Abstract
Microbial therapeutics, which include gut biotics and fecal transplantation, are interventions designed to improve the gut microbiome. Gut biotics can be considered as the administration of direct microbial populations. The delivery of this can be done through live microbial flora, certain food like fiber, microbial products (metabolites and elements) obtained through the fermentation of food products, or as genetically engineered substances, that may have therapeutic benefit on different health disorders. Dietary intervention and pharmacological supplements with gut biotics aim at correcting disruption of the gut microbiota by repopulating with beneficial microorganism leading to decrease in gut permeability, inflammation, and alteration in metabolic activities, through a variety of mechanisms of action. Our understanding of the pharmacokinetics of microbial therapeutics has improved with in vitro models, sampling techniques in the gut, and tools for the reliable identification of gut biotics. Evidence from human studies points out that prebiotics, probiotics and synbiotics have the potential for treating and preventing mental health disorders, whereas with paraprobiotics, proteobiotics and postbiotics, the research is limited at this point. Some animal studies point out that gut biotics can be used with conventional treatments for a synergistic effect on mental health disorders. If future research shows that there is a possibility of synergistic effect of psychotropic medications with gut biotics, then a gut biotic or nutritional prescription can be given along with psychotropics. Even though the overall safety of gut biotics seems to be good, caution is needed to watch for any known and unknown side effects as well as the need for risk benefit analysis with certain vulnerable populations. Future research is needed before wide spread use of natural and genetically engineered gut biotics. Regulatory framework for gut biotics needs to be optimized. Holistic understanding of gut dysbiosis, along with life style factors, by health care providers is necessary for the better management of these conditions. In conclusion, microbial therapeutics are a new psychotherapeutic approach which offer some hope in certain conditions like dementia and depression. Future of microbial therapeutics will be driven by well-done randomized controlled trials and longitudinal research, as well as by replication studies in human subjects.
Keywords: Gut biotics, Probiotics, Prebiotics, Synbiotics, Paraprobiotics proteobiotics, Postbiotics, Life style factors, Psychiatric diseases, Neurocognitive disorders
Introduction
The human gut microbiome is the collective genome and microbial cellular or structural elements and metabolites of the microorganisms inhabiting the gastrointestinal (GI) tract and now considered as a virtual organ [1]. Within the GI tract of humans, resides the largest population of microorganisms, known as the gut microbiota (GM) [2]. Metabolites that are produced by the GM help with the physiological and metabolic processes as well as immunity against pathogens. In the elderly, different factors like polypharmacy, decreased gut mobility, and malnutrition can cause changes in the GM [3]. When there is a disruption of the microbial balance, this is known as gut dysbiosis. It can have wide range of consequences and associated with different diseases [4]. Physiologically significant products derived from GM through their interaction with diet and the host can enter into the circulation from the GI tract and can affect distant organs [5]. In 2018, Bambury et al proposed a pathway for discovery of microbial therapeutics in mental health, known as psychobiotics [6]. A possible link exists between the GM and brain processes and behavior through the microbiota-gut-brain axis [7]. The microbiome is also affected by diet and exercise, which may have an affect on the mood and cognition [8, 9]. Thus, the GM may serve as a target for treatment through the use of microbial therapeutics or microbiota-based treatments including gut biotics and fecal transplantation [10].
What are gut biotics and justification for new terminology?
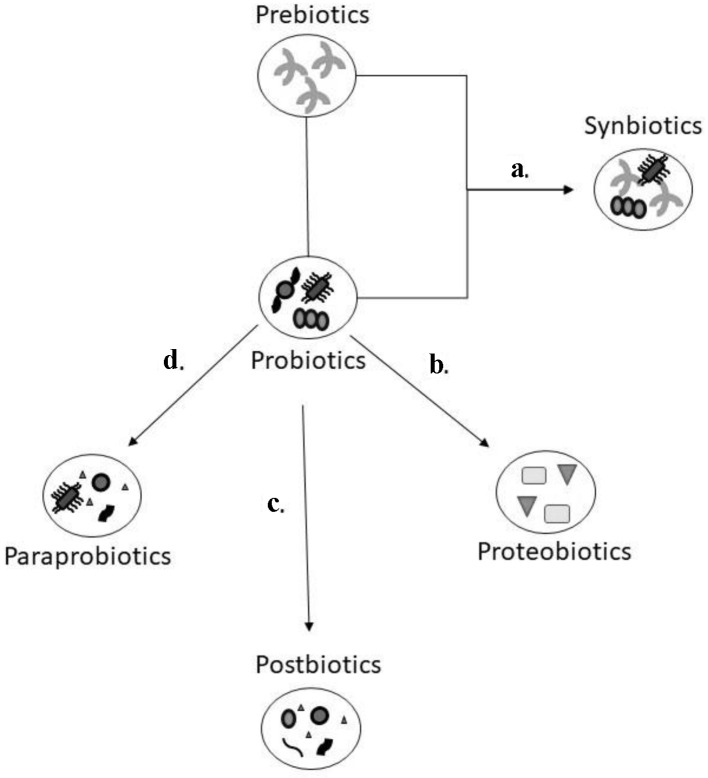
The authors of this paper are coining a new term called gut biotics. Gut biotics are either food constituents that can affect the gut microbes or live microbial administration or its products for therapeutic purposes. A schematic outlining about the various gut biotics can be found in Figure 1. Gut biotics serve as a type of microbial therapy to help balance the human intestinal microorganisms in numerous medical conditions including mental health and cognitive disorders. Genetically engineered gut biotics are available and may have similar therapeutic benefits. In mental health, gut biotics can affect the synthesis of neuroactive compounds or neurotransmitters.
Figure 1.
Schematic model of the various gut biotics. Prebiotics are dietary fibre that help with the growth of probiotics which are microorganisms that can provide a health benefit. The combination of prebiotics and probiotics (a) is referred as synbiotics as it is felt there is a synergistic effect due to this combination. Postbiotics, paraprobiotics, and proteobiotics are considered non-viable bacterial products or metabolic by products from probiotic microbes. The natural metabolites through the process of fermentation (b) are isolated from the culture media of probiotics which are identified as proteobiotics. When probiotics are treated through sonication, chemical, or enzymatic processes (c), they are broken down to functional bioactive compounds, referred to as postbiotics. Using radiation, heating, pasteurization and other inactivation methods (d), probiotics are converted to non-viable microbial cells known as paraprobiotics.
Why there is a need for new terminology called gut biotics?
The paper written by Dinan et al (2013) defined psychobiotics as probiotics and prebiotics that have a mental health benefit [11], whereas Sarkar et al (2016) mentioned psychotrophics and antibiotics contributing to mental health symptoms and disorders should also be considered as psychobiotics [12]. Based on these two above mentioned definitions, there is a no consensus about the use of the term psychobiotics.
Whereas the authors of this paper think that gut biotics is a better terminology for food products and microbial therapeutics which have broader clinical benefits, including in mental health, by repopulating the GM. It is a microbe or microbiome based therapeutic intervention which may help in the treatment or prevention of mental health disorders.
In this scoping review, the different types of gut biotics are discussed in detail with an overview of pharmacological actions, safety concerns, and regulatory issues, as well as their role in neurocognitive and mental health disorders. As well, the effect of life style factors on the GM and possibly on microbial therapeutics has also been included. This manuscript discusses about the available evidence for a possible novel therapy in different mental health disorders.
Methodology and Search Criteria
A literature search was performed using the electronic databases MEDLINE (1966 - July 2021), EMBASE and SCOPUS (1965 - July 2021), PscyhInfo (1966 - July 2021) and DARE (1966 - July 2021). The main search items were mental health, anxiety, depression, bipolar disorder (BP), schizophrenia, post-traumatic stress disorder (PTSD), obsessive-compulsive disorder (OCD), dementia, Alzheimer’s, probiotics, prebiotics, synbiotics, paraprobiotics, postbiotics, proteobiotics, fecal transplantation, and life style factors. Non-English articles were excluded.
Different Types of Gut Biotics
Probiotics
Probiotics are non-pathogenic microorganisms, including bacteria and yeast, seen in certain types of foods or supplements and can positively influence human health (Supplementary Material 1, www.jocmr.org). The World Health Organization defines probiotics as live microorganisms which when administered in adequate amount confer a health benefit on the host [13].
The main types of probiotic bacteria typically belong to the Lactobacillus genus, including the species: acidophilus, sporogenes, plantarum, rhamnosum, delbrueck, reuteri, fermentum, lactus, cellobiosus, brevis; the Bifidobacterium genus, which include: bifidum, infantis, longum, thermophilum, animalis; the Streptococcus genus, primarily the species: lactis, cremoris, alivarius, intermedius as well as other genus consisting of Leuconostoc, Pediococcus, Propionibacterium Bacillus and Enterococcus (specifically E. faecium). The other probiotic microorganisms are yeast and molds which include Saccharomyces cerevisiae, S. boulardii, Aspergillus niger, A. oryzue, and Candida pintolopesii [14]. These supplements may carry the microorganism to the large intestine for better effects, either in the form of pills, capsules, or liquids. Different fermented food products like yogurt, pickles, kefir or tea like kombucha are the source for probiotics and optimize microbial flora in humans. Non-dairy probiotics can be used in lactose intolerant subjects [15], and engineered probiotics are also available [16].
Site of action of probiotics
Probiotics can act throughout the GI tract as well as outside the GI system. Within the GI tract, it can interact with the intestinal microbes directly or by enzymatic activity, or on the intestinal mucosa or epithelium, and can alter the intestinal barrier function as well as the mucosal immune system. Outside the GI tract, probiotics can act on the brain, liver, and other organs [17].
Side effects of probiotics
No major side effects are seen with probiotic foods and supplements. In the first few days after starting therapy, some subjects may have mild stomach upset, diarrhea, or flatulence (passing gas) and bloating. Rarely, they may trigger allergic reactions. Foods like sauerkraut and kimchi (fermented cabbage) can cause headache due to their richness in amines. In some it can cause lactose intolerance, so non-dairy probiotic food can be an option to overcome this side effect [15].
Safety of probiotics
The United States Food and Drug Administration consider probiotics as live biotherapeutic products and associate four possible safety concerns with probiotics which are listed as: 1) systemic infections; 2) deleterious metabolic activities; 3) excessive immune stimulation in susceptible individuals; and 4) gene transfer [18].
The trials so far showed mixed results regarding systemic infections in immunocompromised individuals, subjects with cardiac valvular disorders, central venous catheters, and also with short bowel syndrome; thus, caution is needed in these groups. There is a case report of a 58-year-old diabetic with multiple co-morbidities developing a fungemia and sepsis by S. boulardii used as a supportive therapy for diarrhea [19]. There are other reports of this rare complication occurring, typically in immunocompromised individuals [20]. Another example includes a 23-year-old Turkish man with a history of diabetes insipidus and a bicuspid aortic valve developing Lactobacillus endocarditis; the strain was isolated from yogurt where he was consuming 1.5 L of yogurt and sour milk a day [21]. A 64-year-old male with previous history of mitral valve prolapse with regurgitation developed endocarditis from taking freeze-dried probiotic capsules containing L. rhamnosus after dental surgery [22]. Another report discussed the case of a 74-year-old man with lymphocytic leukemia who developed septicemia after ingestion of Bacillus subtilis spores, commonly found in probiotics in Italy [22]. A clinical trial studying probiotic in severe pancreatitis (the PROPATRIA trial) found an unexpected increase in mortality with probiotic treated patients. However, in this trial, there are concerns regarding the design and analysis of this study [23]. Based on these reports, some caution may be warranted when probiotics are used in immunocompromised subjects and in patients with heart valvular conditions.
Van den Nieuwboer in their systematic review reported no increased risk of adverse events with probiotic or synbiotic treatment in immunocompromised adults. Of a total of 57 clinical studies that enrolled 2,563 participants, 22% of studies did not report safety concerns, which is a limitation of this systematic review [24]. In this study, patients were classified as immunocompromised if critically ill, underwent a recent surgery, and had an organ or autoimmune disease, or human immunodeficiency virus (HIV) positive. From this systematic review, no major safety concerns or adverse events were related to the use of probiotics or synbiotics. In fact, adverse events occurred less in the treatment group compared to the immunocompromised controls [24]. The study results are similar to the evidence by Didari et al (2014) which indicates that probiotics are safe but suggested to do risk-benefit analysis for specific cases [25]. Despite studies showing safety with probiotics, caution is needed in certain population groups as discussed above [26, 27].
In contrary to the above mentioned information, a meta-analysis study of 30 clinical trials and 2,972 patients, probiotics and synbiotic mixtures showed significant reduction in infections (relative risk (RR): 0.80, 95% confidence interval (CI): 0.68 - 0.95, P = 0.009), ventilator associated pneumonia (RR: 0.74, 95% CI: 0.61 - 0.90) and found no effect on mortality [28]. A recent systematic review and meta-analysis of randomized clinical trials (RCTs) showed that probiotic use was associated with a significant lower incidence of ventilator associated pneumonia (RR: 0.70, 95% CI: 0.56 - 0.88, P = 0.002, and in-hospital mortality (odds ratio (OR): 0.73, 95% CI: 0.54 - 0.98, P = 0.04) [29]. Another recent meta-analysis by Fan et al (2019) showed synbiotics have the potential to reduce in-hospital and intensive care unit (ICU) mortality [30]. Evidence from these studies points out that probiotics are reasonably safe in immunocompetent subjects.
Prebiotics
Prebiotics have been described as non-digestible dietary components that can reach the colon intact and can promote the growth and activity of positive bacteria of the GI tract [31]. The original definition has recently been adapted to include any substrate that the host microorganisms can use resulting in health benefit [32]. This updated terminology allows the inclusion of other substances such as polyphenols and polyunsaturated fatty acids along with the typical carbohydrates, as long as there is evidence of action on the host microorganisms [33]. In 2008, the International Scientific Association of Probiotics and Prebiotics (ISAPP) defined “dietary prebiotics as a selectively fermented ingredient that results in specific changes in the composition and/or activity of the gastrointestinal microbiota, thus conferring benefit(s) upon host health” [32, 34].
Prebiotics can be obtained through a variety of different foods, such as fruits and vegetables as well as dietary supplements [35] (Supplementary Material 1, www.jocmr.org). They can help with probiotic growth and with the production of short-chain fatty acids (SCFA) [36]. Most prebiotics are oligosaccharides [37] and are indigestible by humans; however, the gut bacteria can utilize them and this substance can restore the composition of the GM and enhance the action of probiotics [38].
Side effects of prebiotics
Flatulence and abdominal discomfort can be observed. This often occurs when large quantities are consumed and can be avoided with reduced amounts.
Safety of prebiotics
Overall prebiotics are deemed safe. However, there are very rare instances of anaphylactic reactions including a case report in a 39-year-old male after intake of salsify and artichoke vegetables that contained oligofructose and inulin [39]. A study by Soh et al in 2015 describes anaphylaxis to galacto-oliogosaccharides (GOS) in atopic patients from South-East Asia. When testing commercial formulations, Vivinal™ (vGOS) and Oligomate™, 30 subjects out of 487 had positive skin prick tests and when given oral challenge, six patients have positive reactions to vGOS and none to Oligomate™ [40]. The authors suggest that the prevalence of the vGOS allergy may be up to 3.5% (95% CI: 2.2-5.5%) among atopic patients from Singapore [40]. Future studies need to monitor for these types of reactions.
Synbiotics
As the name suggests, synbiotics are the combination of prebiotics and probiotics to obtain a synergistic effect (Supplementary Material 1, www.jocmr.org). This combination is known as synbiotic therapy and it is designed to help make the probiotic more effective [41]. Synbiotics can help with restoring gut dysbiosis in many health conditions. This can be achieved through improvement of immune function, reduction of incidence of nosocomial infections and other effects on human health [35, 42]. It was also shown that presence of prebiotics can help protect probiotics from bile acid stress; however, this may vary with different combination of probiotics and prebiotics [43].
Safety and side effects of synbiotics
Reporting of safety data for synbiotics in RCTs is lacking, but trials with individual gut biotics, probiotics and prebiotics showed some side effects and safety concerns as discussed above [44].
Paraprobiotics
Paraprobiotics (or ghost probiotics) are inactivated and non-viable probiotic cells, when taken in adequate amounts, can confer health benefits through antioxidant, anti-inflammatory and through several metabolic and immune pathways [45] (Supplementary Material 1, www.jocmr.org). Current research is exploring the potential benefit of adding paraprobiotics to probiotic containing yogurt. Paraprobiotic yogurt will contain dead Lactobacillus and Bifidobacterium and the advantage of this will be increased stability of the yogurt for a wide range of temperatures and a prolonged shelf life [46], but the functional effect will be similar to probiotic yogurt [47].
Postbiotics
Postbiotics can be considered the metabolic by-products of gut microbes, in particular the crude extracts from probiotics that elicit a biological response, such as protecting the intestinal mucosal barrier [48, 49]. Thermal, irradiation, sonication, and even enzymatic process can lead to cell rupture of the bacteria and fungi when extracted from various fermented foods [50] (Supplementary Material 1, www.jocmr.org). In essence, postbiotics are the by-products of probiotics after feeding on prebiotics and often isolated through gas chromatography [48] (Supplementary Material 2, www.jocmr.org).
Both postbiotics and paraprobiotics have the potential to be used as pharmacological agents in the prevention and treatment of various psychiatric and neurocognitive disorders. Currently there are no interventional studies in humans examining the role of postbiotics in mental health and neurocognitive disorders. Since some studies have suggested caution around the use of probiotics in immunocompromised patients, postbiotics may serve as a safer alternative.
Proteobiotics
The metabolites of probiotics that possess antimicrobial activity through interruption of cell-to-cell communication and interrupting the virulence strategies are known as proteobiotics [51] (Supplementary Material 1, www.jocmr.org). This prevents bacterial colonization by not killing the bacteria that can help to prevent the development of antibiotic resistance [51]. A study has shown effects of proteobiotics from Lactobacillus acidophilus on enterohemorrhagic Escherichia coli (EHEC) O157:H7 infection, in preventing the virulence of this organism [52].
Pharmacology of Microbial Therapeutics
Pharmacokinetics
Absorption, distribution, metabolism, and excretion are the main components of pharmacokinetics with traditional medications. With gut biotics, these principles will be difficult to delineate and is complex. Microbial therapeutics is complex and has a unique pharmacology. Some gut microbes are killed by the acidity in the stomach as well as by the bile flow in the small intestine and permanent colonization and potential translocation is rare. The GM has been found to affect the pharmacokinetics of various drugs either positively or negatively [53].
The method of delivery plays a vital role in the efficacy of gut biotics. The typical mode of oral delivery in the form of tablet, capsule, and powders, may cause decreased bacterial survival due to the tablet processing technique requiring bacteria to tolerate temperature up to 60 °C [54]. With commercial food products, such as cheese, milk, and yogurt, the bacteria are less protected from the hostile acidity of the stomach [55]. A study by Bolla et al (2011) found that freeze dried formulations may be a preferred method of delivery [56]. The beneficial effect of probiotics depends on the survival of probiotic microbiota in the intestines [57]. The survival varies with different gut microbial strains, and with their ability to attach with the gastrointestinal tract mucosa. Most of the microbes are eliminated via feces after a few days [58].
Pharmacodynamics
GM can produce different metabolites which act at distant sites of the body like brain receptors [59-61]. A review by Lyte (2011) expressed the notion that probiotics are capable of synthesizing neuroactive compounds that can affect the GI and psychological health of the host [62]. The author hypothesized a microbial endocrinology-based mechanism where probiotics produce neurochemicals which binds to the receptors on immune and neuronal cells and affect the functions of the GI tract and central nervous system (CNS) [62].
Pharmacomicrobiomics
Microbial biotransformation by xenobiotic metabolism is important and helps in the up and downregulation of the action of medications [5]. This GM and medication interactions depend on many factors like micro-genomics as well as the metabolic potential of the gut microbiome which are now defined as Pharmacomicrobiomics [63]. The main component of the hepatic drug metabolism is oxidation and conjugation reactions, whereas in GM-mediated metabolism, reduction and hydrolysis reactions are commonly seen [64, 65]. Drug-microbe interactions are bi-directional and the effect is seen with microbes and metabolites on the host drug metabolism and is responsible for the drug response or toxicity. With any new drug development, interaction between the medication and GM should be considered, which may alter the current understanding of pharmacokinetics and pharmacodynamics.
Mechanism of action
Mechanisms of action of probiotics, prebiotics and synbiotics can be direct or indirect through modifications of the endogenous flora, enhancement of the epithelial barrier, concomitant inhibition of pathogenic microorganisms and through modulation of the immune system [66, 67]. Peyer’s patches in the gut help with the immune response due to probiotics [68]. An animal study using mice confirmed that probiotic lactic acid bacteria were identified in the dome area of Peyer’s patches 6 - 12 h after their ingestion [69]. Research evidence pointed out that probiotics, prebiotics and synbiotics have an effect on inflammatory and bio-oxidative factors and have a clinical impact on GI chronic diseases [70]. Studies have also shown that probiotics, prebiotics, and diet modification can have a positive effect on brain function and neurochemistry and help to reduce neuroinflammation and amyloidogenesis and thereby prevent Alzheimer’s disease (AD) pathogenesis [71, 72] (Fig. 2).
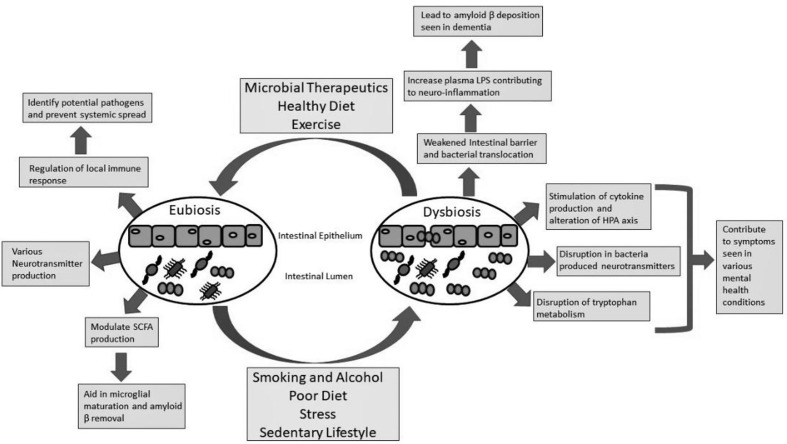
Figure 2.
The role of the gut microbiome in neurocognitive and metal health conditions. In a state of eubiosis, the gut bacteria are controlled by the host immune system to prevent systemic spread. Since many of the gut bacteria have a polysaccharide coating the immune system can recognize potential pathogens from the commensal organism. This can prevent systemic spread and inflammation that has been shown to play a role in various mental health conditions. Different species of bacteria in the gut microbiome can produce different neurotransmitters, such as serotonin through tryptophan metabolism. Short-chain fatty acids (SCFAs) are also produced by different gut bacteria and have been found to play a role in microglial maturation which can help remove amyloid β peptide (the major component in Alzheimer’s disease (AD)). Various factors, such as lifestyle and diet, can lead to microbial dysbiosis. A weakened intestinal barrier can result in bacterial translocation causing increased lipopolysaccharide (LPS) serum levels. The result is systemic and neuroinflammation that can contribute to amyloid β deposition leading to AD. The systemic inflammation can become chronic and lead to an alteration of the hypothalamic pituitary adrenal axis (HPA) and has been linked to various mental health conditions. The disrupted microbiome can affect neurotransmitter production and tryptophan metabolism, again impacting the host’s mental health.
Representative Gut Biotics/Therapeutics Found to Be Useful in Human Studies
Probiotics
Fermented foods
Fermented foods contain probiotic bacteria, yeast and microbial metabolic products [73]. Representative examples of GM targeted fermented foods such as yogurt, kefir, kombucha, sauerkraut, kimchi, tempeh, natto, miso, sour dough bread favor gut diversity which would be considered beneficial for our gut microbes and indirectly for neurocognitive and mental health conditions [74]. In the American Gut Project, subjects who consumed fermented foods one or two times had gut microbe diversity when compared with non-consumers [75]. In the study by Ton et al (2020), probiotic-based kefir intervention for 3 months in AD patients showed satisfactory improvement in memory function in these subjects [76]. In another study, by Mohammadi et al (2016), a benefit with probiotic yogurt but not with conventional yogurt, on mental health disorders was seen [77]. Whereas, a study showed when healthy medical students partook in daily conventional yogurt consumption, they exhibited an increase in alpha diversity of their GM along with reduced stress indicators [78]. However, the benefit of fermented food for treating or preventing depression and anxiety is not well studied [79].
Supplements
Promising human clinical researches with single and multiple probiotic strain specific therapies are seen in neurocognitive and mental health conditions. By targeting the beneficial species of the GM could be a novel approach for management.
Human studies using different strains of probiotics
Neurocognitive disorders
Single strain intervention with Bifidobacterium breve A1 and Lactobacillus plantarum in RCTs with mild cognitive impairment (MCI) subjects showed some benefit [80-82]. Multi-strain probiotic intervention with L. acidophilus, L. casei, Lactobacillus fermentum,and Bifidobacterium fermentum in RCT showed beneficial effect on cognitive function in AD subjects [83]. It was also found that probiotic and selenium co-supplementation resulted in a significant improvement in cognition over a 12-week period in AD patients [84]. A meta- analysis of probiotic studies in MCI and AD by Den et al (2020) showed improved cognitive performance [85]. When examining studies of human subjects, another meta-analysis found that probiotic supplements improved cognitive function in cognitively impaired individuals but not on healthy subjects [86].
Depression
In a study looking at single bacterial strain probiotic interventions, Lacticaseibacillus paracasei strain Shirota showed reduction in symptoms with major depressive and BP [87]. Other studies have examined probiotics with multiple strains, such as Lactobacillus acidophilus, Lactobacillus casei, and Bifidobacterium bifidum [88] or with Bifidobacterium longum and Lactobacillus helveticus [89] which helped improve mood outcomes.
Anxiety
When individuals were treated with a multi-strain probiotic intervention involving B. longom, B. bifidum, Bifidobacterium lactis, and L. acidophilus a reduction in anxiety symptoms was witnessed. It was also found to be useful as an adjunctive therapy along with sertraline [90].
Prebiotics
Food products (high fiber foods)
Oats and barley have been used in foods like bread, biscuits and cereals [91]. There is some evidence that this may help to improve mood and cognition [92, 93]. Fruits and vegetables have high fiber and a meta-analysis study showed reduced risk of cognitive impairment (OR: 0.79; 95% CI: 0.67 - 0.93; P = 0.006) [94] and the Hordaland health study found there was an improvement to different cognitive domains like attention, memory, executive functions, speed of processing, and global cognition [95]. Overall fruits and vegetables play a role in improving cognitive health [96]. Observational studies showed a decreased association of depression with intake of vegetables, fruit, or seaweed, but this is not seen with cereals [97, 98]. A meta-analysis study of observational studies showed reduced risk of incident depression (vegetables: OR: 0.91; 95% CI: 0.87 - 0.96; P < 0.001; fruit: OR: 0.85; 95% CI: 0.81 - 0.90; P < 0.001) [99].
Supplements
Studies on dietary fiber to improve cognition and mood have used prebiotic supplements [33, 100]. The prebiotic supplements are galactooligosaccharides (GOS), fructooligosaccharides (FOS), oligofructose, chicory fiber and inulin. A study by Smith et al (2015) showed an oligofructose-enriched inulin improved memory and mood in healthy volunteers [101].
Limitations
The limitations of the above-mentioned supplements studies in general are the lack of replications studies investigating the same strain of probiotic or type of prebiotics. Information on the right dose and duration of supplement treatment is not clear. Future research should try to address these issues. Future studies should also use nutrient biomarkers to assess the effect of probiotics and prebiotics [102].
Role of Gut Biotics With Psychotherapeutic Medications and Cognitive Enhancing Medications
Psychotropic medications in general can affect the GM and cause dysbiosis, which induce inflammation and neurodegeneration [103]. Co-administration with conventional medications, probiotics have the potential to improve cognition both in normal and AD subjects [83, 104]. An animal study with transgenic mice showed reduced amyloid plaques and improved cognitive performance when administered with pre- and probiotics [105]. GM helps with the expression of hippocampal N-methyl-D-aspartate (NMDA) receptors similar to memantine, whereas this expression is less in germ-free mice [106], and in rats treated with antibiotics [107]. GM, by their anti-inflammatory, metabolic, and pleiotropic effects, reduce the neurodegeneration seen in dementia. Diet rich in probiotics and prebiotics will help to lower the risk of AD and retard the development of neurocognitive decline [108]. Ruminococcus flavefaciens affects gene networks in the brain, suggesting a mechanism for microbial regulation of antidepressant treatment efficiency [109]. Prebiotics have been shown in animal studies to attenuate some of the side effects of antipsychotics, like weight gain [110]. Few animal studies pointed out that probiotic, prebiotic and synbiotic treatment can have some synergistic effect with conventional drug treatments for Alzheimer’s and depression, but more future research is needed in this area especially with human subjects.
Effect of Food and Life Style on Gut Microbiome and Possibly on Microbial Therapeutics
The human gut microbiome is a dynamic virtual organ that is influenced by diet and lifestyle. Lifestyle medicine is a term referring to altering health behaviors to influence factors such as physical activity, diet, tobacco smoking, alcoholism and sleep [111, 112].
Smoking
Intestinal dysbiosis can occur with smoking [113]. The effects of cigarette smoking have been examined and it has shown that it can induce changes in intestinal microbiome along with increasing the permeability of the mucosa and even impair the mucosal immune response [114]. The mechanism of action is not fully understood; however, some of the effect is thought to be due to the harmful compounds found in cigarettes. It was found that nicotine can lead to microbial dysbiosis with an increase in Firmicutes and Proteobacteria while causing a decrease in Bacteroidetes [115]. Smoking cessation helps to improve intestinal dysbiosis to a certain extent [116, 117].
Alcoholism
With alcoholism, drinking amount and unhealthy diet may predict gut dysbiosis. With abstinence over 4 weeks, a study showed reduction with gut dysbiosis. With microbial therapeutics, the study showed an improvement in the speed of recovery of the microbiome [118].
Stress
Stressful life events contribute to mental illness. Psychological stress could lead to altered gut microbial composition [119]. A recent pilot study done in information technology professionals experiencing high stress showed significantly improved stress (self-perceived and job stress), mood and sleep disturbances as well as quality of life after an 8-week intervention of a probiotic Lactobacillus plantarum PS128TM [120].
Sleep deprivation
Sleep deprivation can cause gut dysbiosis [121]. Ingesting probiotics/paraprobiotics may help to improve subjective and objective sleep metrics [122].
Sedentary lifestyle
Individuals with more sedentary lifestyles were shown to have less microbial diversity with more disease-causing species, such as E. coli. However, individuals with more active lifestyles, such as elite athletes, have a greater bacterial diversity, particularly bacteria responsible for producing SCFA [123].
Fast food and sweetened beverages
Fast foods, commercial baked foods and sugar-sweetened beverages have been shown to increase the risk of depression [124, 125]. A study by Zhu et al (2020) found that an individual’s GM can undergo a shift in composition based on their diet, in as short as 4 days. When exposed to a Mediterranean diet that is rich in vegetables and whole grains subjects showed an increase in more fiber-fermenting bacteria, than, when consuming a more fast-food diet (high in fats and carbohydrates), as well as more bile tolerant microbial genera were present [126].
Discussing With the Patient About Gut Biotics
Even though gut biotics have been around for many years, only in the last decade a lot of research is going on this area. Gut biotics are usually found in foods or dietary supplements. Some studies point out that gut biotics may supplement regular treatments, but do not often replace them. Since there are many kinds of gut biotics with different strains and combinations, it is important for patients to talk to their healthcare professional before starting one.
Microbial therapeutics are available as over the counter medications as a non-regulated nutritional supplement or as dietary products. At this point, microbial therapeutics would not replace the current evidence-based pharmacological or psychological treatments. But health care providers treating mental health disorders should consider gut-related principles of therapy with some optimism and caution in their holistic approach [127, 128].
Regulatory Challenges With Gut Biotics
The gut biotics be developed as food products (functional foods or diet supplements) or drug products (biological or microbiome products). Regulatory framework for the development of these products has to be optimized and considered by food and drug regulatory agencies in different countries. The efficacy and safety have to be determined by these agencies with specific guidelines and requirements. According to the US FDA regulations, depending on the intended use of this microbiome, products may be considered as a functional food or dietary supplement which does not require FDA approval before marketing, whereas if it is a drug, it needs FDA approval.
Fecal Transplantation in Neurocognitive and Psychiatric Diseases
A form of microbial therapeutics comes as fecal microbiota transplantation (FMT), which is the transfer of fecal bacteria from a healthy donor to a recipient with the goal of repopulating the GI tract with beneficial bacteria, sharing a similar role as probiotics. FMT has been in use for the treatment of Clostridium difficile infections. Outside of this, research is being done to explore its role in the treatment of metabolic and neurological disorders such as multiple sclerosis, Parkinson’s disease, as well as neurocognitive and psychiatric conditions [129].
To explore the effects of FMT on cognition, a study by D’Amato et al (2020) examined the effects of transplanting the fecal microbiota from aged donor mice to young adult mice. They found that there was a significant impairment in spatial learning and memory, while anxiety, explorative behavior, and locomotor activity remained unchanged in the young adult mice recipients [130]. Changes were also seen with the composition of the gut microbiome, in particular a reduction in SCFA producing bacteria (Lachnospiraceae, Faecalibaculum, and Ruminococcaceae). The authors suggest that age-related shifts in the microbiota may play a role in cognitive decline, and developing techniques to restore “young-like microbiota” may improve cognitive functions in the elderly [130]. It has also been shown that the use of FMT reduced amyloid-β deposition and improved cognitive deficits in mice brains, thus alleviating Alzheimer’s pathology [131]. A case report highlights the improvement seen in an 82-year-old male who received FMT for a C. difficile infection. This individual also showed improved memory and mood up to 6 months post FMT with higher scores on the mini-mental status exam [132].
With psychiatric conditions FMT has been studied with mood disorders, such as anxiety and depression. A study by Zhang et al (2019) found that an FMT from an NLRP3 KO mouse to a chronic unpredictable stress (CUS) mouse helped to reduce depressive-like symptoms [133]. Studies have also shown that when taking FMT from human into mice, these mice can display noticeable behavioral changes. When germ-free rats were given an FMT from depressed human patients, they started to exhibit more depression-like behavior in the form of increased immobility time in the forced swimming test [134]. When mice received FMT from anxious patients with irritable bowel syndrome (IBS), the animals showed an increase in anxious behavior [135]. These animal studies showed both depressive and anxious behavior can be transferred from afflicted individuals to mice.
Current research is being done exploring the role of FMT in treating psychiatric conditions in humans, typically in those with IBS. A study by Huang et al in 2019 found that patients with refractory IBS had improved GI symptoms and reduced anxiety and depression scoring after receiving FMT from healthy donors [136]. A case report of a female patient with major depressive disorder (MDD) showed an improvement in depressive symptoms after an FMT and alteration in the gut microbiome with increased Firmicutes and decreased Bacteroides [137]. More research is needed to explore the role of FMT in other psychiatric conditions.
Gut Biotics in Cognitive Disorders
As an individual ages, there is a shift in the microbiome and decrease in microbial diversity that can result in gut dysbiosis [138]. The microbiota-gut-brain axis along with alterations of the GM and gut dysbiosis has been shown to play a role in MCI and dementia [139]. Prevalence of MCI ranges from 16% to 20% above the age of 60 years [140]. The annual rate of conversion of MCI to dementia is 15% [141]. The prevalence of dementia worldwide in 2015 was 46.8 million and expected to increase to 74.7 million by 2030 [142]. In the brain, neuronal loss with the reduction of trophic factors that affect neurogenesis, along with synaptic plasticity alterations, and in combination with oxidative changes that lead to microglia activation and neuroinflammation which can contribute to cognitive impairment. The microbiota-gut-brain axis involves multiple physiological systems through bidirectional communication [103]. Dysregulation of this axis plays a role in causing AD and its progression. Dysbiosis with reduction in gut bacteria like Lactobacillus and Bifidobacterium can lead to reduced production of neurotransmitter acetylcholine and cause cognitive decline [143]. In AD, abundance in Escherichia and Shigella along with a reduction in Eubacterium rectale microbiota has been studied, resulting in the increase of proinflammatory cytokines leading to changes in neurotransmitters levels [144]. Hence, the modification of the GM may serve as a target to counter this disease process.
Probiotics
Among the risk factors for AD, diet is an important one to consider. Nutritional approaches may prevent and halt the progression of AD [145]. GM through defective mucosal barrier affects the entero-endocrine system and causes pathological proteins in the brain [101]. Newer nutritional therapeutic approaches should be explored. Hence, we want to explore the evidence for probiotics in modulating the cognitive decline. Probiotics through changes in inflammatory reaction, oxidative stress and amyloid-β deposition can prevent the progression of cognitive decline in AD.
Preclinical studies
It was found that mice fed with amyloid-β caused significant changes to the bacterial structure of the GM [146]. When microflora of AD patients was transplanted to gnotobiotic mice, cognitive decline was seen [147]. Microbiome generated amyloid and SCFA through activation of microglia which can help to remove β-amyloid peptide within the brain, which is the main pathological change seen in AD [148]. With neuropsychiatric diseases known as “microgliopathies”, microglial dysfunction is the primary pathogenesis in these conditions [149]. Microglia maturation and function is determined by SCFA and microbiota-derived bacterial fermentation products. Animal studies have found that microglial maturation and function can be restored with the recolonization of gut bacteria [150].
Cognitive impairment can be mitigated through the modification of the GM, with Bifidobacterium breve A1 in Aβ-induced AD mice model [151]. In a study, rats treated with L. plantarum MTCC1325 for 60 days, help to restore the neurotransmitter acetylcholine deficit and also prevents cognitive decline [143]. In an experimental study with the transgenic mouse model of AD, treatment with the probiotic SLAB 51 mixture modified the gut microbiome leading to a change in intestinal metabolite content, including SCFA that helps to improve cognitive function [152]. Studies done in animals with B. longum, B. breve, B. infantis, L. helveticus, L. rhamnosus, L. plantarum, and L. casei were found to be most effective in improving CNS function as well as anxiety, depression, stress and memory [153, 154] (Supplementary Material 3, www.jocmr.org).
Clinical or human studies
One of the targets of probiotic use is to increase the presence of beneficial bacteria which will help to reduce intestinal permeability which in turn decreases lipopolysaccharide (LPS) and reduces inflammatory response [155]. Higher levels of zonulin, a marker of intestinal permeability, have been found to be associated with cognitive decline [156]. As well, insulin resistance, oxidative stress, and inflammatory processes have been shown to contribute to AD, and probiotics can influence these pathways [83]. Supplementation with fermented milk products can activate specific areas of the brain in healthy human females [157]. However, a cross- sectional study showed probiotics can modulate CNS function leading to alleviated cognitive dysfunction [158]. An RCT on patients with memory problems had beneficial effects on cognitive function with supplementation with Bifidobacterium breve A1 [80]. Bifidobacterium breve A1 intake can also suppress the immune reaction and inflammation induced by amyloid in the hippocampus [159]. Two RCTs showed that probiotic supplementation led to a significant improvement in cognitive function in AD with modulation of brain activity [83, 160]. Even in MCI subjects, 12 weeks supplementation of probiotics increased brain-derived neurotrophic factor (BDNF) levels and improved cognitive performance [82].
Meta-analysis data from three RCTs found no benefit of probiotic supplementation on cognitive function when examining 161 AD patients who received Lactobacillus and Bifidobacterium strains (standardized mean difference (SMD): 0.56; 95% CI: -0.06 to 1.18). This result could be due to selection bias of the RCTs used in this study. However, probiotic supplementation improved plasma triglycerides, very-low-density lipoprotein cholesterol, insulin resistance, and plasma malondialdehyde [161]. However, another meta-analysis using all the RCTs from five available studies involving 297 subjects with cognitive decline (either MCI or AD) showed a significant improvement in cognition (SMD: 0.37; 95% CI: 0.14 - 0.61; P = 0.00) [85]. Overall, more evidence from large-scale, longer-term RCT is needed to show the beneficial effect.
Certain strains of probiotics, through the microbiota-gut-brain axis, appear to influence the CNS and behavior. Based on clinical studies, patients suffering from cognitive impairment have been shown to have a decreased abundance of anti-inflammatory bacteria, such as Eubacterium rectale and Bacteroides fragilis, while exhibiting an increased amount of proinflammatory bacteria belonging to the Escherichia and Shigella genus which can contribute to the brain amyloidosis [162, 163]. Supplementation of Lactobacillus and Bifidobacterium has improved both cognitive and mood function [164]. With the current evidence from the research in the field of the gut microbiome and cognitive impairment, probiotics may play a role in the prevention and treatment of AD. The main mechanisms of this beneficial action are likely through the modulation of inflammatory processes, oxidative stress, and other possible mechanisms that help to ameliorate the progression of AD.
Prebiotics on cognition
Prebiotics have been found to influence neurobiology and impact brain function [165]. A recent clinical review found that FOS and inulin (at doses around 5 - 10 g/day) help to improve learning and memory along with behavior. However, this appears to be short-lived, for 4 - 12 weeks, and seen in young, healthy, middle-aged subjects, but not in the elderly [166].
Preclinical studies
A study by Chen et al (2017) found that FOS from Morinda officinalis (perennial slender climbing shrub) showed memory improvement in rodent AD models [167]. While a study by Sun et al (2019) found that in a male APP/PS1 transgenic mice AD model, those treated with a 2% FOS solution for 6 weeks showed improvement in cognitive deficits and increased expression of synapsin I, PSD-95 and GLP-1, as well as decreased phosphorylated JNK level [168]. Another study using E4 FAS mice to model late onset AD found that when animals were supplemented with the prebiotic inulin, a reduction in neuroinflammation was seen due to an increase of SCFAs [169]. Dietary inulin alters the gut microbiome, enhances systemic metabolism and reduces neuroinflammation in an APOE4 mouse model. When Sprague-Dawley rats were treated with chitosan (an oligosaccharide), improvement in cognitive function and a decrease in pro-inflammatory cytokines (tumor necrosis factor-α, interleukin-1β) were found [170].
Clinical studies
Various clinical studies have been done examining the role of prebiotics on cognition. A study by Smith et al (2015) took 47 healthy volunteers and those given 5 g of oligofructose-enriched inulin performed better on recognition memory tasks and showed an increased recall performance (immediate and delayed) [101]. In another study by Prasad et al (2007) examining 61 patients with minimal hepatic encephalopathy and when given 30 - 60 mL of lactulose for 3 months, there was an increase in some cognitive functions and indicators of quality of life, such as emotional and social behavior [171]. It has also been found that certain herbal products, such as dietetic polyphenols, can influence the gut microbiome and help restore dysbiosis and be effective in counteracting the onset of AD [151]. Supplementation with prebiotics can alter microbiota composition and function. Prebiotics form SCFAs and stimulate probiotics, and through antioxidant effect and anti-inflammatory activity, as well as stimulation of BDNF the neurotrophic factor can help in reducing the pathogenesis of AD.
Synbiotics on cognition
The number of studies involving synbiotics is limited. However, one study found that when male Sprague-Dawley rats were given 860 mg/kg of inulin or inulin with E. faecium for 5 weeks, the synbiotic group showed better cognitive performance and a reduction of IL-1β [172].
Similar to preclinical studies, clinical studies with synbiotics on cognition are also limited. It is possible with regular intake of probiotics and prebiotics, along with other nutrients may help to reduce the cognitive decline [108]. A study by Louzada e Ribeiro (2018) found that in 49 elders given 6 g of FOS and a probiotic for 24 weeks, they showed some improvement in depressive symptoms and cognition [173]. To our knowledge, at present no RCTs have been done with synbiotic supplementation in demented subjects.
Other gut biotics
With other gut biotics like paraprobiotics, postbiotics and proteobiotics, lack of research is seen with cognitive impairment. Future research is needed to identify the usefulness of these gut biotics in this disorder.
Future directions
Currently the majority of research examining the role of the gut microbiome and cognitive function has been done using animal models. There is a gap between the observational and interventional studies seen both with animal models and clinical studies of dementia [151]. Only small sample size has been examined and the profiling of the GM composition and functioning has been lacking, thus more longitudinal studies in high-risk population for cognitive impairment are needed [160]. An animal study found that memantine acts synergistically with L. plantarum and helps reduce AD pathology in APPS1 mice [174]. It even has an antimicrobial activity against E. coli, thus suggesting its influence on the GM [175]. Understanding the particular role of certain bacteria species on cognition and also about the molecular mechanisms involved may serve as another means to develop direct therapeutic targets [160].
Gut Biotics in Mood and Psychotic Disorders
Depression and anxiety
MDD has a prevalence of around 7% affecting an estimated 264 million people, and approximately 284 million people suffer from an anxiety disorder [176]. Depression was the most common disorder in 17.1%, followed by panic/anxiety in 11.3%. There is a growing body of literature linking the role of the microbiota-gut-brain axis in depression and anxiety [73]. Thus, modulation of the gut microbiome is being considered as a therapeutic target through the use of prebiotics and probiotics.
Prebiotics
Preclinical studies
In the study by McVey Neufeld et al (2017), prebiotics such as GOS (using 7 g/kg) and polydextrose (PDX) alone or in combination with L. rhamnosus in male Sprague-Dawley rats, showed decreased anxiety-like behavior in these animals [177]. In the study by Szklany et al (2019), pregnant BALB/cByJ mice where given a 3% mixture of short-chain galactooligosaccharides (scGOS) and long-chain fructooligosaccharides (lcFOS) for 11 weeks showed a decrease in anxiety-related repetitive behavior and an improvement in social behavior along with increased expression of BDNF [178]. Another study found that when male Fischer 344 rats where given a variety of prebiotics including GOS (21.23 g/kg), PDX (6.58 g/kg), lactoferrin (1.86 g/kg) and “whey protein concentrate MFGM-10” (15.9 g/kg) for 4 weeks, there was a reduction in anxiety-like behaviors in addition to increased BDNF levels [179].
Clinical studies
The study by Schmidt et al (2015) found that when 45 healthy volunteers were given Bimuno®-GOS for 3 weeks, there was a decrease in salivary cortisol levels and an increase in “positive versus negative attentional vigilance” [180]. In comparison, another study took 81 randomized patients with MDD and gave them GOS for 8 weeks and found no influence on depressive symptoms; however, those receiving the probiotic B. longum and L. helveticus showed improvement in mood symptoms [89]. When 40 patients with moderate depression were given two capsules of FamiLact® with 20 mg/day of fluoxetine for 6 weeks, it was found there was a decrease in Hamilton Rating Scale for depression compared to the placebo group [181]. Another study examined 44 patients with IBS; when treated with Bimuno®-GOS for 3 months, they had lower anxiety scores and self-reported improved quality of life [182]. A study looked at IBS patients found those receiving short-chain FOS for 4 weeks had reduced anxiety scores and increased levels of fecal Bifidobacteria [183]. A study by Talbott et al (2012) looked at 39 healthy women who were given Baker’s yeast β-glucan for 12 weeks and showed an improvement in mood state [184] (Supplementary Material 3, www.jocmr.org).
There have been some human studies as discussed below looking at probiotic consumption and its influence on individuals with increased stress levels. A study by Gruenwald et al investigated the use of probiotics on an individual’s stress tolerance and found they may be effective in helping to reduce stress and exhaustion [185]. Stress can affect GI function and probiotics may help to alleviate GI symptoms brought on by stress [186]. It has also been shown that probiotics can help to reduce anxiety as well as pre-surgery stress in laryngeal cancer patients [187]. Evidence points out that probiotics can improve mood, anxiety, and reduce psychological stress in various psychiatric conditions (Supplementary Material 4, www.jocmr.org).
PTSD
To model PTSD in animals, mice are subjected to chronic social defeat. When mice were treated with Lactobacillus rhamnosus JB-1 for 28 days prior to stress exposure, there was a decrease in anxiety-like behavior and social interaction deficits [188]. However, if mice underwent the stress of chronic social defeat and were treated with Lactobacillus rhamnosus JB-1 or sertraline, there was an increase in both aggressor avoidance and reduced sociability, suggesting a possible detrimental effect [189]. In human subjects, a study by Gocan et al (2012) looked at individuals who developed a combat-related PTSD and monitored symptoms after consumption of fermented soy products. After 6 months of daily ingestion, there was a reduction of anxiety, derealization/detachment, as well as insomnia [190]. Many of the participants were noted to develop flu-like symptoms, skin infections, and even herpes labialis during the beginning of the trial. Further research is needed to look into the potential role the gut biotics may have in the management of patients with PTSD.
OCD
OCD is characterized by the presence of obsessive thoughts that are persistent and intrusive, along with compulsions which are consider repetitive behaviors that are done in response to these obsessions [191]. With regard to treatment, cognitive behavioral therapy (CBT) and pharmacological management with selective serotonins reuptake inhibitors (SSRIs), either alone or in combination, are typically used. When examining the role of gut biotics in the treatment of this condition, a study by Kantak et al (2014) induced OCD-like behavior in mice using RU24960 (a 5HT1A1B receptor agonist) and treated them with either fluoxetine, or a probiotic (Lactobacillus rhamnosus GG), or a neutral substance. The mice treated with the probiotic or fluoxetine showed a reduction in OCD-like behaviors [192]. In another preclinical study, mice were injected with quinpirole hydrochloride (a D2/D3 dopamine agonist) to induce OCD-like behavior [193]. The mice treated with Lactobacillus casei shirota, fluoxetine, or a combination of the two showed improvements in OCD behaviors [193].
One clinical study showed that the administration of Lactobacillus helviticus and Bifidobacterium longum helps to reduce the obsessive-compulsive scores in healthy volunteers [194]. Thus, there is some evidence that probiotics may play a role in the treatment of OCD; however, the research is in its infancy at this time to draw any significant conclusions.
Schizophrenia/schizoaffective
Patients diagnosed with schizophrenia often experience both positive and negative symptoms. The positive symptoms are often characterized by delusions, hallucinations, and disorganized thoughts/speech. In contrast, they may present with negative symptoms including avolition, decreased emotional expression, and a decline in function, both social and occupational. Recent studies have examined the role of the gut microbiome in the disease pathogenesis of schizophrenia [195-197]. A study by Olde et al. (2018) found an increase in microbial diversity in the blood of schizophrenia patients compared to healthy controls [198]. This may suggest influence of gut permeability leading to increased diversity in the blood microbiota in these patients. Thus, treatment of the gut microbiome in schizophrenia patients may be another target in their management, and probiotics may play a role as an adjuvant to conventional antipsychotic therapy.
An animal study using an MIA mouse model of schizophrenia found that the probiotic B. fragilis helped to restore gut permeability, gene expression and interleukin (IL)-6 in the colon, and showed behavioral improvement related to anxiety-like behavior while no change was seen in the sociability of the mice [199].
Patients with schizophrenia tend to have a high prevalence of GI symptoms, which may be related to antipsychotic treatment [200]. Probiotics have been shown to help with constipation [201] that may be seen with some schizophrenia patients due to their treatment. Based on the current evidence, there was no significant change in the positive or negative symptoms of schizophrenia patients treated with probiotic supplementation [202-204]. A case report showed improvement in negative symptoms after 30 days of probiotic treatment [205]. In a study by Okubo et al (2019), patients with schizophrenia receiving a probiotic Bifidobacteriam breve A-1 strain had improved anxiety and depressive symptoms [206]. The use of probiotics can relieve GI discomfort in male schizophrenia patients and also lower Candida albicans antibodies; these antibodies have been found to be elevated in patients with GI afflictions [202]. Immunomodulatory effects have been observed in treatment-resistant patients receiving probiotics in combination with atypical antipsychotics [203]. Further research is needed to examine the role of probiotics in the treatment regimen for patients with schizophrenia.
BP
BP is a chronic mental health condition where patients may experience changes in mood and energy level. Patients with BP have been found to have an altered gut microbiome compared to healthy controls [207, 208]. In particular BP patients have decreased levels of Faecalibacterium, which has been associated with depressive symptoms and often seen in patients with MDD and IBS [209, 210]. Individuals with BP often experience diarrhea and satiety, both of which can be improved with probiotics [211].
Some clinical studies showed that patients with BP, who were treated with probiotic supplementation, had a lower rate of rehospitalization following a recent discharge for mania [212]. It was also observed that individuals with BP treated with probiotics had improved cognitive function [213]. Currently, the research is limited, in this disorder with gut biotics.
Conclusions
Evidence has clearly indicated that gut dysbiosis plays a role in neurocognitive and psychiatric disorders. GM can be administered as gut biotics, dietary interventions and by FMT. Gut biotics are considered as a medicine for many diseases (panpharmacon) like GI and metabolic diseases. Growing evidence points out that it can be helpful with mental health disorders (Supplementary Material 5, www.jocmr.org). It is considered as a bio-therapy, given as food and pharmaceuticals. Food industry uses gut biotics as functional foods and nutraceutical supplements. Changing food behaviors is a desirable direction for gut bacterial management. Our traditional understanding of pharmacokinetics and pharmacodynamics may be altered by GM interactions.
Relationship between gut biotics and improving cognitive and mental health functions is an exciting new area of research. Current data from research studies showed the potential of treating and preventing both neurocognitive and some psychiatric disorders. Gut biotics like probiotics, prebiotics and synbiotics have some benefit in AD and MDD, but more longitudinal studies are needed. To our knowledge, there are no interventional studies using postbiotics, paraprobiotics and proteobiotics on neurocognitive disorders and mental illness. Microbial therapeutics using natural and genetically engineered technology need further research for the management of these conditions. Some studies have pointed out that gut microbe-based therapeutic interventions along with current psychological treatments may have the potential of synergistic effect and have the potential as an adjuvant therapy of mental health disorders. Even though the overall safety seems to be good, caution is needed to watch for any known and unknown side effects as well as the need for risk benefit analysis with certain ill populations. Since some gut biotic products have active substance that is made by a living organism, it should be considered like any other bio-therapeutic intervention, and the risk-benefit ratio should be considered in each patient. Evidence is also pointing out life style factors also influence gut microbes and indirectly microbial therapeutics. A large gap exists with healthcare providers about the understanding and use of gut biotics.
Future research
More clinical research is needed with microbial therapeutics to show evidence that can help to improve balance with mental health. Longitudinal and RCT studies with reproducibility of the results are warranted. For the translation of these findings into clinical practice, further research is needed with good regulatory framework.
Supplementary Material
Types of Gut Biotics.
Methods for the Identification of Gut Biotics.
Selected Animal Studies Exploring the Role of Gut Biotics on Neurocognitive and Mental Health Conditions.
Selected Human Studies Examining Gut Biotics in Neurocognitive and Mental Health Conditions.
Summary of Results From Both Animal and Human Models About the Role of Gut Biotics in Mental Health Conditions.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
The authors declare no potential conflict of interest.
Author Contributions
Both the authors contributed to the conception and drafting of this manuscript as well as revising it. The final draft is approved by both the authors for consideration of publication.
Data Availability
The authors declare that data supporting the findings of this study are available within the article and its supplementary file.
References
- 1.Baquero F, Nombela C. The microbiome as a human organ. Clin Microbiol Infect. 2012;18(Suppl 4):2–4. doi: 10.1111/j.1469-0691.2012.03916.x. [DOI] [PubMed] [Google Scholar]
- 2.Marchesi JR, Ravel J. The vocabulary of microbiome research: a proposal. Microbiome. 2015;3:31. doi: 10.1186/s40168-015-0094-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Claesson MJ, Cusack S, O'Sullivan O, Greene-Diniz R, de Weerd H, Flannery E, Marchesi JR. et al. Composition, variability, and temporal stability of the intestinal microbiota of the elderly. Proc Natl Acad Sci U S A. 2011;108(Suppl 1):4586–4591. doi: 10.1073/pnas.1000097107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carding S, Verbeke K, Vipond DT, Corfe BM, Owen LJ. Dysbiosis of the gut microbiota in disease. Microb Ecol Health Dis. 2015;26:26191. doi: 10.3402/mehd.v26.26191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bik EM, Ugalde JA, Cousins J, Goddard AD, Richman J, Apte ZS. Microbial biotransformations in the human distal gut. Br J Pharmacol. 2018;175(24):4404–4414. doi: 10.1111/bph.14085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bambury A, Sandhu K, Cryan JF, Dinan TG. Finding the needle in the haystack: systematic identification of psychobiotics. Br J Pharmacol. 2018;175(24):4430–4438. doi: 10.1111/bph.14127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mayer EA, Tillisch K, Gupta A. Gut/brain axis and the microbiota. J Clin Invest. 2015;125(3):926–938. doi: 10.1172/JCI76304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kang SS, Jeraldo PR, Kurti A, Miller ME, Cook MD, Whitlock K, Goldenfeld N. et al. Diet and exercise orthogonally alter the gut microbiome and reveal independent associations with anxiety and cognition. Mol Neurodegener. 2014;9:36. doi: 10.1186/1750-1326-9-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, Ling AV. et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505(7484):559–563. doi: 10.1038/nature12820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khoruts A. Targeting the microbiome: from probiotics to fecal microbiota transplantation. Genome Med. 2018;10(1):80. doi: 10.1186/s13073-018-0592-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dinan TG, Stanton C, Cryan JF. Psychobiotics: a novel class of psychotropic. Biol Psychiatry. 2013;74(10):720–726. doi: 10.1016/j.biopsych.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 12.Sarkar A, Lehto SM, Harty S, Dinan TG, Cryan JF, Burnet PWJ. Psychobiotics and the Manipulation of Bacteria-Gut-Brain Signals. Trends Neurosci. 2016;39(11):763–781. doi: 10.1016/j.tins.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morelli L, Capurso L. FAO/WHO guidelines on probiotics: 10 years later. J Clin Gastroenterol. 2012;46(Suppl):S1–2. doi: 10.1097/MCG.0b013e318269fdd5. [DOI] [PubMed] [Google Scholar]
- 14.Fijan S. Microorganisms with claimed probiotic properties: an overview of recent literature. Int J Environ Res Public Health. 2014;11(5):4745–4767. doi: 10.3390/ijerph110504745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Min M, Bunt CR, Mason SL, Hussain MA. Non-dairy probiotic food products: an emerging group of functional foods. Crit Rev Food Sci Nutr. 2019;59(16):2626–2641. doi: 10.1080/10408398.2018.1462760. [DOI] [PubMed] [Google Scholar]
- 16.Aggarwal N, Breedon AME, Davis CM, Hwang IY, Chang MW. Engineering probiotics for therapeutic applications: recent examples and translational outlook. Curr Opin Biotechnol. 2020;65:171–179. doi: 10.1016/j.copbio.2020.02.016. [DOI] [PubMed] [Google Scholar]
- 17.Rijkers GT, Bengmark S, Enck P, Haller D, Herz U, Kalliomaki M, Kudo S. et al. Guidance for substantiating the evidence for beneficial effects of probiotics: current status and recommendations for future research. J Nutr. 2010;140(3):671S–676S. doi: 10.3945/jn.109.113779. [DOI] [PubMed] [Google Scholar]
- 18. FAO/WHO. Guidelines for the evaluation of probiotics in food. Food Nutr. Pap. 2002. https://www.who.int/foodsafety/fs_management/en/probiotic_guidelines.pdf.
- 19.Lestin F, Pertschy A, Rimek D. [Fungemia after oral treatment with Saccharomyces boulardii in a patient with multiple comorbidities] Dtsch Med Wochenschr. 2003;128(48):2531–2533. doi: 10.1055/s-2003-44948. [DOI] [PubMed] [Google Scholar]
- 20.Santino I, Alari A, Bono S, Teti E, Marangi M, Bernardini A, Magrini L. et al. Saccharomyces cerevisiae fungemia, a possible consequence of the treatment of Clostridium difficile colitis with a probioticum. Int J Immunopathol Pharmacol. 2014;27(1):143–146. doi: 10.1177/039463201402700120. [DOI] [PubMed] [Google Scholar]
- 21.Presterl E, Kneifel W, Mayer HK, Zehetgruber M, Makristathis A, Graninger W. Endocarditis by Lactobacillus rhamnosus due to yogurt ingestion? Scand J Infect Dis. 2001;33(9):710–714. doi: 10.1080/00365540110026953. [DOI] [PubMed] [Google Scholar]
- 22.Oggioni MR, Pozzi G, Valensin PE, Galieni P, Bigazzi C. Recurrent septicemia in an immunocompromised patient due to probiotic strains of Bacillus subtilis. J Clin Microbiol. 1998;36(1):325–326. doi: 10.1128/JCM.36.1.325-326.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Besselink MG, Timmerman HM, Buskens E, Nieuwenhuijs VB, Akkermans LM, Gooszen HG, Dutch Acute Pancreatitis Study G. Probiotic prophylaxis in patients with predicted severe acute pancreatitis (PROPATRIA): design and rationale of a double-blind, placebo-controlled randomised multicenter trial [ISRCTN38327949] BMC Surg. 2004;4:12. doi: 10.1186/1471-2482-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van den Nieuwboer M, Brummer RJ, Guarner F, Morelli L, Cabana M, Claasen E. The administration of probiotics and synbiotics in immune compromised adults: is it safe? Benef Microbes. 2015;6(1):3–17. doi: 10.3920/BM2014.0079. [DOI] [PubMed] [Google Scholar]
- 25.Didari T, Solki S, Mozaffari S, Nikfar S, Abdollahi M. A systematic review of the safety of probiotics. Expert Opin Drug Saf. 2014;13(2):227–239. doi: 10.1517/14740338.2014.872627. [DOI] [PubMed] [Google Scholar]
- 26.Hojsak I, Fabiano V, Pop TL, Goulet O, Zuccotti GV, Cokugras FC, Pettoello-Mantovani M. et al. Guidance on the use of probiotics in clinical practice in children with selected clinical conditions and in specific vulnerable groups. Acta Paediatr. 2018;107(6):927–937. doi: 10.1111/apa.14270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boyle RJ, Robins-Browne RM, Tang ML. Probiotic use in clinical practice: what are the risks? Am J Clin Nutr. 2006;83(6):1256–1264. doi: 10.1093/ajcn/83.6.1256. quiz 1446-1257. [DOI] [PubMed] [Google Scholar]
- 28.Manzanares W, Lemieux M, Langlois PL, Wischmeyer PE. Probiotic and synbiotic therapy in critical illness: a systematic review and meta-analysis. Crit Care. 2016;19:262. doi: 10.1186/s13054-016-1434-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Batra P, Soni KD, Mathur P. Efficacy of probiotics in the prevention of VAP in critically ill ICU patients: an updated systematic review and meta-analysis of randomized control trials. J Intensive Care. 2020;8:81. doi: 10.1186/s40560-020-00487-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fan QL, Yu XM, Liu QX, Yang W, Chang Q, Zhang YP. Synbiotics for prevention of ventilator-associated pneumonia: a probiotics strain-specific network meta-analysis. J Int Med Res. 2019;47(11):5349–5374. doi: 10.1177/0300060519876753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gibson GR, Roberfroid MB. Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J Nutr. 1995;125(6):1401–1412. doi: 10.1093/jn/125.6.1401. [DOI] [PubMed] [Google Scholar]
- 32.Gibson GR, Scott KP, Rastall RA, Tuohy KM, Hotchkiss A, Dubert-Ferrandon A, Gareau M. et al. Dietary prebiotics: Current status and new definition. IFIS Functional Foods Bulletin. 2010;7(1):1–19. doi: 10.1616/1476-2137.15880. [DOI] [Google Scholar]
- 33.Davani-Davari D, Negahdaripour M, Karimzadeh I, Seifan M, Mohkam M, Masoumi SJ, Berenjian A. et al. Prebiotics: definition, types, sources, mechanisms, and clinical applications. Foods. 2019;8(3):92. doi: 10.3390/foods8030092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gibson GR, Hutkins R, Sanders ME, Prescott SL, Reimer RA, Salminen SJ, Scott K. et al. Expert consensus document: the International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat Rev Gastroenterol Hepatol. 2017;14(8):491–502. doi: 10.1038/nrgastro.2017.75. [DOI] [PubMed] [Google Scholar]
- 35.Pandey KR, Naik SR, Vakil BV. Probiotics, prebiotics and synbiotics- a review. J Food Sci Technol. 2015;52(12):7577–7587. doi: 10.1007/s13197-015-1921-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Franco-Robles E, Lopez MG. Implication of fructans in health: immunomodulatory and antioxidant mechanisms. ScientificWorldJournal. 2015;2015:289267. doi: 10.1155/2015/289267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bode L. Human milk oligosaccharides: prebiotics and beyond. Nutr Rev. 2009;67(Suppl 2):S183–191. doi: 10.1111/j.1753-4887.2009.00239.x. [DOI] [PubMed] [Google Scholar]
- 38.Su P, Henriksson A, Mitchell H. Prebiotics enhance survival and prolong the retention period of specific probiotic inocula in an in vivo murine model. J Appl Microbiol. 2007;103(6):2392–2400. doi: 10.1111/j.1365-2672.2007.03469.x. [DOI] [PubMed] [Google Scholar]
- 39.Gay-Crosier F, Schreiber G, Hauser C. Anaphylaxis from inulin in vegetables and processed food. N Engl J Med. 2000;342(18):1372. doi: 10.1056/NEJM200005043421814. [DOI] [PubMed] [Google Scholar]
- 40.Soh JY, Huang CH, Chiang WC, Llanora GV, Lee AJ, Loh W, Chin YL. et al. Anaphylaxis to galacto-oligosaccharides - an evaluation in an atopic population in Singapore. Allergy. 2015;70(8):1020–1023. doi: 10.1111/all.12645. [DOI] [PubMed] [Google Scholar]
- 41.Bomba A, Nemcova R, Mudronova D, Guba P. The possibilities of potentiating the efficacy of probiotics. Trends in Food Science & Technology. 2002;13(4):121–126. doi: 10.1016/S0924-2244(02)00129-2. [DOI] [Google Scholar]
- 42.Markowiak P, Slizewska K. Effects of Probiotics, Prebiotics, and Synbiotics on Human Health. Nutrients. 2017;9(9):1021. doi: 10.3390/nu9091021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Adebola OO, Corcoran O, Morgan WA. Synbiotics: the impact of potential prebiotics inulin, lactulose and lactobionic acid on the survival and growth of lactobacilli probiotics. Journal of Functional Foods. 2014;10:75–84. doi: 10.1016/j.jff.2014.05.010. [DOI] [Google Scholar]
- 44.Bafeta A, Koh M, Riveros C, Ravaud P. Harms reporting in randomized controlled trials of interventions aimed at modifying microbiota: a systematic review. Ann Intern Med. 2018;169(4):240–247. doi: 10.7326/M18-0343. [DOI] [PubMed] [Google Scholar]
- 45.Martin R, Langella P. Emerging Health concepts in the probiotics field: streamlining the definitions. Front Microbiol. 2019;10:1047. doi: 10.3389/fmicb.2019.01047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barros CP, Guimaraes JT, Esmerino EA, Duarte MCK, Silva MC, Silva R, Ferreira BM. et al. Paraprobiotics and postbiotics: Concepts and potential applications in dairy products. Current Opinion in Food Science. 2020;32:1–8. doi: 10.1016/j.cofs.2019.12.003. [DOI] [Google Scholar]
- 47.Molaee Parvarei M, Fazeli MR, Mortazavian AM, Sarem Nezhad S, Mortazavi SA, Golabchifar AA, Khorshidian N. Comparative effects of probiotic and paraprobiotic addition on microbiological, biochemical and physical properties of yogurt. Food Res Int. 2021;140:110030. doi: 10.1016/j.foodres.2020.110030. [DOI] [PubMed] [Google Scholar]
- 48.Collado MC, Vinderola G, Salminen S. Postbiotics: facts and open questions. A position paper on the need for a consensus definition. Benef Microbes. 2019;10(7):711–719. doi: 10.3920/BM2019.0015. [DOI] [PubMed] [Google Scholar]
- 49.Aguilar-Toala JE, Garcia-Varela R, Garcia HS, Mata-Haro V, Gonzalez-Cordova AF, Vallejo-Cordoba B, Hernandez-Mendozaa A. Postbiotics: An evolving term within the functional foods field. Trends in Food Science & Technology. 2018;75:105–114. doi: 10.1016/j.tifs.2018.03.009. [DOI] [Google Scholar]
- 50.de Almada CN, Almada CN, Martinez RCR, Sant'Ana AS. Paraprobiotics: Evidences on their ability to modify biological responses, inactivation methods and perspectives on their application in foods. Trends in Food Science & Technology. 2016;58:96–114. doi: 10.1016/j.tifs.2016.09.011. [DOI] [Google Scholar]
- 51.Tarsillo B, Priefer R. Proteobiotics as a new antimicrobial therapy. Microb Pathog. 2020;142:104093. doi: 10.1016/j.micpath.2020.104093. [DOI] [PubMed] [Google Scholar]
- 52.Medellin-Pena MJ, Griffiths MW. Effect of molecules secreted by Lactobacillus acidophilus strain La-5 on Escherichia coli O157:H7 colonization. Appl Environ Microbiol. 2009;75(4):1165–1172. doi: 10.1128/AEM.01651-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Klaassen CD, Cui JY. Review: Mechanisms of How the Intestinal Microbiota Alters the Effects of Drugs and Bile Acids. Drug Metab Dispos. 2015;43(10):1505–1521. doi: 10.1124/dmd.115.065698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Roueche E, Serris E, Thomas G, Perier-Camby L. Influence of temperature on the compaction of an organic powder and the mechanical strength of tablets. Powder Technology. 2006;162(2):138–144. doi: 10.1016/j.powtec.2005.12.005. [DOI] [Google Scholar]
- 55.Govender M, Choonara YE, Kumar P, du Toit LC, van Vuuren S, Pillay V. A review of the advancements in probiotic delivery: Conventional vs. non-conventional formulations for intestinal flora supplementation. AAPS PharmSciTech. 2014;15(1):29–43. doi: 10.1208/s12249-013-0027-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bolla PA, Serradell Mde L, de Urraza PJ, De Antoni GL. Effect of freeze-drying on viability and in vitro probiotic properties of a mixture of lactic acid bacteria and yeasts isolated from kefir. J Dairy Res. 2011;78(1):15–22. doi: 10.1017/S0022029910000610. [DOI] [PubMed] [Google Scholar]
- 57.Bezkorovainy A. Probiotics: determinants of survival and growth in the gut. Am J Clin Nutr. 2001;73(2 Suppl):399S–405S. doi: 10.1093/ajcn/73.2.399s. [DOI] [PubMed] [Google Scholar]
- 58.Marteau P, Shanahan F. Basic aspects and pharmacology of probiotics: an overview of pharmacokinetics, mechanisms of action and side-effects. Best Pract Res Clin Gastroenterol. 2003;17(5):725–740. doi: 10.1016/S1521-6918(03)00055-6. [DOI] [PubMed] [Google Scholar]
- 59.Cohen LJ, Esterhazy D, Kim SH, Lemetre C, Aguilar RR, Gordon EA, Pickard AJ. et al. Commensal bacteria make GPCR ligands that mimic human signalling molecules. Nature. 2017;549(7670):48–53. doi: 10.1038/nature23874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cryan JF, Clarke G, Dinan TG, Schellekens H. A microbial drugstore for motility. Cell Host Microbe. 2018;23(6):691–692. doi: 10.1016/j.chom.2018.05.020. [DOI] [PubMed] [Google Scholar]
- 61.Stilling RM, van de Wouw M, Clarke G, Stanton C, Dinan TG, Cryan JF. The neuropharmacology of butyrate: The bread and butter of the microbiota-gut-brain axis? Neurochem Int. 2016;99:110–132. doi: 10.1016/j.neuint.2016.06.011. [DOI] [PubMed] [Google Scholar]
- 62.Lyte M. Probiotics function mechanistically as delivery vehicles for neuroactive compounds: Microbial endocrinology in the design and use of probiotics. Bioessays. 2011;33(8):574–581. doi: 10.1002/bies.201100024. [DOI] [PubMed] [Google Scholar]
- 63.Rizkallah MR, Saad R, Aziz R. The human microbiome project, personalized medicine and the birth of pharmacomicrobiomics. Current Pharmacogenomics and Personalized Medicine. 2010;8(3):182–193. doi: 10.2174/187569210792246326. [DOI] [Google Scholar]
- 64.Haiser HJ, Turnbaugh PJ. Developing a metagenomic view of xenobiotic metabolism. Pharmacol Res. 2013;69(1):21–31. doi: 10.1016/j.phrs.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Walsh J, Griffin BT, Clarke G, Hyland NP. Drug-gut microbiota interactions: implications for neuropharmacology. Br J Pharmacol. 2018;175(24):4415–4429. doi: 10.1111/bph.14366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Erickson KL, Hubbard NE. Probiotic immunomodulation in health and disease. J Nutr. 2000;130(2S Suppl):403S–409S. doi: 10.1093/jn/130.2.403S. [DOI] [PubMed] [Google Scholar]
- 67.Wu R, Jeffrey M, Johnson-Henry K, Green-Johnson J, Sherman P. Impact of prebiotics, probiotics and gut derived metabolites on host immunity. LymphoSign Journal. 2016;4(1):1–24. doi: 10.14785/lymphosign-2016-0012.. doi: 10.14785/lymphosign-2016-0012.. [DOI] [Google Scholar]
- 68.Claassen E, Van Winsen R, Posno M, Boersma WJ. New and safe "oral" live vaccines based on lactobacillus. Adv Exp Med Biol. 1995;371B:1553–1558. [PubMed] [Google Scholar]
- 69.Gill HS. Probiotics to enhance anti-infective defences in the gastrointestinal tract. Best Pract Res Clin Gastroenterol. 2003;17(5):755–773. doi: 10.1016/S1521-6918(03)00074-X. [DOI] [PubMed] [Google Scholar]
- 70.Plaza-Diaz J, Ruiz-Ojeda FJ, Vilchez-Padial LM, Gil A. Evidence of the anti-inflammatory effects of probiotics and synbiotics in intestinal chronic diseases. Nutrients. 2017;9(6):555. doi: 10.3390/nu9060555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Goyal D, Ali SA, Singh RK. Emerging role of gut microbiota in modulation of neuroinflammation and neurodegeneration with emphasis on Alzheimer's disease. Prog Neuropsychopharmacol Biol Psychiatry. 2021;106:110112. doi: 10.1016/j.pnpbp.2020.110112. [DOI] [PubMed] [Google Scholar]
- 72.Pluta R, Ulamek-Koziol M, Januszewski S, Czuczwar SJ. Gut microbiota and pro/prebiotics in Alzheimer's disease. Aging (Albany NY) 2020;12(6):5539–5550. doi: 10.18632/aging.102930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dimidi E, Cox SR, Rossi M, Whelan K. Fermented foods: definitions and characteristics, impact on the gut microbiota and effects on gastrointestinal health and disease. Nutrients. 2019;11(8):1806. doi: 10.3390/nu11081806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Marco ML, Heeney D, Binda S, Cifelli CJ, Cotter PD, Foligne B, Ganzle M. et al. Health benefits of fermented foods: microbiota and beyond. Curr Opin Biotechnol. 2017;44:94–102. doi: 10.1016/j.copbio.2016.11.010. [DOI] [PubMed] [Google Scholar]
- 75.Taylor BC, Lejzerowicz F, Poirel M, Shaffer JP, Jiang L, Aksenov A, Litwin N. et al. Consumption of fermented foods is associated with systematic differences in the gut microbiome and metabolome. mSystems. 2020;5(2):e00901–19. doi: 10.1128/mSystems.00901-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ton AMM, Campagnaro BP, Alves GA, Aires R, Coco LZ, Arpini CM, Guerra EOT. et al. Oxidative stress and dementia in Alzheimer's patients: effects of synbiotic supplementation. Oxid Med Cell Longev. 2020;2020:2638703. doi: 10.1155/2020/2638703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mohammadi AA, Jazayeri S, Khosravi-Darani K, Solati Z, Mohammadpour N, Asemi Z, Adab Z. et al. The effects of probiotics on mental health and hypothalamic-pituitary-adrenal axis: A randomized, double-blind, placebo-controlled trial in petrochemical workers. Nutr Neurosci. 2016;19(9):387–395. doi: 10.1179/1476830515Y.0000000023. [DOI] [PubMed] [Google Scholar]
- 78.Kato-Kataoka A, Nishida K, Takada M, Kawai M, Kikuchi-Hayakawa H, Suda K, Ishikawa H. et al. Fermented milk containing lactobacillus casei strain Shirota preserves the diversity of the gut microbiota and relieves abdominal dysfunction in healthy medical students exposed to academic stress. Appl Environ Microbiol. 2016;82(12):3649–3658. doi: 10.1128/AEM.04134-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Aslam H, Green J, Jacka FN, Collier F, Berk M, Pasco J, Dawson SL. Fermented foods, the gut and mental health: a mechanistic overview with implications for depression and anxiety. Nutr Neurosci. 2020;23(9):659–671. doi: 10.1080/1028415X.2018.1544332. [DOI] [PubMed] [Google Scholar]
- 80.Kobayashi Y, Kuhara T, Oki M, Xiao JZ. Effects of Bifidobacterium breve A1 on the cognitive function of older adults with memory complaints: a randomised, double-blind, placebo-controlled trial. Benef Microbes. 2019;10(5):511–520. doi: 10.3920/BM2018.0170. [DOI] [PubMed] [Google Scholar]
- 81.Xiao J, Katsumata N, Bernier F, Ohno K, Yamauchi Y, Odamaki T, Yoshikawa K. et al. Probiotic bifidobacterium breve in improving cognitive functions of older adults with suspected mild cognitive impairment: a randomized, double-blind, placebo-controlled trial. J Alzheimers Dis. 2020;77(1):139–147. doi: 10.3233/JAD-200488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hwang YH, Park S, Paik JW, Chae SW, Kim DH, Jeong DG, Ha E. et al. Efficacy and safety of lactobacillus plantarum C29-fermented soybean (DW2009) in individuals with mild cognitive impairment: a 12-week, multi-center, randomized, double-blind, placebo-controlled clinical trial. Nutrients. 2019;11(2):305. doi: 10.3390/nu11020305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Akbari E, Asemi Z, Daneshvar Kakhaki R, Bahmani F, Kouchaki E, Tamtaji OR, Hamidi GA. et al. Effect of probiotic supplementation on cognitive function and metabolic status in Alzheimer's disease: a randomized, double-blind and controlled trial. Front Aging Neurosci. 2016;8:256. doi: 10.3389/fnagi.2016.00256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tamtaji OR, Heidari-Soureshjani R, Mirhosseini N, Kouchaki E, Bahmani F, Aghadavod E, Tajabadi-Ebrahimi M. et al. Probiotic and selenium co-supplementation, and the effects on clinical, metabolic and genetic status in Alzheimer's disease: A randomized, double-blind, controlled trial. Clin Nutr. 2019;38(6):2569–2575. doi: 10.1016/j.clnu.2018.11.034. [DOI] [PubMed] [Google Scholar]
- 85.Den H, Dong X, Chen M, Zou Z. Efficacy of probiotics on cognition, and biomarkers of inflammation and oxidative stress in adults with Alzheimer's disease or mild cognitive impairment - a meta-analysis of randomized controlled trials. Aging (Albany NY) 2020;12(4):4010–4039. doi: 10.18632/aging.102810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lv T, Ye M, Luo F, Hu B, Wang A, Chen J, Yan J. et al. Probiotics treatment improves cognitive impairment in patients and animals: a systematic review and meta-analysis. Neurosci Biobehav Rev. 2021;120:159–172. doi: 10.1016/j.neubiorev.2020.10.027. [DOI] [PubMed] [Google Scholar]
- 87.Otaka M, Kikuchi-Hayakawa H, Ogura J, Ishikawa H, Yomogida Y, Ota M, Hidese S. et al. Effect of Lacticaseibacillus paracasei Strain Shirota on improvement in depressive symptoms, and its association with abundance of actinobacteria in gut microbiota. Microorganisms. 2021;9(5):1026. doi: 10.3390/microorganisms9051026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Akkasheh G, Kashani-Poor Z, Tajabadi-Ebrahimi M, Jafari P, Akbari H, Taghizadeh M, Memarzadeh MR. et al. Clinical and metabolic response to probiotic administration in patients with major depressive disorder: a randomized, double-blind, placebo-controlled trial. Nutrition. 2016;32(3):315–320. doi: 10.1016/j.nut.2015.09.003. [DOI] [PubMed] [Google Scholar]
- 89.Kazemi A, Noorbala AA, Azam K, Eskandari MH, Djafarian K. Effect of probiotic and prebiotic vs placebo on psychological outcomes in patients with major depressive disorder: a randomized clinical trial. Clin Nutr. 2019;38(2):522–528. doi: 10.1016/j.clnu.2018.04.010. [DOI] [PubMed] [Google Scholar]
- 90.Eskandarzadeh S, Effatpanah M, Khosravi-Darani K, Askari R, Hosseini AF, Reisian M, Jazayeri S. Efficacy of a multispecies probiotic as adjunctive therapy in generalized anxiety disorder: a double blind, randomized, placebo-controlled trial. Nutr Neurosci. 2021;24(2):102–108. doi: 10.1080/1028415X.2019.1598669. [DOI] [PubMed] [Google Scholar]
- 91.Shvachko NA, Loskutov IG, Semilet TV, Popov VS, Kovaleva ON, Konarev AV. Bioactive components in oat and barley grain as a promising breeding trend for functional food production. Molecules. 2021;26(8):2260. doi: 10.3390/molecules26082260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kennedy DO, Bonnlander B, Lang SC, Pischel I, Forster J, Khan J, Jackson PA. et al. Acute and chronic effects of green oat (Avena sativa) extract on cognitive function and mood during a laboratory stressor in healthy adults: a randomised, double-blind, placebo-controlled study in healthy humans. Nutrients. 2020;12(6):1598. doi: 10.3390/nu12061598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Alhassani W. The effects of a high fiber beta glucan Talbina meal on emotional well-being among Syrian refugees in metropolitan Detroit (P08-076-19) Curr Dev Nutr. 2019;3(Suppl 1):nzz044.P08-076-19. doi: 10.1093/cdn/nzz044.P08-076-19. [DOI] [Google Scholar]
- 94.Mottaghi T, Amirabdollahian F, Haghighatdoost F. Fruit and vegetable intake and cognitive impairment: a systematic review and meta-analysis of observational studies. Eur J Clin Nutr. 2018;72(10):1336–1344. doi: 10.1038/s41430-017-0005-x. [DOI] [PubMed] [Google Scholar]
- 95.Nurk E, Refsum H, Drevon CA, Tell GS, Nygaard HA, Engedal K, Smith AD. Cognitive performance among the elderly in relation to the intake of plant foods. The Hordaland Health Study. Br J Nutr. 2010;104(8):1190–1201. doi: 10.1017/S0007114510001807. [DOI] [PubMed] [Google Scholar]
- 96.Gehlich KH, Beller J, Lange-Asschenfeldt B, Kocher W, Meinke MC, Lademann J. Fruit and vegetable consumption is associated with improved mental and cognitive health in older adults from non-Western developing countries. Public Health Nutr. 2019;22(4):689–696. doi: 10.1017/S1368980018002525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kim CS, Byeon S, Shin DM. Sources of dietary fiber are differently associated with prevalence of depression. Nutrients. 2020;12(9):2813. doi: 10.3390/nu12092813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Xu H, Li S, Song X, Li Z, Zhang D. Exploration of the association between dietary fiber intake and depressive symptoms in adults. Nutrition. 2018;54:48–53. doi: 10.1016/j.nut.2018.03.009. [DOI] [PubMed] [Google Scholar]
- 99.Matison AP, Mather KA, Flood VM, Reppermund S. Associations between nutrition and the incidence of depression in middle-aged and older adults: A systematic review and meta-analysis of prospective observational population-based studies. Ageing Res Rev. 2021;70:101403. doi: 10.1016/j.arr.2021.101403. [DOI] [PubMed] [Google Scholar]
- 100.Desmedt O, Broers VJV, Zamariola G, Pachikian B, Delzenne N, Luminet O. Effects of prebiotics on affect and cognition in human intervention studies. Nutr Rev. 2019;77(2):81–95. doi: 10.1093/nutrit/nuy052. [DOI] [PubMed] [Google Scholar]
- 101.Smith AP, Sutherland D, Hewlett P. An investigation of the acute effects of oligofructose-enriched inulin on subjective wellbeing, mood and cognitive performance. Nutrients. 2015;7(11):8887–8896. doi: 10.3390/nu7115441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Marx W, Scholey A, Firth J, D'Cunha NM, Lane M, Hockey M, Ashton MM. et al. Prebiotics, probiotics, fermented foods and cognitive outcomes: a meta-analysis of randomized controlled trials. Neurosci Biobehav Rev. 2020;118:472–484. doi: 10.1016/j.neubiorev.2020.07.036. [DOI] [PubMed] [Google Scholar]
- 103.Halverson T, Alagiakrishnan K. Gut microbes in neurocognitive and mental health disorders. Ann Med. 2020;52(8):423–443. doi: 10.1080/07853890.2020.1808239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hort J, Valis M, Angelucci F. Administration of pre/probiotics with conventional drug treatment in Alzheimer's disease. Neural Regen Res. 2020;15(3):448–449. doi: 10.4103/1673-5374.266057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Abraham D, Feher J, Scuderi GL, Szabo D, Dobolyi A, Cservenak M, Juhasz J. et al. Exercise and probiotics attenuate the development of Alzheimer's disease in transgenic mice: Role of microbiome. Exp Gerontol. 2019;115:122–131. doi: 10.1016/j.exger.2018.12.005. [DOI] [PubMed] [Google Scholar]
- 106.Neufeld KA, Kang N, Bienenstock J, Foster JA. Effects of intestinal microbiota on anxiety-like behavior. Commun Integr Biol. 2011;4(4):492–494. doi: 10.4161/cib.15702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wang T, Hu X, Liang S, Li W, Wu X, Wang L, Jin F. Lactobacillus fermentum NS9 restores the antibiotic induced physiological and psychological abnormalities in rats. Benef Microbes. 2015;6(5):707–717. doi: 10.3920/BM2014.0177. [DOI] [PubMed] [Google Scholar]
- 108.Pistollato F, Iglesias RC, Ruiz R, Aparicio S, Crespo J, Lopez LD, Manna PP. et al. Nutritional patterns associated with the maintenance of neurocognitive functions and the risk of dementia and Alzheimer's disease: A focus on human studies. Pharmacol Res. 2018;131:32–43. doi: 10.1016/j.phrs.2018.03.012. [DOI] [PubMed] [Google Scholar]
- 109.Lukic I, Getselter D, Ziv O, Oron O, Reuveni E, Koren O, Elliott E. Antidepressants affect gut microbiota and Ruminococcus flavefaciens is able to abolish their effects on depressive-like behavior. Transl Psychiatry. 2019;9(1):133. doi: 10.1038/s41398-019-0466-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kao AC, Spitzer S, Anthony DC, Lennox B, Burnet PWJ. Prebiotic attenuation of olanzapine-induced weight gain in rats: analysis of central and peripheral biomarkers and gut microbiota. Transl Psychiatry. 2018;8(1):66. doi: 10.1038/s41398-018-0116-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lianov L, Johnson M. Physician competencies for prescribing lifestyle medicine. JAMA. 2010;304(2):202–203. doi: 10.1001/jama.2010.903. [DOI] [PubMed] [Google Scholar]
- 112.Firth J, Solmi M, Wootton RE, Vancampfort D, Schuch FB, Hoare E, Gilbody S. et al. A meta-review of "lifestyle psychiatry": the role of exercise, smoking, diet and sleep in the prevention and treatment of mental disorders. World Psychiatry. 2020;19(3):360–380. doi: 10.1002/wps.20773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Savin Z, Kivity S, Yonath H, Yehuda S. Smoking and the intestinal microbiome. Arch Microbiol. 2018;200(5):677–684. doi: 10.1007/s00203-018-1506-2. [DOI] [PubMed] [Google Scholar]
- 114.Gui X, Yang Z, Li MD. Effect of cigarette smoke on gut microbiota: state of knowledge. Front Physiol. 2021;12:673341. doi: 10.3389/fphys.2021.673341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lee SH, Yun Y, Kim SJ, Lee EJ, Chang Y, Ryu S, Shin H. et al. Association between cigarette smoking status and composition of gut microbiota: population-based cross-sectional study. J Clin Med. 2018;7(9):282. doi: 10.3390/jcm7090282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sublette MG, Cross TL, Korcarz CE, Hansen KM, Murga-Garrido SM, Hazen SL, Wang Z. et al. Effects of smoking and smoking cessation on the intestinal microbiota. J Clin Med. 2020;9(9):2963. doi: 10.3390/jcm9092963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Biedermann L, Zeitz J, Mwinyi J, Sutter-Minder E, Rehman A, Ott SJ, Steurer-Stey C. et al. Smoking cessation induces profound changes in the composition of the intestinal microbiota in humans. PLoS One. 2013;8(3):e59260. doi: 10.1371/journal.pone.0059260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ames NJ, Barb JJ, Schuebel K, Mudra S, Meeks BK, Tuason RTS, Brooks AT. et al. Longitudinal gut microbiome changes in alcohol use disorder are influenced by abstinence and drinking quantity. Gut Microbes. 2020;11(6):1608–1631. doi: 10.1080/19490976.2020.1758010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Stefanaki C, Mastorakos G, Chrousos GP. Gut microbiome-related diseases and therapies Cham. Springer International Publishing; 2021. Gut microbiome and mental stress-related disorders: the interplay of classic and microbial endocrinology; pp. 229–242. [DOI] [Google Scholar]
- 120.Wu SI, Wu CC, Tsai PJ, Cheng LH, Hsu CC, Shan IK, Chan PY. et al. Psychobiotic supplementation of PS128(TM) improves stress, anxiety, and insomnia in highly stressed information technology specialists: a pilot study. Front Nutr. 2021;8:614105. doi: 10.3389/fnut.2021.614105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Smith RP, Easson C, Lyle SM, Kapoor R, Donnelly CP, Davidson EJ, Parikh E. et al. Gut microbiome diversity is associated with sleep physiology in humans. PLoS One. 2019;14(10):e0222394. doi: 10.1371/journal.pone.0222394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Irwin C, McCartney D, Desbrow B, Khalesi S. Effects of probiotics and paraprobiotics on subjective and objective sleep metrics: a systematic review and meta-analysis. Eur J Clin Nutr. 2020;74(11):1536–1549. doi: 10.1038/s41430-020-0656-x. [DOI] [PubMed] [Google Scholar]
- 123.Clauss M, Gerard P, Mosca A, Leclerc M. Interplay Between Exercise and Gut Microbiome in the Context of Human Health and Performance. Front Nutr. 2021;8:637010. doi: 10.3389/fnut.2021.637010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sanchez-Villegas A, Toledo E, de Irala J, Ruiz-Canela M, Pla-Vidal J, Martinez-Gonzalez MA. Fast-food and commercial baked goods consumption and the risk of depression. Public Health Nutr. 2012;15(3):424–432. doi: 10.1017/S1368980011001856. [DOI] [PubMed] [Google Scholar]
- 125.Hu D, Cheng L, Jiang W. Sugar-sweetened beverages consumption and the risk of depression: A meta-analysis of observational studies. J Affect Disord. 2019;245:348–355. doi: 10.1016/j.jad.2018.11.015. [DOI] [PubMed] [Google Scholar]
- 126.Zhu C, Sawrey-Kubicek L, Beals E, Rhodes CH, Houts HE, Sacchi R, Zivkovic AM. Human gut microbiome composition and tryptophan metabolites were changed differently by fast food and Mediterranean diet in 4 days: a pilot study. Nutr Res. 2020;77:62–72. doi: 10.1016/j.nutres.2020.03.005. [DOI] [PubMed] [Google Scholar]
- 127.Spierling SR, Zorrilla EP. Don't stress about CRF: assessing the translational failures of CRF1antagonists. Psychopharmacology (Berl) 2017;234(9-10):1467–1481. doi: 10.1007/s00213-017-4556-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hoffmann DE, Fraser CM, Palumbo F, Ravel J, Rowthorn V, Schwartz J. Probiotics: achieving a better regulatory fit. Food Drug Law J. 2014;69(2):237–272. [PMC free article] [PubMed] [Google Scholar]
- 129.Chinna Meyyappan A, Forth E, Wallace CJK, Milev R. Effect of fecal microbiota transplant on symptoms of psychiatric disorders: a systematic review. BMC Psychiatry. 2020;20(1):299. doi: 10.1186/s12888-020-02654-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.D'Amato A, Di Cesare Mannelli L, Lucarini E, Man AL, Le Gall G, Branca JJV, Ghelardini C. et al. Faecal microbiota transplant from aged donor mice affects spatial learning and memory via modulating hippocampal synaptic plasticity- and neurotransmission-related proteins in young recipients. Microbiome. 2020;8(1):140. doi: 10.1186/s40168-020-00914-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sun J, Xu J, Ling Y, Wang F, Gong T, Yang C, Ye S. et al. Fecal microbiota transplantation alleviated Alzheimer's disease-like pathogenesis in APP/PS1 transgenic mice. Transl Psychiatry. 2019;9(1):189. doi: 10.1038/s41398-019-0525-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hazan S. Rapid improvement in Alzheimer's disease symptoms following fecal microbiota transplantation: a case report. J Int Med Res. 2020;48(6):300060520925930. doi: 10.1177/0300060520925930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zhang Y, Huang R, Cheng M, Wang L, Chao J, Li J, Zheng P. et al. Gut microbiota from NLRP3-deficient mice ameliorates depressive-like behaviors by regulating astrocyte dysfunction via circHIPK2. Microbiome. 2019;7(1):116. doi: 10.1186/s40168-019-0733-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Liu S, Guo R, Liu F, Yuan Q, Yu Y, Ren F. Gut microbiota regulates depression-like behavior in rats through the neuroendocrine-immune-mitochondrial pathway. Neuropsychiatr Dis Treat. 2020;16:859–869. doi: 10.2147/NDT.S243551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.De Palma G, Lynch MD, Lu J, Dang VT, Deng Y, Jury J, Umeh G. et al. Transplantation of fecal microbiota from patients with irritable bowel syndrome alters gut function and behavior in recipient mice. Sci Transl Med. 2017;9(379):eaaf6397. doi: 10.1126/scitranslmed.aaf6397. [DOI] [PubMed] [Google Scholar]
- 136.Huang HL, Chen HT, Luo QL, Xu HM, He J, Li YQ, Zhou YL. et al. Relief of irritable bowel syndrome by fecal microbiota transplantation is associated with changes in diversity and composition of the gut microbiota. J Dig Dis. 2019;20(8):401–408. doi: 10.1111/1751-2980.12756. [DOI] [PubMed] [Google Scholar]
- 137.Cai T, Shi X, Yuan LZ, Tang D, Wang F. Fecal microbiota transplantation in an elderly patient with mental depression. Int Psychogeriatr. 2019;31(10):1525–1526. doi: 10.1017/S1041610219000115. [DOI] [PubMed] [Google Scholar]
- 138.Kohler CA, Maes M, Slyepchenko A, Berk M, Solmi M, Lanctot KL, Carvalho AF. The gut-brain axis, including the microbiome, leaky gut and bacterial translocation: mechanisms and pathophysiological role in Alzheimer's disease. Curr Pharm Des. 2016;22(40):6152–6166. doi: 10.2174/1381612822666160907093807. [DOI] [PubMed] [Google Scholar]
- 139.Li B, He Y, Ma J, Huang P, Du J, Cao L, Wang Y. et al. Mild cognitive impairment has similar alterations as Alzheimer's disease in gut microbiota. Alzheimers Dement. 2019;15(10):1357–1366. doi: 10.1016/j.jalz.2019.07.002. [DOI] [PubMed] [Google Scholar]
- 140.Sachdev PS, Lipnicki DM, Crawford J, Reppermund S, Kochan NA, Trollor JN, Draper B. et al. Risk profiles for mild cognitive impairment vary by age and sex: the Sydney Memory and Ageing study. Am J Geriatr Psychiatry. 2012;20(10):854–865. doi: 10.1097/JGP.0b013e31825461b0. [DOI] [PubMed] [Google Scholar]
- 141.Tokuchi R, Hishikawa N, Kurata T, Sato K, Kono S, Yamashita T, Deguchi K. et al. Clinical and demographic predictors of mild cognitive impairment for converting to Alzheimer's disease and reverting to normal cognition. J Neurol Sci. 2014;346(1-2):288–292. doi: 10.1016/j.jns.2014.09.012. [DOI] [PubMed] [Google Scholar]
- 142.Prince M, Ali GC, Guerchet M, Prina AM, Albanese E, Wu YT. Recent global trends in the prevalence and incidence of dementia, and survival with dementia. Alzheimers Res Ther. 2016;8(1):23. doi: 10.1186/s13195-016-0188-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Nimgampalle M, Kuna Y. Anti-alzheimer properties of probiotic, lactobacillus plantarum MTCC 1325 in Alzheimer's disease induced albino rats. J Clin Diagn Res. 2017;11(8):KC01–KC05. doi: 10.7860/JCDR/2017/26106.10428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Angelucci F, Cechova K, Amlerova J, Hort J. Antibiotics, gut microbiota, and Alzheimer's disease. J Neuroinflammation. 2019;16(1):108. doi: 10.1186/s12974-019-1494-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.McDaniel MA, Maier SF, Einstein GO. "Brain-specific" nutrients: a memory cure? Nutrition. 2003;19(11-12):957–975. doi: 10.1016/S0899-9007(03)00024-8. [DOI] [PubMed] [Google Scholar]
- 146.Dos Santos Guilherme M, Todorov H, Osterhof C, Mollerke A, Cub K, Hankeln T, Gerber S. et al. Impact of acute and chronic amyloid-beta peptide exposure on gut microbial commensals in the mouse. Front Microbiol. 2020;11:1008. doi: 10.3389/fmicb.2020.01008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Fujii Y, Nguyen TTT, Fujimura Y, Kameya N, Nakamura S, Arakawa K, Morita H. Fecal metabolite of a gnotobiotic mouse transplanted with gut microbiota from a patient with Alzheimer's disease. Biosci Biotechnol Biochem. 2019;83(11):2144–2152. doi: 10.1080/09168451.2019.1644149. [DOI] [PubMed] [Google Scholar]
- 148.Zhao Y, Lukiw WJ. Microbiome-generated amyloid and potential impact on amyloidogenesis in Alzheimer's disease (AD) J Nat Sci. 2015;1(7):e138. [PMC free article] [PubMed] [Google Scholar]
- 149.Prinz M, Priller J. Microglia and brain macrophages in the molecular age: from origin to neuropsychiatric disease. Nat Rev Neurosci. 2014;15(5):300–312. doi: 10.1038/nrn3722. [DOI] [PubMed] [Google Scholar]
- 150.Erny D, Hrabe de Angelis AL, Jaitin D, Wieghofer P, Staszewski O, David E, Keren-Shaul H. et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat Neurosci. 2015;18(7):965–977. doi: 10.1038/nn.4030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Mancuso C, Santangelo R. Alzheimer's disease and gut microbiota modifications: The long way between preclinical studies and clinical evidence. Pharmacol Res. 2018;129:329–336. doi: 10.1016/j.phrs.2017.12.009. [DOI] [PubMed] [Google Scholar]
- 152.Bonfili L, Cecarini V, Berardi S, Scarpona S, Suchodolski JS, Nasuti C, Fiorini D. et al. Microbiota modulation counteracts Alzheimer's disease progression influencing neuronal proteolysis and gut hormones plasma levels. Sci Rep. 2017;7(1):2426. doi: 10.1038/s41598-017-02587-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Wang H, Lee IS, Braun C, Enck P. Effect of probiotics on central nervous system functions in animals and humans: a systematic review. J Neurogastroenterol Motil. 2016;22(4):589–605. doi: 10.5056/jnm16018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Tsai YL, Lin TL, Chang CJ, Wu TR, Lai WF, Lu CC, Lai HC. Probiotics, prebiotics and amelioration of diseases. J Biomed Sci. 2019;26(1):3. doi: 10.1186/s12929-018-0493-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Larroya-Garcia A, Navas-Carrillo D, Orenes-Pinero E. Impact of gut microbiota on neurological diseases: Diet composition and novel treatments. Crit Rev Food Sci Nutr. 2019;59(19):3102–3116. doi: 10.1080/10408398.2018.1484340. [DOI] [PubMed] [Google Scholar]
- 156.Wang X, Liu GJ, Gao Q, Li N, Wang RT. C-type lectin-like receptor 2 and zonulin are associated with mild cognitive impairment and Alzheimer's disease. Acta Neurol Scand. 2020;141(3):250–255. doi: 10.1111/ane.13196. [DOI] [PubMed] [Google Scholar]
- 157.Tillisch K, Labus J, Kilpatrick L, Jiang Z, Stains J, Ebrat B, Guyonnet D. et al. Consumption of fermented milk product with probiotic modulates brain activity. Gastroenterology. 2013;144(7):1394–1401. doi: 10.1053/j.gastro.2013.02.043. e1391-1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Saji N, Niida S, Murotani K, Hisada T, Tsuduki T, Sugimoto T, Kimura A. et al. Analysis of the relationship between the gut microbiome and dementia: a cross-sectional study conducted in Japan. Sci Rep. 2019;9(1):1008. doi: 10.1038/s41598-018-38218-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Kobayashi Y, Sugahara H, Shimada K, Mitsuyama E, Kuhara T, Yasuoka A, Kondo T. et al. Therapeutic potential of Bifidobacterium breve strain A1 for preventing cognitive impairment in Alzheimer's disease. Sci Rep. 2017;7(1):13510. doi: 10.1038/s41598-017-13368-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Luc M, Misiak B, Pawlowski M, Stanczykiewicz B, Zablocka A, Szczesniak D, Palega A. et al. Gut microbiota in dementia. Critical review of novel findings and their potential application. Prog Neuropsychopharmacol Biol Psychiatry. 2021;104:110039. doi: 10.1016/j.pnpbp.2020.110039. [DOI] [PubMed] [Google Scholar]
- 161.Kruger JF, Hillesheim E, Pereira A, Camargo CQ, Rabito EI. Probiotics for dementia: a systematic review and meta-analysis of randomized controlled trials. Nutr Rev. 2021;79(2):160–170. doi: 10.1093/nutrit/nuaa037. [DOI] [PubMed] [Google Scholar]
- 162.Sochocka M, Donskow-Lysoniewska K, Diniz BS, Kurpas D, Brzozowska E, Leszek J. The gut microbiome alterations and inflammation-driven pathogenesis of Alzheimer's disease-a critical review. Mol Neurobiol. 2019;56(3):1841–1851. doi: 10.1007/s12035-018-1188-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Cattaneo A, Cattane N, Galluzzi S, Provasi S, Lopizzo N, Festari C, Ferrari C. et al. Association of brain amyloidosis with pro-inflammatory gut bacterial taxa and peripheral inflammation markers in cognitively impaired elderly. Neurobiol Aging. 2017;49:60–68. doi: 10.1016/j.neurobiolaging.2016.08.019. [DOI] [PubMed] [Google Scholar]
- 164.Jiang C, Li G, Huang P, Liu Z, Zhao B. The gut microbiota and Alzheimer's disease. J Alzheimers Dis. 2017;58(1):1–15. doi: 10.3233/JAD-161141. [DOI] [PubMed] [Google Scholar]
- 165.Kao AC, Harty S, Burnet PW. The influence of prebiotics on neurobiology and behavior. Int Rev Neurobiol. 2016;131:21–48. doi: 10.1016/bs.irn.2016.08.007. [DOI] [PubMed] [Google Scholar]
- 166.Serra MC, Nocera JR, Kelleher JL, Addison O. Prebiotic intake in older adults: effects on brain function and behavior. Curr Nutr Rep. 2019;8(2):66–73. doi: 10.1007/s13668-019-0265-2. [DOI] [PubMed] [Google Scholar]
- 167.Chen D, Yang X, Yang J, Lai G, Yong T, Tang X, Shuai O. et al. Prebiotic effect of fructooligosaccharides from Morinda officinalis on Alzheimer's disease in rodent models by targeting the microbiota-gut-brain axis. Front Aging Neurosci. 2017;9:403. doi: 10.3389/fnagi.2017.00403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Sun J, Liu S, Ling Z, Wang F, Ling Y, Gong T, Fang N. et al. Fructooligosaccharides ameliorating cognitive deficits and neurodegeneration in APP/PS1 transgenic mice through modulating gut microbiota. J Agric Food Chem. 2019;67(10):3006–3017. doi: 10.1021/acs.jafc.8b07313. [DOI] [PubMed] [Google Scholar]
- 169.Hoffman JD, Yanckello LM, Chlipala G, Hammond TC, McCulloch SD, Parikh I, Sun S. et al. Dietary inulin alters the gut microbiome, enhances systemic metabolism and reduces neuroinflammation in an APOE4 mouse model. PLoS One. 2019;14(8):e0221828. doi: 10.1371/journal.pone.0221828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Jia S, Lu Z, Gao Z, An J, Wu X, Li X, Dai X. et al. Chitosan oligosaccharides alleviate cognitive deficits in an amyloid-beta1-42-induced rat model of Alzheimer's disease. Int J Biol Macromol. 2016;83:416–425. doi: 10.1016/j.ijbiomac.2015.11.011. [DOI] [PubMed] [Google Scholar]
- 171.Prasad S, Dhiman RK, Duseja A, Chawla YK, Sharma A, Agarwal R. Lactulose improves cognitive functions and health-related quality of life in patients with cirrhosis who have minimal hepatic encephalopathy. Hepatology. 2007;45(3):549–559. doi: 10.1002/hep.21533. [DOI] [PubMed] [Google Scholar]
- 172.Romo-Araiza A, Gutierrez-Salmean G, Galvan EJ, Hernandez-Frausto M, Herrera-Lopez G, Romo-Parra H, Garcia-Contreras V. et al. Probiotics and prebiotics as a therapeutic strategy to improve memory in a model of middle-aged rats. Front Aging Neurosci. 2018;10:416. doi: 10.3389/fnagi.2018.00416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Louzada ER, Ribeiro SML. Synbiotic supplementation, systemic inflammation, and symptoms of brain disorders in elders: A secondary study from a randomized clinical trial. Nutr Neurosci. 2020;23(2):93–100. doi: 10.1080/1028415X.2018.1477349. [DOI] [PubMed] [Google Scholar]
- 174.Wang PX, Deng XR, Zhang CH, Yuan HJ. Gut microbiota and metabolic syndrome. Chin Med J (Engl) 2020;133(7):808–816. doi: 10.1097/CM9.0000000000000696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Yu JY, Zhang B, Peng L, Wu CH, Cao H, Zhong JF, Hoffman J. et al. Repositioning of memantine as a potential novel therapeutic agent against meningitic E. coli-induced pathogenicities through disease-associated Alpha7 cholinergic pathway and RNA sequencing-based transcriptome analysis of host inflammatory responses. PLoS One. 2015;10(5):e0121911. doi: 10.1371/journal.pone.0121911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.G. B. D. Disease. Injury Incidence Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1789–1858. doi: 10.1016/S0140-6736(18)32279-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.McVey Neufeld KA, O'Mahony SM, Hoban AE, Waworuntu RV, Berg BM, Dinan TG, Cryan JF. Neurobehavioural effects of Lactobacillus rhamnosus GG alone and in combination with prebiotics polydextrose and galactooligosaccharide in male rats exposed to early-life stress. Nutr Neurosci. 2019;22(6):425–434. doi: 10.1080/1028415X.2017.1397875. [DOI] [PubMed] [Google Scholar]
- 178.Szklany K, Wopereis H, de Waard C, van Wageningen T, An R, van Limpt K, Knol J. et al. Supplementation of dietary non-digestible oligosaccharides from birth onwards improve social and reduce anxiety-like behaviour in male BALB/c mice. Nutr Neurosci. 2020;23(11):896–910. doi: 10.1080/1028415X.2019.1576362. [DOI] [PubMed] [Google Scholar]
- 179.Mika A, Gaffney M, Roller R, Hills A, Bouchet CA, Hulen KA, Thompson RS. et al. Feeding the developing brain: Juvenile rats fed diet rich in prebiotics and bioactive milk fractions exhibit reduced anxiety-related behavior and modified gene expression in emotion circuits. Neurosci Lett. 2018;677:103–109. doi: 10.1016/j.neulet.2018.01.052. [DOI] [PubMed] [Google Scholar]
- 180.Schmidt K, Cowen PJ, Harmer CJ, Tzortzis G, Errington S, Burnet PW. Prebiotic intake reduces the waking cortisol response and alters emotional bias in healthy volunteers. Psychopharmacology (Berl) 2015;232(10):1793–1801. doi: 10.1007/s00213-014-3810-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Ghorbani Z, Nazari S, Etesam F, Nourimajd S, Ahmadpanah M, Razeghi Jahromi S. The effect of synbiotic as an adjuvant therapy to fluoxetine in moderate depression: A randomized multicenter trial. Archives of Neuroscience. 2018;5(2):e60507. doi: 10.5812/archneurosci.60507. [DOI] [Google Scholar]
- 182.Silk DB, Davis A, Vulevic J, Tzortzis G, Gibson GR. Clinical trial: the effects of a trans-galactooligosaccharide prebiotic on faecal microbiota and symptoms in irritable bowel syndrome. Aliment Pharmacol Ther. 2009;29(5):508–518. doi: 10.1111/j.1365-2036.2008.03911.x. [DOI] [PubMed] [Google Scholar]
- 183.Azpiroz F, Dubray C, Bernalier-Donadille A, Cardot JM, Accarino A, Serra J, Wagner A. et al. Effects of scFOS on the composition of fecal microbiota and anxiety in patients with irritable bowel syndrome: a randomized, double blind, placebo controlled study. Neurogastroenterol Motil. 2017;29(2):e12911. doi: 10.1111/nmo.12911. [DOI] [PubMed] [Google Scholar]
- 184.Talbott SM, Talbott JA. Baker's yeast beta-glucan supplement reduces upper respiratory symptoms and improves mood state in stressed women. J Am Coll Nutr. 2012;31(4):295–300. doi: 10.1080/07315724.2012.10720441. [DOI] [PubMed] [Google Scholar]
- 185.Gruenwald J, Graubaum HJ, Harde A. Effect of a probiotic multivitamin compound on stress and exhaustion. Adv Ther. 2002;19(3):141–150. doi: 10.1007/BF02850270. [DOI] [PubMed] [Google Scholar]
- 186.Diop L, Guillou S, Durand H. Probiotic food supplement reduces stress-induced gastrointestinal symptoms in volunteers: a double-blind, placebo-controlled, randomized trial. Nutr Res. 2008;28(1):1–5. doi: 10.1016/j.nutres.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 187.Yang H, Zhao X, Tang S, Huang H, Zhao X, Ning Z, Fu X. et al. Probiotics reduce psychological stress in patients before laryngeal cancer surgery. Asia Pac J Clin Oncol. 2016;12(1):e92–96. doi: 10.1111/ajco.12120. [DOI] [PubMed] [Google Scholar]
- 188.Bharwani A, Mian MF, Surette MG, Bienenstock J, Forsythe P. Oral treatment with Lactobacillus rhamnosus attenuates behavioural deficits and immune changes in chronic social stress. BMC Med. 2017;15(1):7. doi: 10.1186/s12916-016-0771-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Liu Y, Steinhausen K, Bharwani A, Mian MF, McVey Neufeld KA, Forsythe P. Increased persistence of avoidance behaviour and social deficits with L.rhamnosus JB-1 or selective serotonin reuptake inhibitor treatment following social defeat. Sci Rep. 2020;10(1):13485. doi: 10.1038/s41598-020-69968-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Gocan AG, Bachg D, Schindler AE, Rohr UD. Balancing steroidal hormone cascade in treatment-resistant veteran soldiers with PTSD using a fermented soy product (FSWW08): a pilot study. Horm Mol Biol Clin Investig. 2012;10(3):301–314. doi: 10.1515/hmbci-2011-0135. [DOI] [PubMed] [Google Scholar]
- 191.American Psychiatric Association. Diagnostic and statistical manual of mental disorders (5th ed) Arlington, VA: American Psychiatric Association; 2013. [DOI] [Google Scholar]
- 192.Kantak PA, Bobrow DN, Nyby JG. Obsessive-compulsive-like behaviors in house mice are attenuated by a probiotic (Lactobacillus rhamnosus GG) Behav Pharmacol. 2014;25(1):71–79. doi: 10.1097/FBP.0000000000000013. [DOI] [PubMed] [Google Scholar]
- 193.Sanikhani NS, Modarressi MH, Jafari P, Vousooghi N, Shafei S, Akbariqomi M, Heidari R. et al. The effect of lactobacillus casei consumption in improvement of obsessive-compulsive disorder: an animal study. Probiotics Antimicrob Proteins. 2020;12(4):1409–1419. doi: 10.1007/s12602-020-09642-x. [DOI] [PubMed] [Google Scholar]
- 194.Messaoudi M, Violle N, Bisson JF, Desor D, Javelot H, Rougeot C. Beneficial psychological effects of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in healthy human volunteers. Gut Microbes. 2011;2(4):256–261. doi: 10.4161/gmic.2.4.16108. [DOI] [PubMed] [Google Scholar]
- 195.Zheng P, Zeng B, Liu M, Chen J, Pan J, Han Y, Liu Y. et al. The gut microbiome from patients with schizophrenia modulates the glutamate-glutamine-GABA cycle and schizophrenia-relevant behaviors in mice. Sci Adv. 2019;5(2):eaau8317. doi: 10.1126/sciadv.aau8317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Schwarz E, Maukonen J, Hyytiainen T, Kieseppa T, Oresic M, Sabunciyan S, Mantere O. et al. Analysis of microbiota in first episode psychosis identifies preliminary associations with symptom severity and treatment response. Schizophr Res. 2018;192:398–403. doi: 10.1016/j.schres.2017.04.017. [DOI] [PubMed] [Google Scholar]
- 197.Naidu AS, Bidlack WR, Clemens RA. Probiotic spectra of lactic acid bacteria (LAB) Crit Rev Food Sci Nutr. 1999;39(1):13–126. doi: 10.1080/10408699991279187. [DOI] [PubMed] [Google Scholar]
- 198.Olde Loohuis LM, Mangul S, Ori APS, Jospin G, Koslicki D, Yang HT, Wu T. et al. Transcriptome analysis in whole blood reveals increased microbial diversity in schizophrenia. Transl Psychiatry. 2018;8(1):96. doi: 10.1038/s41398-018-0107-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Hsiao EY, McBride SW, Hsien S, Sharon G, Hyde ER, McCue T, Codelli JA. et al. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell. 2013;155(7):1451–1463. doi: 10.1016/j.cell.2013.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.De Hert M, Dockx L, Bernagie C, Peuskens B, Sweers K, Leucht S, Tack J. et al. Prevalence and severity of antipsychotic related constipation in patients with schizophrenia: a retrospective descriptive study. BMC Gastroenterol. 2011;11:17. doi: 10.1186/1471-230X-11-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Dimidi E, Christodoulides S, Fragkos KC, Scott SM, Whelan K. The effect of probiotics on functional constipation in adults: a systematic review and meta-analysis of randomized controlled trials. Am J Clin Nutr. 2014;100(4):1075–1084. doi: 10.3945/ajcn.114.089151. [DOI] [PubMed] [Google Scholar]
- 202.Severance EG, Gressitt KL, Stallings CR, Katsafanas E, Schweinfurth LA, Savage CLG, Adamos MB. et al. Probiotic normalization of Candida albicans in schizophrenia: A randomized, placebo-controlled, longitudinal pilot study. Brain Behav Immun. 2017;62:41–45. doi: 10.1016/j.bbi.2016.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Tomasik J, Yolken RH, Bahn S, Dickerson FB. Immunomodulatory Effects of Probiotic Supplementation in Schizophrenia Patients: A Randomized, Placebo-Controlled Trial. Biomark Insights. 2015;10:47–54. doi: 10.4137/BMI.S22007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Dickerson FB, Stallings C, Origoni A, Katsafanas E, Savage CL, Schweinfurth LA, Goga J. et al. Effect of probiotic supplementation on schizophrenia symptoms and association with gastrointestinal functioning: a randomized, placebo-controlled trial. Prim Care Companion CNS Disord. 2014;16(1):PCC.13m01579. doi: 10.4088/PCC.13m01579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Nagamine T, Sato N, Seo G. Probiotics reduce negative symptoms of schizophrenia: A care report. International Medical Journal. 2012;19(1):67–68. [Google Scholar]
- 206.Okubo R, Koga M, Katsumata N, Odamaki T, Matsuyama S, Oka M, Narita H. et al. Effect of bifidobacterium breve A-1 on anxiety and depressive symptoms in schizophrenia: A proof-of-concept study. J Affect Disord. 2019;245:377–385. doi: 10.1016/j.jad.2018.11.011. [DOI] [PubMed] [Google Scholar]
- 207.Evans SJ, Bassis CM, Hein R, Assari S, Flowers SA, Kelly MB, Young VB. et al. The gut microbiome composition associates with bipolar disorder and illness severity. J Psychiatr Res. 2017;87:23–29. doi: 10.1016/j.jpsychires.2016.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Coello K, Hansen TH, Sorensen N, Munkholm K, Kessing LV, Pedersen O, Vinberg M. Gut microbiota composition in patients with newly diagnosed bipolar disorder and their unaffected first-degree relatives. Brain Behav Immun. 2019;75:112–118. doi: 10.1016/j.bbi.2018.09.026. [DOI] [PubMed] [Google Scholar]
- 209.Jiang H, Ling Z, Zhang Y, Mao H, Ma Z, Yin Y, Wang W. et al. Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav Immun. 2015;48:186–194. doi: 10.1016/j.bbi.2015.03.016. [DOI] [PubMed] [Google Scholar]
- 210.Sokol H, Seksik P, Furet JP, Firmesse O, Nion-Larmurier I, Beaugerie L, Cosnes J. et al. Low counts of Faecalibacterium prausnitzii in colitis microbiota. Inflamm Bowel Dis. 2009;15(8):1183–1189. doi: 10.1002/ibd.20903. [DOI] [PubMed] [Google Scholar]
- 211.Sherwin E, Sandhu KV, Dinan TG, Cryan JF. May the Force Be With You: The Light and Dark Sides of the Microbiota-Gut-Brain Axis in Neuropsychiatry. CNS Drugs. 2016;30(11):1019–1041. doi: 10.1007/s40263-016-0370-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Dickerson F, Adamos M, Katsafanas E, Khushalani S, Origoni A, Savage C, Schweinfurth L. et al. Adjunctive probiotic microorganisms to prevent rehospitalization in patients with acute mania: A randomized controlled trial. Bipolar Disord. 2018;20(7):614–621. doi: 10.1111/bdi.12652. [DOI] [PubMed] [Google Scholar]
- 213.Reininghaus EZ, Wetzlmair LC, Fellendorf FT, Platzer M, Queissner R, Birner A, Pilz R. et al. The impact of probiotic supplements on cognitive parameters in euthymic individuals with bipolar disorder: a pilot study. Neuropsychobiology. 2020;79:63–70. doi: 10.1159/000492537. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Types of Gut Biotics.
Methods for the Identification of Gut Biotics.
Selected Animal Studies Exploring the Role of Gut Biotics on Neurocognitive and Mental Health Conditions.
Selected Human Studies Examining Gut Biotics in Neurocognitive and Mental Health Conditions.
Summary of Results From Both Animal and Human Models About the Role of Gut Biotics in Mental Health Conditions.
Data Availability Statement
The authors declare that data supporting the findings of this study are available within the article and its supplementary file.