Abstract
L-DOPA therapy in Parkinson’s disease (PD) is limited due to emerging L-DOPA-induced dyskinesia. Research has identified abnormal dopamine release from serotonergic (5-HT) terminals contributing to this dyskinesia. Selective serotonin reuptake inhibitors (SSRIs) or 5-HT receptor (5-HTr) agonists can regulate 5-HT activity and attenuate dyskinesia, but they often also produce a loss of the antiparkinsonian efficacy of L-DOPA. We investigated vilazodone, a novel multimodal 5-HT agent with SSRI and 5-HTr1A partial agonist properties, for its potential to reduce dyskinesia without interfering with the prokinetic effects of L-DOPA, and underlying mechanisms. We assessed vilazodone effects on L-DOPA-induced dyskinesia (abnormal involuntary movements, AIMs) and aberrant responsiveness to corticostriatal drive in striatal medium spiny neurons (MSNs) measured with in vivo single-unit extracellular recordings, in the 6-OHDA rat model of PD. Vilazodone (10 mg/kg) suppressed all subtypes (axial, limb, orolingual) of AIMs induced by L-DOPA (5 mg/kg) and the increase in MSN responsiveness to cortical stimulation (shorter spike onset latency). Both the antidyskinetic effects and reversal in MSN excitability by vilazodone were inhibited by the 5-HTr1A antagonist WAY-100635, demonstrating a critical role for 5-HTr1A in these vilazodone actions. Our results indicate that vilazodone may serve as an adjunct therapeutic for reducing dyskinesia in patients with PD.
Keywords: dopamine, serotonin, L-DOPA, striatum, cortical stimulation, dyskinesia, Parkinson’s disease
1. Introduction
Parkinson’s disease (PD) is a devastating neurodegenerative disorder that is caused by a progressive loss of nigrostriatal dopamine (DA) neurons. Current therapeutic approaches typically focus on restoring central DA function, and treatment with the DA precursor levodopa (L-DOPA) remains the most effective pharmacological strategy to alleviate motor symptoms in PD [1,2,3]. However, long term L-DOPA treatment also produces debilitating motor side effects characterized by involuntary movements known as L-DOPA-induced dyskinesia [4,5,6]. In fact, the incidence of L-DOPA-induced dyskinesia is estimated to reach 90% after 10 years of treatment [7,8,9], which significantly reduces the therapeutic window and quality of life for patients with PD [10,11,12]. Thus, understanding the mechanisms underlying L-DOPA-induced dyskinesia is a critical step to improve L-DOPA therapy.
Several pathophysiological changes after DA denervation and chronic L-DOPA treatment have been identified that contribute to the development and expression of L-DOPA-induced dyskinesia. Many of these involve alterations in corticostriatal functional connectivity and dysregulation of striatal output (for reviews, see e.g., [13,14,15,16]). These cellular and molecular alterations include pre- and postsynaptic changes in the striatum [16]. For example, pronounced postsynaptic modifications occur in medium spiny projection neurons (MSNs), including DA receptor supersensitivity [17], which involves abnormally enhanced activation of intracellular signaling pathways, resulting in aberrant regulation of ion channels and receptors that produces abnormal responsiveness to corticostriatal inputs, as well as altered gene regulation and other effects (e.g., [14,18,19]).
Presynaptic changes associated with L-DOPA-induced dyskinesia include large fluctuations in striatal DA levels upon L-DOPA administration [2,20,21,22]. It is now accepted that these DA fluctuations drive postsynaptic changes in MSNs and L-DOPA-induced dyskinesia (e.g., [16]). These DA surges occur with severe loss of nigrostriatal DA neurons, and it is agreed that such L-DOPA-derived DA is released from other cells [16]. Of particular interest are the serotonergic (5-HT) fibers emanating from the dorsal raphe nucleus (DRN), which have the machinery to convert L-DOPA to DA and then release it [23,24]. These 5-HT fibers increase in density in the DA-depleted striatum (e.g., [25,26,27]). Further findings show that L-DOPA-derived DA released from 5-HT neurons is indeed critical for L-DOPA-induced dyskinesia ([21,22,28,29,30,31]; see [16,32], for recent reviews).
Attempts were made to pharmacologically regulate such abnormal DA release from 5-HT terminals and mitigate L-DOPA-induced dyskinesia by targeting the activity of 5-HT neurons in animal models [32,33]. For example, studies have investigated the use of selective 5-HT reuptake inhibitors (SSRIs), which block the 5-HT transporter [34,35,36,37], and agonists of the 5-HT1A receptor (5-HTr1A), which stimulate 5-HT1A autoreceptors on 5-HT neurons to attenuate their activity and abnormal DA release from their terminals (e.g., [30,37,38,39,40,41,42,43,44]). Results showed that many of these drugs significantly reduced dyskinesia scores in animal models. However, often these agents also diminished the prokinetic effects of L-DOPA, especially at higher doses, thus limiting their potential usefulness (e.g., [36,37,42,43,45]; see [32], for review). Clinical trials have so far been performed with 5-HTr1A agonists [32], and these were typically less successful. For example, sarizotan, a 5-HTr1A full agonist, showed promise in early preclinical [45,46] and clinical studies [47], but was ultimately not found to be superior to placebo in clinical trials [48].
Recent studies investigated a novel SSRI antidepressant, vilazodone, which was approved by the U.S. Food and Drug Administration (FDA) in 2011 [49], as a potential therapeutic to treat L-DOPA-induced dyskinesia. Vilazodone differs from previous agents in that it combines 5-HTr1A partial agonist activity with its SSRI properties [50,51,52]. Early findings showed that vilazodone attenuated development and expression of L-DOPA-induced dyskinesia at doses that did not interfere with L-DOPA’s prokinetic efficacy [53,54]. Moreover, vilazodone also suppressed L-DOPA-induced aberrant gene regulation in striatal MSNs, a molecular correlate of L-DOPA-induced dyskinesia [54].
In the present study, we further investigated the impact of vilazodone on L-DOPA-induced dyskinesia and underlying mechanisms in the unilateral 6-hydroxydopamine (6-OHDA) rat model of PD. For one, we assessed vilazodone effects on the various subtypes of L-DOPA-induced dyskinesia (measured as “abnormal involuntary movements”, AIMs) in this model. Moreover, using in vivo electrophysiological techniques, we investigated the impact of vilazodone on the aberrant responsiveness to corticostriatal drive in striatal MSNs in the dyskinetic state. Furthermore, we determined whether these vilazodone effects were mediated by stimulation of 5-HTr1A. Our results show that vilazodone attenuated all types of L-DOPA-induced AIMs, as well as the increased MSN responsiveness to cortical drive, and that these effects were dependent on 5-HTr1A activation.
2. Results
2.1. Evaluation of 6-OHDA Lesion
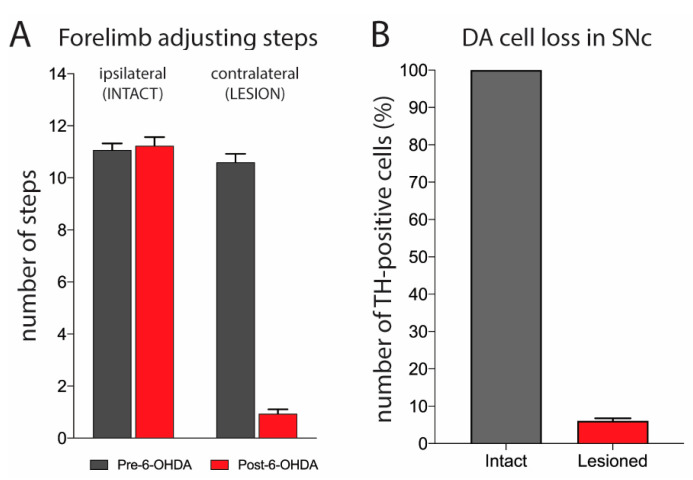
Stepping tests were performed before and after the 6-OHDA lesion to assess the impact of DA cell loss on forelimb movements. Only animals exhibiting a severe loss of forelimb movements, with a drop from a pre-surgery rate of approximately 11 adjusting steps with the forepaw contralateral to the lesion to three or fewer steps at 4 weeks post-surgery, were considered significantly impaired and were included in this study (Figure 1A). This approach has previously been shown to predict near-total DA lesions [55,56,57]. Tyrosine hydroxylase (TH) immunohistochemistry and cell counts were performed to confirm the lesions. The number of DA neurons in the substantia nigra pars compacta (SNc) ipsilateral (lesion) and contralateral (intact) to the side of 6-OHDA infusion was determined. Our results show that rats with three or fewer adjusting steps displayed a loss of DA neurons with a range of 87.9–98.6% (mean ± SEM, 93.95 ± 0.72% of intact side; Figure 1B).
Figure 1.
Unilateral DA lesion by 6-OHDA produces a contralateral forelimb stepping deficit. (A) Forelimb stepping scores (mean ± SEM) in tests performed pre- and four weeks post-surgery. These tests revealed that the 6-OHDA lesion produced a stepping deficit in the forelimb contralateral to the lesion, while stepping with the ipsilateral (intact) forelimb was not affected. The stepping scores given are from the included animals that showed three or fewer adjusting steps with the contralateral forelimb (n = 33). (B) Number of TH-positive cells in the SNc ipsilateral to the lesion, expressed as percentage of the TH-positive cells on the intact side. The included animals displayed a loss of 87.9–98.6% of TH-positive (DA) neurons on the side of the lesion (mean ± SEM, 93.95 ± 0.72% of intact side).
2.2. Vilazodone Suppresses Established AIMs, but Does Not Affect Improved Stepping Performance, after L-DOPA Treatment
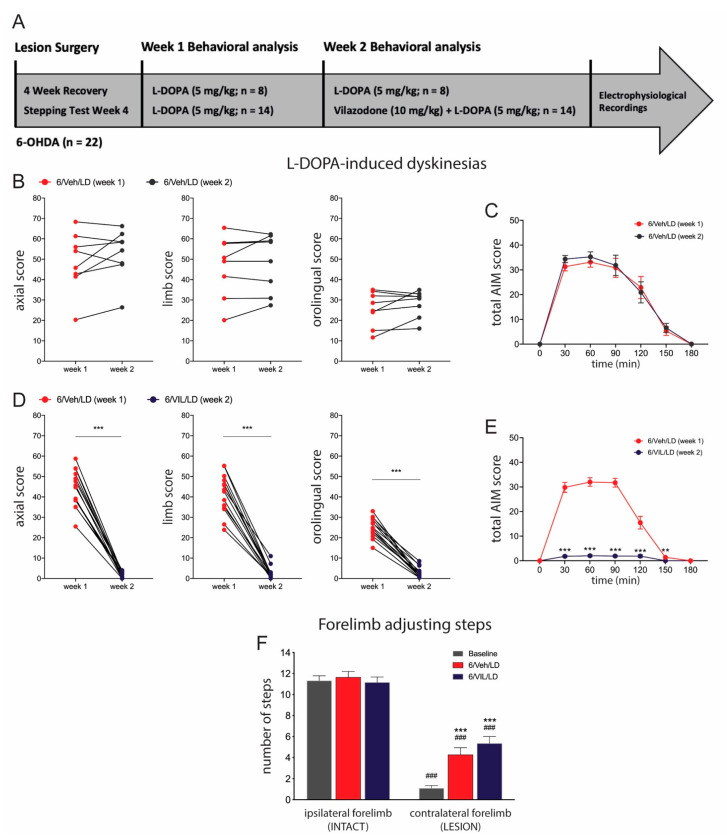
During the first week of drug treatment, all rats received L-DOPA (6/Veh/LD) and displayed axial, limb, and orolingual AIMs on the side of their body contralateral to the 6-OHDA lesion. These AIMs typically lasted for up to 3 h, with a peak in severity occurring around 30–90 min after L-DOPA administration (Figure 2). AIM subtypes were scored on the last three days of the week, and 3-day averages for each subtype and total scores are presented (Figure 2).
Figure 2.
Vilazodone significantly inhibits expression of established AIMs, while not affecting the prokinetic effects of L-DOPA. (A) Experimental design/timeline of study. (B–E) AIMs in 6-OHDA-lesioned rats treated with vehicle (10% cremophor in 0.9% saline) + L-DOPA (5 mg/kg, i.p.) (6/Veh/LD) for two weeks (B,C), and in 6-OHDA-lesioned rats treated with vehicle + L-DOPA (6/Veh/LD) in week 1 followed by vilazodone (10 mg/kg, i.p.) + L-DOPA (6/VIL/LD) in week 2 (D,E). The video analysis revealed that vilazodone co-administration in week 2 significantly attenuated the expression of AIMs compared to L-DOPA-only treatment in week 1, for axial, limb and orolingual AIMs (individual animals) (D) and for total AIM scores (mean ± SEM) across 180 min after L-DOPA administration (E). *** p < 0.001, 6/VIL/LD (week 2) vs. 6/Veh/LD (week 1). (F) Forelimb stepping scores after these drug treatments. Scores (mean ± SEM) from 4 weeks after the lesion (before the start of the treatment protocol, “baseline”) and 1 h after L-DOPA administration on the second day of treatment week 2 are shown. The forelimb stepping test revealed that vilazodone, while inhibiting L-DOPA-induced AIMs, did not negate the prokinetic effects of L-DOPA in stepping behavior (6/VIL/LD vs. 6/Veh/LD, p > 0.05). ### p < 0.001, vs. ipsilateral (INTACT) side; *** p < 0.001, vs. contralateral (LESION) baseline.
In week 2, rats received pretreatment with either vehicle or vilazodone 30 min prior to L-DOPA administration (Figure 2A). In vehicle-pretreated rats (6/Veh/LD, n = 8), AIM scores did not differ between week 2 and week 1, for axial, limb, orolingual, or total AIMs (all Z ≤ −1.26, p > 0.05; Wilcoxon matched-pairs signed-rank test) (Figure 2B,C). In contrast, vilazodone administration before L-DOPA (6/VIL/LD, n = 14) almost completely suppressed established axial, limb, orolingual, and total AIMs, compared to week 1 (all Z = −3.29, p < 0.001) (Figure 2D,E).
To evaluate drug effects on motor performance in these animals, we compared the number of forelimb adjusting steps before the start of the treatment protocol (i.e., 4-week counts, “baseline”) with stepping rates 1 h after L-DOPA administration on the second day (Tue) of treatment week 2 (Figure 2F). Two-factor ANOVA analysis revealed significant main effects of treatment (F(2,91) = 8.940, p < 0.001) and side (ipsilateral vs. contralateral to the lesion) (F(1,91) = 321.1, p < 0.001), with a significant interaction (F(2,91) = 8.798, p < 0.001). Tukey post-hoc tests revealed differences between groups as follows. Stepping with the forelimb contralateral to the 6-OHDA lesion was significantly reduced, compared to ipsilateral stepping, in all three conditions (p < 0.001). After L-DOPA-only treatment (6/Veh/LD), this motor performance was significantly improved (p < 0.001, vs. baseline). Similarly, rats that received vilazodone + L-DOPA co-treatment (6/VIL/LD) displayed a significant improvement in contralateral forelimb adjusting steps (p < 0.001, vs. baseline). These two groups did not differ in their contralateral steps (p > 0.05, 6/Veh/LD vs. 6/VIL/LD) (Figure 2F).
2.3. 5-HTr1A Agonism Mediates Impact of Vilazodone on AIMs
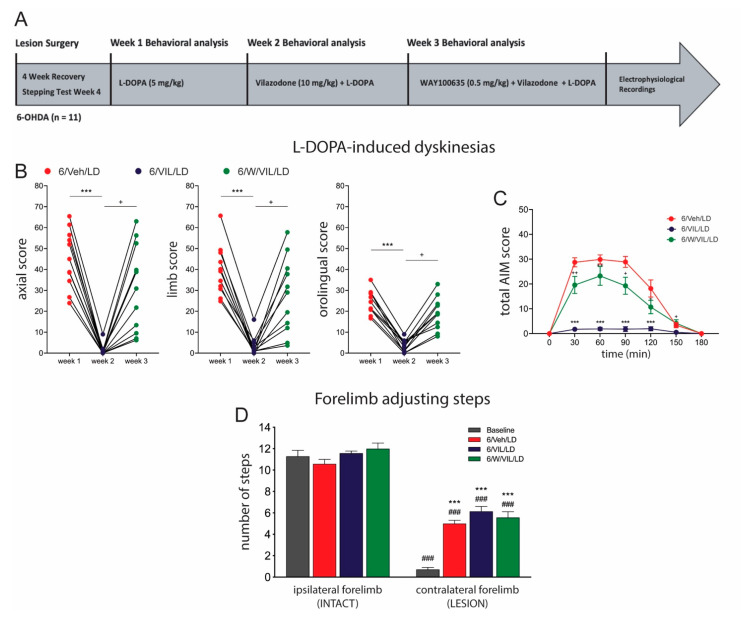
In week 1 of our 5-HTr1A antagonist experiment (Figure 3A), rats (n = 11) received repeated L-DOPA-only treatment (6/Veh/LD) and developed stable AIMs. Friedman ANOVAs showed a significant effect of treatments (all X2(2) > 18.73, p < 0.001). Dunn’s post-hoc tests revealed differences between treatment groups as follows. Consistent with our previous outcomes, vilazodone pretreatment (6/VIL/LD) in week 2 significantly reduced axial, limb, orolingual, and total AIMs compared to L-DOPA-only in week 1 (p < 0.001, vs. 6/Veh/LD) (Figure 3B,C). In contrast, administration of WAY-100635 (WAY), a selective 5-HTr1A antagonist, together with vilazodone and L-DOPA (6/W/VIL/LD) in week 3 significantly attenuated the antidyskinetic efficacy of vilazodone (p < 0.05, vs. 6/VIL/LD; p > 0.05, vs. 6/Veh/LD) (Figure 3B,C).
Figure 3.
The 5-HTr1A antagonist WAY-100635 (WAY) blocks the antidyskinetic effects of vilazodone. (A) Experimental design/timeline of study. (B,C) AIMs in 6-OHDA-lesioned rats treated with vehicle + L-DOPA (5 mg/kg, i.p.) (6/Veh/LD) in week 1, vilazodone (10 mg/kg, i.p.) + L-DOPA (6/VIL/LD) in week 2 and WAY-100635 (0.5 mg/kg, i.p.) + vilazodone + L-DOPA (6/W/VIL/LD) in week 3. The video analysis revealed that vilazodone co-administration (week 2) significantly attenuated the expression of AIMs compared to L-DOPA-only treatment (week 1), whereas WAY co-administration (week 3) significantly inhibited this beneficial effect of vilazodone treatment. This was shown for axial, limb and orolingual AIMs (individual animals) (B) and for total AIM scores (mean ± SEM) across 180 min after L-DOPA administration (C). *** p < 0.001, 6/VIL/LD vs. 6/Veh/LD; + p < 0.05, ++ p < 0.01, 6/W/VIL/LD vs. 6/VIL/LD. (D) Forelimb stepping scores (mean ± SEM) after these drug treatments. Neither vilazodone (6/VIL/LD) nor WAY (6/W/VIL/LD) had any effects on the L-DOPA-induced motor improvements (6/Veh/LD vs. baseline), as assessed by the forelimb stepping test. ### p < 0.001, vs. ipsilateral (INTACT) side; *** p < 0.001, vs. contralateral (LESION) baseline).
Drug effects on motor performance in these animals were assessed with the forelimb stepping test. Two-factor ANOVA analysis of forelimb adjusting steps revealed significant main effects of treatment (F(3,48) = 19.57, p < 0.001) and side (ipsilateral vs. contralateral to the lesion) (F(1,48) = 541.1, p < 0.001), with a significant interaction (F(3,48) = 16.19, p < 0.001). Tukey post-hoc tests revealed the following differences between treatments. Stepping with the forelimb contralateral to the lesion was significantly reduced in all four conditions (p < 0.001). After treatment with L-DOPA-only (6/Veh/LD), vilazodone + L-DOPA (6/VIL/LD) or WAY + vilazodone + L-DOPA (6/W/VIL/LD), this stepping performance was significantly improved (p < 0.001, vs. baseline). Importantly, WAY administration (6/W/VIL/LD) did not alter this therapeutic efficacy as compared to L-DOPA-only treatment (p > 0.05, vs. 6/Veh/LD), or vilazodone + L-DOPA treatment (p > 0.05, vs. 6/VIL/LD) (Figure 3D).
2.4. Effects of Vilazodone and 5-HTr1A Blockade on Striatal MSN Activity in Dyskinetic DA-Depleted Animals
We used in vivo single-unit extracellular recordings to assess drug effects on cortically evoked activity in MSNs of the sensorimotor striatum ipsilateral to the 6-OHDA lesion (Figure 4). While the vast majority (≥95%) of striatal neurons are MSNs, we also infrequently encountered fast-spiking interneurons. These interneurons can be distinguished from MSNs by their short onset latency and duration of action potential responses to low stimulus intensities (<0.95 ms), and burst-like activity following cortical stimulation (see [58,59]). Only cells that exhibited an action potential duration of 1 ms or higher following cortical stimulation were included in this study. Tonically active interneurons typically did not respond to our stimulation protocol.
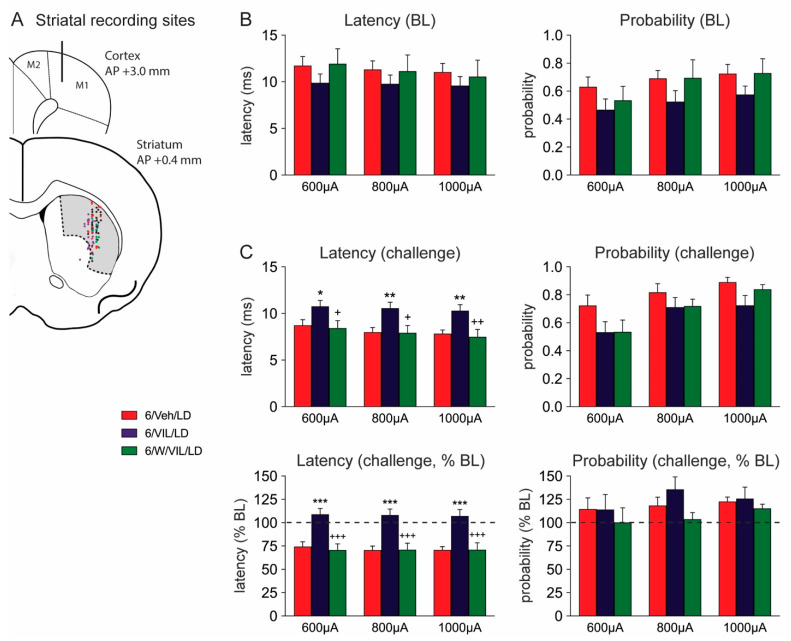
Figure 4.
Vilazodone inhibits L-DOPA effects on spike onset latency after cortical stimulation in striatal MSNs, and this inhibition is mediated by 5-HTr1A stimulation. (A) Schematic illustration of coronal sections through the frontal cortex and middle striatum (approximately at +3.0 and +0.4 mm rostral to bregma) showing the stimulation site in the primary motor cortex (M1), and, collapsed onto one level, the distribution of recording sites in the sensorimotor striatum (shaded) for the different treatment groups (“challenge” units: 6/Veh/LD, red dots; 6/VIL/LD, purple dots; 6/W/VIL/LD, green dots; pooled “baseline” units, black dots). (B) Cortically evoked responses in MSNs of 6-OHDA-lesioned rats recorded before the final drug treatment (“baseline” responses, BL). Displayed are spike onset latency (mean ± SEM) (left) and spike probability (right) in rats that had received a repeated pretreatment with vehicle + L-DOPA (5 mg/kg, i.p.) (6/Veh/LD; n = 11 cells in 5 rats), vilazodone (10 mg/kg, i.p.) + L-DOPA (6/VIL/LD; n = 14 cells in 8 rats), or WAY-100635 (0.5 mg/kg, i.p.) + vilazodone + L-DOPA (6/W/VIL/LD; n = 7 cells in 3 rats). (C) Cortically evoked responses in MSNs of 6-OHDA-lesioned rats recorded after the final drug treatment (“challenge” responses, top). Shown are spike onset latency (left) and spike probability (right) in rats that had received a repeated treatment with vehicle + L-DOPA (5 mg/kg, i.p.) (6/Veh/LD; n = 21 cells in 8 rats), vilazodone (10 mg/kg, i.p.) + L-DOPA (6/VIL/LD; n = 19 cells in 9 rats), or WAY-100635 (0.5 mg/kg, i.p.) + vilazodone + L-DOPA (6/W/VIL/LD; n = 16 cells in 7 rats). Additionally displayed are these data expressed in percentage of baseline values (% BL) (bottom). Vilazodone co-administered with L-DOPA (6/VIL/LD) prevented the L-DOPA-induced decrease in onset latency (6/Veh/LD). WAY administration (6/W/VIL/LD) blocked vilazodone’s ability to attenuate the L-DOPA effect on the onset latency. * p < 0.05, ** p < 0.01, *** p < 0.001, vs. 6/Veh/LD; + p < 0.05, ++ p < 0.01, +++ p < 0.001, vs. 6/VIL/LD.
Cortical stimulation with 400 µA failed to elicit consistent responses in MSNs, and the 400 µA data were thus not included in the statistical analysis. For stimulation intensities of 600, 800 and 1000 µA, in cells recorded before drug administration (“baseline”; 6/Veh/LD, n = 11; 6/VIL/LD, n = 14; 6/W/VIL/LD, n = 7), two-factor ANOVAs showed the following results. Spike onset latency (Figure 4B, left) showed a tendency for a main effect of drug treatment (F(2,87) = 2.143, p = 0.12), no main effect of stimulation intensity (F(2,87) = 0.344, p > 0.05) and no significant interaction (F(4,87) = 0.053, p > 0.05). Analysis of spike probability (Figure 4B, right) revealed a significant main effect of drug treatment (F(2,87) = 3.976, p < 0.05), but no significant main effect of stimulation intensity (F(2,87) = 2.036, p > 0.05) and no significant interaction (F(4,87) = 0.132, p > 0.05). The 6/VIL/LD group showed tendencies for a reduction in onset latency and spike probability compared to the other groups; however, post-hoc tests did not reveal significant differences between individual groups (Figure 4B).
In cells recorded after drug administration (“challenge”; 6/Veh/LD, n = 21; 6/VIL/LD, n = 19; 6/W/VIL/LD, n = 16), two-factor ANOVAs of stimulation-evoked activity demonstrated the following. Spike onset latency (Figure 4C, top left) showed a significant main effect of treatment (F(2,159) = 15.49, p < 0.001), but no significant main effect of stimulation intensity (F(2,159) = 1.130, p > 0.05) and no significant interaction (F(4,159) = 0.064, p > 0.05). Analysis of spike probability (Figure 4C, top right) revealed significant main effects of treatment (F(2,159) = 4.938, p < 0.01) and stimulation intensity (F(2,159) = 9.112, p < 0.001), but no significant interaction (F(4,159) = 0.419, p > 0.05). Tukey post-hoc tests revealed the following differences between treatment groups (“challenge”; Figure 4C, top). Cells in vilazodone + L-DOPA-treated animals displayed a longer onset latency in cortically evoked spikes than cells in vehicle + L-DOPA-treated animals (6/VIL/LD vs. 6/Veh/LD) at 600, 800 and 1000 µA stimulation intensities (all p < 0.05) (Figure 4C, top left). Blocking 5-HTr1A with WAY (0.5 mg/kg) prevented this effect of vilazodone on the onset latency, as WAY treatment significantly reduced the onset latency at all intensities (p < 0.05, 6/W/VIL/LD vs. 6/VIL/LD) to levels observed with L-DOPA-only treatment (p > 0.05, 6/W/VIL/LD vs. 6/Veh/LD). For spike probability (Figure 4C, top right), post-hoc tests did not show significant group differences at any of the 3 current intensities (p > 0.05).
Given the effects of the drug treatments on baseline activity (see above), we also expressed activities recorded after the drug challenge relative to baseline values (percent of baseline; Figure 4C, bottom). Two-factor ANOVAs for these data (“challenge”, % of baseline) revealed, for spike onset latency (Figure 4C, bottom left), a significant main effect of drug treatment (F(2,159) = 38.77, p < 0.001), no significant main effect of stimulation intensity (F(2,159) = 0.066, p > 0.05) and no significant interaction (F(4,159) = 0.041, p > 0.05). For spike probability (Figure 4C, bottom right), no significant main effects of treatment (F(2,159) = 1.949, p > 0.05), stimulation intensity (F(2,159) = 0.892, p > 0.05) or interaction (F(4,159) = 0.307, p > 0.05) were found. Tukey post-hoc tests (Figure 4C, bottom left) revealed that vilazodone + L-DOPA treatment produced a longer spike onset latency than vehicle + L-DOPA treatment (6/VIL/LD vs. 6/Veh/LD) at 600, 800 and 1000 µA stimulation intensities (all p < 0.001), and that blocking 5-HTr1A with WAY completely prevented this effect of vilazodone (p < 0.001, 6/W/VIL/LD vs. 6/VIL/LD; p > 0.05, 6/W/VIL/LD vs. 6/Veh/LD).
In summary, consistent with previous findings demonstrating a significant increase in MSN activity in DA-depleted animals following L-DOPA treatment (e.g., [60,61,62]), our analysis showed that L-DOPA-only treatment (6/Veh/LD) reduced the MSN spike onset latency to 75% of baseline values. Vilazodone reversed this L-DOPA-induced facilitation of MSN responses, and this reversal was blocked by the 5-HTr1A antagonist, WAY-100635.
3. Discussion
The present study investigated, in the 6-OHDA rat model of PD, the antidyskinetic effects of the multimodal serotonergic drug, vilazodone, the 5-HT receptor subtypes involved, and the electrophysiological correlates in the striatum of these drug actions. Our main results can be summarized as follows. First, our findings confirm and extend previous results by us [54] and others [53], demonstrating a powerful inhibitory effect of vilazodone on the various subtypes of L-DOPA-induced dyskinesia (AIMs) observed in this model. Second, importantly, in contrast to other serotonergic modulatory agents, vilazodone co-administration did not compromise the therapeutic efficacy of L-DOPA, as shown by our outcomes in the forelimb stepping test. Third, also in agreement with previous findings [53], these antidyskinetic effects of vilazodone were blocked by the selective 5-HTr1A antagonist WAY-100635, demonstrating a critical role for 5-HTr1A in this vilazodone action. Fourth, in line with the behavioral effects of vilazodone, our in vivo electrophysiological studies revealed that vilazodone prevented the abnormal L-DOPA-induced facilitation of corticostriatal transmission (reflected by a decrease in onset latency of cortically evoked spikes in MSNs), and that these vilazodone effects were also attenuated by blocking 5-HTr1A. These results complement our previous findings showing that vilazodone suppresses abnormal L-DOPA-induced gene regulation in MSNs in this model [54]. Collectively, these findings indicate that vilazodone co-treatment is capable of “normalizing” aberrant MSN activities and corticostriatal transmission that contribute to L-DOPA-induced dyskinesia, and that 5-HT1A serotonin receptors mediate these vilazodone effects.
3.1. Characterization of Dopamine Lesion
The degree of DA cell loss after 6-OHDA infusion was determined by stereological quantification of the number of TH+ cells in the SNc. Our findings show that the DA-depleted animals that were included in this study, after meeting the inclusion criterion of three or fewer contralateral forelimb steps, had a near-total (average >93%) reduction in the TH+ cell numbers in the SNc ipsilateral to the 6-OHDA infusion. This is consistent with previous findings showing that rats with such a robust deficit in stepping performance had a 90% or greater loss of DA cell bodies in the SN [55,56], or an 80–100% loss of DA tissue content [63] or TH immunoreactivity [54,57] in the ipsilateral striatum.
3.2. Vilazodone Attenuates L-DOPA-Induced AIMs, but Does Not Block Prokinetic Effects of L-DOPA
In this study, as in previous studies (e.g., [54,57,64,65]), extensive 6-OHDA-induced striatal DA depletion, followed by repeated daily L-DOPA treatment, produced robust development and expression of AIMs, the rodent equivalent of L-DOPA-induced dyskinesia observed in patients with PD. Our recent work [54,57] demonstrated that L-DOPA given at the relatively low dose of 5 mg/kg once daily for 2–4 weeks was sufficient to induce AIMs in this PD model. Consistent with this finding, in the present study, AIMs emerged as early as one or two days after the first L-DOPA administration, and these AIMs stabilized during the last three days of week 1 and did not further increase between weeks 1 and 2.
A recent study [53] first demonstrated that vilazodone (10 mg/kg), when combined with L-DOPA, significantly reduced established AIMs in 6-OHDA-lesioned rats, an effect we confirmed for our model [54]. In agreement with these outcomes, we here report that vilazodone (10 mg/kg) pretreatment in week 2 almost completely abolished total AIMs as compared to week 1. Moreover, in this study, we provide a detailed analysis of the impact of vilazodone co-administration on the different AIM subtypes (i.e., axial, limb, and orolingual), in addition to the time course of AIM scores across 3 h after L-DOPA administration. Our results demonstrate that all AIM subtypes were dramatically suppressed, with the most robust inhibition seen for axial AIMs and a somewhat lesser effect for limb and orolingual AIMs.
Previous work that assessed SSRIs or 5-HTr1A agonists as antidyskinetic agents found beneficial effects of those compounds, but also reported potentially problematic side effects, including 5-HT syndrome-like effects and a reduction in L-DOPA-induced motor improvement (e.g., [37,38,42,43,47,66]). In contrast, studies investigating vilazodone did not observe symptoms of 5-HT syndrome, even at higher doses [49,53,67]. Moreover, importantly, our findings demonstrate that vilazodone co-administration, at the present intermediate dose (10 mg/kg), which largely suppressed L-DOPA-induced abnormal gene regulation in striatal MSNs [54], did not interfere with the prokinetic efficacy of L-DOPA, as assessed in the forelimb stepping test (present results; [54]), although higher doses may lose some of this advantage [53].
The loss of the prokinetic effects of L-DOPA (e.g., [38,47]) has been attributed to a strong inhibition of 5-HT neurons innervating the striatum following a high-dose treatment with SSRIs or 5-HTr1A full agonists, which might lead to a near-complete shutdown in striatal DA release from 5-HT terminals [68]. It can be speculated that the unique pharmacological profile of vilazodone as an SSRI together with its 5-HTr1A partial agonist property, which is thought to desensitize 5-HTr1A on the serotonergic cell bodies that regulate the firing activity of these neurons [68,69,70], may account for the efficacy seen with this multimodal agent. The impact of vilazodone may be sufficient to moderate serotonergic activity and DA release from these terminals, thus avoiding abnormal DA spikes in the striatum and their molecular [54] and behavioral (AIMs) consequences, without completely shutting down this DA input, and thus enabling prokinetic effects of L-DOPA.
3.3. Vilazodone Attenuates L-DOPA-Induced Facilitation of Corticostriatal Transmission in Dyskinetic Parkinsonian Rats
Hyperexcitability of MSNs has been described as a primary neuropathophysiological correlate of dyskinesia (e.g., [57,61,62,71,72]). In the DA-depleted striatum, DA receptors on MSNs become supersensitive, producing increased responsiveness of MSNs to dopaminergic drugs such as L-DOPA (e.g., [61,72]). This increase in MSN responsiveness after L-DOPA administration is most pronounced in the sensorimotor striatum [54,57,73,74]. We therefore assessed the effects of L-DOPA and vilazodone on cortical stimulation-evoked MSN activity in the sensorimotor striatum.
Consistent with previous work, our results show that chronic L-DOPA treatment produced an increase in MSN responsiveness to cortical stimulation, reflected by a decrease in spike onset latency. Enhanced responsiveness to corticostriatal drive after L-DOPA treatment has been related to aberrant hyperactivation of intracellular signaling pathways in MSNs (e.g., [44,71,72,75,76]). Importantly, in our study, this increase in MSN responsiveness was prevented when vilazodone was combined with L-DOPA treatment, a drug combination that also attenuated abnormal molecular signaling in MSNs [54]. Our findings suggest that vilazodone’s modulatory effects on 5-HT neurons produce a tempered DA release from striatal 5-HT terminals, resulting in an attenuation of hyperactive intracellular signaling, thus allowing a “normalization” of neurophysiological (present study) and molecular [54] activities in these neurons (see also [44]), both critical for avoiding L-DOPA-induced motor abnormalities such as dyskinesia (see [54], for discussion). Future work with cell type-specific experimental manipulations will be necessary to provide more detailed insights into the specific cellular mechanisms underlying the vilazodone effects on striatal neuronal activity.
3.4. The Effects of Vilazodone Are Mediated by 5-HTr1A
Previous work first indicated that vilazodone, a 5-HTr1A partial agonist [50,52], indeed acts via stimulation of 5-HTr1A to inhibit L-DOPA-induced dyskinesia [53]. Our present results confirm and extend these earlier findings by showing that a selective 5-HTr1A antagonist (WAY-100635) strongly attenuated the antidyskinetic effects of vilazodone for all subtypes of AIMs. Moreover, we investigated the impact of blocking 5-HTr1A on the vilazodone effects on cortical stimulation-evoked MSN activities in dyskinetic animals. Our results show that the ability of vilazodone to ameliorate aberrant corticostriatal signaling was inhibited by the 5-HTr1A antagonist, underscoring the importance of 5-HTr1A for vilazodone’s impact on cellular and behavioral effects. These findings thus provide mechanistic insights into the impact of the serotonergic innervation and 5-HTr1A on corticostriatal activation of MSNs in this dyskinetic PD rat model.
Many 5-HT receptor subtypes, including 5-HTr1A, have a fairly wide distribution in the brain [77]. In our study, all drugs, including the 5-HTr1A antagonist, were given systemically, thus precluding conclusions regarding their specific sites of action (receptor location). However, in line with our reasoning, a recent study reported a near-complete suppression in 5-HT neuron firing following administration of another 5-HTr1A agonist (±8-OH-DPAT), an effect that was reversed by WAY-100635 [68]. These findings are consistent with an involvement of the 5-HT transmission to the striatum in vilazodone’s impact on the cellular and behavioral effects of L-DOPA. Future studies using local drug administration will be necessary to ascertain the role of 5-HTr1A on 5-HT neurons in these effects.
4. Conclusions
Our results indicate that vilazodone co-treatment has the ability to “normalize” aberrant corticostriatal transmission and striatal circuit activity following repeated L-DOPA administration via modulating 5-HT activity in a 5-HTr1A-dependent manner. Vilazodone may help temper L-DOPA-mediated DA input by enabling a more physiological-like release of DA from 5-HT terminals in the striatum. This SSRI/5-HTr1A partial agonist thus appears to be superior to agents acting as SSRIs only or as 5-HTr1A full agonists. Future clinical trials will be necessary to confirm vilazodone’s potential clinical efficacy.
5. Materials and Methods
5.1. Animals
Adult male Sprague-Dawley rats (225–249 g upon arrival; Harlan, Indianapolis, IN, USA) were housed 2–3 per cage under standard laboratory conditions (12 h light/dark cycle, lights on at 07:00 h; with ad libitum access to food and water). All procedures met the NIH guidelines for the care and use of laboratory animals and were approved by the Rosalind Franklin University Animal Care and Use Committee (protocol # 17-05; approved on 19 April 2017).
5.2. 6-OHDA-Induced Dopaminergic Lesions and Stepping Test
Two weeks after arrival, rats received a unilateral 6-OHDA lesion. These lesions were performed as described previously [55,57]. Rats were deeply anesthetized using 2–5% isoflurane vapors (Patterson Veterinary, Greeley, CO, USA). They received an injection of desipramine HCl (20 mg/kg, i.p.; in 0.9% saline; Sigma-Aldrich, St Louis, MO, USA) 30 min prior to 6-OHDA administration. A single unilateral infusion of 6-OHDA HBr (Sigma-Aldrich; 8 µg in 4 µL of 0.9% saline containing 0.1% ascorbic acid) was delivered into the right medial forebrain bundle (coordinates, from bregma: AP −4.3 mm, ML −1.6 mm, DV −8.3 mm; [78]) as previously described [55]. The infusion rate was 0.4 µL/min, and the cannula remained in place for an additional 10 min before being retracted.
The 6-OHDA lesion was assessed by performing a forelimb stepping test [79] pre-surgery and then 4 weeks post-surgery. In this test, the rat is held by an experimenter and moved sideways, with its forelimb on the side opposite to the movement direction touching the bench surface. Normally, the rat will perform adjusting steps during this lateral movement, in our settings, typically 10–14 steps [54,55,57]. Following a >90% DA depletion, the number of adjusting steps with the forelimb contralateral to the lesion drops to three steps or fewer, two to four weeks after the 6-OHDA lesion, while stepping with the forelimb ipsilateral to the lesion is unaffected [55,56]. Only rats that displayed a stepping deficit of three or fewer steps with the contralateral forelimb following the 6-OHDA lesion were selected for this study. The lesion was further characterized by measuring TH immunoreactivity (see below).
5.3. Drug Treatments
Starting after a 4-week recovery period, animals with a 6-OHDA lesion that met the inclusion criterion of three or fewer forelimb adjusting steps received drug treatments on five consecutive days/week (Mon–Fri), for two or three weeks. In week 1, all rats received a daily vehicle injection (10% Cremophor EL in 0.9% saline, 2 mL/kg, i.p.; Sigma-Aldrich), followed 30 min later by the L-DOPA (LD) injection (5 mg/kg, i.p., 2 mL/kg; Alfa Aesar, Tewksbury, MA, USA; coadministered with 12.5 mg/kg benserazide HCl; Sigma-Aldrich). In week 2, one cohort received the same treatment of vehicle + L-DOPA as in week 1 (6/Veh/LD; n = 8), and a second cohort received a treatment of vilazodone HCl (VIL) (10 mg/kg, i.p.; Cayman Chemical, Ann Arbor, MI, USA; in 10% Cremophor EL), followed 30 min later by L-DOPA (6/VIL/LD; n = 14).
To assess a potential role for 5-HTr1A in mediating the actions of vilazodone, a third cohort of 6-OHDA-infused rats received vehicle + L-DOPA (6/Veh/LD) in week 1, vilazodone + L-DOPA (6/VIL/LD) in week 2, and in week 3, they received the selective 5-HTr1A antagonist N-[2-[4-(2-methoxyphenyl)-1-piperazinyl]ethyl]-N-2-pyridinyl-cyclo- hexanecarboxamide, 2Z-butenedioate (WAY-100635 (WAY); 0.5 mg/kg, i.p.; Cayman Chemical) 5 min prior to the vilazodone treatment (6/W/VIL/LD; n = 11), in a within-subject design.
5.4. Behavioral Analysis
On the second day of each treatment week, a stepping test was performed 60 min after L-DOPA treatment. Dyskinesias were assessed during the last three days of each treatment week (Wed–Fri), using an established and well-characterized rat dyskinesia scale to measure AIMs [64,65]. Briefly, rats were individually placed in clear plastic cylinders, and AIMs were videotaped and their frequency and severity scored during a 1-min period at 30-min intervals, 30 to 180 min after L-DOPA injection. AIMs are classified as axial, limb (forelimb), or orolingual. Their frequency was assessed using the following scale: 0 = absent; 1 = occasional (1 to 29 s); 2 = frequent (30 to 59 s); 3 = continuous but interrupted by external sensory stimuli; and 4 = continuous, not interrupted by strong sensory stimuli) [64].
Additionally, the AIM severity (amplitude) was assessed as follows: Axial AIMs (1 = 30° angle lateral deviation of head and neck; 2 = 30° < angle ≤ 60° lateral deviation of head and neck; 3 = 60° < angle ≤ 90° lateral deviation of head, neck, and upper trunk; 4 = > 90° angle torsion of head, neck, and trunk, often causing the rat to lose balance), forelimb AIMs (1 = minor involuntary movements of the distal forelimb; 2 = low amplitude movements causing translocation of both distal and proximal forelimb; 3 = involuntary movements of the whole limb including shoulder muscles; 4 = strong, ballism-like limb and shoulder movements), and orolingual AIMs (1 = involuntary movements of the orofacial muscles with no tongue protrusion; 2 = involuntary movements of the orofacial muscles with tongue protrusion).
Blinded scorers were allowed to give partial scores such as 0.5, 1.5, 2.5, and 3.5 in order to increase the accuracy of AIM ratings. A severity score for each AIM subtype was calculated by multiplying frequency and amplitude scores for each assessment period (i.e., 30, 60, 90, 120, 150, and 180 min), and these values were added for a total AIM score for each subtype. An overall total AIM score was calculated by adding total axial, limb, and orolingual scores.
5.5. In Vivo Single-Unit Electrophysiological Recordings
Electrophysiological recordings were performed after the behavioral studies. All animals were maintained on the same daily treatment regimen as in the last week of their behavioral studies and received their last treatment on the day of the recordings. Thus, MSNs were recorded in rats treated with L-DOPA following an injection of either vehicle (6/Veh/LD), vilazodone (6/VIL/LD), or WAY + vilazodone (6/W/VIL/LD). In each group, several MSNs were recorded before the last drug treatment was received to determine “baseline” responses. Responses after the last drug treatment in these animals are designated “challenge” responses; these were recorded 20–180 min after the last drug administration.
Cortically evoked MSN activity was recorded as previously described [55,59,80,81]. Briefly, rats were deeply anesthetized with urethane (1.5 g/kg in physiological saline), and their temperature was maintained at 37 °C using a heating pad. A bipolar cortical stimulation electrode was implanted ipsilateral to the lesion (coordinates, from bregma: AP +3.0 mm, ML –2.5 mm, DV –1.6 mm; [78]) to target the sensorimotor cortex. Cortical local field potentials on the side contralateral to the lesion (AP +3.0 mm, ML +2.5 mm, DV –1.6 mm) were monitored for the presence of slow, large-amplitude waves to ensure that animals were in a deeply anesthetized state during recordings [59]. Recordings began 1 h after electrode implantation.
Microelectrodes for extracellular recordings were manufactured from 2.0 mm outer diameter borosilicate glass capillary tubing (World Precision Instruments, Sarasota, FL, USA) using a vertical micropipette puller (Narishige, Tokyo, Japan). The microelectrode tip was broken to ~1 µm in diameter by pushing against a glass rod, and the electrode was filled with 2 M NaCl solution. Striatal MSN activity was recorded ipsilateral to the cortical stimulation (and lesion) at the following coordinates: AP 0.0 to +0.75 mm, ML –3.3 to –3.9 mm, DV –3.0 to –6.5 mm. These coordinates targeted the sensorimotor striatum, where the most robust L-DOPA-induced pathophysiological changes occur [54,57,74].
MSN activity was assessed across stimulation trials (50 pulses/trial) by measuring probability and onset latency of action potentials evoked by cortical stimulation at four different current intensities (400 µA, 600 µA, 800 µA, and 1000 µA in separate trials) as described previously [80,82]. The order of cortical stimulation intensity was counterbalanced between cells (i.e., either 400–1000 or 1000–400 µA). Cortically evoked MSN action potentials were amplified (Neuro Data Instruments, Delaware Water Gap, PA, USA), filtered, digitized via a Digidata 1440a (Molecular Devices, San Jose, CA, USA), acquired using Axoscope software (Molecular Devices), and analyzed using Clampfit 10 software (Molecular Devices). Upon the completion of the experiment, rats were quickly perfused with 4% paraformaldehyde, and their brains were extracted for postmortem assessment of DA cell loss by TH immunohistochemistry staining and DA cell counting in the SNc, using stereological techniques (described below).
5.6. Tyrosine Hydroxylase Immunohistochemistry
Rat brains were sliced coronally into 50 µm thick sections, using a sliding microtome (SM2010 R, Leica Microsystems, Wetzler, Germany) as previously described [83]. Sections containing the substantia nigra (from bregma: approximately −4.8 to −6.1 mm) were incubated in rabbit anti-TH antibody (1:500; Pel-Freez Biologicals, Rogers, AR, USA) for 24 h followed by a 2-h incubation with biotinylated goat-anti-rabbit secondary antibody (1:200; Vector Laboratories, Burlingame, CA, USA). Sections were then incubated with avidin/biotinylated complex (ABC; Vector Laboratories), and bound complexes were visualized using 3,3′-diaminobenzidine and hydrogen peroxide tablets as previously described [56]. The number of TH-positive neurons was estimated by stereological means (Stereo Investigator, MBF Biosciences, Williston, VT, USA). Briefly, the SNc region in 6 coronal sections (collected at 200 µm intervals) was carefully outlined under 4× magnification using a rat brain atlas [78]. TH+ cells from the SNc on the ipsilateral (lesioned) and contralateral (intact) sides were counted at 100× magnification. Cells were only included if the nucleus and soma were visible and under focus. The extent of the lesion was then calculated as the number of TH+ cells on the lesioned side relative to that on the intact side.
5.7. Statistical Analysis
Statistical analysis was performed using GraphPad Prism version 8.0 (GraphPad Software, San Diego, CA, USA). The differences in AIM scores between treatments in within-subject design experiments were assessed using Wilcoxon matched-pairs signed-rank tests, or Friedman ANOVAs, followed by Dunn’s post-hoc tests to identify differences between individual treatments. Stepping scores were compared with two-factor ANOVAs with Tukey post-hoc tests. For electrophysiological recordings, the differences in spike probability and onset latency of cortically evoked responses were assessed using two-factor ANOVAs, followed by Tukey post-hoc tests to describe differences between individual groups. Differences were considered significant if p < 0.05.
Acknowledgments
We thank Kuei Tseng and Connor Moon for their contributions to this paper.
Author Contributions
Conceptualization, A.R.W. and H.S.; methodology, F.A., F.E.P.-N., A.R. and A.R.W.; investigation, F.A.; formal analysis, F.A. and A.R.W.; writing—original draft preparation, F.A., H.S.; writing—review and editing, A.R.W., F.A., F.E.P.-N., A.R. and H.S.; supervision, A.R.W. and H.S.; project administration, A.R.W. and H.S.; funding acquisition, A.R.W. and H.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported in part by the US National Institutes of Health grant DA046794 (H.S.), and Rosalind Franklin University of Medicine and Science (A.R.W.).
Institutional Review Board Statement
All procedures met the NIH guidelines for the care and use of laboratory animals and were approved by the Rosalind Franklin University Animal Care and Use Committee (protocol # 17-05).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data generated during the current study are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Sample Availability
Not applicable.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Birkmayer W., Hornykiewicz O. The L-3,4-dioxyphenylalanine (DOPA)-effect in Parkinson-akinesia. Wien. Klin. Wochenschr. 1961;73:787–788. [PubMed] [Google Scholar]
- 2.De la Fuente-Fernández R., Sossi V., Huang Z., Furtado S., Lu J.Q., Calne D.B., Ruth T.J., Stoessl A.J. Levodopa-induced changes in synaptic dopamine levels increase with progression of Parkinson’s disease: Implications for dyskinesias. Brain. 2004;127:2747–2754. doi: 10.1093/brain/awh290. [DOI] [PubMed] [Google Scholar]
- 3.Mercuri N.B., Bernardi G. The ‘magic’ of L-Dopa: Why is it the gold standard Parkinson’s disease therapy? Trends Pharm. Sci. 2005;26:341–344. doi: 10.1016/j.tips.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 4.Cotzias G.C., Papavasiliou P.S., Gellene R. L-Dopa in parkinson’s syndrome. N. Engl. J. Med. 1969;281:272. doi: 10.1056/NEJM196907312810518. [DOI] [PubMed] [Google Scholar]
- 5.Mones R.J., Elizan T.S., Siegel G. L-Dopa induced dyskinesias in 152 patients with Parkinson’s disease. Trans. Am. Neurol. Assoc. 1970;95:286–287. [PubMed] [Google Scholar]
- 6.Lane E.L. L-DOPA for Parkinson’s disease-a bittersweet pill. Eur. J. Neurosci. 2019;49:384–398. doi: 10.1111/ejn.14119. [DOI] [PubMed] [Google Scholar]
- 7.Ahlskog J.E., Muenter M.D. Frequency of levodopa-related dyskinesias and motor fluctuations as estimated from the cumulative literature. Mov. Disord. 2001;16:448–458. doi: 10.1002/mds.1090. [DOI] [PubMed] [Google Scholar]
- 8.Hely M.A., Morris J.G., Reid W.G., Trafficante R. Sydney multicenter study of Parkinson’s disease: Non-L-Dopa-responsive problems dominate at 15 years. Mov. Disord. 2005;20:190–199. doi: 10.1002/mds.20324. [DOI] [PubMed] [Google Scholar]
- 9.Huot P., Johnston T.H., Koprich J.B., Fox S.H., Brotchie J.M. The pharmacology of L-DOPA-induced dyskinesia in Parkinson’s disease. Pharmacol. Rev. 2013;65:171–222. doi: 10.1124/pr.111.005678. [DOI] [PubMed] [Google Scholar]
- 10.Damiano A.M., McGrath M.M., Willian M.K., Snyder C.F., LeWitt P.A., Reyes P.F., Richter R.R., Means E.D. Evaluation of a measurement strategy for Parkinson’s disease: Assessing patient health-related quality of life. Qual. Life Res. 2000;9:87–100. doi: 10.1023/A:1008928321652. [DOI] [PubMed] [Google Scholar]
- 11.Chapuis S., Ouchchane L., Metz O., Gerbaud L., Durif F. Impact of the motor complications of Parkinson’s disease on the quality of life. Mov. Disord. 2005;20:224–230. doi: 10.1002/mds.20279. [DOI] [PubMed] [Google Scholar]
- 12.Smith Y., Wichmann T., Factor S.A., DeLong M.R. Parkinson’s disease therapeutics: New developments and challenges since the introduction of levodopa. Neuropsychopharmacology. 2012;37:213–246. doi: 10.1038/npp.2011.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marconi R., Lefebvre-Caparros D., Bonnet A.M., Vidailhet M., Dubois B., Agid Y. Levodopa-induced dyskinesias in Parkinson’s disease phenomenology and pathophysiology. Mov. Disord. 1994;9:2–12. doi: 10.1002/mds.870090103. [DOI] [PubMed] [Google Scholar]
- 14.Cenci M.A., Konradi C. Maladaptive striatal plasticity in L-DOPA-induced dyskinesia. Prog. Brain. Res. 2010;183:209–233. doi: 10.1016/S0079-6123(10)83011-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murer M.G., Moratalla R. Striatal signaling in L-DOPA-induced dyskinesia: Common mechanisms with drug abuse and long term memory involving D1 dopamine receptor stimulation. Front. Neuroanat. 2011;5:51. doi: 10.3389/fnana.2011.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cenci M.A. Molecular mechanisms of L-DOPA-induced dyskinesia. In: Steiner H., Tseng K.Y., editors. Handbook of Basal Ganglia Structure and Function. Elsevier; London, UK: 2016. pp. 857–871. [Google Scholar]
- 17.Klawans H.L., Goetz C., Nausieda P.A., Weiner W.J. Levodopa-induced dopamine receptor hypersensitivity. Trans. Am. Neurol. Assoc. 1977;102:80–83. doi: 10.1002/ana.410020207. [DOI] [PubMed] [Google Scholar]
- 18.Gerfen C.R. D1 dopamine receptor supersensitivity in the dopamine-depleted striatum: Aberrant ERK1/2 signaling. In: Steiner H., Tseng K.Y., editors. Handbook of Basal Ganglia Structure and Function. Elsevier; London, UK: 2010. pp. 491–500. [Google Scholar]
- 19.Calabresi P., Pisani A., Rothwell J., Ghiglieri V., Obeso J.A., Picconi B. Hyperkinetic disorders and loss of synaptic downscaling. Nat. Neurosci. 2016;19:868–875. doi: 10.1038/nn.4306. [DOI] [PubMed] [Google Scholar]
- 20.Abercrombie E.D., Bonatz A.E., Zigmond M.J. Effects of ofl-Dopa on extracellular dopamine in striatum of normal and 6-hydroxydopamine-treated rats. Brain Res. 1990;525:36–44. doi: 10.1016/0006-8993(90)91318-B. [DOI] [PubMed] [Google Scholar]
- 21.Lindgren H.S., Andersson D.R., Lagerkvist S., Nissbrandt H., Cenci M.A. L-DOPA-induced dopamine efflux in the striatum and the substantia nigra in a rat model of Parkinson’s disease: Temporal and quantitative relationship to the expression of dyskinesia. J. Neurochem. 2010;112:1465–1476. doi: 10.1111/j.1471-4159.2009.06556.x. [DOI] [PubMed] [Google Scholar]
- 22.Politis M., Wu K., Loane C., Brooks D.J., Kiferle L., Turkheimer F.E., Bain P., Molloy S., Piccini P. Serotonergic mechanisms responsible for levodopa-induced dyskinesias in Parkinson’s disease patients. J. Clin. Investig. 2014;124:1340–1349. doi: 10.1172/JCI71640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Larsen M.B., Sonders M.S., Mortensen O.V., Larson G.A., Zahniser N.R., Amara S.G. Dopamine transport by the serotonin transporter: A mechanistically distinct mode of substrate translocation. J. Neurosci. 2011;31:6605–6615. doi: 10.1523/JNEUROSCI.0576-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nishijima H., Tomiyama M. What mechanisms are responsible for the reuptake of levodopa-derived dopamine in Parkinsonian striatum? Front. Neurosci. 2016;10:575. doi: 10.3389/fnins.2016.00575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rylander D., Parent M., O’Sullivan S.S., Dovero S., Lees A.J., Bezard E., Descarries L., Cenci M.A. Maladaptive plasticity of serotonin axon terminals in levodopa-induced dyskinesia. Ann. Neurol. 2010;68:619–628. doi: 10.1002/ana.22097. [DOI] [PubMed] [Google Scholar]
- 26.Zeng B.Y., Iravani M.M., Jackson M.J., Rose S., Parent A., Jenner P. Morphological changes in serotoninergic neurites in the striatum and globus pallidus in levodopa primed MPTP treated common marmosets with dyskinesia. Neurobiol. Dis. 2010;40:599–607. doi: 10.1016/j.nbd.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 27.Bédard C., Wallman M.J., Pourcher E., Gould P.V., Parent A., Parent M. Serotonin and dopamine striatal innervation in Parkinson’s disease and Huntington’s chorea. Parkinsonism Relat. Disord. 2011;17:593–598. doi: 10.1016/j.parkreldis.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 28.Arai R., Karasawa N., Geffard M., Nagatsu I. L-DOPA is converted to dopamine in serotonergic fibers of the striatum of the rat: A double-labeling immunofluorescence study. Neurosci. Lett. 1995;195:195–198. doi: 10.1016/0304-3940(95)11817-G. [DOI] [PubMed] [Google Scholar]
- 29.Tanaka H., Kannari K., Maeda T., Tomiyama M., Suda T., Matsunaga M. Role of serotonergic neurons in L-DOPA-derived extracellular dopamine in the striatum of 6-OHDA-lesioned rats. NeuroReport. 1999;10:631–634. doi: 10.1097/00001756-199902250-00034. [DOI] [PubMed] [Google Scholar]
- 30.Carta M., Carlsson T., Kirik D., Björklund A. Dopamine released from 5-HT terminals is the cause of L-DOPA-induced dyskinesia in parkinsonian rats. Brain. 2007;130:1819–1833. doi: 10.1093/brain/awm082. [DOI] [PubMed] [Google Scholar]
- 31.Navailles S., Bioulac B., Gross C., De Deurwaerdère P. Serotonergic neurons mediate ectopic release of dopamine induced by L-DOPA in a rat model of Parkinson’s disease. Neurobiol. Dis. 2010;38:136–143. doi: 10.1016/j.nbd.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 32.Carta M., Björklund A. The serotonergic system in L-DOPA-induced dyskinesia: Pre-clinical evidence and clinical perspective. J. Neural. Transm. 2018;125:1195–1202. doi: 10.1007/s00702-018-1865-5. [DOI] [PubMed] [Google Scholar]
- 33.Lanza K., Bishop C. Serotonergic targets for the treatment of L-DOPA-induced dyskinesia. J. Neural. Transm. 2018;125:1203–1216. doi: 10.1007/s00702-017-1837-1. [DOI] [PubMed] [Google Scholar]
- 34.Yamato H., Kannari K., Shen H., Suda T., Matsunaga M. Fluoxetine reduces L-DOPA-derived extracellular DA in the 6-OHDA-lesioned rat striatum. NeuroReport. 2001;12:1123–1126. doi: 10.1097/00001756-200105080-00015. [DOI] [PubMed] [Google Scholar]
- 35.Bishop C., George J.A., Buchta W., Goldenberg A.A., Mohamed M., Dickinson S.O., Eissa S., Eskow Jaunarajs K.L. Serotonin transporter inhibition attenuates l-DOPA-induced dyskinesia without compromising I-DOPA efficacy in hemi-parkinsonian rats. Eur. J. Neurosci. 2012;36:2839–2848. doi: 10.1111/j.1460-9568.2012.08202.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Conti M.M., Ostock C.Y., Lindenbach D., Goldenberg A.A., Kampton E., Dell’isola R., Katzman A.C., Bishop C. Effects of prolonged selective serotonin reuptake inhibition on the development and expression of L-DOPA-induced dyskinesia in hemi-parkinsonian rats. Neuropharmacology. 2014;77:1–8. doi: 10.1016/j.neuropharm.2013.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fidalgo C., Ko W.K., Tronci E., Li Q., Stancampiano R., Chuan Q., Bezard E., Carta M. Effect of serotonin transporter blockade on L-DOPA-induced dyskinesia in animal models of Parkinson’s disease. Neuroscience. 2015;298:389–396. doi: 10.1016/j.neuroscience.2015.04.027. [DOI] [PubMed] [Google Scholar]
- 38.Kannari K., Kurahashi K., Tomiyama M., Maeda T., Arai A., Baba M., Suda T., Matsunaga M. Tandospirone citrate, a selective 5-HT1A agonist, alleviates L-DOPA-induced dyskinesia in patients with Parkinson’s disease. No Shinkei. 2002;54:133–137. [PubMed] [Google Scholar]
- 39.Tomiyama M., Kimura T., Maeda T., Kannari K., Matsunaga M., Baba M. A serotonin 5-HT1A receptor agonist prevents behavioral sensitization to L-DOPA in a rodent model of Parkinson’s disease. Neurosci. Res. 2005;52:185–194. doi: 10.1016/j.neures.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 40.Bishop C., Taylor J.L., Kuhn D.M., Eskow K.L., Park J.Y., Walker P.D. MDMA and fenfluramine reduce L-DOPA-induced dyskinesia via indirect 5-HT1A receptor stimulation. Eur. J. Neurosci. 2006;23:2669–2676. doi: 10.1111/j.1460-9568.2006.04790.x. [DOI] [PubMed] [Google Scholar]
- 41.Eskow K.L., Gupta V., Alam S., Park J.Y., Bishop C. The partial 5-HT1A agonist buspirone reduces the expression and development of L-DOPA-induced dyskinesia in rats and improves L-DOPA efficacy. Pharmacol. Biochem. Behav. 2007;87:306–314. doi: 10.1016/j.pbb.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 42.Muñoz A., Li Q., Gardoni F., Marcello E., Qin C., Carlsson T., Kirik D., Di Luca M., Björklund A., Bezard E., et al. Study of the antidyskinetic effect of eltoprazine in animal models of levodopa-induced dyskinesia. Mov. Disord. 2013;28:1088–1096. doi: 10.1002/mds.25366. [DOI] [PubMed] [Google Scholar]
- 43.Bézard E., Muñoz A., Tronci E., Pioli E.Y., Li Q., Porras G., Björklund A., Carta M. Anti-dyskinetic effect of anpirtoline in animal models of L-DOPA-induced dyskinesia. Neurosci. Res. 2013;77:242–246. doi: 10.1016/j.neures.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 44.Ghiglieri V., Mineo D., Vannelli A., Cacace F., Mancini M., Pendolino V., Napolitano F., di Maio A., Mellone M., Stanic J., et al. Modulation of serotonergic transmission by eltoprazine in L-DOPA-induced dyskinesia: Behavioral, molecular, and synaptic mechanisms. Neurobiol. Dis. 2016;86:140–153. doi: 10.1016/j.nbd.2015.11.022. [DOI] [PubMed] [Google Scholar]
- 45.Grégoire L., Samadi P., Graham J., Bédard P.J., Bartoszyk G.D., Di Paolo T. Low doses of sarizotan reduce dyskinesias and maintain antiparkinsonian efficacy of l-Dopa in parkinsonian monkeys. Parkinsonism Relat. Disord. 2009;15:445–452. doi: 10.1016/j.parkreldis.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 46.Rosengarten H., Bartoszyk G.D., Quartermain D., Lin Y. The effect of chronic administration of sarizotan, 5-HT1A agonist/D3/D4 ligand, on haloperidol-induced repetitive jaw movements in rat model of tardive dyskinesia. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2006;30:273–279. doi: 10.1016/j.pnpbp.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 47.Goetz C.G., Damier P., Hicking C., Laska E., Müller T., Olanow C.W., Rascol O., Russ H. Sarizotan as a treatment for dyskinesias in Parkinson’s disease: A double-blind placebo-controlled trial. Mov. Disord. 2007;22:179–186. doi: 10.1002/mds.21226. [DOI] [PubMed] [Google Scholar]
- 48.Goetz C.G., Laska E., Hicking C., Damier P., Müller T., Nutt J., Olanow W.C., Rascol O., Russ H. Placebo influences on dyskinesia in Parkinson’s disease. Mov. Disord. 2008;23:700–707. doi: 10.1002/mds.21897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sahli Z.T., Banerjee P., Tarazi F.I. The preclinical and clinical effects of vilazodone for the treatment of major depressive disorder. Expert Opin. Drug Discov. 2016;11:515–523. doi: 10.1517/17460441.2016.1160051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hughes Z.A., Starr K.R., Langmead C.J., Hill M., Bartoszyk G.D., Hagan J.J., Middlemiss D.N., Dawson L.A. Neurochemical evaluation of the novel 5-HT1A receptor partial agonist/serotonin reuptake inhibitor, vilazodone. Eur. J. Pharmacol. 2005;510:49–57. doi: 10.1016/j.ejphar.2005.01.018. [DOI] [PubMed] [Google Scholar]
- 51.Owen R.T. Vilazodone: A new treatment option for major depressive disorder. Drugs Today. 2011;47:531–537. doi: 10.1358/dot.2011.47.7.1622076. [DOI] [PubMed] [Google Scholar]
- 52.Cruz M.P. Vilazodone HCl (Viibryd): A serotonin partial agonist and reuptake inhibitor for the treatment of major depressive disorder. Pharm. Ther. 2012;37:28–31. [PMC free article] [PubMed] [Google Scholar]
- 53.Meadows S.M., Conti M.M., Gross L., Chambers N.E., Avnor Y., Ostock C.Y., Lanza K., Bishop C. Diverse serotonin actions of Vilazodone reduce L-3,4-dihydroxyphenylalanine-induced dyskinesia in hemi-parkinsonian rats. Mov. Disord. 2018;33:1740–1749. doi: 10.1002/mds.100. [DOI] [PubMed] [Google Scholar]
- 54.Altwal F., Moon C., West A.R., Steiner H. The multimodal serotonergic agent vilazodone inhibits L-DOPA-induced gene regulation in striatal projection neurons and associated dyskinesia in an animal model of Parkinson’s disease. Cells. 2020;9:2265. doi: 10.3390/cells9102265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tseng K.Y., Caballero A., Dec A., Cass D.K., Simak N., Sunu E., Park M.J., Blume S.R., Sammut S., Park D.J. Inhibition of striatal soluble guanylyl cyclase-cGMP signaling reverses basal ganglia dysfunction and akinesia in experimental parkinsonism. PLoS ONE. 2011;6:e27187. doi: 10.1371/journal.pone.0027187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jayasinghe V.R., Flores-Barrera E., West A.R., Tseng K.Y. Frequency-dependent corticostriatal disinhibition resulting from chronic dopamine depletion: Role of local striatal cGMP and GABA-AR signaling. Cereb. Cortex. 2017;27:625–634. doi: 10.1093/cercor/bhv241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Padovan-Neto F.E., Patterson S., Voelkner N.M., Altwal F., Beverley J.A., West A.R., Steiner H. Selective regulation of 5-HT1B serotonin receptor expression in the striatum by dopamine depletion and repeated L-DOPA treatment: Relationship to L-DOPA-induced dyskinesias. Mol. Neurobiol. 2020;57:736–751. doi: 10.1007/s12035-019-01739-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mallet N., Le Moine C., Charpier S., Gonon F. Feedforward inhibition of projection neurons by fast-spiking GABA interneurons in the rat striatum in vivo. J. Neurosci. 2005;25:3857–3869. doi: 10.1523/JNEUROSCI.5027-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Padovan-Neto F.E., Cavalcanti-Kiwiatkoviski R., Carolino R.O., Anselmo-Franci J., Del Bel E. Effects of prolonged neuronal nitric oxide synthase inhibition on the development and expression of L-DOPA-induced dyskinesia in 6-OHDA-lesioned rats. Neuropharmacology. 2015;89:87–99. doi: 10.1016/j.neuropharm.2014.08.019. [DOI] [PubMed] [Google Scholar]
- 60.Liang L., DeLong M.R., Papa S.M. Inversion of dopamine responses in striatal medium spiny neurons and involuntary movements. J. Neurosci. 2008;28:7537–7547. doi: 10.1523/JNEUROSCI.1176-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rangel-Barajas C., Silva I., Lopéz-Santiago L.M., Aceves J., Erlij D., Florán B. L-DOPA-induced dyskinesia in hemiparkinsonian rats is associated with up-regulation of adenylyl cyclase type V/VI and increased GABA release in the substantia nigra reticulata. Neurobiol. Dis. 2011;41:51–61. doi: 10.1016/j.nbd.2010.08.018. [DOI] [PubMed] [Google Scholar]
- 62.Ryan M.B., Bair-Marshall C., Nelson A.B. Aberrant striatal activity in parkinsonism and levodopa-induced dyskinesia. Cell Rep. 2018;23:3438–3446. doi: 10.1016/j.celrep.2018.05.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chang J.W., Wachtel S.R., Young D., Kang U.J. Biochemical and anatomical characterization of forepaw adjusting steps in rat models of Parkinson’s disease: Studies on medial forebrain bundle and striatal lesions. Neuroscience. 1999;88:617–628. doi: 10.1016/S0306-4522(98)00217-6. [DOI] [PubMed] [Google Scholar]
- 64.Winkler C., Kirik D., Björklund A., Cenci M.A. L-DOPA-induced dyskinesia in the intrastriatal 6-hydroxydopamine model of parkinson’s disease: Relation to motor and cellular parameters of nigrostriatal function. Neurobiol. Dis. 2002;10:165–186. doi: 10.1006/nbdi.2002.0499. [DOI] [PubMed] [Google Scholar]
- 65.Cenci M.A., Lundblad M. Post- versus presynaptic plasticity in L-DOPA-induced dyskinesia. J. Neurochem. 2006;99:381–392. doi: 10.1111/j.1471-4159.2006.04124.x. [DOI] [PubMed] [Google Scholar]
- 66.Lindenbach D., Palumbo N., Ostock C.Y., Vilceus N., Conti M.M., Bishop C. Side effect profile of 5-HT treatments for Parkinson’s disease and L-DOPA-induced dyskinesia in rats. Br. J. Pharmacol. 2015;172:119–130. doi: 10.1111/bph.12894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Page M.E., Cryan J.F., Sullivan A., Dalvi A., Saucy B., Manning D.R., Lucki I. Behavioral and neurochemical effects of 5-(4-[4-(5-Cyano-3-indolyl)-butyl)-butyl]-1-piperazinyl)-benzofuran-2-carboxamide (EMD 68843): A combined selective inhibitor of serotonin reuptake and 5-hydroxytryptamine(1A) receptor partial agonist. J. Pharmacol. Exp. Ther. 2002;302:1220–1227. doi: 10.1124/jpet.102.034280. [DOI] [PubMed] [Google Scholar]
- 68.Sellnow R.C., Newman J.H., Chambers N., West A.R., Steece-Collier K., Sandoval I.M., Benskey M.J., Bishop C., Manfredsson F.P. Regulation of dopamine neurotransmission from serotonergic neurons by ectopic expression of the dopamine D2 autoreceptor blocks levodopa-induced dyskinesia. Acta Neuropathol. Commun. 2019;7:8. doi: 10.1186/s40478-018-0653-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ashby C.R.J., Kehne J.H., Bartoszyk G.D., Renda M.J., Athanasiou M., Pierz K.A., Seyfried C.A. Electrophysiological evidence for rapid 5-HT₁A autoreceptor inhibition by vilazodone, a 5-HT₁A receptor partial agonist and 5-HT reuptake inhibitor. Eur. J. Pharmacol. 2013;714:359–365. doi: 10.1016/j.ejphar.2013.07.014. [DOI] [PubMed] [Google Scholar]
- 70.El Mansari M., Crnic A., Oosterhof C., Blier P. Long-term administration of the antidepressant vilazodone modulates rat brain monoaminergic systems. Neuropharmacology. 2015;99:696–704. doi: 10.1016/j.neuropharm.2015.09.007. [DOI] [PubMed] [Google Scholar]
- 71.Picconi B., Centonze D., Håkansson K., Bernardi G., Greengard P., Fisone G., Cenci M.A., Calabresi P. Loss of bidirectional striatal synaptic plasticity in L-DOPA–induced dyskinesia. Nat. Neurosci. 2003;6:501–506. doi: 10.1038/nn1040. [DOI] [PubMed] [Google Scholar]
- 72.Aubert I., Guigoni C., Håkansson K., Li Q., Dovero S., Barthe N., Bioulac B.H., Gross C.E., Fisone G., Bloch B., et al. Increased D1 dopamine receptor signaling in levodopa-induced dyskinesia. Ann. Neurol. 2004;57:17–26. doi: 10.1002/ana.20296. [DOI] [PubMed] [Google Scholar]
- 73.Cenci M.A., Lee C.S., Bjorklund A. L-DOPA-induced dyskinesia in the rat is associated with striatal overexpression of prodynorphin- and glutamic acid decarboxylase mRNA. Eur. J. Neurosci. 1998;10:2694–2706. doi: 10.1046/j.1460-9568.1998.00285.x. [DOI] [PubMed] [Google Scholar]
- 74.Girasole A.E., Lum M.Y., Nathaniel D., Bair-Marshall C.J., Guenthner C.J., Luo L., Kreitzer A.C., Nelson A.B. A subpopulation of striatal neurons mediates levodopa-induced dyskinesia. Neuron. 2018;97:787–795. doi: 10.1016/j.neuron.2018.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fienberg A.A., Hiroi N., Mermelstein P.G., Song W.J., Snyder G.L., Nishi A., Cheramy A., O’Callaghan J.P., Miller D.B., Cole D.G., et al. DARPP-32: Regulator of the efficacy of dopaminergic neurotransmission. Science. 1998;281:838–842. doi: 10.1126/science.281.5378.838. [DOI] [PubMed] [Google Scholar]
- 76.Santini E., Valjent E., Usiello A., Carta M., Borgkvist A., Girault J.A., Hervé D., Greengard P., Fisone G. Critical involvement of cAMP/DARPP-32 and extracellular signal-regulated protein kinase signaling in L-DOPA-induced dyskinesia. J. Neurosci. 2007;27:6995–7005. doi: 10.1523/JNEUROSCI.0852-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Barnes N.M., Sharp T. A review of central 5-HT receptors and their function. Neuropharmacology. 1999;38:1083–1152. doi: 10.1016/S0028-3908(99)00010-6. [DOI] [PubMed] [Google Scholar]
- 78.Paxinos G., Watson C. The Rat Brain in Stereotaxic Coordinates. 6th ed. Academic Press; New York, NY, USA: 1998. [Google Scholar]
- 79.Olsson M., Nikkhah G., Bentlage C., Björklund A. Forelimb akinesia in the rat Parkinson model: Differential effects of dopamine agonists and nigral transplants as assessed by a new stepping test. J. Neurosci. 1995;15:3863–3875. doi: 10.1523/JNEUROSCI.15-05-03863.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Threlfell S., Sammut S., Menniti F.S., Schmidt C.J., West A.R. Inhibition of phosphodiesterase 10A increases the responsiveness of striatal projection neurons to cortical stimulation. J. Pharmacol. Exp. Ther. 2009;328:785–795. doi: 10.1124/jpet.108.146332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sammut S., Threlfell S., West A.R. Nitric oxide-soluble guanylyl cyclase signaling regulates corticostriatal transmission and short-term synaptic plasticity of striatal projection neurons recorded in vivo. Neuropharmacology. 2010;58:624–631. doi: 10.1016/j.neuropharm.2009.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ondracek J.M., Dec A., Hoque K.E., Lim S.A., Rasouli G., Indorkar R.P., Linardakis J., Klika B., Mukherji S.J., Burnazi M., et al. Feed-forward excitation of striatal neuron activity by frontal cortical activation of nitric oxide signaling in vivo. Eur. J. Neurosci. 2008;27:1739–1754. doi: 10.1111/j.1460-9568.2008.06157.x. [DOI] [PubMed] [Google Scholar]
- 83.Zhang Y., Meredith G.E., Mendoza-Elias N., Rademacher D.J., Tseng K.Y., Steece-Collier K. Aberrant restoration of spines and their synapses in L-DOPA-induced dyskinesia: Involvement of corticostriatal but not thalamostriatal synapses. J. Neurosci. 2013;33:11655–11667. doi: 10.1523/JNEUROSCI.0288-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data generated during the current study are available from the corresponding author on reasonable request.