Abstract
Purpose:
There is an urgent need to understand the biological factors contributing to the racial survival disparity among women with hormone receptor-positive (HR+), HER2− breast cancer. In this study, we examined the impact of PAM50 subtype on 10-year mortality rate in women with HR+, HER2− breast cancer by race.
Methods:
Women with localized, HR+, HER2− breast cancer diagnosed between 2002 to 2012 from two population-based cohorts were evaluated. Archival tumors were obtained and classified by PAM50 into four molecular subtypes (i.e., luminal A, luminal B, HER2-enriched, and basal-like). The molecular subtypes within HR+, HER2− breast cancers and corresponding 10-year mortality rate were compared between Black and Non-Hispanic White (NHW) women using Cox proportional hazard ratios and survival analysis, adjusting for covariates.
Results:
In this study, 318 women with localized, HR+, HER2− breast cancer were included—227 Black (71%) and 91 NHW (29%). Young Black women (age≤50) had the highest proportion of HR+,non-luminal A tumors (47%), compared to young NHW (10%), older Black women (31%), and older NHW (30%). Overall, women with HR+,non-luminal A subtypes had a higher 10-year mortality rate compared to HR+, luminal A subtypes after adjustment for age, stage and income (HR 4.21 for Blacks, 95% CI=1.74–10.18 and HR 3.44 for NHW, 95% CI=1.31–9.03). Among HR+, non-luminal A subtypes there was however no significant racial difference in 10-yr mortality observed (Black vs. NHW: HR 1.23, 95% CI=0.58–2.58).
Conclusion:
Molecular subtype classification highlights racial disparities in PAM50 subtype distribution among women with HR+, HER2− breast cancer. Among women with HR+, HER2− breast cancer, racial survival disparities are ameliorated after adjusting for molecular subtype.
Keywords: Breast cancer, Racial disparity, Molecular subtype, PAM50
Introduction:
Breast cancer is the most common cancer among women worldwide (1), with variable survival outcomes dependent on tumor biology and race (2, 3). Black women are 30% more likely to die from breast cancer than their Non-Hispanic White (NHW) counterparts, even after controlling for socioeconomic status (4). These racial disparities in breast cancer survival highlight the complex interplay between tumor biology, genomics, patterns of care, and socioeconomic factors (4, 5). Disparities research has largely focused on triple negative breast cancer (TNBC) given the higher prevalence among Black women, particularly those diagnosed at younger ages (2, 6–15). However, hormone receptor-positive (HR+) (i.e., estrogen receptor (ER) and/or progesterone receptor (PR)-positive), human epidermal growth factor receptor 2-negative (HER2−) breast cancer remains the dominant contributor to annual breast cancer deaths worldwide across all racial groups (16–18). Furthermore, even in randomized clinical trials of HR+, HER2− breast cancer patients (19), Black women had worse disease-free survival (DFS) and overall survival (OS) compared with NHW women, despite similar clinical and treatment patterns, which further suggests a biological basis for the racial survival disparity observed (19).
HR+, HER2− breast cancer is the most common immunohistochemical (IHC) subtype across all racial and ethnic groups (2, 18). Given the clinical and biologically heterogenous nature of HR+, HER2− breast cancer, gene expression profiling assays can further refine classification, provide prognostic information, and predict risk of late recurrence beyond standard IHC-classifications (20–23). The 50-gene molecular subtype signature (PAM50) is an assay used to further classify HR+, HER2− breast cancer into four molecular subtypes: luminal A, luminal B, HER2-enriched, and basal-like (24–26). Unlike luminal A tumors, non-luminal A tumors (i.e., luminal B, basal-like and HER2-enriched tumors) have worse survival outcomes (25, 27, 28). Luminal A tumors have a high expression of ER/PR and have the best prognosis due to increased sensitivity of these tumors to endocrine therapies and a naturally indolent course (21). Conversely, luminal B tumors have a lower expression of ER/PR, more aggressive clinical and biological features, and greater likelihood of later recurrences (21, 25). Basal-like HR+, HER2− tumors are thought to behave similar to TNBC with a high expression of ki67, and HER2-enriched tumors have been shown to behave like HER2-amplified tumors (25, 29).
In this study, we sought to determine the role of molecular subtype classification in bridging the racial survival disparity among women with HR+, HER2− breast cancer. We used the PAM50 subtype classification to compare the 10-year mortality rate among a diverse population-based cohort of women with localized, HR+, HER2− breast cancer stratified by PAM50 subtype and race. We hypothesized that racial differences in PAM50 subtype distribution may play a significant role in the racial survival disparity observed among women with HR+, HER2− breast cancer.
Methods:
Study Population
The current study is a pooled analysis of 318 women with localized HR+, HER2− breast cancer who were enrolled in two population-based cohorts: 1) Black Women: Etiology and Survival of Triple-negative Breast Cancers (BEST) Study; and 2) Southern Community Cohort Study (SCCS). The BEST study is a population-based effort consisting of Black women diagnosed with breast cancer ≤ age 50 from 2009 to 2012 retrospectively recruited from the Florida Cancer Registry (30). The SCCS is a prospective cohort study which enrolled 47,920 women aged 40–79 (68% Black) with no history of cancer treatment within 1 year of enrollment from 12 southeastern states between 2002 to 2009 (31). The majority (86%) of SCCS participants were enrolled at Community Health Centers (CHCs), which provide primary health and preventive services to medically underserved and low-income populations; whereas, the remaining participants were enrolled through mail-based general population sampling. Incident cancer cases for SCCS were identified annually through cohort linkage with the 12 state cancer registries (31). All participants in BEST and SCCS provided informed consent and were asked to complete an authorization for release of medical records and tumor samples for verification of clinical information and future analysis. Both study protocols were approved by the Institutional Review Boards at Vanderbilt University and the Department of Health for each statewide cancer registry.
Data collection
Self-reported data on race/ethnicity and annual household income were obtained from participants in both cohorts using a structured questionnaire at study enrollment. Participant and disease characteristics at diagnosis including age, disease stage, tumor size, node status, HR status, and HER2 amplification in the primary tumor were abstracted from state cancer registry data and/or obtained medical records. For SCCS participants, deaths were determined through the state cancer registries and the National Death Index. For BEST participants, deaths were determined through the TransUnion software, and date of death was confirmed and/or collected, where applicable. Overall mortality included death from any cause.
Tissue Sample and RNA Expression Analysis
RNA was extracted from formalin-fixed, paraffin-embedded (FFPE) tumor tissue archival blocks on all participants, to conduct expression profile studies. For BEST participants, breast cancer subtypes were based on the PAM50-based genomic signature using the NanoString nCounter platform through the commercially available Prosigna assay (26). For the SCCS, the PAM50 subtypes were calculated from RNA-sequencing expression data. The PAM50 signature uses the level of expression of 50 target genes plus eight constitutively expressed normalization genes to classify the breast tumors into four distinct molecular subtypes (i.e., luminal A, luminal B, HER2-enriched, and basal-like). Risk of recurrence (ROR) scores, generated through the PAM50 signature, were calculated for both BEST and SCCS participants from PAM50 and RNA-Sequencing data, respectively. This score, which ranges from 0–100, is used to estimate a patient’s probability of disease recurrence by comparing the gene expression profile of the tumor, relative to each of the four PAM50 molecular profiles to determine the degree of similarity (26, 32).
Statistical Analysis
The molecular subtypes within HR+, HER2− breast cancers and corresponding 10-year overall survival was compared between Black and NHW women. Racial differences by categorical variables, such as clinical characteristics and molecular subtypes, were assessed using Pearson chi-square test. A logistic regression model was used to estimate odds ratios (ORs), regressing molecular subtype (HR+,luminal A vs. HR+,non-luminal A) on population subgroups (age, race, stage, and income groups). All statistical tests were two-sided and considered significant at p < 0.05.
To assess differences in mortality across racial groups and breast cancer subtypes, we used univariate and multivariate analyses to compare 10-year mortality rates across the following groups: 1) NHW, HR+,luminal A; 2) NHW, HR+,non-luminal A; 3) Black, HR+,luminal A; and 4) Black, HR+,non-luminal A. Survival was assessed from date of initial diagnosis to date of death or at the 10-year follow-up. HR+,luminal A subtype was used as the reference group, given it is the subtype that shows highest survival, for comparison with HR+,non-Luminal A subtypes. Survival rates of luminal A and HR+,non-luminal A tumors stratified by race were estimated using Kaplan-Meier survival analysis. Multivariable Cox proportional hazard model was performed to account for the impact of potential confounding variables, including age of diagnosis, stage at diagnosis, and annual household income, along with an interaction term between race and subtype.
Results:
Study participants comprised 318 women with Stage I-III HR+, HER2− breast cancer, including 227 Black (71%) and 91 NHW women (29%), of which 64% were luminal A, 23% luminal B, 10% basal-like, and 3% HER2-enriched. Clinical and pathological characteristics among participants is summarized in Table 1.
Table 1:
Patient characteristics stratified by race/ethnicity
| Patient Characteristics | Total (N=318) |
BEST* (N=96) |
SCCS | |
|---|---|---|---|---|
| NHW (N=91) |
||||
| Age, years (%) | ||||
| ≤ 50 | 128 (40) | 96 (100) | 22 (17) | 10 (11) |
| > 50 | 190 (60) | - | 19 (83) | 81 (89) |
| Income, annual household (%) | ||||
| < $25,000 | 196 (64) | 34 (39) | 100 (76) | 62 (70) |
| ≥ $25,000 | 110 (36) | 53 (61) | 31 (24) | 26 (30) |
| Missing | 12 | 9 | - | 3 |
| Stage (%) | ||||
| I | 147 (52) | 42 (50) | 55 (49) | 50 (58) |
| II | 105 (37) | 33 (40) | 43 (38) | 29 (34) |
| III | 30 (11) | 8 (10) | 15 (13) | 7 (8) |
| Missing | 36 | 13 | 18 | 5 |
| PAM50 Subtype (%) | ||||
| HR+,luminal A | 203 (64) | 52 (54) | 85 (65) | 66 (73) |
| HR+,non-luminal A | 115 (36) | 44 (46) | 46 (35) | 25 (27) |
| Luminal B | 79 (23) | 27 (28) | 28 (21) | 18 (20) |
| Basal | 33 (10) | 15 (16) | 13 (10) | 5 (5) |
| HER2-enriched | 9 (3) | 2 (2) | 5 (4) | 2 (2) |
| ROR score, 0–100 (%) | ||||
| < 10 (low) | 81 (25) | 28 (29) | 26 (20) | 27 (30) |
| 10–62 (intermediate) | 155 (49) | 36 (38) | 69 (53) | 50 (55) |
| > 62 (high) | 2 (26) | 32 (33) | 36 (27) | 14 (15) |
BEST study only included Black women ≤ 50 years
Abbreviations: Non-Hispanic White (NHW), Risk of recurrence (ROR).
Young Black women (age ≤ 50) had the highest proportion of HR+,non-luminal A tumors (47%), compared to young NHW (10%), older Blacks (31%), and older NHW (30%). However, Black race was not associated with higher odds of having a HR+,non-luminal A subtype after adjusting for age, stage, and income (OR 1.18; p=0.60) (Table 2). Compared with NHW women, Black women had higher ROR scores (p=0.027) (Figure 1). After adjustment for race, age, and income, women with locally advanced breast cancer (i.e. Stage III) were 4.02 times more likely to have HR+,non-luminal A tumors (p = 0.001).
Table 2:
Multivariable Logistic Regression (HR+,Luminal A vs. HR+,Non-Luminal A)
| Patient Characteristics | HR+,Luminal A (N=203) |
HR+,Non-Luminal A (N=115) |
Adjusted Odds Ratios (95% CI) |
|---|---|---|---|
| Race and age, % | |||
| Age ≤ 50 | |||
| NHW | 9 (90) | 1 (10) | 1.0 |
| Black | 62 (53) | 56 (47) | 7.5 (0.9 – 65.1) |
| Age > 50 | |||
| NHW | 57 (70) | 24 (30) | 1.0 |
| Black | 75 (69) | 34 (31) | 0.9 (0.4 – 1.7) |
| Income, annual household (%) | |||
| < $25,000 | 125 (64) | 71 (36) | 1.0 |
| ≥ $25,000 | 68 (62) | 42 (38) | 1.11 (0.63 – 1.96) |
| Stage (%) | |||
| I | 110 (75) | 37 (25) | 1.0 |
| II | 59 (56) | 46 (44) | 2.38 (1.37 – 4.15) |
| III | 13 (43) | 17 (57) | 4.02 (1.73 – 9.36) |
Abbreviation: Non-Hispanic White (NHW)
Model is adjusted for stage at diagnosis (I, II, III) and annual household income (< $25,000, ≥ $25,000). Luminal A was used as the referent group.
Figure 1:
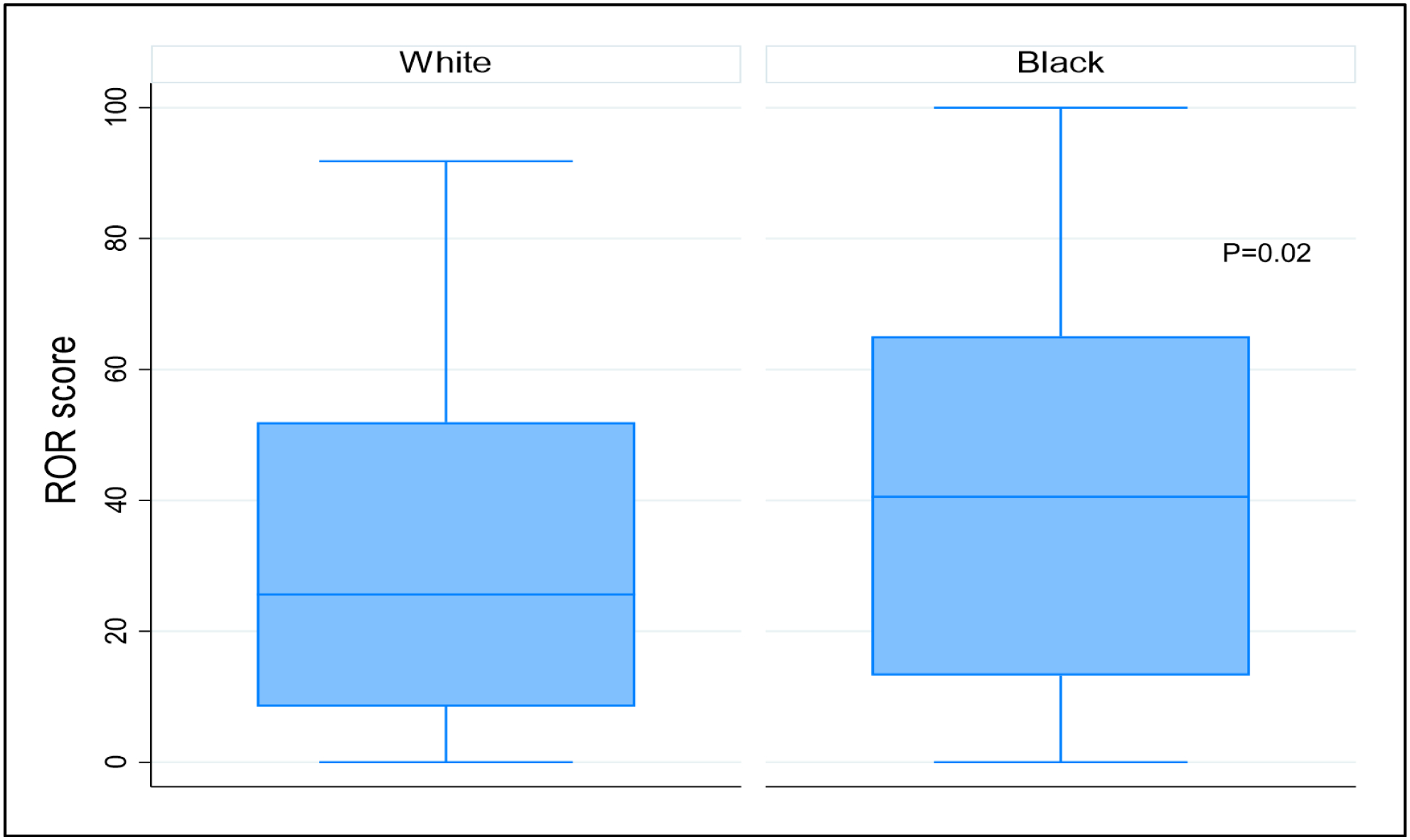
Risk of Recurrence (ROR) scores by Race
During the 10-year period of observation, study participants had 78% OS (70 deaths among 381 cases). Black women had similar OS (79%) compared to NHW women (76%) (p=0.6). Kaplan-Meier survival curves for OS by PAM50 subtype are presented in Figure 2. Among women with HR+, HER2− breast cancer, there was significant variation in OS (p = <0.001): luminal A (87%), luminal B (60%), basal-like (70%), and HER2-enriched (56%) (Figure 2). After stratification by race and PAM50 subtype, the “Black, luminal A” subgroup had the highest 10-year OS and the “NHW, HR+,non-luminal A” subgroup had the lowest 10-year OS (Figure 3). However, after controlling for potential confounders, the “Black, HR+,non-luminal A” group had a higher 10-year mortality rate with a hazard ratio of 4.21 (95% CI=1.74–10.18) compared to the “NHW, HR+,non-luminal A” group with a hazard ratio 3.44 (95% CI=1.31–9.03) (Table 3). Among HR+, non-luminal A subtypes there was however no significant racial difference in 10-yr mortality observed (Black vs. NHW: HR 1.23, 95% CI=0.58–2.58).
Figure 2:
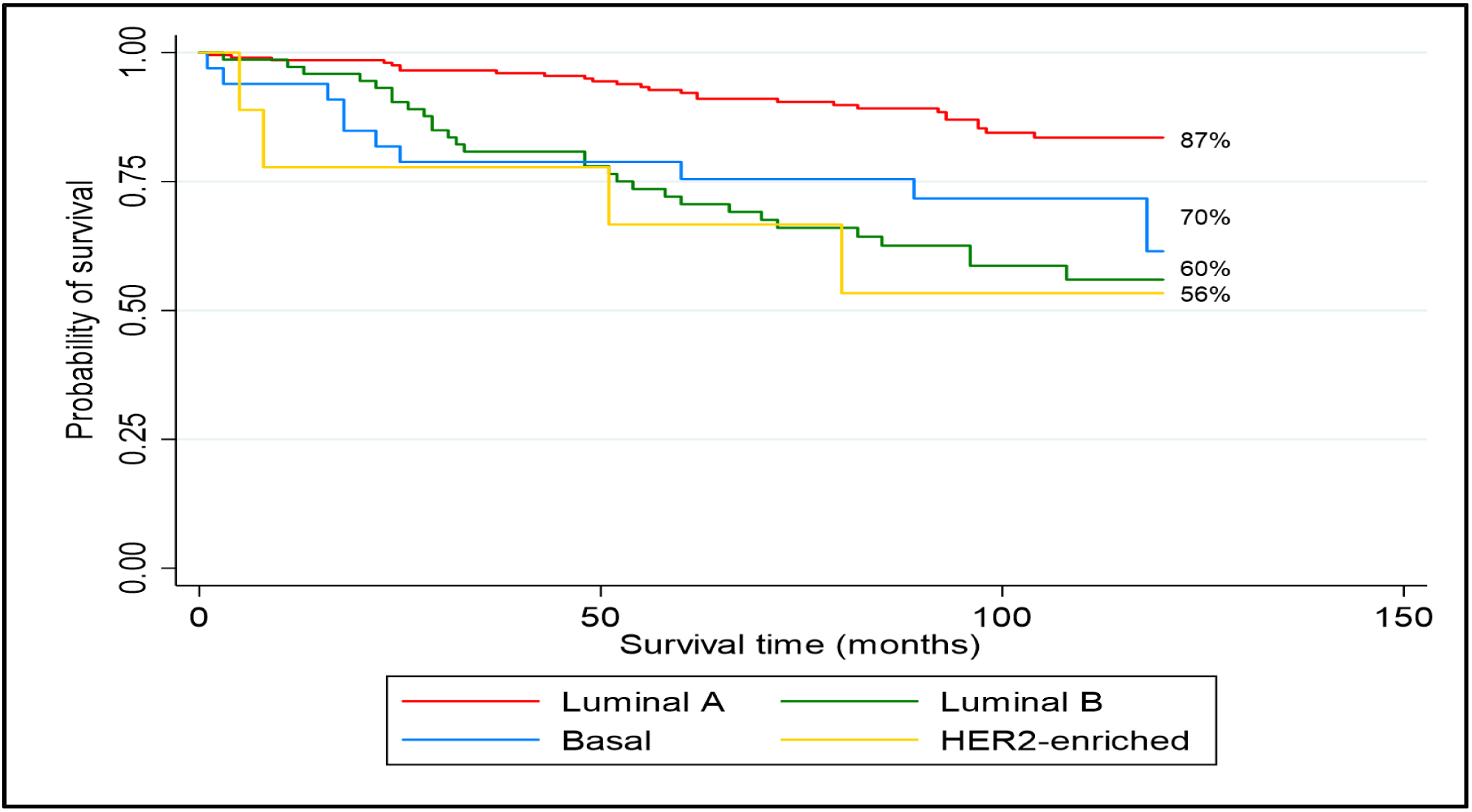
10-year Overall Survival by PAM50 subtype
Figure 3:
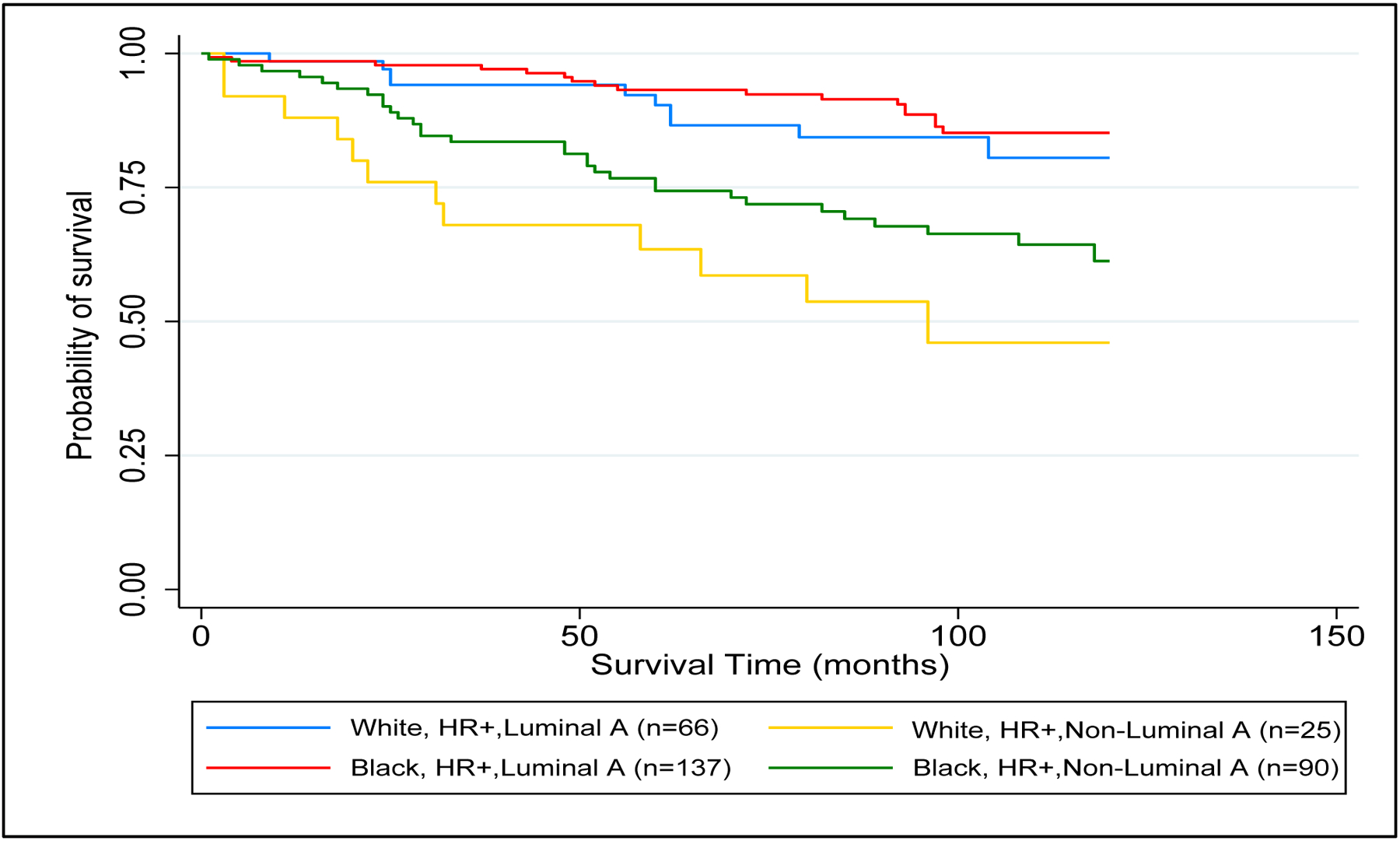
10-year Overall Survival by PAM50 subtype and Race
Table 3:
10-year overall mortality
| Patient Characteristics | Adjusted Hazards Ratio | 95% CI | P |
|---|---|---|---|
| Race/subtype classification | |||
| NHW, HR+,luminal A | 1.0 | - | |
| NHW, HR+,non-luminal A | 3.44 | 1.31–9.03 | 0.01 |
| Black, HR+,luminal A | 1.20 | 0.48–2.95 | 0.70 |
| Black, HR+,non-luminal A | 4.21 | 1.74–10.18 | 0.001 |
| Age, years | |||
| ≤ 50 | 1.0 | - | |
| > 50 | 2.85 | 1.48–5.47 | 0.002 |
| Income, annual household | |||
| < $25,000 | 1.0 | - | |
| ≥ $25,000 | 0.36 | 0.18–0.73 | 0.005 |
| Stage (%) | |||
| I | 1.0 | - | |
| II | 1.86 | 1.01–3.46 | 0.05 |
| III | 4.55 | 2.21–9.35 | <0.001 |
Abbreviations: Non-Hispanic White (NHW)
Model is adjusted for age, stage at diagnosis (I, II, III), and annual household income (< $25,000, ≥ $25,000).
Discussion:
This study examined the biological heterogeneity of HR+, HER2− breast cancer by race. To our knowledge, this is amongst the first studies to evaluate the impact of molecular subtype classification on the racial survival disparity in HR+, HER2− breast cancer. Our findings that the majority of HR+, HER2− tumors are luminal A is consistent with prior reports (23, 33). Patients with tumors that are HR+, luminal A subtype usually belong to the low risk category according to the PAM50 ROR classification and do not benefit from (neo)adjuvant chemotherapy (34, 35). On the other hand, HR+,non-luminal tumors have been associated with endocrine independence, chemosensitivity and poor outcomes (29). Consistent with prior studies (29, 33), HR+,non-luminal A tumors were less commonly observed in our overall study population.
Although young Black patients in our study had a higher likelihood of HR+,non-luminal A subtypes, our findings did not reach statistical significance. This finding may be due to limited power given the small sample size of young NHW and would be important to confirm through additional studies. Among HR+,non-luminal A tumors, the basal-like subtype is a rare entity, with some studies reporting rates as low as 0.8% and as high as 7.9% (23, 33). In contrast to prior studies (23, 29, 33), basal-like tumors comprised a higher percentage, especially among Black women in whom we observed a frequency of 12%. Similar to prior studies to suggest racial differences in molecular subtype distribution (6, 33), our results show a significant racial disparity with a higher frequency (16%) of basal-like HR+, HER2− breast cancer observed among young Black women with no reported cases observed in young NHW women. The aforementioned study by Troester et al included 208 Black women with HR+, HER2− breast cancer, which is similar to our sample size (N=227) however only 89 (42.8%) patients were diagnosed with breast cancer ≤ age 50 in comparison to 128 (56.4%) in our study (33). To our knowledge this is the highest prevalence of basal-like HR+, HER2− breast cancer seen in a study population, and may be explained by the oversampling of young Black women (52%) in the BEST cohort. Basal-like and HER2-enriched tumors are thought to have lower expression of ER/PR, however recent studies have shown that these subtypes can also be identified among tumors with high ER/PR expression (29). Among HR+,non-luminal A tumors, the HER2-enriched subtype had the worst OS. Our findings are similar to prior studies which have showed that HER2-enriched tumors have worse clinical outcomes with higher risk of recurrence and tumors that are thought to reflect endocrine independency (29, 36). Prior studies have suggested that HER2− tumors that are HER2-enriched by gene expression profiling are particularly sensitive to HER2 targeting agents (37–39). This may be clinically relevant given the need to better identify patients who may benefit from a more tailored approach for improving clinical outcomes across racial groups.
Similar to prior studies (27, 29, 40), our data suggests that gene expression profiling provides clinically relevant prognostic information beyond the traditional IHC classifications. Several studies have shown inferior survival outcomes among women with HR+,non-luminal A subtypes regardless of nodal involvement (27, 28, 40–42). In our study, as expected, women with HR+,luminal A tumors had lower mortality rates regardless of race. On the other hand, HR+,non-luminal A tumors had higher mortality rates across both racial groups, with a non-significant higher mortality rate observed among Black women. The difference between the simple (i.e. KM plot) and multiple cox proportional hazard model is expected and was likely due to a higher proportion of young Black women ≤ 50yo (92% vs. 8%) and higher Stage III breast cancers among Black women (77% vs. 23%) compared to NHW women in our study.
Our data showed a distinct separation in mortalitysurvival rates dependent on molecular subtype, and this difference was not significantly modified by race in the HR+,non-luminal A group. The difference in survival detected in our analysis differs from a previous population-based analysis conducted in the Life after Cancer Epidemiology (LACE) and Pathways studies (6) that suggests inferior breast cancer survival rates for all Black women regardless of molecular subtype, however, no difference in OS. Important differences between our study and LACE/Pathways are race and age composition, where LACE/Pathways included predominantly (>75%) older women and fewer than 10% were Black (N=128). In addition, LACE/Pathways survival data is not limited to HR+, HER2− women, with 49% HR+, HER2− cases in their population. Furthermore, differences between these two studies may reflect differences in regional geographic trends, population genetics, socioeconomic status, lifestyle factors and comorbidities. LACE/Pathways participants were predominantly from high socioeconomic backgrounds whereas our study population included predominantly (>60%) low income participants (annual household income < $25,000).
There is an urgent need to further understand the biological factors contributing to the racial survival disparity among women with HR+, HER2− breast cancer. In the era of precision oncology with advances in gene expression profiling to tailor therapeutic options, there is a need to ensure future studies have adequate representation across all racial/ethnic groups. Such studies can provide a better understanding of the biology of breast cancer across different racial and ethnic groups with the potential to narrow the existing breast cancer survival disparity.
The current study has several strengths including being amongst the first studies conducted across the Southeastern United States to study the impact of molecular subtype classification on the racial survival disparity in HR+, HER2− breast cancer. Prior studies have shown that regional racial variations in breast cancer mortality exist, with rising or unchanged death rates in southern or midwestern states among Black women (18, 43), underscoring the need for studies to better understand the contributing factors to this known racial disparity. Furthermore, our study population included amongst the largest collection of Black women with HR+, HER2− breast cancer with molecular subtyping information available. Despite these strengths, there remain some limitations, including the difference in patient characteristics across the two studies. The differences in the proportion of basal-like subtypes may be explained by the inclusion and exclusion criteria of the BEST study, which only recruited Black women diagnosed with breast cancer at or below 50 years of age; whereas the SCCS recruited both Black and NHW women who were diagnosed with breast cancer predominantly over the age of 50. Furthermore, the majority (86%) of SCCS participants were enrolled at CHCs, which provide primary health and preventive services to medically underserved and low-income populations; whereas the BEST study recruited participants from both academic and community centers who had higher annual household incomes. The overrepresentation of a low-income population may not allow for generalization of our findings across all socioeconomic groups.
In conclusion, HR+, HER2− breast cancer usually leads to more favorable clinical outcomes than TNBC partly due to endocrine targeted therapies. Despite these therapeutic advances, more women continue to die from HR+, HER2− breast cancer than from any other breast cancer subtype (16–18). Our findings indicate that molecular subtype classification contributes to the differences in survival observed among women with HR+, HER2− breast cancer. Our results suggest that tumor gene expression profiling is important to identify the aggressive, HR+,non-luminal A tumors overrepresented among Black women which may contribute to the racial survival disparities. Future research may leverage the molecular subtype differences within HR+, HER2− breast cancer which has tremendous potential to improve prognostication across racial groups.
Funding:
Sonya Reid’s research was supported by two NIH training grants (5T32CA160056-08 and 5K12CA090625-20).
Footnotes
Availability of data: The datasets are available from the corresponding author after reasonable request.
The authors declare no potential conflicts of interest.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: a cancer journal for clinicians. 2018;68(6):394–424. [DOI] [PubMed] [Google Scholar]
- 2.Society AC. Breast Cancer Facts & Figures 2019–2020. Atlanta: American Cancer Society, Inc. 2019. [Google Scholar]
- 3.Knutsvik G, Stefansson I, Aziz S, Arnes J, Eide J, Collett K, et al. Evaluation of Ki67 Expression across Distinct Categories of Breast Cancer Specimens: A Population-Based Study of Matched Surgical Specimens, Core Needle Biopsies and Tissue Microarrays. PLoS One. 2014;9:e112121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davis MB, Newman LA. Breast Cancer Disparities: How Can We Leverage Genomics to Improve Outcomes? Surgical oncology clinics of North America. 2018;27(1):217–34. [DOI] [PubMed] [Google Scholar]
- 5.Daly B, Olopade OI. A perfect storm: How tumor biology, genomics, and health care delivery patterns collide to create a racial survival disparity in breast cancer and proposed interventions for change. CA Cancer J Clin. 2015;65(3):221–38. [DOI] [PubMed] [Google Scholar]
- 6.Kroenke CH, Sweeney C, Kwan ML, Quesenberry CP, Weltzien EK, Habel LA, et al. Race and breast cancer survival by intrinsic subtype based on PAM50 gene expression. Breast cancer research and treatment. 2014;144(3):689–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jiagge E, Chitale D, Newman LA. Triple-Negative Breast Cancer, Stem Cells, and African Ancestry. The American Journal of Pathology. 2018;188(2):271–9. [DOI] [PubMed] [Google Scholar]
- 8.Walsh SM, Zabor EC, Stempel M, Morrow M, Gemignani ML. Does race predict survival for women with invasive breast cancer? Cancer. 2019;125(18):3139–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carey LA, Perou CM, Livasy CA, Dressler LG, Cowan D, Conway K, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. Jama. 2006;295(21):2492–502. [DOI] [PubMed] [Google Scholar]
- 10.Lund MJ, Butler EN, Bumpers HL, Okoli J, Rizzo M, Hatchett N, et al. High prevalence of triple-negative tumors in an urban cancer center. Cancer. 2008;113(3):608–15. [DOI] [PubMed] [Google Scholar]
- 11.Yang XR, Sherman ME, Rimm DL, Lissowska J, Brinton LA, Peplonska B, et al. Differences in risk factors for breast cancer molecular subtypes in a population-based study. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2007;16(3):439–43. [DOI] [PubMed] [Google Scholar]
- 12.Olopade OI, Ikpatt FO, Dignam JJ, Khramtsov A, Tetriakova M, Grushko T, et al. Intrinsic Gene Expression subtypes correlated with grade and morphometric parameters reveal a high proportion of aggressive basal-like tumors among black women of African Ancestry. Journal of Clinical Oncology. 2004;22(14S):9509. [Google Scholar]
- 13.Aziz H, Hussain F, Sohn C, Mediavillo R, Saitta A, Hussain A, et al. Early onset of breast carcinoma in African American women with poor prognostic factors. Am J Clin Oncol. 1999;22(5):436–40. [DOI] [PubMed] [Google Scholar]
- 14.Newman LA. Breast cancer in African-American women. Oncologist. 2005;10(1):1–14. [DOI] [PubMed] [Google Scholar]
- 15.American Cancer Society I Cancer Facts & Figures 2012 [Internet]. American Cancer Society, Inc.; 2012. [Available from: http://www.cancer.org/acs/groups/content/@epidemiologysurveilance/documents/document/acspc-031941.pdf. [Google Scholar]
- 16.Warner ET, Tamimi RM, Hughes ME, Ottesen RA, Wong YN, Edge SB, et al. Racial and Ethnic Differences in Breast Cancer Survival: Mediating Effect of Tumor Characteristics and Sociodemographic and Treatment Factors. J Clin Oncol. 2015;33(20):2254–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O’Brien KM, Cole SR, Tse C-K, Perou CM, Carey LA, Foulkes WD, et al. Intrinsic breast tumor subtypes, race, and long-term survival in the Carolina Breast Cancer Study. Clinical cancer research : an official journal of the American Association for Cancer Research. 2010;16(24):6100–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Howlader NNA, Krapcho M, Miller D, Brest A, Yu M, Ruhl J, Tatalovich Z, Mariotto, Lewis DR, Chen HS, Feuer EJ, Cronin KA (eds). SEER Cancer Statistics Review, 1975–2016, National Cancer Institute. https://seercancergov/csr/1975_2016/, based on November 2018 SEER data submission, posted to the SEER web site, April 2019. 2019. [Google Scholar]
- 19.Albain K, Gray R, Sparano J, Makower D, Pritchard K, Hayes D, et al. Abstract GS4–07: Race, ethnicity and clinical outcomes in hormone receptor-positive, HER2-negative, node-negative breast cancer: results from the TAILORx trial. Cancer research. 2019;79(4 Supplement):GS4–07-GS4-. [Google Scholar]
- 20.Network. NCC. Breast Cancer. (Version3.2020) [Accessed March 1, 2020]. Available from: https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf. 2020.
- 21.Prat A, Ellis MJ, Perou CM. Practical implications of gene-expression-based assays for breast oncologists. Nature Reviews Clinical Oncology. 2012;9(1):48–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cancer Genome Atlas N Comprehensive molecular portraits of human breast tumours. Nature. 2012;490(7418):61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheang MCU, Martin M, Nielsen TO, Prat A, Voduc D, Rodriguez-Lescure A, et al. Defining Breast Cancer Intrinsic Subtypes by Quantitative Receptor Expression. The Oncologist. 2015;20(5):474–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perou CM, Sørlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, et al. Molecular portraits of human breast tumours. Nature. 2000;406(6797):747–52. [DOI] [PubMed] [Google Scholar]
- 25.Prat A, Pineda E, Adamo B, Galvan P, Fernandez A, Gaba L, et al. Clinical implications of the intrinsic molecular subtypes of breast cancer. Breast. 2015;24 Suppl 2:S26–35. [DOI] [PubMed] [Google Scholar]
- 26.Parker JS, Mullins M, Cheang MCU, Leung S, Voduc D, Vickery T, et al. Supervised risk predictor of breast cancer based on intrinsic subtypes. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27(8):1160–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prat A, Parker JS, Fan C, Cheang MC, Miller LD, Bergh J, et al. Concordance among gene expression-based predictors for ER-positive breast cancer treated with adjuvant tamoxifen. Ann Oncol. 2012;23(11):2866–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ellis MJ, Suman VJ, Hoog J, Lin L, Snider J, Prat A, et al. Randomized phase II neoadjuvant comparison between letrozole, anastrozole, and exemestane for postmenopausal women with estrogen receptor-rich stage 2 to 3 breast cancer: clinical and biomarker outcomes and predictive value of the baseline PAM50-based intrinsic subtype--ACOSOG Z1031. J Clin Oncol. 2011;29(17):2342–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cejalvo JM, Pascual T, Fernández-Martínez A, Brasó-Maristany F, Gomis RR, Perou CM, et al. Clinical implications of the non-luminal intrinsic subtypes in hormone receptor-positive breast cancer. Cancer treatment reviews. 2018;67:63–70. [DOI] [PubMed] [Google Scholar]
- 30.Bonner D, Cragun D, Reynolds M, Vadaparampil ST, Pal T. Recruitment of a Population-Based Sample of Young Black Women with Breast Cancer through a State Cancer Registry. Breast J. 2016;22(2):166–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Signorello LB, Hargreaves MK, Steinwandel MD, Zheng W, Cai Q, Schlundt DG, et al. Southern community cohort study: establishing a cohort to investigate health disparities. J Natl Med Assoc. 2005;97(7):972–9. [PMC free article] [PubMed] [Google Scholar]
- 32.Nielsen T, Wallden B, Schaper C, Ferree S, Liu S, Gao D, et al. Analytical validation of the PAM50-based Prosigna Breast Cancer Prognostic Gene Signature Assay and nCounter Analysis System using formalin-fixed paraffin-embedded breast tumor specimens. BMC Cancer. 2014;14(1):177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Troester MA, Sun X, Allott EH, Geradts J, Cohen SM, Tse C-K, et al. Racial Differences in PAM50 Subtypes in the Carolina Breast Cancer Study. J Natl Cancer Inst. 2018;110(2):176–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sun Z, Prat A, Cheang MCU, Gelber RD, Perou CM. Chemotherapy benefit for “ER-positive” breast cancer and contamination of Nonluminal subtypes—waiting for TAILORx and RxPONDER. Annals of Oncology. 2015;26(1):70–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fernandez-Martinez A, Pascual T, Perrone G, Morales S, Rodriguez J, González-Rivera M, et al. Limitations in predicting PAM50 intrinsic subtype and risk of relapse score with Ki67 in estrogen receptor-positive HER2-negative breast cancer. Oncotarget. 2017;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prat A, Brase JC, Cheng Y, Nuciforo P, Pare L, Pascual T, et al. Everolimus plus Exemestane for Hormone Receptor-Positive Advanced Breast Cancer: A PAM50 Intrinsic Subtype Analysis of BOLERO-2. Oncologist. 2019;24(7):893–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Prat A, Bianchini G, Thomas M, Belousov A, Cheang MCU, Koehler A, et al. Research-Based PAM50 Subtype Predictor Identifies Higher Responses and Improved Survival Outcomes in HER2-Positive Breast Cancer in the NOAH Study. Clinical Cancer Research. 2014;20(2):511. [DOI] [PubMed] [Google Scholar]
- 38.Prat A, Carey LA, Adamo B, Vidal M, Tabernero J, Cortés J, et al. Molecular features and survival outcomes of the intrinsic subtypes within HER2-positive breast cancer. J Natl Cancer Inst. 2014;106(8):dju152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carey L, Barry W, Pitcher B, Hoadley K, Cheang M, Anders C, et al. Gene expression signatures in pre- and post-therapy (Rx) specimens from CALGB 40601 (Alliance), a neoadjuvant phase III trial of weekly paclitaxel and trastuzumab with or without lapatinib for HER2-positive breast cancer (BrCa). Journal of Clinical Oncology. 2014;32:506-. [Google Scholar]
- 40.Prat A, Cheang MCU, Galván P, Nuciforo P, Paré L, Adamo B, et al. Prognostic Value of Intrinsic Subtypes in Hormone Receptor–Positive Metastatic Breast Cancer Treated With Letrozole With or Without Lapatinib. JAMA Oncology. 2016;2(10):1287–94. [DOI] [PubMed] [Google Scholar]
- 41.Prat A, Pineda E, Adamo B, Galván P, Fernández A, Gaba L, et al. Clinical implications of the intrinsic molecular subtypes of breast cancer. The Breast. 2015;24:S26–S35. [DOI] [PubMed] [Google Scholar]
- 42.Caan BJ, Sweeney C, Habel LA, Kwan ML, Kroenke CH, Weltzien EK, et al. Intrinsic Subtypes from the PAM50 Gene Expression Assay in a Population-Based Breast Cancer Survivor Cohort: Prognostication of Short- and Long-term Outcomes. Cancer Epidemiology Biomarkers & Prevention. 2014;23(5):725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.DeSantis CE, Fedewa SA, Goding Sauer A, Kramer JL, Smith RA, Jemal A. Breast cancer statistics, 2015: Convergence of incidence rates between black and white women. CA: A Cancer Journal for Clinicians. 2016;66(1):31–42. [DOI] [PubMed] [Google Scholar]


