Abstract
Purpose:
To determine the incidence of venous thromboembolism (VTE) and define clinical risk factors associated with the development of new-onset VTE in patients receiving neoadjuvant chemotherapy (NACT) for ovarian cancer (OC).
Methods:
An institutional ovarian cancer database was used to identify all OC patients receiving NACT from 04/2015–09/2018. VTE events were recorded and included clinically diagnosed deep venous thrombosis (DVT) and/or pulmonary embolism (PE). The incidence of VTE events was categorized according to treatment phases (P): P0) First visit/prior to induction of NACT; P1) during NACT before interval debulking surgery (IDS); P2) intraoperative through day 28 post-IDS; P3) during adjuvant chemotherapy.
Results:
A total of 290 patients were identified during the study period. Seventy-five (25.9%) developed a VTE at some point from time of presentation through the peri-operative period. Forty (13.8%) presented with VTE prior to initiation of NACT. An additional 27 (11.6%) developed a VTE during NACT (P1); 6 (3.9%) during the intraoperative and 28-day post-operative period (P2); and 2 (1.3%) during the adjuvant period (P3). The overall VTE rate was 25.9% (n=75). FIGO stage IV disease was the only factor associated with increased risk for a new-onset VTE [Odds Ratio (OR): 3.9 (95% Confidence Interval [CI]=1.2–13.6; p=0.03].
Conclusions:
Patients receiving NACT for advanced OC are at extremely high risk for developing thromboembolic events, either at initial presentation or during induction of NACT, a treatment phase that is traditionally without use of prophylactic anticoagulation. Since Khorana scoring is not predictive in this population, clinicians might need to consider increased screening or use of prophylactic anticoagulation in patients receiving NACT for OC, particularly in advanced metastatic disease.
Keywords: Ovarian Cancer, Neoadjuvant Therapy, Venous Thromboembolism, Survival
INTRODUCTION
Venous thromboembolism (VTE), which includes deep venous thrombosis (DVT) and pulmonary embolism (PE), is a common problem in patients with cancer (1). This procoagulant state can be the result of the malignancy itself, the cancer treatment, and/or patient characteristics (2). Several cancer types are known to have an elevated risk of VTE such as pancreatic, gastric, lung, and ovarian cancer (OC) (3, 4). The thrombogenic potential of these tumors has been reported to be associated with disease volume (5, 6). The risk of VTE in cancer patients is also dependent on patient-related factors such as older age and underlying comorbidities (7).
OC patients who are poor surgical candidates or have initially unresectable disease are considered for neoadjuvant chemotherapy (NACT) (8, 9). Most of these patients carry a high-risk profile for VTE, including high tumor burden, poor performance status, and/or multiple comorbidities (10). Two prior studies have shown a VTE rate of more than 20% in OC patients undergoing NACT (11, 12). These studies also demonstrated that VTE development is highest at the induction of NACT. Current guidelines suggest using the Khorana score to stratify which patients are at elevated risk for VTE and might benefit from prophylactic anticoagulation, but there is no specific recommendation for patients receiving systemic chemotherapy in a neoadjuvant setting (13, 14).
The present study aimed to evaluate the incidence of VTE in OC patients receiving NACT, and to define clinical risk factors associated with the development of new-onset VTE in this population.
METHODS
Study Design
This study was approved by the Institutional Review Board at Memorial Sloan Kettering Cancer Center (MSKCC) (Protocol #17–430). This is a single-center retrospective cohort study of patients with newly diagnosed, pathologically verified ovarian, fallopian tube, or primary peritoneal cancer who sought medical and/or surgical care at MSKCC and were recommended to receive NACT from April 2015 through September 2018. Patients on aspirin were included. STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) guidelines were used to report study findings (15).
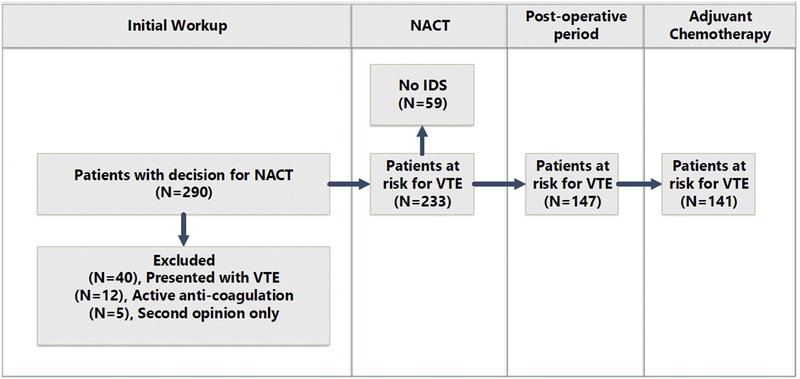
An institutional ovarian cancer database that tracks all consecutive patients seen by either the gynecologic surgery or gynecologic medical oncology services was used to retrieve study data. NACT was defined as receipt of at least one cycle of chemotherapy prior to planned cytoreductive surgery (CRS). The tumor stage was defined at pathological diagnosis based on imaging using the International Federation of Gynecology and Obstetrics (FIGO) staging system (16). Electronic medical records were reviewed to determine the indication for NACT. Indications for NACT were categorized as follows: 1) extent of disease not amenable to surgery; 2) patient comorbidity preventing surgery; 3) other. Further details for the utilization of NACT in patients with advanced ovarian cancer at MSKCC can be found in a previous publication (8). A VTE event was defined as an event that was a symptomatic or image-detected (via computed tomography (CT) scan or Doppler ultrasonography) proximal DVT of the lower or upper-limbs and/or PE. Splanchnic thrombotic events, arterial and superficial thromboses were not included in VTE incidence. We report the number of and timing of the first VTEs. Surgical status was defined as having undergone or not having undergone interval debulking surgery (IDS) at any point during treatment with first-line chemotherapy. The incidence of thrombotic events in patients was categorized according to treatment phases (P): P0) First visit/prior to induction of NACT; P1) During NACT prior to IDS; P2) Intra-operative and 28-days after IDS; P3) During adjuvant chemotherapy. The incidence of VTE at presentation was calculated from the total study cohort. Rates of new onset VTE events at other treatment phases were calculated from the remaining subjects who had not yet had a clinically detectable VTE but were considered at risk for developing VTE. The Khorana score risk assessment tool for predicting the risk of chemotherapy-associated VTE was calculated by the disease type, hematological factors and body mass index (BMI) (17).The Khorana score was calculated for each patient at initial presentation to our institution at time of first complete blood count. Patients on chronic anti-coagulation at presentation (n=12), seeking only a second opinion (n=5), or presented with VTE at initial presentation (n=40) were excluded from the treatment phase analysis (Figure 1). The MSKCC institutional policy follows guidelines for prolonged post-operative thromboprophylaxis, with an injection of low-molecular-weight heparin for 28 days in patients following CRS for OC.
Figure 1.
Study flowchart
Statistical Analysis
Descriptive statistics were provided. The differences between groups were tested using Fisher’s Exact test for categorical variables and the Wilcoxon Rank-Sum test for continuous variables. Continuous variables are reported as median, with range. Categorical variables are described as frequency and percentage. The Fisher’s exact test or Chi-square test was applied to identify the association between clinical variables and the new onset of VTE in univariable analysis. Statistical analyses were performed using the SPSS software (v. 23; SPSS Inc., Chicago, IL, USA). All calculated p-values were two-sided, and p-values <0.05 were considered statistically significant.
RESULTS
Two hundred and ninety OC patients receiving NACT were identified within the study period. Of this cohort, 75 (25.9%) experienced at least one episode of VTE. The highest rate of events (N=40 VTE events, 13.8%) was at first visit/prior to induction of NACT. After the patients were seen by a physician and guided to diagnostic work-up, the second peak of VTE events occurred during NACT (n=27, 9% of whole patient cohort). Demographics and clinicopathological findings of the 233 women who underwent NACT and had not yet experienced a VTE at initial presentation, are presented in Table 1. The median age of the patients was 69.1 (range 42.6–92.6) years; the predominant stage was stage IV disease (69.1%); and the predominant histology was high-grade serous carcinoma (83.3%). The most common indication for NACT was initially unresectable disease. At the time of presentation, 102 patients (43.8%) had a baseline Khorana score of 1, 91 patients (39.1%) had a baseline Khorana score of 2, and 40 patients (16.8%) had baseline Khorana scores of 3 or greater.
Table 1.
Demographics and clinical features of the study cohort (N=233)
| Age, years [Median (range)] | 69.1 | (42.6–92.6) |
| FIGO Stage | ||
| IIIA | 1 | (0.4) |
| IIIB | 3 | (1.3) |
| IIIC | 67 | (28.8) |
| IV | 161 | (69.1) |
| Histology | ||
| High Grade Serous Carcinoma | 194 | (83.3) |
| Mullerian Carcinoma | 29 | (12.4) |
| Carcinosarcoma | 4 | (1.7) |
| Clear Cell Carcinoma | 2 | (0.9) |
| Low grade serous | 2 | (0.9) |
| Other | 2 | (0.9) |
| Genetic testing | ||
| Not Tested | 50 | (21.5) |
| Negative | 144 | (61.8) |
| Positive | 33 | (14.2) |
| BRCA1 | 20 | (8.6) |
| BRCA2 | 13 | (5.6) |
| VUS | 6 | (2.6) |
| NACT Indications | ||
| Unresectable disease | 200 | (85.8) |
| Comorbidity | 30 | (12.9) |
| Other | 3 | (1.3) |
Data are expressed as n (%) unless otherwise specified
NACT neo-adjuvant chemotherapy; VUS variant of uncertain significance; FIGO The International Federation of Gynecology and Obstetrics; BRCA Breast Cancer gene
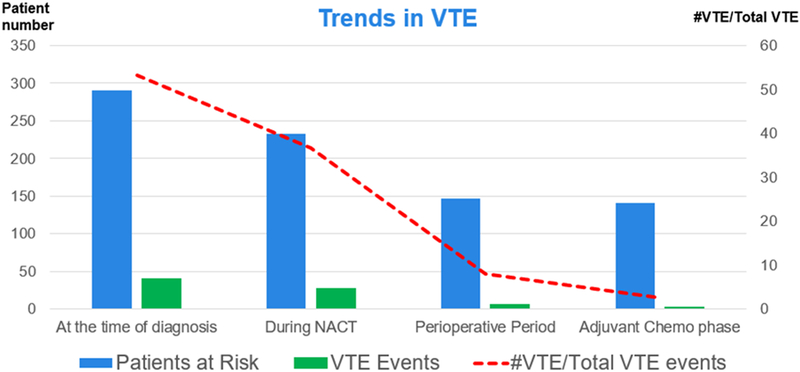
Of the 233 women undergoing NACT (P1), who had not yet experienced a VTE, an additional 27 (11.5%) had a VTE. Fifty-nine of these 233 patients could not undergo IDS and 7 of them were diagnosed with a VTE during the NACT phase. An additional 6 initial VTE events were observed in 174 patients who had undergone IDS. Two initial VTE events were noted in patients receiving adjuvant chemotherapy following IDS (Table 2 and Figure 2).
Table 2.
Initial thromboembolic events
| New onset thromboembolic events | N | (%) |
|---|---|---|
| Total (N=290) | 75 | (25.9) |
| Phase 0: First visit/prior to induction of NACT | 40 | (13.8) |
| Phase 1: during NACT before interval debulking surgery (IDS); | 27 | (9.3) |
| Phase 2: intraoperative through day 28 post-IDS | 6 | (2.1) |
| Phase 3: During adjuvant chemotherapy | 2 | (0.7) |
Figure 2.
The timing and trend of thromboembolic events in patients receiving neo-adjuvant chemotherapy
The clinical features of the new-onset VTE events are shown in Table 3. A total of 35 (15%) patients experienced a new-onset VTE during the time between initiation of NACT until the end of first-line treatment. Nineteen (8.2%) patients had an upper or lower extremity DVT, 10 (4.3%) had a PE, and 6 (2.6%) had synchronous DVT and PE. One-fourth of patients who experienced a VTE event were diagnosed via imaging alone. Leg swelling (40%) was the most common clinical finding in symptomatic patients. Univariate analysis was performed to evaluate the association of clinical factors with new-onset VTE events during NACT (Table 4). FIGO stage IV disease was the only risk factor associated with an increased risk of VTE during NACT (odds ratio (OR): 3.9 (95%CI 1.2–13.6; p=0.03). There was no association between the occurrence of a new VTE and patient’s age, tumor histology, or germline mutation status or Khorana scores. There was no difference in the rates of VTE during NACT between patients that had a Khorana score of less than 2 versus Khorana scores equal to or greater than 2 (40.7% vs 59.3%; p=0.8, respectively.) Median follow-up at the time of analysis was 27 months (range 1–64)
Table 3.
Clinical features of new onset thromboembolic events after presentation (N=233)
| N= | (%) | |
|---|---|---|
| Initial VTE Events, developing after cancer presentation | 35 | |
| Time between diagnosis of cancer and VTE, weeks [Median (range)] | 12 | (1–42) |
| VTE locations per total new onset cases(N=35) | ||
| Extremity DVT | 19 | (8.2) |
| PE | 10 | (4.3) |
| DVT+PE | 6 | (2.6) |
| Presentation symptoms for new onset VTEs | ||
| Incidental | 9 | 25.6 |
| Symptomatic | 26 | 74.4 |
| Leg swelling | 14 | 40.0 |
| Back pain | 3 | 8.5 |
| Shortness of breath | 3 | 8.5 |
| Tachycardia | 3 | 8.5 |
| Failure to thrive | 1 | 2.8 |
| Unknown | 2 | 5.7 |
Data are expressed as n (%) unless otherwise specified
NACT neo-adjuvant chemotherapy; VTE venous thromboembolism; DVT deep venous thrombosis; PE Pulmonary Emboli
Table 4.
Univariate analysis of factors associated with new onset venous thromboembolism during NACT (N=233)
| Patients at risk | New onset VTE | OR (95% CI) | p value | |
|---|---|---|---|---|
| All | 233 | 27 | ||
| Age(years) | ||||
| <70 | 126(54.1) | 19(70.4) | 0.45(0.19–1.09) | 0.99 |
| ≥70 | 107(59.9) | 8(29.6) | ||
| Histology | ||||
| Serous | 196(84.1) | 22(81.5) | 0.89(0.29–2.3) | 0.78 |
| Non-Serous | 37(15.9) | 5(18.5) | ||
| FIGO Stage | ||||
| III | 71(30.6) | 3(11.1) | 3.9(1.2–13.6) | 0.03 |
| IV | 161(69.4) | 24(88.9) | ||
| Germline mutation | ||||
| No mutation | 144(78.7) | 17(77.3) | 1.1(0.38–3.19) | 0.78 |
| Positive for BRCA 1 or 2 | 33(21.1) | 5(22.7) | ||
| Khorana Score | ||||
| <2 | 103(44.2) | 11(40.7) | 1.17(0.5–2.7) | 0.84 |
| ≥2 | 130(55.8) | 16(59.3) | ||
Data are expressed as n (%) unless otherwise specified
NACT, neo-adjuvant chemotherapy; VTE, venous thromboembolism; OR, Odds ratio; FIGO, The International Federation of Gynecology and Obstetrics; BRCA, Breast Cancer gene
DISCUSSION
In the present study, 25.9% of all OC patients presenting for NACT had a VTE, either at initial presentation or during the period from induction of NACT until the completion of first-line therapy. Most cases of new-onset VTEs occurred during the NACT induction phase. Our data is consistent with that of previous publications (11, 12, 18) reporting the highest rate of VTE events at initial diagnosis and during NACT. Our data is also consistent with previous data showing a strong association between the risk of VTE and stage IV metastatic disease or high tumor burden (19, 20) (5, 21). Another important finding of the present study (as shown in previous studies) is that, in our cohort, perioperative VTE event rates were low (3.9% in the present series) (11, 12, 14). This may reflect the beneficial impact of perioperative thromboprophylaxis in surgical oncology patients, or the fact that a high percentage of patients had experienced a VTE prior to surgery and were already receiving therapeutic anticoagulation.
The Khorana predictive model for cancer-associated VTE assigns 1 point to patients with gynecologic cancer and additional points based on BMI >35 kg/m2, platelets >350 000/μL, hemoglobin <10 hgb, and white blood cell count >11 000/ng(17). The aim of the Khorana model is to stratify patients at high risk of thrombosis versus those at low risk, in order to facilitate prophylactic anticoagulation. Per National Comprehensive Cancer Network (NCCN) guidelines, patients with a total Khorana score of 2 or higher are at high risk for VTE (>7%) and could be considered for outpatient thromboprophylaxis (14). On the other hand, the present study revealed that OC patients receiving NACT have an almost three times higher risk of VTE than the highest risk population in the Khorana algorithm, and therefore the Khorana predictive model would not be applicable to our cohort as 40% of the new onset VTE events occurred in patients with Khorana scores of less than 2. In our cohort of OC patients undergoing NACT, the risk of new onset VTE after initial diagnosis is so high (9% of the entire cohort and 11.5% of patients at risk) that it might be justified to consider prophylactic anticoagulation for all of them. Future research should consider increased screening in this population or routine use of prophylactic anticoagulation.
Previous studies have also shown high rates of VTE in OC patients undergoing NACT, independent of the Khorana Score. Greco and colleagues reported very high rates of VTE in OC patients receiving NACT, independent of the Khorana Score (low/intermediate-risk score (10.7%); high-risk score (11.9%)) (11). A prospective cohort study from Denmark evaluated the incidence of symptomatic and incidental VTE in patients with suspected epithelial OC (22). Eighty-two percent of the patients in that study who experienced a VTE had a Khorana score >3; however, the study population included patients with benign and borderline tumors as well as invasive carcinomas.
In the AVERT and CASSINI trials, cancer patients with Khorana scores of >2 were enrolled and randomized to prophylactic anticoagulation (23, 24). However, this threshold for prophylactic anticoagulation may not be sufficient in our patient population because of the inherently high risk of VTE in patients with OC. The Khorana predictive system relies on hematological markers, which are mostly within normal limits at initial presentation in patients with OC. The differences in performances of the Khorana predictive score system may be associated with the significantly increased baseline VTE risk of patients receiving NACT for OC (frail patients with high tumor burden receiving carboplatin- and taxane-based chemotherapy) that masks the association of VTE with other factors (25). Nevertheless, clinicians should be aware of the significantly increased risk of VTE in patients receiving NACT for OC, particularly in the presence of metastatic disease. These observations led us to consider the need for standard use of prophylactic anticoagulation in patients receiving NACT for OC, as the utilization of NACT is increasing significantly (26).
This study has some limitations. The study retrospective design did not allow us to demonstrate whether the patients had developed a VTE after diagnosis but before initiation of chemotherapy, as patients are not screened for VTEs at the time of chemotherapy induction unless they are symptomatic. High risk of VTE is often the basis for determining that a patient should undergo NACT, so there is inherent confounding, and we would expect this group of patients to have higher VTE rates. We did not report types/schedules of chemotherapy or use of targeted therapies such as bevacizumab, which may influence VTE rates.
The study also has strengths and advantages, including the advantage of sequencing VTE events in a timeline (which was required to define the incidence rates in relation to phases of care). The institutional ovarian database was initiated in 2015 and tracks all patients seen with an ovarian complaint from the time of the initial visit; this minimizes the risk of inadvertently omitting some patients (9). The diagnostic work-up of patients, and treatment management, were done by a multidisciplinary team using institutional guidelines to provide coordinated care.
In summary, patients receiving NACT for advanced OC are at high risk for clinically detectable thromboembolic events. Further research regarding the selection criteria and timing of thromboprophylaxis for this vulnerable patient population is warranted, as the highest risk for VTE is observed in a phase of treatment that is traditionally without prophylactic anticoagulation. Although the risk of VTE events is highest before the patients present, and therefore it is impossible to diagnose or treat, future studies should explore the safety, oncologic outcomes, cost, and quality of life for patients requiring prophylactic anticoagulation during neoadjuvant treatment.
Highlights.
The overall risk of venous thromboembolism (VTE) in ovarian cancer (OC) patients receiving NACT was >25%.
The highest rate was observed at initial presentation and during the induction of NACT.
FIGO stage IV disease was the only risk factor associated with an increased risk for VTE.
Prophylactic anticoagulation should be considered in OC patients receiving NACT, especially those with metastatic disease.
ACKNOWLEDGEMENTS
We would like to thank the ovarian database team and all the women who underwent treatment at Memorial Sloan Kettering Cancer Center.
FUNDING: This study was funded in part through the NIH/NCI Support Grant P30 CA008748.
DISCLOSURES: NRA reports the following, outside the submitted work: grant from Stryker/Novadaq (paid to institution); grant from Olympus (paid to institution); grant from GRAIL (paid to institution). Memorial Sloan Kettering Cancer Center (MSK) has financial interests relative to GRAIL. As a result of these interests, MSK could ultimately potentially benefit financially from the outcomes of this research. DSC reports the following, outside the submitted work: Bovie Medical Co. (Medical Advisory Board; stock options); Verthermia Inc. (now Apyx Medical Corp.) (Medical Advisory Board; stock options); Biom’Up (Medical Advisory Board Meeting 4/19/2019; personal fees); Intuitive Surgical, Inc. (recent stock owner; sold Dec. 2018); TransEnterix, Inc. (recent stock owner; sold Dec. 2018). KLR reports the following, outside the submitted work: other from Intuitive Surgical Inc. (airfare to a survivorship conference, where she spoke). ELJ reports the following, outside the submitted work: Summit Biomedical, grant funding.
Footnotes
CONFLICTS OF INTEREST: None declared
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Mulder FI, Horvath-Puho E, van Es N, van Laarhoven H, Pedersen L, Moik F, et al. Venous thromboembolism in cancer patients: a population-based cohort study. Blood. 2020. [DOI] [PubMed] [Google Scholar]
- 2.Ay C, Pabinger I, Cohen AT. Cancer-associated venous thromboembolism: Burden, mechanisms, and management. Thromb Haemost. 2017;117(2):219–30. [DOI] [PubMed] [Google Scholar]
- 3.Walker AJ, Card TR, West J, Crooks C, Grainge MJ. Incidence of venous thromboembolism in patients with cancer - a cohort study using linked United Kingdom databases. Eur J Cancer. 2013;49(6):1404–13. [DOI] [PubMed] [Google Scholar]
- 4.Blom JW, Vanderschoot JP, Oostindier MJ, Osanto S, van der Meer FJ, Rosendaal FR. Incidence of venous thrombosis in a large cohort of 66,329 cancer patients: results of a record linkage study. J Thromb Haemost. 2006;4(3):529–35. [DOI] [PubMed] [Google Scholar]
- 5.Timp JF, Braekkan SK, Versteeg HH, Cannegieter SC. Epidemiology of cancer-associated venous thrombosis. Blood. 2013;122(10):1712–23. [DOI] [PubMed] [Google Scholar]
- 6.Weeks KS, Herbach E, McDonald M, Charlton M, Schweizer ML. Meta-Analysis of VTE Risk: Ovarian Cancer Patients by Stage, Histology, Cytoreduction, and Ascites at Diagnosis. Obstet Gynecol Int. 2020;2020:2374716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khorana AA, Francis CW, Culakova E, Kuderer NM, Lyman GH. Frequency, risk factors, and trends for venous thromboembolism among hospitalized cancer patients. Cancer. 2007;110(10):2339–46. [DOI] [PubMed] [Google Scholar]
- 8.Mueller JJ, Zhou QC, lasonos A, O'Cearbhaill RE, Alvi FA, El Haraki A, et al. Neoadjuvant chemotherapy and primary debulking surgery utilization for advanced-stage ovarian cancer at a comprehensive cancer center. Gynecol Oncol. 2016;140(3):436–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu YL, Filippova OT, Zhou Q, lasonos A, Chi DS, Zivanovic O, et al. Characteristics and survival of ovarian cancer patients treated with neoadjuvant chemotherapy but not undergoing interval debulking surgery. J Gynecol Oncol. 2020;31(1):e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vergote I, du Bois A, Amant F, Heitz F, Leunen K, Harter P. Neoadjuvant chemotherapy in advanced ovarian cancer: On what do we agree and disagree? Gynecol Oncol. 2013;128(1):6–11. [DOI] [PubMed] [Google Scholar]
- 11.Greco PS, Bazzi AA, McLean K, Reynolds RK, Spencer RJ, Johnston CM, et al. Incidence and Timing of Thromboembolic Events in Patients With Ovarian Cancer Undergoing Neoadjuvant Chemotherapy. Obstet Gynecol. 2017;129(6):979–85. [DOI] [PubMed] [Google Scholar]
- 12.Salinaro JR, McQuillen K, Stemple M, Boccaccio R, Ehrisman J, Lorenzo AM, et al. Incidence of venous thromboembolism among patients receiving neoadjuvant chemotherapy for advanced epithelial ovarian cancer. Int J Gynecol Cancer. 2020;30(4):491–7. [DOI] [PubMed] [Google Scholar]
- 13.Key NS, Khorana AA, Kuderer NM, Bohlke K, Lee AYY, Arcelus JI, et al. Venous Thromboembolism Prophylaxis and Treatment in Patients With Cancer: ASCO Clinical Practice Guideline Update. J Clin Oncol. 2020;38(5):496–520. [DOI] [PubMed] [Google Scholar]
- 14.National Comprehensive Cancer Network. Cancer-Associated Venous Thromboembolic Disease (Version 1.2020) [Available from: https://www.nccn.org/professionals/physician_gls/pdf/vte.pdf. [DOI] [PubMed]
- 15.von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453–7. [DOI] [PubMed] [Google Scholar]
- 16.Prat J, Oncology FCoG. Staging classification for cancer of the ovary, fallopian tube, and peritoneum. Int J Gynaecol Obstet. 2014;124(1):1–5. [DOI] [PubMed] [Google Scholar]
- 17.Khorana AA, Kuderer NM, Culakova E, Lyman GH, Francis CW. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood. 2008;111(10):4902–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chavan DM, Huang Z, Song K, Parimi LRH, Yang XS, Zhang X, et al. Incidence of venous thromboembolism following the neoadjuvant chemotherapy regimen for epithelial type of ovarian cancer. Medicine (Baltimore) 2017;96(42):e7935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cronin-Fenton DP, Sondergaard F, Pedersen LA, Fryzek JP, Cetin K, Acquavella J, et al. Hospitalisation for venous thromboembolism in cancer patients and the general population: a population-based cohort study in Denmark, 1997–2006. Br J Cancer. 2010;103(7):947–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chew HK, Wun T, Harvey D, Zhou H, White RH. Incidence of venous thromboembolism and its effect on survival among patients with common cancers. Arch Intern Med. 2006;166(4):458–64. [DOI] [PubMed] [Google Scholar]
- 21.Otten HM, Mathijssen J, ten Cate H, Soesan M, Inghels M, Richel DJ, et al. Symptomatic venous thromboembolism in cancer patients treated with chemotherapy: an underestimated phenomenon. Arch Intern Med. 2004;164(2):190–4. [DOI] [PubMed] [Google Scholar]
- 22.Kahr HS, Christiansen OB, Grove A, Iyer V, Torp-Pedersen C, Knudsen A, et al. Venous thromboembolism in epithelial ovarian cancer. A prospective cohort study. Thromb Res. 2019;181:112–9. [DOI] [PubMed] [Google Scholar]
- 23.Carrier M, Abou-Nassar K, Mallick R, Tagalakis V, Shivakumar S, Schattner A, et al. Apixaban to Prevent Venous Thromboembolism in Patients with Cancer. N Engl J Med. 2019;380(8):711–9. [DOI] [PubMed] [Google Scholar]
- 24.Khorana AA, Soff GA, Kakkar AK, Vadhan-Raj S, Riess H, Wun T, et al. Rivaroxaban for Thromboprophylaxis in High-Risk Ambulatory Patients with Cancer. N Engl J Med. 2019;380(8):720–8. [DOI] [PubMed] [Google Scholar]
- 25.Angelini D, Khorana AA. Risk Assessment Scores for Cancer-Associated Venous Thromboembolic Disease. Semin Thromb Hemost. 2017;43(5):469–78. [DOI] [PubMed] [Google Scholar]
- 26.Horner W, Peng K, Pleasant V, Brackmann M, Ebott J, Gutfreund R, et al. Trends in surgical complexity and treatment modalities utilized in the management of ovarian cancer in an era of neoadjuvant chemotherapy. Gynecol Oncol. 2019;154(2):283–9. [DOI] [PubMed] [Google Scholar]