Abstract
Improved enzyme-linked immunosorbent assay (ELISA) methods have been developed for the determination of femtomole amounts of mycothiol (MSH), the main low-molecular-weight thiol in mycobacteria. The immunoassays utilize an affinity-purified rabbit polyclonal antibody that is highly specific for the pseudodisaccharide moiety of MSH. MSH was first biotinylated by the thiol-specific reagent 3-(N-maleimidopropionyl)biocytin. The MSH-biotin adduct was then captured with immobilized avidin and detected with anti-MSH antibody (biotin-capture ELISA) or was captured with immobilized anti-MSH antibody and detected with alkaline phosphatase-labelled avidin (MSH-capture ELISA). The MSH-capture ELISA was the most sensitive method, measuring as little as 0.3 fmol of MSH. Methods for biotinylating MSH directly from Mycobacterium spp. are described. The MSH-capture ELISA was tested for the detection of M. avium seeded in human urine or cerebrospinal fluid samples and for screening mutant M. smegmatis strains to detect MSH production.
Mycobacteria and most actinomycetes, but not other prokaryotes or eukaryotes, produce mycothiol (MSH [12]), a low-molecular-weight thiol with the structure 1-d-myo-inosityl-2-(N-acetyl-l-cysteinyl)amido-2-deoxy-α-d-glucopyranoside (13, 19, 20). MSH has antioxidant properties superior to those of glutathione (GSH), the antioxidant thiol found in most eukaryotes and some prokaryotes (13), and is the cofactor for an NAD/“cofactor”-dependent formaldehyde dehydrogenase found in actinomycetes, where it serves in a detoxification role analogous to that of GSH in the NAD/GSH-dependent formaldehyde dehydrogenase of GSH-producing organisms (11, 16). Thus, MSH may serve both as a stable intracellular storage form of cysteine and as an essential cofactor for oxidative stress response and detoxification enzymes in a manner analogous to that of GSH in GSH-producing organisms. Recent studies have reported on the biosynthesis of MSH (1, 3). The enzymes involved in the metabolism of MSH may represent new targets for drugs directed against tuberculosis and other mycobacterial infections (12). The development of sensitive and specific methods for the detection of MSH is important for research on MSH metabolism and for use in the clinical diagnosis of mycobacterial infections. MSH analysis has previously relied on derivatization with thiol-specific fluorescent-labelling reagents followed by high-performance liquid chromatography (HPLC) detection (6, 14), but this methodology is expensive and time-consuming, lacks the sensitivity needed to be clinically useful in the diagnosis of mycobacterial infection, and lacks the versatility needed for a variety of applications, such as the screening for MSH production by individual bacterial colonies.
We previously described an immunoassay for the detection of MSH that relies on thiol-specific chemistry for the immobilization of MSH and the subsequent detection by means of a highly specific antibody to MSH (21). In brief, this assay involves the lysis of mycobacterial cells, the capture of MSH by bovine serum albumin (BSA) activated with maleimide, binding to the immobilized MSH by an affinity-purified polyclonal anti-MSH antibody, and conventional secondary antibody detection. The useful range of this assay was about 0.1 to 1.0 pmol per well. We now report new immunoassay architectures that offer much increased sensitivity and versatility of application. MSH was first reacted with 3-(N-maleimidopropionyl)biocytin (MPB) to produce biotinylated MSH (MS-MPB). The greatest sensitivity was achieved with an MSH-capture enzyme-linked immunosorbent assay (ELISA) in which MS-MPB was captured by anti-MSH antibody previously bound onto a protein A-coated microtiter plate, and the immobilized MS-MPB was detected by alkaline phosphatase-labelled avidin. We have successfully used the MSH-capture ELISA to detect Mycobacterium avium cells in human body fluids (urine or cerebrospinal fluid [CSF]) and as a convenient screening method to assay MSH production by mutant M. smegmatis strains.
MATERIALS AND METHODS
Reagents and materials.
Immulon-4 HBX 96-well flat-bottom microtiter plates and plate-sealing tape were purchased from Dynex Technologies, Inc. (Chantilly, Va.). Centricon-100 spin filters were obtained from Amicon, Inc. (Beverly, Mass.). Deglycosylated avidin (ImmunoPure NeutrAvidin) was purchased from Pierce (Rockford, Ill.), and Tween 20 was purchased from Bio-Rad Laboratories (Hercules, Calif.). Goat anti-rabbit immunoglobulin G (IgG) (whole molecule) secondary antibody [F(ab′)2 fragments conjugated to bovine intestinal alkaline phosphatase], Staphylococcus aureus soluble protein A (Sigma P 6031), avidin (ExtrAvidin) alkaline phosphate conjugate, bovine serum albumin fraction V (Sigma A 4503), fish skin (Teleostean) gelatin, N-biotinoyl-N′-(6-maleimidohexanoyl)hydrazide (MHB), MPB, and p-nitrophenyl phosphate (pNPP) were purchased from Sigma Chemical Co. (St. Louis, Mo.). High-purity, heavy-metal-free dithiothreitol (DTT) was obtained from Calbiochem (La Jolla, Calif.). Middlebrook 7H9 and 7H10 culture media and oleate-albumin-dextrose-catalase (OADC) supplement were obtained from Difco Laboratories (Detroit, Mich.). All other reagents were reagent grade or higher purity. Reagent-grade chloroform (Mallinckrodt) and HPLC-grade acetonitrile (Fisher) are moderately toxic solvents and were handled with appropriate ventilation.
Bacterial cultures.
M. smegmatis mc26 and mc2155 were kindly provided by J. Davies (University of British Columbia, Vancouver, British Columbia, Canada). Mutant strains I64 and 49 are chemical mutants of M. smegmatis mc2155 shown by monobromobimane labelling and HPLC analysis (12, 14) to produce 0.05 and <0.004 μmol of MSH per g (residual dry weight), respectively, of mycothiol. M. avium NJH 9141 was obtained from the University of California at San Diego (UCSD) Medical Center. M. smegmatis was grown at 37°C in Middlebrook 7H9 (broth or agar) supplemented with 0.05% (wt/vol) Tween 80 and 0.4% (wt/vol) glucose or on Middlebrook 7H10 agar supplemented with 0.05% (wt/vol) Tween 80 and 0.4% (wt/vol) glucose with or without OADC supplementation. M. avium was grown in Middlebrook 7H9 broth supplemented with OADC and 0.05% (wt/vol) Tween 80.
Body fluid specimens.
All cerebrospinal specimens were excess samples from routine clinical specimens obtained at the UCSD Medical Center. Urine samples were obtained from a healthy donor.
Antibody preparation.
The primary antibody to MSH was prepared as described previously (21). Briefly, purified MSH from M. smegmatis was conjugated to keyhole limpet hemocyanin by treatment with maleimidobenzoyl-N-hydroxysulfosuccinimide ester and rabbits immunized with the MSH-keyhole limpet hemocyanin conjugates. IgG fractions from sera were isolated by ammonium sulfate precipitation in two steps (33 and 50% saturation) and tested for MSH-specific antibodies by dot blots to MSH conjugated to ovalbumin by means of the nonhomologous cross-linker N-succinimidyl-3-(2-pyridyldithio)propionate (21), and MSH-specific antibodies were isolated by affinity chromatography to MSH linked by reaction with an epoxy group on a 12-atom spacing arm to a Sepharose resin matrix. Affinity-purified anti-MSH was stored in aliquots frozen at −20°C and thawed immediately before use.
Preparation of biotinylated MSH standards.
To produce MS-MPB suitable for use as a standard, 5.0 μl of 3.7 mM pure MSH in 0.1% trifluoroacetic acid in water was added to 12.3 μl of 1.5 mM MPB in dimethyl sulfoxide–acetonitrile–100 mM phosphate buffer, pH 7.0 (1:4:5). The mixture was allowed to react for 1 h at room temperature. Unreacted MSH was measured by titration with 5,5′-dithio-bis(2-nitrobenzoic acid) (5) to determine the extent of biotinylation. The standard was stored at −70°C and was diluted for use to a concentration of 1.0 μM in Tris-buffered saline (0.1 M Tris, 0.15 M NaOH, pH 7.3) containing 0.04% (wt/vol) sodium azide, 0.05% (vol/vol) Tween 20, and 0.1% (wt/vol) BSA (TBSTB).
MSH-capture ELISA.
Generally, for each 96-well plate, 8 wells received only the blocking solution and all of the washes. Four of these were used as a blank; the remaining four were controls used for measuring known amounts of the reporter enzyme (Sigma alkaline phosphatase-labelled ExtrAvidin). Unless noted, all washes involved filling wells to the top (400 μl/well) with wash buffer followed by gentle aspiration to drain the wells. First, 100 μl of a 6-ng/μl protein A solution in Tris-buffered saline (pH 7.3) containing 0.04% (wt/vol) sodium azide (TBS) was added to each well of an Immulon-4 HBX microtiter plate, which was then sealed with tape and incubated for 5 h at 37°C. The wells were thoroughly drained by aspiration with gentle vacuum and blocked with 400 μl of a 1% (vol/vol) solution of fish skin gelatin in TBS for 1 h at room temperature. The wells were drained, 100 μl of freshly thawed rabbit anti-MSH IgG (2 ng/μl in TBSTB) was added per well, and the plate was resealed and incubated at least overnight (>14 h) at 4°C. The wells were drained and washed once with TBS containing 0.05% (vol/vol) Tween 20 (TBST). The antigens (standards or unknowns), diluted as necessary in order to contain an identical percentage of CH3CN, were added to the wells. The plate was sealed and incubated for 3.5 h at 37°C. Wells were drained and washed once with TBST. Then, 100 μl of alkaline phosphatase-labelled avidin at a concentration of 0.5 ng/μl in TBSTB was added per well. The plate was sealed and incubated for 1 h at room temperature. Wells were drained and washed once with 400 μl of TBST and three times with 200 μl of TBST per well. The first three wash volumes were removed by sharply tapping the inverted plate, and the last wash volume was removed by aspiration. Alkaline phosphatase-labelled avidin (2 μl of a 0.5-ng/μl solution in TBSTB) was added to two of the control wells that received only the blocking solution and wash buffers; the final two control wells received 2 μl of TBSTB. The plate was then developed with 200 μl per well of freshly made pNPP solution (1 mg/ml in 1 M diethanolamine [pH 9.8] containing 0.4 mM MgCl2). For an endpoint reading, development was stopped by the addition of 50 μl of 4 M NaOH per well. For time-point readings, no stop solution was added to the wells. The plate was read at 405 nm on a microplate reader (model EL311; Bio-Tek Instruments, Winooski, Vt.).
Determination of the dissociation constant Kd for antigen-antibody equilibria in solution.
Affinity-purified rabbit polyclonal anti-MSH antibody prepared as described above was diluted to 0.2 μM in phosphate-buffered saline (PBS; pH 7.2), and two additional 10-fold dilutions in PBS were made from this to give 0.02 and 0.002 μM solutions. The MS-MPB standard was similarly diluted to 0.2, 0.02, and 0.002 μM in PBS. Three microfuge tubes each received 400 μl of anti-MSH solution and 400 μl of MS-MPB solution of equivalent molarity to produce final concentrations of 10−7, 10−8, and 10−9 M, respectively. In parallel, control samples were made containing MS-MPB and PBS instead of antibody solution. The tubes were vortexed well and incubated at room temperature to allow the antigen-antibody interaction to occur; at 10 min, 1 h, and 3 h, duplicate 100-μl aliquots were removed from each tube, transferred to prechilled Centricon-100 (100-kDa molecular mass cutoff) spin filters, and centrifuged for 15 min at 1,000 × g. At the 3-h time point, the remaining 10−7 M antibody-antigen sample was diluted in two 10-fold steps (to give 1:10 and 1:100 dilutions); at 2 min, 20 min, and 3 h after this dilution step, duplicate 100-μl aliquots were removed from the 1:10 and 1:100 dilutions, transferred to prechilled Centricon-100 spin filters, and centrifuged 15 min at 1,000 × g. Aliquots of the filtrates were diluted as necessary and analyzed as described above in order to determine the amount of MS-MPB not bound by antibody and thus to estimate the dissociation constant Kd.
Biotinylation of MSH from M. avium cells in body fluids.
M. avium was harvested at early- to mid-log-phase growth and diluted in fresh medium to give concentrations ranging from ∼3 × 103 to 3 × 104 CFU in a volume of 10 μl. Human CSF (several pooled samples) or urine was filter sterilized through a 0.45-μm-pore-size filter prior to experiments. Sterile-filtered CSF was divided into two portions, one of which (referred to as enriched CSF) received the addition of 1% (vol/vol) glycerol and 0.5% (wt/vol) glucose. To each microfuge tube was added 10 μl of cell suspension and 990 μl of sterile-filtered urine, CSF, or enriched CSF. The tubes were capped, vortexed, and centrifuged for 10 min at 13,000 × g, and 990 μl of supernatant was carefully removed without disturbing the pelleted cells. To the residual 10 μl in each tube was added, in the following order: 100 μl of 10 mM phosphate buffer (pH 7.2), 3 μl of 80 mM EGTA (pH 8.6), and 2.4 μl of 600 mM phosphate buffer (pH 10.7). The final pH of this mixture was 8.0. A 10 mM solution of MPB in dimethyl sulfoxide was prepared shortly before use. This was diluted immediately before the reaction to 6 μM in water-saturated CHCl3 at room temperature, and 120 μl of this solution was added to each sample tube. The tubes were capped, vortexed, and immediately incubated in a water bath for 10 min at 37°C with further vortexing at 5-min intervals. Then, 5 μl of a freshly made solution of 175 μM 2-mercaptoethanol was added to each tube to react with excess MPB; the tubes were capped, shaken, and incubated a further 5 min at 37°C. The samples were stored overnight at 4°C before analysis by ELISA. Before analysis, the tubes were centrifuged 1 min at 13,000 × g, and 100 μl of the top aqueous phase in each tube was carefully removed for analysis.
Microtiter plate screening of cells.
M. smegmatis cells were grown to early logarithmic phase in Middlebrook 7H9 medium supplemented with 0.4% (wt/vol) glucose and 0.05% (vol/vol) Tween 80 and diluted in fresh medium to an initial concentration of 108 CFU/ml; the cells were then further diluted in series as required. To each well of an Immulon-4 microtiter plate was added a 100-μl aliquot of diluted cell suspensions. A 10 mM solution of MPB in dimethyl sulfoxide was prepared shortly before use. This was diluted immediately before the reaction to 6 μM in room temperature CH3CN. Additions of the reagents to the microtiter plate were made by means of a multichannel pipetter. To each well was added 20 μl of 0.1 M Na2HPO4 (pH unadjusted), followed by 120 μl of 6 μM MPB in CH3CN. The plate was covered and incubated in a water bath at 60°C for 15 min. To block unreacted MPB, 20 μl of 60 μM aqueous 2-mercaptoethanol was added to each well to give a final volume of 260 μl per well; the plate was covered, incubated a further 5 min at 60°C, and stored overnight at 4°C prior to analysis by MSH-capture ELISA. The ELISA plate wells were coated with protein A, fish skin gelatin, and anti-MSH antibody, and washed once with TBST as described above for MSH-capture ELISA. The drained wells each received 75 μl of TBS and 25 μl of the biotinylated cell extracts so that each contained 9.6% of the original biotinylated cell extract in 12.5% CH3CN. Standards were also applied in 12.5% acetonitrile in TBS. The rest of the analysis was performed as described above.
RESULTS
The first avidin/biotin-based immunoassay tested was a biotin-capture ELISA based on the capture of MS-MPB on an avidin-coated microtiter plate and detection of bound MS-MPB by anti-MSH antibody as described previously (21). The biotin-capture ELISA used in the present study was more sensitive than the original assay (21) and had the advantage that the acetonitrile used to extract cells increased the assay response (data not shown). Another thiol-specific biotinylation reagent, MHB, was tested as an alternative to MPB. However, biotin-capture ELISA of standard samples of MS-MHB under the same conditions used for MB-MPB standards revealed a fourfold-lower sensitivity for this assay. Thus, MPB was used in the further development of the immunoassays. The MS-MPB standard was found to be stable for at least 1 year, as indicated by ELISA measurements, when stored refrigerated as a 1.0 μM solution in TBSTB. Further testing of the biotin-capture ELISA revealed interference by thiol-MPB derivatives other than MS-MPB, which limited its utility.
An immunoassay architecture based upon capture of MS-MPB to immobilized anti-MSH antibody (MSH-capture ELISA) proved more successful. To assess the limitations of an MSH-capture assay, we examined the binding characteristics of the rabbit anti-MSH IgG to be used in the assay. Antibody and MS-MPB were incubated in PBS at 10−7 or 10−8 M each for 3 h and assayed for free MS-MPB by biotin-capture ELISA after ultrafiltration. A 3-h equilibrated 10−7 M sample was diluted to 10−8 and 10−9 M to allow dissociation of the antibody-antigen complex, and samples were assayed over a 3-h period. A mean estimate for Kd of 40 ± 17 nM was obtained from the equilibrated samples, and a value of koff of ∼1 × 10−4 s−1 was estimated from the dissociation assays. The latter value indicates that the loss of antigen during plate washings should not be a problem.
In the MSH-capture ELISA rabbit anti-MSH IgG was used to capture MS-MPB, and the immobilized biotin residue was detected by avidin-alkaline phosphatase. Direct binding of anti-MSH antibody to the wells by nonspecific adsorption was tested in a preliminary experiment and, as expected, gave poor sensitivity (data not shown). Protein A was selected as an alternative method for immobilization of the primary antibody, and this proved highly satisfactory. The final MSH-capture ELISA protocol was arrived at after experiments that varied the incubation times; the amounts of protein A, primary antibody, and alkaline phosphatase-labelled avidin; and the concentration of acetonitrile in the antigen binding buffer.
The MSH-capture ELISA proved to be highly reproducible and sensitive. Because of the low background (A405 of <0.05 after 1 h of pNPP development with phosphate buffer), development time may be extended to more than an hour if necessary without a serious increase in noise. The MSH-capture ELISA has a working range (primarily dependent on development time) of at least 0.3 to 2,000 fmol of MS-MPB. We consistently were able to detect 0.3 fmol of MS-MPB standard with a signal-to-noise ratio of 2.0 at 60 min of development.
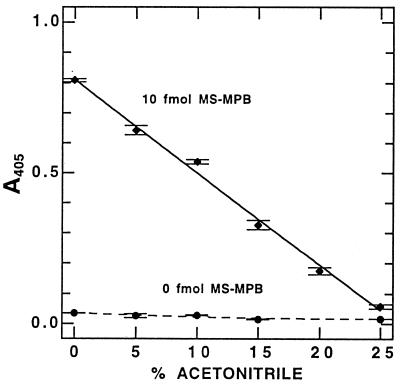
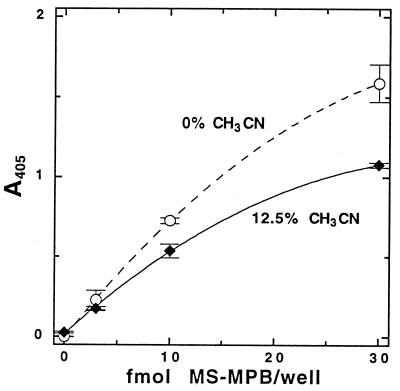
We examined the effects of acetonitrile concentration in the antigen binding step to determine the impact of residual acetonitrile used in cell lysis (12, 14). Binding of MS-MPB by the protein A–anti-MSH complex was inhibited by acetonitrile (Fig. 1), but we also observed a slight decrease in background with the use of small percentages (<15%) of acetonitrile in the binding buffer. The acetonitrile concentration giving the optimal signal-to-noise ratio (taking into account the dilution factor needed to decrease acetonitrile concentration from the 50% in the cell lysis solution) was found to be 10 to 15% (vol/vol). In practice, 12.5% acetonitrile (i.e., a fourfold dilution of the cell extracts into aqueous buffer) was generally used. Figure 2 shows a typical standard curve with MS-MPB in 12.5% acetonitrile in TBS.
FIG. 1.
Effect of acetonitrile content of the binding buffer (TBS) on signal from MS-MPB standards in the MSH-capture ELISA. Data shown are means and ranges of duplicate samples with 30 min of development.
FIG. 2.
MSH-capture ELISA: typical standard curves for MS-MPB in 12.5% acetonitrile in TBS (used for samples biotinylated by the acetonitrile extraction procedure) or in 22 mM phosphate buffer (pH 8.0) without acetonitrile (used for samples biotinylated by the chloroform extraction procedure). Data shown are from 40 min of development. Error bars indicate the standard deviation from the mean (n = 3).
The need to dilute samples prepared by extraction in 50% acetonitrile fourfold prior to analysis led us to explore alternative two-phase lysis-biotinylation protocols. A suitable method was found that involved the addition of an equal volume of a solution of MPB in water-saturated chloroform to the cell suspension, followed by mixing and heating the mixture to 37°C. After centrifugation, a sample of the aqueous layer is removed for ELISA from the resulting two-phase mixture, with most of the cellular debris remaining at the interface. Samples can then be analyzed with no dilution required. Figure 2 includes a typical standard curve with MS-MPB in 22 mM phosphate buffer (pH 8.0) in the absence of acetonitrile. The efficiency of low-molecular-weight thiol extraction by this procedure was tested by DTNB titration and was found to be equivalent to that obtained by warm acetonitrile lysis (data not shown). This method was tested with cultured M. smegmatis and found to be satisfactory, allowing >80% of the cell extract to be used for ELISA analysis of each sample. This treatment with chloroform was shown to kill mycobacterial cells (i.e., no colony formation occurred after 28 days of incubation at 37°C when 5 × 105 CFU M. avium were plated after chloroform treatment).
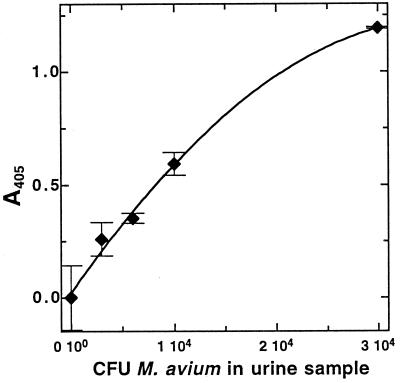
This extraction procedure was used to analyze urine samples that had been seeded with known numbers (3 × 103 to 3 × 104 CFU per sample) of M. avium cells. The results of this analysis are shown in Fig. 3. MSH could be detected in samples containing as few as 3 × 103 CFU of M. avium. MSH recovered from M. avium seeded into urine by this protocol was 68% of that found by direct analysis of samples from the same stock of cells in phosphate buffer without centrifugation (data not shown). Independent analysis by plating indicated a similar recovery from centrifugation and resuspension of the cells, so most of the ∼35% loss can be attributed to the centrifugation step.
FIG. 3.
Results of an MSH-capture ELISA analysis of urine seeded with known amounts of M. avium NJH 9141 and biotinylated by the chloroform extraction method. Data shown are from 40 min of development. Error bars indicate the standard deviation from the mean (n = 3).
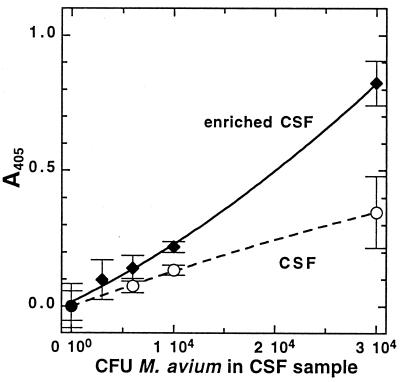
The same method was tested with CSF samples that had been seeded with M. avium cells. The results of this experiment are shown in Fig. 4. We had observed in several experiments (data not shown) that the addition of glycerol and glucose to the mycobacterial cell suspension, even for brief incubation times (∼10 min), roughly doubled the amount of MSH detected, and this effect was also seen in the CSF analyses. MSH could be measured in samples containing as few as 104 CFU of M. avium in enriched CSF.
FIG. 4.
Results of an MSH-capture ELISA analysis of CSF with (enriched CSF) or without the addition of 1% (vol/vol) glycerol and 0.5% (wt/vol) glucose, seeded with known amounts of M. avium NJH 9141, and biotinylated by the chloroform extraction method. Data shown are from 40 min of development. Error bars indicate standard deviation from the mean (n = 3).
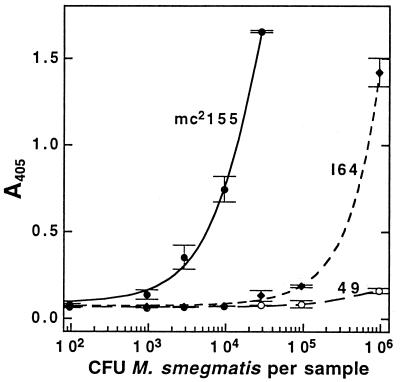
Finally, we evaluated the MSH-capture ELISA as a method for screening individual bacterial clones for MSH production. A microtiter plate format for such a screen appeared to offer advantages (e.g., a standardized grid layout and quantitation of results) over the membrane-based immunoassay we had previously used for this purpose (21). We tested a simplified acetonitrile lysis-biotinylation procedure with strains of M. smegmatis of known MSH content. Independent determinations of the MSH content of the parent strain mc2155 and of the mutant strains I64 and 49 by monobromobimane and HPLC analysis (12) established their MSH content during early-log-phase growth as 10, 0.05, and <0.004 μmol per g (residual dry weight), respectively. The immunoassay results for these strains (Fig. 5) indicated that mc2155 produces about 103-fold more MSH than mutant 49 and 102-fold more than mutant I64, a finding in reasonable accord with the previous results.
FIG. 5.
Results of an MSH-capture ELISA screen of known numbers of M. smegmatis biotinylated in a microtiter plate by the acetonitrile extraction method. Samples were applied in 12.5% acetonitrile in TBS. Data shown are means and ranges of duplicate samples with 60 min of development.
DISCUSSION
Avidin/biotin-based immunoassays are popular because of the extremely high affinity (Kd of ∼10−15 M) of avidin for biotin and the fact that the molecules to be detected frequently can be biotinylated or avidinated without a loss of binding or biological activity (2). The choice of biotinylation reagent can be important, and a sufficiently long spacer arm is required for optimal biotin binding by avidin. We found that MSH derivatized with MPB gave a fourfold-stronger signal than MSH derivatized with MHB, possibly because the linking arm of MPB is two atoms longer than that of MHB. The presence of the hydrophilic carboxylate group in MPB, but not in MHB, may also be important in facilitating the capture by avidin and the binding of MSH antibody.
The first immunoassay architecture we developed (biotin-capture ELISA) provided improved sensitivity compared to the maleimide-BSA-based ELISA (21), but it also had some unusual characteristics and ultimately proved inferior to the MSH-capture ELISA. The MSH-capture ELISA proved capable of measuring 0.3 fmol of MS-MPB, more than 2 orders of magnitude less than our BSA-maleimide capture method (21) and 4 orders of magnitude less than our HPLC method (12, 14). The MSH-capture assay was adversely affected by high levels of acetonitrile, and samples extracted by the acetonitrile protocol must be diluted at least fourfold in order to obtain good sensitivity. However, samples could also be extracted with chloroform, resulting in a biotinylated aqueous phase containing MS-MPB that can be easily separated from the organic phase and analyzed without dilution. The MSH-capture ELISA was highly reproducible and useful over a wide range of MSH levels.
Results of MSH-capture ELISA analysis of human urine and CSF samples seeded with known amounts of M. avium demonstrated that the MSH-capture immunoassay may potentially be useful in the rapid diagnosis of mycobacterial infections such as tuberculous meningitis (TBM) (22) or urinary tract tuberculosis (7). Culturing CSF for mycobacteria remains the “gold standard” for the diagnosis of TBM in spite of the slowness, lack of sensitivity, and unreliability of this method, serious problems for “a disease in which rapid and precise diagnosis is a therapeutic necessity” (22). Newer rapid diagnostics include those based on nucleic acid amplification (9, 17) and gas chromatographic detection of tuberculostearic acid (4, 10), but these are unlikely to be adapted to an inexpensive rapid assay suitable for use in developing countries where the bulk of TBM cases occur (22). In our experiments we used M. avium NJH 9141, which contains 2 to 3 μmol of MSH per g (residual dry weight) (12). M. tuberculosis contains two- to sixfold more MSH than does M. avium (12), and thus the MSH-capture ELISA should be able to detect even fewer cells of M. tuberculosis. With M. avium our assay is already as sensitive as the acid-fast smear which requires a minimum of 5 × 103 to 5 × 104 mycobacteria per ml of specimen for detection (8). Because CSF samples from a large number of patients with TBM contain between 100 and 5,000 mycobacteria per ml, fewer than 20% of these patients are acid-fast smear positive, although 80% are positive by culture (18). With further improvements in sensitivity, the MSH-capture ELISA could prove superior to the acid-fast smear as a rapid, economical means for detecting mycobacterial infection and identifying samples for more extensive testing.
Elaboration of the role of MSH in mycobacteria has been facilitated by the isolation of mutants in MSH biosynthesis (15) by using the immunoblotting techniques developed earlier (21). However, the amounts of bacteria transferred to the membrane for immunoblotting can be variable, and this sometimes results in false-negative results. Thus, it was desirable to have a more quantitative assessment for screening large numbers of clones. The method described here for directly extracting mycobacteria in microtiter plates with 50% acetonitrile coupled with MSH-capture ELISA of the extracts represents a marked improvement. We now culture mycobacterial mutants as single clones in microtiter plates, estimate the cell numbers from the optical density, extract with acetonitrile, and assay by MSH-capture ELISA. Data from A405 readings over a period of 5 to 60 min allow assessment of the MSH content and facilitate the identification of clones with normal and deficient MSH biosynthesis.
ACKNOWLEDGMENTS
We thank Joseph Aguilera for technical assistance with the screening of mycobacteria and Darwin Berg for use of a microplate reader.
This research was funded by grants AI36971 and AA11393 from the National Institutes of Health.
REFERENCES
- 1.Anderberg S J, Newton G L, Fahey R C. Mycothiol biosynthesis and metabolism: cellular levels of potential intermediates in the biosynthesis and degradation of mycothiol in Mycobacterium smegmatis. J Biol Chem. 1998;273:30391–30397. doi: 10.1074/jbc.273.46.30391. [DOI] [PubMed] [Google Scholar]
- 2.Bayer E A, Wilchek M. The avidin-biotin system. In: Diamandis E P, Christopoulos T K, editors. Immunoassay. San Diego, Calif: Academic Press, Inc.; 1996. pp. 237–267. [Google Scholar]
- 3.Bornemann C, Jardine M A, Spies H S C, Steenkamp D J. Biosynthesis of mycothiol: elucidation of the sequence of steps in Mycobacterium smegmatis. Biochem J. 1997;325:623–629. doi: 10.1042/bj3250623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brooks J B, Syriopoulou V, Butler W R, Saroglow G, Karydis K, Almenoff P L. Development of a quantitative chemical ionization gas chromatography-mass spectrometry method to detect tuberculostearic acid in body fluids. J Chromatogr B Biomed Sci Appl. 1998;712:1–10. doi: 10.1016/s0378-4347(98)00158-3. [DOI] [PubMed] [Google Scholar]
- 5.Ellman G L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82:70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 6.Fahey R C, Newton G L. Determination of low-molecular-weight thiols using monobromobimane fluorescent labeling and high-performance liquid chromatography. Methods Enzymol. 1987;143:85–96. doi: 10.1016/0076-6879(87)43016-4. [DOI] [PubMed] [Google Scholar]
- 7.Goldfarb D S, Saiman L. Tuberculosis of the genitourinary tract. In: Rom W N, Garay S M, editors. Tuberculosis. Boston, Mass: Little, Brown & Co.; 1996. pp. 609–622. [Google Scholar]
- 8.Kent P T, Kubica G P. Public health mycobacteriology: a guide for the level III laboratory. Atlanta, Ga: Centers for Disease Control, U.S. Department of Health and Human Services; 1985. [Google Scholar]
- 9.Lang A M, Feris-Iglesias J, Pena C, Sanchez J F, Stockman L, Rys P, Roberts G D, Henry N K, Persing D H, Cockerill F R., III Clinical evaluation of the Gen-Probe Amplified Direct Test for detection of Mycobacterium tuberculosis complex organisms in cerebrospinal fluid. J Clin Microbiol. 1998;36:2191–2194. doi: 10.1128/jcm.36.8.2191-2194.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mayakova T I, Kuznetsova E E, Kovaleva M G, Plyusnin S A. Gas chromatographic-mass spectrometric study of lipids and rapid diagnosis of Mycobacterium tuberculosis. J Chromatogr B Biomed Appl. 1995;672:133–137. doi: 10.1016/0378-4347(95)00192-l. [DOI] [PubMed] [Google Scholar]
- 11.Misset-Smits M, van Ophem P W, Sakuda S, Duine J A. Mycothiol, 1-O-(2′-(N-acetyl-l-cysteinyl)amido-2′-deoxy-α-d-glucopyranosyl)-d-myo-inositol, is the factor of NAD-factor-dependent formaldehyde dehydrogenase. FEBS Lett. 1997;409:221–222. doi: 10.1016/s0014-5793(97)00510-3. [DOI] [PubMed] [Google Scholar]
- 12.Newton G L, Arnold K, Price M S, Sherrill C, delCardayre S B, Aharonowitz Y, Cohen G, Davies J, Fahey R C, Davis C. Distribution of thiols in microorganisms: mycothiol is a major thiol in most actinomycetes. J Bacteriol. 1996;178:1990–1995. doi: 10.1128/jb.178.7.1990-1995.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Newton G L, Bewley C A, Dwyer T J, Horn R, Aharonowitz Y, Cohen G, Davies J, Faulkner D J, Fahey R C. The structure of U17 isolated from Streptomyces clavuligerus and its properties as an antioxidant thiol. Eur J Biochem. 1995;230:821–825. doi: 10.1111/j.1432-1033.1995.0821h.x. [DOI] [PubMed] [Google Scholar]
- 14.Newton G L, Fahey R C, Cohen G, Aharonowitz Y. Low-molecular-weight thiols in streptomycetes and their potential role as antioxidants. J Bacteriol. 1993;175:2734–2742. doi: 10.1128/jb.175.9.2734-2742.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Newton G L, Unson M D, Anderberg S J, Aguilera J A, Oh N N, delCardayré S B, Av-Gay Y, Fahey R C. Characterization of Mycobacterium smegmatis mutants defective in 1-d-myo-inosityl-2-amino-2-deoxy-α-d-glucopyranoside and mycothiol biosynthesis. Biochem Biophys Res Commun. 1999;255:239–244. doi: 10.1006/bbrc.1999.0156. [DOI] [PubMed] [Google Scholar]
- 16.Norin A, van Ophem P W, Piersma S R, Persson B, Duine J A, Jornvall H. Mycothiol-dependent formaldehyde dehydrogenase, a prokaryotic medium-chain dehydrogenase-reductase, phylogenetically links different eukaryotic alcohol dehydrogenases: primary structure, conformational modelling and functional correlations. Eur J Biochem. 1997;248:282–289. doi: 10.1111/j.1432-1033.1997.00282.x. [DOI] [PubMed] [Google Scholar]
- 17.Pfyffer G E, Kissling P, Jahn E M, Welscher H M, Salfinger M, Weber R. Diagnostic performance of amplified Mycobacterium tuberculosis direct test with cerebrospinal fluid, other nonrespiratory, and respiratory specimens. J Clin Microbiol. 1996;34:834–841. doi: 10.1128/jcm.34.4.834-841.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raviglione M C, O’Brien R J. Tuberculosis. In: Fauci A S, Braunwald E, Isselbacher K J, Wilson J D, Martin J D, Kasper D L, Hauser S L, Longo D L, editors. Harrison’s principles of internal medicine. 14th ed. New York, N.Y: McGraw-Hill Co., Inc.; 1996. pp. 1004–1014. [Google Scholar]
- 19.Sakuda S, Zhou Z-Y, Yamada Y. Structure of a novel disulfide of 2-(N-acetylcysteinyl)amido-2-deoxy-α-l-glucopyranolsyl-myo-inositol produced by Streptomyces sp. Biosci Biotech Biochem. 1994;58:1347–1348. doi: 10.1271/bbb.58.1347. [DOI] [PubMed] [Google Scholar]
- 20.Spies H S C, Steenkamp D J. Thiols of intracellular pathogens. Identification of ovothiol A in Leishmania donovani and structural analysis of a novel thiol from Mycobacterium bovis. Eur J Biochem. 1994;224:203–213. doi: 10.1111/j.1432-1033.1994.tb20013.x. [DOI] [PubMed] [Google Scholar]
- 21.Unson M D, Newton G L, Davis C, Fahey R C. An immunoassay for the detection and quantitative determination of mycothiol. J Immunol Methods. 1998;214:29–39. doi: 10.1016/s0022-1759(98)00034-9. [DOI] [PubMed] [Google Scholar]
- 22.Zuger A, Lowy F D. Tuberculosis of the brain, meninges, and spinal cord. In: Rom W N, Garay S M, editors. Tuberculosis. Boston, Mass: Little, Brown & Co.; 1996. pp. 541–556. [Google Scholar]