Abstract
Enhancer-derived RNAs (eRNAs) are a new class of long noncoding RNA that have roles in modulating enhancer-mediated gene transcription, which ultimately influences phenotypic outcomes. We recently published the first study mapping genome-wide eRNA expression in the male mouse cortex during ischemic stroke and identified 77 eRNAs that were significantly altered following a 1 h middle cerebral artery occlusion (MCAO) and 6 h of reperfusion, as compared to sham controls. Knockdown of one such stroke-induced eRNA - eRNA_06347 - resulted in significantly larger infarcts, demonstrating a role for eRNA_06347 in modulating the post-stroke pathophysiology in males. In the current study, we applied quantitative real-time PCR to evaluate whether the 77 eRNAs identified in the male cortex also show altered expression in the post-stroke female cortex. Using age-matched and time-matched female mice, we found that only a subset of the 77 eRNAs were detected in the post-stroke female cortex. Of these, only a small fraction showed similar temporal expression characteristics as males, including eRNA_06347 which was highly induced in both sexes. Knockdown of eRNA_06347 in the female cortex resulted in significantly increased infarct volumes that were closely matched to those in males, indicating that eRNA_06347 modulates the post-stroke pathophysiology similarly in males and females. This suggests a common underlying role for eRNA_06347 in the two sexes. Overall, this is the first study to evaluate eRNA expression and perturbation in the female cortex during stroke, and present a comparative analysis between males and females. Our findings show that eRNAs have sex-dependent and sex-independent expression patterns that may be of significance to the pathophysiological responses to stroke in the two sexes.
Keywords: Ischemic stroke, noncoding RNA, eRNA, sex differences, mouse
1. Introduction
Enhancers are cis-regulatory elements in the genome that interact with promoters to control gene transcription. The recent discovery of abundant, stimulus-dependent transcription of noncoding enhancer RNAs (eRNAs), and the demonstrated roles for some eRNAs in mediating enhancer-promoter interactions highlights the functional importance of eRNAs and raises important questions about their influence on gene modulation and phenotypic outcomes1-4. Emerging data suggests that eRNAs are expressed and active in a wide variety of cell- and tissue-types in development and disease5-12, suggesting that eRNAs have widespread biological roles. In the brain, eRNA expression has been shown to exhibit regional specificity13, indicating region-dependent functional relevance. Recently, we published the first report on stroke-induced eRNA expression changes in the cerebral cortex and identified the effects of eRNA perturbation on post-stroke brain damage14. This study established the expression and functional importance of eRNAs in ischemic brain injury.
Although our recent study provided valuable insights on stroke-related eRNAs, these insights were limited to the male cerebral cortex. Sex as a biological variable is an important consideration for the future success and clinical translation of biomedical research. In ischemic stroke, emerging data have shown that the molecular mechanisms driving cell death and brain damage differ between the sexes15. Previous work has revealed significant differences in cellular responses16, regulatory cells17, regulatory protein activity18 and microRNA expression19-21 between males and females during ischemic stroke. Thus, it is likely that sex-dependent differences may also extend to eRNA expression and function. To date, there are no published reports exploring sex-dependent differences in eRNA expression in the post-ischemic brain. To address this gap in knowledge, in the current study we evaluated eRNA expression in the post-stroke adult female cortex with a focus on the 77 differentially altered eRNAs that we previously identified in males14. Using qPCR, we quantified the relative expression of these eRNAs in females at multiple time-points of reperfusion as compared to sham controls, and cross-compared the results to those in time-matched males. Using a loss-of-function strategy against eRNA_06347, which was robustly and equivalently induced in both males and females, we evaluated its effects on post-stroke infarct volumes in the female brain, and cross-compared the outcome to that in males. Overall, this is the first study to report eRNA expression patterns in the female cerebral cortex during stroke and presents the first comparative analysis of the expression differences and effects of eRNA perturbation in the male versus female brains.
2. Materials & Methods
2.1. Animals
The experiments were carried out in 3-months-old female C57BL/6N mice weighing on average 20.5 g and 3-months-old male C57BL/6N mice weighing on average 22.7 g (Taconic Biosciences, USA) and cared for in accordance with the Guide for the Care and Use of Laboratory Animals, US Department of Health and Human Services Publication Number 86-23 (revised 1986). All experimental protocols were approved by the Intuitional Animal Care and Use Committee of Seton Hall University. Animals were housed in a temperature, humidity, and light-controlled environment with ad libitum access to food and water.
2.2. Estrous Cycle Monitoring
To control for differences in circulating hormones, female mice underwent estrous cycle monitoring using vaginal cytology. Animals were grouped for experiments in the diestrus stage given that it is the longest, and has the lowest circulating levels of estrogen and progesterone22-24. The monitoring was conducted as follows: Daily vaginal smears were collected from each mouse for a period of two and a half weeks to determine accurate cycling pattern. Under standard lighting conditions, the estrous cycle was closely monitored via vaginal lavage. Using a clean microscope slide (Globe Scientific, Catalog number 1324W) and a pipette containing 0.1 mL of physiological saline, the vaginal cavity was gently lavaged and fluid was drawn back into the pipette tip. The fluid was smeared evenly onto the slide in a thin layer, allowed to air dry, stained with Toluidine Blue 0.5 g/dL, and observed under a bright-field microscope to determine the differential cell profiles of the four estrous stages. Animals receiving infusions of antisense oligos via osmotic pump implantation were grouped in the estrus stage to allow for the completion of the pre-treatment period before transitioning to the diestrus stage for induction of transient focal ischemia. Females undergoing only transient focal ischemia were grouped in the diestrus stage and underwent ischemia induction immediately.
2.3. Transient Focal Ischemia
Animals were randomly assigned to experimental groups via coin toss. Transient focal ischemia was induced using the intraluminal filament model of middle cerebral artery occlusion (MCAO) as described previously14, 25. Briefly, animals were anesthetized under 5% isoflurane on a pre-warmed heating pad and arterial occlusion was achieved using a sterile 6-0 silicon-coated filament (210-μm tip diameter, Doccol Corporation, USA) that was inserted through the common carotid artery and advanced through the internal carotid artery (ICA) up to the middle cerebral artery (MCA). Blood flow was monitored via Laser Doppler Flowmetry to ensure successful occlusion. Body temperature was monitored continuously. The incision was sutured and the animals were returned to pre-warmed cages for recovery. After 1h of occlusion, the filament was removed, the incision was sutured, and mice were returned to their cages with ad libitum access to food and water. Reperfusion was allowed for 6h, 12h, or 24h. For sham groups, the midline neck incision was done to expose the ICA, however no filament was inserted to occlude the MCA. At the reperfusion endpoints, neurological severity scoring after MCAO was performed as follows: 0 = no neurological deficit (normal); 1 = mild deficit (failure to completely extend contralateral forepaw); 2 = moderate deficit (circling contralateral to infarct); 3 = severe deficit (falling contralateral to infarct); 4 = very severe deficit (no spontaneous movement). The neurological scores are presented in Supplementary Table 1. Animals exhibiting no neurological deficit or revealing hemorrhage were excluded from the study. Remaining animals were anesthetized using isoflurane, and euthanized via decapitation for cortical tissue isolation, or underwent transcardial perfusion prior to whole brain isolation.
2.4. In Vivo eRNA Knockdown
The eRNA knockdown was achieved via intracerebral ventricular (ICV) infusion of sequence-specific locked nucleic acid (LNA) GapmeR oligos as described previously14. The negative control groups received scrambled oligos and the experimental groups received anti-eRNA oligos. The sequences of the oligos are: eRNA_06347: 5’-GATTTGGAATTGCTAG-3’; negative control: 5’-AACACGTCTATACGC-3’. The oligos were resuspended in artificial CSF at a standardized concentration of 8.3 pmole/μL and a 100 μL volume was loaded into each osmotic pump (Alzet 10003D, DURECT Corporation, USA) connected to a brain infusion cannula via a catheter (Alzet Brain Infusion Kit 3, DURECT Corporation, USA). The preloaded pumps were primed at 37°C overnight. After priming, the animals were anesthetized using isoflurane and the brain infusion cannula was implanted stereotaxically into the lateral ventricle (Bregma: 0.2mm posterior, 0.9 mm lateral, and 2/5 mm dorsoventral) via a burr hole. Perpetual infusion of the oligos was sustained at the rate of 1 μL/h for 48 h, followed by a 1h MCAO surgery, and reperfusion for 24 h. At the reperfusion endpoints, neurological scoring was performed and the animals were euthanized for tissue collection.
2.5. Infarct Volume Estimation
At the 24 h reperfusion endpoint, the mice were anesthesized via 5% isoflurane and then transcardially perfused with ice-cold 1X PBS, followed by perfusion with fresh ice-cold 4% paraformaldehyde (PFA). The brains were isolated immediately and post-fixed in 4% PFA overnight at 4°C, followed by cryoprotection with 30% sucrose for 48-72 h at 4°C. Cryopreserved brains were sectioned on a Cryostar NX50 instrument (Thermo Fisher Scientific, USA) to generate 20-μm-thick whole-brain coronal sections mounted on pre-charged microscope slides (Globe Scientific, USA). For infarct visualization, cresyl violet histological staining was performed. The infarct volumes were quantified using ImageJ (National Institutes of Health, USA), and corrected for edema using Swanson’s formula. The final volumes were cross-compared between the study groups to determine sex-based differences.
2.6. Total RNA Isolation
At the 6h reperfusion endpoint (n=4/group), the animals were euthanized and the cortical tissues from the ipsilateral hemispheres were isolated and immediately stored at −80°C. The tissues were used for total RNA isolation using the mirVana Isolation Kit (Thermo Fisher Scientific, USA) as per the manufacturer’s protocol. Isolated RNA was evaluated for quantity and purity using Qubit 2.0 (Thermo Fisher Scientific, USA).
2.7. Real-Time PCR
Total RNA from four biological replicates per group was DNAse-I treated and converted into single-stranded cDNA using the Maxima H Minus First Strand cDNA Synthesis Kit (Thermo Fisher Scientific, USA) and used for gene expression evaluations on a Step-One Real-Time System (Applied Biosystems, USA). For each reaction, 10 ng of cDNA per sample was incorporated with gene-specific forward and reverse primers and amplified using iTaq Universal SYBR Green Supermix (Bio-Rad Laboratories, USA) in a 20 μL reaction under standard cycling conditions. Expression of 18s rRNA was evaluated as a housekeeping control. All reactions were performed in duplicate.
2.8. Statistics
Statistical analyses were performed using GraphPad Prism 7.0. Analyses between two independent groups were performed using unpaired t-test for normally distributed data or Welch’s t-test for data with unequal variances. The data are expressed as mean ± SD with a significance threshold of p<0.05.
3. Results
3.1. Expression patterns of the eRNAs in the post-stroke female cortex
We recently reported the first genome-wide evaluation of eRNAs in the male mouse cerebral cortex in response to stroke. In that study, we identified 77 eRNAs whose expression was significantly induced at 6 h of reperfusion, many of which showed continued induction through 12 h and 24 h of reperfusion. To determine whether these eRNAs were also expressed in the post-stroke female cortex, in the current study we evaluated their expression levels using quantitative real-time PCR (qPCR) in age-matched females undergoing a 1 h MCAO and 6, 12 or 24 h of reperfusion (n = 4/group). At 6 h of reperfusion, 20 eRNAs were significantly induced (fold-change >2.0; p<0.05) as compared to sham; at 12 h of reperfusion, 23 eRNAs were significantly altered as compared to sham; and at 24 h of reperfusion, 13 eRNAs were significantly altered as compared to sham (Fig. 1). Comparing the expression patterns of the eRNAs across the three time-points, we observed that only 12 of the eRNAs that were significantly induced at 6 h of reperfusion remained significantly induced at 12 h of reperfusion, and only 6 eRNAs remained significantly induced at 24 h of reperfusion (Table 1). Notably, 11 eRNAs that were either not detected or not significantly altered at 6 h of reperfusion, were significantly altered at 12 h of reperfusion (Supplementary Table 2); and seven eRNAs that were either not detected or not significantly altered at 6 h of reperfusion were significantly altered at 24 h of reperfusion (Supplementary Table 3). This indicated delayed induction of the eRNAs after stroke. Of these, only one eRNA – eRNA_0066834 – was commonly altered at both 12 and 24 h of reperfusion. Further, we found that a small subset of the eRNAs was exclusively altered at each time-point: four eRNAs at 6h, two eRNAs at 12h, and one eRNA at 24h of reperfusion (Supplementary Tables 4-6).
Figure 1:
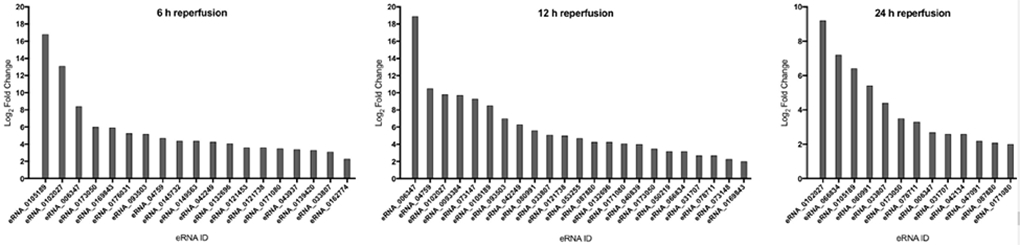
Differentially expressed eRNAs in the post-stroke female cortex at multiple time-points of reperfusion. Expression levels of significantly altered eRNAs determined using qPCR in the post-stroke female cortex at 6, 12 and 24 h of reperfusion, as compared to sham. Values represent average fold-changes determined using the ΔΔCT method from four biological replicates per group, tested in duplicate. X-axis: eRNA ID; Y-axis: Log2 fold-change.
Table 1:
Post-stroke eRNA expression at 6, 12 and 24 h of reperfusion in the female cortex.
| eRNA ID | Log2 Fold Change | ||
|---|---|---|---|
| 6 h | 12 h | 24 h | |
| eRNA_00105189 | 16.8 | 8.5 | 6.4 |
| eRNA_00102027 | 13.1 | 9.8 | 9.2 |
| eRNA_0006347 | 8.4 | 18.9 | 2.7 |
| eRNA_00173050 | 6.0 | 3.5 | 3.5 |
| eRNA_00169843 | 5.9 | 2.0 | - |
| eRNA_00176031 | 5.3 | - | - |
| eRNA_00093503 | 5.2 | 7.0 | 1.1 |
| eRNA_00004759 | 4.7 | 10.5 | - |
| eRNA_00145732 | 4.4 | 1.2 | - |
| eRNA_00149563 | 4.4 | 1.5 | - |
| eRNA_00042249 | 4.3 | 6.3 | - |
| eRNA_00132696 | 4.1 | 4.3 | - |
| eRNA_00121453 | 3.6 | - | - |
| eRNA_00121738 | 3.6 | 5.0 | 1.1 |
| eRNA_00171080 | 3.5 | 4.1 | 2.0 |
| eRNA_00043937 | 3.4 | - | - |
| eRNA_00139420 | 3.3 | - | - |
| eRNA_00033807 | 3.1 | 5.1 | 4.4 |
| eRNA_00162774 | 2.3 | 1.8 | - |
| eRNA_00149153 | 2.1 | 1.4 | - |
3.2. Differential analysis of eRNA expression patterns in the male versus female cortex
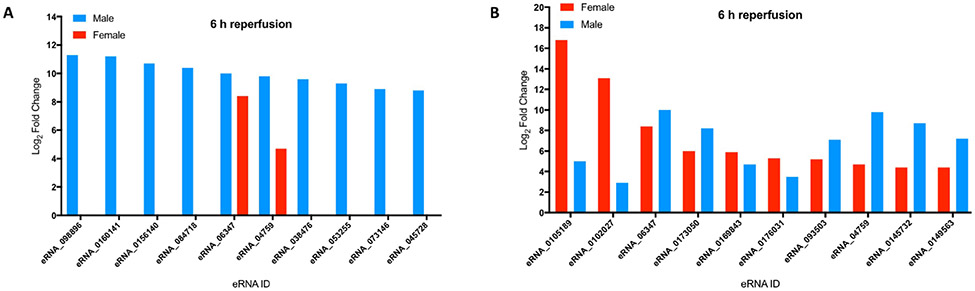
Next, we conducted an analysis of the eRNA expression differences between males and females using data from males that we published previously14. At 6 h of reperfusion, although 20 of the 77 eRNAs were significantly induced in females, the magnitude of their induction varied widely between the two sexes. At the 6 h time-point, of the 10 most highly induced eRNAs in males, only two eRNAs were induced in females, and the remaining eight were undetected (Fig. 2A). Conversely, of the 10 most highly induced eRNAs in females, eight eRNAs showed similar magnitude of induction as that in males. Notably, however, the top two eRNAs in females were induced at a substantially lower magnitude in males (Fig. 2B). Across the three reperfusion time-points, 32 eRNAs were continuously expressed in males at 6, 12 and 24 h of reperfusion, whereas only six eRNAs were continuously expressed in females (Table 2). Only three of these eRNAs were commonly expressed between the sexes. Together, these data show that there are substantial differences in the magnitude and temporal expression patterns of the eRNAs between the two sexes.
Figure 2:
Cross-comparison of the most highly expressed eRNAs in the male and female cortices at 6 h of reperfusion. A) A comparison of the 10 most highly expressed eRNAs in the post-stroke male cortex at 6h reperfusion vis-à-vis their expression levels in the time-matched female cortex. Only two eRNAs – eRNA_6347 and eRNA_04759 – were commonly expressed in both the sexes. B) A comparison of the 10 most highly expressed eRNAs in the post-stroke female cortex at 6h reperfusion vis-à-vis their expression levels in the time-matched male cortex. All ten eRNAs showed significant induction in both sexes, but differed in the magnitude of their expression between the sexes. X-axis: eRNA ID; Y-axis: Log2 fold-change.
Table 2:
eRNAs continuously expressed at all reperfusion time-points in the male and female cortices.
| Log2 Fold Change (Males) |
Log2 Fold Change (Females) |
|||||
|---|---|---|---|---|---|---|
| eRNAs | 6 h | 12 h | 24 h | 6 h | 12 h | 24 h |
| eRNA_098896 | 11.3 | 11.1 | 8.5 | - | - | - |
| eRNA_0160141 | 11.2 | 10 | 8.6 | - | - | - |
| eRNA_0156140 | 10.7 | 9.7 | 8.5 | - | - | - |
| eRNA_084718 | 10.4 | 10 | 9.3 | - | - | - |
| eRNA_06347 | 10 | 8.8 | 6.5 | 8.4 | 18.9 | 2.7 |
| eRNA_04759 | 9.8 | 9.0 | 7.2 | - | - | - |
| eRNA_038476 | 9.6 | 8.5 | 7.0 | - | - | - |
| eRNA_073146 | 8.9 | 8.5 | 7.9 | - | - | - |
| eRNA_0179541 | 8.8 | 8.9 | 7.7 | - | - | - |
| eRNA_045728 | 8.8 | 9.4 | 7.6 | - | - | - |
| eRNA_058357 | 8.8 | 7.9 | 6.6 | - | - | - |
| eRNA_093384 | 8.7 | 8.7 | 7.2 | - | - | - |
| eRNA_098684 | 8.6 | 7.3 | 8.6 | - | - | - |
| eRNA_0121738 | 8.1 | 7.3 | 8.4 | - | - | - |
| eRNA_0171080 | 8.1 | 7.4 | 6.3 | 3.5 | 4.1 | 2.0 |
| eRNA_0164305 | 7.9 | 7.2 | 5.9 | - | - | - |
| eRNA_023536 | 7.8 | 8.8 | 7.9 | - | - | - |
| eRNA_069619 | 7.8 | 7.7 | 7.0 | - | - | - |
| eRNA_046040 | 7.6 | 7.0 | 6.6 | - | - | - |
| eRNA_025428 | 7.4 | 6.3 | 5.9 | - | - | - |
| eRNA_0129583 | 7.2 | 7.5 | 5.6 | - | - | - |
| eRNA_090334 | 7.2 | 7.0 | 6.1 | - | - | - |
| eRNA_0137819 | 7.1 | 7.6 | 7.0 | - | - | - |
| eRNA_034386 | 7.1 | 6.8 | 7.1 | - | - | - |
| eRNA_018069 | 7 | 8.4 | 7.1 | - | - | - |
| eRNA_073148 | 7 | 6.3 | 5.8 | - | - | - |
| eRNA_0178579 | 6.9 | 6.2 | 5.9 | - | - | - |
| eRNA_027428 | 6.9 | 7.2 | 7.5 | - | - | - |
| eRNA_0105189 | 5 | 4.6 | 3.8 | 16.8 | 8.5 | 6.4 |
| eRNA_0169843 | 4.7 | 4.2 | 2.8 | - | - | - |
| eRNA_073147 | 3.6 | 3.4 | 2.6 | - | - | - |
| eRNA_047091 | 3.3 | 2.6 | 2.3 | - | - | - |
| eRNA_0102027 | - | - | - | 13.1 | 9.8 | 9.2 |
| eRNA_0173050 | - | - | - | 6.0 | 3.5 | 3.5 |
| eRNA_033807 | - | - | - | 3.1 | 5.1 | 4.4 |
Despite these differences, however, there exist some strong similarities that may underlie conserved pathophysiological outcomes in males and females. One such similarity is the expression pattern of eRNA_06347. This eRNA is the fifth most highly induced eRNA in the male cortex and the third most highly induced eRNA in the female cortex. Further, it is one of only three eRNAs that were robustly and continuously expressed at all three time-points of reperfusion in both the sexes. In our previous study, knockdown of eRNA_06347 in the male mouse cortex resulted in significantly increased infarct volumes as compared to controls. Given the highly similar expression patterns of eRNA_06347 in males and females, and its demonstrated effect on the post-stroke pathophysiology in males, we selected eRNA_06347 for knockdown in female mice to evaluate its effects in the post-stroke female cortex.
3.3. Effect of eRNA_06347 knockdown on the post-stroke infarct volume in the female cortex
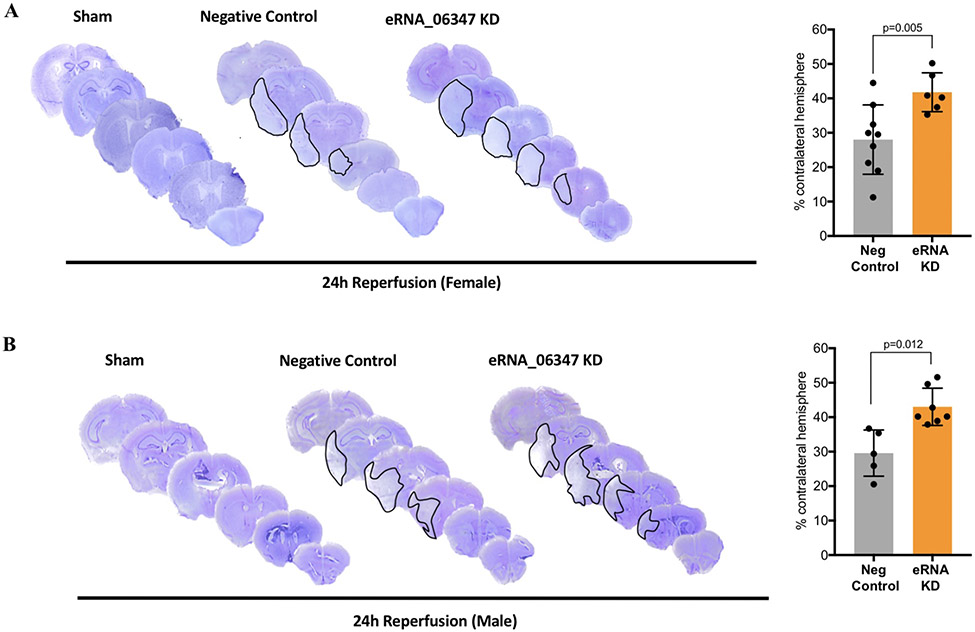
To identify the effects of eRNA_06347 perturbation in the female cortex, we followed an identical pre-treatment knockdown protocol as done previously in males14. Separate cohorts of mice received either anti-eRNA_06347 oligos or negative control oligos for 48 h, followed by a 1 h MCAO (n=11/group). The sham groups received negative control oligos followed by sham surgery. At 24 h of reperfusion the animals were euthanized and the infarct volumes were quantified using cresyl violet-stained coronal brain sections. Of the 11 animals in the negative control group, one animal had a hemorrhage and another animal died. These two animals were excluded from the study, resulting in a final sample size of n=9. In the eRNA_06347 knockdown group, three animals died and two animals had no infarcts, and were excluded from the study, resulting in a final sample size of n=6. These final datasets were used for differential analyses between the groups. In comparison to sham, both the negative control group and the eRNA_06347 knockdown group that received a stroke exhibited distinct infarcts. However, the eRNA_06347 knockdown group exhibited significantly larger infarct volumes as compared to negative controls, with an average infarct volume of 41.78% as compared to 28.00% in the negative control group (Fig. 3A). This demonstrated that, as in males, perturbation of eRNA_06347 in the female cortex resulted in significantly worsened infarct outcomes after a stroke.
Figure 3:
Effect of eRNA_06347 knockdown on cortical infarct volumes in male and female mice. Representative cresyl violet-stained sections in A) sham, negative control (n=9/group) and eRNA_06347 knockdown groups (n=6/group) in female mice (left panel). Quantitation of the infarct volumes shows an average increase of 13.78% in the eRNA_06347 KD group versus the negative control group (right panel). B) sham, negative control (n=5/group) and eRNA_06347 knockdown groups (n=7/group) in male mice from our previous study14 (left panel). Quantitation of the infarct volumes shows an average increase of 13.63% in the eRNA_06347 KD group versus the negative control group (right panel). KD: knockdown. Data were analyzed using Welch’s t-test and are expressed as mean ± SD.
3.4. The effect of eRNA_06347 knockdown on post-stroke infarct volumes in the male versus female cortex
Next, we compared the infarct volume changes in response to eRNA_06347 knockdown in the male versus female cortex (Fig. 3B). Comparing the negative control groups alone, males exhibited a slightly higher average infarct volume of 29.40% as compared to 28.00% in females (p = 0.73). Similarly, in the eRNA_06347 knockdown groups, males exhibited slightly higher infarct volumes at 43.03% as compared to 41.78% in females (p = 0.69). When normalized to their respective negative controls, the males and females showed average increases in infarct volumes of 13.63% and 13.78%, respectively. These highly similar outcomes in both the sexes show that eRNA_06347 has a consistent, sex-independent role in modulating the post-stroke pathophysiology and suggests that the mechanisms and pathways underlying the actions of eRNA_06347 may be conserved in males and females.
4. Discussion
Sex-based differences are an increasingly important consideration in the biomedical sciences, and particularly so in ischemic stroke because it is well-established that the post-stroke pathophysiology and outcomes vary considerably between males and females17, 18, 26-29. Clarifying these differences has implications for potential stroke therapies18, 20, 30. Because noncoding RNAs have emerged as an important component of the post-stroke molecular response relatively recently, studies on sex-based differences in this area are limited. To date, differential expression and function of noncoding RNAs in stroke has been largely focused on microRNAs19-21, 31, which are among the earliest, and therefore most commonly studied, type of noncoding RNA. In the current study, we present the first insights into sex-based differences in stroke for a different type of noncoding RNA – the eRNA.
We previously showed that eRNA expression peaks early after onset of stroke injury, and at 6 h of reperfusion we identified 77 eRNAs that were significantly induced in the male mouse cortex14. Because immediate-early gene expression underlies secondary processes that ultimately shape phenotypic outcomes, the early and robustly induced eRNAs might have a role in shaping the subsequent post-stroke outcome. Apropos to this, we observed that knockdown of one such eRNA – eRNA_06347 – resulted in significantly worsened infarcts, which indicated that eRNA_06347 may be involved in the innate neuroprotective response. Given this background, our main goal in this study was to evaluate the expression of the pre-validated 77 stroke-induced eRNAs in the post-stroke female cortex to determine whether there were any sex-based similarities or differences that might further our understanding of sex-based differences in noncoding RNA expression.
The results showed that there were stark differences in the eRNA expression profiles in the male versus female cortex. Comparing just the 6 h time-points, less than a third of the 77 eRNAs that were significantly altered in males were also altered in females, as compared to sham. However, looking at the later time-points of 12 h and 24 h of reperfusion in females, we found that a number of additional eRNAs began exhibiting significantly altered expression, showing that these eRNAs had a delayed induction in the female cortex as compared to the male cortex. Comparing the magnitude of expression of the commonly altered eRNAs in the two sexes, we found that the vast majority of eRNAs showed large differences in expression levels between the sexes. Whether these differences have biological and functional ramifications is unknown. One caveat in this regard is that the male eRNAs were detected using RNA-seq, which is more sensitive than the qPCR method applied to females. This might distort the actual differences to some extent. A future study incorporating a genome-wide evaluation of eRNAs via RNA-seq in females might narrow some of these apparent gaps in expression between the two sexes.
Extending the analysis further, when we evaluated eRNA expression across a longitudinal time-frame to include all three time-points of reperfusion, we found that 32 of the 77 eRNAs remained continuously altered in males, whereas only six eRNAs remained continuously altered in females at all three time-points. Of these, three eRNAs were commonly induced in both the sexes. Given that the half-life of RNAs is generally just a few hours32, such a robust and continuous alteration shows that these eRNAs are either exceptionally stable or expressly transcribed by the cells, which suggests biological importance. Notably, one of the three commonly induced eRNAs in this category was eRNA_06347, the perturbation of which we had previously linked to worsened post-stroke infarct volumes14. In line with those observations, we found that eRNA_06347 perturbation in the female cortex also resulted in worsened post-stroke infarct volumes and, importantly, the magnitude of this effect was highly similar to that in males. Taken together, these data strongly support a functional, and potentially similar, role for eRNA_06347 in males and females. In future studies, we will investigate this link further.
Overall, our study shows that eRNAs are robustly altered in response to stroke in both males and females. While there are a number of expression differences between the two sexes that may drive differential outcomes, there are also many similarities that may underlie certain core innate responses to stroke in a sex-independent manner. Why these differences arise – whether due to hormones or other factors – remains to be studied. What other eRNAs are altered in the post-stroke female cortex beyond the 77 eRNAs that we examined also remains to be studied. These limitations of our study will be addressed in future projects encompassing genome-wide RNA-sequencing for unbiased identification of stroke-responsive eRNAs in females, and hormonal-manipulation protocols for investigating the effects of circulating hormones on the differential eRNA expression profiles between the sexes. The functional roles and mechanisms of the eRNAs will also be investigated in future studies.
In summary, our study lays the groundwork for further examinations of sex-based differences in eRNA expression and paves the path for future investigations into eRNA function incorporating sex as a biological variable.
Supplementary Material
Ischemic stroke robustly alters the expression of eRNAs in the female cerebral cortex.
The vast majority of eRNAs examined exhibited sex-dependent expression patterns during ischemic stroke.
A small subset of eRNAs exhibited highly similar expression patterns in the male and female cortex during ischemic stroke.
The perturbation of an eRNA commonly expressed in males and females resulted in very similar infarct volume changes in both the sexes, suggesting a sex-independent functional role.
This is the first study to report sex-based eRNA expression and function in ischemic stroke.
Sources of Funding:
This work was supported by the National Institute Of Neurological Disorders And Stroke of the National Institutes of Health under Award Number R01NS115835 to Ashutosh Dharap.
Footnotes
Disclosures/Conflict of Interest: None.
'Declarations of interest: none'.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kim TK, Hemberg M, Gray JM, et al. Widespread transcription at neuronal activity-regulated enhancers. Nature. May 13 2010;465(7295):182–7. doi: 10.1038/nature09033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schaukowitch K, Joo JY, Liu X, Watts JK, Martinez C, Kim TK. Enhancer RNA facilitates NELF release from immediate early genes. Mol Cell. October 02 2014;56(1):29–42. doi: 10.1016/j.molcel.2014.08.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ding M, Liu Y, Liao X, Zhan H, Huang W. Enhancer RNAs (eRNAs): New Insights into Gene Transcription and Disease Treatment. J Cancer. 2018:2334–40. vol. 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carullo NVN, Simon RC, Salisbury AJ, et al. Enhancer RNAs are necessary and sufficient for activity-dependent neuronal gene transcription. 2018-July-17 2018;doi: 10.1101/270967 [DOI] [Google Scholar]
- 5.Carullo NVN, Day JJ. Genomic Enhancers in Brain Health and Disease. Genes (Basel). January 14 2019;10(1)doi: 10.3390/genes10010043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaikkonen MU, Spann NJ, Heinz S, et al. Remodeling of the enhancer landscape during macrophage activation is coupled to enhancer transcription. Mol Cell. August 8 2013;51(3):310–25. doi: 10.1016/j.molcel.2013.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li W, Notani D, Ma Q, et al. Functional Importance of eRNAs for Estrogen-dependent Transcriptional Activation Events. Nature. June 27 2013;498(7455):516–20. doi: 10.1038/nature12210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Melo CA, Drost J, Wijchers PJ, et al. eRNAs are required for p53-dependent enhancer activity and gene transcription. Mol Cell. February 7 2013;49(3):524–35. doi: 10.1016/j.molcel.2012.11.021 [DOI] [PubMed] [Google Scholar]
- 9.Hsieh CL, Fei T, Chen Y, et al. Enhancer RNAs participate in androgen receptor-driven looping that selectively enhances gene activation. Proc Natl Acad Sci U S A. May 20 2014;111(20):7319–24. doi: 10.1073/pnas.1324151111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mirtschink P, Bischof C, Pham MD, et al. Inhibition of the Hypoxia-Inducible Factor 1α-Induced Cardiospecific HERNA1 Enhance-Templated RNA Protects From Heart Disease. Circulation. June 11 2019;139(24):2778–92. doi: 10.1161/circulationaha.118.036769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arner E, Daub CO, Vitting-Seerup K, et al. Transcribed enhancers lead waves of coordinated transcription in transitioning mammalian cells. Science. February 27 2015;347(6225):1010–4. doi: 10.1126/science.1259418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Le Gras S, Keime C, Anthony A, et al. Altered enhancer transcription underlies Huntington's disease striatal transcriptional signature. Sci Rep. February 22 2017;7:42875. doi: 10.1038/srep42875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yao P, Lin P, Gokoolparsadh A, Assareh A, Thang MW, Voineagu I. Coexpression networks identify brain region-specific enhancer RNAs in the human brain. Nat Neurosci. August 2015;18(8):1168–74. doi: 10.1038/nn.4063 [DOI] [PubMed] [Google Scholar]
- 14.Bhattarai S, Akella A, Gandhi A, Dharap A. Modulation of Brain Pathology by Enhancer RNAs in Cerebral Ischemia. Mol Neurobiol. April 2021;58(4):1482–1490. doi: 10.1007/s12035-020-02194-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Manwani B, McCullough LD. Sexual dimorphism in ischemic stroke: lessons from the laboratory. Womens Health (Lond). May 2011;7(3):319–39. doi: 10.2217/whe.11.22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bodhankar S, Lapato A, Chen Y, Vandenbark AA, Saugstad JA, Offner H. Role for microglia in sex differences after ischemic stroke: importance of M2. Metabolic brain disease. December 2015;30(6):1515–29. doi: 10.1007/s11011-015-9714-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seifert HA, Benedek G, Liang J, et al. Sex differences in regulatory cells in experimental stroke. Cell Immunol. August 2017;318:49–54. doi: 10.1016/j.cellimm.2017.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dotson AL, Wang J, Chen Y, et al. Sex differences and the role of PPAR alpha in experimental stroke. Metabolic brain disease. June 2016;31(3):539–47. doi: 10.1007/s11011-015-9766-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lusardi TA, Murphy SJ, Phillips JI, et al. MicroRNA responses to focal cerebral ischemia in male and female mouse brain. Frontiers in molecular neuroscience. 2014;7:11. doi: 10.3389/fnmol.2014.00011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sohrabji F, Selvamani A. Sex differences in miRNA as therapies for ischemic stroke. Neurochem Int. July 2019;127:56–63. doi: 10.1016/j.neuint.2018.10.021 [DOI] [PubMed] [Google Scholar]
- 21.Banerjee A, Chokkalla AK, Shi JJ, et al. Microarray Profiling Reveals Distinct Circulating miRNAs in Aged Male and Female Mice Subjected to Post-stroke Social Isolation. Neuromolecular Med. June 2021;23(2):305–314. doi: 10.1007/s12017-020-08622-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Byers SL, Wiles MV, Dunn SL, Taft RA. Mouse Estrous Cycle Identification Tool and Images. PLoS One. 2012. vol. 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cora MC, Kooistra L, Travlos G. Vaginal Cytology of the Laboratory Rat and Mouse: Review and Criteria for the Staging of the Estrous Cycle Using Stained Vaginal Smears. research-article. 2015-March-03 2015;doi: 10.1177/0192623315570339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Westwood FR. The Female Rat Reproductive Cycle: A Practical Histological Guide to Staging:. review-article. 2008-May-08 2008;doi: 10.1177/0192623308315665 [DOI] [PubMed] [Google Scholar]
- 25.Shahjouei S, Cai PY, Ansari S, et al. Middle Cerebral Artery Occlusion Model of Stroke in Rodents: A Step-by-Step Approach. J Vasc Interv Neurol. January 2016;8(5):1–8. [PMC free article] [PubMed] [Google Scholar]
- 26.Kim T, Chelluboina B, Chokkalla AK, Vemuganti R. Age and sex differences in the pathophysiology of acute CNS injury. Neurochem Int. July 2019;127:22–28. doi: 10.1016/j.neuint.2019.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Choleris E, Galea LAM, Sohrabji F, Frick KM. Sex differences in the brain: Implications for behavioral and biomedical research. Neurosci Biobehav Rev. February 2018;85:126–145. doi: 10.1016/j.neubiorev.2017.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Girijala RL, Sohrabji F, Bush RL. Sex differences in stroke: Review of current knowledge and evidence. Vasc Med. April 2017;22(2):135–145. doi: 10.1177/1358863X16668263 [DOI] [PubMed] [Google Scholar]
- 29.Dykstra-Aiello C, Sharp FR, Jickling GC, et al. Alternative Splicing of Putative Stroke/Vascular Risk Factor Genes Expressed in Blood Following Ischemic Stroke Is Sexually Dimorphic and Cause-Specific. Front Neurol. 2020;11:584695. doi: 10.3389/fneur.2020.584695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sohrabji F, Park MJ, Mahnke AH. Sex differences in stroke therapies. J Neurosci Res. January 2017;95(1-2):681–691. doi: 10.1002/jnr.23855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Selvamani A, Sohrabji F. Mir363-3p improves ischemic stroke outcomes in female but not male rats. Neurochem Int. July 2017;107:168–181. doi: 10.1016/j.neuint.2016.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen CYA, Ezzeddine N, Shyu AB. Messenger RNA Half-Life Measurements in Mammalian Cells. Methods Enzymol. 2008;448:335–57. doi: 10.1016/s0076-6879(08)02617-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.