Abstract
Cystic fibrosis (CF) predisposes patients to bacterial colonization and infection of the lower airways. Several species belonging to the genus Burkholderia are potential CF-related pathogens, but microbiological identification may be complicated. This situation is not in the least due to the poorly defined taxonomic status of these bacteria, and further validation of the available diagnostic assays is required. A total of 114 geographically diverse bacterial isolates, previously identified in reference laboratories as Burkholderia cepacia (n = 51), B. gladioli (n = 14), Ralstonia pickettii (n = 6), B. multivorans (n = 2), Stenotrophomonas maltophilia (n = 3), and Pseudomonas aeruginosa (n = 11), were collected from environmental, clinical, and reference sources. In addition, 27 clinical isolates putatively identified as Burkholderia spp. were recovered from the sputum of Dutch CF patients. All isolates were used to evaluate the accuracy of two selective growth media, four systems for biochemical identification (API 20NE, Vitek GNI, Vitek NFC, and MicroScan), and three different PCR-based assays. The PCR assays amplify different parts of the ribosomal DNA operon, either alone or in combination with cleavage by various restriction enzymes (PCR-restriction fragment length polymorphism [RFLP] analysis). The best system for the biochemical identification of B. cepacia appeared to be the API 20NE test. None of the biochemical assays successfully grouped the B. gladioli strains. The PCR-RFLP method appeared to be the optimal method for accurate nucleic acid-mediated identification of the different Burkholderia spp. With this method, B. gladioli was also reliably classified in a separate group. For the laboratory diagnosis of B. cepacia, we recommend parallel cultures on blood agar medium and selective agar plates. Further identification of colonies with a Burkholderia phenotype should be performed with the API 20NE test. For final confirmation of species identities, PCR amplification of the small-subunit rRNA gene followed by RFLP analysis with various enzymes is recommended.
Pulmonary colonization with Burkholderia cepacia is associated with a poor clinical prognosis for patients with cystic fibrosis (CF) (23). A relatively constant fraction of CF patients in Western European countries appear to be colonized with this usually multiple-antibiotic-resistant organism. However, the prevalence in individual CF centers may differ widely due to epidemic bursts of infection and problems with the identification of the microorganism. Interestingly, in some CF patients, long-term colonization can occur without an adverse effect on lung function (8). On the other hand, some individuals deteriorate rapidly after colonization, and death may occur within 1 to 6 months (8). There is also evidence that particular clonal isolates of B. cepacia can be easily transmitted from person to person (12, 25, 31, 32). Separating B. cepacia-colonized or -infected patients from other CF patients has been used to prevent bacterial spread, but this practice has a severe social and psychological impact on the patients and their family members (10). In The Netherlands, colonization with B. cepacia is considered a contraindication for lung transplantation. However, this point of view is not internationally acknowledged (19). Because of the serious implications of the identification of B. cepacia in patients with CF, microbiological diagnosis should be carried out as accurately as possible. However, the isolation and reliable identification of B. cepacia have been complicated and difficult (6, 9, 15, 21). Although the pathogenicity of the closely related species B. gladioli has not been definitely assessed, reliable pathogenicity studies can only be performed once precise species identification can be achieved in the routine microbiology laboratory.
B. cepacia has recently been described as a complex of multiple genetic types, or genomovars: B. cepacia (genomovar I), B. multivorans (genomovar II), genomovars III and IV (36), and the closely related new species B. vietnamiensis (formerly genomovar IV) (4, 9, 33). Strains of all of these genomovars, B. vietnamiensis, and B. gladioli have been isolated from CF patients. Several of the Burkholderia spp. are of commercial importance because they can be used as biopesticides, as plant growth promoters, or for the degradation of environmental pollutants (4, 18, 22, 33).
In The Netherlands, the identification of putative B. cepacia isolates is generally performed by conventional biochemical analyses. Different institutions use different approaches; even when essentially the same diagnostic scheme has been applied, several discrepancies have become apparent upon close evaluation and comparison of the diagnostic data obtained. Moreover, the lack of a well-evaluated and generally accepted diagnostic “gold standard” technique is considered a significant limitation by all of those involved in the management of and care for CF patients. In this communication, we address the lack of gold standard microbiology techniques and describe the results of conventional and molecular identification procedures applied to a large and diverse collection of Burkholderia strains. Finally, a method for the optimal identification of B. cepacia and other clinically relevant Burkholderia spp. is suggested on the basis of the results of this comparative study.
MATERIALS AND METHODS
Collection and initial identification of strains.
Several strains of B. cepacia, B. gladioli, Ralstonia pickettii (formerly B. pickettii [39]), B. multivorans, Stenotrophomonas maltophilia, and Pseudomonas aeruginosa were obtained from expert microbiology centers in Germany, Canada, France, Sweden, and The Netherlands. The characteristics of the strains are given in Table 1. Note that some of the reference strains obtained from the different participants are the same. B. cepacia ATCC 25416, for instance, was obtained from three centers. The strain used in our Rotterdam laboratory was obtained directly from the American Type Culture Collection and, as such, provided an accurate species control specimen. Table 1 summarizes the methods of identification used in the strain contributor home laboratories.
TABLE 1.
Geographical, clinical, and microbiological characteristics of the strain collectiona
| Origin | Organism | No. of isolates | Source | Method of identification by the study participants |
|---|---|---|---|---|
| RIVMb | B. cepacia | 2 | Sputa, lung biopsy | 16S rRNA sequence |
| B. gladioli | 5 | CF patient | Biochemical | |
| S. maltophilia | 3 | Sputa | ||
| University of British Columbia, Vancouver, British Columbia, Canada | B. cepacia | 4 | CF patient | API 20NE, biochemical (15, 23) |
| 1 | Soil (ATCC 25416) | |||
| B. multivorans | 1 | CF patient | ||
| 1 | Soil (ATTC 17616) | |||
| B. gladioli | 2 | CF patient, CGD | ||
| 1 | Onion (ATCC 10248) | |||
| Sweden | B. cepacia | 4 | CCUG 788, CCUG 12691, CCUG 9631, CCUG 36978 | |
| R. pickettii | 2 | CCUG 3314, CCUG 3318 | ||
| B. gladioli | 2 | CCUG 1782, CCUG 2115 | ||
| Medizinische Hochschule, Hannover, Germany | B. cepacia | 33 | CF patient | SpeI fragment pattern and/or sequencing of 16S rRNA, BCSA, OFPBL, API 20NE |
| 1 | Environment | |||
| B. gladioli | 2 | CF patient | ||
| Universite Paul Sabatier, Toulouse, France | B. cepacia | 3 | ATCC 25416, ATCC 17759, ATCC 25609 | |
| B. gladioli | 2 | ATCC 10248, ATCC 19302 | ||
| AZR; SKZc | B. cepacia | 2 | ATCC 25416, ATCC 17759 | |
| 1 | SKMM | |||
| P. aeruginosaf | 11 | ATCC | MicroScan and PC480-PC1250 PCR | |
| CF patient | ||||
| R. pickettii | 4 | CF patient | MicroScan and API 20NE, BCSA | |
| Environment | ||||
| Unspecified | 19 | CF patient | PC480-PC1250 PCR | |
| LVFd | Unspecified | 2 | Patient | |
| 2 | CF patient | Biochemical | ||
| AZUe | Unspecified | 4 | CF patient | API 20NE |
For B. cepacia, 10 reference strains, 2 environmental isolates, and 37 clinical strains were included in the present analysis. For B. gladioli, these figures are 4, 1, and 4, respectively. One clinical isolate and one environmental isolate of B. multivorans are included. For P. aeruginosa, 11 reference and clinical strains were studied. Two R. pickettii reference strains and four clinical isolates of this species were analyzed. Finally, 25 “unidentified” bacterial isolates were included. The SpeI fragment patterns were generated by pulsed-field gel electrophoresis of DNA macrorestriction fragments. SKMM, Foundation for Quality Control in Medical Microbiology, Groningen, The Netherlands; ATCC, American Type Culture Collection, Manassas, Va.; CCUG, Culture Collection of the University of Goteborg, Goteborg, Sweden; CGD, chronic granulomatous disease.
National Institute of Public Health and the Environment RIVM, Bilthoven, The Netherlands.
AZR, Erasmus University Medical Center Rotterdam EMCR, Rotterdam, The Netherlands; SKZ, Sophia Children’s Hospital, Rotterdam, The Netherlands.
Laboratory for Public Health Friesland, Leeuwarden, The Netherlands.
University Hospital Utrecht, Utrecht, The Netherlands.
P. aeruginosa strains were not isolated from CF patients and did not have a mucoid phenotype.
All strains were characterized by a combination of microbiological, biochemical, and molecular methods at a single, central laboratory (Department of Medical Microbiology and Infectious Diseases, Erasmus University Medical Centre Rotterdam EMCR, Rotterdam, The Netherlands). All individual assays were performed batchwise by a single researcher to prevent experimental day-to-day and person-to-person variability.
Presumptive identification with selective culture media.
All strains were subcultured on B. cepacia selective agar (BCSA) (15) and oxidation-fermentation base-polymyxin B-bacitracin-lactose medium (OFPBL; Becton-Dickinson, Heidelberg, Germany) (38). The plates were incubated for 5 days at 30 or 37°C. Growth on BCSA is considered indicative of B. cepacia. Colonies on OFPBL were suspected of being B. cepacia if they were yellow as a result of lactose oxidation in the presence of the bromothymol blue indicator. The natural yellow pigment of bacterial strains other than B. cepacia may lead to misidentification and a false-positive result, however.
Biochemical testing.
All bacterial isolates were characterized on the basis of phenotypic characteristics by use of four commercial assays. Prior to inoculation into these systems, a subculture was made on a brucella blood agar plate (bioMerieux, Marcy l’Etoile, France), which was incubated at 37°C for 18 h. Oxidase production was determined by means of a dipstick oxidase test (Difco, Detroit, Mich.).
API 20NE.
For all strains, a cell suspension having a 0.5 McFarland optical density (MF) standard was made with 0.85% NaCl. Appropriate amounts of this material were added to the wells of an API 20NE strip (bioMerieux), some of which were covered with mineral oil. The strips were incubated for 48 h at 30°C, and the results were recorded by visual inspection and scored with an APILAB PLUS software package as proposed by the manufacturer.
Vitek analysis.
For identification, two different Vitek cards (from bioMerieux at location given above and at s’-Hertogenbosch, The Netherlands) were used. One card is routinely used for the identification of gram-negative bacteria (GNI), and the other card has been developed for the industrial identification of nonfermenters (NFC). The latter is not commercially available yet. For all strains, a suspension having a 1.0 MF standard was made with 0.45% NaCl. The cards, filled with the suspensions, were placed in a specific tray, which was placed in the Vitek combined reader-incubator. Identification with the NFC was performed by use of the equipment of the Dutch bioMerieux representative in s’-Hertogenbosch, The Netherlands.
MicroScan analysis.
For identification by use of MicroScan technology (Dade International, West Sacramento, Calif.), the so-called “negative urine combo type 1” was used. This test format also assays the susceptibility of strains to antibiotics frequently used against urinary tract infections and/or infections caused by gram-negative bacteria. A cell suspension having a 0.5 MF standard was made with 0.45% NaCl. One hundred microliters of this suspension was pipetted into a tube with water containing pluronic acid to avoid air bubbling. The MicroScan kit was filled with the cell suspension and placed in a WalkAway reader-incubator for overnight processing.
PCR analyses.
PCR-mediated identification was performed by means of three methods directed to various parts of the ribosomal gene operon (26, 29, 35, 37). Prior to DNA isolation, strains were grown overnight at 37°C on brucella blood agar plates. One to three colonies were suspended in 25 mM Tris-HCl (pH 8.0)–10 mM EDTA–50 mM glucose and treated with proteinase K and 10% sodium dodecyl sulfate (SDS). DNA was purified by affinity chromatography with guanidine hydrothiocyanate and Celite (Janssen Pharmaceuticals, Beerse, Belgium) (5). The DNA concentration was estimated by electrophoresis with 1% agarose gels (Hispanagar; Sphaero Q, Leiden, The Netherlands) in the presence of known quantities of lambda DNA as references. The PCR mixtures used for all amplifications contained 10 mM Tris-HCl (pH 9.0), 50 mM KCl, 2.5 mM MgCl2, 0.01% gelatin, 0.1% Triton X-100, 0.2 mM respective deoxyribonucleotide triphosphate, 2 U of Taq polymerase (Sphaero Q), and 50 pmol of each primer. Amplification of DNA was performed with a model 60 thermocycler (Biomed, Theres, Germany). Amplicons were analyzed by electrophoresis with 1% agarose gels in the presence of a 100-bp DNA ladder for size assessment (Gibco/BRL Life Technologies, Breda, The Netherlands).
Two diagnostic PCR assays were performed; the first was done with primers PC1 and PC2 (35). This assay amplifies the ribosomal internal transcribed spacer (ITS) spanning the distance between the 16S and the 23S ribosomal genes. Approximately 300 ng of DNA was added to a PCR mixture. Amplification involved an initial denaturation of 2 min at 94°C, followed by 30 cycles of 30 s at 94°C, 30 s at 68°C, and 1 min at 72°C. A final extension of 30 min at 72°C was performed. The target sequence comprised 323 bp. In the second diagnostic PCR assay, primers PC480 and PC1250 were used (26). This amplification reaction, targeting the 16S ribosomal gene, should result in the specific detection of B. cepacia. Approximately 150 ng of DNA was added to a standard PCR mixture. Amplification was performed according to the following scheme: initial denaturation of 5 min at 96°C, followed by 25 cycles of 15 s at 96°C, 30 s at 52°C, and 1.5 min at 70°C. A final extension of 5 min at 70°C was included. The target sequence was 770 bp long.
PCR-RFLP.
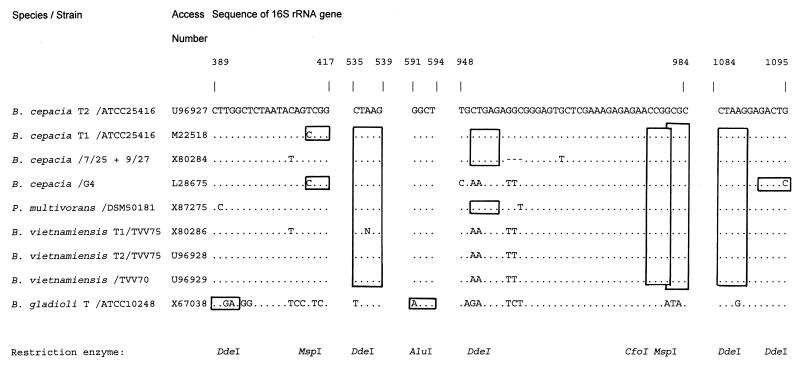
Amplification of the 16S rRNA gene, followed by restriction enzyme-mediated fragmentation of the amplicon (restriction fragment length polymorphism [RFLP] analysis), should result in Burkholderia sp.-specific banding patterns (29, 37). Approximately 50 ng of DNA was added to a PCR mixture as described above. PCR was performed with primers rD1 and fD1 and a 35-cycle program of 2 min at 95°C, 30 s at 42°C, and 4 min at 72°C, with a final extension of 20 min at 72°C. The amplicons, approximately 1,500 bp long, were analyzed and quantified on a 1% agarose gel (Hispanagar). After amplification, the samples were treated with four different restriction enzymes (AluI, CfoI, MspI, and DdeI; Boehringer GmbH, Mannheim, Germany). These restriction enzymes were selected on the basis of homology searches performed for Burkholderia sp.-specific 16S rRNA sequences available through GenBank (see Fig. 2). One microliter of enzyme mixture containing 1 U of restriction enzyme and the appropriate buffer components was added to 9 μl of the amplified product. The mixture was incubated for 2 h at 37°C. The sizes of the restriction fragments were documented by electrophoresis, ethidium bromide staining, UV transillumination, and photography.
FIG. 2.
Prediction of different restriction sites in the ribosomal genes for Burkholderia spp. Numbering identifies nucleotides in the consensus small-subunit rRNA gene sequences available through GenBank. Restriction sites are highlighted by boxes, and the nature of the restriction enzyme is indicated by the appropriate abbreviation.
RESULTS
Culture-based assays.
All of the B. cepacia and B. gladioli strains were positive in the oxidase assay. The results of growth analyses with selective agar plates are shown in Table 2. Forty-eight of 50 strains of B. cepacia grew on both BCSA and OFPBL plates. The two isolates not growing on BCSA plates were gentamicin-sensitive B. cepacia, a result which implies that gentamicin susceptibility may not be a 100% reliable species discriminator. These isolates were derived from a single German CF patient, showed identical random amplified polymorphic DNA patterns, were negative for the B. cepacia epidemicity marker, and belonged to the B. cepacia genomovar III group (16).
TABLE 2.
Growth of Burkholderia spp. and other gram-negative bacilli on two selective media
| Organisma | No. of isolates | No. (%) growing on:
|
|
|---|---|---|---|
| BCSA | OFPBL | ||
| B. cepacia | 50 | 48 (96) | 50 (100) |
| B. gladioli | 14 | 6 (43) | 8 (57) |
| R. pickettii | 2 | 2 (100) | 0 (0) |
| B. multivorans | 2 | 2 (100) | 2 (100) |
| S. maltophilia | 3 | 0 (0) | 1 (33) |
| P. aeruginosa | 11 | 0 (0) | 0 (0) |
Identification as provided by the different strain contributors.
Biochemical identification.
It must be mentioned explicitly that the taxons B. gladioli and B. multivorans are not included in the databases of any of the assays used here. The results of biochemical assays are shown in Table 3. Overall, the API 20NE and the Vitek GNI were the most accurate. The API 20NE gave a doubtful but essentially correct identification (“low level of discrimination”) for 6% of the strains; for only 2% of the strains could the system not provide a bacterial identification at all. The Vitek GNI could not identify 10% of the tested strains. None of the systems was able to identify B. gladioli correctly and efficiently. The API 20NE identified 36% of these strains as B. cepacia. The Vitek GNI identified 21% of B. gladioli strains as B. cepacia, whereas the Vitek NFC and the MicroScan scored 50% and none, respectively, of 14 strains of B. gladioli as B. cepacia. The MicroScan identified 43% of the strains as nonfermenters, and for 57% of the strains an incorrect identification was given. The two strains from Canada which were presented as B. multivorans were identified as B. cepacia by all four systems. All systems could reliably identify two strains of R. pickettii, the P. aeruginosa strains, and two strains of S. maltophilia. The Vitek NFC approach did not correctly identify one of the S. maltophilia strains tested.
TABLE 3.
Comparison of four systems of biochemical identification for Burkholderia spp. and various other bacterial speciesa
| Organism | No. of isolates | System | No. (%) of isolates with the following identification:
|
|||
|---|---|---|---|---|---|---|
| Correctb | Incomplete | Incorrect | Unidentified | |||
| B. cepacia | 50 | Vitek GNI | 45 (90) | 0 (0) | 0 (0) | 5 (10) |
| Vitek NFC | 34 (68) | 0 (0) | 11 (22) | 5 (10) | ||
| API 20NE | 45 (90) | 3 (6) | 1 (2) | 1 (2) | ||
| MicroScan | 34 (68) | 0 (0) | 16 (32) | 0 (0) | ||
| B. gladioli | 14 | Vitek GNI | 0 (0) | 0 (0) | 6 (43) | 8 (57) |
| Vitek NFC | 0 (0) | 0 (0) | 9 (64) | 5 (56) | ||
| API 20NE | 0 (0) | 0 (0) | 14 (100) | 0 (0) | ||
| MicroScan | 0 (0) | 0 (0) | 14 (100) | 0 (0) | ||
| R. pickettii | 6 | Vitek GNI | 6 (100) | 0 (0) | 0 (0) | 0 (0) |
| Vitek NFC | 4 (100) | 0 (0) | 0 (0) | 0 (0) | ||
| API 20NE | 4 (66) | 1 (17) | 0 (0) | 1 (17) | ||
| MicroScan | 6 (100) | 0 (0) | 0 (0) | 0 (0) | ||
| B. multivorans | 2 | Vitek GNI | 2 (100) | 0 (0) | 0 (0) | 0 (0) |
| Vitek NFC | 2 (100) | 0 (0) | 0 (0) | 0 (0) | ||
| API 20NE | 2 (100) | 0 (0) | 0 (0) | 0 (0) | ||
| MicroScan | 2 (100) | 0 (0) | 0 (0) | 0 (0) | ||
| S. maltophilia | 3 | Vitek GNI | 3 (100) | 0 (0) | 0 (0) | 0 (0) |
| Vitek NFC | 2 (66) | 0 (0) | 0 (0) | 1 (33) | ||
| API 20NE | 3 (100) | 0 (0) | 0 (0) | 0 (0) | ||
| MicroScan | 3 (100) | 0 (0) | 0 (0) | 0 (0) | ||
| P. aeruginosa | 11 | Vitek GNI | 11 (100) | 0 (0) | 0 (0) | 0 (0) |
| Vitek NFC | 10 (91) | 0 (0) | 0 (0) | 1 (9) | ||
| API 20NE | 11 (100) | 0 (0) | 0 (0) | 0 (0) | ||
| MicroScan | 11 (100) | 0 (0) | 0 (0) | 0 (0) | ||
| Unspecified clinical isolatesc | ||||||
| B. cepacia | 20 | Vitek GNI | 9 (45) | 5 (25) | 1 (5) | 5 (25) |
| Vitek NFC | 8 (40) | 0 (0) | 0 (0) | 12 (60) | ||
| API 20NE | 16 (80) | 3 (15) | 1 (5) | 0 (0) | ||
| MicroScan | 5 (25) | 0 (0) | 15 (75) | 0 (0) | ||
| P. aeruginosa | 5 | Vitek GNI | 1 (20) | 2 (40) | 2 (40) | 0 (0) |
| Vitek NFC | 2 (40) | 0 (0) | 0 (0) | 3 (60) | ||
| API 20NE | 4 (80) | 0 (0) | 1 (20) | 0 (0) | ||
| MicroScan | 0 (0) | 0 (0) | 5 (100) | 0 (0) | ||
Note that B. gladioli is not mentioned in the databases of the systems.
Identification to the species level with a probability of more than 80%.
For the evaluation of this group of isolates, PCR-RFLP was considered the gold standard for identification.
PCR assays.
The results shown in Table 4 and Figure 1 summarize the experimental data obtained with the PCR-RFLP procedure. The diagnostic PCRs with primers PC480-PC1250 and PC1-PC2 could not accurately differentiate B. cepacia from B. gladioli (Table 4). The PCR with the PC1-PC2 primer combination, whose major drawback is that it lacks sensitivity for B. cepacia, provided a positive result for only 22 of 50 B. cepacia strains (sensitivity, 44%). The specificity of this PCR was good, since no positive results were encountered among the different B. gladioli strains. Although the PC480-PC1250 PCR correctly recognized all strains from both species (100% sensitivity), it did not distinguish between B. cepacia and B. gladioli. Moreover, other species may produce positive signals as well (e.g., Alcaligenes spp. [unpublished observation]), rendering this diagnostic application useless.
TABLE 4.
Results of PCR-mediated identification
| Organisma | No. of strains | No. (%) identified with the following primer pair:
|
PCR-RFLP resultsd
|
||
|---|---|---|---|---|---|
| PC1-PC2b | PC480-PC1250c | Type | No. (%) of strains with the indicated type | ||
| B. cepacia | 50 | 22 (44) | 50 (100) | AAAB | 20 (40) |
| AAAA | 19 (38) | ||||
| ABBB | 11 (22) | ||||
| B. gladioli | 14 | 0 | 14 (100) | BBBC | 10 (71) |
| BBEC | 4 (29) | ||||
| R. pickettii | 6 | 0 | 0 | DDDE | 2 (100) |
| B. multivorans | 2 | 0 | 2 (100) | AAAA | 2 (100) |
| S. maltophilia | 3 | 0 | 0 | CCCD | 3 (100) |
| P. aeruginosa | 11 | 0 | 0 | EEFH | 3 (27) |
| FEFH | 6 (55) | ||||
| GFFH | 2 (18) | ||||
| Clinical isolatee | 27 | 2 (7) | 21 (78) | AAAA | 18 (67) |
| AAAB | 2 (7) | ||||
| EEFH | 5 (19) | ||||
| Other | 2 (7) | ||||
Identification as provided by the strain contributors.
Increased level of discordance for B. cepacia compared to results with PC1250-PC480, but correctly negative for the other species.
Apparently suited for identifying both Burkholderia species; however, non-Burkholderia species also may be identified.
Order of restriction enzymes used: AluI, CfoI, MspI, and DdeI.
RFLP was considered the gold standard for identification.
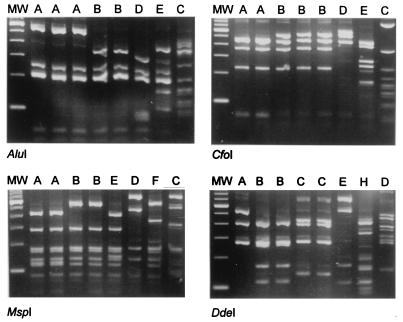
FIG. 1.
PCR-RFLP analysis of Burkholderia spp. and other gram-negative bacilli. The four panels display the results obtained by restriction of small-subunit rRNA amplicons with the restriction endonucleases indicated below the panels. The lanes show the RFLP types found in each enzyme assay. DNA templates were derived from the following strains (from left to right) (RFLP type): B. cepacia Dutch patient isolate (AAAA), B. cepacia ATCC 25416 (AAAB), B. cepacia H134-6 (ABBB), B. gladioli ATCC 10248 (BBBC), B. gladioli RIVM 95-665 (BBEC), R. pickettii CCUG3314 (DDDE), P. aeruginosa ATCC 27853 (EEFH), and S. maltophilia RIVM 96-330 (CCCD). Lane MW, molecular weight (MW) standard.
RFLP typing of ribosomal amplicons turned out to be the most appropriate technique for discriminating B. cepacia from B. gladioli. All B. gladioli strains were found identical when AluI and DdeI digests were considered (type BC). For B. cepacia, the AluI pattern was characteristic (type A). These assays were diagnostically accurate and corroborated the initial laboratory identification. On the other hand, the RFLP analyses demonstrated intraspecies heterogeneity (types AAAA, AAAB, and ABBB for B. cepacia, for instance). Interestingly, several of these single RFLP patterns were shared by the two species (A and B patterns for MspI, for instance). The RFLP patterns obtained for the other species clearly differed from the B. cepacia and B. gladioli patterns (e.g., types DDDE, EEFH, and CCCD). Clearly, strains identified as B. cepacia were genetically heterogeneous, as were P. aeruginosa strains (types EEFH, FEFH, and GFFH). It is interesting to note that 19 of the B. cepacia strains generated a type AAAA PCR-RFLP pattern, which is identical to that recorded for the B. multivorans strains. This finding is in agreement with the recent proposals of Vandamme et al. (36), who separated B. multivorans from the bulk group of B. cepacia as a separate type. Type AAAA is also encountered among clinical isolates, substantiating the fact that isolates belonging to this specific genomovar have colonizing and/or pathogenic potential.
Analysis of clinical Burkholderia sp. isolates.
Twenty of 27 (74%) of the clinical isolates that were suspected of being B. cepacia appeared to be genuine B. cepacia when PCR-RFLP was considered the gold standard for identification. This finding implies that the different Dutch strain contributors possibly misidentified at least seven strains, five of which were identified as P. aeruginosa by RFLP. This figure is even higher when the other forms of identification are considered. Based on Vitek NFC analysis, for instance, 12 of 20 B. cepacia strains (60%) would not have been identified correctly. It must be emphasized here that the Vitek NFC has not yet been qualified for laboratory-based medical microbiological diagnostic procedures. The API 20NE misidentified only a single strain, underscoring its excellent diagnostic potential. Not all strain contributors misidentified the strains; since most of the strains were isolated from CF patients and showed aberrant characteristics, the microbiologists involved wanted to exclude the possibility of B. cepacia carriage.
DISCUSSION
The genetic defect causing CF predisposes patients to an aberrant pulmonary susceptibility to infectious disease. Several of the bacterial species encountering a suitable ecological niche in the lungs of CF patients are especially pathogenic for the host. B. cepacia, a microorganism originally identified as the causative agent of soft rot of onions, is a well-known representative of this particular group; in 20% of patients who become colonized with this species, the rapidly fatal B. cepacia syndrome may occur (11). Microbiological detection of B. cepacia appears to be complicated, and laboratory proficiency testing was particularly disappointing in the past (34). Difficulties and inaccuracies at the level of laboratory procedures have been documented even when simple plating procedures are used for screening (15). Mix-ups with relatively nonpathogenic bacterial species have been described (21), and even commercially available diagnostic tests have failed (6). Also, the taxonomy of Burkholderia spp. is still evolving, and the pathogenic potential of the diverse species and subspecies for humans has not been elucidated yet (10). Major biological and genetic diversity among isolates of B. cepacia subspecies cultured from the same environmental source can be demonstrated (7). Variation in the flagellin genes of B. cepacia can be demonstrated for subdivisions within the species as well (14). In addition, it has been demonstrated that clinical isolates of B. cepacia may have characteristics of B. gladioli (3), a species for which some records of severe infections exist as well (1, 13, 17, 20, 28, 30). The issues mentioned here warrant in-depth studies on the value of the currently available diagnostic tools for the identification of B. cepacia.
In the present communication, we tried to define the best procedures for diagnosing the presence of Burkholderia spp. in the sputum of CF patients. A collection of Burkholderia strains was obtained from several reference laboratories. Based on our results, we suggest the use of either BCSA or OFPBL plates for the initial isolation of B. cepacia directly from clinical material. The sensitivity of these growth media appeared to be excellent (96 and 100%, respectively); the specificity, however, was not 100%. Because of the growth of species other than B. cepacia on selective agar, such agar cannot be used for the definitive identification of a strain as B. cepacia but can provide a useful first screen, as stated previously (15). In the presence of colonization or infection by B. gladioli, many strains will not be detected if this procedure is used as the single diagnostic assay.
It was quite striking to find that the automated assays, such as Vitek and MicroScan, performed with varying but consistently insufficient accuracy. Only the Vitek GNI reliably identified most of the B. cepacia strains, but it encountered major problems with B. gladioli strains. Improvement in both cards and software is certainly needed for all automated systems currently available. The major outcome of the present analysis is the fact that molecular identification by PCR-RFLP analysis is superior to the biochemical and microbiological species identification procedures used here, although it should be emphasized that the API 20NE performed satisfactorily, as documented previously (15, 27). No international gold standard for the routine laboratory identification of clinically relevant Burkholderia species exists to date, so the definition of accurate sensitivities and specificities for the tests used in the present communication remains a topic for future investigations. The PCR-RFLP procedure was found to be in excellent agreement with strain identification provided by the different study participants.
Genetic mosaicism has been proposed on the basis of phenotypic studies for B. cepacia and B. gladioli (3). On the basis of the currently known small-subunit rRNA gene sequences (Fig. 2) and the PCR-RFLP test, intraspecific polymorphism can be anticipated and noted, respectively. Depending on the restriction enzyme, species-specific RFLP patterns or RFLP patterns that are shared between the two species can be observed. Whether this observation relates to genetic exchange or mere coincidence, based on lack of variability in the ribosomal region that is targeted by the enzyme, requires additional DNA sequencing studies. In the present study, no clearly overlapping characteristics were noted among the diverse types of the strains and the tests that were performed. Only for a B. cepacia strain with type ABBB was the PCR with primer pair PC1-PC2 positive, although exceptions may be encountered in the future. It would be interesting to determine whether the RFLPs coincide with the epidemicity and pathogenicity of the strain (24, 25).
For adequate characterization of the strains of Burkholderia spp. described in this report, we suggest that the PCR-RFLP procedure be used as the most definitive means of identification. However, it must be emphasized that PCR-based methods are not generally available to the clinical microbiologist. We recommend that microbiologists initiate characterization with a diagnostic agar followed by API 20NE. Routine microbiology procedures should be used prior to the tests mentioned above in order to exclude all nonrelevant bacterial species.
The PCR-RFLP procedure seems to be a reliable tool for discriminating strains of B. cepacia versus B. gladioli. This technique should facilitate more detailed studies on the clinical relevance of the latter species in CF and other diseases. Whereas previously a comparison of the endotoxicity of lipopolysaccharide isolated from both species was a measure of clinical relevance (10), now precise species identification can replace superficial lipopolysaccharide characteristics for a more species-defining feature. In order to make another important step forward, the DNA assays used here and those newly described in the literature (2) should be adapted for PCR directly from sputum samples.
ACKNOWLEDGMENTS
We thank David Speert and Deborah Henry (Research Centre, Department of Pediatrics, University of British Columbia, Vancouver, Canada) for providing several reference strains and for their support during the preparation of the manuscript. We gratefully acknowledge the involvement of the medical microbiologists Peter de Man (Department of Medical Microbiology, Sint Franciscus Gasthuis, Rotterdam, The Netherlands) and Johan Mouton (Department of Medical Microbiology, Canisius Wilhelmina Hospital, Nijmegen, The Netherlands) in providing several of the strains. We thank Anja Senneker (bioMerieux, s’-Hertogenbosch, The Netherlands) for help with the use of the Vitek NFC and for putting these cards at our disposal free of charge. We thank Sabine Deelen for incidental help with strain cultivation.
REFERENCES
- 1.Barker P M, Wood R E, Gilligan P H. Lung infection with Burkholderia gladioli in a child with cystic fibrosis: acute clinical and spirometric deterioration. Pediatr Pulmonol. 1997;23:123–125. doi: 10.1002/(sici)1099-0496(199702)23:2<123::aid-ppul9>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 2.Bauernfeind A, Schneider I, Jungwirth R, Roller C. Discrimination of Burkholderia species detectable in cystic fibrosis patients by PCR. J Clin Microbiol. 1998;36:2748–2751. doi: 10.1128/jcm.36.9.2748-2751.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baxter I A, Lambert P A, Simpson I N. Isolation from clinical sources of Burkholderia cepacia possessing characteristics of Burkholderia gladioli. J Antimicrob Chemother. 1997;39:169–175. doi: 10.1093/jac/39.2.169. [DOI] [PubMed] [Google Scholar]
- 4.Bevinino A, Tabacchioni S, Chiarini L, Carusi M V, del Gallo M, Visca P. Phenotypic comparison between rhizosphere and clinical isolates of Burkholderia cepacia. Microbiology. 1994;140:1069–1077. doi: 10.1099/13500872-140-5-1069. [DOI] [PubMed] [Google Scholar]
- 5.Boom R, Sol C J A, Salimans M M M, Jansen C L, Wertheim-van Dillen P M E, van der Noordaa J. Rapid and simple method for purification of nucleic acids. J Clin Microbiol. 1990;28:495–503. doi: 10.1128/jcm.28.3.495-503.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burdge D R, Noble M A, Campbell M E, Krell V L, Speert D P. Xanthomonas maltophilia misidentified as Pseudomonas cepacia in cultures of sputum from patients with cystic fibrosis: a diagnostic pitfall with major clinical implications. Clin Infect Dis. 1995;20:445–448. doi: 10.1093/clinids/20.2.445. [DOI] [PubMed] [Google Scholar]
- 7.Di Cello F, Bevivino A, Chiarini L, Fani R, Paffetti D, Tabacchioni S, Dalmastri C. Biodiversity of a Burkholderia cepacia population isolated from the maize rhizosphere at different plant growth stages. Appl Environ Microbiol. 1997;63:4485–4493. doi: 10.1128/aem.63.11.4485-4493.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gilligan P H. Microbiology of airway disease in patients with cystic fibrosis. Clin Microbiol Rev. 1991;4:35–51. doi: 10.1128/cmr.4.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gillis M, van Van T, Bardin R, Goor M, Hebbar P, Willems A, Segers P, Kersters K, Heulin T, Fernandez M P. Polyphasic taxonomy in the genus Burkholderia leading to an amended description of the genus and proposition of Burkholderia vietnamiensis sp. nov. for N2-fixing isolates from rice in Vietnam. Int J Syst Bacteriol. 1995;45:274–289. [Google Scholar]
- 10.Govan J R W, Deretic V. Microbial pathogenesis in cystic fibrosis: mucoid Pseudomonas aeruginosa and Burkholderia cepacia. Microbiol Rev. 1996;60:539–574. doi: 10.1128/mr.60.3.539-574.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Govan J R W, Hughes J E, Vandamme P. Burkholderia cepacia: medical, taxonomic and ecological issues. J Med Microbiol. 1996;45:395–407. doi: 10.1099/00222615-45-6-395. [DOI] [PubMed] [Google Scholar]
- 12.Govan J R W, Brown P H, Maddison J, Doherty C J, Nelson J W, Dodd M, Greening A P, Webb A K. Evidence for transmission of Pseudomonas cepacia by social contact in cystic fibrosis. Lancet. 1993;342:15–19. doi: 10.1016/0140-6736(93)91881-l. [DOI] [PubMed] [Google Scholar]
- 13.Graves M, Robin T, Chipman A M, Wong J, Khashe S, Janda J M. Four additional cases of Burkholderia gladioli infection with microbiological correlates and review. Clin Infect Dis. 1997;25:838–842. doi: 10.1086/515551. [DOI] [PubMed] [Google Scholar]
- 14.Hales B A, Morgan J A W, Hart C A, Winstanley C. Variation in flagellin genes and proteins of Burkholderia cepacia. J Bacteriol. 1998;180:1110–1118. doi: 10.1128/jb.180.5.1110-1118.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Henry D A, Campbell M E, LiPuma J J, Speert D P. Identification of Burkholderia cepacia isolates from patients with cystic fibrosis and use of a simple new selective medium. J Clin Microbiol. 1997;35:614–619. doi: 10.1128/jcm.35.3.614-619.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Henry, D. A. Personal communication.
- 17.Hoare S, Cant A J. Chronic granulomatous disease presenting as severe sepsis due to Burkholderia gladioli. Clin Infect Dis. 1996;23:411. doi: 10.1093/clinids/23.2.411. [DOI] [PubMed] [Google Scholar]
- 18.Holmes A, Govan J, Goldstein R. Agriculture use of Burkholderia (Pseudomonas) cepacia: a threat to human health? Emerg Infect Dis. 1998;4:221–227. doi: 10.3201/eid0402.980209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kanji S S, Tapson V, Davis R D, Madden J, Browning I. Infections in patients with cystic fibrosis following lung transplantation. Chest. 1997;112:924–930. doi: 10.1378/chest.112.4.924. [DOI] [PubMed] [Google Scholar]
- 20.Khan S U, Gordon S M, Stillwell P C, Kirby T J, Arroliga A C. Empyema and bloodstream infection caused by Burkholderia gladioli in a patient with cystic fibrosis after lung transplantation. Pediatr Infect Dis J. 1996;15:637–639. doi: 10.1097/00006454-199607000-00020. [DOI] [PubMed] [Google Scholar]
- 21.Kiska D L, Kerr A, Jones M C, Carracciolo J A, Eskridge B, Jordan M, Miller S, Hughes D, King N, Gilligan P H. Accuracy of four commercial systems for identification of Burkholderia cepacia and other gram-negative nonfermenting bacilli recovered from patients with cystic fibrosis. J Clin Microbiol. 1996;34:886–891. doi: 10.1128/jcm.34.4.886-891.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Landa A S, Sipkema F M, Weijma J, Beenackers A A, Dolfing J, Janssen D B. Cometabolic degradation of trichloroethylene by Pseudomonas cepacia G4 in a chemostat with toluene as the primary substrate. Appl Environ Microbiol. 1994;60:3368–3374. doi: 10.1128/aem.60.9.3368-3374.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.LiPuma J J. Burkholderia cepacia: management issues and new insights. Clin Chest Med. 1998;19:473–486. doi: 10.1016/s0272-5231(05)70094-0. [DOI] [PubMed] [Google Scholar]
- 24.Mahenthiralingam E, Simpson D A, Speert D P. Identification and characterization of a novel DNA marker associated with epidemic Burkholderia cepacia strains recovered from patients with cystic fibrosis. J Clin Microbiol. 1997;35:808–816. doi: 10.1128/jcm.35.4.808-816.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mahenthiralingam E, Campbell M E, Henry D A, Speert D P. Epidemiology of Burkholderia cepacia infection in patients with cystic fibrosis: analysis by randomly amplified polymorphic DNA fingerprinting. J Clin Microbiol. 1996;34:2914–2920. doi: 10.1128/jcm.34.12.2914-2920.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O’Callaghan E M, Tanner M S, Boulnois G J. Development of a PCR probe test for identifying Pseudomonas aeruginosa and Pseudomonas (Burkholderia) cepacia. J Clin Pathol. 1994;47:222–226. doi: 10.1136/jcp.47.3.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pitt T L, Govan J R W. Pseudomonas cepacia and cystic fibrosis. PHLS Microbiol Dig. 1993;10:69–72. [Google Scholar]
- 28.Ross J, Holland S M, Gill V J, DeCarlo E S, Gallin J I. Severe Burkholderia (Pseudomonas) gladioli infection in chronic granulomatous disease: report of two successfully treated cases. Clin Infect Dis. 1995;21:1291–1293. doi: 10.1093/clinids/21.5.1291. [DOI] [PubMed] [Google Scholar]
- 29.Segonds C, Heulin T, Marty N, Chabanon G. Differentiation of Burkholderia species by PCR-restriction fragment analysis of the 16S rRNA gene and application to cystic fibrosis isolates. J Clin Microbiol. 1999;37:2201–2208. doi: 10.1128/jcm.37.7.2201-2208.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shin J H, Kim S H, Shin M G, Suh S P, Ryang D W, Jeong M H. Bacteremia due to Burkholderia gladioli: case report. Clin Infect Dis. 1997;25:1264–1265. doi: 10.1086/516973. [DOI] [PubMed] [Google Scholar]
- 31.Smith D L, Gumery L B, Smith E G, Stableforth D E, Kaufmann M E, Pitt T L. Epidemic of Pseudomonas cepacia in an adult cystic fibrosis unit: evidence of person-to-person transmission. J Clin Microbiol. 1993;31:3017–3022. doi: 10.1128/jcm.31.11.3017-3022.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sun L, Jiang R, Steinbach S, Holmes A, Campanelli C, Forstner J, Sajjan U, Tan Y, Riley M, Goldstein R. The emergence of a highly transmissible lineage of abl+ Pseudomonas (Burkholderia) cepacia causing CF centre epidemics in North America and Britain. Nat Med. 1995;1:661–665. doi: 10.1038/nm0795-661. [DOI] [PubMed] [Google Scholar]
- 33.Tabacchioni S, Visca P, Chiarini L, Bevinino A, di Serio C, Fancelli S, Fani R. Molecular characterisation of rhizosphere and clinical isolates of Burkholderia cepacia. Res Microbiol. 1995;146:531–542. doi: 10.1016/0923-2508(96)80559-6. [DOI] [PubMed] [Google Scholar]
- 34.Tablan O C, Carson L A, Cusick L B, Bland L A, Martone W J, Jarvis W R. Laboratory proficiency test results on use of selective media for isolating Pseudomonas cepacia from simulated sputum specimens of patients with cystic fibrosis. J Clin Microbiol. 1987;25:485–487. doi: 10.1128/jcm.25.3.485-487.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tyler S D, Strathdee C A, Rozee K R, Johnson W M. Primers designed to differentiate pathogenic pseudomonads on the basis of the sequencing of genes coding for 16S-23S rRNA internal transcribed spacers. Clin Diagn Lab Immunol. 1995;4:448–453. doi: 10.1128/cdli.2.4.448-453.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vandamme P, Holmes G, Vancanneyt M, Coenye T, Hoste B, Coopman R, Revets H, Lauers S, Gillis M, Kersters K, Govan J R V. Occurrence of multiple genomovars of Burkholderia cepacia in cystic fibrosis patients and proposal of Burkholderia multivorans sp. nov. Int J Syst Bacteriol. 1997;47:1188–1200. doi: 10.1099/00207713-47-4-1188. [DOI] [PubMed] [Google Scholar]
- 37.Weisburg W G, Barns S M, Pelletier D A, Lane D J. 16S ribosomal DNA amplification for phylogenetic study. J Bacteriol. 1991;173:697–703. doi: 10.1128/jb.173.2.697-703.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Welch D F, Muszynski M J, Pai C H, Marcon M J, Hribar M M, Gilligan P H, Matsen J M, Ahlin P A, Hilman B C, Chartrand S A. Selective and differential medium for recovery of Pseudomonas cepacia from the respiratory tracts of patients with cystic fibrosis. J Clin Microbiol. 1987;25:1730–1734. doi: 10.1128/jcm.25.9.1730-1734.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yabuuchi E, Kosako Y, Yano I, Hotta H, Nishiuchi Y. Transfer of two Burkholderia and an Alcaligenes species to Ralstonia gen. nov.: proposal of Ralstonia pickettii (Ralston, Palleromi and Doudoroff 1973) comb. nov., Ralstonia solanacearum (Smith 1896) comb. nov. and Ralstonia eutropha (Davis 1969) comb. nov. Microbiol Immunol. 1995;39:897–904. doi: 10.1111/j.1348-0421.1995.tb03275.x. [DOI] [PubMed] [Google Scholar]