Abstract
Several studies have demonstrated the harmful effects of chronic kidney disease (CKD) on the audiovestibular system. Through a time-to-event analysis, we aimed to compare the association of CKD with sudden sensorineural hearing loss (SSNHL), and Ménière’s disease against a control population without CKD. We used a total of 1,025,340 patients from the Korean National Health Insurance Service database from 2002 to 2013. The CKD group (n = 2572) included patients diagnosed with CKD more than three times between January 2003 and December 2005. The non-CKD group (n = 5144) consisted of two patients without CKD for every patient with CKD. Each patient was monitored until December 2013. We calculated the incidence, survival rate, and hazards ratio (HR) of SSNHL and Ménière’s disease. In the CKD group, the incidence of SSNHL and Ménière’s disease was 1.39 and 3.64 per 1000 person-years, respectively. Patients with CKD showed an adjusted HR of 2.15 and 1.45 for SSNHL and Ménière’s disease, respectively. Middle-aged patients with CKD were associated with a higher incidence of developing SSNHL and Ménière’s disease than those without CKD. Female patients with CKD had a higher risk of developing SSNHL; however, there was no significant difference in the risk of Ménière’s disease in patients with CKD according to sex. Our findings suggest that CKD is associated with an increased incidence of SSNHL and Ménière’s disease. Therefore, audiovestibular surveillance should be considered in patients with CKD.
Subject terms: Diseases, Health care, Risk factors, Urology
Introduction
Chronic kidney disease (CKD) is defined as abnormalities of kidney structure or function that are present for > 3 months and have an impact on health1. The global prevalence of CKD is estimated to range from 11 to 13%, and it may be more common than diabetes mellitus (DM) with an estimated prevalence of approximately 8%2. According to the 2012 Kidney Disease Improving Global Outcomes clinical practice guidelines, CKD is divided into five classes based on the value of the glomerular filtration rate (GFR). Increasing evidence has shown that CKD is an independent risk factor for cardiovascular disease, and there is an inverse relationship between GFR and risk of cardiovascular disease2–5. Additionally, prior studies have revealed that decreased renal function might be a predictor of hospitalization, cognitive dysfunction, and poor quality of life3,6–8. Patients with CKD could suffer from several otorhinolaryngological problems, including sensorineural hearing loss, epistaxis, candidiasis, halitosis, xerostomia, dysgeusia, and lip, and thyroid cancers9.
Among otorhinolaryngological complications, dysfunction of the audiovestibular system is one of the most common problems10,11. The etiology of these complications in patients with CKD may be due to several structural and functional similarities between the kidney and the inner ear12. Moreover, disturbances in water and electrolyte homeostasis can affect the endolymphatic fluid, which may induce endolymphatic hydrops13. Finally, some drugs such as loop diuretics and aminoglycosides used in the treatment of patients with CKD are well-known for their ototoxicity14. To date, only a few studies using population-based data have demonstrated a relationship between CKD and dysfunction of the audiovestibular system15,16. One study has shown that patients with CKD are at a higher risk of developing sudden sensorineural hearing loss (SSNHL) than the general population15. Another study has demonstrated that patients with CKD are at an increased risk of developing vestibular dysfunction in comparison to healthy populations16. Thus, CKD may be considered a risk factor for SSNHL or Ménière’s disease. However, studies on the relationship between CKD and SSNHL or Ménière’s disease are still lacking.
Therefore, to determine a significant link between CKD and SSNHL or Ménière’s disease, we investigated the prospective hazard of SSNHL and Ménière’s disease in patients with CKD using a nationwide representative sample from the National Sample Cohort (NSC) 2002–2013 data provided by the Korea National Health Insurance Service (KNHIS) in South Korea.
Results
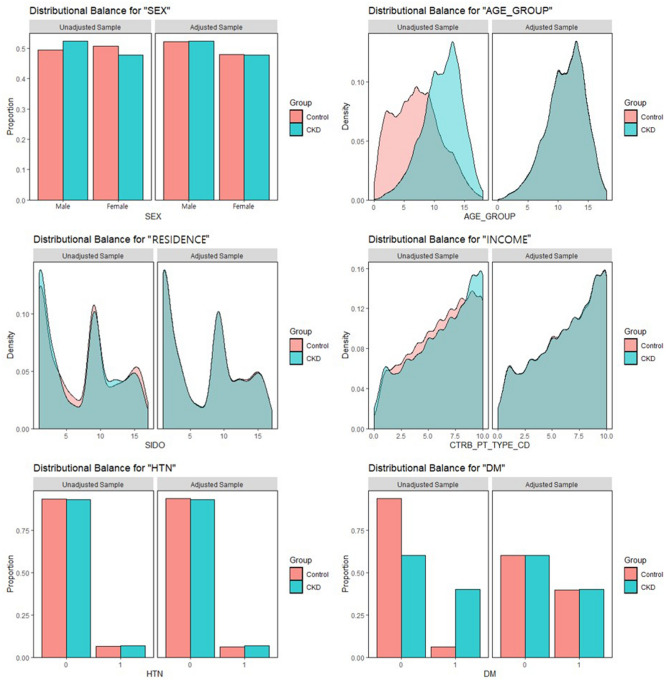
We selected 2572 patients with CKD and 5144 comparison participants (non-CKD) using propensity score matching. The details of the study population and group characteristics are presented in Table 1. We observed no significant differences between the CKD and comparison groups regarding sex, age, residential area, household income, hypertension (HTN), or diabetes mellitus (DM). This indicates that group matching was performed appropriately. Additionally, to indicate good matching, we presented the balance plot before and after matching (Fig. 1). This showed a good matching balance along with our sensitivity analysis.
Table 1.
Characteristics of the study participants.
| Variables | Comparison (n = 5144) | Chronic kidney disease (n = 2572) | P value |
|---|---|---|---|
| Sex | 0.917 | ||
| Male | 2682 (52.1%) | 1345 (52.3%) | |
| Female | 2462 (47.9%) | 1227 (47.7%) | |
| Ages (years) | 0.970 | ||
| < 45 | 1408 (27.4%) | 709 (27.6%) | |
| 45–64 | 2409 (46.8%) | 1197 (46.5%) | |
| > 64 | 1327 (25.8%) | 666 (25.9%) | |
| Residence | 0.983 | ||
| Seoul (metropolitan) | 1247 (24.2%) | 625 (24.3%) | |
| 2nd area (other metropolitan) | 1300 (25.3%) | 654 (25.4%) | |
| 3rd area | 2597 (50.5%) | 1293 (50.3%) | |
| Household income | 0.987 | ||
| Low (0–30%) | 1044 (20.3%) | 522 (20.3%) | |
| Middle (30–70%) | 1885 (36.6%) | 938 (36.5%) | |
| High (70–100%) | 2215 (43.1%) | 1112 (43.2%) | |
| Hypertension | 0.261 | ||
| No | 4820 (93.7%) | 2392 (93.0%) | |
| Yes | 324 (6.3%) | 180 (7.0%) | |
| Diabetes mellitus | 0.967 | ||
| No | 3094 (60.1%) | 1545 (60.1%) | |
| Yes | 2050 (39.9%) | 1027 (39.9%) | |
SSNHL, Sudden sensorineural hearing loss; Seoul, a largest metropolitan; 2nd area, other metropolitan cities; 3rd area, other areas.
Figure 1.
Balance plot for 6 variables before and after matching.
In this study, we examined a total of 78,915.7 person-years and 78,453.3 person-years of SSNHL and Ménière’s disease, respectively, which included 56,559.9 person-years and 56,172.7 person-years in the comparison group, and 22,355.8 person-years and 22,280.6 person-years in the CKD group, respectively. Our results showed that the incidence of SSNHL and Ménière’s disease per 1000 person-years was 1.39 and 3.64, respectively, for the CKD group, compared to 0.60 and 3.38, respectively, for the comparison group.
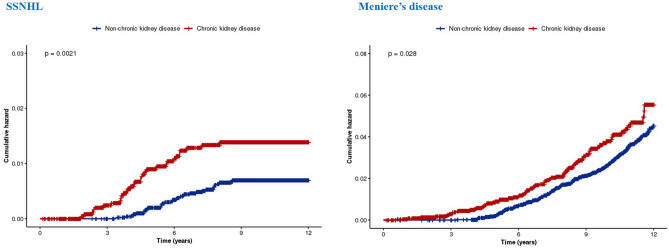
We analyzed the hazards ratios (HR) for the development of SSNHL and Ménière’s disease during the 8-year follow-up period using univariate and multivariate Cox regression models; these findings are presented in Tables 2 and 3. After adjusting for sociodemographic factors (sex, age, residential area, household income, hypertension [HTN], and DM), we observed that patients with CKD had a significant association with the prospective development of SSNHL (adjusted HR = 2.15, 95% confidence interval [CI] [1.32–3.51]) and Ménière’s disease (adjusted HR = 1.45, 95% CI [1.11–1.89]). Additionally, we observed that HTN and DM were significantly associated with the prospective development of SSNHL (HTN, adjusted HR = 2.38, 95% CI [1.17–4.85]; DM, adjusted HR = 3.18, 95% CI [1.87–5.42]), whereas the adjusted HR of Ménière’s disease was significantly increased among the females and older age groups (women, adjusted HR = 1.48, 95% CI [1.16–1.88]; patients aged > 64 years, adjusted HR = 2.94, 95% CI [2.00–4.31]). Moreover, the reverse Kaplan–Meier survival curves and the results of log-rank tests are presented in Fig. 2, which depicts the cumulative incidences of SSNHL and Ménière’s disease in the patient and comparison groups.
Table 2.
Incidence per 1000 person-years and hazards ratio (95% confidence interval) of sudden sensorineural hearing loss.
| Variables | N | Case | Person-years | Incidence | Unadjusted HR (95% CI) | P value | Adjusted HR (95% CI) | P value |
|---|---|---|---|---|---|---|---|---|
| Group | ||||||||
| Comparison group | 5144 | 34 | 56,559.9 | 0.60 | 1 (ref) | 1 (ref) | ||
| CKD group | 2572 | 31 | 22,355.8 | 1.39 | 2.11 (1.30–3.43)** | 0.003 | 2.15 (1.32–3.51)** | 0.002 |
| Sex | ||||||||
| Male | 4027 | 37 | 40,408.2 | 0.92 | 1 (ref) | 1 (ref) | ||
| Female | 3689 | 28 | 38,507.5 | 0.73 | 0.81 (0.49–1.32) | 0.388 | 0.79 (0.48–1.30) | 0.352 |
| Ages (years) | ||||||||
| < 45 | 2117 | 16 | 22,783.0 | 0.70 | 1 (ref) | 1 (ref) | ||
| 45–64 | 3606 | 34 | 37,789.3 | 0.90 | 1.30 (0.71–2.35) | 0.394 | 0.99 (0.53–1.83) | 0.965 |
| > 64 | 1993 | 15 | 18,343.4 | 0.82 | 1.18 (0.58–2.38) | 0.653 | 0.89 (0.43–1.84) | 0.748 |
| Residence | ||||||||
| Seoul | 1872 | 16 | 19,202.5 | 0.83 | 1 (ref) | 1 (ref) | ||
| 2nd area | 1954 | 15 | 20,064.3 | 0.75 | 0.90 (0.44–1.82) | 0.768 | 0.93 (0.46–1.89) | 0.849 |
| 3rd area | 3890 | 34 | 39,648.9 | 0.86 | 1.03 (0.57–1.87) | 0.915 | 0.99 (0.54–1.80) | 0.972 |
| Household income | ||||||||
| Low (0–30%) | 1566 | 11 | 15,696.4 | 0.70 | 1 (ref) | 1 (ref) | ||
| Middle (30–70%) | 2823 | 26 | 29,105.5 | 0.89 | 1.29 (0.64–2.60) | 0.483 | 1.26 (0.62–2.55) | 0.525 |
| High (70–100%) | 3327 | 28 | 34,113.8 | 0.82 | 1.18 (0.59–2.38) | 0.634 | 1.09 (0.54–2.20) | 0.812 |
| Hypertension | ||||||||
| No | 7212 | 56 | 73,764.4 | 0.76 | 1 (ref) | 1 (ref) | ||
| Yes | 504 | 9 | 5151.3 | 1.75 | 2.33 (1.16–4.72)* | 0.018 | 2.38 (1.17–4.85)* | 0.016 |
| Diabetes mellitus | ||||||||
| No | 4639 | 22 | 48,118.6 | 0.46 | 1 (ref) | 1 (ref) | ||
| Yes | 3077 | 43 | 30,797.1 | 1.40 | 3.09 (1.85–5.17)*** | < 0.001 | 3.18 (1.87–5.42)*** | < 0.001 |
CKD, Chronic kidney disease; SSNHL, Sudden sensorineural hearing loss; HR, Hazard ratio; CI, Confidence interval. *P < 0.05, **P < 0.010, and ***P < 0.001.
Table 3.
Incidence per 1000 person-years and hazards ratio (95% confidence interval) of Meniere’s disease.
| Variables | N | Case | Person-years | Incidence | Unadjusted HR (95% CI) | P value | Adjusted HR (95% CI) | P value |
|---|---|---|---|---|---|---|---|---|
| Group | ||||||||
| Comparison group | 5144 | 190 | 56,172.7 | 3.38 | 1 (ref) | 1 (ref) | ||
| CKD group | 2572 | 81 | 22,280.6 | 3.64 | 1.34 (1.03–1.75)* | 0.028 | 1.45 (1.11–1.89)** | 0.006 |
| Sex | ||||||||
| Male | 4027 | 111 | 40,304.9 | 2.75 | 1 (ref) | 1 (ref) | ||
| Female | 3689 | 160 | 38,148.4 | 4.19 | 1.48 (1.16–1.88)** | 0.002 | 1.48 (1.16–1.88)** | 0.002 |
| Age (years) | ||||||||
| < 45 | 2117 | 41 | 22,794.3 | 1.80 | 1 (ref) | 1 (ref) | ||
| 45–64 | 3606 | 143 | 37,632.3 | 3.80 | 2.13 (1.51–3.02)*** | < 0.001 | 2.06 (1.45–2.94)*** | < 0.001 |
| > 64 | 1993 | 87 | 18,026.7 | 4.83 | 2.98 (2.05–4.32)*** | < 0.001 | 2.94 (2.00–4.31)*** | < 0.001 |
| Residence | ||||||||
| Seoul | 1872 | 53 | 19,147.5 | 2.77 | 1 (ref) | 1 (ref) | ||
| 2nd area | 1954 | 72 | 19,928.0 | 3.61 | 1.31 (0.92–1.87) | 0.134 | 1.39 (0.98–1.99) | 0.068 |
| 3rd area | 3890 | 146 | 39,377.9 | 3.71 | 1.35 (0.99–1.85) | 0.062 | 1.34 (0.98–1.84) | 0.068 |
| Household income | ||||||||
| Low (0–30%) | 1566 | 52 | 15,658.4 | 3.32 | 1 (ref) | 1 (ref) | ||
| Middle (30–70%) | 2823 | 104 | 28,970.4 | 3.59 | 1.06 (0.76–1.48) | 0.731 | 1.14 (0.81–1.58) | 0.457 |
| High (70–100%) | 3327 | 115 | 33,824.5 | 3.40 | 1.01 (0.73–1.40) | 0.962 | 1.01 (0.73–1.41) | 0.937 |
| Hypertension | ||||||||
| No | 7212 | 254 | 73,305.2 | 3.46 | 1 (ref) | 1 (ref) | ||
| Yes | 504 | 17 | 5148.1 | 3.30 | 0.94 (0.57–1.53) | 0.797 | 0.88 (0.54–1.45) | 0.625 |
| Diabetes mellitus | ||||||||
| No | 4639 | 141 | 47,895.4 | 2.94 | 1 (ref) | 1 (ref) | ||
| Yes | 3077 | 130 | 30,557.9 | 4.25 | 1.46 (1.15–1.86)** | 0.002 | 1.25 (0.98–1.60) | 0.074 |
CKD, Chronic kidney disease; SSNHL, Sudden sensorineural hearing loss; HR, Hazard ratio; CI, Confidence interval. *P < 0.05, **P < 0.010, and ***P < 0.001.
Figure 2.
Risk of development of SSNHL and Ménière’s disease among individuals with and without chronic kidney disease. SSNHL, sudden sensorineural hearing loss.
Next, we performed subgroup analyses to examine the association between CKD and the development of SSNHL or Ménière’s disease according to age and sex. After adjusting for other variables, we observed that the prospective development of SSNHL and Ménière’s disease was only significantly related to the middle-aged CKD group (45–64 years; SSNHL, adjusted HR: 2.36, 95% CI [1.29–4.62]; Ménière’s disease, adjusted HR: 1.50, 95% CI [1.04–2.14]; Table 4). Additionally, we observed that female patients with CKD were more strongly associated with the development of SSNHL than male patients (Table 5). However, there was no significant relationship between Ménière’s disease and CKD according to sex (Table 4).
Table 4.
Hazard ratios of sudden sensorineural hearing loss or Meniere’s disease by age between patients with and without chronic kidney disease.
| Variables | N | Case | Person–years | Incidence | Unadjusted HR (95% CI) | P value | Adjusted HR (95% CI) | P value |
|---|---|---|---|---|---|---|---|---|
| SSNHL | ||||||||
| Age (years) < 45 | ||||||||
| Comparison group | 1408 | 8 | 6948.9 | 0.51 | 1 (ref) | 1 (ref) | ||
| CKD group | 709 | 8 | 15,834.1 | 1.15 | 2.08 (0.78–5.54) | 0.143 | 2.05 (0.77–5.48) | 0.152 |
| Age (years) 45–64 | ||||||||
| Comparison group | 2409 | 17 | 27,034.5 | 0.63 | 1 (ref) | 1 (ref) | ||
| CKD group | 1197 | 17 | 10,754.8 | 1.58 | 2.27 (1.16–4.45)* | 0.017 | 2.36 (1.20–4.62)* | 0.012 |
| Age (years) > 64 | ||||||||
| Comparison group | 1327 | 9 | 13,691.3 | 0.66 | 1 (ref) | 1 (ref) | ||
| CKD group | 666 | 6 | 4652.1 | 1.29 | 1.82 (0.64–5.11) | 0.259 | 1.92 (0.68–5.42) | 0.220 |
| Meniere’s Diseases | ||||||||
| Age (years) < 45 | ||||||||
| Comparison group | 1408 | 28 | 15,850.1 | 1.77 | 1 (ref) | 1 (ref) | ||
| CKD group | 709 | 13 | 6944.2 | 1.87 | 1.21 (0.62–2.36) | 0.572 | 1.22 (0.63–2.38) | 0.561 |
| Age (years) 45–64 | ||||||||
| Comparison group | 2409 | 98 | 26,914.0 | 3.61 | 1 (ref) | 1 (ref) | ||
| CKD group | 1197 | 45 | 10,718.3 | 4.20 | 1.48 (1.03–2.12)* | 0.033 | 1.50 (1.04–2.14)* | 0.029 |
| Age (years) > 64 | ||||||||
| Comparison group | 1327 | 64 | 13,408.6 | 4.77 | 1 (ref) | 1 (ref) | ||
| CKD group | 666 | 23 | 4618.1 | 4.98 | 1.46 (0.90–2.37) | 0.130 | 1.50 (0.92–2.45) | 0.102 |
SSNHL, Sudden sensorineural hearing loss; CKD, Chronic kidney disease; CI, Confidence interval (*P < 0.05).
Table 5.
Hazard ratios of sudden sensorineural hearing loss or Meniere’s disease by sex between patients with and without chronic kidney disease.
| Variables | N | Case | Person-years | Incidence | Unadjusted HR (95% CI) | P value | Adjusted HR (95% CI) | P value |
|---|---|---|---|---|---|---|---|---|
| SSNHL | ||||||||
| Sex Male | ||||||||
| Comparison group | 2682 | 20 | 28,926.4 | 0.69 | 1 (ref) | 1 (ref) | ||
| CKD group | 1345 | 17 | 11,481.8 | 1.41 | 1.98 (1.04–3.79)* | 0.038 | 1.99 (1.04–3.80)* | 0.037 |
| Sex Female | ||||||||
| Comparison group | 2462 | 14 | 27,633.5 | 0.51 | 1 (ref) | 1 (ref) | ||
| CKD group | 1227 | 14 | 10,874.0 | 1.29 | 2.30 (1.09–4.82)* | 0.028 | 2.44 (1.16–5.14)* | 0.019 |
| Meniere’s Diseases | ||||||||
| Sex Male | ||||||||
| Comparison group | 2682 | 81 | 28,823.3 | 2.81 | 1 (ref) | 1 (ref) | ||
| CKD group | 1345 | 30 | 11,481.6 | 2.61 | 1.17 (0.76–1.79) | 0.470 | 1.30 (0.85–1.99) | 0.231 |
| Sex Female | ||||||||
| Comparison group | 2462 | 109 | 27,349.4 | 3.99 | 1 (ref) | 1 (ref) | ||
| CKD group | 1227 | 51 | 10,799.0 | 4.72 | 1.47 (1.05–2.07)* | 0.025 | 1.56 (1.11–2.20)* | 0.010 |
SSNHL, Sudden sensorineural hearing loss; CKD, Chronic kidney disease; CI, Confidence interval (*P < 0.05).
Discussion
CKD is a major global health burden due to its high prevalence, economic cost, and harmful effects on other organs. In this longitudinal study, we examined the association between CKD and SSNHL or Ménière’s disease in 2572 patients with CKD and 5144 sociodemographically matched comparison participants, whose data were extracted from a nationwide 8-year longitudinal cohort database of 1,025,340 South Korean patients. Interestingly, we observed that patients with CKD had a significantly increased incidence of SSNHL and Ménière’s disease, with an HR of 2.15 and 1.45, respectively, after adjusting for sociodemographic factors and the presence of comorbidities. Moreover, we observed that middle-aged patients with CKD had a significantly increased risk of SSNHL and Ménière’s disease. Further, the risk of developing SSNHL was higher in female patients with CKD than in male patients.
Research has shown that patients with CKD are prone to developing otologic symptoms related to audiovestibular dysfunction. These symptoms are often permanent, difficult to control, and have a significantly negative influence on the patient’s quality of life. Among the described audiovestibular dysfunctions, the possible cause linking CKD with SSNHL and Ménière’s disease remains unclear. However, the nephrons of the kidney and the stria vascularis of the cochlea show very similar anatomical, physiological, and pharmacological characteristics17. Additionally, antibodies formed against the nephrons may be immunologically deposited in the stria vascularis of the cochlea18,19. Moreover, CKD-related electrolytic and osmotic alterations that affect the cochlea can influence the labyrinth13. Furthermore, hemodialysis and renal transplantation may induce electrolyte disturbances and osmotic alterations in the inner ear, resulting in sensorineural hearing loss, tinnitus, and vertigo9. Prolonged hemodialysis may also result in the accumulation of amyloid in the inner ear tissues20. Thus, despite the exact pathogenesis of SSNHL and Ménière’s disease in patients with CKD remaining unclear, the suggested potential etiologic factors may explain the relationship between CKD and the subsequent development of SSNHL and Ménière’s disease.
To the best of our knowledge, this is the first study to investigate the association of developing SSNHL and Ménière’s disease in patients with CKD. Interestingly, we observed that CKD resulted in a significantly increased risk of SSNHL and Ménière’s disease. Consistent with our results, previous studies have shown that patients with CKD are at greater risk of tinnitus, SSNHL, and vestibular dysfunction than the normal population15,16,21. However, we also observed that middle-aged patients with CKD exhibited significantly increased risks of SSNHL and Ménière’s disease. Previous epidemiological studies have shown that SSNHL and Ménière’s disease have peak ages ranging between 30 and 60 years22,23. Thus, we consider that in middle-aged patients, the neuro-otologic organs may be more vulnerable to disturbances in water and electrolyte homeostasis. Additionally, we detected an increased risk of SSNHL in female patients with CKD compared to male patients. It remains unclear why female patients with CKD exhibited a significantly increased risk of SSNHL. However, another population-based study in South Korea showed that SSNHL has a slight female preponderance24; thus, we thought our finding may be due to this different sex preponderance.
This study has several unique strengths. First, we used a large national population-based database, which enabled us to effectively analyze all incidences of SSNHL and Ménière’s disease. Second, our cohort had a relatively long follow-up period (8 years). Third, the inclusion criteria of this study were based on an established diagnostic code, with the additional requirement of pure-tone audiometry for diagnosis. Finally, a prior study for validation of the KNHIS-NSC data revealed that the prevalence of 20 major diseases was similar for each year; thus, the reliability of the KNHIS-NSC data was defined as “fair to good.” Therefore, our findings suggest that CKD increases the risk of SSNHL and Ménière’s disease.
Our study also has some notable limitations. First, we could not obtain any specific personal health data, including body mass index, GFR level, lipid profiles, and information regarding behavioral risk factors, such as smoking or alcohol consumption. Second, SSNHL and Ménière’s disease were diagnosed based on the diagnostic code, which might be less accurate compared to the data obtained from medical charts that included details such as the medical history, imaging findings, or audiometry results. Third, we could not access the specific data such as the severity of hearing and vestibular impairment due to lacking in our registry; therefore, we were unable to investigate whether CKD influenced the severity of SSNHL and Ménière’s disease. Finally, family history, genetic conditions, and radiographic findings for SSNHL (such as enlarged vestibular aqueducts) could affect the potential for SSNHL and Ménière’s disease. However, in this cohort study, we could not include these variables as control variables, because our national insurance service does not cover these findings. Future clinical studies that investigate a wider range of factors and diagnostic criteria are needed to provide additional evidence for the link between CKD and SSNHL or Ménière’s disease.
In conclusion, this study investigated the possible link between CKD and the prospective development of SSNHL and Ménière’s disease. Interestingly, we observed that patients with CKD had a significantly higher risk of developing SSNHL and Ménière’s disease than non-CKD patients during an 8-year follow-up period. This finding suggests that CKD may be a risk factor for the development of SSNHL and Ménière’s disease; therefore, clinicians should consider patients with CKD to be at a high risk of developing SSNHL and Ménière’s disease and take specific measures to reduce the risk of developing these sequelae.
Methods
Data source and study population
This nationwide propensity score-matched cohort study was reviewed and approved by the Institutional Review Board of Hallym Medical University Chuncheon Sacred Hospital (Chuncheon, Korea). The Institutional Review Board of Hallym Medical University Chuncheon Sacred Hospital waived the written informed consent for this study due to the KNHIS-NSC dataset consists of de-identified secondary data for research purposes. This study adhered to the tenets of the Declaration of Helsinki. All citizens in Korea are obligated to enroll in the KNHIS, and a centralized large database provides access to nearly all data of the health insurance system. Therefore, the KNHIS contains reimbursement records from all medical facilities, including hospitals, private clinics, and public centers in South Korea. Claims are accompanied by data regarding diagnostic codes, procedures, prescription drugs, personal information about the patient, information about the hospital, the direct medical costs of both inpatient and outpatient care, and dental services. This study utilized the data of a representative sample of 1,025,340 adults from the 2002–2013 KNHIS-NSC in South Korea. This dataset accounted for approximately 2.2% of the South Korean population in 200225,26. Stratified random sampling was performed using 1476 strata by age (18 groups), sex (2 groups), and income level (41 groups: 40 health insurance groups and 1 medical aid beneficiary) among the South Korean population. There were no duplicated or omitted patient health care records as all South Korean residents receive a unique identification number at birth.
Study setting and participants
In this study, all disease diagnostic codes were identified using the International Classification of Diseases, 10th revision (ICD-10), Clinical Modification codes. The study design was a retrospective, nationwide propensity score-matched cohort study. The CKD group included all patients who received inpatient or outpatient care for an initial diagnosis of CKD (N18.1–5, N18.9) between January 2002 and December 2005. To further improve the accuracy of the CKD definition, we only included patients who had been diagnosed with CKD more than three times between 2002 and 2005. Patients were excluded if they (1) were diagnosed with SSNHL or Ménière’s disease between 2002 and 2005, (2) died as a result of any cause between 2002 and 2005 or as a result of an accident after 2006, and (3) were aged < 18 years. In the total 1,025,340 patient datasets, the number of CKD patients was identified using the ICD-10 code (N18.1–5, N18.9). Before selection, there were 11,382 CKD patients in the overall population (2002–2013). Among them, there were 3090 CKD patients in the period we decided to observe (2002–2005). A group of 2572 CKD patients was finally determined, excluding deaths and patients previously diagnosed with SSNHL and Ménière’s disease. The comparison group (non-CKD) comprised randomly selected propensity score-matched patients without CKD from the remaining cohort registered in the database (two for each patient with CKD). These patients were matched with patients with CKD for sociodemographic factors (age, sex, residential area, and household income), comorbidities, and the year of enrollment (CKD diagnosis). Eventually, 2572 and 5144 participants were enrolled in the CKD and comparison groups, respectively. All patients were monitored for the development of SSNHL or Ménière’s disease until December 2013. In this study, the endpoints of the study are the event (SSNHL or Ménière’s disease) or all-cause mortality. However, if patients had no events and were alive on December 31, 2013 (the final following period of this database) we censored this time point.
Study outcome
The risk of SSNHL or Ménière’s disease development was the main study outcome. Incidence of SSNHL or Ménière’s disease during the follow-up was defined as the presence of ICD-10 codes H91.2 (SSNHL) or H81.0 (Ménière’s disease) with additional pure-tone audiometry more than twice.
Independent variables
The study population was divided into two groups according to sex, three groups according to age (< 45, 45–64, and > 64 years), three groups according to household income (low: 0–30%, middle: 30–70%, and high: 70–100% of the median), and three groups according to area of residence (Seoul: the largest metropolitan region in South Korea, 2nd area: other metropolitan cities in South Korea, and 3rd area: small cities and rural areas). We also analyzed HTN and DM as comorbidities using the ICD-10 codes and prescription lists from the KNHIS-NSC database. HTN was defined as a previous diagnosis of hypertension (I10–11) and the use of antihypertensive drugs. DM was defined as a previous DM diagnosis (E10–14) and the use of one or more oral hypoglycemic agents or insulin. We defined the presence of comorbidities as any diagnoses of these codes between 2003 and 2004 prior to the diagnosis of SSNHL or Ménière’s disease.
Statistical analysis
The risks of SSNHL and Ménière’s disease were compared between the CKD and comparison groups as person-years at risk, which were defined as the duration between either the date of CKD diagnosis or January 1, 2003 (for the comparison group), and the patient’s respective endpoint. Incidence rates per 1000 person-years for SSNHL or Ménière’s disease were obtained by dividing the number of patients with incidence of specific diseases by person-years at risk. Person-years consisted of the following 3 cases: (1) In the case of death, the number of years from the date of initial diagnosis of CKD to the date of death; (2) In the case of side effects, the number of years from the first diagnosis of CKD to the date of the first diagnosis of side effects; (3) If there are no side effects, the number of years from the date of initial diagnosis of CKD to ‘2013-12-31’, the study endpoint. To identify whether CKD increased the risk of occurrence of specific diseases, we used Cox proportional hazards regression analyses to calculate the HRs and 95% CIs, adjusted for other predictor variables. All statistical analyses were performed using R (version 3.5.0; R Foundation for Statistical Computing, Vienna, Austria), with a significance level of 0.05.
Author contributions
D.-K.K. had the research idea and conceived the study design; J.Y.K. and D.-K.K. performed data acquisition; J.Y.K., S.L., and J.C. performed data analysis/interpretation; statistical analysis: S.L., J.C., G.S., and D-K.K. performed the statistical analysis; D-K.K. prepared the original draft; J.Y.K. and D.-K.K. were responsible for funding acquisition. The review and editing of the manuscript was supervised by all authors, under the mentorship of J.Y.K. and D-K.K. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute, funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HI17C2412 to Jong-Yeup Kim and 2021R1C1C1005746 to Dong-Kyu Kim).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Jong-Yeup Kim and Suehyun Lee.
References
- 1.Webster AC, Nagler EV, Morton RL, Masson P. Chronic kidney disease. Lancet. 2017;389:1238–1252. doi: 10.1016/S0140-6736(16)32064-5. [DOI] [PubMed] [Google Scholar]
- 2.Hill NR, et al. Global prevalence of chronic kidney disease—A systematic review and meta-analysis. PLoS ONE. 2016;11:e0158765. doi: 10.1371/journal.pone.0158765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N. Engl. J. Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 4.Kundhal K, Lok CE. Clinical epidemiology of cardiovascular disease in chronic kidney disease. Nephron. Clin. Pract. 2005;101:c47–c52. doi: 10.1159/000086221. [DOI] [PubMed] [Google Scholar]
- 5.Foley RN. Clinical epidemiology of cardiovascular disease in chronic kidney disease. J. Ren. Care. 2010;36:4–8. doi: 10.1111/j.1755-6686.2010.00171.x. [DOI] [PubMed] [Google Scholar]
- 6.Etgen T, Chonchol M, Förstl H, Sander D. Chronic kidney disease and cognitive impairment: A systematic review and meta-analysis. Am. J. Nephrol. 2012;35:474–482. doi: 10.1159/000338135. [DOI] [PubMed] [Google Scholar]
- 7.Zammit AR, Katz MJ, Bitzer M, Lipton RB. Cognitive impairment and dementia in older adults with chronic kidney disease: A review. Alzheimer Dis. Assoc. Disord. 2016;30:357–366. doi: 10.1097/WAD.0000000000000178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perlman RL, et al. Quality of life in chronic kidney disease (CKD): A cross-sectional analysis in the Renal Research Institute-CKD study. Am. J. Kidney Dis. 2005;45:658–666. doi: 10.1053/j.ajkd.2004.12.021. [DOI] [PubMed] [Google Scholar]
- 9.Krajewska WJ, Krajewski W, Zatoński T. Otorhinolaryngological dysfunctions induced by chronic kidney disease in pre- and post-transplant stages. Eur. Arch. Otorhinolaryngol. 2020;277:1575–1591. doi: 10.1007/s00405-020-05925-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meena RS, Aseri Y, Singh BK, Verma PC. Hearing loss in patients of chronic renal failure: A study of 100 cases. Indian J. Otolaryngol. Head Neck Surg. 2012;64:356–359. doi: 10.1007/s12070-011-0405-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jamaldeen J, Basheer A, Sarma AC, Kandasamy R. Prevalence and patterns of hearing loss among chronic kidney disease patients undergoing haemodialysis. Australas. Med. J. 2015;8:41–46. doi: 10.4066/AMJ.2015.2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peyvandi A, Roozbahany NA. Hearing loss in chronic renal failure patient undergoing hemodialysis. Indian J. Otolaryngol. Head Neck Surg. 2013;65:537–540. doi: 10.1007/s12070-011-0454-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gabr TA, Kotait MA, Okda HI. Audiovestibular functions in chronic kidney disease in relation to haemodialysis. J. Laryngol. Otol. 2019;133:592–599. doi: 10.1017/S0022215119001415. [DOI] [PubMed] [Google Scholar]
- 14.Mitschke H, Schmidt P, Zazgornik J, Kopsa H, Pils P. Effect of renal transplantation on uremic deafness: A long-term study. Audiology. 1977;16:530–534. doi: 10.3109/00206097709080024. [DOI] [PubMed] [Google Scholar]
- 15.Kang SM, Lim HW, Yu H. Idiopathic sudden sensorineural hearing loss in dialysis patients. Ren. Fail. 2018;40:170–174. doi: 10.1080/0886022X.2018.1450760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jung DJ, Lee KY, Do JY, Kang SH. Chronic kidney disease as a risk factor for vestibular dysfunction. Postgrad. Med. 2017;129:649–652. doi: 10.1080/00325481.2017.1338493. [DOI] [PubMed] [Google Scholar]
- 17.Quick CA, Fish A, Brown C. The relationship between cochlea and kidney. Laryngoscope. 1973;83:1469–1482. doi: 10.1288/00005537-197309000-00007. [DOI] [PubMed] [Google Scholar]
- 18.Gatland D, Tucker B, Chalstrey S, Keene M, Baker L. Hearing loss in chronic renal failure-hearing threshold changes following haemodialysis. J. R. Soc. Med. 1991;84:587–589. doi: 10.1177/014107689108401006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thodi C, Thodis E, Danielides V, Pasadakis P, Vargemezis V. Hearing in renal failure. Nephrol. Dial. Transplant. 2006;21:3023–3030. doi: 10.1093/ndt/gfl472. [DOI] [PubMed] [Google Scholar]
- 20.Scarpioni R, Ricardi M, Albertazzi V, De Amicis S, Rastelli F, Zerbini L. Dialysis-related amyloidosis: Challenges and solutions. Int. J. Nephrol. Renovasc. Dis. 2016;9:319–328. doi: 10.2147/IJNRD.S84784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shih CP, et al. Increased risk of tinnitus in patients with chronic kidney disease: A nationwide, population-based cohort study. PLoS ONE. 2017;12:e0183192. doi: 10.1371/journal.pone.0183192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Megighian D, Bolzan M, Barion U, Nicolai P. Epidemiological considerations in sudden hearing loss: A study of 183 cases. Arch. Otorhinolaryngol. 1986;243:250–253. doi: 10.1007/BF00464440. [DOI] [PubMed] [Google Scholar]
- 23.Bruderer SG, Bodmer D, Stohler NA, Jick SS, Meier CR. Population-based study on the epidemiology of Ménière’s disease. Audiol Neurootol. 2017;22:74–82. doi: 10.1159/000475875. [DOI] [PubMed] [Google Scholar]
- 24.Kim SH, Kim SJ, Im H, Kim TH, Song JJ, Chae SW. A trend in sudden sensorineural hearing loss: Data from a population-based study. Audiol. Neurootol. 2017;22:311–316. doi: 10.1159/000485313. [DOI] [PubMed] [Google Scholar]
- 25.Seong SC, et al. Cohort profile: the National Health Insurance Service-National Health Screening Cohort (NHIS-HEALS) in Korea. BMJ Open. 2017;7:e016640. doi: 10.1136/bmjopen-2017-016640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee J, Lee JS, Park SH, Shin SA, Kim K. Cohort profile: The National Health Insurance Service-National Sample Cohort (NHIS-NSC), South Korea. Int. J. Epidemiol. 2017;46:e15. doi: 10.1093/ije/dyv319. [DOI] [PubMed] [Google Scholar]