Abstract
Background and Aims: Currently, it is difficult to predict the reversibility of renal function and to discriminate renal parenchymal injury in cirrhotic patients with acute kidney injury (AKI). The aim of this study is to evaluate whether urine N-acetyl-β-d-Glucosaminidase (NAG) can predict the survival and response to terlipressin in cirrhotic patients with AKI. Methods: Two hundred sixty-two cirrhotic consecutive patients who developed AKI were prospectively enrolled from 11 tertiary medical centers in Korea between 2016 to 2019. AKI was defined as an increase in serum Cr (SCr) of 0.3 mg/dL or a 50% increase in baseline SCr. Patients diagnosed with hepatorenal syndrome (HRS-AKI) were treated with terlipressin plus albumin. Results: The patients were 58.8 ± 12.9 years old on average and were predominantly male (72.5%). The mean MELD score was 25.3 ± 9.1. When classified according to the AKI phenotype, there were 119 pre-renal, 52 acute tubular necrosis, 18 miscellaneous, and 73 HRS-AKI patients. However, the urine NAG was not effective at discriminating AKI phenotypes, except for HRS-AKI. The baseline urine NAG increased as the baseline AKI stage increased (p < 0.001). In addition, within the same AKI stage, the urine NAG values were significantly lower in the AKI-resolved group than in the unresolved group. The urine NAG level was significantly lower in living patients compared with those who died or who underwent a liver transplant within 3 months (p = 0.005). In the multivariate analysis, the increased urine NAG was a significant risk factor for the 3-month transplant-free survival (TFS) rate, especially in patients with Child–Pugh class ≤ B or MELD < 24. The urine NAG did not predict the response to terlipressin treatment in patients with HRS. Conclusions: Urine NAG is strongly associated with the severity of AKI in patients with liver cirrhosis and is useful for predicting the 3-month TFS.
Keywords: acute kidney injury, hepatorenal syndrome, N-acetyl-β-d-Glucosaminidase
1. Introduction
Occurring in 40–50% of hospitalized patients, acute kidney injury (AKI) is a common complication in patients with advanced liver cirrhosis [1,2]. Numerous studies have established that kidney function plays a major role in the prognosis of cirrhosis [3]. Firstly, it is related to the increasing development of other complications, including variceal bleeding, spontaneous bacterial peritonitis, and hepatic encephalopathy for about 1.2–1.5 times [4]. Secondly, AKI shortens the survival time of patients with liver cirrhosis and is a common cause of mortality [3]. Referring to previous studies, even stage 1 AKI can be life-threatening in advanced liver cirrhosis. If AKI does not improve during the early stages of cirrhosis, the 3-month mortality rate can reach 32% [5]. Therefore, early identification and treatment may facilitate recovery from AKI, thereby reducing mortality in cirrhosis patients.
Serum creatinine (SCr) is the most widely used tool for the diagnosis of AKI [6]. Although SCr is the simplest tool to use in clinical practice, its accuracy in patients with cirrhosis has been questioned. A recent study showed that renal dysfunction is greatly underestimated on the basis of creatinine levels in cirrhotic patients [7]. As SCr may not be as competent as expected for predicting the prognosis of AKI patients, an alternative is required.
The definition and management of hepatorenal syndrome (HRS-AKI), the most life-threatening form of AKI, have changed as we have gained new knowledge into the pathophysiology [8]. While terlipressin plus albumin is the standard treatment for HRS-AKI, it only works in about half of treated patients. To date, the predictive factors associated with terlipressin response are a high baseline SCr, high bilirubin, and lack of increase in mean arterial pressure during treatment [9]. Given these facts, clinicians of late have focused on finding new biomarkers for the accurate diagnosis of AKI, differentiation of the type of AKI, and the prediction of terlipressin response in HRS-AKI.
The usefulness of biomarkers such as Neutrophil Gelatinase Associated Lipocalin (NAGL), interleukin-18, kidney injury molecule-1, and L-FABP have been studied in patients with cirrhosis [10]. Unfortunately, these biomarkers have non-specific increases under systemic inflammation, urinary tract infection, etc., and the ability to distinguish between HRS-AKI and acute tubular necrosis (ATN) is modest. Urine N-acetyl-β-d-glucosaminidase (NAG) is a lysosomal enzyme present dominantly in proximal tubules. It has a high molecular weight (140 kDa) and cannot penetrate to the glomerulus, meaning it is unlikely to be increased due to damage outside the kidney. Urine NAG has been proven to be clinically effective for the prediction of acute renal failure, post cardiac surgery, and in primary glomerulonephritis [11]. Despite this clear clinical relevance, little is known about how well urine NAG predicts the prognosis and treatment response in cirrhotic patients with AKI. The aim of this study was to evaluate the usefulness of the urine biomarker NAG for predicting the survival and response to terlipressin in patients with liver cirrhosis and established AKI or HRS-AKI.
2. Materials and Methods
2.1. Patients and Study Protocol
This is a multicenter, prospective cohort study involving 11 tertiary referral hospitals during the period of April 2017 to August 2019. The study protocol was registered at ClinicalTrials.gov (registration number NCT03530761, accessed on 13 August 2021). Reporting of the study conforms to the Consolidated Standards of Reporting Trials (CONSORT) 2010 statement. The inclusion criteria were as follows: (1) hospitalized patients with liver cirrhosis who were over 19 years old, (2) accompanied by acute kidney injury or hepatorenal syndrome, (3) able to urinate, and (4) agreed to the study. Exclusion criteria were as follows: (1) patients with active bleeding (e.g., variceal bleeding) within last 7 days from enrollment, (2) prior or current history of hepatocellular carcinoma (HCC), (3) hypersensitivity to terlipressin, (4) chronic kidney disease, (5) acute on chronic liver failure without liver cirrhosis at enrollment, (6) inadequate urine sample for examination, (7) pregnant and lactating patients, or (8) those who did not agree to participate in the study. The study protocol was approved by the Institutional Review Board of each hospital (IRB number; SCHBC 2017-02-005-003). The study protocol conformed to the ethical guidelines of the World Medical Association’s Declaration of Helsinki. All participants of the provided written informed consent. All authors had access to the study data and reviewed and approved the final manuscript.
2.2. Data Collection and Measurement of Urine NAG
In all of the patients, the baseline urine NAG was measured before the administration of albumin. In the case of HRS patients receiving terlipressin, urine NAG levels were rechecked 3 days after the standard treatment of terlipressin plus albumin. Urine NAG was measured using a colorimetric assay with a commercially available kit (N-Assay L NAG Nittobo, Nittobo Medical Co. Ltd., Tokyo, Japan) and automatic blood analyzer (Accute (TBA-40FR), TOSHIBA-CANON, Tochigi, Japan). Other clinical information including baseline demographics, laboratory data, and clinical course were collected prospectively.
2.3. Definition, Management, Outcomes, and Follow-Up
The definition of AKI and HRS followed the current definition of ICA-AKI criteria [6]. AKI was defined as an increase in serum creatinine of more than 0.3 mg/dL within 48 h or a 50% increase from baseline. The phenotypes of the AKI were classified into four groups as follows [12,13,14]: (1) pre-renal AKI, history of acute hypovolemia such as diuretics, bleeding, or diarrhea; (2) ATN [8,15,16], fractional excretion of sodium (FeNa) > 2% or urine sodium > 40 mEq/L or urine osmolarity < 350 mOsm/L; (3) HRS-AKI was defined by (i) diagnosis of cirrhosis and ascites, (ii) AKI according to ICA-AKI criteria [6], (iii) no response after two consecutive days of diuretic withdrawal and plasma volume expansion with 1 g/kg albumin, (iv) absence of shock, (v) no current or recent use of nephrotoxic drugs, and (vi) no macroscopic signs of structural kidney injury, absence of microhematuria, and normal findings on renal ultrasonography; and (4) miscellaneous, not included in any of the above three groups.
Management of AKI or HRS followed the standard treatment of the current guidelines [6,17,18], including fluid resuscitation, withdrawal of nephrotoxic drug or diuretics, or volume expansion with albumin.
Primary outcomes were a 3-month transplant-free survival in all AKI patients and response rate to terlipressin therapy in HRS-AKI patients. Secondary outcomes were overall survival, regression of AKI, and recurrence of AKI. The index date was defined as the date when an AKI or HRS occurred. The follow-up period was calculated from the index date to the day when the outcome of interest occurred or the last follow-up date.
2.4. Sample Size Calculation
Given the lack of previous research into this topic, we referred to a related study reporting the area-under-the curve (AUC) for urine biomarkers in patients with AKI, as it relates to the overall mortality (AUC for mortality was 0.75) [19]. We conservatively hypothesized that urine NAG would have a similar predictability. The estimated sample size was 220 patients, with an alpha value of 0.05 and a power of 80%. Considering a 10% drop out rate, a total of 245 patients were required. In patients with HRS-AKI, a subgroup of this study, the sample size was calculated based on the response to terlipressin therapy. According to a previous study, predicting the ability to the response rate of terlipressin was reported to have an AUC of 0.85 [20]. The resulting estimated sample size was a subgroup of 50 patients, with an alpha value of 0.05 and power of 80%. Considering a 10% drop out rate, a total of 56 patients with HRS-AKI were required.
2.5. Statistical Analysis
Frequencies and percentages were used for the descriptive statistics. Statistical differences between groups were investigated using χ2 test and Student’s t-test. Patient survival probability was estimated using the Kaplan–Meier method, and differences between the curves were compared using the log-rank test. The main analysis tool used for survival was the Cox proportional hazards model. Multivariate analysis was performed to determine the effect of urine NAG on liver transplantation or death. In order to minimize the influence of confounding factors, unadjusted (Model 1), age, sex (Model 2), MELD score at baseline (Model 3), and c-reactive protein at baseline (Model 4) were serially adjusted in a Cox proportional hazard model. The confounding factor was selected as items that had a significant effect on liver transplantation or death in univariable analysis (p < 0.10) and clinically relevant. In addition, stratification analysis according to the MELD score, Child–Pugh class, and degree ascites were presented. All of the statistical analyses were performed using R (version 4.0.2, The R Foundation for Statistical Computing, Vienna, Austria), SPSS software (ver. 23.0; SPSS Inc., Chicago, IL, USA). Statistical significance was defined at p < 0.05.
3. Results
3.1. Baseline Characteristics and AKI Phenotype
The baseline demographic and clinical characteristics are summarized in Table 1. A total of 262 patients (all hospitalized) were analyzed, including 190 (72.5%) males. The patients were 58.8 ± 12.9 years old on average and had a mean body mass index (BMI) of 24.1 ± 4.2 kg/m2. The etiology of liver cirrhosis consisted mainly of alcohol (83.2%) and viral (16.8%). The median follow-up duration was 241 (IQR 40–388) days. Most patients enrolled in the study had deteriorated liver function. The mean Child–Pugh score was 9.8 ± 2.5 and the mean MELD score was 25.3 ± 9.1. AKI was in stage 1 for 135 patients (51.5%) (stage 1A 2.3%, stage 1B 49.2%), stage 2 for 79 patients (30.2%), and stage 3 for 48 patients (18.3%); among these, 73 (27.9%) were HRS-AKI.
Table 1.
Baseline characteristics and AKI phenotype.
| Variable | Total (N = 262) |
Pre-Renal (N = 119) |
ATN (N = 52) |
Miscellaneous (N = 18) |
HRS-AKI (N = 73) |
p |
|---|---|---|---|---|---|---|
| Age (year) | 58.8 ± 12.9 | 59.0 ± 13.1 | 60.6 ± 13.7 | 62.6 ± 13.0 | 56.1 ± 11.6 | 0.121 |
| Male (n, %) | 190 (72.5%) | 96 (80.7%) | 38 (73.1%) | 10 (55.6%) | 46 (63.0%) | 0.020 |
| Body mass index (kg/m2) | 24.0 ± 4.2 | 24.0 ± 4.1 | 23.7 ± 4.6 | 24.0 ± 4.3 | 24.2 ± 4.1 | 0.931 |
| Etiology (n, %) | 0.418 | |||||
| Viral | 44 (16.8%) | 15 (12.6%) | 10 (19.2%) | 4 (22.2%) | 15 (20.5%) | |
| Non-viral | 218 (83.2%) | 104 (87.4%) | 42 (80.8%) | 14 (77.8%) | 58 (79.5%) | |
| Current alcohol drinking (n, %) | 100 (38.1%) | 42 (35.3%) | 14 (26.9%) | 6 (33.3%) | 25 (34.2%) | 0.209 |
| Diabetes (n, %) | 87 (33.2%) | 38 (31.9%) | 21 (40.4%) | 4 (22.2%) | 24 (32.9%) | 0.517 |
| Prior use of diuretics (n, %) | 152 (58.0%) | 71 (59.7%) | 27 (51.9%) | 13 (72.2%) | 41 (56.2%) | 0.472 |
| Prior use of beta blocker (n, %) | 55 (20.9%) | 27 (22.7%) | 12 (23.1%) | 3 (16.7%) | 13 (17.8%) | 0.803 |
| AKI stage (n, %) | <0.001 | |||||
| Stage I | 135 (51.5%) | 75 (63.0%) | 25 (48.1%) | 13 (72.2%) | NA | |
| Stage II | 79 (30.1%) | 30 (25.2%) | 19 (36.5%) | 4 (22.2%) | NA | |
| Stage III | 48 (18.4%) | 14 (11.8%) | 8 (15.4%) | 1 (5.6%) | NA | |
| HRS-AKI (n, %) | 73 (27.8%) | 0 (0%) | 0 (0%) | 0 (0%) | 73 (100%) | <0.001 |
| Ascites (n, %) | 0.081 | |||||
| No | 84 (32.1%) | 36 (30.3%) | 25 (48.1%) | 6 (33.3%) | 17 (23.3%) | |
| Mild | 47 (17.9%) | 21 (17.6%) | 10 (19.2%) | 4 (22.2%) | 12 (16.4%) | |
| Moderate to severe | 131 (50.0%) | 62 (52.1%) | 17 (32.7%) | 8 (44.4%) | 44 (60.3%) | |
| Hepatic encephalopathy (n, %) | 0.125 | |||||
| No | 199 (76.0%) | 88 (73.9%) | 46 (88.5%) | 16 (88.9%) | 49 (67.1%) | |
| Grade I to II | 26 (9.9%) | 14 (11.8%) | 2 (3.8%) | 1 (5.6%) | 9 (12.3%) | |
| Grade III to IV | 37 (14.1%) | 17 (14.3%) | 4 (7.7%) | 1 (5.6%) | 15 (20.5%) | |
| Child–Pugh class (n, %) | 0.002 | |||||
| A | 28 (10.7%) | 10 (8.4%) | 11 (21.2%) | 5 (27.8%) | 2 (2.7%) | |
| B | 88 (33.6%) | 43 (36.1%) | 20 (38.5%) | 3 (16.7%) | 22 (30.1%) | |
| C | 146 (55.7%) | 66 (55.5%) | 21 (40.4%) | 10 (55.6%) | 49 (67.1%) | |
| Child–Pugh score | 9.8 ± 2.4 | 9.9 ± 2.3 | 8.6 ± 2.5 | 8.8 ± 2.6 | 10.6 ± 2.2 | <0.001 |
| MELD score | 25.2 ± 9.1 | 24.1 ± 7.9 | 22.4 ± 8.6 | 21.1 ± 9.1 | 30.2 ± 9.3 | <0.001 |
| Vital sign | ||||||
| Systolic blood pressure (mmHg) | 116 ± 19 | 114 ± 18 | 120 ± 20 | 119 ± 17 | 114 ± 19 | 0.255 |
| Diastolic blood pressure (mmHg) | 69 ± 14 | 69 ± 12 | 70 ± 11 | 74 ± 12 | 69 ± 17 | 0.486 |
| Mean blood pressure (mmHg) | 85 ± 14 | 84 ± 13 | 87 ± 14 | 89 ± 13 | 84 ± 17 | 0.435 |
| Heart rate (beats per minute) | 86 ± 17 | 87 ± 18 | 83 ± 17 | 86 ± 17 | 85 ± 16 | 0.517 |
| Laboratory findings | ||||||
| White blood cell (/μL) | 9748 ± 6898 | 10,727 ± 7691 | 8589 ± 6606 | 6173 ± 3228 | 9860 ± 3562 | 0.032 |
| Hemoglobin (g/dL) | 9.7 ± 2.2 | 9.7 ± 2.3 | 9.9 ± 2.6 | 10.2 ± 1.4 | 9.5 ± 2.1 | 0.542 |
| Platelet (103/μL) | 104 ± 60 | 105 ± 63 | 113 ± 61 | 104 ± 64 | 98 ± 55 | 0.602 |
| hs-CRP (mg/dL) | 3.2 ± 4.3 | 3.5 ± 3.8 | 2.8 ± 4.9 | 2.4 ± 4.2 | 3.1 ± 4.5 | 0.676 |
| Albumin (g/dL) | 2.7 ± 0.6 | 2.6 ± 0.6 | 3.0 ± 0.7 | 2.8 ± 0.8 | 2.5 ± 0.4 | 0.002 |
| BUN (mg/dL) | 42 ± 23 | 40 ± 22 | 35 ± 17 | 31 ±15 | 55 ±25 | <0.001 |
| Total bilirubin (mg/dL) | 8.4 ± 10.1 | 8.2 ± 10.1 | 6.6 ± 9.0 | 3.9 ± 5.0 | 11.1 ± 11.3 | 0.016 |
| AST (U/L) | 162 ± 621 | 136 ± 192 | 296 ± 1320 | 169 ± 474 | 107 ± 177 | 0.363 |
| ALT (U/L) | 84 ± 349 | 64 ± 117 | 149 ± 708 | 64 ± 120 | 75 ± 240 | 0.510 |
| Serum sodium (mmol/L) | 132 ± 7 | 132 ± 8 | 133 ± 5 | 133 ± 6 | 130 ± 6 | 0.112 |
| Creatinine_baseline (mg/dL) | 1.05 ± 0.36 | 1.01 ± 0.26 | 0.92 ± 0.22 | 1.06 ± 0.29 | 1.19 ± 0.51 | <0.001 |
| Creatinine_enrollment (mg/dL) | 2.27 ± 0.87 | 1.99 ± 0.52 | 2.01 ± 0.58 | 1.89 ± 0.76 | 3.02 ±1.07 | <0.001 |
| Prothrombin time (INR) | 1.85 ± 0.85 | 1.78 ± 0.69 | 1.71 ± 0.75 | 1.78 ±1.20 | 2.06 ± 0.99 | 0.078 |
| Urine NAG (mg/dL) | 28.31 ± 45.23 | 26.36 ± 37.06 | 16.06 ± 23.40 | 13.22 ± 16.52 | 43.92 ± 65.52 | 0.002 |
When classified according to the AKI phenotype, there were 119 pre-renal, 52 ATN, 18 miscellaneous, and 73 HRS-AKI patients. A comparison of the baseline characteristics according to the AKI phenotype is presented in Table 1. The urine NAG level was higher in the HRS-AKI group than in the other three groups (43.92 ± 65.52 mg/dL, p = 0.002). In the remaining three groups, except for HRS-AKI, the urine NAG level was 26.36 ± 37.06 mg/dL in the pre-renal group, 16.06 ± 23.40 mg/dL in the ATN group, and 13.22 ± 16.52 mg/dL in the miscellaneous group, showing no significant difference between groups (p = 0.076). Differences in transplant free survival (TFS) according to AKI phenotypes were compared, but no differences were found between the groups (log ran p = 0.477) (Supplementary Figure S1).
3.2. Relationship between Urine NAG and AKI Stage
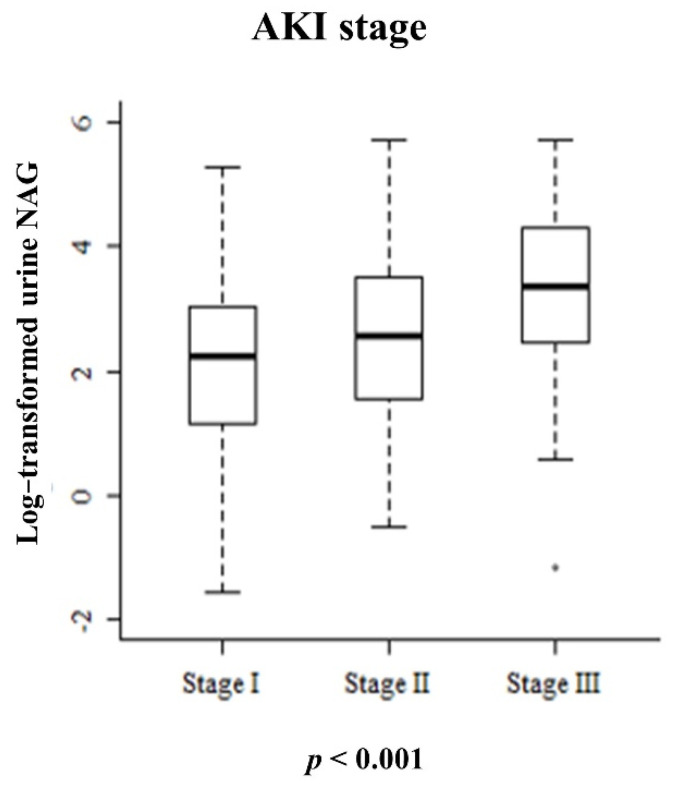
The mean urine NAG value was 28.31 ± 45.23 mg/dL. Urine NAG increased as the baseline AKI stage increased. The mean urine NAG values according to each AKI stage were 17.22 ± 24.66, 32.11 ± 52.71, and 53.23 ± 63.27 mg/dL, respectively, which were significantly different (Figure 1, p < 0.01). The baseline values of the urine NAG were compared between 87 patients with diabetes and the remaining patients without diabetes, but there was no clinically significant difference.
Figure 1.
Boxplot comparing the mean urine NAG according to AKI stage.
3.3. Relationship of Urine NAG and Change of AKI Status
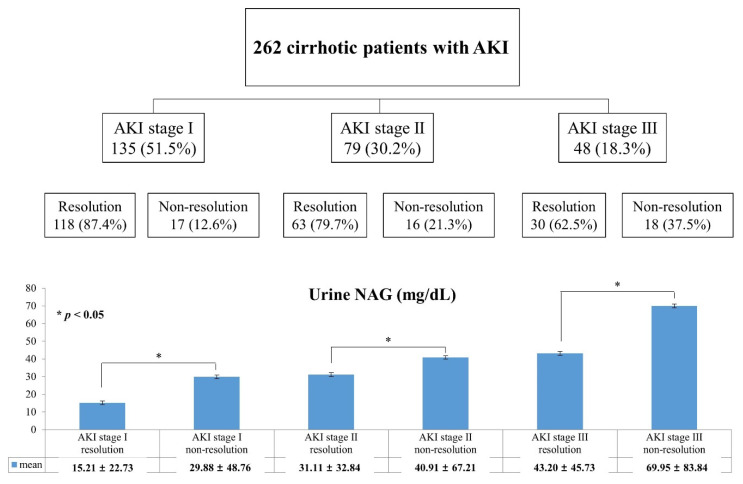
Figure 2 depicts the change of AKI status during follow-up. The patients with regressed AKI were 118 (87.4%) at stage 1, 63 (79.7%) at stage 2, and 30 (62.5%) at stage 3. As the AKI stage increased, the proportion who showed regression significantly decreased (p < 0.01). Notably, the urine NAG values were significantly lower in the resolved group than in the unresolved group within each AKI stage (AKI stage I, 15.21 ± 22.73 vs. 29.88 ± 48.76 mg/dL, p = 0.027; AKI stage II, 31.11 ± 32.84 vs. 40.91 ± 67.21 mg/dL, p = 0.043; and AKI stage III, 43.20 ± 45.73 vs. 69.95 ± 83.84 mg/dL, p = 0.032). This result suggests that urine NAG is useful for predicting who can recover from AKI.
Figure 2.
Flowchart of AKI status and urine NAG according to AKI regression. (* p < 0.05).
During the follow-up period, 93 (35.5%) patients had AKI recurrence. However, no difference in urine NAG was observed according to the recurrence of AKI (Supplementary Figure S2, p = 0.180). The logistic regression analysis also showed that the urine NAG level was not associated with the recurrence of AKI (Supplementary Table S1).
3.4. Relationship between Urine NAG and 3-Months Transplant-Free Survival
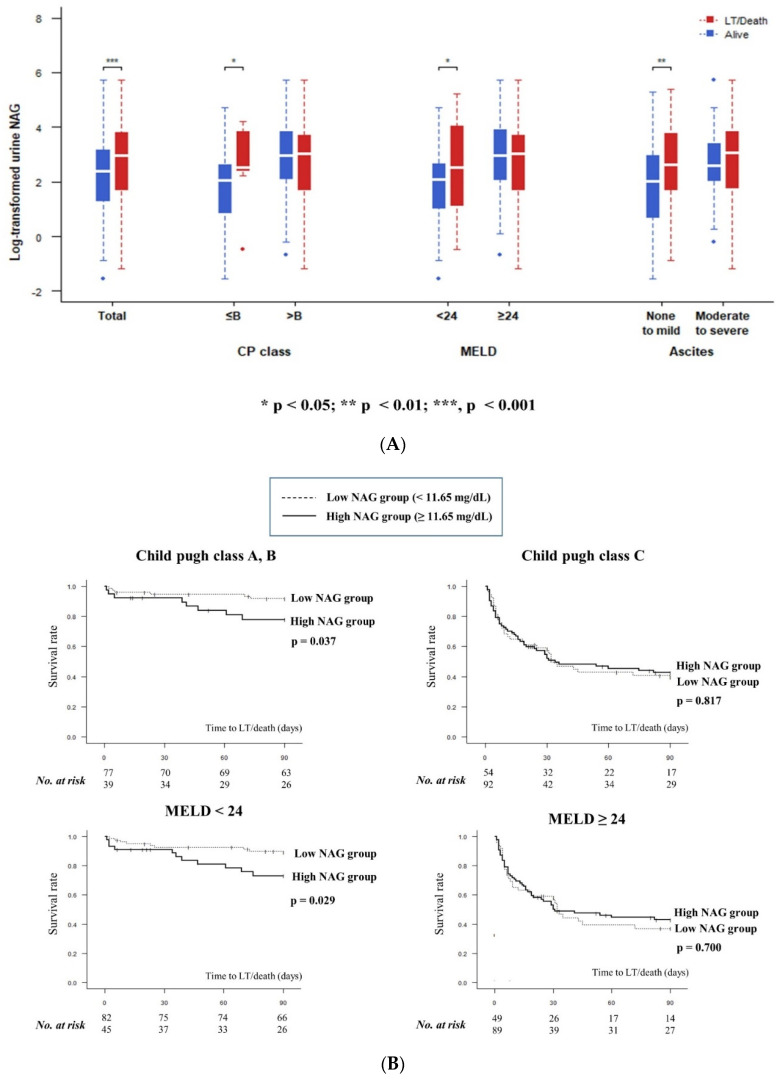
During the 3-month follow-up period, 95 patients died or received liver transplants. The baseline characteristic comparison of the two groups is presented in Table 2. Patients who experienced death or liver transplant within 3 months showed significantly higher urine NAG than those who did not (38.80 ± 55.90 vs. 22.34 ± 36.73 mg/dL, p = 0.024, Supplementary Figure S3). The multivariate analysis also showed that urine NAG was a significant prognostic marker of death or transplantation within 3 months (Table 3). Of note, urine NAG showed more predictive power in the patients with preserved liver function. The performance of urine NAG for predicting the 3-month TFS was slightly different according to the severity of liver disease. Among the patients with a relatively good liver function, those who were alive had lower urine NAG values compared with those who were not. In patients with deteriorated liver function, such as those with Child–Pugh class C, MELD score over 24, or those with significant ascites, no significant difference in urine NAG values according to clinical outcomes were found (Figure 3A, Supplementary Table S2). Looking at the Kaplan–Meier curve, high urine NAG is related to an increased risk of poor transplant-free survival in patients with Child–Pugh class A or B, or with a MELD below 24 (Figure 3B).
Table 2.
Comparison of baseline characteristics according to clinical outcomes.
| Variable | LT/Death in 3-Months (N = 95) |
Alive (N = 167) |
p |
|---|---|---|---|
| Age (year) | 60.32 ± 12.66 | 57.96 ± 13.04 | 0.157 |
| Male (n, %) | 71 (74.74%) | 119 (71.26%) | 0.644 |
| Liver transplantation (n, %) | 13 (13.68%) | 0 | 0.999 |
| Death (n, %) | 82 (86.31%) | 0 | 0.999 |
| AKI stage (n, %) | 0.001 | ||
| Stage I | 35 (36.84%) | 100 (59.88%) | |
| Stage II | 36 (37.89%) | 43 (25.75%) | |
| Stage III | 24 (25.26%) | 24 (14.37%) | |
| HRS-AKI (n, %) | 36 (37.89%) | 37 (22.16%) | 0.010 |
| Ascites (n, %) | 0.021 | ||
| No | 22 (23.16%) | 62 (37.13%) | |
| Mild | 15 (15.79%) | 32 (19.16%) | |
| Moderate to severe | 58 (61.05%) | 73 (43.71%) | |
| Hepatic encephalopathy (n, %) | <0.001 | ||
| No | 59 (62.11%) | 140 (83.83%) | |
| Grade I to II | 15 (15.79%) | 11 (6.59%) | |
| Grade III to IV | 21 (22.11%) | 16 (9.58%) | |
| Child–Pugh class (n, %) | <0.001 | ||
| A | 3 (3.16%) | 25 (14.97%) | |
| B | 11 (11.58%) | 77 (46.11%) | |
| C | 81 (85.26%) | 65 (38.92%) | |
| Child–Pugh score | 11.37 ± 2.11 | 8.90 ± 2.23 | <0.001 |
| MELD score | 31.30 ± 8.48 | 21.84 ± 7.56 | <0.001 |
| Laboratory findings | |||
| White blood cell (/μL) | 12,270.20 ± 7648.55 | 8314.59 ± 5999.27 | <0.001 |
| Platelet (103/μL) | 96.21 ± 58.20 | 109.65 ± 61.75 | 0.047 |
| hs-CRP (mg/dL) | 4.08 ± 4.52 | 2.73 ± 4.13 | <0.001 |
| Albumin (g/dL) | 2.52 ± 0.54 | 2.86 ± 0.69 | <0.001 |
| BUN (mg/dL) | 51.18 ± 25.12 | 38.21 ± 21.38 | <0.001 |
| Total bilirubin (mg/dL) | 13.25 ± 11.34 | 5.70 ± 8.31 | <0.001 |
| AST (U/L) | 159.09 ± 273.73 | 164.76 ± 750.93 | <0.001 |
| ALT (U/L) | 89.84 ± 234.14 | 81.07 ± 401.41 | 0.003 |
| Serum sodium (mmol/L) | 129.79 ± 8.25 | 133.51 ± 6.21 | <0.001 |
| Creatinine_baseline (mg/dL) | 1.03 ± 0.37 | 1.06 ± 0.36 | 0.392 |
| Creatinine_enrollment (mg/dL) | 2.46 ± 0.87 | 2.17 ± 0.86 | 0.010 |
| Prothrombin time (INR) | 2.37 ± 1.03 | 1.55 ± 0.52 | <0.001 |
| Urine NAG (mg/dL) | 38.80 ± 55.90 | 22.34 ± 36.73 | 0.005 |
Table 3.
Multivariate analysis of the effect of urine NAG on the incidence of LT/death.
| Category | N | Model 1 | Model 2 | Model 3 | Model 4 |
|---|---|---|---|---|---|
| OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | ||
| LT/Death in 3-months | |||||
| Total | 262 | 1.008 (1.002–1.015) ** | 1.010 (1.004–1.017) ** | 1.003 (0.997–1.010) | 1.003 (0.996–1.010) |
| MELD | |||||
| <24 | 127 | 1.028 (1.010–1.052) ** | 1.039 (1.016–1.065) ** | 1.036 (1.011–1.063) ** | 1.034 (1.008–1.062) * |
| ≥24 | 135 | 1.000 (0.994–1.007) | 1.002 (0.996–1.009) | 1.000 (0.993–1.007) | 1.000 (0.993–1.007) |
| Child–Pugh class | |||||
| ≤B | 116 | 1.022 (1.001–1.044) * | 1.033 (1.009–1.059) ** | 1.029 (1.003–1.055) * | 1.028 (1.002–1.054) * |
| >B | 146 | 1.002 (0.996–1.008) | 1.004 (0.997–1.011) | 1.001 (0.994–1.008) | 1.001 (0.994–1.008) |
| Ascites | |||||
| None to mild | 131 | 1.008 (0.999–1.018) | 1.009 (0.999–1.019) | 1.000 (0.988–1.012) | 1.000 (0.988–1.012) |
| Moderate to severe | 131 | 1.007 (1.000–1.017) | 1.009 (1.001–1.020) * | 1.004 (0.995–1.016) | 1.003 (0.994–1.013) |
* p < 0.05; ** p < 0.01. Model 1: unadjusted. Model 2: adjusted for age and sex. Model 3: Model 2 plus MELD score at baseline. Model 4: Model 3 plus CRP at baseline.
Figure 3.
Urine NAG and liver disease severity: (A) comparison of urine NAG values; (B) transplant-free survival according to urine NAG.
3.5. Urine NAG and Terlipressin Treatment Response in HRS-AKI
Of the total AKI patients, 73 patients (27.86%) were classified as HRS-AKI. The baseline characteristics of these patients are presented in Table 1. There was no significant relationship between the clinical outcome and urine NAG value in HRS-AKI patients. There was no difference in urine NAG according to AKI regression (p = 0.663), AKI recurrence (p = 0.216), 3-month TFS (p = 0.689), and overall survival (p = 0.868) (Supplementary Figure S4).
One of our main interests in HRS-AKI patients was whether this biomarker could predict the response to terlipressin treatment. Urine NAG tended to decrease on day 3 in the terlipressin-response group, but it was not statistically significant (p = 0.383, Supplementary Figure S5). The logistic regression analysis also showed that the urine NAG level was not effective at predicting the response to terlipressin in patients with HRS-AKI (Supplementary Table S3).
4. Discussion
This multicenter, prospective study included more than 200 patients and showed that urine NAG is associated with baseline AKI stage and the short-term prognosis of cirrhotic patients with AKI. Our results indicate that in patients with established AKI, the urinary NAG activity is a useful surrogate for the severity of AKI and that it has prognostic utility.
AKI is still often missed in patients with liver cirrhosis, while the pathophysiology is still being actively studied. Since the ICA criteria for patients with liver cirrhosis was first introduced in 2005, the definitions and pathophysiology of AKI and HRS have continued to evolve as the version was updated in 2015 and 2019 [6,8,21]. It has been found that the increase of SCr is more important than the absolute value of SCr, and that inflammation plays an important role in AKI/HRS pathophysiology outside of the traditional hemodynamic changes [6,8]. As the research progresses, biomarker studies for detecting AKI early and differentiating between prerenal azotemia and ATN are being actively conducted.
Currently, the most representative biomarkers are NGAL and IL-18. Previous reports have shown that the presence of ATN significantly increases the level of these renal biomarkers compared with the prerenal azotemia [22,23]. However, NGAL and IL-18 have several disadvantages as they increase during non-AKI situations and are insufficient to differentiate HRS from ATN [10,24]. Ideal prerequisites for any clinically useful AKI marker include the following: (1) the biomarker should only be found in the AKI state, (2) the degree of elevation is related to the severity, (3) the value should be higher for ATN than for either PRA or HRS, and (4) it should be accessible in the clinic and testing costs should be as low as creatinine testing.
In this context, NAG could be a good candidate as a biomarker for AKI. NAG is a large molecule (130 kDa) and cannot penetrate the glomerulus [11,25]. Therefore, it is only elevated during renal tubular injury, suggesting that it is more specific for discriminating ATN. On top of this, the concentrations of NAG were significantly higher in patients with AKI compared with the controls or with chronic kidney disease [26,27]. Thanks to its proven usefulness for post cardiac surgery and more generally for AKI patients without cirrhosis, it is now commercially available and relatively inexpensive compared with other biomarkers. Testing costs are as low as $10 [11,28].
There is only a one retrospective study on the usefulness of NAG in patients with cirrhosis, which was conducted in decompensated cirrhotic patients [29]. However, the number of patients was relatively small and the proportion of patients with AKI was too low, so it was insufficient to confirm the usefulness of NAG in AKI patients. As far as we know, our study is the first prospective study in patients with liver cirrhosis who have already developed AKI or HRS.
The first finding in our study was that urine NAG was associated with short term survival in AKI patients. This was clinically significant, even after correcting for other factors such as liver function. This means that the higher the NAG level, the higher the mortality rate. This is similar to other studies, where a high NAG has been reported to be associated with dialysis or death in ARF patients without liver cirrhosis [11]. Although NGAL and IL-18 have demonstrated their usefulness in predicting the prognosis of cirrhosis patients and in patients with acute-on-chronic liver failure, both markers are not widely used in clinical practice [22]. It is difficult for hepatologists to predict which patients will recover from AKI and which will not. Therefore, for AKI patients requiring rapid diagnosis and treatment, NAG is clearly more predictive than Cr. In other words, our results suggest that patients with increased urine NAG are likely to have a poor prognosis and will not to respond to treatment, making timely liver transplant a priority.
The second finding of our study is that the information provided by urine NAG is not very useful in patients with highly advanced decompensated cirrhosis. This makes sense as the liver function itself is a more important factor for determining prognosis than renal function in patients with advanced decompensated liver cirrhosis. For this reason, Cr has a lower priority than the previous items [30]. The most commonly used predictors reported in the literature are in the order of bilirubin, prothrombin time, albumin, ascites, and age, which is the same as for our study [30]. In addition, urine NAG is known to reflect mild, subclinical renal tubular damage better than severe tubular damage [31,32]. Therefore, it is sensible it showed high predictive power during the early stages of liver cirrhosis. Thus, we would like to recommend liver transplantation or best supportive care for these patients with severe grade (Child–Pugh class C or with high MELD score) liver cirrhosis [1,33].
Finally, our study found that there was no predictive power of urine NAG in patients with HRS-AKI receiving terlipressin. Similarly, urine NGAL failed to predict the response to terlipressin treatment in patients with HRS [24]. We presume that there are three reasons NAG failed to predict the terlipressin response. In HRS-AKI, systemic inflammation and systemic circulatory dysfunction was found to be important for pathophysiology [8]. Because the urine NAG level is generally not associated with systemic inflammation, it appears that the predictive power of NAG is diminished in HRS-AKI patients. As a second hypothesis, it is possible that a significant number of patients diagnosed with HRS-AKI actually overlapped with ATN, as HRS and ATN are on a continuous spectrum [6]. In these heterogeneous groups, prediction of the response to treatment may vary depending on the timing and degree of ATN manifestation. Finally, as the treatment response was not the primary aim of this study, there is a possibility of beta error due to an insufficient sample size, and the patients events occurred outside the hospital, so there may have been a difference in the sampling time. Perhaps we more meaningful results would be produced if serial sampling is possible in hospitalized patients. To date, clinical factors such as serum bilirubin, early increase in arterial pressure, and the development of hypotension have been reported as predictors of the response to terlipressin [34,35], but favorable results have not been reported in the biomarker field.
As with all studies, there are limitations to our research. First, contrary to the results of other biomarkers, urine NAG was not effective at discriminating AKI phenotypes, except for HRS-AKI. In previous studies, the AKI phenotype was reported to be pre-renal 48%, ATN 12%, and HRS-AKI 29% [24], and it is almost similar to the classification in our study (pre-renal 45.4%, ATN 19.8%, and HRS-AKI 28%). As renal biopsy was not done in the patients with suspected ATN, we tried to discriminate it indirectly through FeNa, urine sediment, urine osmolality, and urine sodium. While these are commonly used metrics, it is well known that these clinical indicators are less accurate in cirrhotic patients [36,37,38,39]. Moreover, there might be significant overlap between HRS-AKI and ATN, and classifying the exact type of AKI may be clinically inaccurate in this heterogeneous group. As a result, we think that this inaccurate classification may have lowered the phenotype predictive power of urine NAG. Second, we measured the urine NAG at baseline and on day 3 after treatment in order to predict the response to terlipressin. In fact, when the measurement should be performed for the highest accuracy has yet to be validated. In other research, measurement on the second day after the diagnosis of HRS is recommended, but more research is needed in the future [40].
Clinically, we expect urine NAG to be used as a diagnostic tool to predict the prognosis of patients with AKI and HRS. Urine NAG can help identify patients who need to prioritize a liver transplant rather than pharmacological treatment.
In conclusion, this is the first study to demonstrate the clinical utility of urine NAG as a short-term prognostic marker in cirrhotic patients with AKI or HRS-AKI. A more precise estimation of the severity of AKI and the ability to predict clinical outcomes using urine NAG can potentially facilitate targeted therapies for patients who have advanced liver cirrhosis.
Acknowledgments
We gratefully acknowledge the technical support of the Bommedical Corporation (Seoul, Korea).
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/jcm10194328/s1. Table S1: Logistic regression analysis for AKI recurrence in patients with AKI. Table S2: Urine NAG depending upon liver disease severity. Table S3: Baseline characteristics of HRS-AKI. Table S4: Logistic regression analysis for response to terlipressin therapy in patients with HRS-AKI. Figure S1: Transplant free survival according to AKI phenotype. Figure S2: Boxplot comparing mean urine NAG according to AKI recurrence. Figure S3: Urine NAG level according to clinical outcomes. Figure S4: Boxplot comparing mean urine NAG between groups (HRS group). Figure S5: Relationship between delta urine NAG and response to terlipressin.
Author Contributions
J.-J.Y., conceptualization, formal analysis, investigation, writing—original draft, review, and editing; J.H.K. (Jung Hyun Kwon), investigation and writing—original draft; Y.S.K., investigation and methodology; S.W.N., investigation; J.W.P., investigation and methodology; H.Y.K., investigation and methodology; C.W.K., investigation; S.K.S., investigation; Y.E.C., investigation; E.S.J., investigation; S.-H.J., investigation; J.W.L., conceptualization and investigation; D.S.S., investigation; J.M.Y., investigation; S.W.L., investigation; H.L.L., investigation; Y.K.J., investigation; H.J.Y., investigation; B.L., statistical analysis; J.H.K. (Ju Hyun Kim), conceptualization and formal analysis; S.G.K. conceptualization, data curation, investigation, methodology, writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by Gyeong-In Area, Korean Association for the Study of the Liver, and was supported by the Soonchunhyang University research fund.
Institutional Review Board Statement
The study protocol was approved by the Institutional Review Board of each hospital (IRB number; SCHBC 2017-02-005-003). The study protocol conformed to the ethical guidelines of the World Medical Association’s Declaration of Helsinki. All participants provided written informed consent.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available upon request from the corresponding author.
Conflicts of Interest
No conflict of interest to declare.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Biggins S.W., Angeli P., Garcia-Tsao G., Gines P., Ling S.C., Nadim M.K., Wong F., Kim W.R. Diagnosis, Evaluation, and Management of Ascites, Spontaneous Bacterial Peritonitis and Hepatorenal Syndrome: 2021 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2021;74:1014–1048. doi: 10.1002/hep.31884. [DOI] [PubMed] [Google Scholar]
- 2.Thapa P., Kc S., Hamal A.B., Sharma D., Khadka S., Karki N., Jaishi B., Tiwari P.S., Vaidya A., Karki A. Prevalence of Acute Kidney Injury in Patients with Liver Cirrhosis. JNMA J. Nepal Med. Assoc. 2020;58:554–559. doi: 10.31729/jnma.5147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wong F. Acute kidney injury in liver cirrhosis: New definition and application. Clin. Mol. Hepatol. 2016;22:415–422. doi: 10.3350/cmh.2016.0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Belcher J.M., Garcia-Tsao G., Sanyal A.J., Bhogal H., Lim J.K., Ansari N., Coca S.G., Parikh C.R., Consortium T.-A. Association of AKI with mortality and complications in hospitalized patients with cirrhosis. Hepatology. 2013;57:753–762. doi: 10.1002/hep.25735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bucsics T., Krones E. Renal dysfunction in cirrhosis: Acute kidney injury and the hepatorenal syndrome. Gastroenterol. Rep. (Oxf.) 2017;5:127–137. doi: 10.1093/gastro/gox009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Angeli P., Gines P., Wong F., Bernardi M., Boyer T.D., Gerbes A., Moreau R., Jalan R., Sarin S.K., Piano S., et al. Diagnosis and management of acute kidney injury in patients with cirrhosis: Revised consensus recommendations of the International Club of Ascites. J. Hepatol. 2015;62:968–974. doi: 10.1016/j.jhep.2014.12.029. [DOI] [PubMed] [Google Scholar]
- 7.Yoo J.J., Kim S.G., Kim Y.S., Lee B., Lee M.H., Jeong S.W., Jang J.Y., Lee S.H., Kim H.S., Kim Y.D., et al. Estimation of renal function in patients with liver cirrhosis: Impact of muscle mass and sex. J. Hepatol. 2019;70:847–854. doi: 10.1016/j.jhep.2018.12.030. [DOI] [PubMed] [Google Scholar]
- 8.Angeli P., Garcia-Tsao G., Nadim M.K., Parikh C.R. News in pathophysiology, definition and classification of hepatorenal syndrome: A step beyond the International Club of Ascites (ICA) consensus document. J. Hepatol. 2019;71:811–822. doi: 10.1016/j.jhep.2019.07.002. [DOI] [PubMed] [Google Scholar]
- 9.Mindikoglu A.L., Pappas S.C. Predictors of Response to Terlipressin in Hepatorenal Syndrome. Clin. Gastroenterol. Hepatol. 2018;16:1174. doi: 10.1016/j.cgh.2018.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ariza X., Sola E., Elia C., Barreto R., Moreira R., Morales-Ruiz M., Graupera I., Rodriguez E., Huelin P., Sole C., et al. Analysis of a urinary biomarker panel for clinical outcomes assessment in cirrhosis. PLoS ONE. 2015;10:e0128145. doi: 10.1371/journal.pone.0128145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liangos O., Perianayagam M.C., Vaidya V.S., Han W.K., Wald R., Tighiouart H., MacKinnon R.W., Li L., Balakrishnan V.S., Pereira B.J., et al. Urinary N-acetyl-beta-(d)-glucosaminidase activity and kidney injury molecule-1 level are associated with adverse outcomes in acute renal failure. J. Am. Soc. Nephrol. 2007;18:904–912. doi: 10.1681/ASN.2006030221. [DOI] [PubMed] [Google Scholar]
- 12.Piano S., Rosi S., Maresio G., Fasolato S., Cavallin M., Romano A., Morando F., Gola E., Frigo A.C., Gatta A., et al. Evaluation of the Acute Kidney Injury Network criteria in hospitalized patients with cirrhosis and ascites. J. Hepatol. 2013;59:482–489. doi: 10.1016/j.jhep.2013.03.039. [DOI] [PubMed] [Google Scholar]
- 13.Francoz C., Nadim M.K., Durand F. Kidney biomarkers in cirrhosis. J. Hepatol. 2016;65:809–824. doi: 10.1016/j.jhep.2016.05.025. [DOI] [PubMed] [Google Scholar]
- 14.Fagundes C., Barreto R., Guevara M., Garcia E., Sola E., Rodriguez E., Graupera I., Ariza X., Pereira G., Alfaro I., et al. A modified acute kidney injury classification for diagnosis and risk stratification of impairment of kidney function in cirrhosis. J. Hepatol. 2013;59:474–481. doi: 10.1016/j.jhep.2013.04.036. [DOI] [PubMed] [Google Scholar]
- 15.Miller T.R., Anderson R.J., Linas S.L., Henrich W.L., Berns A.S., Gabow P.A., Schrier R.W. Urinary diagnostic indices in acute renal failure: A prospective study. Ann. Intern. Med. 1978;89:47–50. doi: 10.7326/0003-4819-89-1-47. [DOI] [PubMed] [Google Scholar]
- 16.Moreau R., Lebrec D. Acute renal failure in patients with cirrhosis: Perspectives in the age of MELD. Hepatology. 2003;37:233–243. doi: 10.1053/jhep.2003.50084. [DOI] [PubMed] [Google Scholar]
- 17.Sanchez L.O., Francoz C. Global strategy for the diagnosis and management of acute kidney injury in patients with liver cirrhosis. United Eur. Gastroenterol. J. 2021;9:220–228. doi: 10.1177/2050640620980713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin. Pract. 2012;120:c179–c184. doi: 10.1159/000339789. [DOI] [PubMed] [Google Scholar]
- 19.Park M.Y., Choi S.J., Kim J.K., Hwang S.D., Lee Y.W. Urinary cystatin C levels as a diagnostic and prognostic biomarker in patients with acute kidney injury. Nephrology. 2013;18:256–262. doi: 10.1111/nep.12037. [DOI] [PubMed] [Google Scholar]
- 20.Nazar A., Pereira G.H., Guevara M., Martin-Llahi M., Pepin M.N., Marinelli M., Sola E., Baccaro M.E., Terra C., Arroyo V., et al. Predictors of response to therapy with terlipressin and albumin in patients with cirrhosis and type 1 hepatorenal syndrome. Hepatology. 2010;51:219–226. doi: 10.1002/hep.23283. [DOI] [PubMed] [Google Scholar]
- 21.Peron J.M., Bureau C., Gonzalez L., Garcia-Ricard F., de Soyres O., Dupuis E., Alric L., Pourrat J., Vinel J.P. Treatment of hepatorenal syndrome as defined by the international ascites club by albumin and furosemide infusion according to the central venous pressure: A prospective pilot study. Am. J. Gastroenterol. 2005;100:2702–2707. doi: 10.1111/j.1572-0241.2005.00271.x. [DOI] [PubMed] [Google Scholar]
- 22.Puthumana J., Ariza X., Belcher J.M., Graupera I., Gines P., Parikh C.R. Urine Interleukin 18 and Lipocalin 2 Are Biomarkers of Acute Tubular Necrosis in Patients with Cirrhosis: A Systematic Review and Meta-analysis. Clin. Gastroenterol. Hepatol. 2017;15:1003–1013.e3. doi: 10.1016/j.cgh.2016.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ariza X., Graupera I., Coll M., Sola E., Barreto R., Garcia E., Moreira R., Elia C., Morales-Ruiz M., Llopis M., et al. Neutrophil gelatinase-associated lipocalin is a biomarker of acute-on-chronic liver failure and prognosis in cirrhosis. J. Hepatol. 2016;65:57–65. doi: 10.1016/j.jhep.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 24.Huelin P., Sola E., Elia C., Sole C., Risso A., Moreira R., Carol M., Fabrellas N., Bassegoda O., Juanola A., et al. Neutrophil Gelatinase-Associated Lipocalin for Assessment of Acute Kidney Injury in Cirrhosis: A Prospective Study. Hepatology. 2019;70:319–333. doi: 10.1002/hep.30592. [DOI] [PubMed] [Google Scholar]
- 25.Bazzi C., Petrini C., Rizza V., Arrigo G., Napodano P., Paparella M., D’Amico G. Urinary N-acetyl-beta-glucosaminidase excretion is a marker of tubular cell dysfunction and a predictor of outcome in primary glomerulonephritis. Nephrol. Dial. Transpl. 2002;17:1890–1896. doi: 10.1093/ndt/17.11.1890. [DOI] [PubMed] [Google Scholar]
- 26.Han W.K., Waikar S.S., Johnson A., Betensky R.A., Dent C.L., Devarajan P., Bonventre J.V. Urinary biomarkers in the early diagnosis of acute kidney injury. Kidney Int. 2008;73:863–869. doi: 10.1038/sj.ki.5002715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Han W.K., Bonventre J.V. Biologic markers for the early detection of acute kidney injury. Curr. Opin. Crit. Care. 2004;10:476–482. doi: 10.1097/01.ccx.0000145095.90327.f2. [DOI] [PubMed] [Google Scholar]
- 28.Katagiri D., Doi K., Honda K., Negishi K., Fujita T., Hisagi M., Ono M., Matsubara T., Yahagi N., Iwagami M., et al. Combination of two urinary biomarkers predicts acute kidney injury after adult cardiac surgery. Ann. Thorac. Surg. 2012;93:577–583. doi: 10.1016/j.athoracsur.2011.10.048. [DOI] [PubMed] [Google Scholar]
- 29.Kim T.H., Lee H.A., Seo Y.S., Lee Y.R., Yim S.Y., Lee Y.S., Suh S.J., Jung Y.K., Kim J.H., An H., et al. Assessment and prediction of acute kidney injury in patients with decompensated cirrhosis with serum cystatin C and urine N-acetyl-beta-d-glucosaminidase. J. Gastroenterol. Hepatol. 2019;34:234–240. doi: 10.1111/jgh.14387. [DOI] [PubMed] [Google Scholar]
- 30.D’Amico G., Garcia-Tsao G., Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: A systematic review of 118 studies. J. Hepatol. 2006;44:217–231. doi: 10.1016/j.jhep.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 31.Wellwood J.M., Ellis B.G., Price R.G., Hammond K., Thompson A.E., Jones N.F. Urinary N-acetyl-beta-d-glucosaminidase activities in patients with renal disease. Br. Med. J. 1975;3:408–411. doi: 10.1136/bmj.3.5980.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kang H.K., Kim D.K., Lee B.H., Om A.S., Hong J.H., Koh H.C., Lee C.H., Shin I.C., Kang J.S. Urinary N-acetyl-beta-d-glucosaminidase and malondialdehyde as a markers of renal damage in burned patients. J. Korean Med. Sci. 2001;16:598–602. doi: 10.3346/jkms.2001.16.5.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.European Association for the Study of the Liver Electronic address, e.e.e.; European Association for the Study of the, L. EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J. Hepatol. 2018;69:406–460. doi: 10.1016/j.jhep.2018.03.024. [DOI] [PubMed] [Google Scholar]
- 34.Boyer T.D., Sanyal A.J., Garcia-Tsao G., Blei A., Carl D., Bexon A.S., Teuber P., Terlipressin Study G. Predictors of response to terlipressin plus albumin in hepatorenal syndrome (HRS) type 1: Relationship of serum creatinine to hemodynamics. J. Hepatol. 2011;55:315–321. doi: 10.1016/j.jhep.2010.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vinit S., Kumar A., Sharma P., Bansal R., Tyagi P., Bansal N., Singla V., Kumar M., Ranjan P., Sachdeva M., et al. Predictors of Response and Outcome to Terlipressin in Patients With Hepatorenal Syndrome. Clin. Gastroenterol. Hepatol. 2015;13:1383. doi: 10.1016/j.cgh.2015.04.036. [DOI] [Google Scholar]
- 36.Pepin M.N., Bouchard J., Legault L., Ethier J. Diagnostic performance of fractional excretion of urea and fractional excretion of sodium in the evaluations of patients with acute kidney injury with or without diuretic treatment. Am. J. Kidney Dis. 2007;50:566–573. doi: 10.1053/j.ajkd.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 37.Cabrera J., Arroyo V., Ballesta A.M., Rimola A., Gual J., Elena M., Rodes J. Aminoglycoside nephrotoxicity in cirrhosis. Value of urinary beta 2-microglobulin to discriminate functional renal failure from acute tubular damage. Gastroenterology. 1982;82:97–105. doi: 10.1016/0016-5085(82)90129-9. [DOI] [PubMed] [Google Scholar]
- 38.Belcher J.M., Garcia-Tsao G., Sanyal A.J., Thiessen-Philbrook H., Peixoto A.J., Perazella M.A., Ansari N., Lim J., Coca S.G., Parikh C.R., et al. Urinary biomarkers and progression of AKI in patients with cirrhosis. Clin. J. Am. Soc. Nephrol. 2014;9:1857–1867. doi: 10.2215/CJN.09430913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carvounis C.P., Nisar S., Guro-Razuman S. Significance of the fractional excretion of urea in the differential diagnosis of acute renal failure. Kidney Int. 2002;62:2223–2229. doi: 10.1046/j.1523-1755.2002.00683.x. [DOI] [PubMed] [Google Scholar]
- 40.Gines P., Sola E., Angeli P., Wong F., Nadim M.K., Kamath P.S. Hepatorenal syndrome. Nat. Rev. Dis. Primers. 2018;4:23. doi: 10.1038/s41572-018-0022-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available upon request from the corresponding author.