Abstract
Background:
Patients undergoing surgical procedures are vulnerable to repetitive evoked or ongoing nociceptive barrage. Using functional near infrared spectroscopy, we aimed to evaluate the cortical hemodynamic signal power changes during ongoing nociception in healthy awake volunteers and in surgical patients under general anesthesia. We hypothesized that ongoing nociception to heat or surgical trauma would induce reductions in the power of cortical low frequency hemodynamic oscillations in a similar manner as previously reported using functional magnetic resonance imaging for ongoing pain.
Methods:
Cortical hemodynamic signals during noxious stimuli from the fontopolar cortex were evaluated in two groups: Group 1: A healthy/conscious group (n=15, all males) where ongoing noxious and innocuous heat stimulus was induced by a contact thermode to the dorsum of left hand; and Group 2: A patient/unconscious group (n=13, 3 males) receiving general anesthesia undergoing knee surgery. The fractional power of low frequency hemodynamic signals was compared across stimulation conditions in the healthy awake group, and between patients who received standard anesthesia and those who received standard anesthesia with additional regional nerve block.
Results:
A reduction of the total fractional power in both groups: specifically, a decrease in slow-5 frequency band (0.01–0.027Hz) of oxygenated hemoglobin concentration changes over the frontopolar cortex was observed during ongoing noxious stimuli in the healthy awake group (paired t-test p=0.017, effect size=0.70), and during invasive procedures in the surgery group (paired t-test p=0.003, effect size=2.16). The reduction was partially reversed in patients who received a regional nerve block that likely diminished afferent nociceptive activity (two-sample t-test p=0.002, effect size=2.34).
Conclusions:
These results suggest common power changes in slow-wave cortical hemodynamic oscillations during ongoing nociceptive processing in conscious and unconscious states. The observed signal may potentially promote future development of a surrogate signal to assess ongoing nociception under general anesthesia.
Introduction
While general anesthesia induces unresponsiveness and amnesia, the extent of analgesia remains a concern1,2. Previous animal and human investigations suggested that nociceptive/pain processing in the central nervous system (CNS) could be maintained under anesthesia3–5 or even enhanced by some general anesthetics6,7. Repetitive nociceptive signaling following invasive surgical procedures and resultant inflammation leading to hyperalgesia and central sensitization8,9 which may contribute to postoperative pain and potential initiation of pain chronification (e.g., post-surgical neuropathic pain, reported by 10% to 50% of surgical patients)10,11.
Prior studies with functional magnetic resonance imaging revealed specific patterns of power alterations in low frequency blood-oxygenation-level-dependent signal oscillations (typically between 0.01 and 0.20Hz) when pain becomes persistent12–18. A significant reduction in the power of infra-slow oscillations during acute 5-minute and 20-minute noxious stimuli has been reported in healthy awake subjects19. These power decreases were seen to particularly involve key regions of the default mode network such as the medial prefrontal cortex, the precuneus, the posterior cingulate cortex and the inferior parietal cortex. Low frequency oscillations of hemodynamic signals are thought to represent an intrinsic component of neural activity20. The power spectra alterations during ongoing pain may reflect a reorganization of brain connectivity due to continuous nociceptive input through peripheral pain pathways, which disrupts intrinsic resting state dynamics. Importantly, animal21,22 and human models23,24 showed that the low frequency oscillations were observable during anesthesia. However, the amplitudes of the hemodynamic oscillations might be enhanced or attenuated depending on the types of anesthetics and stimulation.
Using functional near infrared spectroscopy, our group has reported similar responses to transient nociceptive stimuli in awake25, sedated26 and anesthetized state3. While nociceptive activation of the CNS occurs with deep general anesthesia, responses to nociceptive stimuli may not be easily detected clinically but are observed using functional imaging and neurophysiological monitoring4,27. Thus, there is a need to provide a continuous monitoring of nociceptive signals that occurs from surgical interventions under anesthesia. Sharing a similar physiological basis as functional magnetic resonance imaging, functional near infrared spectroscopy measures cortical hemodynamic changes and has been previously employed to detect amplitude and power alterations in low frequency oscillations28,29. We focus primarily on the frontopolar cortex, a cortical region that is involved in high level integration of nociceptive information30 and easy to access in the operating room with the functional near infrared spectroscopy imaging technique. We hypothesized that (1) ongoing nociception (heat in healthy volunteers and surgical induced trauma) will induce similar reductions in the power of frontopolar cortex low frequency hemodynamic oscillations; and (2) the low frequency power reductions will be mitigated in surgical patients by the addition of a regional anesthetic blockade (a known analgesic effect) when compared with standard inhalational anesthesia.
Methods
Definitions
The international association for the study of pain defines pain as “An unpleasant sensory and emotional experience associated with, or resembling that associated with, actual or potential tissue damage”, which is an individual and subjective experience. Nociception is described as “observable activity in the nervous system in response to an adequate stimulus”. Nociceptive stimuli used in this study (thermal or surgical incision) produce activations in pain pathways (e.g., spino-thalamo-cortical) that result in the individual’s interpretation of “pain”. Under anesthesia, the unconscious state precludes the definition of “pain” since a subjective report is not possible. We use the term “pain” for subjective feeling, “painful stimuli” for the externally applied heat stimulus particularly in the awake volunteer group, and “nociception” or “nociceptive signals” to describe the measurable brain response to such stimuli.
Subjects
This study was approved by the Institutional Review Board (IRB) of Boston Children’s Hospital, and conformed to the ethical standards of human research as defined by the Helsinki Accord and the international association for the study of pain. Two subject populations were recruited: Group 1 - healthy volunteers to evaluate awake nociceptive brain responses to external painful stimuli; and Group 2 - anesthetized patients, otherwise healthy, undergoing routine knee surgery to evaluate nociception under anesthesia. The subject recruitment and data collection lasted two years from 2017 to 2019. Knee surgery patients were recruited as part of our functional near infrared spectroscopy program to study brain response to nociception under anesthesia3,26. Written informed consents were obtained from the participants and their legal guardians (if a minor) in both groups prior to functional near infrared spectroscopy measures. The exclusion criteria included a history of neurological trauma or psychiatric disorders, muscular disease, diabetes and smoking. In addition, participants who were unable to keep the head still (awake) for at least three consecutive minutes or whose scalp or hair did not permit sufficient penetration of optical lights were excluded from this study.
The rationale for the two study groups was (a) in prior studies we had observed similar changes in the sensory cortex and the frontopolar cortex to evoked stimuli in adult patients under sedation26 and pediatric patients under anesthesia3; (b) the notion of functional near infrared spectroscopy signal for ongoing nociception under anesthesia needed to be first defined in awake subjects. Our thesis is that nociceptive pathways are intact and that similar patterns of activation would be present even in the context of analgesia. With respect to the latter, opioids do not completely block nociceptive signals produced by significant nociceptive activators31 such as surgical incisions. Therefore, the use of a different group (awake) of individuals was to evaluate signal processing using a parallel format for determining “ongoing pain/nociception”.
Experimental Procedures
Awake (Group 1):
Using ongoing heat as induced stimulus, we set up the following specific null hypotheses for the healthy awake volunteer study: (1) the power of measured low frequency oscillations during ongoing heat pain (noxious) would not be statistically different than that during resting state or ongoing heat warm (innocuous); (2) from a non-stimulating period to a stimulating period (heat pain or heat warm), time-frequency analysis would not reveal significant changes in the power of measured low frequency hemodynamic signal oscillations. Three data acquisition sessions were performed in a sequential order: a resting-state session, a heat warm session, and a heat pain session, see Fig. 1A for an overview of the study design. During the resting state session, the participant was simply asked to sit still in a chair for six minutes with their eyes open and no additional task or stimulation was given. Prior to the heat warm or heat pain session, the subjective pain perception levels were determined for each participant by applying continuous thermal stimulations with varying intensities to the dorsum of the left hand with a Medoc Thermal Sensory Analyzer (Medoc Ltd., Ramat Yishai, Israel). The participant was asked to report when the thermal stimulation resulted in a perception score of 3 or 5 on a 0–10 scale, with the score 3/10 being described as “the participant should be strongly aware of the stimulus but should just feel comfortably warm and no pain at all” (heat warm) and the score 5/10 being “the participant should feel moderately painful but the pain should be tolerable for at least five minutes with no breath-holding, sweating or any retreat reactions” (heat pain). The heat intensities corresponding to these two perception levels were documented and were used in the next heat warm or heat pain session. During the stimulation sessions, the participant was placed in a resting period for the first 100 seconds, followed by a five-minute stimulation period with a continuous thermal stimulus applied to the same area of the left hand using either the 3/10 intensity (in the heat warm session) or the 5/10 intensity (in the heat pain session). Another 100 seconds of resting period recording was collected at the end of the stimulation period to capture signal changes during the recovery phase.
Figure 1.
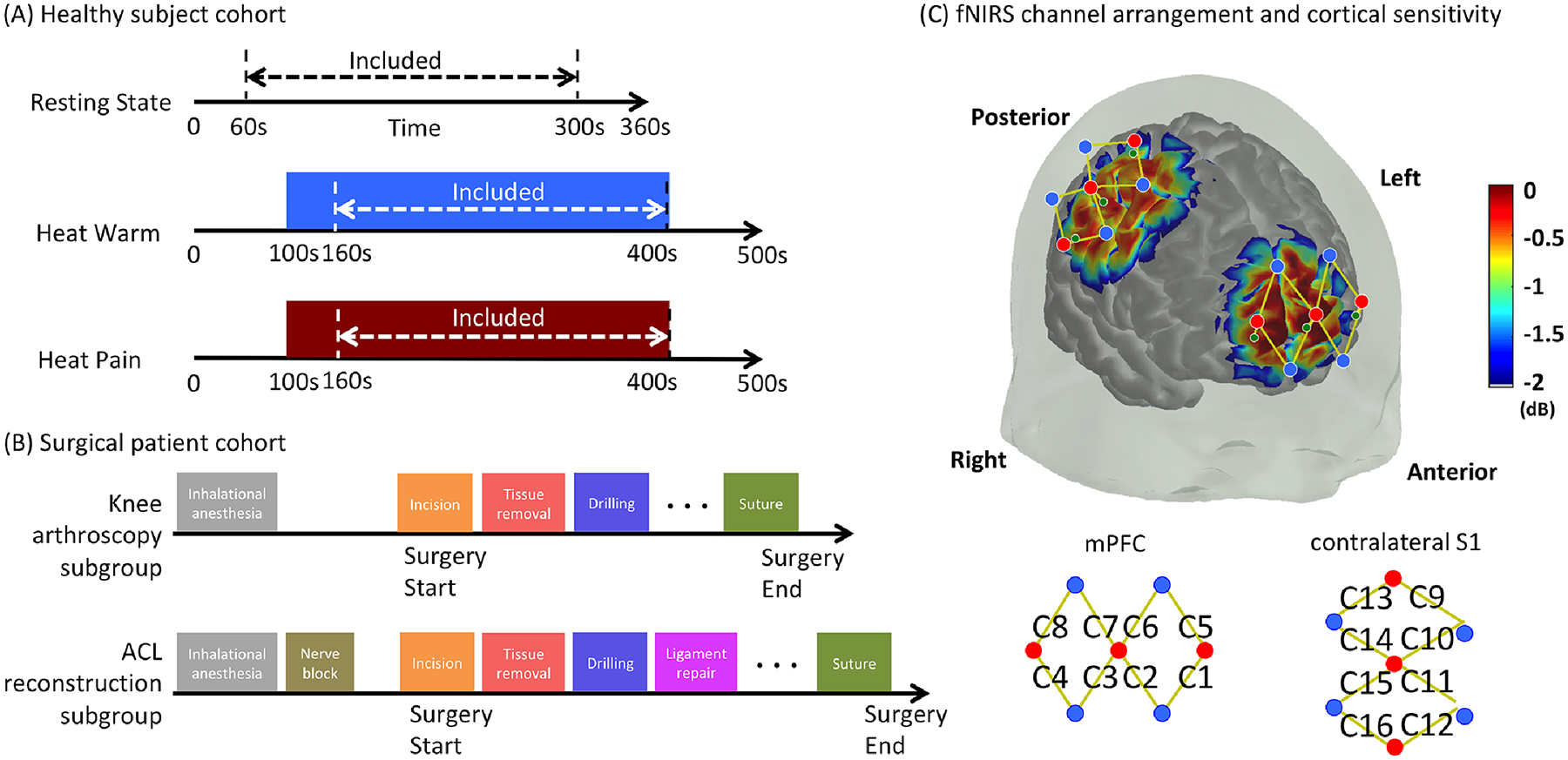
Overview of study design and functional near infrared spectroscopy cortical coverage. (A) Overview of recording sessions in the healthy awake volunteer group. (B) Sketch plot of the subgroups and the surgical procedures. (C) The arrangement of light emitters (red dots), light detectors (blue dots) and the formed channels (yellow curves). The corresponding sensitivity matrix is shown directly on the cortex.
Anesthetized (Group 2):
The specific null hypotheses set up for the anesthetized patient group were as follows: (1) the power level of the measured cortical low frequency oscillations during major invasive surgical procedures would not be significantly different in patients without additional analgesic control than in those with additional analgesic control; (2) time-frequency analysis of cortical low frequency oscillation power would not detect statistically significant power changes following major invasive surgical procedures compared with the pre-procedure baseline in surgical patients with and without additional analgesic control. To test these hypotheses, the recruited knee surgery patients were first assigned into one of the two following study subgroups depending on the type of scheduled surgery and analgesic level: a knee arthroscopy surgery subgroup and an anterior cruciate ligament reconstruction surgery subgroup. This surgery patient group was chosen particularly because of the similarities in the early procedures of both types of the surgeries, which involved an orthopedist (LM) making small incisions, removing soft tissues and inserting probing arthroscope into the knee joint of the patient for diagnosis or treatment (Fig.1B). Anesthesia was standardized for all the patients, which was induced with propofol and fentanyl and was maintained by sevoflurane in air and oxygen. Additional nerve block was performed on patients in the anterior cruciate ligament reconstruction surgery subgroup (but not on regular knee arthroscopy surgery patients) for pain control, such as femoral nerve block and adductor canal block. The similar early surgical procedures but distinct analgesia levels between the two subgroups provided us with an ideal model to study the effect of ongoing pain on brain dynamics under general anesthesia. For each patient, functional near infrared spectroscopy data were acquired for the entire surgical session. The timing of surgical events that were likely to induce major nerve or tissue damage (and therefore likely led to significant ongoing nociceptive inputs) was marked directly in the data.
Data Collection and Pre-Processing:
A multichannel continuous wave functional near infrared spectroscopy system (CW7, TechEn Inc., Milford, USA) utilizing laser diodes at 690 and 830 nm was used to collect brain hemodynamic data for both the healthy volunteer group and the surgical patient group. Caps of different sizes were used to fit the participant’s head. Fig.1C depicts the detailed montage design used in this study and the corresponding detection sensitivity profile. As described above, our primary region of interest was determined to be the frontopolar cortex. Three functional near infrared spectroscopy light emitters and four light detectors were placed over this region of interest to form a total of eight channels with a normal emitter-detector distance of 3 centimeters to cover the lateral (channels C1, C4, C5 and C8) and medial portions (channels C2, C3, C6 and C7) of the frontopolar cortex. For healthy awake volunteers, another eight channels (channels C9 to C16) were installed to cover the primary somatosensory cortex, a classical region that is known to be within the lateral (sensory) nociceptive pathway. However, it was not possible to maintain optode contact and obtain reliable functional near infrared spectroscopy signals from somatosensory cortex channels in surgical patients who were kept in a supine position. Therefore, no somatosensory cortex signal was included in our study of patients under anesthesia. An additional short separation light detector was installed 8 millimeters away from each light emitter to capture the signal changes from extracerebral layers such as the skin, scalp and skull. For healthy volunteers, the locations of the functional near infrared spectroscopy optodes were digitized after data collection with a 3D digitizer (Polhemus patriot, Colchester, VT) for a post-hoc analysis to make sure that the inter-subject variability of optode locations was reasonable (result not shown here).
The acquired functional near infrared spectroscopy data were pre-processed using the open source Matlab (Mathworks, Natick, USA) toolbox Homer2. The raw optical signals, sampled at 25 Hz, were first transformed to optical density changes by taking the logarithm of the data. Detection of motion artifacts was conducted on the optical density time courses using the automatic motion artifact detection module of Homer2 (with a standard deviation threshold of 50 and amplitude change threshold of 5). We then manually inspected the time courses for remaining sudden drifts and shape spikes in the data. Any participant’s data that contained motion artifacts were not selected for further analysis. The optical density data were filtered between 0.01 Hz and 0.50 Hz with a 3rd order Butterworth band pass filter, and were then converted into oxygenated hemoglobin and deoxygenated hemoglobin concentration changes using the modified Beer-Lambert Law with a partial pathlength factor of 6. For each normal channel (i.e. a pair of emitter and detector placed at a distance of 3 cm), a linear regression model was established to remove physiological interference that potentially comes from superficial layers, using the time course of a short separation channel (i.e. a pair of emitter and detector placed at a distance of 0.8cm) that showed the highest correlation with that of the normal channel as a regressing covariate as well as a constant regressor32. The residuals of the regression analysis were used for subsequent data analysis.
Frequency Analysis for Healthy Awake Volunteers
Power spectrum analysis was first performed on oxygenated hemoglobin concentration change data to investigate the differences in the power of low frequency oscillations between resting and stimulation conditions. In this study, we specifically chose oxygenated hemoglobin over deoxygenated hemoglobin due to the relatively higher signal-to-noise ratio in delineating the low frequency oscillations of cerebral hemodynamics33. A schematic of this “static” approach is provided in Fig. 2A. The oxygenated hemoglobin time course during the time period of interest of each channel in each subject was first transformed to the frequency domain using the Fast Fourier Transform. In the volunteer group, the time period of interest was defined as the data from 60 second after the start of task (rest, heat warm or heat pain) to end of task, lasting a total duration of 240 seconds. The first minute of the stimulation time was not included in the transformation to avoid any evoked, transient brain response to the heat stimulus (e.g. a salience response) before a stable feeling of ongoing heat warm or pain was established. Power spectral density was then computed on the time period of interest as the square of the Fast Fourier Transform amplitude at each frequency component. We employed Buzsáki’s nomenclature to divide the functional near infrared spectroscopy data frequency range (i.e. 0.01–0.5 Hz) into five distinct sub-bands: slow-5 (0.01–0.027 Hz), slow-4 (0.027–0.073 Hz), slow-3 (0.073–0.198 Hz), slow-2 (0.198–0.25 Hz), and slow-1 (0.25–0.5 Hz). The power spectral density components of each sub-band was added together, and was then normalized to the summed power of the whole frequency range (0.01–0.5 Hz) to obtain the band-wise fractional power. By normalization, fractional power showed improved sensitivity and specificity to detect spontaneous brain fluctuations by suppressing non-neuronal physiological interferences34.
Figure 2.
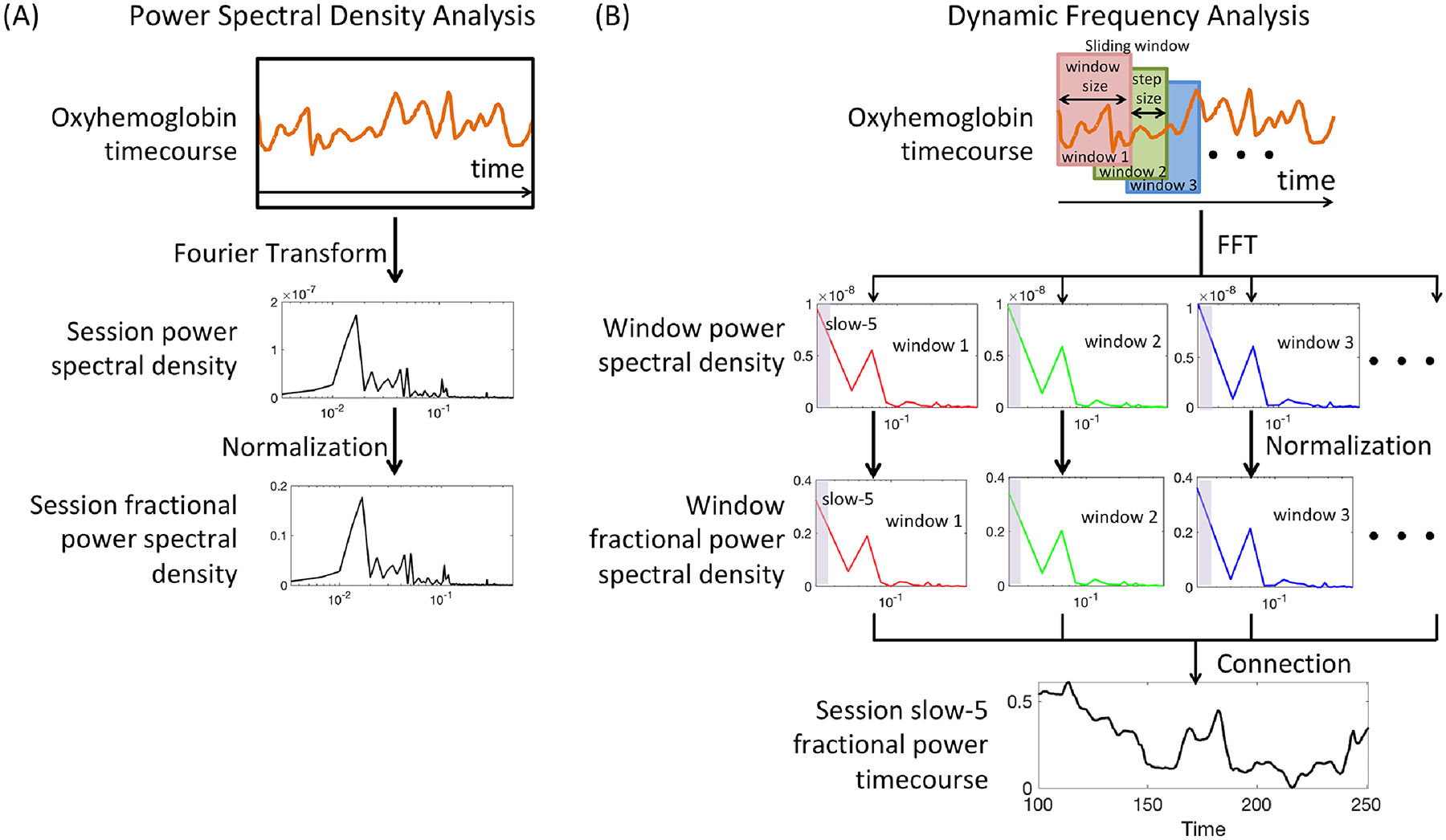
Sketch plot of the frequency analysis pipeline. (A) Power Spectral Density analysis. The entire functional near infrared spectroscopy channel timecourse within the time period of interest was used to generate the power spectral density plot. (B) Dynamic frequency analysis. A sliding window approach was used to generate a session fractional power timecourse of a specific frequency band (e.g. slow-5).
Taking advantage of the high temporal resolution of functional near infrared spectroscopy, we then performed dynamic frequency analysis to allow observation of the time-varying patterns in the frequency power properties within a single session before, during and after a stimulus was applied. The dynamic frequency analysis was conducted using a sliding window approach, with a window size of 50s and a step size 1s respectively. More specifically, the first estimates of the total fractional power in each of the five sub-bands were calculated based on the first 50 seconds of a session (resting, heat warm or heat pain). As the window slid from the first 50 seconds to the end, these estimates of total fractional power became a time course with a temporal resolution of 1s. Previous work using functional magnetic resonance imaging suggested different window sizes from 40 to 100 seconds for dynamic power or connectivity analysis35. In this analysis, we used a window size of 50s to balance the number of data samples within each window and the sensitivity to detect short-term power changes. Moreover, additional tests using different window size such as 25s or 100s presented similar results (not shown here).
Frequency Analysis for Anesthetized Patients
A central difficulty to compare fractional power spectral density between the knee arthroscopy patient subgroup (subgroup without nerve block) and the anterior cruciate ligament reconstruction patient subgroup (subgroup with nerve block) is that, despite the similarities in early procedure types, there were significant differences in surgery towards the end regarding the total duration of surgery, the type and number of late surgical events, etc. In general, anterior cruciate ligament reconstruction surgeries lasted much longer than arthroscopy surgeries and involved more invasive procedures such as the ligament removal and replacement (see Fig.1B), which might intrinsically result in a much higher load of ongoing nociceptive inputs in the anterior cruciate ligament reconstruction patient towards the end of surgery and therefore would not provide a fair comparison on the effect of nerve block treatment between the two subgroups. For the sake of simplicity in this power spectral density analysis, we decided to focus on just one major type of surgical event, soft tissue removal, which normally appeared at the early stages of both types of surgeries. The time period of interest was then selected as a five-minute window when soft tissue removal was first applied and occupied at least 50% of the window (i.e. lasted for more than 2.5 minutes in total). As in the awake volunteer group, the first minute of the window was excluded to remove the transient brain response to the event. The remaining four minutes of oxygenated hemoglobin data of each patient were then converted to frequency domain and were used to generate fractional power of sub-bands within the low frequency range with a similar approach described in the above section.
Dynamic frequency analysis was performed on the surgical data of all patients from 100 seconds before the first incision to the end of the surgery to generate time courses of total fractional power changes in each sub-band. An additional 3rd order Butterworth low pass filter thresholding at 0.01Hz and a 2nd order infinite impulse response notch filter at 0.005Hz were applied to remove high frequency noise and intrinsic oscillations seen in the time courses. We defined a fractional power response function of each channel for each patient by averaging the changes associated with all marked surgical events in the fractional power time course from 5s before the onset of the event to 250s after the event onset. Notably, the resolution of the fractional power response function was 1s (as step size = 1s in the sliding window approach) and each second of fractional power change in the response function corresponded to 50s of temporal data in the oxygenated hemoglobin concentration change time course (as window size = 50s). For example, fractional power value at the zero point (t = 0) in a response function was generated with the oxygenated hemoglobin temporal data from 50s before the event onset to the onset time. Prior to averaging across events, the fractional power change of each event was normalized to the total fractional power levels over the 5s before the zero point.
Statistical Analysis
Statistical analysis plan was made prior to data collection and assessment, and the statistical analysis was carried out using Matlab. The extracted fractional power values of sub-bands across healthy awake volunteers (Group 1) or anesthetized surgical patients (Group 2) were presented as mean ± standard deviation. The differences in fractional power between and within heat stimulation sessions in Group 1 and between surgery subgroups in Group 2 were presented with effect size, p-values (see below for description of conducted statistical tests), and 95% CI of the difference. In this study, the effect size was evaluated using Cohen’s d for matched samples (for within-subject comparisons) and for independent samples (for between subgroup comparisons).
Healthy awake volunteer data: Based on our hypothesis that ongoing heat pain will induce reductions in the power of frontopolar cortex low frequency hemodynamic oscillations, we compared the fractional power of each sub-band across stimulation conditions for each functional near infrared spectroscopy channel with a two-tailed paired t-test as in previous studies13,15,19,36. Normality of the data was assessed with the Kolmogorov-Smirnov test to make sure no significant evidence of departures was detected. The null hypothesis was that there were no statistically significant changes in the fractional power from a less stimulated condition to a more stimulated condition (e.g. resting to warm, resting to pain and warm to pain). For each sub-band, Type I error from multiple comparisons was controlled by conducting False Discovery Rate correction on p-values of lateral and medial channels at α = 0.05.
In the dynamic frequency analysis, we defined baseline fractional power as the mean power averaged from the initial resting periods of a session in the dynamic power time course, and stimulation fractional power as the total fractional power averaged from the last four minutes of the heat warm or heat pain stimulation periods (i.e. the same periods used in the above fractional power spectral density analysis). For each sub-band, we conducted two-tailed paired t-test between the baseline fractional power and the stimulation fractional power to test the null hypothesis that there were no statistically significant decreases in slow-5 and other sub-bands from the resting period to the stimulation period. False discovery rate corrections were applied to ensure a false discovery rate of less than 5%.
Anesthetized patient data: Similar to the healthy awake data analysis, the fractional power spectral density power of each sub-band generated from the time period of interest was compared between the knee arthroscopy surgery subgroup and the anterior cruciate ligament reconstruction subgroup using a two-tailed two-sample t-test. False discovery rate correction at an alpha value of 0.05 was applied on the p-values obtained from the comparisons of multiple channels to reduce Type I errors.
In the dynamic frequency analysis, we conducted two-tailed paired t-tests after the generation of the fractional power response function for each functional near infrared spectroscopy channel between each time point of the response function and the value at the zero point to test the null hypothesis that the fractional power at a particular time point was not significantly reduced following a surgical event compared with the initial state (i.e. the zero point) for patients undergoing knee arthroscopy or anterior cruciate ligament reconstruction surgeries. For each of the five sub-bands, channels that showed p-values less than 0.05 after false discovery rate correction were reported.
Results
Dataset
Based on a priori power analysis using our previous surgery data3, a total of 28 healthy volunteers and 28 surgical patients were evaluated for inclusion in the analysis. In the healthy awake volunteer group, datasets were excluded due to participants could not tolerant the pain stimulus (2 datasets), head size too large (1 dataset), thick or dark hair (3 datasets), and motion artifacts (7 datasets). For surgical patients, datasets were excluded due to system failure (1 dataset), medical emergency during surgery (1 dataset), short surgeries containing insufficient length of ongoing invasive procedures (3 datasets), optodes lost of contact or blocked by hair during surgery (3 datasets), high level of physiological noises (3 datasets), and motion artifacts due to frequent body movements by the surgeon or nurses (5 datasets). Table 1 summarizes the demographic information and the testing conditions of the remaining participants in our study, including fifteen healthy young adult participants (all males, age: 25.5 ± 5.5 years old, age range from 20 to 39) in the awake state (Group 1) and thirteen teenagers and young adults (3 males, age: 17.5 ± 3.8 years old, age range from 13 to 25) in the anesthetized state (Group 2). These healthy volunteers served as their own controls in the comparisons of functional near infrared spectroscopy signals across the three different conditions (resting, heat warm and heat pain). Notably, the temperature and the actual sensory perception level applied during the ongoing heat warm stimulation (innocuous) were significantly lower than those during the heat pain stimulation (noxious) (both p < 0.0001). In the surgical patient group, six patients (2 males, age: 18.7 ± 3.3 years old, age range from 15 to 23) underwent regular knee arthroscopic surgery without additional analgesic control, while the other seven patients (1 male, age: 16.6 ± 4.2 years old, age range from 13 to 25) received anterior cruciate ligament reconstruction surgery and a nerve block conducted prior to the start of the surgery. The detailed anesthesia information of each patient is included in Supplementary Table 1. For the patients, sensory testing was conducted by anesthesiologists in the postoperative period, and a clinical opinion on the efficacy of the nerve block was provided. Two sample Wilcoxon rank sum tests revealed no statistically significant difference in gender (p = 0.874), age (p = 0.280) or surgery lateralization (p = 0.767) between the knee arthroscopy subgroup and the anterior cruciate ligament reconstruction subgroup. Those factors were therefore not included as nuisance variables in our statistical tests of fractional power differences in the following sections.
Table 1.
Demographic information and conditions of the participants in this study
| Healthy awake volunteers (Group 1) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| # | Gender | Age | Innocuous heat (°C) | Noxious heat (°C) | Innocuous heat sensory perception level, post-session evaluation (0 – 10) | Noxious heat sensory perception level, post-session evaluation (0 – 10) | |||
| H1 | Male | 34 | 43 | 45 | 3 | 6 | |||
| H2 | Male | 26 | 40 | 45 | 2.5 | 6.5 | |||
| H3 | Male | 22 | 40 | 44 | 3 | 5 | |||
| H4 | Male | 22 | 40 | 46 | 2 | 7 | |||
| H5 | Male | 21 | 43 | 45 | 3 | 5 | |||
| H6 | Male | 20 | 42 | 46 | 3 | 4.8 | |||
| H7 | Male | 27 | 40 | 44 | 3 | 5 | |||
| H8 | Male | 22 | 38 | 44 | 3 | 5 | |||
| H9 | Male | 33 | 40 | 45 | 2.5 | 4.5 | |||
| H10 | Male | 23 | 40 | 46 | 2 | 6.5 | |||
| H11 | Male | 23 | 40 | 46 | 3 | 5 | |||
| H12 | Male | 39 | 41 | 46 | 3 | 5 | |||
| H13 | Male | 23 | 40 | 44 | 3 | 5 | |||
| H14 | Male | 23 | 40 | 46 | 3 | 5.5 | |||
| H15 | Male | 24 | 40 | 44 | 3 | 5 | |||
| 15 M 0 F | 25.5 ± 5.5 | 40.5 ± 1.3 | 45.1 ± 0.9 | 2.8 ± 0.4 | 5.4 ± 0.7 | ||||
| Anesthetized surgical patients (Group 2) | |||||||||
| # | Gender | Age | Knee surgery lateralization | Surgical procedures | Surgery duration (min) | Nerve block effectiveness | |||
| Knee arthroscopy subgroup (without nerve block) | |||||||||
| P2 | Female | 15 | Right | Knee arthroscopy with lateral meniscus repair and saucerization of discoid meniscus | 26 | ||||
| P5 | Female | 19 | Left | Lateral medial femoral condyle osteochondritis dissecans lesion fixation and drilling | 49 | ||||
| P8 | Female | 16 | Right | Knee arthroscopy, patella maltracking, scar tissue, bone spur at the notch, lateral release, chondroplasty | 27 | ||||
| P10 | Male | 23 | Left | Knee arthroscopic loose body removal, lateral femoral condyle chondroplasty, partial synovectomy with plica excision and microfracture of lateral femoral condyle | 37 | ||||
| P12 | Female | 17 | Right | Excision of fibrotic medial plica and partial lateral release under arthroscopic control | 22 | ||||
| P13 | Male | 22 | Right | Arthroscopy with partial medial meniscectomy | 25 | ||||
| 2 M 4 F | 18.7 ± 3.3 | 4 Right 2 Left | 31 ± 10 | ||||||
| Anterior cruciate ligament reconstruction subgroup (with nerve block) | |||||||||
| P1 | Female | 13 | Left | Knee arthroscopic aided anterior cruciate ligament reconstruction with hamstring autograft | 55 | Effective | |||
| P3 | Female | 18 | Left | Knee arthroscopic aided anterior cruciate ligament reconstruction with hamstring autograft | 46 | Partial | |||
| P4 | Female | 17 | Right | Anterior cruciate ligament tear, anterior horn lateral meniscus tear, complex radial tear of medial meniscus, medial compartment osteoarthritis and grade II chondromalacia medial femoral condoyle and medial tibial condyle. | 39 | Effective | |||
| P6 | Female | 13 | Right | Arthroscopy with anterior cruciate ligament reconstruction using autologous hamstring graft and trephination of the medial meniscus | 91 | Effective | |||
| P7 | Male | 14 | Right | Anterior cruciate ligament reconstruction with iliotibial band | 61 | Effective | |||
| P9 | Female | 16 | Left | Knee arthroscopy and bridge-enhanced anterior cruciate ligament Repair anterior cruciate ligament Repair | 37 | Partial | |||
| P11 | Female | 25 | Left | Knee arthroscopic aided anterior cruciate ligament reconstruction with hamstring autograft and lateral meniscus repair | 93 | Partial | |||
| 1 M 6 F | 16.6 ± 4.2 | 3 Right 4 Left | 60 ± 23 | ||||||
Evaluation of Ongoing Noxious and Innocuous Stimuli in Healthy Awake Individuals
Between-session comparison (PSD analysis):
Fig. 3A depicts the fractional power spectral density plots from our analysis of frontopolar cortex signals collected from the fifteen participants in awake conditions. Two-tailed paired t-tests revealed two channels located in the lateral frontopolar cortex (C5 and C8) that showed statistically significant reductions in the total fractional power of slow-5 band during ongoing noxious heat stimulation period compared with resting state or ongoing innocuous heat period (Fig. 3B, C5, slow-5 total fractional power during rest: 0.508 ± 0.189, during innocuous heat: 0.458 ± 0.139, during noxious heat: 0.357 ± 0.188, for resting vs. noxious heat Cohen’s d = 0.68, p = 0.020 and 95% CI of the difference = [0.028 0.275], for innocuous vs. noxious heat Cohen’s d = 0.79, p = 0.009 and 95% CI = [0.030 0.173]; C8, during rest: 0.477 ± 0.182, during innocuous heat: 0.469 ± 0.196, during noxious heat: 0.333 ± 0.139, for resting vs. noxious heat Cohen’s d = 0.71, p = 0.016 and 95% CI = [0.031 0.257], for innocuous vs. noxious heat Cohen’s d = 0.84, p = 0.006 and 95% CI = [0.046 0.227]). On the other hand, no significant difference was seen between resting state and ongoing innocuous heat stimulation period (C5, for resting vs. innocuous heat Cohen’s d = 0.28, p = 0.294, 95% CI = [−0.147 0.048]; C8, for resting vs. innocuous heat Cohen’s d = 0.03, p = 0.902, 95% CI = [−0.135 0.120]), highlighting the specificity of the slow-5 fractional power decrease to the perception of ongoing pain. The power spectral density analysis over the other low frequency bands (i.e. slow-4 to slow-1) did not show any statistically significant difference among the three testing conditions at a false discovery rate-corrected p-value less than 0.05.
Figure 3.
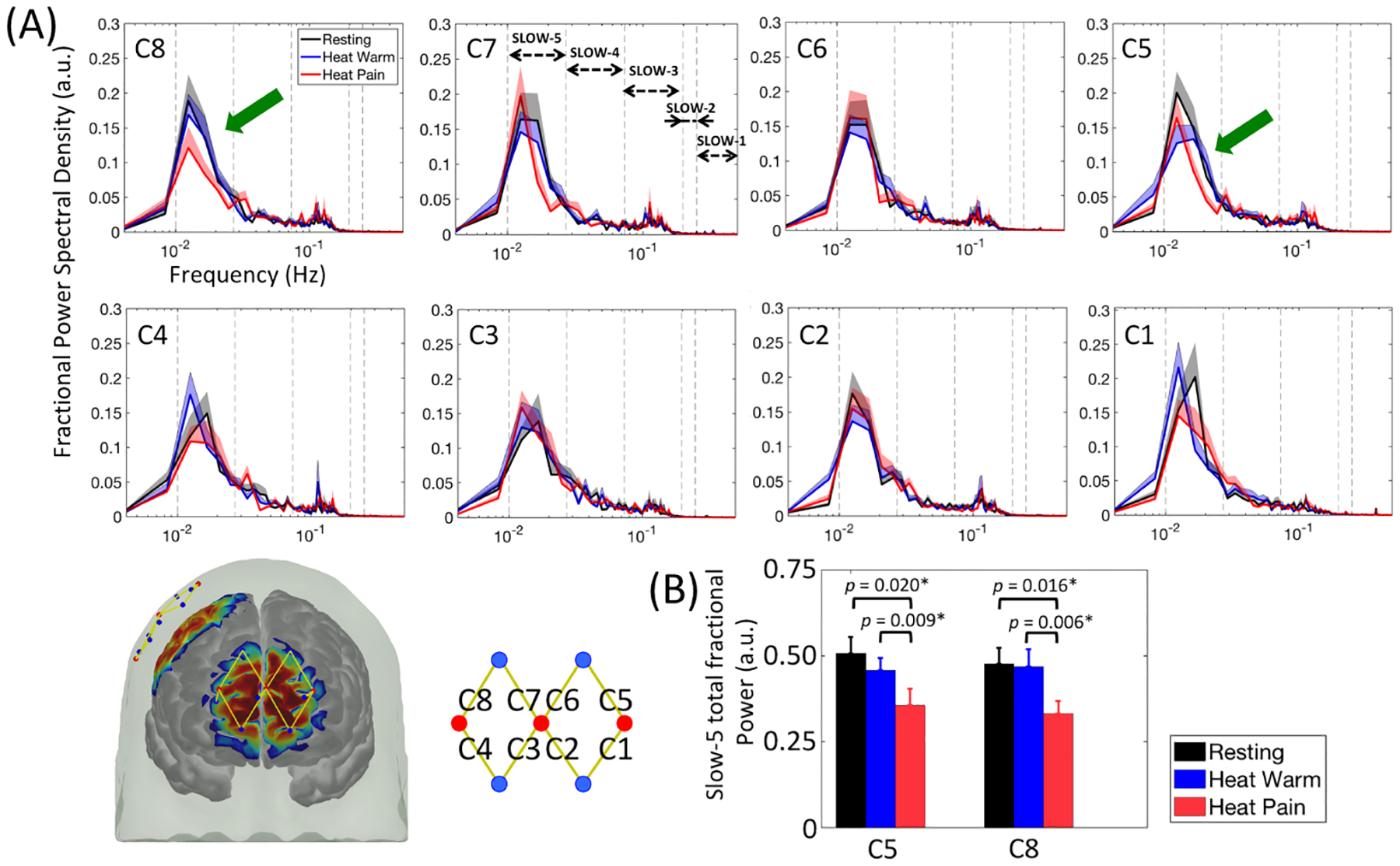
Power spectral density analysis of frontopolar cortex signals during ongoing innocuous (heat warm) and noxious (heat pain) stimuli in healthy awake volunteers. (A) Reductions in slow-5 band fractional power were identified in C5 and C8 (green arrows). Errorbars indicate the standard error of the mean. (B) Bar plots and the p-values from two-tailed paired t-tests of the slow-5 fractional power values for C5, C8 and an average of C5 and C8. Significant p-values after false discovery rate correction are marked with asterisks.
Within-session comparison (dynamic analysis):
We further explored the dynamic nature of the low frequency oscillation power changes by plotting the time course of slow-5 fractional power within each of the three sessions. In the same two channels, i.e., C5 and C8 (Fig. 4A), we observed a notable decrease in slow-5 fractional power from around 100s following the induction of ongoing noxious heat stimulation. Such reduced slow-5 fractional power was maintained for the entire 5-min stimulation period, and gradually went back to the normal after the withdrawal of stimulus at 400s. Statistical significance was obtained from two-tailed paired t-tests comparing the slow-5 fractional power levels during the stimulation period and the pre-stimulation resting period for channel C2 and C8 after false discovery rate correction (Fig. 4B, C2 noxious heat session, slow-5 total fractional power during pre-stimulation resting: 0.415 ± 0.118, during ongoing noxious heat stimulation: 0.309 ± 0.132, Cohen’s d = 0.66, p = 0.023 and 95% CI of the difference = [0.017 0.195]; C8 noxious heat session, slow-5 total fractional power during pre-stimulation resting: 0.375 ± 0.136, during ongoing noxious heat stimulation: 0.293 ± 0.090, Cohen’s d = 0.70, p = 0.017 and 95% CI of the difference = [0.017 0.146]). The dynamic slow-5 fractional power during the resting state session and the innocuous heat session were oscillating in most cases, with no clear trend of decrease corresponding to the stimulation paradigm. Interestingly, the slow-5 band of C8 showed a trend of fractional power increase that was temporally synchronized with innocuous heat stimulation. However, two-tailed t-tests revealed that such increase was non-significant (p = 0.057). The dynamic frequency power time courses of the other frontopolar cortex channels mostly showed similar oscillating patterns, however no statistical significance was found in the power changes following the stimulation period (see Supplementary Fig. S1).
Figure 4.
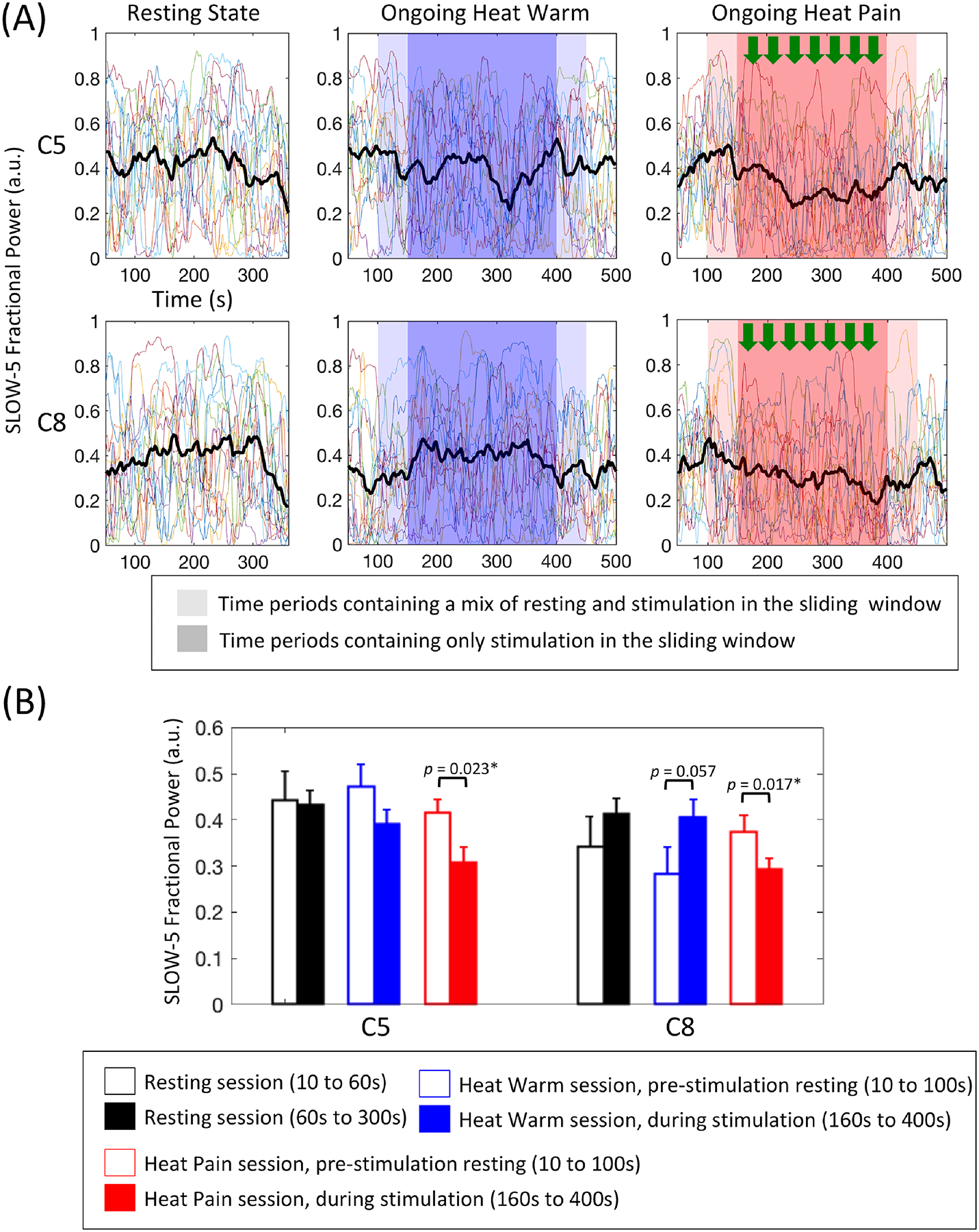
Dynamic frequency analysis results of within-session slow-5 fractional power changes. (A) The slow-5 fractional power timecourses of the resting state session, ongoing innocuous heat stimulation session and ongoing noxious heat stimulation session of all participants (individual curves) and the mean changes averaged across participants (black bold curves). Decreases in slow-5 fractional power were seen following the induction of ongoing noxious stimuli (green arrows). (B) Bar plots and p-values comparing the slow-5 fractional power level during the stimulation with their initial pre-stimulation levels. Significant p-values after false discovery rate correction are marked with asterisks. Errorbars show the standard error of the mean.
Clear functional near infrared spectroscopy signals with no motion artifact were obtained from the right somatosensory area in eight participants. The fractional power spectral density plots and the slow-5 power time courses of the eight somatosensory cortex channels revealed no significant changes in the power of low frequency bands during ongoing noxious stimulation compared with resting state or innocuous stimulation period, see Supplementary Fig. S2 and Fig. S3.
Evaluation of Ongoing Nociceptive Stimuli in Anesthetized Individuals undergoing Knee Surgery:
To demonstrate the quality of data collected in the operating room and surgery procedures, Fig. 5A shows an example of pre-processed oxygenated hemoglobin concentration change traces from a medial frontopolar cortex channel (C3) in a patient undergoing knee arthroscopy (without nerve block, Patient 5) and another patient undergoing anterior cruciate ligament reconstruction surgery (with nerve block, Patient 4). For each patient, we recorded the onset and offset time of major invasive surgical events, including incision, soft tissue removal, surgical hammering, microfracture drilling, ligament replacement (in anterior cruciate ligament reconstruction surgery) and suture. As noted above, functional near infrared spectroscopy signals collected from somatosensory cortex channels were generally noisy due to poor scalp-optode contact and hair contamination. Moreover, somatosensory cortex results in healthy awake individuals suggested no significant frequency power changes associated with ongoing heat stimulation. Therefore, we focused on the analysis of frontopolar cortex signals in the evaluation of ongoing nociceptive inputs during surgery.
Figure 5.
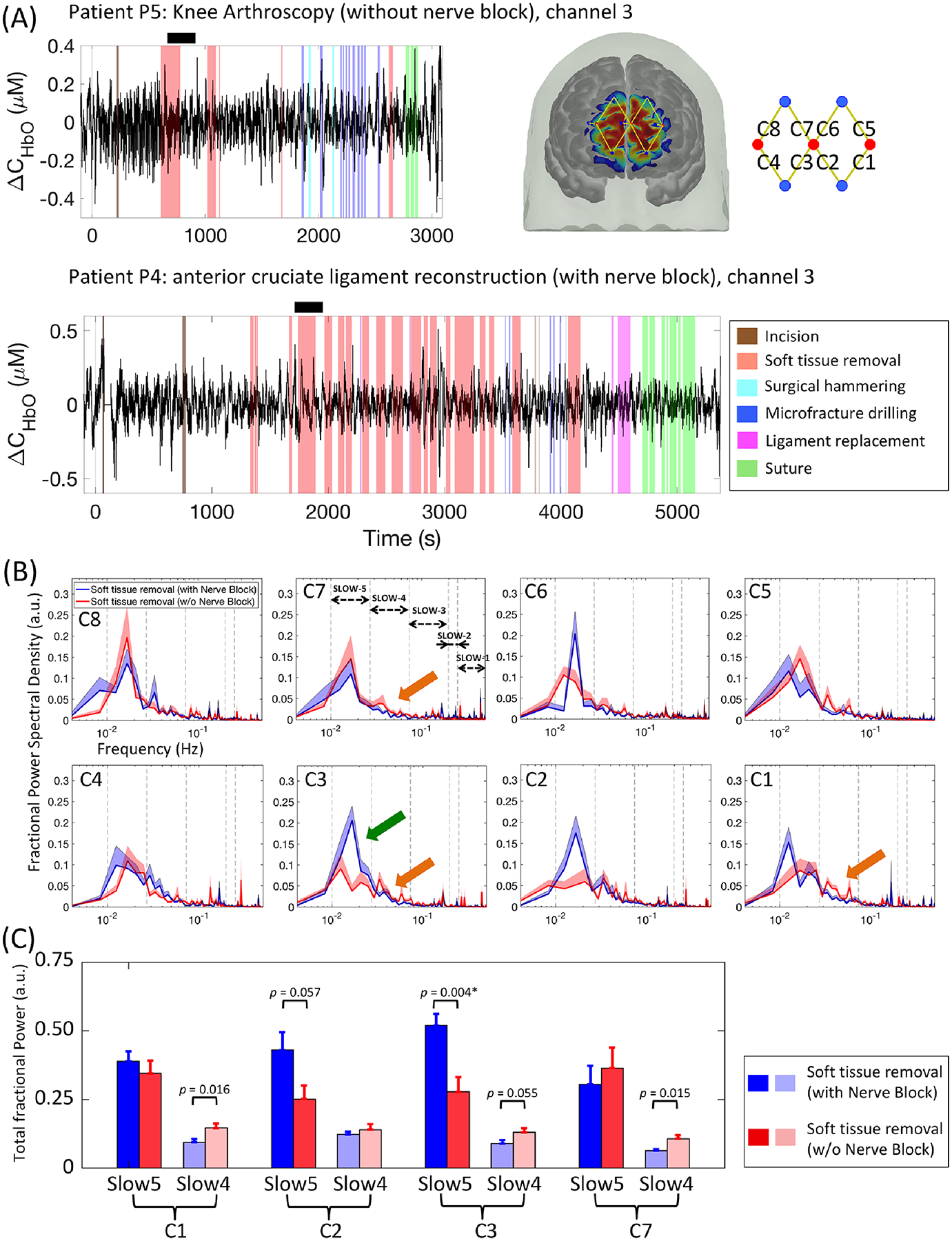
Power spectral density analysis of frontopolar cortex signals during the first removal of soft tissue in surgical patients under general anesthesia. (A) Demonstrations of functional near infrared spectroscopy signal quality (oxygenated hemoglobin concentration changes) and surgical procedures in two example patients: P5 (above) and P4 (below). (B) Patients without additional nerve block showed significant lower slow-5 fractional power compared with those with nerve block in C2 and C3 (green arrows). Interestingly, increased fractional power of slow-4 band was also noticed in patients without nerve block in C1, C3 and C7. Errorbars show the standard error of the mean. (C) Bar plots and the p-values from two-tailed two sample t-tests of the slow-5 and slow-4 fractional power values. Significant p-values after false discovery rate correction are marked with asterisks.
Between-group comparison (power spectral density analysis):
Considering the variability in surgical duration and procedures between the two surgery subgroups, we generated the fractional power spectral density plots of the same 4-min period (i.e., following the first major soft tissue removal event) in each patient (Fig. 5B, see methods section above). Two-tailed two-sample t-tests revealed significantly lower fractional power in slow-5 band of the medial frontopolar cortex channel C3 in patients without nerve block compared to patients with pre-conducted nerve block (Fig. 5C, anterior cruciate ligament reconstruction patients receiving additional nerve block: slow-5 total fractional power during soft tissue removal: 0.520 ± 0.113; knee arthroscopy patients receiving only standard anesthesia: slow-5 during soft tissue removal: 0.277 ± 0.130; for patients with vs. without nerve block Cohen’s d = 2.01, p = 0.004, 95% CI of the difference = [0.095 0.391]). Interestingly, although not a primary focus of this study, patients without nerve block also exhibited higher fractional power in slow-4 band in channels C1 and C7 (C1, patients with additional nerve block: slow-4 during soft tissue removal: 0.205 ± 0.063, patients with only standard anesthesia slow-4 during soft tissue removal: 0.321 ± 0.085, for patients with vs. without nerve block Cohen’s d = 1.58, p = 0.016, 95% CI = [−0.207 −0.026]; C7, patients with additional nerve block slow-4: 0.137 ± 0.042, patients with standard anesthesia slow-4: 0.232 ± 0.073, for patients with vs. without nerve block Cohen’s d = 1.61, p = 0.015, 95% CI = [−0.166 −0.023]). The analysis on other low frequency bands (slow-3, slow-2, and slow-1) did not reveal significant differences between subgroups.
Comparison of fractional power response function to surgical events (dynamic analysis):
Fig. 6A takes channel C3 as an example and shows the slow-5 and slow-4 dynamic fractional power change time courses during the entire surgery of the same two patients depicted in Fig. 5A. From Fig. 6, many of the recorded invasive surgical events were seen to be associated with a decrease in the slow-5 fractional power. However, the amplitude of the changes seemed to be generally larger in patients without nerve block (e.g. patient 5) than in patients with nerve block (patient 4). This was confirmed by the extracted fractional power response function from simple averaging of the temporal changes of all recorded surgical events (Fig. 6B). In patients with no nerve block, channel C3 showed a statistically significant drop (p < 0.05 after false discovery rate correction) in slow-5 fractional power mostly from 129s to 187s in the response function (corresponding to the real time oxygenated hemoglobin concentration changes from 79s to 187s as a Fast Fourier Transform window size of 50s was used) compared with the fractional power level at t = 0 in the response function whose value was generated with the oxygenated hemoglobin concentration changes of the 50s window preceding the surgical event onset (C3, normalized slow-5 total fractional power at t = 0 of the fractional power response function: 0.017 ± 0.010, slow-5 between 129 s and 187 s of the response function: −0.235 ± 0.115, for slow-5 normalized fractional power during baseline vs. responding time Cohen’s d = 2.16, p = 0.003, 95% CI of the difference = [0.129 0.375]). Such trend of decreases of slow-5 fractional power was also seen in channels C6 and C7 (with no statistical significance after false discovery rate correction) but was not present in any of the channels from the patients with pre-conducted nerve block. In fact, tests between the two surgery subgroups revealed significantly lower slow-5 fractional power in C3 from 124 s to 168 s of the response function in the no nerve block subgroup compared with the nerve block subgroup (C3, patients with standard anesthesia slow-5 normalized total fractional power between 124 s and 168 s of the fractional power response function: −0.219 ± 0.113, patients with nerve block slow-5 between 124 s and 168 s of the response function: −0.090 ± 0.145, for slow-5 normalized fractional power during responding time between the two subgroups Cohen’s d = 2.34, p = 0.002, 95% CI of the difference = [0.147 0.469]). On the other hand, while some large increases in slow-4 fractional power following surgical events could be seen particularly in channels C3 and C7 of the subgroup without nerve block, statistical analysis revealed no significant difference at a false discovery rate-corrected p < 0.05 in pre- vs. post-onset periods or between subgroups (Supplementary Fig. S4 and S5).
Figure 6.
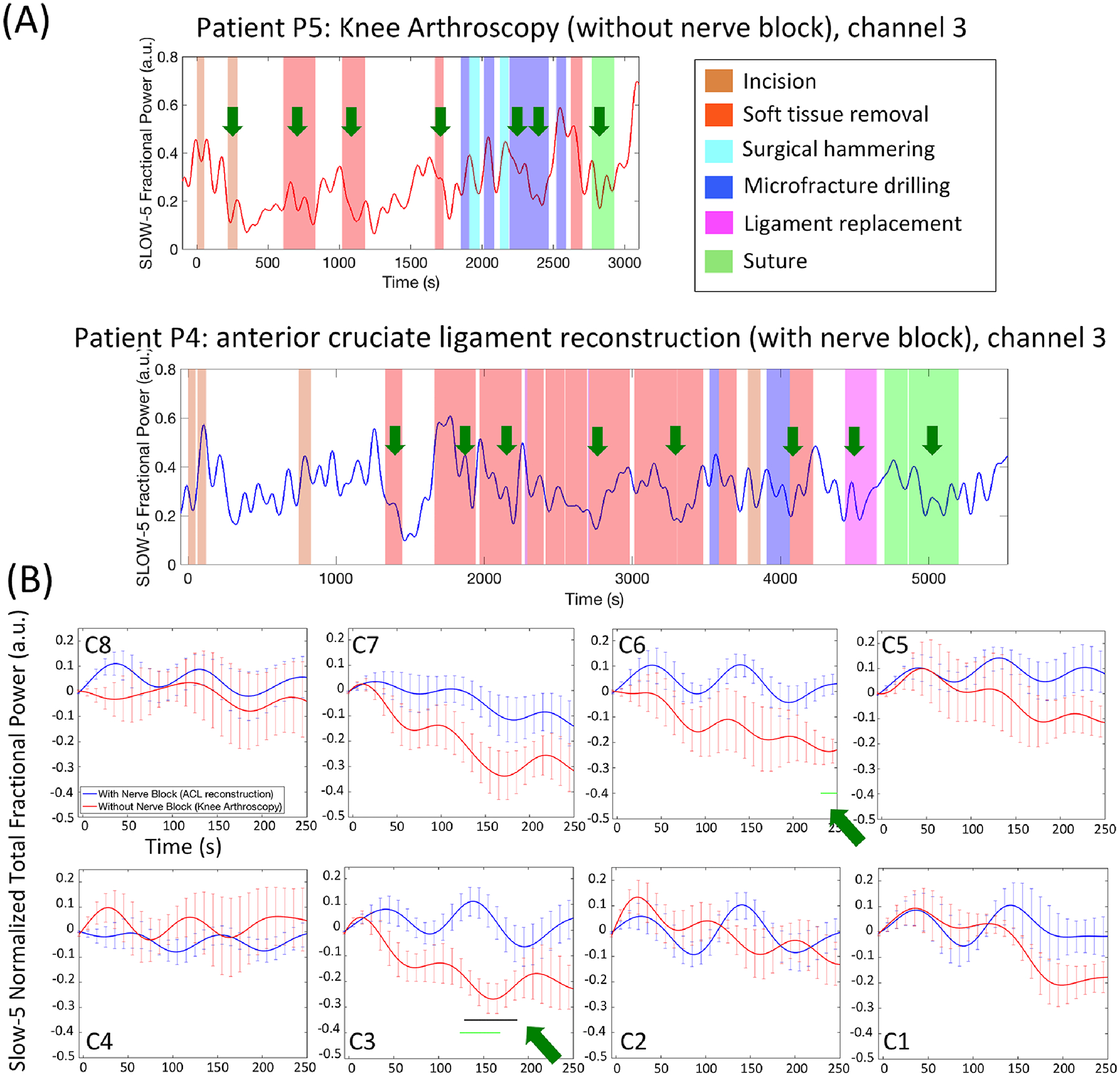
Dynamic frequency analysis of frontopolar cortex signals in anesthetized patients. (A) The slow-5 fractional power timecourses during the entire surgery in the two same example patients as in Fig.5A. Reductions in the slow-5 fractional power were seen following recorded invasive surgical procedures (green arrows). (B) Fractional power response function to recorded invasive surgical events in each frontopolar cortex channel. The response functions were first averaged across all recorded events of a single patient then across all patients. The errorbars indicate standard error of the mean. Three channels (C3, C6 and C7) showed significant results. The black horizontal curves depict the time points where slow-5 fractional power was significantly lower than the initial zero point (t = 0) of the same patient (p < 0.05, two-tailed paired t-tests after false discovery rate correction), while the green horizontal curves depict the time points where slow-5 fractional power was significantly lower in the no nerve block patient subgroup compared with the nerve block subgroup (p < 0.05, two-tailed two-sample t-tests after false discovery rate correction).
Summary of Results
This study evaluated ongoing pain/nociception with functional near infrared spectroscopy in healthy fully conscious volunteers (Group 1) and also ongoing nociceptive inputs in unconscious anesthetized patients (Group 2). In awake/conscious individuals, ongoing noxious heat produced reductions in the fractional power of slow-5 oscillations over the frontopolar cortex compared with pre-stimulation resting state or ongoing innocuous heat. Similar spectral changes were observed for ongoing nociceptive inputs associated with invasive surgical procedure in anesthetized/unconscious patients undergoing knee surgery. Of interest, a regional nerve block attenuated the slow-5 fractional power decrease in the frontopolar cortex. Using dynamic frequency analysis, we generated time courses and response functions of slow-5 fractional power change during ongoing stimulation in both groups, highlighting the potential of our method to be used for real time evaluation of ongoing pain/nociception.
Discussion
Low frequency oscillation fractional power changes associated with ongoing nociception
The fractional power changes in low frequency hemodynamic oscillations were associated with ongoing pain/nociception for a number of reasons: (1) Slow-5 fractional power decrease over frontopolar cortex were consistent with previous work using functional magnetic resonance imaging studying ongoing acute pain19 and chronic pain including back pain12, limb pain16,18, diabetic neuropathic pain13, trigeminal neuralgia17,37, postherpetic neuralgia14 and migraine15. These studies reported similar power reductions in a low frequency sub-band normally defined within 0.01–0.06 Hz, and the reduction amplitude was correlated with the patient’s pain ratings or other clinical measures14,15,18; (2) Awake volunteer data showed that the loss of fractional power in frontopolar cortex slow-5 signals compared with resting state was only present during ongoing heat pain stimulation; (3) A similar slow-5 fractional power decrease was observed in anesthetized patients undergoing invasive surgical procedures which elicited ongoing nociceptive inputs through direct nerve damage and activation of peripheral nociceptors10. Such alterations in slow-5 fractional power were attenuated by regional peripheral nerve block to diminish afferent nociceptive traffic. These pieces of evidence supported our hypothesis that the observed power reduction was related to altered brain functions associated with ongoing nociceptive inputs. However, it should also be noted that the specificity of the signal to ongoing nociception remains unclear.
Nociceptive processing in conscious vs. unconscious state
The effect of general anesthesia on brain functioning and pain-related networks is far from been fully understood. Depending on the anesthetic agent, dose and induction methods (inhalational or intravenous), commonly reported brain functional changes during loss of consciousness included attenuated response to sensory stimuli in primary and association cortices, break-down of frontoparietal connections, and reduced (but not eliminated) within- and inter-resting state network connectivity strengths38. The preserved networks were reported to be generally low-level sensory networks, while controversial evidence was collected for the default mode network in the literature39–41. Little data has been collected on brain processing of nociceptive inputs in an unconscious vs. conscious state. Using positron emission tomography, Hofbauer et al. evaluated the cerebral blood flow response to brief (5s) warm and mildly painful heat stimuli during propofol-induced loss of consciousness, and reported retained activations in the sensorimotor network but dramatically reduced response in the medial pain system such as the anterior cingulate cortex which is considered to modulate the affective-motivational components of pain43. Interestingly, a recent study4 utilized an increased intensity of the brief noxious stimulus comparable with surgical stimulations, and reported similar patterns of brain activations under deep general anesthesia (by propofol) to those in awake state. Specifically, strong responses in the commonly acknowledged “nociception matrix” or pain connectome were observed including sensorimotor area, insula, anterior cingulate cortex and medial prefrontal regions. In this study, we observed a reduction of low frequency hemodynamic oscillation power in the frontopolar cortex associated with ongoing nociception in both awake and anesthetized states. This seemed to support that the frontopolar cortex functional dynamics may be altered by intense ongoing nociceptive inputs in anesthetized patients in a way similar to awake volunteers experiencing ongoing pain. However, the power decrease was located in distinct frontopolar cortex subregions, which potentially implied differences in functions or connectivity patterns in response to nociception within the frontopolar cortex (i.e. lateral vs. medial as noted in our previous review30) under altered level of consciousness or anesthetic/drug effects. Future studies with matched subject characteristics (e.g. age, gender) and higher spatial resolution imaging techniques are needed to elucidate the spatial pattern of responses to nociception in anesthetized states.
Interpretation of slow-5 power decrease during ongoing nociception
In both awake individuals and anesthetized patients, the reduction of frontopolar cortex fractional power during ongoing noxious stimuli was seen to be specific to the slow-5 frequency range (0.010–0.027Hz). The definition of the sub-bands was adapted from previous electrophysiological data analysis. Center frequencies of the sub-bands follow an arithmetic progression on the natural logarithmic scale44. The sub-bands are likely generated by distinct brain oscillators with specific physiological mechanisms, and higher frequency oscillators were usually more localized while slow oscillators could involve distant brain areas44. Likewise, it may be that the lower frequency hemodynamic signals reflect the regulation of general neuronal excitability and the coordination of distinct large-scale brain networks, whereas the high frequency components (e.g. > 0.1Hz) mediate local functions of the brain45. Indeed, decreases in cortical low frequency oscillation power during persistent pain were often associated with reductions in functional connectivities13,19,37. Therefore, the slow-5 signal power decrease over the frontopolar cortex (but not in the contralateral somatosensory area) may suggest a disruption of the co-functioning among distant brain regions particularly related to the dysfunction of default mode network19 or cognitive46 processing during ongoing nociceptive stimuli. From the CNS, such changes might also be induced by parallel central sensitization that, as we have reported15, results in increased disinhibition of thalamocortical projections36.
Capturing ongoing nociception under general anesthesia
The need for a signal that can provide an objective report of the analgesic state during surgery may be more important than previously considered in the context of ongoing nociceptive processing regarded as “subclinical or unconscious pain”. Prior studies evaluating nociception level and analgesia efficacy under anesthesia usually employed autonomic signals including heart rate, blood pressure, pupil diameter and skin conductance. In this work, we propose a new metric based on functional near infrared spectroscopy-measured hemodynamic signal in the CNS. However, as discussed, ongoing nociceptive transmission may engage both nociceptive specific processes and possibly more complex interaction of “cognitive” or “emotional” circuits47 during the unconscious state. Practical use of the frontopolar cortex slow-5 signal to capture ongoing nociception may require future work for extensive validation of the signal with wider range of stimulus types or clinical conditions, the evaluation of its sensitivity and the specificity profile, as well as the development of analytical methods that could enable more online processing of signal dynamic features.
Caveats
(1) Nociception vs. Pain: We obtained similar frequency signals in response to ongoing pain stimulus in awake volunteers and to surgical events in anesthetized patients. Whether or not the signal in the latter case truly reflected nociceptive processing in an unconscious state could only be speculated (e.g., from sensitivity to analgesic modulation) due to the lack of patient self-report. (2) Study participants: The participants in those two groups were not matched for age or gender, which prevented quantitative comparison of the hemodynamic signals between groups. One way around this issue would be to have surgical patients undergo ongoing heat stimuli prior to surgery, notwithstanding operating room requirements. (3) Sex differences: We omitted specific measures of sex differences48,49. Sex differences were not considered, as we believe they do not contribute to the main theme of the study: Can one measure ongoing nociception under anesthesia? The healthy awake group only provided a metric for ongoing pain in a manner where the stimulus and the subjective responses could be correlated. Although subjective measures could not be obtained from patients under general anesthesia, we could however, compare the brain response to an ongoing noxious stimulus that should match or at least parallel same signal features from awake individuals. While future studies should explore sex and other variables, such studies in anesthetized patients would not provide insights into the nature of the “on” (noxious stimuli activating the brain) or “off” (full analgesic control of afferent nociceptive activity) states. It may be that factors such as sex are more prone to having “on” signals under anesthesia, and therefore these studies may define risk profiles for the anesthetic/surgical event. (4) Sample Size and Statistical Inferences: The sample size is small (N=15 for healthy volunteers and N=13 for surgical patients). This led to difficulties in the statistical inferences of reported effects as they might be driven by confounding factors. A larger sample size is required to further define equivalence (similarities/differences) in the signal. (5) Pre-defined effect size: As a exploratory study, a minimum clinically meaningful effect size was not pre-defined which might weaken our interpretation of the reported differences between sessions and participant groups. (6) Anesthetic Issues: General anesthesia was provided using an inhalational agent (sevoflurane) with intermittent additions of opioid analgesics (fentanyl). The effect of anesthesia or pharmacologic agents on cortical hemodynamics and nociceptive processing was not modeled. Instead, we employed a short separation approach to remove global physiological changes32. Moreover, our work studying the effect of morphine on cortical pain response reported that opioids do not provide complete loss of nociceptive signal31. Under surgery, the nature of tissue damage is likely to be a more potent nociceptive driver. Many gaseous anesthetics, including sevoflurane, have little analgesic effect50.
Conclusion
We observed reductions in the fractional power of slow-5 band in the frontopolar cortex following both ongoing heat pain stimulation in healthy awake volunteers and continued invasive surgical events in anesthetized patients. These changes were seen to be specific to noxious sensory stimuli in healthy awake volunteers and be modulated by analgesic procedures (nerve block) in surgical patients. A surrogate measure of ongoing nociception during surgery may allow for enhanced analgesia during general anesthesia for surgery.
Supplementary Material
Funding Statement:
This study was supported by the National Institutes of Health and the National Institute of General Medical Sciences Grants NIH-NIGMS 1-R01-GM122405 (DB), the Mayday Fund, New York (DB), the Mayday/Herlands Chair of Pain Systems Neuroscience (DB), and the Anesthesia Research Distinguished Trailblazer Award 2018-2019 (KP).
Footnotes
Prior Presentations: Part of the data used in this study were presented at the International Association for the Study of Pain: World Congress, Boston, USA, September 2018 and fNIRS 2018, Tokyo, Japan, October 2018.
Conflicts of Interest: All authors declare no conflict of interest.
References
- 1.Alkire MT, Hudetz AG, Tononi G: Consciousness and Anesthesia. Science 2008; 322:876–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tasbihgou SR, Vogels MF, Absalom AR: Accidental awareness during general anaesthesia – a narrative review. Anaesthesia 2018; 73:112–22 [DOI] [PubMed] [Google Scholar]
- 3.Kussman BD, Aasted CM, Yücel MA, Steele SC, Alexander ME, Boas DA, Borsook D, Becerra L: Capturing Pain in the Cortex during General Anesthesia: Near Infrared Spectroscopy Measures in Patients Undergoing Catheter Ablation of Arrhythmias. PLOS ONE 2016; 11:e0158975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lichtner G, Auksztulewicz R, Velten H, Mavrodis D, Scheel M, Blankenburg F, Dincklage F von: Nociceptive activation in spinal cord and brain persists during deep general anaesthesia. Br J Anaesth 2018; 121:291–302 [DOI] [PubMed] [Google Scholar]
- 5.Zhao F, Williams M, Bowlby M, Houghton A, Hargreaves R, Evelhoch J, Williams DS: Qualification of fMRI as a biomarker for pain in anesthetized rats by comparison with behavioral response in conscious rats. NeuroImage 2014; 84:724–32 [DOI] [PubMed] [Google Scholar]
- 6.Fischer MJM, Leffler A, Niedermirtl F, Kistner K, Eberhardt M, Reeh PW, Nau C: The General Anesthetic Propofol Excites Nociceptors by Activating TRPV1 and TRPA1 Rather than GABAA Receptors. J Biol Chem 2010; 285:34781–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matta JA, Cornett PM, Miyares RL, Abe K, Sahibzada N, Ahern GP: General anesthetics activate a nociceptive ion channel to enhance pain and inflammation. Proc Natl Acad Sci U S A 2008; 105:8784–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Woolf CJ: Central sensitization: Implications for the diagnosis and treatment of pain. Pain 2011; 152:S2–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Williams DA: Phenotypic Features of Central Sensitization. J Appl Biobehav Res 2018; 23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Borsook D, Kussman BD, George E, Becerra LR, Burke DW: Surgically induced neuropathic pain: understanding the perioperative process. Ann Surg 2013; 257:403–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lascelles BDX, Waterman AE, Cripps PJ, Livingston A, Henderson G: Central sensitization as a result of surgical pain: investigation of the pre-emptive value of pethidine for ovariohysterectomy in the rat. Pain 1995; 62:201–12 [DOI] [PubMed] [Google Scholar]
- 12.Baliki MN, Baria AT, Apkarian AV: The cortical rhythms of chronic back pain. J Neurosci Off J Soc Neurosci 2011; 31:13981–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cauda F, Sacco K, Duca S, Cocito D, D’Agata F, Geminiani GC, Canavero S: Altered resting state in diabetic neuropathic pain. PloS One 2009; 4:e4542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gu L, Hong S, Jiang J, Liu J, Cao X, Huang Q, Zeng X, Zhou F, Zhang D: Bidirectional alterations in ALFF across slow-5 and slow-4 frequencies in the brains of postherpetic neuralgia patients. J Pain Res 2018; 12:39–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hodkinson DJ, Wilcox SL, Veggeberg R, Noseda R, Burstein R, Borsook D, Becerra L: Increased Amplitude of Thalamocortical Low-Frequency Oscillations in Patients with Migraine. J Neurosci Off J Soc Neurosci 2016; 36:8026–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Malinen S, Vartiainen N, Hlushchuk Y, Koskinen M, Ramkumar P, Forss N, Kalso E, Hari R: Aberrant temporal and spatial brain activity during rest in patients with chronic pain. Proc Natl Acad Sci 2010; 107:6493–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Y, Xu C, Zhai L, Lu X, Wu X, Yi Y, Liu Z, Guan Q, Zhang X: Spatial-temporal signature of resting-state BOLD signals in classic trigeminal neuralgia. J Pain Res 2017; 10:2741–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou F, Gu L, Hong S, Liu J, Jiang J, Huang M, Zhang Y, Gong H: Altered low-frequency oscillation amplitude of resting state-fMRI in patients with discogenic low-back and leg pain. J Pain Res 2018; 11:165–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alshelh Z, Marciszewski KK, Akhter R, Di Pietro F, Mills EP, Vickers ER, Peck CC, Murray GM, Henderson LA: Disruption of default mode network dynamics in acute and chronic pain states. NeuroImage Clin 2017; 17:222–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duff EP, Johnston LA, Xiong J, Fox PT, Mareels I, Egan GF: The power of spectral density analysis for mapping endogenous BOLD signal fluctuations. Hum Brain Mapp 2008; 29:778–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kannurpatti SS, Biswal BB, Kim YR, Rosen BR: Spatio-temporal characteristics of low-frequency BOLD signal fluctuations in isoflurane-anesthetized rat brain. NeuroImage 2008; 40:1738–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu T-L, Mishra A, Wang F, Yang P-F, Gore JC, Chen LM: Effects of isoflurane anesthesia on resting-state fMRI signals and functional connectivity within primary somatosensory cortex of monkeys. Brain Behav 2016; 6:e00591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kiviniemi VJ, Haanpää H, Kantola J-H, Jauhiainen J, Vainionpää V, Alahuhta S, Tervonen O: Midazolam sedation increases fluctuation and synchrony of the resting brain BOLD signal. Magn Reson Imaging 2005; 23:531–7 [DOI] [PubMed] [Google Scholar]
- 24.Liu X, Lauer KK, Ward BD, Roberts C, Liu S, Gollapudy S, Rohloff R, Gross W, Chen G, Xu Z, Binder JR, Li S-J, Hudetz AG: Propofol attenuates low-frequency fluctuations of resting-state fMRI BOLD signal in the anterior frontal cortex upon loss of consciousness. NeuroImage 2017; 147:295–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peng K, Yücel MA, Aasted CM, Steele SC, Boas DA, Borsook D, Becerra L: Using prerecorded hemodynamic response functions in detecting prefrontal pain response: a functional near-infrared spectroscopy study. Neurophotonics 2018; 5:011018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Becerra L, Aasted CM, Boas DA, George E, Yücel MA, Kussman BD, Kelsey P, Borsook D: Brain measures of nociception using near-infrared spectroscopy in patients undergoing routine screening colonoscopy. Pain 2016; 157:840–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lichtner G, Auksztulewicz R, Kirilina E, Velten H, Mavrodis D, Scheel M, Blankenburg F, Dincklage F von: Effects of propofol anesthesia on the processing of noxious stimuli in the spinal cord and the brain. NeuroImage 2018; 172:642–53 [DOI] [PubMed] [Google Scholar]
- 28.Rosenbaum D, Int-Veen I, Kroczek A, Hilsendegen P, Velten-Schurian K, Bihlmaier I, Fallgatter AJ, Ehlis A-C: Amplitude of low frequency fluctuations (ALFF) of spontaneous and induced rumination in major depression: An fNIRS study. Sci Rep 2020; 10:21520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen Y, Tang J, Chen Y, Farrand J, Craft MA, Carlson BW, Yuan H: Amplitude of fNIRS Resting-State Global Signal Is Related to EEG Vigilance Measures: A Simultaneous fNIRS and EEG Study. Front Neurosci 2020; 14:560878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peng K, Steele SC, Becerra L, Borsook D: Brodmann area 10: Collating, integrating and high level processing of nociception and pain. Prog Neurobiol 2018; 161:1–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peng K, Yücel MA, Steele SC, Bittner EA, Aasted CM, Hoeft MA, Lee A, George EE, Boas DA, Becerra L, Borsook D: Morphine Attenuates fNIRS Signal Associated With Painful Stimuli in the Medial Frontopolar Cortex (medial BA 10). Front Hum Neurosci 2018; 12:394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gagnon L, Perdue K, Greve DN, Goldenholz D, Kaskhedikar G, Boas DA: Improved recovery of the hemodynamic response in diffuse optical imaging using short optode separations and state-space modeling. NeuroImage 2011; 56:1362–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Obrig H, Neufang M, Wenzel R, Kohl M, Steinbrink J, Einhäupl K, Villringer A: Spontaneous low frequency oscillations of cerebral hemodynamics and metabolism in human adults. NeuroImage 2000; 12:623–39 [DOI] [PubMed] [Google Scholar]
- 34.Yang L, Yan Y, Wang Y, Hu X, Lu J, Chan P, Yan T, Han Y: Gradual Disturbances of the Amplitude of Low-Frequency Fluctuations (ALFF) and Fractional ALFF in Alzheimer Spectrum. Front Neurosci 2018; 12:975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zalesky A, Breakspear M: Towards a statistical test for functional connectivity dynamics. NeuroImage 2015; 114:466–70 [DOI] [PubMed] [Google Scholar]
- 36.Alshelh Z, Di Pietro F, Youssef AM, Reeves JM, Macey PM, Vickers ER, Peck CC, Murray GM, Henderson LA: Chronic Neuropathic Pain: It’s about the Rhythm. J Neurosci Off J Soc Neurosci 2016; 36:1008–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang Y, Mao Z, Pan L, Ling Z, Liu X, Zhang J, Yu X: Frequency-specific alterations in cortical rhythms and functional connectivity in trigeminal neuralgia. Brain Imaging Behav 2019; 13:1497–509 [DOI] [PubMed] [Google Scholar]
- 38.Hudetz AG: General Anesthesia and Human Brain Connectivity. Brain Connect 2012; 2:291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bonhomme V, Vanhaudenhuyse A, Demertzi A, Bruno M-A, Jaquet O, Bahri MA, Plenevaux A, Boly M, Boveroux P, Soddu A, Brichant JF, Maquet P, Laureys S: Resting-state Network-specific Breakdown of Functional Connectivity during Ketamine Alteration of Consciousness in Volunteers. Anesthesiology 2016; 125:873–88 [DOI] [PubMed] [Google Scholar]
- 40.Liu X, Li H, Luo F, Zhang L, Han R, Wang B: Variation of the default mode network with altered alertness levels induced by propofol. Neuropsychiatr Dis Treat 2015. doi: 10.2147/NDT.S88156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martuzzi R, Ramani R, Qiu M, Rajeevan N, Constable RT: Functional connectivity and alterations in baseline brain state in humans. NeuroImage 2010; 49:823–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hofbauer RK, Fiset P, Plourde G, Backman SB, Bushnell MC: Dose-dependent Effects of Propofol on the Central Processing of Thermal Pain. Anesthesiology 2004; 100:386–94 [DOI] [PubMed] [Google Scholar]
- 43.Sewards TV, Sewards MA: The medial pain system: Neural representations of the motivational aspect of pain. Brain Res Bull 2002; 59:163–80 [DOI] [PubMed] [Google Scholar]
- 44.Penttonen M, Buzsáki G: Natural logarithmic relationship between brain oscillators. Thalamus Relat Syst 2003; 2:145–52 [Google Scholar]
- 45.Chen JE, Glover GH: BOLD fractional contribution to resting-state functional connectivity above 0.1 Hz. NeuroImage 2015; 107:207–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cheng JC, Bosma RL, Hemington KS, Kucyi A, Lindquist MA, Davis KD: Slow-5 dynamic functional connectivity reflects the capacity to sustain cognitive performance during pain. NeuroImage 2017; 157:61–8 [DOI] [PubMed] [Google Scholar]
- 47.Hashmi JA, Baliki MN, Huang L, Baria AT, Torbey S, Hermann KM, Schnitzer TJ, Apkarian AV: Shape shifting pain: chronification of back pain shifts brain representation from nociceptive to emotional circuits. Brain J Neurol 2013; 136:2751–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bartley EJ, Fillingim RB: Sex differences in pain: a brief review of clinical and experimental findings. Br J Anaesth 2013; 111:52–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pieretti S, Di Giannuario A, Di Giovannandrea R, Marzoli F, Piccaro G, Minosi P, Aloisi AM: Gender differences in pain and its relief. Ann Ist Super Sanita 2016; 52:184–9 [DOI] [PubMed] [Google Scholar]
- 50.Tomi K, Mashimo T, Tashiro C, Yagi M, Pak M, Nishimura S, Nishimura M, Yoshiya I: ALTERATIONS IN PAIN THRESHOLD AND PSYCHOMOTOR RESPONSE ASSOCIATED WITH SUBANAESTHETIC CONCENTRATIONS OF INHALATION ANAESTHETICS IN HUMANS. Br J Anaesth 1993; 70:684–6 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


