Abstract
Stable free radicals are widely used as molecular probes and labels in various biophysical and biomedical research applications of magnetic resonance spectroscopy and imaging. Among these radicals, sterically shielded nitroxides of pyrrolidine series demonstrate the highest stability in biological systems. Here, we suggest new convenient procedure for preparation of 3-carboxy-2,2,5,5-tetraethylpyrrolidine-1-oxyl, a reduction-resistant analog of widely used carboxy-Proxyl, from cheap commercially available reagents with the yield exceeding the most optimistic literature data. Several new spin labels and probes of 2,2,5,5-tetraethylpyrrolidine-1-oxyl series were prepared and reduction of these radicals in ascorbate solutions, mice blood and tissue homogenates was studied.
Keywords: nitroxide, spin label, spin probe, ethynylmagnesium bromide, nitrone, pyrrolidine
1. Introduction
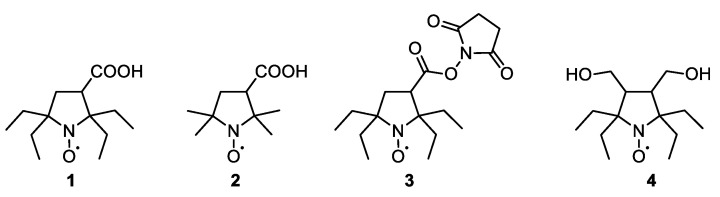
Nitroxide spin labels are playing an important role in application of EPR spectroscopy and imaging in biophysics and structural biology providing useful information about structure and dynamics of biomolecules and mechanisms of their interaction [1,2,3]. Modern achievements in EPR technique allow for structural studies inside living cells [4,5] and visualization of biochemical processes in living tissues [6]. Regretfully, some biogenic molecules and enzymatic systems readily reduce nitroxides to diamagnetic compounds and this hinders application of nitroxide spin labels in biomedical research [7]. Bulky substituents (larger than methyl) adjacent to nitroxide group can retard nitroxides decay in biological systems. So-called sterically shielded nitroxides demonstrate much higher stability to bioreduction compared to conventional tetramethyl nitroxides [8,9]. Moreover, 3-carboxy-2,2,5,5-tetraethylpyrrolidine-1-oxyl (1) (Chart 1) showed slower decay in cytosolic extract than trityl radical [9]. Recently, 2,2,5,5-tetraethyl-2,5-dihydropyrrole-1-oxyls were suggested as spin labels for in-cell applications [10,11,12]. Meanwhile, sterically shielded pyrrolidine-1-oxyls show higher resistance to chemical reduction than similar 2,5-dihydropyrrole-1-oxyls [10,13,14]. In analogy to the widely used nitroxide 3-carboxy-Proxyl (2), 1 can be considered as a valuable precursor of various reduction-resistant spin labels. For example, the N-hydroxysuccinimide ester 3 has been prepared and characterized by Rajca et al. [14]. However, the published synthesis of 1 requires multistep procedures, which give rather low overall yield of this nitroxide. We have recently published a method of synthesis of 3,4-bis-(hydroxymethyl)-2,2,5,5-tetraethylpyrrolidine-1-oxyl (4) from 2-aminobutanoic acid, 3-pentanone, and dimethyl fumarate in four steps with an overall yield of 40% [15]. The previously developed strategy can be adopted for the synthesis of 1 [16]. Here, we describe how 1 can be prepared in six simple steps from cheap commercially available reagents with the yield exceeding the most optimistic literature data. A set of new reduction-resistant 2,2,5,5-tetraethylpyrrolidine-1-oxyls have been synthesized, including analogs of conventional spin labels and probes. Reduction of selected spin probes of 2,2,5,5-tetraethylpyrrolidine series in an ascorbate-containing model system, mice blood and tissue homogenates was studied.
Chart 1.
Structure of nitroxides 1–4.
2. Results and Discussion
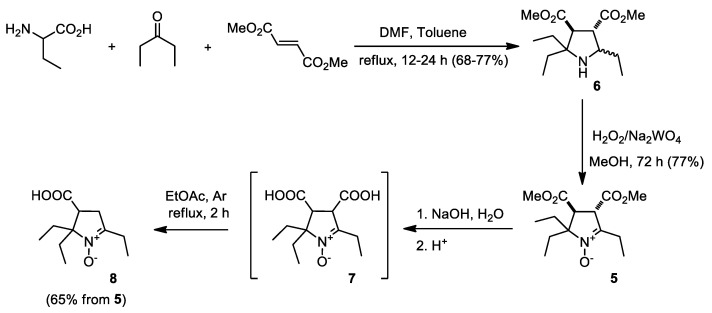
We have recently reported that nitrone 5 can be prepared in two steps, with 52–60% yield from 2-aminobutanoic acid, 3-pentanone and dimethyl fumarate via the three-component domino process, leading to pyrrolidine 6 and following oxidation of the latter with a hydrogen peroxide-tungstate system [15]. Alkali hydrolysis of the ester groups in 5 affords dicarboxylic acid 7, which is unstable, and similarly to β-keto-carboxylic acids, easily undergoes decarboxylation to give 8 (Scheme 1). Thus, the mixture of 7 and 8 was extracted from acidified aqueous solution with ethyl acetate and the extract was heated to reflux for complete decarboxylation of 7. Crystallization from THF gave 8 as colorless crystalline solid, the structure of this compound was unambiguously confirmed by spectral data and element analysis.
Scheme 1.
Synthesis of 8.
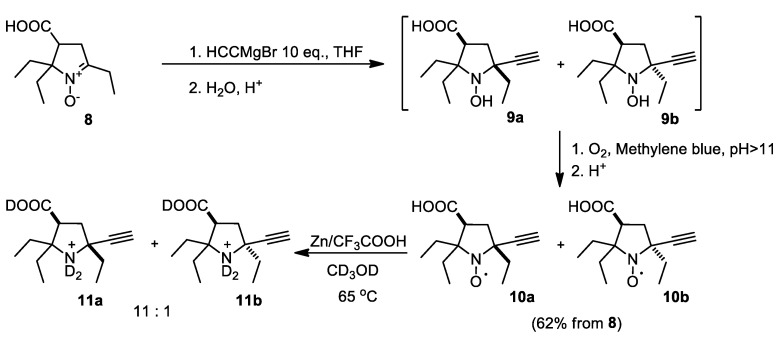
It was shown previously that treatment of 3,4-bis-(hydroxymethyl)-2,5,5-triethyl-1-pyrroline N-oxide with ethylmagnesium bromide does not lead to nucleophilic addition onto nitrone group, presumably due to metalation [15]. In contrast, the reactions of 2,5,5-triethyl-1-pyrroline 1-oxides with less basic vinylmagnesium bromide or allylmagnesium bromide were shown to afford corresponding nitroxides [15,17]. Here, we used even less basic organometallic reagent, i.e., ethynylmagnesium bromide. Acetylene-derived organometallic reagents were successfully used by Hideg et al. in their syntheses of functionalized nitroxides from substituted 1-pyrroline 1-oxides [18,19,20,21]. Nitrone 8 was treated with 10-fold excess of ethynylmagnesium bromide in THF. The conversion was complete after 24 h at ambient temperature. Acidification of the reaction mixture was necessary to extract the products after quenching with water, indicating that carboxylic group remained unaffected. The extract contained a mixture of hydroxylamines and nitroxides due to partial oxidation in aerobic conditions. To achieve complete conversion of hydroxylamine to nitroxide, air was bubbled through the methanol solution in the presence of catalytic amount of methylene blue. The resulting mixture of nitroxides could not be separated using column chromatography. Crystallization from a pentane-toluene mixture afforded yellow crystals of 10a (Scheme 2). The purity of the sample of 10a was confirmed using HPLC and 1H NMR after reduction with Zn in the presence of trifluoroacetic acid in CD3OD (see experimental section and Supplementary Materials).
Scheme 2.
Addition of ethynylmagnesium bromide to nitrone 8.
It is known that depending on conditions, reaction of nitroxides with zinc and carboxylic acid may give hydroxylamine or amine [22]. If the reaction is carried out with excess of zinc at 60–65 °C, the nitroxide group is quantitatively reduced to secondary amine, with carboxylic, mesyloxy, amino, carboxamido, terminal ethylene and acetylene groups remaining unaffected. Reduction of nitroxide group with zinc in the presence of trifluoroacetic acid appeared to be a convenient method for identification and quantification of isomers or by-products in the samples of tetraethyl-substituted nitroxides using 1H NMR. The sample can be prepared for NMR analysis in 15 min from 5–15 mg of nitroxide. The resulting ammonium cation does not form an asymmetric center at the nitrogen atom, showing a single set of signals in 1H NMR spectra for each isomer even if another asymmetric center is present in the molecule. This method gives clear advantages over other methods, allowing for fast quantitative analysis. For comparison, reduction to hydroxylamines is reversible in neutral or basic media, e.g., upon catalytic hydrogenation or in reactions with hydrazines or hydroxylamines. Some hydroxylamines are rapidly oxidized back to nitroxide with air oxygen. Reaction of sterically shielded nitroxides with hydrazines or hydroxylamines is very slow, and it is hard to achieve complete conversion. The presence of a nitroxide in the samples leads to broadening of lines in NMR spectra and complicates the analysis. The hydroxylamines can be stabilized in acidic media, e.g., upon reduction with alcohol in strongly acidic media [23,24]; however, hydroxylammonium cation formation adds an asymmetric center to the molecule, affording a mixture of diastereomers in variable ratio with superposition of signals or broadening in the NMR spectra [25]. Some other reactions, such as conversion to alkoxyamines [26] or reduction to amines with thiols [13,27], never afford a single diamagnetic product with quantitative yield.
The 1H NMR spectrum of the mixture of nitroxides prepared from 8 (after reduction) showed the presence of another nitroxide, presumably the minor isomer 10b. The ratio of isomers estimated from the NMR spectrum (see Table S1 and Figure S1) was 11:1. The IR spectrum of the major product indicated the presence of carboxylic group and terminal acetylene moiety. Stereochemical structure of the major product 10a was determined using X-ray analysis of the single crystal (Figure S2). Predominant formation of 10a obviously occurs due to a nucleophilic attack from the less hindered side of the nitrone group.
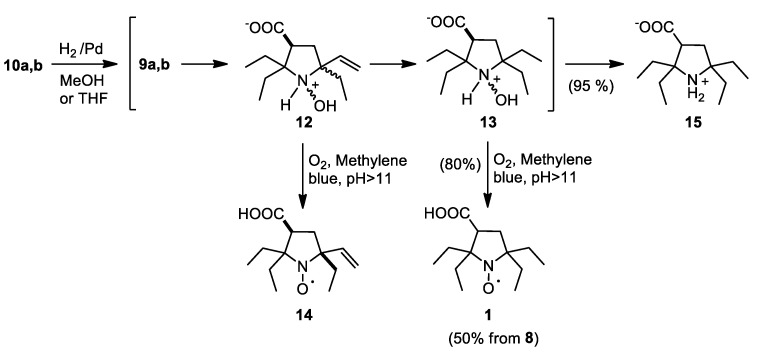
Hydrogenation of a mixture of 10a,b on Pd/C in methanol leads to the formation of crystalline precipitate, presumably consisting of zwitterionic salts 12 and 13, which are moderately soluble in methanol (Scheme 3). As a result, re-oxidation of the reaction mixture with air oxygen in the presence of methylene blue usually gives mixtures of 1 and 14, which are hard to separate using chromatography or crystallization. Careful hydrogenation of 10a,b in methanol allowed to isolate pure 14 (major isomer), but it is hard to achieve complete conversion into 13 without partial reduction of N-hydroxy group to amino. Hydrogenation of 10a,b in methanol without control of absorbed hydrogen gave 15 with nearly quantitative yield. The use of THF instead of MeOH allowed to prevent precipitation of intermediate compounds. After absorption of ca. 2.5 eq. of H2, re-oxidation of the reaction mixture with air oxygen in the presence of methylene blue afforded pure 1 with 80% yield. Noteworthily, we did not observe formation of 15 even upon longer hydrogenation of 10a,b in THF. Since the conversion of 12 to 13 may be incomplete, purity of samples of 1 was always verified using 1H NMR after reduction with Zn/CF3COOH system. The overall yield of 1 from 2-aminobutanoic acid, 3-pentanone and dimethyl fumarate, thus, exceeds 19%, which is far higher than the yield described in the literature [14].
Scheme 3.
Hydrogenation of 10a,b and synthesis of 1.
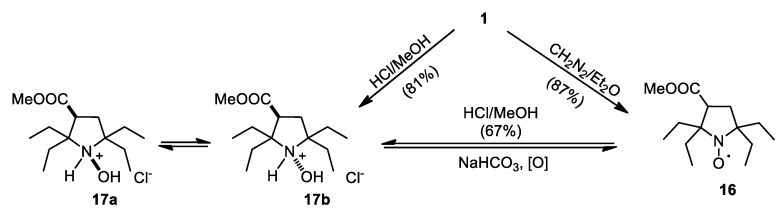
Treatment of 1 with MeOH-HCl afforded ester hydrochloride 17 (Scheme 4). This compound can be prepared via reaction of 1 with diazomethane and subsequent reduction in MeOH-HCl mixture. NMR spectra of 17 revealed two sets of signals with different intensity, clearly showing formation of two diastereomeric cations 17a and 17b. Basification of 17 under aerobic conditions gave 16 due to rapid oxidation of the free base.
Scheme 4.
Synthesis and interconversion of esters 16 and 17.
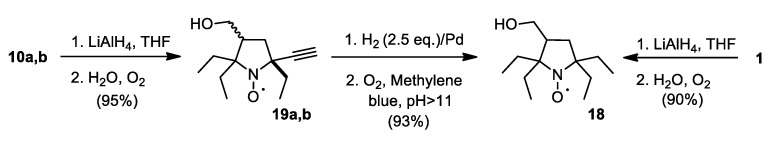
Conversion of 1 into spin label 3 capable of attachment to primary amino groups have been reported earlier [14]. Reduction of the carboxylic group in 1 opens a pathway to another set of spin labels and probes (Scheme 5). Carbinol 18 was prepared via two different pathways; the best overall yield (from 8) has been achieved using reduction of 10a,b with LiAlH4 with subsequent hydrogenation and oxidation.
Scheme 5.
Synthesis of 18.
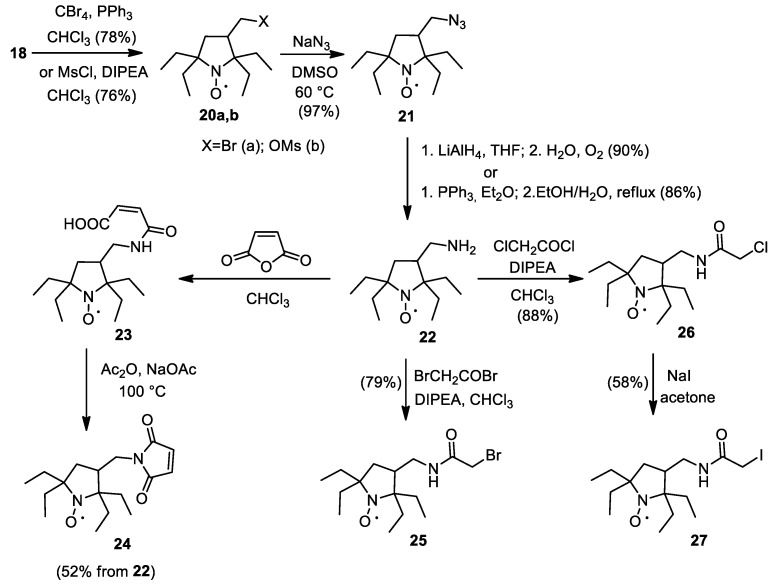
Appel reaction is a useful method for one-step conversion of nitroxide carbinols into corresponding bromides [28,29]. Treatment of 18 with CBr4 and PPh3 afforded 20a with 78% yield (Scheme 6). Reaction of 18 with methanesulfonyl chloride in the presence of a base gives similar yield of corresponding mesylate 20b. Heating of 20a,b with sodium azide in DMSO gives 21. Reduction of azido group gave aminomethyl derivative 25, a key compound for the synthesis of spin labels 24–27.
Scheme 6.
Synthesis of spin labels.
The maleimide spin label 24 was prepared in a two-step procedure according to the literature protocol for similar pyrroline spin labels [11,30]. Acylation of 22 with bromoacetyl bromide or chloroacetyl chloride afforded 25 and 26; the latter was converted into iodoacetamido spin label 27 upon treatment with NaI in acetone.
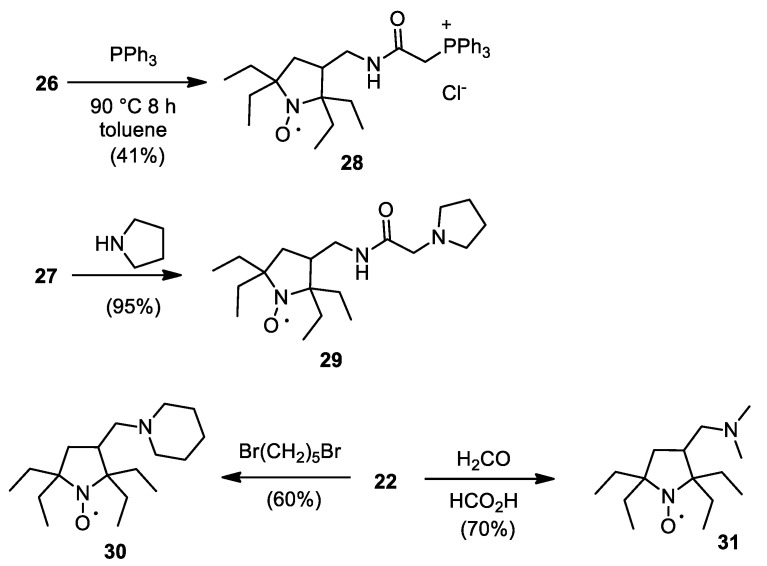
The feasibility of nitroxides for in vivo EPRI, MRI and Overhauser MRI studies have been demonstrated in numerous papers (see, for example, [31,32,33,34]). For these applications, both decay of nitroxides and their physical clearance from the tissue into the blood stream play an important role. Intracellular targeting is one of the efficient methods to increase retention in tissues [35,36]. If this technique is used, reduction-resistant nitroxides, which showed high lifetimes in cytosol extracts [9], may have an important advantage over conventional tetramethyl-substituted spin probes, which are known to undergo rapid reduction inside cells. Triphenylphosphonium group is known to provide efficient intracellular accumulation via transmembrane electrostatic potential-driven mechanism [37,38]. 2-(Triphenylphosphonio)acetamido derivatives of nitroxides were successfully used both in living tissue imaging and in treatment of oxidative stress-related pathologies [38,39,40,41]. Heating of 26 with excess of triphenylphosphine in toluene afforded reduction-resistant mitochondria-targeted nitroxide 28 (Scheme 7).
Scheme 7.
Synthesis of nitroxide triphenylphosphonium salt and tertiary amines.
Moderately basic amino groups can also provide higher retention/intracellular accumulation due to transmembrane potential or ion-trapping effect [42]. Some nitroxides with tertiary amino groups showed high efficacy as both MRI contrast agents and radioprotectors [34,43]. In this work, we tried several different methods for the preparation of reduction-resistant nitroxides with tertiary amino groups. Spin label 27 readily reacts with pyrrolidine to give 29 with nearly quantitative yield. Heating of 22 with 1.2 excess of dibromopentane gave a mixture, and a major product 30 was isolated with 60% yield. An Eschweiler–Clarke reaction afforded 31 with 70% yield.
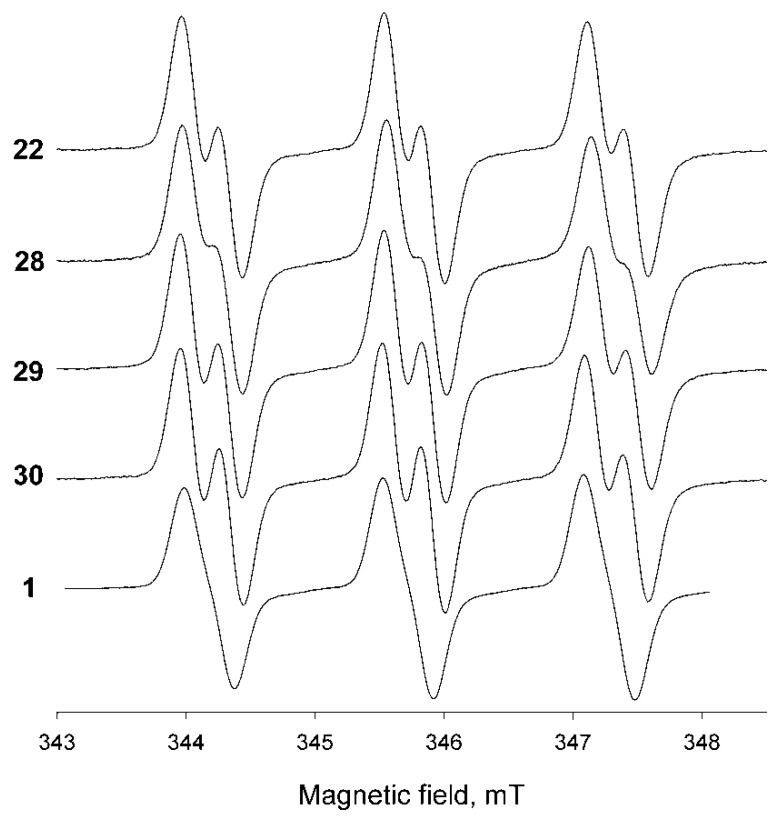
Parameters of EPR spectra, partition coefficients octanol/water and rate constants of reduction with ascorbate of nitroxides 1, 22 and 28–30 are listed in the Table 1. Kinetics measurements were performed in buffer at pH 7.4. Spectra recordings and partition coefficient measurements were performed in distilled water. The EPR spectra of all the nitroxides are represented by broadened triplet of doublets with additional large hyperfine splitting constant (hfc) of 0.26–0.3 mT on one of methylene hydrogens (Figure 1, cf. [14,15,44,45]).
Table 1.
Parameters of EPR spectra of nitroxides solutions in water, partition coefficients octanol/water and second-order rate constants for the initial rates of reduction of nitroxides 1, 22 and 28–30 (0.3–0.4 mM) with ascorbate (100–300 mM) in 50 mM phosphate buffer, pH 7.4, 25 °C in the presence of glutathione 2 mM.
Figure 1.
X-band EPR spectra of the radicals 1, 22 and 28–30 in the oxygen-free aqueous solution. Radical concentration—0.4 mM. Spectrometer settings: modulation amplitudes—0.05–0.06 mT, microwave power—5 mW, total spectrum acquisition time—2 min.
Partition coefficients of the spin probes, defined as a ratio of concentrations in octanol to water Kp, vary from 5 to 70, indicating that the nitroxides may be capable of permeating cells. The new spin probes showed rather high resistance to reduction with ascorbic acid, and the rate constants kred were similar to that of 1 [9,14].
Low rates of reduction of 2,2,5,5-tetraethyl-substituted pyrrolidine nitroxides with biogenic reductants make them promising probes for biomedical research using magnetic resonance techniques. However, enzymatic reduction of nitroxides inside cells can make a major contribution to their decay in tissues; therefore, the relative stability of the spin probes in tissues may differ from that in ascorbate system [7]. To estimate the stability of new spin probes in living systems, we investigated their decay in blood and homogenates of brain, kidney and liver. The concentration of biogenic reductants in blood plasma is relatively low, not exceeding 100 μM for ascorbate [46] and GSH [47]. The main cellular component of blood, erythrocytes, is characterized by low metabolic activity and does not contain mitochondria. The kidney and liver are the main organs that perform removal of exogenous substances from blood, and this function requires high content of redox enzymatic systems and cellular antioxidants. Moreover, the liver is extremely rich in mitochondria due to its critical metabolic function in the body, with each hepatocyte containing 1000–2000 mitochondria [48], while mitochondrion content in different parts of the kidney varies within a broad range [49]. The brain (central nervous system) has an extraordinarily high metabolic rate, because neurons need large amounts of ATP for the maintenance of ionic gradients across the cell membranes and for neurotransmission, and most neuronal ATP is generated by oxidative metabolism in mitochondria [50].
For this model study, we chose 22 and 28. The former one has a basic primary amino group which mainly exists in protonated cationic form at physiological pH. This nitroxide is the most hydrophilic and should have the lowest permeability into cells and mitochondria. The latter, 28, has somewhat higher Kp, but it has lipophilic triphenylphosphonium cationic group. Similar compounds are capable of permeation through cellular membranes and accumulate in cells and mitochondria via a transmembrane potential-driven mechanism [37].
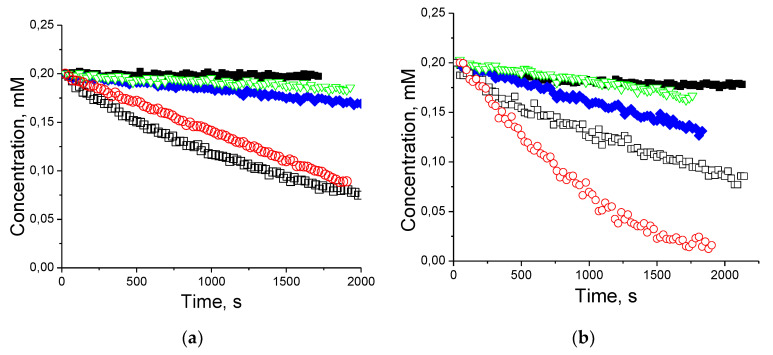
Both radicals showed high stability in blood, where the content of reducing agents is low (Figure 2). No decay of 22 was observed in 30 min, while concentration of 28 underwent ca. 10% decrease and plateaus in 10 min. A somewhat faster decay of 28 may be the result of its reaction with reductants inside the blood cells.
Figure 2.
The decay of nitroxides 22 (a) and 28 (b) in murine blood (■), and in homogenates of the brain (♦), kidney (□), liver (○) and heart (▽).
The tissue homogenates were prepared from organs frozen in liquid nitrogen. This method implies disruption of cellular membranes; however, small organelles, such as mitochondria, may remain intact. Therefore, the reduction of nitroxides in tissue homogenates should be caused both by cellular reductants and enzymatic systems released into solution after partial destruction of membranes, as well as by intact mitochondria.
The EPR measurements revealed a drastic difference in the observed kinetics of the nitroxides decay in homogenates of different organs. Both nitroxides expectedly showed the fastest decay in homogenates of liver and kidney, slower decay in brain and minor decay in heart muscle (Figure 2). Faster decay of 28 as compared to 22 in homogenates of brain and liver presumably result from targeted accumulation of the former in the remaining intact mitochondria. Taking into account the ascorbate content in tissues [46] and reduction rate constant shown in the Table 1, nearly 50% decay of 22 in half an hour cannot be explained by non-enzymatic nitroxide reduction. The recent study by Babić et al. showed that NADPH-dependent destruction by cytochrome P450 may be a major pathway of sterically shielded piperidine nitroxides decay in the presence of hepatic microsomal fraction [51]. The behavior observed here for 22 and 28 is in agreement with the above-mentioned finding. Indeed, cytochromes P450 are expressed in the liver and other organs, including the brain [52] and kidney [53], where they can contribute significantly to local metabolism. In contrast, muscles are known to have low level of xenobiotic metabolism activity [54]. Moreover, P450s are expressed in mitochondria too [55]; therefore, the accumulation of 28 in mitochondria may result in faster decay of this nitroxide compared to 22.
3. Materials and Methods
The nitrone 5 was prepared according to the literature protocols [15]. IR spectra were acquired on an FT-IR spectrometer in KBr and are reported in wave numbers (cm−1). Reactions were monitored by TLC carried out using UV light 254 nm, 1% aqueous permanganate and 10% solution of phosphomolibdic acid in ethanol and/or Dragendorff reagent as visualizing agents. Column chromatography was performed on silica gel 60 (70−230 mesh). 1H NMR spectra were recorded at 300, 400 or 500 MHz, and 13C NMR spectra were recorded at 75, 100 or 125 MHz. 1H and 13C chemical shifts (δ) were internally referenced to the residual solvent peak. For analysis and structure assignments of nitroxides, the samples were subjected to reduction with the zinc/CF3COOH system before recording of the NMR spectra as described below.
3.1. Preparation of samples for 1H NMR from nitroxides
A suspension of zinc dust (100 mg) in a solution of a nitroxide (10–15 mg) in CD3OD (0.4 mL) in a small glass vial was heated to reflux upon vigorous stirring and trifluoroacetic acid (0.1 mL) was added dropwise. The mixture was stirred for 10–15 min and the solution was transferred into an NMR tube through a pipette tip with tightly inserted paper filter. The vial was rinsed with a small portion of CD3OD or CDCl3, and this solution was filtered into the same NMR tube until the normal NMR sample volume was reached.
3.2. Synthesis
3-Carboxy-2,2,5-triethyl-3,4-dihydro-2H-pyrrole 1-oxide (8)
A 4 M solution of sodium hydroxide in water (10 mL) was added to a solution of nitrone 5 (2.85 g, 10 mmol) in methanol (15 mL). The mixture was allowed to stand at room temperature for 24 h. Then, methanol was evaporated in vacuum and water residue was cooled to 5 °C. The cold solution was neutralized with cold 1M solution of sulfuric acid in water (20 mL) and extracted with ethyl acetate (3 × 10 mL). The organic phase was dried with Na2SO4 and filtered off. The yellow solution was heated to reflux under argon for 2 h (TLC control on silica gel, ethyl acetate/methanol/acetic acid—100/10/1). After that, the red solution was allowed to stand at -20 °C for 24 h. The pink crystalline precipitate of nitrone 8 was collected (1.40 g, 65%) and used for the next step without purification. Nitrone 8 was recrystallized from THF for characterization: colorless crystals, m.p. 145–146 °C. IR (KBr) νmax: 1697 (C=O). 1H NMR (400 MHz; CDCl3, δ): 0.70 (t, Jt = 7.4 Hz, 3H), 0.83 (t, Jt = 7.4 Hz, 3H), 1.08 (t, Jt = 7.7 Hz, 3H), 1.72–1.90 (m, 3H), 1.99 (dt, Jd= 14.3 Hz, Jt = 7.4 Hz, 1H), 2.49 (dt, Jd= 15.8 Hz, Jt = 7.7 Hz, 1H), 2.64 (dt, Jd = 15.8 Hz, Jt = 7.7 Hz, 1H), 2.66 (dd, Jd1 = 18.7 Hz, Jd2 = 9.6 Hz, 1H), 3.06 (dd, Jd1 = 18.7 Hz, Jd2 = 8.9 Hz, 1H), 3.19 (dd, Jd1= 9.6 Hz, Jd2= 8.9 Hz, 1H), 13.26 (br, 1H). 13C{1H} NMR (100 MHz, CDCl3, δ): 7.3, 7.9, 9.3, 20.2, 27.9, 29.5, 31.4, 41.4, 81.8, 155.0, 171.9. Anal. Calcd for C11H19NO3: C, 61.95; H, 8.98; N, 6.57. Found: C, 62.19; H, 8.65; N, 6.62.
3-Carboxy-5-ethynyl-2,2,5-triethylpyrrolidine-1-oxyls (10a and 10b)
A powder of nitrone 8 (10.6 g, 50 mmol) was added to a 0.5–1 M solution of ethynylmagnesium bromide in THF (500 mL) upon stirring. The mixture was allowed to stand at room temperature for 24 h (TLC control on silica gel, ethyl acetate/methanol/acetic acid—100/10/1), then quenched with water and acidified with 1 M solution of sulfuric acid in water (250–500 mL) to pH 3–4. Then, organic phase was washed with brine and dried with Na2SO4 and filtered off. The THF was distilled off in vacuum and residue was dissolved in methanol (200 mL). A 1 M solution of sodium hydroxide in water (60 mL) was added to the mixture. Then, the methylene blue (6 mg, 0.02 mmol) was added to the mixture and the air was bubbled until the solution turned dark blue. The methanol was distilled off in vacuum, while the remaining aqueous solution was washed with ethyl acetate, acidified with 1 M solution of sulfuric acid in water (32 mL) to pH < 4 and extracted with ethyl acetate (3 × 50 mL). The organic phase was dried with Na2SO4 and the solvent was evaporated in vacuum. The residue was triturated with diethyl ether, and yellowish crystalline precipitate of 10a,b was collected, 7.3 g (62%) of orange crystals, m.p. 100–108 °C. IR (KBr) νmax: 3263 (≡C-H), 1730 (C=O), 2112 (C≡CH). Anal. Calcd for C13H20NO3: C, 65.52; H, 8.46; N, 5.88. Found: C, 65.66; H, 8.53; N, 5.85. 1H NMR (400 MHz; CD3OD/CDCl3, Zn/CF3COOH system δ): 10a: 0.97 (t, Jt = 7.4 Hz, 3H), 1.06 (t, Jt = 7.4 Hz, 3H), 1.16 (t, Jt = 7.3 Hz, 3H), 1.81 (dq, Jd = 14.8 Hz, Jq = 7.4 Hz, 1H), 1.86 (dq, Jd = 14.8 Hz, Jq = 7.4 Hz, 1H), 2.02–2.17(m, 3H), 2.24 (dq, Jd = 7.3 Hz, Jq = 14.2 Hz, 1H), 2.44 (dd, Jd1 = 13.9 Hz, Jd2 = 8.9 Hz, 1H), 2.61 (dd, Jd1 = 13.9 Hz, Jd2 = 7.5 Hz, 1H), 3.25 (s, 1H), 3.37 (dd, Jd1 = 8.9 Hz, Jd2 = 7.5 Hz, 1H); 10b: 2.34 (dd, Jd1 = 13.7 Hz, Jd2= 6.6 Hz, 1H), 2.85 (dd, Jd1 = 13.7 Hz, Jd2 = 10.5 Hz, 1H), 3.07 (s, 1H), 3.07 (dd, Jd1 = 10.5, Jd2 = 6.6 Hz, 1H), ratio of 10a:10b—11:1.The sample of pure 10a for X-ray analysis was prepared via slow condensation of pentane in toluene solution of 10a,b at 5 °C.
3-Carboxy-2,2,5,5-tetraethylpyrrolidine-1-oxyl (1)
The hydrogenation was performed in analogy to the earlier described procedure [12]. A solution of 10a,b (2.38 g, 10 mmol) in THF (10 mL) was placed in the reaction vessel equipped with magnetic stirrer and connection line to gasometer filled with hydrogen. The catalyst (Pd/C, 4%, 50 mg) was added and the system was purged with hydrogen and closed. The mixture was vigorously stirred until hydrogen absorption ceased (ca. 5 h, 0.6 L of hydrogen absorbed), after which the catalyst was filtered off and washed with THF. The solution was diluted with methanol (20 mL) and 1 M solution of sodium hydroxide in water (11 mL) was added to the mixture. The methylene blue (6 mg, 0.02 mmol) was added to the solution and the air was bubbled until the solution turned dark blue. The methanol was distilled off in vacuum and the remaining aqueous solution was washed with ethyl acetate. The water phase was then acidified to pH < 4 with 1 M solution of sulfuric acid in water (6 mL) and extracted with ethyl acetate (3 × 30 mL). The organic phase was dried with Na2SO4, the solvent was evaporated in vacuum and the residue was triturated with diethyl ether to give yellowish crystalline precipitate of 1 (1.9 g, 80%), orange crystals, m.p. 100–103 °C (lit. 103–106 °C [11]). IR (KBr) νmax: 1709 (C=O). Anal. Calcd for C13H24NO3: C, 64.43; H, 9.98; N, 5.78. Found: C, 64.41; H, 9.88; N, 5.78. 1H NMR (400 MHz; CD3OD/CDCl3, Zn/CF3COOH system δ): 0.96 (t, Jt = 7.4 Hz, 3H), 0.98 (t, Jt = 7.4 Hz, 3H), 0.99 (t, Jt = 7.4 Hz, 3H), 1.05 (t, Jt = 7.4 Hz, 3H), 1.72 (dq, Jd = 14.4 Hz, Jq = 7.4 Hz, 1H), 1.78–2.09 (m, 7H), 2.26 (dd, Jd1 = 14.2 Hz, Jd2 = 8.1 Hz, 1H), 2.30 (dd, Jd1 = 14.2 Hz, Jd2 = 9.3 Hz, 1H), 3.12 (dd, Jd1= 8.1 Hz, Jd2 = 9.3 Hz, 1H).
3-Carboxy-5-vinyl-2,2,5-triethylpyrrolidine-1-oxyl (14)
Isolated after hydrogenation in methanol for 3 h (ca. 60% of hydrogen absorbed) as described above for 1. 14: m.p. 80–83 °C, Anal. Calcd for C13H22NO3: C, 64.97; H, 9.23; N, 5.83. Found: C, 65.15; H, 9.34; N, 5.66. IR (KBr) νmax: 1731, 1709 (C=O); 3084 (H-C = ); 1637 (C=C). 1H NMR (400 MHz; CD3OD/CDCl3, Zn/CF3COOH system δ): 0.77 (t, Jt = 7.4 Hz, 3H), 0.84 (t, Jt = 7.4 Hz, 3H), 0.85 (t, Jt = 7.4 Hz, 3H), 1.61–2.06 (m, 6H), 2.21 (dd, Jd1 = 14.2 Hz, Jd2 = 4.5 Hz, 1H), 2.56 (dd, Jd1 = 14.2 Hz, Jd2 = 8.0 Hz, 1H), 3.06 (dd, Jd1 = 8.0 Hz, Jd2 = 4.5 Hz, 1H), 5.28 (d, Jd = 17.4 Hz, 1H), 5.36 (d, Jd = 10.8 Hz, 1H), 6.00 (dd, Jd1 = 17.4 Hz, Jd2= 10.8 Hz, 1H).
3-Carboxy-2,2,5,5-tetraethylpyrrolidine (15)
A solution of 10a,b (0.238 g, 1 mmol) in methanol (3 mL) was placed in the reaction vessel equipped with a magnetic stirrer and connection line to gasometer filled with hydrogen. The catalyst (Pd/C, 4%, 200 mg) was added and the system was purged with hydrogen and closed. The mixture was vigorously stirred until hydrogen absorption ceased (ca. 6 h). The white crystalline precipitate formed and then disappeared in the reaction mixture during the hydrogenation. The catalyst was filtered off and washed with methanol, the solvent was distilled off in vacuum affording 15, colorless crystalline powder, 215 mg (95%), m.p. 113–114 °C (EtOH-Et2O); IR (KBr) νmax: 3293, 3151 (hydrogen bonded NH, COOH), 1633, 1578 (C=O). Anal. Calcd for C13H25NO2×H2O: C, 63.64; H, 11.09; N, 5.71. Found: C, 63.38; H, 10.97; N, 5.67. 1H NMR (300 MHz; CD3OD/CDCl3, δ): 0.75–0.96 (m, 12H), 1.47–1.61 (m, 1H), 1.63–1.90 (m, 6H), 1.91–2.12 (m, 3H), 2.74 (t, Jt = 7.2 Hz, 1H). 13C{1H} NMR (125 MHz; CD3OD/CDCl3, δ): 7.6, 7.9, 8.0, 8.1, 24.8, 28.0, 28.7, 29.5, 38.7, 52.8, 69.0, 72.5, 176.4.
3-Methoxycarbonyl-2,2,5,5-tetraethylpyrrolidine-1-oxyl (16)
The solution of CH2N2 in Et2O (20 mL) was prepared form N-nitroso-N-methylurea (1.16 g, 11.3 mmol), H2O (0.5 mL) and KOH (0.95 g, 17 mmol) and added to the solution of 1 (1.36 g, 5.6 mmol) in dry Et2O (20 mL). The mixture was stirred at ambient temperature for 5 h. The solvent was evaporated in vacuum and residue was separated using column chromatography on silica gel (hexane:EtOAc—9:1, Rf = 0.35), yielding 16, 1.25 g (87%) orange oil. UV (EtOH) λmax (logε): 237 (4.94). IR (neat) νmax: 1740 (C=O). Anal. Calcd for C14H26NO3: C, 65.59; H, 10.22; N, 5.46. Found: C, 65.50; H, 10.27; N, 5.46.
1-Hydroxy-3-methoxycarbonyl-2,2,5,5-tetraethylpyrrolidine hydrochloride (17)
Method A (from 16). To the solution of 16 (170 mg, 0.66 mmol) in MeOH (1 mL), the saturated solution of dry HCl in MeOH (4 mL) was added in one portion. The mixture was allowed to stand at room temperature for 24 h until discoloration. The solvent was evaporated in vacuum and the residue was triturated with dry Et2O (5 mL). The colorless precipitate of 17 was filtered off (130 mg, 67%) and recrystallized from isopropanol, m.p. 148–150 °C (isopropanol). IR (KBr) νmax: 1737 (C=O). Anal. Calcd for C14H28ClNO3: C, 57.23; H, 9.61; Cl, 12.06; N, 4.77. Found: C, 57.09; H, 9.81; N, 4.86; Cl, 11.92. 1H NMR (300 MHz; CD3OD + CDCl3, δ): 0.83 (t, Jt = 7.4 Hz, 3H), 0.85 (t, Jt = 7.4 Hz, 3H), 0.87 (t, Jt = 7.4 Hz, 3H), 0.93 (t, Jt = 7.4 Hz, 3H), 1.28 (dq, Jd = 13.2 Hz, Jq = 7.4 Hz, 1H), 1.04–1.40 (m, 7H), 1.85 (dd, Jd1 = 13.3 Hz, Jd2 = 7.3 Hz, 1H), 2.07 (dd, Jd1 = 13.3 Hz, Jd2 = 11.5 Hz, 1H), 2.09 (dd, Jd1 = 11.5 Hz, Jd2 = 7.3 Hz, 1H), 3.67 (s, 3H). 13C{1H} NMR (75 MHz; CDCl3, δ): 7.8, 7.9, 8.1, 8.3, 8.4, 21.2, 24.2, 25.3, 25.8, 27.2, 27.9, 28.4, 28.5; 33.9, 34.1, 45.2, 47.5; 52.7, 52.6, 77.7, 77.8 79.7, 80.8, 169.1, 170.2.
Method B (from 1). To the solution of 1 (500 mg, 2.06 mmol) in MeOH (1 mL), the saturated solution of dry HCl in MeOH (7 mL) was added in one portion. The mixture was allowed to stand at room temperature for 72 h. The solvent was evaporated in vacuum and the residue was triturated with dry Et2O (5 mL). The colorless precipitate of 17 was filtered off, yield 550 mg (91%).
3-Hydroxymethyl-5-ethinyl-2,2,5-triethylpyrrolidine-1-oxyl (19a,b) mixture of isomers
The solution of 10a,b (2.38 g, 10 mmol) in THF (10 mL) was added dropwise to a stirred solution of LiAlH4 (0.57 g, 15 mmol) in THF (10 mL). The mixture was stirred at ambient temperature for 1 h, and then the flask was cooled to 5–10 °C in a cold-water bath and quenched with water. The organic phase was separated via decantation, and the remaining viscous suspension was washed with THF (3 × 50 mL). The extract was dried with Na2CO3 and filtered. The solvent was evaporated in vacuum and the residue was triturated with hexane-diethyl ether mixture and orange crystalline precipitate of 19a,b was collected, 2.12g (95%) of orange crystal, m.p. 76–77 °C (dec). IR (KBr) νmax: 3465 (O–H), 3242 (≡C-H), 2108 (C≡CH). Anal. Calcd for C13H22NO2: C, 69.61; H, 9.89; N, 6.24. Found: C, 69.33; H, 9.44; N, 5.95. 1H NMR (500 MHz; CD3OD/CDCl3, Zn/CF3COOH system δ): 0.93 (t, Jt = 7.4 Hz, 3H), 0.95 (t, Jt = 7.2 Hz, 3H), 1.11 (t, Jt = 7.4 Hz, 3H), 1.62–1.75 (m, 3H), 1.82–1.97 (m, 4H), 2.57 (tt, Jt1 = 5.1 Hz, Jt2 = 7.6 Hz, 1H), 2.62 (dd, Jd1 = 12.9 Hz, Jd2 = 7.6 Hz, 1H), 2.88 (s, 1H), 3.15 (d, Jd = 5.1 Hz, 2H).
3-Hydroxymethyl-2,2,5,5-tetraethylpyrrolidine-1-oxyl (18)
Method A. Reduction of 1 with LiAlH4 as described above afforded (18), yield 90%, orange oil, IR (neat) νmax: 3425 (O-H). Anal. Calcd for C13H26NO2: C, 68.38; H, 11.48; N, 6.13. Found: C, 68.37; H, 11.55; N, 6.45. 1H NMR (300 MHz; CD3OD/CDCl3, Zn/CF3COOH system δ): 0.92 (t, Jt = 7.4 Hz, 3H), 0.93 (t, Jt = 7.4 Hz, 3H), 0.95 (t, Jt = 7.4 Hz, 3H), 0.96 (t, Jt = 7.4 Hz, 3H), 1.64–1.95 (m, 9H), 2.24 (dd, Jd1 = 13.5, Jd2 = 7.0 Hz, 1H), 2.41 (dddd, Jd1 = 10.2 Hz, Jd2 = 7.3 Hz, Jd3 = 6.9 Hz, Jd4 = 5.5 Hz, 1H), 3.57 (dd, Jd1 = 10.9 Hz, Jd2 = 7.3 Hz, 1H), 3.70 (dd, Jd1 = 10.9 Hz, Jd2 = 5.5 Hz, 1H).
Method B. Hydrogenation of 19a,b was carried out according to the procedure described above for 1. The mixture of the nitroxide (2.0 g, 9 mmol), catalyst (Pd/C, 4%, 50 mg) and methanol (10 mL) was stirred in hydrogen atmosphere. When hydrogen absorption ceased (ca. 0.6 L of hydrogen absorbed), the catalyst was filtered off, and the air was bubbled through the solution. The methanol was distilled off in vacuum and residue was dissolved in 50 mL of ethyl acetate. The organic phase was washed with 0.1 M sulfuric acid solution (3 × 30 mL). The organic phase was separated and was dried with Na2CO3 and filtered off. The solvent was evaporated to give 18, 1.9 g (93%).
3-Bromomethyl-2,2,5,5-tetraethylpyrrolidine-1-oxyl (20a)
The solution of PPh3 (0.6 g, 2.3 mmol) in dry CH2Cl2 (2 mL) was added dropwise to a stirred cold solution of 18 (0.45 g, 2 mmol) and CBr4 (0.73 g, 2.2 mmol) in dry CH2Cl2 (2 mL). The mixture was stirred at ambient temperature for 1 h. The solvent was evaporated in vacuum and residue was separated using column chromatography on silica gel (hexane:EtOAc—13:1), yielding 20a: 0.45 g (78%) of orange oil, IR (neat) νmax: 648, 594 (C-Br). Anal. Calcd for C13H25NOBr: C, 53.61; H, 8.65; N, 4.81; Br, 27.44. Found: C, 53.20; H, 8.87; N, 4.74; Br, 27.24.
3-Mesyloxymethyl-2,2,5,5-tetraethylpyrrolidine-1-oxyl (20b)
The solution of DIPEA (0.8 g, 6.2 mmol) in dry CHCl3 (5 mL) was added dropwise to a stirred mixture of the nitroxide 18 (1.33 g, 5.8 mmol) and MsCl (0.7 g, 6.1 mmol). The mixture was stirred at ambient temperature for 2 h, then washed with water (3 × 30 mL). The organic phase was separated and was dried with MgSO4 and filtered, the solvent was evaporated and residue was separated using column chromatography on silica gel (chloroform), yielding 20b: 1.35 g (76%), orange oil, IR (neat) νmax: 1357, 1176 (S=O). Anal. Calcd for C14H28NO4S: C, 54.87; H, 9.21; N, 4.57; S, 10.46. Found: C, 54.40; H, 9.02; N, 4.33; S, 10.03. 1H NMR (300 MHz; CD3OD/CDCl3, Zn/CF3COOH system δ): 0.96 (t, Jt = 7.4 Hz, 3H), 0.97 (t, Jt = 7.4 Hz,3H), 1.00 (t, Jt=7.4 Hz, 3H), 1.01 (t, Jt=7.4 Hz, 3H), 1.68–2.07 (m, 9H), 2.31 (dd, Jd1=13.3 Hz, Jd2=6.9 Hz,1H), 2.72 (dddd, Jd1=10.7 Hz, Jd2=7.4 Hz, Jd3=6.9 Hz, Jd4=6.5 Hz, 1H), 3.11 (s, 3H), 4.24 (dd, Jd1=10.0 Hz, Jd2= 7.4 Hz, 1H), 4.37 (dd, Jd1 = 10.0 Hz, Jd2 = 6.5 Hz, 1H).
3-Azidomethyl-2,2,5,5-tetraethylpyrrolidine-1-oxyl (21).
The mixture of the nitroxide 20a or 20b (16.5 mmol), NaN3 (5.4 g, 82 mmol) and DMSO (100 mL) was stirred at 60 °C for 30 h. The mixture was diluted with water (100 mL) and was extracted with hexane (3 × 50 mL). The organic phase was separated and was dried with Na2CO3 and filtered off. The solvent was evaporated and residue was separated using column chromatography on silica gel (hexane:EtOAc—13:1), yielding 21: 4.0 g (97%), orange oil, IR (neat) νmax: 2098 (N3). Anal. Calcd for C13H25N4O: C, 61.63; H, 9.95; N, 22.11. Found: C, 62.10; H, 9.95; N, 22.01.
3-Aminomethyl-2,2,5,5-tetraethylpyrrolidine-1-oxyl (22)
Method A. The solution of nitroxide 21 (4.0 g, 16 mmol) in THF (20 mL) was added dropwise to a stirred mixture of LiAlH4 (0.9g, 24 mmol) in THF (50 mL). The mixture was stirred at ambient temperature for 1 h, and then the flask was placed into a cold-water bath and quenched with water. The organic phase was separated via decantation, and the remaining viscous suspension was washed with THF (3 × 30 mL); the extract was dried with Na2CO3 and filtered. The solvent was evaporated, yielding 22, 3.24 g (90%), orange oil, IR (neat) νmax: 3377 (N-H). HRMS (EI/DFS) m/z: [M]+calcd for C13H27N2O 227.2118, found 227.2116. 1H NMR (300 MHz; CD3OD/CDCl3, Zn/CF3COOH system δ): 0.92 (t, Jt = 7.4 Hz, 3H), 0.94 (t, Jt = 7.4 Hz, 3H), 0.97 (t, Jt = 7.4 Hz, 3H), 0.98 (t, Jt = 7.4 Hz, 3H), 1.60–2.03 (m, 9H), 2.40 (dd, Jd1 = 13.3 Hz, Jd2 = 6.6 Hz, 1H), 2.66 (dddd, Jd1 = 11.9 Hz, Jd2 = 11.1 Hz, Jd3 = 6.6 Hz, Jd4 = 3.4 Hz, 1H), 2.98 (t, Jt = 11.9 Hz, 1H), 3.17 (dd, Jd1 = 12.0 Hz, Jd2 = 3.4 Hz, 1H).
Method B. Triphenylphosphine (0.45 g, 1.7 mmol) was added to the solution of azide 21 (0.23 g, 0.9 mmol) in dry diethyl ether. The reaction mixture was stirred and heated under reflux for 2 h. The solvent was evaporated under reduced pressure and 50% aqueous ethanol (10 mL) was added to the residue. The resulted mixture was heated under reflux with stirring for 8 h. The ethanol was distilled off under reduced pressure and the water layer was acidified with 3% aqueous hydrochloric acid to pH = 3 and extracted with chloroform (5 × 5 mL). The water layer was basified with sodium carbonate to pH ~ 9–10, extracted with chloroform (5 × 5 mL), and the extract was dried with sodium carbonate, and solvent was evaporated under reduced pressure. The residue was purified by column chromatography on Al2O3 (eluent—CHCl3) to give 22 (0.17 g, 86%).
3-Maleimidomethyl-2,2,5,5-tetraethylpyrrolidine-1-oxyl (24)
The solution of maleic anhydride (0.2 g, 2 mmol) in chloroform (10 mL) was added dropwise to a stirred solution of the nitroxide 22 (0.34 g, 1.5 mmol) in chloroform (10 mL), and stirred at ambient temperature for 24 h. Then, the NaOAc (0.5, 6 mmol) and Ac2O (10.4 g, 102 mmol) were added and the mixture was stirred in an oil bath at 110 °C for 3 h. The reaction mixture was concentrated in vacuum and the residue was subjected to column chromatography on silica gel (chloroform), yielding 24: 0.24 g (52%), orange crystals, m.p. 83–84 °C (from hexane). IR (KBr) νmax: 1701 (C=O). Anal. Calcd for C17H27N2O3: C, 66.42; H, 8.85; N, 9.11. Found: C, 66.69; H, 9.25; N, 9.08.
3-Bromoacetamidomethyl-2,2,5,5-tetraethylpyrrolidine-1-oxyl (25)
A mixture of amine 22 (0.11 g, 0.5 mmol) with DIPEA (97 μL, 0.6 mmol) in dry CHCl3 was cooled to 0 °C in an ice bath. After that, a solution of bromoacetyl bromide (46 μL, 0.5 mmol) in dry CHCl3 was added dropwise, and the reaction mixture was allowed to stand at room temperature for 3 h. The solution was carefully washed with water three times and dried with Na2SO4. The solvent was removed under reduced pressure and the residue was subjected to column chromatography on silica gel, eluent CHCl3 afforded 25 (0.13 g, 79%) as an orange oil. IR (neat) νmax: 1658 (C=O), 1552 (N-H). HRMS (EI/DFS) m/z [M]+calcd for C15H28BrN2O2 347.1329, found 347.1333.
3-Chloroacetamidomethyl-2,2,5,5-tetraethylpyrrolidine-1-oxyl (26)
The solution of 2-chloroacetyl chloride (0.27 g, 2.4 mmol) in CHCl3 (10 mmol) was added dropwise to a stirred cold solution of the nitroxide 22 (0.50 g, 2.2 mmol) and DIPEA (0.43 g, 3.3 mmol) in CHCl3 (20 mL). The mixture was stirred at ambient temperature for 1 h. The mixture was washed with water (3 × 20 mL). The organic phase was separated and was dried with Na2SO4 and filtered off. The solvent was evaporated and residue was separated using column chromatography on silica gel (hexane:EtOAc—1:1), yielding 26: 0.64 g (88%) of orange oil, IR (neat) νmax: 1666 (C=O). Anal. Calcd for C15H28N2O2Cl: C, 59.29; H, 9.29; N, 9.22; Cl, 11.67. Found: C, 59.36; H, 9.12; N, 8.75; Cl, 11.81.
3-Iodoacetamidomethyl-2,2,5,5-tetraethylpyrrolidine-1-oxyl (27)
The mixture of 26 (0.64 g, 2.1 mmol) and NaI (1 g, 6.3 mmol) in acetone (15 mL) was stirred at ambient temperature for 48 h. The precipitate was filtered off. The solvent was evaporated and the residue was diluted with water (20 mL) and extracted with diethyl ether (3 × 20 mL). The organic phase was separated and dried with MgSO4. The solvent was evaporated in vacuum and the residue was triturated with hexane, and precipitate of 27 was collected, 0.48 g (58%), orange crystals, m.p. 83.9 °C (dec, from diethyl ether). IR (KBr) νmax: 1643 (C=O). Anal. Calcd for C15H28N2O2I: C, 45.58; H, 7.14; N, 7.09; I, 32.10. Found: C, 45.30; H, 7.06; N, 6.85; I, 32.21. HRMS (EI/DFS) m/z: [M]+calcd for C15H28N2O2127I 395.1190, found 395.1194.
3-(2-Triphenylphosphonio)acetamidomethyl-2,2,5,5-tetraethylpyrrolidine-1-oxyl chloride (28)
A mixture of 29 (0.305 g, 1 mmol), triphenylphosphine (0.6 g, 2.3 mmol) and toluene (5 mL) was heated to reflux under nitrogen for 5 h and then left at 50 °C overnight. The solvent was distilled off in vacuum and the residue was purified using column chromatography (silica gel, eluent chloroform:ethanol—50:1). The collected fractions of 30 were concentrated in vacuum, while the residue was dissolved in a mixture of diethyl ether and benzene 1:1 and left at 10 °C for crystallization. The precipitate of 30 monohydrate was filtered off, washed with cold diethyl ether and dried. The yield was 0.240 g (41%). Pale yellowish crystals m.p. 108 °C (dec.). IR (KBr) νmax: 1670 (C=O). Anal. Calcd for C15H28N2O2I: C, 67.85; H, 7.76; N, 4.80; Cl, 6.07; P, 5.30. Found: C, 67.64; H, 8.04; N, 4.50; Cl, 6.01; P, 5.16. 1H NMR (after reduction with Zn/CF3COOH) (400 MHz; CD3OD-CF3COOH, δ): 0.80 (m, 12H), 1.54 (m, 5H), 1.68 (m, 4H), 1.95 (dd Jd1 = 7 Hz, Jd2 = 13.5 Hz, 1H), 2.27 (m, 1H), 3.00 (dd Jd1 = 13.5 Hz, Jd2 = 10.5 Hz, 1H) 3.18 (dd Jd1 = 13.5 Hz, Jd2 = 4 Hz, 1H), 4.50 (m, partly exchanged), 7.59 (m, 12H), 7.71 (m, 3H).
3-(2-(Pyrrolidin-1-yl)acetamidomethyl)-2,2,5,5-tetraethylpyrrolidine-1-oxyl (29)
A mixture of 27 (0.11 g, 0.3 mmol) and pyrrolidine (0.1 g, 1.4 mmol) in dry chloroform (10 mL) was stirred at ambient temperature for 1 h. The mixture was washed with water (3 × 20 mL). The organic phase was separated and was dried with Na2CO3 and filtered off. The solvent was evaporated and residue was separated using column chromatography on silica gel (methanol:EtOAc—1:9), yielding 29, 0.09 g (95%) orange oil. IR (neat) νmax: 1662 (C=O). HRMS (EI/DFS) m/z: [M]+calcd for C19H36N3O2 338.2802, found 338.2798. 1H NMR (300 MHz; CD3OD/CDCl3, Zn/CF3COOH system): 0.90 (t, Jt = 7.4Hz, 3H), 0.92 (t, Jt = 7.4 Hz, 3H), 0.97 (t, Jt = 7.4 Hz, 3H), 0.99 (t, Jt= 7.4 Hz, 3H), 1.62–1.97 (m, 14H), 2.38–2.54 (m, 1H), 2.94–3.25 (m, 3H), 3.39–3.51 (m, 1H), 3.59–3.81 (m, 1H), 3.97 (s, 2H).
3-((Piperidin-1-yl)methyl)-2,2,5,5-tetraethylpyrrolidine-1-oxyl (30)
The mixture of amine 22 0.20 g, 0.9 mmol), 1,5-dibromopentane (0.24 g, 1.1 mmol) and potassium carbonate (0.62 g, 4.5 mmol) in acetonitrile (10 mL) was stirred at 60 °C. The precipitate was filtered off. The solvent was evaporated and the residue was separated using column chromatography on silica gel (hexane:EtOAc—1:1), yielding 33, 0.15 g (60%), orange oil. HRMS (EI/DFS) m/z: [M]+calcd for C18H35N2O 295.2744, found 295.2741. IR (neat) νmax: 1461 (C-H). 1H NMR (300 MHz; CD3OD/CDCl3, Zn/CF3COOH system δ): 0.91 (t, Jt = 7.4 Hz, 3H), 0.92 (t, Jt = 7.4 Hz, 3H), 0.96 (t, Jt = 7.4 Hz, 3H), 0.98 (t, Jt = 7.4 Hz, 3H), 1.36–1.53 (br, 1H), 1.61–1.98 (m, 14H), 2.40 (dd, Jd1 = 13.6 Hz, Jd2= 6.7 Hz, 1H), 2.70–2.92 (m, 3H), 3.10 (dd, Jd1 = 13.2 Hz, Jd2 = 2.3 Hz, 1H), 3.21 (dd, Jd1 = 13.2 Hz, Jd2 = 11.1 Hz, 1H), 3.50–3.67 (m, 2H).
3-(Dimethylaminomethyl)-2,2,5,5-tetraethylpyrrolidine-1-oxyl (31)
The mixture of amine 22 (0.15 g, 0.6 mmol), 20% formaldehyde water solution (0.5 mL, 3.3 mmol) and formic acid (0.308 g, 6.7 mmol) was stirred at 50 °C for 4 h. The mixture was diluted with sodium hydrocarbonate solution (10 mL) and was extracted with ether (3 × 10 mL). The organic phase was separated and dried with Na2CO3 and filtered off. The solvent was evaporated and residue was separated using column chromatography on silica gel (methanol:EtOAc—1:9), yielding 31, 0.12g (70%). HRMS (EI/DFS) m/z: [M]+ calcd for C15H31N2O 255.2431, found 255.2433. IR (neat) νmax: 1461 (C-H).HRMS (EI/DFS) m/z: [M]+calcd for C15H31N2O 255.2431, found 255.2433., 1H NMR (300 MHz; CD3OD/CDCl3, Zn/CF3COOH system δ): 0.93 (t, Jt = 7.4 Hz, 3H), 0.94 (t, Jt = 7.4 Hz, 3H), 0.98 (t, Jt = 7.4 Hz, 3H), 1.00 (t, Jt = 7.4 Hz, 3H), 1.65–2.01 (m, 9H), 2.39 (dd, Jd1 = 13.4 Hz, Jd2 = 6.3 Hz, 1H), 2.64–2.77 (m, 1H), 2.92 (s, 6H), 3.22 (dd, Jd1 = 12.8 Hz, Jd2 = 2.7 Hz, 1H), 3.32 (dd, Jd1 = 12.8 Hz, Jd2 = 11.2 Hz, 1H).
3.3. EPR Measurements and Kinetics
The EPR spectra in water were recorded on a Bruker ER-200D-SRC spectrometer in 50 µL glass capillary for 0.2–0.4 mM radical solutions degassed via bubbling with argon. Spectrometer settings: frequency, 9.87 GHz; microwave power, 5.0 mW; modulation amplitude, 0.05 mT; time constant, 50–100 ms; and conversion time, 5.12 ms. For kinetic measurements in water, stock solutions of nitroxide, ascorbic acid and glutathione in phosphate buffer (5 mM, pH 7.4) were prepared, and pH was adjusted to 7.4 with NaOH or HCl. All the solutions were deoxygenated with argon, carefully and quickly mixed in a small tube to attain final concentrations (nitroxide, 0.2–04 mM; GSH, 2 mM; and ascorbate, 100–300 mM) and were placed into an EPR capillary (50 μL). The capillary was sealed on both sides and placed into the EPR resonator. The decay of amplitude of the low-field component of the EPR spectrum was followed to obtain the kinetics. The initial part of the decay curves (up to 200 min) was used for fitting. Kinetics of the decay was fitted to a monoexponential function to calculate the first-order rate constants. Then, these constants were divided by the concentration of ascorbic acid to calculate the second-order reaction constants (Figure S3). Partition coefficients in a water–octanol mixture were estimated from the amplitudes of the EPR spectra of a nitroxide in water after extensive shaking with different portions of added octanol and separation using centrifugation.
The measurements of kinetics of nitroxide decay in blood and tissue homogenates were performed using an Elexsys E540 X-band spectrometer in a 50 µL glass capillary for 0.2 mM solutions, with the following spectrometer settings: frequency, 9.87 GHz; centerfield, 350.6 mT; sweep range, 10 mT; microwave power, 2.0 mW; modulation amplitude, 0.1 mT; time constant, 10.24 ms; and conversion time, 20.48 ms.
The stock solutions of the nitroxides 25 and 31 in water (1 mM) were prepared, and pH was adjusted to 7.0 with NaOH. A total of 2 μL of the nitroxide solution was added to 98 μL of the blood or tissue homogenate, giving a nitroxide concentration equal to 0.2 mM. The resulting mixture of nitroxide with blood or tissue homogenate was transferred to the glass capillary (50 μL) and the latter was placed into the EPR resonator. The decay of the double integral of the EPR spectrum was followed to obtain the kinetics.
3.4. Animals
Male five-month-old C57BL/6 mice were obtained from the Breeding Experimental Animal Laboratory of the Institute of Cytology and Genetics (ICG), Siberian Division of the Russian Academy of Sciences (Novosibirsk, Russia). All the experiments were carried out according to Animal Care Regulations of the Institute of Cytology and Genetics (ICG), Novosibirsk. Animals were sacrificed by decapitation, blood was collected into a microcentrifuge tube with heparin (final concentration 5%), gently rocked by hand and snap-frozen with liquid nitrogen immediately after collection. Organ tissue (brain, heart, liver and kidney) was excised on ice, rinsed with ice-cold 10 mM PBS (pH 7.4), sheared with scissors and homogenized in PBS (1/1 by weight) on ice using electric homogenizer fitted with a Teflon head. The homogenates were then additionally mixed with PBS (1/1 by weight, final dilution 1/3) to decrease viscosity for further aspiration into glass capillaries, and snap-frozen in liquid nitrogen. Before measurement, 10 mM aqueous solutions of the nitroxide probes were prepared ex tempore, adjusted to physiological pH (7.0) with NaOH and mixed with thawed homogenates (final concentration 0.2 mM). Homogenates were then loaded into 50 μL glass capillaries, which were then sealed with plasticine and inserted into the chamber of the EPR spectrometer. Time elapsed between thawing and beginning of the recording was 2 min.
4. Conclusions
Here, we again demonstrated the benefits of previously suggested strategy [15] for the synthesis of sterically shielded pyrrolidine nitroxides. The general approach implies assembling of 2,5,5-substituted pyrrolidine derivatives, subsequent oxidation to nitrone and addition of organometallic reagent. The use of ethynylmagnesium bromide in this reaction allowed to retain carboxylic group and to prepare 3-carboxy-2,2,5,5-tetraethylpyrrolidine-1-oxyl (1) with much better overall yield as compared to literature data. This method was used to prepare a set of new spin labels and probes, which showed higher resistance to reduction with ascorbate than 1. Fast decay of the new spin probes in homogenates of liver and kidney and slow decay in homogenate of heart implies possible participation of cytochrome P450 in nitroxide decay, in analogy to the mechanism suggested by Babić et al. [51].
5. Patents
Part of this work is protected with a Russian patent [16].
Acknowledgments
We thank the personnel of the Multi-Access Center of SB RAS for recording of IR, UV, NMR and HRMS spectra and for performing X-ray and elemental analyses.
Supplementary Materials
IR, UV and NMR spectra, HPLC, X-ray analysis and kinetics measurement data are available online at https://www.mdpi.com/article/10.3390/molecules26195761/s1.
Author Contributions
Synthesis, S.A.D., M.S.U., I.F.Z., D.A.M. and Y.F.P.; EPR measurements: Y.I.G. and D.A.P.; analysis: E.I.C.; X-ray analysis, Y.V.G.; animal handling, M.A.T.; writing, S.A.D. and I.A.K.; supervision, E.G.B. and I.A.K. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Russian Foundation for Basic Research (18-53-76003 within the framework of the ERA.Net RUS+ project ST2017-382: NanoHyperRadicals) (Synthesis, spectra and kinetic measurements in model systems) and partly by the Ministry of Science and Higher Education of Russia (project number 14.W03.31.0034) (kinetics in blood and tissues).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article or supplementary materials.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds 1, 8, 16, 18, 24, 25, 30 and 31 are available from the authors.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hubbell W.L., López C.J., Altenbach C., Yang Z. Technological advances in site-directed spin labelling. Curr. Opin. Struct. Biol. 2013;23:725–733. doi: 10.1016/j.sbi.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roser P., Schmidt M.J., Drescher M., Summerer D. Site-directed spin labeling of proteins for distance measurements in vitro and in cells. Org. Biomol. Chem. 2016;14:5468–5476. doi: 10.1039/C6OB00473C. [DOI] [PubMed] [Google Scholar]
- 3.Endeward B., Marko A., Denysenkov V.P., Sigurdsson S.T., Prisner T.F. Advanced EPR methods for studying conformational dynamics in nucleic acids. Methods Enzymol. 2015;564:403–425. doi: 10.1016/bs.mie.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 4.Bonucci A., Ouari O., Guigliarelli B., Belle V., Mileo E. In-cell EPR: Progress towards structural studies inside cells. ChemBioChem. 2019;21:451–460. doi: 10.1002/cbic.201900291. [DOI] [PubMed] [Google Scholar]
- 5.Widder P., Schuck J., Summerer D., Drescher M. Combining site-directed spin labeling in vivo and in-cell EPR distance determination. Phys. Chem. Chem. Phys. 2020;22:4875–4879. doi: 10.1039/C9CP05584C. [DOI] [PubMed] [Google Scholar]
- 6.Jugniot N., Duttagupta I., Rivot A., Massot P., Cardiet C., Pizzoccaro A., Jean M., Vanthuyne N., Franconi J.-M., Voisin P., et al. An elastase activity reporter for Electronic Paramagnetic Resonance (EPR) and Overhauser-enhanced Magnetic Resonance Imaging (OMRI) as a lineshifting nitroxide. Free. Radic. Biol. Med. 2018;126:101–112. doi: 10.1016/j.freeradbiomed.2018.08.006. [DOI] [PubMed] [Google Scholar]
- 7.Kocherginsky N., Swartz H. Nitroxide Spin Labels—Reactions in Biology and Chemistry. CRC Press; Boca Raton, FL, USA: 1995. [Google Scholar]
- 8.Marx L., Chiarelli R., Guiberteau T., Rassat A. A comparative study of the reduction by ascorbate of 1,1,3,3-tetraethylisoindolin-2-yloxyl and of 1,1,3,3-tetramethylisoindolin-2-yloxyl. J. Chem. Soc. Perkin Trans. 1. 2000;2000:1181–1182. doi: 10.1039/b001891k. [DOI] [Google Scholar]
- 9.Jagtap A.P., Krstic I., Kunjir N.C., Hänsel R., Prisner T.F., Sigurdsson S.T. Sterically shielded spin labels for in-cell EPR spectroscopy: Analysis of stability in reducing environment. Free Radic. Res. 2014;49:78–85. doi: 10.3109/10715762.2014.979409. [DOI] [PubMed] [Google Scholar]
- 10.Wang Y., Paletta J.T., Berg K., Reinhart E., Rajca S., Rajca A. Synthesis of unnatural amino acids functionalized with sterically shielded pyrroline nitroxides. Org. Lett. 2014;16:5298–5300. doi: 10.1021/ol502449r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karthikeyan G., Bonucci A., Casano G., Gerbaud G., Abel S., Thomé V., Kodjabachian L., Magalon A., Guigliarelli B., Belle V., et al. A bioresistant nitroxide spin label for in-cell EPR spectroscopy: In vitro and in oocytes protein structural dynamics studies. Angew. Chem. 2018;130:1380–1384. doi: 10.1002/ange.201710184. [DOI] [PubMed] [Google Scholar]
- 12.Bleicken S., Assafa T.E., Zhang H., Elsner C., Ritsch I., Pink M., Rajca S., Jeschke G., Rajca A., Bordignon E. gem-Diethyl pyrroline nitroxide spin labels: Synthesis, EPR characterization, rotamer libraries and biocompatibility. ChemistryOpen. 2019;8:1057–1065. doi: 10.1002/open.201900119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kirilyuk I.A., Polienko Y.F., Krumkacheva O.A., Strizhakov R.K., Gatilov Y.V., Grigor’ev I.A., Bagryanskaya E.G. Synthesis of 2,5-bis(spirocyclohexane)-substituted Nitroxides of Pyrroline and Pyrrolidine series, including Thiol-specific spin label: An analogue of MTSSL with long relaxation time. J. Org. Chem. 2012;77:8016–8027. doi: 10.1021/jo301235j. [DOI] [PubMed] [Google Scholar]
- 14.Paletta J.T., Pink M., Foley B., Rajca S., Rajca A. Synthesis and reduction kinetics of sterically shielded pyrrolidine nitroxides. Org. Lett. 2012;14:5322–5325. doi: 10.1021/ol302506f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dobrynin S.A., Glazachev Y.I., Gatilov Y.V., Chernyak E.I., Salnikov G.E., Kirilyuk I.A. Synthesis of 3,4-bis(hydroxymethyl)-2,2,5,5-tetraethylpyrrolidin-1-oxyl via 1,3-dipolar cycloaddition of azomethine ylide to activated alkene. J. Org. Chem. 2018;83:5392–5397. doi: 10.1021/acs.joc.8b00085. [DOI] [PubMed] [Google Scholar]
- 16.Dobrynin S.A., Khoroshunova Y.V., Kirilyuk I.A. Method of Producing 2,2,5,5-Tetramethyl-3-Carboxypyrrolidine-1-Oxyl. No. 2702331. RU Patent. 2019 June 25;
- 17.Lampp L., Morgenstern U., Merzweiler K., Imming P., Seidel R.W. Synthesis and characterization of sterically and electrostatically shielded pyrrolidine nitroxide radicals. J. Mol. Struct. 2019;1182:87–94. doi: 10.1016/j.molstruc.2019.01.015. [DOI] [Google Scholar]
- 18.Baracz N.M., Hankovszky O.H., Sar S.P., Jerkovich G., Hideg K. Synthesis of alkynyl-substituted pyrrolidin-1-yloxyl radicals from 1-pyrroline n-oxide nnitrones and alkynylmagnesium bromides. Synthesis. 1996;1996:204–208. doi: 10.1055/s-1996-4199. [DOI] [Google Scholar]
- 19.Sár C.P., Jekö J., Fajer P., Hideg K. Synthesis and reactions of new alkynyl substituted nitroxide radicals. Synthesis. 1999;1999:1039–1045. doi: 10.1055/s-1999-3503. [DOI] [Google Scholar]
- 20.Sar S.P., Osz E., Jeko J., Hideg K. Synthesis of spiro[pyrolidine-2,2-adamantane] nitrones and nitroxides. Synthesis. 2005;2005:255–259. doi: 10.1055/s-2004-834937. [DOI] [Google Scholar]
- 21.Hideg K., Balog M., Abé C., Kálai T., Steinhoff H.-J., Jekő J. Synthesis of new paramagnetic fatty acids and lipophilic spin labels. Synthesis. 2007;2007:1663–1670. doi: 10.1055/s-2007-966067. [DOI] [Google Scholar]
- 22.Rey P., Ramasseul R., Rassat A. A useful protecting group in the preparation of amino-nitroxides. J. Chem. Soc. Chem. Commun. 1976;3:83–84. doi: 10.1039/C39760000083. [DOI] [Google Scholar]
- 23.Sosnovsky G., Cai Z. A study of the Favorskii rearrangement with 3-bromo-4-oxo-2,2,6,6-tetramethylpiperidine-1-oxyl. J. Org. Chem. 1995;60:3414–3418. doi: 10.1021/jo00116a029. [DOI] [Google Scholar]
- 24.Polienko Y.F., Kuprikova N.M., Parkhomenko D.A., Gatilov Y.V., Chernyak E.I., Kirilyuk I.A. Synthesis of 2,5-bis(spirocyclohexane)-substituted nitroxides: New spin labeling agents. Tetrahedron. 2020;81:131915. doi: 10.1016/j.tet.2020.131915. [DOI] [Google Scholar]
- 25.Yan’shole V.V., Kirilyuk I.A., Grigor’ev I.A., Morozov S.V., Tsentalovich Y.P. Antioxidative properties of nitroxyl radicals and hydroxyamines in reactions with triplet and deaminated kynurenine. Russ. Chem. Bull. 2010;59:66–74. doi: 10.1007/s11172-010-0046-y. [DOI] [Google Scholar]
- 26.Chalmers B.A., Morris J.C., Fairfull-Smith K.E., Graingerb R.S., Bottle S.E. A novel protecting group methodology for syntheses using nitroxides. Chem. Commun. 2013;49:10382–10384. doi: 10.1039/C3CC46146G. [DOI] [PubMed] [Google Scholar]
- 27.Murayama K., Yoshioka T. Studies on stable free radicals. III. Reactions of stable nitroxide radicals with s-radicals derived from benzenethiols and thiamine. Bull. Chem. Soc. Jpn. 1969;42:1942–1947. doi: 10.1246/bcsj.42.1942. [DOI] [Google Scholar]
- 28.Bálint J., Kiss V., Egri G., Kálai T., Demeter Á., Balog M., Fogassya E., Hideg K. Kinetic resolution of 1-oxyl-3-hydroxymethyl-2,2,5,5-tetramethylpyrrolidine derivatives by lipase-catalyzed enantiomer selective acylation. Tetrahedron Asymmetry. 2004;15:671–679. doi: 10.1016/j.tetasy.2003.12.026. [DOI] [Google Scholar]
- 29.Úr G., Kálai T., Balog M., Bognár B., Gulyás-Fekete G., Hideg K. Synthesis of new pyrroline nitroxides with ethynyl functional group. Synth. Commun. 2015;45:2122–2129. doi: 10.1080/00397911.2015.1066391. [DOI] [Google Scholar]
- 30.Barratt M.D., Davies A.P., Evans M.T.A. Maleimide and Isomaleimide Pyrrolidine-Nitroxide Spin Labels. Eur. J. Biochem. 1971;24:280–283. doi: 10.1111/j.1432-1033.1971.tb19682.x. [DOI] [PubMed] [Google Scholar]
- 31.Bacic G., Pavićević A., Peyrot F. In vivo evaluation of different alterations of redox status by studying pharmacokinetics of nitroxides using magnetic resonance techniques. Redox Biol. 2015;8:226–242. doi: 10.1016/j.redox.2015.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kosem N., Naganuma T., Ichikawa K., Morales N.P., Yasukawa K., Hyodo F., Yamada K., Utsumi H. Whole-body kinetic image of a redox probe in mice using overhauser-enhanced MRI. Free Radic. Biol. Med. 2012;53:328–336. doi: 10.1016/j.freeradbiomed.2012.04.026. [DOI] [PubMed] [Google Scholar]
- 33.Davis R.M., Mitchell J.B., Krishna M.C. Nitroxides as cancer imaging agents. Anti-Cancer Agents Med. Chem. 2011;11:347–358. doi: 10.2174/187152011795677526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Matsumoto K.-I., Hyodo F., Matsumoto A., Koretsky A.P., Sowers A.L., Mitchell J.B., Krishna M.C. High-resolution mapping of tumor redox status by magnetic resonance imaging using nitroxides as redox-sensitive contrast agents. Clin. Cancer Res. 2006;12:2455–3462. doi: 10.1158/1078-0432.CCR-05-2747. [DOI] [PubMed] [Google Scholar]
- 35.Rosen G.M., Burks S.R., Kohr M.J., Kao J.P.Y. Synthesis and biological testing of aminoxyls designed for long-term retention by living cells. Org. Biomol. Chem. 2005;3:645–648. doi: 10.1039/b415586f. [DOI] [PubMed] [Google Scholar]
- 36.Legenzov E.A., Muralidharan S., Woodcock L.B., Eaton G.R., Eaton S.S., Rosen G.M., Kao J.P.Y. Designing molecular probes to prolong intracellular retention: Application to nitroxide spin probes. Bioconjugate Chem. 2016;27:2923–2930. doi: 10.1021/acs.bioconjchem.6b00595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Murphy M.P., Smith R.A.J. Targeting antioxidants to mitochondria by conjugation to lipophilic cations. Annu. Rev. Pharmacol. Toxicol. 2007;47:629–656. doi: 10.1146/annurev.pharmtox.47.120505.105110. [DOI] [PubMed] [Google Scholar]
- 38.Zielonka J., Joseph J., Sikora A., Hardy M., Ouari O., Vasquez-Vivar J., Cheng G., Lopez M., Kalyanaraman B. Mitochondria-targeted Triphenylphosphonium-based compounds: Syntheses, mechanisms of action, and therapeutic and diagnostic applications. Chem. Rev. 2017;117:10043–10120. doi: 10.1021/acs.chemrev.7b00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lazarova D., Shibata S., Ishii I., Zlateva G., Zhelev Z., Aoki I., Bakalova R. Imaging of redox-imbalance and oxidative stress in kidney in vivo, induced by dietary cholesterol. Biotechnol. Biotechnol. Equip. 2019;33:294–301. doi: 10.1080/13102818.2019.1573153. [DOI] [Google Scholar]
- 40.Dikalova A.E., Bikineyeva A.T., Budzyn K., Nazarewicz R.R., McCann L., Lewis W., Harrison D.G., Dikalov S.I. Therapeutic targeting of mitochondrial superoxide in hypertension. Circ. Res. 2010;107:106–116. doi: 10.1161/CIRCRESAHA.109.214601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dikalova S.I., Kirilyuk I.A., Dikalova A.E. Antihypertensive effect of mitochondria-targeted proxyl nitroxides. Redox Biol. 2014;4:355–362. doi: 10.1016/j.redox.2014.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goldman S.D.B., Funk R.S., Rajewski R.A., Krise J.P. Mechanisms of amine accumulation in, and egress from, lysosomes. Bioanalysis. 2009;1:1445–1459. doi: 10.4155/bio.09.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Davis R.M., Sowers A.L., DeGraff W., Bernardo M., Thetford A., Krishna M.C., Mitchell J.B. A novel nitroxide is an effective brain redox imaging contrast agent and in vivo radioprotector. Free Radic. Biol. Med. 2011;51:780–790. doi: 10.1016/j.freeradbiomed.2011.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bobko A.A., Kirilyuk I.A., Gritsan N.P., Polovyanenko D.N., Grigor’ev I.A., Khramtsov V.V., Bagryanskaya E.G. EPR and quantum chemical studies of the pH-sensitive imidazoline and imidazolidine nitroxides with bulky substituents. Appl. Magn. Reson. 2010;39:437–451. doi: 10.1007/s00723-010-0179-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhurko I.F., Dobrynin S., Gorodetskii A.A., Glazachev Y.I., Rybalova T.V., Chernyak E.I., Asanbaeva N., Bagryanskaya E.G., Kirilyuk I.A. 2-butyl-2-tert-butyl-5,5-diethylpyrrolidine-1-oxyls: Synthesis and properties. Molecules. 2020;25:845. doi: 10.3390/molecules25040845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Iwama M., Amano A., Shimokado K., Maruyama N., Ishigami A. Ascorbic acid levels in various tissues, plasma and urine of mice during aging. J. Nutr. Sci. Vitaminol. 2012;58:169–174. doi: 10.3177/jnsv.58.169. [DOI] [PubMed] [Google Scholar]
- 47.Anderson M.E., Meister A. Dynamic state of glutathione in blood plasma. J. Biol. Chem. 1980;255:9530–9533. doi: 10.1016/S0021-9258(18)43421-7. [DOI] [PubMed] [Google Scholar]
- 48.Wiesner R.J., Ruegg J.C., Morano I. Counting target molecules by exponential polymerase chain reaction: Copy number of mitochondrial DNA in rat tissues. Biochem. Biophys. Res. Commun. 1992;183:553–559. doi: 10.1016/0006-291X(92)90517-O. [DOI] [PubMed] [Google Scholar]
- 49.Soltoff S.P. ATP and the regulation of renal cell function. Annu. Rev. Physiol. 1986;48:9–31. doi: 10.1146/annurev.ph.48.030186.000301. [DOI] [PubMed] [Google Scholar]
- 50.Kann O., Kovács R. Mitochondria and neuronal activity. Am. J. Physiol. Physiol. 2007;292:C641–C657. doi: 10.1152/ajpcell.00222.2006. [DOI] [PubMed] [Google Scholar]
- 51.Babic N., Orio M., Peyrot F. Unexpected rapid aerobic transformation of 2,2,6,6-tetraethyl-4-oxo(piperidin-1-yloxyl) radical by cytochrome P450 in the presence of NADPH: Evidence against a simple reduction of the nitroxide moiety to the hydroxylamine. Free Radic. Biol. Med. 2020;156:144–156. doi: 10.1016/j.freeradbiomed.2020.05.021. [DOI] [PubMed] [Google Scholar]
- 52.Hedlund E., Gustafsson J.A., Warner M. Cytochrome P450 in the brain: A review. Curr. Drug Metab. 2001;2:245–263. doi: 10.2174/1389200013338513. [DOI] [PubMed] [Google Scholar]
- 53.Knights K.M., Rowland A., Miners J.O. Renal drug metabolism in humans: The potential for drug-endobiotic interactions involving cytochrome P450 (CYP) and UDP-glucuronosyltransferase (UGT) Br. J. Clin. Pharmacol. 2013;76:587–602. doi: 10.1111/bcp.12086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Crosbie S.J., Blain P.G., Williams F.M. An investigation into the role of rat skeletal muscle as a site for xenobiotic metabolism using microsomes and isolated cells. Hum. Exp. Toxicol. 1997;16:138–145. doi: 10.1177/096032719701600302. [DOI] [PubMed] [Google Scholar]
- 55.Omura T. Mitochondrial P450s. Chem. Biol. Interact. 2006;163:86–93. doi: 10.1016/j.cbi.2006.06.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are contained within the article or supplementary materials.