Abstract
Sex differences in brain anatomy have been described from early childhood through late adulthood, but without any clear consensus among studies. Here, we applied a machine learning approach to estimate ‘Brain Sex’ using a continuous (rather than binary) classifier in 162 boys and 185 girls aged between 5 and 18 years. Changes in the estimated sex differences over time at different age groups were subsequently calculated using a sliding window approach. We hypothesized that males and females would differ in brain structure already during childhood, but that these differences will become even more pronounced with increasing age, particularly during adolescence. Overall, the classifier achieved a good performance, with an accuracy of 80.4% and an AUC of 0.897 across all age groups. Assessing changes in the estimated sex with age revealed a growing difference between the sexes with increasing age. That is, the very large effect size of d=1.2 which was already evident during childhood increased even further from age 11 onward, and eventually reached an effect size of d=1.6 at age 17. Altogether these findings suggest a systematic sex difference in brain structure already during childhood, and a subsequent increase of this difference during adolescence.
Keywords: adolescence, brain, childhood, development, machine learning, puberty, relevance vector, sex
1. Introduction
An ever growing interest in sex differences in the brain has resulted in a vast amount of literature on this topic (for reviews see Cosgrove et al., 2007; Giedd et al., 2012; Jancke, 2018; Lenroot and Giedd, 2010; Luders and Kurth, 2020; Luders and Toga, 2010; Sacher et al., 2013). While it remains unclear which parts of the brain differ and in what way exactly, the lack of consensus between studies does not necessarily imply that observed sex differences are spurious and incidental, or that a distinction into “male” and “female” brains is impossible. For example, when assessing brain patterns using multivariate machine learning techniques (instead of focusing on a specific brain feature using univariate traditional analyses), male-female distinctions have been established with classification accuracies between 69% and 93% (Anderson et al., 2019; Chekroud et al., 2016; Del Giudice et al., 2016; Rosenblatt, 2016; Tunc et al., 2016).
Importantly, sex differences in the brain do not only exist during adulthood, but are already present earlier in life (Berenbaum and Beltz, 2011, 2016; Cosgrove et al., 2007; Giedd et al., 1999; Giedd et al., 2012; Hines, 2010; Lenroot and Giedd, 2006, 2010; Luders and Toga, 2010; Sacher et al., 2013). In fact, male and female brains have been reported to differ significantly in newborns and babies (Benavides et al., 2019; Gilmore et al., 2007), with more and more sex differences becoming evident during childhood and adolescence (Giedd et al., 2012; Gur and Gur, 2016; Herting and Sowell, 2017; Tunc et al., 2016; Vijayakumar et al., 2018). This seems to suggest that males and females might be distinguishable based on sex differences in brain anatomy already early in life, with the sex gap further widening over the years. At this point, however, the degree of such an age-dependent sex difference as well as the trajectory of the gap widening is largely unknown.
The present study was designed to assess the male-female separability in brain anatomy in the developing brain. For this purpose, a multivariate machine learning algorithm was applied in 347 healthy children and adolescents (162 boys and 185 girls) between the age of 5 and 18. Given that individual brains may show different degrees of “maleness” or “femaleness”, we used a classifier that yielded a continuous probabilistic estimate for being male/female, rather than a binary classifier. We hypothesized that male and female brains can be distinguished with considerable accuracy already in childhood, indicating sex differences in brain structure before puberty. We furthermore expected that the continuous classifier would yield increasingly dissimilar estimates of sex in boys and girls in later years (thus indicating more pronounced sex differences in brain structure with increasing age). Adolescence, for example, might present itself as a period where dissimilarities in brain structure increase disproportionally due to the influence of puberty with higher levels of circulating sex hormones, which may render the brain more masculine (more feminine, respectively).
2. Methods
2.1. Subjects and Image acquisition
The study included 347 subjects (162 boys; 185 girls) aged between 4.9 and 18.6 years (mean ± SD: 11.2 ± 3.8 years). All subjects were selected from the NIH Pediatric MRI Repository created by the NIH MRI Study of Normal Brain Development (https://www.bic.mni.mcgill.ca/nihpd_info/info2/index.html), which is described in detail elsewhere (Evans, 2006). Of the 432 subjects available in this repository, 56 subjects were excluded given the low resolution of their MRI images (>2mm3) resulting from an altered scanning protocol to reduce motion. In addition, 28 subjects were excluded due to insufficient image quality and improper segmentation, and one subject was excluded because MRI-related information was missing. Informed consent was obtained from parents and adolescents, and assents were obtained from the children. All protocols and procedures were approved by the relevant Institutional Review Board at each pediatric study center and at each coordinating center (Evans, 2006); additional local ethics approval for the data analysis was obtained from the University of Auckland (UoA) ethics committee (Protocol No. 022375). T1-weighted images of the brain were obtained at six sites on 1.5 Tesla systems from General Electrics (GE) or Siemens Medical Systems using a 3D T1-weighted spoiled gradient recalled (SPGR) echo sequence with the following parameters: TR = 22–25 ms, TE = 10–11 ms, excitation pulse = 30°, refocusing pulse = 180°, field of view: anterior-posterior = 256 mm; left-right = 160–180 mm (for details see Evans, 2006). On Siemens scanners the voxel size was 1 × 1 × 1 mm3, whereas on GE scanners it was 1 × 1 × 1.5 mm3 (Evans, 2006).
2.2. Data Preprocessing
The T1-weighted images were preprocessed using SPM8 (http://www.fil.ion.ucl.ac.uk/spm) and the VBM8 toolbox (http://dbm.neuro.uni-jena.de/vbm.html). Given that the investigated population consisted of children with a mean age of 11.2 years, customized tissue probability maps in MNI space were calculated using the TOM8 Toolbox (Wilke et al., 2008). Using these customized tissue probability maps, all brain images were corrected for magnetic field inhomogeneities and tissue-classified into gray matter, white matter, and cerebrospinal fluid. The segmentation procedure was based on maximum a posteriori estimations (Rajapakse et al., 1997) and used a partial volume estimation algorithm (Tohka et al., 2004), a spatially adapting non-linear means denoising filter (Manjon et al., 2010), as well as a hidden Markov Random Field model (Cuadra et al., 2005). The resulting gray and white matter partitions were spatially normalized to MNI space using 12-parameter affine transformations, which effectively resulted in a correction for overall brain size, while preserving individual differences in local size and shape. Subsequently, the normalized tissue segments were smoothed using an 8 mm FWHM Gaussian Kernel, and spatial resolution was set to 8 mm (Franke et al., 2010). Finally, a principal component analysis (PCA) was performed for further data reduction using the Matlab Toolbox for Dimensionality Reduction (http://ict.ewi.tudelft.nl/~lvandermaaten/Home.html), as described previously (Franke et al., 2010). To treat training and test samples separately, the loadings for the PCA were estimated from each training set prior to training the classifier and applied to the respective test set for prediction.
2.3. Estimating ‘Brain Sex’
The approach is based on machine learning using Relevance Vector Regression (RVR) (Tipping, 2001) and uses “The Spider” (https://people.kyb.tuebingen.mpg.de/spider/main.html) within MATLAB (The MathWorks, Natick. MA). It operates on the same principles as the BrainAGE framework, which is described in detail elsewhere (Franke et al., 2012; Franke et al., 2010). Briefly, the inputs are the voxel-wise intensities of the preprocessed gray and white matter segments after the PCA (see Section 2.2), and the output is the ‘Brain Sex’ estimate. ‘Brain Sex’ is a brain-specific number on a scale in which “0” indicates a 100% female brain and “1” indicates a 100% male brain. That is, rather than forcing a binary classification (male / female), this classifier yields a continuous probabilistic estimate of the degree of “maleness” or “femaleness”1. For example, a ‘Brain Sex’ estimate of “0.7” would indicate a brain that is more male than female, while a ‘Brain Sex’ estimate of “0.4” would indicate a brain that is more female than male. Training of the RVR machine and prediction of sex in the dataset was achieved using a 10-fold cross validation that was repeated nine times using different random permutations of the dataset. The resulting ten probabilistic ‘Brain Sex’ estimates per subject were then averaged and used as the input for the statistical analysis. The raw estimates for each sex by age are shown in the Supplementary Figure.
2.4. Statistical Analysis
2.4.1. Classifier Performance and Possible Effect of Scanner
To assess the predictive quality of the classifier, the individual ‘Brain Sex’ estimates were used to calculate the receiver-operator characteristic (ROC). In addition, the individual ‘Brain Sex’ estimates were rounded up or down to either 1 (male) or 0 (female). This binarized classification was then assessed for classification accuracy (in reference to the real biological sex). As brain images of the study sample were acquired on different scanners, the potential effect of scanner was assessed in addition by treating the ‘Brain Sex’ estimates as the dependent variable, scanner as the independent variable, and sex as well as age as covariates.
2.4.2. Age-related Changes of ‘Brain Sex’: Trajectory over the entire Age Span
To assess the effects of age on the ‘Brain Sex’ estimates, we calculated the effect size of the estimated mean sex differences at different age groups. Specifically, we employed a sliding window approach with a window length of three years to create 13 different age groups between 5 and 17 years (i.e., 1st group: 5–7 years, 2nd group: 6–8 years, 3rd group: 7–9 years, etc.). Information on the sex distribution in each “window” is provided in the Supplementary Table. Subsequently, we calculated the effect size of the estimated sex difference for each of these age groups as Cohen’s d using a general linear model with the ‘Brain Sex’ estimate as the dependent variable and the biological sex as the independent variable. In addition, based on the resulting effect sizes of the estimated sex difference for each age group, we calculated the trajectory using a non-parametric cubic smoothing spline (Fjell et al., 2010; Fjell et al., 2013; Ziegler et al., 2012). The optimal smoothing parameter for the smoothing spline was established by calculating the adjusted R2 for a wide range of smoothing parameters and choosing the parameter that resulted in the maximum adjusted R2. The point of maximum acceleration of the trajectory was determined from its second derivative.
2.4.3. Age-related Changes of ‘Brain Sex’: Comparing the Extremes of Age 6 and Age 17
To further assess and quantify changes in the estimated ‘Brain Sex’ between age 6 and age 17, we calculated the interaction between sex and the two age groups, using the ‘Brain Sex’ estimates as the dependent variable and the interaction between biological sex (male / female) and age group (6 years / 17 years) as the independent variable. In addition, AUC and prediction accuracy were calculated for these two age groups (6 years / 17 years).
3. Results
3.1. Classifier Performance and Possible Effect of Scanner
Across the entire age span, the sex classification was 80.4% accurate, and the receiver-operating characteristic of the predictions had an AUC of 0.897. These measures indicate a suitable classification performance and a reliable distinction between the sexes based on brain anatomy across the age range examined. The different scanners had no effect on the sex classification (F=0.151, p=0.963).
3.2. Age-related Changes of ‘Brain Sex’: Trajectory over the Entire Age Span
Overall, between the ages 6 and 17, the differentiation between male and female brains increased over time as indicated by the age-specific markers as well as the fitted trajectory line (Figure 1, top panel)2. Importantly, the sex differentiation was already evident at age 6 with a very large effect size (Cohen’s d=1.2). It continued to increase over the years and peaked at age 17 (Cohen’s d=1.6). The maximum of acceleration occurred at age 11 (see green arrow).
Figure 1:
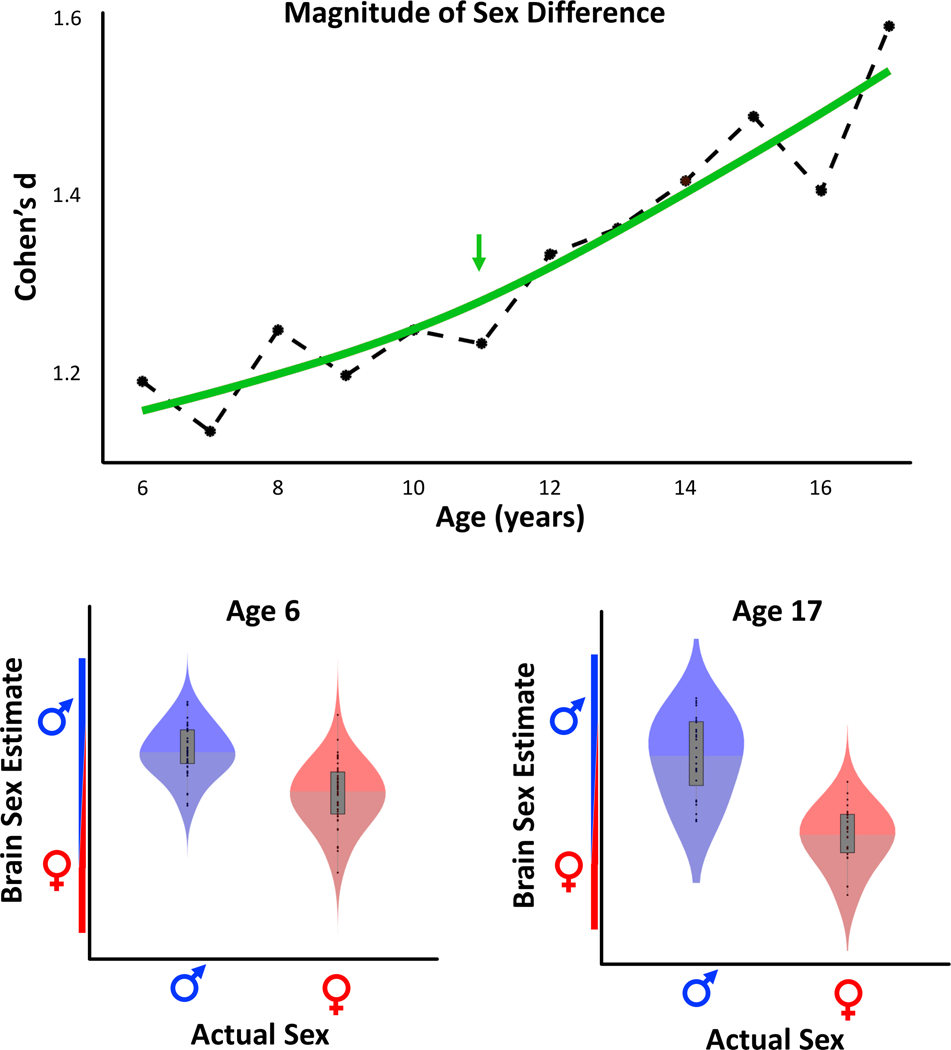
Age-specific sex differences. The top graph shows the magnitude of the difference between the ‘Brain Sex’ estimates in boys and girls (calculated as Cohen’s d) between age 6 and age 17 as markers connected by a dashed line. The fitted trajectory as determined by cubic smoothing splines is depicted in green, and the point of maximal acceleration is marked with a green arrow. The bottom graphs show the ‘Brain Sex’ estimates in relation to the actual sex for males (blue) and females (red) at age 6 and age 17. Note that the increased sex difference at age 17 corresponds to more homogeneous ‘Brain Sex’ estimates within each sex (particularly in girls) at age 17, which result in a reduced overlap between the predicted maleness / femaleness.
3.3. Age-related Changes of ‘Brain Sex’: Comparing the Extremes of Age 6 and Age 17
When comparing the predictions between age 6 and age 17 (Figure 1, bottom panel), the increased sex difference in the older sample corresponds to a decreased overlap between predicted “femaleness” and “maleness”. In other words, fewer boys and girls at age 17 (Figure 1, bottom right) have a similar ‘Brain Sex’ estimate than at age 6 (Figure 1, bottom left). This observation was confirmed by a significant interaction between sex and age (d=0.289, T=1.703, p=0.045), confirming that brains of boys and girls at age 17 are less similar than at age 6. This conclusion is further supported by an increase in AUC and accuracy from age 6 (AUC=0.86, accuracy=79.1%) to age 17 (AUC=0.95, accuracy=84.6%).
Of note, this decreasing overlap between the sexes with increasing age is driven both by a shift towards the male and female extremes as well as by more homogeneous estimates, particularly in girls. Specifically, the mean ‘Brain Sex’ estimate for boys increased from 0.65 to 0.68, while the mean ‘Brain Sex’ estimate for girls decreased from 0.25 to 0.23 (with 0 being female and 1 being male). In boys, the overall range of the ‘Brain Sex’ estimate decreased slightly between age 6 and 17 (0.14–1.10 vs. 0.31–1.17), whereas in girls it decreased considerably (−0.50–0.98 vs. −0.2–0.59).
4. Discussion
Using a multivariate machine learning approach, we observed a good separability between males and females based on brain anatomy. This observation replicates previous studies that reported a high accuracy in distinguishing between the sexes using machine learning (Anderson et al., 2019; Chekroud et al., 2016; Del Giudice et al., 2016; Rosenblatt, 2016; Tunc et al., 2016). Overall, the current results support the notion that male and female brains are anatomically different throughout childhood and adolescence.
4.1. Sex Differences before Puberty
Interestingly, the observed sex difference was already evident at the age of six, which corroborates other studies that have described sex differences in brain structure in children, babies, and even newborns (Benavides et al., 2019; Giedd et al., 2012; Gilmore et al., 2007; Lenroot and Giedd, 2010). These findings suggest an influence of early developmental factors, such as steroid hormones. The idea that exposure to sex hormones during development has a permanent organizational effect on the brain is well-established, both in animals (Phoenix et al., 1959) and humans (for review see Berenbaum and Beltz, 2011, 2016; Hines, 2010). For example, the so-called “organizational-activational hypothesis” (Arnold, 2009; MacLusky and Naftolin, 1981; Phoenix et al., 1959) suggests that chromosomal sex determines gonadal sex as well as the release of respective sex hormones resulting in a feminization or masculinization of the body, including the brain (McCarthy and Arnold, 2011; Phoenix et al., 1959). The outcomes of more recent studies point to more complex interrelations between brain structure, hormonal effects, gene expression and epigenetic modifications caused by environmental influences (Arnold and Burgoyne, 2004; Arnold and Chen, 2009; Carruth et al., 2002; De Vries et al., 2002; McCarthy and Arnold, 2011), but still posit that sexual differentiation begins in utero.
4.2. Sex Differences during Adolescence
While the sex difference was already evident at age 6, it increased even more during adolescence between age 11 and age 17 – a time frame that is most commonly associated with puberty (Blakemore et al., 2010). It is now generally accepted that the increase of sex hormones during puberty has activational and organizing effects on the brain, resulting in significant changes in brain structure, including sex differences, even though the results from imaging studies remain somewhat conflicting (for reviews see Arnold, 2020; Blakemore et al., 2010; Giedd et al., 2012; Herting and Sowell, 2017; Lenroot and Giedd, 2010; McCarthy and Arnold, 2011; Sisk and Foster, 2004; Sisk and Zehr, 2005; Vijayakumar et al., 2018). For example, white matter was observed to increase faster in males compared to females during puberty (De Bellis et al., 2001; Lenroot et al., 2007), but also see contrasting findings with respect to the corpus callosum in particular (Chavarria et al., 2014; Luders et al., 2010). Gray matter changes, on the other hand, are often described as following an inverted U-shape in both males and females, with the peak occurring close to the onset of puberty (Forkert et al., 2016; Giedd et al., 1999; Giedd et al., 2012; Krongold et al., 2017; Lenroot and Giedd, 2006, 2010; Lenroot et al., 2007), but also see contrasting findings when focusing on cortical gray matter only (Mills et al., 2016; Tamnes et al., 2017; Walhovd et al., 2017). However, as the onset of puberty occurs earlier on average in girls than in boys, gray matter changes may still follow different trajectories in both sexes (Lenroot et al., 2007). In addition, gray matter was reported to change differently in boys and girls depending on the brain region, albeit findings are not always consistent. For example, while some studies report the female hippocampus to increase in volume during puberty (Giedd et al., 1996; Hu et al., 2013; Neufang et al., 2009; Satterthwaite et al., 2014), others report it to decrease (Blanton et al., 2012; Bramen et al., 2011). Nevertheless, while single brain structures may show a considerable variance and thus at times fail to reflect sex-specific changes during puberty, the overall pattern of brain anatomy may indeed become more sexually dimorphic when assessed using a multivariate approach (Rosenblatt, 2016). In other words, the whole might be greater (aka more telling) than the sum of its parts, as also reflected in the outcomes of previous machine learning studies (Anderson et al., 2019; Chekroud et al., 2016; Del Giudice et al., 2016; Rosenblatt, 2016; Tunc et al., 2016).
4.3. Summary and Implications for Future Research
Altogether, the current findings are in good agreement with the outcomes of prior studies and further enhance the notion that adolescence and puberty exert sex-specific effects on the brain. In the past, sex differences have been frequently assessed in a binary way. Binary views seem to imply that males and females are either fundamentally different (in the sense of a dimorphism) or not. In contrast, the current study used a continuous classifier to distinguish between boys and girls, and we observed an accurate separability between the sexes (even before the onset of puberty). This suggests that sex differences do not manifest in the form of an always and ever present clear-cut sexual dimorphism. This conclusion is also in line with current models of sexual differentiation of the brain (Arnold, 2020; Arnold and Burgoyne, 2004; Arnold and Chen, 2009; Carruth et al., 2002; De Vries et al., 2002; McCarthy and Arnold, 2011) pointing to a complex interaction between hormonal, genetic, epigenetic, as well as location- and time-specific effects that may result in a variability of “maleness” or “femaleness” across individuals. To better understand the driving biological mechanisms, future research, ideally longitudinal in nature, is needed to follow up on our current findings by linking measures of ‘Brain Sex’ to actual hormonal levels and developmental stages from early childhood to adulthood (Vijayakumar et al., 2018). Moreover, by including cognitive and behavioral measures, follow-up studies will determine whether there is a link between the degree of male / female brain structure and (sex-typical) cognition and behavior.
Supplementary Material
Acknowledgements
EL is funded by the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health under award number R01HD081720. Data used in the preparation of this article were obtained from the NIH Pediatric MRI Data Repository created by the NIH MRI Study of Normal Brain Development. This is a multisite, longitudinal study of typically developing children from ages newborn through young adulthood conducted by the Brain Development Cooperative Group and supported by the National Institute of Child Health and Human Development, the National Institute on Drug Abuse, the National Institute of Mental Health, and the National Institute of Neurological Disorders and Stroke (Contract #s N01-HD02-3343, N01-MH9-0002, and N01-NS-9-2314, −2315, −2316, −2317, −2319 and −2320). A complete list of the participating sites and respective study investigators can be found here: http://pediatricmri.nih.gov/nihpd/info/participating_centers.html. Dataset identifier(s): [NIMH Data Repositories Study identification number or associated Digital Object Identifier 10.15154/1518487]. This manuscript reflects the views of the authors and may not reflect the opinions or views of the NIH.
Footnotes
It must be noted that other classifiers can also be used to obtain continuous classifications. However, we intentionally chose the relevance vector machine as it has proven to be reliable and stable (Franke et al., 2012; Franke et al. 2010), both across a wide age range (including children) and different scanners.
The age range of the sample was 5–18 years but given the ‘sliding window’ approach, the findings are presented for age 6 (5–7 years), age 7 (6–8 years) … and age 17 (16–18 years).
References
- Anderson NE, Harenski KA, Harenski CL, Koenigs MR, Decety J, Calhoun VD, Kiehl KA, 2019. Machine learning of brain gray matter differentiates sex in a large forensic sample. Hum Brain Mapp 40, 1496–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold AP, 2009. The organizational-activational hypothesis as the foundation for a unified theory of sexual differentiation of all mammalian tissues. Horm Behav 55, 570–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold AP, 2020. Sexual differentiation of brain and other tissues: Five questions for the next 50 years. Horm Behav 120, 104691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold AP, Burgoyne PS, 2004. Are XX and XY brain cells intrinsically different? Trends Endocrinol Metab 15, 6–11. [DOI] [PubMed] [Google Scholar]
- Arnold AP, Chen X, 2009. What does the “four core genotypes” mouse model tell us about sex differences in the brain and other tissues? Front Neuroendocrinol 30, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benavides A, Metzger A, Tereshchenko A, Conrad A, Bell EF, Spencer J, Ross-Sheehy S, Georgieff M, Magnotta V, Nopoulos P, 2019. Sex-specific alterations in preterm brain. Pediatr Res 85, 55–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berenbaum SA, Beltz AM, 2011. Sexual differentiation of human behavior: effects of prenatal and pubertal organizational hormones. Front Neuroendocrinol 32, 183–200. [DOI] [PubMed] [Google Scholar]
- Berenbaum SA, Beltz AM, 2016. How Early Hormones Shape Gender Development. Curr Opin Behav Sci 7, 53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore SJ, Burnett S, Dahl RE, 2010. The role of puberty in the developing adolescent brain. Hum Brain Mapp 31, 926–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanton RE, Cooney RE, Joormann J, Eugene F, Glover GH, Gotlib IH, 2012. Pubertal stage and brain anatomy in girls. Neuroscience 217, 105–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramen JE, Hranilovich JA, Dahl RE, Forbes EE, Chen J, Toga AW, Dinov ID, Worthman CM, Sowell ER, 2011. Puberty influences medial temporal lobe and cortical gray matter maturation differently in boys than girls matched for sexual maturity. Cereb Cortex 21, 636–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carruth LL, Reisert I, Arnold AP, 2002. Sex chromosome genes directly affect brain sexual differentiation. Nat Neurosci 5, 933–934. [DOI] [PubMed] [Google Scholar]
- Chavarria MC, Sanchez FJ, Chou YY, Thompson PM, Luders E, 2014. Puberty in the corpus callosum. Neuroscience 265, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chekroud AM, Ward EJ, Rosenberg MD, Holmes AJ, 2016. Patterns in the human brain mosaic discriminate males from females. Proc Natl Acad Sci U S A 113, E1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove KP, Mazure CM, Staley JK, 2007. Evolving knowledge of sex differences in brain structure, function, and chemistry. Biol Psychiatry 62, 847–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuadra MB, Cammoun L, Butz T, Cuisenaire O, Thiran JP, 2005. Comparison and validation of tissue modelization and statistical classification methods in T1-weighted MR brain images. IEEE Trans Med Imaging 24, 1548–1565. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Keshavan MS, Beers SR, Hall J, Frustaci K, Masalehdan A, Noll J, Boring AM, 2001. Sex differences in brain maturation during childhood and adolescence. Cereb Cortex 11, 552–557. [DOI] [PubMed] [Google Scholar]
- De Vries GJ, Rissman EF, Simerly RB, Yang LY, Scordalakes EM, Auger CJ, Swain A, Lovell-Badge R, Burgoyne PS, Arnold AP, 2002. A model system for study of sex chromosome effects on sexually dimorphic neural and behavioral traits. J Neurosci 22, 9005–9014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Giudice M, Lippa RA, Puts DA, Bailey DH, Bailey JM, Schmitt DP, 2016. Joel et al. ’s method systematically fails to detect large, consistent sex differences. Proc Natl Acad Sci U S A 113, E1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans AC, 2006. The NIH MRI study of normal brain development. Neuroimage 30, 184–202. [DOI] [PubMed] [Google Scholar]
- Fjell AM, Walhovd KB, Westlye LT, Ostby Y, Tamnes CK, Jernigan TL, Gamst A, Dale AM, 2010. When does brain aging accelerate? Dangers of quadratic fits in cross-sectional studies. Neuroimage 50, 1376–1383. [DOI] [PubMed] [Google Scholar]
- Fjell AM, Westlye LT, Grydeland H, Amlien I, Espeseth T, Reinvang I, Raz N, Holland D, Dale AM, Walhovd KB, Alzheimer Disease Neuroimaging I, 2013. Critical ages in the life course of the adult brain: nonlinear subcortical aging. Neurobiol Aging 34, 2239–2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forkert ND, Li MD, Lober RM, Yeom KW, 2016. Gray Matter Growth Is Accompanied by Increasing Blood Flow and Decreasing Apparent Diffusion Coefficient during Childhood. AJNR Am J Neuroradiol 37, 1738–1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke K, Luders E, May A, Wilke M, Gaser C, 2012. Brain maturation: Predicting individual BrainAGE in children and adolescents using structural MRI. Neuroimage 63, 1305–1312. [DOI] [PubMed] [Google Scholar]
- Franke K, Ziegler G, Kloppel S, Gaser C, 2010. Estimating the age of healthy subjects from T1-weighted MRI scans using kernel methods: exploring the influence of various parameters. Neuroimage 50, 883–892. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC, Rapoport JL, 1999. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci 2, 861–863. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Raznahan A, Mills KL, Lenroot RK, 2012. Review: magnetic resonance imaging of male/female differences in human adolescent brain anatomy. Biol Sex Differ 3, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN, Vaituzis AC, Hamburger SD, Lange N, Rajapakse JC, Kaysen D, Vauss YC, Rapoport JL, 1996. Quantitative MRI of the temporal lobe, amygdala, and hippocampus in normal human development: ages 4–18 years. J Comp Neurol 366, 223–230. [DOI] [PubMed] [Google Scholar]
- Gilmore JH, Lin W, Prastawa MW, Looney CB, Vetsa YS, Knickmeyer RC, Evans DD, Smith JK, Hamer RM, Lieberman JA, Gerig G, 2007. Regional gray matter growth, sexual dimorphism, and cerebral asymmetry in the neonatal brain. J Neurosci 27, 1255–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur RE, Gur RC, 2016. Sex differences in brain and behavior in adolescence: Findings from the Philadelphia Neurodevelopmental Cohort. Neurosci Biobehav Rev 70, 159–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herting MM, Sowell ER, 2017. Puberty and structural brain development in humans. Front Neuroendocrinol 44, 122–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hines M, 2010. Sex-related variation in human behavior and the brain. Trends Cogn Sci 14, 448–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S, Pruessner JC, Coupe P, Collins DL, 2013. Volumetric analysis of medial temporal lobe structures in brain development from childhood to adolescence. Neuroimage 74, 276–287. [DOI] [PubMed] [Google Scholar]
- Jancke L, 2018. Sex/gender differences in cognition, neurophysiology, and neuroanatomy. F1000Res 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krongold M, Cooper C, Bray S, 2017. Modular Development of Cortical Gray Matter Across Childhood and Adolescence. Cereb Cortex 27, 1125–1136. [DOI] [PubMed] [Google Scholar]
- Lenroot RK, Giedd JN, 2006. Brain development in children and adolescents: insights from anatomical magnetic resonance imaging. Neurosci Biobehav Rev 30, 718–729. [DOI] [PubMed] [Google Scholar]
- Lenroot RK, Giedd JN, 2010. Sex differences in the adolescent brain. Brain Cogn 72, 46–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenroot RK, Gogtay N, Greenstein DK, Wells EM, Wallace GL, Clasen LS, Blumenthal JD, Lerch J, Zijdenbos AP, Evans AC, Thompson PM, Giedd JN, 2007. Sexual dimorphism of brain developmental trajectories during childhood and adolescence. Neuroimage 36, 1065–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luders E, Kurth F, 2020. Structural differences between male and female brains. In: Lanzenberger R, Kranz GS, Savic I (Eds.), Sex Differences in Neurology and Psychiatry. Elsevier. [DOI] [PubMed] [Google Scholar]
- Luders E, Thompson PM, Toga AW, 2010. The development of the corpus callosum in the healthy human brain. J Neurosci 30, 10985–10990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luders E, Toga AW, 2010. Sex differences in brain anatomy. In: Savic I (Ed.), Prog Brain Res. Elsevier, pp. 3–12. [DOI] [PubMed] [Google Scholar]
- MacLusky NJ, Naftolin F, 1981. Sexual differentiation of the central nervous system. Science 211, 1294–1302. [DOI] [PubMed] [Google Scholar]
- Manjon JV, Coupe P, Marti-Bonmati L, Collins DL, Robles M, 2010. Adaptive non-local means denoising of MR images with spatially varying noise levels. J Magn Reson Imaging 31, 192–203. [DOI] [PubMed] [Google Scholar]
- McCarthy MM, Arnold AP, 2011. Reframing sexual differentiation of the brain. Nat Neurosci 14, 677–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills KL, Goddings AL, Herting MM, Meuwese R, Blakemore SJ, Crone EA, Dahl RE, Guroglu B, Raznahan A, Sowell ER, Tamnes CK, 2016. Structural brain development between childhood and adulthood: Convergence across four longitudinal samples. Neuroimage 141, 273–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufang S, Specht K, Hausmann M, Gunturkun O, Herpertz-Dahlmann B, Fink GR, Konrad K, 2009. Sex differences and the impact of steroid hormones on the developing human brain. Cereb Cortex 19, 464–473. [DOI] [PubMed] [Google Scholar]
- Phoenix CH, Goy RW, Gerall AA, Young WC, 1959. Organizing action of prenatally administered testosterone propionate on the tissues mediating mating behavior in the female guinea pig. Endocrinology 65, 369–382. [DOI] [PubMed] [Google Scholar]
- Rajapakse JC, Giedd JN, Rapoport JL, 1997. Statistical approach to segmentation of single-channel cerebral MR images. IEEE Trans Med Imaging 16, 176–186. [DOI] [PubMed] [Google Scholar]
- Rosenblatt JD, 2016. Multivariate revisit to “sex beyond the genitalia”. Proc Natl Acad Sci U S A 113, E1966–1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacher J, Neumann J, Okon-Singer H, Gotowiec S, Villringer A, 2013. Sexual dimorphism in the human brain: evidence from neuroimaging. Magn Reson Imaging 31, 366–375. [DOI] [PubMed] [Google Scholar]
- Satterthwaite TD, Vandekar S, Wolf DH, Ruparel K, Roalf DR, Jackson C, Elliott MA, Bilker WB, Calkins ME, Prabhakaran K, Davatzikos C, Hakonarson H, Gur RE, Gur RC, 2014. Sex differences in the effect of puberty on hippocampal morphology. J Am Acad Child Adolesc Psychiatry 53, 341–350 e341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisk CL, Foster DL, 2004. The neural basis of puberty and adolescence. Nat Neurosci 7, 1040–1047. [DOI] [PubMed] [Google Scholar]
- Sisk CL, Zehr JL, 2005. Pubertal hormones organize the adolescent brain and behavior. Front Neuroendocrinol 26, 163–174. [DOI] [PubMed] [Google Scholar]
- Tamnes CK, Herting MM, Goddings AL, Meuwese R, Blakemore SJ, Dahl RE, Guroglu B, Raznahan A, Sowell ER, Crone EA, Mills KL, 2017. Development of the Cerebral Cortex across Adolescence: A Multisample Study of Inter-Related Longitudinal Changes in Cortical Volume, Surface Area, and Thickness. J Neurosci 37, 3402–3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tipping ME, 2001. Sparse bayesian learning and the relevance vector machine. J Mach Learn Res 1, 211–244. [Google Scholar]
- Tohka J, Zijdenbos A, Evans A, 2004. Fast and robust parameter estimation for statistical partial volume models in brain MRI. Neuroimage 23, 84–97. [DOI] [PubMed] [Google Scholar]
- Tunc B, Solmaz B, Parker D, Satterthwaite TD, Elliott MA, Calkins ME, Ruparel K, Gur RE, Gur RC, Verma R, 2016. Establishing a link between sex-related differences in the structural connectome and behaviour. Philos Trans R Soc Lond B Biol Sci 371, 20150111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayakumar N, Op de Macks Z, Shirtcliff EA, Pfeifer JH, 2018. Puberty and the human brain: Insights into adolescent development. Neurosci Biobehav Rev 92, 417–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walhovd KB, Fjell AM, Giedd J, Dale AM, Brown TT, 2017. Through Thick and Thin: a Need to Reconcile Contradictory Results on Trajectories in Human Cortical Development. Cereb Cortex 27, 1472–1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilke M, Holland SK, Altaye M, Gaser C, 2008. Template-O-Matic: a toolbox for creating customized pediatric templates. Neuroimage 41, 903–913. [DOI] [PubMed] [Google Scholar]
- Ziegler G, Dahnke R, Jancke L, Yotter RA, May A, Gaser C, 2012. Brain structural trajectories over the adult lifespan. Hum Brain Mapp 33, 2377–2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


