Abstract
Microbial etiology of diarrhea is a significant cause of death, especially in children in developing countries. The presence of microbes that are resistant to current treatment options for diarrhea suggests the need to find newer antimicrobial agents for treatment. Therefore, this study focused on investigating the antimicrobial effect of some Ghanaian chewing sticks commonly used for oral hygiene, Azadirachta indica, Garcinia afzelii, and Garcinia kola, against selected diarrhea-causing organisms. From the stem and bark of each plant, 70% methanolic extract was experimented on Salmonella and Shigella species, namely, Shigella sonnei, Shigella flexeneri, Salmonella typhinirium enterica, Salmonella typhi attenuated, and Klebsiella oxytoca for microbial susceptibility using the agar well diffusion method. Additionally, the antioxidant profile of the methanolic extracts were investigated using 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical, 2,2'-azino-bis (3-ethylbenzthiazoline-6-sulphonic) acid (ABTS) scavenging activities, and ferric-reducing antioxidant potential (FRAP) assays, while the total polyphenolic content was determined using the Folin–Ciocalteau reagent. G. afzelii and A. indica stem demonstrated the highest antimicrobial effect, inhibiting the growth of all test organisms. Additionally, the extracts demonstrated high antioxidant potential and were found to possess significant amounts of phenolic compounds. Therefore, methanolic extracts of G. afzelii and A. indica stem are promising candidates for the identification of safe novel compounds to mitigate diarrheal diseases.
1. Introduction
Diarrheal disease is a significant global health problem that occurs in approximately 1.7 billion children yearly and is responsible for the death of around 525,000 children annually. After malaria, diarrheal disease is the leading cause of malnutrition in children less than five years of age and the second leading cause of mortality globally [1].
Diarrhea refers to a condition in which an individual may pass three or more liquid or loose stools per day. It occurs due to imbalances in the mechanisms regulating the secretion and absorption of water and electrolyte in the gut leading to dehydration [2, 3]. These imbalances can be caused by microorganisms (bacteria and viruses), helminths, toxins, diet, and allergic reactions [4]. Diarrheal disease is a medical emergency; hence, oral rehydration therapy, reduction in gut motility using antispasmodic drugs, and the use of antisecretory agents and drugs that inhibit secretion of prostaglandins, as well as antibiotic therapy, have proven to be very useful clinical interventions [5]. Despite the usefulness of these interventions in most cases, these therapies are not without discomforting side effects such as dry mouth, headaches, nausea, constipation, and drowsiness, which limit their usage [6, 7]. Additionally, a high prevalence of resistant strains of diarrheal pathogens has been observed in many developing countries where there is little restriction on the use of antibiotics and where the incidence of diarrhea-associated death in childhood is high [8, 9]. Hence, there is a need for the identification of newer therapeutic agents with fewer side effects and increased potency.
In developing countries, there is an increased reliance on natural product remedies for the management of both infectious and noninfectious diseases [10, 11]. Natural products are also seeing increased patronage even in the developed world [12]. In many African settings, these natural products are used to treat several medical conditions in folk medicine. These natural products have to be the source for the production of many orthodox medicines that are currently in use [11]. In 2012, Newman and Cragg reported that about half of the approved drugs over three decades were either directly or indirectly derived from natural products [13], due mainly to the high levels of phenol, alkaloid, saponin, and flavonoid secondary metabolites in such natural products [14]. Thus, investigating the ability of isolates from plant-based products to act as treatment options for different disease conditions is an important starting point in the search for newer therapeutic interventions for diverse disease states.
In Ghana, some selected roots, stems, and twigs of numerous plants have been used as chewing sticks for oral hygiene since time immemorial. Recent research has shown that these chewing sticks possess antimicrobial activity against selected oral disease-causing microbes such as S. mutans, S. aureus, and E. coli (unpublished data). Based on these observations and those from previously published results from Nigeria and Tanzania that these chewing sticks showed inhibitory effects against both Gram-positive and -negative bacteria [15, 16], we investigated the ability of these chewing sticks to exert inhibitory effects against common diarrhea-causing microbes since childhood diarrhea is still an important public health problem in Ghana [17]. Additionally, in bacteria-associated diarrhea, the microbial signals released by the infected microbes led to a substantive production of reactive oxygen and/or nitrogen species by the host to exert bactericidal effects on the infecting microbes [18]. These free radicals exert oxidative stress on the hosts by the lipid peroxidation mechanism leading to molecular damage and consequently tissue and organ failure, hence suggesting that therapeutic interventions that limit the production of these reactive species may prevent tissue damage induced by these enteric microbial infections. Some plant-based products are an important source of antioxidant molecules that scavenge for free radicals. Furthermore, the antibiotic properties of plant products cannot be overemphasized [19], thus making them an important target for finding therapeutic interventions for diarrhea and other enteric infections. This study aimed to carry out a preliminary investigation to determine if extracts from three commonly used chewing sticks in Ghana possessed any antimicrobial effects against selected diarrhea-causing bacteria as well as investigate the antioxidant potential of these isolates.
2. Materials and Methods
2.1. Study Plants and Preexperimental Preparations
The plants used in this study were Azadirachta indica, Garcinia afzelii, and Garcinia kola. For each plant, the stem and bark were used. Prior to obtaining the extract, the plant specimens were washed thoroughly with sterile distilled water. The washed plant specimens were air-dried for at least a month. The dried specimens were macerated into coarse pieces.
2.2. 70% Methanolic Extraction of Crude Plant Products
In a sterile conical glass jar, 2 L of 70% methanol solution was added to 200 g of each macerated plant sample. The plants samples were soaked for 48 hours with intermittent shaking. The methanol solvent filtrate was evaporated at 45°C in preweighed glass bottles in an oven.
2.3. Determination of Antimicrobial Activity
The test isolates, Shigella sonnei (DSM 5570), Shigella flexneri, Salmonella typhi enterica sub sp. enterica (ATCC19585), Salmonella typhinirium attenuated, and Klebsiella oxytoca (ATCC 13182), were obtained from the microbiology unit of the Department of Biomedical Sciences, University of Health and Allied Sciences. The agar diffusion method as previously used [20] and modified [21] was used. The test organisms were subcultured on Mueller-Hinton agar at 37°C between 18 and 24 hours. An inoculum (500 µL) was adjusted to 1.5 × 108 CFU/mL using 0.5 McFarland standard. On each Mueller-Hinton agar plate, each test organism was evenly spread using a sterile microbiological loop. The plate was incubated at room temperature (approx. 25°C) for 10 minutes. Subsequently, seven wells (diameter: 8 mm) were aseptically created into the agar medium. Into each of the wells, 100 µL (5 mg/mL) of stems and backs of Azadirachta indica, Garcinia afzelii, and Garcinia kola extracts were aseptically dispensed into six individual wells while 30 µL of a reference drug (chloramphenicol (CPL; concentration–5 mg/mL)) was dispensed aseptically into the seventh well. The Mueller-Hinton agar plates were incubated aerobically between 18 and 24 hours. The zones of inhibitions of the plant extracts were measured and compared to those of the reference drug.
2.4. Profiling of the Antioxidant Scavenging Activity of the Plant Extracts
The antioxidant activity of the study plants was evaluated using previously published protocols [22]. The antioxidant properties of the plant extracts were determined using the 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity, 2, 2'-azino-bis (3-ethylbenzthiazoline-6-sulphonic) acid (ABTS) scavenging activity, ferric-reducing antioxidant potential (FRAP), and determination of total phenolic content.
2.4.1. Determining DPPH Radical Scavenging Activity
The DPPH scavenging activity was determined by adding 200 µL (mg/mL) of each plant extract to 800 µL of 0.1 mmol/L of DPPH. The extract-DPPH mixture was incubated at room temperature for 30 minutes. Subsequently, the absorbance of the mixture was read spectrophotometrically at 517 nm using a VERSAmax microplate reader (Molecular Devices, San Jose, USA). Using the formula % scavenging [DPPH] = [(A0 − A1)/A0] x 100%, where A0 = the absorbance of the blank and A1 = the absorbance in the presence of the sample extract or standard (ascorbic acid), the % radical scavenging activity was calculated. Mean value from duplicate experiments was recorded. This protocol has been used in a recent study (Dravie et al., 2020).
2.4.2. Determining ABTS Scavenging Activity
ABTS scavenging activity was determined by adding 200 µL (mg/mL) of the plant extracts to 800 µl of ABTS solution. The mixture was incubated at room temperature (25°C) for 10 minutes. Subsequently, the absorbance of the mixture was read spectrophotometrically at 734 nm using a VERSAmax microplate reader (Molecular Devices, San Jose, USA). The radical scavenging activity of the plant extracts was determined as recently published [22].
2.4.3. The FRAP Assay
The FRAP assay was performed using the FRAP working reagent previously published [22]. In brief, 100 μL (mg/mL) of the plant extracts was added to 3 mL of FRAP working reagent, incubated thermostatically for 30 minutes at 37°C. Using the VERSAmax microplate reader (Molecular Devices, San Jose, USA), the absorbance was measured at 593 nm. The absorbance of the plant extract corrected by the blank absorbance was proportional to the FRAP value. This protocol has been used in a recent study [22].
2.5. Estimation of Total Phenolic Content
The protocol for the determination of the phenolic content in the plant extracts has been published [22]. In brief, 0.5 g of the plant extract was dissolved in 5.0 mL methanol, followed by addition of 0.5 mL Folin–Ciocalteau reagent for 5 minutes. The mixture was incubated for 1 hour after adding 1 mL of 1 N sodium carbonate. The absorbance was read at 765 nm. Absorbance reading of each assay was converted to concentration using a calibration curve prepared using 100 µg/mL, 200 µg/mL, 400 µg/mL, 600 µg/mL, 800 µg/mL, and 1000 µg/mL solutions of gallic acid in methanol.
2.6. Data Analysis
Zones of inhibition of plant extracts were measured in millimeters and compared to those of the reference drug. Data were presented as mean ± SEM. Tabular and graphical presentation were used to present data.
3. Results
Analysis of zones of inhibition indicated that the microorganisms examined were susceptible to G. afzelii and G. indica stem, whereas none of the microorganisms examined except for S. sonnei was susceptible to G. kola stem. G. kola and A. indica barks displayed no antimicrobial activity against S. typhimurium enterica. Additionally, the A. indica stem did not inhibit the growth of S. flexneri (Table 1).
Table 1.
Antimicrobial activity of chewing stick extracts against selected microorganisms.
| Organism | Zone of inhibition (mm) | ||||||
|---|---|---|---|---|---|---|---|
| A. indica | G. afzelii | G. kola | CPL | ||||
| Stem | Bark | Stem | Bark | Stem | Bark | ||
| S. sonnei (DSM 5570) | 13 | 4 | 10 | 12 | 3 | 9 | 24 |
| S. flexneri | 14 | 0 | 18 | 16 | 0 | 11 | 19 |
| S. typhi | 9 | 14 | 10 | 13 | 0 | 11 | 29 |
| S. typhimurium enterica sub sp. (ATCC19585) | 15 | 0 | 12 | 19 | 0 | 0 | 28 |
| K. oxytoca (ATCC13182) | 15 | 8 | 13 | 14 | 0 | 14 | 31 |
The extracts demonstrated significantly high DPPH radical scavenging activity (Figure 1). Both A. indica stem and G. kola bark exhibited the highest DPPH radical scavenging effect, which was as high as 89.58%. This was followed by G. afzelii stem (87.08%), A. indica bark (86.25%), G. kola stem (85.00%), and G. afzelii bark (81.25%). The ascorbic acid standard recorded 94.17% inhibition (Figure 1).
Figure 1.
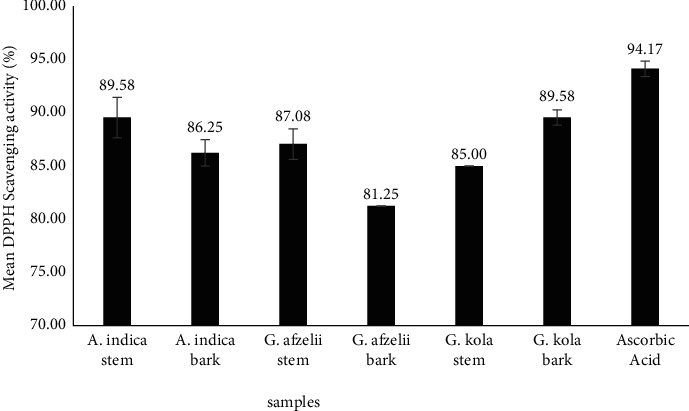
DPPH scavenging activity of chewing stick extracts and standard ascorbic acid.
The extracts used in this study were found to quench ABTS free radicals significantly in a manner comparable to the ascorbic acid standard (98.83%) (Figure 2). G. kola stem demonstrated the highest activity by scavenging about 99.53% ABTS radicals. This was followed by G. afzelii stem and G. kola bark (99.30%), A. indica bark (99.06%), G. afzelii bark (98.12%), A. indica stem (96.01%).
Figure 2.
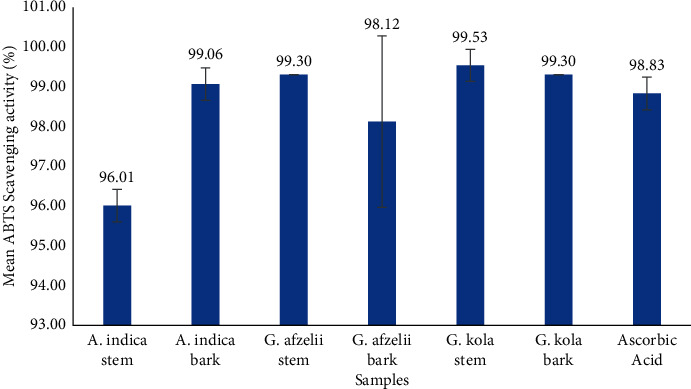
ABTS radical scavenging activity of chewing stick extracts and standard ascorbic acid.
Furthermore, extracts from the chewing sticks examined in this study demonstrated exceedingly high FRAP values (Table 2). The highest FRAP value was recorded for A. indica bark (2.29 ± 0.15). This was followed by G. afzelii bark (2.24 ± 0.05), G. kola bark (1.85 ± 0.15), G. afzelii stem (1.64 ± 0.18), G. kola stem (1.61 ± 0.01), and A. indica stem (1.26 ± 0.07).
Table 2.
Mean FRAP values of chewing stick extracts compared to standard ascorbic acid.
| Samples | A. indica stem | A. indica bark | G. afzelii stem | G. afzelii bark | G. kola stem | G. kola bark | Ascorbic acid |
|---|---|---|---|---|---|---|---|
| Mean FRAP value | 1.26 ± 0.07 | 2.29 ± 0.15 | 1.64 ± 0.18 | 2.24 ± 0.05 | 1.61 ± 0.01 | 1.85 ± 0.15 | 2.00 ± 0.00 |
Except for the A. indica stem, all extracts demonstrated appreciable total phenolic contents in gallic acid equivalence (GAE) (Figure 3). The highest phenolic content was observed in G. afzelii bark extract (75.38 µgGAE/g), followed by G. kola bark (71.93 µgGAE/g), G. afzelii stem (68.15 µgGAE/g), A. indica bark (67.28 µgGAE/g), G. kola stem (62.67 µgGAE/g), and A. indica stem (30.33 µgGAE/g).
Figure 3.
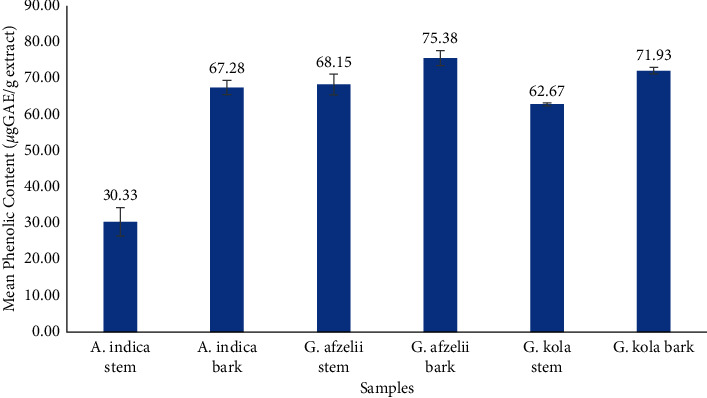
Total phenolic contents of chewing stick extracts.
4. Discussion
Due to the increasing prevalence of antibiotic resistance and the toxicity associated with synthetic drugs products, several studies have attempted to find plant products with antimicrobial properties due to their tolerability on the body. In this study, the antimicrobial effects of methanolic extract of three different plants, namely, Azadirachta indica, Garcinia afzelii, and Garcinia kola, commonly used as chewing sticks in Ghana for oral hygiene against some diarrhea-causing pathogens are evaluated. These organisms included two Shigella spp., S. sonnei and S. flexneri, which have been associated with shigellosis with symptoms of diarrhea, fever, vomiting, and stomach cramps. Worldwide prevalence of shigellosis is 164.7 million people annually, with greater than 95% of the cases occurring in developing countries [23]. Antimicrobial effects on other microorganisms such as Salmonella typhi enterica, Salmonella typhinirium attenuated, L. innocua, and K. oxytoca which have been associated with different forms of diarrheal diseases in the literature were also examined [24–26].
Our results showed that G. afzelii and A. indica stem inhibited the growth of the entire microorganism examined to varying degrees using the agar well diffusion assay. In a related study, a similar inhibitory effect of G. afzelii and A. indica stem against S. aureus and E. coli was observed (unpublished data). These two microbes have also been associated with diarrhea [27, 28]. G. kola stem showed the least antimicrobial activity inhibiting the growth of only one of the Shigella species.
Since all extracts demonstrated antibacterial effects to various extents, it can be concluded that each chewing stick sample may possess various inhibitors of bacteria, hence the observed biological activities. Previously, Ashie et al. [29] investigated the microbial pathogens associated with acute childhood diarrhea within a Ghanaian setting, and Salmonella spp. and Shigella spp. were found to be the most prevalent bacterial species associated with diarrhea. Our results showing an inhibitory effect of the chewing stick extracts on these two microbes as well as other diarrhea-causing microbes is, therefore, of important public health consequence since it may lead to the potential isolation of bioactive compounds that can be used in the treatment of diarrhea.
Antioxidants are molecules that scavenge free radicals and are important for maintaining good health. In response to an infected microbe, the body releases a host of reactive oxygen and nitrogen species to fight the infection; however, excessive production of these free radicals can result in tissue damage which leads to other complications [18]. Maintaining a good balance of oxidants and antioxidants is very essential for maintaining enteric health. The results of the present study reveal that the chewing stick extracts possess exceptionally high antioxidant effects in both the DPPH and the ABTS assays.
The 2,2-diphenyl-1-picrylhydrazyl (DPPH) is a known nitrogen-centred free radical. Therefore, any products with adequate antioxidant activity can scavenge for a significant amount of this radical and, hence, may be useful to reduce oxidative stress in organisms. We demonstrated that all the plant extracts were able to significantly reduce the initial amount of the DPPH free radical in the solution. One attribute of antioxidants is to scavenge for proton radical [30]. Therefore, continuing usage of these plants as chewing stick could reduce oxidative stress in humans. Additionally, these plants could provide useful compounds for formulation of antioxidants for human use. Furthermore, the initial level of 2, 2'-azino-bis (3-ethylbenzthiazoline-6-sulphonic) acid (ABTS) scavenging activity was reduced by the plant extracts. This cationic radical is able to react with most antioxidants to reduce their bioavailability in vivo [31].
In this experimental study, we investigated the transformation of ferric (Fe3+) to ferrous (Fe2+) iron as a measure of the reductive potential of the various extracts. Again, all extracts used in this study demonstrated significant ferric iron-reducing antioxidant potential. This potential makes the strong antioxidants [32]. Reducing properties have been linked to the presence of reductone, which demonstrates antioxidant properties by breaking chain reactions and are also potent inhibitors of peroxide formation. Some peroxides such as H2O2 have been linked to tissue injury and cell toxicity, and hence, there is a need to adequately control their levels by reduction. From our results, the exceptionally high antioxidant effects demonstrated by the various chewing sticks present them as candidates for the extraction of novel antioxidant compounds.
We also found out that the plant extracts contain high levels of phenolic compounds. Meanwhile, most of the antimicrobial properties of plants are attributed to phenol and flavonoid secondary metabolites. Also, phenols have been shown to exhibit strong antioxidant activity [33]. Taken together, G. afzelii and A. indica have been found to inhibit the growth of S. sonnei, S. flexeneri, S. typhinirium enterica, S. typhi attenuated, and K. oxytoca and, hence, may reduce incidences of diarrhea when chewed continually. Additionally, these plants were found to possess high antioxidant activity. Therefore, even though these plants are chewed for the purses of oral hygiene, users may unknowingly be protecting themselves from diarrhea-causing pathogens and, at the same time, neutralizing free radicals in the body. These properties of the plants are important for maintaining better human health and preventing diseases.
5. Conclusions
Our results show that A. indica and G. afzelii stem, which are commonly used chewing sticks in Ghana, possess potent antimicrobial activity against common diarrhea-causing microorganisms. All extracts examined also contained significant antioxidant activity. The high phenolic content of the plants may be responsible for the high antioxidant and potent antimicrobial activities observed in the study. These chewing sticks, therefore, can be further studied in the future to elucidate the actual compound producing the observed biological activity. Finally, the continual use of these plants for oral hygiene must be encouraged.
Data Availability
All data are in the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.WHO. Diarrhoeal Disease . Geneva, Switzerland: WHO; 2017. https://www.who.int/news-room/fact-sheets/detail/diarrhoeal-disease . [Google Scholar]
- 2.Camilleri M., Sellin J. H., Barrett K. E. Pathophysiology, evaluation, and management of chronic watery diarrhea. Gastroenterology . 2017;152(3):515–532. doi: 10.1053/j.gastro.2016.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Organization W. H. Diarrhoeal Disease . Geneva, Switzerland: WHO; 2017. https://www.who.int/news-room/fact-sheets/detail/diarrhoeal-disease . [Google Scholar]
- 4.Whyte L. A., Jenkins H. R. Pathophysiology of diarrhoea. Paediatrics and Child Health . 2012;22(10):443–447. doi: 10.1016/j.paed.2012.05.006. [DOI] [Google Scholar]
- 5.Barr W., Smith A. Acute diarrhea. American Family Physician . 2014;89(3):180–189. [PubMed] [Google Scholar]
- 6.Onoja S. O., Ihejirika G. Q., Nwankudu O. N., Omeh Y. N., Ezeja M. I. Antidiarrheal and antioxidant activities of methanol extract of Bryophyllum pinnatum Leaf harvested from south-eastern Nigeria in mice. Journal of Pharmacy . 2018;2018:6. doi: 10.1155/2018/6810620.6810620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee K. J. Pharmacologic agents for chronic diarrhea. Intestinal Research . 2015;13(4):p. 306. doi: 10.5217/ir.2015.13.4.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Humphries R. M., Schuetz A. N. Antimicrobial susceptibility testing of bacteria that cause gastroenteritis. Clinics in Laboratory Medicine . 2015;35(2):313–331. doi: 10.1016/j.cll.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 9.Okeke I. N., Aboderin O. A., Byarugaba D. K., Ojo K. K., Opintan J. A. Growing problem of multidrug-resistant enteric pathogens in Africa. Emerging Infectious Diseases . 2007;13(11):1640–1646. doi: 10.3201/eid1311.070674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ameade E. P. K., Ibrahim M., Ibrahim H. S., Habib R. H., Gbedema S. Y. Concurrent use of herbal and orthodox medicines among residents of Tamale, Northern Ghana, who patronize hospitals and herbal clinics. Evidence Based Complementary and Alternative Medicine . 2018;2018:8. doi: 10.1155/2018/1289125.1289125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Veeresham C. Natural products derived from plants as a source of drugs. Journal of Advanced Pharmaceutical Technology and Research . 2012;3(4) doi: 10.4103/2231-4040.104709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bent S. Herbal medicine in the United States: review of efficacy, safety, and regulation. Journal of General Internal Medicine . 2008;23(6):854–859. doi: 10.1007/s11606-008-0632-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Newman D. J., Cragg G. M. Natural products as sources of new drugs over the 30 years from 1981 to 2010. Journal of Natural Products . 2012;75(3):311–335. doi: 10.1021/np200906s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Singh B., Bhat T. K., Singh B. Potential therapeutic applications of some antinutritional plant secondary metabolites. Journal of Agricultural and Food Chemistry . 2003;51(19):5579–5597. doi: 10.1021/jf021150r. [DOI] [PubMed] [Google Scholar]
- 15.Akande T. A., Ajao A. T. Chemotherapeutic values of four Nigerian chewing sticks on bacteria isolates of dental infection. Global Journal of Science Frontier Research . 2011;11:90–96. [Google Scholar]
- 16.Khan M. N., Ngassapa O., Matee M. I. N. Antimicrobial activity of Tanzanian chewing sticks against oral pathogenic microbes. Pharmaceutical Biology . 2000;38(3):235–240. doi: 10.1076/1388-0209(200007)3831-sft235. [DOI] [PubMed] [Google Scholar]
- 17.Tetteh J., Takramah W. K., Ayanore M. A., Adoliba Ayanore A., Bisung E., Alamu J. Trends for diarrhea morbidity in the Jasikan District of Ghana: estimates from district level diarrhea surveillance data, 2012–2016. Journal of Tropical Medicine . 2018;2018:10. doi: 10.1155/2018/4863607.4863607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bhattacharyya A., Chattopadhyay R., Mitra S., Crowe S. E. Oxidative stress: an essential factor in the pathogenesis of gastrointestinal mucosal diseases. Physiological Reviews . 2014;94(2):329–354. doi: 10.1152/physrev.00040.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mekinić I. G., Skroza D., Ljubenkov I., Katalinić V., Šimat V. Antioxidant and antimicrobial potential of phenolic metabolites from traditionally used mediterranean herbs and spices. Foods . 2019;8(11):p. 579. doi: 10.3390/foods8110579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perez C. Antibiotic assay by agar-well diffusion method. Acta Biologiae et Medicinae Experimentalis . 1990;15:113–115. [Google Scholar]
- 21.Ahmad I., Beg A. Z. Antimicrobial and phytochemical studies on 45 Indian medicinal plants against multi-drug resistant human pathogens. Journal of Ethnopharmacology . 2001;74(2):113–123. doi: 10.1016/s0378-8741(00)00335-4. [DOI] [PubMed] [Google Scholar]
- 22.Dravie E. E., Kortei N. K., Essuman E. K., Tettey C. O., Boakye A. A., Hunkpe G. Antioxidant, phytochemical and physicochemical properties of sesame seed (Sesamum indicum L) Scientific African . 2020;8 doi: 10.1016/j.sciaf.2020.e00349.e00349 [DOI] [Google Scholar]
- 23.Duran C., Nato F., Dartevelle S., et al. Rapid diagnosis of diarrhea caused by Shigella sonnei using dipsticks; comparison of rectal swabs, direct stool and stool culture. PLoS One . 2013;8(11) doi: 10.1371/journal.pone.0080267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wotzka S. Y., Nguyen B. D., Hardt W.-D. Salmonella Typhimurium diarrhea reveals basic principles of enteropathogen infection and disease-promoted DNA exchange. Cell Host & Microbe . 2017;21(4):443–454. doi: 10.1016/j.chom.2017.03.009. [DOI] [PubMed] [Google Scholar]
- 25.Mehmood H., Marwat A. D. J. K., Khan N. A. J. Invasive Listeria monocytogenes gastroenteritis leading to stupor, bacteremia, fever, and diarrhea: a rare life-threatening condition. Journal of investigative medicine high impact case reports . 2017;5(2) doi: 10.1177/2324709617707978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheng V. C. C., Yam W.-C., Tsang L.-L., et al. Epidemiology of Klebsiella oxytoca-associated diarrhea detected by Simmons citrate agar supplemented with inositol, tryptophan, and bile salts. Journal of Clinical Microbiology . 2012;50(5):1571–1579. doi: 10.1128/jcm.00163-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lane A. B., Copeland N. K., Onmus-Leone F., Lawler J. V. Methicillin-resistant staphylococcus aureus as a probable cause of antibiotic-associated enterocolitis. Case Reports in Infectious Diseases . 2018;2018:3. doi: 10.1155/2018/3106305.3106305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nataro J. P., Kaper J. B. Diarrheagenic Escherichia coli. Clinical Microbiology Reviews . 1998;11(1):142–201. doi: 10.1128/cmr.11.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ashie G. K., Mutocheluh M., Owusu M., et al. Microbial pathogens associated with acute childhood diarrhoea in Kumasi, Ghana. BMC Research Notes . 2017;10(1):p. 264. doi: 10.1186/s13104-017-2578-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Adedapo A. A., Jimoh F. O., Afolayan A. J., Masika P. J. Antioxidant activities and phenolic contents of the methanol extracts of the stems of Acokanthera oppositifolia and Adenia gummifera. BMC Complementary and Alternative Medicine . 2008;8(1):p. 54. doi: 10.1186/1472-6882-8-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nenadis N., Tsimidou M. Observations on the estimation of scavenging activity of phenolic compounds using rapid 1,1-diphenyl-2-picrylhydrazyl (DPPH ) tests. Journal of the American Oil Chemists Society . 2002;79(12):p. 1191. doi: 10.1007/s11746-002-0626-z. [DOI] [Google Scholar]
- 32.Thakral J., Borar S., Kalia A. N. Antioxidant potential fractionation from methanol extract of aerial parts of Convolvulus arvensis Linn. (Convolvulaceae) International Journal of Pharmaceutical Sciences and Drug Research . 2010;2(3):219–223. [Google Scholar]
- 33.Cook N., Samman S. Flavonoids--Chemistry, metabolism, cardioprotective effects, and dietary sources. The Journal of Nutritional Biochemistry . 1996;7(2):66–76. doi: 10.1016/0955-2863(95)00168-9. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are in the manuscript.


