Abstract
The Lyme disease spirochete Borrelia burgdorferi sensu stricto downregulates outer surface protein A (OspA) and upregulates outer surface protein C (OspC) during tick feeding. The switching of these proteins correlates with increased spirochetal infectivity for the mammal. We examined the effect of temperature on differential expression of OspA and OspC by B. burgdorferi cocultivated with a cell line isolated from the vector tick Ixodes scapularis. The effect of incubation at 31, 34, or 37°C on expression of OspA and OspC by B. burgdorferi JMNT and N40 was analyzed by indirect fluorescent-antibody microscopy, polyacrylamide gel electrophoresis, and immunoblotting. The amount of OspA relative to the amount of flagellin was highest in spirochetes cocultivated with tick cells at 31°C and declined with increasing temperature in both strains. OspC production was enhanced in spirochetes cocultivated with tick cells at 37°C. Spirochetes grown axenically in BSK-H medium also produced more OspC at 37°C, but OspA content was not appreciably affected by temperature. Our findings indicate that temperature, along with cultivation in a tick cell culture system, plays a role in the differential expression of OspA and enhances differential expression of OspC by spirochetes.
The Lyme disease spirochete Borrelia burgdorferi sensu stricto cycles in nature between small mammals and Ixodes ticks. This ability to invade and infect two physiologically quite divergent hosts involves alterations in the outer surface protein (Osp) composition of B. burgdorferi, most notably, outer surface protein A (OspA) and outer surface protein (OspC). These changes occur while spirochetes are being transmitted from the tick to the mammalian host. Factors such as temperature (7, 29–31) and blood meal (23, 29) have been demonstrated to influence synthesis of several B. burgdorferi proteins. One of the most notable effects is an increase in OspC production and a decline in OspA production in spirochetes during nymphal attachment and feeding (6, 8–10, 25, 29). This differential expression of OspA and OspC during tick feeding coincides with an increase in the infectivity of spirochetes for the mammalian host (23, 24). OspA has been shown to be expressed in significant amounts in most B. burgdorferi strains in culture (2, 4, 10, 17, 32). An increase in temperature enhanced the expression of OspC in spirochetes cultured in BSK medium, whereas no effect on OspA was noted (7).
The aim of this research was to compare OspA and OspC expression in B. burgdorferi sensu stricto cocultivated with tick (Ixodes scapularis) cells with those cultivated axenically in BSK-H medium. We also examined how temperature affects the expression of OspA and OspC in B. burgdorferi in the two culture systems. Here we report that temperature modulates the expression of both OspA and OspC in B. burgdorferi cocultivated with I. scapularis cells.
(This work is part of the Ph.D. dissertation of Marygorret Obonyo.)
MATERIALS AND METHODS
Spirochete strains and cultivation.
Strains JMNT (14, 22) and N40 (3) of B. burgdorferi sensu stricto (passage 3), previously maintained at 34°C, were stored frozen in liquid nitrogen. Before each experiment, frozen spirochetes were thawed at 37°C, transferred to complete BSK-H (Sigma, St. Louis, Mo.) medium supplemented with 6.3% rabbit serum (Gibco, Grand Island, N.Y.) and 1.4% gelatin (Difco, Detroit, Mich.), and incubated at 34°C for 4 days prior to cocultivation with tick cells or axenic cultivation in BSK-H medium.
I. scapularis tick cell line ISE6 (18) was used. The line was maintained in L15B (19) medium containing 5% heat-inactivated fetal bovine serum (Sigma), 10% tryptose phosphate broth (Difco), and 1% lipoprotein cholesterol concentrate (ICN Pharmaceuticals, Inc., Costa Mesa, Calif.). Cells, seeded into 24-well tissue culture plates (1 ml/well) or 25-cm2 tissue culture flasks (5 ml/flask), were grown at 31°C until the cell density reached 2.5 × 106 cells/ml (usually by 7 days). Spirochetes, previously grown in BSK-H medium, were counted with a Petroff-Hausser bacterial counting chamber (Hausser Scientific, Horsham, Pa.) and were diluted in L15BS medium (13, 19) (1.25 × 107 borreliae/ml). The spent tick cell culture medium (L15B) was replaced with an equivalent volume of spirochetes in L15BS medium (13, 19), and cultures were incubated at 31, 34, or 37°C. For comparison, spirochetes cultured axenically were diluted to 105 spirochetes/ml in fresh complete BSK-H medium and were incubated at 31, 34, or 37°C.
Microscopic evaluation.
Cells were suspended with a Pasteur pipette, centrifuged onto glass microscope slides (Shandon Southern Instruments, Pittsburgh, Pa.), air dried, and fixed in methanol. Some slides were stained with 4% Giemsa (Gibco) solution and others were used for indirect fluorescent-antibody (IFA) microscopy. For IFA microscopy, cells were incubated with monoclonal antibody (MAb) H5332 (2) (provided by Tom Schwan, Rocky Mountain Laboratories, National Institutes of Health) at 37°C for 30 min. Thereafter, slides were washed once in phosphate-buffered saline (PBS) containing 5 mM MgCl2 and were then reacted for 30 min at 37°C with rhodamine-conjugated anti-mouse immunoglobulin G (Pierce, Rockford, Ill.). The slides were washed once in PBS containing MgCl2 (5 mM) and were examined by fluorescence microscopy.
SDS-PAGE and immunoblotting.
Spirochetes grown axenically in BSK-H medium at 31°C were harvested after 7 days, and those grown at 34 or 37°C were passaged into fresh medium when they reached the mid-logarithmic phase of growth (4 days) and were harvested 3 days later. Spirochetes were counted with a bacterial counting chamber and were harvested by centrifugation (3,000 × g) for 30 min at 4°C. The resulting pellet was resuspended in Hank’s balanced salt solution (HBSS) and was recentrifuged (3,000 × g for 30 min at 4°C). After a second wash with HBSS and a second round of centrifugation, pelleted spirochetes were resuspended in 2× sodium dodecyl sulfate (SDS) reducing buffer and were lysed by boiling for 5 min. Spirochetes grown with ISE6 cells in 25-cm2 tissue culture flasks were suspended by pipetting and were separated from ISE6 cells by centrifugation (170 × g) at ambient temperature for 5 min; those spirochetes remaining in suspension were counted and were harvested as stated above for spirochetes cultured axenically in BSK-H medium.
Whole-cell lysates (2 × 106 to 4 × 106 spirochetes/lane) were subjected to a discontinuous SDS-polyacrylamide gel electrophoresis (PAGE) (15) with a 12% acrylamide separating gel. Electrophoresis was done at a constant voltage of 190 V with the Mini-PROTEAN II system (Bio-Rad, Hercules, Calif.). After electrophoresis the proteins were visualized by staining the gels with Rapid Coomassie blue stain (Diversified Biotech, Boston, Mass.).
For immunoblot analyses, gel-separated proteins were transferred to polyvinylidene difluoride membranes (Immobilon-P; Millipore Corporation, Bedford, Mass.) with the Mini-PROTEAN II system (Bio-Rad) at 100 V for 1 h. Following transfer, the membranes were blocked with 5% nonfat dry milk–0.2% Tween 20 in PBS for 2 h, washed three times in PBS, and reacted overnight at 4°C with a mixture of mouse MAbs, MAbs H5332 (2), H4610 (26), and H9724 (1) (all provided by Tom Schwan, Rocky Mountain Laboratories, National Institutes of Health), which are specific for OspA, OspB, and flagellin, respectively, and MAb L22 2B8, which is specific for OspC (32). After incubation with primary antibodies, the blots were washed three times in PBS and were incubated for 1 h with the secondary antibody, goat anti-mouse antibody conjugated to horseradish peroxidase (Pierce). After three 10-min washes of the blots in PBS, bound antibody was visualized with 3,3′,5,5′-tetramethylbenzidine (Kirkegaard & Perry Laboratories, Inc., Gaithersburg, Md.) by standard methods.
Gels and immunoblots were scanned and the OspA and OspC band intensities were determined with Kodak Digital Science Image Analysis Software (Eastman Kodak Co., Rochester, N.Y.). These digitized images were used to acquire molecular mass data and determine the staining intensity for each protein (OspA, OspC, or flagellin). The amount of flagellin was used as our standard to compare the differential expression of OspA and OspC, since flagellin expression was not influenced by temperature. Digitized images were used to estimate the relative amounts of OspA and OspC to the amount of flagellin. For ease of comparison, band intensities for flagellin were normalized to a value of 1.0.
RESULTS
The availability of I. scapularis cell lines (21) made it possible for us to experimentally analyze the effect of temperature on Osp expression of B. burgdorferi cocultivated with tick cells. We had previously demonstrated that spirochetes would not grow in L15BS without tick cells (14) and that tick cells did not survive in BSK for more than 2 days (13). When we initiated these experiments, we considered that the changes in Osp expression were not rapid heat shock responses which occur within a few hours (31). Therefore, borreliae were exposed to cells and temperature changes for 5 to 7 days to allow induction of the OspA-OspC response. Variability in OspC expression with time of cultivation has been reported, even at those temperatures that strongly induce OspC expression (29). We reduced this variability by using borrelial inocula that had an identical thermal prehistory (34°C) to initiate our experimental cultures.
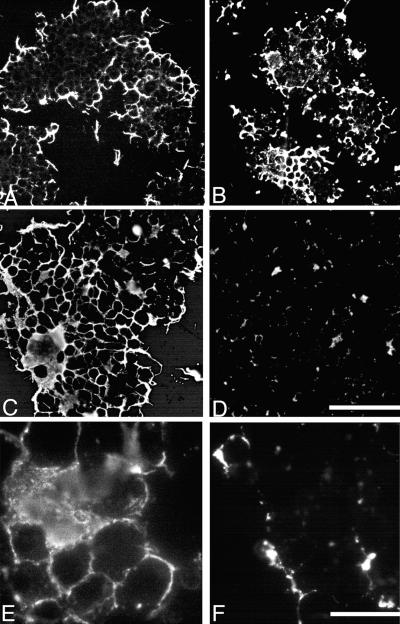
Phase-contrast microscopy revealed that B. burgdorferi markedly disrupted ISE6 cell layers by day 5 in cultures grown at 34 or 37°C. By day 7, when spirochetes were harvested for SDS-PAGE and immunoblot analyses, none of the tick cells were adherent in cultures maintained at 34 or 37°C, while approximately 30% of the tick cells were adherent in cultures maintained at 31°C. Control cells cultured axenically in L15BS remained normal and adherent. Clusters of spirochetes were attached to the tick cells and were found in the spaces between the cells (Fig. 1). The influence of temperature on OspA expression of borreliae cocultivated with tick cells was also examined by IFA microscopy. Spirochetes cocultivated with tick cells at 31 or 37°C for 48 h reacted strongly with the OspA-specific MAb (Fig. 2A and B). By day 5 the OspA-specific MAb reactivity of borreliae cultured at 31°C was strong (Fig. 2C), while in those spirochetes cultured at 37°C the amount of MAb binding had declined noticeably (Fig. 2D). Moreover, at 31°C, spirochetes reacted uniformly with the MAb (Fig. 2E), while at 37°C OspA expression was patchy (Fig. 2F). Spirochetes cocultivated with the I. scapularis cells at 34°C did not show a noticeable change in OspA expression within the 7 days.
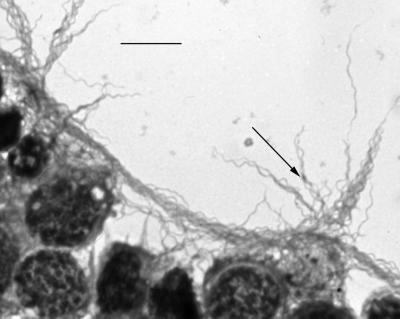
FIG. 1.
Giemsa-stained B. burgdorferi JMNT (arrowhead) attached to tick cells (ISE6 cells). Bar, 10 μm.
FIG. 2.
Epifluorescent image of rhodamine-labeled B. burgdorferi JMNT attached to tick cells (ISE6 cells). The cells were harvested at selected times and centrifuged onto microscope slides, and the spirochetes reacted with MAb to OspA. Panels A to D are all at the same magnification (bar in panel D, 100 μm). (A) Cells grown at 31°C after 48 h. (B) Cells grown at 37°C and harvested after 48 h. (C) Cells grown at 31°C and harvested after 5 days. (D) Cells grown at 37°C and harvested after 5 days. Panels E and F are at the same magnification (bar, in panel F, 20 μm). (E) Cells grown at 31°C and harvested after 5. (F) Cells grown at 37°C and harvested after 5 days.
To quantify relative differences in the protein expression of spirochetes cultured at 31, 34, or 37°C at the time of maximal tick cell layer disruption, we harvested spirochetes for SDS-PAGE and immunoblot analysis. Because SDS-PAGE profiles showed no major differences in borreliae harvested on day 5 or day 7 (data not shown), spirochetes cocultivated for 7 days with ISE6 cells were harvested for all subsequent analyses.
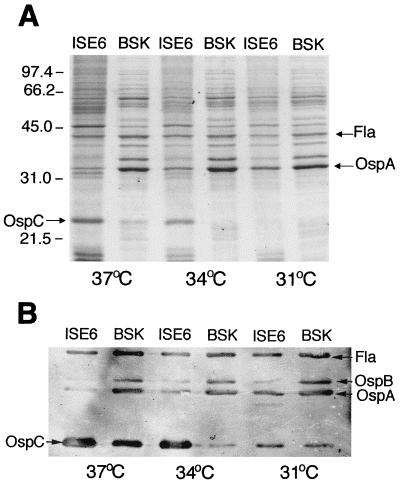
We compared the differential expression of OspA and OspC by B. burgdorferi JMNT and N40 cocultivated with tick cells or cultured axenically. Because expression of flagellin is not influenced by temperature (29, 31), the amount of OspA or OspC was related to the amount of flagellin as the standard in each lane. Coomassie blue staining indicated that strain JMNT cocultivated with tick cells expressed high levels of OspC at 34 and 37°C but not at 31°C (Fig. 3A). OspC production in spirochetes cocultivated with tick cells at 34 or 37°C was greater than that in spirochetes cultured in BSK-H medium. OspA production was greater when spirochetes were grown axenically in BSK-H medium than when they were cocultivated with tick cells. Furthermore, at 37°C there was a concomitant decline in OspA production. As expected, the amount of flagellin was not affected by temperature or cocultivation with tick cells. To confirm our SDS-PAGE observations we did an immunoblot analysis using a mixture containing MAbs to OspA, OspB, OspC, and flagellin. Reactivity with MAb L22 2B8 showed that OspC was present at high levels in JMNT cocultivated with tick cells at 37°C as well as in spirochetes cultured axenically at 37°C (Fig. 3B). At 34°C the spirochetes cocultivated with ISE6 cells also expressed high levels of OspC, while much less OspC was present on spirochetes cultured axenically at 34°C. Borreliae grown at 31°C in either system also produced OspC, but the signal was weaker. The results obtained with MAbs to OspA and OspB indicated that spirochetes cocultivated with tick cells at 37°C expressed low levels of OspA and OspB, whereas spirochetes cocultivated with tick cells at 31°C expressed more OspA and OspB. Borreliae cultured axenically produced high levels of OspA and OspB at all three temperatures. Similar SDS-PAGE and immunoblotting data were obtained with strain N40. OspC production by strain N40 cocultivated with tick cells at 34 or 37°C was also greater than that by spirochetes cultured in BSK-H medium. Again, OspA production was highest for spirochetes grown axenically in BSK-H medium. At 37°C there was a decline in OspA production by N40 cocultivated with tick cells. Our immunoblot analysis with the MAb mixture confirmed our SDS-PAGE observations.
FIG. 3.
Analysis of B. burgdorferi JMNT by SDS-PAGE with Rapid Coomassie blue staining (A) or immunoblotting (B) with MAbs to OspA, OspB, OspC, and flagellin as probes. Spirochetes were cocultivated with a vector tick cell line (ISE6 cells) or were grown axenically in BSK medium at 37, 34, or 31°C. Molecular mass markers (in kilodaltons) are shown on the left.
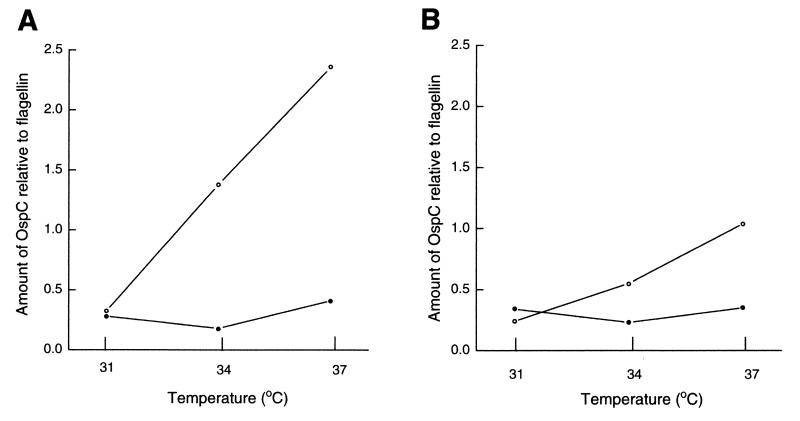
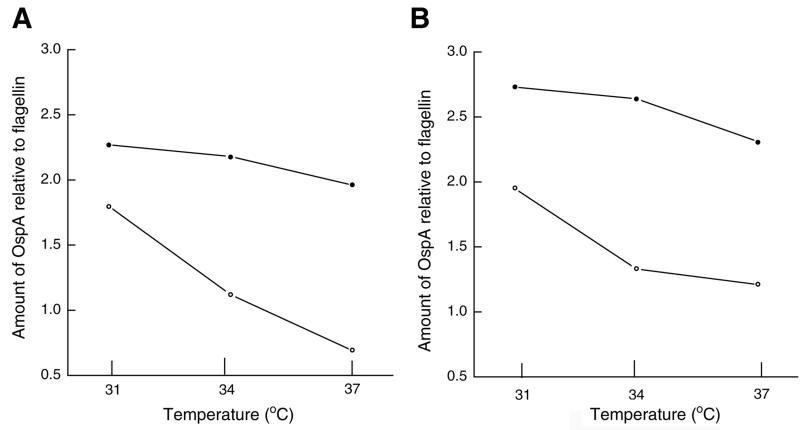
To confirm the differential expressions of OspA and OspC, we analyzed our gels and immunoblots using image analysis software. This data analysis confirmed that OspC production increased with temperature in both strains (strains JMNT and N40) cocultivated with tick cells (Fig. 4). For both strains the level of OspA production declined with an increase in temperature and the amount of OspA relative to the amount of flagellin was lowest in borreliae cocultivated with tick cells at 37°C (Fig. 5).
FIG. 4.
Amount of OspC relative to amount of flagellin in B. burgdorferi JMNT (A) and N40 (B). Spirochetes were cocultivated with tick cells (open circles) or were grown axenically in BSK-H medium (closed circles) at 31, 34, or 37°C. The gels were scanned to determine OspA, OspC, and flagellin band intensities by using Kodak Image Analysis Software. Flagellin was used as a reference protein, and the ratio of the amount of a protein band to the amount of flagellin was used to generate these graphs.
FIG. 5.
Amount of OspA relative to amount of flagellin in B. burgdorferi JMNT (A) and N40 (B). Spirochetes were cocultivated with tick cells (open circles) or were grown axenically in BSK-H medium (closed circles) at 31, 34, or 37°C. Gels were scanned to determine OspA, OspC, and flagellin band intensities by using Kodak Image Analysis Software. Flagellin was used as a reference protein, and the ratio of the amount of a protein band to the amount of flagellin was used to generate these graphs.
DISCUSSION
Successful transmission of the Lyme disease spirochete (B. burgdorferi) from ticks to mammalian hosts, and vice versa, presumably requires the induction of major physiological changes in the spirochete. Temporal changes in the protein composition of the spirochete’s outer surface, mainly in OspA and OspC, during this transition has led to the hypothesis that these Osps are pivotal to infectivity for the mammalian host and survival in the vector (27). Spirochetes within the gut of unfed and questing ticks express mainly OspA but little or no OspC (29) and are confined mainly to the luminal surfaces of gut cells (5, 33). Spirochetes in homogenates prepared from unfed ticks are not infectious for mammals (23, 24). When an infected tick feeds on a mammalian host several physiological changes occur and the tick’s body temperature increases from ambient temperature to more than 34°C (20). During the blood meal the spirochetes start to migrate from the gut to invade the salivary glands and ultimately the mammalian host via the saliva (9, 33). The infectivity of spirochetes for the mammalian host increases during the tick’s blood meal (23). Several studies have demonstrated a downregulation of spirochetal OspA and upregulation of OspC during the early stages of the tick’s blood meal (6, 8, 10, 25, 29). Downregulation of OspA expression occurs within 72 h of feeding, and it is estimated that only one-third of the borreliae in the gut and salivary glands are OspA positive at that time (10).
Our results indicate that a tick cell culture system is a good model system for reproducing in vitro those events that occur while spirochetes are in the tick. Expression of spirochetal Osps in feeding ticks differs from what is observed in BSK medium, as suggested by the host immune response, which is directed against OspA and OspB after needle inoculation (25, 28). The differential expression of OspA and OspC that we observed for B. burgdorferi cocultivated with I. scapularis cells was similar to that observed for borreliae resident within feeding I. scapularis ticks. We observed a decline in OspA production and an increase in OspC production which was more pronounced in spirochetes cocultivated with tick cells at 37°C than in those cultured axenically in BSK-H medium at the same temperature. Our IFA microscopy, SDS-PAGE, and immunoblotting results all revealed this decline in OspA production. OspC production was enhanced at 37°C in both strains (strains JMNT and N40) cocultivated with tick cells. These results agree with those from previous studies that have shown the upregulation of OspC production in B. burgdorferi cultured at higher temperatures and its decline at lower temperatures (7, 29, 31). However, a temperature-induced decline in OspA production was not reported in those studies. Our results suggest that, along with temperature, tick cell contact or other culture parameters, e.g., osmolality, may be involved in the modulation of spirochetal expression of OspA. Manipulation of the tick cell system therefore should be the next step in the search for answers about the regulation of Osp expression. Answers to such questions could provide clues about transmission mechanisms in ticks during attachment and feeding. Such studies could provide information on the molecular mechanisms controlling the Osp phenotypes of the spirochetes that we observe in nature and assist us in understanding and predicting the efficacy of Lyme disease vaccines based on recombinant Osps. The role of OspA in the adhesion of spirochetes to tick cells remains to be determined. MAb H5332, which is directed against OspA, did not interfere with the binding of B. burgdorferi to I. scapularis cells (14). Also, mutants of B. burgdorferi sensu stricto that lack OspA have been found to adhere well to cultured I. scapularis cells (12). This would suggest that, at least in vitro, OspA does not solely mediate binding of spirochetes to tick cell membranes. Furthermore, our results indicate that while spirochetes had downregulated OspA at 37°C they still had detectable levels of OspA by immunoblotting. These results were not unexpected because Osps, once they are present, will persist on the spirochetal surface for several divisions (31).
Our interpretation of the differential expression of OspA and OspC is based on investigations with North American strains of B. burgdorferi sensu stricto. In Europe, at least three different genospecies of B. burgdorferi sensu lato that are pathogenic for humans exist, and the three genospecies display different patterns of Osp expression in culture and in the vector tick. Borrelia afzelii, for example, has been shown to express both OspA and OspC in unfed nymphs of Ixodes ricinus (11, 16). However, as reported for B. burgdorferi sensu stricto in I. scapularis, B. afzelii also downregulates OspA, while it continues to express OspC in feeding I. ricinus (16). Further investigations with strains from different genospecies are needed to clarify the function and dynamics of OspA and OspC expression in both the tick and the mammalian host.
ACKNOWLEDGMENTS
We thank Russell Johnson for support and helpful suggestions. We thank Tom Schwan (Rocky Mountain Laboratories, National Institute of Health) for providing us with MAbs H5332, H4610, and H9724 against OspA, OspB, and flagellin, respectively. We thank Stephen Barthold (Center for Comparative Medicine, University of California, Davis) for providing strain N40.
This work was supported by Public Health Service grant AR37909 (to T.J.K.).
REFERENCES
- 1.Barbour A G, Hayes S F, Heiland R A, Schrumpf M E, Tessier S L. A Borrelia-specific monoclonal antibody binds to a flagellar epitope. Infect Immun. 1986;52:549–554. doi: 10.1128/iai.52.2.549-554.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barbour A G, Tessier S L, Todd W J. Lyme disease spirochetes and ixodid tick spirochetes share a common surface antigenic determinant defined by a monoclonal antibody. Infect Immun. 1983;41:795–804. doi: 10.1128/iai.41.2.795-804.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barthold S W, Moody K D, Terwilliger G A, Duray P H, Jacoby R O, Steere A C. Experimental Lyme arthritis in rats infected with Borrelia burgdorferi. J Infect Dis. 1988;157:842–846. doi: 10.1093/infdis/157.4.842. [DOI] [PubMed] [Google Scholar]
- 4.Benach J L, Coleman J L, Golightly M G. A murine IgM monoclonal antibody binds an antigenic determinant in outer surface protein A, an immunodominant basic protein of Lyme disease spirochete. J Immunol. 1988;140:265–272. [PubMed] [Google Scholar]
- 5.Burgdorfer W, Barbour A G, Hayes S F, Benach J L, Grunwaldt E, Davis J P. Lyme disease—a tick-borne spirochetosis? Science. 1982;216:1317–1319. doi: 10.1126/science.7043737. [DOI] [PubMed] [Google Scholar]
- 6.Burkot T R, Piesman J, Wirtz R A. Quantitation of the Borrelia burgdorferi outer surface protein A in Ixodes scapularis: fluctuations during the tick cycle, doubling times, and loss while feeding. J Infect Dis. 1994;170:883–889. doi: 10.1093/infdis/170.4.883. [DOI] [PubMed] [Google Scholar]
- 7.Cluss R G, Boothby J T. Thermoregulation of protein synthesis in Borrelia burgdorferi. Infect Immun. 1990;58:1038–1042. doi: 10.1128/iai.58.4.1038-1042.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coleman J L, Gebbia J A, Piesman J, Degen J L, Bugge T H, Benach J L. Plasminogen is required for efficient dissemination of B. burgdorferi in ticks and for enhancement of spirochetemia in mice. Cell. 1997;89:1111–1119. doi: 10.1016/s0092-8674(00)80298-6. [DOI] [PubMed] [Google Scholar]
- 9.de Silva A M, Fikrig E. Growth and migration of Borrelia burgdorferi in Ixodes ticks during blood feeding. Am J Trop Med Hyg. 1995;53:397–404. doi: 10.4269/ajtmh.1995.53.397. [DOI] [PubMed] [Google Scholar]
- 10.de Silva A M, Telford III S R, Brunet L R, Barthold S W, Fikrig E. Borrelia burgdorferi OspA is an arthropod-specific transmission-blocking Lyme disease vaccine. J Exp Med. 1996;183:271–275. doi: 10.1084/jem.183.1.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fingerle V, Hauser U, Liegl G, Petko B, Preac-Mursic V, Wilske B. Expression of outer surface proteins A and C of Borrelia burgdorferi in Ixodes ricinus. J Clin Microbiol. 1995;33:1867–1869. doi: 10.1128/jcm.33.7.1867-1869.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kurtti, T. J. Unpublished data.
- 13.Kurtti T J, Munderloh U G, Ahlstrand G G, Johnson R C. Borrelia burgdorferi in tick cell culture: growth and cellular adherence. J Med Entomol. 1988;25:256–261. doi: 10.1093/jmedent/25.4.256. [DOI] [PubMed] [Google Scholar]
- 14.Kurtti T J, Munderloh U G, Krueger D E, Johnson R C, Schwan T G. Adhesion to and invasion of cultured tick (Acarina: Ixodidae) cells by Borrelia burgdorferi (Spirochaetales: Spirochaetaceae) and maintenance of infectivity. J Med Entomol. 1993;30:586–596. doi: 10.1093/jmedent/30.3.586. [DOI] [PubMed] [Google Scholar]
- 15.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 16.Leuba-Garcia S, Martinez R, Gern L. Expression of outer surface proteins A and C of Borrelia afzelii in Ixodes ricinus ticks and in the skin of mice. Zentbl Bakteriol Parasitenkd Infektionskr Hyg Abt 1 Orig. 1998;287:475–484. doi: 10.1016/s0934-8840(98)80187-4. [DOI] [PubMed] [Google Scholar]
- 17.Montgomery R R, Malawista S E, Feen K J M, Bockenstedt L K. Direct demonstration of antigenic substitution of Borrelia burgdorferi ex vivo: exploration of the paradox of the early immune response to outer surface proteins A and C in Lyme disease. J Exp Med. 1996;183:261–269. doi: 10.1084/jem.183.1.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Munderloh, U. G. Unpublished data.
- 19.Munderloh U G, Kurtti T J. Formulation of medium for tick cell culture. Exp Appl Acarol. 1989;7:219–229. doi: 10.1007/BF01194061. [DOI] [PubMed] [Google Scholar]
- 20.Munderloh U G, Kurtti T G. Cellular and molecular interrelationships between ticks and prokaryotic tick-borne pathogens. Annu Rev Entomol. 1995;40:221–243. doi: 10.1146/annurev.en.40.010195.001253. [DOI] [PubMed] [Google Scholar]
- 21.Munderloh U G, Liu Y, Wang M, Chen C, Kurtti T J. Establishment, maintenance and description of cell lines from the tick Ixodes scapularis. J Parasitol. 1994;80:533–543. [PubMed] [Google Scholar]
- 22.Munderloh U G, Park Y-J, Dioh J M, Fallon A M, Kurtti T J. Plasmid modifications in a tick-borne pathogen, Borrelia burgdorferi, cocultured with tick cells. Insect Mol Biol. 1993;1:195–203. doi: 10.1111/j.1365-2583.1993.tb00092.x. [DOI] [PubMed] [Google Scholar]
- 23.Piesman J. Dynamics of Borrelia burgdorferi transmission by nymphal Ixodes dammini ticks. J Infect Dis. 1993;167:1082–1085. doi: 10.1093/infdis/167.5.1082. [DOI] [PubMed] [Google Scholar]
- 24.Piesman J, Oliver J R, Sinsky R J. Growth kinetics of the Lyme disease spirochete (Borrelia burgdorferi) in vector ticks (Ixodes dammini) Am J Trop Med Hyg. 1990;42:352–357. doi: 10.4269/ajtmh.1990.42.352. [DOI] [PubMed] [Google Scholar]
- 25.Roehrig J T, Piesman J, Hunt A R, Keen M G, Happ C M, Johnson B J B. The hamster immune response to tick-transmitted Borrelia burgdorferi differs from the response to needle-inoculated, cultured organisms. J Immunol. 1992;149:3648–3653. [PubMed] [Google Scholar]
- 26.Rosa P E, Schwan T, Hogan D. Recombination between genes encoding major outer surface proteins A and B of Borrelia burgdorferi. Mol Microbiol. 1992;6:3031–3040. doi: 10.1111/j.1365-2958.1992.tb01761.x. [DOI] [PubMed] [Google Scholar]
- 27.Schwan T G. Ticks and Borrelia: model systems for investigating pathogen-arthropod interactions. Infect Agents Dis. 1996;5:167–181. [PubMed] [Google Scholar]
- 28.Schwan T G, Kime K K, Schrumpf M E, Coe J E, Simpson W J. Antibody response in white-footed mice (Peromyscus leucopus) experimentally infected with the Lyme disease spirochete (Borrelia burgdorferi) Infect Immun. 1989;57:3445–3451. doi: 10.1128/iai.57.11.3445-3451.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schwan T G, Piesman J, Golde W T, Dolan M C, Rosa P A. Induction of an outer surface protein on Borrelia burgdorferi during tick feeding. Proc Natl Acad Sci USA. 1995;92:2909–2913. doi: 10.1073/pnas.92.7.2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stevenson B, Bono J L, Schwan T G, Rosa P. Borrelia burgdorferi Erp proteins are immunogenic in mammals infected by tick bite, and their synthesis is inducible in cultured bacteria. Infect Immun. 1998;66:2648–2654. doi: 10.1128/iai.66.6.2648-2654.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stevenson B, Schwan T, Rosa P A. Temperature-related differential expression of antigens in the Lyme disease spirochete, Borrelia burgdorferi. Infect Immun. 1995;63:4535–4539. doi: 10.1128/iai.63.11.4535-4539.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wilske B, Jauris-Heipke S, Lobentazer R, Pradel I, Preac-Mursic V, Roessler D, Soutschek E, Johnson R C. Phenotypic analysis of the outer surface protein C (OspC) of Borrelia burgdorferi sensu lato by monoclonal antibodies: relationship to genospecies and OspA serotype. J Clin Microbiol. 1995;33:103–109. doi: 10.1128/jcm.33.1.103-109.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zung J L, Lewengrub S, Rudzinska M A, Spielman A, Telford S R, Piesman J. Fine structural evidence for the penetration of the Lyme disease spirochete Borrelia burgdorferi through the gut and salivary tissues of Ixodes dammini. Can J Zool. 1989;67:1737–1748. [Google Scholar]