Abstract
Women are ubiquitously exposed to non-persistent endocrine disrupting chemicals (EDCs) from food contact materials and personal care products. Understanding the impacts of exposure to these chemicals on pregnancy and long-term health outcomes in women is a critical area of research that has been largely overlooked. This brief review focuses on the epidemiological literature exploring associations of non-persistent EDCs – including phthalates, parabens, bisphenols, and triclosan – with maternal pregnancy outcomes and long-term health outcomes in women. We focus on the challenges of this research, particularly assessing non-persistent EDC exposures, aspects of study design, and analyses. We conclude by reviewing the best practices for non-persistent EDC research with regards to pregnancy and women’s health. Though limited, we found some evidence indicating that exposure to non-persistent EDCs is associated with pregnancy health. However, findings from these studies have been inconsistent and require corroboration. Recent studies have also proposed that non-persistent EDC exposures in pregnancy may adversely affect postnatal maternal health. To date, only a few studies have been conducted and have only focused on postpartum weight. More research is needed in this area to inform efforts to promote optimal health across the lifespan of women.
Keywords: endocrine disrupting chemicals, maternal health, pregnancy, women
Introduction
As many as 86% of women will give birth at least once in their lifetime (2016). The very acts of achieving and maintaining a pregnancy are important determinants of women’s long-term health (Dassanayake, et al. 2020, Rich-Edwards, et al. 2013, Walker, et al. 2020). For example, pregnancy complications, including gestational diabetes mellitus (GDM) and pregnancy hypertension/preeclampsia, are associated with long-term maternal metabolic changes, such as adiposity, insulin resistance, type 2 diabetes, high blood pressure, and dyslipidemia (Dassanayake, et al. 2020, Rich-Edwards, et al. 2013, Walker, et al. 2020). Current clinical recommendations in pregnancy generally focus on healthy pregnancy and offspring outcomes, but little is understood about modifiable lifestyle factors in pregnancy that could safeguard women from developing chronic diseases long after they deliver.
Pregnancy is a period of heightened susceptibility to environmental stressors (Boyles, et al. 2020) and one modifiable lifestyle factor is exposure to endocrine disrupting chemicals (EDCs). EDCs are characterized by their ability to dysregulate endocrine pathways critical for hormonal homeostasis. Biomonitoring data of non-persistent EDCs, such as phthalates, phenols, and parabens, from the National Health and Nutrition Examination Survey (NHANES) indicate that women have ubiquitous exposure to many chemicals found in personal care products (Bellavia, et al. 2019), often at higher levels than men, likely due to their greater use of cosmetics and other personal care products (Braun, et al. 2014, Calafat, et al. 2010). These EDCs are found in lotions, cosmetics, perfumes, sunscreens, hair products, feminine hygiene products, and soaps (Table 1). In addition, some of these chemicals (e.g., phthalates and bisphenols) originate from the diet due to their use in food processing and packaging, or are found in consumer products or household furnishings, resulting in ingestion of dust and contaminated food, as well as dermal absorption.
Table 1:
Metabolites and uses of phthalates, triclosan, bisphenol A, and their replacements.
| Compound | Metabolitesa | Uses in Commerce |
|---|---|---|
|
| ||
| DEHPb | MEHP, MECPP, MEHHP, & MEOHP | Plasticizer used in PVC plastics, food packaging, and plastic medical tubing & bags. |
| DiNCH | MHiNCH | Plasticizer used in PVC plastics, food packaging, medical tubing/bags (DEHP Replacement). |
| DEHTP | MEHHTP & MECPTP | Plasticizer used in PVC plastics, food packaging, medical tubing/bags (DEHP Replacement). |
| BBzPb | MBzP | Plasticizer used in vinyl flooring, adhesives, food packaging, synthetic leather, & toys. |
| DEP | MEP | Scent retainer or emollient used in personal care products (cosmetics, lotions, & perfumes). Used as a medication excipient. |
| DnBP & DiBPb | MnBP & MiBP | Scent retainer in personal care products (cosmetics, lotions, & perfumes). Used as a medication excipient. Plasticizer found in some cellulose plastics, & adhesives. |
| Triclosanc | Triclosan | Antimicrobial compound used in soaps, personal care products, toothpaste, kitchen utensils, clothes, cleaning products. |
| Triclocarbanc | Triclocarban | Antimicrobial compound used in personal care & consumer products (Triclosan alternative). |
| Bisphenol A | BPA | Monomer used to manufacture polycarbonate plastics, resins, food cans linings, dental fillings, & medical equipment. Developer in thermal receipts. |
| Bisphenol F & Bisphenol S | BPF & BPS | Monomer used to manufacture food can linings, plastics, resins, and food packaging. Used in cleaning products, industrial solvents, lacquer, varnish, & adhesives. Developer in thermal receipts. (BPA Replacements) |
| Parabens | BP, EP, MP, PP | Antibacterial compound used in medications, personal care products, and foods. |
| Benzophenones | Benzophenones 1 & 3 | Ultraviolet light filter used in sunscreens, cosmetics, consumer product packaging, sunglasses, and sports equipment. |
All of these EDC metabolites are measured in urine and are either the parent compound or metabolic by-products of the parent compound
In the United States, the Consumer Product Safety Improvement Act of 2008 restricted the used of these phthalates in children’s toys and care articles to <0.1%.
The United States Food and Drug Administration has prohibited the use of triclosan and triclocarban in hand soaps and sanitizers.
Abbreviations: Di-2-ethylhexyl phthalate (DEHP), di-isodecyl/isononly phthalate (DiDP/DiNP), di-n/i-butyl phthalate (DnBP/DiBP), butylbenzyl phthalate (BBzP), diethyl phthalate (DEP), 1,2-cyclo-hexane dicarboxylic acid, diisononyl ester (DiNCH), bis(2-ethylhexyl) terephthalate (DEHTP), Bisphenols A, F, S (BPA, BPF, BPS). Mono-2-ethylhexyl phthalate (MEHP), mono-2-ethyl-5-carboxypentyl phthalate (MECPP), mono-2-ethyl-5-hydroxyhexyl phthalate (MEHHP), mono-2-ethyl-5-oxohexyl phthalate (MEOHP), cyclohexane-1,2-dicarboxylic acid monohydroxy isononyl ester (MHiNCH), mono-2-ethylhydroxyhexyl terephthalate (MEHHTP), mono-2-ethyl-5-carboxypentyl terephthalate (MECPTP), mono-benzyl phthalate (MBzP), monoethyl (MEP), & mono-n/i-butyl (MnBP/MiBP) phthalate. Ethyl-, methyl-, butyl, and propyl-parabens (BP, EP, MP, & PP).
In pregnancy, exposure to several EDCs has been associated with poor pregnancy outcomes (e.g. preterm birth) and long-term consequences for infant and child health (Karwacka, et al. 2017, Philippat, et al. 2017). However, several recent observational studies have identified associations between exposure to some non-persistent EDCs in pregnancy and adverse maternal outcomes, including maternal glucose disruption (James-Todd, et al. 2018, James-Todd, et al. 2016, Shaffer, et al. 2019, Yang, et al. 2020) and maternal thyroid and sex hormone concentrations in pregnancy (Johns, et al. 2015). These dysregulations in pregnancy can have long-term deleterious consequences for women’s health. Yet, little is known about the contribution of EDC exposures in pregnancy for maternal health after parturition.
This review will evaluate the impacts of non-persistent EDCs (phthalates, parabens, phenols, and triclosan) on women’s health, with a special focus on pregnancy as a critical window of exposure for women’s long-term health. We will specifically focus on non-persistent EDCs because women may be able to modify their exposure to these chemicals through behavioral changes (e.g., changing or reducing personal care product use or dietary modifications) (Harley, et al. 2016). We posit that the associations of non-persistent EDCs with women’s health may be partially mediated by EDC-targeted disruptions to maternal health during pregnancy, but current limitations in study design, EDC exposure measurements, and statistical methods to this point make it difficult to definitively conclude this. In this review, we first briefly discuss pregnancy history and women’s long-term health to set the stage for pregnancy outcomes as an important view into the future health of women. Second, we discuss patterns of associations of exposure to non-persistent EDCs in pregnancy in relation to pregnancy outcomes and highlight the challenges that contribute to the difficulty in establishing a cohesive picture of non-persistent EDC exposure and pregnancy outcomes. Third, we explore in more detail the limited literature on non-persistent EDC exposure in pregnancy and maternal health after pregnancy. Lastly, we provide guidance for moving this research field forward in order to achieve the ultimate goal of identifying pregnancy-related factors that will protect women’s long-term health.
Methods
For this narrative review, we searched PubMed using keywords including (but not limited to) pregnant, EDCs, specific classes of EDCs (e.g. phthalates, bisphenol), post-natal, and post-partum. We selected epidemiologic studies that illustrated challenges or best practices in designing and interpreting the exposure-outcome relationships of interest and attempted to summarize associations by class of chemical. We included articles that were published before January 2021. For all studies reviewed here, we only described associations that were adjusted for important confounders, including sociodemographic characteristics, lifestyle factors, and health conditions (specific example of such details can be found in Table 2).
Table 2.
Studies of non-persistent endocrine disrupting chemical exposures during pregnancy and maternal postpartum weight gain.
| Reference | Cohort and Location | Sample Size | Chemicals Measured | Urine Samples and Dilution Correction | Analytic Treatment of Metabolites | Statistical Modeling | Outcome | Covariates | Results |
|---|---|---|---|---|---|---|---|---|---|
| Rodriguez-Carmona et al, 2019 | Early Life Exposure in Mexico to Environmental Toxicants Project, Mexico City, Mexico | Original subset of ELEMENT: 250, Analyzed subset: 178 |
• MEHP • MEHHP • MEOHP • MECPP • MBzP • MCPP • MnBP • MiBP • MEP |
Collected once in each trimester, women had to contribute at least one urine sample to be included Specific gravity |
Summed metabolites of parent compounds DEHP (MEHP, MEHHP, MEOHP, and MECPP) and DnBP (MnBP and MiBP). Single-pollutant models of metabolites or sums of parent compounds |
Path analysis that included log-transformed phthalate metabolite concentrations across pregnancy | Annual weight change in kg. Weight was measured up to five times in the first year postpartum and twice during follow-up visits 5.2–10.7 years later |
• Maternal age • Education • Energy intake |
• One log-unit increase in mCPP was associated with annual weight increase of 0.33 kg (95% CI: 0.09, 0.56). • One log-unit increase in MBzP was associated with weight decrease of 0.21 kg (95% CI: −0.38, −0.03) |
| Perng,et al, 2020 | Early Life Exposure in Mexico to Environmental Toxicants Project Mexico City, Mexico | Original subset of ELEMENT: 250, Analyzed subset: 167 |
• BPA • MEHP • MEHHP • MEOHP • MECPP • MBzP • MCPP • MnBP • MiBP • MEP |
Collected once in each trimester, women had to contribute at least one urine sample to be included Specific gravity |
Summed metabolites of parent compounds DEHP (MEHP, MEHHP, MEOHP, and MECPP) and DnBP (MnBP and MiBP). Single-pollutant models of metabolites or sums of parent compounds |
Mixed effects linear models that incorporated repeated measures of weight | Average annual rate of weight change in kg through one year postpartum. Weight was measured up to four times post-partum. Positive beta estimates indicate slower weight change while negative indicate faster weight change |
• Maternal age • Parity • Height • First trimester body mass index • Gestational age at first trimester visit • Maternal smoking during pregnancy • Breast feeding duration • Birthweight |
• One log-unit increase in BPA was associated with 0.68 kg/year slower weight change (95%CI: 0.07,1.29) • One log-unit increase in MBzP was associated with 0.73 kg/year slower weight change (95%CI: 0.08,1.39). • One log-unit increase in MCPP was associated with 0.79 kg/year slower weight change (95%CI: 0.14, 1.43). • One log-unit increase in ∑DEHP was associated with 1.00 kg/year slower weight change (95%CI: 0.04, 1.61). • One log-unit increase in ∑DBP was associated with 0.79 kg/year slower weight change (95% CI: 0.16, 1.42). |
| Philips et al, 2020 | Generation R study, Rotterdam, the Netherlands | Original subset: 1,405, Analyzed subset: 1,192 |
• BPA • BPS • BPF • Phthalic acid • MMP • MEP • MiBP • MnBP • MEHP • MEHPP • MEOHP MECPP • MCMHP • MBzP • MCPP • MHpP |
Collected three times during pregnancy, used early and mid-pregnancy samples only Creatinine |
Summed bisphenols (BPA, BPS, BPF) Summed metabolites of parent compound DEHP (MEHP, MEHHP, MEOHP, MCMHP, and MECPP) Summed metabolite of parent compound DNOP (MCPP, MOP, and MCHpP) Summed high molecular weight phthalates (∑DEHP, MCPP, MBzP, MHxP, MHpP).Summed low molecular weight phthalates (MMP, MEP, MnBP, MiBP).Phthalic acid was used as a proxy for total burden of phthalate exposure |
Multivariable linear models | Weight change (g) from pre-pregnancy to 6 years postpartum. | • Maternal age • Parity • Ethnicity • Education • Dietary caloric intake • Pre-pregnancy body mass index • Maternal smoking during pregnancy • Maternal alcohol use during pregnancy |
• One log-unit increase in BPA was associated with weight increase of 364 g (95%CI: 10, 718) 6 years postpartum • One log-unit increase in phthalic acid was associated with 734 g increase in weight (95%CI: 273, 1196). • One log-unit increase in low molecular weight phthalate concentration was associated with 678 g increase in weight (95%CI: 328, 1029). • One log-unit in high molecular weight phthalate metabolite concentration was associated with 724 g increase in weight(95%CI: 233, 1061). • One log-unit increase in ∑DEHP was associated with 588 g increase in weight (95% CI: 115, 1061). • One log-unit increase in ∑DNOP was associated with 840 g increase in weight (95%CI: 347, 1332) |
Abbreviations: Bisphenols A, F, S (BPA, BPF, BPS). Confidence Interval (CI), Di-2-ethylhexyl phthalate (DEHP), di-n-butyl phthalate (DnBP), di-n-octyl phthalate (DNOP) mono-2-ethylhexyl phthalate (MEHP), mono-2-ethyl-5-carboxypentyl phthalate (MECPP), mono-2-ethyl-5-hydroxyhexyl phthalate (MEHHP), mono-2-ethyl-5-oxohexyl phthalate (MEOHP), mono-[(2-carboxymethyl)hexyl]phthalate (MCMHP), mono-benzyl phthalate (MBzP), monoethyl (MEP), & mono-n/i-butyl (MnBP/MiBP) phthalate, mono(3-carboxypropyl)phthalate (MCPP), mono-hexylphthalate (MHxP), monomethylphthalate (MMP), mono-2-heptylphthalate (MHpP), monocotyl phthalate (MOP), mono-(−7-carboxy-n-heptyl) phthalate (MCHpP)
Pregnancy outcomes as an important view into the future health of women
Pregnancy is a time of significant physiological change that is experienced by most women during their lifetime. This unique period can also be accompanied by health complications that offer a warning for maternal health risks and long-term outcomes. Thus, it is critical to identify interventions that prevent pregnancy complications and support women’s health in the future. Several recent reviews suggest that various pregnancy pathologies, as well as the act of being pregnant, may be a “stress test” – in that pregnancy may serve as a first glance into potential long-term health outcomes in women (Ananth 2014, Cunningham and LaMarca 2018, Dassanayake, et al. 2020, Durnwald 2015, Leslie and Briggs 2016, Rich-Edwards, et al. 2013, Troisi, et al. 2018). Specifically, pregnancy outcomes such as ischemic placental disease, shortened gestation/preterm birth, GDM, and pregnancy hypertensive disorders have been shown to have consistent, positive associations with later cardiovascular disease and mortality in women. Several reviews have noted associations of GDM with the subsequent development of type 2 diabetes mellitus, with the greatest risk of developing type 2 diabetes mellitus occurring within five years of a pregnancy complicated by GDM (Dassanayake, et al. 2020, Rich-Edwards, et al. 2013). Evidence also suggests that extremely shortened gestation (20–31 weeks) is associated with subsequent risk of type 2 diabetes mellitus in the ten years after pregnancy (James-Todd, et al. 2013). A recent meta-analysis noted that associations of GDM and cancer varied depending on the geographic location of study participants and the type of cancer studied, with breast cancer being the most commonly studied cancer in this context (Wang, et al. 2020); although another recent meta-analysis found no overall association between gestational hypertension or preeclampsia and breast cancer (Sun, et al. 2018). Together, this body of work suggests that factors that impact pregnancy outcomes in women may have lasting consequences for their health, making this a susceptible period of life (Leslie and Briggs 2016, Rich-Edwards, et al. 2013).
Non-persistent EDCs in pregnancy and maternal pregnancy health
Non-persistent EDCs dysregulate pathways governed by hormones. Given the indispensable role of hormones in establishing and maintaining a pregnancy, there has been keen interest in the potential for non-persistent EDCs to interrupt pregnancy and fetal development. However, much of this research has considered how various maternal morbidities impact fetal or child outcomes but have not specifically addressed the consequences of exposure for the health of the mother. Recent studies have begun to address these gaps. Among epidemiological studies examining associations of non-persistent EDCs and pregnancy health outcomes, the most commonly studied pregnancy health outcome is GDM. Other important pregnancy health outcomes that have been studied in relation to non-persistent EDC exposures include preeclampsia, other pregnancy hypertensive disorders, and gestational weight gain.
Gestational Diabetes Mellitus
Recently, several epidemiological studies have explored associations between non-persistent EDCs and GDM, which reported mixed results. In relation to GDM, impaired glucose tolerance, and increased blood glucose concentrations, three studies reported positive associations with monoehtyl phthalate (MEP), monobutyl phthalate (MBP), and monocarboxyoctyl phthalate (MCOP) (James-Todd, et al. 2018, James-Todd, et al. 2016, Shaffer, et al. 2019) and negative associations with monocarboxyoctyl phthalate (MCOP) (Shaffer, et al. 2019), monoisobutyl phthalate (MiBP) (James-Todd, et al. 2018), and the sum of di-2-ethylhexyl phthalate (DEHP) metabolites (James-Todd, et al. 2016). MEP was the only metabolite consistently and positively associated with GDM, impaired glucose tolerance, and blood glucose concentration across these studies. However, another study reported no associations between phthalate metabolites and either GDM or impaired glucose tolerance (Shapiro, et al. 2015). Studies evaluating associations of phenols and parabens with pregnancy glucose complications are similarly mixed (Bellavia, et al. 2019, Fisher, et al. 2018, Li, et al. 2019b, Liu, et al. 2019, Ouyang, et al. 2018, Shapiro, et al. 2018, Shapiro, et al. 2015, Yang, et al. 2020, Zhang, et al. 2019). The interpretation and synthesis of results across studies are challenged by differences in exposure assessment and outcome definitions used to characterize pregnancy glucose homeostasis endpoints. Additional inconsistencies in findings across studies may be related to the use of single pollutant models that may produce statistically significant estimates that are the result of multiple testing.
Preeclampsia and pregnancy hypertensive disorders
The epidemiological literature of associations between non-persistent EDCs and preeclampsia/pregnancy hypertensive disorders is sparse. Of available studies, bisphenol A (BPA) was most consistently associated with preeclampsia. Urinary BPA concentrations at 10 weeks gestation were associated with a 1.53-fold (95% CI: 1.04, 2.25) risk of preeclampsia (Cantonwine, et al. 2016), and women with high serum concentrations of BPA at 16–20 weeks had 16.46-fold (95% CI: 5.42, 49.85) increase in odds of pre-eclampsia compared to women with low concentrations (Ye, et al. 2017). However, serum BPA levels are subject to exogenous contaminations and are unlikely to be a useful biomarker of exposure (Calafat, et al. 2013, Calafat, et al. 2015), thus it is difficult to interpret the findings from this study.
Associations between phthalate metabolites and preeclampsia are not consistent across studies. MEP, mono(3-carboxypropyl) phthalate (MCPP), and the metabolites of DEHP were associated with increased risk of preeclampsia, while MiBP was associated with a decreased risk (Cantonwine, et al. 2016). However, MiBP measured in the first trimester was associated with elevated diastolic blood pressure only among women carrying male fetuses (Han, et al. 2020). In another study, the butyl benzyl phthalate (BBzP) metabolite monobenzyl phthalate (MBzP) was positively associated with diastolic blood pressure throughout gestation, and was also associated with an increased risk of a combined outcome of pregnancy hypertension, preeclampsia, eclampsia, and HELLP syndrome (Werner, et al. 2015). As with gestational diabetes, it is difficult to draw conclusions about these associations because of the variability in the measures used to identify pregnancy hypertensive disorders, the timing of exposure assessment, and the use of single pollutant models that may result in significant findings simply from chance. Additionally, the limited number of studies makes it difficult to discern patterns of associations.
Gestational weight gain
Both inadequate and excessive weight gain during pregnancy can have lasting implications for mothers and children (Kominiarek and Peaceman 2017, Oken, et al. 2007). Several studies have explored the associations between phthalate metabolites and gestational weight gain, also with mixed results. The phthalate metabolite MEP has been shown to be associated with gestational weight gain across multiple studies, but the direction of the association is not consistent. MEP was associated with 2.17 (95% CI: 0.98, 4.79) fold higher odds of excessive weight gain (James-Todd, et al. 2016), whereas women with inadequate gestational weight gain were shown to have lower concentrations of MEP (Li, et al. 2019a). Another study reported 1.18 (95% CI: 1.01, 1.39) fold higher odds of inadequate gestational weight gain with increasing low molecular weight phthalate concentrations prior to 18 weeks gestation (with MEP being the major component of the sum of low molecular weight phthalates in this study) (Philips, et al. 2020b). In addition to the common EDC-related challenges in study design that will be discussed in later sections, some of the inconsistency may be due to the variation in the associations across the outcome – such that the relationship between phthalates and gestational weight gain may differ in women with low vs. high gain. This is suggested by findings that higher MEP concentrations were associated with greater BMI change at the 75th percentile of early gestational weight gain (Bellavia, et al. 2017). This study also found associations of phthalate metabolites MCPP, MBzP, and sum of DEHP metabolites were associated with greater change in early pregnancy BMI among women whose BMI change was in the 50th and 75th percentiles, whereas the sum of DEHP metabolites was negatively associated with BMI change among women whose BMI change was in the 25th percentile (Bellavia, et al. 2017).
Fewer studies have investigated parabens and phenols in relation to gestational weight gain. Methyl-, ethyl-, and propyl paraben were all associated with first trimester gestational weight gain, but the association was strongest among women who were classified as overweight or obese (Wen, et al. 2020). One study found that bisphenol concentrations were generally associated with lower pregnancy weight gain. Early pregnancy (less than 18 weeks gestation) BPA concentrations (log-unit increase) were associated with 132 gram (g) lower weight gain from mid- to late- pregnancy (95%CI: −231g, −34g), and higher concentrations of BPA replacement bisphenol S (BPS) was associated with 261g lower weight gain across pregnancy (95% CI: −466g, −56g) (Philips, et al. 2020b). As with previous outcomes, the varied time frame of exposure assessment and the differences in timing of outcome ascertainment contribute to the challenges in synthesizing the results. These studies suggest that identifying a relevant window for both exposure and outcome is important for research that seeks to understand associations of non-persistent EDCs and gestational weight gain.
Shortened gestational length
In addition to the immediate risks associated with shortened gestation (for both mother and baby), shorter gestational length (or preterm birth) has also been associated with long-term maternal health outcomes, including greater risk of developing type 2 diabetes and cardiovascular disease (James-Todd, et al. 2013, Tanz, et al. 2019). The associations of pregnancy exposure to EDCs (including non-persistent EDCs) with shortened gestational length have been reviewed extensively (Ferguson and Chin 2017, Marie, et al. 2015). Overall, the associations of non-persistent EDCs and shortened gestation or preterm birth are not consistent, with the exception of triclosan exposure for which most studies found no statistically significant associations with shortened gestation (Ding, et al. 2017, Huo, et al. 2018, Jamal, et al. 2020, Khoshhali, et al. 2020). Consistency of association is lacking for summed DEHP metabolites and shortened gestation, which have been associated with increased risk of preterm birth (Ferguson, et al. 2014), decreased risk of shortened gestation (Chin, et al. 2019), increased risk of long gestation (Adibi, et al. 2009), and no association with gestation duration (Shoaff, et al. 2016). The associations of BPA and parabens with shortened gestation and preterm birth are similarly conflicting. Understanding associations of non-persistent EDCs and length of gestation endpoints may be limited by the low prevalence of preterm birth and the characterization of preterm birth as a binary variable; this may decrease the sensitivity of a study to detect an association. Alternatively, if the effects of non-persistent EDCs on gestational length are non-linear, the use of linear regression models for gestational age at birth will mischaracterize the association. Though it is possible that EDC exposure has the potential to impact maternal health by disrupting the length of gestation, substantially more research is needed to address the inconsistencies in prior studies.
Non-persistent EDCs and maternal health after pregnancy
Pregnancy has lasting consequences for women’s health, so there is reason to posit that chemical exposures in pregnancy will impact women long after they give birth.
Hormonal disruption in pregnancy and women’s long-term health
One likely mechanism behind this hypothesis is that EDC exposure in pregnancy alters maternal hormones, which impacts women’s health long after pregnancy (Figure 1). Observational studies in pregnant women suggest that phthalates, phenols, and parabens may alter concentrations of estrogen, androgens, or both (Aker, et al. 2019, Johns, et al. 2015, Sathyanarayana, et al. 2017), and a prospective case-control study found that higher gestational estrogen concentrations were associated with increased risk of breast cancer in mothers after 38 years of follow-up (Cohn, et al. 2017). Changes in estrogens, as well as androgens, may also be implicated in cardiovascular disease and osteoporosis, which are prevalent in post-menopausal women (Lello, et al. 2015); if these are caused by hormone disruption during pregnancy warrants further investigation. Data on the ability of non-persistent EDCs to bind to thyroid receptors are mixed (Paul-Friedman, et al. 2019, Zoeller 2007), but observational studies suggest that these same EDCs are associated with altered maternal triiodothyronine (T3) and thyroxine (T4) concentrations (Derakhshan, et al. 2019, Johns, et al. 2016, Romano, et al. 2018). Around 5–9% of women with a thyroid disorder in pregnancy may experience postpartum thyroiditis within the first year postpartum that can lead to permanent hypothyroidism, increasing the likelihood of fertility problems for subsequent pregnancies and more severe menopause symptoms during midlife (Kennedy, et al. 2010). Altered thyroid hormone and thyroid stimulating hormone (TSH) concentrations later in life may also be associated with increased risk of cardiovascular disease, although additional studies are needed to confirm this association (Cappola, et al. 2019). Similarly, thyroid hormones and TSH are involved in maintaining bone health, and deviations from normal thyroid hormone concentrations, especially post-menopause, may be associated with increased risk of osteoporosis (Delitala, et al. 2020). Whether non-persistent EDCs modulate thyroid receptor in pregnancy warrants further investigation.
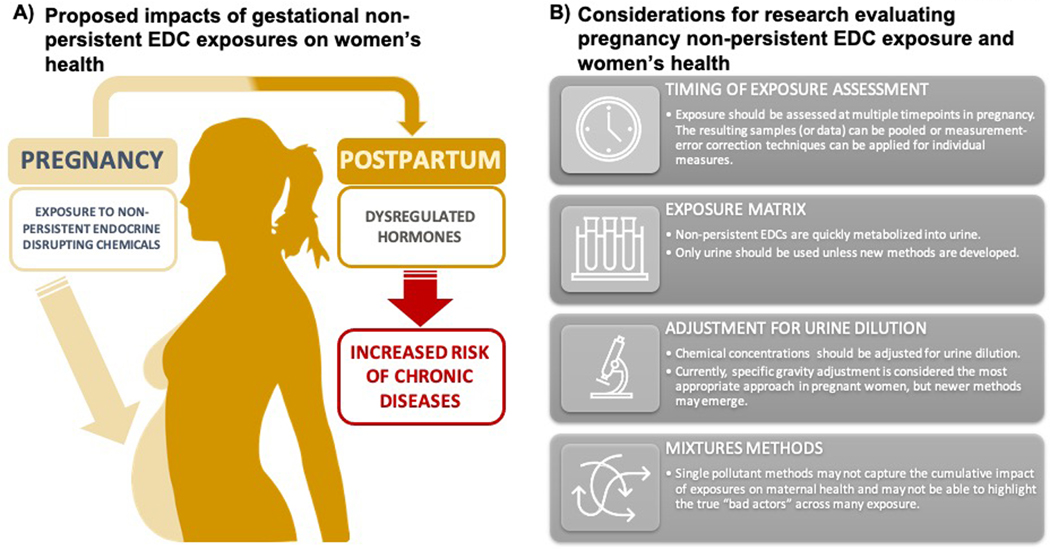
Figure 1: Proposed role of non-persistent endocrine disrupting chemical (EDCs) exposures in women’s long-term health (A) and future directions (B).
The current review outlines the potential for non-persistent EDC exposures in pregnancy to impact women’s health long after they give birth. One potential mechanism that would support this hypothesis is the role of maternal hormones as mediators of the relationship between non-persistent EDC exposure and women’s long-term health. Though substantially more data are needed to support this hypothesis, experimental and human epidemiological studies have found that non-persistent EDC exposure in pregnancy dysregulates maternal hormones in pregnancy. These disturbances in pregnancy can impact numerous health outcomes in women, including cancer development, gynecologic health, cardiometabolic outcomes, and likely numerous others. Given the inconsistencies in the current literature related to non-persistent EDC exposures and women’s health, several important methodological considerations should be kept in mind for future studies that aim to connect gestational exposures to women’s long-term health.
Pregnancy exposure to non-persistent EDCs and maternal long-term weight gain
To date, the epidemiological evidence that exposure to non-persistent EDCs during pregnancy is associated with long-term maternal health is limited, and mainly focuses on postpartum weight change with inconclusive results (Table 2). One study found that pregnancy concentrations of the plasticizers MCPP and MBzP were associated with annual weight change (through 10 years postpartum), but in opposite directions; MCPP was associated with annual gains of 0.33 kg/year, while MBzP was associated with decreases of 0.21 kg/year (Rodríguez-Carmona, et al. 2019). Another study found that BPA, total phthalic acid, low molecular weight phthalates, and high molecular weight phthalates were associated with weight gains ranging from 364 to 734 g six years postpartum (Philips, et al. 2020a). As will be discussed later, these inconsistencies may be due to differences in timing of exposure assessment (both during pregnancy and postpartum). However, a key challenge for interpretation and synthesis of the information from these studies is the differences in how phthalate exposures were statistically analyzed; the first study approximated parent compound exposure, while the second study used total body burden of phthalates and categorized them based on molecular weight. Another recent study noted that BPA and phthalate metabolites measured across pregnancy were associated with slower weight loss through one-year postpartum, with effects ranging from 0.6 to 1.0 kg/year slower for an interquartile range change in metabolite concentration (Perng, et al. 2020).
Non-persistent EDC exposure in pregnancy and other long-term health outcomes in mothers
We found no epidemiological studies investigating non-persistent EDC exposures during pregnancy and other postpartum health outcomes in women, such as heart disease or type 2 diabetes. One study estimated lifetime risk of breast cancer from exposure to non-persistent EDCs from plastic water bottles during pregnancy and found the risk to be minimal (Jeddi, et al. 2016), but the literature linking pregnancy exposure to EDCs and cancer is also sparse. One review has hypothesized a link between pregnancy exposure to persistent EDCs and breast cancer (Terry, et al. 2019), but the impact of pregnancy exposure to non-persistent EDCs has been insufficiently studied.
Current limitations and best practices
Inconsistent results across studies make it difficult to determine if EDC exposure during pregnancy increases long-term chronic disease susceptibility in women. There are still considerable gaps in our knowledge of the long-term consequences of these exposures on women’s health during and after pregnancy. The study of women’s health in the context of non-persistent EDC exposures shares many challenges with other EDC-related research areas, but also has several unique considerations. It is also important to note that while the non-persistent EDCs reviewed here have been shown to have unique mechanisms of action in experimental models (Strakovsky and Schantz 2018), they are all classified as endocrine disrupting chemicals that share common exposure sources in human populations and are therefore more similar to each other than they are to other classes of chemicals. Therefore, the challenges described below related to study design must be addressed first before the field can move forward to asking chemical-specific mechanistic questions using epidemiologic studies.
Challenges in non-persistent EDCs research
As with most epidemiological studies of non-persistent EDCs, the field is challenged by the nature of the exposure. Metabolism and excretion of non-persistent EDCs is rapid, with half-lives ranging from hours to a few days (Frederiksen, et al. 2007, Moos, et al. 2016, Sandborgh-Englund, et al. 2006, Vandenberg, et al. 2007). Therefore, measurement of non-persistent chemical concentrations is highly dependent on timing (Lassen, et al. 2013), with studies recommending that exposure is best characterized by obtaining and pooling samples over a period of days or weeks during periods relevant to disease etiology (Vernet, et al. 2019, Vernet, et al. 2018). Alternatively, measurement-error correction techniques can be employed (e.g., regression calibration) (Jackson-Browne, et al. 2019). Several studies we noted here used single urine samples to characterize exposure, and a few studies of outcomes that occurred before birth (GDM for example) used exposure measures from urine samples obtained after the outcome had already occurred.
Along with the need to carefully consider timing of exposure assessment, the matrix in which these chemicals are measured is important, and urine is the optimal matrix for measuring most of these chemicals (Calafat, et al. 2013, Calafat, et al. 2015). Many of the studies included here evaluated chemical concentrations in urine, but a few used alternative matrices such as meconium (Baker, et al. 2020), and serum (Ye, et al. 2017). Serum and plasma are particularly poor choices for measuring non-persistent EDCs because of contamination from medical equipment and low/transient concentrations of the chemicals (Calafat, et al. 2013, Koch and Calafat 2009). Future studies should continue to use urine biomarkers unless more appropriate biomarkers are developed.
With regards to measuring non-persistent EDC concentration in urine, adjustment for urine dilution is another important consideration. In pregnancy especially, variation in individual hydration levels can affect non-persistent EDC metabolite concentrations in urine. Without adjustment for urine dilution, hydration may confound or obscure associations of non-persistent EDC metabolite concentrations with the outcomes of interest. Some studies suggest the urinary dilution measure of specific gravity is most appropriate in pregnant women (Lee, et al. 2020, MacPherson, et al. 2018). Therefore, it is important to consider both the selection of the urinary dilution measure (e.g. creatinine, specific gravity) (Lee, et al. 2020, MacPherson, et al. 2018) and the statistical approach to adjustment (O’Brien, et al. 2016).
Most studies reviewed here relied on single pollutant models or sums of related metabolites to characterize parent compound exposure. However, as discussed in the 2015 National Institute of Environmental Health Sciences workshop entitled “Statistical Approaches for Assessing Health Effects of Environmental Chemical Mixtures in Epidemiology Studies”, exposure to individual EDCs does not occur in isolation, and there are methods to address several questions related to exposure to chemical mixtures (Braun, et al. 2016). For example, methodologies are available to isolate the effect of an individual compound, to assess the cumulative effects of compound mixtures, and to evaluate interactions between various compounds in a mixture (Braun and Gray 2017). A recent study in 130 cases of preterm birth and 352 random controls assessed whether individual phthalate metabolites or their combination was associated with gestational age at delivery (Boss, et al. 2018). Authors reported that each interquartile range (IQR) increase in log- mono-2-ethyl-5-carboxypentyl phthalate (MECPP), was associated with a higher hazard ratio (HR) for preterm birth (HR: 1.2; 95%CI: 1.1, 1.3) and a 1.2% (95% CI: 0.3, 2.1%) decrease in gestational age at birth. However, when an environmental risk score was calculated from weighted sum of phthalate metabolites, compared to the first quartile, being in the fourth quartile of the environmental risk score was associated with a HR for preterm birth of 1.4 (95% CI: 1.2, 1.8) and a 2.6% (95% CI: 0.8, 4.3%) decrease in gestational age – suggesting that exposure to a cumulative mixture of phthalates elevated prematurity risk to a greater extent compared to the single pollutants (Boss, et al. 2018). Therefore, future studies should consider evaluating non-persistent EDCs as mixtures to better model exposure in human populations.
Challenges in study design specific to research evaluating non-persistent EDCs in pregnancy and pregnancy outcomes or maternal postnatal health
As with all epidemiologic studies, valid study design is critical in research of non-persistent EDCs and women’s health outcomes, especially becausethere are likely periods of heightened susceptibility during pregnancy for many of the health outcomes mentioned in this review. Although there are many statistical methods for identifying periods of heightened susceptibility (Buckley, et al. 2019), it is also important to develop studies that already account for the temporality of the exposure-outcome relationships within the design. Cross-sectional studies, though convenient, lack temporality of the exposure-outcome association, which is why conducting prospective cohort studies is critical. However, it can be challenging to design prospective studies as the disease latency and the critical timing of exposure are often unknown in pregnancy and long-term health. Additionally, prospective studies need to consider how exposure and other health behaviors change over time. To address this, some cohort studies developed protocols to collect multiple urine samples across pregnancy, but may miss timepoints of heightened susceptibility for an outcome, especially around the time of implantation, as women are often recruited into research after they conceive (Chin, et al. 2019). This makes it challenging to evaluate associations of non-persistent EDC concentrations with early pregnancy outcomes, including implantation failure and early pregnancy loss. Preconception studies can sometimes overcome this limitation by capturing maternal non-persistent EDC concentrations during the preconception and early pregnancy windows, and several have demonstrated associations of non-persistent EDCs with early pregnancy loss (Messerlian, et al. 2016, Toft, et al. 2012). However, results from preconception cohorts may be difficult to interpret since women participating in these studies are often seeking fertility treatment. Unless the infertility is due to the male partner (and women are able to conceive naturally/with the use of intrauterine insemination), results from these studies may not be generalizable to naturally conceived pregnancies.
While early pregnancy will always be difficult to capture, an important approach for addressing inconsistencies in exposure assessment is for future studies to collect exposure data across multiple gestational timepoints, especially during critical windows of heightened susceptibility to the effects of non-persistent EDC (Pacyga, et al. 2021). With this approach, exposure can first be assessed in a cross-pregnancy pool, and then in more defined windows of susceptibility, if warranted. Another important consideration specifically related to evaluating exposure in pregnancy and long-term health outcomes is to capture exposure several times postnatally – including at the time of the health outcome measures. While challenging, it is critical to understand whether the EDC-outcome relationship is related to past (pregnancy) or current EDC exposure. Also potentially important, but infrequently studied, is paternal nonpersistent EDC exposure in the preconception period and its contribution to maternal health outcomes. Limited research also indicates paternal non-persistent EDC concentrations may be associated with shortened gestation (Mustieles, et al. 2020), but the paternal contribution has largely been overlooked for other conditions. As a result, future research may need to evaluate paternal contribution to women’s health outcomes. Careful consideration of these study design challenges is necessary to move the field of non-persistent EDCs and women’s health forward.
Conclusions
In this review, we noted a growing body of literature surrounding exposure to non-persistent EDCs during pregnancy and pregnancy outcomes in mothers, but it is thus far difficult to interpret. In contrast, recent studies have only begun to suggest that non-persistent EDC exposures in pregnancy adversely affect postnatal maternal health, but this is based on a limited number of studies focused on post-natal weight change. Therefore, while there is reason to posit that pregnancy exposures to non-persistent EDCs could impact women’s long-term health, we conclude that the current literature warrants substantial corroboration. To move the field forward, best practices using a sensitive-periods framework, valid epidemiological study design, and mixtures models, are needed in future research to maximize our ability to draw conclusions about the effects of non-persistent EDCs on women’s health. Use of these best practices will be crucial to unravelling the non-persistent EDC exposures that most impact future health outcomes in women.
Acknowledgments
Funding sources: This publication was made possible by the National Institute for Environmental Health Sciences (NIH/NIEHS) grants ES024795 and ES032227. This project was also supported by the USDA National Institute of Food and Agriculture and Michigan AgBioResearch.
Dr. Braun served as an expert witness in litigation related to perfluorooctanonic acid contamination in drinking water in New Hampshire. Any funds he received from this arrangement were/are paid to Brown University and cannot be used for his direct benefit (e.g., salary/fringe, travel, etc.).
Footnotes
Declaration of Interest: Authors have no conflicts of interest to disclose.
References cited
- Adibi JJ, Hauser R, Williams PL, Whyatt RM, Calafat AM, Nelson H, Herrick R, and a SH 2009. Maternal urinary metabolites of Di-(2-Ethylhexyl) phthalate in relation to the timing of labor in a US multicenter pregnancy cohort study. Am J Epidemiol 169 1015–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aker AM, Ferguson KK, Rosario ZY, Mukherjee B, Alshawabkeh AN, Calafat AM, Cordero JF, and Meeker JD 2019. A repeated measures study of phenol, paraben and Triclocarban urinary biomarkers and circulating maternal hormones during gestation in the Puerto Rico PROTECT cohort. Environmental Health 18 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ananth CV 2014. Ischemic placental disease: a unifying concept for preeclampsia, intrauterine growth restriction, and placental abruption. Semin Perinatol 38 131–132. [DOI] [PubMed] [Google Scholar]
- Baker BH, Wu H, Laue HE, Boivin A, Gillet V, Langlois MF, Bellenger JP, Baccarelli AA, and Takser L 2020. Methylparaben in meconium and risk of maternal thyroid dysfunction, adverse birth outcomes, and Attention-Deficit Hyperactivity Disorder (ADHD). Environ Int 139 105716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellavia A, Chiu YH, Brown FM, Mínguez-Alarcón L, Ford JB, Keller M, Petrozza J, Williams PL, Ye X, Calafat AM, et al. 2019. Urinary concentrations of parabens mixture and pregnancy glucose levels among women from a fertility clinic. Environmental Research 168 389–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellavia A, Hauser R, Seely EW, Meeker JD, Ferguson KK, McElrath TF, and James-Todd T 2017. Urinary phthalate metabolite concentrations and maternal weight during early pregnancy. International Journal of Hygiene and Environmental Health 220 1347–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boss J, Zhai J, Aung MT, Ferguson KK, Johns LE, McElrath TF, Meeker JD, and Mukherjee B 2018. Associations between mixtures of urinary phthalate metabolites with gestational age at delivery: a time to event analysis using summative phthalate risk scores. Environmental Health 17 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyles AL, Beverly BE, Fenton SE, Jackson CL, Jukic AMZ, Sutherland VL, Baird DD, Collman GW, Dixon D, Ferguson KK, et al. 2020. Environmental Factors Involved in Maternal Morbidity and Mortality. Journal of Women’s Health. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM, C Gennings, Hauser R, and Webster TF 2016. What Can Epidemiological Studies Tell Us about the Impact of Chemical Mixtures on Human Health? Environmental Health Perspectives 124 A6–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM, and Gray K 2017. Challenges to studying the health effects of early life environmental chemical exposures on children’s health. PLOS Biology 15 e2002800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM, Just AC, Williams PL, Smith KW, Calafat AM, and Hauser R 2014. Personal care product use and urinary phthalate metabolite and paraben concentrations during pregnancy among women from a fertility clinic. Journal of Exposure Science and Environmental Epidemiology 24 459–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley JP, Hamra GB, and Braun JM 2019. Statistical Approaches for Investigating Periods of Susceptibility in Children’s Environmental Health Research. Current Environmental Health Reports 6 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calafat AM, Koch HM, Swan SH, Hauser R, Goldman LR, Lanphear BP, Longnecker MP, Rudel RA, Teitelbaum SL, Whyatt RM, et al. 2013. Misuse of blood serum to assess exposure to bisphenol A and phthalates. Breast Cancer Resesarch 15 403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calafat AM, Longnecker MP, Koch HM, Swan SH, Hauser R, Goldman LR, Lanphear BP, Rudel RA, Engel SM, Teitelbaum SL, et al. 2015. Optimal Exposure Biomarkers for Nonpersistent Chemicals in Environmental Epidemiology. Environ Health Perspect 123 A166–A168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calafat AM, Ye X, Wong LY, Bishop AM, and Needham LL 2010. Urinary concentrations of four parabens in the U.S. population: NHANES 2005–2006. Environ Health Perspect 118 679–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantonwine DE, Meeker JD, Ferguson KK, Mukherjee B, Hauser R, and McElrath TF 2016. Urinary Concentrations of Bisphenol A and Phthalate Metabolites Measured during Pregnancy and Risk of Preeclampsia. Environmental Health Perspectives 124 1651–1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappola AR, Desai AS, Medici M, Cooper LS, Egan D, Sopko G, Fishman GI, Goldman S, Cooper DS, Mora S, et al. 2019. Thyroid and Cardiovascular Disease: Research Agenda for Enhancing Knowledge, Prevention, and Treatment. Thyroid 29 760–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin HB, Jukic AM, Wilcox AJ, Weinberg CR, Ferguson KK, Calafat AM, McConnaughey DR, and Baird DD 2019. Association of urinary concentrations of early pregnancy phthalate metabolites and bisphenol A with length of gestation. Environmental Health 18 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn BA, Cirillo PM, Hopper BR, and Siiteri PK 2017. Third Trimester Estrogens and Maternal Breast Cancer: Prospective Evidence. Journal of Clinical Endocrinololgy and Metabolism 102 3739–3748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham MW Jr., and LaMarca B 2018. Risk of cardiovascular disease, end-stage renal disease, and stroke in postpartum women and their fetuses after a hypertensive pregnancy. American Journal of Physiology- Regulatory, Integrative and Comparative Physioloogy 315 R521–r528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dassanayake M, Langen E, and Davis MB 2020. Pregnancy Complications as a Window to Future Cardiovascular Disease. Cardiology in Review 28 14–19. [DOI] [PubMed] [Google Scholar]
- Delitala AP, Scuteri A, and Doria C 2020. Thyroid Hormone Diseases and Osteoporosis. Journal of Clinical Medicine 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derakhshan A, Shu H, Peeters RP, Kortenkamp A, Lindh CH, Demeneix B, Bornehag CG, and Korevaar TIM 2019. Association of urinary bisphenols and triclosan with thyroid function during early pregnancy. Environment International 133 105123. [DOI] [PubMed] [Google Scholar]
- Ding G, Wang C, Vinturache A, Zhao S, Pan R, Han W, Chen L, Wang W, Yuan T, Gao Y, et al. 2017. Prenatal low-level phenol exposures and birth outcomes in China. Science of The Total Environment 607–608 1400–1407. [DOI] [PubMed] [Google Scholar]
- Durnwald C 2015. Gestational diabetes: Linking epidemiology, excessive gestational weight gain, adverse pregnancy outcomes, and future metabolic syndrome. Seminars in Perinatology 39 254–258. [DOI] [PubMed] [Google Scholar]
- Ferguson KK, and Chin HB 2017. Environmental chemicals and preterm birth: Biological mechanisms and the state of the science. Curr Epidemiol Rep 4 56–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson KK, McElrath TF, Ko YA, Mukherjee B, and Meeker JD 2014. Variability in urinary phthalate metabolite levels across pregnancy and sensitive windows of exposure for the risk of preterm birth. Environment International 70 118–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher BG, Frederiksen H, Andersson AM, Juul A, Thankamony A, Ong KK, Dunger DB, Hughes IA, and Acerini CL 2018. Serum Phthalate and Triclosan Levels Have Opposing Associations With Risk Factors for Gestational Diabetes Mellitus. Frontiers in Endocrinology 9 99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederiksen H, Skakkebaek NE, and Andersson A-M 2007. Metabolism of phthalates in humans. Molecular Nutrition & Food Research 51 899–911. [DOI] [PubMed] [Google Scholar]
- Han X, Li J, Wang Y, Xu S, Li Y, Liu H, Zhou Y, Zhao H, Fang J, Cai Z, et al. 2020. Association between phthalate exposure and blood pressure during pregnancy. Ecotoxicology and Environmental Safety 189 109944. [DOI] [PubMed] [Google Scholar]
- Harley KG, Kogut K, Madrigal DS, Cardenas M, Vera IA, Meza-Alfaro G, She J, Gavin Q, Zahedi R, Bradman A, et al. 2016. Reducing Phthalate, Paraben, and Phenol Exposure from Personal Care Products in Adolescent Girls: Findings from the HERMOSA Intervention Study. Environmental Health Perspectives 124 1600–1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huo W, Xia W, Wu C, Zhu Y, Zhang B, Wan Y, Zhou A, Qian Z, Chen Z, Jiang Y, et al. 2018. Urinary level of triclosan in a population of Chinese pregnant women and its association with birth outcomes. Environ Pollut 233 872–879. [DOI] [PubMed] [Google Scholar]
- Jackson-Browne MS, Papandonatos GD, Chen A, Yolton K, Lanphear BP, and Braun JM 2019. Early-life triclosan exposure and parent-reported behavior problems in 8-year-old children. Environ Int 128 446–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamal A, Rastkari N, Dehghaniathar R, Nodehi RN, Nasseri S, Kashani H, Shamsipour M, and Yunesian M 2020. Prenatal urinary concentrations of environmental phenols and birth outcomes in the mother-infant pairs of Tehran Environment and Neurodevelopmental Disorders (TEND) cohort study. Environmental Research 184 109331. [DOI] [PubMed] [Google Scholar]
- James-Todd TM, Chiu YH, Messerlian C, Minguez-Alarcon L, Ford JB, Keller M, Petrozza J, Williams PL, Ye X, Calafat AM, et al. 2018. Trimester-specific phthalate concentrations and glucose levels among women from a fertility clinic. Environmental Health 17 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James-Todd TM, Karumanchi SA, Hibert EL, Mason SM, Vadnais MA, and Hu FB 2013. Gestational Age, Infant Birth Weight, and Subsequent Risk of Type 2 Diabetes in Mothers: Nurses’ Health Study II. Preventing Chronic Disease 10 E156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James-Todd TM, Meeker JD, Huang T, Hauser R, Ferguson KK, Rich-Edwards JW, McElrath TF, and Seely EW 2016. Pregnancy urinary phthalate metabolite concentrations and gestational diabetes risk factors. Environment International 96 118–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeddi MZ, Rastkari N, Ahmadkhaniha R, and Yunesian M 2016. Endocrine disruptor phthalates in bottled water: daily exposure and health risk assessment in pregnant and lactating women. Environmental Monitoring and Assessment 188 534. [DOI] [PubMed] [Google Scholar]
- Johns LE, Ferguson KK, McElrath TF, Mukherjee B, and Meeker JD 2016. Associations between Repeated Measures of Maternal Urinary Phthalate Metabolites and Thyroid Hormone Parameters during Pregnancy. Environmental Health Perspectives 124 1808–1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns LE, Ferguson KK, Soldin OP, Cantonwine DE, Rivera-González LO, Del Toro LV, Calafat AM, Ye X, Alshawabkeh AN, Cordero JF, et al. 2015. Urinary phthalate metabolites in relation to maternal serum thyroid and sex hormone levels during pregnancy: a longitudinal analysis. Reproductive Biology and Endocrinology 13 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karwacka A, Zamkowska D, Radwan M, and Jurewicz J 2017. Exposure to modern, widespread environmental endocrine disrupting chemicals and their effect on the reproductive potential of women: an overview of current epidemiological evidence. Human Fertility 1–24. [DOI] [PubMed] [Google Scholar]
- Kennedy RL, Malabu UH, Jarrod G, Nigam P, Kannan K, and Rane A 2010. Thyroid function and pregnancy: before, during and beyond. Journal of Obstetrics and Gynaecology 30 774–783. [DOI] [PubMed] [Google Scholar]
- Khoshhali M, Amin MM, Fatehizadeh A, Ebrahimi A, Taheri E, and Kelishadi R 2020. Impact of prenatal triclosan exposure on gestational age and anthropometric measures at birth: A systematic review and meta-analysis. Journal of Research in Medical Sciences 25 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch HM, and Calafat AM 2009. Human body burdens of chemicals used in plastic manufacture. Philosophical Transactions of the Royal Society B- Biological Sciences 364 2063–2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kominiarek MA, and Peaceman AM 2017. Gestational weight gain. Am J Obstet Gynecol 217 642–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassen TH, Frederiksen H, Jensen TK, Petersen JH, Main KM, Skakkebæk NE, Jørgensen N, Kranich SK, and Andersson A-M 2013. Temporal variability in urinary excretion of bisphenol A and seven other phenols in spot, morning, and 24-h urine samples. Environmental Research 126 164–170. [DOI] [PubMed] [Google Scholar]
- Lee G, Kim S, Park H, Lee J, Lee JP, Kho Y, Choi G, Park J, Worakhunpiset S, Moon HB, et al. 2020. Variability of urinary creatinine, specific gravity, and osmolality over the course of pregnancy: Implications in exposure assessment among pregnant women. Environ Res 110473. [DOI] [PubMed] [Google Scholar]
- Lello S, Capozzi A, and Scambia G 2015. Osteoporosis and cardiovascular disease: an update. Gynecological Endocrinology 31 590–594. [DOI] [PubMed] [Google Scholar]
- Leslie MS, and Briggs LA 2016. Preeclampsia and the Risk of Future Vascular Disease and Mortality: A Review. Journal of Midwifery & Women’s Health 61 315–324. [DOI] [PubMed] [Google Scholar]
- Li J, Qian X, Zhao H, Zhou Y, Xu S, Li Y, Xiang L, Shi J, Xia W, and Cai Z 2019a. Determinants of exposure levels, metabolism, and health risks of phthalates among pregnant women in Wuhan, China. Ecotoxicology and Environmental Safety 184 109657. [DOI] [PubMed] [Google Scholar]
- Li Y, Xu S, Li Y, Zhang B, Huo W, Zhu Y, Wan Y, Zheng T, Zhou A, Chen Z, et al. 2019b. Association between urinary parabens and gestational diabetes mellitus across prepregnancy body mass index categories. Environmental Research 170 151–159. [DOI] [PubMed] [Google Scholar]
- Liu W, Zhou Y, Li J, Sun X, Liu H, Jiang Y, Peng Y, Zhao H, Xia W, Li Y, et al. 2019. Parabens exposure in early pregnancy and gestational diabetes mellitus. Environment International 126 468–475. [DOI] [PubMed] [Google Scholar]
- MacPherson S, Arbuckle TE, and Fisher M 2018. Adjusting urinary chemical biomarkers for hydration status during pregnancy. J Expo Sci Environ Epidemiol 28 481–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marie C, Vendittelli F, and Sauvant-Rochat MP 2015. Obstetrical outcomes and biomarkers to assess exposure to phthalates: A review. Environment International 83 116–136. [DOI] [PubMed] [Google Scholar]
- Messerlian C, Wylie BJ, Mínguez-Alarcón L, Williams PL, Ford JB, Souter IC, Calafat AM, Hauser R, and Study T Earth 2016. Urinary Concentrations of Phthalate Metabolites and Pregnancy Loss Among Women Conceiving with Medically Assisted Reproduction. Epidemiology (Cambridge, Mass.) 27 879–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moos RK, Angerer J, Dierkes G, Brüning T, and Koch HM 2016. Metabolism and elimination of methyl, iso- and n-butyl paraben in human urine after single oral dosage. Archives of Toxicology 90 2699–2709. [DOI] [PubMed] [Google Scholar]
- Mustieles V, Zhang Y, Yland J, Braun JM, Williams PL, Wylie BJ, Attaman JA, Ford JB, Azevedo A, Calafat AM, et al. 2020. Maternal and paternal preconception exposure to phenols and preterm birth. Environment International 137 105523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien KM, Upson K, Cook NR, and Weinberg CR 2016. Environmental Chemicals in Urine and Blood: Improving Methods for Creatinine and Lipid Adjustment. Environmental Health Perspectives 124 220–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oken E, Taveras EM, Kleinman KP, Rich-Edwards JW, and Gillman MW 2007. Gestational weight gain and child adiposity at age 3 years. Am J Obstet Gynecol 196 322 e321–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang F, Tang N, Zhang HJ, Wang X, Zhao S, Wang W, Zhang J, and Cheng W 2018. Maternal urinary triclosan level, gestational diabetes mellitus and birth weight in Chinese women. Science of The Total Environment 626 451–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacyga DC, Gardiner JC, Flaws JA, Li Z, Calafat AM, Korrick SA, Schantz SL, and Strakovsky RS 2021. Maternal phthalate and phthalate alternative metabolites and urinary biomarkers of estrogens and testosterones across pregnancy. Environ Int 155 106676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul-Friedman K, Martin M, Crofton KM, Hsu CW, Sakamuru S, Zhao J, Xia M, Huang R, Stavreva DA, Soni V, et al. 2019. Limited Chemical Structural Diversity Found to Modulate Thyroid Hormone Receptor in the Tox21 Chemical Library. Environ Health Perspect 127 97009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perng W, Kasper NM, Watkins DJ, Sanchez BN, Meeker JD, Cantoral A, Solano-González M, Tellez-Rojo MM, and Peterson K 2020. Exposure to Endocrine-Disrupting Chemicals During Pregnancy Is Associated with Weight Change Through 1 Year Postpartum Among Women in the Early-Life Exposure in Mexico to Environmental Toxicants Project. Journal of Women’s Health. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pew Research Center. They’re Waiting Longer, but U.S. Women Today More Likely to Have Children than a Decade Ago. 2016. [Google Scholar]
- Philippat C, Nakiwala D, Calafat AM, Botton J, De Agostini M, Heude B, Slama R, and EM-CS Group 2017. Prenatal Exposure to Nonpersistent Endocrine Disruptors and Behavior in Boys at 3 and 5 Years. Environ Health Perspect 125 097014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philips EM, Jaddoe VWV, Deierlein A, Asimakopoulos AG, Kannan K, Steegers EAP, and Trasande L 2020a. Exposures to phthalates and bisphenols in pregnancy and postpartum weight gain in a population-based longitudinal birth cohort. Environment International 144 106002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philips EM, Santos S, Steegers EAP, Asimakopoulos AG, Kannan K, Trasande L, and Jaddoe VWV 2020b. Maternal bisphenol and phthalate urine concentrations and weight gain during pregnancy. Environment International 135 105342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich-Edwards JW, Fraser A, Lawlor DA, and Catov JM 2013. Pregnancy Characteristics and Women’s Future Cardiovascular Health: An Underused Opportunity to Improve Women’s Health? Epidemiologic Reviews 36 57–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Carmona Y, Cantoral A, Trejo-Valdivia B, Téllez-Rojo MM, Svensson K, Peterson KE, Meeker JD, Schnaas L, Solano M, and Watkins DJ 2019. Phthalate exposure during pregnancy and long-term weight gain in women. Environmental Research 169 26–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano ME, Eliot MN, Zoeller RT, Hoofnagle AN, Calafat AM, Karagas MR, Yolton K, Chen A, Lanphear BP, and Braun JM 2018. Maternal urinary phthalate metabolites during pregnancy and thyroid hormone concentrations in maternal and cord sera: The HOME Study. International Journal of Hygiene and Environmental Health 221 623–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandborgh-Englund G, Adolfsson-Erici M, Odham G, and Ekstrand J 2006. Pharmacokinetics of Triclosan Following Oral Ingestion in Humans. Journal of Toxicology and Environmental Health, Part A 69 1861–1873. [DOI] [PubMed] [Google Scholar]
- Sathyanarayana S, Butts S, Wang C, Barrett E, Nguyen R, Schwartz SM, Haaland W, Swan SH, and T Team 2017. Early Prenatal Phthalate Exposure, Sex Steroid Hormones, and Birth Outcomes. Journal of Clinical Endocrinology and Metabolism 102 1870–1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaffer RM, Ferguson KK, Sheppard L, James-Todd T, Butts S, Chandrasekaran S, Swan SH, Barrett ES, Nguyen R, Bush N, et al. 2019. Maternal urinary phthalate metabolites in relation to gestational diabetes and glucose intolerance during pregnancy. Environment International 123 588–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro GD, Arbuckle TE, Ashley-Martin J, Fraser WD, Fisher M, Bouchard MF, Monnier P, Morisset AS, Ettinger AS, and Dodds L 2018. Associations between maternal triclosan concentrations in early pregnancy and gestational diabetes mellitus, impaired glucose tolerance, gestational weight gain and fetal markers of metabolic function. Environmental Research 161 554–561. [DOI] [PubMed] [Google Scholar]
- Shapiro GD, Dodds L, Arbuckle TE, Ashley-Martin J, Fraser W, Fisher M, Taback S, Keely E, Bouchard MF, Monnier P, et al. 2015. Exposure to phthalates, bisphenol A and metals in pregnancy and the association with impaired glucose tolerance and gestational diabetes mellitus: The MIREC study. Environment International 83 63–71. [DOI] [PubMed] [Google Scholar]
- Shoaff JR, Romano ME, Yolton K, Lanphear BP, Calafat AM, and Braun JM 2016. Prenatal phthalate exposure and infant size at birth and gestational duration. Environmental Research 150 52–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strakovsky RS, and Schantz SL 2018. Using Experimental Models to Assess Effects of Bisphenol A (BPA) and Phthalates on the Placenta: Challenges and Perspectives. Toxicol Sci 166 250–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun M, Fan Y, Hou Y, and Fan Y 2018. Preeclampsia and maternal risk of breast cancer: a meta-analysis of cohort studies. The Journal of Maternal-Fetal & Neonatal Medicine 31 2484–2491. [DOI] [PubMed] [Google Scholar]
- Tanz LJ, Stuart JJ, Williams PL, Missmer SA, Rimm EB, James-Todd TM, and Rich-Edwards JW 2019. Preterm Delivery and Maternal Cardiovascular Disease Risk Factors: The Nurses’ Health Study II. Journal of Women’s Health 28 677–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry MB, Michels KB, Brody JG, Byrne C, Chen S, DJ Jerry, Malecki KMC, Martin MB, Miller RL, Neuhausen SL, et al. 2019. Environmental exposures during windows of susceptibility for breast cancer: a framework for prevention research. Breast Cancer Research 21 96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toft G, Jönsson BAG, Lindh CH, Jensen TK, Hjollund NH, Vested A, and Bonde JP 2012. Association between Pregnancy Loss and Urinary Phthalate Levels around the Time of Conception. Environmental Health Perspectives 120 458–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troisi R, Bjørge T, Gissler M, Grotmol T, Kitahara CM, Myrtveit Sæther SM, Ording AG, Sköld C, Sørensen HT, Trabert B, et al. 2018. The role of pregnancy, perinatal factors and hormones in maternal cancer risk: a review of the evidence. Journal of Internal Medicine 283 430–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg LN, Hauser R, Marcus M, Olea N, and Welshons WV 2007. Human exposure to bisphenol A (BPA). Reproductive Toxicology 24 139–177. [DOI] [PubMed] [Google Scholar]
- Vernet C, Philippat C, Agier L, Calafat AM, Ye X, Lyon-Caen S, Hainaut P, Siroux V, Schisterman EF, and Slama R 2019. An Empirical Validation of the Within-subject Biospecimens Pooling Approach to Minimize Exposure Misclassification in Biomarker-based Studies. Epidemiology 30 756–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernet C, Philippat C, Calafat AM, Ye X, Lyon-Caen S, Siroux V, Schisterman EF, and Slama R 2018. Within-Day, Between-Day, and Between-Week Variability of Urinary Concentrations of Phenol Biomarkers in Pregnant Women. Environmental Health Perspectives 126 037005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker E, Flannery O, and Mackillop L 2020. Gestational diabetes and progression to type two diabetes mellitus: missed opportunities of follow up and prevention? Primary Care Diabetes 14 698–702. [DOI] [PubMed] [Google Scholar]
- Wang Y, Yan P, Fu T, Yuan J, Yang G, Liu Y, and Zhang ZJ 2020. The association between gestational diabetes mellitus and cancer in women: A systematic review and meta-analysis of observational studies. Diabetes & Metabolism 46 461–471. [DOI] [PubMed] [Google Scholar]
- Wen Q, Zhou Y, Wang Y, Li J, Zhao H, Liao J, Liu H, Li Y, Cai Z, and Xia W 2020. Association between urinary paraben concentrations and gestational weight gain during pregnancy. Journal of Exposure Science and Environmental Epidemiology 30 845–855. [DOI] [PubMed] [Google Scholar]
- Werner EF, Braun JM, Yolton K, Khoury JC, and Lanphear BP 2015. The association between maternal urinary phthalate concentrations and blood pressure in pregnancy: The HOME Study. Environmental Health 14 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Wang H, Du H, Xu L, Liu S, Yi J, Chen Y, Jiang Q, and He G 2020. Serum Bisphenol A, glucose homeostasis, and gestational diabetes mellitus in Chinese pregnant women: a prospective study. Environmental Science and Pollution Research. [DOI] [PubMed] [Google Scholar]
- Ye Y, Zhou Q, Feng L, Wu J, Xiong Y, and Li X 2017. Maternal serum bisphenol A levels and risk of pre-eclampsia: a nested case–control study. European Journal of Public Health 27 1102–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Xia W, Liu W, Li X, Hu J, Zhang B, Xu S, Zhou Y, Li J, Cai Z, et al. 2019. Exposure to Bisphenol a Substitutes and Gestational Diabetes Mellitus: A Prospective Cohort Study in China. Frontiers in Endocrinology 10 262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoeller RT 2007. Environmental chemicals impacting the thyroid: targets and consequences. Thyroid 17 811–817. [DOI] [PubMed] [Google Scholar]