Abstract
Background
Venous thromboembolism is a common complication after hip fractures. However, there are no reliable laboratory assays to identify patients at risk for venous thromboembolic (VTE) events after major orthopaedic surgery.
Question/purposes
(1) Are rotational thromboelastometry (ROTEM) findings associated with the presence or development of symptomatic VTE after hip fracture surgery? (2) Were any other patient factors associated with the presence or development of symptomatic VTE after hip fracture surgery? (3) Which ROTEM parameters were the most accurate in terms of detecting the association of hypercoagulability with symptomatic VTE?
Methods
This retrospective study was conducted over a 13-month period. In all, 354 patients with femoral neck and peritrochanteric fractures who underwent hip hemiarthoplasty or cephallomedullary nailing were assessed for eligibility. Of those, 99% (349 of 354) were considered eligible for the study, 1% (3 of 354) of patients were excluded due to coagulation disorders, and another 1% (2 of 354) were excluded because they died before the postoperative ROTEM analysis. An additional 4% (13 of 354) of patients were lost before the minimum study follow-up of 3 months, leaving 95% (336 of 354) for analysis. A ROTEM analysis was performed in all patients at the time of their hospital admission, within hours of the injury, and on the second postoperative day. The patients were monitored for the development of symptoms indicative of VTE, and the gold standard tests for diagnosing VTE, such as CT pulmonary angiography or vascular ultrasound, were selectively performed only in symptomatic patients and not routinely in all patients. Therefore, this study evaluates the association of ROTEM with only clinically evident VTE events and not with all VTE events. ROTEM results did not affect the clinical surveillance of the study group and the decision for further work up. To determine whether ROTEM findings were associated with the presence or development of symptomatic VTE, ROTEM parameters were compared between patients with and without symptomatic VTE. To establish whether any other patient factors were associated with the presence or development of symptomatic VTE after hip fracture surgery, clinical parameters and conventional laboratory values were also compared between patients with and without symptomatic VTE. Finally, to determine which ROTEM parameters were the most accurate in terms of detecting the association of hypercoagulability with symptomatic VTE, the area under the curve (AUC) for certain cut off values of ROTEM parameters was calculated.
Results
We found several abnormal ROTEM values to be associated with the presence or development of symptomatic VTE. The preoperative maximum clot firmness was higher in patients with clinically evident VTE than in patients without these complications (median [interquartile range] 70 mm [68 to 71] versus 65 mm [61 to 68]; p < 0.001). The preoperative clot formation time was lower in patients with clinically evident VTE than those without clinically evident VTE (median 61 seconds [58 to 65] versus 70 seconds [67 to 74]; p < 0.001), and also the postoperative clot formation time was lower in patients with clinically evident VTE than those without these complications (median 52 seconds [49 to 59] versus 62 seconds [57 to 68]; p < 0.001). Increased BMI was also associated with clinically evident VTE (odds ratio 1.26 [95% confidence interval 1.07 to 1.53]; p < 0.001). We found no differences between patients with and without clinically evident VTE in terms of age, sex, smoking status, comorbidities, and preoperative use of anticoagulants. Lastly, preoperative clot formation time demonstrated the best performance for detecting the association of hypercoagulability with symptomatic VTE (AUC 0.89 [95% CI 0.81 to 0.97]), with 81% (95% CI 48% to 97%) sensitivity and 86% (95% CI 81% to 89%) specificity for clot formation time ≤ 65 seconds.
Conclusion
ROTEM’s performance in this preliminary study was promising in terms of its association with symptomatic VTE. This study extended our earlier work by demonstrating that ROTEM has a high accuracy in detecting the level of hypercoagulability that is associated with symptomatic VTE. However, until its performance is validated in a study that applies a diagnostic gold standard (such as venography, duplex/Doppler, or chest CT) in all patients having ROTEM to confirm its performance, ROTEM should not be used as a regular part of clinical practice.
Level of Evidence
Level IV, diagnostic study.
Introduction
In the surgical setting, major orthopaedic procedures such as those to treat hip fractures confer a high risk of postoperative venous thromboembolism (VTE) and affect nearly 600,000 patients in the United States, with an incidence of up to 7.5% for pulmonary embolism [2, 3, 27]. Any attempt to develop reliable predictive scores for VTE based on clinical parameters has led to complex scoring systems with a low predictive performance [1, 15]. Additionally, conventional coagulation tests cannot identify the changes in the coagulation mechanism that are associated with VTE; therefore, they cannot be used as predictive indicators of these complications. The development of reliable laboratory tests that can identify patients at a high risk for VTE early could guide thromboprophylaxis and decrease the incidence of these devastating complications. There are several post-traumatic mechanisms such as endothelial damage that are involved in trauma-induced hypercoagulability after hip fractures [18, 22, 28, 29]. Rotational thromboelastometry (ROTEM) can provide detailed information about the coagulation mechanism and can identify specific abnormalities in the overall coagulation cascade, which can be the key in detecting the hypercoagulability that develops after hip fractures and leads to VTE [16]. It has been shown in a previous study that ROTEM analysis can reliably detect this higher coagulation activity after hip fractures and surgical treatment, even when this is undetectable by conventional coagulation assays [28].
Even though previous research has found that ROTEM parameters are associated with trauma-induced coagulopathy due to hip fractures, their association with symptomatic thromboembolic events in patients with hip fractures is still unknown [11, 28]. Although the trauma-induced hypercoagulopathy that can be detected by ROTEM is probably directly associated with the development of thromboembolic complications in patients with hip fractures, it has yet to be proven whether ROTEM results are associated with thromboembolism, since the accuracy of a method to detect a clinical manifestation can be lower than its accuracy to detect the pathophysiologic culprit of this clinical manifestation.
We therefore asked: (1) Are rotational thromboelastometry (ROTEM) findings associated with the presence or development of symptomatic VTE after hip fracture surgery? (2) Were any other patient factors associated with the presence or development of symptomatic VTE after hip fracture surgery? (3) Which ROTEM parameters were the most accurate in terms of detecting the association of hypercoagulability with symptomatic VTE?
Patients and Methods
Study Design and Participants
This retrospective study was conducted over a 13-month period (July 2019 to August 2020). In all, 354 patients with femoral neck and peritrochanteric fractures who underwent hip hemiarthoplasty or cephallomedullary nailing at the Department of Orthopaedic Surgery of the Attikon University Hospital were assessed for eligibility. Of those, 99% (349 of 354) were considered eligible for the study, 1% (3 of 354) were excluded due to coagulation disorders (one patient with Factor V Leiden and two with hemophilia), and another 1% (2 of 354) because they had died (heart failure/myocardial infarction) before the second postoperative day and thus before the postoperative ROTEM analysis. An additional 4% (13 of 354) of patients were lost before the minimum study follow-up of 3 months, leaving 95% (336 of 354) for analysis (Fig. 1). The median age of the 336 patients who underwent hip fracture surgery was 79 years; 49% (163 of 336) of patients were men and 51% (173 of 336) were women. Forty-three percent (145 of 336) of patients sustained femoral neck fractures, and 57% (191 of 336) had peritrochanteric fractures. Thirty-nine percent (131 of 336) of patients were operated on within 24 hours of admission, and 61% (205 of 336) more than 24 hours after admission. Three percent (11 of 336) of patients presented with postoperative symptomatic VTE during the 3-month follow-up period; 2% (6 of 336) of patients had deep vein thrombosis, and 1% (5 of 336) had pulmonary embolism. Of patients operated on within 24 hours of admission, 3% (4 of 131) developed symptomatic VTE, and 3% (7 of 205) of those operated on more than 24 hours after admission developed symptomatic VTE. One patient developed clinically evident VTE during the first postoperative week, three patients during the second postoperative week, three during the third week, two during the fourth week, one during the seventh week, and one during the ninth week. Two percent (8 of 336) of patients died (three patients because of heart failure/myocardial infarction, four patients because of pulmonary infections and sepsis, and one patient because of malignant neoplasm) during the follow-up period. Seventy-eight patients used anticoagulant medications before their injury, including antiplatelets in 56 patients, vitamin K antagonists in three patients, and novel oral anticoagulants in 19 patients. The use of chronic anticoagulant medications did not differ between patients with VTE and those without (Table 1; Supplemental Digital Content 1, http://links.lww.com/CORR/A574).
Fig. 1.
Flowchart of the study population.
Patients who developed VTE symptoms underwent CT pulmonary angiography for the diagnosis of pulmonary embolism or vascular ultrasound for diagnosis of deep vein thrombosis. A preoperative ROTEM analysis was performed in all patients within hours of the injury during their admission to the hospital in the emergency department. A postoperative ROTEM analysis was also performed on the second postoperative day. The time interval between the two ROTEM analyses and the gold standard studies for VTE varied from 3 days to 9 weeks, based on the time of development of clinical VTE symptoms.
Test Methods
In this study, we evaluated only clinically evident pulmonary embolism or deep vein thrombosis. The patients were monitored clinically for the development of VTE symptoms, and the gold standard tests for diagnosis of VTE such as CT pulmonary angiography or vascular ultrasound were selectively performed only in symptomatic patients and not routinely for all patients. We did so because we wanted to evaluate the association of the coagulation status as reflected by ROTEM results with only those VTE events that are most commonly diagnosed in the clinical practice. Therefore, the ROTEM results and their evaluated accuracy in this study reflect their association with only clinically evident VTE events, and not with the overall number of VTE events, which could be substantially higher. The results of ROTEM analysis did not affect the clinical surveillance of the study population or the conduct of any diagnostic studies for VTE. Although the gold standard studies for diagnosis of VTE were performed only in symptomatic patients and not routinely in all patients, there was a very low threshold in ordering and performing such studies for patients with VTE symptoms.
The ROTEM analysis included the extrinsic thromboelastometry (EXTEM) and intrinsic thromboelastometry (INTEM) assays. The EXTEM assays refers to the extrinsic pathway of the coagulation cascade that is initiated by contact with the tissue factor after injury, while the INTEM assay refers to the intrinsic pathway of the cascade activated by blood-borne proteins. Low–molecular weight heparins (LMWHs) mainly affect the intrinsic pathway of coagulation, therefore, they mainly affect the results of INTEM assay. ROTEM analysis was performed preoperatively at the time of patient admission to the hospital in the emergency department, within the first few hours after the injury. The preoperative ROTEM analysis was performed to evaluate the baseline coagulation status of patients without thromboprophylaxis. Subsequently, a second ROTEM analysis including an INTEM assay was performed on the second postoperative day after the administration of the third dose of LMWH. This postoperative time was selected based on the pharmacokinetics of LMWH indicating that steady state levels of LMWH are achieved after the third dose [5]. Thus, although conducting ROTEM at the time of VTE diagnosis would allow a more direct investigation of the association between hypercoagulability and thromboembolism, the obtained postoperative ROTEM values on the second postoperative day still reflect the established coagulation profile for these patients during the critical postoperative period of the first few weeks, which is when VTE usually occurs.
For the ROTEM analysis, 2 mL of whole blood were collected for the ROTEM analysis. Citrated tubes were immediately filled with drawn blood and analyzed in a ROTEM analyzer within 90 minutes of blood collection. It has been shown that ROTEM values remain unchanged for blood samples collected in citrated tubes and stored at room temperature for up to 6 hours [14, 25]. The analysis was performed on a ROTEM analyzer (Tem Innovations GmbH) as described [24]. The following EXTEM and INTEM parameters were measured: clotting time (in seconds), the time from the beginning of the measurement until the formation of a clot 2 mm in amplitude of clot firmness; clot formation time (in seconds), the time from clotting time (amplitude of 2 mm) until a clot firmness of 20 mm was achieved; amplitude was recorded at 10 minutes (A10, in mm); alpha angle (a°), the angle between the central line (x axis) and the tangent of the thromboelastometry tracing at the amplitude point of 2 mm, describing the kinetics of clot formation; maximum clot firmness (in mm), the final strength of the clot; and the lysis index at 60 minutes (LI60, %), which is the percentage of remaining clot stability in relation to the maximum clot firmness after the 60-minute observation period after clotting time, indicating the speed of fibrinolysis. Since the clotting time and clot formation time describe similar aspects of the coagulation process, as well as the maximum clot firmness and the amplitude clot firmness at 10 minutes, these parameters could be used selectively for monitoring patients depending on their accuracy. We decided to include an INTEM analysis because there is some evidence, although limited, that the effect of LMWH on the coagulation mechanism can be detected using INTEM parameters [4, 26]. Therefore, this method may be more suitable than an EXTEM analysis as a monitoring tool to evaluate whether potential adjustments in LMWH doses will lead to adequate anticoagulation and more effective thromboprophylaxis.
Clinical Care
Patients underwent surgery the day after their admission to the hospital or in the following days, after discontinuation of chronic anticoagulation medications. Tranexamic acid was not used in our study population, and transfusion with red blood cell units was indicated for patients with hemoglobin levels lower than 8 g/dL or for patients with any signs of hemodynamic instability or anemia. All patients received thromboprophylaxis for 30 days postoperatively, including LMWH (4500 IUs of tinzaparin once daily). Patients who were receiving anticoagulation medications before surgery continued their medications after the 30-day period of LMWH. The LMWH dose was adjusted for patients with renal insufficiency and glomerular filtration rate (GFR) < 20 mL/min (2500 IUs for patients 30-50 kg, 3500 IUs for patients 50-150 kg, and 50 IUs/kg for patients < 30 kg or > 150 kg).
Primary and Secondary Study Outcomes
Our primary study goal was to assess whether rotational thromboelastometry (ROTEM) findings are associated with the presence or development of symptomatic VTE after hip fracture surgery. For our evaluation, we recorded and compared the preoperative and postoperative ROTEM results between patients with and without VTE. Moreover, we studied the independent association between ROTEM results and development of symptomatic VTE controlled for age, gender, smoking status, comorbidities, type of fracture, chronic use of anticoagulants, and BMI.
Our secondary study goals were to identify any other patient factors associated with the presence or development of symptomatic VTE after hip fracture surgery and to find which ROTEM parameters are the most accurate in terms of detecting the association of hypercoagulability with symptomatic VTE. To evaluate whether there were any other patient factors associated with symptomatic VTE, the patient clinical parameters, the fracture type, and the results of preoperative conventional laboratory tests including international normalized ratio, prothrombin time, activated partial thromboplastin time, and platelet count were recorded and compared between patients with and without VTE.
Ethical Approval
The study complied with all the relevant national regulations and institutional policies and was in accordance with the tenets of the Helsinki Declaration. It was approved by the institutional review board of our hospital (ref. number: 501/19-07-2019), and although the study was considered to have a minimal risk for patients since the blood samples for the ROTEM analysis were collected as part of routinely performed blood draws and no additional blood draws were performed, written consent was obtained from every patient.
Statistical Analysis
The statistical analysis included descriptive statistics of the study population, which are presented as medians and interquartile ranges (IQR) or frequencies with percentages when appropriate. Patients who died before the postoperative blood draw for ROTEM analysis were excluded from the study. We had no missing data for the patients analyzed. We did not perform a power analysis as this is not always necessary or feasible in observational studies. We determined the sample size based on practical considerations (time, availability of eligible patients, and cost), but we also aimed to reach a similar or even higher sample size as compared with relevant studies [11]. We compared the demographics, conventional laboratory values, and ROTEM parameters between patients with postoperative clinically evident VTE and those without. The two groups were compared using the nonparametric Wilcoxon rank sum test for continuous parameters and the chi-square test for categorical variables. To further investigate the relationship between ROTEM parameters and clinically evident VTE (adjusted for gender, smoking status, Charlson comorbidity index, fracture type, chronic use of anticoagulants, and BMI), a logistic regression analysis was performed. Receiver operating characteristic curves and their respective areas under the receiver operating characteristic curves (AUCs) were also used as an overall measure of test performance to evaluate and quantify the ability of these ROTEM parameters to identify patients at risk of clinically evident VTE. The receiver operating characteristic curves of the corresponding ROTEM parameters were statistically compared using the Hanley and McNeil [10] method to evaluate whether any of these parameters were more accurate in detection of the association between hypercoagulopathy and symptomatic VTE. The optimal cutoff values were identified with the Youden index. For the statistical analysis, STATA version 15.0 (Stata Corp) software was used. For all tests, a p value lower than 0.05 indicated statistical significance.
Results
Are ROTEM Findings Associated with the Presence or Development of Symptomatic VTE After Hip Fracture Surgery?
We found several abnormal thromboelastometry values to be associated with the presence or development of symptomatic VTE. These included preoperative and postoperative maximum clot firmness, clot formation time, and amplitude of clot firmness at 10 minutes (A10), indicating that in patients with clinically evident VTE, thrombus formation is a faster process and the final thrombus has stronger properties. Specifically, the preoperative INTEM clot formation time was lower in patients with clinically evident VTE than in those without clinically evident VTE (median [IQR] 61 seconds [58 to 65] versus 70 seconds [67 to 74]; p < 0.001) (Table 1), and the postoperative INTEM clot formation time was lower in patients with clinically evident VTE than those without these complications (median 52 seconds [49 to 59] versus 62 seconds [57 to 68]; p < 0.001) (Table 2). The preoperative INTEM maximum clot firmness was higher in patients with clinically evident VTE than those without clinically evident VTE (median 70 mm [68 to 71] versus 65 mm [61 to 68]; p < 0.001) (Table 1), and the postoperative INTEM maximum clot firmness was higher in patients with clinically evident VTE than those without these complications (median 78 mm [75 to 79] versus 71 mm [65 to 74]; p < 0.001) (Table 2). The preoperative INTEM A10 was higher in patients with clinically evident VTE than in those without clinically evident VTE (median 62 mm [60 to 64] versus 59 mm [56 to 63]; p = 0.03) (Table 1), and the postoperative INTEM A10 was higher in patients with clinically evident VTE than those without these complications (median 67 mm [64 to 71] versus 61 mm [57 to 66]; p < 0.001) (Table 2). After controlling for potential confounding variables such as gender, age, smoking status, and BMI, we found that symptomatic VTE was associated with lower preoperative EXTEM clot formation time (odds ratio 0.79 [95% confidence interval 0.71 to 0.89]; p < 0.001), higher EXTEM maximum clot firmness (OR 1.24 [95% CI 1.10 to 1.40]; p < 0.001), higher EXTEM A10 (OR 1.18 [95% CI 1.03 to 1.35]; p = 0.01), lower INTEM clot formation time (OR 0.69 [95% CI 0.55 to 0.82]; p < 0.001), and higher INTEM maximum clot firmness (OR 1.18 [95% CI 1.04 to 1.34]; p = 0.009), as well as with lower INTEM clot formation time (OR 0.86 [95% CI 0.78 to 0.94]; p = 0.002), higher INTEM maximum clot firmness (OR 1.29 [95% CI 1.11 to 1.50]; p = 0.001), and higher INTEM A10 (OR 1.25 [95% CI 1.08 to 1.45]; p = 0.002) on the second postoperative day (Table 3). To evaluate whether the fracture type affected the association between ROTEM findings and the development of clinically evident VTE, we examined the particular interaction term in the regression models representing the differential effect by fracture type. We found no significant results; however, we consider that our analysis was underpowered for such analyses.
Table 1.
Preoperative EXTEM and INTEM parameters among patients with VTE and those without
| Variables | VTE | No VTE | Difference of medians | p value |
| EXTEM CT in s | 60 (57-61) | 61 (56-66) | 1 | 0.28 |
| EXTEM CFT in s | 76 (69-79) | 85 (82-89) | 9 | < 0.001 |
| EXTEM A10 in mm | 57 (54-59) | 53 (50-56) | 4 | 0.004 |
| EXTEM MCF in mm | 70 (69-72) | 65 (61-68) | 5 | < 0.001 |
| EXTEM alpha angle in ° | 73 (70-76) | 73 (71-77) | 0 | 0.57 |
| EXTEM LI60 in % | 90 (89-95) | 92 (90-96) | 2 | 0.37 |
| INTEM CT in s | 177 (175-182) | 182 (178-185) | 5 | 0.12 |
| INTEM CFT in s | 61 (58-65) | 70 (67-74) | 9 | < 0.001 |
| INTEM A10 in mm | 62 (60-64) | 59 (56-63) | 3 | 0.03 |
| INTEM MCF in mm | 70 (68-71) | 65 (61-68) | 5 | < 0.001 |
| INTEM alpha angle in ° | 79 (74-80) | 77 (73-81) | 2 | 0.96 |
| INTEM LI60 in % | 92 (87-95) | 89 (85-94) | 3 | 0.32 |
Data are presented as medians and interquartile ranges; CT = clotting time; CFT = clot formation time; A10 = clot amplitude at 10 minutes; MCF = maximum clot firmness; LI60 = lysis index at 60 minutes.
Table 2.
INTEM parameters on the second postoperative day in patients with VTE and those without
| Variables | VTE | No VTE | Difference of medians | p value |
| INTEM CT in s | 176 (174-180) | 178 (175-184) | 2 | 0.30 |
| INTEM CFT in s | 52 (49-59) | 62 (57-68) | 10 | < 0.001 |
| INTEM A10 in mm | 67 (64-71) | 61 (57-66) | 6 | < 0.001 |
| INTEM MCF in mm | 78 (75-79) | 71 (65-74) | 7 | < 0.001 |
| INTEM alpha angle in ° | 79 (78-79) | 77 (74-80) | 2 | 0.14 |
| INTEM LI60 in % | 89 (88-91) | 92 (88-94) | 3 | 0.37 |
Data are presented as medians and interquartile ranges; CT = clotting time; CFT = clot formation time; A10 = clot amplitude at 10 minutes; MCF = maximum clot firmness; LI60 = lysis index at 60 minutes.
Table 3.
Results of the multivariable logistic regression analysis for VTE as the dependent variable, with ROTEM parameters, age, sex, BMI, Charlson comorbidity index, smoking status, surgery (peritrochanteric vs femoral neck fracture), and chronic anticoagulant use as independent variables
| VTE | |||
| Variables | OR | 95% CI | p value |
| Preoperative EXTEM CFT | 0.79 | 0.71-0.89 | < 0.001 |
| Preoperative EXTEM MCF | 1.24 | 1.10-1.40 | < 0.001 |
| Preoperative EXTEM A10 | 1.18 | 1.03-1.35 | 0.01 |
| Preoperative INTEM CFT | 0.69 | 0.55-0.82 | < 0.001 |
| Preoperative INTEM MCF | 1.18 | 1.04-1.34 | 0.009 |
| Postoperative INTEM CFT | 0.86 | 0.78-0.94 | 0.002 |
| Postoperative INTEM MCF | 1.29 | 1.11-1.50 | 0.001 |
| Postoperative INTEM A10 | 1.25 | 1.08-1.45 | 0.002 |
CFT = clot formation time; A10 = clot amplitude at 10 minutes; MCF = maximum clot firmness.
Were Any Other Patient Factors Associated with the Presence or Development of Symptomatic VTE After Hip Fracture Surgery?
We found that increased BMI of patients was associated with the presence or development of symptomatic VTE. More specifically, patients who developed symptomatic VTE were more likely to have a higher BMI compared with those who did not develop symptomatic VTE (OR 1.26 [95% CI 1.07 to 1.53]; p < 0.001). We found no differences between patients with and without clinically evident VTE in terms of age, sex, smoking status, comorbidities, and preoperative use of anticoagulants (Table 1; Supplemental Digital Content 1, http://links.lww.com/CORR/A574). We also found no differences between the conventional preoperative coagulation parameters of patients who developed clinically evident VTE and those who did not (Table 2; Supplemental Digital Content 2, http://links.lww.com/CORR/A575).
Which ROTEM Parameters Were the Most Accurate in Terms of Detecting the Association of Hypercoagulability with Symptomatic VTE?
Preoperative INTEM clot formation time, preoperative EXTEM clot formation time, and postoperative INTEM maximum clot firmness demonstrated the highest levels of accuracy in terms of association with a symptomatic VTE event. Specifically, preoperative INTEM clot formation time demonstrated the best accuracy (AUC 0.89 [95% CI 0.81 to 0.97]), followed by preoperative EXTEM clot formation time (AUC 0.88 [95% CI 0.81 to 0.96]) and postoperative INTEM maximum clot firmness (AUC 0.85 [95% CI 0.80 to 0.90]) (Fig. 2). Based on these parameters, a preoperative INTEM clot formation time value ≤ 65 seconds had 81% (95% CI 48% to 97%) sensitivity and 86% (95% CI 81% to 89%) specificity to detect symptomatic VTE, preoperative EXTEM clot formation time ≤ 81 seconds had 90% (95% CI 58% to 99%) sensitivity and 76% (95% CI 70% to 80%) specificity to detect symptomatic VTE, and postoperative INTEM maximum clot firmness ≥ 75 mm had 100% (95% CI 71% to 100%) sensitivity and 75% (95% CI 70% to 79%) specificity to detect symptomatic VTE (Table 4). Moreover, a comparison of the respective AUC values of the best three ROTEM parameters showed that there was no difference among them (p = 0.60); thus, preoperative EXTEM and INTEM clot formation time and postoperative INTEM maximum clot firmness did not differ in terms of accuracy in detecting the association with symptomatic VTE.
Fig. 2.
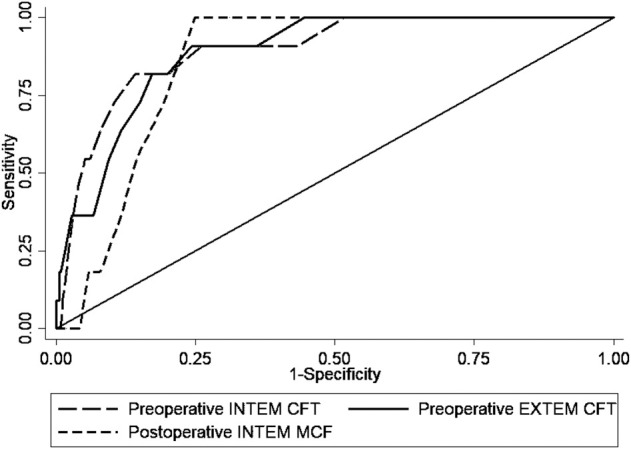
The areas under the receiver operating characteristic curve of preoperative INTEM and EXTEM clot formation time (CFT) and postoperative INTEM maximum clot firmness (MCF) for the diagnosis of VTE complications.
Table 4.
Accuracy of ROTEM parameters and platelet count for the association with clinically evident VTE
| Parametera | Area under the curve (95% CI) | Optimal cutoff | Sensitivity (%) | Specificity (%) |
| Preoperative EXTEM A10 | 0.75 (0.61-0.88) | ≥ 57 | 73 | 80 |
| Preoperative EXTEM MCF | 0.84 (0.75-0.92) | ≥ 69 | 81 | 79 |
| Preoperative EXTEM CFT | 0.88 (0.81-0.96) | ≤ 81 | 90 | 76 |
| Preoperative INTEM A10 | 0.68 (0.56-0.79) | ≥ 60 | 91 | 53 |
| Preoperative INTEM MCF | 0.79 (0.71-0.87) | ≥ 67 | 90 | 62 |
| Preoperative INTEM CFT | 0.89 (0.81-0.97) | ≤ 65 | 81 | 86 |
| Postoperative INTEM A10 | 0.81 (0.71-0.90) | ≥ 64 | 91 | 65 |
| Postoperative INTEM MCF | 0.85 (0.80-0.90) | ≥ 75 | 100 | 75 |
| Postoperative INTEM CFT | 0.80 (0.69-0.91) | ≤ 56 | 78 | 73 |
The area under the curve and optimal cutoff (Youden index) values with corresponding sensitivity and specificity are presented; A10 = clot amplitude at 10 minutes; MCF = maximum clot firmness; CFT = clot formation time.
Preoperative INTEM clot formation time, preoperative EXTEM clot formation time, and postoperative INTEM maximum clot firmness demonstrated the highest levels of accuracy.
Discussion
Although postoperative thromboprophylaxis has been proven to be an effective preventive measure, the incidence of perioperative thromboembolism for hip fractures is still high. The conventional coagulation parameters such as prothrombin time, activated partial thromboplastin time, and international normalized ratio assess only a specific aspect of the coagulation cascade, essentially giving a snapshot evaluation of this cascade; they cannot identify the changes in the coagulation mechanism that are associated with VTE. On the contrary, ROTEM is a bedside laboratory method that can provide more detailed information about the coagulation mechanism because it can evaluate several specific aspects of the hemostatic mechanism through a dynamic analysis of all elements of clot formation and breakdown [16]. Therefore, it has the potential to detect the hypercoagulability that develops after hip fractures and leads to VTE. Indeed, we found that several abnormal thromboelastometry values were associated with the presence or development of symptomatic VTE, with preoperative clot forming time and postoperative maximum clot firmness having the highest accuracy in terms of their association with a symptomatic VTE event.
Limitations
An important limitation of our study is that although in some other studies the gold standard tests for VTE diagnosis such as CT angiography or vascular ultrasound were performed routinely to detect all VTE events (subclinical and clinical events), we performed the gold standard tests selectively to evaluate only the clinically evident VTE events [7, 12]. It must be noted that selective use of the gold standard tests is expected to result in an overestimation of the accuracy of ROTEM and in an underestimation of the true number of all VTE events (clinical and subclinical events). Our goal was to assess the accuracy of ROTEM to detect the hypercoagulability that is associated with only those VTE events that are detected by the most common clinical practice, which is monitoring patients for symptoms indicative of VTE and then conducting gold standard tests. Readers should interpret our results with caution, keeping in mind that our results refer only to clinically evident VTE events. We must note that ROTEM is a novel laboratory method that is not supposed to replace any imaging method like CT pulmonary angiography or vascular ultrasound for VTE diagnosis; therefore, we did not perform a direct comparison with the accuracy of CT pulmonary angiography or vascular ultrasound. This is a preliminary study and future studies should re-evaluate ROTEM with consistent doppler/Duplex and chest CT in all patients to give a better sense of this test’s actual properties. Another important limitation is that a report of the pool VTE incidence can be misleading since VTE includes two entities with different clinical implications; pulmonary embolism can be fatal and deep vein thrombosis is usually clinically trivial. However, in our study, the overall number of the VTE events (n = 11) was almost evenly divided—six patients with deep vein thrombosis and five patients with pulmonary embolism—therefore, the pool VTE incidence is indicative for both entities.
Also, since the gold standard tests for VTE diagnosis were not performed at the same time as the ROTEM analysis, a preexisting VTE at the time of the preoperative and postoperative ROTEM data points could not be ruled out. Therefore, the ROTEM findings that were identified in this study could be simply diagnostic of the clot that had already formed and not associated with future clots, just as a positive ultrasound correlates strongly with a clot but does not predict one. Even though this may be true for some cases, the fact that preoperative ROTEM results were obtained within only a few hours of injury, and it was unlikely that a thrombus already formed, and because they were associated with symptomatic VTE events indicates that in most cases, our significant ROTEM findings revealed a hypercoagulable profile that was associated with subsequent clot formation and not with a preexisting one. Last, we must note that the proportion of symptomatic thromboembolic complications in our study was slightly higher than reported [8, 13], which may be explained by the relatively high BMI and comorbidity index in our study population. However, we performed an adjusted analysis through logistic regression to ensure these variables did not confound our results.
Are ROTEM Findings Associated with the Presence or Development of Symptomatic VTE After Hip Fracture Surgery?
We found that several abnormal thromboelastometry values were associated with the presence or development of symptomatic VTE in patients treated for hip fractures. Specifically, patients with symptomatic VTE had higher preoperative and postoperative maximum clot firmness and amplitude of clot firmness at 10 minutes than did patients without such complications. Additionally, patients with symptomatic VTE had lower preoperative and postoperative clot formation time values. Our results align with most other studies that used thromboelastography to evaluate the role of viscoelastic studies for the detection of postoperative or posttraumatic VTE [6, 9, 11, 19, 20, 30]. Wilson et al. [30] evaluated 250 patients with proximal femoral fractures and found that there were differences in thromboelastography parameters between patients with deep vein thrombosis and those without, revealing a higher coagulation index for patients in whom VTE developed. Similarly, Gary et al. [9] assessed 1818 patients and found that increased maximum amplitude of clot firmness values were independent predictors of thromboembolic complications. More specifically, patients with severe extremity trauma who had maximum amplitude of clot firmness ≥ 65 mm and maximum amplitude of clot firmness ≥ 72 mm at admission were 3.6 and 6.7 times, respectively, more likely to develop in-hospital VTE. On the contrary, Parameswaran et al. [19] evaluated preoperative thromboelastography parameters in 101 patients who underwent TKA or THA, and the authors were unable to establish a correlation between thromboelastography-based hypercoagulability and VTE. However, all these studies used thromboelastography, whereas our study investigated the performance of rotational thromboelastometry for the association with symptomatic VTE.
Were Any Other Patient Factors Associated with the Presence or Development of Symptomatic VTE after Hip Fracture Surgery?
We found that increased BMI was associated with symptomatic VTE. This finding is supported by other studies that also found that obesity was associated with an increased rate of postoperative VTE [17, 23]. Sloan et al. [23] reviewed and analyzed a large national database and concluded that overweight or obese patients had a higher rates of pulmonary embolism after primary THA or TKA. In another retrospective, case-control study, Mantilla et al. [17] tried to identify risk factors for clinically relevant VTE. They found that obese patients (BMI > 30 kg/m2) were 3.4 times more likely to develop VTE compared with controls. Possible causes for this increased risk for postoperative VTE in obese patients include limited postoperative mobility, ineffectiveness of mechanical prophylaxis, and high levels of procoagulant inflammatory markers [23].
Which ROTEM Parameters Were the Most Accurate in Terms of Detecting the Association of Hypercoagulability with Symptomatic VTE?
We found that preoperative INTEM clot forming time, preoperative EXTEM clot forming time, and postoperative INTEM maximum clot firmness demonstrated the highest levels of accuracy in terms of their association with a symptomatic event. The accuracy of ROTEM for the detection of postoperative or posttraumatic VTE has been investigated in another study. Hincker et al. [11] investigated the predictive performance of ROTEM parameters for postoperative VTE in 313 patients who underwent major noncardiac surgeries. In that study, the authors used a preoperative ROTEM analysis including EXTEM, INTEM, and FIBTEM activators, and they found that preoperative INTEM A10 had the largest AUC value (0.75). The respective AUC values in our study were higher than those of Hincker et al. [11], probably reflecting the homogeneity in our study population because Hincker et al. [11] enrolled patients with different types of surgery. Even though the apparent accuracy of ROTEM in this study is probably greater than its actual accuracy because we elected to perform the gold standard tests only in symptomatic patients, our findings indicate that ROTEM has the potential to identify patients at high risk for developing clinically evident VTE complications. However, ROTEM should not be used in routine clinical practice until a more robust study using duplex/Doppler and/or chest CT in all patients who received ROTEM is performed.
Conclusion
The findings of this study indicate that ROTEM has the ability to identify patients who are at high risk of VTE after hip fractures. The ROTEM analysis had a high accuracy in detecting the hypercoagulable state associated with VTE; therefore, its use as a monitoring test for such complications may be valuable, and there is the potential to develop ROTEM-based preventive strategies such as increased anticoagulation doses or more aggressive mobilization protocols for patients who are at a high risk for VTE, as shown by their ROTEM results. Although the goal of our study was not to assess or establish a change in thromboprophylaxis protocols, we chose to include ROTEM parameters that can be affected by blood levels in patients who have taken LMWH; thus, future studies may evaluate a dose-adjustment protocol guided by INTEM ROTEM results for preventing VTE.
Supplementary Material
Footnotes
Each author certifies that neither he nor she, nor any member of his or her immediate family, has funding or commercial associations (consultancies, stock ownership, equity interest, patent/licensing arrangements, etc.) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research® editors and board members are on file with the publication and can be viewed on request.
Ethical approval for this study was obtained from the Attiko Hospital, Athens, Greece (ref. number: 501/19-07-2019).
This work was performed at the First Department of Orthopaedics and the Laboratory of Haematology and Blood Bank Unit of Attiko Hospital, Athens, Greece.
Contributor Information
Dimitrios V. Papadopoulos, Email: di_papadopoulos@yahoo.gr.
Ioannis G. Trikoupis, Email: gtrikoupis@hotmail.com.
Konstantina A. Tsante, Email: ktsante@yahoo.com.
Andreas F. Mavrogenis, Email: afm@otenet.gr.
Panagiotis Koulouvaris, Email: info@drkoulouvaris.gr.
Daniele Piovani, Email: dpiovani@hotmail.com.
Anastasios G. Kriebardis, Email: akrieb@uniwa.gr.
Argyri Gialeraki, Email: agialer@med.uoa.gr.
Georgios K. Nikolopoulos, Email: gnikopoulos@gmail.com.
Stefanos Bonovas, Email: sbonovas@gmail.com.
Panayiotis J. Papagelopoulos, Email: pjporthopedic@gmail.com.
Argirios E. Tsantes, Email: atsantes@yahoo.com.
References
- 1.Anderson FA, Jr, Spencer FA. Risk factors for venous thromboembolism. Circulation . 2003;107:I9-I16. [DOI] [PubMed] [Google Scholar]
- 2.Berquist D, Efsing HO, Hallbook T, Hedlund T. Thromboembolism after elective and post-traumatic hip surgery—a controlled prophylactic trial with dextran 70 and low-dose heparin. Acta Chir Scand . 1979;145:213-218. [PubMed] [Google Scholar]
- 3.Brown W, Lunati M, Maceroli M, et al. Ability of thromboelastography to detect hypercoagulability: a systematic review and meta-analysis. J Orthop Trauma . 2020;34:278-286. [DOI] [PubMed] [Google Scholar]
- 4.Christensen T, Vad H, Pedersen S, et al. Coagulation profile in patients undergoing video-assisted thoracoscopic lobectomy: a randomized, controlled trial. PLoS One. 2017;12:e0171809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Connelly C, Van P, Hart K, et al. Thrombelastography-based dosing of enoxaparin for thromboprophylaxis in trauma and surgical patients. JAMA Surg. 2016;151:e162069. [DOI] [PubMed] [Google Scholar]
- 6.Cotton BA, Minei KM, Radwan ZA, et al. Admission rapid thrombelastography predicts development of pulmonary embolism in trauma patients. J Trauma Acute Care Surg . 2012;72:1470-1475. [DOI] [PubMed] [Google Scholar]
- 7.Eriksson B, Borris L, Friedman R, et al. Rivaroxaban versus enoxaparin for thromboprophylaxis after hip arthroplasty. N Engl J Med. 2008; 358:2765-2775. [DOI] [PubMed] [Google Scholar]
- 8.Falck-Ytter Y, Francis CW, Johanson NA, et al. Prevention of VTE in orthopedic surgery patients: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest . 2012;141:e278S-e325S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gary JL, Schneider PS, Galpin M, Radwan Z, Munz JW, Achor TS. Can thrombelastography predict venous thromboembolic events in patients with severe extremity trauma? J Orthop Trauma. 2016;30:294-298. [DOI] [PubMed] [Google Scholar]
- 10.Hanley JA, McNeil BJ. A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology. 1983;148:839-843. [DOI] [PubMed] [Google Scholar]
- 11.Hincker A, Feit J, Sladen R, Wagener G. Rotational thromboelastometry predicts thromboembolic complications after major non-cardiac surgery. Critical Care. 2014;18:549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kakkar A, Brenner B, Dahl O, et al. Extended duration rivaroxaban versus short-term enoxaparin for the prevention of venous thromboembolism after total hip arthroplasty: a doubleblind, randomised controlled trial. Lancet . 2008;372:31-39. [DOI] [PubMed] [Google Scholar]
- 13.Kim J. Deep vein thrombosis prophylaxis after total hip arthroplasty in Asian patients. Hip Pelvis. 2018;30:197-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lang T, Bauters A, Braun SL, et al. Multi-centre investigation on reference ranges for ROTEM thromboelastometry. Blood Coagul Fibrinolysis. 2005;16:301-310. [DOI] [PubMed] [Google Scholar]
- 15.Lowe GD, Haverkate F, Thompson SG, et al. Prediction of deep vein thrombosis after elective hip replacement surgery by preoperative clinical and haemostatic variables: the ECAT DVT study. European Concerted Action on Thrombosis. Thromb Haemost. 1999;81:879-886. [PubMed] [Google Scholar]
- 16.Luddington RJ. Thrombelastography/thromboelastometry. Clin Lab Haematol. 2005;27:81-90. [DOI] [PubMed] [Google Scholar]
- 17.Mantilla CB, Horlocker TT, Schroeder DR, Berry DJ, Brown DL. Risk factors for clinically relevant pulmonary embolism and deep venous thrombosis in patients undergoing primary hip or knee arthroplasty. Anesthesiology. 2003;99:552-560. [DOI] [PubMed] [Google Scholar]
- 18.Muntz J. Thromboprophylaxis in orthopedic surgery: how long is long enough? Am J Orthop (Belle Mead NJ) . 2009;38:394-401. [PubMed] [Google Scholar]
- 19.Parameswaran A, Krishnamoorthy VP, Oommen AT, et al. Is preoperative assessment of coagulation profile with thrombelastography (TEG) useful in predicting venous thromboembolism (VTE) following orthopaedic surgery? J Clin Orthop Trauma. 2016;7:225-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park MS, Martini WZ, Dubick MA, et al. Thromboelastography as a better indicator of postinjury hypercoagulable state than prothrombin time or activated partial thromboplastin time. J Trauma . 2009;67:266-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Plotkin AJ, Wade CE, Jenkins DH, et al. A reduction in clot formation rate and strength assessed by thrombelastography is indicative of transfusion requirements in patients with penetrating injuries. J Trauma . 2008;64:S64-S68. [DOI] [PubMed] [Google Scholar]
- 22.Schreiber MA. Coagulopathy in the trauma patient. Curr Opin Crit Care . 2005;11:590-597. [DOI] [PubMed] [Google Scholar]
- 23.Sloan M, Sheth N, Lee G. Is obesity associated with increased risk of deep vein thrombosis or pulmonary embolism after hip and knee arthroplasty? A large database study. Clin Orthop Relat Res . 2019;477:523-532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sokou R, Piovani D, Konstantinidi A, et al. A risk score for predicting the incidence of hemorrhage in critically ill neonates: development and validation study. Thromb Haemost. 2021;121:131-139. [DOI] [PubMed] [Google Scholar]
- 25.Theusinger OM, Nurnberg J, Asmis LM, Seifert B, Spahn DR. Rotation thromboelastometry (ROTEM) stability and reproducibility over time. Eur J Cardiothorac Surg . 2010;37:677-683. [DOI] [PubMed] [Google Scholar]
- 26.Thomas O, Larsson A, Tynngård N, Schott U. Thromboelastometry versus free-oscillation rheometry and enoxaparin versus tinzaparin: an in-vitro study comparing two viscoelastic haemostatic tests’ dose-responses to two low molecular weight heparins at the time of withdrawing epidural catheters from ten patients after major surgery. BMC Anesthesiol . 2015;24;15:170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thromboembolic Risk Factors (THRIFT) Consensus Group. Risk of and prophylaxis for venous thromboembolism in hospital patients. Br Med J . 1992;305:567-573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsantes AG, Trikoupis IG, Papadopoulos DV, et al. Higher coagulation activity in hip fracture patients: a case-control study using rotational thromboelastometry. Int J Lab Hematol . [Published online ahead of print November 24, 2020]. DOI: 10.1111/ijlh.13409. [DOI] [PubMed]
- 29.Wei KL, Lin CJ, Lai KA. Changes in coagulatory profile after orthopedic surgery. J Formos Med Assoc. 1995;94:541-547. [PubMed] [Google Scholar]
- 30.Wilson D, Cooke EA, McNally MA, Wilson HK, Yeates A, Mollan RA. Changes in coagulability as measured by thrombelastography following surgery for proximal femoral fracture. Injury. 2001;32:765-770. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



