Abstract
Hyperglycemia is a condition with high glucose levels that may result in dyslipidemia. In severe cases, this alteration may lead to diabetic retinopathy. Numerous drugs have been approved by officials to treat these conditions, but usage of any synthetic drugs in the long term will result in unavoidable side effects such as kidney failure. Therefore, more emphasis is being placed on natural ingredients due to their bioavailability and absence of side effects. In regards to this claim, promising results have been witnessed in the usage of Ipomoea batatas (I. batatas) in treating the hyperglycemic and dyslipidemic condition. Thus, the aim of this paper is to conduct an overview of the reported effects of I. batatas focusing on in vitro and in vivo trials in reducing high glucose levels and regulating the dyslipidemic condition. A comprehensive literature search was performed using Scopus, Web of Science, Springer Nature, and PubMed databases to identify the potential articles on particular topics. The search query was accomplished based on the Boolean operators involving keywords such as (1) Beneficial effect OR healing OR intervention AND (2) sweet potato OR Ipomoea batatas OR traditional herb AND (3) blood glucose OR LDL OR lipid OR cholesterol OR dyslipidemia. Only articles published from 2011 onwards were selected for further analysis. This review includes the (1) method of intervention and the outcome (2) signaling mechanism involved (3) underlying mechanism of action, and the possible side effects observed based on the phytoconstiuents isolated. The comprehensive literature search retrieved a total of 2491 articles using the appropriate keywords. However, on the basis of the inclusion and exclusion criteria, only 23 articles were chosen for further review. The results from these articles indicate that I. batatas has proven to be effective in treating the hyperglycemic condition and is able to regulate dyslipidemia. Therefore, this systematic review summarizes the signaling mechanism, mechanism of action, and phytoconstituents responsible for those activities of I. batatas in treating hyperglycemic based on the in vitro and in vivo study.
Keywords: Ipomoea batatas, extraction, hyperglycemia, diabetic retinopathy, dyslipidemia, mechanism of action, signaling pathway
1. Introduction
The Ipomoea batatas (I. batatas) which is also commonly known as sweet potato is a plant that belongs to the Convolvulaceae family [1]. It is a perennial herbaceous vine that produces edible leaves and storage roots and can grow well on marginal lands. In this type of crop, asexual propagation is the most common form of dispersal, which results in a natural reduction of the genetic pool due to a lack of gene exchange [2]. I. batatas is a common staple root crop across the world as it produces the most edible energy per hectare and more than 6000 types of morphological variation of the leaf have been documented to date [3]. According to food and agriculture organizations of the United Nations in 2014, I. batatas is the sixth most consumed food crop worldwide, with a production of 178 million tons [4] and with China being the leading country in producing sweet potato [5]. The extraction of I. batatas contains a wide range of phenotypic variability [6] and bioactive compounds with acidic properties [7] and radical scavengers [8].
Additionally, in only the leaf, 130 known metabolites have been discovered through a simple extraction process [2]. Of those, phenolic compounds, such as mono caffeoylquinic acid, dicaffeoylquinic acid, and 3,4,5-tri caffeoylquinic acid, flavonoids including quercetin, myricetin, luteolin, and apigenin, and anthocyanins, are predominantly found in the leaf extracts [1]. In particular, dicaffeoylquinic acid and 3,4,5-tri caffeoylquinic acid possess anti-diabetic capacity and can inhibit lipid peroxidation [1]. Specifically, isolation of the caffeic acid derivatives, anthocyanosides, flavonoids, and arabinogalactan-protein from the I. batatas is said to be an effective anti-diabetic agent [9].
1.1. Hyperglycemia
Hyperglycemia is a condition in which the blood plasma contains an abnormal amount of glucose. It occurs when the body is unable to produce enough insulin or develops resistance to insulin. Hyperglycemia is when the blood glucose is greater than 125 mg/dL when fasting [10]. Hyperglycemia is the hallmark of diabetes mellitus, a chronic condition characterized by increased levels of glucose and an altered level of usual metabolic process in lipid and protein [11]. Many organs can be affected in the case of uncontrolled glucose levels. However, under hyperglycemic conditions, most cells can adjust to the rate of intracellular transport of glucose and protect the intracellular milieu from undesirable effects. However, certain cells, such as neural cells, endothelial cells, and β-cells, fail to stimulate glucose afflux regulation and equilibrate their intracellular glucose level to the extracellular concentrations, making them more vulnerable to the effects of hyperglycemia [12]. In hyperglycemic conditions, the production of nicotinamide adenine dinucleotide (NADH) production will be increased as a consequence of the increased level of glucose flux in the glycolytic pathway, causing a surge in the production of pyruvates and acetyl-CoA [13].
Since NADH is an electron carrier, an excessive level of NADPH will stress the mitochondrial electron transport chain which may not be inhibited by glucose-6-phosphate. As a result, the more glucose is consumed, the more glucose 6-phosphate (G6P) is produced, which is then broken down through glycolysis and the Krebs cycle, resulting in the uncontrolled formation of more NADH [14], eventually leading to the activation of NADPH oxidase. The enzymatic source of reactive oxygen species in the retina is NADPH oxidase, which is involved in the development of diabetic retinopathy. As a consequence, in a hyperglycemic state, microvascular complications of the retina can develop, which may induce oxidative damage in the retina, leading to the leakage of the tiny blood vessels, and act as signaling molecules to stimulate neovascularization, resulting in new fragile vessels [15].
1.2. Dyslipidemia
Dyslipidemia or lipid abnormalities are characterized by an increase in plasma cholesterol, triglycerides (TGs) in the range >200 mg/dL, or a reduction in high-density lipoproteins (HDL) cholesterol (<40 mg/dL) [16]. It is a complex disorder that involves central and specific organ mechanisms [17]. These include single or multiple gene mutations that result in either overproduction or defective clearance of triglycerides and low-density lipoproteins (LDL), or in the underproduction or excessive clearance of HDL. Similarly, other factors including a sedentary lifestyle along with excess consumption of calories, saturated fat, cholesterol, and trans fat diet may trigger this disorder [18]. Low-density lipoprotein cholesterol (LDL-C) components appear extremely small and compact, and more vulnerable to oxidation in the presence of hypertriglyceridemia (hyper-TG). Glycation of LDL-C is promoted by hyperglycemia, while the simultaneous glycation and oxidation of LDL-C will eventually raise the atherogenicity of LDL-C [19].
A recent cohort study shows that the prevalence rate of hypercholesterolemia in Malaysia in the years 2006 to 2012 (TC ≥ 240 mg/dL) was 44.9% while ethnic Malay tops the list with 51% [20]. Interestingly, a strong correlation was discovered between rising cholesterol levels in insulin-dependent diabetics and the severity of diabetic retinopathy. This means that people with high levels of total cholesterol or LDL-C in their serum are more likely to develop retinal hard exudate. This happens when the lipoproteins start to leak out from the retinal capillaries into the extracellular space of the retina, causing the formation of hard exudate in the retina [21].
2. Methods
2.1. Search Strategy
The search strategy was done based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines described elsewhere [22]. A systematic review for the literature search was performed to identify relevant articles on the therapeutic potential of I. batatas leaves in hyperglycemia and dyslipidemia in regards to diabetic retinopathy. A literature search was performed comprehensively in a few selected databases such as Scopus, Web of Science, Springer Nature, and PubMed. The literature search was strictly selected for any publications related to the topic from 2011 onwards. The search query was done based on the Boolean operators, as described elsewhere [23]. The set of keywords are as follows: (1) Beneficial effect OR healing OR intervention AND (2) sweet potato OR Ipomoea batatas OR traditional herb AND (3) blood glucose OR LDL OR lipid OR cholesterol OR dyslipidemia.
2.2. Inclusion Criteria
Only research articles based on in vitro and in vivo studies that discussed effects, therapeutic potentials, signaling mechanisms, and mechanism of action were chosen to be further analyzed. The chosen articles had to be written in the English language with abstracts. The selected studies had to evaluate at least the following essential measures: (1) methods of intervention and outcome (2) signaling mechanisms involved (3) underlying mechanisms of action, and the possible side effects observed. Moreover, the reference was limited to within the last 10 years of publication, starting from January 2011 until June 2021.
2.3. Exclusion Criteria
Conference papers, thesis dissertations, review papers, manuscripts that were not written in English language, papers that did not have abstracts and articles that did not meet the criteria of the above-mentioned inclusion criteria were excluded. Studies focusing on I. batatas in clinical trials or other diseases, such as carcinoma, were also excluded from this review.
2.4. Data Extraction and Management
According to the inclusion criteria of this paper, all the published manuscripts were screened thoroughly. To ensure the guideline was followed, the titles and abstracts of each chosen articles were reviewed. Then, the full text was read to rule out articles that did not fulfill the inclusion criteria. Three independent reviewers (R.N., H.B., and M.D.Y.) did the initial screening of the titles and the abstracts of all identified records for potentially relevant studies. Any disagreement was discussed between the authors. The fourth reviewer (M.K.H.) was consulted to settle any form of disagreement that may arise. The obtained results include (1) the subject type, (2) dosage of treatment, (3) method of extraction, (4) follow up duration, (5) study outcome of the parameter assessed, and (6) the end result of the study. No conflict of interest was found among the reviewers during the data extraction process.
2.5. Strategy for Data Extraction
The findings of this literature review focus on in vivo trials of I. batatas in hyperglycemia and dyslipidemia in relation to the dosage of the treatment and the outcome observed. This is tabulated in Section 3. In Section 4, the analysis of the results is presented considering the mechanism of action and the signaling pathway involved in combating hyperglycemia and dyslipidemia with regards to diabetic retinopathy with some suggestions for future applications in standardized approaches that have been described extensively.
3. Results
3.1. Literature Search
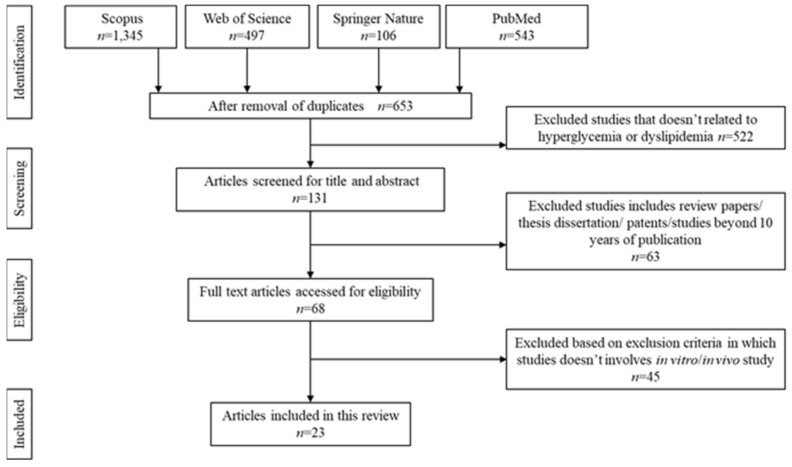
The literature search was able to identify 2491 articles that were potentially relevant to the title. Upon detection of duplication, 1838 articles were removed. Upon thoroughly screening the title and abstracts, 522 articles were removed. Due to unmatched content based on the inclusion criteria stated above, another additional 63 articles were excluded. Another 45 articles were rejected upon full-text screening either due to the constraint sample size or involvement of clinical trials. Deep screening allowed us to identify the final 23. The flow chart of the screening, identification, and the reasons for exclusion are summarized in Figure 1. Data extraction was done based on the finalized articles selection, as shown in Table 1 and Table 2.
Figure 1.
Identification and screening for literature search.
Table 1.
Effect of I. batatas on hyperglycemic condition.
| Author | Type of Subject | Dosage of Treatment |
Extraction Method | Follow Up Duration | Findings | Conclusion |
|---|---|---|---|---|---|---|
| Niwa et al., 2011 [24] | Male Wistar rats injected with Streptozotocin | 5 g/kg/day for 8 weeks | Not stated | 1st, 3rd, 5th, and 7th week |
|
I. batatas exhibits anti-hyperglycemic effects through the p38 MAP kinase signaling pathway. |
| Pal et al., 2015 [25] | Male albino Sprague Dawley rats injected with Streptozotocin | 100 mg/kg/day for 30 days | Ethanol and aqueous | 7th, 14th, 21st, and 28th day |
|
Aqueous extraction of I. batatas is an effective anti- hyperglycemic agent. |
| Lin et al., 2017 [26] | CN1927, CYY98 of sweet potato leaf extract (SPLE), and 40 °C oven-dried TN64 | Not relevant | Ethanol | - |
|
SPLE improves TNF-α -induced insulin resistance by activating insulin signaling. |
| Tahir et al., 2018 [27] | Male Wistar rats induced with Alloxan monohydrate | 4 g/kg/day for 15 days | Methanol and aqueous | 3rd, 6th, 9th, 12th, and 15th day |
|
I. batatas decreases diabetic complications by decreasing calcium and magnesium level in various organs. |
| Akhtar et al., 2018 [28] | Male Wistar rats induced with Alloxan monohydrate | 4 g/kg/day for 14 days | Methanol | 3rd, 6th, 9th, 12th, and 15th day |
|
Methanol extraction of I. batatas is an effective anti-hyperglycemic agent. |
| Kamal et al., 2018 [29] | Male Wistar rats induced with Alloxan monohydrate | 4 g/kg/day for 14 days | Aqueous | 3rd, 6th, 9th, 12th, and 15th day |
|
Aqueous extraction of I. batatas is an effective anti-hyperglycemic agent in older aged subjects. |
| Omodamiro et al., 2018 [30] | Adult female albino rats | 1000 mg/kg/day or 750 mg/kg/day or 500 mg/kg/day or 250 mg/kg/day for 7 days | Methanol | Every 2 days |
|
Methanol extraction of 500 mg/kg is an optimum dose for anti-hyperglycemic. |
| Rafiu et al., 2018 [31] | Adult male Wister strains albino rats induced with Streptozotocin | 400 mg/kg/day for 28 days. | Aqueous | Every day |
|
Aqueous extraction of 400 mg/kg is an optimum dose for anti-hyperglycemic. |
| Rafiu et al., 2018 [32] | Adult male Wister strain albino rats induced with Streptozotocin | 400 mg/kg/day for 28 days. | Aqueous | Every 2 days |
|
I. batatas is able to reverse hyperglycemic condition. |
| Almoraie, 2019 [33] | Adult male Wister albino rats induced with Streptozotocin | 200 mg/kg/day for 4 weeks | Aqueous | Not stated |
|
I. batatas is able to reverse hyperglycemic condition, hyperinsulinemia, oxidative stress, inflammatory and histopathological changes in pancreas. |
| Novrial et al., 2020 [34] | Male Sprague Dawley rats induced with Streptozotocin | 0.25 g/kg/day or 0.8 g/kg/day or 2.5 g/kg/day for 14 days | Ethanol | 6th and 14th day |
|
I. batatas is an effective hyperglycemic agent. |
| Refaat et al., 2020 [35] | Adult male albino Sprague Dawley rats induced with Alloxan monohydrate | 2.5% or 5% of diet for 28 days | Powdered form (freeze dried) | 7th, 14th, 21st, and 28th day |
|
5% of grinded I. batatas leaves in diet is an effective anti-hyperglycemic agent. |
| Shih et al., 2020 [36] | Male mice induced with Streptozotocin | 0.5% or 5% of diet for 8 weeks | Powdered form (freeze dried) | Once a week |
|
I. batatas exhibits anti- hyperglycemic effects by stimulating the regeneration of pancreatic islet and insulin resistance. |
| Jiang et al., 2020 [37] | Male mice induced with Streptozotocin | 500 mg/kg/day for 8 weeks | Ethanol | Once a week |
|
Sweet potato extract stimulates glycolysis and reduces gluconeogenesis. |
| Luo et al., 2021 [38] | Mice | Not specified | Not specified | Not specified |
|
I. batatas exhibits anti-hyperglycemic effects through PI3K and glycogen synthase kinase-3β signaling pathway. |
Table 2.
Effect of I. batatas on dyslipidemia.
| Author | Type of Subject | Dosage of Treatment | Extraction Method | Follow up Duration |
Findings | Conclusion |
|---|---|---|---|---|---|---|
| Park et al., 2012 [39] | Male Sprague Dawley rats fed with high-fat diet (HFD) | 5% of total diet for 4 weeks | Fleshed | 4th week |
|
Sweet potato extraction is an effective agent to enhance fecal lipid excretion. |
| Jawi et al., 2015 [40] | Local Balinese male rabbits fed with high cholesterol diet | 4 mL/kg/day for 60 days | Aqueous | 60th day |
|
Sweet potato extract is able to reduce cholesterol content. |
| Kurata et al., 2017 [41] | Male Sprague Dawley rats fed with HFD | 0%, 1%, 3%, and 5% of total diet for 35 days | freeze-dried powder | 35th day |
|
I. batatas is able to stimulate lipid metabolism. |
| Nasoetion et al., 2019 [42] | Broiler chicks fed with crude protein diet | 25 mL/kg/day and 50 mL/kg/day for 35 days | Aqueous | Weekly for 35 days |
|
Sweet potato extract is able to reduce fat deposition. |
| Nur et al., 2019 [43] | Zebrafish (Danio rerio) fed with HFD | 80 ppm, 120 ppm, and 160 ppm for 40 days | Ethanol | Weekly for 40 days |
|
Sweet potato extract is able to reduce fat deposition. |
| Heriwijaya et al., 2020 [44] | White male Wister rats fed with high cholesterol diet | 3 cc, 6 cc, and 9cc for 12 weeks | Aqueous | 12th week |
|
Purple sweet potato leaf extract is able to reverse hyperlipidemia condition |
| Khairani et al., 2020 [45] | Male Mus Musculus mice fed with HFD | 0.3 mL/day, 0.5 mL/day, and 1.0 mL/day for 9 weeks | Aqueous | 9th week |
|
0.3 mL/20 g/bw is the optimum dosage of the extract to exhibit maximum effect. |
| Ntchapda et al., 2021 [46] | Male Wistar rats fed with HFD | 400 mg/kg, 500 mg/kg, and 600 mg/kg for 4 weeks | Aqueous | 4th week |
|
I. batatas is able to reverse hyperlipidemia condition. |
3.2. The Diabetic Retinopathy as a Consequence of Hyperglycemia
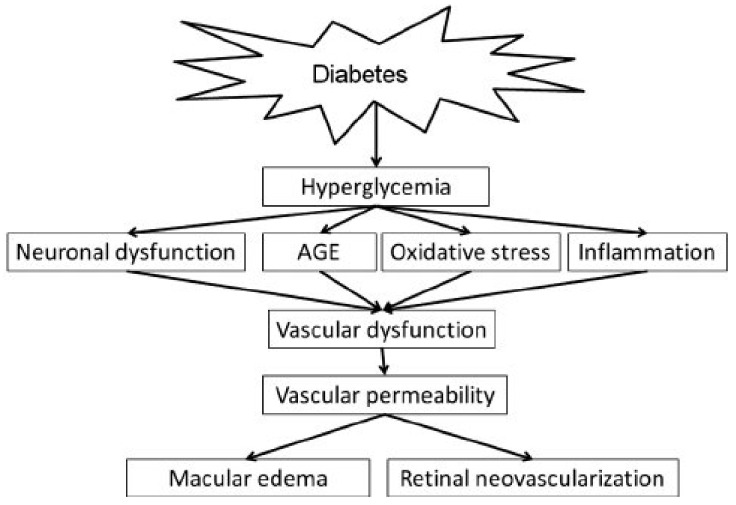
Diabetic retinopathy arises as a complication of hyperglycemia and dyslipidemia. Particularly, the hyperglycemic conditions can induce microvascular damage through multiple pathways. This includes the hexosamine pathway, protein kinase C (PKC) pathway, polyol pathway, and the accumulation of advanced glycation end products (AGEs). However, the outcome of all these pathways manifests in a similar pathology which starts with alteration in the normal flow of blood in the retina [47].
A consequence of high blood glucose is nitric oxide, which is a vasodilator. As a result, the retinal blood vessels will start to dilate and the normal flow of blood to the retina is altered. Thus, metabolic autoregulation is initiated in the retina which may stimulate retinal metabolism [48]. Uncontrolled dilation of the blood vessel will eventually weaken the capillary wall [49]. In this case, the intraluminal pressure will become unbalanced, leading to the formation of microaneurysm [50], and thus the drastic apoptotic of pericytes [49]. Loss of pericytes triggers the formation of acellular capillaries in which the tubes are produced with the basement membrane, causing the capillary to occlude [51]. Over time, this vessel blockage will increase their permeability, which in turn will stimulate endothelial cell proliferation in the intravessel. As a corollary, neovascularization occurs, resulting in hemorrhages and vision loss [52].
On the contrary, the correlation between dyslipidemia and diabetic retinopathy is interrelated with AGE and the PKC pathway in the hyperglycemic condition. In the PKC pathway, glucose flux is raised via the glycolysis process. As a result, the key activator of PKC, de novo synthesis of diacylglycerol (DAG) will be stimulated. Consequently, the accumulation of long-chain fatty acids will be transformed into DAG as well, which will eventually upregulate PKC [53]. After, proteins in the extracellular matrix (ECM) will undergo differential synthesis causing the ECM to remodel. Along with these changes, there will be a sudden increase in the release of angiogenic factors and endothelial cells, leading to the dysfunction of leukocytes. Hence, the capillary is blocked and the normal blood flow to the retina is altered, resulting in vision loss [54].
Similarly, AGE is closely associated with lipids and the development of vision loss. Non-enzymatic interactions between reducing sugars and lipoproteins produce AGE. In hyperglycemic condition, the level of AGE is increased drastically due to the availability of excessive glucose [55]. In a highly oxidative environment such as the retina, the accumulation of lipid and modification of protein will cause an accumulation of lipoxidation end products (ALEs), leading to the loss of pericytes, which in turn contributes to vascular complication, particularly in the retina [56]. Figure 2 summarizes the pathway of hyperglycemia leading to diabetic retinopathy.
Figure 2.
The pathophysiology of diabetic retinopathy as a consequence of high blood glucose level. Picture reproduced from Shin et al., (2014) [57]. Permission adapted from https://creativecommons.org/licenses/by-nc-sa/3.0/.
3.3. Mechanism of Ipomoea batatas in Hyperglycemia and Dyslipidemia
The primary reason for I. batatas’s healing capability is mainly due to its bioactive compounds such as flavonoids and phenols. In this context, flavonoids are able to promote glucose absorption in peripheral tissue and enhance insulin secretion via the modulation of pleiotropic mechanisms [58]. The sequence of pleiotropic mechanisms includes stimulation of glucagon-like peptide-1 (GLP-1), which may enhance autonomic nerve activation and cause a rise in portal GLP-1. As a result, glucose synthesis via portal GLP-1 receptors will be hindered. At the same time, islet activities will be inhibited. Thus, the inactivation of locally produced intact GLP-1 in the islets is prevented. This may increase insulin secretion while suppressing glucagon secretion and possibly reducing islet inflammation simultaneously [59]. Certainly, being a natural antioxidant, flavone compounds in I. batatas extract are able to suppress reactive oxygen species (ROS) [60]. It has been hypothesized that the buildup of ROS due to persistent hyperglycemic condition is a major factor in the apoptosis of pancreatic β-cells in diabetes type 1 and insulin resistance in diabetes type 2 [61]. In addition, quercetin triggers the regeneration of β-cells in the pancreas, causing an increase in insulin secretion [62]. This is because quercetin has the ability to regulate Ca2+ fluxes. This in turn increases the intracellular concentration of Ca2+. As a result, the cellular pathway involving insulin secretion will be stimulated, reducing the possibility of insulin resistance, acting as an anti-diabetic agent [63].
Likewise, it is likely that the insoluble dietary fiber in I. batatas prevented lipid absorption in the small intestine [41]. By altering lipid absorption and transport, flavonoids may help to ameliorate dyslipidemia. At the gastrointestinal level, flavonoids enhance a drastic reduction of fat absorption. This is achieved via the regulation of different enzymes involved in lipid metabolism and the expression of transcription factors involved in triglyceride and cholesterol synthesis, such as sterol regulatory element-binding proteins (SREBP-1) and (SREBP-2). Through this mechanism, flavonoids could lower plasma triglycerides, total cholesterol, LDL cholesterol, and increase HDL cholesterol [64]. A similar mechanism is witnessed in the quercetin compound. In this case, by reducing pancreatic lipase activity, the quercetin reduces intestine dietary fat absorption. In addition, quercetin was found to inhibit cholesterol absorption through epithelial cholesterol transporters and reduce triglyceride absorption through epithelial fatty acid transporters such as fatty acid transport protein 4 (FATP4) [65]. Table 1 and Table 2 summarize the effect of I. batatas on hyperglycemic condition and dyslipidemia.
3.4. Signaling Mechanism of Ipomoea batatas in Reducing Hyperglycemic Condition
Ipomoea batatas is able to ameliorate hyperglycemic and regulate dyslipidemia via various signaling mechanisms. For example, it has been hypothesized that I. batatas has the capacity to lower tumor necrosis factor alpha (TNF-α) levels while also decreasing the expression of p38 mitogen-activated protein kinase (p38 MAPK) [24], a protein kinase implicated in β-cell death as well as influencing cellular responses to cytokines. Overexpression of TNF-α can activate the kinase by upregulating p38 phosphorylation, reducing the expression of Bcl-2 and Bax, an apoptosis regulator of β cells [66]. In this, inhibition of the p38 pathway hinders the downregulation of Bcl-2 and Bax which reduces blood glucose levels by enhancing the role of endogenous antiapoptotic Bcl proteins, such as Bcl-2 and Bcl-xL, thus suppressing the cell response to glucose [67].
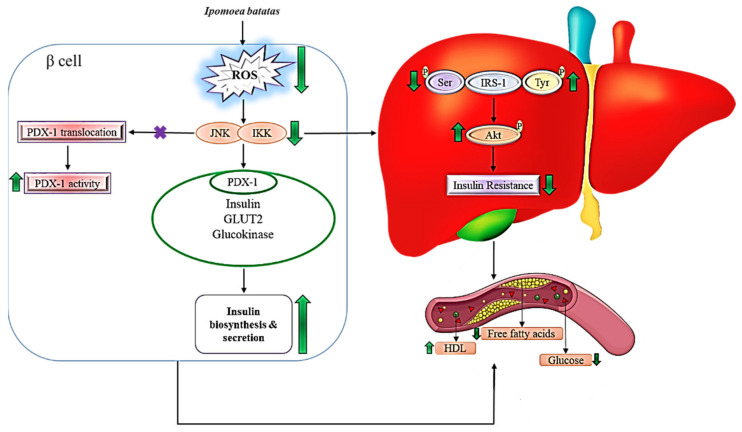
Moreover, flavonoids in I. batatas improves glucose uptake by the cells via phosphoinositide 3-kinase (PI3K)/Akt and adenosine monophosphate-activated protein kinase (AMPK). The PI3K signaling pathway is a significant factor in the translocation of the glucose transporter (GLUT) protein from intracellular compartments to the plasma membrane, as it is a signal transduction system downstream of an insulin receptor (IR). The activation of various phosphorylation-dephosphorylation cascades occurs when insulin-IR binds within the cells. When this happens, the intracellular subunit of IRs autophosphorylates, the tyrosine kinase is activated, which catalyzes repeated phosphorylation of the IR substrate (IRS) proteins. In contrast, disruption in IRS protein phosphorylation or impaired PI3K recruitment from the cytosol, which results in PI3K inactivation, causes insulin resistance, followed by diabetes. In this situation, the flavonoids from I. batatas are able to activate Akt [36]. Activation of Akt further stimulates the translation of GLUT4, which is mainly expressed in the insulin-responsive tissues. As a result, insulin stimulates GLUT4 translocation from the intracellular locations to the cell surface, which enhances glucose absorption in cells, thereby reducing glucose levels in the plasma [68]. Figure 3 explains the signaling mechanism of I. batatas in reducing high glucose level and regulation of the dyslipidemic condition.
Figure 3.
Mechanism of action of Ipomoea batatas in reducing hyperglycemia and regulating dyslipidemic condition.
Similarly, a high level of glucose may cause the accumulation of ROS in β-cells. Accumulation of ROS may activate the c-Jun N-terminal kinase (JNK) pathway which may cause pancreatic and duodenal homeobox-1 (PDX-1) to translocate from the nucleus to the cytoplasm, resulting in a decrease in PDX-1 activity. This will eventually reduce the expression of the insulin gene. Thus, the normal insulin biosynthesis process is hindered [69]. In addition, the presence of quercetin in I. batatas ensures proper regulation of Ca2+ concentration in insulinoma cells. This usually correlates with TNF-α accumulation. This pathway may be activated by increasing the glucose concentrations, as in hyperglycemia. This is because a high level of TNF-α in insulinoma cells will raise the concentration of Ca2+cytosolic, which in turn stimulates calpain and calcineurin [70]. Activated calcineurin mediates dephosphorylation of the Bcl-2 associated with the agonist of cell death (BAD) protein. Such events will promote caspase activation, which could lead to pancreatic β-cell death via Ca2+ channel activation, thereby inhibiting the secretion of insulin [71]. On the contrary, calcineurin indirectly promotes insulin release by stimulating insulin gene expression. This action is mediated by the calcineurin-dependent dephosphorylation and activation of the transcription factor known as the nuclear factor of activated T cells (NFAT). However, calcineurin B1 deficiency may contribute to the development of diabetes mellitus as a result of insufficient insulin synthesis as age progresses. The results show that calcineurin can promote both anti- and pro-apoptotic processes in the same islet cell. Although calcineurin deficiency can lead to diabetes, research demonstrates that calcineurin inhibition is necessary to protect against cytokine-induced β-cell death [72].
3.5. Signaling Mechanism of Ipomoea batatas in Regulating Dyslipidemia
The presence of active compounds such as polyphenols in I. batatas is known to be an essential element in regulating dyslipidemia. It has been hypothesized that polyphenols could suppress fat accumulation via the downregulation lipogenic pathway particularly via the downregulation of SREBP-1c and its downstream molecules, specifically lipogenic genes such as acetyl-CoA carboxylase (ACC) and stearoyl-CoA desaturase (SCD) [73]. When the lipogenic genes, ACC and SCD, are being suppressed, the concentration of malonyl-CoA will be reduced. As a result, carnitine palmitoyl transferase (CPT)-1 will be activated, enhancing the β-oxidation of fatty acids [74]. Thus, the transport of non-esterified fatty acids to the liver will be inhibited, leading to the reduction in the triglyceride synthesis [75]. Additionally, deleting or suppressing ACC further decreases fat mass and increases insulin sensitivity via the lowering of malonyl-CoA levels [76]. Meanwhile, SCD reduces fat accumulation by catalyzing the biosynthesis of monounsaturated fatty acids from saturated fatty acids [77].
4. Discussion
The findings obtained in this review are shown in Table 1, highlighting the beneficial effect of I. batatas as an anti-hyperglycemia agent [24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,66,67,68,69,70,71,72,73,74,75,76,77] and also a good regulator in dyslipidemic conditions [39,40,42,43,44,45,46]. A positive outcome in reducing plasma glucose level is witnessed as early as six days after treating diabetic rats with I. batatas extract [34], while 28 days of continuous treatment with I. batatas extract is considered an ideal duration for the extract to exhibit a maximum level of effect. In regards to this statement, three experimental studies conducted for up to 28 days observed regeneration of β-cell mass [24]. A minimum amount of 100 mg seems to be effective in revealing I. batatas healing ability in hyperglycemic conditions [25]. Meanwhile, the findings obtained by all the researchers are almost identical to one another. In this case, all the studies reviewed show that I. batatas extract, regardless of the method of extraction, are able to reduce plasma glucose level, glycosylated protein, and increased level of insulin [24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,66,67,68,69,70,71,72,73,74,75,76,77].
A study done by Niwa et al. in 2011 shows that a decreased level of the 8-OHdG marker in serum decreased formation of nitrotyrosine in the aorta [24]. 8-OHdGs is an important marker indicating severe-form diabetes, which is produced due to DNA oxidation in cells. This may lead to other secondary complications such as micro or macrovascular complications, most likely diabetic retinopathy [78]. Reduction of 8-OHdG level indicates I. batatas has the ability to hinder deoxyribonucleic acid (DNA) oxidation in β-cells. On the other hand, nitrotyrosine is an indicator for the formation of peroxynitrite which is considered a potent nitrating and oxidant agent. Therefore, a high level of nitrotyrosine may deplete the antioxidant defense and hinder the normal role of certain enzymes, which may trigger direct cytotoxic effects to endothelial cells, leading to apoptosis of fibroblasts and myocytes in the heart [79]. The outcome observed by Niwa et al., 2011 [24] show the protective ability of I. batatas anti-apoptotic agent. Similarly, Pal et al., 2015 observed an inhibitory effect on α-glucosidase in rats fed with ethanol extracted I. batatas [77]. Generally, α-glucosidase is an exotype carbohydrase and a membrane-bound enzyme found in the epithelium of the small intestine that catalyzes the hydrolytic breakdown of oligosaccharides into absorbable monosaccharides to aid glucose absorption by the small intestine. In other words, α-glucosidase catalyzes the release of glucose from the substrate’s non-reducing end. Hence, inhibition of α-glucosidase hinders the rise in glucose levels in plasma [80].
Nonetheless, I. batatas extract has proven to be a potent antioxidant. This claim was further proven when Lin et al., 2017 observed a high iron-chelating capability [26]. It is obvious that ROS accumulation mediates apoptosis of pancreatic islet cells, leading to a low level of insulin secretion, as β-cells are very susceptible to oxidative damage [81]. Systemic iron overload may dysregulate glucose metabolism [82] and generate an excessive amount of ROS via Fenton reaction [83]. Meantime, Tahir et al., 2018 witnessed a reduction in Ca2+ in various organs upon force-feeding streptozotocin-induced rats [27]. This shows that I. batatas has the ability to inhibit insulin resistivity, poor insulin sensitivity, and impaired glucose tolerance which is a complication of a high level of Ca2+ in serum [84]. Moreover, Akhtar et al., 2018 [28] and Kamal et al., 2018 [29] noticed a drastic reduction in SGPT and SGOT levels in the laboratory assessment. Both SGPT and SGOT are an indicator of tissue strain in the liver due to a high sugar level. A similar result was identified by Refaat et al., 2020 [35], Shih et al., 2020 [36], and Jiang et al., 2020 [37] who observed a reduction in ALT, AST, and ALP. The outcome shows the capability of I. batatas extract to exhibit a protective effect against the liver from ROS due to a high level of glucose.
Nevertheless, investigations done by Rafiu et al., 2018 [32], Almoraie 2019 [33], and Jiang et al., 2020 [37] showed that I. batatas could increase hepatic enzyme activity, specifically SOD, catalase, and GPx, and decrease the level of MDA in hepatocytes. With oxidative stress implicated in the pathogenesis of diabetic patients, reduction in the SOD, catalase, and GPx are a common pathology. Interestingly, all the findings in this review support the statement that these positive effects of I. batatas that are exhibited through several signaling mechanisms. For instance, Niwa et al., 2011 [24] conclude that I. batatas exhibits anti-hyperglycemic effects through the p38 MAP kinase signaling pathway while Luo et al., 2021 [37] state that the effects are manifested via PI3K and the glycogen synthase kinase-3β signaling pathway. However, in both pathways debated in their study, the pathway leads to the suppression of cell response to glucose, thereby reducing the glucose level in the plasma.
Conversely, all laboratory evaluations, as shown in Table 2, demonstrate almost similar outcomes on the role of I. batatas in regulating dyslipidemic conditions. One study chosen to be reviewed in this article shows that 0.3 mL of aqueous extraction is enough to disclose the underlying mechanism of I. batatas in regulating such conditions [39,40,42,43,44,45,46]. They notice that there is a decreased level of triglyceride, total cholesterol, and glucose in plasma while the level of HDL starts to rise over time. Although there is not much evidence on the underlying signaling mechanism involved, a recent study speculated that this could be due to the presence of I. batatas bioactive compounds such as flavonoids, quercetin, and polyphenols that are able to suppress fat accumulation via the downregulation of lipogenic pathway [73].
5. Safety Concern and Dosage Recommendation
Ipomoea batatas has been classified as a medicinal food. However, safety concerns in consuming I. batatas have still not been considered in certain conditions. For instance, I. batatas contains oxalic acid, an organic compound which may form oxalates stone in the urinary tract in dehydration condition [85]. In in vivo trials, no toxicity was encountered up to 5000 mg/kg of I. batatas extract ingestion. However, long-term administration of I. batatas extract at doses greater than 1000 mg/kg has been shown to have deleterious effects on the liver and kidney [86]. In contrast, clinical trials showed no serious adverse effects. However, I. batatas is not recommended for those with a hypersensitivity reaction, as those patients have a high possibility to develop generalized urticaria, hypotension, edema, dizziness, vomiting, sensation of tickling in the throat, and loss of consciousness upon consuming I. batatas [87].
6. Conclusions and Future Perspective
In conclusion, I. Batatas are a very versatile vegetable that can be used as a medication substitute. It is effective in treating hyperglycemia, and its activity is found to be higher than that of diabinese, a commonly used diabetes drug. Just within 8 weeks of I. batatas consumption, pancreatic cell function is readily increased, lipid levels reduced, insulin resistance starts to diminish, and reduced glycemic index is witnessed. The results prove that I. batatas could potentially be used to treat the hyperglycemic and dyslipidemic conditions. However, clinical trials are still lacking in evidence since not many clinical trials have been done on this extract. Research reveals no clinical data regarding the use of I. batatas in treating dyslipidemia, although a Cochrane review indicates that 4 g/day is safe for up to 5 months for hyperglycemic treatment. Yet, the long-term effect has not been studied yet. Therefore, it is suggested to further explore the potential of I. batatas in human trials for its potential role in bioprospecting and drug discovery in treating hyperglycemic and dyslipidemic conditions in future.
Author Contributions
Conceptualization, R.N. and M.D.Y.; methodology, R.N.; software, H.B.; validation, F.O., Z.A.Z. and M.K.H.; formal analysis, R.N.; investigation, R.N.; resources, H.B.; data curation, F.O.; writing—original draft preparation, R.N.; writing—review and editing, R.N.; visualization, M.D.Y.; supervision, H.B.; project administration, M.K.H.; funding acquisition, Z.A.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Fundamental (FRGS), grant number: “FRGS/1/2019/SKK10/UPM/02/2 (04-01-19-2133FR)” in the form of database subscription.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zhang C., Liu D., Wu L., Zhang J., Li X., Wu W. Chemical Characterization and Antioxidant Properties of Ethanolic Extract and Its Fractions from Sweet Potato (Ipomoea batatas L.) Leaves. Foods. 2019;9:15. doi: 10.3390/foods9010015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Drapal M., Rossel G., Heider B., Fraser P.D. Metabolic diversity in sweet potato (Ipomoea batatas, Lam.) leaves and storage roots. Hortic. Res. 2019;6:1–9. doi: 10.1038/s41438-018-0075-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gupta S., Rosenthal D.M., Stinchcombe J.R., Baucom R.S. The remarkable morphological diversity of leaf shape in sweet potato (Ipomoea batatas): The influence of genetics, environment, and G×E. New Phytol. 2020;225:2183–2195. doi: 10.1111/nph.16286. [DOI] [PubMed] [Google Scholar]
- 4.Food & Agriculture Organizations of the United Nations Crops. [(accessed on 3 April 2021)]. Available online: http://www.fao.org/faostat/en/#data/QC.
- 5.Fu Z.F., Tu Z.C., Zhang L., Wang H., Wen Q.H., Huang T. Antioxidant activities and polyphenols of sweet potato (Ipomoea batatas L.) leaves extracted with solvents of various polarities. Food Biosci. 2016;15:11–18. doi: 10.1016/j.fbio.2016.04.004. [DOI] [Google Scholar]
- 6.Meents A.K., Chen S.P., Reichelt M., Lu H.H., Bartram S., Yeh K.W., Mithöfer A. Volatile DMNT systemically induces jasmonate-independent direct anti-herbivore defense in leaves of sweet potato (Ipomoea batatas) plants. Sci. Rep. 2019;9:1–13. doi: 10.1038/s41598-019-53946-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.He J., Qin L. Growth and photosynthetic characteristics of sweet potato (Ipomoea batatas) leaves grown under natural sunlight with supplemental LED lighting in a tropical greenhouse. J. Plant Physiol. 2020;252:153239. doi: 10.1016/j.jplph.2020.153239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jang Y., Koh E. Antioxidant content and activity in leaves and petioles of six sweet potato (Ipomoea batatas L.) and antioxidant properties of blanched leaves. Food Sci. Biotechnol. 2019;28:337–345. doi: 10.1007/s10068-018-0481-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee C.-L., Lee S.-L., Chen C.-J., Chen H.-C., Kao M.-C., Liu C.-H., Chen J.-Y., Lai Y.-T., Wu Y.-C. Characterization of Secondary Metabolites from Purple Ipomoea batatas Leaves and Their Effects on Glucose Uptake. Molecules. 2016;21:745. doi: 10.3390/molecules21060745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nyirjesy S.C., Sheikh S., Hadjiliadis D., De Leon D.D., Peleckis A.J., Eiel J.N., Kubrak C., Stefanovski D., Rubenstein R.C., Rickels M.R., et al. β-Cell secretory defects are present in pancreatic insufficient cystic fibrosis with 1-hour oral glucose tolerance test glucose ≥155 mg/dL. Pediatr. Diabetes. 2018;19:1173–1182. doi: 10.1111/pedi.12700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marcovecchio M.L. Complications of acute and chronic hyperglycemia. US Endocrinol. 2017;13:17–21. doi: 10.17925/USE.2017.13.01.17. [DOI] [Google Scholar]
- 12.Duan W., Shen X., Lei J., Xu Q., Yu Y., Li R., Wu E., Ma Q. Hyperglycemia, a neglected factor during cancer progression. Biomed Res. Int. 2014;2014 doi: 10.1155/2014/461917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yan L.-J. Pathogenesis of chronic hyperglycemia: From reductive stress to oxidative stress. J. Diabetes Res. 2014;2014 doi: 10.1155/2014/137919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bonora M., Patergnani S., Rimessi A., de Marchi E., Suski J.M., Bononi A., Giorgi C., Marchi S., Missiroli S., Poletti F., et al. ATP synthesis and storage. Purinergic Signal. 2012;8:343–357. doi: 10.1007/s11302-012-9305-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peng J.J., Xiong S.Q., Ding L.X., Peng J., Xia X.B. Diabetic retinopathy: Focus on NADPH oxidase and its potential as therapeutic target. Eur. J. Pharmacol. 2019;853:381–387. doi: 10.1016/j.ejphar.2019.04.038. [DOI] [PubMed] [Google Scholar]
- 16.Kopin L., Lowenstein C.J. Dyslipidemia. Ann. Intern. Med. 2017;167:ICT82–ICT95. doi: 10.7326/AITC201712050. [DOI] [PubMed] [Google Scholar]
- 17.Hammer S.S., Busik J.V. The role of dyslipidemia in diabetic retinopathy. Vision Res. 2017;139:228–236. doi: 10.1016/j.visres.2017.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patni N., Ahmad Z., Wilson D.P. Genetics and Dyslipidemia. In: Feingold K.R., Anawalt B., Boyce A., Chrousos G., de Herder W.W., Dhatariya K., Dungan K., Grossman A., Hershman J.M., Hofland J., et al., editors. Endocrinology. National Center for Biotechnology Information, U.S. National Library of Medicine; Bethesda, MD, USA: 2020. pp. 1–12. [Google Scholar]
- 19.Mancini G.B.J., Hegele R.A., Leiter L.A. Dyslipidemia. Can. J. Diabetes. 2018;42:S178–S185. doi: 10.1016/j.jcjd.2017.10.019. [DOI] [PubMed] [Google Scholar]
- 20.Jamal R., Zakaria S.Z.S., Kamaruddin M.A., Jalal N.A., Ismail N., Kamil N.M., Abdullah N., Baharudin N., Hussin N.H., Othman H., et al. Cohort Profile: The Malaysian Cohort (TMC) project: A prospective study of non-communicable diseases in a multi-ethnic population. Int. J. Epidemiol. 2014;44:423–431. doi: 10.1093/ije/dyu089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chang Y.C., Wu W.C. Dyslipidemia and diabetic retinopathy. Rev. Diabet. Stud. 2013;10:121–132. doi: 10.1900/RDS.2013.10.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hutton B., Salanti G., Caldwell D.M., Chaimani A., Schmid C.H., Cameron C., Ioannidis J.P.A., Straus S., Thorlund K., Jansen J.P., et al. The PRISMA Extension Statement for Reporting of Systematic Reviews Incorporating Network Meta-analyses of Health Care Interventions: Checklist and Explanations. Ann. Intern. Med. 2015;162:777–784. doi: 10.7326/M14-2385. [DOI] [PubMed] [Google Scholar]
- 23.Grewal A., Kataria H., Dhawan I. Literature search for research planning and identification of research problem. Indian J. Anaesth. 2016;60:635–639. doi: 10.4103/0019-5049.190618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Niwa A., Tajiri T., Higashino H. Ipomoea batatas and Agarics blazei ameliorate diabetic disorders with therapeutic antioxidant potential in streptozotocin-induced diabetic rats. J. Clin. Biochem. Nutr. 2011;48:194–202. doi: 10.3164/jcbn.10-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pal S., Gautam S., Mishra A., Maurya R., Srivastava A.K. Antihyperglycemic and Antidyslipidemic Potential of Ipomoea Batatas Leaves in Validated Diabetic Animal Models. [(accessed on 7 August 2021)];Int. J. Pharm. Pharm. Sci. 2015 7:176–186. Available online: https://innovareacademics.in/journals/index.php/ijpps/article/view/5603. [Google Scholar]
- 26.Lin K.-H., Low P.-Y., Chao P.-Y., Shih M.-C., Chiang M.-C., Lai Y.-C., Wu S.-B. Antioxidant Properties and Glucose Uptake Effect of Ethanol Extracts from Different Sweet Potato Leaves Prepared by Lyophilization and Oven- Drying at 40 °C. Curr. Nutr. Food Sci. 2017;13:227–236. doi: 10.2174/1573401313666170222120700. [DOI] [Google Scholar]
- 27.Tahir I.M., Akhter N., Parveen D.A., Mehboob H., Saleem S., Munir N., Shah S.M.-A., Zaheer J., Khan F.S., Sultana S., et al. Effects of methanolic and aqueous extracts of Ipomoea batatas L on mineral contents level (calcium and magnesium) in alloxan-induced diabetic rats. Pak. J. Pharm. Sci. 2018;31:2077–2083. [PubMed] [Google Scholar]
- 28.Akhtar N., Akram M., Daniyal M., Ahmad S. Evaluation of antidiabetic activity of Ipomoea batatas L. extract in alloxan-induced diabetic rats. Int. J. Immunopathol. Pharmacol. 2018;32:1–6. doi: 10.1177/2058738418814678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kamal S., Akhter N., Khan S.G., Kiran S., Farooq T., Akram M., Shah S.M.A., Tahir I.M., Akhlaq M., Said F., et al. Anti-diabetic activity of aqueous extract of Ipomoea batatas L. in alloxan induced diabetic Wistar rats and its effects on biochemical parameters in diabetic rats. Pakistan J. Pharm. Sci. 2018;31:1539–1548. [PubMed] [Google Scholar]
- 30.Omodamiro O., Omodamiro R. Evaluation of Hypoglycemic and Hypolipidemic Potentials of Sweet Potato on a Wistar Albino Rat. Am. J. Adv. Drug Deliv. 2018;6 doi: 10.21767/2321-547x.1000024. [DOI] [Google Scholar]
- 31.Rafiu A., Luka C. Effects of Aqueous Extract of Ipomoea batatas Leaf on Blood Glucose, Kidney Functions and Hematological Parameters of Streptozotocin-Induced Diabetic Rats. J. Res. Diabetes Metab. 2018;4:004–009. [Google Scholar]
- 32.Rafiu A.A., Luka C. Evaluation of the Antidiabetic Property of Aqueous Extract of Ipomoea batatas Leaf on Hyperglycemia, Hyperlipidemia, Blood Electrolytes, and Enzymatic Antioxidants of Streptozotocin-Induced Diabetic Rats. J. Res. Diabetes Metab. 2018;4:022–026. [Google Scholar]
- 33.Almoraie N.M. The Role of Ipomoea Batatas Leaves Extract on the Treatment of Diabetes Induced by Streptozotocin. Pharmacophore. 2019;10:14–20. [Google Scholar]
- 34.Novrial D., Soebowo S., Widjojo P. Protective effect of Ipomoea batatas L leaves extract on histology of pancreatic langerhans islet and beta cell insulin expression of rats induced by streptozotocin. Molekul. 2020;15:48–55. doi: 10.20884/1.jm.2020.15.1.563. [DOI] [Google Scholar]
- 35.Refaat W.A., El-Kholie E.M., Abo El-Nasr A.F. Possible Effects of Sweet Potato Leaves, Roots and their Mixture Feeding on Alloxan-Induced Diabetic Ratsm. J. Home Econ. 2020;30:1–18. [Google Scholar]
- 36.Shih C.K., Chen C.M., Varga V., Shih L.C., Chen P.R., Lo S.F., Shyur L.F., Li S.C. White sweet potato ameliorates hyperglycemia and regenerates pancreatic islets in diabetic mice. Food Nutr. Res. 2020;64:1–11. doi: 10.29219/fnr.v64.3609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jiang T., Shuai X., Li J., Yang N., Deng L., Li S., He Y., Guo H., Li Y., He J. Protein-Bound Anthocyanin Compounds of Purple Sweet Potato Ameliorate Hyperglycemia by Regulating Hepatic Glucose Metabolism in High-Fat Diet/Streptozotocin-Induced Diabetic Mice. J. Agric. Food Chem. 2020;68:1596–1608. doi: 10.1021/acs.jafc.9b06916. [DOI] [PubMed] [Google Scholar]
- 38.Luo D., Mu T., Sun H. Sweet potato (Ipomoea batatas L.) leaf polyphenols ameliorate hyperglycemia in type 2 diabetes mellitus mice. Food Funct. 2021 doi: 10.1039/D0FO02733B. [DOI] [PubMed] [Google Scholar]
- 39.Park J.H., Choi S.Y., Lee K.W., Kim S.S., Cho K.D., Han C.K. Effect of diets with red yeast sweet potato powder supplement on fecal amount and lipid metabolism in rats fed a high-fat diet. J. Korean Soc. Food Sci. Nutr. 2012;41:487–493. doi: 10.3746/jkfn.2012.41.4.487. [DOI] [Google Scholar]
- 40.Jawi I.M., Indrayani A.W., Sutirta-Yasa I.W.P. Aqueous Extract of Balinese Purple Sweet Potato (Ipomoea Batatas L.) Prevents Oxidative Stress and Decreases Blood Interleukin-1 in Hypercholesterolemic Rabbits. Bali Med. J. 2015;4:37–40. [Google Scholar]
- 41.Kurata R., Kobayashi T., Ishii T., Niimi H., Niisaka S., Kubo M., Kishimoto M. Influence of sweet potato (Ipomoea batatas L.) leaf consumption on rat lipid metabolism. Food Sci. Technol. Res. 2017;23:57–62. doi: 10.3136/fstr.23.57. [DOI] [Google Scholar]
- 42.Nasoetion M.H., Atmomarsono U., Sunarti D., Suthama N. Growth performance and lipid profile of broilers fed different levels of purple sweet potato extract and raised under different stocking densities. Livest. Res. Rural Dev. 2019;31:97. [Google Scholar]
- 43.Nur A., Karina Riskawati Y., Widodo E., Ratnawati R., Firdausy A., Kusuma Dewi L., Karina M. Effect of Purple Sweet Potato Extract (Ipomoea batatas L) on Zebrafish (Danio rerio) By Diet Induced Obesity. Res. J. Life Sci. 2019;6:83–93. doi: 10.21776/ub.rjls.2019.006.02.2. [DOI] [Google Scholar]
- 44.Heriwijaya I.P.P.D., Jawi I.M., Satriyasa B.K. Uji efektivitas ekstrak air daun ubi jalar ungu (Ipomoea batatas) terhadap profil lipid tikus putih jantan galur wistar yang diinduksi pakan dislipidemia. Intisari Sains Medis. 2020;11:452. doi: 10.15562/ism.v11i2.584. [DOI] [Google Scholar]
- 45.Khairani A.F., Islami U., Anggun Syamsunarno M.R., Lantika U.A. Synbiotic purple sweet potato yogurt ameliorate lipid metabolism in high fat diet mice model. Biomed. Pharmacol. J. 2020;13:175–184. doi: 10.13005/bpj/1874. [DOI] [Google Scholar]
- 46.Ntchapda F., Tchatchouang F.C., Miaffo D., Maidadi B., Vecchio L., Talla R.E., Bonabe C., Seke Etet P.F., Dimo T. Hypolipidemic and anti-atherosclerogenic effects of aqueous extract of Ipomoea batatas leaves in diet-induced hypercholesterolemic rats. J. Integr. Med. 2021;19:243–250. doi: 10.1016/j.joim.2021.02.002. [DOI] [PubMed] [Google Scholar]
- 47.Safi S.Z., Qvist R., Kumar S., Batumalaie K., Ismail I.S. Bin Molecular mechanisms of diabetic retinopathy, general preventive strategies, and novel therapeutic targets. Biomed Res. Int. 2014;2014:801269. doi: 10.1155/2014/801269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bek T. Diameter Changes of Retinal Vessels in Diabetic Retinopathy. Curr. Diab. Rep. 2017;17:82. doi: 10.1007/s11892-017-0909-9. [DOI] [PubMed] [Google Scholar]
- 49.Hammes H.P., Lin J., Renner O., Shani M., Lundqvist A., Betsholtz C., Brownlee M., Deutsch U. Pericytes and the pathogenesis of diabetic retinopathy. Diabetes. 2002;51:3107–3112. doi: 10.2337/diabetes.51.10.3107. [DOI] [PubMed] [Google Scholar]
- 50.Wiley H.E., Ferris F.L. Nonproliferative Diabetic Retinopathy and Diabetic Macular Edema. In: Stephen J.R., SriniVas R.S., Wiedemann P., Wilkinson C.P., Schachat A.P., editors. Retina. Volume 2. Elsevier Inc.; Los Angeles, CA, USA: 2012. pp. 940–968. [Google Scholar]
- 51.Beltramo E., Porta M. Pericyte Loss in Diabetic Retinopathy: Mechanisms and Consequences. Curr. Med. Chem. 2013;20:3218–3225. doi: 10.2174/09298673113209990022. [DOI] [PubMed] [Google Scholar]
- 52.Penn J.S., Madan A., Caldwell R.B., Bartoli M., Caldwell R.W., Hartnett M.E. Vascular endothelial growth factor in eye disease. Prog. Retin. Eye Res. 2008;27:331–371. doi: 10.1016/j.preteyeres.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Aiello L.P. The potential role of PKC β in diabetic retinopathy and macular edema. Surv. Ophthalmol. 2002;47:S263–S269. doi: 10.1016/S0039-6257(02)00391-0. [DOI] [PubMed] [Google Scholar]
- 54.Neve A., Cantatore F.P., Maruotti N., Corrado A., Ribatti D. Extracellular matrix modulates angiogenesis in physiological and pathological conditions. Biomed Res. Int. 2014;2014:756078. doi: 10.1155/2014/756078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Singh V.P., Bali A., Singh N., Jaggi A.S. Advanced glycation end products and diabetic complications. Korean J. Physiol. Pharmacol. 2014;18:1–14. doi: 10.4196/kjpp.2014.18.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Augustine J., Troendle E.P., Barabas P., McAleese C.A., Friedel T., Stitt A.W., Curtis T.M. The Role of Lipoxidation in the Pathogenesis of Diabetic Retinopathy. Front. Endocrinol. 2021;11:1146. doi: 10.3389/fendo.2020.621938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shin E.S., Sorenson C.M., Sheibani N. Diabetes and Retinal Vascular Dysfunction. J. Ophthalmic Vis. Res. 2014;9:362–373. doi: 10.4103/2008-322X.143378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pandey K.B., Rizvi S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev. 2009;2:270–278. doi: 10.4161/oxim.2.5.9498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Omar B., Ahrén B. Pleiotropic mechanisms for the glucose-lowering action of DPP-4 inhibitors. Diabetes. 2014;63:2196–2202. doi: 10.2337/db14-0052. [DOI] [PubMed] [Google Scholar]
- 60.Hue S.-M., Boyce A.N., Somasundram C. Antioxidant activity, phenolic and flavonoid contents in the leaves of different varieties of sweet potato (Ipomoea batatas) Aust. J. Crop Sci. 2012;6:375–380. [Google Scholar]
- 61.Bondeva T., Wolf G. Reactive oxygen species in diabetic nephropathy: Friend or foe? Nephrol. Dial. Transplant. 2014;29:1998–2003. doi: 10.1093/ndt/gfu037. [DOI] [PubMed] [Google Scholar]
- 62.Soares J.M.D., Leal A.E.B.P., Silva J.C., Almeida J.R.G.S., Oliveira H.P. De Influence of flavonoids on mechanism of modulation of insulin secretion. Pharmacogn. Mag. 2017;13:639–646. doi: 10.4103/pm.pm_87_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Al-Ishaq R.K., Abotaleb M., Kubatka P., Kajo K., Büsselberg D. Flavonoids and their anti-diabetic effects: Cellular mechanisms and effects to improve blood sugar levels. Biomolecules. 2019;9:430. doi: 10.3390/biom9090430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zeka K., Ruparelia K., Arroo R., Budriesi R., Micucci M. Flavonoids and Their Metabolites: Prevention in Cardiovascular Diseases and Diabetes. Diseases. 2017;5:19. doi: 10.3390/diseases5030019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Makino R., Takano K., Kita K., Nishimukai M. Influence of long-term feeding of high-fat diet on quercetin and fat absorption from the small intestine in lymph duct-cannulated rats. Biosci. Biotechnol. Biochem. 2018;82:2007–2011. doi: 10.1080/09168451.2018.1498726. [DOI] [PubMed] [Google Scholar]
- 66.Wei X., Gu N., Feng N., Guo X., Ma X. Inhibition of p38 mitogen-activated protein kinase exerts a hypoglycemic effect by improving β cell function via inhibition of β cell apoptosis in db/db mice. J. Enzyme Inhib. Med. Chem. 2018;33:1494–1500. doi: 10.1080/14756366.2018.1477138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Luciani D.S., White S.A., Widenmaier S.B., Saran V.V., Taghizadeh F., Hu X., Allard M.F., Johnson J.D. Bcl-2 and Bcl-xL suppress glucose signaling in pancreatic β-cells. Diabetes. 2013;62:170–182. doi: 10.2337/db11-1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Stöckli J., Fazakerley D.J., James D.E. GLUT4 exocytosis. J. Cell Sci. 2011;124:4147–4159. doi: 10.1242/jcs.097063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kaneto H., Katakami N., Matsuhisa M., Matsuoka T.A. Role of reactive oxygen species in the progression of type 2 diabetes and atherosclerosis. Mediators Inflamm. 2010;2010:1–11. doi: 10.1155/2010/453892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yang J., Wang C., Guan Y. Cytokines in the progression of pancreatic -cell dysfunction. Int. J. Endocrinol. 2010;2010:515136–515146. doi: 10.1155/2010/515136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Alejandro E.U., Kalynyak T.B., Taghizadeh F., Gwiazda K.S., Rawstron E.K., Jacob K.J., Johnson J.D. Acute insulin signaling in pancreatic beta-cells is mediated by multiple Raf-1 dependent pathways. Endocrinology. 2010;151:502–512. doi: 10.1210/en.2009-0678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ranta F., Düfer M., Stork B., Wesselborg S., Drews G., Häring H.U., Lang F., Ullrich S. Regulation of calcineurin activity in insulin-secreting cells: Stimulation by Hsp90 during glucocorticoid-induced apoptosis. Cell. Signal. 2008;20:1780–1786. doi: 10.1016/j.cellsig.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 73.Musilová J., Bystrick J., Árvay J., Harangózo L. Polyphenols and phenolic acids in sweet potato (Ipomoea batatas L.) roots. Potravin. Slovak J. Food Sci. 2017;11:82–87. doi: 10.5219/705. [DOI] [Google Scholar]
- 74.Longo N., Frigeni M., Pasquali M. Carnitine transport and fatty acid oxidation. Biochim. Biophys. Acta Mol. Cell Res. 2016;1863:2422–2435. doi: 10.1016/j.bbamcr.2016.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dessì M., Noce A., Bertucci P., di Villahermosa S.M., Zenobi R., Castagnola V., Addessi E., Di Daniele N. Atherosclerosis, Dyslipidemia, and Inflammation: The Significant Role of Polyunsaturated Fatty Acids. Int. Sch. Res. Not. 2013;2013:1–13. doi: 10.1155/2013/191823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Liu T., Gou L., Yan S., Huang T. Inhibition of acetyl-CoA carboxylase by PP-7a exerts beneficial effects on metabolic dysregulation in a mouse model of diet-induced obesity. Exp. Ther. Med. 2020;20:521–529. doi: 10.3892/etm.2020.8700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ravaut G., Légiot A., Bergeron K.F., Mounier C. Monounsaturated fatty acids in obesity-related inflammation. Int. J. Mol. Sci. 2021;22:330. doi: 10.3390/ijms22010330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nishikawa T., Sasahara T., Kiritoshi S., Sonoda K., Senokuchi T., Matsuo T., Kukidome D., Wake N., Matsumura T., Miyamura N., et al. Evaluation of urinary 8-hydroxydeoxy-guanosine as a novel biomarker of macrovascular complications in type 2 diabetes. Diabetes Care. 2003;26:1507–1512. doi: 10.2337/diacare.26.5.1507. [DOI] [PubMed] [Google Scholar]
- 79.Ceriello A., Quagliaro L., Catone B., Pascon R., Piazzola M., Bais B., Marra G., Tonutti L., Taboga C., Motz E. Role of hyperglycemia in nitrotyrosine postprandial generation. Diabetes Care. 2002;25:1439–1443. doi: 10.2337/diacare.25.8.1439. [DOI] [PubMed] [Google Scholar]
- 80.Kumar S., Narwal S., Kumar V., Prakash O. α-glucosidase inhibitors from plants: A natural approach to treat diabetes. Pharmacogn. Rev. 2011;5:19–29. doi: 10.4103/0973-7847.79096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Swaminathan S., Fonseca V.A., Alam M.G., Shah S.V. The role of iron in diabetes and its complications. Diabetes Care. 2007;30:1926–1933. doi: 10.2337/dc06-2625. [DOI] [PubMed] [Google Scholar]
- 82.Shaaban M., Dawod A.E., Nasr M. Role of iron in diabetes mellitus and its complications. Menoufia Med. J. 2016;29:11. doi: 10.4103/1110-2098.178938. [DOI] [Google Scholar]
- 83.Bystrom L.M., Guzman M.L., Rivella S. Iron and reactive oxygen species: Friends or foes of cancer cells? Antioxidants Redox Signal. 2014;20:1917–1924. doi: 10.1089/ars.2012.5014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhu J., Xun P., Bae J.C., Kim J.H., Kim D.J., Yang K., He K. Circulating calcium levels and the risk of type 2 diabetes: A systematic review and meta-analysis. Br. J. Nutr. 2019;122:376–387. doi: 10.1017/S0007114519001430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mohanraj R., Sivasankar S. Sweet potato (Ipomoea batatas [L.] Lam)—A valuable medicinal food: A review. J. Med. Food. 2014;17:733–741. doi: 10.1089/jmf.2013.2818. [DOI] [PubMed] [Google Scholar]
- 86.Imafidon K., Durojaiye M., Daniel B. Safety Assessment of Ipomoea Batatas (L.). Lam Leaf Extract, A Traditional Haematinic; In Male Wistar Rats. J. Ghana Sci. Assoc. 2015;16:45–51. [Google Scholar]
- 87.Dutta S. Sweet Potatoes for Diabetes Mellitus: A Systematic Review. Pharmacophore. 2015;6:72. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.