Abstract
Healthcare is undergoing a digital transformation, and the Centers for Medicare & Medicaid Services (CMS) aims to help providers navigate the clinical quality improvement landscape. In December 2017, CMS launched the Electronic Clinical Quality Measure (eCQM) Strategy Project. This article consists of 2 parts. The first part describes stakeholder outreach aimed to identify burdens and recommendations related to eCQM implementation and reporting. The second part describes how these burdens were addressed by CMS and how to engage in the digital transformation journey.
Six themes emerged from the stakeholder feedback: Alignment, Value, Development Process, Implementation and Reporting Processes; EHR certification process; and Communication, Education, and Outreach. CMS and its partners addressed over 100 recommendations to improve the eCQM development, implementation, and reporting experience by creating implementation strategies. This included the development of new tools, such as the Measure Collaboration (MC) Workspace and ongoing testing of Fast Healthcare Interoperability Resources (FHIR)-based standards for quality measurement.
CMS is sharing this summary of the eCQM Strategy Project to reflect CMS’ interest in stakeholder engagement and burden reduction, increase awareness of available resources, and encourage continued engagement throughout this digital transformation in quality reporting.
Keywords: electronic clinical quality measures, quality reporting, clinical quality measurement, value-based purchasing program, fast healthcare interoperability resources (FHIR)
INTRODUCTION
Electronic clinical quality measures (eCQM) use data electronically extracted from health information technology systems to measure the quality of healthcare provided.1 Centers for Medicare & Medicaid Services (CMS) uses eCQMs in quality reporting and value-based purchasing programs. The number of eCQMs a health system reports is determined by the requirements of individual CMS quality programs. For instance, in the CMS Hospital Inpatient Quality Reporting Program, hospitals are required to report on 4 eCQMs, and in the CMS Merit-Based Incentive Payment System, using the EHR Reporting option, eligible clinicians may report on up to 6 eCQMs.2,3 In response to stakeholder feedback about the burdens of eCQM implementation and reporting, CMS launched the eCQM Strategy Project in the fall of 2017 .
The eCQM Strategy Project evaluated technical end user eCQM requirements with the goal of streamlining these requirements, reducing unnecessary burden, increasing efficiencies, and improving the beneficiary experience. Through this project, CMS aimed to identify stakeholder burdens with eCQMs and recommendations to improve their experiences. Recommendations are varying types of actions to solve or mitigate reported burdens. Table 1 shows a high-level timeline of the eCQM Strategy Project that provided CMS with frontline perspectives of eCQM implementation and reporting burden, and recommendations for improvement in using eCQMs in CMS quality reporting programs.
Table 1.
High-level eCQM strategy project timeline
| Time Frame | eCQM Strategy Project Activities |
|---|---|
| October 2017–November 2017 |
|
| December 2017–March 2018 |
|
| April 2018–June 2018 |
|
| July 2018–July 2020 |
|
STAKEHOLDER ENGAGEMENT
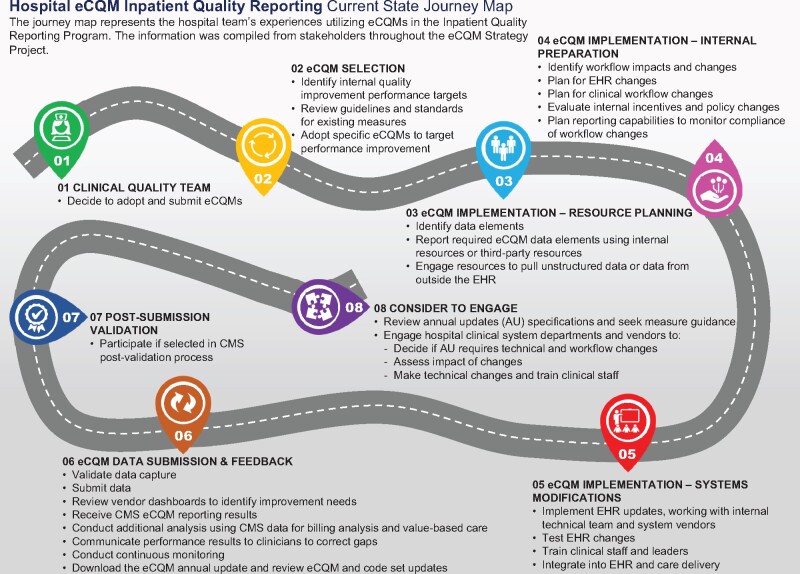
CMS wanted to hear directly from stakeholders, so the eCQM Strategy Project team engaged with stakeholders across the digital quality measurement community using a human-centered design approach to learn about their experiences with eCQM activities. At the onset of the project, the team received training from a human-centered design expert to ensure each step of the project involved end users and focused on improving their experiences with eCQMs. Journey maps were developed and provided a visual representation of stakeholder experiences with eCQMs. Figure 1 represents the current state journey map for hospitals reporting eCQMs in the CMS Inpatient Quality Reporting Program. The approach consisted of 4 main steps: 1) an environmental scan, 2) a workshop, 3) site visits, and 4) iterative stakeholder validation and refinement through conference sessions and focus groups.
Figure 1.
Hospital eCQM inpatient quality reporting current state journey map.
The team performed an environmental scan reviewing public comments, issues raised in CMS help desk systems, and documentation from previously held federal eCQM kaizen events to learn more about stakeholder experiences with eCQMs. The eCQM kaizens involved stakeholders from federal agencies, healthcare organizations, health information technology vendors, and measure developers working together to identify issues with eCQMs and potential process improvements. The environmental scan findings reflected perspectives of clinicians, hospital staff, and health information technology (IT) vendors sharing burden associated with data mapping, lack of transparency, confusing processes, and quality measures that may not be relevant to their patient populations.
The team facilitated an in-person workshop with CMS quality program staff, and other stakeholder representatives, from phases of the eCQM life cycle. Using wall posters depicting flowcharts of current state eCQM development, implementation, and reporting processes, attendees validated processes and provided insight on the burdens identified through the environmental scan. This insight provided context for learning about the eCQM experience through site visits to hospital and clinician organizations.
The team performed 17 site visits to organizations in the Northeast, Mid-Atlantic, and Midwest regions listening to clinicians, clinical quality consultants, health IT analysts, and executives share their experiences with eCQMs. At least 2 project team members attended each site visit, with 1 person serving as the note taker. The notes were transcribed and reviewed by the team for completeness. The transcriptions were then imported into NVivo qualitative data analysis software. Two staff members performed manual coding and thematic analysis of the site visit findings. The team focused on feedback regarding burdens. A comment describing a difficult aspect of an eCQM-related process was classified as a burden. Site visit participants also shared recommendations for improvement to a reported burden.
FEEDBACK FROM THE FIELD: KEY THEMES
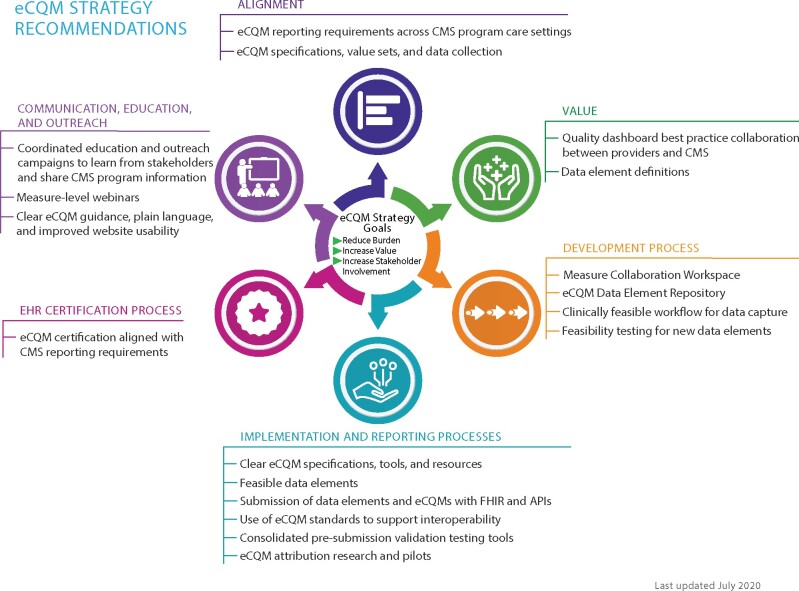
After CMS and the project team analyzed stakeholder feedback and identified 6 key themes, the burdens and recommendations for improvement were reviewed with CMS quality program staff and supporting contractors. These groups identified additional recommendations with possible solutions or actions that may mitigate burdens reported. The project team compiled the key themes, burdens, recommendations, and possible solutions or actions and facilitated gallery-walk discussion sessions at the 2018 and 2019 Health Information and Management Systems Society (HIMSS) Conference, and the 2019 CMS Quality Conference to gain feedback and validation from a broader group of stakeholders. Once validated, the team proposed 117 recommendations, 71 of which CMS led creating implementation strategies for. The eCQM Strategy Recommendations depicted in Figure 2 present the 6 themes identified through the process, and the associated high-level recommendations.
Figure 2.
eCQM strategy recommendations.
Alignment
Value
Development Process
Implementation and Reporting Processes
EHR Certification Process
Communication, Education, and Outreach
The Alignment recommendations address the perceived lack of alignment across CMS programs, other payers, and regulatory agencies potentially leading to increased time and effort complying with different program requirements and technical specifications.
The Value recommendations address burdens with reporting on eCQMs that providers believe may not be relevant to them, do not contribute to their quality initiatives, and do not accurately represent the care they provide to their patients. The recommendations include creating flexible opportunities to recommend relevant quality measures and to provide feedback on measures earlier in the development process.
The Development Process recommendations address the burdens related to the length of time and lack of clarity of the eCQM development process. Measure developers and health IT vendors recommended a collaborative eCQM development environment to improve transparency for future measure needs and improve access to testing data to streamline the eCQM development life cycle.
The Implementation and Reporting Process recommendations address the complex eCQM workflows resulting from: 1) difficulty interpreting the eCQM specifications and discerning which data elements must be captured in the clinician workflow; 2) documentation required for eCQM reporting that does not directly support patient care; and 3) multiple submission mechanisms and formats resulting in delays, poor submitter feedback, and lack of usability and consistency.
Hospitals and clinicians expected the EHR Certification Process to ensure accurate and successful eCQM calculation, reporting, and submission to CMS. In practice, they encountered issues with eCQM data submissions to CMS, because EHR certification and CMS reporting requirements were not aligned.
A recurring topic across all other themes was the need for improved Communications, Education, and Outreach. Stakeholders requested plain language, simplified eCQM-related materials, increased consistency of content found on CMS quality program sites, and education and outreach focused on implementation and reporting eCQMs.
CMS ACTIVITIES TO REDUCE eCQM REPORTING BURDEN
From July 2018 to July 2020, the eCQM Strategy Project team collaborated with and communicated feedback to CMS policy owners to implement recommendations or develop action plans. As of July 2020, CMS has addressed, implemented, or have a plan of action in place for 114 of the 117 total recommendations. As the ultimate owner of burden reduction activities, CMS continues to explore the 3 remaining recommendations related to provider attribution and how provider attribution may impact measure calculations.
Table 2 provides a listing of a subset of 15 recommendations with corresponding burden reduction activities and goals, and additional detail on several selected activities is provided below. This subset was selected based on relevance to readers and level of stakeholder interest at conferences, webinar Q&As, and focus groups.
Table 2.
Burden reduction activities
| Theme and Burden Reported | Recommendation | Solution or Innovation to Mitigate Reported Burden | Target Audience to Benefit from Recommendation | Goal |
|---|---|---|---|---|
| Alignment: eCQM quality reporting requirements are not aligned across CMS and federal agencies. | Improve intra- and inter-agency collaboration and align eCQM reporting requirements. | Expanded eCQM governance group membership | Federal partners across Department of Health and Human Services, Department of Defense, and Department of Veterans Affairs | Improved interagency collaboration by sharing quality measurement initiatives and experience across federal health agencies. |
|
Alignment: Inconsistent measure specifications and value sets are burdensome to implement and report. |
Align measure specifications, data elements, and value sets. | Established Value Set Workgroup | Clinicians, hospitals, health IT vendors, measure developers | Harmonization of value sets and providing guidelines to improve the usability and clarity of value sets across eCQMs and CDS. |
|
Value: Lack of awareness of using clinical dashboards for eCQM tracking. |
Increase awareness of best practices related to clinical dashboards. | Delivered a clinical dashboard best practices webinar4 | Clinicians, hospitals, health IT vendors | Increase awareness of best practices and value of using clinical dashboards to support quality improvement initiatives. |
|
Value: eCQMs are not meaningful and do not assist hospitals and clinicians in their quality improvement initiatives. |
Include clinicians in the eCQM development process to help prioritize new eCQM development. | Developed Measure Collaboration (MC) Workspace eCQM concept module5 | Clinicians, hospitals, health IT vendors, measure developers, CMS | Improves transparency of measure development process and provides capability to submit eCQM concept ideas to CMS. |
|
Development Process: Lengthy eCQM development process. |
Identify alternative approaches for accelerating critical measure development processes. | Identified opportunities to streamline the measure development life cycle | Clinicians, hospitals, health IT vendors, measure developers, CMS | Achieve time savings in the measure development process by using the CMS Measures Inventory Tool Environmental Scan and leveraging the MC Workspace. |
|
Development Process: Decreased clinical participation in the eCQM development process at all points in the life cycle. |
Create a collaborative workspace to increase engagement in CMS measure development. | Launched the MC Workspace, New eCQM Clinical Workflow, and eCQM Test Results modules | Clinicians, hospitals, health IT vendors, measure developers, CMS | Provides a collaborative portal to promote transparency and better interaction across stakeholder communities that develop, implement, and report eCQMs. |
|
Implementation and Reporting Processes: Implementing eCQMs is time and resource intensive to map data and determine workflow requirements. |
Provide a repository that provides easy-to-understand definitions of existing measures including data mapping for every data element. | Developed the MC Workspace eCQM Data Element Repository (DERep)6 | Clinicians, hospitals, health IT vendors, measure developers | Provide data mapping support to improve accurate capture and calculation of eCQMs. |
|
Implementation and Reporting Processes: Reconfiguring the same workflows across multiple organizations is not cost-effective or efficient. |
Prepare a series of measure-level implementation webinars with best practices that includes review of data capture and consistent data mapping practices. | Delivered series of measure-level webinars | Clinicians, hospitals, health IT vendors | Help stakeholders understand data capture, data mapping, and common workflows. |
|
Implementation and Reporting Processes: Stakeholders shared help desk concerns citing poor response times, contradictory responses, and cumbersome workflow. |
Improve eCQM support websites and help desks. | Review and alignment of eCQM help desk processes across CMS quality programs | Clinicians, hospitals, health IT vendors | Improve customer experience with eCQM help desk support in the ONC Project Tracking System (Jira).7 |
|
Implementation and Reporting Processes: Multiple tools for submission are burdensome and some EHRs are not able to submit QRDAs. |
Improve Tools, Standards, and Processes for eCQM reporting. | Launched CMS FHIR for Quality Reporting Initiative for Planning and Testing8 | Clinicians, hospitals, health IT vendors | Explore a transition to FHIR-based quality reporting to simplify and align reporting specifications across CMS programs and with other nongovernment entities. |
|
Implementation and Reporting Processes: Cumbersome quality reporting submission systems. |
Streamline the login processes and improved error reports. | CMS Hospital Quality Reporting team launched the Next Generation System9 | Clinicians, hospitals, health IT vendors | Reduce burden with testing and submitting quality reports for the Hospital Inpatient Quality Reporting program. |
|
EHR Certification: The certification process does not ensure that a provider will be able to successfully report and submit its eCQM data from its Certified Electronic Health Record Technology to CMS. |
Improve alignment between eCQM certification and eCQM reporting and submission requirements. | Partnered with ONC to achieve this alignment | ONC, health IT vendors | Reduce the number of errors with eCQM data submission to CMS by requiring health IT products conform to CMS submission requirements.10 |
|
EHR Certification: Stakeholders report errors with eCQM data submissions to CMS. |
Provide instance of a tool replicating CMS submission requirements. | Developed the Cypress Validation Utility Plus (CVU+)11 | Health IT vendors | Reduce the number of errors with eCQM data submission to CMS by allowing health IT vendors to test synthetic QRDA Category I and Category III documents for conformance to CMS submission requirements. |
|
Communication, Education, Outreach: There is lack of awareness of available eCQM resources a lack of understanding of how and when to participate in measure development, testing, or troubleshooting technical issues throughout the eCQM life cycle. |
Provide simplified resources and increase awareness of eCQM resources and participation opportunities. | Encouraged Use of Plain Language to Simplify eCQM-Related Materials Across CMS eCQM Contractors | Clinicians, hospitals, health IT vendors, measure developers | Improve readability and consistency of eCQM materials produced by CMS contractors, partners, and federal health agencies. |
|
Communication, Education, Outreach: Hospital stakeholders shared challenges locating confusing or contradictory eCQM requirements on multiple program sites. |
Improve CMS Quality Program resources through consistent formatting of eCQM sections of CMS program sites. | Aligned eCQM-Related References Across CMS Quality Reporting Program Sites | Clinicians, hospitals, health IT vendors, measure developers | Improve accuracy and consistency of eCQM references and eCQM related materials on CMS, federal health agency, and industry websites. |
NEW TOOLS AND RESOURCES
CMS would like to leverage advancements in data standards and technology to help reduce burden associated with quality measurement while achieving desired improvements to patient care. In response to stakeholder feedback from the eCQM Strategy Project, CMS continues to develop tools and resources intended to simplify quality reporting.
Mechanism to suggest or share feedback on measures under development
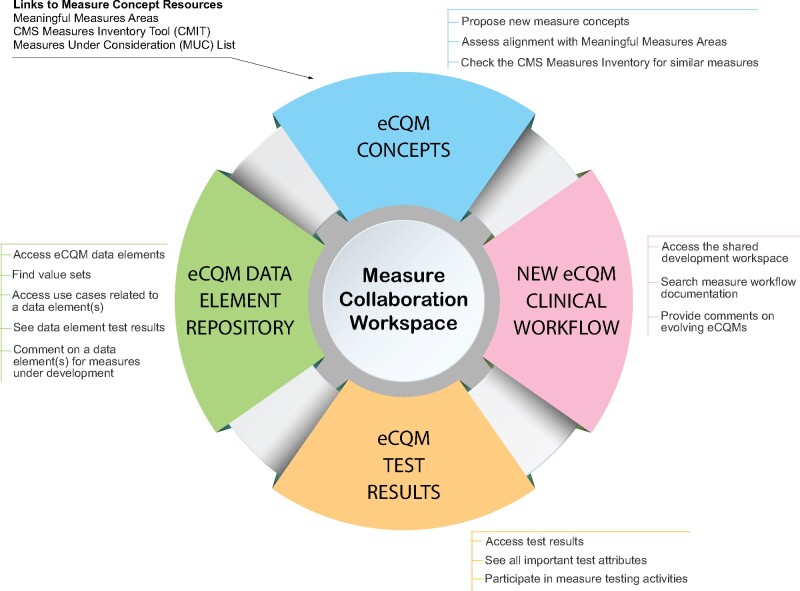
The Measure Collaboration (MC) Workspace is a CMS website that brings together interconnected resources, tools, and processes to promote transparency and better interaction across stakeholder communities that develop, implement, and report eCQMs.12 The MC Workspace gives users who could not participate in formal eCQM testing processes an opportunity to provide input on eCQM development and impact to their workflows. Figure 3 depicts the 4 modules of the MC Workspace and their purposes.
Figure 3.
Measure collaboration workspace.
Resources for eCQM implementation support
The eCQM Data Element Repository (DERep) was deployed in December 2018 as the first module of the MC Workspace in response to stakeholder feedback requesting eCQM data definitions to help with data mapping challenges.6 The eCQM DERep centralizes data element definitions for all eCQMs used in CMS Quality Reporting Programs. The searchable eCQM DERep is designed to save time previously spent navigating between applications and documents gathering data element information needed to perform data mapping. At conference demonstrations, stakeholders shared positive feedback appreciating centralized access to eCQM information from the eCQM specification, the Quality Data Model, and the Value Set Authority Center.
Mechanism to contribute to and test future digital quality measurement standards
CMS began Fast Healthcare Interoperability Resources (FHIR) testing for quality reporting to simplify and streamline the eCQM reporting process. CMS partnered with the Da Vinci Project Data Exchange for Quality Measurement (DEQM) team to explore a transition to FHIR-based quality reporting for eCQMs. The Da Vinci Project is a community of payer and provider leaders working to enable value-based care and solve interoperability challenges. The team is testing the feasibility of using the FHIR standard to simplify and align reporting specifications across CMS programs and other nongovernment entities, to reduce dependency on vendor support for help and to resolve challenges smaller practices experience with reporting with the Quality Reporting Document Architecture (QRDA).13
Resources for CMS eCQM quality reporting submission burdens
The team worked with the Office of the National Coordinator for Health IT (ONC) to ensure health IT certification criteria aligns with the CMS QRDA Implementation Guide (IG). ONC published the 21st Century Cures Act: Interoperability, Information Blocking, and the ONC Health IT Certification Program in May 2020 which replaced the Health Level Seven International (HL7) QRDA standard requirements in the 2015 edition “Clinical Quality Measures—report” criterion in §170.315(c)(3) with requirements for certified health IT to support the CMS QRDAIGs. This alignment is anticipated to reduce errors that providers encounter when submitting quality data to CMS.10
WHERE TO LEARN MORE
Building on the eCQM Strategy Project, CMS continues to engage with stakeholders to provide support and obtain feedback through communication, education, and outreach channels. Those interested in reviewing more detailed information about the eCQM Strategy Project can access the eCQM Strategy Project Outcomes Report on the Electronic Clinical Quality Improvement (eCQI) Resource Center. The eCQI Resource Center website is sponsored and maintained by CMS within the US Department of Health and Human Services and provides a centralized “one-stop shop” for stakeholders engaged in electronic quality improvement drawing from existing resources wherever possible to minimize duplication of resources and efforts across programs and websites.14 The site contains resources and information put forth by federal agencies—CMS, ONC, the National Library of Medicine, the Agency for Healthcare Research and Quality—and provides links to external tools to meet the needs of stakeholders throughout their eCQI efforts.
The eCQI Resource Center hosts 2 important references: 1) eCQM 101—Getting Started with eCQMs for Quality Reporting Programs information sheet provides an overview of what eCQMs are and how they are used, and 2) eCQM Communications Resources document includes a listing of resources with descriptions and contact information.15,16
NEXT STEPS AND WAYS TO GET INVOLVED
To achieve digital transformation in quality reporting, CMS continues to support hands-on development and testing of FHIR for quality reporting at HL7 FHIR “connectathons” to promote interoperability, simplify quality reporting processes, and align clinical decision support and quality measures standards. The HL7 website for the Da Vinci project use cases for Data Exchange for Quality Measurement and Gaps In Care and Information provides opportunities to get involved and contribute to standards development or testing.17,18 CMS continues to support research to understand provider attribution challenges associated with eCQM use and reporting and identifying feasible solutions.18 CMS also encourages stakeholder engagement in strategy development designed to achieve digital transformation across CMS quality reporting programs. The federal government and private stakeholders provide opportunities for stakeholders to actively engage in eCQI efforts through open meetings, public comment periods, expert panels, and educational events. The eCQI Resource Center includes resources for role-based engagement with the eCQI community.19 CMS supports the electronic clinical quality improvement objectives and strategies outlined in the 2020-2025 Federal Health IT Strategic Plan.20
FUNDING
This work was supported by the Centers for Medicare and Medicaid Services contract number 75FCMC18D0047.
AUTHOR CONTRIBUTIONS
All authors contributed substantially to the project design or analysis of data, participated in the drafting and editing of the manuscript, and approved the final version for submission. All authors agree to be available for questions regarding all aspects of the work.
DATA AVAILABILITY STATEMENT
The data underlying this article will be shared on reasonable request to the corresponding author.
CONFLICT OF INTEREST STATEMENT
None declared.
NOTICE
This document was produced for the US Government under Contract Number 75FCMC18D0047, and is subject to Federal Acquisition Regulation Clause 52.227-14, Rights in Data-General.
No other use other than that granted to the US Government, or to those acting on behalf of the US Government under that Clause, is authorized without the express written permission of The MITRE Corporation.
For further information, please contact The MITRE Corporation, Contracts Management Office, 7515 Colshire Drive, McLean, VA 22102-7539, (703) 983-6000.
© 2020 The MITRE Corporation.
ACKNOWLEDGMENTS
The authors thank CMS Executive Leadership, their federal partners, the eCQM Governance Group, the Da Vinci Project Community, the health systems that shared their time and experience, and stakeholders across the eCQM community for their support and insight.
REFERENCE LIST
- 1.Centers for Medicare & Medicaid Services. Electronic Clinical Quality Measures. https://ecqi.healthit.gov/ecqms. Accessed October 21, 2020.
- 2.Centers for Medicare and Medicaid Services. Fiscal Year 2022 Hospital Inpatient Quality Reporting Program Guide. In:2019.
- 3.Centers for Medicare & Medicaid Services. Quality Payment Program: Quality Measures Requirements. 2020. https://qpp.cms.gov/mips/quality-measures. Accessed October 21, 2020.
- 4.Centers for Medicare & Medicaid Services. Use of Clinical Dashboards to Drive Performance Improvement for eCQMs. 2019; https://www.qualityreportingcenter.com/en/inpatient-quality-reporting-programs/hospital-inpatient-quality-reporting-iqr-program/2019-events/ecqm07312019/. Accessed October 21, 2020.
- 5.Centers for Medicare & Medicaid Services. Measure Collaboration (MC) Workspace: Electronic Clinical Quality Measure (eCQM) Concepts. https://ecqi.healthit.gov/mc-workspace-2/ecqm-concepts. Accessed October 21, 2020.
- 6.Centers for Medicare & Medicaid Services. Measure Collaboration (MC) Workspace: Electronic Clinical Quality Measure (eCQM) Data Element Repository (DERep). https://ecqi.healthit.gov/mc-workspace-2/data-element-repository. Accessed October 21, 2020.
- 7.Office of the National Coordinator for Health Information Technology. ONC Project Tracking System: eCQM Issue Tracker. https://oncprojectracking.healthit.gov/support/projects/CQM/summary. Accessed October 21, 2020.
- 8.Centers for Medicare & Medicaid Services. Advancing Technology for Quality Reporting at CMS: Burden Reduction and FHIR. 2020; https://ecqi.healthit.gov/sites/default/files/Advancing-Tech-for-Qual-Reporting-at-CMS-FHIR-Virtual-Presentation-June-2020-508.pdf. Accessed October 21, 2020.
- 9.Centers for Medicare & Medicaid Services. eCQM Next Generation of Hospital Quality Reporting: Navigation Guide. 2019; https://www.qualityreportingcenter.com/globalassets/iqr_resources/september-2019/ecqm_-next-generation-of-hqr-navigation-guide_fall-2019_vfinalu508u.pdf. Accessed October 21, 2020.
- 10.Office of the National Coordinator for Health Information Technology (ONC) Department of Health and Human Services. 21st Century Cures Act: Interoperability, Information Blocking, and the ONC Health IT Certification Program. In. Vol 85 FR 256422020:25642–25961.
- 11.Centers for Medicare & Medicaid Services. eCQI Resource Center: CVU. https://ecqi.healthit.gov/tool/cvu. Accessed October 21, 2020.
- 12.Centers for Medicare & Medicaid Services. Measure Collaboration Workspace. https://ecqi.healthit.gov/mc-workspace-2. Accessed October 21, 2020.
- 13.Centers for Medicare & Medicaid Services. eCQI Resource Center: QRDA - Quality Reporting Document Architecture. https://ecqi.healthit.gov/qrda. Accessed October 21, 2020.
- 14.Centers for Medicare & Medicaid Services. Electronic Clinical Quality Improvement (eCQI) Resource Center. https://ecqi.healthit.gov/. Accessed October 21, 2020.
- 15.Centers for Medicare & Medicaid Services. eCQM 101 - Getting Started with Electronic Clinical Quality Measures for Quality Reporting Programs. 2019; https://ecqi.healthit.gov/system/files/eCQM_101_Getting_Started_with_Electronic_Clinical_3.20.2019_508.pdf. Accessed October 21, 2020.
- 16.Centers for Medicare & Medicaid Services. CMS Electronic Clinical Quality Measure (eCQM) Communication Resources. 2020; https://ecqi.healthit.gov/sites/default/files/eCQMCommunication%20Resources-2020-508.pdf. Accessed October 21, 2020.
- 17.Health Level Seven International Da Vinci Project. Da Vinci Use Cases: Data Exchange for Quality Measures (DEQM). https://confluence.hl7.org/pages/viewpage.action?pageId=21857600. Accessed October 21, 2020.
- 18.Health Level Seven International Da Vinci Project. Da Vinci Use Cases: Gaps In Care & Information. https://confluence.hl7.org/pages/viewpage.action?pageId=66929644. Accessed October 21, 2020.
- 19.Centers for Medicare & Medicaid Services. eCQI Resource Center: Engage in Electronic Clinical Quality Improvement (eCQI). https://ecqi.healthit.gov/engage-electronic-clinical-quality-improvement-ecqi. Accessed October 21, 2020.
- 20.Office of the National Coordinator for Health Information Technology (ONC) Office of the Secretary Department of Health and Human Services. 2020-2025 Federal Health IT Strategic Plan. 2020; https://www.healthit.gov/sites/default/files/page/2020-10/Federal%20Health%20IT%20Strategic%20Plan_2020_2025.pdf. Accessed October 21, 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.