Abstract
Aim
To assess nurses’ ability to observe newborn behaviour after in situ training provided by caregivers with advanced practice certification in the Newborn Individualized Developmental Care and Assessment Program (NIDCAP).
Design
Prospective observational study.
Methods
Twelve nurses viewed 20‐min films showing the behaviour of 10 premature newborns before, during and after the usual caregiving. The behaviour was rated on an observation sheet with 88 items distributed into six systems. The responses were compared to the reference ratings established by two professionals certified for this programme.
Results
Despite less accurate observations during care and for some components, the nurses generally showed a satisfactory ability to observe newborn behaviour after training by NIDCAP expert professionals. The dissemination of observation skills among caregivers may result in an improved quality of patient care and better communication among professionals in a department of neonatology.
Keywords: advanced practice, infant behaviour, neonatal care, Newborn Individualized Developmental Care and Assessment Program (NIDCAP), nurse, nurses–patient interaction, premature infant, staff development, video technology
1. INTRODUCTION
The concept of developmental care recognizes the physical, psychological and emotional vulnerabilities of premature babies and their families and is focused on minimizing potential short‐ and long‐term complications associated with a hospital stay in the neonatal intensive care unit (NICU; Montirosso et al., 2012). Several aspects of care are involved, grouped into five core measures: sleep protection; assessment and management of stress and pain; developmentally supportive activities including positioning, feeding and skin‐to‐skin (kangaroo) care; family‐centred care and promotion of a healing environment (Coughlin et al., 2009).
The Newborn Individualized Developmental Care and Assessment Program (NIDCAP) is designed to integrate all aspects of neurodevelopmental care based on a reading of each preterm infant's behavioural cues in a family‐focused care approach (Als & McAnulty, 2011). NIDCAP is an evidence‐based, comprehensive, internationally recognized programme that improves outcomes for premature infants, with reported benefits for length of hospitalization and neurodevelopment at 9 months (Ohlsson & Jacobs, 2013). It also increases maternal closeness and involvement in infant care and parental confidence in caregiving (Als et al., 2003; Kleberg et al., 2007; Nelson & Bedford, 2016; Sannino et al., 2016).
1.1. Background
Our department of neonatal medicine began moving towards the practice of developmental care in 2001 and was awarded NIDCAP Level II in 2007. Since then, NIDCAP has been supervised by a steering committee that meets quarterly and is composed of the head of the department, nurse managers, staff members with advanced practice certification, clinical psychologists, a psychomotor therapist and occasionally a member of the hospital management.
One of the challenges of the NIDCAP approach is performing formalized, naturalistic observations of an infant before, during and after a caregiving procedure, as this is the cornerstone of programme effectiveness. The training of NIDCAP professionals is long, usually 2 to 3 years, and demanding, requiring personal work, self‐assessment and a series of behavioural observations initially guided by a certified trainer before autonomous advanced practice is reached (Als & McAnulty, 2011). Therefore, in most nurseries or centres that have adopted this programme, 5%–10% of the staff members are certified as NIDCAP professionals, limiting full observations of only the most vulnerable patients (Westrup, 2015). This is also the case in our department, which has a capacity of 59 beds and a team that includes 10 paediatricians and 120–130 nurses, in line with the national decree. After the last training sequence of 2016–2020, seven staff members have obtained advanced practice certification, including three paediatricians, two nurses, one psychologist and one physiotherapist.
The number and regularity of observations is one of the steering committee's concerns. The low number of full NIDCAP observations prompted the committee to create a network of referent nurses for developmental care (RNDCs) in 2010. The RNDC network is composed of volunteer nurses who have undergone training in individualized developmental care, including the use of a behavioural observation sheet. The training lasts 2 days and is provided by the two nurses certified in 2007. Training opportunities are regularly offered to ensure that a minimum of 10 referent nurses are in the network. Changes have also been made to the NIDCAP Observation Sheet to clarify certain items. These modifications are not intended to replace the NIDCAP Sheet, which remains the reference for formal observations in the department, but to encourage individualized care in daily practice.
While several surveys suggest that having staff trained in observation positively influences developmental care uptake in units (Hamilton & Redshaw, 2009; Pierrat et al., 2016), no study has provided factual data on the impact of disseminating observation skills to uncertified caregivers after NIDCAP implementation in a neonatal unit or department. Determining whether this competence could be acquired by our RNDC network members was, however, an essential step in achieving our objective of increasing the number and regularity of observations in our department.
2. THE STUDY
2.1. Aims
The main objective of this study was to determine whether this in situ, non‐formal training programme is indeed able to transfer a satisfactory capacity to observe newborn behaviour to RNDCs. The secondary objective was to determine the factors associated with a nurse's adequate observation of infant behaviour.
2.2. Design
This prospective observational study assessed RNDCs' ability to observe the behaviour of premature newborns on standardized films, using a specific collection form.
2.3. Participants
2.3.1. Neonates
Films of neonates hospitalized in a neonatal unit were made in the department of neonatal medicine. The inclusion criteria were the following: (a) gestational age <33 weeks, (b) birth weight <1500 g and (c) postnatal age between 1–60 days. The only criterion of non‐inclusion was the refusal by the holders of parental authority to give their signed authorization to film their infant.
Written information was posted in the department's family reception room, explaining the objective of the study and encouraging interested parents to make themselves known to a staff member. The parents’ applications were centralized by the two nurses with NIDCAP advanced practice certification, who selected among them based on the study recruitment protocol. Recruitment had to ensure an equal number of newborns in the following gestational age categories: 24–27, 28–30 and >30 weeks. Within each category, at least one film had to be made during the first week of life, at least one film between 8–30 days and at least one film between 31–60 days. This distribution ensured the observation of a wide range of medical situations, that is dependence on different types of ventilatory and/or nutritional assistance and a sufficient diversity of care sequences, like aspiration, feeding, changing diapers, bathing, weighing or other anthropometric measurements, and kangaroo mother care.
All filming was done by the same two nurses with NIDCAP advanced practice certification using material loaned by the hospital's communications department. Both nurses were always present for the care interventions but did not participate. As filming progressed, regular checking ensured that none of the care sequences listed above were missing. The two nurses then collaborated with the communications department to assemble the film sequences into a single film, according to the following charter: all films should last 20 min and include three sequences each lasting 6–7 min, clearly identified by three inlays bearing the words “before care,” “during care” and “after care.”
2.3.2. Nurses
The 12 nurses had 2 days of training in June 2013 and have been using the observation sheet regularly ever since. They had 10.3 (7.2, 13.5) years of professional experience in neonatology.
2.4. Data collection
The behavioural observation form used in this study is an adaptation of the French translation of the NIDCAP Observation Sheet (Als et al., 1986). Modifications were based on comments made by department nurses to facilitate the use of an observation grid for daily care. The structure of this form was then validated by the steering committee for internal use exclusively. This form was used in this study because it was better mastered by the RNDCs than the NIDCAP Observation Sheet.
Eighty‐eight components, distributed into six systems, are used to describe newborn behaviour (Table 1).
TABLE 1.
Behavioural observation sheet used in the study
| Var | Var | ||
|---|---|---|---|
| Autonomic nervous system | Lifeless or unresponsive | 0 | |
| Respiratory rate >60 or <40 rpm | 0.2 | Deep sleep | 0 |
| Deep breathing <60 rpm | 0.1 | Light sleep with frequent awakening | 0.55 |
| Change in respiratory rate >20 rpm | 0.2 | Drowsy alertness | 0.15 |
| Breathing pauses | 0.5 | Hypoactive or hypoalert behaviour | 0 |
| Apnoea | 0 | Hypervigilance | 0 |
| Chest retraction | 0.25 | Worried on awakening | 0.6 |
| Sinking with rising abdomen | 0.4 | Awakened and calm | 0.45 |
| Change in heart rate >20 bpm | 0 | Frantic and uncontrollable activity | 0.1 |
| Bradycardia <80 bpm | 0 | Agitation with self‐regulation | 0.35 |
| Tachycardia >180 bpm | 0 | Strained fussing or crying | 0.5 |
| Regular heart rate | 0 | Rhythmical robust crying | 0 |
| Decrease in blood oxygen saturation (SpO2) | 0 | Interactional system | |
| Pallor | 0.2 | Gaze aversion | 0 |
| Skin marbling | 0.15 | Floating or wandering eyes | 0.35 |
| Dark skin areas | 0.2 | Frowning | 0.75 |
| Cyanosis | 0 | Grimacing | 0.75 |
| Erythrosis | 0.15 | Yawning | 0.55 |
| Pink complexion | 0.1 | Whimpering | 0.1 |
| Change in skin colour | 0.4 | Grunting | 0.1 |
| Hiccups | 0.25 | Relaxed face | 0.5 |
| Sneezing | 0.05 | Gaze | 0.25 |
| Coughing | 0.1 | Directed gaze | 0.25 |
| Regurgitation (spit‐up) | 0.1 | Smiling | 0.2 |
| Nausea | 0.05 | Speech movements, cooing, gurgling | 0 |
| Abdominal wall contraction | 0.1 | Self‐regulation system | |
| Flatulence | 0 | Protective manoeuvres | 0.8 |
| Abdominal distension | 0.55 | Grasping attempt | 0.5 |
| Tremoring | 0.5 | Grasping | 0.5 |
| Startling | 0.3 | Searching support with legs | 0.6 |
| Motor system | Foot clasping | 0.35 | |
| Hypotonia | 0.6 | Hand clasping | 0.5 |
| Hypertonia | 0.1 | Hands to face | 0.4 |
| Adequate tonus | 0.2 | Hands to mouth | 0.3 |
| Extended arms | 0.75 | Oral system | |
| Flexed or tucked arms | 0.55 | Lip movements | 0.2 |
| Extended legs | 0.8 | Suck search | 0.45 |
| Flexed or tucked legs | 0.7 | Turning towards stimulation | 0.2 |
| Squirming | 0.55 | Withdrawal from stimulation | 0 |
| Arched back or opisthotonus | 0.3 | Mouth closing | 0.25 |
| Tucked trunk and limbs | 0.6 | Finger or hand sucking | 0.15 |
| Rare movements | 0.3 | Vigorous sucking | 0.3 |
| Sudden and disordered movements | 0.6 | Weak sucking | 0.15 |
| Smooth and synchronous movements | 0.35 | Licking | 0.05 |
| Sleep‐wake system | Rooting | 0 | |
| Sudden and disorganized transitions | 0.05 | Coordinated sucking, swallowing and breathing | 0.2 |
| Progressive or harmonious transitions | 0.45 | Uncoordinated sucking, swallowing and breathing | 0 |
Refer to Methods and Results sections for the variability (Var) of each component.
The autonomic nervous system (29 items) integrates the breathing, colour, visceral and respiratory items of the NIDCAP Sheet. Numerical values have been added to clarify the notions of fast, slow, regular and irregular respiratory or heart rate. The description of the infant's colour has been reorganized into four items. Most of the visceral and respiratory signs have been kept with reformulation.
The motor system (13 items) includes almost all the items in the NIDCAP Sheet. Some have been reworded, for example hypotonia instead of flaccid arms and legs and hypertonia instead of stretch/drawn. Three items to describe normal tonus and rare or sudden and disordered movements have been added. The search for support with legs has been placed in the self‐regulation system.
The sleep‐wake system (14 items) describes these states in concrete detail, whereas the NIDCAP Sheet uses an abstract classification in 13 stages. Our observation form includes a range of states from sleeping to wakefulness and patterns of transition from state to state, with stress or stability indicators for alertness, activity and crying.
The interactional system (12 items) refers to the attention items on the NIDCAP Sheet. Most of the items have been kept, sometimes with reformulations, for example grunt for fuss, relaxed face for face open and gaze for looking. Two items of facial description on the NIDACP Sheet, grimace and smile, are now positioned in this system.
The self‐regulation system (eight items) combines defence behaviours, such as protective manoeuvres and hands to face, and regulatory behaviours, such as grasping and clasping. These items were present in the description of the face and limbs on the NIDCAP Sheet.
In the oral system (12 items), we placed complementary facial description items from the NIDCAP Sheet. Several behaviours have been added to prompt a more precise description of the oral sphere and the coordination of sucking, swallowing and breathing.
2.5. Ethical consideration
The study protocol was approved by the South Mediterranean IV Ethics Committee (reference: CPP 15.09.03sc). Both parents gave written consent to participate in this study with their newborn. Participation consisted of having their infant's behaviour filmed over a single period of up to 1.5 hr, with the understanding that they might also be filmed if they were providing the care themselves. The parents also authorized the possible transfer of the recordings to a secure site for the sole purpose of carrying out the study.
2.6. Data analysis
2.6.1. Reference rating of the film sequences
The reference for our behavioural observation form for each film was developed by two professionals with advanced practice certification, a paediatrician and a paediatric nurse, both members of the steering committee. They each completed three forms to describe the newborn's behaviour before, during and after the care intervention. The separation of the three sequences was clearly indicated on each film. For all the films, there were discrepancies in the ratings of these two experts and, according to the protocol, they were resolved by a third certified expert, also a member of the steering committee. The third expert rated each film on the observation forms, blinded to the results of the other two. The final decision about a discrepancy was resolved as follows: the third expert agreed with one of the first two experts, for a simple majority.
2.6.2. Rating of films by the RNDCs
Each patient film was assigned a random number. The 12 RNDCs had personal access codes to a server to view the films, with the order of the films for each nurse also determined by a random list. The nurses were free to watch the films when they wished and in the manner that suited them best, within a period of approximately 1 year after opening the server. To standardize the conditions for rating the films, the server was programmed to allow viewing of each film at most two times. Responses to the behavioural items on the observation forms were formatted as present/absent in the electronic case report form.
2.6.3. Statistical analysis
All analyses were conducted by the Department of Research and Medical Information of our hospital, using statistical software (SAS Enterprise Guide, version 7.13; SAS Institute).
Sample size calculation
To calculate the sample size, we made an estimation of the required number of films to be rated. From an expected rate of correct rating of at least 80%, a standard deviation of 12%, and accuracy of ±2.5%, 89 ratings, that is eight films per nurse, were needed. To take into account the data correlation due to each nurse rating several films, the sample size was increased by applying an inflation factor. This factor was defined as (1 + [m − 1] ρ), where m is the number of ratings per nurse (=8) and ρ is the correlation between ratings for the same nurse (=0.04), giving an inflation coefficient of 1.3, that is an increase of 27 ratings and a total number of (89 + 27) 116 ratings. As each film would be rated by 12 nurses, it was necessary to include and film 10 neonates to obtain 120 ratings.
Primary outcome
The ability to accurately observe newborn behaviour was assessed by comparing the RNDC ratings of the films to the reference ratings. RNDC ratings were assumed to be accurate if the median rate was at least 80%. To calculate this median rate and its interquartile range (Q25, Q75), the proportions of correct answers were estimated for each nurse on all the items in each film sequence—that is before, during and after the care—and all the neonates, and were then averaged.
Secondary outcomes
The correct answer rates were calculated for each system to determine whether some were more difficult than others. The rates were estimated for each nurse for all the items of each system, and all newborns and were then averaged (median, Q25, Q75, min‐max). Overall comparisons between systems were evaluated with the Friedman test. In cases of significant difference, paired comparisons were made for all the systems, with Bonferroni correction to account for the multiplicity of tests.
A similar analysis was made for each item to determine the relative difficulty of answering correctly. Based on the median values, heat maps were generated to identify the most concordant or discordant items with the reference ratings (Salanti et al., 2011), using a green gradient code for a value of at least 80%.
A variability score was established for each item according to the following method. The absence of a change indicated an identical answer on the item for the three sequences of the same film, that is before, during and after care. A single change of answer between the sequences indicated a moderate change. A change in the answer for each of the sequences indicated a strong change. The variability score for each item for the 10 films was calculated according to the empirical formula: (0 * number of absence of change) + (1 * number of moderate changes) + (2 * number of strong changes). The absolute score, which could vary theoretically from 0–20, was expressed as a numerical value between 0–1 from the reference answers. A variability score and a correct answer rate were calculated for each system. The correlation between the variability score of each item and the correct answer rate was assessed for each system by the Spearman correlation coefficient.
3. RESULTS
3.1. Infants
The median gestational age and birth weight of the 10 newborns were, respectively, 30 (25.9, 31.4) weeks and 1,125 (920, 1,280) g. At the moment of filming, postnatal age, corrected gestational age and weight were 19 (6, 46) days, 32.5 (30.9, 34.9) weeks, and 1,273 (1,080, 1,500) g. The main characteristics of the pregnancy, newborns and care performed are shown in Table 2.
TABLE 2.
Characteristics of pregnancy and newborns at the moment of the filmed sequences
| Pregnancy | |
| Antenatal steroids‐ 2 BM doses | 7 (70) |
| Antenatal steroids‐ 1 BM dose | 3 (30) |
| Singleton | 8 (80) |
| Respiratory care | |
| Invasive ventilation | 1 (10) |
| CPAP or HFNC | 6 (60) |
| Spontaneous ventilation | 3 (30) |
| Epicutaneocava catheter | 7 (70) |
| Nasogastric tube | 9 (90) |
| Feeding | |
| Breastfeeding or bottle feeding only | 1 (10) |
| Nasogastric tube only | 8 (80) |
| Both, including use of supplemental feeding tube device | 1 (10) |
| Milk type | |
| Human milk | 7 (70) |
| Preterm formula | 3 (30) |
| Type of bed | |
| Incubator | 7 (70) |
| Radiant table | 1 (10) |
| Cradle | 2 (20) |
| Care provided | |
| Aspiration | 2 (20) |
| Bathing | 1 (10) |
| Breastfeeding | 3 (30) |
| Facial cleaning (eyes, mouth) | 3 (30) |
| Gastric tube placement | 1 (10) |
| Kangaroo mother care | 4 (40) |
| Diaper change and/or dressing | 6 (60) |
| Temperature taking | 3 (30) |
| Weighing and/or other anthropometric measurements | 3 (30) |
| Parental presence | |
| Two parents | 3 (30) |
| One parent | 6 (60) |
| No parent | 1 (10) |
Values are numbers (%). Several care interventions may have been done in the same film.
Abbreviations: BM, betamethasone; CPAP, continuous positive airway pressure; HFNC, high flow nasal cannula.
3.2. Film viewing
The films were produced and then viewed by the 12 RNDCs between December 2015–February 2017. Each film was seen two (1, 2) times by the nurses before they filled in the forms.
3.3. Primary outcome
The median rate of correct answers of the 12 RNDCs was 83.2 (81.5, 84.4)%, with extreme values of 77.0% and 84.8%. The rate of correct answers for the entire sheet was lower during care compared to before or after care (Table 3).
TABLE 3.
Correct answer rate (%) for the whole sheet and the systems, according to care intervention, for the 12 nurses during the viewing of the 10 test films
| Sequence | Before | During | After | p* |
|---|---|---|---|---|
| Whole sheet |
85.4 (83.2, 86.4) [81.3–86.6] |
77.8 (75.5, 79.5) [67.3–81.0] |
85.8 (85.1, 87.3) [82.4–88.0] |
<.001 |
| Autonomic nervous |
85.0 (83.6, 86.9) 1 [81.4–89.3] |
77.8 (76.4, 80.2) [71.0–83.4] |
88.8 (87.8, 90.2)3 [86.9–92.4] |
<.001 |
| Motor |
77.7 (73.4, 80.0) [69.2–86.2] |
76.2 (70.8, 79.6) [52.3–85.4] |
76.6 (75.8, 79.6) [73.8–82.3] |
.20 |
| Sleep‐wake |
87.1 (84.3, 90.0) 1 [81.4–92.9] |
76.4 (72.5, 79.0) [66.4–82.1] |
87.9 (85.7, 90.4)3 [82.9–92.1] |
<.001 |
| Interactional |
86.3 (84.2, 90.0) 1 [81.7–91.7] |
76.3 (72.1, 79.2) [70.8–84.2] |
81.3 (77.5, 83.3) [70.8–88.3] |
.002 |
| Self‐regulation |
74.4 (70.7, 80.0) [66.3–83.8] |
70.7 (64.4, 75.7) [40.0–80.0] |
85 (82.5, 88.8)4 [78.8–91.3] |
<.001 |
| Oral |
93.4 (91.7, 95.0)2 [90.8–95.8] |
85.4 (82.5, 87.5)2 [79.2–90.0] |
91.3 (88.3, 92.5)5 [84.2–94.2] |
<.001 |
Values are median (Q25, Q75) [minimal‐maximal].
p*: comparison between the three sequence using Friedman's test.
p < .05 versus motor, self‐regulation, and oral systems, 2 p < .05 versus all other systems, 3 p < .05 versus motor and interactional systems, 4 p < .05 versus motor and oral systems,5 p < .05 versus motor, interactional and self‐regulation systems using Bonferroni correction.
3.4. Secondary outcomes
3.4.1. Correct answer rate for the systems according to the film sequence (Table 3)
Before the intervention, the correct answer rate was highest for the oral system compared to all other systems. It was lowest for the self‐regulation and motor systems, with no difference between them.
During the care intervention, the correct answer rate remained higher for the oral system compared to all other systems.
After the care intervention, the correct answer rate was again higher for the oral system. It was the lowest for the motor system.
3.4.2. Correct answer rate according to the items
For the autonomic nervous system, 18 items out of 29 (62%) reached an optimal rate of correct answers, that is >80% for all sequences. The lowest scores were obtained for the items assessing respiration, that is extreme respiratory rates, changes in respiratory rate and breathing pauses. Two items—pink complexion and tremoring—were scored <80% on the three assessments (Figure 1a).
FIGURE 1.
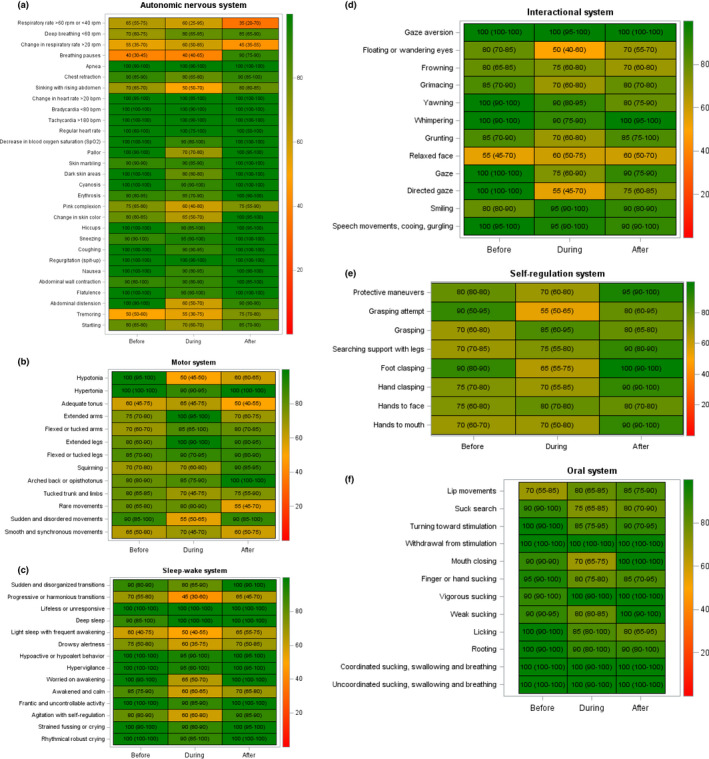
Heat maps of the 88 items of the behavioural observation sheet. The colour scale represents the median values of the correct answers for the 12 nurses and 10 films. The scale ranges from 0 (red, indicating an answer is always false)–100 (green, indicating an answer is always true). The value in each box indicates the median value of the answer in comparison with the reference answer. (a) Autonomic nervous system (29 items); (b) Motor system (13 items); (c) Sleep‐wake system (14 items); (d) Interactional system (12 items); (e) Self‐regulation system (8 items); (f) Oral system (12 items)
For the motor system, optimal scores were obtained for only 4/13 (31%) items. Two items—adequate tone and smooth movements—were scored suboptimally in all three sequences (Figure 1b).
For the sleep‐wake system, eight out of 14 items (58%) were scored satisfactorily for all sequences. Three items—progressive transitions, light sleep and drowsy alertness—were scored suboptimally for all three sequences (Figure 1c).
For the interactional system, the scores were optimal for all sequences for five out of 12 items (42%). The lowest score was observed for the relaxed face item (Figure 1d).
For the self‐regulation system, none of the eight items had an optimal score for all sequences. The lowest score was obtained for grasping attempt (Figure 1e).
For the oral system, nine out of 12 items (78%) had an optimal score for all sequences. Three items—lip movements, suck search and mouth closing—were scored suboptimally for a single sequence (Figure 1f).
3.4.3. Correct answer rate according to item variability
Table 1 shows the variability of each item for the 10 films. The median value was 0.2 (0.08, 0.50), with extreme values of 0 and 0.8. The variability score for items grouped into systems is shown in Table 4. This score was different between systems (p < .01), with greater variability for the motor system and the self‐regulation system. The correct answer rate was also different between systems (p = .02), with lower rates for these two systems. Inverse correlations were found between the correct answer rate and the variability score for three systems with low item variability: the autonomic nervous, sleep‐wake and interactional systems.
TABLE 4.
Variability (%) and correct answer rate of items grouped into systems
| Variability score | Correct answer rate (%) | ρ | p | |
|---|---|---|---|---|
| Autonomic nervous |
0.10 (0.00, 0.25) [0.00–0.55] |
93.3 (75.0, 96.7) [56.7–1.0] |
−0.79 | <.001 |
| Motor |
0.55 (0.30, 0.60) 1 [0.10–0.80] |
78.3 (68.3, 85.0) 2 [11.7–96.7] |
0.15 | .63 |
| Sleep‐wake |
0.13 (0.00, 0.45) [0.00–0.60] |
90.0 (75.0, 96.7) [58.3–1.0] |
−0.76 | .002 |
| Interactional |
0.25 (0.10, 0.53) [0.00–0.75] |
82.5 (74.2, 91.7) [58.3–1.0] |
−0.72 | .008 |
| Self‐regulation |
0.50 (0.38, 0.55) 1 [0.30–0.80] |
78.3 (75.8, 82.5) 2 [73.3–83.3] |
0.14 | .75 |
| Oral |
0.18 (0.03, 0.23) [0.00–0.45] |
90.0 (86.7, 98.3) [78.3–1.0] |
0.47 | .12 |
Values are median (Q25, Q75) [minimal‐maximal]. ρ: Spearman correlation coefficient and p: null probability associated.
p < .01 versus autonomic nervous and oral systems.
p < .01 versus oral system, using Bonferroni correction.
4. DISCUSSION
This study based on video simulation suggested the satisfactory ability of the RNDCs to correctly observe the behaviour of newborns after training by NIDCAP professionals and regular use of the behavioural observational sheet. It also underlined the general difficulty of observing infants while giving care, and the difficulties of observing certain components or systems, especially if they varied throughout caregiving.
Although NIDCAP has changed the NICU practices and environment and increased parental involvement (Als et al., 2003; Greisen et al., 2009; Pierrat et al., 2016), few studies have provided factual data on the dissemination of observation skills to uncertified caregivers after programme implementation in a neonatal unit or department. According to the Edmonton NIDCAP trial, approximately 20% of the NICU staff members had been educated to provide NIDCAP‐based care, and the results suggested that this education had effectively imparted to nurses the ability to understand and respond to preterm infant behaviour (Peters et al., 2009). Conversely, our results were observed in the context of in‐house training and guidance for daily practice provided occasionally by NIDCAP‐trained staff members.
The views on the NIDCAP care plans expressed by the nurses were sometimes associated with the feeling that preparing them was time‐consuming (Mosqueda et al., 2013; van der Pal et al., 2007). Our results suggest that the RNDCs had acquired competence in observing newborns, which theoretically may have enabled them to adjust their caregiving to the infant's developmental status. Previous investigations have suggested a link between individualized developmental care and physiological stability, which may reduce the time spent mainly on nursing care interventions (Brown & Heermann, 1997; Stevens et al., 1996; Westrup et al., 2002).
The significant decrease in the rate of overall correct answers on the whole form during care is important because behavioural observations during caregiving provide the basis for recommendations to minimize stress and optimize an infant's development (Als & McAnulty, 2011). According to the NIDCAP approach, the caregiver should first objectively observe behaviour during the input represented by the care and then interpret it in terms of approach or withdrawal. One can speculate that the RNDCs at least partially overinterpreted the film sequences instead of strictly observing the different components. Indeed, most were aware of the significant distress or pain associated with routine nursing in these patients. Viewing films of infants also triggers caretaking behaviour towards the infant, with amplified motivation in stress conditions in women compared to men (Probst et al., 2017). Our sample included only women, who might have felt judged in their professional skills and, as a result, been exposed to stress.
Most of the components of the autonomic nervous system are part of traditional patient monitoring, and it was unsurprising that most of them were correctly observed in all sequences. We were, however, surprised by inaccurate answers for components like respiratory rate and its changes, and breathing pauses. In formal NIDCAP observations, breathing is the focus of meticulous clinical observation, which may involve careful counting of respiratory movements, whereas RNDCs might be more likely to pick up this information from continuous cardiorespiratory monitoring, as in daily NICU practice. Recognition of sleep‐wake states and sleep patterns is also a concern in day‐to‐day monitoring to determine the optimal periods for feeding and care. The form that we used described different states of consciousness, which were generally well recognized by the nurses. A few items, however, raised questions. Reliability in observing harmonious sleep‐wake transition types was poor, and light sleep with frequent awakening was also misidentified. Several works have highlighted the rapid changes of state in these patients, notably occurring for subtle to moderate environmental variations (Kuhn et al., 2013; Zores et al., 2018). The interactional system exclusively included a facial repertoire, which allows subtle and complex human expressions from birth (Als & McAnulty, 2011). In NICUs, all pain scales focus on facial expression, which is considered highly discriminating for the detection and assessment of pain intensity (Milesi et al., 2010). Consistently, components referring to static cues, such as floating eyes and relaxed face, were generally rated less accurately than those referring to dynamic cues and based on facial musculature, such as smiling and yawning.
The answers were suboptimal for two systems: motor and self‐regulation. Components including muscle tone may be difficult to assess only by viewing films, as they are better appreciated by coupling vision and physical contact with the infant, as during smooth manipulation (Goo et al., 2018). Self‐regulation is by definition an interpretation of the infant's behavioural signals. Proper reading of the components probably requires advanced observation experience in order to individualize the strategies used by the infants to calm themselves and relax even after mild stress (Vandenberg, 2007).
The oral system is not individualized in the model of the synactive organization of behavioural development (Als et al., 2005), but components appear on the NIDCAP Behavioral Observation Sheet and were grouped and completed under this terminology. It could be expected that answers in this domain would reach the highest scores regardless of the sequence, as infant feeding is a basic concern taught widely in nurse educational programmes.
The main objective in assessing item variability during the films was to determine whether variability would be a source of error in the answers. The unit of measure for a correct answer was therefore the item and not the RNDC, as for the other analyses. The hypothesis of variability as a source of error was proved correct because the two systems with the highest item variability had the lowest correct answer rates and, conversely, for three out of four systems with low item variability, the inverse correlation was found. These results may be instructive for research and teaching in the field of behavioural observation of the newborn.
4.1. Study limitations
The number of films was modest, with only one showing an infant supported with invasive mechanical ventilation. A recent study performed in our department underlined the priority given to noninvasive ventilation in the management of premature infants, the duration of invasive ventilation currently representing only 6% of the total duration of respiratory support (Habas et al., 2020).
The nurses selected for this study were only those trained and chaperoned by their peers with advanced practice certification. The regulations on the nurse‐to‐infant ratio in an approximately 60‐bed department of neonatology mean that our network of RNDCs represented only 10% of the nursing workforce. The contribution of these professionals is nevertheless critical in ensuring the dissemination of skills in newborn observation and the practice of developmental care among the staff (Hamilton & Redshaw, 2009).
Our study was not carried out within the context of a relationship with an infant and his or her family, and we cannot infer results that would have been obtained in the very different environment of real life. We chose film observation because it offered a standardized evaluation framework, and “in vivo” evaluation is now needed to confirm these results observed under conditions of “simulated reality.”
The NIDCAP Observation Sheet has a very solid theoretical foundation (Als & McAnulty, 2011). Our in‐house form was inspired by this tool but does not have comparable validity. Thus, its use is limited to promoting patient observation and facilitating communication between NIDCAP certified and non‐certified professionals in our department. Nevertheless, it was selected for the study because the RNDCs have used it regularly since training in individual developmental care. This factor limits the generalization of our methods to other departments or units that might want to evaluate the dissemination of newborn observation skills among professionals.
Finally, while our study suggests that the RNDCs had acceptable skills in behavioural observation, it did not assess their ability to write the narrative reports that share an understanding of the infant's development and proposals for individualized care plans with both parents and other caregivers. These observational reports are nevertheless paramount for supporting ongoing brain development (Als et al., 2003, 2004, 2012; Buehler et al., 1995).
5. CONCLUSION
This film simulation study suggested that NIDCAP education is able to prompt the generation of tools and organization within a department of neonatology and the dissemination of relevant observations of newborn behaviour beyond the nucleus of certified professionals. This may result in an improved quality of patient care and better communication among staff. This educational device could also help identify, on an individual basis, the infants who need formal observations in a team with limited resources in NIDCAP experts.
CONFLICT OF INTEREST
The authors have no financial relationships and no potential conflicts of interest relevant to this article to disclose.
AUTHOR CONTRIBUTION
Laurence Chandebois, Sabine Durand, Renaud Mesnage and Gilles Cambonie conceptualized and designed the study and drafted the manuscript. Erika Nogué and Nicolas Nagot carried out the statistical analyses and reviewed the manuscript. Catherine Bouschbacher, Florence Masson and Laurence Chandebois established the reference behavioural observation forms for each film and reviewed the manuscript. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
ACKNOWLEDGEMENTS
We thank all the parents and their infants for their participation in this study. We also thank the Communications Department of Montpellier University Hospital for its invaluable assistance in producing the films and to Ms Bentz for her support in drafting the grant. We are also very indebted to Dr Joy Browne (University of Colorado, USA) and Dr Nathalie Ratinsky (University of Western Brittany, France) for providing NIDCAP training to the Department of Neonatology of Montpellier University Hospital. We extend our warm thanks to the members of the NIDCAP steering committee, which included, in addition to the authors of the article, Stella Penez, Patricia Fournier and Anne Gimenez (nurse managers), Anne Lemaitre and Karine Bertran de Balanda clinical (clinical psychologists), Nicole Boulay (psychomotor therapist), Guillaume du Chaffaut (member of the hospital management) and the RNDCs who participated in this study: Claire Alcacer, Marie Pierre Atienza, Delphine Beuque, Eloise Carbonneau, Laetitia Ciotoli, Florence Dartigues, Agnes Fabre, Alexandra Galabru, Laurence Galant, Julie Graille, Stephanie Riera, and Elodie Tavernier.
Chandebois L, Nogue E, Bouschbacher C, et al. Dissemination of newborn behavior observation skills after Newborn Individualized Developmental Care and Assessment Program (NIDCAP) implementation. Nurs Open. 2021;8:3547–3557. 10.1002/nop2.904
Funding information
All phases of this study were supported by Montpellier University Hospital (Grant: Appel à projets 2015 infirmiers et psychologues).
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Als, H. , Butler, S. , Kosta, S. , & McAnulty, G. (2005). The Assessment of Preterm Infants' Behavior (APIB): Furthering the understanding and measurement of neurodevelopmental competence in preterm and full‐term infants. Mental Retardation and Developmental Disabilities Research Reviews, 11(1), 94–102. 10.1002/mrdd.20053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Als, H. , Duffy, F. H. , McAnulty, G. , Butler, S. C. , Lightbody, L. , Kosta, S. , Weisenfeld, N. I. , Robertson, R. , Parad, R. B. , Ringer, S. A. , Blickman, J. G. , Zurakowski, D. , & Warfield, S. K. (2012). NIDCAP improves brain function and structure in preterm infants with severe intrauterine growth restriction. Journal of Perinatology, 32(10), 797–803. 10.1038/jp.2011.201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Als, H. , Duffy, F. H. , McAnulty, G. B. , Rivkin, M. J. , Vajapeyam, S. , Mulkern, R. V. , Warfield, S. K. , Huppi, P. S. , Butler, S. C. , Conneman, N. , Fischer, C. , & Eichenwald, E. C. (2004). Early experience alters brain function and structure. Pediatrics, 113(4), 846–857. 10.1542/peds.113.4.846 [DOI] [PubMed] [Google Scholar]
- Als, H. , Gilkerson, L. , Duffy, F. H. , Mcanulty, G. B. , Buehler, D. M. , Vandenberg, K. , Sweet, N. , Sell, E. , Parad, R. B. , Ringer, S. A. , Butler, S. C. , Blickman, J. G. , & Jones, K. J. (2003). A three‐center, randomized, controlled trial of individualized developmental care for very low birth weight preterm infants: Medical, neurodevelopmental, parenting, and caregiving effects. Journal of Developmental and Behavioral Pediatrics, 24(6), 399–408. 10.1097/00004703-200312000-00001 [DOI] [PubMed] [Google Scholar]
- Als, H. , Lawhon, G. , Brown, E. , Gibes, R. , Duffy, F. H. , McAnulty, G. , & Blickman, J. G. (1986). Individualized behavioral and environmental care for the very low birth weight preterm infant at high risk for bronchopulmonary dysplasia: Neonatal intensive care unit and developmental outcome. Pediatrics, 78(6), 1123–1132. [PubMed] [Google Scholar]
- Als, H. , & McAnulty, G. B. (2011). The newborn individualized developmental care and assessment program (NIDCAP) with kangaroo mother care (KMC): Comprehensive care for preterm infants. Current Women’s Health Reviews, 7(3), 288–301. 10.2174/157340411796355216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, L. D. , & Heermann, J. A. (1997). The effect of developmental care on preterm infant outcome. Applied Nursing Research, 10(4), 190–197. 10.1016/s0897-1897(97)80572-1 [DOI] [PubMed] [Google Scholar]
- Buehler, D. M. , Als, H. , Duffy, F. H. , McAnulty, G. B. , & Liederman, J. (1995). Effectiveness of individualized developmental care for low‐risk preterm infants: Behavioral and electrophysiologic evidence. Pediatrics, 96(5 Pt 1), 923–932. [PubMed] [Google Scholar]
- Coughlin, M. , Gibbins, S. , & Hoath, S. (2009). Core measures for developmentally supportive care in neonatal intensive care units: Theory, precedence and practice. Journal of Advanced Nursing, 65(10), 2239–2248. 10.1111/j.1365-2648.2009.05052.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goo, M. , Tucker, K. , & Johnston, L. M. (2018). Muscle tone assessments for children aged 0 to 12 years: A systematic review. Developmental Medicine and Child Neurology, 60(7), 660–671. 10.1111/dmcn.13668 [DOI] [PubMed] [Google Scholar]
- Greisen, G. , Mirante, N. , Haumont, D. , Pierrat, V. , Pallás‐Alonso, C. R. , Warren, I. , Smit, B. J. , Westrup, B. , Sizun, J. , Maraschini, A. , & Cuttini, M. (2009). Parents, siblings and grandparents in the Neonatal Intensive Care Unit. A survey of policies in eight European countries. Acta Paediatrica, 98(11), 1744–1750. 10.1111/j.1651-2227.2009.01439.x [DOI] [PubMed] [Google Scholar]
- Habas, F. , Durand, S. , Milési, C. , Mesnage, R. , Combes, C. , Gavotto, A. , Picaud, J. C. , & Cambonie, G. (2020). 15‐Year trends in respiratory care of extremely preterm infants: Contributing factors and consequences on health and growth during hospitalization. Pediatric Pulmonology, 55, 1946–1954. 10.1002/ppul.24774 [DOI] [PubMed] [Google Scholar]
- Hamilton, K. E. , & Redshaw, M. E. (2009). Developmental care in the UK: A developing initiative. Acta Paediatrica, 98(11), 1738–1743. 10.1111/j.1651-2227.2009.01431.x [DOI] [PubMed] [Google Scholar]
- Kleberg, A. , Hellström‐Westas, L. , & Widström, A. M. (2007). Mothers' perception of newborn individualized developmental care and assessment program (NIDCAP) as compared to conventional care. Early Human Development, 83(6), 403–411. 10.1016/j.earlhumdev.2006.05.024 [DOI] [PubMed] [Google Scholar]
- Kuhn, P. , Zores, C. , Langlet, C. , Escande, B. , Astruc, D. , & Dufour, A. (2013). Moderate acoustic changes can disrupt the sleep of very preterm infants in their incubators. Acta Paediatrica, 102(10), 949–954. 10.1111/apa.12330 [DOI] [PubMed] [Google Scholar]
- Milesi, C. , Cambonie, G. , Jacquot, A. , Barbotte, E. , Mesnage, R. , Masson, F. , Pidoux, O. , Ferragu, F. , Thevenot, P. , Mariette, J.‐B. , & Picaud, J.‐C. (2010). Validation of a neonatal pain scale adapted to the new practices in caring for preterm newborns. Archives of Disease in Childhood Fetal and Neonatal Edition, 95(4), F263–F266. 10.1136/adc.2008.144758 [DOI] [PubMed] [Google Scholar]
- Montirosso, R. , De Prete, A. , Bellù, R. , Tronick, E. , Borgatti, R. , & Neonatal Adequate Care for Quality of Life (NEO‐ACQUA) Study Group . (2012). Level of NICU quality of developmental care and neurobehavioral performance in very preterm infants. Pediatrics, 129(5), e1129–e1137. 10.1542/peds.2011-0813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosqueda, R. , Castilla, Y. , Perapoch, J. , de la Cruz, J. , López‐Maestro, M. , & Pallás, C. (2013). Staff perceptions on Newborn Individualized Developmental Care and Assessment Program (NIDCAP) during its implementation in two Spanish neonatal units. Early Human Development, 89(1), 27–33. 10.1016/j.earlhumdev.2012.07.013 [DOI] [PubMed] [Google Scholar]
- Nelson, A. M. , & Bedford, P. J. (2016). Mothering a preterm infant receiving NIDCAP care in a level III newborn intensive care unit. Journal of Pediatric Nursing, 31(4), e271–e282. 10.1016/j.pedn.2016.01.001 [DOI] [PubMed] [Google Scholar]
- Ohlsson, A. , & Jacobs, S. E. (2013). NIDCAP: A systematic review and meta‐analyses of randomized controlled trials. Pediatrics, 131(3), e881–e893. 10.1542/peds.2012-2121 [DOI] [PubMed] [Google Scholar]
- Peters, K. L. , Rosychuk, R. J. , Hendson, L. , Coté, J. J. , McPherson, C. , & Tyebkhan, J. M. (2009). Improvement of short‐ and long‐term outcomes for very low birth weight infants: Edmonton NIDCAP trial. Pediatrics, 124(4), 1009–1020. 10.1542/peds.2008-3808 [DOI] [PubMed] [Google Scholar]
- Pierrat, V. , Coquelin, A. , Cuttini, M. , Khoshnood, B. , Glorieux, I. , Claris, O. , Durox, M. , Kaminski, M. , Ancel, P.‐Y. , Arnaud, C. , & EPIPAGE‐2 Neurodevelopmental Care Writing Group . (2016). Translating neurodevelopmental care policies into practice: The experience of neonatal ICUs in France‐The EPIPAGE‐2 cohort study. Pediatric Critical Care Medicine, 17(10), 957–967. 10.1097/PCC.0000000000000914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Probst, F. , Meng‐Hentschel, J. , Golle, J. , Stucki, S. , Akyildiz‐Kunz, C. , & Lobmaier, J. S. (2017). Do women tend while men fight or flee? Differential emotive reactions of stressed men and women while viewing newborn infants. Psychoneuroendocrinology, 75, 213–221. 10.1016/j.psyneuen.2016.11.005 [DOI] [PubMed] [Google Scholar]
- Salanti, G. , Ades, A. E. , & Ioannidis, J. P. (2011). Graphical methods and numerical summaries for presenting results from multiple‐treatment meta‐analysis: An overview and tutorial. Journal of Clinical Epidemiology, 64(2), 163–171. 10.1016/j.jclinepi.2010.03.016 [DOI] [PubMed] [Google Scholar]
- Sannino, P. , Giannì, M. L. , De Bon, G. , Fontana, C. , Picciolini, O. , Plevani, L. , Fumagalli, M. , Consonni, D. , & Mosca, F. (2016). Support to mothers of premature babies using NIDCAP method: A non‐randomized controlled trial. Early Human Development, 95, 15–20. 10.1016/j.earlhumdev.2016.01.016 [DOI] [PubMed] [Google Scholar]
- Stevens, B. , Petryshen, P. , Hawkins, J. , Smith, B. , & Taylor, P. (1996). Developmental versus conventional care: A comparison of clinical outcomes for very low birth weight infants. The Canadian Journal of Nursing Research, 28(4), 97–113. [PubMed] [Google Scholar]
- van der Pal, S. M. , Maguire, C. M. , Cessie, S. L. , Veen, S. , Wit, J. M. , Walther, F. J. , & Bruil, J. (2007). Staff opinions regarding the Newborn Individualized Developmental Care and Assessment Program (NIDCAP). Early Human Development, 83(7), 425–432. 10.1016/j.earlhumdev.2007.03.007 [DOI] [PubMed] [Google Scholar]
- Vandenberg, K. A. (2007). Individualized developmental care for high risk newborns in the NICU: A practice guideline. Early Human Development, 83(7), 433–442. 10.1016/j.earlhumdev.2007.03.008 [DOI] [PubMed] [Google Scholar]
- Westrup, B. (2015). Family‐centered developmentally supportive care: The Swedish example. Archives De Pediatrie, 22(10), 1086–1091. 10.1016/j.arcped.2015.07.005 [DOI] [PubMed] [Google Scholar]
- Westrup, B. , Stjernqvist, K. , Kleberg, A. , Hellström‐Westas, L. , & Lagercrantz, H. (2002). Neonatal individualized care in practice: A Swedish experience. Seminars in Neonatology, 7(6), 447–457. 10.1053/siny.2002.0150 [DOI] [PubMed] [Google Scholar]
- Zores, C. , Dufour, A. , Pebayle, T. , Dahan, I. , Astruc, D. , & Kuhn, P. (2018). Observational study found that even small variations in light can wake up very preterm infants in a neonatal intensive care unit. Acta Paediatrica, 107(7), 1191–1197. 10.1111/apa.14261 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


