Abstract
Alcohol abuse induces changes in microglia morphology and immune function, but whether microglia initiate or simply amplify the harmful effects of alcohol exposure is still a matter of debate. Here, we determine microglia function in acute and voluntary drinking behaviors using a colony-stimulating factor 1 receptor inhibitor (PLX5622). We show that microglia depletion does not alter the sedative or hypnotic effects of acute intoxication. Microglia depletion also does not change the escalation or maintenance of chronic voluntary alcohol consumption. Transcriptomic analysis revealed that although many immune genes have been implicated in alcohol abuse, down-regulation of microglia genes does not necessitate changes in alcohol intake. Instead, microglia depletion and chronic alcohol result in compensatory upregulation of alcohol-responsive, reactive astrocyte genes, indicating astrocytes may play a role in regulation of these alcohol behaviors. Taken together, our behavioral and transcriptional data indicate that microglia are not the primary effector cell responsible for regulation of acute and voluntary alcohol behaviors. Because microglia depletion did not regulate acute or voluntary alcohol behaviors, we hypothesized that these doses were insufficient to activate microglia and recruit them to an effector phenotype. Therefore, we used a model of repeated immune activation using polyinosinic:polycytidylic acid (poly(I:C)) to activate microglia. Microglia depletion blocked poly(I:C)-induced escalations in alcohol intake, indicating microglia regulate drinking behaviors with sufficient immune activation. By testing the functional role of microglia in alcohol behaviors, we provide insight into when microglia are causal and when they are consequential for the transition from alcohol use to dependence.
Keywords: alcohol, astrocytes, microglia, neuroimmune, PLX5622, transcriptome
1. INTRODUCTION
Microglia are resident brain parenchymal cells that are essential mediators of development, homeostasis, and disease.1 In addition to their role as the primary effector cells in the brain, microglia play an important role in development and learning by regulating synaptic pruning, neurogenesis, and synaptic plasticity.1–3 Dysregulation of microglia programs in disease results in loss of homeostatic function and a transition to aberrant responses to local and environmental cues such an inappropriate phagocytosis or chronic immune activation.1,4 Microglia dysregulation is implicated in the initiation and progression of alcohol use disorder (AUD) and alcohol abuse.5 Despite implications of their importance, little is known about whether the phenotypical transformation of microglia is causative for pathogenesis or is a consequence of AUD.
Alcohol activates microglia leading to potentiated subsequent microglial responses to alcohol exposure, suggesting alcohol exposure may recruit microglia to an effector cell phenotype.6 This may occur because alcohol directly activates microglia to increase expression of proinflammatory chemokines and cytokines, and these inflammatory mediators have been shown to directly regulate alcohol behaviors.7,8 Chronic alcohol consumption also induces microglia proliferation in rodent models of alcohol abuse and in brains from postmortem individuals with AUD.6,9,10 This increase in microglia proliferation is accompanied by morphological changes in microglia reflective of a proinflammatory phenotype.6,11 Transcriptome profiling of microglia from the prefrontal cortex of mice chronically consuming alcohol found altered expression of immune response and immune activation genes, suggesting alcohol enhances microglia-specific immune responses.12 Blockade of microglia immune response by minocycline alters alcohol-induced motor impairment,13 decreases alcohol self-administration in mice,14 and attenuates withdrawal-induced anxiety and relapse drinking in rats.15 Furthermore, depletion of microglia prevents proinflammatory gene expression after acute withdrawal from binge alcohol, indicating microglia are partly necessary for the brain’s proinflammatory response that underlies binge drinking.16 Because pharmacological inhibition of microglia immune response regulates multiple alcohol behaviors, we hypothesized that microglia may be the critical mediators of alcohol behaviors.
Therefore, targeting microglia may provide a novel therapeutic strategy to reduce immune response and ultimately alcohol abuse. One way to target microglia is to pharmacologically inhibit colony-stimulating factor 1 receptor (CSF1R). Since CSF1R knockout mice are born without microglia and mice lacking either of its two ligands, colony-stimulating factor 1 (CSF1) or interleukin 34 (IL-34), also have reduced microglial number, this suggests that microglia are fully dependent upon CSF1R signaling for survival.17,18 Inhibition of CSF1R by the drug PLX5622 leads to near-complete elimination of all microglia from the central nervous system (CNS) within 7 to 21 days.19 Moreover, microglia remain eliminated for the duration of PLX5622 treatment allowing for temporal control over microglia populations in the adult CNS.19 Microglia depletion using CSF1R inhibition has been widely used and shown to improve microglial Aβ-plaque association, cognitive effects due to Alzheimer disease, and promote brain recovery after brain injury or neuronal loss.20 However, microglia depletion can also worsen progression of prion disease, ischemic injury, and flavivirus-induced encephalitis, suggesting that microglia have distinct and sometimes opposing roles in different disease states.20
Here, we tested if microglia regulate alcohol behaviors directly by using a potent CSF1R inhibitor to deplete microglia. Surprisingly, although microglia have been implicated in both the brain’s response to alcohol and regulation of alcohol behaviors, we found that microglia depletion did not alter acute intoxication phenotypes or voluntary alcohol consumption. We utilized RNA sequencing to profile the transcriptome in response to voluntary alcohol consumption and microglia depletion. We identified a microglia depletion signature as well as a compensatory upregulation of alcohol-responsive astrocyte genes that have been implicated in regulation of alcohol consumption. Therefore, we conclude that microglia activation is a consequence of low dose and voluntary alcohol consumption but is not causal for regulation of these alcohol behaviors. Because microglia depletion did not regulate acute or voluntary alcohol behaviors, we hypothesized that acute or voluntary doses were insufficient to recruit microglia to an effector phenotype. Therefore, we used a model of repeated viral immune activation—administration of polyinosinic:polycytidylic acid (poly(I:C)) induces proinflammatory gene expression, activates microglia, and alters both voluntary and ethanol self-administration drinking behaviors.21–23 Our poly(I:C) model of repeated immune activation increases voluntary alcohol intake over time, suggesting micro-glial recruitment may be crucial to how neuroimmune-induced escalations occur.22 We found microglia depletion blocked poly(I:C)–induced escalations in alcohol intake, indicating microglia regulate drinking behaviors with sufficient immune activation. Together, this suggests that although microglia have been implicated in alcohol abuse, microglia do not regulate all alcohol behaviors. Other cell types, such as astrocytes, may be critical mediators of acute intoxication and voluntary alcohol behaviors.
2. MATERIALS AND METHODS
2.1. Mice
All procedures in this study were approved by the University of Texas at Austin Institutional Animal Care and Use Committee. All behavioral experiments were performed in male C57BL/6J mice; for additional housing information, see Supporting Information.
2.2. Drugs
The CSF1R inhibitor PLX5622 was provided by Plexxikon Inc (Berkeley, CA) formulated at a dose of 1200 ppm in RMH1800 Diet by Research Diets (New Brunswick, NJ). Control diet (RMH 1800) was also provided by Research Diets (New Brunswick, NJ). Injectable alco-hol (Aaper Alcohol and Chemical, Shelbyville, KY) solutions were pre-pared in 0.9% saline (20%, v/v). Gaboxadol (Sigma-Aldrich, St. Louis, MO) was dissolved in 0.9% saline. All drugs were injected intraperito-neally (i.p.). We validated PLX5622 formulated in RMH1800 Diet functioned in both hippocampus and in the medial prefrontal cortex (mPFC) (Figures S1-S2). Poly(I:C) HMW obtained from Invivogen (San Diego, CA) was prepared as previously described.22 Briefly, poly(I:C)(5 mg/kg, i.p.) was administered every 4 days during no alcohol access periods. For the control injection, 0.9% saline (volume matched) was administered to control groups.
2.3. Two-bottle choice every-other-day procedure
Intermittent (every-other-day [EOD]) access to alcohol increases voluntary drinking in and mice.24,25 Mice were given EOD access to alcohol (15% v/v) and water for 24-hour sessions, and water only was offered on off days. The side placement of the alcohol bottles was alternated with each alcohol session. The quantity of alcohol consumed was calculated as g/kg body weight/24 h. Total fluid intake was calculated as g/kg body weight/24 h (n = 10/group).
2.4. Loss of righting reflex
Sensitivity to the depressant effects of alcohol (3.6 g/kg, i.p.) or gaboxadol (55 mg/kg, i.p.) was determined using the loss of righting reflex (LORR) (sleep time) assay in mice. These drug doses were based on previous studies.26 When mice became ataxic, they were placed in the supine position in V-shaped plastic troughs until they were able to right themselves three times within 30 seconds. Sleep time was defined as the time from being placed in the supine position until they regained their righting reflex.
2.5. Rotarod
Mice were trained on a fixed speed rotarod (Economex; Columbus Instruments, Columbus, OH) at 5 rpm, and training was considered complete when mice were able to remain on the rotarod for 60 seconds. Every 15 minutes after injection of alcohol (2 g/kg, i.p.) or diazepam (5 mg/kg, i.p.), mice were placed back on the rotarod and latency to fall was measured until mice were able to remain on the rotarod for 60 seconds.
2.6. Brain collection
For immunohistochemistry experiments, mice were anesthetized with isoflurane, transcardially perfused with 0.9% saline until cleared of blood and then perfused with freshly prepared 4% paraformaldehyde PFA in phosphate-buffered saline. Then the brain was removed and postfixed in 4% PFA at 4C for 24 hours followed by cryoprotection for 24 hours at 4C in 20% sucrose. Brains were then placed in a plas-tic mold containing optimum cutting temperature compound (VWR, Radnor, PA) and quickly frozen in isopentane on dry ice. For the RNA-sequencing experiment, the brains were quickly harvested and the mPFC rapidly dissected before being snap frozen in liquid nitrogen.
2.7. RNA isolation and sequencing
Total RNA was isolated as previously described27 and submitted to the Genomic Sequencing and Analysis Facility at the University of Texas at Austin for RNA-sequencing analysis. Additional sequencing and bioinformatics information can be found in the Supporting Information. Raw and processed sequencing data from this study have been deposited in the Gene Expression Omnibus under the accession number GSE132516.
2.8. Immunohistochemistry
Immunohistochemistry was performed as previously described28; see Supporting Information for detailed protocols. Briefly, free-floating sections were stained for IBA1 and in the mPFC and hippocampus. Sections were imaged and analyzed as previously described28; see Supporting Information for additional details.
2.9. Flow cytometric analysis
Mice were treated with either control diet or PLX5622 diet, and then the brains, spleens, and liver dissected and myeloid populations were assessed by flow cytometry as previously described.29 See Supporting Information for detailed protocols and a list of antibodies used.
2.10. Statistical analysis
With the exception of RNA-sequencing analyses, data are reported as mean ± SEM values, unless otherwise noted. The statistics software program GraphPad Prism (GraphPad Software, Inc, La Jolla, CA) was used to perform two-way analyses of variance (ANOVAs), Welch t tests. Drinking data were analyzed by repeated measures two-way ANOVA and Bonferroni post hoc tests. Grubbs test (α = 0.05) was used to detect potential outliers. Welch t tests (two-tailed) were used for alcohol sedation data, FACS data, and immunohistochemical data.
3. RESULTS
3.1. Microglia do not regulate alcohol-induced sedation or motor incoordination
Prior studies demonstrate that treatment with minocycline, which attenuates microglia activation, protects mice from acute alcohol-induced sedation and motor impairment.13 Therefore, we hypothesized that microglia depletion would also result in reduced duration of ethanol (EtOH)–induced LORR and faster recovery from EtOH-induced motor incoordination in a rotarod test. To test this hypothesis, we depleted microglia using the CSF1R inhibitor PLX5622 for 21 days and then measured the duration of LORR following EtOH injection. For EtOH (3.6 g/kg), there was no difference in duration or latency of LORR between microglia-depleted and microglia-intact mice (P = 0.87) (Figure 1A-B). Moderate doses of alcohol cause motor incoordination by potentiation of GABAergic activity.30 Therefore, we additionally tested if microglia depletion would alter the effects of GABAergic sedatives. There was no difference in duration or latency of LORR after injection of gaboxadol (55 mg/kg) (Figure 1C-D). Acute administration of EtOH (2 g/kg) produced motor incoordination in microglia-depleted and microglia-intact mice, as measured by the rotarod test. There were no differences in the recovery from EtOH-induced impairment (Figure 1E). No differences in the recovery from diazepam-induced motor incoordination were found between microglia-depleted and microglia-intact mice (Figure 1F). Microglia depletion in these groups of mice was verified using immunohistochemistry (Figure S3). Taken together, this suggests that microglia do not directly regulate the sedative or motor incoordination effects of alcohol or GABAergic sedatives.
FIGURE 1.
Microglia do not regulate alcohol-induced sedation or motor incoordination. Male C57BL/6J mice were placed on either control diet or PLX5622 diet for 21 days prior to behavioral testing to achieve >95% microglia depletion and then maintained on either diet for the remainder of behavioral testing. A, Ethanol-induced loss of righting reflex (LORR) in microglia-intact versus microglia-depleted mice. B, Ethanol-induced latency to LORR in microglia-intact versus microglia-depleted mice. C, Gaboxadol-induced LORR in microglia-intact versus microglia-depleted mice. D, Gaboxadol-induced latency to LORR in microglia-intact versus microglia-depleted mice. E, Ethanol-induced motor incoordination in microglia-intact versus microglia-depleted mice. F, Diazepam-induced motor incoordination in microglia-intact versus microglia-depleted mice. Values represent mean ± SEM, n = 10/group. LORR data were analyzed by Welch t test. Motor incoordination data were analyzed by repeated measures two-way ANOVA. EtOH, ethanol; LORR, loss of righting reflex.
3.2. Microglia depletion does not alter escalation or maintenance of voluntary alcohol intake
Prior studies suggest that the activation of microglia influences acute behavioral responses to alcohol, including heavy drinking, withdrawal, and the transition to alcohol dependence.5 Therefore, we hypothsized that microglia regulate escalation and maintenance of voluntary alcohol intake. To test this hypothesis, we first depleted microglia using PLX5622 for 21 days, and then mice began EOD drinking for 44 days (Figure 2A). As shown in Figure 2, microglia depletion did not alter alcohol consumption (Figure 2B, Ftreatment[14,31] = 0.0012, P = 0.97) or preference (Figure 2C, Ftreatment[14,31] = 0.22, P = 0.65). Total fluid intake was unchanged by microglia depletion (Figure 2D, Ftreatment[14,31] = 0.37, P = 0.54). Microglia depletion was verified using immunohistochemistry (Figure S4). Bodyweight was also unchanged by microglia depletion (Figure S4). Together, this indicated that depletion of microglia does not regulate escalation of alcohol intake or preference.
FIGURE 2.
Microglia do not regulate escalation nor maintenance of alcohol intake. A, Male C57BL/6J mice were on PLX5622 diet for 21 days prior to beginning drinking to achieve >95% microglia depletion and then maintained on PLX5622 diet for 44 days while undergoing an every-other-day two-bottle choice (2BC) procedure. B, EtOH consumption (g/kg/24 h); C, EtOH preference; D, total fluid intake (g/kg/24 h). E, Male C57BL/6J mice were allowed to escalate and establish a drinking baseline on an every-other-day 2BC procedure for 22 days, and then mice started either PLX5622 diet or control diet while continuing every-other-day 2BC for 14 days. Microglia were repopulated while mice continued every-other-day 2BC for 6 days. F, EtOH consumption (g/kg/24 h); G, EtOH preference; H, total fluid intake (g/kg/24 h). Values represent mean ± SEM, n = 10/group. Data were analyzed by repeated measures two-way ANOVA. EtOH, ethanol; 2BC, two-bottle choice.
We next tested if microglia are necessary for maintenance of chronic alcohol consumption. Mice escalated drinking on an EOD procedure and drank until a stable baseline had been met (~22 days)(Figure 2E). We then depleted microglia for 14 days while mice continued EOD drinking (Figure 2E). Microglia depletion did not change alcohol consumption (Figure 2F, Ftreatment(14,27) = 0.32, P = 0.57) or preference (Figure 2G, Ftreatment(14,27) = 1.24, P = 0.28). Total fluid intake did not change with microglia depletion (Figure 2H, Ftreatment(14,27) = 1.44, P = 0.24). We then repopulated microglia by placing mice on control diet (RMH 1800) to determine if the presence of microglia would change drinking behavior. Repopulation trended toward reduced alcohol consumption (Figure 2F, Ftreatment(14,27) = 4.32, P = 0.054) and total fluid intake (Figure 2H, Ftreatment(14,27) = 4.56, P = 0.50) but did not change alcohol preference (Figure 2G, Ftreatment(14,27) = 1.66, P = 0.22). Microglia depletion and repopulation were verified using immunohistochemistry (Figure S5). Microglia depletion did not alter body weight throughout the experiment (Figure S5). Together, this indicated that microglia do not regulate either escalation or maintenance of voluntary alcohol consumption—raising the possibilities that microglia are not the primary effector cells required for voluntary alcohol intake and voluntary drinking does not produce sufficient neuroimmune activation to recruit microglia-mediated escalations in alcohol intake.
3.3. Microglia depletion downregulates microglial gene expression even after chronic alcohol consumption
To investigate transcriptional responses to both voluntary alcohol consumption and microglia depletion, we utilized 3’UTR biased transcriptome sequencing (3’Tag-seq). In response to microglia depletion and voluntary alcohol consumption, a total of 619 genes were differentially expressed in the mPFC (P < 0.05) (Figure S6). Genes were equally downregulated (323 genes) and upregulated (297 genes) by PLX5622 treatment (Figure S6). In general, the genes downregulated by microglia depletion had the largest fold changes and smallest P values. As expected, microglia marker genes such as Csf1r (colony-stimulating factor 1 receptor) and Laptm5 (lysosomal protein trans-membrane 5) were significantly downregulated by microglia depletion. Microglia depletion and chronic alcohol consumption also altered gene expression in other CNS cell types such as astrocytes and neurons. Specifically, microglia depletion upregulated expression of reactive astrocyte markers and astrocytic genes implicated in alcohol abuse27,32 (Table S1). Many neuronal genes were also upregulated after microglia depletion and related to modulation of chemical synaptic transmission (GO:0050804) (Figure S7). Many of these neuronal genes have been also previously implicated in AUD, such as Bdnf (brain-derived neurotrophic factor) (Figure S7).33 Together, this suggests microglia depletion and chronic alcohol consumption alter microglia genes, but there are also significant changes the expression of other CNS cell types. A complete list of differentially expressed genes is listed in Table S1.
To identify discrete groups of coexpressed genes showing transcriptional differences between microglia-intact and microglia-depleted mice consuming alcohol, we constructed a coexpression network. As previously shown for complex diseases, coexpression networks permit analysis of gene expression variation related to multiple disease-related traits.34 We assessed module eigengene relation-ship to microglia depletion and alcohol intake, providing a complementary assessment to that performed in the standard differential expression analysis.
The comparison between alcohol-consuming microglia-depleted versus microglia-intact mice revealed one network module, module 8 (M8) that highly correlated with microglia depletion treatment (584 genes, r = −0.73, P = 6e-04) (Figure S8). The coexpressed genes in M8 were downregulated with microglia depletion (Figure 3A). We found that M8 had significant enrichment in cell activation involved in immune response (GO:0002623) and was highly enriched in microglia cell type markers (Figure 3B,E). GO enrichment of downregulated myeloid leukocyte immune response was consistent with RNA sequencing of whole brain after microglia depletion (Figure 3E).35,36 A further advantage of network analysis over standard analysis of differential expression is that it allows one to infer functional relevance of genes based on their network position. Indeed, the hubs of M8, genes with the highest rank of M8 membership, were C1qb (complement C1q B chain), C1qc, Laptm5, and Csf1r (Figure 3C-D) (Table S2). All these genes are microglia markers,37 reinforcing that M8 is a “microglia depletion signature.” Moreover, the hub gene Csf1r is the pharmacological target of PLX5622, validating that manipulation of hub genes within this network does not change voluntary alcohol consumption. Interestingly, no immune response genes previously implicated in regulating alcohol consumption (e.g., Il6, B2m [beta-2 microglobulin], Ctss [cathepsin S], and Tlr4 [toll-like receptor 4]) were enriched in the M8 module, suggesting that these immune response genes may be more important to regulation of drinking behavior compared with microglial marker genes. Together, these data suggest that alterations in myeloid cell function is a primary effect of microglia depletion treatment even after voluntary alcohol consumption.
FIGURE 3.
Gene coexpression module associated with microglia depletion signature after chronic alcohol intake. A, Module 8 (M8) relative eigengene expression values between treatments. B, Enrichment (y-axis) for the cell-type specific genes (x-axis) belonging to the coexpression module. C, M8 hub genes, genes most strongly correlated with the module eigengene value. D, Visualization of the top 30 connections for M8. Size of the circle represents magnitude of log2 fold change. E, Relevant gene ontology categories enriched in M8.
3.4. Microglia depletion and chronic alcohol consumption enhance reactive astrocyte and neuronal gene expression
Although microglia depletion alone does not produce proinflammatory responses such as astrogliosis,19,35,36 a previous study found that microglia depletion alters the function of other cell types in the CNS such as astrocytes and neurons.16 Our differential expression analysis suggested changes in both astrocytic and neuronal gene expression after depletion and chronic alcohol consumption. Therefore, we additionally investigated potential compensatory modules that were upregulated by microglia depletion treatment.
One module of coexpressed genes that was upregulated after microglia depletion and significantly negatively correlated with alcohol consumption was module 17 (M17, 310 genes, r = −0.58, P = 0.01)(Figures 4A and S8 and Table S2). Coexpressed genes in M17 were highly enriched in neuron-related genes (Figure 4B). There was only one highly connected gene (i.e., hub gene) within this module, Syn2, which has previously been implicated in AUD (Figure 4C-D).38,39 Further reinforcing that neuronal processes may be altered after microglia depletion, this module was enriched in processes related to neurotransmitter transport (GO:0006836), including genes such as Slc6a17 (solute carrier family 6 member 17), which belongs to a family of solute carriers that are responsible for the presynaptic uptake of most neurotransmitters. Therefore, microglia depletion in the context of chronic alcohol consumption may alter ethanol-induced neuroadaptations, although these neuroadaptations do not change voluntary drinking behavior.
FIGURE 4.
Gene coexpression module associated with neuronal gene expression changes after microglia depletion and chronic alcohol intake. A, Module 17 (M17) relative eigengene expression values between treatments. B, Enrichment (y-axis) for the cell-type specific genes (x-axis) belonging to the coexpression module. C, M17 hub gene, gene most strongly correlated with the module eigengene value. D, Visualization of the top 30 connections for M17. Size of the circle represents magnitude of log2 fold change. E, Relevant gene ontology categories enriched in M17.
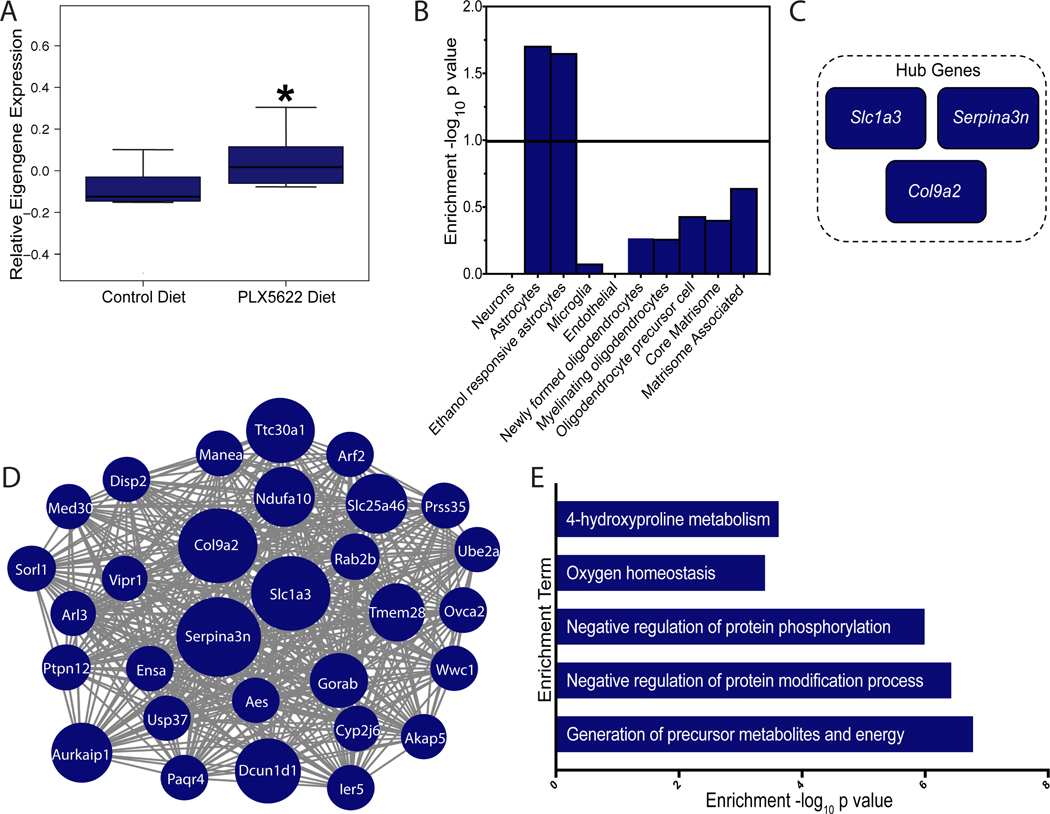
Another module of coexpressed genes that was significantly upregulated after microglia depletion and positively correlated with alcohol consumption was module 22 (M22, 117 genes, r = 0.82, P = 3e-05) (Figures 5A and S8 and Table S2). M22 was enriched in processes related to generation of precursor metabolites and energy (GO:0006091) and also showed a significant enrichment in reactive astrocyte cell type markers (i.e., Slc1a3, Gfap [glial acidic fibrillary protein], and Serpina3n [serine protease inhibitor 3]) (Figure 5B,E and Table S2). Furthermore, M22 had enrichment in genes associated with transcriptional changes in astrocytes after voluntary drinking31 (Figure 5B). The most highly connected genes (ie, hub genes) within M22 related to astrocyte control of glutamate reuptake homeostasis and reactive astrocyte activation (Figure 5C-D, Slc1a3 and Serpina3n), suggesting compensatory astrocyte activation in alcohol-consuming mice may prevent microglia depletion-related changes in alcohol intake. We validated M22 by verifying that microglia depletion also increases protein levels of GFAP-positive reactive astrocytes in the mPFC, as the transcriptome data predicts. As shown in Figure S9, after microglia depletion, there is a significant increase in density and integrated intensity of GFAP protein in the mPFC, supporting the hypothesis that there is a compensatory upregulation of reactive astrocytes after microglia depletion and alcohol consumption. Given the role of astrocytes in regulating synaptic activity, glutamate homeostasis, and inflammation—all critical components of the transition from alcohol abuse to dependence—astrocytes may be an important effector cell that regulates these alcohol behaviors.
FIGURE 5.
Gene coexpression module associated with compensatory reactive astrocytes after microglia depletion and chronic alcohol intake. A, Module 22 (M22) relative eigengene expression values between treatments. B, Enrichment (y-axis) for the cell-type specific genes (x-axis) belonging to the coexpression module. C, M22 hub genes, genes most strongly correlated with the module eigengene value. D, Visualization of the top 30 connections for M22. Size of the circle represents magnitude of log2 fold change. E, Relevant gene ontology categories enriched in M22.
3.5. Microglia depletion prevents poly(I:C)–induced escalation in alcohol intake
Because microglia depletion did not regulate acute or voluntary alcohol behaviors, we hypothesized that acute or voluntary doses were insufficient to recruit microglia to an effector cell phenotype. Therefore, we used a model of repeated viral immune activation using poly(I:C)—which has been shown to activate microglia and alter drinking behavior.21–23 This poly(I:C) model increases voluntary alcohol intake over time in a voluntary drinking procedure, leading to the hypothesis that microglial recruitment to an effector cell phenotype may be crucial to how neuroimmune-induced escalations in alcohol intake occur.22 Mice were given PLX5622 diet for 21 days to deplete 95% of microglia. Then mice were injected with poly(I:C) (5 mg/kg) every 4 days during EOD drinking while continuing to deplete microglia (Figure 6A). Consistent with previous studies, poly(I:C) escalated alcohol intake over time in microglia-intact mice.22 However, microglia depletion prevented poly(I:C)–induced escalations in alcohol intake (Ftreatment(14,29) = 0.79, P = 0.38) and preference (Ftreatment(14,29) = 2.83, P = 0.11) (Figure 6B-C). There was no difference in total fluid intake between poly(I:C)–treated microglia-intact and microglia-depleted mice (Ftreatment(14,29) = 0.15, P = 0.69), suggesting an alcohol-specific effect (Figure 6D). Microglia depletion was verified using immunohistochemistry (Figures 6E-F and S10). Microglia depletion also prevented poly(I:C) suppression of body weight (Ftreatment(14,27) = 6.71, P = 0.02) (Figure 6G). Therefore, microglia are necessary for neuroimmune-induced increases in alcohol intake, suggesting microglia do regulate alcohol behaviors but only after immune activation has occurred.
FIGURE 6.
Microglia depletion prevents poly(I:C)–induced escalations in alcohol intake. A, Male C57BL/6J mice were on PLX5622 diet for 21 days prior to beginning drinking to achieve >95% depletion and then maintained on PLX5622 diet for 44 days while undergoing an every-other-day two-bottle choice procedure with poly(I:C) injections every 4 days. B, EtOH consumption (g/kg/24 h); C, EtOH preference; D, total fluid intake (g/kg/24 h). E, Representative images of effect of microglia depletion on IBA1 staining (20×, scale bar = 50 μm). F, Quantification of microglia depletion. G, Effect of PLX5622 diet and poly(I:C) on body weight. Values represent mean ± SEM, drinking data: n = 10/group, immunohistochemistry: n = 3/group, body weight: n = 10/group. Drinking data were analyzed by repeated measures two-way ANOVA with Bonferroni post hoc testing (*P < 0.05, **P < 0.0021). Immunohistochemical data were analyzed by Welch t test (*P < 0.05, **P < 0.01, ***P < 0.001). Body weight data were analyzed by repeated measures two-way ANOVA and Bonferroni post hoc tests (*P < 0.05, **P < 0.01, ***P < 0.001). EtOH, ethanol.
3.6. PLX5622 depletes other peripheral immune cell populations
A concern of using a CSF1R inhibitor is that CSF1R is a marker for multiple types of myeloid cells, including peripheral macrophages and monocytes. Therefore, to determine specificity of PLX5622 for microglia, mice were placed on PLX5622 diet for 21 days to deplete microglia, and then changes in immune cell populations were assessed by flow cytometry. As shown in Figure 7, treatment with PLX5622 diet results in a decrease in macrophages and Gr1lo monocytes in circulation as well as in the spleen and liver. In addition, PLX5622 treatment depleted granulocytes from circulation, but not from the spleen or liver. PLX5622 treatment did not deplete dendritic cells or Gr1hi monocytes (Figure S11). PLX5622 treatment also did not alter splenic myeloid populations, such as macrophage, monocyte, or granulocyte populations (Figure S11). As shown in Figures S1-S2, microglia depletion after 21 days was >95% in both mPFC and hippocampus. One concern is that PLX5622 treatment depleted liver macrophages, which could affect ethanol metabolism. However, it has been shown that liver hepatocytes metabolize the vast majority of ingested alcohol.40 Taken together, CSF1R inhibition using PLX5622 has a primary effect depleting microglia; however, other peripheral myeloid cells are significantly depleted by CSF1R inhibition and their contribution to the effects observed on behavior cannot be discounted.
FIGURE 7.
PLX5622 diet depletes other peripheral macrophage and monocyte populations. C57BL/6J male mice were on either control diet or PLX5622 diet for 21 days to deplete microglia. Spleen, blood, and liver were collected from both groups. A, Representative flow cytometry plots of total CD45+, live, and myeloid (CD11c+ and/or CD11b+) cells that are macrophages (CD64+F4/80+) (top) or monocytes (CSF1R+CD11b+) from the spleens of control diet– and PLX5622 diet–treated mice. B, Representative flow cytometry plots of total CD45+, live, and myeloid (CD11c+ and/or CD11b+) cells that are macrophages (CD64+F4/80+) (top) or monocytes (CSF1R+CD11b+) from the blood of control diet– and PLX5622 diet–treated mice. C, Representative flow cytometry plots of total CD45+, live, and myeloid (CD11c+ and/or CD11b+) cells that are macrophages (CD64+F4/80+) (top) or monocytes (CSF1R+CD11b+Gr1lo) from the livers of control diet– and PLX5622 diet–treated mice. D-I, Graphs show mean ± SEM of accumulative data (n = 3/group). Graphs show the frequency of myeloid cells that are CD64+F4/80+ macrophages from the (D) spleens, (E) blood, and (F) livers of the indicated treatment groups. Graphs show the frequency of myeloid cells that are CSF1R+CD11b+Gr1lo monocytes from the (G) spleens, (H) blood, and (I) livers of the indicated treatment groups. Data were analyzed by Welch t test (*P < 0.05, **P < 0.01, ***P < 0.001).
4. DISCUSSION
Although many studies suggest microglia activation may be important for alcohol abuse, we define the alcohol-related behaviors that are dependent on microglia. We found microglia regulate specific behaviors such as neuroimmune-induced escalations in alcohol intake but not all alcohol behaviors. Specifically, microglia depletion did not alter the sedative or hypnotic effects of acute intoxication. Microglia depletion also did not alter the escalation or maintenance of chronic voluntary alcohol consumption. Transcriptional analysis after chronic alcohol and microglia depletion determined that although many immune genes have been implicated in AUD, downregulation of microglia genes does not necessitate changes in alcohol behavior. Moreover, microglia depletion may not change alcohol intake because of compensatory upregulation of alcohol-responsive and reactive astrocyte genes. On the basis of our microglia depletion behavioral and transcriptional data, we hypothesized that acute and voluntary doses of ethanol were insufficient to recruit microglia to an effector phenotype. Microglia depletion prevented poly(I:C)–induced escalations in alcohol intake, suggesting additional immune activation is crucial to recruiting microglia to a regulatory role. These results demonstrate that different alcohol behaviors are regulated by different glial cell types, suggesting that microglia and astrocytes respond to specific environmental cues that recruit them to an effector cell phenotype.
Microglia depletion did not alter GABA- or alcohol-induced sedation or motor incoordination. This is in direct contrast to knockout studies of various inflammatory mediators and innate immune pathway components (e.g., Il1ra, Tlr2, and Ccl2 [C-C motif chemokine ligand 2]), which regulate the sedative and motor inco-ordination effects of alcohol.7,13,26 In this context, our results suggest that inflammatory mediators from cells other than microglia are critical regulators of acute intoxication phenotypes. Astrocytes, the most abundant type of CNS glia, also have the ability to respond to and participate in immune responses in the CNS.41 Astrocytes express NF-kB, a crucial transcription factor in any inflammatory response.41 High dose of alcohol increases NF-kB activity in human astroglial cultures, suggesting that alcohol may directly modulate astrocyte immune function.42 This alcohol-induced astrocyte immune response may also regulate the sedative effects of alcohol. Astrocytes highly express Ccl2, and knockout of Ccl2 extended alcohol-induced LORR, suggesting that an astrocyte-derived inflammatory mediator regulates the sedative effects of alcohol.7 Moreover, astrocytes are the primary source of extracellular adenosine, and acute increases in adenosine following alcohol exposure partly mediate the ataxic and hypnotic effects of acute alcohol exposure.43 Future studies will address if silencing astrocyte function regulates the sedatives effects of alcohol.
It has been hypothesized that positive feedback cycles of proinflammatory immune signaling promote excessive alcohol drinking. As the main immune effector cell in the brain, it was reasonable to propose that microglia regulate the inflammatory response to chronic alcohol. However, microglia depletion did not alter escalation or maintenance of chronic alcohol consumption. Transcriptomic analysis revealed a “microglia depletion” signature, indicating that PLX5622 downregulates microglia immune response and myeloid function genes as predicted. Many of the microglia marker genes within this “microglia depletion” signature overlapped with the alcohol-responsive microglia-specific transcriptome,12 suggesting that our 3’Tag-seq method captured microglia-specific transcriptional changes after chronic alcohol. Many of these microglial genes also overlapped with genes downregulated after microglia depletion and binge ethanol, suggesting a commonality in how CSF1R inhibition alters micro-glial gene expression in the brain.16 However, despite enrichment of microglia genes and overlap with the alcohol-responsive microglia transcriptome, coexpressed genes related to microglia depletion were not enriched in differentially expressed genes previously implicated in AUD or in regulating alcohol consumption (e.g., Ctss, B2m, Tlr4, and Il6).8,34 Indeed, no genes in the innate immune pathways previously linked with regulating alcohol consumption were enriched in this module.7,8 Therefore, we conclude downregulation of microglia genes is not sufficient to block the inflammatory effects of alcohol that control alcohol behavior in this voluntary procedure. We suggest that other sources of innate immune signaling and secreted inflammatory factors may regulate voluntary alcohol intake—indicating that another cell type may be more important for mediating escalations in voluntary alcohol intake.
As mentioned above, astrocytes are crucial regulators of innate and adaptive immune responses.41 Network analysis revealed that microglia depletion and chronic alcohol consumption upregulate astrocyte-specific genes in module 22, suggesting compensatory astrocyte activation. Many of the coexpressed genes within this module were related to pan-reactive astrocytes implicated in neurodegenerative diseases.32 Genes within this module also significantly overlapped with the alcohol-responsive astrocyte transcriptome, indicating an enrichment of alcohol-responsive astrocyte genes that may regulate alcohol intake.31 Previous studies have shown that under homeostatic and disease states, microglial-secreted cytokines (IL-1α, C1q, and TNFα [tumor necrosis factor alpha]) induce A1 reactive astrocyte formation, indicating that microglia promote astrocyte activation.32 However, our results suggest that alcohol can directly activate astrocytes to induce a neuroinflammatory phenotype in the absence of microglia. Recent studies suggest that astrocytes may also regulate alcohol behaviors. Transgenic mice overexpressing Ccl2 in astrocytes have slightly reduced alcohol consumption,44 whereas astrocyte-specific gap junction inhibition transiently increases alcohol preference and motivation for operant ethanol self-administration—establishing a functional role for astrocytes in regulation of alcohol behaviors.45 Future studies will use chemogenetic and genetic tools to deter-mine if astrocytes regulate voluntary alcohol behavior.
In addition to enhancing reactive astrocyte expression, microglia depletion also broadly changed neuronal gene expression, specifically upregulating neuronal genes related to regulation of synaptic transmission. A previous study also found decreased neuronal genes after microglia depletion and chronic binge ethanol.16 In our study, many of the upregulated neuronal genes related to regulation of synaptic transmission and included genes previously implicated in AUD such as Syn2 (synapsin II), Homer1 (homer protein homolog 1), and Bdnf.33,39,46 Syn2 was the hub gene for the neuronal module M17, suggesting it may play a regulatory role in both alcohol behaviors and the transcriptional response to microglia depletion. Syn2 codes for a neuronal phosphoprotein that coats synaptic vesicles, binds to the cytoskeleton, and regulates neurotransmitter release. Previous studies show Syn2 expression is decreased in the caudate putamen of inbred alcohol-preferring versus inbred alcohol non-preferring rats, and its expression is increased by intracranial operant ethanol self-adminis-tration.39 In postmortem brain from alcohol-dependent individuals, there is also downregulation of Syn2.34,38 Our gene expression data found that microglia depletion upregulated expression of Syn2 and other neuronal genes implicated in AUD, suggesting that depletion may be providing a neuroprotective effect for ethanol-induced neuroadaptations. Future studies, using electrophysiological recordings will investigate how microglia depletion changes synaptic function and if microglia depletion prevents ethanol-induced changes in synaptic function.
Chronic alcohol abuse in humans results in long-lasting immune gene expression changes and chronic immune activation.47 Previous studies demonstrate high-dose binge alcohol exposure potentiates a subsequent microglia response leading to microglial activation.6 When we initiated exogenous immune activation with poly(I:C) during alcohol consumption, microglia depletion blocked neuroimmune-induced escalations in alcohol intake, suggesting that microglia are critical mediators of alcohol behavior after sufficient immune activation. A remaining question is if this effect is specific to viral immune activation or if microglia more generally mediate other neuroimmune-induced escalations in drinking.
How might microglia depletion prevent these poly(I:C)–induced escalations in alcohol intake? It has been well established that microglial release of cytokines and chemokines modulates neuronal signaling.48 Poly(I:C) and ethanol alter expression of inflammatory genes as well as glutamatergic genes.21,22 Poly(I:C) alone also increases hippocampal extracellular glutamate.49 Moreover, administration of the mGluR2/3 agonist LY379268 blocked the effects of poly(I:C) on ethanol self-administration, suggesting a role for glutamatergic regulation of poly(I:C)’s effect on drinking.21 Many of the chemokines and cytokines increased by chronic alcohol and poly(I:C) alter the balance between neuronal excitation and inhibition via complementary mechanisms on both glutamatergic and GABAergic signaling.48 For instance, CCL2 enhances total neuronal excitability by promoting excitatory synaptic transmission in glutamatergic neurons.50 Therefore, additional studies utilizing RNA-sequencing techniques to deter-mine gene expression changes after poly(I:C) and microglia depletion will provide important insights into how poly(I:C) escalates alcohol intake and why microglia depletion rescues this behavioral phenotype.
Another context in which microglia may be critical mediators of alcohol behaviors is high-dose alcohol exposure, such as binge drinking or alcohol dependence. Microglia depletion prevented inflammatory gene expression after acute binge alcohol exposure, suggesting microglia could regulate binge-like drinking.16 Gene expression studies from individuals with AUD and mouse models of alcohol dependence all show strong enrichment of innate immune pathways, including genes highly enriched in microglia (e.g., B2m, Ctss, and Il6) and microglial-specific upregulation of inflammatory pathways.27,47 Additionally, mouse models of alcohol dependence increase microglia proliferation, similar to individuals with AUD.9,10 Taken together, this suggests microglia play a key role in the development of alcohol dependence. Future investigations will need to determine if high-dose alcohol recruits microglia to an effector phenotype to regulate binge-like drinking and alcohol dependence.
We hypothesize that different alcohol behaviors recruit and are regulated by specific glial cell types. Astrocytes may play a more critical role in the sedative effects of acute intoxication and escalation of voluntary intake, whereas microglia regulate neuroimmune-induced escalations in voluntary intake. Together, this suggests that microglia and astrocytes respond to specific environmental cues, depending on the type of alcohol exposure, that recruit them to an effector cell phenotype. Therefore, more targeted pharmacotherapies, accounting for glial cell type and type of alcohol exposure, may represent novel treatment strategies for alcohol abuse.
Finally, our work raises another important question. What factors in the microenvironment are necessary for recruitment of astrocytes versus microglia to an effector cell phenotype? Our previous work demonstrated that astrocytes and microglia have unique gene expression changes, differentiating them from other CNS cell types after alcohol exposure.12,27,31 However, the type of alcohol exposure also determines the type and magnitude of gene expression changes in astrocytes and microglia, suggesting that the microenvironment signals are unique in each type of alcohol behavior.27,31 These findings warrant future studies to determine what signals from the microenvironment are necessary to recruit astrocytes or microglia to regulate specific alcohol behaviors.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the National Institutes of Health/National Institute of Alcohol Abuse and Alcoholism [U01 AA020926, P01 AA020683, AA013520, AA0006399, AA025499, and AA012404] and support from the American Cancer Society to LIRE ([128265-RSG-158-01-CSM) and TAT (Postdoctoral Fellowship, 126584-PF-14-216-01-TBF). The authors would like to thank Andrey Rymar at Plexxikon Inc for all of his assistance helping secure chow formulation and drug distribution.
Footnotes
SUPPORTING INFORMATION
Additional supporting information may be found in the Supporting Information section.
CONFLICT OF INTEREST
The authors report no biomedical financial interests or potential conflicts of interest.
REFERENCES
- 1.Li Q, Barres BA. Microglia and macrophages in brain homeostasis and disease. Nat Rev Immunol. 2018;18(4):225–242. [DOI] [PubMed] [Google Scholar]
- 2.Paolicelli RC, Ferretti MT. Function and dysfunction of microglia dur-ing brain development: consequences for synapses and neural cir-cuits. Front Synaptic Neurosci. 2017;9:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parkhurst CN, Yang G, Ninan I, et al. Microglia promote learning-dependent synapse formation through brain-derived neurotrophic factor. Cell. 2013;155(7):1596–1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ayata P, Badimon A, Strasburger HJ, et al. Epigenetic regulation of brain region-specific microglia clearance activity. Nat Neurosci. 2018; 21(8):1049–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Henriques JF, Portugal CC, Canedo T, Relvas JB, Summavielle T, Socodato R. Microglia and alcohol meet at the crossroads: microglia as critical modulators of alcohol neurotoxicity. Toxicol Lett. 2018;283:21–31. [DOI] [PubMed] [Google Scholar]
- 6.Marshall SA, Geil CR, Nixon K. Prior binge ethanol exposure potenti-ates the microglial response in a model of alcohol-induced neu-rodegeneration. Brain Sci. 2016;6:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blednov YA, Bergeson SE, Walker D, Ferreira VM, Kuziel WA, Harris RA. Perturbation of chemokine networks by gene deletion alters the reinforcing actions of ethanol. Behav Brain Res. 2005;165 (1):110–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blednov YA, Ponomarev I, Geil C, Bergeson S, Koob GF, Harris RA. Neuroimmune regulation of alcohol consumption: behavioral valida-tion of genes obtained from genomic studies. Addict Biol. 2012;17(1):108–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.He J, Crews FT. Increased MCP-1 and microglia in various regions of the human alcoholic brain. Exp Neurol. 2008;210(2):349–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Siemsen BD, Landin JD, McFaddin JA, Hooker KN, Chandler LJ, Scofield MD. Chronic Intermittent Ethanol Exposure Differentially Alters Microglial Morphology in the Prelimbic Cortex and Nucleus Accumbens Core. Biorxiv. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marshall SA, McClain JA, Kelso ML, Hopkins DM, Pauly JR, Nixon K. Microglial activation is not equivalent to neuroinflammation in alcohol-induced neurodegeneration: the importance of microglia phe-notype. Neurobiol Dis. 2013;54:239–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McCarthy GM, Farris SP, Blednov YA, Harris RA, Mayfield RD. Micro-glial-specific transcriptome changes following chronic alcohol con-sumption. Neuropharmacology. 2018;128:416–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu Y, Lousberg EL, Moldenhauer LM, et al. Attenuation of microglial and IL-1 signaling protects mice from acute alcohol-induced sedation and/or motor impairment. Brain Behav Immun. 2011;25(Suppl 1): S155–S164. [DOI] [PubMed] [Google Scholar]
- 14.Agrawal RG, Hewetson A, George CM, Syapin PJ, Bergeson SE. Min-ocycline reduces ethanol drinking. Brain Behav Immun. 2011;25(Suppl 1):S165–S169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gajbhiye SV, Tripathi RK, Petare AU, Potey AV, Shankar A. Min-ocycline in alcohol withdrawal induced anxiety and alcohol relapse in rats. Curr Clin Pharmacol. 2018;13:65–72. [DOI] [PubMed] [Google Scholar]
- 16.Walter TJ, Crews FT. Microglial depletion alters the brain neuroimmune response to acute binge ethanol withdrawal. J Neuroinflammation. 2017;14:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Erblich B, Zhu L, Etgen AM, Dobrenis K, Pollard JW. Absence of col-ony stimulation factor-1 receptor results in loss of microglia, disrupted brain development and olfactory deficits. PLoS ONE. 2011; 6:e26317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li J, Chen K, Zhu L, Pollard JW. Conditional deletion of the colony stimulating factor-1 receptor (c-fms proto-oncogene) in mice. Genesis. 2006;44:328–335. [DOI] [PubMed] [Google Scholar]
- 19.Dagher NN, Najafi AR, Kayala KM, et al. Colony-stimulating factor 1 receptor inhibition prevents microglial plaque association and improves cognition in 3xTg-AD mice. J Neuroinflammation. 2015; 12:139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Han J, Zhu K, Zhang XM, Harris RA. Enforced microglial depletion and repopulation as a promising strategy for the treatment of neuro-logical disorders. Glia. 2019;67(2):217–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Randall PA, Vetreno RP, Makhijani VH, Crews FT, Besheer J. The toll-like receptor 3 agonist poly(I:C) induces rapid and lasting changes in gene expression related to glutamatergic function and increases etha-nol self-administration in rats. Alcohol Clin Exp Res. 2019;43:48–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Warden AS, Azzam M, DaCosta A, et al. Toll-like receptor 3 activation increases voluntary alcohol intake in C57BL/6J male mice. Brain Behav Immun. 2019;77:55–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhu X, Levasseur PR, Michaelis KA, Burfeind KG, Marks DL. A distinct brain pathway links viral RNA exposure to sickness behavior. Sci Rep. 2016;6:29885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hwa LS, Chu A, Levinson SA, Kayyali TM, DeBold JF, Miczek KA. Per-sistent escalation of alcohol drinking in C57BL/6J mice with intermit-tent access to 20% ethanol. Alcohol Clin Exp Res. 2011;35:1938–1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Melendez RI. Intermittent (every-other-day) drinking induces rapid escalation of ethanol intake and preference in adolescent and adult C57BL/6J mice. Alcohol Clin Exp Res. 2011;35:652–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blednov YA, Black M, Benavidez JM, Da Costa A, Mayfield J, Harris RA. Sedative and motor incoordination effects of ethanol in mice lacking CD14, TLR2, TLR4, or MyD88. Alcohol Clin Exp Res. 2017;41:531–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Erickson EK, Blednov YA, Harris RA, Mayfield RD. Glial gene net-works associated with alcohol dependence. Sci Rep. 2019;9:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Warden A, Truitt J, Merriman M, et al. Localization of PPAR isotypes in the adult mouse and human brain. Sci Rep. 2016;6:27618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Funk KE, Klein RS. CSF1R antagonism limits local restimulation of antiviral CD8(+) T cells during viral encephalitis. J Neuroinflammation. 2019;16:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hanchar HJ, Dodson PD, Olsen RW, Otis TS, Wallner M. Alcohol-induced motor impairment caused by increased extrasynaptic GABA(A) receptor activity. Nat Neurosci. 2005;8(3):339–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Erickson EK, Farris SP, Blednov YA, Mayfield RD, Harris RA. Astro-cyte-specific transcriptome responses to chronic ethanol consump-tion. Pharmacogenomics J. 2018;18:578–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liddelow SA, Guttenplan KA, Clarke LE, et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature. 2017;541 (7638):481–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Logrip ML, Barak S, Warnault V, Ron D. Corticostriatal BDNF and alcohol addiction. Brain Res. 2015;1628(Pt A):60–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ponomarev I, Wang S, Zhang L, Harris RA, Mayfield RD. Gene coexpression networks in human brain identify epigenetic modifica-tions in alcohol dependence. J Neurosci. 2012;32(5):1884–1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Elmore MR, Najafi AR, Koike MA, et al. Colony-stimulating factor 1 receptor signaling is necessary for microglia viability, unmasking a microglia progenitor cell in the adult brain. Neuron. 2014;82(2):380–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang Y, Xu Z, Xiong S, et al. Repopulated microglia are solely derived from the proliferation of residual microglia after acute deple-tion. Nat Neurosci. 2018;21(4):530–540. [DOI] [PubMed] [Google Scholar]
- 37.Zhang Y, Chen K, Sloan SA, et al. An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cere-bral cortex. J Neurosci. 2014;34:11929–11947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grebb JA, Greengard P. An analysis of synapsin II, a neuronal phos-phoprotein, in postmortem brain tissue from alcoholic and neuropsy-chiatrically ill adults and medically ill children and young adults. Arch Gen Psychiatry. 1990;47(12):1149–1156. [DOI] [PubMed] [Google Scholar]
- 39.Rodd ZA, Bertsch BA, Strother WN, et al. Candidate genes, pathways and mechanisms for alcoholism: an expanded convergent functional genomics approach. Pharmacogenomics J. 2007;7:222–256. [DOI] [PubMed] [Google Scholar]
- 40.Clemens DL. Use of cultured cells to study alcohol metabolism. Alco-hol Res Health. 2006;29(4):291–295. [PMC free article] [PubMed] [Google Scholar]
- 41.Colombo E, Farina C. Astrocytes: key regulators of neu-roinflammation. Trends Immunol. 2016;37:608–620. [DOI] [PubMed] [Google Scholar]
- 42.Davis RL, Syapin PJ. Ethanol increases nuclear factor-kappa B activity in human astroglial cells. Neurosci Lett. 2004;371:128–132. [DOI] [PubMed] [Google Scholar]
- 43.Diamond I, Nagy L, Mochly-Rosen D, Gordon A. The role of adeno-sine and adenosine transport in ethanol-induced cellular tolerance and dependence. Possible biologic and genetic markers of alcoholism. Ann N Y Acad Sci. 1991;625:473–487. [DOI] [PubMed] [Google Scholar]
- 44.Bray JG, Reyes KC, Roberts AJ, Ransohoff RM, Gruol DL. Synaptic plasticity in the hippocampus shows resistance to acute ethanol exposure in transgenic mice with astrocyte-targeted enhanced CCL2 expression. Neuropharmacology. 2013;67:115–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bull C, Freitas KC, Zou S, et al. Rat nucleus accumbens core astro-cytes modulate reward and the motivation to self-administer ethanol after abstinence. Neuropsychopharmacology. 2014;39(12):2835–2845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Meyers JL, Salling MC, Almli LM, et al. Frequency of alcohol con-sumption in humans; the role of metabotropic glutamate receptors and downstream signaling pathways. Transl Psychiatry. 2015;5:e586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kapoor M, Wang JC, Farris SP, et al. Analysis of whole genome-transcriptomic organization in brain to identify genes associated with alcoholism. Transl Psychiatry. 2019;9:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Prieto GA, Cotman CW. Cytokines and cytokine networks target neu-rons to modulate long-term potentiation. Cytokine Growth Factor Rev. 2017;34:27–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hunsberger HC, Konat GW, Reed MN. Peripheral viral challenge ele-vates extracellular glutamate in the hippocampus leading to seizure hypersusceptibility. J Neurochem. 2017;141(3):341–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Duan L, Zhang XD, Miao WY, et al. PDGFRbeta cells rapidly relay inflammatory signal from the circulatory system to neurons via che-mokine CCL2. Neuron. 2018;100:183, e188–200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.