in Korea: First data from the Korean Multicentre Bronchiectasis Audit and Research Collaboration registry and comparison with other international registries. Respirology. 2021;26:619–621. doi: 10.1111/resp.14059. [DOI] [PubMed] [Google Scholar]
- 7.Dhar R., Singh S., Talwar D., Mohan M., Tripathi S.K., Swarnakar R., Trivedi S., Rajagopala S., D’Souza G., Padmanabhan A., et al. Bronchiectasis in India: Results from the European Multicentre Bronchiectasis Audit and Research Collaboration (EMBARC) and Respiratory Research Network of India Registry. Lancet Glob. Health. 2019;7:e1269–e1279. doi: 10.1016/S2214-109X(19)30327-4. [DOI] [PubMed] [Google Scholar]
- 8.Ravimohan S., Kornfeld H., Weissman D., Bisson G.P. Tuberculosis and lung damage: From epidemiology to pathophysiology. Eur. Respir. Rev. 2018;27:170077. doi: 10.1183/16000617.0077-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roberts H.R., Wells A.U., Milne D.G., Rubens M.B., Kolbe J., Cole P.J., Hansell D.M. Airflow obstruction in bronchiectasis: Correlation between computed tomography features and pulmonary function tests. Thorax. 2000;55:198–204. doi: 10.1136/thorax.55.3.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Milliron B., Henry T.S., Veeraraghavan S., Little B.P. Bronchiectasis: Mechanisms and Imaging Clues of Associated Common and Uncommon Diseases. Radiographics. 2015;35:1011–1030. doi: 10.1148/rg.2015140214. [DOI] [PubMed] [Google Scholar]
- 11.Wang H., Ji X.-B., Li C.-W., Lu H.-W., Mao B., Liang S., Cheng K.-B., Bai J.-W., Martinez-Garcia M.A., Xu J.-F. Clinical characteristics and validation of bronchiectasis severity score systems for post-tuberculosis bronchiectasis. Clin. Respir. J. 2018;12:2346–2353. doi: 10.1111/crj.12911. [DOI] [PubMed] [Google Scholar]
- 12.Al-Harbi A., Al-Ghamdi M., Khan M., Al-Rajhi S., Al-Jahdali H. Performance of Multidimensional Severity Scoring Systems in Patients with Post-Tuberculosis Bronchiectasis. Int. J. Chronic Obstr. Pulm. Dis. 2020;ume 15:2157–2165. doi: 10.2147/COPD.S261797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Polverino E., Goeminne P.C., McDonnell M.J., Aliberti S., Marshall S.E., Loebinger M.R., Murris M., Cantón R., Torres A., Dimakou K., et al. European Respiratory Society guidelines for the management of adult bronchiectasis. Eur. Respir. J. 2017;50:1700629. doi: 10.1183/13993003.00629-2017. [DOI] [PubMed] [Google Scholar]
- 14.Hill A.T., Sullivan A.L., Chalmers J.D., De Soyza A., Elborn J.S., Floto R.A., Grillo L., Gruffydd-Jones K., Harvey A., Haworth C.S., et al. British Thoracic Society Guideline for bronchiectasis in adults. Thorax. 2019;74:1–69. doi: 10.1136/thoraxjnl-2018-212463. [DOI] [PubMed] [Google Scholar]
- 15.Choi J.K., Paek D., Lee J.O. Normal Predictive Values of Spirometry in Korean Population. Tuberc. Respir. Dis. 2005;58:230–242. doi: 10.4046/trd.2005.58.3.230. [DOI] [Google Scholar]
- 16.Chung S.J., Kim H.I., Yang B., Kim T., Sim Y.S., Kang H.K., Kim S.H., Yoon H.J., Choi H., Lee H. Impact of the severity of restrictive spirometric pattern on nutrition, physical activity, and quality of life: Results from a nationally representative data-base. Sci. Rep. 2020;10:19672. doi: 10.1038/s41598-020-76777-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sim Y.S., Lee J.-H., Lee W.-Y., Suh D.I., Oh Y.-M., Yoon J.-S., Lee J.H., Cho J.H., Kwon C.S., Chang J.H. Spirometry and Bronchodilator Test. Tuberc. Respir. Dis. 2017;80:105–112. doi: 10.4046/trd.2017.80.2.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reiff D.B., Wells A.U., Carr D.H., Cole P.J., Hansell D.M. CT findings in bronchiectasis: Limited value in distinguishing between idiopathic and specific types. Am. J. Roentgenol. 1995;165:261–267. doi: 10.2214/ajr.165.2.7618537. [DOI] [PubMed] [Google Scholar]
- 19.Chalmers J.D., Goeminne P., Aliberti S., McDonnell M.J., Lonni S., Davidson J., Poppelwell L., Salih W., Pesci A., Dupont L.J., et al. The Bronchiectasis Severity Index. An International Derivation and Validation Study. Am. J. Respir. Crit. Care Med. 2014;189:576–585. doi: 10.1164/rccm.201309-1575OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martinez-Garcia M.A., de Gracia J., Vendrell Relat M., Giron R.M., Maiz Carro L., de la Rosa Carrillo D., Olveira C. Mul-tidimensional approach to non-cystic fibrosis bronchiectasis: The FACED score. Eur. Respir. J. 2014;43:1357–1367. doi: 10.1183/09031936.00026313. [DOI] [PubMed] [Google Scholar]
- 21.Hill A.T., Haworth C.S., Aliberti S., Barker A., Blasi F., Boersma W., Chalmers J.D., De Soyza A., Dimakou K., Elborn J.S., et al. Pulmonary exacerbation in adults with bronchiectasis: A consensus definition for clinical research. Eur. Respir. J. 2017;49:1700051. doi: 10.1183/13993003.00051-2017. [DOI] [PubMed] [Google Scholar]
- 22.Kim H.K., Lee H., Kim S.H., Choi H., Lee J.H., Lee J.S., Lee S.W., Oh Y.M. Validation of the Korean Version of the Bron-chiectasis Health Questionnaire. Tuberc. Respir. Dis. (Seoul) 2020;83:228–233. doi: 10.4046/trd.2020.0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boaventura R., Sibila O., Agusti A., Chalmers J.D. Treatable traits in bronchiectasis. Eur. Respir. J. 2018;52:1801269. doi: 10.1183/13993003.01269-2018. [DOI] [PubMed] [Google Scholar]
- 24.Jeong H.J., Lee H., Carriere K.C., Kim J.H., Han J.-H., Shin B., Jeong B.-H., Koh W.-J., Kwon O.J., Park H.Y. Effects of long-term bronchodilators in bronchiectasis patients with airflow limitation based on bronchodilator response at baseline. Int. J. Chronic Obstr. Pulm. Dis. 2016;11:2757–2764. doi: 10.2147/COPD.S115581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee S.Y., Lee J.S., Lee S.W., Oh Y.M. Effects of treatment with long-acting muscarinic antagonists (LAMA) and long-acting beta-agonists (LABA) on lung function improvement in patients with bronchiectasis: An observational study. J. Thorac. Dis. 2021;13:169–177. doi: 10.21037/jtd-20-1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim H.J., Baek S., Kim H.J., Lee J.S., Oh Y.-M., Lee S.-D., Lee S.W. The Impact of Smoking on Airflow Limitation in Subjects with History of Asthma and Inactive Tuberculosis. PLOS ONE. 2015;10:e0125020. doi: 10.1371/journal.pone.0125020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spinou A., Chalmers J.D. Using Airway Clearance Techniques in Bronchiectasis: Halfway There. Chest. 2020;158:1298–1300. doi: 10.1016/j.chest.2020.07.062. [DOI] [PubMed] [Google Scholar]
- 28.O’Neill K., O’Donnell A.E., Bradley J.M. Airway clearance, mucoactive therapies and pulmonary rehabilitation in bronchi-ectasis. Respirology. 2019;24:227–237. doi: 10.1111/resp.13459. [DOI] [PubMed] [Google Scholar]
- 29.Huang H.-L., Cheng M.-H., Lu P.-L., Shu C.-C., Wang J.-Y., Wang J.-T., Chong I.-W., Lee L.-N. Epidemiology and Predictors of NTM Pulmonary Infection in Taiwan—A Retrospective, Five-Year Multicenter Study. Sci. Rep. 2017;7:16300. doi: 10.1038/s41598-017-16559-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang B., Ryu J., Kim T., Jo Y.S., Kim Y., Park H.Y., Kang Y.A., Lee S.J., Lee O.-J., Moon J.-Y., et al. Impact of Bronchiectasis on Incident Nontuberculous Mycobacterial Pulmonary Disease. Chest. 2021;159:1807–1811. doi: 10.1016/j.chest.2020.12.020. [DOI] [PubMed] [Google Scholar]
- 31.Choi H., Kim S.-Y., Kim D.H., Huh H.J., Ki C.-S., Lee N.Y., Lee S.-H., Shin S., Shin S.J., Daley C.L., et al. Clinical Characteristics and Treatment Outcomes of Patients with Acquired Macrolide-Resistant Mycobacterium abscessus Lung Disease. Antimicrob. Agents Chemother. 2017;61:e01146-17. doi: 10.1128/aac.01146-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available upon reasonable request from the corresponding author.