Abstract
Purpose
To evaluate the efficacy of Lactobacillus paracasei CNCM I-1572 (L. casei DG®) in both prevention of symptomatic recurrences and improvement of quality of life in patients with chronic bacterial prostatitis (CBP).
Methods
Patients with CBP attending a single Urological Institution were enrolled in this phase IV study. At enrollment, all patients were treated with antibiotics in agreement with EAU guidelines and then were treated with L. casei DG® (2 capsules/day for 3 months). Clinical and microbiological analyses were carried out before (enrollment, T0) and 6 months (T2) after the treatment. Both safety and adherence to the treatment were evaluated 3 months (T1) after the enrollment. NIH Chronic Prostatitis Symptom Index (CPSI), International Prostate Symptom Score (IPSS) and Quality of Well-Being (QoL) questionnaires were used. The outcome measures were the rate of symptomatic recurrence, changes in questionnaire symptom scores and the reduction of antibiotic use.
Results
Eighty-four patients were included. At T2, 61 patients (72.6%) reported a clinical improvement of symptoms with a return to their clinical status before symptoms. A time dependent improvement in clinical symptoms with significant changes in NIH-CPSI, IPSS and QoL (mean difference T2 vs T0: 16.5 ± 3.58; − 11.0 ± 4.32; + 0.3 ± 0.09; p < 0.001), was reported. We recorded that L. casei DG® treatment induced a statistically significant decrease in both (p < 0.001) symptomatic recurrence [1.9/3 months vs 0.5/3 months] and antibiotic use [− 7938 UDD]. No clinically relevant adverse effects were reported.
Conclusions
L. casei DG® prevents symptomatic recurrences and improves the quality of life in patients with CBP, reducing the antibiotic use.
Introduction
Even though if the prevalence of chronic bacterial prostatitis (CBP), category II according to the National Institutes of Health (NIH) classification, ranges in Europe between 7 and 14% of all cases with prostatitis [1], the impact on patient’s quality of life is high [2, 3]. A antibiogram-driven long-term treatment with fluoroquinolones represents the gold standard therapy of CBP, but nowadays the need to improve the adherence to antibiotic stewardship programs forced us to re-think the approach to CBP. Several authors reported that the pathogenesis of CP seems related to the presence of bacterila biofilm [2] while clinical symptoms with the prostate inflammation mediated by several cytokines, e.g., Interleukin-8 [3–5]. On the other hand, other authors suggested that probiotics, in particular Lacobacillus strains, could modulate the inflammatory pathway regulating the bowel inflammatory status [6, 7], suggesting a role in prostatic diseases, too [8]. In agreement with this, Vicari et al., documented that probiotics play a role in the management of patients affected by CBP and irritable bowel syndrome but at the best of our knowledge, no study addressed the therapeutic role of Lactobacilli in the management of CBP patients evaluating its role in the decrease of antibiotic use [8]. Therefore, here we evaluated the efficacy of Lactobacillus paracasei CNCM I-1572 (L. casei DG®) in both the prevention of symptomatic recurrences and the improvement of the quality of life in CBP patients.
Patients and methods
Study design and participants
We performed a clinical, single center, phase IV study on CBP patients from January 2019 up to December 2019. This study was approved by the local Ethic Committee (approval protocol number 258, 2019) and its was conducted in compliance with the Institutional Review Board/Human Subjects Research Committee requirements and with the Declaration of Helsinki and with the Guidelines for Good Clinical Practice criteria. Before the beginning of the study, the enrolled patients or legal guardians signed the informed consent.
Population inclusion criteria
In agreement with our previous studies [9], we enclosed men > 18 year and < 45 year, with symptoms related to CBP for at least 3 months and a positive Meares–Stamey 4-glass test with first voided urine, midstream urine, expressed prostatic secretion and a post-prostatic massage urine culture, which had to be ≥ 10 [3] colony forming units (CFU)/mL of uropathogens. Patients who had recently (< 4 weeks) undergone oral or parental treatment or who were currently using prophylactic antibiotic drugs were excluded. All patients with positive tests for atypical or sexually transmitted pathogens, such as Chlamydia trachomatis, Ureaplasmaurealiticum, or Neisseria gonorrhoeae were also excluded. To obtain a homogenous group to analyze the following bacteria were considered as uropathogens, in agreement with Trinchieri: enteric Gram-negative rods; enterococci, Staphylococcus saprophyticus; and group B streptococci [10]. Finally, all patients with clinically significant intestinal disease were excluded, to obtain results on efficacy of probiotic therapy on chronic bacterial prostatitis. Patients who did not sign the informed consent were also excluded.
Experimental protocol
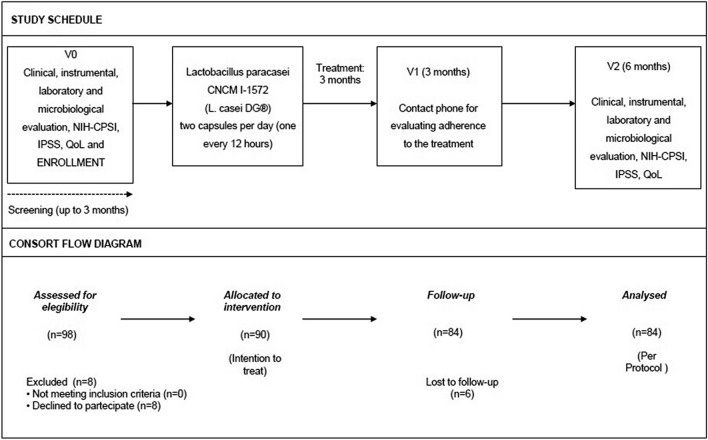
At the enrolment, all patients were treated with antibacterial agents in agreement with European Association of Urology (EAU) guidelines [11], to obtain infection free status at baseline (T0) and then they received a treatment with L. casei DG®1 capsule/12 h for 3 months. At the first follow-up time point (3 months, T1), all patients were telephonically contacted to evaluate both the compliance and the safety of the treatment. Clinical and microbiological analyses were carried out at the enrolment (T0) and 6 months after the discontinuation of L. casei DG® (T2 time point). In these periods, the patients were asked to fill out the NIH Chronic Prostatitis Symptom Index (CPSI), International Prostatic Symptom Score (IPSS) and Quality of Well-Being (QoL) questionnaires. All patients underwent urologic visit and microbiological evaluation in presence of clinical recurrence, also. The study schedule and study flow chart are reported in Fig. 1.
Fig. 1.
Study schedule and CONSORT flow diagram
End-points
The primary efficacy end-point of this trial was the statistically significant improvement (p < 0.05) of clinical symptoms at T2 vs T0. The secondary end-point of efficacy was the statistically significant decrease (p < 0.05) in the use of antimicrobial drugs. The reduction of antibiotic use was calculated under the following criteria: the number of Used Daily Dose (UDD) of antibiotics at T0 was compared respect to T2 [UDDT2 –UDDT0; results < 0 the decrease of antibiotic use is relevant] in agreement with the paper of Monnet et al. [12] that documented a strong correlation between the Defined DD of antibiotic and the antimicrobial prescription. All patients enrolled in this study have been previously included in a prospective dedicated data-base (Advanced PROSTATitis DataBase, Microsoft Access format) [13]. The primary safety end-point was the presence/absence of significant differences in the rate of adverse drug effects between T2 and T0. In agreement with our previous paper, the Naranjo probability test was used to evaluate the correlation between adverse drug reaction and treatment [14].
Clinical and microbiological definitions
Clinical efficacy was considered as being asymptomatic for at least 2 weeks. Clinical failure was defined as the persistence of clinical symptoms after treatment or the suspension of therapy for significant reported adverse effects. In addition, spontaneously reported adverse events or those noted by the investigator were recorded during the whole study period. The Meares–Stamey test was carried-out only in patients with symptomatic recurrence. All positive patients to Meares–Stamey test for uropathogens were treated with an alternative antibiotic depending on the organism and its susceptibility profile. Microbiological culture was carried out in accordance with the methods described in our previous papers [1, 2, 9, 13]. In brief, bacteria isolated from all samples were cultivated aerobically in Columbia blood agar (BioMerieux, Italy) and in a 10% CO2 atmosphere in Columbia CNA agar (BioMerieux; BD, Italy). They were identified and characterized biochemically using the species identification cards of the Vitek II semi-automated System for Microbiology-BioMerieux; antibiotic chemosensitivity has been carried-out using Vitek II semi-automated System for Microbiology (BioMerieux) [6]. Positive urine cultures had colony counts > 10 [5] UFC/mL [1, 2, 9, 13].
Questionnaires and urological examinations
The validated Italian versions of the NIH Chronic Prostatitis Symptom Index (NIH-CPSI) [15] and the International Prostate Symptom Score (IPSS) [16] were administered to each patient and self-completed. The questionnaire was administered at the patient’s arrival at the Centre and the results collected in the dedicated database. Moreover, patient quality of life was measured using an Italian version of the Quality of Well-Being, a validated, multi-attribute health scale [17]. In accordance with the study by Nickel et al., prostatitis-like symptoms were considered significant at a pain score of ≥ 4. The NIH-CPSI was also used in determining clinical therapy efficacy [18].
Composition and formulation of probiotics used in this trial
The probiotic preparation consisted of a gelatine capsule containing at least 24 billion viable cells of the bacterial strain L. casei DG® (Lactobacillus paracasei CNCM I-1572), Enterolactis® plus (SOFAR S.p.A., Trezzano Rosa, Milan, Italy) deposited at Institute Pasteur of Paris with number I1572. Probiotic capsules were delivered in aluminium boxes sealed with a plastic cap containing desiccant salts.
Statistical inference
This study has been planned as prospective phase IV study. To obtain clinically significant results to analyze, sample size calculation was based on the following assumptions: difference in terms of recurrence between enrollment (at the end of antibiotic treatment period) and follow-up visit (6 months): −1/3 months ± 1; α error level, 0.05 two-sided; statistical power, 80%; anticipated effect size, Cohen’s d = 0.5. The sample size calculation yielded 80 individuals. Considering a drop-out rate of at least 10%, the final sample size has been set to 90 patients. At baseline, the independent sample two-tailed t test was used to compare variables. For categorical parameters, chi-square test was applied. Changes from baseline to end of therapy were analyzed using ranked one-way analysis of variance (ANOVA) with a term for treatment group. The Shapiro-Wilks’s test for normality has been used. Data were reported as means ± standard deviation (SD). For all statistical comparisons, significance was considered as p < 0.05. All reported p values are two-sided. All statistical analyses were performed using SPSS 11.0 for Apple-Macintosh (SPSS, Inc., Chicago, Illinois). No placebo run-in period was considered necessary for the treatment of those patients showing Meares-Stamey test positivity. All data recorded in this study, i.e., anamnestic, clinical, and laboratory data, containing sensitive information were deidentified to ensure analysis of anonymous data only. This process was performed by non-medical staff using dedicated software.
Results
Ninety-eight patients were screened and admitted to this study. Eight patients (8.2%) refused to be enrolled, while 90 patients were enrolled (Intention To Treat group—ITT) (91.8%). 6 patients were lost (6.7%) to the follow-up and 84 completed the study (Per Protocol group—PP).
Baseline characteristics (T0)
Anamnestic, clinical, microbiological and questionnaires data at the enrolled patients are reported in Table 1.
Table 1.
Demographic, clinical, laboratory and microbiological patient’s data at the enrolment time
| Total or mean (SD* or %) | |
|---|---|
| Patients | 84 |
| Age | 36.1 ± 6.8 |
| Educational qualification | |
| Primary School | – |
| High School | 63 (74.9) |
| University | 21 (25.1) |
| Sexual behaviour | |
| 1 partner | 79 (94.1) |
| > 1 partners | 5 (3.9) |
| Contraceptive use | |
| Condom | 43 (51.2) |
| Coitus interruptus | 41 (48.8) |
| Start of CBP # history (months) | 23.9 ± 5.9 |
| Symptoms Score at baseline | |
| NIH-CPSI§ | 20.2 ± 2.3 |
| IPSS° | 18.4 ± 3.4 |
| QoL‡ | 0.57 ± 0.1 |
| Clinical presentation | |
| Burning | 52 (62.5) |
| Tenesmus | 16 (19.1) |
| Painful micturition | 69 (82.1) |
| Dysuria + Frequency | 38 (45.2) |
| Urgency | 22 (26.2) |
| Previous treatments (> 4 weeks before enrolment) | |
| Alpha-blockers | 8 (0.9) |
| Antibiotics | 84 (100) |
| Anti-inflammatory drugs | 24 (28.5) |
| Phytotherapy | 30 (35.7) |
| Antibiotics + Phytotherapy | 55 (65.4) |
| Antibiotics + Anti-inflammatory | 23 (27.8) |
| Microbiological findings | |
| Positive Meares–Stamey test | 84 (100) |
| Escherichia coli | 47 (55.9) |
| Enterococcus faecalis | 25 (29.7) |
| Other uropathogens | 12 (14.4) |
| Klebsiella spp. | 6 (50.0) |
| Serratia spp. | 4 (33.3) |
| Entrobacter spp. | 2 (16.7) |
| No growth | 0 (–) |
The table shows all baseline characteristics and clinical parameters at visit 0. SD* = Standard Deviation; CBP# = Chronic Bacterial Prostatitis; NIH-CPSI§ = NIH Chronic Prostatitis Symptom Index; IPSS° = International Prostate Symptoms Score; QoL‡ = Quality of Well-Being questionnaires
Adherence and adverse events (T1)
All patients correctly took all doses without any discontinuation, showing compliance with the study protocol of 100%. Two patients had mild adverse effects (mild dyspepsia) that did not require treatment suspension. No severe adverse effects have been reported.
Follow-up 6 months (T2)
At the end of follow-up period, 61 patients (72.6%) reported a significant clinical improvement (first safety end-point). A statistically significant reduction of symptomatic recurrence rate has been reported between T2 and T0 [1.9/3 months vs 0.5/3 months (p < 0.001)]. At T2, we documented significant changes in the score of NIH-CPSI, IPSS and QoL compared to T0 (mean difference: -16.5 ± 3.58; -11.0 ± 4.32; + 0.3 ± 0.09; p < 0.001; p < 0.001; p < 0.001, respectively) (Fig. 2). Table 2 reports the mean change differences from T2 to T0. The UDD at T0 was 9,525.6, while at T2 was 1,587.6 with a statistically significant difference in terms of antibiotics used [− 7938 (p < 0.001)] (secondary efficacy end-point). The Table 3 shows all microbiological findings in patients with symptomatic recurrence and mean antimicrobial resistance profiles of all bacterial isolates at enrolment and follow-up visit.
Fig. 2.
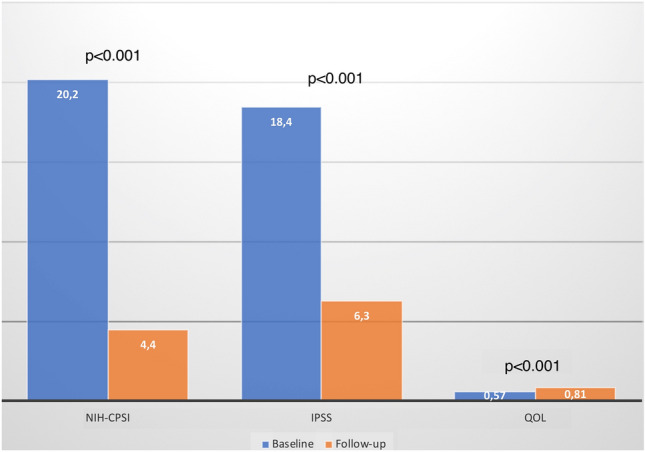
NIH-CPSI, IPSS and QoL scores at baseline and follow-up evaluations
Table 2.
Questionnaire results at the 6 months follow-up visit
| Pre-treatment | Post-treatment | p | |
|---|---|---|---|
| Mean (SD*) | Mean (SD*) | ||
| NIH-CPSI# | |||
| pre-treatment | 20.2 ± 2.3 | 4.4 ± 2.1 | < 0.001 |
| Mean difference | − 16.5 ± 3.58 | ||
| IPSS° | |||
| pre-treatment | 18.4 ± 3.4 | 6.3 ± 2.4 | < 0.001 |
| Mean difference | − 11.0 ± 4.3 | ||
| QoL‡ | |||
| pre-treatment | 0.57 ± 0.1 | 0.81 ± 0.1 | < 0.001 |
| Mean difference | + 0.3 ± 0.09 | ||
The table shows all questionnaire results at the follow-up visit. SD* = Standard Deviation; NIH-CPSI# = NIH Chronic Prostatitis Symptom Index; IPSS° = International Prostate Symptoms score; QoL‡ = Quality of Well-Being questionnaires
Table 3.
Microbiological findings in patients with symptomatic recurrence and mean antimicrobial resistance profiles of all bacterial isolates at enrolment and follow-up visit
| Patients with symptomatic recurrence at T2 time point | 23 (27.4%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Antimicrobial agents tested | |||||||||
| Resistance (%) | |||||||||
| GM | CPFX | CTX | LVFX | IPM | PIPC/TAZ | SMX/TMP | ABPC | VCM | |
| Isolated bacteria at T0 (baseline) | |||||||||
| Escherichia coli (14) | 7.1 | 57.1 | 0 | 57.1 | 0 | 0 | 21.4 | 42.8 | – |
| Enterococcus faecalis (9) | 66.6 | 55.5 | 22.2 | 33.3 | 11.1 | 11.1 | 33.3 | 33.3 | 0 |
| Antimicrobial agents tested | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Resistance (%) | ||||||||||
| GM | CPFX | CTX | LVFX | IPM | PIPC/TAZ | SMX/TMP | ABPC | VCM | ||
| Isolated bacteria at T2 time point (6 months) | ||||||||||
| Escherichia coli (10) | 30 | 70 | 10 | 70 | 0 | 0 | 30 | 40 | – | |
| Enterococcus faecalis (12) | 58.3 | 33.3 | 8.3 | 33.3 | 0 | 0 | 25 | 16.6 | 0 | |
| Klebsiella oxytoca (1) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | – | – | |
The table shows all microbiological findings in patients with symptomatic recurrence and mean antimicrobial resistance profiles of all bacterial isolates at T0 and T2 time point. GM = gentamicin; CPFX = ciprofloxacin; CTX = cefotaxime; LVFX = levofloxacin; IPM = imipenem; PIPC/TAZ = piperacillin/tazobactam; SMX/TMp = sulfamethoxazole-trimethoprim; ABPC = ampicillin; VCM = vancomycin
Discussion
In this study, we demonstrated for the first time, that in patients with CBP, the treatment with L. casei DG® prevents the symptomatic recurrences, improving the quality of life and reducing the antibiotic use. Moreover, we demonstrated full treatment compliance, as no study discontinuations were recorded. The high compliance is related to the low frequency of adverse events and the efficacy of the treatment in terms of quality of life improvement.
Results in the context of previous studies
CBP continues to pose a treatment challenge for all urologists and for these reasons, a lot of non-standardized treatment schedule, sometimes in off-label way, were offered to the patients. Promising results are emerging from studies focusing on the microbiota of patients. Recent data acquisition about the role of the microbiome in healthy humans, allowed us to understand that there exists interplay and symbiotic relationships between our bodies and the microorganisms colonizing the gastro-intestinal system [19]. Recent studies indicated that the microbiome can influence prostate inflammation in relation to benign prostate conditions such as prostatitis/chronic pelvic pain syndrome and benign prostatic hyperplasia, as well as in prostate cancer [20]. Starting from these considerations, the reduction of antibiotics and the maintenance of normal gut homeostasis should be considered also in the management of bacterial prostatitis. Vicari and co-workers enrolled a total of 106 infertile male patients affected by CBP and irritable bowel syndrome [8]. All patients underwent rifaximin treatment in combination with probiotics containing multiple bacterial strains and compared the clinical results with a no treatment control group [8]. They concluded that a long-term treatment with rifaximin and probiotics is effective in lowering the progression of prostatitis into more complicated forms of male accessory gland infections [8]. Unlike Vicari’s study, in our study, we enrolled patients without any intestinal disease and we treated them with probiotics only. On the other hand, another Italian group, demonstrated that a 6 months treatment with probiotics plus Vaccinium Macracarpon and Lyciumbarbarum L., reduces the number of symptomatic episodes and improves the quality of life [21]. The efficacy of probiotics in the management of CBP could be related to the immune modulation of the bowel epithelium with the suppression of the low-grade inflammation [8, 22] and with the decrease of uropathogens spreading through the bowel mucosa. Here, we selected a specific probiotic strain, L. casei DG®, that has been demonstrated to modulate the intestinal microbial ecosystems of healthy adults and patients affected by inflammatory bowel disease, and to influence host immune response via its unique polysaccharide capsule [23–25]. L. casei DG® has also been demonstrated as therapeutic potential for several dysfunctions and pathological conditions such as increasing the efficacy of antibiotic eradication therapy against H. pylori [26].
Strengths and limitations of this study
Even if our results are encouraging, this study shows several limitations to consider. Firstly, the number of enrolled patients. Even if the number need to treat is correctly calculated is very important to highlight that the efficacy and safety of probiotics should be evaluated with a long-term follow-up, to discover delayed adverse side effects. However, taking into consideration the studies evaluating the long-term therapy with L. casei DG® in other medical setting, we could consider this treatment safe also in CBP [27]. The use of a short-term antibiotic treatment period should not be considered a limitation of the study. In accordance with Bjerklund Johansen et al., who stated that the minimum duration of antibiotic treatment should be 2–4 weeks, we chosen this 14-day treatment course [27]. Finally, the lack of a control group should be considered a limitation of the study. Moreover, the role of a possible placebo effect should be considered as a factor influencing the patients’ outcome. However, in this Phase IV study we considered the following end-points: treatment efficacy defined as improvement of clinical symptoms at T2 vs T0, and a decrease in the use of antimicrobial drugs at T2 vs T0. In this sense, even though we did not consider a control group in our study design, the efficacy of L. casei DG® has been demonstrated by performing a paired comparison between pre- (T0) and post-treatment (T2) outcomes, in line with Thomas Jaeger [28]. Moreover, comparison of the results of this study with a cohort of historical controls, assessed 6 months after the end of antibacterial treatment suggests that L. casei DG® may have concurred to a sustained reduction of NIH-CPSI and IPSS scores (19.8 ± 1.9 and 17.9 ± 3.3 in the historical cohort), and may have had an influence in decreasing the recurrence rate (1.7/3 months) and the antibiotic usage[9,13]. However, future studies with randomized and blinded design are needed to confirm these results.
Implications for clinical practice
The results of this study should be read in the light of the continuing increase of resistant bacterial strains and the necessity to find new strategies to reduce the use of antibiotics in CBP. Our results suggest that L. casei DG® reduces both the symptomatic recurrence and the use of antibiotics. Therefore, the use of L. casei DG® after antibiotic treatment represents a valid tool to improve the antibiotic stewardship in urological setting. In an economic perspective, our findings suggest a reduction in direct costs related to the reduction of antibiotic daily dose and indirect costs related to the patients’ well-being (less lost working days, less stress and anxiety), even if we did not revaluate this economic outcome.
Conclusions
In patients with CBP, L. casei DG® is able to prevent symptomatic recurrences, improving the quality of life and reducing the antibiotic use. Future larger clinical trials with a randomized and blinded design are needed to confirm these results especially in terms of economic perspectives.
Acknowledgements
We are grateful to Professor John Denton (Department of Modern Philology, University of Florence) for manuscript language revision.
Author contributions
TC, LG, EC data collecting and analyzing; TC, LG, FC AP manuscript writing; RB, GD, GM, VM, TEBJ, supervision.
Funding
None.
Compliance with ethical standards
Conflict of interest
Tommaso Cai and Alessandro Palmieri received travelling and speaking grants from SOFAR Company.
Ethical approval
Approval protocol number 258, 2019.
Informed consent
Before the beginning of the study, the enrolled patients or legal guardians signed the informed consent. All data recorded in this study, i.e., anamnestic, clinical, and laboratory data, containing sensitive information were deidentified to ensure analysis of anonymous data only. This process was performed by non-medical staff using dedicated software.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bartoletti R, Cai T, Mondaini N, et al. Prevalence, incidence estimation, risk factors and characterization of chronic prostatitis/chronic pelvic pain syndrome in urological hospital outpatients in Italy: results of a multi center case-control observational study. J Urol. 2007;178(6):2411–2415. doi: 10.1016/j.juro.2007.08.046. [DOI] [PubMed] [Google Scholar]
- 2.Bartoletti R, Cai T, Nesi G, Albanese S, Meacci F, Mazzoli S, Naber K. (2014) The impact of biofilm-producing bacteria on chronic bacterial prostatitis treatment: results from a longitudinal cohort study.World J Urol 32(3):737–742. [DOI] [PubMed]
- 3.Magri V, Boltri M, Cai T, Colombo R, Cuzzocrea S, De Visschere P, Giuberti R, Granatieri CM, Latino MA, Larganà G, Leli C, Maierna G, Marchese V, Massa E, Matteelli A, Montanari E, Morgia G, Naber KG, Papadouli V, Perletti G, Rekleiti N, Russo GI, Sensini A, Stamatiou K, Trinchieri A, Wagenlehner FME. Multidisciplinary approach to prostatitis. Arch Ital Urol Androl. 2019;90(4):227–248. doi: 10.4081/aiua.2018.4.227. [DOI] [PubMed] [Google Scholar]
- 4.Penna G, Mondaini N, Amuchastegui S, Degli Innocenti S, Carini M, Giubilei G, Fibbi B, Colli E, Maggi M, Adorini L. Seminal plasma cytokines and chemokines in prostate inflammation: interleukin 8 as a predictive biomarker in chronic prostatitis/chronicpelvicpainsyndrome and benignprostatichyperplasia. Eur Urol. 2007;51(2):524–533. doi: 10.1016/j.eururo.2006.07.016. [DOI] [PubMed] [Google Scholar]
- 5.Cai T, Verze P, La Rocca R, Palmieri A, Tiscione D, Luciani LG, Mazzoli S, Mirone V, Malossini G. The Clinical Efficacy of Pollen Extract and Vitamins on Chronic Prostatitis/Chronic Pelvic Pain Syndrome Is Linked to a Decrease in the Pro-Inflammatory Cytokine Interleukin-8. World J Mens Health. 2017;35(2):120–128. doi: 10.5534/wjmh.2017.35.2.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tsilingiri K, Barbosa T, Penna G, et al. Probiotic and postbiotic activity in health and disease: comparison on a novel polarised ex-vivo organ culture model. Gut. 2012;61:1007–1015. doi: 10.1136/gutjnl-2011-300971. [DOI] [PubMed] [Google Scholar]
- 7.Compare D, Rocco A, Coccoli P, Angrisani D, Sgamato C, Iovine B, Salvatore U, Nardone G. Lactobacillus Casei DG and Its Postbiotic Reduce the Inflammatory Mucosal Response: An Ex-Vivo Organ Culture Model of Post-Infectious Irritable Bowel Syndrome. BMC Gastroenterol. 2017;17(1):53. doi: 10.1186/s12876-017-0605-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vicari E, La Vignera S, Castiglione R, Condorelli RA, Vicari LO, Calogero AE. Chronic Bacterial Prostatitis and Irritable Bowel Syndrome: Effectiveness of Treatment With Rifaximin Followed by the Probiotic VSL#3. Asian J Androl. 2014;16(5):735–739. doi: 10.4103/1008-682X.131064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cai T, Mazzoli S, Bechi A, et al. Serenoarepens associated with Urticadioica (ProstaMEV) and curcumin and quercitin (FlogMEV) extracts are able to improve the efficacy of prulifloxacin in bacterial prostatitis patients: results from a prospective randomised study. Int J Antimicrob Agents. 2009;33(6):549–553. doi: 10.1016/j.ijantimicag.2008.11.012. [DOI] [PubMed] [Google Scholar]
- 10.Trinchieri A. Role of levofloxacin in the treatment of urinary tract infections. Arch Ital UrolAndrol2001;73:105–13. [PubMed]
- 11.Bonkat G, Bartoletti R, Cai T et al. EAU guidelines on urological infections (2019). https://uroweb.org/guideline/urological-infections
- 12.Monnet DL, Mölstad S, Cars O. Defined daily doses of antimicrobials reflect antimicrobial prescriptions in ambulatory care. J AntimicrobChemother. 2004;53(6):1109–1111. doi: 10.1093/jac/dkh230. [DOI] [PubMed] [Google Scholar]
- 13.Cai T, Pisano F, Nesi G, Magri V, Verze P, Perletti G, Gontero P, Mirone V, Bartoletti R. Chlamydia trachomatis versus common uropathogens as a cause of chronic bacterial prostatitis: is there any difference? results of a prospective parallel-cohort study. Investig Clin Urol. 2017;58(6):460–467. doi: 10.4111/icu.2017.58.6.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gallelli L, Ferreri G, Colosimo M, Pirritano D, Flocco MA, Pelaia G, Maselli R, De Sarro GB. Retrospective analysis of adverse drug reactions to bronchodilators observed in two pulmonary divisions of Catanzaro. Italy Pharmacol Res. 2003;47(6):493–499. doi: 10.1016/S1043-6618(03)00003-3. [DOI] [PubMed] [Google Scholar]
- 15.Giubilei G, Mondaini N, Crisci A, Raugei A, Lombardi G, Travaglini F, et al. The Italian version of the National Institutes of Health Chronic prostatitis symptom index. Eur Urol. 2005;47:805–811. doi: 10.1016/j.eururo.2004.12.025. [DOI] [PubMed] [Google Scholar]
- 16.Badia X, Garcia-Losa M, Dal-Re R. Ten-language translation and harmonizationof the International Prostate Symptom Score: developing a methodology for multinational clinical trials. Eur Urol. 1997;31:129–140. doi: 10.1159/000474438. [DOI] [PubMed] [Google Scholar]
- 17.Kaplan RM, Bush JW, Berry CC: Health status: types of validity and the index of wellbeing. Health Serv Res 1976;11: 478–507 [PMC free article] [PubMed]
- 18.Nickel JC, Downey J, Hunter D, Clark J. Prevalence of prostatitis-like symptoms population based study using the National Institutes of Health chronic prostates symptoms index. J Urol. 2001;165:843–845. [PubMed] [Google Scholar]
- 19.David LA, Maurice CF, Carmody RN, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505:559–563. doi: 10.1038/nature12820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Porter CM, Shrestha E, Peiffer LB, Sfanos KS. The microbiome in prostate inflammation and prostate cancer. Prostate Cancer Prostatic Dis. 2018;21(3):345–354. doi: 10.1038/s41391-018-0041-1. [DOI] [PubMed] [Google Scholar]
- 21.Chiancone F, Carrino M, Meccariello C, Pucci L, Fedelini M, Fedelini P.The Use of a Combination of Vaccinium Macracarpon, Lyciumbarbarum L. and Probiotics (Bifiprost®) for the Prevention of Chronic Bacterial Prostatitis: A Double-Blind Randomized Study.Urol Int. 2019;103(4):423–426. [DOI] [PubMed]
- 22.Camilleri M. Probiotics and irritable bowel syndrome: rationale, putative mechanisms, and evidence of clinical efficacy. J Clin Gastroenterol. 2006;40:264–269. doi: 10.1097/00004836-200603000-00020. [DOI] [PubMed] [Google Scholar]
- 23.Ferrario C, Taverniti V, Milani C, Fiore W, Laureati M, De Noni I, Stuknyte M, Chouaia B, Riso P, Guglielmetti S. Modulation of Fecal Clostridiales Bacteria and Butyrate by Probiotic Intervention with Lactobacillus paracasei DG Varies among Healthy Adults. J Nutr. 2014;144(11):1787–1796. doi: 10.3945/jn.114.197723. [DOI] [PubMed] [Google Scholar]
- 24.Cremon C, Guglielmetti S, Gargari G, Taverniti V, Castellazzi AM, Valsecchi C, Tagliacarne C, Fiore W, Bellini M, Bertani L, Gambaccini D, Cicala M, Germanà B, Vecchi M, Pagano I, Barbaro MR, Bellacosa L, Stanghellini V, Barbara G. Effect of Lactobacillus paracasei CNCM I-1572 on symptoms, gut microbiota, short chain fatty acids, and immune activation in patients with irritable bowel syndrome: A pilot randomized clinical trial. United European Gastroenterol J. 2018;6(4):604–613. doi: 10.1177/2050640617736478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Balzaretti S, Taverniti V, Guglielmetti S, Fiore W, Minuzzo M,Ngo HN, Ngere JB, Sadiq S, Humphreys PN, Laws AP. A novel rhamnose-rich hetero-exopolysaccharide isolated from Lactobacillus paracasei DG activates THP-1 human monocytic cells. Appl Environ Microbiol. 2017; 17;83(3). pii: e02702–16 [DOI] [PMC free article] [PubMed]
- 26.Paoluzi OA, Del Vecchio BG, Visconti E, Coppola M, Fontana C, Favaro M, Pallone F. Low efficacy of levofloxacin-doxycycline-based third-line triple therapy for Helicobacter pylori eradication in Italy. World J Gastroenterol. 2015;21(21):6698–6705. doi: 10.3748/wjg.v21.i21.6698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bjerklund Johansen TE, Grüneberg RN, Guibert J, Hofstetter A, Lobel B, Naber KG, et al. The role of antibiotics in the treatment of chronic prostatitis: a consensus statement. Eur Urol. 1998;34:457–466. doi: 10.1159/000019784. [DOI] [PubMed] [Google Scholar]
- 28.Thomas Jaeger and Ravi Deshpande. Choosing a Phase IV Study Design. Phase 4 Health Inc. Canadian Pharmaceutical Marketing/Winter 2003 available at: http://www.stacommunications.com/journals/pdfs/cpm/cpmwinter03/phasefour.pdf