SUMMARY
Even with strict implementation of preventive measures, surgical site infections (SSIs) remain among the most prevalent health care-associated infections. New strategies to prevent SSIs would thus have a huge impact, also in light of increasing global rates of antimicrobial drug resistance. Considering the indispensable role of innate immune cells in host defense in surgical wounds, enhancing their function may represent a potential strategy for prevention of SSIs. Trained immunity is characterized by metabolic, epigenetic, and functional reprogramming of innate immune cells. These functional changes take place at multiple levels, namely, at the level of bone marrow precursors, circulating innate immune cells, and resident tissue macrophages. Experimental studies have shown that induction of trained immunity can protect against various infections. Increasing evidence suggests that it may also lower the risk and severity of SSIs. This may occur through several different mechanisms. First, trained immunity enhances local host defense against soft tissue infections, including those caused by Staphylococcus aureus, the most common cause of SSIs. Second, training effects on nonimmune cells such as fibroblasts have been shown to improve wound repair. Third, trained immunity may prevent or reverse the postoperative immunoparalysis that contributes to risk of infections following surgery. There are multiple approaches to inducing trained immunity, such as vaccination with the bacillus Calmette-Guérin (BCG) tuberculosis vaccine, topical administration of β-glucan, or treatment with the Toll-like receptor 7 agonist imiquimod. Clinical-experimental studies should establish if and how induction of trained immunity can best help prevent SSIs and what patient groups would most benefit.
KEYWORDS: postoperative immunosuppression, surgical site infections, tissue repair, trained immunity
INTRODUCTION
Surgical site infections (SSIs) are infections of the soft tissues and organs after surgery (1). SSIs arise upon disruption of the balance between host defense mechanisms and bacterial load or virulence (2). Many risk factors contributing to the disruption of this delicate balance have been identified. These risk factors are generally divided into patient-related and procedure-related risk factors (Table 1). Strategies for the prevention of SSIs focus on both sides of this balance by improving host defense and also by reducing the risk of bacterial contamination.
TABLE 1.
Risk factors for surgical site infectionsa
| Risk factor type | Risk factors |
|---|---|
| Patient related | Increased age, nutritional status, diabetes mellitus, obesity, alcoholism, smoking, immunosuppression (primary or secondary immunodeficiencies, immunosuppressive drugs, chemotherapy), history of skin or soft tissue infection, recent radiotherapy, preoperative albumin < 3.5 mg/dl, total bilirubin > 1.0 mg/dl, coexistent infection at a remote body site, colonization with microorganisms |
| Procedure related | |
| Preoperative | Inadequate skin preparation; hair removal method; inappropriate antibiotic choice, timing, and dosing; length of preoperative stay |
| Procedure | Increased complexity of procedure, long duration of operation, blood transfusion, surgical technique, poor hemostasis, failure to eliminate dead space, tissue trauma, poor glycemic control, hypothermia, inadequate oxygenation, breach in asepsis |
| Facility, equipment and personnel | Inadequate ventilation, increased operating room traffic, contaminated environmental surfaces, nonsterile equipment, inadequate gloving, inappropriate surgical scrub |
| Postoperative | Higher wound classification, surgical drains, foreign material in the surgical site |
Despite strict implementation of preventive measures, SSIs remain one of the most prevalent health care-associated infections. In the United States, almost 2% of surgical procedures are complicated with an SSI (3). This adds up to annual 290,000 SSIs in the United States alone (4). SSIs are a substantial cause of morbidity, prolonged hospitalization, readmission, and death. SSIs are therefore a prominent burden for the health care system, with extensive clinical and economic impact (5). In addition, SSIs are increasingly caused by antimicrobial-resistant pathogens, and surgical patients present more commonly with comorbidities (6, 7). This increases the challenge and costs of treating SSIs and emphasizes the need for additional preventive measures (8, 9).
Trained immunity or innate immune memory can possibly form a new strategy for the prevention of SSIs. Trained immunity is a recently described process that indicates the nonspecific immune memory of the innate immune system. Training of innate immune cells is mediated by the activation of pattern recognition receptors by an initial inflammatory stimulus (e.g., β-glucan, bacillus Calmette-Guérin [BCG], oxidized low-density lipoprotein [oxLDL], urate). This leads to epigenetic reprogramming and metabolic rewiring of innate immune cells, resulting in functional changes that enable a stronger immune response to secondary stimuli, thereby enhancing host protection (10). Considering the role of the innate immune system in host defense of surgical wounds (11), trained immunity might provide insights for new preventive measures for SSIs. Therefore, the purpose of this review is to evaluate the feasibility of trained immunity induction for prevention of SSIs.
First, protection by trained immunity against bacterial infections—specifically against surgical site infections—is discussed. Second, the effects of trained immunity on wound healing are debated. Lastly, we will consider the effects of training on postoperative immunosuppression and propose several ideas for inducing trained immunity for prevention of SSIs.
MOLECULAR MECHANISMS OF TRAINED IMMUNITY
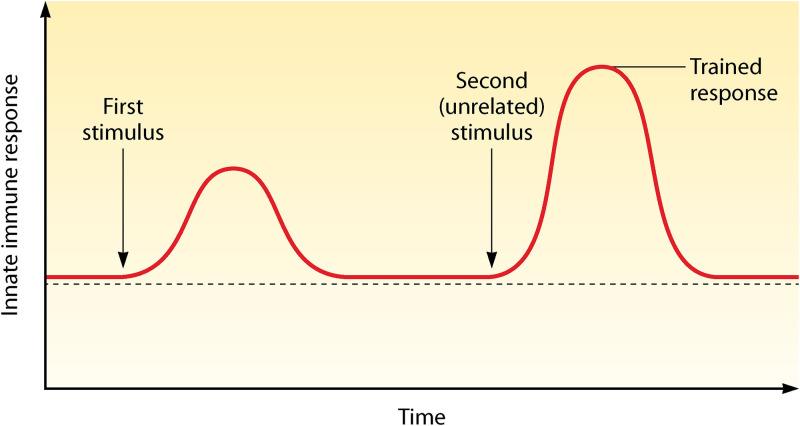
The immune system is classically divided into the innate and adaptive immune systems. The innate immune system consists of both myeloid (e.g., macrophages, monocytes, neutrophils) and lymphoid cells (e.g., NK cells, γδ T cells, innate lymphoid cells) and is characterized as a rapid, nonspecific responder to insults. The adaptive immune system (T and B cells) responds much slower, but in a highly specific way, with the ability to generate immunological memory and protection against recurrent infections. However, this general view has been challenged by several studies showing that innate immune cells are able to mount a nonspecific immunological memory, which is called “trained immunity” (Fig. 1) (12).
FIG 1.
Trained immunity response. Trained immunity is defined as an increased nonspecific innate immune response to (unrelated) pathogens after exposure to an initial stimulus.
Trained immunity or innate immune memory is mediated via epigenetic, metabolic, and functional reprogramming of innate immune cells. An initial inflammatory stimulus (e.g., vaccination [BCG], pathogen [β-glucan], oxLDL) leads to activation of pattern recognition receptors (PRRs) by pathogen-associated molecular patterns (PAMPs) or damage-associated molecular patterns (DAMPs) (13). Following activation, PRRs initiate intracellular pathways that result in expression of genes important for host defense. After the initial stimulus is eliminated, gene transcription returns to baseline. Nevertheless, sustained changes are induced in innate immune cells by metabolic rewiring and epigenetic reprogramming (10). These changes result in an enhanced accessibility of particular genes, allowing more robust gene expression upon a subsequent encounter to (unrelated) pathogens (14, 15), with an increase in proinflammatory cytokine production (interleukin-1 beta [IL-1β], IL-6, tumor necrosis factor alpha [TNF-α]) and microbicidal function. Thus, training of innate immune cells leads to an increased responsiveness to secondary infections.
Epigenetic Reprogramming
The increased responsiveness of innate immune cells is regulated through epigenetic changes, predominantly by histone modifications. Methylation or acetylation of histone tails at promoter and associated enhancer regions of inflammatory genes results in an open, transcriptionally permissive configuration of chromatin (16). The persistence of histone modifications after the initial insult underlies the more efficient induction of these genes upon secondary encounter to (unrelated) pathogens. Histone marks associated with an enhanced accessibility of genes in trained immunity are histone 3 lysine 4 methylation (H3K4me1), histone 3 lysine 4 trimethylation (H3K4me3), and histone 3 lysine 27 acetylation (H3K27ac) (15–17). The recently discovered latent enhancers also seem to play a part in innate immune memory. Latent enhancers are regions in the genome lacking histone mark H3K4me1, which is characteristic for enhancers, but acquire this mark in response to lipopolysaccharide (LPS) stimulation. A fraction of these latent enhancers retains H3K4me1 after stimulation and contributes to stronger activation of innate immune cells after restimulation (18, 19).
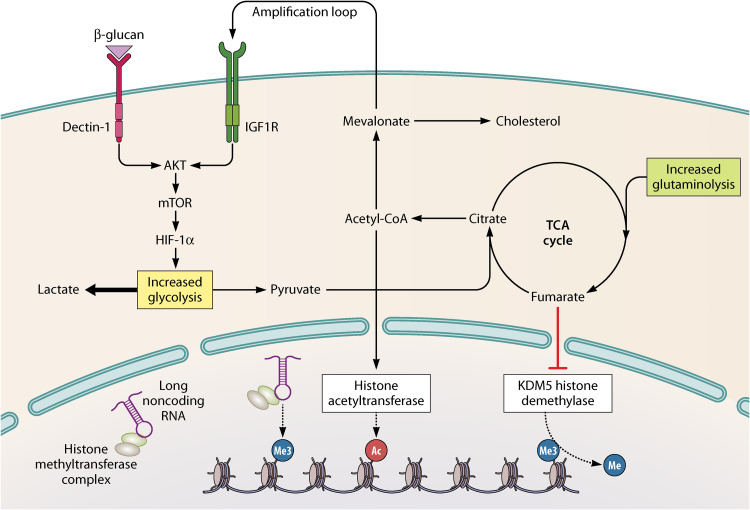
Recently, a class of long noncoding RNAs called “immune gene-priming lncRNAs” (IPLs) were shown to be responsible for accumulation of H3K4me3 at promoter sites of inflammatory genes in trained immunity. IPLs bind to histone methyltransferase complexes and direct these complexes to inflammatory genes to facilitate H3K4me3 epigenetic priming (Fig. 2). IPLs were shown to be upregulated upon training of innate immune cells (20). To conclude, there are various levels at which epigenetic mechanisms mediate trained immunity.
FIG 2.
Intertwist of epigenetics and metabolism in trained immunity. Upon recognition of specific ligands by pattern recognition receptors, intracellular pathways are induced that lead to the upregulation of several metabolic pathways, including glycolysis, glutaminolysis, and cholesterol synthesis. Levels of certain metabolites—citrate and fumarate—are increased and influence epigenetic enzymes, resulting in histone marks associated with trained immunity. In addition, acetyl coenzyme A (acetyl-CoA) is converted into mevalonate, which creates an amplification loop by activating insulin-like growth factor 1 receptor (IGF1R). Long noncoding RNAs are able to bind histone methyltransferase complexes. They guide these complexes to genes and facilitate accumulation of histone 3 lysine 4 trimethylation (H3K4me3) marks. HIF1α, hypoxia-inducible factor 1 alpha; mTOR, mechanistic target of rapamycin; TCA cycle, tricarboxylic acid cycle.
Metabolic Rewiring
Several metabolic pathways are induced in trained immunity, including glycolysis, glutaminolysis, and cholesterol synthesis (Fig. 2). The rewiring of cellular metabolism not only results in increased energy production but also contributes to epigenetic reprogramming by accumulation of metabolites modulating the activity of epigenetic enzymes. These metabolic changes are epigenetically mediated (21, 22), hence showing the interaction of epigenetics and metabolism in innate immune memory.
Upregulation of glycolysis is mediated by the Akt/mechanistic target of rapamycin (mTOR)/hypoxia-inducible factor 1 alpha (HIF1α) pathway (21, 22). Trained monocytes revealed increased glucose consumption, lactate production, and oxygen consumption rate, reflecting an induction of glycolysis and oxidative phosphorylation (22, 23). Lactate was shown to improve gene accessibility by inhibiting histone deacetylase activity (24). As glycolysis is focused mainly on lactate production, upregulation of glutaminolysis is thought to replenish the tricarboxylic acid (TCA) cycle (25). Concentrations of the TCA cycle metabolites citrate, succinate, malate, fumarate, and 2-hydroxyglutarate are increased in trained monocytes (25). Succinate and fumarate were shown to have a stabilizing effect on HIF-1α (26, 27). In addition, fumarate influences epigenetic reprogramming by inhibiting the activity of the KDM5 family of histone demethylases. Citrate is converted into acetyl coenzyme A (acetyl-CoA) in the cytosol, which acts as an acetyl donor for histone acetylation (28). Acetyl-CoA can enter the cholesterol synthesis pathway to increase production of the metabolite mevalonate. Mevalonate can, subsequently, induce an amplification loop by activating insulin-like growth factor 1 receptor (29). The importance of these pathways is confirmed by abrogation of trained immunity upon inhibition of glutaminolysis, cholesterol synthesis, or glycolysis (21, 22, 25, 29). Of interest, although similar pathways are induced in β-glucan- and BCG-induced trained immunity, there are distinct differences in the metabolic rewiring. This is probably due to different PAMPs and intracellular pathways involved in activation of the cells.
Cell Populations Involved in Trained Immunity
Monocytes/macrophages and NK cells are the cell populations described most often as being able to develop innate immune memory phenotypes. Recently, however, neutrophils were also discovered to have memory properties. Neutrophils isolated from BCG-vaccinated individuals showed enhanced killing capacity following a subsequent challenge with Candida albicans. Moreover, BCG vaccination increased the accessibility to proinflammatory genes in blood-derived neutrophils (30).
Innate immune memory was shown to be maintained in monocytes and neutrophils for several months, although these cells have a much shorter life span (17, 30). This paradox is explained by the fact that training also takes place at the level of hematopoietic stem and progenitor cells (HSPCs) (31–33). Exposure to BCG or β-glucan resulted in epigenetic changes in HSPCs and enhanced myelopoiesis in the bone marrow. More importantly, educated HSPCs were shown to generate trained monocytes/macrophages. As shared progenitor cells of macrophages and neutrophils—granulocyte macrophage progenitors—are increased and reprogrammed upon training (31), it is reasonable to assume that the same is true for neutrophils. Interestingly, neutrophil numbers are significantly increased in BCG-vaccinated infants days after vaccination, as proof of increased granulopoiesis (32). Moreover, genes involved in the development and function of neutrophils were upregulated in HSPCs (32), and a subset of multipotent progenitor cells polarized toward the myeloid lineage showed high expression of several granulocytic markers (33).
TRAINED IMMUNITY AND PROTECTION AGAINST BACTERIAL INFECTIONS
Surgical site infections are caused predominantly by bacteria, mostly by Staphylococcus aureus, Escherichia coli, coagulase-negative staphylococci, Enterococcus faecalis, and Pseudomonas aeruginosa (34). The relative importance of various pathogens has remained quite stable over the years (35) but differs by surgery type and surgical wound class (clean, clean-contaminated, contaminated, dirty-infected). This section reviews the evidence for protection against bacterial infections by trained immunity.
Human Studies
Emerging data suggest the existence of trained immunity effects in humans. First, a large number of epidemiological studies have shown nonspecific protective effects of live vaccines such as BCG, measles vaccine, and oral polio vaccine against infections other than the disease vaccinated for (36). Studies in West Africa showed a 45 to 59% reduction in overall mortality in children vaccinated with BCG, which is a much bigger decrease than could be explained by protection against tuberculosis (37–39). Several randomized controlled trials have been performed to confirm these nonspecific effects of BCG; they report conflicting results, which may be explained by differences in infectious exposure, maternal BCG vaccination, and study endpoints between high- and low-income settings (40–44).
Second, strong evidence supports that the nonspecific effects of BCG are partly mediated via innate immune memory. In severe combined immunodeficiency mice that lack T and B cells, BCG showed 100% protection against a lethal dose of C. albicans (17). In humans, multiple studies have shown BCG vaccination to increase proinflammatory cytokine responses to ex vivo stimulation with other pathogens (17, 45–49). Moreover, BCG vaccination induced epigenetic reprogramming of human monocytes, resulting in an increased accessibility to inflammatory genes (17, 46). Lastly, BCG vaccination showed protection in models of controlled human infection with yellow fever and malaria (46, 50).
In humans, limited clinical evidence suggests that trained immunity protects against bacterial infections. The protection by trained immunity against viral infections is being extensively studied, especially since the start of the coronavirus infection 2019 (COVID-19) pandemic (46, 51, 52). To our best knowledge, there are, however, no human studies regarding the protection of innate immune memory against bacterial infections. Protection against bacterial infections has only been suggested, as BCG vaccination was shown to decrease sepsis-related hospitalization rate and mortality (40, 53), which is nearly always caused by bacteria. Of interest, a recently published trial in elderly patients showed a significant decrease in the incidence of infections upon BCG vaccination (49). This decrease was mainly attributable to prevention of respiratory infections. Infections more commonly caused by bacteria (e.g., sepsis, pneumoniae, skin infections) were not significantly decreased, probably because the study was insufficiently powered to prove these differences.
Animal Studies
Animal studies provide compelling evidence for protection against bacterial infections by trained immunity (Table 2). Multiple studies demonstrate that training mice with different microbial ligands protects against subsequent infection with S. aureus (21, 54–59). In addition, trained immunity was shown to protect mice against a range of other bacteria, including Mycobacterium tuberculosis (60), Listeria monocytogenes (58, 61), Yersinia pestis (61), Streptococcus pneumoniae (62, 63), Klebsiella pneumoniae (64), Streptococcus pyogenes (65), Escherichia coli (58, 63, 65, 66), Pseudomonas aeruginosa (58, 65), and Enterococcus faecalis (65). Most bacteria were administered systemically, but studies showed that trained immunity can also protect from infections of the lungs (58, 60–63), peritoneum (58, 61), joint (65), muscle (66, 67), and skin (68–70).
TABLE 2.
Overview of protection by trained immunity in murine models described for secondary bacterial infectionsa
| Bacterium | Route of administration of bacteria | Murine model | Training agent | Time between last dose of training agent and bacterial challenge | Survival | Bacterial titers | Histopathological changes | Expt repeated | Notes | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| Staphylococcus aureus | i.v. | 129 Sv mice | β-glucan i.v. | 4 days | ↑ | + | 54 | |||
| i.v. | AKR/J mice | β-glucan i.v. | 4 days | ↑ | ↓ renal necrosis | − | 55 | |||
| i.v. | C57BL/6J mice | Zymosan i.p. | 3 days | ↑ | ↓ in blood | − | 58 | |||
| i.v. | CD2F1 mice | PCA-2 yeast cells i.v. | 2 wks | ↑ | − | 56 | ||||
| i.v. | CDF1 mice | BCG i.p. | 2 wks | ↑ | − | 57 | ||||
| Bacteria-laden K wire in a bone | C57BL/6 mice | Zymosan i.v. | 2 wks | ↓ in joint | − | Similar results with LPS i.v. as training agent and/or when challenged with i.v. S. aureus | 65 | |||
| s.c. in both flanks | Rag1−/− mice in BALB/c background | S. aureus s.c. in right flank | 6 wks | ↓ in skin | ↓ size of abscesses | − | 68 | |||
| Mycobacterium tuberculosis | i.n. | BALB/c mice | β-glucan i.p. | 4 days | ↑ | ↓ in lung | − | 60 | ||
| Listeria monocytogenes | i.v. | C57BL/6J mice | Zymosan i.p. | 3 days | ↑ | ↓ in blood, spleen, liver, and kidney | + | 58 | ||
| + | 61 | |||||||||
| i.p. | C57BL/6J mice | γHV68 i.n. | 4 wks | ↑ | ↓ in spleen and liver | + | 61 | |||
| Yersinia pestis | i.n. | C57BL/6J mice | γHV68 i.n. | 4 wks | ↓ in spleen and lungs | + | 61 | |||
| Streptococcus pneumoniae | i.n. | C57BL/6J mice | MTBVAC s.c. | 9 wks | ↑ | ↓ in blood | + | 62 | ||
| i.t. | BALB/c mice | Respiratory adenovirus infection i.n. | 4 wks | ↑ | ↓ in lungs and spleen | + | 63 | |||
| Escherichia coli | Bacteria-laden suture in muscle | Swiss Webster mice | BCG s.c. | 13 days | ↓ at site of challenge | + | 66 | |||
| Bacteria-laden K wire in a bone | C57BL/6 mice | Zymosan i.v. | 2 wks | ↓ in joint | − | Similar results with LPS i.v. as training agent and/or when challenged with i.v. E. coli | 65 | |||
| i.p. | C57BL/6J mice | Zymosan i.p. | 3 days | ↑ | − | 58 | ||||
| Pseudomonas aeruginosa | i.n. | C57BL/6J mice | Zymosan i.p. | 3 days | ↑ | − | 58 | |||
| Bacteria-laden K wire in a bone | C57BL/6 mice | Zymosan i.v. | 2 wks | ↓ in joint | − | Similar results with LPS i.v. as training agent and/or when challenged with i.v. P. aeruginosa | 65 | |||
| Streptococcus pyogenes | Bacteria-laden K wire in a bone | C57BL/6 mice | Zymosan i.v. | 2 wks | ↓ in joint | − | Similar results with LPS i.v. as training agent and/or when challenged with i.v. S. pyogenes | 65 | ||
| Enterococcus faecalis | Bacteria-laden K wire in a bone | C57BL/6 mice | Zymosan i.v. | 2 wks | ↓ in joint | − | Similar results with LPS i.v. as training agent and/or when challenged with i.v. E. faecalis | 65 | ||
| Klebsiella pneumoniae | Bacteria-laden suture in muscle | Swiss Webster mice | MDP s.c. | 1 day | ↓ in blood | − | 64 |
BCG, bacillus Calmette-Guérin; CpG ODN, synthetic oligodeoxynucleotides expressing CpG motif; i.t., intratracheally; i.n., intranasally; i.p., intraperitoneally; i.v., intravenously; LPS, lipopolysaccharide; MDP, muramyl dipeptide; s.c., subcutaneously; ↑, significantly increased; ↓, significantly decreased; −, not mentioned; +, performed in study.
Yet, there are two caveats to these animal studies. First, many of these studies lack experimental detail, which makes it difficult to estimate risk of bias. Second, the effect presented by some animal studies could be due to priming instead of trained immunity, with priming defined as applying a stimulus to change the functional state of a cell and applying a second stimulus when gene transcription has not returned to basal levels yet (as opposed to trained immunity, where the second stimulus is applied only once gene transcription is back to basal) (71). In an in vitro model with human monocytes, gene transcriptional levels return to normal after 3 to 7 days. It is unknown how this translates to animal models, but it might take longer due to the continuing presence of a training agent in the body and the induction of trained immunity at the level of the bone marrow. In some studies, secondary challenge was done within a week after primary infection. Protective effects reported by these studies might therefore be due to priming rather than trained immunity. Nevertheless, protective effects were also reported in mice that were challenged 1 month or longer after the first stimulus. These more lasting effects are likely to be the result of trained immunity. That said, in vitro models of trained immunity clearly have their own limitations, and animal studies are a very important tool to study trained immunity and its protective effects, strongly supporting the hypothesis that trained immunity can help protect against bacterial infections.
TRAINED IMMUNITY AND HOST DEFENSE IN SURGICAL WOUNDS
Trained immunity is likely to contribute to protection against bacterial infections. Still, its protective features in specific compartments remain unclear. This section addresses the role of innate immune cells in tissue repair and describes the contribution of trained immunity to protect against bacterial infections of surgical wounds.
Wound Repair and Involvement of Innate Immune System
Cutaneous wound repair is generally divided into three phases that partly overlap in time and space: inflammation, proliferation, and remodeling (72, 73). The inflammatory phase is characterized by clot formation and infiltration of leukocytes to cleanse the wound area of bacteria and necrotic tissue. During the proliferation phase, reepithelialization, angiogenesis, and formation of granulation tissue are responsible for covering and filling the wound area to restore tissue integrity. Remodeling, the last phase of wound healing that can take up to a year after injury, involves reorganization of the connective tissue and wound contraction, with scar formation often the endpoint of wound healing.
Focusing on the inflammatory phase, neutrophils and monocytes/macrophages are the main immune cells in cutaneous wound repair (11). Other immune cells with a function in tissue repair are mast cells, γδ T cells, and TH1/TH2 cells, the last two mentioned being mostly involved in the proliferation and remodeling phase (11). Neutrophils are recruited to the site of injury and phagocytose invading pathogens and necrotic tissue (74). Monocytes enter the site of injury a few days later and ingest cell debris, including neutrophil remnants, and remaining pathogens (72). Although resident macrophages contribute to the inflammatory response to some extent, most macrophages found at the site of injury are derived from blood monocytes and recruited by chemokine release (75).
Protection against Surgical Site Infections
The concept of immunostimulating therapies for prevention of surgical infections has been studied in the past. Around 1980, multiple so-called “biologic response modifiers” were examined, among which were BCG and muramyl dipeptide (MDP). These agents were studied in an animal model of surgical infection involving intramuscular insertion of a suture laden with bacteria. When administered 13 days before E. coli infection, BCG was shown to significantly reduce the number of viable bacteria isolated from adjacent tissue to the suture (66). Moreover, multiple studies have reported positive effects of MDP on survival and local bacterial recovery from a Klebsiella pneumoniae infection (64, 67, 76, 77). However, considering that MDP was administered only 24 h before the bacterial infection, these effects are more likely the result of direct stimulation and priming instead of trained immunity processes.
However, recent studies have shown that trained immunity protects against bacterial infections in skin and soft tissue, which, apart from the surgical incision and tissue manipulation, resembles a surgical site infection. Mice which were previously infected with S. aureus showed a significantly reduced bacterial burden and smaller skin lesions upon subsequent infection 3 to 6 weeks later (68–70, 78). Considering that these effects were also present in Rag1−/− and Rag2−/− mice, which lack both T and B cell function, protection is afforded by innate immune memory (68, 70). Moreover, primed Rag2−/− γc−/− mice, which lack mature T, B, and NK cells, showed increased bacterial killing upon secondary infection (69), indicating the negligible role of NK cells in protection against surgical site infection.
“Trained” Innate Immune Cells in Surgical Wounds
Tissue-resident macrophages and bone marrow-derived monocytes (BMDM) are likely to be the major effector cells involved in the innate immune memory targeting skin and soft tissue infections (69, 78). Bone marrow-derived macrophages, cultured from bone marrow cells of mice primed with a subcutaneous injection of S. aureus, showed enhanced intracellular killing of S. aureus and were able to transfer their memory phenotype to naive mice (78). This indicates the existence of “trained” bone marrow monocyte/macrophage progenitors, which were able to generate “trained” bone marrow-derived macrophages but can be expected to also give rise to “trained” bone marrow-derived blood monocytes, which can infiltrate the site of skin injury. There also seemed to be a more localized protection, as mice showed smaller lesions on the previously infected flank than on the naive flank (although both flanks showed smaller lesions than those of naive mice) (68, 78). This might be explained by the presence of primed resident macrophages in the previously infected flank. Priming mice with an intradermal injection of S. aureus did not result in primed BMDM (69). Instead, the memory effect was strictly dependent on resident macrophages. Resident macrophages showed an altered transcriptional signature upon intradermal injection with S. aureus, which was associated with increased resistance to secondary infection. Interestingly, trained immunity effects were also seen in Ccr2−/− mice, exhibiting defective monocyte recruitment, but were abolished when resident macrophages were depleted from the skin (69). To conclude, both BMDM and resident macrophages can acquire innate immune memory and can, independently of one another, provide protection against infection.
Innate immune memory in neutrophils has only recently been described (30) and has not yet been studied in wound infection models. Still, one can expect a contribution of “trained” neutrophils to protection against SSIs, as they are—similar to bone marrow-derived monocytes—likely derived from reprogrammed hematopoietic stem cells, and neutrophils are recruited in large numbers to sites of skin injury. Future studies are needed to confirm this hypothesis.
Trained Immunity-Induced Protection against Surgical Site Infection with S. aureus
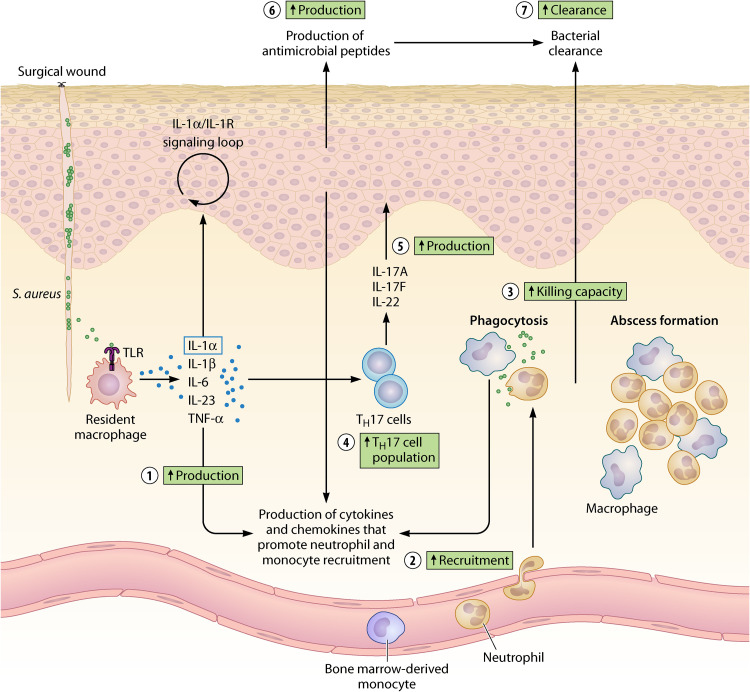
The well-described immune response against S. aureus skin infection provides insights into how “trained” innate immune cells might improve host defense. Upon surgical incision, bacteria migrate from the skin and colonize the wound bed. S. aureus is recognized by Toll-like receptor 2 (TLR2) on resident macrophages or other skin-resident immune cells. These cells produce numerous cytokines (IL-1α, IL-1β, IL-6, TNF-α, IL-23) and chemokines, thereby promoting neutrophil and monocyte recruitment (79, 80). Cytokine production also stimulates TH17 cells and innate lymphoid cells 3 to release IL-17A, IL-17F, and IL-22. These cytokines induce continuous production of neutrophil-attracting chemokines and antimicrobial peptides by keratinocytes. Production of neutrophil-attracting chemokines is also stimulated by the release of IL-1α, which initiates an IL-1α–IL-1R signaling loop in keratinocytes (79, 80). Infiltration of neutrophils at the site of injury promotes neutrophil abscess formation, which is required for bacterial control and clearance (79, 80). While neutrophils in the skin already decrease 2 days after the infection, macrophages are present for a longer period of time, being responsible for abscess resolution (81). Both cells continue secreting cytokines and chemokines to enhance the recruitment of more effector cells.
Trained immunity enhances several immunological processes involved in host defense against S. aureus (Fig. 3). Upon training, resident macrophages showed an elevated cytokine production when exposed to S. aureus (69), which probably leads to an increased recruitment of innate effector cells (as reported in references 68, 69, and 78). “Trained” innate immune cells might also shape the response of other cells, as primed mice showed an increase in the TH17 cell population and in IL-17A and IL-22 levels in the skin (68, 78), which possibly explains the increased production of antimicrobial peptides upon training (68). Furthermore, “trained” neutrophils and BMDM have shown increased cytokine production and antimicrobial function (although not yet demonstrated at the single macrophage or neutrophil level in a primed wound infection model) (15, 21, 30, 58, 60). These increased pathways in the immune response against S. aureus likely contribute to enhanced clearance of bacteria from a surgical wound.
FIG 3.
Trained immunity enhances local host defense against S. aureus in a surgical wound. Trained immunity improves the immune response against S. aureus in several ways. “Trained” resident macrophages produce more proinflammatory cytokines (1) upon encounter with S. aureus. This results in an increased recruitment of innate immune cells to the site of injury (2). “Trained” neutrophils and bone marrow-derived monocytes show increased killing capacity upon exposure to a pathogen (3), although not yet proven in a wound infection model. Moreover, the TH17 cell population (4) and production of TH17-derived cytokines, i.e., IL-17A and IL-22, in the skin increases upon training (5). This probably induces enhanced release of antimicrobial peptides by keratinocytes (6). All of these processes contribute to an increased clearance of bacteria from the surgical wound (7).
To sum up, preliminary evidence shows that trained immunity can protect against SSIs. This protection is likely to be afforded by the infiltration of “trained” innate immune cells in the surgical wound and the presence of “trained” resident innate immune cells. Notwithstanding, local factors might diminish trained immunity effects on prevention of SSIs. Migration of “trained” innate immune cells might be impeded by the presence of blood or necrotic tissue in the surgical wound. Then again, “trained” innate immune cells might contribute to enhanced debridement of the surgical wound. Moreover, the effect of a pathogen itself on trained immunity should be considered, as a recent study showed that virulent Mycobacterium tuberculosis can reprogram hematopoietic stem cells (HSCs) and impair development of trained immunity (82). However, to our best knowledge, there are no studies associating the pathogens involved in SSIs with impairment of trained immunity.
TRAINED TISSUE REPAIR
Macrophages not only contribute to the inflammatory response but also produce several growth factors that stimulate proliferation of nonimmune cells (e.g., keratinocytes, fibroblasts) and synthesis of extracellular matrix (83, 84). The general thought is that macrophages acquire different phenotypes during tissue repair (proinflammatory, pro-wound healing, profibrotic, anti-inflammatory, antifibrotic) linked to their different functions, which is critical for the successful sequence of phases in tissue repair (83). Neutrophils were, until recently, thought of as having only proinflammatory functions. Neutrophils have now been shown to also contribute to the resolution of inflammation by releasing growth factors and promoting angiogenesis (85). Thus, as both neutrophils and macrophages are believed to be involved in more than just the inflammatory phase following tissue injury, training of innate immune cells in a surgical wound might influence the overall process of tissue repair.
It is difficult to predict the effects of trained immunity on tissue repair, as this is mediated by complex interactions between stimulating and inhibitory mediators. Excessive or inappropriate induction of trained immunity could exacerbate tissue damage and has been shown to play a role in cardiovascular disease (86) and hypothesized to play a role in autoimmune and autoinflammatory diseases (87). Exposure of monocytes to oxLDL was shown to induce a long-lasting proinflammatory phenotype in macrophages (86). It is unknown how training of macrophages will affect their phenotype and function during wound repair. Decreased presence of anti-inflammatory macrophages or uncontrolled production of inflammatory mediators is thought to contribute to development of chronic wounds and pathological scar formation (83). On the other hand, it could be argued that training in a surgical wound might benefit tissue repair. Training of innate immune cells could result in an increased responsiveness of innate immune cells to infection, with an effective and rapid elimination of pathogens. This would prevent excessive inflammation and facilitate succession to the proliferation phase of wound healing. Indeed, primed mice showed a reduction in bacterial burden, infiltrating neutrophils, and skin IL-1β concentrations 5 days after a recurrent intradermal S. aureus infection (69). The same model showed an increase in skin-infiltrating neutrophils and blood monocytes 4 h after challenge with S. aureus. More importantly, three similar studies reported a decrease in skin lesion severity in primed mice when exposed to recurrent S. aureus soft tissue infection (68, 70, 78).
Training nonimmune cells in the surgical wound could possibly improve wound repair. Trained immunity has mainly been described in a subset of innate immune cells and their progenitors. However, recent studies show that nonimmune cells, including mesenchymal stem cells, fibroblasts, endothelial cells, and skin epithelial stem cells, also have memory-like features (88, 89). Importantly, two studies provide in vivo evidence for the beneficial effect of the memory function of nonimmune cells on wound repair. Epithelial stem cells (EpSCs) were primed with 5% imiquimod cream (a TLR7 agonist) (90). Upon wounding, inflammation-recovered skin showed enhanced wound closure, even when the initial stimulus was applied 180 days earlier. This effect was also seen when mice were primed with epidermal abrasion wounding or infection with C. albicans. Similar to mechanisms described for trained immunity, epithelial cell memory was associated with alterations at the chromatin level, giving EpSCs enhanced accessibility to key stress response genes upon secondary challenge. Of interest, primed EpSCs showed a more accessible chromatin state of inflammatory genes, suggesting enhancement of anti-infectious properties of nonimmune cells upon training. Subcutaneous adipose tissue mesenchymal stem cells (AD-MSCs) were primed with LPS and injected in rats undergoing skin flap surgery (91). Rats treated with LPS-primed AD-MSCs showed a significant decrease in skin flap necrosis, indicating an acceleration of wound healing. Epigenetic mechanisms were suggested to underlie the memory function of AD-MSCs.
Taken together, evidence suggests that training of immune and nonimmune cells in a surgical wound might result in enhanced tissue repair. This is referred to as “trained tissue repair” (92), which indicates the capability of immune and nonimmune cells to enhance tissue repair after exposure to a previous inflammatory insult. Still, one should consider the risk of inappropriately activating trained immunity and delaying wound repair. The mechanisms underlying excessive activation of trained immunity need further study, to add to our understanding of regulating and safely deploying trained immunity as a strategy for prevention of SSIs.
TRAINED IMMUNITY AND POSTOPERATIVE IMMUNOSUPPRESSION
Postoperative immunosuppression is commonly seen after surgery. It results from a dysregulated immune response, involving neurohormonal, immunological, and hemodynamic factors (93, 94). Broadly described, surgical trauma induces a local hyperinflammatory response, which contributes to inducing repair processes and eliminating invading pathogens. However, to protect surrounding tissues against inflammatory damage, compensatory anti-inflammatory responses are elicited (95). This mainly affects cell-mediated immunity and results in an overall immunosuppressed state (93, 94). Undoubtedly, the immune responses involved in postoperative immunosuppression show similarities with sepsis, as also being defined by the simultaneous presence of proinflammatory and anti-inflammatory responses (96).
Due to impairment of cell-mediated immunity, postoperative immunosuppression is associated with an increased susceptibility to infection (97, 98). In sepsis-induced immunosuppression, the functions of both tissue-resident and blood immune cells are impaired (99, 100). Since tissue-resident immune cells play an important part in the immune response against invading pathogens in a surgical wound (101), impairment of their function will contribute to an increased susceptibility to SSIs. Thus, trained immunity might be able to prevent SSIs by decreasing postoperative immunosuppression. We envision two possibilities on how trained immunity might affect postoperative immunosuppression, partly based on evidence from studies of sepsis.
First, postoperative immunosuppression might be decreased by the increased responsiveness of “trained” innate immune cells in the surgical wound. Admittedly, with the simultaneous presence of hyperinflammation and immunosuppression, one could suggest that increased proinflammatory cytokine production associated with trained immunity would elicit a deepened immunosuppressive state. Indeed, multiple studies have shown that an exaggerated IL-6 response is associated with subsequent development of postoperative complications (102, 103). However, to our best knowledge, evidence causally linking IL-6 expression to poor postoperative outcomes is lacking and IL-6 could merely be a marker of excessive inflammation due to defective clearance of damaged tissue and pathogens. Based on earlier data regarding the role of cytokines in host defense during sepsis (104), we propose that trained immunity would limit immunosuppression by enhancing antimicrobial properties and clearance of damaged tissue, thereby limiting unnecessary or inefficient inflammation that can be deleterious (Fig. 4).
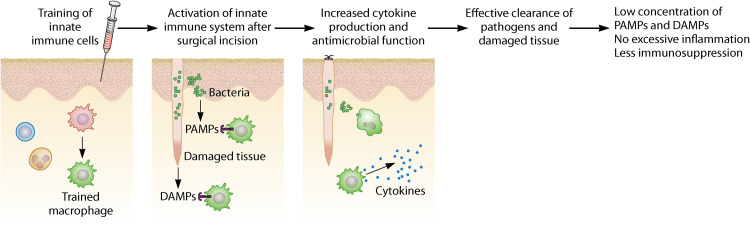
FIG 4.
Trained immunity decreases immunosuppression by inducing an effective innate immune response. The “trained” innate immune system becomes activated by the extensive release of damage-associated molecular patterns (DAMPs) from damaged tissue. In addition, the relatively small release of pathogen-associated molecular patterns (PAMPs) from invading pathogens contributes to eliciting an immune response. Upon recognition of DAMPs and PAMPs, “trained” innate immune cells show an increased responsiveness, thereby resulting in an effective clearance of pathogens and damaged tissue. This results in a low concentration of PAMPs and DAMPs in the surgical wound, thereby preventing excessive inflammation and severe immunosuppression.
Second, trained immunity might decrease postoperative immunosuppression by reversing surgery-induced tolerance of innate immune cells. In the postoperative period, monocyte deactivation can occur. This is characterized by reduced human leukocyte antigen DR (HLA-DR) expression and diminished production of proinflammatory cytokines upon LPS stimulation (also referred to as immunoparalysis or tolerance) (105–107). Monocyte deactivation has been extensively studied in sepsis and simulated in experiments by LPS administration to induce tolerance. These experiments showed LPS-induced tolerance, as opposed to training, to be mediated by silencing of inflammatory genes by epigenetic modifications (14). Interferon gamma was shown to partially recover metabolic function, cytokine production, and HLA-DR expression on monocytes, indicating reversal of tolerance (99, 108, 109). Similarly, training with β-glucan was able to reverse LPS-induced tolerance in monocytes (110).
In postoperative immunosuppression, we hypothesize that extensive tissue damage following major surgery can induce a state of tolerance in innate immune cells by prolonged or high exposure to DAMPs. Indeed, the levels of several DAMPs are increased after tissue injury (by trauma or surgery) and are negatively correlated with HLA-DR expression (111, 112). Moreover, studies have shown that DAMPs induce tolerance in monocytes via epigenetic changes (113, 114), similar to LPS-induced tolerance. As LPS- and DAMP-induced tolerance are both mediated via epigenetic changes and show largely similar gene expression profiles (14, 113), trained immunity might also reverse DAMP-induced tolerance.
Still, postoperative immunosuppression is complex and incompletely understood. The first step into further research could be the isolation of innate immune cells from surgical patients to study tolerance mechanisms and possible reversal by trained immunity.
HOW TO INDUCE TRAINED IMMUNTIY FOR PREVENTION OF SURGICAL SITE INFECTIONS
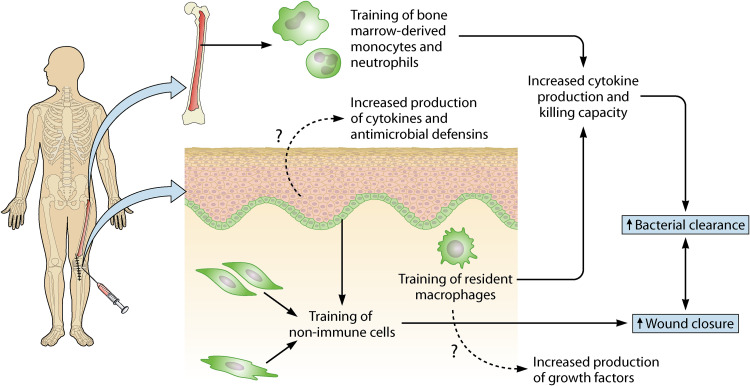
Innate immune memory takes place at the level of bone marrow precursors (central innate immune memory) and at the level of resident macrophages (peripheral innate immune memory). As mentioned before, the induction of trained immunity in both niches provides, independently of one another, protection against skin and soft tissue infection (68, 69). Training of both levels simultaneously seems to increase protection even more (as seen by a greater protection in a previously infected flank than in a naive flank) (68, 78). Thus, it is important to bear in mind which levels of training are induced by administration of a stimulus. Ideally, this trained immunity-inducing stimulus would be administered at the (future) site of injury and could induce long-term responses of resident (non)immune cells and of bone marrow precursors (Fig. 5).
FIG 5.
Administration of stimuli at the (future) site of injury can induce central innate immune memory, peripheral innate immune memory, and/or trained tissue repair. Central innate immune memory is induced by training of bone marrow precursors, resulting in “trained” blood monocytes and neutrophils. Peripheral innate immune memory arises upon training of resident macrophages. By increasing their effector functions, “trained” innate immune cells enhance bacterial clearance. Trained tissue repair is mediated by training of nonimmune cells (e.g., epithelial stem cells, mesenchymal stem cells) and results in enhanced wound closure. As the cells involved often have multiple functions, we hypothesize a contribution of “trained” keratinocytes to host defense by increasing their production in cytokines and antimicrobial defensins. Moreover, “trained” macrophages might contribute to trained tissue repair by an increase in their production of growth factors.
Training agents can be administered before or after surgery. When administered just before or after surgery, effects would be, strictly speaking, due to priming instead of training, as the interval between training agent and surgically inflicted damage is too short for activity of innate immune cells to return to basal levels. The effect of the second stimulus would therefore be additive to the immune response (71). This perhaps has the potential advantage of reversing surgery-induced tolerance of innate immune cells, as discussed in the previous section. The time interval between training and surgery or vice versa needs to be the subject of further research.
Here, we propose several simple and cost-effective ways to induce trained immunity for prevention of SSIs. First, BCG has been well studied for the induction of trained immunity in humans and has proven to be safe for use in immunocompetent individuals and in those with prior BCG vaccination (115, 116). BCG can be delivered as an intradermal injection in the upper arm before or after surgery, as is standard protocol. This has been shown to train bone marrow precursors (32). One could consider administration of BCG at a (future) site of injury, as this might induce training of resident (non)immune cells in the skin. Local application of BCG is not uncommon, with BCG as a standard immunotherapy for superficial bladder cancer and as an option for intralesional therapy in cutaneous melanoma (117, 118). As training with a live attenuated virus, such as BCG, is not considered safe in severely immunocompromised patients, killed gamma-irradiated BCG might be an alternative (119). The limitations of BCG can be represented by the fact that only 50% of individuals respond with an effective induction of trained immunity after BCG vaccination (46, 50).
Second, topical or oral administration of β-glucan might be a potent preventive measure against SSIs. Topical application of β-glucan on wounds has been extensively studied (120). In humans, the application of β-glucan on diabetic ulcers has been shown to increase wound healing (121), mediated by an increased proliferation of fibroblasts and keratinocytes (120). In addition, topical application of β-glucan has been associated with an induced TNF-α release in wound site macrophages (122). As such, β-glucan’s application on surgical wounds might increase local host defense. Importantly, the safety of topical application of β-glucan on chronic wounds is confirmed by several human clinical studies (121, 123, 124).
Oral administration of β-glucan might also be an attractive method to induce training in surgical patients, as it is inexpensive and well tolerated. In mice, oral glucan administration was shown to significantly increase survival upon secondary infection with S. aureus or C. albicans (125). However, in humans, ingestion of 1,000 mg β-glucan once daily for a week did not result in enhanced innate immune responses (126), possibly because β-glucan was not able to cross into the circulation of the subjects in this study.
Lastly, host defense in a surgical wound might be increased by topical application of imiquimod (TLR7 agonist). Imiquimod was shown to train skin epithelial stem cells and enhance wound closure (90). This raises the question whether imiquimod can also induce innate immune memory. Topical application of imiquimod was shown to induce local TNF-α and alpha interferon (IFN-α) release (127). More importantly, topical application of imiquimod significantly increased immunogenicity against the influenza vaccine, indicating a priming effect (128). A small study in which imiquimod was applied on surgical wounds reported no serious adverse events, albeit local skin reactions were reported (129).
In addition, there are several other training agents: muramyl dipeptide, CpG oligodeoxynucleotides, mevalonate, oxLDL, and uric acid (130). These might also be worth considering for prevention of SSIs. In addition, recent studies have suggested a role for gut microbiota in induction of trained immunity (131, 132). This raises the question whether changing the composition of the gut microbiota before surgery can perhaps change the microbe-associated molecular patterns or metabolites to which innate immune cells are exposed and thereupon induce innate immune memory.
CONCLUSIONS
Trained immunity provides a compelling concept to be exploited for additional prevention of SSIs. Multiple mechanisms seem to be involved in the protection against SSIs by trained immunity. First, trained immunity enhances local host defense against infections in the skin and soft tissue, which, apart from the surgical incision and tissue manipulation, resemble surgical site infections. Protection in the skin is afforded by “trained” bone marrow-derived monocytes and resident macrophages, but “trained” neutrophils might also contribute. Second, the increased responsiveness of innate immune cells in a surgical wound results in a rapid clearance of pathogens and damaged tissue. This might prevent excessive inflammation and, as such, promotes tissue repair and limits immunodepression. Third, tissue repair is possibly enhanced after exposure to a previous inflammatory insult, also termed trained tissue repair. This is (partly) mediated by training of nonimmune cells, which act by enhancing wound closure. Trained tissue repair restores the mechanical barrier more quickly and prevents invasion of pathogens. Lastly, trained immunity might counter surgery-induced tolerance of innate immune cells.
Future research should focus on expanding knowledge of these mechanisms. Animal models can help study the “trained” immune response in surgical wounds—specifically the role of “trained” neutrophils—and to study β-glucan, BCG, and imiquimod as preventive measures against SSIs. The wound infection model described by Stratford et al. or by Lamont et al. can be used as a murine model of surgical site infection (64, 133). Nevertheless, considering the differences in mouse and human immunology, the most important line of study will be the clinical translation from animals to humans. Hopefully, this review can inspire future preclinical and clinical studies in this area and contribute to new preventive measures for surgical site infections.
ACKNOWLEDGMENTS
M.G.N. was supported by a Spinoza grant from the Netherlands Organization for Scientific Research and a European Research Council (ERC) advanced grant (no. 833247).
M.G.N. and L.A.B.J. are scientific founders of Trained Therapeutics Discovery. All other authors declare no competing interests.
Biographies

Lieke ter Steeg is an M.Sc. student of medicine at Radboudumc who has done the groundwork for this review as part of her M.Sc. research elective and who aspires to be a clinician-scientist in internal medicine or clinical microbiology. She is interested in the clinical translation of “trained immunity” and the development of new treatment options for infectious and inflammatory diseases.

Jorge Domínguez-Andrés, Pharm.D., Ph.D., is a scientific researcher at the Radboud University Medical Center in Nijmegen, the Netherlands. He received his Pharm.D. from the University of Salamanca in Spain and completed his biomedical qualifications at the Hôpital Nord Laennec in Nantes (France) and at the University of Navarra (Spain). He received his Ph.D. at the University Autónoma of Madrid (Spain). In 2017, he joined the Radboud University Medical Center in Nijmegen. He studies the role of specific metabolites in the modulation of the immune response against pathogens and the elucidation of the molecular mechanisms controlling the inflammatory response to develop a new generation of vaccines with broader effects. He has worked in this field for 8 years.

Mihai G. Netea was born and studied medicine in Cluj-Napoca, Romania. He completed his Ph.D. at Radboud University, Nijmegen, the Netherlands, on studies investigating the cytokine network in sepsis. After working as a postdoc at the University of Colorado, he returned to Nijmegen, where he finished his clinical training as an infectious disease specialist and where he currently heads the Division of Experimental Medicine, Department of Internal Medicine, Nijmegen University Nijmegen Medical Center. He is mainly interested in understanding the factors influencing the variability of human immune responses, the biology of sepsis, and immunoparalysis in severe infections and in studying the memory traits of innate immunity.

Leo A. B. Joosten studied Biochemistry and Pathobiology at Radboud University (Nijmegen, the Netherlands). He has been an associate professor of Experimental Medicine at Radboudumc (Nijmegen, the Netherlands) and a visiting professor at the University Federal Goiania (Goiania, Brazil). He currently holds a position as a pathobiologist and full professor of mechanisms of inflammatory diseases at Radboud University (Nijmegen, the Netherlands) and is a visiting professor at the University of Alabama at Birmingham (Birmingham, AL, USA) and Faculdade Polícia Militar (Goiania, Brazil). His research for the past 10 years has focused on the role of cytokines in the pathogenesis of chronic inflammation and aimed to find novel inhibitors to treat patients with these inflammatory diseases. He has also been studying “trained immunity”; his main interests are the effects of different metabolites on trained immunity and the mechanisms underlying trained immunity.

Reinout van Crevel studied Medicine at the University of Amsterdam and conducted his training in infectious diseases and his Ph.D. at Radboud University Medical Center. He previously lived and worked in Indonesia, leading a research and implementation program on HIV (2006–2011). He now works as head of the Infectious Disease Division and professor in Global Health at Radboud and is an honorary professor at Oxford University and the University of Indonesia, Jakarta. He combines his patient care, teaching, and research in the Netherlands with collaborative research in Indonesia, Uganda, and Tanzania. Since 1998, his main research interest has been tuberculosis, where he aims to integrate patient studies and laboratory sciences to understand the “biology” and improve diagnosis and management of Mycobacterium tuberculosis infection and severe and diabetes-associated active tuberculosis. Since 2011, he has also been studying “trained immunity,” the topic of this paper, and its translation to clinical practice.
REFERENCES
- 1.National Healthcare Safety Network. 2021. Surgical site infection event (SSI). CDC, Atlanta, GA.
- 2.Mangram AJ, Horan TC, Pearson ML, Silver LC, Jarvis WR. 1999. Guideline for prevention of surgical site infection, 1999. Hospital Infection Control Practices Advisory Committee. Infect Control Hosp Epidemiol 20:250–278; quiz 279–280. 10.1086/501620. [DOI] [PubMed] [Google Scholar]
- 3.Mu Y, Edwards JR, Horan TC, Berrios-Torres SI, Fridkin SK. 2011. Improving risk-adjusted measures of surgical site infection for the national healthcare safety network. Infect Control Hosp Epidemiol 32:970–986. 10.1086/662016. [DOI] [PubMed] [Google Scholar]
- 4.Klevens RM, Edwards JR, Richards CL, Jr, Horan TC, Gaynes RP, Pollock DA, Cardo DM. 2007. Estimating health care-associated infections and deaths in U.S. hospitals. Public Health Rep 122:160–166. 10.1177/003335490712200205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Turner MC, Migaly J. 2019. Surgical site infection: the clinical and economic impact. Clin Colon Rectal Surg 32:157–165. 10.1055/s-0038-1677002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Owens CD, Stoessel K. 2008. Surgical site infections: epidemiology, microbiology and prevention. J Hosp Infect 70(Suppl 2):3–10. 10.1016/S0195-6701(08)60017-1. [DOI] [PubMed] [Google Scholar]
- 7.van Leersum NJ, Janssen-Heijnen MLG, Wouters MWJM, Rutten HJT, Coebergh JW, Tollenaar RAEM, Lemmens VEPP. 2013. Increasing prevalence of comorbidity in patients with colorectal cancer in the South of the Netherlands 1995–2010. Int J Cancer 132:2157–2163. 10.1002/ijc.27871. [DOI] [PubMed] [Google Scholar]
- 8.Vargas-Alzate CA, Higuita-Gutiérrez LF, López-López L, Cienfuegos-Gallet AV, Jiménez Quiceno JN. 2018. High excess costs of infections caused by carbapenem-resistant Gram-negative bacilli in an endemic region. Int J Antimicrob Agents 51:601–607. 10.1016/j.ijantimicag.2017.12.012. [DOI] [PubMed] [Google Scholar]
- 9.Engemann JJ, Carmeli Y, Cosgrove SE, Fowler VG, Bronstein MZ, Trivette SL, Briggs JP, Sexton DJ, Kaye KS. 2003. Adverse clinical and economic outcomes attributable to methicillin resistance among patients with Staphylococcus aureus surgical site infection. Clin Infect Dis 36:592–598. 10.1086/367653. [DOI] [PubMed] [Google Scholar]
- 10.Netea MG, Joosten LAB, Latz E, Mills KHG, Natoli G, Stunnenberg HG, O'Neill LAJ, Xavier RJ. 2016. Trained immunity: a program of innate immune memory in health and disease. Science 352:aaf1098. 10.1126/science.aaf1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eming SA, Krieg T, Davidson JM. 2007. Inflammation in wound repair: molecular and cellular mechanisms. J Invest Dermatol 127:514–525. 10.1038/sj.jid.5700701. [DOI] [PubMed] [Google Scholar]
- 12.Netea MG, Quintin J, van der Meer JW. 2011. Trained immunity: a memory for innate host defense. Cell Host Microbe 9:355–361. 10.1016/j.chom.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 13.Kumar H, Kawai T, Akira S. 2011. Pathogen recognition by the innate immune system. Int Rev Immunol 30:16–34. 10.3109/08830185.2010.529976. [DOI] [PubMed] [Google Scholar]
- 14.Foster SL, Hargreaves DC, Medzhitov R. 2007. Gene-specific control of inflammation by TLR-induced chromatin modifications. Nature 447:972–978. 10.1038/nature05836. [DOI] [PubMed] [Google Scholar]
- 15.Quintin J, Saeed S, Martens JHA, Giamarellos-Bourboulis EJ, Ifrim DC, Logie C, Jacobs L, Jansen T, Kullberg BJ, Wijmenga C, Joosten LAB, Xavier RJ, van der Meer JWM, Stunnenberg HG, Netea MG. 2012. Candida albicans infection affords protection against reinfection via functional reprogramming of monocytes. Cell Host Microbe 12:223–232. 10.1016/j.chom.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saeed S, Quintin J, Kerstens HH, Rao NA, Aghajanirefah A, Matarese F, Cheng SC, Ratter J, Berentsen K, van der Ent MA, Sharifi N, Janssen-Megens EM, Ter Huurne M, Mandoli A, van Schaik T, Ng A, Burden F, Downes K, Frontini M, Kumar V, Giamarellos-Bourboulis EJ, Ouwehand WH, van der Meer JW, Joosten LA, Wijmenga C, Martens JH, Xavier RJ, Logie C, Netea MG, Stunnenberg HG. 2014. Epigenetic programming of monocyte-to-macrophage differentiation and trained innate immunity. Science 345:1251086. 10.1126/science.1251086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kleinnijenhuis J, Quintin J, Preijers F, Joosten LA, Ifrim DC, Saeed S, Jacobs C, van Loenhout J, de Jong D, Stunnenberg HG, Xavier RJ, van der Meer JW, van Crevel R, Netea MG. 2012. Bacille Calmette-Guerin induces NOD2-dependent nonspecific protection from reinfection via epigenetic reprogramming of monocytes. Proc Natl Acad Sci USA 109:17537–17542. 10.1073/pnas.1202870109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ostuni R, Piccolo V, Barozzi I, Polletti S, Termanini A, Bonifacio S, Curina A, Prosperini E, Ghisletti S, Natoli G. 2013. Latent enhancers activated by stimulation in differentiated cells. Cell 152:157–171. 10.1016/j.cell.2012.12.018. [DOI] [PubMed] [Google Scholar]
- 19.Kaikkonen MU, Spann NJ, Heinz S, Romanoski CE, Allison KA, Stender JD, Chun HB, Tough DF, Prinjha RK, Benner C, Glass CK. 2013. Remodeling of the enhancer landscape during macrophage activation is coupled to enhancer transcription. Mol Cell 51:310–325. 10.1016/j.molcel.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fanucchi S, Fok ET, Dalla E, Shibayama Y, Börner K, Chang EY, Stoychev S, Imakaev M, Grimm D, Wang KC, Li G, Sung WK, Mhlanga MM. 2019. Immune genes are primed for robust transcription by proximal long noncoding RNAs located in nuclear compartments. Nat Genet 51:138–150. 10.1038/s41588-018-0298-2. [DOI] [PubMed] [Google Scholar]
- 21.Cheng S-C, Quintin J, Cramer RA, Shepardson KM, Saeed S, Kumar V, Giamarellos-Bourboulis EJ, Martens JHA, Rao NA, Aghajanirefah A, Manjeri GR, Li Y, Ifrim DC, Arts RJW, van der Veer BMJW, van der Meer BMJW, Deen PMT, Logie C, O'Neill LA, Willems P, van de Veerdonk FL, van der Meer JWM, Ng A, Joosten LAB, Wijmenga C, Stunnenberg HG, Xavier RJ, Netea MG. 2014. mTOR- and HIF-1α-mediated aerobic glycolysis as metabolic basis for trained immunity. Science 345:1250684. 10.1126/science.1250684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arts RJW, Carvalho A, La Rocca C, Palma C, Rodrigues F, Silvestre R, Kleinnijenhuis J, Lachmandas E, Gonçalves LG, Belinha A, Cunha C, Oosting M, Joosten LAB, Matarese G, van Crevel R, Netea MG. 2016. Immunometabolic pathways in BCG-induced trained immunity. Cell Rep 17:2562–2571. 10.1016/j.celrep.2016.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Keating ST, Groh L, van der Heijden CDCC, Rodriguez H, Dos Santos JC, Fanucchi S, Okabe J, Kaipananickal H, van Puffelen JH, Helder L, Noz MP, Matzaraki V, Li Y, de Bree LCJ, Koeken VACM, Moorlag S, Mourits VP, Domínguez-Andrés J, Oosting M, Bulthuis EP, Koopman WJH, Mhlanga M, El-Osta A, Joosten LAB, Netea MG, Riksen NP. 2020. The Set7 lysine methyltransferase regulates plasticity in oxidative phosphorylation necessary for trained immunity induced by β-glucan. Cell Rep 31:107548–107548. 10.1016/j.celrep.2020.107548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Latham T, Mackay L, Sproul D, Karim M, Culley J, Harrison DJ, Hayward L, Langridge-Smith P, Gilbert N, Ramsahoye BH. 2012. Lactate, a product of glycolytic metabolism, inhibits histone deacetylase activity and promotes changes in gene expression. Nucleic Acids Res 40:4794–4803. 10.1093/nar/gks066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arts RJ, Novakovic B, Ter Horst R, Carvalho A, Bekkering S, Lachmandas E, Rodrigues F, Silvestre R, Cheng SC, Wang SY, Habibi E, Gonçalves LG, Mesquita I, Cunha C, van Laarhoven A, van de Veerdonk FL, Williams DL, van der Meer JW, Logie C, O'Neill LA, Dinarello CA, Riksen NP, van Crevel R, Clish C, Notebaart RA, Joosten LA, Stunnenberg HG, Xavier RJ, Netea MG. 2016. Glutaminolysis and fumarate accumulation integrate immunometabolic and epigenetic programs in trained immunity. Cell Metab 24:807–819. 10.1016/j.cmet.2016.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Serra-Pérez A, Planas AM, Núñez-O'Mara A, Berra E, García-Villoria J, Ribes A, Santalucía T. 2010. Extended ischemia prevents HIF1alpha degradation at reoxygenation by impairing prolyl-hydroxylation: role of Krebs cycle metabolites. J Biol Chem 285:18217–18224. 10.1074/jbc.M110.101048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tannahill GM, Curtis AM, Adamik J, Palsson-McDermott EM, McGettrick AF, Goel G, Frezza C, Bernard NJ, Kelly B, Foley NH, Zheng L, Gardet A, Tong Z, Jany SS, Corr SC, Haneklaus M, Caffrey BE, Pierce K, Walmsley S, Beasley FC, Cummins E, Nizet V, Whyte M, Taylor CT, Lin H, Masters SL, Gottlieb E, Kelly VP, Clish C, Auron PE, Xavier RJ, O’Neill LAJ. 2013. Succinate is an inflammatory signal that induces IL-1β through HIF-1α. Nature 496:238–242. 10.1038/nature11986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gut P, Verdin E. 2013. The nexus of chromatin regulation and intermediary metabolism. Nature 502:489–498. 10.1038/nature12752. [DOI] [PubMed] [Google Scholar]
- 29.Bekkering S, Arts RJW, Novakovic B, Kourtzelis I, van der Heijden C, Li Y, Popa CD, Ter Horst R, van Tuijl J, Netea-Maier RT, van de Veerdonk FL, Chavakis T, Joosten LAB, van der Meer JWM, Stunnenberg H, Riksen NP, Netea MG. 2018. Metabolic induction of trained immunity through the mevalonate pathway. Cell 172:135–146.e9. 10.1016/j.cell.2017.11.025. [DOI] [PubMed] [Google Scholar]
- 30.Moorlag S, Rodriguez-Rosales YA, Gillard J, Fanucchi S, Theunissen K, Novakovic B, de Bont CM, Negishi Y, Fok ET, Kalafati L, Verginis P, Mourits VP, Koeken V, de Bree LCJ, Pruijn GJM, Fenwick C, van Crevel R, Joosten LAB, Joosten I, Koenen H, Mhlanga MM, Diavatopoulos DA, Chavakis T, Netea MG. 2020. BCG vaccination induces long-term functional reprogramming of human neutrophils. Cell Rep 33:108387. 10.1016/j.celrep.2020.108387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mitroulis I, Ruppova K, Wang B, Chen LS, Grzybek M, Grinenko T, Eugster A, Troullinaki M, Palladini A, Kourtzelis I, Chatzigeorgiou A, Schlitzer A, Beyer M, Joosten LAB, Isermann B, Lesche M, Petzold A, Simons K, Henry I, Dahl A, Schultze JL, Wielockx B, Zamboni N, Mirtschink P, Coskun Ü, Hajishengallis G, Netea MG, Chavakis T. 2018. Modulation of myelopoiesis progenitors is an integral component of trained immunity. Cell 172:147–161.e12. 10.1016/j.cell.2017.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cirovic B, de Bree LCJ, Groh L, Blok BA, Chan J, van der Velden W, Bremmers MEJ, van Crevel R, Händler K, Picelli S, Schulte-Schrepping J, Klee K, Oosting M, Koeken V, van Ingen J, Li Y, Benn CS, Schultze JL, Joosten LAB, Curtis N, Netea MG, Schlitzer A. 2020. BCG vaccination in humans elicits trained immunity via the hematopoietic progenitor compartment. Cell Host Microbe 28:322–334.e5. 10.1016/j.chom.2020.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaufmann E, Sanz J, Dunn JL, Khan N, Mendonça LE, Pacis A, Tzelepis F, Pernet E, Dumaine A, Grenier JC, Mailhot-Léonard F, Ahmed E, Belle J, Besla R, Mazer B, King IL, Nijnik A, Robbins CS, Barreiro LB, Divangahi M. 2018. BCG educates hematopoietic stem cells to generate protective innate immunity against tuberculosis. Cell 172:176–190.e19. 10.1016/j.cell.2017.12.031. [DOI] [PubMed] [Google Scholar]
- 34.Weiner-Lastinger LM, Abner S, Edwards JR, Kallen AJ, Karlsson M, Magill SS, Pollock D, See I, Soe MM, Walters MS, Dudeck MA. 2020. Antimicrobial-resistant pathogens associated with adult healthcare-associated infections: summary of data reported to the National Healthcare Safety Network, 2015–2017. Infect Control Hosp Epidemiol 41:1–18. 10.1017/ice.2019.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Young PY, Khadaroo RG. 2014. Surgical site infections. Surg Clin North Am 94:1245–1264. 10.1016/j.suc.2014.08.008. [DOI] [PubMed] [Google Scholar]
- 36.Benn CS, Netea MG, Selin LK, Aaby P. 2013. A small jab—a big effect: nonspecific immunomodulation by vaccines. Trends Immunol 34:431–439. 10.1016/j.it.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 37.Garly ML, Martins CL, Balé C, Baldé MA, Hedegaard KL, Gustafson P, Lisse IM, Whittle HC, Aaby P. 2003. BCG scar and positive tuberculin reaction associated with reduced child mortality in West Africa. A non-specific beneficial effect of BCG? Vaccine 21:2782–2790. 10.1016/s0264-410x(03)00181-6. [DOI] [PubMed] [Google Scholar]
- 38.Roth A, Gustafson P, Nhaga A, Djana Q, Poulsen A, Garly ML, Jensen H, Sodemann M, Rodriques A, Aaby P. 2005. BCG vaccination scar associated with better childhood survival in Guinea-Bissau. Int J Epidemiol 34:540–547. 10.1093/ije/dyh392. [DOI] [PubMed] [Google Scholar]
- 39.Kristensen I, Aaby P, Jensen H. 2000. Routine vaccinations and child survival: follow up study in Guinea-Bissau, West Africa. BMJ 321:1435–1438. 10.1136/bmj.321.7274.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aaby P, Roth A, Ravn H, Napirna BM, Rodrigues A, Lisse IM, Stensballe L, Diness BR, Lausch KR, Lund N, Biering-Sørensen S, Whittle H, Benn CS. 2011. Randomized trial of BCG vaccination at birth to low-birth-weight children: beneficial nonspecific effects in the neonatal period? J Infect Dis 204:245–252. 10.1093/infdis/jir240. [DOI] [PubMed] [Google Scholar]
- 41.Jayaraman K, Adhisivam B, Nallasivan S, Krishnan RG, Kamalarathnam C, Bharathi M, McSharry B, Namachivayam SP, Shann F, Boopalan SI, David P, Bhat BV. 2019. Two randomized trials of the effect of the Russian strain of bacillus Calmette-Guérin alone or with oral polio vaccine on neonatal mortality in infants weighing <2000 g in India. Pediatr Infect Dis J 38:198–202. 10.1097/INF.0000000000002198. [DOI] [PubMed] [Google Scholar]
- 42.Stensballe LG, Ravn H, Birk NM, Kjærgaard J, Nissen TN, Pihl GT, Thøstesen LM, Greisen G, Jeppesen DL, Kofoed PE, Pryds O, Sørup S, Aaby P, Benn CS. 2019. BCG vaccination at birth and rate of hospitalization for infection until 15 months of age in Danish children: a randomized clinical multicenter trial. J Pediatric Infect Dis Soc 8:213–220. 10.1093/jpids/piy029. [DOI] [PubMed] [Google Scholar]
- 43.Kjærgaard J, Birk NM, Nissen TN, Thøstesen LM, Pihl GT, Benn CS, Jeppesen DL, Pryds O, Kofoed PE, Aaby P, Greisen G, Stensballe LG. 2016. Nonspecific effect of BCG vaccination at birth on early childhood infections: a randomized, clinical multicenter trial. Pediatr Res 80:681–685. 10.1038/pr.2016.142. [DOI] [PubMed] [Google Scholar]
- 44.Stensballe LG, Sørup S, Aaby P, Benn CS, Greisen G, Jeppesen DL, Birk NM, Kjærgaard J, Nissen TN, Pihl GT, Thøstesen LM, Kofoed P-E, Pryds O, Ravn H. 2017. BCG vaccination at birth and early childhood hospitalisation: a randomised clinical multicentre trial. Arch Dis Child 102:224–231. 10.1136/archdischild-2016-310760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kleinnijenhuis J, Quintin J, Preijers F, Benn CS, Joosten LA, Jacobs C, van Loenhout J, Xavier RJ, Aaby P, van der Meer JW, van Crevel R, Netea MG. 2014. Long-lasting effects of BCG vaccination on both heterologous Th1/Th17 responses and innate trained immunity. J Innate Immun 6:152–158. 10.1159/000355628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Arts RJW, Moorlag S, Novakovic B, Li Y, Wang SY, Oosting M, Kumar V, Xavier RJ, Wijmenga C, Joosten LAB, Reusken C, Benn CS, Aaby P, Koopmans MP, Stunnenberg HG, van Crevel R, Netea MG. 2018. BCG vaccination protects against experimental viral infection in humans through the induction of cytokines associated with trained immunity. Cell Host Microbe 23:89–100.e5. 10.1016/j.chom.2017.12.010. [DOI] [PubMed] [Google Scholar]
- 47.Jensen KJ, Larsen N, Biering-Sørensen S, Andersen A, Eriksen HB, Monteiro I, Hougaard D, Aaby P, Netea MG, Flanagan KL, Benn CS. 2015. Heterologous immunological effects of early BCG vaccination in low-birth-weight infants in Guinea-Bissau: a randomized-controlled trial. J Infect Dis 211:956–967. 10.1093/infdis/jiu508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Koeken V, de Bree LCJ, Mourits VP, Moorlag SJ, Walk J, Cirovic B, Arts RJ, Jaeger M, Dijkstra H, Lemmers H, Joosten LA, Benn CS, van Crevel R, Netea M. 2020. BCG vaccination in humans inhibits systemic inflammation in a sex-dependent manner. J Clin Invest 130:5591–5602. 10.1172/JCI133935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Giamarellos-Bourboulis EJ, Tsilika M, Moorlag S, Antonakos N, Kotsaki A, Domínguez-Andrés J, Kyriazopoulou E, Gkavogianni T, Adami M-E, Damoraki G, Koufargyris P, Karageorgos A, Bolanou A, Koenen H, van Crevel R, Droggiti D-I, Renieris G, Papadopoulos A, Netea MG. 2020. Activate: randomized clinical trial of BCG vaccination against infection in the elderly. Cell 183:315–323.e9. 10.1016/j.cell.2020.08.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Walk J, de Bree LCJ, Graumans W, Stoter R, van Gemert GJ, van de Vegte-Bolmer M, Teelen K, Hermsen CC, Arts RJW, Behet MC, Keramati F, Moorlag S, Yang ASP, van Crevel R, Aaby P, de Mast Q, van der Ven A, Stabell Benn C, Netea MG, Sauerwein RW. 2019. Outcomes of controlled human malaria infection after BCG vaccination. Nat Commun 10:874. 10.1038/s41467-019-08659-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Moorlag S, Arts RJW, van Crevel R, Netea MG. 2019. Non-specific effects of BCG vaccine on viral infections. Clin Microbiol Infect 25:1473–1478. 10.1016/j.cmi.2019.04.020. [DOI] [PubMed] [Google Scholar]
- 52.Netea MG, Giamarellos-Bourboulis EJ, Domínguez-Andrés J, Curtis N, van Crevel R, van de Veerdonk FL, Bonten M. 2020. Trained immunity: a tool for reducing susceptibility to and the severity of SARS-CoV-2 infection. Cell 181:969–977. 10.1016/j.cell.2020.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.de Castro MJ, Pardo-Seco J, Martinón-Torres F. 2015. Nonspecific (heterologous) protection of neonatal BCG vaccination against hospitalization due to respiratory infection and sepsis. Clin Infect Dis 60:1611–1619. 10.1093/cid/civ144. [DOI] [PubMed] [Google Scholar]
- 54.Marakalala MJ, Williams DL, Hoving JC, Engstad R, Netea MG, Brown GD. 2013. Dectin-1 plays a redundant role in the immunomodulatory activities of β-glucan-rich ligands in vivo. Microbes Infect 15:511–515. 10.1016/j.micinf.2013.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Di Luzio NR, Williams DL. 1978. Protective effect of glucan against systemic Staphylococcus aureus septicemia in normal and leukemic mice. Infect Immun 20:804–810. 10.1128/iai.20.3.804-810.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bistoni F, Vecchiarelli A, Cenci E, Puccetti P, Marconi P, Cassone A. 1986. Evidence for macrophage-mediated protection against lethal Candida albicans infection. Infect Immun 51:668–674. 10.1128/iai.51.2.668-674.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sher NA, Chaparas SD, Greenberg LE, Bernard S. 1975. Effects of BCG, Corynebacterium parvum, and methanol-extraction residue in the reduction of mortality from Staphylococcus aureus and Candida albicans infections in immunosuppressed mice. Infect Immun 12:1325–1330. 10.1128/iai.12.6.1325-1330.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ciarlo E, Heinonen T, Théroude C, Asgari F, Le Roy D, Netea MG, Roger T. 2020. Trained immunity confers broad-spectrum protection against bacterial infections. J Infect Dis 222:1869–1881. 10.1093/infdis/jiz692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kokoshis PL, Williams DL, Cook JA, Di Luzio NR. 1978. Increased resistance to Staphylococcus aureus infection and enhancement in serum lysozyme activity by glucan. Science 199:1340–1342. 10.1126/science.628841. [DOI] [PubMed] [Google Scholar]
- 60.Moorlag S, Khan N, Novakovic B, Kaufmann E, Jansen T, van Crevel R, Divangahi M, Netea MG. 2020. β-Glucan induces protective trained immunity against Mycobacterium tuberculosis infection: a key role for IL-1. Cell Rep 31:107634. 10.1016/j.celrep.2020.107634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Barton ES, White DW, Cathelyn JS, Brett-McClellan KA, Engle M, Diamond MS, Miller VL, Virgin HW, IV.. 2007. Herpesvirus latency confers symbiotic protection from bacterial infection. Nature 447:326–329. 10.1038/nature05762. [DOI] [PubMed] [Google Scholar]
- 62.Tarancón R, Domínguez-Andrés J, Uranga S, Ferreira AV, Groh LA, Domenech M, González-Camacho F, Riksen NP, Aguilo N, Yuste J, Martín C, Netea MG. 2020. New live attenuated tuberculosis vaccine MTBVAC induces trained immunity and confers protection against experimental lethal pneumonia. PLoS Pathog 16:e1008404. 10.1371/journal.ppat.1008404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yao Y, Jeyanathan M, Haddadi S, Barra NG, Vaseghi-Shanjani M, Damjanovic D, Lai R, Afkhami S, Chen Y, Dvorkin-Gheva A, Robbins CS, Schertzer JD, Xing Z. 2018. Induction of autonomous memory alveolar macrophages requires T cell help and is critical to trained immunity. Cell 175:1634–1650.e17. 10.1016/j.cell.2018.09.042. [DOI] [PubMed] [Google Scholar]
- 64.Lamont PM, Schrodt GR, Galland RB, Kaufman CM, Cheadle WG, Polk HC, Jr.. 1984. Enhancement of the local inflammatory response to bacterial infection by muramyl dipeptide. Br J Exp Pathol 65:319–325. [PMC free article] [PubMed] [Google Scholar]
- 65.Zhu H, Lin J, Wei H, Bao B, Gao T, Zheng X. 2020. Does training innate immunity confer broad-spectrum protection against bone and joint infection in a mouse model? Clin Orthop Relat Res 478:2670–2681. 10.1097/CORR.0000000000001461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fagelman KM, Flint LM, Jr, McCoy MT, Polk HC, Jr, Trachtenberg LS. 1981. Simulated surgical wound infection in mice: effect of stimulation on nonspecific host defense mechanisms. Arch Surg 116:761–764. 10.1001/archsurg.1981.01380180021005. [DOI] [PubMed] [Google Scholar]
- 67.Cobb JP, Brown CM, Brown GL, Polk HC, Jr.. 1986. Muramyl dipeptide protects decomplemented mice from surgically-induced infection. Int J Immunopharmacol 8:799–803. 10.1016/0192-0561(86)90017-2. [DOI] [PubMed] [Google Scholar]
- 68.Chan LC, Chaili S, Filler SG, Miller LS, Solis NV, Wang H, Johnson CW, Lee HK, Diaz LF, Yeaman MR. 2017. Innate immune memory contributes to host defense against recurrent skin and skin structure infections caused by methicillin-resistant Staphylococcus aureus. Infect Immun 85:e00876-16. 10.1128/IAI.00876-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Feuerstein R, Forde AJ, Lohrmann F, Kolter J, Ramirez NJ, Zimmermann J, Gomez de Agüero M, Henneke P. 2020. Resident macrophages acquire innate immune memory in staphylococcal skin infection. Elife 9:e55602. 10.7554/eLife.55602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wong Fok Lung T, Monk IR, Acker KP, Mu A, Wang N, Riquelme SA, Pires S, Noguera LP, Dach F, Gabryszewski SJ, Howden BP, Prince A. 2020. Staphylococcus aureus small colony variants impair host immunity by activating host cell glycolysis and inducing necroptosis. Nat Microbiol 5:141–153. 10.1038/s41564-019-0597-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Divangahi M, Aaby P, Khader SA, Barreiro LB, Bekkering S, Chavakis T, van Crevel R, Curtis N, DiNardo AR, Dominguez-Andres J, Duivenvoorden R, Fanucchi S, Fayad Z, Fuchs E, Hamon M, Jeffrey KL, Khan N, Joosten LAB, Kaufmann E, Latz E, Matarese G, van der Meer JWM, Mhlanga M, Moorlag SJCFM, Mulder WJM, Naik S, Novakovic B, O'Neill L, Ochando J, Ozato K, Riksen NP, Sauerwein R, Sherwood ER, Schlitzer A, Schultze JL, Sieweke MH, Benn CS, Stunnenberg H, Sun J, van de Veerdonk FL, Weis S, Williams DL, Xavier R, Netea MG. 2021. Trained immunity, tolerance, priming and differentiation: distinct immunological processes. Nat Immunol 22:2–6. 10.1038/s41590-020-00845-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Reinke JM, Sorg H. 2012. Wound repair and regeneration. Eur Surg Res 49:35–43. 10.1159/000339613. [DOI] [PubMed] [Google Scholar]
- 73.Singer AJ, Clark RAF. 1999. Cutaneous wound healing. N Engl J Med 341:738–746. 10.1056/NEJM199909023411006. [DOI] [PubMed] [Google Scholar]
- 74.Wang J. 2018. Neutrophils in tissue injury and repair. Cell Tissue Res 371:531–539. 10.1007/s00441-017-2785-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Davies LC, Jenkins SJ, Allen JE, Taylor PR. 2013. Tissue-resident macrophages. Nat Immunol 14:986–995. 10.1038/ni.2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Galland RB, Trachtenberg LS, Rynerson N, Polk HC, Jr.. 1982. Nonspecific enhancement of resistance to local bacterial infection in starved mice. Arch Surg 117:161–164. 10.1001/archsurg.1982.01380260041007. [DOI] [PubMed] [Google Scholar]
- 77.Lamont PM, Maier KG, Melton L, Polk HC, Jr.. 1987. Stimulatory effects of muramyl dipeptide upon neutrophils isolated from a local bacterial infection. Br J Exp Pathol 68:655–661. [PMC free article] [PubMed] [Google Scholar]
- 78.Chan LC, Rossetti M, Miller LS, Filler SG, Johnson CW, Lee HK, Wang H, Gjertson D, Fowler VG, Jr, Reed EF, Yeaman MR, Group MSI, MRSA Systems Immunobiology Group . 2018. Protective immunity in recurrent Staphylococcus aureus infection reflects localized immune signatures and macrophage-conferred memory. Proc Natl Acad Sci USA 115:E11111–E11119. 10.1073/pnas.1808353115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Krishna S, Miller LS. 2012. Innate and adaptive immune responses against Staphylococcus aureus skin infections. Semin Immunopathol 34:261–280. 10.1007/s00281-011-0292-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Miller LS, Cho JS. 2011. Immunity against Staphylococcus aureus cutaneous infections. Nat Rev Immunol 11:505–518. 10.1038/nri3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Feuerstein R, Seidl M, Prinz M, Henneke P. 2015. MyD88 in macrophages is critical for abscess resolution in staphylococcal skin infection. J Immunol 194:2735–2745. 10.4049/jimmunol.1402566. [DOI] [PubMed] [Google Scholar]
- 82.Khan N, Downey J, Sanz J, Kaufmann E, Blankenhaus B, Pacis A, Pernet E, Ahmed E, Cardoso S, Nijnik A, Mazer B, Sassetti C, Behr MA, Soares MP, Barreiro LB, Divangahi M. 2020. M. tuberculosis reprograms hematopoietic stem cells to limit myelopoiesis and impair trained immunity. Cell 183:752–770.e22. 10.1016/j.cell.2020.09.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wynn TA, Vannella KM. 2016. Macrophages in tissue repair, regeneration, and fibrosis. Immunity 44:450–462. 10.1016/j.immuni.2016.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rappolee DA, Mark D, Banda MJ, Werb Z. 1988. Wound macrophages express TGF-alpha and other growth factors in vivo: analysis by mRNA phenotyping. Science 241:708–712. 10.1126/science.3041594. [DOI] [PubMed] [Google Scholar]
- 85.Peiseler M, Kubes P. 2019. More friend than foe: the emerging role of neutrophils in tissue repair. J Clin Invest 129:2629–2639. 10.1172/JCI124616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bekkering S, Quintin J, Joosten LA, van der Meer JW, Netea MG, Riksen NP. 2014. Oxidized low-density lipoprotein induces long-term proinflammatory cytokine production and foam cell formation via epigenetic reprogramming of monocytes. Arterioscler Thromb Vasc Biol 34:1731–1738. 10.1161/ATVBAHA.114.303887. [DOI] [PubMed] [Google Scholar]
- 87.Arts RJW, Joosten LAB, Netea MG. 2018. The potential role of trained immunity in autoimmune and autoinflammatory disorders. Front Immunol 9:298. 10.3389/fimmu.2018.00298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hamada A, Torre C, Drancourt M, Ghigo E. 2018. Trained immunity carried by non-immune cells. Front Microbiol 9:3225. 10.3389/fmicb.2018.03225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Crowley T, Buckley CD, Clark AR. 2018. Stroma: the forgotten cells of innate immune memory. Clin Exp Immunol 193:24–36. 10.1111/cei.13149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Naik S, Larsen SB, Gomez NC, Alaverdyan K, Sendoel A, Yuan S, Polak L, Kulukian A, Chai S, Fuchs E. 2017. Inflammatory memory sensitizes skin epithelial stem cells to tissue damage. Nature 550:475–480. 10.1038/nature24271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Liu GY, Liu Y, Lu Y, Qin YR, Di GH, Lei YH, Liu HX, Li YQ, Wu C, Hu XW, Duan HF. 2016. Short-term memory of danger signals or environmental stimuli in mesenchymal stem cells: implications for therapeutic potential. Cell Mol Immunol 13:369–378. 10.1038/cmi.2015.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hajishengallis G, Li X, Mitroulis I, Chavakis T. 2019. Trained innate immunity and its implications for mucosal immunity and inflammation. Adv Exp Med Biol 1197:11–26. 10.1007/978-3-030-28524-1_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Menger MD, Vollmar B. 2004. Surgical trauma: hyperinflammation versus immunosuppression? Langenbecks Arch Surg 389:475–484. 10.1007/s00423-004-0472-0. [DOI] [PubMed] [Google Scholar]
- 94.Baumann H, Gauldie J. 1994. The acute phase response. Immunol Today 15:74–80. 10.1016/0167-5699(94)90137-6. [DOI] [PubMed] [Google Scholar]
- 95.Munford RS, Pugin J. 2001. Normal responses to injury prevent systemic inflammation and can be immunosuppressive. Am J Respir Crit Care Med 163:316–321. 10.1164/ajrccm.163.2.2007102. [DOI] [PubMed] [Google Scholar]
- 96.van der Poll T, van de Veerdonk FL, Scicluna BP, Netea MG. 2017. The immunopathology of sepsis and potential therapeutic targets. Nat Rev Immunol 17:407–420. 10.1038/nri.2017.36. [DOI] [PubMed] [Google Scholar]
- 97.Angele MK, Faist E. 2002. Clinical review: immunodepression in the surgical patient and increased susceptibility to infection. Crit Care 6:298–305. 10.1186/cc1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wakefield CH, Carey PD, Foulds S, Monson JR, Guillou PJ. 2005. Changes in major histocompatibility complex class II expression in monocytes and T cells of patients developing infection after surgery. Br J Surg 80:205–209. 10.1002/bjs.1800800224. [DOI] [PubMed] [Google Scholar]
- 99.Leentjens J, Kox M, Koch RM, Preijers F, Joosten LA, van der Hoeven JG, Netea MG, Pickkers P. 2012. Reversal of immunoparalysis in humans in vivo: a double-blind, placebo-controlled, randomized pilot study. Am J Respir Crit Care Med 186:838–845. 10.1164/rccm.201204-0645OC. [DOI] [PubMed] [Google Scholar]
- 100.Boomer JS, To K, Chang KC, Takasu O, Osborne DF, Walton AH, Bricker TL, Jarman SD, 2nd, Kreisel D, Krupnick AS, Srivastava A, Swanson PE, Green JM, Hotchkiss RS. 2011. Immunosuppression in patients who die of sepsis and multiple organ failure. JAMA 306:2594–2605. 10.1001/jama.2011.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kupper TS, Fuhlbrigge RC. 2004. Immune surveillance in the skin: mechanisms and clinical consequences. Nat Rev Immunol 4:211–222. 10.1038/nri1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Baigrie RJ, Lamont PM, Kwiatkowski D, Dallman MJ, Morris PJ. 2005. Systemic cytokine response after major surgery. Br J Surg 79:757–760. 10.1002/bjs.1800790813. [DOI] [PubMed] [Google Scholar]
- 103.Longbottom ER, Torrance HDT, Owen HC, Fragkou PC, Hinds CJ, Pearse RM, O’Dwyer MJ. 2016. Features of postoperative immune suppression are reversible with interferon gamma and independent of interleukin-6 pathways. Ann Surg 264:370–377. 10.1097/SLA.0000000000001484. [DOI] [PubMed] [Google Scholar]
- 104.Netea MG, van der Meer JW, van Deuren M, Kullberg BJ. 2003. Proinflammatory cytokines and sepsis syndrome: not enough, or too much of a good thing? Trends Immunol 24:254–258. 10.1016/s1471-4906(03)00079-6. [DOI] [PubMed] [Google Scholar]
- 105.Handy JM, Scott AJ, Cross AM, Sinha P, O’Dea KP, Takata M. 2010. HLA-DR expression and differential trafficking of monocyte subsets following low to intermediate risk surgery. Anaesthesia 65:27–35. 10.1111/j.1365-2044.2009.06161.x. [DOI] [PubMed] [Google Scholar]
- 106.Lachmann G, von Haefen C, Kurth J, Yuerek F, Spies C. 2018. Innate immunity recovers earlier than acquired immunity during severe postoperative immunosuppression. Int J Med Sci 15:1–9. 10.7150/ijms.21433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Volk HD, Thieme M, Heym S, Döcke WD, Ruppe U, Tausch W, Manger D, Zuckermann S, Golosubow A, Nieter B. 1991. Alterations in function and phenotype of monocytes from patients with septic disease—predictive value and new therapeutic strategies. Behring Inst Mitt 1991:208–215. [PubMed] [Google Scholar]
- 108.Cheng SC, Scicluna BP, Arts RJ, Gresnigt MS, Lachmandas E, Giamarellos-Bourboulis EJ, Kox M, Manjeri GR, Wagenaars JA, Cremer OL, Leentjens J, van der Meer AJ, van de Veerdonk FL, Bonten MJ, Schultz MJ, Willems PH, Pickkers P, Joosten LA, van der Poll T, Netea MG. 2016. Broad defects in the energy metabolism of leukocytes underlie immunoparalysis in sepsis. Nat Immunol 17:406–413. 10.1038/ni.3398. [DOI] [PubMed] [Google Scholar]
- 109.Döcke WD, Randow F, Syrbe U, Krausch D, Asadullah K, Reinke P, Volk HD, Kox W. 1997. Monocyte deactivation in septic patients: restoration by IFN-gamma treatment. Nat Med 3:678–681. 10.1038/nm0697-678. [DOI] [PubMed] [Google Scholar]
- 110.Novakovic B, Habibi E, Wang S-Y, Arts RJW, Davar R, Megchelenbrink W, Kim B, Kuznetsova T, Kox M, Zwaag J, Matarese F, van Heeringen SJ, Janssen-Megens EM, Sharifi N, Wang C, Keramati F, Schoonenberg V, Flicek P, Clarke L, Pickkers P, Heath S, Gut I, Netea MG, Martens JHA, Logie C, Stunnenberg HG. 2016. β-Glucan reverses the epigenetic state of LPS-induced immunological tolerance. Cell 167:1354–1368.e14. 10.1016/j.cell.2016.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Timmermans K, Kox M, Vaneker M, van den Berg M, John A, van Laarhoven A, van der Hoeven H, Scheffer GJ, Pickkers P. 2016. Plasma levels of danger-associated molecular patterns are associated with immune suppression in trauma patients. Intensive Care Med 42:551–561. 10.1007/s00134-015-4205-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Leijte GP, Custers H, Gerretsen J, Heijne A, Roth J, Vogl T, Scheffer GJ, Pickkers P, Kox M. 2018. Increased plasma levels of danger-associated molecular patterns are associated with immune suppression and postoperative infections in patients undergoing cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Front Immunol 9:663. 10.3389/fimmu.2018.00663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Austermann J, Friesenhagen J, Fassl SK, Petersen B, Ortkras T, Burgmann J, Barczyk-Kahlert K, Faist E, Zedler S, Pirr S, Rohde C, Müller-Tidow C, von Köckritz-Blickwede M, von Kaisenberg CS, Flohé SB, Ulas T, Schultze JL, Roth J, Vogl T, Viemann D. 2014. Alarmins MRP8 and MRP14 induce stress tolerance in phagocytes under sterile inflammatory conditions. Cell Rep 9:2112–2123. 10.1016/j.celrep.2014.11.020. [DOI] [PubMed] [Google Scholar]
- 114.Aneja R, Odoms K, Dunsmore K, Shanley TP, Wong HR. 2006. Extracellular heat shock protein-70 induces endotoxin tolerance in THP-1 cells. J Immunol 177:7184–7192. 10.4049/jimmunol.177.10.7184. [DOI] [PubMed] [Google Scholar]
- 115.World Health Organization. 2012. Information sheet: observed rate of vaccine reactions Bacille Calmette-Guérin (BCG) vaccine. WHO, Geneva, Switzerland. [Google Scholar]
- 116.Hatherill M, Geldenhuys H, Pienaar B, Suliman S, Chheng P, Debanne SM, Hoft DF, Boom WH, Hanekom WA, Johnson JL. 2014. Safety and reactogenicity of BCG revaccination with isoniazid pretreatment in TST positive adults. Vaccine 32:3982–3988. 10.1016/j.vaccine.2014.04.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Coit DG, Thompson JA, Albertini MR, Barker C, Carson WE, Contreras C, Daniels GA, DiMaio D, Fields RC, Fleming MD, Freeman M, Galan A, Gastman B, Guild V, Johnson D, Joseph RW, Lange JR, Nath S, Olszanski AJ, Ott P, Gupta AP, Ross MI, Salama AK, Skitzki J, Sosman J, Swetter SM, Tanabe KK, Wuthrick E, McMillian NR, Engh AM. 2019. Cutaneous melanoma, version 2.2019, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 17:367–402. 10.6004/jnccn.2019.0018. [DOI] [PubMed] [Google Scholar]
- 118.Babjuk M, Burger M, Compérat EM, Gontero P, Mostafid AH, Palou J, van Rhijn BWG, Rouprêt M, Shariat SF, Sylvester R, Zigeuner R, Capoun O, Cohen D, Escrig JLD, Hernández V, Peyronnet B, Seisen T, Soukup V. 2019. European Association of Urology guidelines on non-muscle-invasive bladder cancer (TaT1 and carcinoma in situ)—2019 update. Eur Urol 76:639–657. 10.1016/j.eururo.2019.08.016. [DOI] [PubMed] [Google Scholar]
- 119.Arts RJ, Blok BA, Aaby P, Joosten LA, de Jong D, van der Meer JW, Benn CS, van Crevel R, Netea MG. 2015. Long-term in vitro and in vivo effects of γ-irradiated BCG on innate and adaptive immunity. J Leukoc Biol 98:995–1001. 10.1189/jlb.4MA0215-059R. [DOI] [PubMed] [Google Scholar]
- 120.Majtan J, Jesenak M. 2018. β-Glucans: multi-functional modulator of wound healing. Molecules 23:806. 10.3390/molecules23040806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zykova SN, Balandina KA, Vorokhobina NV, Kuznetsova AV, Engstad R, Zykova TA. 2014. Macrophage stimulating agent soluble yeast β-1,3/1,6-glucan as a topical treatment of diabetic foot and leg ulcers: a randomized, double blind, placebo-controlled phase II study. J Diabetes Invest 5:392–399. 10.1111/jdi.12165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Roy S, Dickerson R, Khanna S, Collard E, Gnyawali U, Gordillo GM, Sen CK. 2011. Particulate β-glucan induces TNF-α production in wound macrophages via a redox-sensitive NF-κβ-dependent pathway. Wound Repair Regen 19:411–419. 10.1111/j.1524-475X.2011.00688.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Karaaslan O, Kankaya Y, Sungur N, Kocer U, Sedat Cuzdan S, Sahin B, Uysal A. 2012. Case series of topical and orally administered β-glucan for the treatment of diabetic wounds: clinical study. J Cutan Med Surg 16:180–186. 10.1177/120347541201600308. [DOI] [PubMed] [Google Scholar]
- 124.King B, Barrett S, Cutting KF. 2017. Clinical evaluation of a bioactive beta-glucan gel in the treatment of 'hard-to-heal' wounds. J Wound Care 26:58–63. 10.12968/jowc.2017.26.2.58. [DOI] [PubMed] [Google Scholar]
- 125.Rice PJ, Adams EL, Ozment-Skelton T, Gonzalez AJ, Goldman MP, Lockhart BE, Barker LA, Breuel KF, DePonti WK, Kalbfleisch JH, Ensley HE, Brown GD, Gordon S, Williams DL. 2005. Oral delivery and gastrointestinal absorption of soluble glucans stimulate increased resistance to infectious challenge. J Pharmacol Exp Ther 314:1079–1086. 10.1124/jpet.105.085415. [DOI] [PubMed] [Google Scholar]
- 126.Leentjens J, Quintin J, Gerretsen J, Kox M, Pickkers P, Netea MG. 2014. The effects of orally administered beta-glucan on innate immune responses in humans, a randomized open-label intervention pilot-study. PLoS One 9:e108794. 10.1371/journal.pone.0108794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Imbertson LM, Couture AM, Gibson SJ, Smith RMA, Miller RL, Reiter MJ, Wagner TL, Tomai MA, Beaurline JM. 1998. Cytokine induction in hairless mouse and rat skin after topical application of the immune response modifiers imiquimod and S-28463. J Invest Dermatol 110:734–739. 10.1046/j.1523-1747.1998.00174.x. [DOI] [PubMed] [Google Scholar]
- 128.Hung IF, Zhang AJ, To KK, Chan JF, Li P, Wong TL, Zhang R, Chan TC, Chan BC, Wai HH, Chan LW, Fong HP, Hui RK, Kong KL, Leung AC, Ngan AH, Tsang LW, Yeung AP, Yiu GC, Yung W, Lau JY, Chen H, Chan KH, Yuen KY. 2016. Topical imiquimod before intradermal trivalent influenza vaccine for protection against heterologous non-vaccine and antigenically drifted viruses: a single-centre, double-blind, randomised, controlled phase 2b/3 trial. Lancet Infect Dis 16:209–218. 10.1016/S1473-3099(15)00354-0. [DOI] [PubMed] [Google Scholar]
- 129.Berman B, Frankel S, Villa AM, Ramirez CC, Poochareon V, Nouri K. 2005. Double-blind, randomized, placebo-controlled, prospective study evaluating the tolerability and effectiveness of imiquimod applied to postsurgical excisions on scar cosmesis. Dermatol Surg 31:1399–1403. 10.2310/6350.2005.31204. [DOI] [PubMed] [Google Scholar]
- 130.Mulder WJM, Ochando J, Joosten LAB, Fayad ZA, Netea MG. 2019. Therapeutic targeting of trained immunity. Nat Rev Drug Discov 18:553–566. 10.1038/s41573-019-0025-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Negi S, Das DK, Pahari S, Nadeem S, Agrewala JN. 2019. Potential role of gut microbiota in induction and regulation of innate immune memory. Front Immunol 10:2441. 10.3389/fimmu.2019.02441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.McCoy KD, Burkhard R, Geuking MB. 2019. The microbiome and immune memory formation. Immunol Cell Biol 97:625–635. 10.1111/imcb.12273. [DOI] [PubMed] [Google Scholar]
- 133.Stratford AF, Zoutman DE, Davidson JS. 2002. Effect of lidocaine and epinephrine on Staphylococcus aureus in a guinea pig model of surgical wound infection. Plast Reconstr Surg 110:1275–1279. [DOI] [PubMed] [Google Scholar]
- 134.World Health Organization. 2018. Global guidelines for the prevention of surgical site infection, 2nd ed. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 135.Ban KA, Minei JP, Laronga C, Harbrecht BG, Jensen EH, Fry DE, Itani KM, Dellinger EP, Ko CY, Duane TM. 2017. American College of Surgeons and Surgical Infection Society: surgical site infection guidelines, 2016 update. J Am Coll Surg 224:59–74. 10.1016/j.jamcollsurg.2016.10.029. [DOI] [PubMed] [Google Scholar]