Abstract
Co-evolutionary arms races are fertile ground for innovation. The strong selective pressures exerted by mobile genetic elements (MGEs) on their prokaryotic hosts exemplify this, yielding robust technologies such as restriction enzymes and CRISPR-Cas nucleases. Clustered, regularly interspaced short palindromic repeats (CRISPR) and CRISPR-associated (Cas) genes, a diverse family of prokaryotic adaptive immune systems, have emerged as a biotechnological tool and therapeutic. The discovery of protein inhibitors of CRISPR-Cas systems, called “anti-CRISPRs” (Acrs), enables the development of more controllable and precise CRISPR-Cas tools. The applications of Acr proteins for post-translational control of CRISPR-Cas systems in prokaryotic and mammalian cells, organisms, and ecosystems will be discussed here.
CRISPR-Cas systems provide prokaryotes with adaptive immunity from their pathogens. These multi-protein (Class 1) or single protein (Class 2) nuclease complexes use a guide or CRISPR RNA (gRNA or crRNA) to target invasive nucleic acids. The specificity and easily programmable nature of CRISPR-Cas nucleases have been repurposed for various molecular, biotechnological, and medical applications, including gene editing, gene knockouts, and gene regulation1,2. However, despite the revolutionary advantages offered by the growing CRISPR-Cas toolbox, several challenges remain for the efficacy and safety of these technologies. For example, Cas nuclease activity in cells during in vivo or ex vivo editing can lead to off-target effects3, unexpected on-target effects4, cellular toxicity5,6, and immunogenicity7,8—all of which need to be addressed for the development of safe genome editing applications.
Anti-CRISPR (Acr) proteins are a collective arsenal of natural bona fide CRISPR-Cas antagonists encoded by diverse MGEs, such as plasmids and phages, that inhibit CRISPR-Cas immune function at various stages9,10. To date, 45 non-homologous anti-CRISPR proteins (24 for Class 1 CRISPR-Cas, 21 for Class 2) have been discovered, with distinct mechanisms and structures and no significant sequence similarities to each other11–13. Distinct acr genes can often be found next to each other, which has enabled their discovery14. The ability of many Acr proteins to directly interfere with CRISPR-Cas functions in heterologous hosts provides genetically encodable, post-translational regulation for CRISPR-Cas derived technologies.
Characterized Acr proteins inhibit CRISPR-Cas function by interacting directly with a Cas protein to prevent target DNA binding, cleavage, crRNA loading, or effector complex formation (table 1, Fig. 1). Some Acr proteins that inhibit Type I CRISPR-Cas systems, for example, interact with the crRNA-guided Cascade complex and prevent DNA binding, while others prevent recruitment of the Cas3 nuclease15. The mechanisms elucidated for Type II Acr proteins have presented similar conclusions, where a direct interaction with Cas9 limits DNA binding through steric occlusion16,17 or prevents the activation of the HNH nuclease domain—allowing DNA binding but blocking target cleavage18. Interestingly, the Cas12a inhibitor AcrVA1 operates through an enzymatic mechanism, cleaving the gRNA when bound to Cas12a12.
Table 1.
Summary of identified anti-CRISPR mechanisms
| Type | Mechanism | Subtype inhibited | Acr Name | Cas Ortholog | References |
|---|---|---|---|---|---|
| I | DNA binding | I-F | AcrIF1 | PaeCascade (I-F), PecCascade (I-F) | 15,81,86 |
| AcrIF2 | PaeCascade (I-F), PecCascade (I-F) | 15,81,86 | |||
| AcrIF4 | PaeCascade (I-F) | 86 | |||
| AcrIF10 | PaeCascade (I-F), PecCascade (I-F) | 81,88 | |||
| I-D | AcrID1 | SisCas10d (I-D) | 89 | ||
| I-E | AcrIE1 | PaeCas3 (I-E) | 32 | ||
| I-F | AcrIF3 | PaeCas3 (I-F) | 15,86 | ||
| ? | I-C | AcrIC1 | PaeCascade/Cas3 (I-C) | 75 | |
| I-E | AcrIE2–7 | PaeCascade/Cas3 (I-E) | 75,90 | ||
| I-F | AcrIF5–9 | PaeCascade/Cas3 (I-F) | 15,75,81 | ||
| II | DNA binding | II-A | AcrIIA2* | SpyCas9, LmoCas9 | 16,20 |
| AcrIIA4* | SpyCas9, LmoCas9 | 16,20 | |||
| AcrIIA6* | St1Cas9 | 83,91 | |||
| II-C | AcrIIC3* | NmeCas9, Nme2Cas9, HpaCas9, SmuCas9 | 18,21,92,93 | ||
| AcrIIC4* | NmeCas9, Nme2Cas9, HpaCas9, SmuCas9 | 92 | |||
| AcrIIC5* | NmeCas9, HpaCas9, SmuCas9 | 92 | |||
| Guide loading | AcrIIC2* | NmeCas9, SmuCas9, HpaCas9 | 21,92,94,95 | ||
| DNA cleavage | AcrIIC1* | NmeCas9, Nme2Cas9, CjeCas9, GeoCas9, SmuCas9, HpaCas9 | 18,21,92,95 | ||
| II-A | AcrIIA11* | SpyCas9, TdeCas9 | 85 | ||
| ? | II-A | AcrIIA1 | LmoCas9, SpyCas9 | 20,96 | |
| AcrIIA3 | LmoCas9, SpyCas9 | 20 | |||
| AcrIIA5* | St1Cas9, St3Cas9, SpyCas9 | 83,97 | |||
| AcrIIA7–10 | SpyCas9 | 84 | |||
| III | ? | III-B | AcrIIIB1 | SisCmr-α; SisCmr-γ | 98 |
| V | DNA binding | V-A | AcrVA1* | MbCas12a, AsCas12a, LbCas12a, FnCas12a | 12,75,82,99 |
| AcrVA4* | MbCas12a, LbCas12a | 12,82,99 | |||
| AcrVA5* | MbCas12a, LbCas12a | 13,82 | |||
| ? | AcrVA2 | MbCas12a | 75 | ||
| AcrVA3.1† | MbCas12a, PaeCascade/Cas3 (I-C) | 75 | |||
| VI | ? | VI-B | Csx27# | BzoCas13b, PbuCas13b | 80 |
Acr protein functions in human cells.
Csx27 has anti-CRISPR function but appears to be a Cas protein likely serving a regulatory role.
Some Acrs inhibit multiple subtypes. AcrVA3.1 has been shown to inhibit V-A and I-C CRISPR-Cas systems. AcrIE4-IF7, a fusion of AcrIE4 and AcrIF7, has been shown to inhibit both I-F and I-E subtypes75.
Figure 1. Stages of CRISPR-Cas immunity and mechanisms of Anti-CRISPR function.

a. The stages of CRISPR-Cas immunity: During adaptation, a fragment of the invading nucleic acid is incorporated as a new spacer (purple) in the CRISPR array. In the biogenesis stage, the CRISPR array is transcribed into a long precursor CRISPR RNA (pre-crRNA) that is processed by Cas proteins into single crRNAs (purple, yellow, and green) that act as guides for Cas effector nucleases (blue). During interference, the crRNA-guided effector nucleases survey the cell in search of a cognate sequence, leading to target cleavage upon binding. b. Mechanisms for anti-CRISPR (Acr) function. Targeted phages and other MGEs can bypass CRISPR-Cas immunity by expressing Acr proteins. Although the inhibitory functions of the Acr proteins characterised to date are highly diverse, they can be broadly classified into two main mechanisms of action: i) Target DNA binding inhibitors: eg. AcrIIA4 (red) occludes Cas9’s PAM recognition domain, AcrIIC3 (blue) forces Cas9 dimerization, AcrVA1 (yellow) cleaves Cas12a’s crRNA, AcrVA5 (green) inhibits Cas12a via post translational acetylation (Ac), and AcrIF1 (vermilion) prevents target DNA binding by binding to Cascade. ii) DNA cleavage inhibitors: eg. AcrIIC1 (orange) disables Cas9 by binding to the HNH nuclease domain, and AcrIE1 (pink) blocks DNA cleavage by binding to Cas3.
Acr proteins are named for the system that they inhibit in the order in which they were discovered19. For example, the widely used AcrIIA4 protein was the fourth Type II-A Acr protein discovered. Several Acr proteins have already proven successful at regulating gene editing activities in different cell types, most notably two SpyCas9 inhibitors (AcrIIA2 and AcrIIA4)20 and two NmeCas9 inhibitors (AcrIIC1 and AcrIIC3)21. Here we discuss these proteins and others as useful technologies for regulating CRISPR-Cas-derived tools in both bacterial and eukaryotic cells. Additionally, we highlight the use of Acr proteins for inhibiting gene drives and controlling catalytically dead CRISPR-Cas-based applications, and we propose potential future applications. For more details on the current CRISPR-Cas toolkit, we refer readers to other recent reviews1,22.
Applications of Acr proteins in prokaryotes
CRISPR-Cas is a powerful tool for many prokaryotic applications23. Cas9-based editing has been employed in numerous bacteria, from model organisms like Escherichia coli24 to industrially relevant species belonging to Clostridium, Lactobacillus, or Streptomyces genera25. Endogenous CRISPR-Cas systems can also be used for editing applications, as approximately 40% of all sequenced, culturable bacteria and 87% of archaea carry a CRISPR-Cas system of some type26. In these applications, Acr proteins have dual utility: i) identifying strains where rampant endogenous CRISPR-Cas inhibition suggests that editing will not be effective, and ii) enhancing temporal control, which can enable editing of bacterial and phage genomes where not previously possible (Fig 2A).
Figure 2. Applications of acrs in prokaryotes.
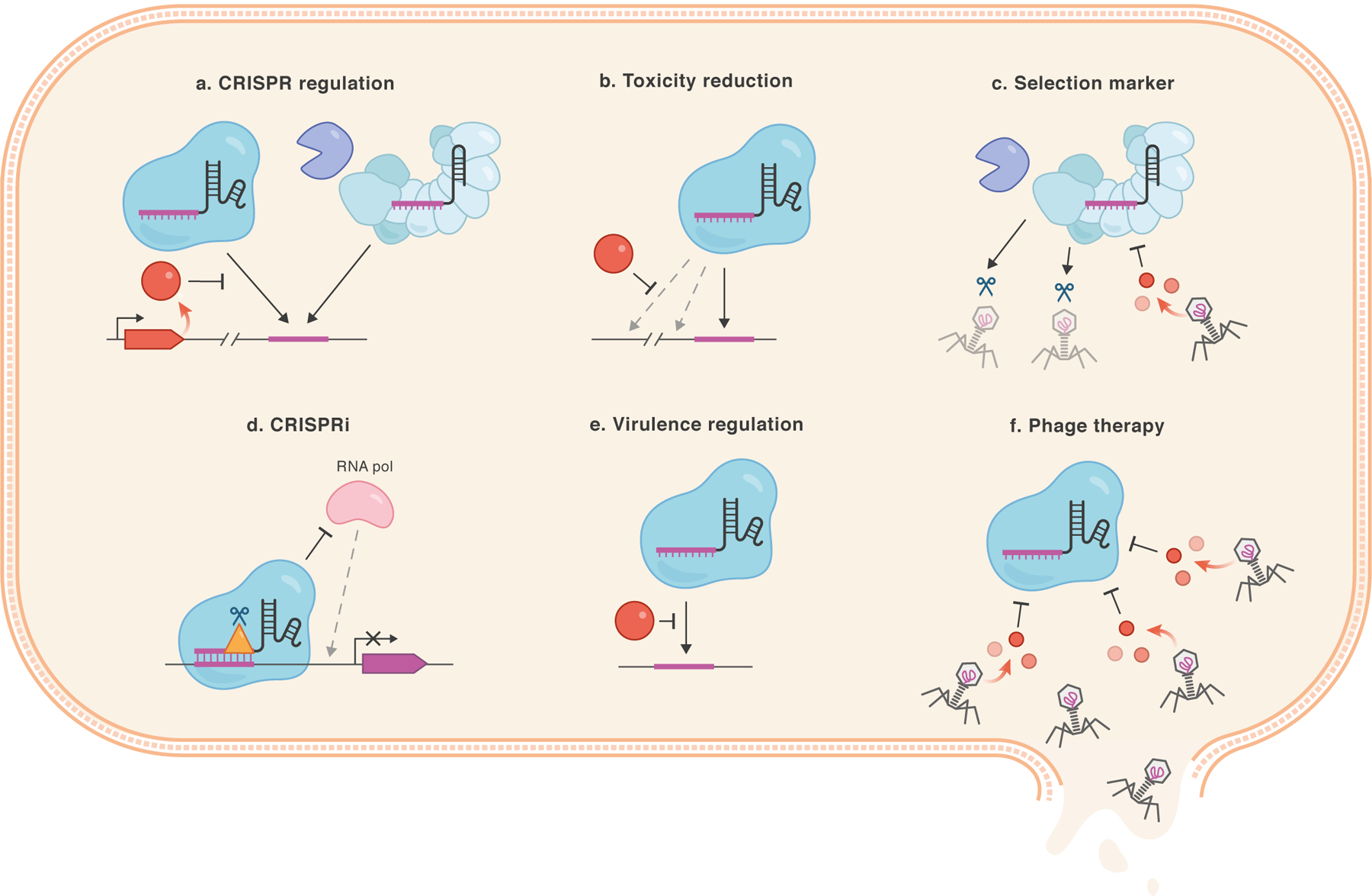
acrs have the potential to serve a wide variety of functions in bacteria. a. Acr proteins (red circle) may block editing by endogenous or exogenous CRISPR-Cas systems. b. Acr proteins (red circle) may help reduce the occurrence of any unwanted genomic editing events that contribute to toxicity. c. acrs can serve as selectable markers in the genetic editing of bacteriophages. d. Acr proteins (e.g. AcrIIC1, AcrIF3, AcrIE1, yellow triangle) can disable the endonuclease activity of the effector nuclease, thereby repurposing it as a CRISPR interference system for gene knockdown applications. e. CRISPR-Cas machinery can contribute to bacterial virulence, for example, through binding to genomic DNA. Administration of Acr proteins may prevent these non-canonical functions of CRISPR-Cas systems. f. The viral expression of Acr proteins can potentially broaden the host range of phage therapeutics by inhibiting CRISPR-Cas immunity.
In general, there are two routes to achieving genomic editing or targeted gene repression with CRISPR-Cas in bacteria: one can either introduce an entire exogenous system into the given organism27, or reprogram an endogenous system by expressing a self-targeting crRNA28. Chromosomal targeting with both strategies kill bacteria at high efficiencies, with genome edited cells constituting a fraction of survivor cells. The presence of Acr proteins could greatly impede the high efficiency of these processes. In fact, genomically encoded Acr proteins are highly prevalent in bacteria; >30 % of Pseudomonas aeruginosa strains carrying a CRISPR-Cas system also encode one or more cognate acr genes (with the number likely to be higher as more of these gene families are identified)29. Similarly, Acr proteins that inhibit Cas9 are found in >50% of Listeria monocytogenes strains encoding Cas920, indicating that this may present a frequent obstacle when attempting to use CRISPR-Cas systems to edit or kill bacteria. If a bacterial strain encodes an acr gene against an endogenous CRISPR system, then utilizing an exogenous system for editing may be a more productive approach. In bacteria that naturally have Cas9 orthologues, such as L. monocytogenes, the commonly used SpyCas9 protein may not be a viable approach due to the presence of acr genes that inhibit it. The continued identification of anti-CRISPR proteins and mechanisms to inhibit or block their activity are needed to broadly enable CRISPR-based editing in bacteria.
Beyond their identification, Acr proteins can also be directly used to enhance microbial gene-editing strategies (Fig. 2A–C). For example, the low transformation efficiencies of many microbes limit one’s ability to recover transformants after expressing a genome-targeting crRNA. The controlled expression of Acr proteins (potentially on the same construct expressing the synthetic crRNA) may mitigate the toxic effects of genomic targeting, enabling stable transformation (Fig. 2B). Upon repression or de-induction of Acr protein production, editing can commence from a larger starting population. Additionally, utilizing acr genes as selection markers to confer resistance against native CRISPR-Cas systems can provide a novel route for the engineering of viruses, for which there are a paucity of selectable markers (Fig. 2C). This strategy was recently employed to knock out genes in a difficult-to-engineer archaeal virus Sulfolobus islandicus rod-shaped virus 2 (SIRV2)30. acrID1 was used to replace a gene of interest, thereby providing positive selection for edited viruses when challenged with the native Sulfolobus islandicus Type I-D CRISPR-Cas system.
CRISPR-Cas systems have previously been engineered to repress gene expression in bacteria31. Although these CRISPR interference (CRISPRi) systems have mostly utilized exogenous catalytically “dead” Class 2 enzymes, endogenous Type I CRISPR-Cas systems can be repurposed to achieve specific gene silencing28. This would require, however, the inactivation or deletion of the effector nuclease (Cas3 in this case). AcrIF3 and AcrIE1 prevent Cas3 recruitment to the surveillance complex at the genomic target15,32, thereby “activating” CRISPRi in the absence of any genome manipulation (Fig. 2D). Similarly, broad spectrum AcrIIC1 allows Cas9 to bind DNA but prevents cleavage, likely enabling CRISPRi in bacteria with II-C CRISPR systems18.
Acr proteins may also be useful in antibacterial applications. As CRISPR-Cas systems have been proposed to regulate bacterial virulence33, Acr proteins could disrupt the CRISPR-dependent virulence mechanisms of these bacterial pathogens (Fig. 2E). Non-canonical Cas functions, however, may not be inhibited by phage proteins; therefore, investigation of this prediction is needed.
Lastly, acr genes may also be used to augment phage therapy approaches, as they can expand phage host range (Fig. 2F). With the global propagation of antibiotic-resistant bacteria, phage therapy has reemerged as an alternative method for combating bacterial infections34; however, many bacterial pathogens are naturally equipped with active CRISPR-Cas systems that may limit the efficacy of phage-based therapeutic approaches. Because of their small size, an arsenal of Acr proteins could be engineered into therapeutic phages to combat CRISPR-based phage resistance, leading to improved efficacy for these antibacterial strategies.
Applications of Acr proteins in eukaryotes
CRISPR-Cas systems have been heterologously expressed in many eukaryotic systems, including fungal35, plant36, and mammalian cells37,38. Cas9 and Cas12a have primarily been used due to their ease of programmability and expression in many hosts39,40. However, strategies to limit and/or control Cas nuclease activity are limited. Moreover, Cas nucleases have been shown to cause variable degrees of off-target editing3,41,42, which could be remedied with “off-switches”. Strategies to prevent off-target editing have generally focused on limiting nuclease activity and expression, such as injecting pre-formed ribonucleoprotein (RNP) complexes42–44, introducing additional regulatory domains45,46, or mutating Cas947,48. Although these strategies have reduced off-target effects, they have important limitations. Regulatory domains can significantly increase the size of Cas9 and often require additional ligands. High-fidelity Cas9 variants can work well for certain guides and delivery modalities but are not universally efficacious and require extensive engineering for each Cas orthologue47–49. Although limiting the duration of CRISPR-Cas activity with RNP delivery can be effective for reducing off-target effects, this strategy is not adaptable for genetically encoded systems, such as gene drives and in vivo delivery via viral vectors. Moreover, the duration of RNP activity cannot be tightly controlled without an additional level of regulation.
The delayed introduction of an Acr protein presents a flexible and tunable mechanism to limit off-target editing while utilizing the wild-type version of the Cas enzyme. For example, delivery of AcrIIA4, either encoded on a plasmid or as purified protein, six hours after the introduction of Cas9 RNPs was found to reduce off-target editing in human cells50. This was efficacious for cells using different sgRNAs that target ß-globin (HBB) and vascular endothelial growth factor A (VEGFA) with reported off-target sites. The underlying assumption is that off-target edits begin to accrue after a majority of on-target editing has occurred (Fig. 3). If so, there should be an ideal and empirically measurable moment where anti-CRISPR activity is optimal, which may vary by cell type and target.
Figure 3. Applications and regulation of Acr proteins.

Anti-CRISPRs have been used to regulate CRISPR-Cas systems in many different scenarios, including gene editing, gene regulation, epigenetic modification, and DNA imaging. Acr proteins can in turn be regulated using inducible promoters, tissue-specific miRNAs, light, and small molecules to achieve rapid and dynamic control of CRISPR-Cas activity.
The relatively small size of most Acr proteins (~50–200 amino acids) also makes them promising CRISPR-Cas modulators during delivery in situ via adeno-associated viral (AAV) vectors. These vectors are commonly employed to deliver genes to cells within specific tissues but are tightly limited on cargo size. Regulation of Cas nuclease activity in tissues is particularly important, given reports of off-target effects and cytotoxicity associated with excessive nuclease activity3,5,6,51. Indeed, cytotoxicity and poor engraftment outcomes for CD34+ ex vivo hematopoietic stem cells (HSCs) expressing Cas9 have been reported6. The delivery of AcrIIA2 and AcrIIA4 on a single adenovirus vector two days after Cas9 significantly improved engraftment outcomes in mice with on-target editing rates unaffected. Another study recently reported successful editing of the CEP290 gene in photoreceptor cells in mice and non-human primates using AAV-mediated delivery of SaCas9 and sgRNA52. Interestingly, this study reported robust SaCas9 expression in the eye up to 40 weeks after a single dose, even though editing was complete after ~1 week. The persistence of Cas9 expression indicates that a mechanism to inactivate Cas9 would be advantageous, given that the enzyme is presumably active. This could be especially important for more promiscuous sgRNAs or for more immunocompetent tissues than the eye. Progress towards Acr deployment in vivo has recently been made with AcrIIC3 successfully inhibiting Nme2Cas9 editing in mice, without any apparent toxicity53. Delivery of AcrIIC3 and the sgRNA on one AAV with Nme2Cas9 on another led to near-complete inhibition of editing in the heart and liver. Moreover, a miRNA-based strategy to prevent AcrIIC3 production in specific tissues was successful.
CRISPR-Cas systems are increasingly being harnessed to alter gene expression without cleavage1. dCas9 or dCas12a have been fused to various functional domains, including transcriptional activators, repressors, and epigenetic modifiers1 (Fig. 3). These functional domains are then recruited to specific sites by the dCas-guide complex. Because most characterized Acr proteins prevent Cas proteins from binding DNA, they have been used to spatially and temporally regulate these processes and confirm that they result from Cas-dependent activity. For example, AcrIIA4 was recently used to inactivate a dCas9-Tet1 demethylase fusion and demonstrated the remarkable persistence of demethylation in the absence of continued dCas9-Tet1 activity54 (Fig. 3). Similarly, dCas9 has also been fused to fluorescent reporters to visualize chromatin dynamics in a sequence-specific manner1,55. AcrIIC3 was shown to prevent NmeCas9-GFP localization to telomeres in human cells21. Given reports of high background fluorescence from free-floating CRISPR complexes56, Acr proteins can be used to determine if their localization patterns are specifically due to target binding (Fig 3).
Fusion variants of Cas9 and Cas12a have also been developed for applications other than gene regulation. Base editors, which consist of a catalytically impaired Cas protein fused to a nucleotide deaminase, convert one nucleotide base pair to another at specific sites without inducing double-stranded breaks57–59. Given recent reports of off-target base editing of RNA in some systems60,61, Acr proteins may be a useful reagent for this and other recruitment efforts to mechanistically dissect off-target events mediated by the fused enzyme compared to Cas9 itself.
The ability of some Acr proteins to prevent target binding by Cas proteins has also been harnessed for biosensors and synthetic gene circuits (Fig 3). For example, a biosensor that couples induction of anti-CRISPR with enhancement of fluorescent expression was constructed in Saccharomyces cerevisiae62. In this case, AcrIIA2 and AcrIIA4 were expressed from galactose- or ß-estradiol-inducible promoters in a system where dCas9::sgRNA complexes are programmed to constitutively repress green fluorescent protein (eGFP) transcription, enabling a simple readout to detect these molecules. Another genetic circuit was developed in mammalian cells using dCas9 fused to VPR, a transcriptional activator domain63. Expression of dCas9-VPR simultaneously induced expression of both GFP and AcrIIA4, which subsequently bound dCas9-VPR and prevented further GFP activation. This feedback loop generated a pulse of fluorescence, demonstrating the utility of acrs for creating dynamic gene circuits.
Application of Acr proteins for gene drives
The advent of CRISPR-Cas9-based technologies has accelerated the potential for ecological engineering through the use of “gene drives,” which spread engineered traits within a population through a super-Mendelian mechanism64 (Fig. 4A,B). Gene drives often feature a transgenic organism with chromosomally encoded Cas9 that is programmed to target the homologous region on the sister chromosome. When the targeted region repairs the cut using the drive sequence as a template, Cas9 and its associated cargo become encoded on both chromosomes. Gene drives have the potential to greatly benefit human health in various ways, including curtailing insect-borne diseases such as malaria or dengue65,66, eliminating invasive species, and increasing agricultural sustainability67. However, gene drives have been met with calls for caution68, as they could have unforeseen consequences or be co-opted for nefarious purposes, leading to large-scale devastation. For these reasons, multiple robust safety measures are needed before gene drive technologies can be employed in the wild.
Figure 4. Utilization of Acr proteins for controlling gene drives.

a. In Mendelian inheritance, a heterozygous mutant allele (marked with blue), is inherited in 25% of the offspring when mated with a homozygous non-mutant. b. The presence of a gene-drive element containing the Cas9 endonuclease rapidly spreads the Cas9 allele throughout the population. c. A population of Acr-expressing homozygotes inhibiting Cas9 can impede the spread of the gene drive construct (the presence of the Acr is denoted with a red circle). A scenario is depicted where a population expressing an Acr is protected from a gene drive. It is assumed that the gene-drive expressing individuals will mate with engineered Acr-expressing individuals.
Acr proteins currently present the most direct and broadly acting (i.e. independent of sgRNA sequence) method for inhibiting or modulating drive strength and could be deployed concomitantly with or after a gene drive (Fig 4C). It was recently demonstrated that both AcrIIA2 and AcrIIA4 are able to inhibit gene drives at varying levels, with AcrIIA4 showing > 99.9% suppression in a yeast model system69. Interestingly, AcrIIA2 was slightly weaker at inhibiting gene drive, consistent with early work demonstrating that AcrIIA2 is weaker that AcrIIA4 in some contexts17,20. This study also revealed that the exact level of gene drive inhibition is titratable depending on specific mutations within the acr genes as well as their levels of expression. Using a mutant Acr or a natural variant that does not completely inhibit Cas9 to weaken a drive may provide the ideal scenario for achieving drive persistence by avoiding strong selection towards complete inactivation. To rapidly halt the spread of an ongoing drive, an alternative Cas enzyme could be employed to drive the acr gene through the population and thereby reverse the effect of the original. Acr proteins thus present a means for finely-tuned control of gene drives, which may enable safe deployment of this promising technology. Future work in animal-based gene drives will be needed to test Acr efficacy, specifically whether mating a gene drive animal with an anti-CRISPR animal returns inheritance to Mendelian frequencies or has unforeseen outcomes.
Applications of Acr proteins in vitro
Acr proteins have also been employed as lab reagents. In one case, CRISPR-Cas9 RNPs were detected and quantified using AcrIIA4 as an immobilized capture ligand70. Fixing AcrIIA4 on glassy carbon electrodes enabled the specific detection of sgRNA-loaded Cas9 using electrochemical, colorimetric, and fluorescent readouts, which can be used to to measure Cas9 delivery efficacy and persistence in biological samples. In another case, Acr proteins were used to facilitate adenoviral vector production71. A helper-dependent adenovirus was engineered to express Cas9 for genome editing and self-cleavage after transduction into cells, thereby allowing editing but preventing Cas9 persistence. To prevent self-cleavage during vector production prior to transduction, expression of AcrIIA2 and AcrIIA4 was combined with Cas9 mRNA downregulation. Acr proteins can similarly be used to inactivate Cas protein in other systems where leaky Cas activity is detrimental or confounding.
Regulation of Acr proteins
Multiple strategies have been developed to modulate Cas9 expression and activity1, including inducible promoters72 and destabilization domains that allow expression only in the presence of certain ligands46. Other strategies control the functionality of Cas9 protein using light45,73 or small molecules46,74. These strategies have shown efficacy for SpyCas9 regulation; however, they have not been adapted to all of the other natural and engineered Cas variants that differ in size, fidelity, and PAM motif. Engineering all nucleases to include these regulatory domains or finding potent small molecules to inhibit them remains a formidable challenge. Fortunately, Acr proteins have been found for most major Cas proteins and some can inhibit more than one Cas ortholog (e.g. AcrIIA4, AcrIIA5, AcrIIC1, or AcrVA1). They can therefore be used to inhibit multiple Cas orthologs or different engineered variants of the same ortholog without modifying each nuclease. Furthermore, “regulating the regulator” of CRISPR-Cas activity will likely provide tighter on-off control than directly regulating the Cas enzyme, since one can induce the Acr protein, which represses Cas activity, instead of relying on a leaky basal off-state.
In order to achieve more rapid and dynamic control of CRISPR-Cas activity, several methods for regulating anti-CRISPR expression and activity have been discovered or developed, including i) transcriptional, ii) post-transcriptional, iii) optogenetic, and iv) ligand-based strategies63,75–77(Fig 3). (i) Anti-CRISPR-associated (aca) genes have recently been shown to repress acr transcription in bacteria by binding to the native acr- promoter78. These aca genes are usually encoded within the same operon as acr genes and provide a means to downregulate initially high levels of acr transcription. In heterologous systems, acr genes have been transcriptionally regulated using inducible promoters17,62,75; however, temporal regulation could also be achieved with promoters expressed at a specific stage of the cell cycle or in response to cellular events. (ii) Post-transcriptional regulation of anti-CRISPRs using tissue-specific microRNAs has recently been developed to control Cas9. This was achieved by modifying the 3’ UTR of acrIIA4 and acrIIC3 transcripts to include binding sites for microRNAs (miRNAs) that are highly expressed in liver cells53,76,79. These miRNAs effectively downregulate AcrIIA4 and AcrIIC3 in hepatocytes, where Cas9 activity is desired, but allow it to inhibit Cas9 in other cell types. This strategy effectively inhibited in vivo Nme2Cas9 editing in heart tissue but allowed editing in liver cells53. (iii) A photo-controllable AcrIIA4 variant was developed by inserting the LOV2 domain from Avena sativa phototrophin-1 into an AcrIIA4 surface-exposed loop and optimizing for functionality77. In the absence of light, AcrIIA4-LOV2 is able to bind and inhibit SpyCas9; however, upon photoexcitation, the LOV2 domain loses its structural conformation and causes AcrIIA4 to misfold and lose affinity for Cas9. (iv) A ligand-inducible Acr protein was also recently developed by fusing AcrIIA4 to a destabilization domain (DD)63. In the presence of Shield-1, the DD is stabilized, allowing AcrIIA4 to maintain structural integrity and inhibit Cas9. In the absence of Shield-1, AcrIIA4 misfolds and becomes degraded, thereby liberating Cas9. In this study, DD-AcrIIA4 inhibited a dCas9-VPR transcriptional activator in an inducible manner. Interestingly, fusing dCas9-VPR directly to a destabilization domain did not render dCas9 inducible, perhaps due to limitations imposed by the VPR domain. This finding demonstrates the value of using inducible Acr proteins to control Cas proteins that may not be amenable to direct regulation or further engineering.
Conclusions
Moving forward, we anticipate that the discovery of Acr proteins can match the discovery and development of new CRISPR-Cas systems. MGEs encoding acr genes have yet to be identified for several CRISPR-Cas types and subtypes, most notably the Type VI system. The CRISPR associated protein Csx27 has been shown to repress Type VI-B function80, however, phages and plasmids may also encode inhibitors for Cas13. The discovery of acrs for these systems will provide regulatory tools for Cas proteins and may reveal mechanistic novelty that have yet to be uncovered for these systems. The recent discovery of a catalytic Acr protein that functions at sub-stoichiometric levels12 suggests that similarly novel and potent CRISPR inhibitory mechanisms are waiting to be identified. Lastly, anti-CRISPRs that directly interfere with other stages of CRISPR immunity such as crRNA processing and spacer acquisition, rather than targeting, have yet to be reported.
Additional work must also be done to determine if Acr proteins are safe and effective off-switches in vivo. Although Acr proteins have been shown to inhibit editing by Cas9 in mice53, it is not known if they ever induce toxicity or provoke a host response, which must be determined for their safe implementation in animals. The efficacy of Acr proteins for preventing off-target editing or halting gene drives in animals also remains to be demonstrated. The applications described here for CRISPR-Cas systems and their antagonists represent an early stage for these technologies, and much innovation is likely to come.
Informational Box 1: Anti-CRISPR discovery
Likely as a consequence of the fast-paced evolutionary arms race, Acr proteins display little significant sequence or structural similarities with each other or proteins of known function. Apart from sharing a typically low molecular weight, Acr proteins lack a common denominator, rendering de novo prediction challenging. Current discovery approaches have utilized the clustering of acr genes together into “acr-loci”, typically in the vicinity of conserved anti-CRISPR associated (aca) transcriptional regulators14,81. An alternative discovery strategy is based on the detection of “self-targeting” genomes − that is, genomes in which there are CRISPR spacers that match MGEs integrated in the same genome (e.g. prophages)20,75,82. In principle, the resultant autoimmune state should trigger cell death, and consequently, tolerance to self-targeting suggests that the integrated MGEs encode acr genes. Additionally, acrs have been found by screening lytic phages to find those that evade CRISPR-Cas immune targeting83 and through metagenomics screens84,85. The functional verification of putative acrs is subsequently carried out using in vivo or in vitro functional assays of CRISPR interference inhibition20,75,82,86,87. Future work in this area will likely feature novel screening approaches focused on a specific mode-of-action, algorithms for anti-CRISPR prediction (e.g. machine learning), and the thorough characterization of the spectrum of activity for Acr proteins. Additionally, the identification of novel inhibitory mechanisms, such as catalytic Acr proteins, (e.g. AcrVA1 and AcrVA5)12,13 will likely prove highly useful.
Informational Box 2: Advantages of Anti-CRISPRs
Although many strategies for regulating CRISPR-Cas activity have been developed, Acr proteins have several features that make them well suited for certain applications, such as:
Genetically encodable. acr genes can be encoded and delivered on vectors to cells in vivo or used to halt gene drives in situ. Because they are separate from the CRISPR-Cas system, they can be deployed to shut off or maintain the desired dynamic range of Cas activity as needed. This can be used to continually protect cells from editing, finely tune the amount and duration of Cas activity, and limit undesired background levels in inducible CRISPR-Cas systems.
Broad-spectrum. Many Acr proteins, such as AcrIIA5, AcrIIC1, and AcrVA1, have been found to inhibit multiple orthologs of its target18,75,82,83. This broad-spectrum activity can be used to regulate multiple natural and engineered variants of CRISPR-Cas systems without re-engineering each Cas protein.
Diverse in strength and mechanism. Multiple Acr proteins have been discovered to target the same nuclease yet vary substantially in their size, strength of inhibition, and mechanism of action9,11. Acr proteins can accordingly be selected and optimized (or weakened) according to the needs of the assay (Table 1).
Easy to use. Acr proteins can easily be integrated into a wide range of in vivo and in vitro systems using a standard molecular biology toolkit, without the need for expensive ligands, equipment, or protein engineering. The direct mode-of-action for many characterized Acr proteins ensure that they function in heterologous hosts. They are also complementary to many existing strategies of regulation.
Informational Box 3: Limitations of Anti-CRISPRs
Although Acr proteins have several features that make them well-suited for certain applications, they have drawbacks that make them less suited for others.
Additional component. Although external regulation with Acr proteins can be beneficial, it also introduces an extra component to the system. The use of genetically encoded acrs may necessitate an additional vector or increase vector size. For transient inhibition, purified Acr protein can be used instead; however, unlike small molecule inhibitors of Cas974, Acr proteins are not cell permeable and must be delivered into cells.
Slow reversability. Although the degree of CRISPR-Cas inhibition can be titrated with Acr proteins of varying potency, a single inhibition event is not readily reversible without additional engineering. Stochiometric CRISPR-Cas inhibition by Acr proteins can be overcome by increasing the amount of Cas protein or decreasing Acr expression, but this may be slower than other regulatory methods, such as small molecules or optogenetics directly acting on Cas enzymes. Fortunately, light- and ligand-inducible variants of AcrIIA4 have been engineered to improve reversability and temporal control63,77, but this has yet to be developed for other Acr proteins.
Potential toxicity or immunogenicity. Two Acr proteins have been expressed in mice without causing apparent tissue damage53, but it is not yet known if they interact with other host proteins or provoke a host response. Expression of some Acr proteins has displayed toxicity75. Additional parameters must be considered in vivo, including Acr protein stability, optimal expression levels, and potential for “off-target” interactions.
Acknowledgments:
The authors thank Marina Pinilla-Redondo, who made the figures for this manuscript. Anti-CRISPR research in the Bondy-Denomy lab was supported by the University of California San Francisco Program for Breakthrough in Biomedical Research, funded in part by the Sandler Foundation, and an NIH Office of the Director Early Independence Award DP5-OD021344, R01GM127489, and by DARPA HR0011-17-2-0043. NDM was supported by NIH F32GM133127, BC was supported by the Eötvös National Scholarship of Hungary and a Marie Skłodowska-Curie Actions Individual Global Fellowship (number 844093) of the Horizon 2020 Research Program of the European Commission. RPR was funded by Joint Programming Initiative-Antimicrobial Resistance (JIP-AMR; DARWIN project, #7044-00004B), the Innovation Fund Denmark (Trojan Horse Project, #5157-00005B).
Footnotes
Competing Interests:
J.B.-D. is a scientific advisory board member of SNIPR Biome and Excision Biotherapeutics and a scientific advisory board member and co-founder of Acrigen Biosciences. R.P.R. is a consultant for Ancilia Inc.
References:
- 1.Adli M The CRISPR tool kit for genome editing and beyond. Nat Commun 1–13 (2018). doi: 10.1038/s41467-018-04252-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hsu PD, Lander ES & Zhang F Development and applications of CRISPR-Cas9 for genome engineering. Cell 157, 1262–1278 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hsu PD et al. DNA targeting specificity of RNA-guided Cas9 nucleases. Nat. Biotechnol 31, 827–832 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee H & Kim J-S Unexpected CRISPR on-target effects. Nature biotechnology 36, 703–704 (2018). [DOI] [PubMed] [Google Scholar]
- 5.Ihry RJ et al. p53 inhibits CRISPR-Cas9 engineering in human pluripotent stem cells. Nature Medicine 24, 939–946 (2018). [DOI] [PubMed] [Google Scholar]
- 6.Li C et al. HDAd5/35++ Adenovirus Vector Expressing Anti-CRISPR Peptides Decreases CRISPR/Cas9 Toxicity in Human Hematopoietic Stem Cells. Mol Ther Methods Clin Dev 9, 390–401 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study first demonstrated that acr genes delivered into cells ex vivo can reduce Cas9-associated cytotoxicity and improve engraftment outcomes.
- 7.Chew WL et al. A multifunctional AAV–CRISPR–Cas9 and its host response. Nat. Methods 13, 868–874 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang D et al. Adenovirus-Mediated Somatic Genome Editing of Pten by CRISPR/Cas9 in Mouse Liver in Spite of Cas9-Specific Immune Responses. Hum. Gene Ther 26, 432–442 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Borges AL, Davidson AR & Bondy-Denomy J The Discovery, Mechanisms, and Evolutionary Impact of Anti-CRISPRs. Annu Rev Virol 4, 37–59 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stanley SY & Maxwell KL Phage-Encoded Anti-CRISPR Defenses. Annu. Rev. Genet 52, 445–464 (2018). [DOI] [PubMed] [Google Scholar]
- 11.Trasanidou D et al. Keeping crispr in check: diverse mechanisms of phage-encoded anti-crisprs. FEMS Microbiology Letters 366, 1709 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Knott GJ et al. Broad-spectrum enzymatic inhibition of CRISPR-Cas12a. Nat. Struct. Mol. Biol 26, 315–321 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dong L et al. An anti-CRISPR protein disables type V Cas12a by acetylation. Nat. Struct. Mol. Biol 26, 308–314 (2019). [DOI] [PubMed] [Google Scholar]
- 14.Pawluk A, Davidson AR & Maxwell KL Anti-CRISPR: discovery, mechanism and function. Nat. Rev. Microbiol 16, 12–17 (2018). [DOI] [PubMed] [Google Scholar]
- 15.Bondy-Denomy J et al. Multiple mechanisms for CRISPR-Cas inhibition by anti-CRISPR proteins. Nature 526, 136–139 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study identified multiple mechanisms of inhibition via direct interactions with Cas proteins for the first discovered Acr proteins.
- 16.Dong D et al. Structural basis of CRISPR-SpyCas9 inhibition by an anti-CRISPR protein. Nature 546, 436–439 (2017). [DOI] [PubMed] [Google Scholar]; This work identified the first mechanism and structure of a Cas9 inhibitor, showing AcrIIA4 binds the PAM-interacting motif.
- 17.Jiang F et al. Temperature-Responsive Competitive Inhibition of CRISPR-Cas9. Mol. Cell 73, 601–610.e5 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harrington LB et al. A Broad-Spectrum Inhibitor of CRISPR-Cas9. Cell 170, 1224–1233.e15 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bondy-Denomy J et al. A Unified Resource for Tracking Anti-CRISPR Names. CRISPR J 1, 304–305 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rauch BJ et al. Inhibition of CRISPR-Cas9 with Bacteriophage Proteins. Cell 168, 150–158.e10 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study reported the first Acr proteins that inhibit SpyCas9 and demonstrated the efficacy of AcrIIA2 and AcrIIA4 in human cells.
- 21.Pawluk A et al. Naturally Occurring Off-Switches for CRISPR-Cas9. Cell 167, 1829–1838.e9 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study identified the first Acr proteins that inhibit NmeCas9 and demonstrated their efficacy in human cells.
- 22.Pickar-Oliver A & Gersbach CA The next generation of CRISPR-Cas technologies and applications. Nat. Rev. Mol. Cell Biol 20, 490–507 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Choi KR & Lee SY CRISPR technologies for bacterial systems: Current achievements and future directions. Biotechnol. Adv 34, 1180–1209 (2016). [DOI] [PubMed] [Google Scholar]
- 24.Jiang Y et al. Multigene editing in the Escherichia coli genome via the CRISPR-Cas9 system. Appl. Environ. Microbiol 81, 2506–2514 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luo ML, Leenay RT & Beisel CL Current and future prospects for CRISPR-based tools in bacteria. Biotechnol. Bioeng 113, 930–943 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Makarova KS et al. An updated evolutionary classification of CRISPR-Cas systems. Nat. Rev. Microbiol 13, 722–736 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jiang W, Bikard D, Cox D, Zhang F & Marraffini LA RNA-guided editing of bacterial genomes using CRISPR-Cas systems. Nat. Biotechnol 31, 233–239 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luo ML, Mullis AS, Leenay RT & Beisel CL Repurposing endogenous type I CRISPR-Cas systems for programmable gene repression. Nucleic Acids Res. 43, 674–681 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Belkum A et al. Phylogenetic Distribution of CRISPR-Cas Systems in Antibiotic-Resistant Pseudomonas aeruginosa. MBio 6, e01796–15 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mayo-Muñoz D et al. Anti-CRISPR-Based and CRISPR-Based Genome Editing of Sulfolobus islandicus Rod-Shaped Virus 2. Viruses 10, 695–17 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study demonstrated the use of anti-CRISPRs as selectable markers in viral genome engineering.
- 31.Qi LS et al. Repurposing CRISPR as an RNA-guided platform for sequence-specific control of gene expression. Cell 152, 1173–1183 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pawluk A et al. Disabling a Type I-E CRISPR-Cas Nuclease with a Bacteriophage-Encoded Anti-CRISPR Protein. MBio 8, 43 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Louwen R, Staals RHJ, Endtz HP, van Baarlen P & van der Oost J The role of CRISPR-Cas systems in virulence of pathogenic bacteria. Microbiol. Mol. Biol. Rev 78, 74–88 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nobrega FL, Costa AR, Kluskens LD & Azeredo J Revisiting phage therapy: new applications for old resources. Trends in Microbiology 23, 185–191 (2015). [DOI] [PubMed] [Google Scholar]
- 35.Muñoz IV, Sarrocco S, Malfatti L, Baroncelli R & Vannacci G CRISPR-Cas for Fungal Genome Editing: A New Tool for the Management of Plant Diseases. Front Plant Sci 10, 135 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Langner T, Kamoun S & Belhaj K CRISPR Crops: Plant Genome Editing Toward Disease Resistance. Annu Rev Phytopathol 56, 479–512 (2018). [DOI] [PubMed] [Google Scholar]
- 37.Jinek M et al. RNA-programmed genome editing in human cells. eLife 2, e00471 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mali P et al. RNA-guided human genome engineering via Cas9. Science 339, 823–826 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Swarts DC & Jinek M Cas9 versus Cas12a/Cpf1: Structure-function comparisons and implications for genome editing. Wiley Interdiscip Rev RNA 9, e1481 (2018). [DOI] [PubMed] [Google Scholar]
- 40.Yao R et al. CRISPR-Cas9/Cas12a biotechnology and application in bacteria. Synth Syst Biotechnol 3, 135–149 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kleinstiver BP et al. Genome-wide specificities of CRISPR-Cas Cpf1 nucleases in human cells. Nat. Biotechnol 34, 869–874 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim D et al. Genome-wide analysis reveals specificities of Cpf1 endonucleases in human cells. Nat. Biotechnol 34, 863–868 (2016). [DOI] [PubMed] [Google Scholar]
- 43.Kim S, Kim D, Cho SW, Kim J & Kim JS Highly efficient RNA-guided genome editing in human cells via delivery of purified Cas9 ribonucleoproteins. Genome Res. 24, 1012–1019 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lin S, Staahl BT, Alla RK & Doudna JA Enhanced homology-directed human genome engineering by controlled timing of CRISPR/Cas9 delivery. eLife 3, 1314–13 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nihongaki Y, Kawano F, Nakajima T & Sato M Photoactivatable CRISPR-Cas9 for optogenetic genome editing. Nat. Biotechnol 33, 755–760 (2015). [DOI] [PubMed] [Google Scholar]
- 46.Senturk S et al. Rapid and tunable method to temporally control gene editing based on conditional Cas9 stabilization. Nat Commun 8, 14370 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kleinstiver BP et al. High-fidelity CRISPR–Cas9 nucleases with no detectable genome-wide off-target effects. Nature 529, 490–495 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Slaymaker IM et al. Rationally engineered Cas9 nucleases with improved specificity. Science 351, 84–88 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen JS et al. Enhanced proofreading governs CRISPR-Cas9 targeting accuracy. Nature 550, 407–410 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shin J et al. Disabling Cas9 by an anti-CRISPR DNA mimic. Sci Adv 3, e1701620 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study first demonstrated that AcrIIA4 can reduce off-target editing while maintaining on-target editing in human cells.
- 51.Yang S, Li S & Li X-J Shortening the Half-Life of Cas9 Maintains Its Gene Editing Ability and Reduces Neuronal Toxicity. CellReports 25, 2653–2659.e3 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Maeder ML et al. Development of a gene-editing approach to restore vision loss in Leber congenital amaurosis type 10. Nature Medicine 25, 229–233 (2019). [DOI] [PubMed] [Google Scholar]
- 53.Lee J et al. Tissue-restricted Genome Editing in vivo Specified by MicroRNA-repressible Anti-CRISPR Proteins. RNA rna.071704.119 (2019). doi: 10.1261/rna.071704.119 [DOI] [PMC free article] [PubMed] [Google Scholar]; This study is the first demonstration of Cas9 inhibition in mice.
- 54.Liu XS et al. Rescue of Fragile X Syndrome Neurons by DNA Methylation Editing of the FMR1 Gene. Cell 172, 979–992.e6 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen B et al. Dynamic imaging of genomic loci in living human cells by an optimized CRISPR/Cas system. Cell 155, 1479–1491 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wu X, Mao S, Ying Y, Krueger CJ & Chen AK Progress and Challenges for Live-cell Imaging of Genomic Loci Using CRISPR-based Platforms. Genomics Proteomics Bioinformatics (2019). doi: 10.1016/j.gpb.2018.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Komor AC, Kim YB, Packer MS, Zuris JA & Liu DR Programmable editing of a target base in genomic DNA without double-stranded DNA cleavage. Nature 533, 420–424 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gaudelli NM et al. Programmable base editing of A•T to G•C in genomic DNA without DNA cleavage. Nature 551, 464–471 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li X et al. Base editing with a Cpf1-cytidine deaminase fusion. Nat. Biotechnol 36, 324–327 (2018). [DOI] [PubMed] [Google Scholar]
- 60.Zuo E et al. Cytosine base editor generates substantial off-target single-nucleotide variants in mouse embryos. Science 364, 289–292 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jin S et al. Cytosine, but not adenine, base editors induce genome-wide off-target mutations in rice. Science 364, 292–295 (2019). [DOI] [PubMed] [Google Scholar]
- 62.Li J, Xu Z, Chupalov A & Marchisio MA Anti-CRISPR-based biosensors in the yeast S. cerevisiae. 1–14 (2018). doi: 10.1186/s13036-018-0101-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nakamura M et al. Anti-CRISPR-mediated control of gene editing and synthetic circuits in eukaryotic cells. Nat Commun 10, 194 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study demonstrates many applications of Acr proteins in eukaryotic cells, including “write protecting” cells from futher editing, CRISPR-based gene regulation circuits, and ligand-inducible AcrIIA4.
- 64.Burt A Site-specific selfish genes as tools for the control and genetic engineering of natural populations. Proc. Biol. Sci 270, 921–928 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gantz VM et al. Highly efficient Cas9-mediated gene drive for population modification of the malaria vector mosquito Anopheles stephensi. Proc. Natl. Acad. Sci. U.S.A 112, E6736–43 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hammond A et al. A CRISPR-Cas9 gene drive system targeting female reproduction in the malaria mosquito vector Anopheles gambiae. Nat. Biotechnol 34, 78–83 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Esvelt KM, Smidler AL, Catteruccia F & Church GM Concerning RNA-guided gene drives for the alteration of wild populations. eLife 3, 20131071 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Akbari OS et al. BIOSAFETY. Safeguarding gene drive experiments in the laboratory. Science 349, 927–929 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Basgall EM et al. Gene drive inhibition by the anti-CRISPR proteins AcrIIA2 and AcrIIA4 in Saccharomyces cerevisiae. Microbiology (Reading, Engl.) 164, 464–474 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study first demonstrated the ability of AcrIIA2 and AcrIIA4 to halt gene drives in yeast.
- 70.Johnston RK et al. Use of anti-CRISPR protein AcrIIA4 as a capture ligand for CRISPR/Cas9 detection. Biosens Bioelectron 141, 111361 (2019). [DOI] [PubMed] [Google Scholar]
- 71.Palmer DJ, Turner DL & Ng P Production of CRISPR/Cas9-Mediated Self-Cleaving Helper-Dependent Adenoviruses. Mol Ther Methods Clin Dev 13, 432–439 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dow LE et al. Inducible in vivo genome editing with CRISPR-Cas9. Nat. Biotechnol 33, 390–394 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hemphill J, Borchardt EK, Brown K, Asokan A & Deiters A Optical Control of CRISPR/Cas9 Gene Editing. J. Am. Chem. Soc 137, 5642–5645 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Maji B et al. A High-Throughput Platform to Identify Small-Molecule Inhibitors of CRISPR-Cas9. Cell 177, 1067–1079.e19 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Marino ND et al. Discovery of widespread type I and type V CRISPR-Cas inhibitors. Science 362, 240–242 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hoffmann MD et al. Cell-specific CRISPR-Cas9 activation by microRNA-dependent expression of anti-CRISPR proteins. Nucleic Acids Res. 2, e00471 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bubeck F et al. Engineered anti-CRISPR proteins for optogenetic control of CRISPR-Cas9. Nat. Methods 15, 924–927 (2018). [DOI] [PubMed] [Google Scholar]; This study reported an optogenetic AcrIIA4 variant that can be inactivated in cells using light.
- 78.Stanley SY et al. Anti-CRISPR-Associated Proteins Are Crucial Repressors of Anti-CRISPR Transcription. Cell 178, 1452–1464.e13 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hirosawa M, Fujita Y & Saito H Cell-Type-Specific CRISPR Activation with MicroRNA-Responsive AcrllA4 Switch. ACS Synth Biol 8, 1575–1582 (2019). [DOI] [PubMed] [Google Scholar]
- 80.Smargon AA et al. Cas13b Is a Type VI-B CRISPR-Associated RNA-Guided RNase Differentially Regulated by Accessory Proteins Csx27 and Csx28. Mol. Cell 65, 618–630.e7 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pawluk A et al. Inactivation of CRISPR-Cas systems by anti-CRISPR proteins in diverse bacterial species. Nat Microbiol 1, 16085 (2016). [DOI] [PubMed] [Google Scholar]
- 82.Watters KE, Fellmann C, Bai HB, Ren SM & Doudna JA Systematic discovery of natural CRISPR-Cas12a inhibitors. Science 9, eaau5138 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hynes AP et al. Widespread anti-CRISPR proteins in virulent bacteriophages inhibit a range of Cas9 proteins. Nat Commun 9, 2919 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Uribe RV et al. Discovery and Characterization of Cas9 Inhibitors Disseminated across Seven Bacterial Phyla. Cell Host and Microbe 25, 233–241.e5 (2019). [DOI] [PubMed] [Google Scholar]
- 85.Forsberg KJ et al. Functional metagenomics-guided discovery of potent Cas9 inhibitors in the human microbiome. eLife 8, 1709 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bondy-Denomy J, Pawluk A, Maxwell KL & Davidson AR Bacteriophage genes that inactivate the CRISPR/Cas bacterial immune system. Nature 493, 429–432 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study identified the first phage proteins with anti-CRISPR function.
- 87.Wandera KG et al. An enhanced assay to characterize anti-CRISPR proteins using a cell-free transcription-translation system. Methods (2019). doi: 10.1016/j.ymeth.2019.05.014 [DOI] [PubMed] [Google Scholar]
- 88.Guo TW et al. Cryo-EM Structures Reveal Mechanism and Inhibition of DNA Targeting by a CRISPR-Cas Surveillance Complex. Cell 171, 414–426.e12 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.He F et al. Anti-CRISPR proteins encoded by archaeal lytic viruses inhibit subtype I-D immunity. Nat Microbiol 1–11 (2018). doi: 10.1038/s41564-018-0120-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pawluk A, Bondy-Denomy J, Cheung VHW, Maxwell KL & Davidson AR A new group of phage anti-CRISPR genes inhibits the type I-E CRISPR-Cas system of Pseudomonas aeruginosa. MBio 5, e00896–e00896–14 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fuchsbauer O et al. Cas9 Allosteric Inhibition by the Anti-CRISPR Protein AcrIIA6. Mol. Cell (2019). doi: 10.1016/j.molcel.2019.09.012 [DOI] [PubMed] [Google Scholar]
- 92.Lee J et al. Potent Cas9 Inhibition in Bacterial and Human Cells by AcrIIC4 and AcrIIC5 Anti-CRISPR Proteins. MBio 9, 1239 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sun W et al. Structures of Neisseria meningitidis Cas9 Complexes in Catalytically Poised and Anti-CRISPR-Inhibited States. Mol. Cell (2019). doi: 10.1016/j.molcel.2019.09.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Thavalingam A et al. Inhibition of CRISPR-Cas9 ribonucleoprotein complex assembly by anti-CRISPR AcrIIC2. Nat Commun 10, 2806–11 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhu Y et al. Diverse Mechanisms of CRISPR-Cas9 Inhibition by Type IIC Anti-CRISPR Proteins. Mol. Cell 74, 296–309.e7 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ka D, An SY, Suh J-Y & Bae E Crystal structure of an anti-CRISPR protein, AcrIIA1. Nucleic Acids Res. 46, 485–492 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hynes AP et al. An anti-CRISPR from a virulent streptococcal phage inhibits Streptococcus pyogenes Cas9. Nat Microbiol 2, 1374–1380 (2017). [DOI] [PubMed] [Google Scholar]
- 98.Bhoobalan-Chitty Y, Johansen TB, Di Cianni N & Peng X Inhibition of Type III CRISPR-Cas Immunity by an Archaeal Virus-Encoded Anti-CRISPR Protein. Cell 179, 448–458.e11 (2019). [DOI] [PubMed] [Google Scholar]
- 99.Zhang H et al. Structural Basis for the Inhibition of CRISPR-Cas12a by Anti-CRISPR Proteins. Cell Host and Microbe 25, 815–826.e4 (2019). [DOI] [PubMed] [Google Scholar]


