Abstract
Background:
Little is known about environmental food allergen exposure on school surfaces.
Objective:
Compare the distribution of major food allergens in floor dust and table wipe samples from elementary schools, and dust samples from students’ homes.
Methods:
In this sub-study of the School Inner-City Asthma Study-II, 103 table wipe samples and 98 floor dust samples from cafeterias and classrooms in 18 elementary schools were analyzed for milk, peanut, cashew, hazelnut and egg by multiplex array. Home kitchen floor and bed dust samples from 90 students were also analyzed.
Results:
Food allergens were detectable in schools, but at significantly lower levels than in homes (p<0.001). In schools, milk and peanut were detected in all table wipe samples; milk and egg were detected in all floor dust samples. Cafeteria table wipe samples contained significantly higher levels of milk, peanut, hazelnut, and egg, compared with classrooms. Cafeteria floor dust samples contained higher levels milk than classrooms. Peanut-restrictive policies did not consistently reduce environmental peanut exposure in schools. Peanut allergen was lower in dust from homes of students with peanut allergy (n=5) compared to without (n=85) (p<0.001). Reassuringly, peanut allergen in the schools of peanut-allergic students was not significantly different than in their homes.
Conclusion:
Food allergens were readily detectable on tables and floors in elementary schools, but at levels lower than in students’ homes. For peanut-allergic students, the levels of detectable peanut in their schools were not higher than their homes. The low levels of detectable food allergens in school environments are unlikely to result in severe allergic reactions.
Keywords: food allergen, food allergy, inner-city, school, exposure, environment, multiplex array for foods
Introduction
The prevalence of IgE-mediated food allergy (FA) is increasing, with milk, egg, peanut, and tree nuts being the most common triggers in pediatric patients.1, 2 Small amounts of these allergens can accumulate in the dust which collects on table surfaces and floors. Cutaneous contact to peanut in home dust has been associated with an increased risk of peanut sensitization, especially in children with atopic dermatitis.3, 4 For people with established FA, reactions from food protein in the environment is a concern.5 Prior studies have evaluated environmental food allergen exposures in homes, restaurants, and airplanes.6–8 Children spend the majority of their days in school, and little is known about food allergen exposure in the school environment. To date, US studies evaluating environmental food exposure in schools have focused exclusively on peanut.9, 10 In 2004, Perry et al. reported that Ara h 1 was detected by monoclonal ELISA on only one water fountain, and not on desks or cafeteria tables, in six schools.9 In contrast, in 2017, Sheehan et al. found that whole peanut protein was detectable by polyclonal ELISA in 100% of vacuumed floor dust samples from 18 elementary schools. Despite studies evaluating peanut exposure in schools, there is little understanding of the distribution of non-peanut environmental food protein levels and how they compare across US homes and schools. Furthermore, there is little evidence that food allergen-restrictive policies reduce allergic reactions or improve quality of life.11–13
The major objectives of this study were to: 1) evaluate the detection rates and quantify levels of the major food allergens: milk, peanut, tree nuts and egg, in table wipes and floor dust in schools; 2) determine the difference in food allergen levels in cafeterias compared to classrooms; and 3) compare distribution of food allergen levels across home and school environments of children with and without IgE-mediated FA.
Materials & Methods
School Inner-City Asthma Study II (SICAS-II)
This was a sub-study of SICAS-II, a prospective study intervening upon school-specific environmental risk factors for asthma (ClinicalTrials.gov Identifier: NCT02291302).14, 15 Boston Children’s Hospital Institutional Review Board and the participating school systems approved the protocol. All 18 elementary schools from the first two years of SICAS-II were included and samples were collected prior to the SICAS-II intervention. All schools allowed students to bring peanut and tree nut-containing foods, but 13 individual classrooms restricted peanut. Ninety students had dust samples collected from their home kitchen floors and beds.
Sample collection
Wipes from 103 tables/desks were analyzed for food allergen, one from each of the 86 classrooms and 17 cafeterias. One square foot on a table/desk was wiped with filter paper (Kimwipes 4×8, Kimberly-Clark, Roswell, GA) moistened with endotoxin-free extract (phosphate buffered saline, pH 7.4, containing 1% Tween-20, Sigma-Aldrich, St Louis, MO).16 Floor dust samples from the same room as the table/desk wipe samples were also collected, as previously described,17, 18 of which 98 (95.1%) had sufficient quantity of dust for analysis (16 from cafeterias, 82 from classrooms). School samples were obtained after student dismissal. One sample from the bed and one sample from the kitchen floor were collected from the homes of 90 students. Floor and bed samples were obtained per standardized protocol using a vacuum (Oreck model BB870-AD, Oreck, Nashville, TN) with dust collector (DACI laboratory, Johns Hopkins University, Baltimore, MD).19 Table wipe samples were extracted as previously described.16 Dust samples were sieved and extracted for allergen measurement.
Measurement of Food Allergens
Food allergens were measured using a multiplex array for indoor allergens (MARIA) which has been used to assess indoor allergen exposure in SICAS.20, 21 It measured seven food allergens simultaneously (Luminex xMAP, Luminex Corp., Austin TX) using the same monoclonal antibody pairs that have been used in ELISA.22 Mass spectrometry calibration standards showed ≥95% purity.
Table wipe and dust samples were analyzed for milk (Bos d 5-β-lactoglobulin), peanut (Ara h 3, Ara h 6), cashew (Ana o 3), hazelnut (Cor a 9) and egg (Gal d 1-ovomucoid, Gal d 2-ovalbumin). Hazelnut and cashew were the tree nuts selected, as their measurement had been previously validated using multiplex array. Table wipe extracts were analyzed undiluted and at 1/5 and 1/20 dilutions. For samples that contained high allergen levels, Bos d 5 was diluted and analyzed at 1/10, 1/100 and 1/10,000. The lower limits of detection (LLOD) for wipe and dust samples respectively, were: Bos d 5, 0.02 ng/ml and 0.004 μg/g; Ara h 3, 0.06 ng/ml and 0.013 μg/g; Ara h 6, 0.01 ng/ml and 0.002 μg/g; Ana o 3, 0.02 ng/ml and 0.004 μg/g; Cor a 9, 0.02 ng/ml and 0.004 μg/g; Gal d 1, 0.98 ng/ml and 0.218 μg/g; and Gal d 2, 0.02 ng/ml and 0.004 μg/g. For samples below the LLOD, the value was set to the LLOD for data analysis.
Determination of FA Status and Allergen Exposure in Schools and Homes
In SICAS-II, students with asthma were screened and recruited from elementary schools. After obtaining consent, trained interviewers administered a comprehensive baseline survey to the child’s caregiver that included detailed questions regarding FA. The FA section included assessment of symptoms experienced within one hour of food ingestion. Our definition of IgE-mediated FA was consistent with other studies and included cutaneous, gastrointestinal, respiratory, and cardiovascular symptoms.23 Subjects were not considered to have FA if reported symptoms were not consistent with IgE-mediated FA. Caregivers were asked about food allergen exposures in their child’s school and home. None of the schools or homes restricted milk or egg. Thirteen caregivers reported that their child’s classroom was peanut-free. All caregivers of children with peanut allergy (n=5) reported that their homes were peanut-free.
Statistical Analyses
Statistical analysis was performed using IBM SPSS Statistics for Windows, Version 24.0. (Armonk, NY). Continuous data for allergen measurements are expressed as medians and interquartile ranges. Spearman correlation was used. Independent-samples Kruskal-Wallis was used to compare distribution of each specific food allergen across locations, and Mann-Whitney U test was used for pairwise comparison. Generalized linear models (gamma family, log link), were used to compare differences between allergen levels with the following dichotomous factors: 1) school vs. home, 2) food-allergic participant vs. non-food allergic participant, and 3) kitchen/cafeteria (traditional food consumption areas) vs. bedroom/classroom. We retained interaction terms if p<0.10. A p-value <0.05 was considered statistically significant. Significance values were adjusted by the Bonferroni correction for multiple tests.
Results
Detection and quantification of food allergens in schools
Detection rates and food allergen levels from table wipe and settled floor dust samples are described in Table I. Milk was detected in 100% of table wipe and floor dust samples and present at the highest concentration, with a median level of 53.3 ng/mL in table wipe samples, and 11.6 μg/g in dust samples. Peanut and egg allergens were prevalent, however at low levels of exposure. Ara h 6 was detected in 100% of the table wipe samples and 90.8% of the dust samples. Gal d 2, was detected in 73.8% of the table wipe samples and 100% of the floor dust samples. Hazelnut was the least commonly detected. There were no strong correlations between levels of specific allergens in table wipes and floor dust collected from matched rooms, but moderate correlation for milk (r=0.452, p<0.001).
Table I.
Food allergens detected in elementary school table wipes and floor dust.
| Table Wipe Samples (N=103) | Floor Dust Samples (N=98) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Allergen Levels (ng/mL) | Allergen Levels (μg/g) | ||||||||
| Allergen by Component | Detectable, % (N) | 25th Percentile | Median | 75th Percentile | Detectable, % (N) | 25th Percentile | Median | 75th Percentile | |
| Milk | Bos d 5 | 100% (103) | 5.0 | 53.3 | 306.7 | 100% (98) | 3.6 | 11.6 | 30.7 |
| Peanut | Ara h 3 | 87.4% (90) | 0.11 | 0.31 | 0.75 | 48.0% (47) | ND | ND | 0.058 |
| Ara h 6 | 100% (103) | 0.13 | 0.28 | 0.55 | 90.8% (89) | 0.011 | 0.024 | 0.069 | |
| Cashew | Ana o 3 | 98.1%(101) | 0.06 | 0.10 | 0.20 | 65.3% (64) | ND | 0.008 | 0.019 |
| Hazelnut | Cor a 9 | 22.3% (23) | ND | ND | ND | 33.7% (33) | ND | ND | 0.008 |
| Egg | Gal d 1 | 55.3% (57) | ND | 1.15 | 2.87 | 75.6% (74) | 0.23 | 0.50 | 1.17 |
| Gal d 2 | 73.8% (76) | ND | 0.14 | 0.45 | 100% (98) | 0.73 | 1.97 | 4.80 | |
ND=Not detectable (value below lower limit of detection).
Comparison between food allergen levels in school cafeterias and classrooms
We compared differences between the distribution of food allergens in cafeterias and classrooms (Table II). Table wipe samples from cafeterias, contained significantly higher levels of milk, Bos d 5 (p=0.006), peanut, Ara h 3 (p=0.001), hazelnut, Cor a 9 (p=0.027), and egg, Gal d 1 (p=0.033) compared with those from classrooms. Floor dust samples from cafeterias contained significantly higher levels milk allergen than those from classrooms, (72.8 μg/g vs 8.5 μg/g, p=0.001), presumably due to frequent spillage of milk. There were no significant differences in the distribution of the remaining food allergens in floor dust between classrooms and cafeterias.
Table II:
Comparison of food allergen exposure in school cafeterias and classrooms.
| Table Wipe Samples (N=103) | Floor Dust Samples (N=98) | ||||||
|---|---|---|---|---|---|---|---|
| Median (IQR) (ng/mL) | Median (IQR) (μg/g) | ||||||
| Allergen by Component | Cafeteria (N=17) | Classroom (N=86) | p | Cafeteria (N=16) | Classroom (N=82) | p | |
| Milk | Bos d 5 | 673.8 (57–5999) | 28.6 (4.73–261.0) | 0.006 | 72.8 (12.3–123.0) | 8.5 (3.1–24.7) | 0.001 |
| Peanut | Ara h 3 | 0.86 (0.42–4.71) | 0.24 (0.10–0.58) | 0.001 | 0.03 (ND–0.08) | ND (ND–0.05) | 0.157 |
| Ara h 6 | 0.28 (0.12–0.43) | 0.28 (0.14–0.55) | 0.965 | 0.04 (0.02–0.15) | 0.02 (0.01–0.06) | 0.146 | |
| Cashew | Ana o 3 | 0.12 (0.07–0.19) | 0.10 (0.06–0.19) | 0.499 | 0.005 (ND–0.035) | 0.009 (ND–0.020) | 0.412 |
| Hazelnut | Cor a 9 | ND (ND–0.04) | ND (ND–ND) | 0.027 | ND (ND–0.30) | C ND (ND–0.008) | 0.337 |
| Egg | Gal d 1 | 1.66 (1.2–6.74) | 0.98 (ND–2.63) | 0.033 | 0.57 (0.24–1.32) | 0.48 (0.22–1.12) | 0.462 |
| Gal d 2 | 0.61 (0.05–4.56) | 0.13 (ND–0.35) | 0.053 | 1.51 (0.60–3.10) | 2.16 (0.76–4.84) | 0.332 | |
Milk, peanut (Ara h 3), hazelnut, and egg (Gal d 1) levels were higher in the cafeteria table wipe samples. Milk levels were also significantly higher in floor dust samples.
ND=Not detectable (value below lower limit of detection).
Peanut allergen levels in peanut-restricted classrooms versus non-restricted classrooms
Table wipe samples from classrooms that restricted peanut (n=13) had significantly lower Ara h 6 than samples from classrooms that did not restrict peanut (n=69) (median=0.15 vs 0.31, p=0.03), however, there was no significant difference in median Ara h 3 (0.18 versus 0.23, p=0.31). Furthermore, in floor dust samples, median Ara h 3 was significantly higher in the dust from classrooms that restricted peanut allergen than those that did not (median=0.056 vs 0.013, p=0.002). Median Ara h 6 was also higher in peanut-restricted rooms than those that were not peanut-restricted (0.045 vs 0.018), but this did not reach statistical significance, p=0.12.
Correlation between components of the same food and between different foods in school samples
We next evaluated the degree of correlation between different food allergen components measured within the same sample (Figure 1). Peanut components correlated in table wipes (r=0.555, p<0.001) and floor dust (r=0.706, p<0.001). Egg components also correlated in table wipes (r=0.464, p<0.001) and floor dust (r=0.708, p<0.001). There were notable relationships between food allergens from different foods quantified in matched table wipe (Figure 1A) and floor dust samples (Figure 1B). There was strong correlation between milk and egg in table wipe (r=0.641, p<0.001) and dust samples (r=0.527, p<0.001). There were strong correlations between levels of peanut components and the measured tree nuts in table wipes and floor dust samples.
Figure 1. Correlation coefficients between food allergens in the elementary school environment.
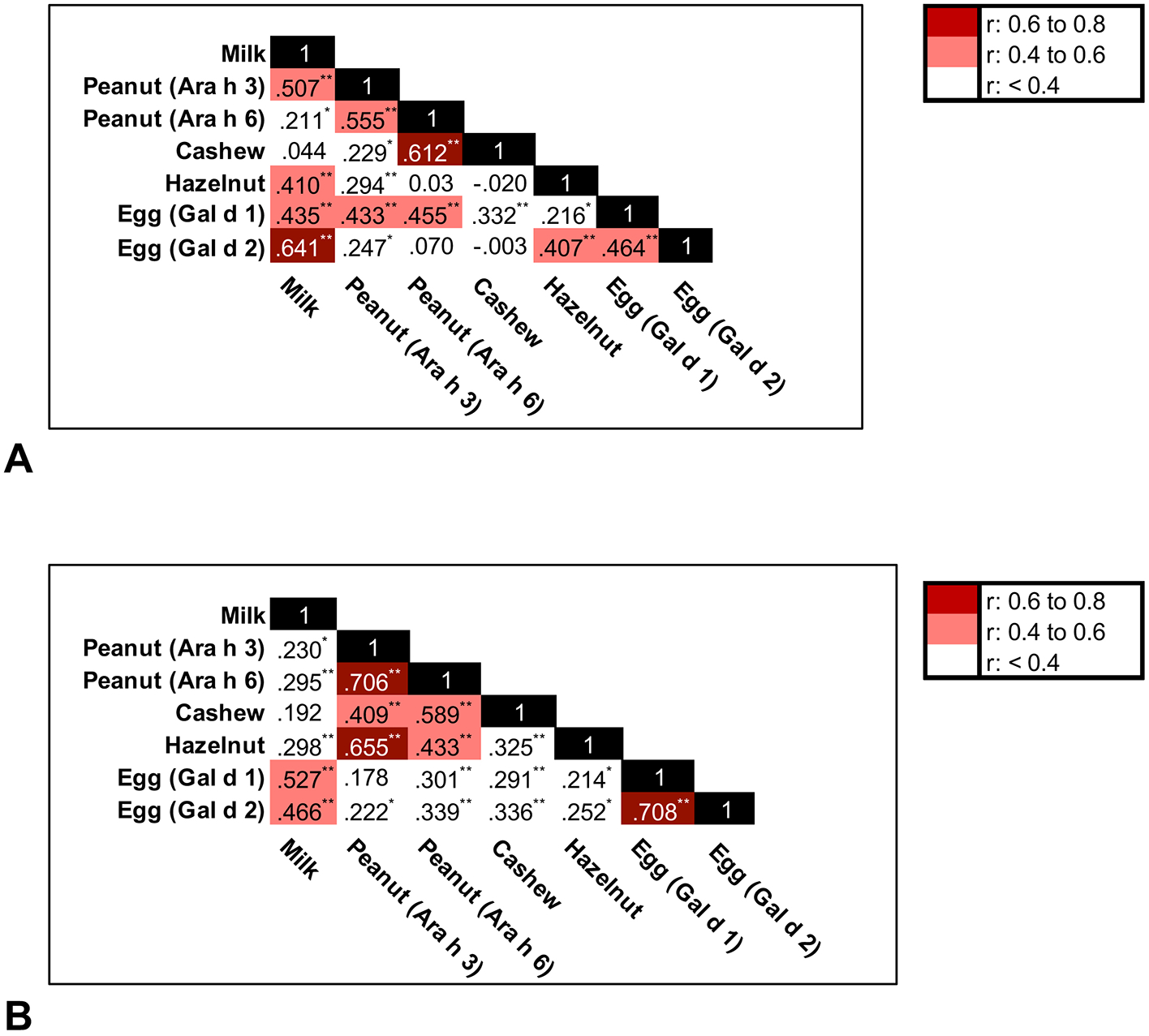
(A) Table wipe samples (N=103), and (B) Floor dust samples (N=98). Correlation significant *P<0.05, **P<0.01.
Food allergen exposure in home kitchen and bed dust
After analyzing levels of common food allergens in the schools, we evaluated levels of food allergens in students’ homes. Food allergens were commonly detected in dust samples from homes (Table III): >97% of kitchen floor and bed samples contained detectable milk, peanut, and egg. Egg (Gal d 2) was the most prevalent allergen in kitchens and was present at the highest level. It was significantly higher in kitchen samples compared to bed samples (p<0.001). In contrast, Ara h 3 was significantly higher in bed samples than kitchen samples (p=0.035). There was no significant difference between kitchens and beds for the remaining allergens. Components within the same food were strongly correlated (Figure 2). There was moderate correlation between milk and egg, and between peanut and tree nuts.
Table III.
Detection rates for food allergens in home dust samples (kitchen and bed) and levels of allergen detected.
| Home Dust Samples (N=180) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Kitchen Floor (N=90) | Bed (N=90) | |||||||||
| Allergen Levels (μg/g) | Alle rgen Levels (μg/g) | |||||||||
| Allergen by Component | Detectable % (N) | 25th Percentile | Median | 75th Percentile | Detectable % (N) | 25th Percentile | Median | 75th Percentile | p | |
| Milk | Bos d 5 | 97.8% (88) | 8.44 | 29.57 | 91.58 | 100% (90) | 8.56 | 19.74 | 50.55 | 0.914 |
| Peanut | Ara h 3 | 90% (81) | 0.039 | 0.18 | 1.23 | 93.3% (84) | 0.22 | 0.76 | 2.07 | 0.035 |
| Ara h 6 | 97.8% (88) | 0.19 | 0.045 | 0.61 | 97.8% (88) | 0.10 | 0.33 | 0.96 | 0.480 | |
| Cashew | Ana o 3 | 82.2% (74) | 0.005 | 0.017 | 0.11 | 96.7% (87) | 0.011 | 0.033 | 0.084 | 0.174 |
| Hazelnut | Cor a 9 | 48.9% (44) | ND | ND | 0.24 | 60% (54) | ND | 0.008 | 0.054 | 0.661 |
| Egg | Gal d 1 | 97.8% (88) | 4.15 | 11.31 | 30.7 | 100% (90) | 1.04 | 2.73 | 4.86 | <0.001 |
| Gal d 2 | 100% (90) | 11.47 | 74.08 | 259.3 | 100% (90) | 2.58 | 8.97 | 20.26 | <0.001 | |
ND=Not detectable (value below lower limit of detection).
Figure 2. Correlation coefficients between food allergens in home floor dust samples of elementary school students.
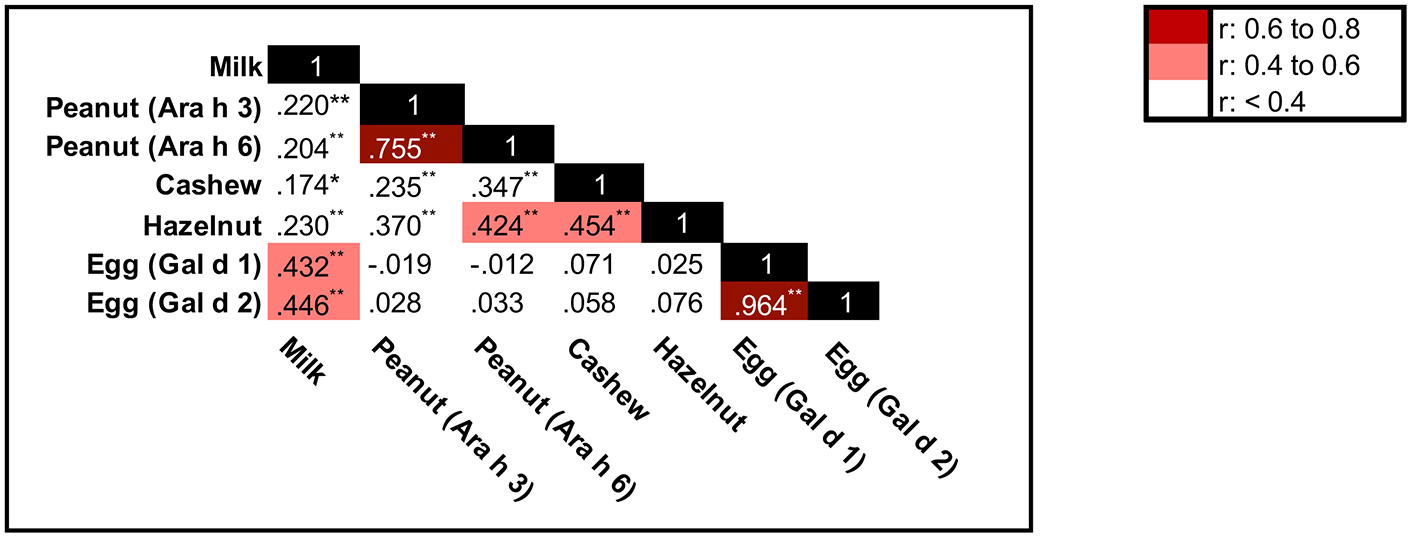
(N=180). Correlation significant *P<0.05, **P<0.01.
Comparison of food allergen levels in dust across school and home environments
Overall, school samples contained significantly lower levels of each food allergen respectively compared with home samples (p<0.001 for all allergens) (Figure 3). Using generalized linear models, compared to classrooms, home kitchen samples contained almost 5 times more milk allergen (95% CI 201–962%, p<0.001), and bed samples contained two times more (95% CI 74–515%, p<0.001). Peanut (Ara h 6) was 41 times higher in homes compared with schools (95% CI 702% to 21840%, p<0.001). Egg (Gal d 2) was 98 times higher in home kitchens compared with levels detected in schools (95% CI 4379–21788%, p<0.001). Beds contained more than 2 times the level of Gal d 2 found in schools (95% CI 66–710%, p<0.001). The significant differences based on location varied by allergen (Figure 3).
Figure 3: Food Allergen Levels Across Home and School Locations.
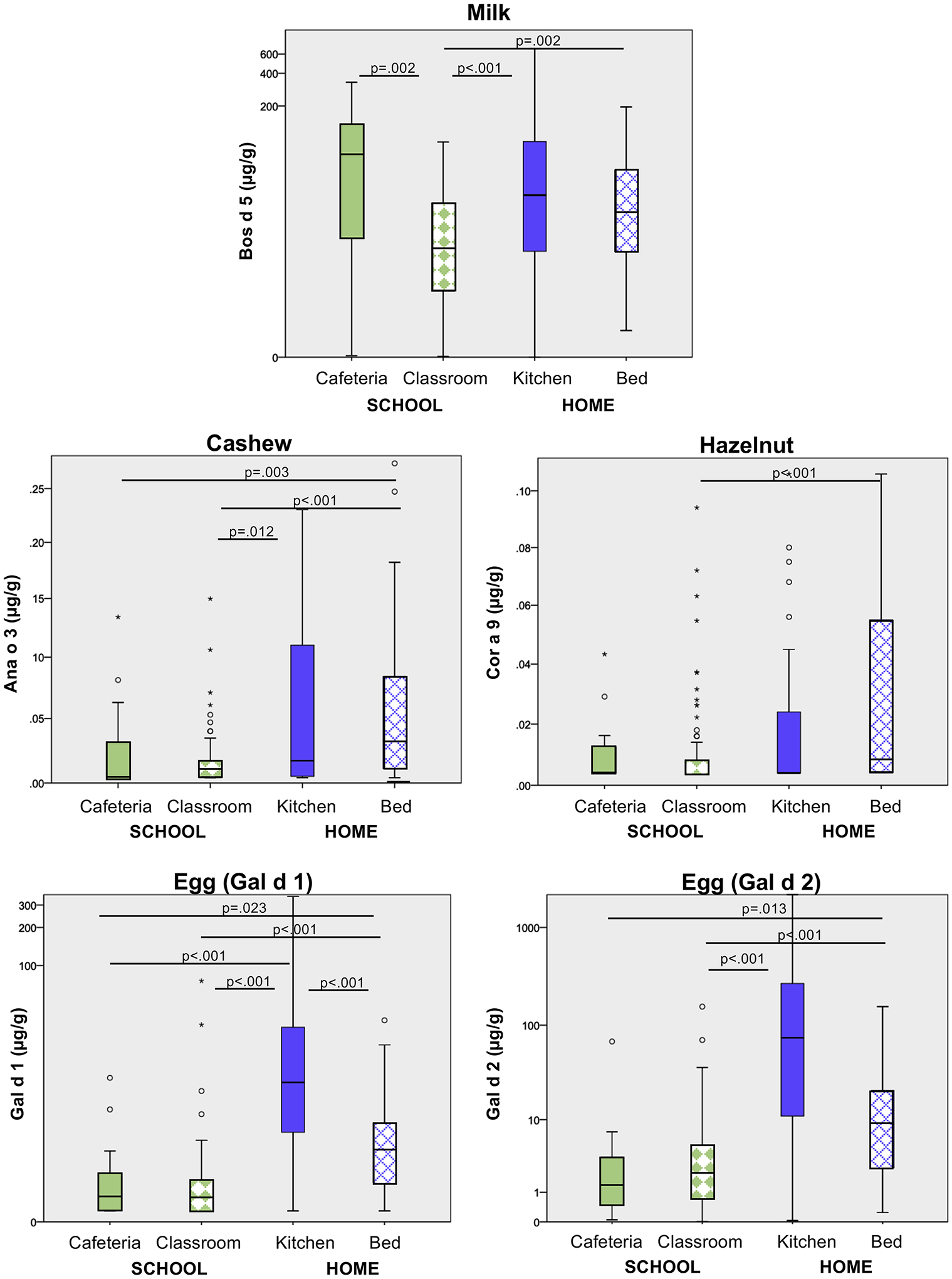
There were 98 school samples (16 cafeteria and 82 classroom) and 180 home samples (90 from the kitchen and bed, respectively). Only significant differences shown. For each of the allergens, the distribution of food allergen detected from homes was significantly higher than the schools (p<0.001 for all). Pairwise comparison by Mann Whitney U test.
Food allergen levels in the environments of food allergic children
There was a significant difference in the distribution of peanut allergen found in the environments of those with (n=5) and without (n=85) IgE-mediated peanut allergy. All caregivers of peanut-allergic students reported that their homes were peanut-restricted. Ara h 3 was lower in the kitchens of those with peanut allergy compared to those without peanut allergy (p=0.020). Ara h 3 and Ara h 6 were significantly lower in the beds of those with peanut allergy than those without (p<0.001 and p=0.001, respectively). For the children with peanut allergy, there was no significant difference in peanut allergen level across school and home environments (Figure 4). Overall, Ara h 3 was 44 times higher in homes without a peanut allergic child compared to school locations (95% CI 1525–12589%, p<0.001).
Figure 4: Distribution of peanut allergen in environments of those with and without peanut allergy.
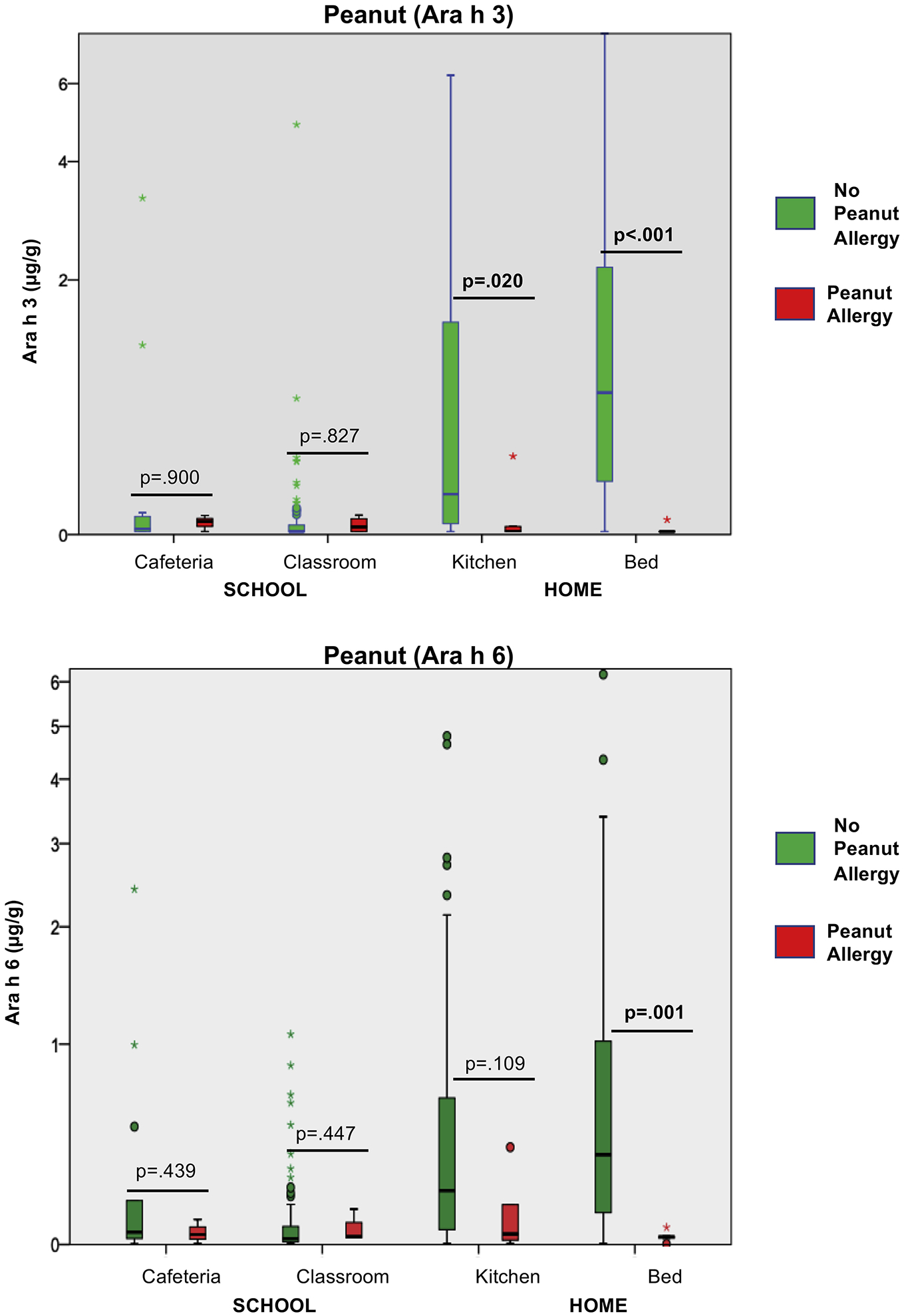
Ara h 3 and Ara h 6 in floor dust from environments of children with (n=5) and without (n=85) peanut allergy. Ara h 3 was significantly lower in the kitchens and beds of those with peanut allergy than those without. There was no significant difference between distribution of allergen in the home and school environments in children with peanut allergy (Ara h 3, p=.616) (Ara h 6, p=.843). Pairwise comparison by Mann Whitney U test.
Despite caregivers reporting their children having IgE-mediated milk allergy (n=5), and egg allergy (n=4), no families restricted milk or egg from their homes. We found no significant differences in distribution of milk and egg allergens across environments of those with and without those allergies, respectively.
Discussion
This comprehensive study conducted in inner-city elementary schools in the northeastern US found that common food allergens are readily detectable in dust collected from table surfaces and floors. However, the levels are low, and risk of anaphylaxis from touching or inhaling foods is very small. Almost all cases of reported food-induced anaphylaxis in schools have resulted from ingestion and not from casual contact with environmental surfaces.24 This represents the first analysis of environmental food allergen exposure in US schools for foods other than peanut and illustrates how allergen levels vary across the school environment. For most of the foods analyzed, table wipe samples from cafeterias contained significantly higher levels of food allergens compared with wipes from classrooms. However, there was little difference in allergen levels from these two locations in dust samples. Although several classrooms restricted peanut, peanut allergen levels were not consistently lower in these classrooms than in classrooms that did not restrict peanut. Food allergen levels in the school were significantly lower than levels in the home. Reassuringly, there was no difference between the level of peanut allergen detected in schools and in the homes of peanut-allergic students.
US studies that have evaluated environmental school food allergen exposure have focused exclusively on peanut,9, 10 possibly because the inadvertent ingestion of peanut by allergic individuals is the most common cause of fatal food-allergic reactions.25, 26 The current study confirmed that while peanut protein was commonly detected in schools, so were tree nuts, milk and egg. Milk was detected in 100% of school samples and in the highest levels. For comparison with other environmental allergens found in the school environment, the median level of milk protein in floor dust from schools in our study was 11.6 μg/g, while a prior investigation of environmental allergens in schools showed that the median levels of mouse, cat and dog were 0.90, 0.23 and 0.11 μg/g respectively.27 The clinical implications of this relatively higher level of milk exposure are unclear, but significantly less than that expected to cause a systemic allergic reaction if ingested. Dairy is a substantial component of the diet in many children and is readily available in schools through subsidized programs.28–30
As expected, we detected a variety of food allergens in the cafeteria samples, and for most of the allergens, levels were higher on table wipes from cafeterias than from classrooms. Surprisingly, except for milk, there was no significant difference between the amount of allergen detected in floor dust samples from these two school locations. There is little known about the transfer or the eradication of food allergens in the environment for foods other than peanut. While gross spillage of peanut can be easily cleaned,9 complete elimination of detectable peanut protein can be difficult and peanut is easily transferred throughout the environment.6 Students’ hands and shoes, or cleaning agents like mops and brooms can inadvertently transfer food allergens throughout the school. Differences in cleaning tools and solvents used on tables compared to floors may underscore the discrepancy between food allergen levels detected in table wipe versus floor dust samples, and account for variation across the schools and homes studied.
There was strong correlation between components from the same food within the same sample type (e.g., Ara h 3 and Ara h 6 in floor dust). There were also significant correlations between different foods. Milk and egg levels correlated in matched samples, while peanut and tree nut levels also correlated significantly. Milk and egg are often consumed together in baked goods, and peanuts and tree nuts are often consumed in nut mixtures or protein bars.
Peanut levels were low in schools overall, but not consistently lower in samples from peanut-restricted classrooms, perhaps due to difficulty enforcing these restrictions, or the easy transfer of peanut allergen.31–33 From a broader lens, understanding the impact on peanut-restricted classrooms on the quality of life of students and families is an essential area of research. Unfortunately, there is little data available regarding the impact of allergen-restricted zones. Studies to date have not consistently found a benefit in terms of decreasing risk of reaction or improving quality of life.11–13 Evidence-based analyses of how school food allergen policies mitigate environmental food allergen exposure across multiple school environments and sample types, and possible impact on the frequency or severity of allergic reactions, are warranted.
Food allergy diagnosis is life-altering for children and their families. Balancing appropriate avoidance of the ingestion of known triggers with continued engagement in school activities is challenging.34 Despite data on peanut demonstrating that casual contact or inhalation does not constitute a significant risk for severe reactions,9, 35 perceived risk is higher for some families. Integrity of the skin barrier may play an important role in the development of cutaneous reactions to food proteins. In two studies of peanut-allergic children, peanut products were applied to the skin. No systemic reactions occurred and a minority of subjects developed minor local cutaneous reactions.35, 36 There are several reports of anaphylaxis from cutaneous exposure to milk.37–41 In three cases, milk-based creams were applied to an impaired to skin barrier.37, 39, 41 These frank cutaneous exposures are undoubtedly more dangerous than the low-level environmental exposures we detected, and systemic reactions to food allergen, when it is not explicitly ingested, are exceptionally rare.42
Many school food allergy policy decisions may be made out of fear, misunderstanding of food allergy pathophysiology or risks, or based on anecdotal reports of severe allergic reactions.9 Our study provides important real-world data that it is highly improbable that enough environmental food allergen in schools could be ingested to precipitate a systemic reaction, or that cutaneous exposure to minute amounts of these food proteins could cause a clinical reaction.
Given the low levels of food allergen detected in our study, it is highly unlikely that environmental exposure to food allergen in schools could cause a significant allergic reaction. The threshold dose that elicits an objective reaction varies, from about 0.5 mg up to 8,000–10,000 mg.43, 44 A randomized, double-blind, placebo-controlled food challenge of 14 peanut-allergic subjects found objective reactions to as low as 2 mg peanut protein, but subjective symptoms at doses as low as 100 μg.45 The amount of peanut allergen detected in the schools was significantly lower than this level and unlikely to cause allergic reaction, even for the most sensitive.
Theoretically, low levels of cutaneous exposure to food allergen may precipitate the development of allergic sensitization, especially in susceptible individuals such as those with atopic dermatitis. This has been previously shown for peanut.3, 4 The “dual allergen exposure hypothesis” suggests that low-dose, cutaneous exposure may be sensitizing, while oral exposure could be tolerizing.46–48 Health outcomes associated with these surface exposures were not within the scope of this study, but remain an important area of research.
There is a lack of data available regarding the detection of non-peanut foods on US school surfaces and how this compares to levels in homes. Two Norwegian studies reported detection of codfish and egg white allergens in students’ home and school environments. In both studies, higher levels were detected in homes.49, 50 Our study echoes these findings, and demonstrates that food allergen levels were significantly lower in schools than in homes. However, we found significantly lower Ara h 3 and Ara h 6 levels in the homes of those with peanut allergy. Peanut allergen levels in the school environments of peanut-allergic students were not significantly different than the levels found in their homes.
This study has several limitations. As a sub-analysis of the School Inner-City Asthma Study-II, it was not specifically designed to evaluate the differences in food allergen levels in the home and school of food-allergic children versus nonallergic children. Furthermore, samples from allergen-restricted tables were not specifically collected. Results may not be generalizable as it was conducted in an inner-city, northeastern elementary school population. There were few food-allergic participants, which may limit our ability to detect a difference between allergen levels from environments of those with and without FA. This may explain why findings were at times significant for Ara h 3 or Ara h 6, but not both when expected. Allergen restriction in specific classrooms was reported by caregivers, which introduces bias. Although it was not possible to associate our data with reports of allergic reaction in the schools, this is an important area of future study.
In conclusion, the high rates of detection of food allergens highlight the variety and extent of environmental food allergen exposures on surfaces in cafeterias and classrooms. Levels of food allergen were lower in school environments compared to students’ homes. Anaphylactic reactions in schools are rare,11 suggesting these low levels of environmental exposures in schools are unlikely to result in severe allergic reactions. Although sample size of peanut-restricted classrooms was small, we did not find that peanut-restrictive policies consistently reduced environmental peanut exposure in schools. Our findings may offer guidance that food allergy policies can be most impactful by focusing on minimizing oral ingestions of food allergens, rather than attempting to eliminate potential surface contact exposures. Further research is warranted to better understand the clinical implications of environmental exposures to food allergens and the impact of restrictive food allergen policies in schools.
Highlights Box:
What is already known about this topic?
Food allergen proteins are detectable in table wipes and vacuumed floor samples in inner-city US elementary schools.
What does this article add to our knowledge?
Milk, egg, peanut, and tree nut allergens are readily detectable in environmental samples from US elementary schools, but at lower levels than in students’ homes.
How does this study impact current management guidelines?
Further investigation is needed to determine the clinical implications and possible impact on school policies.
Funding:
This research is supported by the National Institutes of Health T32 AI007512 (Maciag), K23 AI143962 (Bartnikas), K23 AI104780 (Sheehan), R01 AI073964, R01 AI073964-02S1, K24 AI106822, U10 HL098102, U01 AI110397, R01 HL137192, U19AR06952 (Phipatanakul). This work was also funded in part by a grant from the European Union 7th Framework, Project No. FP7-KBBE-2012-6-312147 (Indoor Biotechnologies).
This work was conducted with support from Harvard Catalyst | The Harvard Clinical and Translational Science Center (National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health Award UL1 TR001102) and financial contributions from Harvard University and its affiliated academic healthcare centers. The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic healthcare centers, or the National Institutes of Health.
Conflicts of Interest:
W. Phipatanakul is a consultant advisory for Teva, Genentech, Novartis, GSK and Regeneron, for asthma-related therapeutics. M. Chapman receives grant support from the National Institute of Allergy and Infectious Diseases and is a co-owner and employee of Indoor Biotechnologies. S. Filep is an employee of Indoor Biotechnologies. The rest of the authors declare that they have no relevant conflicts of interest.
Abbreviations:
- FA
food allergy
- MARIA
multiplex array for indoor allergens
- SICAS-II
School Inner-City Asthma Study II
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Sicherer SH, Sampson HA. Food allergy. J Allergy Clin Immunol 2010; 125:S116–25. [DOI] [PubMed] [Google Scholar]
- 2.Sicherer SH, Sampson HA. Food allergy: A review and update on epidemiology, pathogenesis, diagnosis, prevention, and management. J Allergy Clin Immunol 2018; 141:41–58. [DOI] [PubMed] [Google Scholar]
- 3.Brough HA, Liu AH, Sicherer S, Makinson K, Douiri A, Brown SJ, et al. Atopic dermatitis increases the effect of exposure to peanut antigen in dust on peanut sensitization and likely peanut allergy. J Allergy Clin Immunol 2015; 135:164–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brough HA, Simpson A, Makinson K, Hankinson J, Brown S, Douiri A, et al. Peanut allergy: effect of environmental peanut exposure in children with filaggrin loss-of-function mutations. J Allergy Clin Immunol 2014; 134:867–75.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bollinger ME, Dahlquist LM, Mudd K, Sonntag C, Dillinger L, McKenna K. The impact of food allergy on the daily activities of children and their families. Ann Allergy Asthma Immunol 2006; 96:415–21. [DOI] [PubMed] [Google Scholar]
- 6.Brough HA, Makinson K, Penagos M, Maleki SJ, Cheng H, Douiri A, et al. Distribution of peanut protein in the home environment. J Allergy Clin Immunol 2013; 132:623–9. [DOI] [PubMed] [Google Scholar]
- 7.Brough HA, Santos AF, Makinson K, Penagos M, Stephens AC, Douiri A, et al. Peanut protein in household dust is related to household peanut consumption and is biologically active. J Allergy Clin Immunol 2013; 132:630–8. [DOI] [PubMed] [Google Scholar]
- 8.Jin JJ, Dorn JM, Yunginger J, Ott NL. Ara h 2 is detectable on surfaces of commercial airplanes. J Allergy Clin Immunol Pract 2019; 7:659–61.e2. [DOI] [PubMed] [Google Scholar]
- 9.Perry TT, Conover-Walker MK, Pomes A, Chapman MD, Wood RA. Distribution of peanut allergen in the environment. J Allergy Clin Immunol 2004; 113:973–6. [DOI] [PubMed] [Google Scholar]
- 10.Sheehan WJ, Brough HA, Makinson K, Petty CR, Lack G, Phipatanakul W. Distribution of peanut protein in school and home environments of inner-city children. J Allergy Clin Immunol 2017; 140:1724–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bartnikas LM, Huffaker MF, Sheehan WJ, Kanchongkittiphon W, Petty CR, Leibowitz R, et al. Impact of school peanut-free policies on epinephrine administration. J Allergy Clin Immunol 2017; 140:465–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nguyen-Luu NU, Ben-Shoshan M, Alizadehfar R, Joseph L, Harada L, Allen M, et al. Inadvertent exposures in children with peanut allergy. Pediatr Allergy Immunol 2012; 23:133–9. [DOI] [PubMed] [Google Scholar]
- 13.Cherkaoui S, Ben-Shoshan M, Alizadehfar R, Asai Y, Chan E, Cheuk S, et al. Accidental exposures to peanut in a large cohort of Canadian children with peanut allergy. Clin Transl Allergy 2015; 5:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Phipatanakul W, Koutrakis P, Coull BA, Kang CM, Wolfson JM, Ferguson ST, et al. The School Inner-City Asthma Intervention Study: Design, rationale, methods, and lessons learned. Contemp Clin Trials 2017; 60:14–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Phipatanakul W School Inner-City Asthma Intervention Study (SICAS-2). 2020.
- 16.Kanchongkittiphon W, Sheehan WJ, Friedlander J, Chapman MD, King EM, Martirosyan K, et al. Allergens on desktop surfaces in preschools and elementary schools of urban children with asthma. Allergy 2014; 69:960–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Phipatanakul W, Bailey A, Hoffman EB, Sheehan WJ, Lane JP, Baxi S, et al. The school inner-city asthma study: design, methods, and lessons learned. J Asthma 2011; 48:1007–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smedje G, Norback D, Edling C. Asthma among secondary schoolchildren in relation to the school environment. Clin Exp Allergy 1997; 27:1270–8. [PubMed] [Google Scholar]
- 19.Mitchell H, Senturia Y, Gergen P, Baker D, Joseph C, McNiff-Mortimer K, et al. Design and methods of the National Cooperative Inner-City Asthma Study. Pediatr Pulmonol 1997; 24:237–52. [DOI] [PubMed] [Google Scholar]
- 20.King EM, Filep S, Smith B, Platts-Mills T, Hamilton RG, Schmechel D, et al. A multi-center ring trial of allergen analysis using fluorescent multiplex array technology. J Immunol Methods 2013; 387:89–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Permaul P, Hoffman E, Fu C, Sheehan W, Baxi S, Gaffin J, et al. Allergens in urban schools and homes of children with asthma. Pediatr Allergy Immunol 2012; 23:543–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoyt AEW, Chapman MD, King EM, Platts-Mills TAE, Steinke JW. Food allergen component proteins are not detected in early-childhood vaccines. J Allergy Clin Immunol Pract 2018; 6:677–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Friedlander JL, Sheehan WJ, Baxi SN, Kopel LS, Gaffin JM, Ozonoff A, et al. Food allergy and increased asthma morbidity in a School-based Inner-City Asthma Study. J Allergy Clin Immunol Pract 2013; 1:479–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Waserman S, Cruickshank H, Hildebrand KJ, Mack D, Bantock L, Bingemann T, et al. Prevention and management of allergic reactions to food in child care centers and schools: Practice guidelines. J Allergy Clin Immunol 2021; 147:1561–78. [DOI] [PubMed] [Google Scholar]
- 25.Bock SA, Munoz-Furlong A, Sampson HA. Further fatalities caused by anaphylactic reactions to food, 2001–2006. J Allergy Clin Immunol 2007; 119:1016–8. [DOI] [PubMed] [Google Scholar]
- 26.Yunginger JW, Sweeney KG, Sturner WQ, Giannandrea LA, Teigland JD, Bray M, et al. Fatal food-induced anaphylaxis. Jama 1988; 260:1450–2. [PubMed] [Google Scholar]
- 27.Sheehan WJ, Permaul P, Petty CR, Coull BA, Baxi SN, Gaffin JM, et al. Association Between Allergen Exposure in Inner-City Schools and Asthma Morbidity Among Students. JAMA Pediatr 2017; 171:31–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Keast DR, Fulgoni VL 3rd, Nicklas TA, O’Neil CE. Food sources of energy and nutrients among children in the United States: National Health and Nutrition Examination Survey 2003–2006. Nutrients 2013; 5:283–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Robson SM, Khoury JC, Kalkwarf HJ, Copeland K. Dietary intake of children attending full-time child care: What are they eating away from the child-care center? J Acad Nutr Diet 2015; 115:1472–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pediatrics AAo. American Academy of Pediatrics Committee on Nutrition. In: Kleinman RE GF, editors., editor. Pediatric Nutrition. 7th ed. ed. Elk Grove Village, IL; 2014. [Google Scholar]
- 31.Özen A, Boran P, Torlak F, Karakoç-Aydıner E, Barış S, Karavuş M, et al. School Board Policies on Prevention and Management of Anaphylaxis in İstanbul: Where Do We Stand? Balkan Med J 2016; 33:539–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kao LM, Wang J, Kagan O, Russell A, Mustafa SS, Houdek D, et al. School nurse perspectives on school policies for food allergy and anaphylaxis. Ann Allergy Asthma Immunol 2018; 120:304–9. [DOI] [PubMed] [Google Scholar]
- 33.Muñoz VL. ‘Everybody has to think - do I have any peanuts and nuts in my lunch?’ School nurses, collective adherence, and children’s food allergies. Sociol Health Illn 2018; 40:603–22. [DOI] [PubMed] [Google Scholar]
- 34.Herbert LJ, Mehta P, Sharma H. Mealtime behavior among parents and their young children with food allergy. Ann Allergy Asthma Immunol 2017; 118:345–50. [DOI] [PubMed] [Google Scholar]
- 35.Simonte SJ, Ma S, Mofidi S, Sicherer SH. Relevance of casual contact with peanut butter in children with peanut allergy. J Allergy Clin Immunol 2003; 112:180–2. [DOI] [PubMed] [Google Scholar]
- 36.Wainstein BK, Yee A, Jelley D, Ziegler M, Ziegler JB. Combining skin prick, immediate skin application and specific-IgE testing in the diagnosis of peanut allergy in children. Pediatr Allergy Immunol 2007; 18:231–9. [DOI] [PubMed] [Google Scholar]
- 37.Jarmoc LM, Primack WA. Anaphylaxis to cutaneous exposure to milk protein in a diaper rash ointment. Clin Pediatr (Phila) 1987; 26:154–5. [DOI] [PubMed] [Google Scholar]
- 38.Tabar AI, Alvarez MJ, Echechipia S, Acero S, Garcia BE, Olaguibel JM. Anaphylaxis from cow’s milk casein. Allergy 1996; 51:343–5. [DOI] [PubMed] [Google Scholar]
- 39.Liccardi G, De Falco F, Gilder JA, D’Amato M, D’Amato G. Severe systemic allergic reaction induced by accidental skin contact with cow milk in a 16-year-old boy. A case report. J Investig Allergol Clin Immunol 2004; 14:168–71. [PubMed] [Google Scholar]
- 40.Porcaro F, Caminiti L, Crisafulli G, Guglielmo F, Pajno GB. Anaphylaxis to cutaneous exposure to bovine colostrum based cream. Asian Pac J Allergy Immunol 2019; 37:9–11. [DOI] [PubMed] [Google Scholar]
- 41.Lecks HI. Anaphylaxis from milk protein in diaper ointment. Jama 1980; 244:1560. [PubMed] [Google Scholar]
- 42.Bartnikas LM, Sicherer SH. Fatal Anaphylaxis: Searching for Lessons from Tragedy. J Allergy Clin Immunol Pract 2020; 8:334–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Taylor SL, Crevel RW, Sheffield D, Kabourek J, Baumert J. Threshold dose for peanut: risk characterization based upon published results from challenges of peanut-allergic individuals. Food Chem Toxicol 2009; 47:1198–204. [DOI] [PubMed] [Google Scholar]
- 44.Taylor SL, Hefle SL, Bindslev-Jensen C, Bock SA, Burks AW Jr., Christie L, et al. Factors affecting the determination of threshold doses for allergenic foods: how much is too much? J Allergy Clin Immunol 2002; 109:24–30. [DOI] [PubMed] [Google Scholar]
- 45.Hourihane JB, Kilburn SA, Nordlee JA, Hefle SL, Taylor SL, Warner JO. An evaluation of the sensitivity of subjects with peanut allergy to very low doses of peanut protein: a randomized, double-blind, placebo-controlled food challenge study. J Allergy Clin Immunol 1997; 100:596–600. [DOI] [PubMed] [Google Scholar]
- 46.Bartnikas LM, Gurish MF, Burton OT, Leisten S, Janssen E, Oettgen HC, et al. Epicutaneous sensitization results in IgE-dependent intestinal mast cell expansion and food-induced anaphylaxis. J Allergy Clin Immunol 2013; 131:451–60.e1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Strid J, Hourihane J, Kimber I, Callard R, Strobel S. Epicutaneous exposure to peanut protein prevents oral tolerance and enhances allergic sensitization. Clin Exp Allergy 2005; 35:757–66. [DOI] [PubMed] [Google Scholar]
- 48.Du Toit G, Sampson HA, Plaut M, Burks AW, Akdis CA, Lack G. Food allergy: Update on prevention and tolerance. J Allergy Clin Immunol 2018; 141:30–40. [DOI] [PubMed] [Google Scholar]
- 49.Dotterud LK, Van TD, Kvammen B, Dybendal T, Elsayed S, Falk ES. Allergen content in dust from homes and schools in northern Norway in relation to sensitization and allergy symptoms in schoolchildren. Clin Exp Allergy 1997; 27:252–61. [PubMed] [Google Scholar]
- 50.Dybendal T, Elsayed S. Dust from carpeted and smooth floors. VI. Allergens in homes compared with those in schools in Norway. Allergy 1994; 49:210–6. [DOI] [PubMed] [Google Scholar]


