Abstract
Purpose
To investigate whether eosinophils and other white blood cell subtypes could be used as response and prognostic markers to anti-Programmed cell Death-1 or anti-PD-Ligand-1 treatments in non-small cell lung cancer patients.
Methods
We retrospectively analyzed data from the NSCLC patients consecutively treated at our hospital with a PD-1/PD-L1 inhibitor in monotherapy for advanced disease. A total of 191 patients were evaluated at three time-points to investigate any relation between tumor response and WBC counts.
Results
Baseline WBC and subtypes did not differ according to the type of response seen under treatment. A higher relative eosinophil count (REC) correlated with more objective responses (p = 0.019 at t1 and p = 0.014 at t2; OR for progression = 0.54 and 0.53, respectively) independently of the smoking status, PD-L1 status, and immune-related toxicity (IRT). Higher REC was also associated with a longer duration of treatment (p = 0.0096). Baseline absolute neutrophil count was prognostic (p = 0.049). At t1 relative lymphocytes, absolute and relative neutrophils, and neutrophil-to-lymphocyte ratio were prognostic (p = 0.044, p = 0.014, p = 0.0033, and p = 0.029, respectively).
Conclusion
Our results show that in NSCLC patients anti-PD-1/PD-L1 therapy induces an early increase only in blood eosinophils, more prominent in responding patients and independent of the smoking status, PD-L1 status, and IRT. Eosinophils are also associated with a longer duration of treatment. Furthermore, our data support a prognostic role of neutrophils, lymphocytes, and their ratio for NSCLC patients with advanced disease treated with PD(L)-1 blockade.
Keywords: White blood cells, PD-1 inhibitors, Non-small cell lung cancer, Prognostic marker, Predictive marker, Checkpoint inhibitors
Introduction
The use of immune checkpoint inhibitors (ICI) for non-small-cell lung cancer (NSCLC) is increasing. Currently validated indications include advanced and locally advanced disease [1]. One of the challenges regarding ICI lies in the evaluation of objective response to these drugs. Classically, response evaluation relies on radiological criteria based on the Response Evaluation Criteria In Solid Tumors (RECIST) [2]. However, in the setting of ICI, these criteria seem imperfect. Indeed, several atypical response patterns like pseudoprogression have been observed that make radiological evaluation less clear than it is with chemotherapy [3]. In the search for additional evaluation tools, white blood cell (WBC) count has been investigated, among others, in melanoma and in NSCLC patients treated with Programmed cell Death (PD) Ligand (L)-1 inhibitors [4–7]. Some reports also mention a potential prognostic role of WBC subtypes and/or their ratio for various malignancies among which NSCLC [5, 7–9]. We previously reported a retrospective study investigating peripheral blood eosinophil counts as a parameter in the evaluation of response in NSCLC patients receiving PD-1 blockers [10]. In the present study we first aimed to investigate whether the results obtained in our former cohort could be confirmed. Then, we compared the potential predictive value of different subtypes of WBC and investigated the prognostic value of baseline WBC subtypes.
Material and Methods
Patients
All consecutive cases of advanced stage NSCLC were collected from our internal cancer registry from August 1st, 2015 to September 30th, 2019. A computer-based search was performed with the following inclusion criteria: (1) use of an anti-PD-1 or anti-PD-L1 agent (pembrolizumab at 2 mg/kg/3 weeks during the early access program (EAP) and then at 200 mg/3 weeks; nivolumab at 3 mg/kg/2 weeks during the EAP and then at 240 mg/2 weeks; atezolizumab at 1200 mg/3 weeks; durvalumab at 10 mg/kg/2 weeks) or (2) a pathological diagnosis of NSCLC for which the patient was registered in the electronic treatment prescription system. For the 388 patients identified the following exclusion criteria were applied: histology other than NSCLC (n = 27), missing laboratory values (n = 15), loss of follow-up (n = 22); early treatment discontinuation, i.e., before the second evaluation (n = 106) due to death, toxicity, and progressive disease without death or patient’s will; ongoing treatment (n = 7) or chemotherapy combined with anti-PD-1 (n = 20). Based on this, 191 patients were included in the present analysis.
Data Collection
We collected the following data: (i) patient characteristics: age at the start of immunotherapy, gender, smoking status, concomitant obstructive airway disease, use of inhaled or oral corticoids and the reason for it (underlying respiratory condition, immune-related toxicity (IRT), other), date of death, and baseline Eastern Cooperative Oncology Group (ECOG)-Performance Status (PS); (ii) lung cancer characteristics: histology, stage of disease, line of treatment of the anti-PD (L)-1, PD-L1 expression level, based on immunohistochemistry (monoclonal antibody clone 22C3 with Automated Stainer, Dako), and presence or absence of a mutation based on next-generation sequencing analysis and ALK immunohistochemistry; (iii) treatment characteristics: dates of the start of treatment [t0], first evaluation (t1) and second evaluation (t2), immunotherapeutic agent, response at t1 and t2 using the RECIST criteria v1.1, immune-related toxicity (IRT), and duration of treatment; (iv) biological variables: total WBC counts and differential WBC counts (neutrophils, lymphocytes, eosinophils; absolute and relative) at t0, t1, and t2.
Response Evaluation
A total of 191 patients were assessed for tumor response based on the RECIST criteria v 1.1 at two time-points (t1, t2; 8 to 12 weeks interval in between) and compared with baseline data. We describe patients as responders (R; for complete or partial response), stable (S), or progressive (P). We focused on the first two radiological evaluations as the majority of objective responses occur in the first two months of treatment (t1) with PD-1 blockers in monotherapy for NSCLC [11, 12]. We extended the evaluation period to the second radiological evaluation (t2) in order to include the patients showing a non-significant response at t1 further evolving toward progression or response.
Duration of Treatment
Duration of treatment with anti-PD (L)-1 drugs was calculated from the time of first administration until the last recorded dose administration (data cut-off December 5th, 2019) and expressed in weeks.
Overall Survival
Overall survival (OS) was defined as the time between the first dose of PD (L)-1 blocker and the date of death from any cause and expressed in months. If still alive at data cut-off (December 5th, 2019) the patient was censored.
Statistical Analyses
Biological variables were studied as continuous variables and are described as medians and interquartile ranges. Qualitative data are described using frequencies and percentages. For the analyses on biological variables logarithmic analyses were performed (translated logarithm log (. +1) for the relative eosinophil count (REC) in percentage and log (. +0.01) for the absolute eosinophil count (AEC) in 103cells/mm3). Univariate logistic regression analyses were performed with determination of the Odds ratio (OR), with confidence interval (CI) at 95% and p-values. Survival was calculated, expressed in months, and reported with Kaplan–Meier curves, and Cox regression models were used to analyze the impact of the different variables on the survival and reported as Hazard Ratio (HR), with CI at 95% and p-values. Results were considered significant with an uncertainty level of 5% (p < 0.05). Calculations were made with the help of SAS software (version 9.4) and graphs with R software (version 3.6.2).
Results
Patient Characteristics
A total of 191 patients were included in the study (Table 1). Approximately two-thirds of the patients were male with a large majority (94.8%) of (former) smokers and in good performance status (PS; 92.7% PS 0–1). Slightly more than half of the patients presented with a chronic obstructive airway disease at the time of PD(L)-1 blocker initiation but only 10.5% used inhaled corticoids and none used oral corticoids during the study period. The predominant histology was adenocarcinoma (55.5%). The majority of patients (69.7%) had stage IV disease at the time of treatment with PD(L)-1 blockade. Most of the patients (67.7%) received an anti-PD(L)-1 antibody in second or later line of treatment.
Table 1.
Patient's characteristics
| Characteristic | Total (n = 191) | Number (%) |
|---|---|---|
| Age-years | ||
| Median | 66 | |
| Range | 42–85 | |
| Gender | 191 | |
| Male | 122 (63.9) | |
| Female | 69 (36.1) | |
| Smoking status | 191 | |
| Non-smoker | 10 (5.2) | |
| Former smoker | 117 (61.3) | |
| Current smoker | 64 (33.5) | |
| Obstructive airway disease | 191 | |
| None | 83 (43.5) | |
| COPD | 88 (46.1) | |
| Asthma | 20 (10.4) | |
| Inhaled corticosteroids | 191 | |
| No | 171 (89.5) | |
| Yes | 20 (10.5) | |
| ECOG-PS | 191 | |
| 0 | 26 (13.6) | |
| 1 | 151 (79.1) | |
| 2+ | 14 (7.3) | |
| Histology | 191 | |
| Adenocarcinoma | 106 (55.5) | |
| NOS | 7 (3.7) | |
| Squamous cell carcinoma | 72 (37.7) | |
| LCNE carcinoma | 6 (3.1) | |
| Oncogenic driver | 119 | |
| None | 77 (64.7) | |
| EGFR | 3 (2.5) | |
| ALK | 0 (0) | |
| Other | 19 (16) | |
| Unknown | 20 (16.8) | |
| Disease stage | 191 | |
| II | 2 (1.0) | |
| III | 56 (29.3) | |
| IV | 133 (69.7) | |
| PDL-1 category | 191 | |
| 1 | 64 (33.5) | |
| 2 | 26 (13.6) | |
| 3 | 29 (15.2) | |
| 4 | 72 (37.7) | |
| IT line stage IV | 133 | |
| 1L | 43 (32.3) | |
| 2L+ | 90 (67.3) | |
| IT Agent | 191 | |
| Nivolumab | 100 (52.3) | |
| Pembrolizumab | 58 (30.4) | |
| Durvalumab | 22 (11.5) | |
| Atezolizumab | 11 (5.8) |
Smoking status: as registered at the start of PD(L)-1 blockade. Obstructive airway disease: COPD Chronic obstructive pulmonary disease, ECOG-PS Eastern Cooperative Oncology Group-Performance Status. Histology: NOS not otherwise specified. Oncogenic driver: EGFR Epidermal growth factor receptor (Tumor Hotspot Mastr kit, Illumina MiSeq), ALK Anaplastic lymphoma kinase (monoclonal antibody with Automated Stainer Benchmark, Roche), other BRAF, KRAS, and PIK3CA mutations (Tumor Hotspot Mastr kit, Illumina MiSeq), unknown No NGS or EGFR/ALK testing done, no At least no EGFR mutation/ALK rearrangement identified. Disease stage: according to the TNM 7th classification. PD-L1 category: 1 ≥ 50%, 2 1–49%, 3 < 1%, 4 Unknown. IT line stage IV: line of treatment for the PD(L)-1 blockade: 1L First line and 2L + Second or later line
White Blood Cell Counts Over Time Under PD(L)-1 Blockade
Baseline WBC and subtypes did not differ between responding, stable, and progressive patients. Among the studied biological variables only eosinophils rose under PD(L)-1 inhibition between the start of treatment and the time of first or second evaluation (p < 0.0001) (Table 2).
Table 2.
Kinetics of white blood cell counts over time
| t0 | t1 | t2 | p-value | |
|---|---|---|---|---|
|
WBC 103cell/mm3 |
8.47 ± 3.70 | 8.09 ± 3.16 | 8.56 ± 4.94 | 0.70 |
| Eosinophils % | 2.34 ± 2.00ab | 3.38 ± 2.79a | 3.29 ± 2.83b | <0.0001 |
| 103cell/mm3 | 0.19 ± 0.20ab | 0.27 ± 0.27a | 0.29 ± 0.40b | <0.0001 |
| Lymphocytes % | 20.16 ± 9.67 | 20.66 ± 8.54 | 20.41 ± 9.50 | 0.43 |
| 103cell/mm3 | 1.56 ± 0.75 | 1.55 ± 0.62 | 1.58 ± 0.70 | 0.43 |
| Neutrophils % | 67.13 ± 11.93 | 65.68 ± 9.81 | 65.65 ± 13.32 | 0.20 |
| 103cell/mm3 | 5.90 ± 3.28 | 5.47 ± 2.85 | 5.99 ± 4.72 | 0.15 |
| NLR | 4.64 ± 3.83 | 4.38 ± 5.26 | 0.20 |
t0 Pre-treatment, t1 First evaluation, t2 Second evaluation. Comparisons made with Scheffé’s test between t0–t1 (pa < 0.0001) and t0–t2 (pb < 0.0001). WBC: white blood cells. NLR neutrophils-to-lymphocytes ratio
Response
At the time of first evaluation 51 (26.7%) of the 191 patients were responders (R), 103 (53.9%) stable (S), and 37 (19.4%) progressive patients (P). At t2, we found 64 R (33.5%), 67 S (35.1%), and 60 P (31.4%). We found 3 patients (4.7%) showing progression at t1 but response at t2, so-called pseudoprogression. Five R (8.3%) became P at t2.
Higher response rates were noted for high PD-L1 expression (i.e., >50%; p = 0.0001 at t1 and p = 0.0031 at t2), pembrolizumab use (p < 0.0001 at t1 and p = 0.0096 at t2), and former smokers (p = 0.024; OR = 2.88).
Regarding biological variables none of the baseline values predicted the response at t1 or at t2. Responders had a significantly higher REC than progressive patients at t1 (p = 0.019 with OR = 0.54) and at t2 (p = 0.014 with OR = 0.53). By univariate analysis (two-way factorial ANOVA) PD-L1 status (p = 0.18 for REC and p = 0.067 for AEC), smoking status (p = 0.43 for REC and p = 0.13 for AEC) and immune-related toxicity (IRT) (p = 0.87 for REC and p = 0.93 for AEC) had no influence on eosinophil levels. No biological variable other than eosinophils was predictive of the response at t1 or at t2 (Table 3).
Table 3.
Biological variables according to the type of response at t2
| Responders | Stable | Progressive | ||||
|---|---|---|---|---|---|---|
| WBC | ||||||
| t0 | 8.53 (5.92–10.61) | 7.76 (6.22–18.49) | 7.68 (6.04–10.36) | |||
| t1 | 6.82 (5.74–8.98) | 7.79 (5.94–8.60) | 7.66 (6.25–9.42) | |||
| t2 | 6.63 (5.66–8.89) | 7.58 (6.54–9.13) | 8.52 (6.55–10.61) | |||
| Eosinophils | AEC | REC | AEC | REC | AEC | REC |
| t0 | 0.14 (0.08–0.28) | 1.85 (0.90–3.40) | 0.13 (0.09–0.23) | 1.90 (1.00–3.10) | 0.12 (0.06–0.21) | 1.65 (0.80–8.70) |
| t1 | 0.22 (0.14–0.35) | 3.1 (2.05–4.75)* | 0.2 (0.11–0.30) | 2.9 (1.50–4.00)* | 0.19 (0.10–0.34) | 2.6 (1.30–3.60) |
| t2 | 0.24 (0.15–0.39) | 3.55 (1.85–5.50) | 0.22 (0.13–0.30) | 2.50 (1.80–3.70) | 0.13 (0.06–0.31) | 1.90 (0.80–3.80) |
| Neutrophils | ANC | RNC | ANC | RNC | ANC | RNC |
| t0 | 5.51 (3.75–7.70) | 68.75 (60.40–74.55) | 5.06 (3.96–6.87) | 66.60 (60.20–74.20) | 5.31 (3.88–7.59) | 68.95 (62.65–74.00) |
| t1 | 4.54 (3.67–5.77) | 64.45 (56.75–69.70) | 5.27 (3.76–5.98) | 66.90 (62.00–72.10) | 5.30 (4.03–6.63) | 67.15 (60.85–74.65) |
| t2 | 4.14 (3.19–5.77) | 63.90 (52.70–68.50) | 5.07 (4.23–6.31) | 67.60 (59.50–74.00) | 5.95 (4.26–7.77) | 69.35 (62.90–80.15) |
| Lymphocytes | ALC | RLC | ALC | RLC | ALC | RLC |
| t0 | 1.62 (1.13–2.02) | 19.80 (14.25–25.15) | 1.33 (1.04–1.85) | 18.20 (13.60–24.40) | 1.51 (1.09–1.89) | 17.55 (14.40–25.10) |
| t1 | 1.55 (1.10–1.86) | 21.25 (15.95–26.40) | 1.45 (1.10–1.82) | 19.90 (15.00–24.40) | 1.48 (1.01–1.85) | 19.95 (12.85–24.65) |
| t2 | 1.67 (1.17–2.06) | 22.20 (16.75–29.30) | 1.46 (1.15–1.84) | 18.50 (13.80–24.90) | 1.29 (1.03–1.89) | 16.80 (10.75–24.95) |
WBC White blood cells (.103cell/mm3), AEC Absolute eosinophil count (.103cell/mm3), REC Relative eosinophil count (%), ANC Absolute neutrophil count (.103cell/mm3), RNC Relative neutrophil count (%), ALC Absolute lymphocyte count (.103cell/mm3), RLC Relative lymphocyte count (%). Responders (n = 64), stable (n = 67), progressive (n = 60) patients: according to the RECIST criteria (see materials and methods). Results expressed as medians and interquartile ranges. Logistic regression analysis; p-value vs. progressive. *p < .05
Toxicity
The overall rate for IRT was 24.1%. Most IRT was of low intensity, requiring no immunosuppressive therapy. Indeed, only 12 out of 191 patients (6.3%) required oral corticoids (OCS) for the control of their IRT. Skin (22/191 patients, 11.6%), thyroid (9 patients, 4.7%), joints (5 patients, 2.6%), and lungs (5 patients, 2.6%) were the most frequently involved. For the durvalumab subgroup we identified significantly more IRT (40.9% reported at t1 or t2 vs. 21.9% for non-durvalumab drugs, p = 0.017), a higher use of OCS (18.1% vs. 4.7%, p = 0.0057), and higher pulmonary and thyroid toxicity (13.6% for both in the durvalumab group, compared to 1.2% and 3.6% for patients receiving non-durvalumab drugs, respectively; p = 0.0037). There was no correlation between baseline WBC subtypes and toxicity and no correlation between toxicity and response.
Duration of Treatment
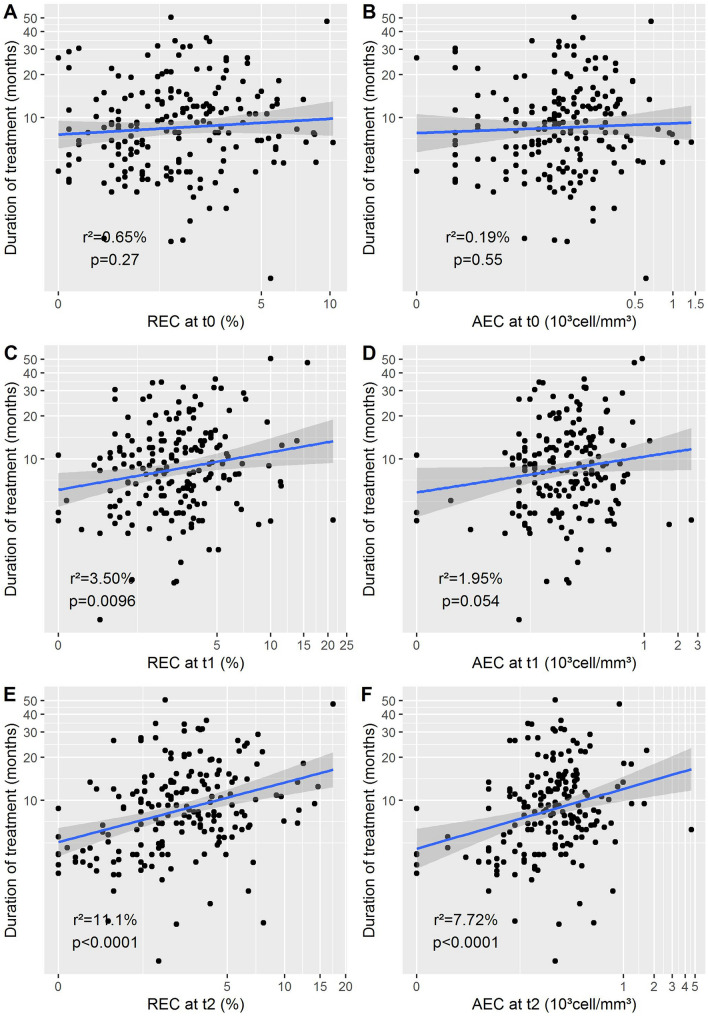
At the time of first evaluation a higher REC and a lower ANC were associated with a longer duration of treatment (p = 0.0096 and p = 0.021, respectively) (Fig. 1). At t2 all biological variables were predictive for the duration of treatment (data not shown).
Fig. 1.
Eosinophils and duration of treatment. Logarithmic scale representation for relative eosinophil counts (REC) and absolute eosinophil counts (AEC). t0: before treatment; t1: at time of first evaluation; t2: at time of second evaluation
Overall Survival
The median OS was 18.8 months with 98 patients (51.3%) alive at data cut-off. No clinico-pathological feature was prognostic in this cohort. The OS was longer in patients responding at t1 (p < 0.0001) with medians of OS of 30.4 months for responders, 19.9 months for stable, and 12.8 months for progressive patients. A lower baseline absolute neutrophil count (ANC) correlated with longer OS (p = 0.049) while at t1, the relative lymphocyte count (RLC), relative neutrophil count (RNC), ANC, and neutrophil-to-lymphocyte ratio (NLR) were correlated with OS (p = 0.044, p = 0.014, p = 0.0033, and p = 0.029, respectively) (Table 4).
Table 4.
Risk of death in the 191 patients according to biological variables at t1
| Variable | t1 values | p-values | HR | 95% CI |
|---|---|---|---|---|
| White blood cells (103cells/mm3) | ||||
| Alive | 7.28 (5.74–8.90) | 0.094 | 1.78 | 0.91–3.51 |
| Dead | 7.80 (6.32–8.97) | |||
| Eosinophils (%) | ||||
| Alive | 2.90 (1.90–4.00) | 0.081 | 0.70 | 0.47–1.04 |
| Dead | 2.80 (1.30–4.10) | |||
| Eosinophils (103cells/mm3) | ||||
| Alive | 0.21 (0.12–0.33) | 0.22 | 0.84 | 0.64–1.10 |
| Dead | 0.20 (0.11–0.33) | |||
| Lymphocytes (%) | ||||
| Alive | 20.85 (15.90–25.50) | 0.044* | 0.64 | 0.42–0.99 |
| Dead | 19.10 (13.80–23.00) | |||
| Lymphocytes (103cells/mm3) | ||||
| Alive | 1.56 (1.10–1.88) | 0.39 | 0.81 | 0.50–1.31 |
| Dead | 1.47 (1.09–1.81) | |||
| Neutrophils (%) | ||||
| Alive | 64.10 (59.40–69.20) | 0.014* | 1.03 | 1.00–1.05 |
| Dead | 68.90 (60.80–73.60 | |||
| Neutrophils (103cells/mm3) | ||||
| Alive | 4.62 (3.54–5.87) | 0.0033* | 1.11 | 1.04–1.20 |
| Dead | 5.21 (4.11–6.32) | |||
| NLR | ||||
| Alive | 2.96 (2.38–4.27) | 0.029* | 1.46 | 1.04–2.06 |
| Dead | 3.56 (2.67–5.35) | |||
Results expressed as medians and interquartile ranges. Alive (n = 98)/dead (n = 93): as recorded at data cut-off (see Materials and methods). HR Hazard ratio for death. CI Confidence interval. NLR Neutrophils to lymphocytes ratio. *significant p-value (< 0.05)
Discussion
In this cohort of advanced stage NSCLC patients treated with PD(L)-1 blockade, PD-L1 expression levels and smoking history were associated with response, confirming earlier data [13, 14]. Pembrolizumab was associated with more responses, as it was the only drug used in patients with high PD-L1 expression levels. Regarding biological data we noted an early rise only in eosinophils. Moreover, a higher proportion of eosinophils was associated with an early response and with a longer duration of treatment. Neutrophils, lymphocytes, and their ratio, either at baseline or early in the course of treatment, appeared to be prognostic.
The role of eosinophils in tumors is still a matter of debate. In various tumor types in vitro data and preclinical models show direct and indirect anti-tumor effects [15, 16] but also pro-tumorigenic effects [17–20]. Neutrophils can, like eosinophils, have both anti- and pro-tumor functions [21, 22].
The prognostic and predictive value of blood biomarkers and more specifically WBC and their subtypes in patients treated with ICI have been reported in several tumor types, e.g., colorectal cancer [23], breast cancer [24, 25], prostate cancer [26], melanoma [4, 27–29], and NSCLC [7]. However, these studies lack homogeneity: absolute vs. relative WBC counts, single vs. composite markers, and continuous vs. categorized variables. Weide and colleagues proposed a prognostic model based on categorized serum lactate dehydrogenase (LDH), WBC count, and clinical characteristics [4]. The risk of death was 2.4-fold (p = 0.003) and 2.2-fold (p < 0.001) for patients with pre-treatment RLC < 17.5% and REC < 1.5%, respectively. In part based on these results Tanizaki and colleagues studied the prognostic and predictive value of peripheral blood biomarkers in a population of NSCLC patients treated with nivolumab for advanced disease (n = 137) [7]. They found a strong association between baseline low (<7.5 cells/mL) ANC, high (>1.0 cells/mL) absolute lymphocyte count (ALC) and high (>0.15 cells/mL) AEC and higher response rates, progression-free survival (PFS), and OS. In those two studies, authors used categorized variables, i.e., AEC > or < 0.15 cells/mL and REC > or < 1.5%. We, however, considered the variables as continuous. Keeping this in mind, in our cohort a higher proportion in eosinophils at the time of the first evaluation (t1) were associated with a higher chance of objective response to treatment at t1 and t2. In our series, this was independent of the smoking history, PD-L1 status, and immune-related toxicity (IRT). We could, however, not identify a cut-off value for REC at t1 with satisfying sensitivity for discriminating responders from stable and from progressive patients at t2 (32.8% sensitivity and 81.9% specificity for a cut-off of 5.3% REC, p-value = 0.0137). Our study also emphasizes the association between blood eosinophils and the durability of clinical benefit for NSCLC patients, as expressed by the duration of treatment. A series of melanoma patients comfort these findings with response to ipilimumab correlated with an early rise in eosinophils [27].
In contrast to other series, toxicity in our cohort was not correlated with a higher probability of response and also not with raised eosinophils. Several retrospective studies [30–32] and one prospective report [33] showed an association between early IRT for advanced NSCLC and outcome. Although these studies are small sized and mostly lack pathological correlation, there is some rationale to explain this link: similarity between tumor antigens and self-antigens leading to cross-reactivity of T cells that are reactivated by the ICI [34], pre-existing autoimmunity with reactivation of T cells primarily directed at self-antigens [35] or B-cell reactivation through PD-1 blockade [36]. The fact that we did not find a correlation with response may be due to the retrospective nature of the study with incomplete data collection during patients’ follow-up. On the other hand, a correlation between eosinophilia (i.e., AEC > 0.5 cells/mL) and immune-related toxicity (p = 0.0042) has been demonstrated in a retrospective series including 146 patients with various solid tumor types treated with anti-PD(L)-1 [37]. As a correlation between eosinophils and response to ICI and between eosinophils and toxicity under ICI were shown, it is tempting to think that both clinical results (response and toxicity) are two sides of one phenomenon: immune (re-)activation. This, however, remains to be formally proven.
Some authors found a prognostic value of baseline eosinophils [4, 7]. This was not the case in our series. However, we found a clear association between eosinophils and response to treatment and between response and OS. The lack of prognostic value of REC at t0 may be due to small sample size when compared to the work of Weide and colleagues. Moreover, the prognostic value of baseline neutrophils, lymphocytes, and neutrophils/lymphocytes ratio (NLR) demonstrated in our work supports the findings of several authors [5, 8, 38]. Illustratively, the prognostic value of the iSEND model (immunotherapy, Sex, ECOG-PS, NLR, and Delta NLR) is being investigated in a prospective manner after it showed its value as a predictive tool for patients with advanced NSCLC treated with nivolumab [8). In earlier stages of disease a study on operated NSCLC specimen revealed an inverse correlation between neutrophils and CD8 + cytotoxic T cells [39].
An additional interesting finding of the present study is that blood eosinophils are the only WBC subtype displaying a rise during the first six months of anti-PD (L)-1 therapy for NSCLC, data that are in keeping with results from a large French cohort and from our previously published data [10, 40]. Further studies will have to explore why this rise is transient and whether raised eosinophils in responders are a consequence of or a trigger for immune anti-tumor activation.
Conclusion
In this study patients receiving PD(L)-1 blockade for advanced NSCLC and showing a raised proportion of eosinophils at the time of first evaluation were more likely to show an objective response according to the RECIST criteria at the time of second evaluation, regardless of smoking history, PD-L1 status, and IRT. A higher REC also correlated with a longer duration of treatment. We could, however, not identify a clear cut-off value to propose eosinophils as a predictive biomarker. It seems necessary to identify the underlying mechanism(s) leading to a rise in blood eosinophils in patients deriving clinical benefit from anti-PD(L)-1 drugs. Further results of this cohort support the prognostic role of neutrophils, lymphocytes, and their ratio, either at baseline or early in the course of treatment.
Author Contribution
AS collected the data, contributed to the statistical analyses, and wrote the article. MH conducted the statistical analyses and contributed to the redaction of the manuscript. RA collected the data. JLC, RL, and BD reviewed the manuscript and contributed to its final version. All authors read and approved the final manuscript.
Funding
This work did not receive the support of any organization or association.
Data Availability
The datasets supporting the conclusions of this article are included within the article.
Code Availability
Not applicable.
Declarations
Conflict of interest
All authors declare to have no conflict of interest with the submitted work.
Ethical Approval
This study was approved by the Ethics Committee of the CHU de Liège with reference number 2019/239.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Planchard D, Popat S, Kerr K, Novello S, Smit EF, Faivre-Finn C, et al (2019) Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol [Internet] 30(5):863–870. Available from: https://www.ncbi.nlm.nih.gov/pubmed/30715168 [DOI] [PubMed]
- 2.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer [Internet] 45(2):228–247. Available from: https://www.ncbi.nlm.nih.gov/pubmed/19097774 [DOI] [PubMed]
- 3.Borcoman E, Kanjanapan Y, Champiat S, Kato S, Servois V, Kurzrock R, et al (2019) Novel patterns of response under immunotherapy. Ann Oncol [Internet] 30(3):385–396. Available from: https://www.ncbi.nlm.nih.gov/pubmed/30657859 [DOI] [PubMed]
- 4.Weide B, Martens A, Hassel JC, Berking C, Postow MA, Bisschop K, et al (2016) Baseline biomarkers for outcome of melanoma patients treated with pembrolizumab. Clin Cancer Res [Internet] 22(22):5487–5496. Available from: https://www.ncbi.nlm.nih.gov/pubmed/27185375 [DOI] [PMC free article] [PubMed]
- 5.Bagley SJ, Kothari S, Aggarwal C, Bauml JM, Alley EW, Evans TL, et al (2017) Pretreatment neutrophil-to-lymphocyte ratio as a marker of outcomes in nivolumab-treated patients with advanced non-small-cell lung cancer. Lung Cancer [Internet] 106:1–7. Available from: https://www.ncbi.nlm.nih.gov/pubmed/28285682 [DOI] [PubMed]
- 6.Diem S, Schmid S, Krapf M, Flatz L, Born D, Jochum W, et al (2017) Neutrophil-to-Lymphocyte ratio (NLR) and Platelet-to-Lymphocyte ratio (PLR) as prognostic markers in patients with non-small cell lung cancer (NSCLC) treated with nivolumab. Lung Cancer [Internet] 111:176–181. Available from: https://www.ncbi.nlm.nih.gov/pubmed/28838390 [DOI] [PubMed]
- 7.Tanizaki J, Haratani K, Hayashi H, Chiba Y, Nakamura Y, Yonesaka K, et al (2018) Peripheral blood biomarkers associated with clinical outcome in non-small cell lung cancer patients treated with nivolumab. J Thorac Oncol [Internet] 13(1):97–105. Available from: https://www.ncbi.nlm.nih.gov/pubmed/29170120 [DOI] [PubMed]
- 8.Park W, Kwon D, Saravia D, Desai A, Vargas F, El Dinali M, et al (2018) Developing a predictive model for clinical outcomes of advanced non-small cell lung cancer patients treated with nivolumab. Clin Lung Cancer [Internet] 19(3):280.e4–288.e4. Available from: https://www.ncbi.nlm.nih.gov/pubmed/29336998 [DOI] [PubMed]
- 9.Park W, Mezquita L, Okabe N, Chae YK, Kwon D, Saravia D, et al. Association of the prognostic model iSEND with PD-1/L1 monotherapy outcome in non-small-cell lung cancer. Br J Cancer [Internet]. 2020;122(3):340–7. Available from: https://www.ncbi.nlm.nih.gov/pubmed/31761899 [DOI] [PMC free article] [PubMed]
- 10.Sibille A, Henket M, Corhay JL, Louis R, Duysinx B (2020) Clinical benefit to programmed death-1 inhibition for non-small-cell lung cancer is associated with higher blood eosinophil levels. Acta Oncol [Internet] 59(3):257–259. Available from: https://www.ncbi.nlm.nih.gov/pubmed/31755328 [DOI] [PubMed]
- 11.Brahmer J, Reckamp KL, Baas P, Crinò L, Eberhardt WE, Poddubskaya E, et al (2015) Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med [Internet] 373(2):123–135. Available from: https://www.ncbi.nlm.nih.gov/pubmed/26028407 [DOI] [PMC free article] [PubMed]
- 12.Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, et al (2015) Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med [Internet] 373(17):1627–1639. Available from: https://www.ncbi.nlm.nih.gov/pubmed/26412456 [DOI] [PMC free article] [PubMed]
- 13.Herbst RS, Baas P, Kim DW, Felip E, Pérez-Gracia JL, Han JY, et al (2016) Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet [Internet] 387(10027):1540–1550. Available from: https://www.ncbi.nlm.nih.gov/pubmed/26712084 [DOI] [PubMed]
- 14.Mazieres J, Drilon A, Lusque A, Mhanna L, Cortot AB, Mezquita L, et al (2019) Immune checkpoint inhibitors for patients with advanced lung cancer and oncogenic driver alterations: Results from the IMMUNOTARGET registry. Ann Oncol. [DOI] [PMC free article] [PubMed]
- 15.Carretero R, Sektioglu IM, Garbi N, Salgado OC, Beckhove P, Hämmerling GJ (2015) Eosinophils orchestrate cancer rejection by normalizing tumor vessels and enhancing infiltration of CD8(+) T cells. Nat Immunol [Internet] 16(6):609–617. Available from: https://www.ncbi.nlm.nih.gov/pubmed/25915731 [DOI] [PubMed]
- 16.Simon SCS, Utikal J, Umansky V (2019) Opposing roles of eosinophils in cancer. Cancer Immunol Immunother [Internet] 68(5):823–833. Available from: https://www.ncbi.nlm.nih.gov/pubmed/30302498 [DOI] [PMC free article] [PubMed]
- 17.Zaynagetdinov R, Sherrill TP, Gleaves LA, McLoed AG, Saxon JA, Habermann AC, et al (2015) Interleukin-5 facilitates lung metastasis by modulating the immune microenvironment. Cancer Res [Internet] 75(8):1624–1634. Available from: https://www.ncbi.nlm.nih.gov/pubmed/25691457 [DOI] [PMC free article] [PubMed]
- 18.Astigiano S, Morandi B, Costa R, Mastracci L, D’Agostino A, Ratto GB, et al (2005) Eosinophil granulocytes account for indoleamine 2,3-dioxygenase-mediated immune escape in human non-small cell lung cancer. Neoplasia [Internet] 7(4):390–396. Available from: https://www.ncbi.nlm.nih.gov/pubmed/15967116 [DOI] [PMC free article] [PubMed]
- 19.Kratochvill F, Neale G, Haverkamp JM, de Velde LA, Smith AM, Kawauchi D, et al (2015) TNF Counterbalances the Emergence of M2 Tumor Macrophages. Cell Rep [Internet] 12(11):1902–1914. Available from: https://www.ncbi.nlm.nih.gov/pubmed/26365184 [DOI] [PMC free article] [PubMed]
- 20.Puxeddu I, Berkman N, Nissim Ben Efraim AH, Davies DE, Ribatti D, Gleich GJ, et al (2009) The role of eosinophil major basic protein in angiogenesis. Allergy [Internet] 64(3):368–374. Available from: https://www.ncbi.nlm.nih.gov/pubmed/19120069 [DOI] [PubMed]
- 21.Fridlender ZG, Sun J, Kim S, Kapoor V, Cheng G, Ling L, et al (2009) Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell [Internet] 16(3):183–194. Available from: https://www.ncbi.nlm.nih.gov/pubmed/19732719 [DOI] [PMC free article] [PubMed]
- 22.Coffelt SB, Wellenstein MD, de Visser KE (2016) Neutrophils in cancer: neutral no more. Nat Rev Cancer [Internet] 16(7):431–446. Available from: https://www.ncbi.nlm.nih.gov/pubmed/27282249 [DOI] [PubMed]
- 23.Wei Y, Zhang X, Wang G, Zhou Y, Luo M, Wang S, et al (2018) The impacts of pretreatment circulating eosinophils and basophils on prognosis of stage I-III colorectal cancer. Asia Pac J Clin Oncol [Internet] 14(5):e243–e251. Available from: https://www.ncbi.nlm.nih.gov/pubmed/29532611 [DOI] [PubMed]
- 24.Ownby HE, Roi LD, Isenberg RR, Brennan MJ (1983) Peripheral lymphocyte and eosinophil counts as indicators of prognosis in primary breast cancer. Cancer [Internet] 52(1):126–130. Available from: https://www.ncbi.nlm.nih.gov/pubmed/6850535 [DOI] [PubMed]
- 25.Gündüz S, Göksu SS, Arslan D, Tatli AM, Uysal M, Gündüz UR, et al (2015) Factors affecting disease-free survival in patients with human epidermal growth factor receptor 2-positive breast cancer who receive adjuvant trastuzumab. Mol Clin Oncol [Internet] 3(5):1109–1112. Available from: https://www.ncbi.nlm.nih.gov/pubmed/26623060 [DOI] [PMC free article] [PubMed]
- 26.McNeel DG, Gardner TA, Higano CS, Kantoff PW, Small EJ, Wener MH, et al (2014) A transient increase in eosinophils is associated with prolonged survival in men with metastatic castration-resistant prostate cancer who receive sipuleucel-T. Cancer Immunol Res [Internet] 2(10):988–999. Available from: https://www.ncbi.nlm.nih.gov/pubmed/25189164 [DOI] [PMC free article] [PubMed]
- 27.Gebhardt C, Sevko A, Jiang H, Lichtenberger R, Reith M, Tarnanidis K, et al. Myeloid cells and related chronic inflammatory factors as novel predictive markers in melanoma treatment with ipilimumab. Clin Cancer Res [Internet]. 2015;21(24):5453–5459. Available from: https://www.ncbi.nlm.nih.gov/pubmed/26289067 [DOI] [PubMed]
- 28.Moreira A, Leisgang W, Schuler G, Heinzerling L (2017) Eosinophilic count as a biomarker for prognosis of melanoma patients and its importance in the response to immunotherapy. Immunotherapy [Internet] 9(2):115–121. Available from: https://www.ncbi.nlm.nih.gov/pubmed/28128709 [DOI] [PubMed]
- 29.Heppt M V, Heinzerling L, Kähler KC, Forschner A, Kirchberger MC, Loquai C, et al (2017) Prognostic factors and outcomes in metastatic uveal melanoma treated with programmed cell death-1 or combined PD-1/cytotoxic T-lymphocyte antigen-4 inhibition. Eur J Cancer [Internet] 82:56–65. Available from: https://www.ncbi.nlm.nih.gov/pubmed/28648699 [DOI] [PubMed]
- 30.Haratani K, Hayashi H, Chiba Y, Kudo K, Yonesaka K, Kato R, et al (2018) Association of immune-related adverse events with nivolumab efficacy in non-small-cell lung cancer. JAMA Oncol [Internet] 4(3):374–378. Available from: https://www.ncbi.nlm.nih.gov/pubmed/28975219 [DOI] [PMC free article] [PubMed]
- 31.Hasan Ali O, Diem S, Markert E, Jochum W, Kerl K, French LE, et al (2016) Characterization of nivolumab-associated skin reactions in patients with metastatic non-small cell lung cancer. Oncoimmunology [Internet] 5(11):e1231292. Available from: https://www.ncbi.nlm.nih.gov/pubmed/27999741 [DOI] [PMC free article] [PubMed]
- 32.Osorio JC, Ni A, Chaft JE, Pollina R, Kasler MK, Stephens D, et al (2017) Antibody-mediated thyroid dysfunction during T-cell checkpoint blockade in patients with non-small-cell lung cancer. Ann Oncol [Internet] 28(3):583–589. Available from: https://www.ncbi.nlm.nih.gov/pubmed/27998967 [DOI] [PMC free article] [PubMed]
- 33.Teraoka S, Fujimoto D, Morimoto T, Kawachi H, Ito M, Sato Y, et al (2017) Early immune-related adverse events and association with outcome in advanced non–small cell lung cancer patients treated with nivolumab: a prospective cohort study. J Thorac Oncol [DOI] [PubMed]
- 34.Cui J, Bystryn JC (1995) Melanoma and vitiligo are associated with antibody responses to similar antigens on pigment cells. Arch Dermatol [Internet] 131(3):314–318. Available from: https://www.ncbi.nlm.nih.gov/pubmed/7887661 [PubMed]
- 35.Yoest JM (2017) Clinical features, predictive correlates, and pathophysiology of immune-related adverse events in immune checkpoint inhibitor treatments in cancer: a short review. Immunotargets Ther [Internet] 6:73–862. Available from: https://www.ncbi.nlm.nih.gov/pubmed/29067284 [DOI] [PMC free article] [PubMed]
- 36.Zhang M, Xia L, Yang Y, Liu S, Ji P, Wang S, et al (2019) PD-1 blockade augments humoral immunity through ICOS-mediated CD4. Int Immunopharmacol [Internet] 66:127–138. Available from: https://www.ncbi.nlm.nih.gov/pubmed/30448635 [DOI] [PubMed]
- 37.Krishnan T, Tomita Y, Roberts-Thomson R (2020) A retrospective analysis of eosinophilia as a predictive marker of response and toxicity to cancer immunotherapy. Futur Sci OA 6(10). [DOI] [PMC free article] [PubMed]
- 38.Wang Z, Zhan P, Lv Y, Shen K, Wei Y, Liu H, et al (2019) Prognostic role of pretreatment neutrophil-to-lymphocyte ratio in non-small cell lung cancer patients treated with systemic therapy: a meta-analysis. Transl Lung Cancer Res [Internet] 8(3):214–226. Available from: https://www.ncbi.nlm.nih.gov/pubmed/31367535 [DOI] [PMC free article] [PubMed]
- 39.Kargl J, Busch SE, Yang GH, Kim KH, Hanke ML, Metz HE, et al (2017) Neutrophils dominate the immune cell composition in non-small cell lung cancer. Nat Commun [Internet 8:14381. Available from: https://www.ncbi.nlm.nih.gov/pubmed/28146145 [DOI] [PMC free article] [PubMed]
- 40.Bernard-Tessier A, Jeanville P, Champiat S, Lazarovici J, Voisin AL, Mateus C, et al (2017) Immune-related eosinophilia induced by anti-programmed death 1 or death-ligand 1 antibodies. Eur J Cancer [Internet] 81:135–137. Available from: https://www.ncbi.nlm.nih.gov/pubmed/28624693 [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets supporting the conclusions of this article are included within the article.
Not applicable.