Abstract
Lewis basic salts promote benzyltrimethylsilane coupling with (hetero)aryl nitriles, sulfones and chlorides as a new route to 1,1-diarylalkanes. This method combines the substrate modularity and selectivity characteristic of cross-coupling with the practicality of a base-promoted protocol. In addition, a Lewis base strategy enables a complementary scope to existing methods, employs stable and easily prepared organosilanes and achieves selective arylation in the presence of acidic functional groups. The utility of this method is demonstrated by the synthesis of pharmaceutical analogues and its use in multicomponent reactions.
Graphical abstract
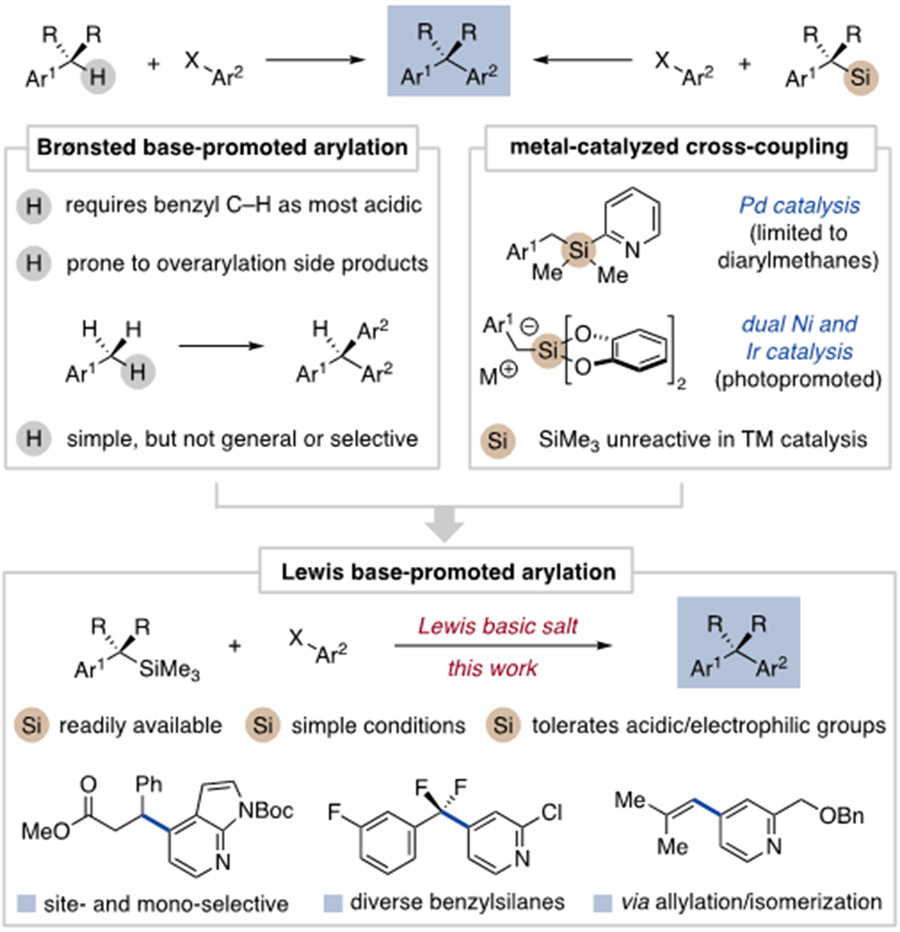
1,1-Diarylalkanes are valuable compounds often prepared by coupling functionalized benzylic reagents with aromatic electrophiles.1 In practice, the benzylic coupling partner and mechanism for achieving C–C bond formation define the scope and suitability of a given method. A widely used strategy is transition metal-catalyzed coupling of aryl (pseudo)halides with benzyl magnesium, zinc and boron compounds.2,3 This approach enables robust and predictable reactivity often at the expense of using reactive benzylic reagents prepared in situ. Significant recent effort has been focused on alternative coupling partners and strategies to increase the efficiency and scope of 1,1-diarylalkane synthesis.1,4-6
Benzylic deprotonation represents one such attractive strategy that generates carbanion intermediates for metal-catalyzed7 and catalyst-free8 reactions with aryl electrophiles (Figure 1, left). Direct deprotonative arylation is perhaps ideal as no catalyst is needed and only inexpensive reagents are used. However, this approach often leads to multiarylation side products and typically requires acidic pronucleophiles such as diarylmethanes.8 Deprotonative activation also limits the coupling scope to relatively simple substrates in which the most acidic proton is at the desired benzylic position.
Figure 1.
Background and motivation for Lewis base-promoted arylation reactions of organotrimethylsilanes.
We sought a new benzylic arylation method that blends the modularity and selectivity of cross-coupling with the practicality of a base-promoted protocol. This drew our attention toward Lewis base activation of Lewis acidic benzyl compounds, an underdeveloped approach for aryl Csp2-Csp3 coupling.9 In this regard, benzyltrimethylsilanes could be ideal coupling partners as they are air stable, non-hygroscopic and easily accessed in great diversity.10 Furthermore, distinct synthetic routes are available to complex benzyltrimethylsilanes that cannot be used to access analogous organometallic reagents.11 To date, the high stability of benzyltrimethylsilanes has rendered them unreactive in metal-catalyzed cross-coupling12 and thus their use in arylation reactions is limited.13 More specialized silanes are required to overcome this challenge in conjunction with Pd- and metallaphotoredox-catalyzed methodology (Figure 1, right).14
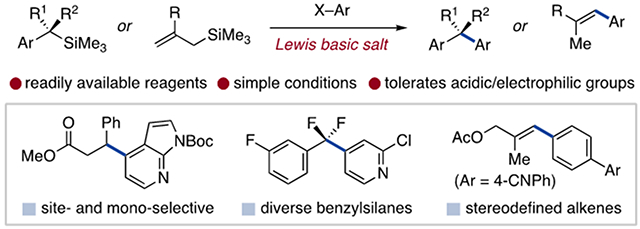
We herein report that Lewis basic salts promote the direct coupling of benzyltrimethylsilanes to a range of aromatic electrophiles (Figure 1, bottom). Benzylic arylation outcompetes potential anionic side reactions to enable monoselective coupling in the presence of acidic and electrophilic functional groups. This strategy can be extended to other organosilanes and reaction sequences, including the tandem arylation/isomerization of allylsilanes as a new route to alkenyl arenes. Thus, Lewis base-promoted arylation provides a practical coupling protocol with a reaction scope that complements established methods.
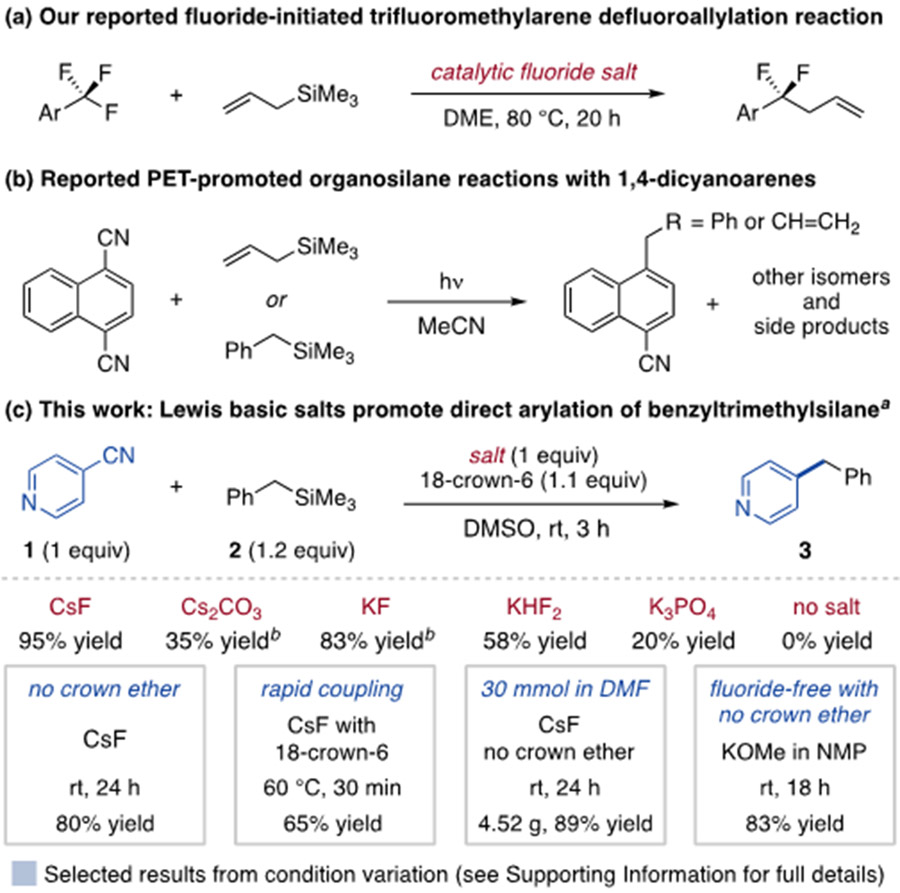
We recently reported the monoselective defluoroallylation of trifluoromethylarenes enabled by fluoride activation of allyltrimethylsilanes (Scheme 1a).15 This reaction is proposed to operate through an anionic allyl intermediate that undergoes single electron transfer (SET) to the trifluoromethylarene, leading to C–F bond cleavage and allylation of the resulting difluorobenzylic radical. This sequence has similarities to photoinduced electron transfer (PET) allylation of 1,4-dicyanoarenes using allyltrimethylsilane, namely SET prior to C–C bond formation.16 Benzyltrimethylsilane has also been examined in PET studies, although these reactions suffer from low regioselectivity and side product formation while requiring use of ultraviolet light (Scheme 1b).16a,17 Based on these precedents, we hypothesized Lewis base activation of organotrimethylsilanes could promote their direct coupling with aromatic electrophiles beyond trifluoromethylarenes.
Scheme 1. Reported organosilane reactions with aryl electrophiles and development of base-promoted arylation.
aYields determined by 1H NMR spectroscopy; 18-crown-6 added as a 1M solution in THF. 18 h time for salts other than CsF. bYields improve to 57% and 84% at 100 °C without 18-crown-6 for Cs2CO3 and KF, respectively.
To test this hypothesis, we examined the reaction of 4-cyanopyridine (1) with benzyltrimethylsilane (2) and found 18-crown-6-ligated cesium fluoride promotes monoselective coupling in 3 h at room temperature (rt) in DMSO (95% yield, Scheme 1c). Less basic anions, including carbonate, bifluoride and phosphate salts, promote arylation in moderate yields. Conditions in the boxes of Scheme 1c show the ability to adjust reaction parameters depending on priority, ranging from the use of fluoride-free salts without 18-crown-6 to short reaction times or large reaction scale.
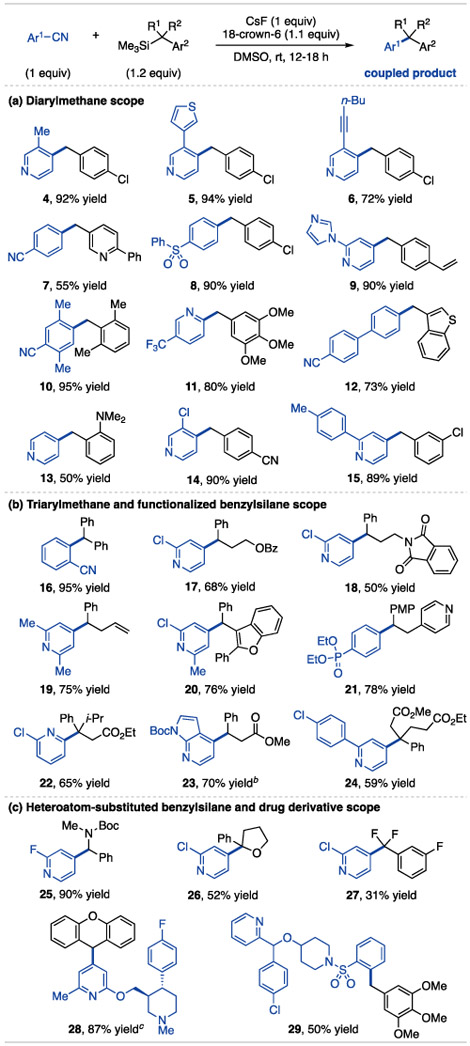
Table 1 contains a product scope for benzyltrimethylsilane coupling with cyanoarenes using CsF and 18-crown-6 in DMSO. Primary, secondary and tertiary benzylsilanes react with 2- and 4-cyanopyridines and electron-deficient cyanobenzenes (Table 1a and b). The products feature redox-active and electrophilic aryl substituents such as alkynes (6), styrenes (9), nitriles (7, 10, 12, 16), sulfones (8), trifluoromethyl groups (11) and activated halides (17, 18, 20, 22, 25). Acidic and electrophilic functional groups, including alkyl benzoates (17), phthalimides (18), alkenes (19), alkyl pyridines (19-21) and esters (22-24) are also tolerated. Table 1c shows products of α-heteroatom benzylsilanes (25-27) and with paroxetine (28) and bepotastine (29) drug substructures. Product 27, derived from an α,α-difluorobenzyltrimethyl-silane prepared via trifluoromethylarene defluorosilylation, illustrates a benzylic coupling partner unique to this method.11a,18 In sum, the scope features substitution patterns and functionalities that are difficult to access or not tolerated in alternative arylation strategies.
Table 1.
Product scope using cyanoarene electrophiles.a
|
Isolated yields from reactions using 1.0 mmol of cyanoarene; 18-crown-6 added as a 1M solution in THF.
1.5 equiv of silane.
2.0 equiv of silane.
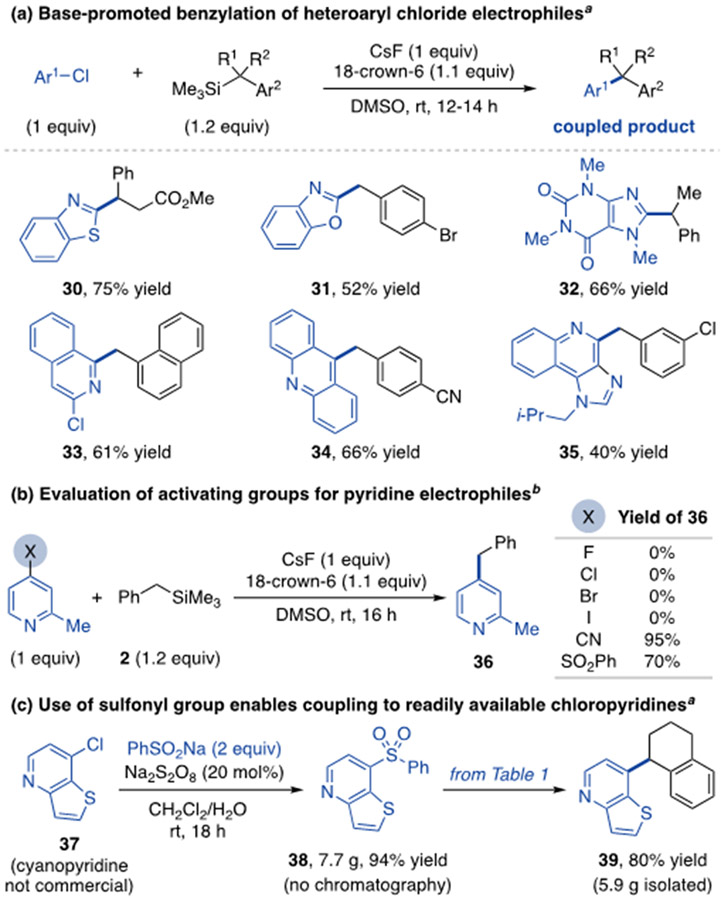
We next examined aryl electrophiles that do not generate cyanide byproducts (Scheme 2a). 2-Chloro-1,3-azoles are effective coupling partners (30-32), as are chlorides with extended π-systems, such as 1,3-dichloroisoquinoline (33), 9-chloroacridine (34) and the 2-chloroquinoline derivative of the anti-tumor drug imiquimod (35). Although 4-halopyridines do not react under these conditions, 4-sulfonylpyridines provide good yields (Scheme 2b).19 To show the benefits of this finding, 4-chloropyridine 37, for which the 4-cyano congener is not commercially available, was converted to sulfone 38 on multigram scale without chromatography (Scheme 2c).20 Benzylsilane coupling to 38 under the standard conditions without crown ether yielded 5.9 g of diarylalkane 39. Thus, base-promoted benzylation is applicable to heteroaryl halides either directly or after sulfonyl group installation.
Scheme 2. Expansion of aryl electrophile scope.
aIsolated product yields. bYields determined by 1H NMR spectroscopy of crude reaction mixtures. 18-Crown-6 added as a 1M solution in THF.
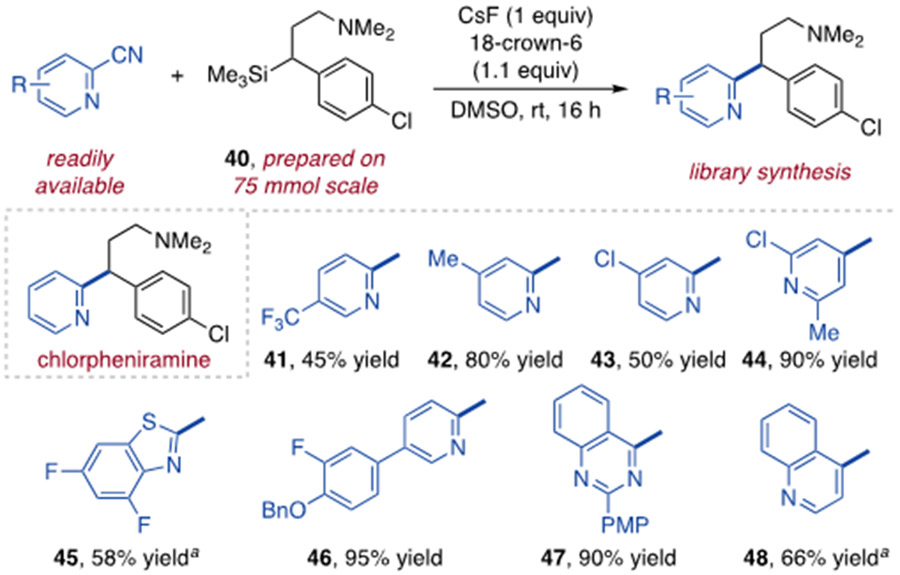
This method can facilitate access to 1,1-diarylalkane compound libraries from abundantly available cyano and chloroarenes. We selected the antihistamine chlorpheniramine to demonstrate this concept, for which the corresponding benzylsilane precursor 40 can be readily prepared on 75 mmol scale (Figure 2).21 Coupling of 40 with eight arene electrophiles generates diverse chlorpheniramine analogues, including trifluoromethyl- (41), methyl- (42), halo- (43, 44) and aryl-substituted (46) variants. A 2-chloro-1,3-benzothiazole (45), a 4-cyanoquinazoline (47) and 4-chloroquinoline (48) also react to access greater structural variety.
Figure 2.
Synthesis of chlorpheniramine analogues. Yields shown are of purified products. 18-Crown-6 added as a 1M solution in THF. a Chloroarene substrate used.
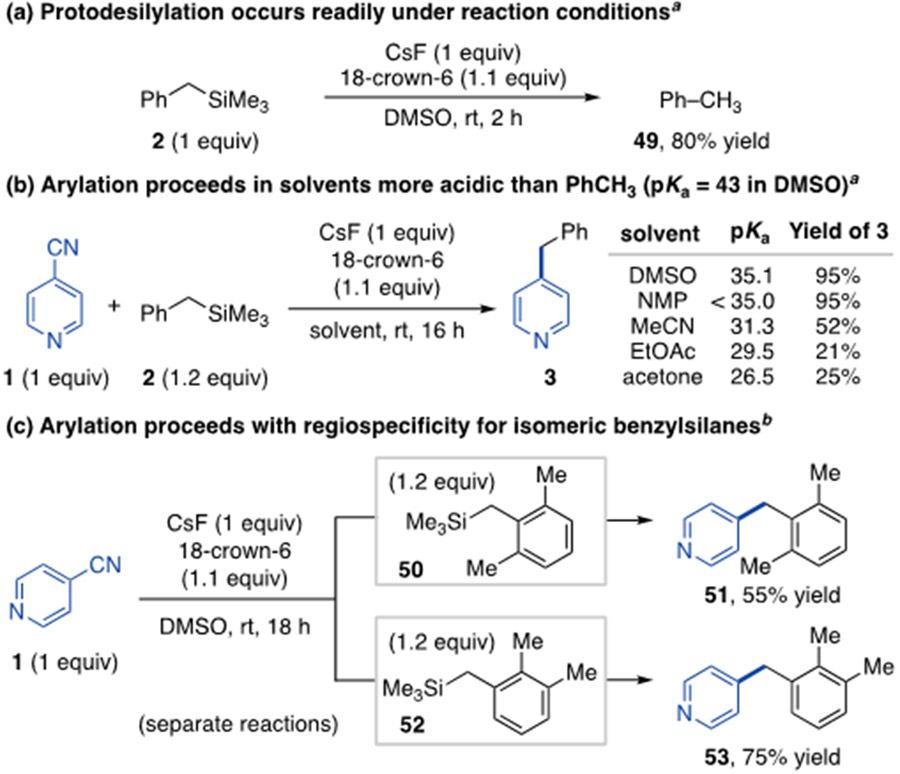
We next performed studies on the reaction selectivity for arylation over other anionic processes. When the aryl electrophile is removed from the standard conditions, toluene forms in 80% yield after 2 h (Scheme 3a).22 This suggests benzylic protonation is a competing pathway with arylation; however, it is interesting to note that benzylation of 4-cyanopyridine occurs in solvents significantly more acidic than toluene (Scheme 3b).23,24 Furthermore, separate reactions of two benzylsilane isomers (50 and 52) led to regioselective arylation for the original position of the −SiMe3 group (Scheme 3c). These results demonstrate arylation occurs preferentially over potential proton transfer events.25 An important implication is that deprotonation of acidic diarylalkane products is minimized, thus preventing multiarylation side reactions. These findings also illustrate critical advantages of a Lewis base-promoted arylation method, as a Brønsted base approach would not generate benzylic carbanions in the presence of more acidic protons, and would likely lead to multiarylation and poor selectivities in substrates with multiple benzylic positions.26
Scheme 3. Investigation of benzylic arylation selectivity.
aYields determined by 1H NMR spectroscopy of crude reaction mixture. bIsolated product yields. 18-Crown-6 added as 1M solution in THF.
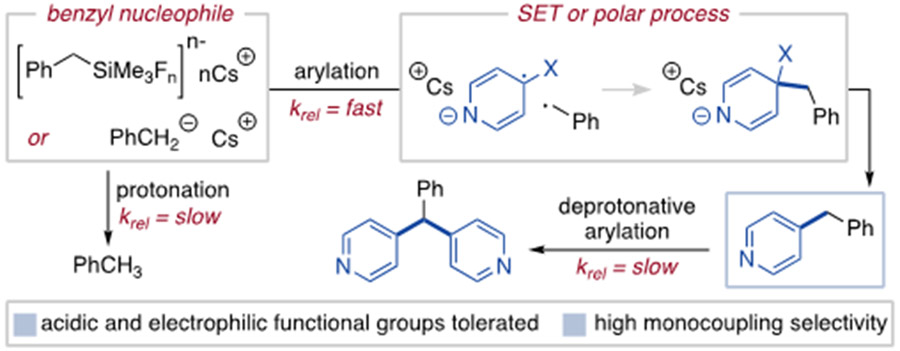
To explain the high arylation selectivity, we propose an anionic benzylic intermediate25 undergoes rapid aromatic substitution via a polar or SET-based mechanism (Figure 3).27 The SET mechanism is the base-promoted analogous pathway to PET reactions of organosilanes with 1,4-dicyanoarenes.17,28,29 A polar process is also plausible as cyano- and sulfonylarenes can participate in typical addition-elimination substitution reactions.30 Distinguishing between these processes is known to be challenging for addition of anionic reagents to similar electrophiles31,32 and we have made observations explainable by both pathways.33 The coupling mode may also be substrate dependent, although arylation uniformly outcompetes other anionic reactions as monoselectivity occurs for all reported substrates.34
Figure 3.
Potential pathways and rationale for selective arylation.
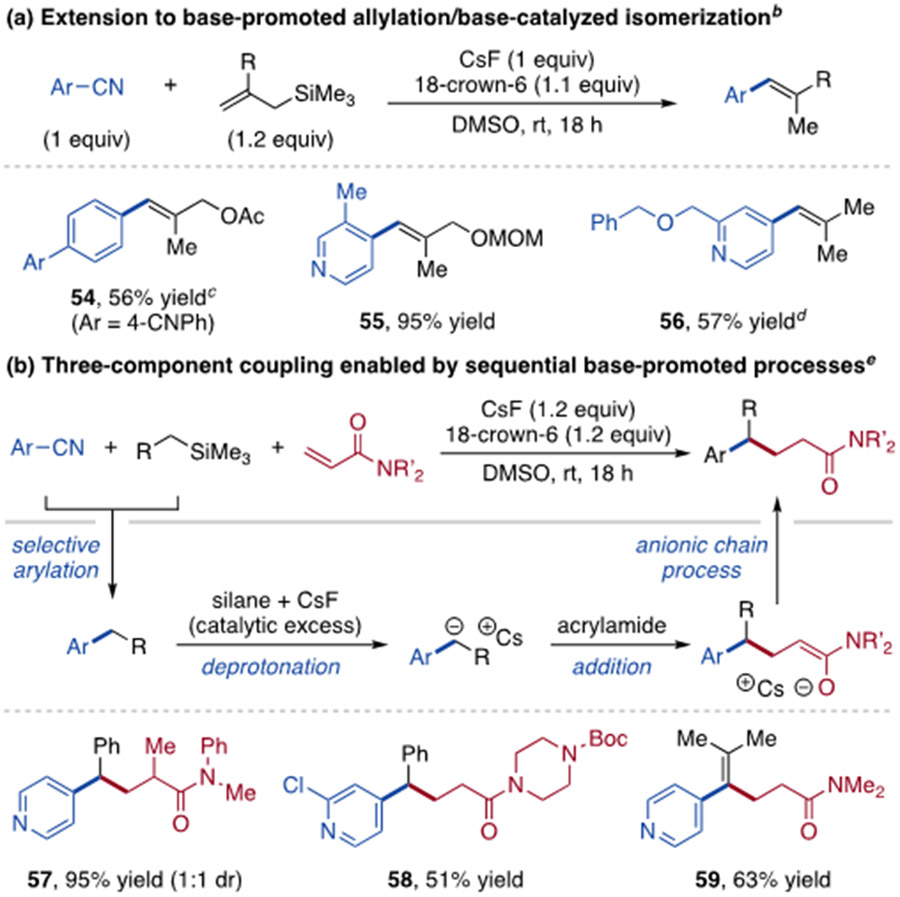
From these studies, we realized organosilane arylation could be incorporated into tandem base-promoted reaction sequences. First, we found allyltrimethylsilanes react to form allyl arene intermediates that undergo stereoselective isomerization to aryl alkenes 54, 55 and 56 (Scheme 4a).35,36
Scheme 4. Expanded scope using new coupling partners.a.
aYields are of purified product; diastereoselectivities determined by 1H NMR spectroscopy; 18-crown-6 added as 1M solution in THF. b>10:1 alkene E:Z ratios observed. cReaction performed at 60 °C. dCorresponding 4-phenylsulfonyl pyridine used as substrate. eArCN (1 equiv), organosilane (1.2 equiv) and acrylamide (1-2 equiv) used.
Next, we proposed a three-component coupling process between organosilanes, aryl electrophiles and Michael acceptors. We hypothesized selective benzylic arylation would occur and the remaining catalytic organosilane/fluoride combination could initiate a Michael addition reaction (Scheme 4b).37 Thus, γ,γ-diaryl amides 57 and 58 can be prepared via this strategy. Using methallyltrimethylsilane, tetrasubstituted alkene 59 forms through three selective base-promoted processes (arylation, addition and alkene isomerization).
In conclusion, Lewis basic salts provide a practical means of engaging benzyl- and allyltrimethylsilanes in arylation reactions. This approach enables regio- and monoselective access to 1,1-diarylalkane and aryl alkene products with complementary scope to existing methods. The strategic application of multiple base-promoted processes also facilitates advanced coupling sequences, a prospect we continue to explore.
Supplementary Material
ACKNOWLEDGMENT
Research reported in this publication was supported by the National Institute of General Medical Sciences of the National Institutes of Health under award number R35GM138350. We thank the National Institutes of Health for a postdoctoral fellowship for T. W. R. (F32GM140567) and Colorado State University for startup funding. The content is solely the responsibility of the authors and does not necessarily reflect the official views of the National Institutes of Health. We thank Shawn Wright (CSU) for initial studies on allyltrimethylsilane arylation reactions and Professor Yiming Wang (Pittsburgh) for input on this manuscript.
Footnotes
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website. Detailed experimental procedures, characterization data, and NMR spectra for all compounds (PDF).
REFERENCES
- (1).(a) For reviews, see: Mondal S; Panda G Synthetic methodologies of achiral diarylmethanols, diaryl and triarylmethanes (TRAMs) and medicinal properties of diaryl and triarylmethanesan overview. RSC Adv. 2014, 4, 28317–28358. [Google Scholar]; (b) Belal M; Lu X; Yin G Recent advances in the synthesis of 1,1-diarylalkanes by transition-metal catalysis. Sci. China Chem 2021, 64, 513–533. [Google Scholar]; (c) Nambo M; Crudden CM Recent Advances in the Synthesis of Triarylmethanes by Transition Metal Catalysis. ACS Catal. 2015, 5, 4734–4742. [Google Scholar]; (d) Xu J; Bercher OP; Talley MR; Watson MP Nickel-Catalyzed, Stereospecific C–C and C–B Cross-Couplings via C–N and C–O Bond Activation. ACS Catal. 2021, 11, 1604–1612. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Kshatriya R; Jejurkar VP; Saha S Advances in The Catalytic Synthesis of Triarylmethanes (TRAMs). Eur. J. Org. Chem 2019, 2019, 3818–3841. [Google Scholar]; (f) Jia T; Cao P; Liao J Enantioselective synthesis of gem-diarylalkanes by transition metal-catalyzed asymmetric arylations (TMCAAr). Chem. Sci 2018, 9, 546–559. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Moon PJ; Lundgren RJ Metal-Catalyzed Ionic Decarboxylative Cross-Coupling Reactions of C(sp3) Acids: Reaction Development, Mechanisms, and Application. ACS Catal. 2020, 10, 1742–1753. [Google Scholar]
- (2).(a) For examples, see: Negishi E; King AO; Okukado N Selective carbon-carbon bond formation via transition metal catalysis. 3. A highly selective synthesis of unsymmetrical biaryls and diarylmethanes by the nickel- or palladium-catalyzed reaction of aryl- and benzylzinc derivatives with aryl halides. J. Org. Chem 1977, 42, 1821–1823. [Google Scholar]; (b) Metzger A; Melzig L; Despotopoulou C; Knochel P Pd-Catalyzed Cross-Coupling of Functionalized Organozinc Reagents with Thiomethyl-Substituted Heterocycles. Org. Lett 2009, 11, 4228–4231. [DOI] [PubMed] [Google Scholar]; (c) Hirohisa O; Hideki Y; Koichiro O Cobalt-catalyzed Cross-coupling Reaction of Chloropyridines with Grignard Reagents. Chem. Lett 2004, 33, 1240–1241. [Google Scholar]; (d) Endo K; Ishioka T; Ohkubo T; Shibata T One-Pot Synthesis of Symmetrical and Unsymmetrical Diarylmethanes via Diborylmethane J. Org. Chem 2012, 77, 7223–7231. [DOI] [PubMed] [Google Scholar]
- (3).(a) Several reports describe the arylation of benzyl zinc reagents without a transition metal catalyst: Quinio P; Roman DS; León T; William S; Karaghiosoff K; Knochel P Transition-Metal-Free Cross-Coupling of Aryl and N-Heteroaryl Cyanides with Benzylic Zinc Reagents. Org. Lett 2015, 17, 4396–4399. [DOI] [PubMed] [Google Scholar]; (b) Shiota T; Yamamori T Regioselective Reactions of Organozinc Reagents with 2,4-Dichloroquinoline and 5,7-Dichloropyrazolo[1,5-a]pyrimidine. J. Org. Chem 1999, 64, 453–457. [Google Scholar]
- (4).(a) For example reactions of aryl halides with styrenes via benzylic metal intermediates, see: Friis SD; Pirnot MT; Buchwald SL Asymmetric Hydroarylation of Vinylarenes Using a Synergistic Combination of CuH and Pd Catalysis. J. Am. Chem. Soc 2016, 138, 8372–8375. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Logan KM; Smith KB; Brown MK Copper/Palladium Synergistic Catalysis for the syn- and anti-Selective Carboboration of Alkenes. Angew. Chem. Int. Ed 2015, 54, 5228–5231. [DOI] [PubMed] [Google Scholar]
- (5).(a) For examples of cross-electrophile coupling, see: Ackerman LKG; Anka-Lufford LL; Naodovic M; Weix DJ Cobalt co-catalysis for cross-electrophile coupling: diarylmethanes from benzyl mesylates and aryl halides. Chem. Sci 2015, 6, 1115–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Poremba KE; Kadunce NT; Suzuki N; Cherney AH; Reisman SE Nickel-Catalyzed Asymmetric Reductive Cross-Coupling To Access 1,1-Diarylalkanes. J. Am. Chem. Soc 2017, 139, 5684–5687. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Charboneau DJ; Barth EL; Hazari N; Uehling MR; Zultanski SL A Widely Applicable Dual Catalytic System for Cross-Electrophile Coupling Enabled by Mechanistic Studies. ACS Catal. 2020, 21, 12642–12656. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Guo P; Wang K; Jin W-J; Xie H; Qi L; Liu X-Y; Shu X-Z Dynamic Kinetic Cross-Electrophile Arylation of Benzyl Alcohols by Nickel Catalysis. J. Am. Chem. Soc 2021, 143, 513–523. [DOI] [PubMed] [Google Scholar]
- (6).(a) For representative arylation reactions that proceed through benzylic radicals, see: Vasilopoulos A; Zultanski SL; Stahl SS Feedstocks to Pharmacophores: Cu-Catalyzed Oxidative Arylation of Inexpensive Alkylarenes Enabling Direct Access to Diarylalkanes. J. Am. Chem. Soc 2017, 139, 7705–7708. [DOI] [PubMed] [Google Scholar]; (b) Zhang W; Chen P; Liu G Copper-Catalyzed Arylation of Benzylic C–H bonds with Alkylarenes as the Limiting Reagents. J. Am. Chem. Soc 2017, 139, 7709–7712. [DOI] [PubMed] [Google Scholar]; (c) Hoshikawa T; Inoue M Photoinduced direct 4-pyridination of C(sp3)–H Bonds. Chem. Sci 2013, 4, 3118–3123. [Google Scholar]; (d) Qvortrup K; Rankic DA; MacMillan DWC A General Strategy for Organocatalytic Activation of C–H Bonds via Photoredox Catalysis: Direct Arylation of Benzylic Ethers. J. Am. Chem. Soc 2014, 136, 626–629. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Gao L; Wang G; Cao J; Chen H; Gu Y; Liu X; Cheng X; Ma J; Li S Lewis Acid-Catalyzed Selective Reductive Decarboxylative Pyridylation of N-Hydroxyphthalimide Esters: Synthesis of Congested Pyridine-Substituted Quaternary Carbons. ACS Catal. 2019, 9, 10142–10151. [Google Scholar]; (f) Tellis JC; Primer DN; Molander GA Single-electron transmetalation in organoboron cross-coupling by photoredox/nickel dual catalysis. Science 2014, 345, 433–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).(a) For examples, see: Sha S-C; Tcyrulnikov S; Li M; Hu B; Fu Y; Kozlowski MC; Walsh PJ Cation-π Interactions in the Benzylic Arylation of Toluenes with Bimetallic Catalysts. J. Am. Chem. Soc 2018, 40, 12415–12423. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Jiang H; Sha S-C; Jeong SA; Manor BC; Walsh PJ Ni(NIXANTPHOS)-Catalyzed Mono-Arylation of Toluenes with Aryl Chlorides and Bromides. Org. Lett 2019, 21, 1735–1739. [DOI] [PubMed] [Google Scholar]; (c) Burton PM; Morris JA Palladium-Catalyzed Benzylic Arylation of 2-Methyl Azaarenes. Org. Lett 2010, 12, 5359–5361. [DOI] [PubMed] [Google Scholar]; (d) Zhang S Hu B; Zheng Z; Walsh PJ Palladium-Catalyzed Triarylation of sp3 C–H Bonds in Heteroarylmethanes: Synthesis of Triaryl(heteroaryl)methanes. Adv. Synth. Catal 2018, 360, 1493–1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).(a) For examples, see: Wei X; Qu B; Zeng X; Savoie J; Fandrick KR; Desrosiers J-N; Tcyrulnikov S; Marsini MA; Buono FG; Li Z; Yang B-S; Tang W; Haddad N; Gutierrez O; Wang J; Lee H; Ma S; Campbell S; Lorenz JC; Eckhardt M; Himmelsbach F; Peters S; Patel ND; Tan Z; Yee NK; Song JJ; Roschangar F; Kozlowski MC; Senanayake CH Sequential C-H Arylation and Enantioselective Hydrogenation Enables Ideal Asymmetric Entry to the Indenopiperidine Core of an 11β-HSD-1 Inhibitor. J. Am. Chem. Soc 2016, 138, 15473–15481. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Li M; Berritt S; Matuszewski L; Deng G; Pascual-Escudero A; Panetti GB; Poznik M; Yang X; Chruma JJ; Walsh PJ Transition-Metal-Free Radical C(sp3)–C(sp2) and C(sp3)–C(sp3) Coupling Enabled by 2-Azaallyls as Super-Electron-Donors and Coupling-Partners. J. Am. Chem. Soc 2017, 139, 16327–16333. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Ji X; Huang T; Wu W; Liang F; Cao S LDA-Mediated Synthesis of Triarylmethanes by Arylation of Diarylmethanes with Fluoroarenes at Room Temperature. Org. Lett 2015, 17, 5096–5099. [DOI] [PubMed] [Google Scholar]; (d) Lanin YL; Huel C; Legraverend M; Aubertin A-M; Bisagni E Syntheses of 4-Benzylpyridones via Nucleophilic Aromatic Substitutions. Synthesis 2001, 12, 1806–1811. [Google Scholar]; (e) Dyker G; Muth O Synthesis of Methylene- and Methine-Bridged Oligopyridines. Eur. J. Org. Chem 2004, 2004, 4319–4322. [Google Scholar]
- (9).For a recent report on alkoxide-promoted arylation of tertiary benzylic boronates, see: Takeda M; Nagao K; Ohmiya H Transition-Metal-Free Cross-Coupling by Using Tertiary Benzylic Organoboronates. Angew. Chem. Int. Ed 2020, 59, 22460–22464. [DOI] [PubMed] [Google Scholar]
- (10).(a) For example routes to benzyltrimethylsilanes: Das M; O’Shea DF Synthesis and application of benzyl-TMS derivatives as bench stable benzyl anion equivalents. Tetrahedron 2013, 69, 6448–6460. [Google Scholar]; (b) Li W; Gao G; Yang C; Xia W Direct oxidation of the C(sp2)–C(sp3) bond from benzyltrimethylsilanes to phenols. Chem. Commun 2017, 53, 5291–5293. [DOI] [PubMed] [Google Scholar]; (c) Takahashi H; Hossain KM; Nishihara Y; Shibata T; Takagi K Synthesis of Functionalized Benzylsilanes from Arylzinc Compounds and (Iodomethyl)trimethylsilane by Means of a Novel Rh Catalysis. J. Org. Chem 2006, 71, 671–675. [DOI] [PubMed] [Google Scholar]
- (11).(a) For examples, see: Utsumi S; Katagiri T; Uneyama K Cu-deposits on Mg metal surfaces promote electron transfer reactions. Tetrahedron 2012, 68, 1085–1091. [Google Scholar]; (b) Kundu PK; Ghosh SK Magnesium-induced regiospecific C-silylation of suitably substituted enoates and dienoates. Tetrahedron 2010, 66, 8562–8568. [Google Scholar]; (c) Zhang T; Zhang Z; Nishiyama Y; Maekawa H Facile and highly selective silylation of vinylpyridines at the β-olefinic carbon by magnesium-promoted reduction. Tetrahedron 2016, 72, 2293–2299. [Google Scholar]
- (12).(a) Grimaud L; Jutand A Role of Fluoride Ions in Palladium-Catalyzed Cross-Coupling Reactions. Synthesis 2017, 49, 1182–1189. [Google Scholar]; (b) Hiyama T; Minami Y; Mori A Transition-Metal-Catalyzed Cross-coupling of Organosilicon Compounds. In Organosilicon Chemistry: Novel Approaches and Reactions; Hiyama T; Oestreich M, Eds.; Wiley-VCH: location, 2020; pp 281–332. See also ref. 14a. [Google Scholar]
- (13).(a) For benzyltrimethylsilane arylation via aryl C–H substitution, see: Wu Y; Bouvet S; Izquierdo S; Shafir A Synthesis of Polysubstituted Iodoarenes Enabled by Iterative Iodine-Directed para and ortho C–H Functionalization. Angew. Chem. Int. Ed 2019, 58, 2617–2621. [DOI] [PubMed] [Google Scholar]; (b) Dong J; Wang X; Wang Z; Song H; Liu Y; Wang Q Metal-, photocatalyst-, and light-free late-stage C–H alkylation of N-heteroarenes with organotrimethylsilanes using persulfate as a stoichiometric oxidant. Org. Chem. Front 2019, 6, 2902–2906. [Google Scholar]; (c) Puthanveedu M; Vasiliki V; Antonchick AP Catalytic Selective Metal-Free Cross-Coupling of Heteroaromatic N-Oxides with Organosilanes. Org. Lett 2019, 21, 3407–3411. [DOI] [PubMed] [Google Scholar]
- (14).(a) Itami K; Mineno M; Kamei T; Yoshida J A General and Straightforward Route toward Diarylmethanes. Integrated Cross-Coupling Reactions Using (2-Pyridyl)silylmethylstannane as an Air-Stable, Storable, and Versatile Coupling Platform. Org. Lett 2002, 4, 3635–3638. [DOI] [PubMed] [Google Scholar]; (b) Corcé V; Chamoreau L-M; Derat E; Goddard J-P; Ollivier C; Fensterbank L Silicates as Latent Alkyl Radical Precursors: Visible-Light Photocatalytic Oxidation of Hypervalent Bis-Catecholato Silicon Compounds. Angew. Chem. Int. Ed 2015, 54, 11414–11418. [DOI] [PubMed] [Google Scholar]; (c) Jouffroy M; Primer DN; Molander GA Base-Free Photoredox/Nickel Dual-Catalytic Cross-Coupling of Ammonium Alkylsilicates. J. Am. Chem. Soc 2016, 138, 475–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Luo C; Bandar JS Selective Defluoroallylation of Trifluoromethylarenes. J. Am. Chem. Soc 2019, 141, 14120–14125. [DOI] [PubMed] [Google Scholar]
- (16).(a) For representative studies, see: Mizuno K; Ikeda M; Otsuji Y A novel photosubstitution of dicyanobenzenes by allylic and benzylic silanes. Tetrahedron Lett 1985, 26, 461–464. [Google Scholar]; (b) Kazuhisa N; Kazuhiko M; Yoshio O Photosubstitution of Dicyanobenzenes by Allylic Silanes, Germanes, and Stannanes via Photoinduced Electron Transfer. Bull. Chem. Soc. Jpn 1993, 66, 2371–2379. [Google Scholar]; (c) For a related recent method: Liu Y; Li H; Chiba S Photoinduced Cross-Coupling of Aryl Iodides with Alkenes. Org. Lett 2021, 23, 427–432. [DOI] [PubMed] [Google Scholar]
- (17).(a) For representative studies, see: Kazuhiki M; Kiyotaro T; Masahiro Y; Yoshio O Photoarylmethylation of 1,4-Dicyanonaphthalene by Use of Group 14 Organometallic Compounds. Chem. Lett 1988, 17, 145–148. [Google Scholar]; (b) Toshiyuki T; Kazuhiko M; Isao H; Yoshio O Photoinduced Electron-Transfer Reactions of Arylmethyl-Substituted 14 Group Compounds: Photoarylmethylation and Photooxygenation. Bull. Chem. Soc. Jpn 1993, 66, 3747–3754. [Google Scholar]; For a related photoredox-catalyzed oxidative [1,2]-Brook rearrangement/arylation reaction, see: Deng Y; Liu Q; Smith AB III. Oxidative [1,2]-Brook Rearrangements Exploiting Single-Electron Transfer: Photoredox-Catalyzed Alkylations and Arylations. J. Am. Chem. Soc 2017, 139, 9487–9490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Other products from benzyltrimethylsilanes prepared via reductive trimethylsilylation are 17, 18, 21-24, 30 and those in Figure 2.
- (19).The observation that 4-halopyridines do not undergo benzylation is consistent with a coupling process that is not metal-catalyzed.
- (20).Nguyen VD; Nguyen VT; Haug GC; Dang HT; Arman HD; Ermler WC; Larionov OV Rapid and Chemodivergent Synthesis of N-Heterocyclic Sulfones and Sulfides: Mechanistic and Computational Details of the Persulfate-Initiated Catalysis. ACS Catal. 2019, 9, 4015–4024. [Google Scholar]
- (21).(a) For other routes to chlorpheniramine analogues, see: Dean KJ; Summers RL; Lehane AM; Martin RE; Barrow RA Chlorpheniramine Analogues Reverse Chloroquine Resistance in Plasmodium falciparum by Inhibiting PfCRT. ACS Med. Chem. Lett 2014, 5, 576–581. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Dong CD; Wang X; Pei Z; Shen R Metal-Free Denitrogenative C–C Couplings of Pyridotriazoles with Boronic Acids To Afford α-Secondary and α-Tertiary Pyridines. Org. Lett 2019, 21, 4148–4152. [DOI] [PubMed] [Google Scholar]
- (22).(a) For examples of Lewis base-promoted benzyltrimethylsilane addition reactions, see references 10a, 13c and Yao W; Li R; Jiang H; Han D An Additive-Free, Base-Catalyzed Protodesilylation of Organosilanes. J. Org. Chem 2018, 83, 2250–2255. [DOI] [PubMed] [Google Scholar]; (b) Reich HJ Mechanism of C–Si Bond Cleavage Using Lewis Bases (n → σ*). In Lewis Base Catalysis in Organic Synthesis; Vedejs E; Denmark SE Eds.; Wiley-VCH: Weinheim, 2016; pp 233–280. [Google Scholar]
- (23).As detailed in references 13 and 22, benzyltrimethylsilane addition reactions are typically performed in nonacidic solvents. These reactions typically require a strong Lewis base, such as fluoride or alkoxide salts, in contrast to reactivity observed in Scheme 1c.
- (24).Bordwell FG Equilibrium acidities in dimethyl sulfoxide solution. Acc. Chem. Res 1988, 21, 456–463. [Google Scholar]
- (25).Lewis base-promoted reactions of benzyl and allylsilanes have been proposed to react through penta- and hexacoordinate silicate as well as carbanion intermediates. For a review, see ref 22b.
- (26).To demonstrate this, deprotonative silylation of 1,2,3-trimethylbenzene produces a 6:1 mixture of 52:50, with no obvious way to favor 50 as the major product. Subjection of the 6:1 mixture of 52:50 to the arylation conditions results in an analogous 6:1 ratio of products 53:51. See Supporting Information for full details.
- (27).(a) For reviews on SET in substitution reactions, see: Rossi RA; Pierini AB; Peñeñory AB Nucleophilic Substitution Reactions by Electron Transfer. Chem. Rev 2003, 103, 71–167. [DOI] [PubMed] [Google Scholar]; (b) Zhang N; Samanta SR; Rosen BM; Percec V Single Electron Transfer in Radical Ion and Radical-Mediated Organic, Materials and Polymer Synthesis. Chem. Rev 2014, 114, 5848–5958. [DOI] [PubMed] [Google Scholar]
- (28).(a) Measured reduction potentials of benzyl radicals and cyanoarenes span a similar range that is sensitive to the substrate identity, see: Sim BA; Milne PH; Griller D; Wayner DDM Thermodynamic Significance of ρ+ and ρ− from Substituent Effects on the Redox Potentials of Arylmethyl Radicals. J. Am. Chem. Soc 1990, 112, 6636–6638. [Google Scholar]; (b) Mcdevitt P; Vittimberga BM The electron transfer reactions of cyano substituted pyridines and quinolines with thermally generated diphenyl ketyl. J. Heterocycl. Chem 1990, 27, 1903–1908. [Google Scholar]; (c) Mori Y; Sakaguchi Y; Hayashi H Magnetic Field Effects on Chemical Reactions of Biradical Radical Ion Pairs in Homogeneous Fluid Solvents. J. Phys. Chem. A 2000, 104, 4896–4905. [Google Scholar]
- (29).Cyanoarenes are commonly used in redox substitution reactions; see refs 6c-e and: Leifart D; Studer A; The Persistent Radical Effect in Organic Synthesis. Angew. Chem. Int. Ed 2020, 59, 74–108. [DOI] [PubMed] [Google Scholar]
- (30).(a) Thompson AD; Huestis MP Cyanide Anion as a Leaving Group in Nucleophilic Aromatic Substitution: Synthesis of Quaternary Centers at Azine Heterocycles. J. Org. Chem 2013, 78, 762–769. [DOI] [PubMed] [Google Scholar]; (b) Wei X; Zhang C; Wang Y; Zhan Q; Qiu G; Fan L; Yin G Decyanative Cross-Coupling of Cyanopyrimidines with O-, S-, and N-Nucleophiles: A Route to Alkoxylpyrimidines, Aminopyrimidines and Alkylthiopyrimidines. Eur. J. Org. Chem 2019, 2019, 7142–7150. [Google Scholar]; (c) Barlin GB; Brown WV Useful Reactions of Nucleophiles with Some Methylsulphonyl Derivatives of Nitrogen Heterocycles. J. Chem. Soc 1967, 2473–2476. [Google Scholar]
- (31).For examples of nucleophilic benzylation reactions of activated cyano and sulfonylarenes that have been proposed to proceed via either pathway, see references 3a, 8a, 9 and Lei Y; Yang J; Qi R; Wang S; Wang R; Xu Z Arylation of benzyl amines with aromatic nitriles. Chem. Comm 2018, 54, 11881–11884. [DOI] [PubMed] [Google Scholar]
- (32).(a) For discussions on one and two electron modes of addition in SNAr reactions, see: Terrier F Other SNAr Substitution Pathways. In Modern Nucleophilic Aromatic Substitution; Wiley-VCH, Weinheim, 2013, pp 423–463. [Google Scholar]; (b) Pross A The single electron shift as a fundamental process in organic chemistry: the relationship between polar and electron-transfer pathways. Acc. Chem. Res 1985, 18, 212–219. [Google Scholar]; (c) Percec V; Clough RS; Grigoras M; Rinaldi PL; Litman VE Reductive dehalogenation versus substitution in the polyetherification of 4,4'-dihalodiphenyl sulfones with bisphenolates. Macromolecules 1993, 26, 3650–3662. [Google Scholar]; (d) Bacaloglu R; Bunton CA; Ortega F Single-electron transfer in aromatic nucleophilic addition and substitution in aqueous media. J. Am. Chem. Soc 1988, 110, 3503–3512. [Google Scholar]
- (33).A discussion of observations made that pertain to potential mechanistic pathways are described in the Supporting Information.
- (34).(a) We note that a potential alternative pathway involves initial addition to the nitrile followed by rearrangement; see: Miller JA; Dankwardt JW; Penney JM Nickel Catalyzed Cross-Coupling and Amination Reactions of Aryl Nitriles. Synthesis 2003, 11, 1643–1648. [Google Scholar]; (b) For a discussion of a related pathway for sulfur-based electrophiles, see: Dean WM; Šiaučiulis M; Storr TE; Lewis W; Stockman RA Versatile C(sp2)–C(sp3) Ligand Couplings of Sulfoxides for the Enantioselective Synthesis of Diarylalkanes. Angew. Chem. Int. Ed 2016, 55, 10013–10016. [DOI] [PubMed] [Google Scholar]
- (35).(a) For allyltrimethylsilane coupling reactions, see: Akram MO; Mali PS; Patil NT Cross-Coupling Reactions of Aryldiazonium Salts with Allylsilanes under Merged Gold/Visible-Light Photoredox Catalysis. Org. Lett 2017, 19, 3075–3078. [DOI] [PubMed] [Google Scholar]; (b) De Carolis M; Protti S; Fagnoni M; Albini A Metal-Free Cross-Coupling Reactions of Aryl Sulfonates and Phosphates through Photoheterolysis of Aryl–Oxygen Bonds. Angew. Chem. Int. Ed 2005, 44, 1232–1236. [DOI] [PubMed] [Google Scholar]
- (36).(a) For related isomerization observed during allylsilane arylation reactions, see: Vorbüggen H; Krolikiewcz K Conversion of heterocyclic N-oxides into α-alkylated heterocycles trimethylsilanol as leaving group — IV. Tetrahedron Lett. 1983, 24, 889–890. [Google Scholar]; (b) Denmark SE; Werner NS Cross-Coupling of Aromatic Bromides with Allylic Silanolate Salts. J. Am. Chem. Soc 2008, 130, 16382–16393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Yamashita Y; Kobayashi S Catalytic Carbon–Carbon Bond-Forming Reactions of Weakly Acidic Carbon Pronucleophiles Using Strong Brønsted Bases as Catalysts. Chem. Eur. J 2018, 24, 10–17. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.