Abstract
BACKGROUND AND AIMS
Microscopic colitis is an increasingly common cause of watery diarrhea. Several classes of medications have been associated with microscopic colitis in prior studies.
METHODS
This was a case-control study of patients referred for elective, outpatient colonoscopy for diarrhea. Patients were excluded for inflammatory bowel disease, C. difficile, or other infectious diarrhea. Colon biopsies were reviewed by the study pathologist and patients were classified as microscopic colitis cases or non-microscopic colitis controls.
RESULTS
The study population included 110 microscopic colitis cases and 252 controls. The cases were older, better educated and more likely to be female. Cases reported a greater number of loose, watery, or liquid stools, nocturnal stools, more urgency and weight loss compared to controls. There was no association with proton pump inhibitors (PPIs), adjusted OR (aOR) 0.66, 95% CI 0.38-1.13, or nonsteroidal anti-inflammatory drugs, aOR 0.68, 95% CI 0.40-1.17. Cholecystectomy was less common in cases, aOR 0.33, 95% CI 0.17-0.64, but microscopic colitis cases had more frequent bowel movements following cholecystectomy.
CONCLUSION
Compared to similar patients with diarrhea, cases with microscopic colitis were not more likely to have taken previously implicated medications. They had more diarrhea following cholecystectomy suggesting that bile may play a role in symptoms or etiology. We conclude that the appropriate choice of controls is crucial to understanding risk factors for microscopic colitis.
Keywords: Colitis, Microscopic/epidemiology, Humans, Diarrhea/epidemiology
Graphical Abstract
INTRODUCTION
Microscopic colitis is currently a common cause of watery diarrhea in older adults, although it is increasingly recognized in younger individuals.1 The condition is called “microscopic colitis” because the mucosa appears grossly normal. Under the microscope, however, there may be a thickened collagen band (collagenous colitis) or a lymphocytic infiltration (lymphocytic colitis).2 Both collagenous and lymphocytic colitis share features of intraepithelial lymphocytes, surface epithelial damage, and increased lamina propria inflammation. Lymphocytic and collagenous colitis, are generally considered to be subtypes of the same disease based on the histologic overlap, parallel increase in incidence, similar clinical presentation, and identical response to therapy.3
The incidence of microscopic colitis has been increasing and is now thought to be comparable to or exceed inflammatory bowel disease.4–8 Some of the increased incidence is due to greater recognition of the disease.9 The etiology of microscopic colitis is unknown. The disease is widely considered to be an abnormal immune reaction to luminal antigens in predisposed hosts.10 The concept of a luminal factor is supported by the fact that the disease resolves with diversion of the fecal stream,11 and recurs when continuity is restored.11,12 Reported triggers are drugs,10 smoking13, 14 and autoimmunity.15
Published evidence about risk factors for microscopic colitis has been limited by small numbers of cases, retrospective data collection and case series designs. In order to avoid the limitations of prior research, we conducted a case-control study comparing patients with microscopic colitis to patients with other causes of diarrhea in order to learn more about associations with medications.
METHODS
Design
We designed a case-control study of patients who were referred to The University of North Carolina Hospitals for elective outpatient colonoscopy for diarrhea. UNC Hospitals is a safety net hospital serving a disproportionate share of indigent patients. At the time of study enrollment, status as a microscopic colitis case or a non-microscopic control was not known. All data were prospectively collected.
Recruitment and enrollment
We identified possible participants using The Carolina Data Warehouse for Health, a central real-time data repository that contains clinical, research, and administrative data sourced from the UNC Health Care System electronic medical record. Patients were excluded if the indication for colonoscopy was not diarrhea or possible microscopic colitis, or if they had a diagnosis of inflammatory bowel disease, C. difficile or another infectious diarrhea. Patients who were nominally eligible for inclusion were mailed an introductory letter and study brochure.
On the day of the colonoscopy, a research assistant asked the patients the average number of bowel movements per day and stool form based on the Bristol Stool Form Scale.16 Eligibility was based on stool consistency, not stool number. To be eligible, the patient had to report a Bristol Stool Form type 5, 6, 7 (mushy, loose, watery) during the prior week. Eligible subjects who agreed to the study signed consent, HIPAA, and Storing Biological Specimens with Identifying Information forms.
Colonoscopies were performed by faculty gastroenterologists and supervised fellows. If the colonoscopy revealed gross inflammation, the patient was excluded. Clinical biopsies were sent to the surgical pathology laboratory where they were reviewed by one of the faculty pathologists. If the clinical biopsies showed neutrophilic colitis or eosinophilic colitis, the patient became ineligible.
There were some occasions when the research assistant was not available at the time of the colonoscopy to consent a potentially eligible patient (missed group). The interviewer contacted the patient by phone to verify eligibility and to obtain consent for the interview and the use of clinical biopsies. The research pathologist reviewed the slides to establish the pathologic diagnosis.
In order to enrich the number of microscopic colitis patients in the study, once each month. we used a custom query tool to identify all patients with a new pathological diagnosis of microscopic colitis. These patients were contacted and consented in a similar fashion as the missed group described previously. There was no age restriction for these patients.
Pathologic review
The research pathologist (JTW) reviewed the clinical slides and classified patients as having microscopic colitis or not. The pathologist was not aware of patient symptoms or diagnosis. When the research pathologist noted patchy lymphocytes, the diagnosis was categorized as “indeterminate microscopic colitis”. For this analysis, we excluded indeterminate cases. There were too few indeterminate cases (14) for a separate analysis, and including them in either the microscopic colitis case group or the control group would lead to misclassification.
Interviews
Patients were given the option to complete the interview using a web-based survey or phone survey. Those who indicated an interest in the web-based survey were sent a personalized link with reminders on days 5, 10, and 13. Those who had not completed the survey in 20 days were called by the telephone interviewer to attempt to complete the interview. The major sections of the questionnaire were: demographics, smoking, medical history medications taken in the last year, reproductive history (women), microscopic colitis disease activity, and irritable bowel and bloating questions (Rome Foundation-licensed agreement with Rome Foundation October 24, 2017). For medications, we asked patients how many weeks they took the medication during the year prior to their colonoscopy.
Data analysis
Data analysis was conducted using Stata 17.0 (Stata Corp, College Station, TX). Data analysis began by inspecting the distributions of variables, excluding implausible values and grouping categorical variables using logical cut points. Exposure variables were examined one-by-one in univariate analyses using chi square (categorical) or t-tests (continuous). Model building was informed by a directed acyclic graph.17 We used multivariable models to calculate odds ratios and 95% confidence intervals (CI) for exposure variables adjusted for age, sex, and education. In a sensitivity analysis we excluded patients with microscopic colitis who were identified from pathology reports.
Informed consent
The study was approved by the Institutional Review Board at the University of North Carolina. All patient gave informed consent. All authors had access to the study data and reviewed and approved the final manuscript.
RESULTS
Patients were eligible for the study between April 1, 2015 and December 22, 2020. During that interval, 1008 patients were nominally eligible. There were 176 who cancelled their colonoscopy appointment, 161 who were ineligible before or after the colonoscopy, 99 who were missed in the endoscopy suite because the research assistant was not available, and 196 who refused. Overall, 376 consented for the study and were interviewed. We excluded 14 with indeterminate disease leaving 362 for the present analysis, 110 microscopic colitis cases and 252 non-microscopic colitis controls. Among the 110 cases there were 34 who were identified from path reports.
Descriptive characteristics of the study population are shown in Table 1. The cases were older than controls, mean age 63.2 (standard deviation (SD) 12.7, interquartile range 40-83) vs 54.5 (SD 11.9, interquartile range 36-78). Marital status was not different. The overall study population was predominantly white. The cases were more likely to be white race than controls (96.2 vs 85.7%). The cases were more likely to be women than controls (86.2% vs 69.8%). The study participants were well educated, 51.0% had college degrees or study beyond college. The cases were better educated than controls. History of ever smoking cigarettes was not different between the two groups, but controls were more likely to be current smokers (21.0% vs 11.3). The mean body mass index (BMI) was higher for controls (BMI 29.5 (SD 7.2) than cases (BMI 25.7 (SD 6.4)). When adjusted for age, education, sex, race, smoking, BMI and marital status we found that age, education, sex and BMI were significantly different. Subsequent analyses were adjusted for age, education and sex. We did not adjust for BMI because the cases were more likely to report weight loss since the diarrhea began and we assumed that lower BMI was a consequence of the disease. Smoking was not adjusted because it was not associated with outcome after controlling for age.
Table 1.
Characteristics of the study population
| Cases n=110 |
Controls n=252 |
|||||
|---|---|---|---|---|---|---|
|
|
||||||
| N or mean | (% or SD | N or mean | (% or SD) | Adjusted Odds Ratio† | 95% CI | |
| Age, years (mean, SD) | 63.2 | (12.7 SD) | 54.5 | (11.8) | 1.06 | 1.03-1.08 |
| Married | 75 | (70.8) | 146 | (66.7) | 0.58 | 0.30-1.09 |
| White race | 102 | (96.2) | 186 | (85.7) | 2.33 | 0.74-7.36 |
| Sex (female) | 94 | (86.2) | 176 | (69.8) | 3.58 | 1.72-7.42 |
| College education and beyond | 70 | (66.0) | 97 | (44.2) | 2.78 | 1.50-5.17 |
| Current smoker | 12 | (11.3) | 46 | (21.0) | 1.43 | 0.60-3.42 |
| BMI (mean, SD) | 25.7 | (6.4 SD) | 29.5 | (7.2 SD) | 0.93 | 0.89-0.97 |
Adjusted for age, marital status, race, sex, education, smoking and BMI
Cotter et al developed a scoring system to measure microscopic colitis severity.18 As shown in Table 2, cases reported a greater number of loose, watery or liquid stools, nocturnal stools, and more urgency. The cases were more likely to have lost weight (aOR 2.42, 95% CI 1.36-4.29).
Table 2.
Symptoms reported by microscopic colitis cases and non-microscopic colitis controls
| Cases (n=110) |
Controls (n=252) |
Adjusted odds ratio† | ||||
|---|---|---|---|---|---|---|
|
|
||||||
| No. | (%) | No. | (%) | 95% CI | ||
| Number of loose, watery or liquid bowel movements each day during week before colonoscopy. | ||||||
| 0-3 per day | 33 | (34.4) | 107 | (57.5) | 3.31 | 1.84-5.98 |
| >4 per day | 63 | (65.6) | 79 | (42.5) | ||
| Awaken from sleep to have a bowel movement during week before colonoscopy. | 59 | (61.5) | 93 | (50.3) | 1.96 | 1.10-3.46 |
| Abdominal pain during week before colonoscopy | 50 | (52.1) | 126 | (67.4) | 0.79 | 0.44-1.41 |
| Rush to the bathroom to have a bowel movement during week before colonoscopy | 89 | (92.7) | 152 | (81.3) | 3.32 | 1.32-8.34 |
| Lost weight since diarrhea began | 62 | (65.2) | 79 % | (42.5) | 2.42 | 1.36–4.29 |
| Accidental bowel leakage during month before colonoscopy | 75 | (68.2) | 168 | (66.7) | 0.95 | 0.72-1.26 |
Adjusted for age, sex, education
Because the cases had more diarrhea than the controls we did an exploratory analysis to assess whether the number of liquid stools in the week prior to colonoscopy could have distorted the results. We calculated crude odds ratios for case-control status and demographic characteristics and compared the estimates to similar odds ratios adjusted for number of liquid stools. We found no differences (not shown).
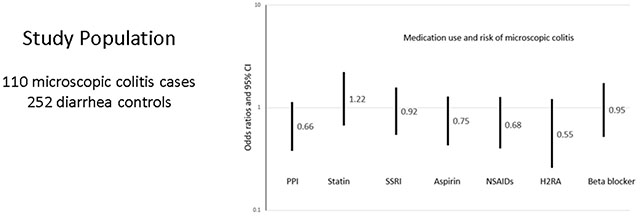
We next examined medication classes previously reported to be associated with microscopic colitis including proton pump inhibitors (PPIs), statins, selective serotonin reuptake inhibitors (SSRIs), aspirin, nonsteroidal anti-inflammatory drugs (NSAIDs), non-aspirin pain relievers, H2 receptor antagonists and beta blockers. As shown in Table 3, none of the implicated drugs was taken more frequently in microscopic colitis case. PPIs were less commonly used by cases (31.1%), than controls (45.6%), p=0.01, but the difference was not significant after controlling for confounders (adjusted odds ratio (aOR) 0.65, 95% confidence interval (CI) 0.38-1.13). NSAIDs were also less frequent in cases (39.6%) than controls (51.6%), p = 0.04, but there was no difference in the adjusted model (aOR 0.68, 95% CI 0.40-1.17).
Table 3.
Medication use in the past year, microscopic colitis cases and non-microscopic colitis controls
| Cases n=110 |
Controls n=252 |
|||||
|---|---|---|---|---|---|---|
|
|
||||||
| Taken in the past year | No. | (%) | No. | (%) | Adjusted odds ratio† | 95% CI |
| PPI | 33 | (31.1) | 98 | (45.6) | 0.66 | 0.38-1.13 |
| Statin | 36 | (34.0) | 60 | (27.8) | 1.22 | 0.67-2.23 |
| SSRI | 44 | (41.5) | 92 | (43.0) | 0.92 | 0.54-1.57 |
| Aspirin | 42 | (39.6) | 89 | (41.2) | 0.75 | 0.43-1.29 |
| NSAIDS | 42 | (39.6) | 111 | (51.6) | 0.68 | 0.40-1.27 |
| Non-aspirin pain reliever | 49 | (46.2) | 103 | (47.7) | 0.93 | 0.55-1.56 |
| H2 RA | 11 | (10.4) | 40 | (18.5) | 0.55 | 0.26-1.21 |
| Beta blocker | 27 | (25.5) | 60 | (28.3) | 0.95 | 0.52-1.74 |
PPI proton pump inhibitor, SSRI selective serotonin reuptake inhibitor, NSAIDs non-steroidal anti-inflammatory drugs, H2 RA histamine 2 receptor antagonist
Adjusted for age, sex, education
Because the cases were 10 years older than controls on average, all of the analyses were adjusted for age. As a further precaution, we performed additional analyses. First, we stratified by age quartiles and calculated age-specific odds ratios as well as a Mantel Haenszel summary odds ratio. The results were similar to the age-adjusted estimates. The Mantel Haenszel chi square for homogeneity did not indicate differences by strata. We also constructed 2x2 tables for drug use by case-control status for each age strata. In none of the stratified analyses were cases more likely to take implicated drugs than controls. Finally, we looked to see if drug use was associated with age. Statins were more common in the oldest age quartile but statins were not associated with case-control status in the oldest quartile of age. None of the other drugs were taken more commonly in the elderly. Patients experiencing diarrhea might have changed their medications. To explore that possibility, we restricted the analysis to patients who had taken the medication for at least 48 weeks during the year prior to their colonoscopy. The results were the same.
We repeated the analyses for drugs after excluding the patients who were included in the study from review of pathology reports. There results were not different.
Certain drugs such as PPI’s can cause diarrhea. Patients with diarrhea were recruited for this study, and drug-induced diarrhea could have been differential between cases and controls. To explore this, we repeated the analyses for drugs controlling for PPI use. The estimates were unchanged.
We examined allergic conditions such as hay fever, asthma, eczema, drug allergy, food allergy and medication allergy (Table 4). Hay fever, drug allergies, and medication use for allergic conditions were common. Hay fever was less common in cases (aOR 0.58, 95% CI 0.33-1.02). None of the other allergic conditions was more common in microscopic colitis cases in adjusted analyses, individually or in the aggregate.
Table 4.
Allergic conditions reported by microscopic colitis cases and non-microscopic colitis controls
| Cases n=110 |
Controls n=252 |
|||||
|---|---|---|---|---|---|---|
|
|
||||||
| No | (%) | No. | (%) | Adjusted odds ratio† | 95% CI | |
| Hay fever | 66 | (63.5) | 154 | (71.0) | 0.58 | 0.33 – 1.02 |
| Asthma | 12 | (11.3) | 44 | (20.4) | 0.49 | 0.23 - 1.04 |
| Eczema | 15 | (14.2) | 31 | (14.3) | 1.00 | 0.48 - 2.12 |
| Drug allergy | 58 | (54.7) | 110 | (50.9) | 1.00 | 0.60 - 1.76 |
| Food allergy | 23 | (21.7) | 44 | (20.4) | 0.94 | 0.64 - 1.76 |
| Other allergy | 18 | (17.0) | 36 | (16.6) | 1.11 | 0.48 – 2.12 |
| Took meds for allergy | 58 | (54.7) | 120 | (53.3) | 0.97 | 0.58 – 2.25 |
| Any allergy | 96 | (90.6) | 192 | (88.5) | 1.10 | 0.46 - 2.65 |
Adjusted for age, sex, education
Table 5 presents information on surgical history. The cases were less likely to have had a cholecystectomy (aOR 0.33, 95% CI 0.17-0.64) but not other operations. Because cholecystectomy can lead to bile salt induced diarrhea, we explored the association between stool frequency and cholecystectomy. We categorized the number of loose, watery or liquid stools in the week prior to colonoscopy into approximate quartiles: 0-2, 3, 4-5 and 6 or more loose stools. Patients with a cholecystectomy overall (cases and controls) had a greater number of loose stools (p= 0.03). Supplementary Table 1 shows stratified analyses by case-control status. Cases with microscopic colitis who had a cholecystectomy had a greater number of loose stools than cases without a cholecystectomy (p=0.009). Controls who had a cholecystectomy did not have a larger number of loose bowel movements in the week prior to colonoscopy than controls without a cholecystectomy (p=0.09). Figure 1 depicts loose, watery or liquid bowel movements per day in cases and controls stratified by cholecystectomy. The case group had substantially more loose stools than the controls among patients with prior cholecystectomy.
Table 5.
Surgical history reported by microscopic colitis cases and non-microscopic controls
| Cases n=110 |
Controls n=252 |
|||||
|---|---|---|---|---|---|---|
|
|
||||||
| No. | (%) | No. | (%) | Adjusted odds ratio† | 95% CI | |
| Cholecystectomy | 17 | (16.0) | 75 | (34.6) | 0.33 | 0.17 - 0.64 |
| Appendectomy | 22 | (20.8) | 50 | (23.4) | 0.60 | 0.31 – 1.16 |
| Tubal ligation | 26 | (28.9) | 59 | (38.1) | 0.81 | 0.43 - 1.52 |
| Hysterectomy | 27 | (30.0) | 67 | (43.2) | 0.64 | 0.34 - 1.25 |
| Oophorectomy | 14 | (15.6) | 29 | (19.0) | 0.75 | 0.33 - 1.67 |
Adjusted for age, sex, education
Figure 1.
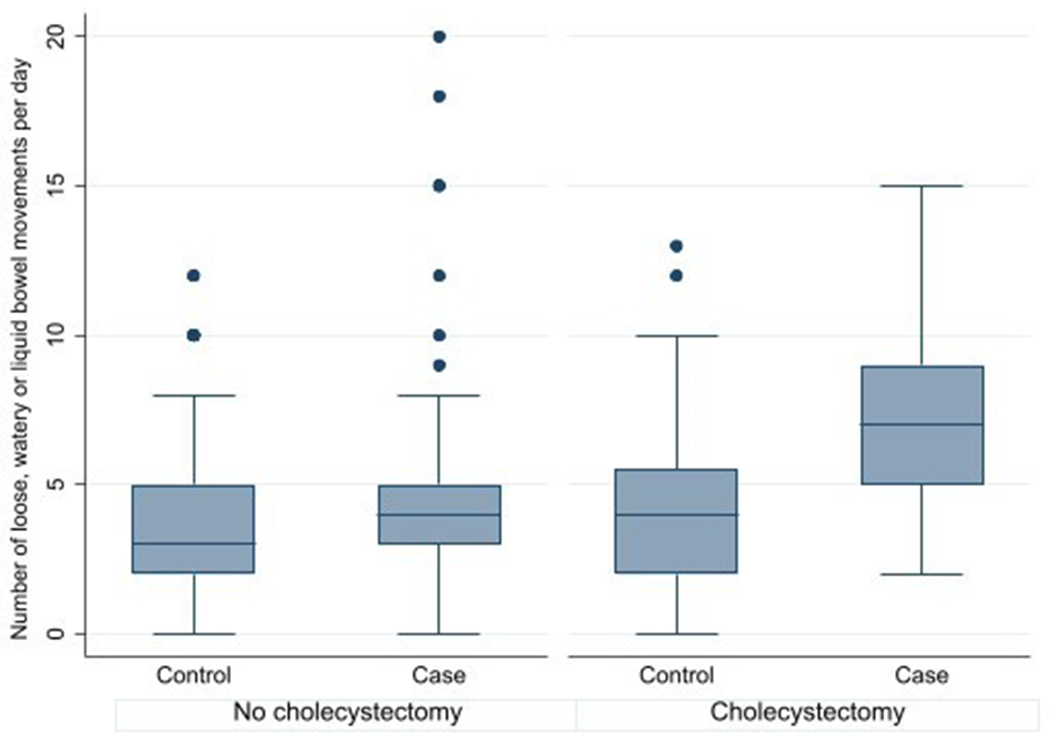
Loose, watery or liquid bowel movements per day in microscopic colitis cases and non-microscopic colitis controls stratified by cholecystectomy
Patients who were eligible for this study were referred for an elective colonoscopy for diarrhea. Those without microscopic colitis (controls) might be more like to have irritable bowel syndrome (IBS). In addition to self-reported IBS, we used the strict Rome IV criteria to classify study subjects.19 As shown in Supplementary Table 2, cases were less likely to have been previously told by a doctor that they had IBS than controls (aOR 0.43, 95% CI 0.21-0.85). Moreover, cases were substantially less likely to have IBS using the Rome IV Criteria for irritable bowel syndrome (aOR 0.19, 95% CI 0.05-0.66). Cases were less likely to satisfy the Rome IV criteria for bloating (aOR 0.62, 95% CI 0.36 – 1.06). The cases were also less likely to report that it had been 6 months or longer since they started feeling bloated or their abdomen looked unusually large, aOR 0.45, 95% CI 0.21-0.97.
In order to explore whether the results in the present study might be explained by a control group of patients with irritable bowel syndrome, we conducted an exploratory analysis of drugs and allergy stratified by IBS. For this analysis we defined IBS as whether a doctor had ever told the patient they had IBS. The number of patients who met the rigid Rome IV IBS criteria was too small (n=35) to support detailed analyses. As shown in Supplementary Table 3, there were 74 patients who reported IBS and 242 with no report of IBS. After adjusting for age, sex and education, there was no difference between non-IBS microscopic colitis cases and non-IBS diarrhea controls for any allergy or any drug.
DISCUSSION
We conducted a large case-control study of patients with diarrhea in order to learn more about microscopic colitis. As expected, the patients with microscopic colitis were older and predominantly women. While all of the patients in the present study had diarrhea, the microscopic colitis patients had worse diarrhea. They had more frequent loose bowel movements each day, more nocturnal stools and more urgency. They were also more likely to lose weight since their diarrhea began. The controls, were more likely to have pain and bloating, symptoms of irritable bowel syndrome. We found that patients with microscopic colitis may be especially susceptible to the effects of bile salts with a larger number of stools following cholecystectomy than controls. There were notable differences from the literature. We found no association with medications or allergy.
Microscopic colitis is the umbrella term for lymphocytic colitis and collagenous colitis. Although sometimes considered separate entities, we have included both lymphocytic and collagenous colitis in the present paper. The decision to combine the groups was motivated by the observation that both forms of microscopic colitis have had parallel increases in incidence, as well as with similar symptoms, age of onset, female predominance, and response to treatment.3,20 When lymphocytic and collagenous colitis are considered separately, the sample size is reduced in each group making it difficult to demonstrate associations. Our study, with 110 well characterized cases, is among the larger studies.
Many classes of drugs have been linked to microscopic colitis including PPIs, NSAIDs, SSRIs, statins and beta blockers. The fact that drugs from so many different classes have been implicated is unusual and hard to explain. Although there is the widespread belief that these medications are responsible for microscopic colitis, the quality of evidence is low as judged by the European Microscopic Colitis Group and United European Gastroenterology.21 Many of the studies lacked a control group.5,12, 22–27 A widely cited paper from Barcelona reported that patients with microscopic colitis were more likely to take SSRIs, NSAIDs, and statins. The controls were 53 patients seen for cataract or sino-nasal surgery.28 The observed effect could be a consequence of the choice of surgery patients as the comparison group. In fact, the study included a second comparison group with watery diarrhea. Compared to the surgical controls, the diarrhea controls were also significantly more likely to take SSRIs and statins, but not NSAIDS or beta blockers. The microscopic colitis patients were not compared to the diarrhea patients.
The largest studies of medication use in microscopic colitis have used a population-based approach to identify cases and community controls. Bondurup at al. identified 3474 patients with lymphocytic colitis and 2277 patients with collagenous colitis using the Danish Pathology Registry, and matched each patient by sex and age to 100 community controls.29 The authors reported associations with PPIs, NSAIDs, statins and SSRIs. However, when they adjusted for more frequent endoscopic examinations, all of the associations were attenuated. Masclee et al. used both community controls and colonoscopy controls.30 The colonoscopies were performed for colon cancer screening and other indications. The number of positive associations was reduced to PPIs and NSAIDs in the colonoscopy controls. These large studies demonstrate that when the controls are more similar to the cases (specifically colonoscopy patients) the drug associations are more limited.
A study from the University of Pennsylvania used a similar design to ours.31 They recruited 26 microscopic colitis cases and 259 controls with diarrhea. None of the commonly implicated drugs – PPIs, SSRIs, statins – was more common in microscopic colitis cases than diarrhea controls in adjusted analyses. Guagnozzi et al. also compared 47 microscopic colitis cases to 317 diarrhea controls.32 There was no association with medications. The patients were younger and had a lower proportion of women than most studies. A recently published study from Columbia University and the Mayo clinic assembled microscopic colitis patients over a decade and matched them with up to two controls who underwent colonoscopy for diarrhea.33 They found an inverse association between microscopic colitis and PPIs, H2 blockers and oral diabetes medications.
Microscopic colitis has been reported to be associated with food allergies and asthma.34,35 We asked patients about a number of allergic conditions such as food and drug allergy, asthma, eczema and hay fever. There were no positive associations with microscopic colitis individually or collectively. Hay fever was more common in controls.
Bile acid malabsorption has been reported in microscopic colitis and microscopic colitis patients with bile acid malabsorption respond to bile salt binding agents.36 Budesonide treatment has been shown to significantly increase 75Se-labelled homocholic acid-taurine retention, a test for bile acid malabsorption and to improve symptom scores.37 Cholecystectomy can increase exposure of the colon to bile acids When we compared the proportions of microscopic colitis patients with prior cholecystectomy to our diarrhea controls, we found that cholecystectomy was more common in the controls (aOR 0.33, 95 CI 0.17-0.64. Because cholecystectomy is a cause of diarrhea, and therefore might lead to referral of our control patients for colonoscopy, we conducted an exploratory analysis that examined stool frequency by case-control status stratified by cholecystectomy. For controls, the number of loose stools was not associated with cholecystectomy status. For microscopic cases, there was a strong association between stool frequency and cholecystectomy. The mechanism could be related to the farnesoid X receptor (FXR). FXR acts as the main nuclear bile acid receptor playing a role in bile acid synthesis and intracellular bile acid accumulation.38 Patients with microscopic colitis have been found to have significant reductions in FXR expression in the colon, possibly making the colonic epithelial cells more susceptible to bile acids and contributing to disease pathogenesis and symptoms.38 Our results provide further support for the belief that patients with microscopic colitis may be particularly susceptible to bile acids or that bile acids may have an etiologic role. The findings also support the use of bile acid binding agents in the management.39
The choice of the comparison group is critically important. In our study, the microscopic colitis population included patients who were referred by their physicians to a single university hospital for chronic diarrhea, underwent colonoscopy and agreed to participate in research that required extra colon biopsies and a telephone or internet interview. The most appropriate controls for these highly selected cases would be individuals drawn from the same population, that is, patients who were referred for chronic diarrhea, underwent a colonoscopy and consented for research. The optimal controls are individuals who would be diagnosed with microscopic colitis at UNC if they had the disease.40 We believe that differences between our study and most prior studies is the fact that our controls had colonoscopies and biopsies for chronic diarrhea.
It is possible that our results are incorrect because the controls are overmatched to the cases. An example of overmatching would be a case-control study of smoking and lung cancer where the controls were patients with chronic obstructive lung disease (also caused by smoking). Such a study would fail to show an association between smoking and lung cancer. In our circumstance, there are two categories of diarrhea patients – microscopic colitis diarrhea (cases) and non-microscopic colitis diarrhea (controls). If a drug, say PPI, were responsible for both microscopic colitis and non-microscopic colitis diarrhea, we would not detect an association between the drug and microscopic colitis. All of the drugs previously implicated in the literature would have to be similarly associated with both microscopic colitis and sufficient diarrhea to lead to colonoscopy. We acknowledge that as a possibility, but think that it is unlikely.
Because of the highly selected nature of the cases and controls, the study is susceptible to bias. If there were factors that influenced referral and drug use differentially between cases and controls, the results would be biased.
Our study had some notable strengths. The study was larger than most. All of our patients had similar symptoms, referral patterns and access to care. In fact, when consented, we did not know whether they had microscopic colitis or not, which makes for a fair comparison. We obtained detailed information on a range of exposures. We adjusted for confounding factors.
The study was limited to a single center and included patients who were very well educated making the study results less generalizable. It is much more important to protect internal validity and avoid bias than to ensure the results are generalizable. The cases were 10 years older than the controls, on average. All analyses were adjusted for age. We also conducted a number of additional analyses to be certain that the age imbalance did not explain the null results. We relied on self-report for exposures. The microscopic colitis cases had more diarrhea than the controls. We did not find evidence that this difference led to biased estimates. There is no reason to believe that there would be differential reporting by cases and controls, but the cases were aware of their microscopic colitis diagnosis at the time of the interview. Recall bias typically exaggerates risk estimates, and our results were generally null. The study was a case-control study. By definition, case-control studies are retrospective. Cases and appropriate controls are selected and past exposures determined. In contrast to case-control studies that rely on data that are already collected, we collected pre-specified data from patients who were enrolled prospectively. There could be ambiguity of the temporal association between exposure and disease. While the sample size was modest, the upper bounds of the 95% confidence intervals preclude a strong effect of any of the exposures.
We found no association between microscopic colitis and medications, autoimmune disease and allergic conditions. Our study differed from most prior studies that included either no controls or community controls. We believe that our choice of diarrhea controls provided a more appropriate comparison population and explains why our results differ from others. Microscopic colitis is an important and growing problem that is likely to become even more important as the population ages. In order to discover the etiology and possible prevention we will need rigorous studies with appropriate design features. Future studies would benefit from prospectively collected data, careful exposure assessment, rigorous pathologic review and collection of biological specimens to begin to unravel the mechanisms for disease development.
Supplementary Material
Grant support:
This research was supported, in part, by grants from the National Institutes of Health (P30 DK034987, R01 DK105114)
Abbreviations:
- aOR
adjusted odds ratio
- BMI
body mass index
- CI
confidence interval
- FXR
farnesoid X receptor
- IBS
irritable bowel syndrome
- NSAIDS
nonsteroidal anti-inflammatory drugs
- PPIs
proton pump inhibitors
- SD
standard deviation
- SSRIs
selective serotonin reuptake inhibitors
Footnotes
Data transparency: Data will not be available to other researchers
Disclosures: None of the authors have financial, professional or personal conflicts of interest.
REFERENCES
- 1.Gentile NM, Khanna S, Loftus EV Jr., et al. The epidemiology of microscopic colitis in olmsted county from 2002 to 2010: a population-based study. Clin Gastroenterol Hepatol 2014;12:838–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roberts ME. Microscopic colitis. Pathologic considerations. J Clin Gastroenterol 2004;38:S18–S26. [DOI] [PubMed] [Google Scholar]
- 3.Rasmussen MA, Munck LK. Systematic review: are lymphocytic colitis and collagenous colitis two subtypes of the same disease - microscopic colitis? Aliment Pharmacol Ther 2012;36:79–90. [DOI] [PubMed] [Google Scholar]
- 4.Bergman D, Clements MS, Khalili H, et al. A nationwide cohort study of the incidence of microscopic colitis in Sweden. Aliment Pharmacol Ther 2019;49:1395–1400. [DOI] [PubMed] [Google Scholar]
- 5.Fumery M, Kohut M, Gower-Rousseau C, et al. Incidence, Clinical Presentation, and Associated Factors of Microscopic Colitis in Northern France: A Population-Based Study. Dig Dis Sci 2017;62:1571–1579. [DOI] [PubMed] [Google Scholar]
- 6.Verhaegh BP, Jonkers DM, Driessen A, et al. Incidence of microscopic colitis in the Netherlands. A nationwide population-based study from 2000 to 2012. Dig Liver Dis 2015;47:30–6. [DOI] [PubMed] [Google Scholar]
- 7.Tong J, Zheng Q, Zhang C, et al. Incidence, prevalence, and temporal trends of microscopic colitis: a systematic review and meta-analysis. Am J Gastroenterol 2015;110:265–76; quiz 277. [DOI] [PubMed] [Google Scholar]
- 8.Weimers P, Ankersen DV, Lophaven S, et al. Incidence and prevalence of microscopic colitis between 2001 and 2016: A Danish nationwide cohort study. J Crohns Colitis 2020. [DOI] [PubMed] [Google Scholar]
- 9.Andrews CN, Beck PL, Wilsack L, et al. Evaluation of endoscopist and pathologist factors affecting the incidence of microscopic colitis. Can J Gastroenterol 2012;26:515–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beaugerie L, Pardi DS. Review article: drug-induced microscopic colitis-proposal for a scoring system and review of the literature. Aliment Pharmacol Ther 2005;22:277–84. [DOI] [PubMed] [Google Scholar]
- 11.Jarnerot G, Tysk C, Bohr J, et al. Collagenous colitis and fecal stream diversion. Gastroenterology 1995;109:449–55. [DOI] [PubMed] [Google Scholar]
- 12.Veress B, Löfberg R, Bergman L. Microscopic colitis syndrome. Gut 1995;36:880–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yen EF, Pokhrel B, Du H, et al. Current and past cigarette smoking significantly increase risk for microscopic colitis. Inflamm Bowel Dis 2012;18:1835–41. [DOI] [PubMed] [Google Scholar]
- 14.Al Momani L, Balagoni H, Alomari M, et al. The association between smoking and both types of microscopic colitis: A systematic review and meta-analysis. Arab J Gastroenterol 2020;21:9–18. [DOI] [PubMed] [Google Scholar]
- 15.Pardi DS, Kelly CP. Microscopic colitis. Gastroenterology 2011;140:1155–65. [DOI] [PubMed] [Google Scholar]
- 16.Lewis SJ, Heaton KW. Stool form scale as a useful guide to intestinal transit time. Scand J Gastroenterol 1997;32:920–4. [DOI] [PubMed] [Google Scholar]
- 17.Etminan M, Collins GS, Mansournia MA. Using Causal Diagrams to Improve the Design and Interpretation of Medical Research. Chest 2020;158:S21–S28. [DOI] [PubMed] [Google Scholar]
- 18.Cotter TG, Binder M, Loftus EV Jr., et al. Development of a Microscopic Colitis Disease Activity Index: a prospective cohort study. Gut 2018;67:441–446. [DOI] [PubMed] [Google Scholar]
- 19.Mearin F, Lacy BE, Chang L, et al. Bowel Disorders. Gastroenterology 2016. [DOI] [PubMed] [Google Scholar]
- 20.Bjornbak C, Engel PJ, Nielsen PL, et al. Microscopic colitis: clinical findings, topography and persistence of histopathological subgroups. Aliment Pharmacol Ther 2011;34:1225–34. [DOI] [PubMed] [Google Scholar]
- 21.Miehlke S, Guagnozzi D, Zabana Y, et al. European guidelines on microscopic colitis: United European Gastroenterology and European Microscopic Colitis Group statements and recommendations. United European Gastroenterol J 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bohr J, Tysk C, Eriksson S, et al. Collagenous colitis: a retrospective study of clinical presentation and treatment in 163 patients. Gut 1996;39:846–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goff JS, Barnett JL, Pelke T, et al. Collagenous colitis: histopathology and clinical course. Am J Gastroenterol 1997;92:57–60. [PubMed] [Google Scholar]
- 24.Olesen M, Eriksson S, Bohr J, et al. Lymphocytic colitis: a retrospective clinical study of 199 Swedish patients. Gut 2004;53:536–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abdo A, Raboud J, Freeman HJ, et al. Clinical and histological predictors of response to medical therapy in collagenous colitis. Am J Gastroenterol 2002;97:1164–8. [DOI] [PubMed] [Google Scholar]
- 26.Simondi D, Pellicano R, Reggiani S, et al. A retrospective study on a cohort of patients with lymphocytic colitis. Rev Esp Enferm Dig 2010;102:381–4. [DOI] [PubMed] [Google Scholar]
- 27.Pardi DS, Ramnath VR, Loftus EV Jr., et al. Lymphocytic colitis: clinical features, treatment, and outcomes. Am J Gastroenterol 2002;97:2829–33. [DOI] [PubMed] [Google Scholar]
- 28.Fernandez-Banares F, Esteve M, Espinos JC, et al. Drug consumption and the risk of microscopic colitis. Am J Gastroenterol 2007;102:324–30. [DOI] [PubMed] [Google Scholar]
- 29.Bonderup OK, Fenger-Grøn M, Wigh T, et al. Drug exposure and risk of microscopic colitis: a nationwide Danish case-control study with 5751 cases. Inflamm Bowel Dis 2014;20:1702–7. [DOI] [PubMed] [Google Scholar]
- 30.Masclee GM, Coloma PM, Kuipers EJ, et al. Increased risk of microscopic colitis with use of proton pump inhibitors and non-steroidal anti-inflammatory drugs. Am J Gastroenterol 2015;110:749–59. [DOI] [PubMed] [Google Scholar]
- 31.Pascua MF, Kedia P, Weiner MG, et al. Microscopic colitis and Medication Use. Clin Med Insights Gastroenterol 2010;2010:11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guagnozzi D, Lucendo AJ, Angueira T, et al. Drug consumption and additional risk factors associated with microscopic colitis: Case-control study. Rev Esp Enferm Dig 2015;107:347–53. [PubMed] [Google Scholar]
- 33.Zylberberg HM, Kamboj AK, De Cuir N, et al. Medication use and microscopic colitis: a multicentre retrospective cohort study. Aliment Pharmacol Ther 2021. [DOI] [PubMed] [Google Scholar]
- 34.Weidenhiller M, Muller S, Schwab D, et al. Microscopic (collagenous and lymphocytic) colitis triggered by food allergy. Gut 2005;54:312–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koskela RM, Niemelä SE, Karttunen TJ, et al. Clinical characteristics of collagenous and lymphocytic colitis. Scand J Gastroenterol 2004;39:837–45. [DOI] [PubMed] [Google Scholar]
- 36.Ung KA, Gillberg R, Kilander A, et al. Role of bile acids and bile acid binding agents in patients with collagenous colitis. Gut 2000;46:170–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bajor A, Kilander A, Gälman C, et al. Budesonide treatment is associated with increased bile acid absorption in collagenous colitis. Aliment Pharmacol Ther 2006;24:1643–9. [DOI] [PubMed] [Google Scholar]
- 38.Torres J, Palmela C, Gomes de Sena P, et al. Farnesoid X Receptor Expression in Microscopic Colitis: A Potential Role in Disease Etiopathogenesis. GE Port J Gastroenterol 2018;25:30–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shor J, Churrango G, Hosseini N, et al. Management of microscopic colitis: challenges and solutions. Clin Exp Gastroenterol 2019;12:111–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rothman KJ. Modern Epidemiology. Boston: Little, Brown and Company, 1986. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


